Abstract
Context
Prepubertal obesity is a well-established predictor of earlier pubertal onset, which is itself a risk factor for poor health and well-being. Identifying specific patterns of weight gain in early life may help explain differential risk for earlier pubertal onset.
Objective
The objective of the study was to examine patterns of weight gain across infancy and early childhood in relation to pubertal onset outcomes.
Design, Setting, and Participants
Participants were 426 girls in the National Institute of Child Health and Human Development Study of Early Child Care and Youth Development, a longitudinal birth cohort of children and their families followed between birth and adolescence.
Main Outcome Measures
Three pubertal onset outcomes were examined, including age at menarche and ages at Tanner stage II for dimensions of breast and pubic hair development.
Results
In infancy (birth to 15 months), greater percent weight gain and higher birthweight predicted earlier pubertal onset for all outcomes (Ps < 0.05). In early childhood (24 months to grade 1), body mass index (BMI) trajectories reflecting BMI values that were persistently high or changed from low to high over time (vs BMI values that were stable at median or low levels), predicted younger ages at menarche and the onset of breast (Ps < 0.05), but not pubic hair (Ps > 0.05), development. All associations were independent of breastfeeding, maternal menarcheal age, and race/ethnicity.
Conclusions
Distinct patterns of early life weight gain predict differential risk for earlier onset puberty. Focusing on these patterns for earlier and more targeted intervention may help lessen life course linkages between prepubertal obesity, accelerated pubertal development, and negative postpubertal outcomes.
Keywords: infancy weight gain, body mass index, obesity, puberty, pubertal onset, age at menarche
Childhood obesity in girls is a well-established predictor of earlier pubertal onset [1, 2], which is itself a risk factor for type 2 diabetes, cardiovascular disease, breast cancer, and early mortality [3-7] and for a variety of poor socioemotional (eg, depression) [8] and behavioral (eg, lower academic achievement) [9] outcomes. However, research has been more limited regarding early life patterns of weight gain that may have differential effects on pubertal development. If such patterns are identified, earlier and more targeted intervention may be possible to help break life course linkages between prepubertal obesity, accelerated pubertal development, and negative postpubertal outcomes.
Two relevant constructs pertain to rates of weight gain in infancy and trajectories of body mass index (BMI) changes in early childhood. In prior studies, “high-risk” patterns have been identified describing rapid or excess weight gain in the first months of life as well as specific patterns of BMI changes that are persistently high or shift from low to high over time [10-14]. These patterns, in turn, have been linked to subsequent risk for obesity and cardiometabolic diseases in adulthood [15-18]. With respect to pubertal development, greater weight gain, especially in infancy, has also been shown to predict earlier pubertal onset [19-23]. However, no studies, to our knowledge, have attempted to link distinct trajectories of BMI changes over the prepubertal period [10] to differential risk for earlier pubertal onset. The current study extends this work by considering both weight gain in infancy and trajectories of BMI changes in early childhood. This integrated approach may provide new insights into the developmental pathways through which such exposures shape long-term health and well-being.
The current study included 426 girls from the landmark National Institute of Child Health and Human Development (NICHD) Study of Early Child Care and Youth Development (SECCYD), a longitudinal investigation of health and development in children. The current study sought to extend prior work in this same sample [10] by examining early life patterns of weight gain in relation to pubertal onset outcomes: age at menarche and ages at Tanner stage (TS) II for dimensions of breast and pubic hair development, marking the initiation of gonadarche and adrenarche, respectively. The study objectives were 2-fold: (1) to examine percent weight gain between birth and age 15 months, adjusted for gestational age and birthweight, in relation to pubertal onset outcomes; and (2) to characterize trajectories of BMI changes between age 24 months and grade 1 in relation to pubertal onset outcomes. High-risk patterns of weight gain, reflecting greater weight gain in infancy and persistently high BMI values or changes in BMI values from low to high over early childhood (vs stable BMI values at median and low levels), were hypothesized to predict earlier pubertal onset, according to all 3 pubertal timing indicators.
Methods
Participants
Subjects in the current study were participants in the NICHD SECCYD, a prospective study of children and their families followed between birth and adolescence [24]. Families were recruited from 10 study sites in the United States: Charlottesville, VA; Irvine, CA; Lawrence, KS; Little Rock, AR; Madison, WI; Morganton, NC; Philadelphia, PA; Pittsburgh, PA; Seattle, WA; and Wellesley, MA. In the first 11 months of 1991, all mother-infant dyads of babies born within preselected 24-hour intervals at participating hospitals were screened. Exclusion criteria were mother <18 years old, non-English speaking, or had a substance use disorder; serious medical problems (mother or infant); lived >1 hour from the study site; child being placed for adoption; concurrent participation in another study; and refusal to participate in initial screening. Additional sampling requirements were imposed (eg, 10% recruitment of single-parent households) to ensure that the sociodemographic composition of the final sample (N = 1364 families; n = 659 girls [48.3%] and n = 705 boys [51.7%]) was similar to the population in the same geographic regions, according to the 1990 US Census. Retained for analysis in the current study were 426 girls (64.6% of the 659 girls originally recruited) who participated in at least 1 assessment of pubertal development between ages 9 and 15.5 years and who agreed to be followed for future assessments. In logistic models regressing the retention indicator onto race/ethnicity and socioeconomic status indicators (maternal and paternal/partner education, family income-to-needs ratio), only maternal education had a significant, independent effect (odds ratio = 1.10; 95% CI, 1.03-1.17; P < 0.01), reflecting higher odds of participation in the pubertal development assessment among girls who had more educated mothers. Informed consent and assent were obtained from the parents and children, respectively. The study was approved by the institutional review boards of each university-based study site, including the Human Subjects Division of the University of Washington.
Measures
Pubertal timing outcomes
A physical examination was performed by trained health care providers each year between child ages 9.5 and 15.5 years [25] to determine stage of sexual maturity using TS criteria [26, 27], augmented by breast bud palpation. As part of this system, photographs depicting typical development were used to compare and rate stage of sexual maturity for breast and pubic hair development, ranging between stage I (prepuberty) and stage V (full sexual maturity) [28]. Girls who were between stages were assigned to the earlier stage [25]. Annual evaluations continued until menarche and full sexual maturity for breast and pubic hair development were reached. Age at menarche was determined by querying the girls and their mothers. Mothers’ reports were used if girls’ reports were missing. Derived from these assessments, 3 indicators of pubertal onset were examined as the primary outcomes of interest: (1) age at menarche; (2) age at onset of breast development (ie, age at TS II); and (3) age at onset of pubic hair development (ie, age at TS II).
Early life weight-related predictors
Percent weight gain in infancy
Infancy weight gain was calculated as the percentage weight gain between birth and 15 months (15-month weight minus birthweight divided by birthweight), using weight (in kilograms) at birth and 15 months. Gestational age (in weeks) was included in all analyses of infancy weight gain.
BMI trajectory in early childhood
BMI values (weight in kilograms/height in meters squared) were calculated from measurements of height and weight at child ages 24, 36, and 54 months, and in grade 1. BMI percentile (BMIP) values were then derived based on the 2000 Centers for Disease Control and Prevention BMI-for-age clinical growth charts for girls. BMIP values were subsequently input into cluster analysis to identify clusters of BMIP trajectories over this period.
All available weight-related assessments in periods of infancy and early childhood were used in the current study. The selection of timepoints was constrained by the design of the NICHD SECCYD.
Covariates
Covariates included breastfeeding history, maternal menarcheal age, and child race/ethnicity. Breastfeeding history was defined as the total number of months of breastfeeding derived from mothers’ reports at child ages 1, 6, 15, 24, and 36 months. Mothers were queried regarding continued breastfeeding and the age of the child when/if breastfeeding was stopped. Maternal menarcheal age (in years) was assessed by self-report of mothers queried at 3 different assessments, from which the mean was computed. Child race/ethnicity was coded according to mother reports in 5 categories: Asian, African American, Latina, “other” race, and White.
Analytical plan
Six accelerated failure time models with Weibull distributions were fit to examine 2 weight-related predictors in relation to each of 3 pubertal onset outcomes. All models were adjusted for covariates. In infancy (models 1-3), weight-related predictors included percent weight gain between birth and age 15 months along with gestational age (in weeks) and birthweight (in kilograms). In early childhood (models 4-6), weight-related predictors included BMIP trajectories over child ages 24, 36, and 54 months and grade 1. All models accommodated left and interval censoring for pubertal onset outcomes derived from TS. Values were left censored for 46.3% and 30.3% of the sample for age at onset of breast and pubic hair development, respectively, and all other values on these outcomes were interval censored. Age at menarche was modeled as uncensored. Factor-change (FC) coefficients were calculated to represent multiplicative effects on pubertal onset outcomes per 1-unit increase in the corresponding predictor. Across all participants and variables included in the analyses, 10.9% of data values were missing. Missing values were multiply imputed to create 30 completed data sets. Parameter and standard errors were estimated by combining results across imputed data sets [29].
Results
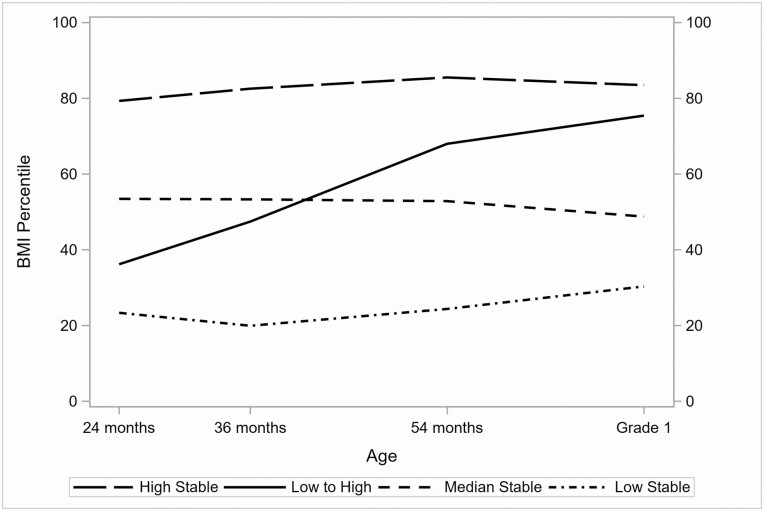
Results of descriptive analyses are reported in Table 1 with respect to the covariates, weight-related prepubertal variables, and pubertal onset outcomes of interest. Results of K-means cluster analysis of BMIP values at 24, 36, and 54 months and grade 1 are reported in Table 2 and Fig. 1. Of note, 4 clusters were identified showing distinct profiles in which trajectories of BMIP (1) were persistently high (“high stable:” 36.2% of the sample; BMIP means 79.3-85.5); (2) changed from low to high (“low to high:” 17.8% of the sample; BMIP means 36.2-75.4); (3) were persistently at near median levels (“median stable:” 23.5% of the sample; BMIP means 48.8-53.5); or (4) were persistently low (“low stable:” 22.5% of the sample; BMIP means 20.0-30.3). These clusters generally aligned with results of prior analyses in the larger SECCYD sample [10].
Table 1.
Sample descriptive statistics
| Covariates | Mean (SE) | Range | n (%) |
|---|---|---|---|
| Breastfeeding, mo | 5.07 (0.30) | 0.0-36.0 | - |
| Maternal menarcheal age, y | 12.75 (0.07) | 9.0-18.0 | - |
| Child race/ethnicity | |||
| Asian (%) | - | - | 5 (1.2) |
| African American (%) | - | - | 47 (11.0) |
| Latina (%) | - | - | 21 (4.9) |
| Other (%) | - | - | 16 (3.8) |
| White (%) | - | - | 337 (79.1) |
| Prepubertal variablesa | |||
| Gestational age, wk | 39.23 (0.07) | 34.0-42.0 | - |
| Preterm, <37 weeks (%) | - | - | 15 (3.5) |
| Birthweight, kg | 3.42 (0.02) | 2.0-5.3 | - |
| Weight, 15 mo, kg | 10.45 (0.06) | 8.0-14.4 | - |
| Infancy weight gain, % | 210.53 (2.32) | 114.5-379.8 | - |
| 24 mo | |||
| BMIP | 52.95 (1.33) | 0.4-99.6 | - |
| Overweight (%) | - | - | 40 (9.4) |
| Obese (%) | - | - | 19 (4.5) |
| 36 mo | |||
| BMIP | 55.32 (1.34) | 1.9-99.7 | - |
| Overweight (%) | - | - | 56 (13.1) |
| Obese (%) | - | - | 20 (4.7) |
| 54 mo | |||
| BMIP | 60.95 (1.31) | 0.2-99.6 | - |
| Overweight (%) | - | - | 60 (14.1) |
| Obese (%) | - | - | 39 (9.2) |
| Grade 1, mean age 6.9 (0.29) | |||
| BMIP | 61.92 (1.26) | 3.8-99.6 | - |
| Overweight, % | - | - | 54 (12.7) |
| Obese, % | - | - | 37 (8.7) |
| Pubertal onset variablesb | Median (SE) | 95% CI | |
| Menarcheal age, y | 12.67 (0.01) | 12.434-12.909 | - |
| Breast onset, TS II | 9.97 (0.02) | 9.622-10.340 | - |
| Pubic hair onset, TS II | 10.57 (0.01) | 10.301-10.850 | - |
Abbreviations: BMIP, body mass index percentile; TS, Tanner stage.
a Infancy weight gain was calculated as the difference in weight in kilograms (15 mo minus birthweight) divided by birthweight. Categories of overweight and obese were defined by BMIP values between 85 and 94.9 and 95+, respectively.
b Median ages for the pubertal onset variables were estimated from accelerated failure time models adjusted for covariates (breastfeeding, maternal menarcheal age, and child race/ethnicity).
Table 2.
Cluster analysis of BMIP trajectories across ages 24, 36, and 54 months, and grade 1
| BMIP trajectory | Variable | Mean (SE) | Min | Max |
|---|---|---|---|---|
| Cluster 1: high stable (n = 154, 36.2%) | BMIP, 24 mo | 79.32 (1.17) | 48.89 | 99.59 |
| BMIP, 36 mo | 82.55 (1.00) | 46.26 | 99.67 | |
| BMIP, 54 mo | 85.51 (0.92) | 50.83 | 99.60 | |
| BMIP, grade 1 | 83.48 (1.01) | 54.55 | 99.57 | |
| Cluster 2: low to high (n = 76, 17.8%) | BMIP, 24 mo | 36.19 (1.85) | 5.68 | 71.57 |
| BMIP, 36 mo | 47.46 (1.87) | 10.92 | 87.69 | |
| BMIP, 54 mo | 68.00 (1.66) | 39.20 | 97.35 | |
| BMIP, grade 1 | 75.44 (1.58) | 52.43 | 99.48 | |
| Cluster 3: median stable (n = 100, 23.5%) | BMIP, 24 mo | 53.46 (1.78) | 18.92 | 91.09 |
| BMIP, 36 mo | 53.33 (1.65) | 11.29 | 85.57 | |
| BMIP, 54 mo | 52.85 (1.56) | 21.53 | 78.02 | |
| BMIP, grade 1 | 48.77 (1.68) | 10.99 | 72.72 | |
| Cluster 4: low stable (n = 96, 22.5%) | BMIP, 24 mo | 23.37 (1.69) | 0.43 | 60.75 |
| BMIP, 36 mo | 19.95 (1.30) | 1.88 | 54.12 | |
| BMIP, 54 mo | 24.39 (1.50) | 0.23 | 55.61 | |
| BMIP, grade 1 | 30.34 (1.67) | 3.78 | 68.16 |
Abbreviations: BMIP, body mass index percentile; max, maximum; min, minimum.
Figure 1.
Means of 4 clusters of BMIP trajectories across ages 24, 36, and 54 months and grade 1.
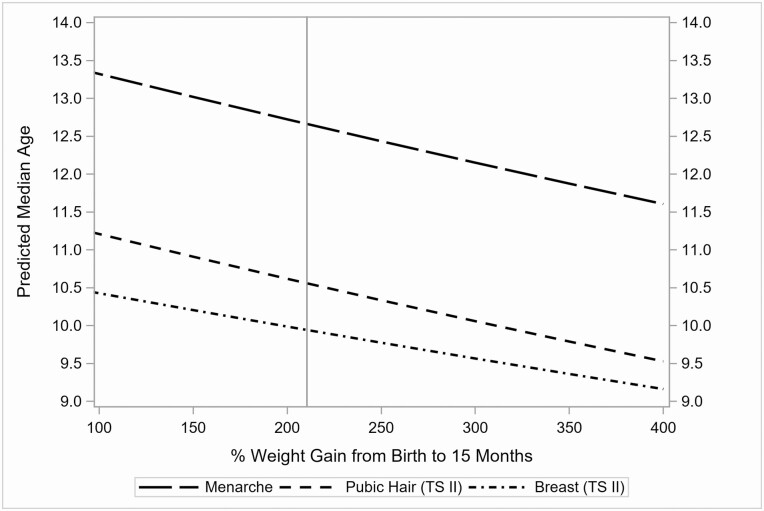
Results of covariate-adjusted survival models are reported in Table 3, estimating effects of predictors in the period of infancy on each pubertal onset outcome (models 1-3). With respect to the weight-related variables in infancy, both greater percent weight gain (between birth and age 15 months) and higher birthweight significantly, and independently, predicted younger ages at all 3 pubertal onset outcomes. Each 100% increase in weight gain (2× birthweight) corresponded to a 4.5% decrease in age at menarche (FC = 0.955; 95% CI, 0.930-0.981; P < 0.001), a 4.2% decrease in age at onset of breast development (FC = 0.958; 95% CI, 0.918-0.999; P < 0.05), and a 5.3% decrease in age at onset of pubic hair development (FC = 0.947; 95% CI, 0.913-0.982; P < 0.01). Figure 2 depicts the model-predicted median ages for each pubertal onset outcome as a function of percent weight gain between birth and age 15 months. In addition, each 1-kilogram increase in birthweight corresponded to a 3.0% decrease in age at menarche (FC = 0.970; 95% CI, 0.947-0.993; P < 0.05), a 5.4% decrease in age at onset of breast development (FC = 0.946; 95% CI, 0.909-0.985; P < 0.01), and 4.4% decrease in age at onset of pubic hair development (FC = 0.956; 95% CI, 0.925-0.987; P < 0.01). Regarding the covariates, maternal menarcheal age and child race/ethnicity also significantly, and independently, predicted all 3 pubertal onset outcomes.
Table 3.
FC coefficients describing effects of weight gain in infancy and BMIP trajectories in early childhood on pubertal onset outcomes, adjusted for covariates
| Age at menarche | Age at breast onset (TS II) | Age at pubic hair onset (TS II) | ||||
|---|---|---|---|---|---|---|
| Infancy | Model 1 | Model 2 | Model 3 | |||
| Predictors | FC | 95% CI | FC | 95% CI | FC | 95% CI |
| Infancy weight gain, %a | 0.955*** | 0.930-0.981 | 0.958* | 0.918-0.999 | 0.947** | 0.913-0.982 |
| Gestational age (wk) | 0.999 | 0.993-1.005 | 0.999 | 0.988-1.010 | 1.005 | 0.997-1.014 |
| Birthweight, kg | 0.970* | 0.947-0.993 | 0.946** | 0.909-0.985 | 0.956** | 0.925-0.987 |
| Covariates | ||||||
| Breastfeeding, mo | 0.999 | 0.998-1.001 | 0.999 | 0.996-1.001 | 0.998 | 0.997-1.000 |
| Maternal menarcheal age, y | 1.018**** | 1.013-1.023 | 1.015** | 1.006-1.024 | 1.013*** | 1.006-1.020 |
| Asian (vs White) | 0.963 | 0.897-1.034 | 0.945 | 0.827-1.081 | 1.000 | 0.908-1.102 |
| African American (vs White) | 0.958** | 0.934-0.984 | 0.926*** | 0.885-0.968 | 0.905**** | 0.872-0.940 |
| Latina (vs White) | 0.972 | 0.936-1.010 | 0.987 | 0.928-1.050 | 0.994 | 0.945-1.045 |
| Other (vs White) | 0.970 | 0.931-1.011 | 0.977 | 0.903-1.056 | 1.013 | 0.959-1.070 |
| Early childhood | Model 4 | Model 5 | Model 6 | |||
| Predictors | FC | 95% CI | FC | 95% CI | FC | 95% CI |
| BMI: high stable (vs low to high)b | 1.004 | 0.981-1.027 | 1.000 | 0.962-1.039 | 1.001 | 0.968-1.034 |
| BMI: high stable (vs median stable) | 0.975* | 0.954-0.995 | 0.944** | 0.912-0.978 | 0.979 | 0.951-1.008 |
| BMI: high stable (vs low stable) | 0.977* | 0.957-0.998 | 0.955* | 0.921-0.990 | 0.976 | 0.948-1.006 |
| BMI: low to high (vs median stable) | 0.971* | 0.947-0.996 | 0.944** | 0.906-0.984 | 0.978 | 0.943-1.015 |
| BMI: low to high (vs low stable) | 0.974* | 0.950-0.999 | 0.955* | 0.916-0.996 | 0.976 | 0.941-1.012 |
| BMI: median stable (vs low stable) | 1.003 | 0.980-1.026 | 1.012 | 0.973-1.052 | 0.997 | 0.965-1.031 |
| Covariates | ||||||
| Breastfeeding, mo | 1.000 | 0.998-1.001 | 0.999 | 0.997-1.002 | 0.999 | 0.997-1.001 |
| Maternal menarcheal age, y | 1.018**** | 1.013-1.023 | 1.014** | 1.005-1.023 | 1.013*** | 1.005-1.020 |
| Asian (vs White) | 0.959 | 0.893-1.030 | 0.962 | 0.847-1.092 | 0.999 | 0.907-1.101 |
| African American (vs White) | 0.957*** | 0.932-0.982 | 0.929** | 0.889-0.971 | 0.909**** | 0.876-0.944 |
| Latina (vs White) | 0.972 | 0.936-1.008 | 0.984 | 0.926-1.045 | 0.998 | 0.949-1.050 |
| Other (vs White) | 0.972 | 0.932-1.013 | 0.986 | 0.911-1.067 | 1.015 | 0.960-1.074 |
Abbreviations: BMI, body mass index; FC, factor change; TS, Tanner stage.
*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
a Percent weight gain between birth and age 15 months.
b BMI percentile trajectory across ages 24, 35, and 54 months, and grade 1.
Figure 2.
Model-predicted median ages for each pubertal onset outcome by percent weight gain between birth and age 15 months. The vertical line represents the mean percent weight gain of 210.5%.
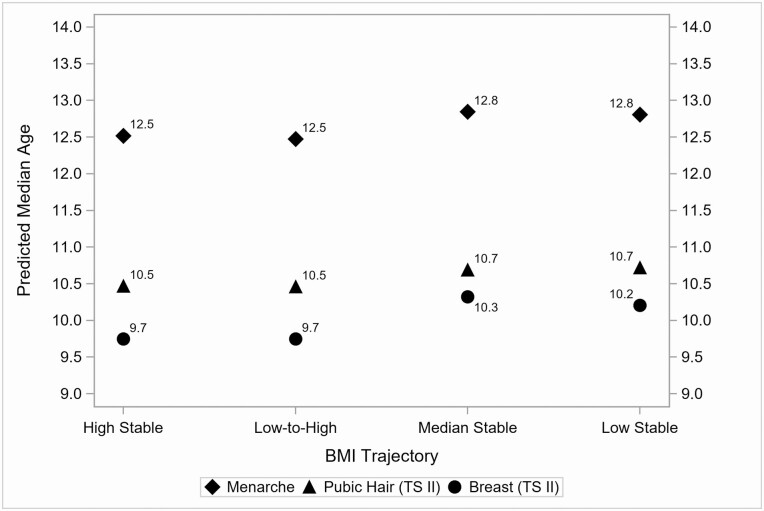
In parallel, results of covariate-adjusted survival models are reported in Table 3, estimating effects of predictors in the period of early childhood on each pubertal timing outcome (models 4-6). With respect to the 4 clusters representing trajectories of BMIP between child ages 24 months and grade 1, each of the hypothesized high-risk clusters (high stable and low to high), when compared with each of the low-risk clusters (median stable and low stable), significantly, and independently, predicted younger median ages at menarche and age at onset of breast development, but not pubic hair development. That is, being in the high stable vs median stable cluster corresponded to a 2.5% decrease in age at menarche (FC = 0.975; 95% CI, 0.954-0.995; P < 0.05) and a 5.6% decrease in age at onset of breast development (FC = 0.944; 95% CI, 0.912-0.978; P < 0.01). Being in the high stable vs low stable cluster corresponded to a 2.3% decrease in age at menarche (FC = 0.977; 95% CI, 0.957-0.998; P < 0.05) and a 4.5% decrease in age at onset of breast development (FC = 0.955; 95% CI, 0.921-0.990; P < 0.05). Similarly, being in the low to high vs median stable cluster corresponded to a 2.9% decrease in age at menarche (FC = 0.971; 95% CI, 0.947-0.996; P < 0.05) and a 5.6% decrease in age at onset of breast development (FC = 0.944; 95% CI, 0.906-0.984; P < 0.01). Being in the low to high v. low stable cluster corresponded to a 2.6% decrease in age at menarche (FC = 0.974; 95% CI, 0.950-0.999; P < 0.05) and a 4.5% decrease in age at onset of breast development (FC = 0.955; 95% CI, 0.916-0.996; P < 0.05). Figure 3 depicts the predicted median ages for each pubertal onset outcome as a function of cluster membership for the BMIP trajectories. Regarding the covariates, maternal menarcheal age and child race/ethnicity again significantly, and independently, predicted all 3 pubertal timing outcomes. For additional clarity, the model-predicted median ages (corresponding to the FC coefficients reported in Table 3) for each pubertal onset outcome are reported in Table 4 at values of infant percent weight gain ranging between 100% (2× birthweight) to 400% (5× birthweight) and for the 4 clusters of early childhood BMIP trajectories.
Figure 3.
Model-predicted median ages for each pubertal onset outcome by BMIP trajectory.
Table 4.
Model-predicted median ages for infant percent weight gain and the 4 early childhood BMIP trajectories
| Age at Menarche | Age at Breast Onset (TS II) | Age at Pubic Hair Onset (TS II) | ||||
|---|---|---|---|---|---|---|
| Median | 95% CI | Median | 95% CI | Median | 95% CI | |
| Infant % weight gain | ||||||
| 100% gain (2× birthweight) | 13.32 | 12.86-13.80 | 10.42 | 9.82-11.07 | 11.21 | 10.70-11.75 |
| 150% gain (2.5× birthweight) | 13.02 | 12.70-13.35 | 10.20 | 9.76-10.67 | 10.91 | 10.55-11.28 |
| 200% gain (3× birthweight) | 12.72 | 12.48-12.97 | 9.99 | 9.63-10.36 | 10.62 | 10.35-10.89 |
| 250% gain (3.5× birthweight) | 12.43 | 12.17-12.70 | 9.77 | 9.39-10.17 | 10.33 | 10.03-10.65 |
| 300% gain (4× birthweight) | 12.15 | 11.79-12.52 | 9.57 | 9.08-10.08 | 10.06 | 9.64-10.49 |
| 350% gain (4.5× birthweight) | 11.87 | 11.40-12.37 | 9.36 | 8.73-10.03 | 9.79 | 9.24-10.37 |
| 400% gain (5× birthweight) | 11.60 | 11.00-12.24 | 9.16 | 8.39-10.01 | 9.53 | 8.84-10.26 |
| Early child BMI trajectories | ||||||
| High stable | 12.52 | 12.26-12.78 | 9.75 | 9.38-10.13 | 10.47 | 10.18-10.77 |
| Low to high | 12.48 | 12.15-12.81 | 9.75 | 9.31-10.21 | 10.46 | 10.07-10.86 |
| Median stable | 12.85 | 12.55-13.15 | 10.32 | 9.91-10.76 | 10.69 | 10.35-11.04 |
| Low stable | 12.81 | 12.51-13.12 | 10.21 | 9.78-10.65 | 10.72 | 10.36-11.09 |
Abbreviations: BMI, body mass index; BMIP, body mass index percentile; TS, Tanner stage.
Discussion
The goal of the current study was to move beyond the examination of childhood obesity to consider nuances in patterns of early life weight gain in relation to pubertal timing. In a relatively large, normative risk sample of girls, findings revealed that both higher rates of weight gain in infancy and high-risk BMIP trajectories in early childhood confer risk for earlier pubertal onset. In addition, in the current sample, 19% and 4% of girls showed a gain in weight at 15 months that was 3.5× their birthweight or greater and 4.0× their birthweight or greater, respectively, indicating a sizable proportion of girls were at risk of experiencing pubertal onset approximately 3.5 to 4.5 and 5.5 to 7.0 months earlier, respectively, than girls with an average weight gain. Similarly, 54% of girls belonged to a high-risk BMIP trajectory indicating more than one-half of the girls in the sample were at risk of experiencing pubertal onset approximately 3.5 to 7.0 months earlier than girls belonging to a low-risk trajectory. Taken together, findings show that high-risk patterns of weight gain were relatively common among the girls and that these patterns predicted meaningful differences in the onset of puberty. Findings warrant the attention of health care providers to increase surveillance in girls who exhibit these patterns and to consider possible interventions to attenuate maladaptive patterns of weight gain beginning as early as infancy.
The current study findings also revealed that higher birthweight predicted younger ages at all 3 pubertal onset outcomes. These findings are consistent with a prior study in which higher birthweight predicted younger ages at menarche and advanced breast and pubic hair development [20] as well as 2 other studies in which higher birthweight predicted advanced breast development [30] and younger menarcheal age [31], although, in the latter study, this association did not reach statistical significance. In contrast, several other studies have reported lower birthweight was associated with earlier pubertal onset [32-34] or that there was no association between birthweight and pubertal onset outcomes [19]. Our study is similar to Wang et al [20] reporting an inverse association between birthweight and age of pubertal onset outcomes. Both samples were US-born and both had similar distributions with respect to gestational age. That is, Wang et al [20] excluded preterm (<37 weeks) infants and, in our study, only 3.5% of infants were preterm. Although we did not exclude preterm infants specifically, the broader selection criteria in the NICHD SECCYD required exclusion of infants with serious medical problems, likely affecting the representation of lower birthweight infants. More research is needed to disentangle this mixed literature, clarifying differences across studies that may account for inconsistent findings, including focusing on study selection criteria and their impact on the distribution of birthweight and gestational age, the sample country of origin, and aspects of the postnatal environment that may offset risks associated with low birthweight, thereby potentially altering the nature of associations between birthweight, patterns of weight gain, and pubertal timing.
The current findings have important implications for understanding the intersection of risk associated with weight gain and obesity and risk associated with pubertal development itself. Understanding these sources of risk and their convergence may help explain the development of insulin resistance and the promotion of processes underlying cardiometabolic diseases. The association between obesity and insulin resistance is well established [35]. In parallel, the physiological changes that typically occur during puberty, even among normal weight children, include increases in insulin resistance [36-38] and a compensatory higher insulin response [39]. The dual impacts of obesity and puberty have been hypothesized to contribute to the development of youth-onset type 2 diabetes [40], which typically emerges during puberty [41]. Other evidence shows that among overweight/obese children, β-cell function deteriorates over the course of puberty [42], reflecting progressive disruptions in insulin secretion and the regulation of blood glucose. Findings in the current study imply that further delineation of problematic weight gain patterns in the prepubertal period may help identify children most vulnerable to pubertal effects on insulin resistance. As well, findings in the current study also imply that such risk may vary by the timing of pubertal onset.
A key strength of the current study was its longitudinal design and life course framework, focusing on developmental pathways that may link early life exposures to adulthood health and well-being. In this context, trajectories of weight gain were examined in separate periods of infancy and early childhood to potentially identify earlier opportunities for intervention and prevention. An additional key strength of the current study was its use of rigorous measurement methodologies. TS was determined by trained health care providers in annual physical examinations to assess dimensions of breast development and pubic hair growth. Real-time self-reports were ascertained from the girls and their mothers to assess age at menarche. Supplementation of TS with breast bud palpation and the use of multiple indicators of pubertal onset further enhanced the reliability and overall quality of the assessments. Finally, the current study incorporated assessments of known confounding factors, maternal menarcheal age and race/ethnicity, and additionally considered the role of breastfeeding measured by querying mothers in real-time over 5 time points.
A key weakness of the current study was a lack of racial/ethnic diversity in the sample. Only 21% of the sample belonged to a non-White racial/ethnic group, limiting the generalizability of the study findings as well as opportunities to test the study hypotheses in separate groups of racial/ethnic minority girls. This limitation is especially problematic because evidence shows there are racial/ethnic differences in both prepubertal obesity and pubertal onset [43, 44], which makes understanding life course models of these associations in racial/ethnic minority girls even more important. An additional weakness was the lack of relevant prepubertal health data in areas of nutrition and physical activity as well as concurrent biomarker data pertaining to variations in growth and pubertal development (eg, leptin, IGF-1, androgens) and emerging health risk indicators (eg, insulin resistance). The current study also lacked data addressing the shared genetic associations between obesity and pubertal development [45], precluding accounting of how shared genetic variance may partially explain the observed associations. Finally, the annual physical examinations in which TS was performed were not initiated until age 9.5 years, leading to some left-censored observations. To address this, accelerated failure time models were fit to accommodate both left and interval censoring.
Future research should focus on the replication of findings in the current study by addressing whether these same patterns of weight gain, including the clustering of BMIP trajectories into 4 distinct profiles, are present in other larger and more diverse samples and whether these patterns have value in predicting the timing of puberty and its sequelae. In addition, this work should be extended to consider trajectories of weight gain and cardiometabolic health in the postpubertal period. Important, unanswered questions pertain to the correspondence between trajectories of prepubertal and postpubertal weight gain. For example, is the continuation of risk in the postpubertal period largely predicted by factors in the prepubertal period? Alternatively, does earlier pubertal onset itself worsen this trajectory, leading to an acceleration of risk in the postpubertal period? Although not addressed directly, prior studies have shown earlier pubertal onset was associated with an increase in postpubertal weight gain and a worsening of cardiovascular risk factor profiles [46, 47], supporting the notion of accelerated postpubertal risk. Another area for future investigation is the identification of behavioral and biological correlates of the weight gain patterns. Correlates that distinguish girls with higher rates of infancy weight gain and high-risk BMIP trajectories may help explain these variations. This information would be particularly useful, for example, in better understanding girls in the low to high BMIP trajectory who exhibited, on average, an increase of almost 40 BMIP points between age 24 months and grade 1.
In conclusion, the current study extended findings relating prepubertal obesity to earlier pubertal timing by examining early life patterns of weight gain in relation to multiple indicators of pubertal onset. The identification of factors that predict pubertal onset is an important goal as earlier pubertal onset is itself a risk factor for a host of poor health, socioemotional, and behavioral outcomes [3-7]. Study findings showed that higher rates of weight gain between birth and 15 months and high-risk BMIP trajectories between 24 months and grade 1 may be risk factors for earlier pubertal onset. This work has important implications for health care providers to increase surveillance in girls who exhibit these patterns and to consider possible interventions to attenuate maladaptive patterns of weight gain beginning as early as infancy. Focusing on problematic patterns of weight gain for earlier and more targeted intervention may help to lessen life course linkages between prepubertal obesity, accelerated pubertal development, and negative postpubertal outcomes.
Acknowledgments
Financial Support: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U10HD025447, R01HD091132) and the National Heart, Lung, and Blood Institute (R01HL130103) at the National Institutes of Health.
Glossary
Abbreviations
- BMI
body mass index
- BMIP
body mass index percentile
- FC
factor change
- NICHD
National Institute of Child Health and Human Development
- SECCYD
Study of Early Child Care and Youth Development
- TS
Tanner stage.
Contributor Information
Maria E Bleil, Email: mbleil@uw.edu, Child, Family, & Population Health Nursing, University of Washington, Seattle, WA 98195, USA.
Bradley M Appelhans, Department of Preventive Medicine, Rush University Medical Center, Chicago, I, L 60612, USA.
Steven E Gregorich, Department of Medicine, University of California San Francisco, San Francisco, CA 94143, USA.
Alexis S Thomas, Child, Family, & Population Health Nursing, University of Washington, Seattle, WA 98195, USA.
Robert A Hiatt, Department of Epidemiology & Biostatistics, University of California San Francisco, San Francisco, CA 94158, USA.
Glenn I Roisman, Institute of Child Development, University of Minnesota, Minneapolis, MN 55455, USA.
Cathryn Booth-LaForce, Child, Family, & Population Health Nursing, University of Washington, Seattle, WA 98195, USA.
Additional Information
Disclosure Statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Data Availability
Data from the National Institute of Child Health and Human Development Study of Early Child Care and Youth Development used in the current study are available on the following website: icpsr.umich.edu/web/ICPSR/series/233.
References
- 1. Biro FM, Greenspan LC, Galvez MP, et al. Onset of breast development in a longitudinal cohort. Pediatrics. 2013;132(6):1019-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Biro FM, Khoury P, Morrison JA. Influence of obesity on timing of puberty. Int J Androl. 2006;29(1):272-7; discussion 286. [DOI] [PubMed] [Google Scholar]
- 3. Cancer CGoHFiB. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13:1141-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. He C, Zhang C, Hunter DJ, et al. Age at menarche and risk of type 2 diabetes: results from 2 large prospective cohort studies. Am J Epidemiol. 2010;171(3):334-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jacobsen BK, Heuch I, Kvåle G. Association of low age at menarche with increased all-cause mortality: a 37-year follow-up of 61 319 Norwegian women. Am J Epidemiol. 2007;166(12):1431-1437. [DOI] [PubMed] [Google Scholar]
- 6. Jacobsen BK, Oda K, Knutsen SF, Fraser GE. Age at menarche, total mortality and mortality from ischaemic heart disease and stroke: the Adventist Health Study, 1976-88. Int J Epidemiol. 2009;38(1):245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McPherson K, Steel CM, Dixon JM. ABC of breast diseases. Breast cancer-epidemiology, risk factors, and genetics. Bmj. 2000;321(7261):624-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Graber JA. Pubertal timing and the development of psychopathology in adolescence and beyond. Horm Behav. 2013;64(2):262-269. [DOI] [PubMed] [Google Scholar]
- 9. Cavanagh SE, Riegle-Crumb C, Crosnoe R. Puberty and the education of girls. Soc Psychol Q. 2007;70(2):186-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bichteler A, Gershoff ET. Identification of children’s BMI trajectories and prediction from weight gain in infancy. Obesity (Silver Spring). 2018;26(6):1050-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li C, Goran MI, Kaur H, Nollen N, Ahluwalia JS. Developmental trajectories of overweight during childhood: role of early life factors. Obesity (Silver Spring). 2007;15(3):760-771. [DOI] [PubMed] [Google Scholar]
- 12. Li YF, Lin SJ, Chiang TL. Timing of rapid weight gain and its effect on subsequent overweight or obesity in childhood: findings from a longitudinal birth cohort study. BMC Pediatr. 2020;20(1):293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pryor LE, Tremblay RE, Boivin M, et al. Developmental trajectories of body mass index in early childhood and their risk factors: an 8-year longitudinal study. Arch Pediatr Adolesc Med. 2011;165(10):906-912. [DOI] [PubMed] [Google Scholar]
- 14. Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123(4):1177-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Antonisamy B, Vasan SK, Geethanjali FS, et al. Weight gain and height growth during infancy, childhood, and adolescence as predictors of adult cardiovascular risk. J Pediatr. 2017;180:53-61.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stettler N, Kumanyika SK, Katz SH, Zemel BS, Stallings VA. Rapid weight gain during infancy and obesity in young adulthood in a cohort of African Americans. Am J Clin Nutr. 2003;77(6):1374-1378. [DOI] [PubMed] [Google Scholar]
- 17. Yuan Y, Chu C, Zheng WL, et al. Body mass index trajectories in early life is predictive of cardiometabolic risk. J Pediatr. 2020;219:31-37.e6. [DOI] [PubMed] [Google Scholar]
- 18. Khuc K, Blanco E, Burrows R, et al. Adolescent metabolic syndrome risk is increased with higher infancy weight gain and decreased with longer breast feeding. Int J Pediatr. 2012;2012:478610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maisonet M, Christensen KY, Rubin C, et al. Role of prenatal characteristics and early growth on pubertal attainment of British girls. Pediatrics. 2010;126(3):e591-e600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Y, Dinse GE, Rogan WJ. Birth weight, early weight gain and pubertal maturation: a longitudinal study. Pediatr Obes. 2012;7(2):101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Juul F, Chang VW, Brar P, Parekh N. Birth weight, early life weight gain and age at menarche: a systematic review of longitudinal studies. Obes Rev. 2017;18(11):1272-1288. [DOI] [PubMed] [Google Scholar]
- 22. Wei J, Liu S, Cheng Y, Yang W, Zhu Z, Zeng L. Association of infant physical development and rapid growth with pubertal onset among girls in rural China. JAMA Netw Open. 2021;4(5):e216831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ong KK, Emmett P, Northstone K, et al. Infancy weight gain predicts childhood body fat and age at menarche in girls. J Clin Endocrinol Metab. 2009;94(5):1527-1532. [DOI] [PubMed] [Google Scholar]
- 24. NICHD Early Child Care Research Network. New York, NY: Guilford Press; 2005. https://www.guilford.com/books/Child-Care-and-Child-Development/The-NICHD-Early-Child-Care-Research-Network/9781593852870/editor [Google Scholar]
- 25. Susman EJ, Houts RM, Steinberg L, et al. Longitudinal development of secondary sexual characteristics in girls and boys between ages 9 1/2 and 15 1/2 years. Arch Pediatr Adolesc Med. 2010;164(2):166-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dorn LD, Dahl RE, Woodward HR, Biro F. Defining the boundaries of early adolescence: a user’s guide to assessing pubertal status and pubertal timing in research with adolescents. Appl Dev Sci. 2006;10(1):30-56. [Google Scholar]
- 27. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herman-Giddens ME, Slora EJ, Wasserman RC, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics. 1997;99(4):505-512. [DOI] [PubMed] [Google Scholar]
- 29. Rubin D. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 30. Olivo-Marston S, Graubard BI, Visvanathan K, Forman MR. Gender-specific differences in birthweight and the odds of puberty: NHANES III, 1988-94. Paediatr Perinat Epidemiol. 2010;24(3):222-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Terry MB, Ferris JS, Tehranifar P, Wei Y, Flom JD. Birth weight, postnatal growth, and age at menarche. Am J Epidemiol. 2009;170(1):72-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sloboda DM, Hart R, Doherty DA, Pennell CE, Hickey M. Age at menarche: influences of prenatal and postnatal growth. J Clin Endocrinol Metab. 2007;92(1):46-50. [DOI] [PubMed] [Google Scholar]
- 33. Sørensen K, Juul A, Christensen K, Skytthe A, Scheike T, Kold Jensen T. Birth size and age at menarche: a twin perspective. Hum Reprod. 2013;28(10):2865-2871. [DOI] [PubMed] [Google Scholar]
- 34. Hvidt JJ, Brix N, Ernst A, Lauridsen LLB, Ramlau-Hansen CH. Size at birth, infant growth, and age at pubertal development in boys and girls. Clin Epidemiol. 2019;11:873-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106(4):473-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med. 1986;315(4):215-219. [DOI] [PubMed] [Google Scholar]
- 37. Ball GDC, Huang TTK, Gower BA, et al. Longitudinal changes in insulin sensitivity, insulin secretion, and beta-cell function during puberty. J Pediatr. 2006;148(1):16-22. [DOI] [PubMed] [Google Scholar]
- 38. Moran A, Jacobs DR Jr, Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48(10):2039-2044. [DOI] [PubMed] [Google Scholar]
- 39. Caprio S, Plewe G, Diamond MP, et al. Increased insulin secretion in puberty: a compensatory response to reductions in insulin sensitivity. J Pediatr. 1989;114(6):963-967. [DOI] [PubMed] [Google Scholar]
- 40. Kelsey MM, Pyle L, Hilkin A, et al. The impact of obesity on insulin sensitivity and secretion during pubertal progression: a longitudinal study. J Clin Endocrinol Metab. 2020;105:2061-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Copeland KC, Zeitler P, Geffner M, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab. 2011;96(1):159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kelly LA, Lane CJ, Weigensberg MJ, Toledo-Corral CM, Goran MI. Pubertal changes of insulin sensitivity, acute insulin response, and β-cell function in overweight Latino youth. J Pediatr. 2011;158(3):442-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Relation of age at menarche to race, time period, and anthropometric dimensions: the Bogalusa Heart Study. Pediatrics. 2002;110(4):e43. [DOI] [PubMed] [Google Scholar]
- 44. Singh GK, Siahpush M, Kogan MD. Rising social inequalities in US childhood obesity, 2003-2007. Ann Epidemiol. 2010;20(1):40-52. [DOI] [PubMed] [Google Scholar]
- 45. Day FR, Perry JR, Ong KK. Genetic regulation of puberty timing in humans. Neuroendocrinology. 2015;102(4):247-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Frontini MG, Srinivasan SR, Berenson GS. Longitudinal changes in risk variables underlying metabolic Syndrome X from childhood to young adulthood in female subjects with a history of early menarche: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2003;27(11):1398-1404. [DOI] [PubMed] [Google Scholar]
- 47. Remsberg KE, Demerath EW, Schubert CM, Chumlea WC, Sun SS, Siervogel RM. Early menarche and the development of cardiovascular disease risk factors in adolescent girls: the Fels Longitudinal Study. J Clin Endocrinol Metab. 2005;90(5):2718-2724. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from the National Institute of Child Health and Human Development Study of Early Child Care and Youth Development used in the current study are available on the following website: icpsr.umich.edu/web/ICPSR/series/233.