Abstract
The life cycle of human immunodeficiency virus type 1 (HIV-1) is intricately related to the activation state of the host cells supporting viral replication. Although cellular activation is essential to mount an effective host immune response to invading pathogens, paradoxically the marked systemic immune activation that accompanies HIV-1 infection in vivo may play an important role in sustaining phenomenal rates of HIV-1 replication in infected persons. Moreover, by inducing CD4+ cell loss by apoptosis, immune activation may further be central to the increased rate of CD4+ cell turnover and eventual development of CD4+ lymphocytopenia. In addition to HIV-1-induced immune activation, exogenous immune stimuli such as opportunistic infections may further impact the rate of HIV-1 replication systemically or at localized anatomical sites. Such stimuli may also lead to genotypic and phenotypic changes in the virus pool. Together, these various immunological effects on the biology of HIV-1 may potentially enhance disease progression in HIV-infected persons and may ultimately outweigh the beneficial aspects of antiviral immune responses. This may be particularly important for those living in developing countries, where there is little or no access to antiretroviral drugs and where frequent exposure to pathogenic organisms sustains a chronically heightened state of immune activation. Moreover, immune activation associated with sexually transmitted diseases, chorioamnionitis, and mastitis may have important local effects on HIV-1 replication that may increase the risk of sexual or mother-to-child transmission of HIV-1. The aim of this paper is to provide a broad review of the interrelationship between immune activation and the immunopathogenesis, transmission, progression, and treatment of HIV-1 infection in vivo.
Following in vitro demonstrations that tumor necrosis factor alpha (TNF-α) greatly enhances the transcription of human immunodeficiency virus type 1 (HIV-1) in chronically infected mononuclear cells (67, 106, 252), it became clear that the pathogenesis of HIV-1 infection and AIDS is intimately related to the activation state of the host immune system. Although immunological activation in response to invading organisms is essential in order to mount an effective host response, paradoxically this may also provide an immunological environment that actually drives viral replication and disease progression in HIV-infected persons. A clear understanding of the effects of immune activation on HIV-1 infection in vivo is therefore crucial to our overall understanding of the immunopathogenesis and mechanisms of transmission of this virus.
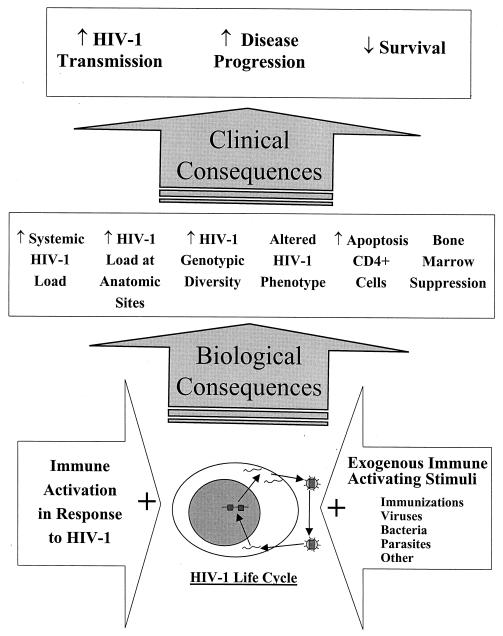
Figure 1 summarizes the broad effects of immune activation on HIV-1 infection in vivo, not only highlighting the impact on the biology of the virus but also indicating the clinical consequences that may potentially result. Immune activation as a result of the host response to either HIV-1 itself or the presence of exogenous stimuli may impact the viral life cycle at the cellular level. This may not only result in increased HIV-1 replication systemically or at localized anatomical sites but may also lead to changes in HIV-1 phenotype and genotype, increase apoptosis of host immune cells, and suppress hematopoietic regeneration. Local immune activation in the genital tract associated with sexually transmitted diseases (STDs) may increase the risk of sexual and mother-to-child transmission of HIV-1, and it is hypothesized that systemic immune activation accelerates disease progression and reduces the survival of HIV-1-infected persons. Immune activation has also been a key theme in therapeutic approaches to the control of HIV-1 replication and elimination of the infection, and the existence of immunologically quiescent mononuclear cells containing latent, integrated provirus has also been identified as one of the major obstacles to achieving a therapeutic cure for this infection in persons receiving highly active antiretroviral therapy (HAART) (60, 103, 362). This paper provides a broad review of the interrelationship between immune activation and the biology, immunopathogenesis, transmission, progression, and treatment of HIV-1 infection in vivo. Consideration of specific antiviral immune mechanisms, however, does not fall within the scope of this review.
FIG. 1.
Consequences of immune activation in HIV-1 infection in vivo. This conceptual diagram highlights the broad consequences of immune activation on the biology of HIV-1 and on lymphoid cell populations in vivo and their subsequent effect on HIV-1 transmission, disease progression, and survival in HIV-1-infected persons.
MECHANISMS BY WHICH CELLULAR ACTIVATION ENHANCES HIV-1 REPLICATION
The life cycle of HIV-1 is intimately related to the activation state of its host cells. HIV-1 is dependent on host cell surface receptor expression for entry, on many cytoplasmic pathways for the afferent and efferent events of its life cycle, and on the transcriptional machinery within the host cell nucleus for viral gene expression.
HIV-1 Cellular Entry
HIV-1 typically enters host cells through the interaction of the viral envelope protein, gp120, with CD4 and a chemokine coreceptor on the surface of the host cells. The β-chemokine receptor, CCR5, is critical in the initial establishment of chronic HIV-1 infection in vivo; transmitted strains predominantly have V3 loop sequences that predict the use of this coreceptor (373), and individuals in whom this receptor is genetically deficient are largely resistant to infection (202). In contrast, disease progression is often associated with the emergence of syncytium-inducing (SI) variants that utilize the CXCR4 α-chemokine receptor (70), although this is not a prerequisite for disease progression (79). The relative expression of these coreceptors determines the relative susceptibility of cells to HIV-1 infection. Typically, CXCR4 is expressed by immunologically naive CD45RA+ T lymphocytes whereas CCR5 is expressed by more activated CD45RO+ T lymphocytes (32, 366). While chemokine receptor expression is strongly linked to cellular activation (32, 257, 366), the regulation of expression is clearly complex and different costimulatory signaling pathways may have reciprocal effects on β-chemokine receptors (reviewed in reference 49).
Immune activation resulting from the presence of opportunistic infections, other inflammatory stimuli, or the antigenic stimulus of HIV-1 infection itself may affect the surface expression of these coreceptors by uninfected mononuclear cells, thereby modulating their susceptibility to HIV-1 infection. For example, Mycobacterium avium infection (341) and exogenous interleukin-2 (IL-2) treatment (350) are each associated with upregulation of CCR5 expression on peripheral blood mononuclear cells in vivo and lipopolysaccharides (LPS) upregulate CXCR4 expression on macrophages in vitro (240). Possibly in response to increasing immune activation, CCR5 expression increases with HIV-1 disease progression in vivo, and this may be an important determinant of the clinical course of infection (80, 285). Furthermore, chronic immune activation associated with the high prevalence of coinfections among HIV-infected persons in Africa may be responsible for the increased CCR5 expression by peripheral blood mononuclear cells in persons living in this region (167), which favors HIV-1 strains that utilize this coreceptor (66).
Certain coinfections in HIV-infected persons may also increase the susceptibility of mononuclear cells to HIV-1 infection by exerting additional effects on viral entry. Cytomegalovirus (CMV) encodes a chemokine receptor homologue, US28, which distantly resembles the human chemokine receptors, CCR5 and CXCR4. This CMV-encoded receptor facilitates the entry of HIV-1 into CD4+ human cell lines (272), although it is not known whether such a mechanism operates in vivo. Human herpesvirus 6 (HHV-6), another common opportunistic infection in HIV-infected persons, induces the expression of the CD4 receptor on γ/δ T lymphocytes (207) and natural killer (NK) cells (209), rendering them susceptible to HIV-1 infection. Similarly, there is evidence that herpes simplex virus type 1 (HSV-1) facilitates the entry of HIV-1 into keratinocytes, which do not express the CD4 receptor. The formation of HSV-1/HIV-1 virion hybrids is thought to mediate this process, and HSV-1-encoded envelope proteins facilitate entry into keratinocytes (154).
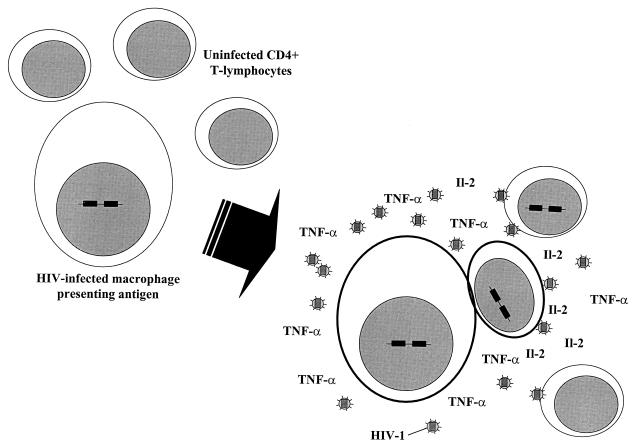
The inflammatory immune response to opportunistic infections also provides a permissive microenvironment for cell-to-cell transmission of HIV-1 (217). Antigen-presenting cells (APCs) are important reservoirs of HIV-1 (220, 224, 331), and during the process of antigen presentation there is intimate association between APCs and lymphocytes (Fig. 2). During this process, intercellular signaling through costimulatory and intercellular adhesion molecules augments the induction of potent cellular activation and proinflammatory cytokine secretion, leading to marked upregulation of HIV-1 transcription in infected cells (233, 259, 305, 306, 351). High local concentrations of HIV-1 and proinflammatory cytokines, together with the state of heightened cellular activation, also provide the ideal microenvironment for intercellular spread and propagation of HIV-1 (217, 305, 306, 351).
FIG. 2.
Replication and intercellular transmission of HIV-1 is increased during antigen presentation. Macrophages serve as long-lived reservoirs of HIV-1 infection. During antigen presentation (both HIV-1 antigens and other antigens), activation of macrophages and CD4+ T lymphocytes leads to potent upregulation of proinflammatory cytokine secretion and HIV-1 transcription. This provides the ideal microenvironment for transmission of HIV-1 to CD4+ cells and for rapid HIV-1 replication in an expanding pool of activated memory (CD45RO+) cells. Selective infection of antigen-specific memory CD4+ cells may also lead to selective loss of this clone of cells.
Upregulation of adhesion molecules during inflammatory processes may further promote virus-induced cell-cell fusion, thereby facilitating the direct spread of virus between cells (156). Furthermore, antigen presentation results in the activation and clonal expansion of CD45RO+ memory CD4+ T lymphocytes, which constitute a pool of susceptible target cells for HIV-1 propagation. Thus, by a variety of means, opportunistic infections and other inflammatory stimuli may facilitate the cellular entry of HIV-1 and virus transmission within the host mononuclear cell pool.
HIV-1 Reverse Transcription
Following entry into the host cell, HIV-1 RNA must undergo reverse transcription to cDNA and then be imported to the nucleus for integration as a provirus into the host genome. HIV-1 replicates preferentially in CD45RO+ memory T lymphocytes rather than the more immature and immunologically quiescent CD45RA+ naive lymphocytes (316, 363). This cell subset selection appears to be due to the inability of HIV-1 to complete reverse transcription in CD45RA+ T cells (369, 370), despite comparable viral entry into the two cell subsets (316). Following virus uptake, the resultant postfusion complex within the cytoplasm is labile, and in the absence of activation signaling the virus loses its capacity to initiate a productive infection (363). However, through the induction of the intracellular transcription factor NF-AT (nuclear factor of activated T cells) (173), for example, proinflammatory cytokine signaling enables the preintegration complex to complete reverse transcription and continue through the viral life cycle (333, 369, 370). Thus, proinflammatory signaling associated with the presence of opportunistic infections and other inflammatory stimuli in the host may facilitate completion of the afferent HIV-1 life cycle in both memory and naive cells.
HIV-1 Proviral Transcription
Cellular transcription factors and the HIV-1 LTR.
HIV-1 proviral transcription is greatly influenced by the state of host cell activation and is regulated by sequences in the 5′ long terminal repeat (LTR) of the viral genome. A number of these regulatory sequences resemble those present in human cellular genes and are able to specifically bind numerous host cell transcription factors. In this way, HIV-1 is able to harness the cellular transcriptional machinery in order to replicate, and this results in the coordination of viral transcription and cellular activation.
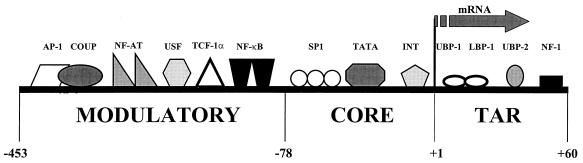
The HIV-1 5′ LTR comprises three functionally discrete regions (reviewed in references 10, and 113) (Fig. 3). The transactivation response region binds the virus-encoded Tat transactivating protein, which is essential to achieving significant levels of virus transcription. The core promoter region mediates a basal level of HIV-1 transcription in response to SP1 transcription factors and TATA binding proteins. However, the key mechanism by which host cellular activation enhances HIV-1 transcription is the recognition of a number of inducible host transcription factors by the modulatory enhancer region, which lies upstream of the core promoter region within the 5′ LTR (reviewed in references 10 and 113).
FIG. 3.
Cellular transcription factors that bind to the HIV-1 LTR. The HIV-1 promoter is functionally divided into the modulatory enhancer region and the core promoter region, which both lie upstream of the transcription start point, and the transactivation response region (TAR), which lies downstream. The interaction of these cellular transcription factors with the HIV-1 promoter results in the tight coordination of HIV-1 replication to the activation state of the host cell.
The modulatory enhancer region contains at least six defined elements that bind cellular transcription factors (113), and the best characterized of these is nuclear- factor-κB (NF-κB) (reviewed in reference 16). This heterodimeric molecule is present in a preformed state in the cytoplasm, complexed with inhibitory IκB proteins. On exposure of the cell to various activating stimuli, including TNF-α, IL-1, LPS, and phorbol esters, phosphorylation of IκBs leads to their dissociation from active NF-κB (reviewed in reference 16). Physiologically active NF-κB then translocates to the nucleus, where it mediates the activation of viral and cellular gene transcription by binding to its specific recognition sequences in the enhancer region (reviewed in reference 10). Other transcription factors may also synergize with NF-κB to further increase transcription (266). For example, in lymphocytes, certain members of the NF-AT family bind to the κB regulatory elements and synergize with NF-κB and Tat in transcriptional activation of HIV-1 (174). The NF-κB pathway represents an important mechanism by which bacteria, viruses, and other inflammatory stimuli may enhance HIV-1 replication.
In vitro studies of the effects of activation of HIV-infected cells of the monocytic lineage show the regulation of viral transcription to be more complex than that observed in lymphocytes. Activation of cells of the monocytic lineage may either increase or decrease HIV-1 transcription, and this depends on a number of factors, including the cell type studied and the stage of differentiation of the cells (26, 276, 347). Also, induction of alpha/beta interferon (IFN-α/β) secretion leads to the suppression of HIV-1 transcription in these cells (161, 347). In vivo, however, macrophages activated in the presence of tuberculosis (TB) are highly productive sources of HIV-1 replication (188, 245, 254).
Cytokines and HIV-1 transcription.
Since HIV-1 replication is closely regulated by the host cell transcriptional machinery, it comes under the influence of a complex network of proinflammatory and immunoregulatory cytokines (42, 105, 106). TNF-α, which plays a pivotal role in HIV-1 pathogenesis (227), induces HIV-1 transcription in both macrophages and T lymphocytes via the NF-κB pathway (67, 106, 227, 252). Other proinflammatory cytokines (IL-1, IL-2, and IL-6) also induce HIV-1 replication (reviewed in reference 273). IL-6 synergizes with TNF-α to enhance HIV-1 replication at transcriptional and posttranscriptional levels in monocytic cells but not lymphocytes (275). IL-1 increases HIV-1 replication in promonocytic cell lines by enhancing TNF-α-mediated induction of NF-κB (128). In addition to these proinflammatory cytokines, it is possible that other host-encoded immune mediators may play a role in the induction of HIV-1 transcription during the inflammatory response (85, 117).
Transactivating DNA viruses and HIV-1 transcription.
It is clear that a wide variety of copathogens may indirectly enhance HIV-1 transcription through proinflammatory cytokine induction. However, certain DNA viruses are also known to encode gene products that directly transactivate the HIV-1 LTR (116) (Table 1). Because of the ability of these DNA viruses to directly induce HIV-1 transcription and their high prevalence as coinfections in HIV-infected persons, much attention has focused on these viruses as potentially important cofactors in HIV-1 disease progression. The impact of these coinfections on HIV-1 load and disease progression in vivo is discussed later in this review.
TABLE 1.
Viruses that enhance HIV-1 replication in vitro through gene products that directly transactivate the HIV-1 LTR
| Virus | Gene product | Reference |
|---|---|---|
| HTLV-1 | Tax | 308 |
| HBV | HBx | 304 |
| CMV | 1E1 | 159 |
| HSV-1 | 1E110, 1E175 | 242 |
| HHV-6 | 112 |
Influence of TH1- and TH2-Type Responses on HIV-1 Infection
In addition to cytokine-induced cellular activation, it has been hypothesized that qualitative T-helper (TH)-type responses may also impact AIDS pathogenesis. A switch in the predominant response from type 1 (TH1) to type 2 (TH2) and production of the associated cytokines may be related to, and even facilitate, disease progression (65, 213). Indeed, some studies have found that TH2 and type 0 (TH0) lymphocyte clones are more permissive to HIV-1 replication in vitro than are TH1 clones (213, 339). More recent studies indicate that HIV-1 disease progression is associated with increasing secretion of IL-10, a defined TH2 cytokine (317, 325). However, these findings and the putative role of modulation of TH responses in HIV pathogenesis remain controversial (99, 132).
The controversy concerning the role of TH-type responses in HIV-1 pathogenesis has, however, raised an important question about whether coinfections that modulate these TH responses might also impact the natural history of HIV-1 in vivo (21, 22). Parasitic diseases, which commonly coinfect HIV-infected persons in developing countries, typically induce a dominant TH2 lymphocyte immune environment that modulates immune responses to other antigens. Thus, mice with schistosomiasis mount TH2 responses when subsequently challenged with nonparasite antigens that normally induce TH1 responses (182, 263, 357). Similarly, schistosomiasis in humans impairs TH1 responses to tetanus toxoid immunization, with cellular production of IFN-γ, a TH1 cytokine, being inversely related to the worm burden (297). Possibly as a consequence of this TH modulation, mice with schistosomiasis show impaired CD8+ cytotoxic lymphocyte activity and reduced clearance of vaccinia virus (1). These observations have led to the hypothesis that TH2 modulation of the immune system resulting from coinfections with parasitic diseases may facilitate HIV-1 replication in coinfected persons (21, 189), although data to support this are lacking.
SYSTEMIC IMMUNE ACTIVATION IN RESPONSE TO HIV-1 INFECTION
The course of HIV-1 infection is characterized by a biphasic viremia. Following the dissemination and propagation of HIV-1 in the lymphoid tissues during primary infection, engagement of the host antiviral immune response coincides with a fall in the plasma virus load to a set-point level (reviewed in reference 260). In the absence of effective antiretroviral therapy, the HIV-1 load progressively increases, with failure of the immune system to control virus replication (295). These viral dynamics are accompanied by marked systemic changes in the immune system.
Primary HIV-1 Infection
Uncontrolled viral replication during primary HIV-1 infection initially leads to activation of a marked cell-mediated immune response with (i) increased numbers of activated CD8+ T lymphocytes expressing CD38, CD45RO, and HLA-DR (73); (ii) increased numbers of NK cells (310); (iii) elevated concentrations of the immune markers soluble CD8 (sCD8), soluble TNF-receptor type II (sTNF-RII), neopterin, and soluble CD30 (sCD30) in blood; and (iv) increased concentrations of the cytokines IFN-γ, TNF-α, and IL-1β (19, 133, 287, 310). Marked early increases in IFN-γ secretion have been found to correlate with oligoclonal expansion of CD8+ T cells, which is a dominant feature in the immune response to primary HIV-1 infection (133); this cellular immune response is later accompanied by the development of the humoral response (179). Elevated levels of immune markers decline as the disease enters the chronic phase (310). Although the majority of productive HIV-1 replication occurs in activated CD4+ lymphocytes, the ability of HIV-1 to also infect relatively inactive, nonreplicating cells (375) may permit the establishment of host infection. Subsequent activation of the immune system may serve to drive viral replication but also ultimately limit viral dissemination.
Chronic HIV-1 Infection
Although the hallmark of HIV-1 infection and AIDS is immunodeficiency resulting from functional and numeric loss of CD4+ T lymphocytes, throughout the course of chronic HIV-1 infection there is also a heightened state of systemic immune activation (14, 20, 214). The envelope glycoprotein gp120 of all HIV-1 subtypes is a potent immunogen (348) and leads to activation of both macrophages and lymphocytes (reviewed in reference 48) and induction of proinflammatory cytokines (169, 230, 286). HIV-1 infection may also prime immune cells for enhanced TNF-α and IL-6 secretion on exposure to bacterial products (23), further leading to greatly heightened immune activation during opportunistic infections.
Cell surface and soluble markers of immune activation.
During chronic HIV-1 infection, elevated numbers of activated CD8+ lymphocytes are present in the peripheral circulation, expressing CD38, HLA-DR, CD57, and CD71 (20, 197, 203, 214), and they play an important role in the host antiviral response (144, 251, 302). CD4+ lymphocytes, although numerically depleted, are also activated, and a significant proportion express HLA-DR and CD25 (214). Increased levels of CD71 are detectable on peripheral B lymphocytes, and production of immunoglobulins G and M is augmented (225).
Concentrations of cytokines and soluble immune activation markers in plasma are stably elevated in HIV-infected persons when measured serially over weeks and months (15), and this observation correlates with the finding that the plasma HIV-1 load is also remarkably stable during the early, asymptomatic phase of infection (53). However, with progression of HIV-1 disease, increasing concentrations of sCD8 (20, 247), soluble CD14 (sCD14) (201), TNF-α (14, 15, 298), and neopterin (reviewed in reference 17) in serum reflect increasing activation of CD8+ lymphocytes and macrophages, respectively.
Elevated concentrations of a variety of other soluble immune markers in serum correlate with disease progression in HIV-infected persons. These include neopterin, β2-microglobulin, sTNF-RII, and soluble CD25 (sCD25) (20, 97, 98, 197, 298, 371). An increase in immune activation generally precedes the inflection point of CD4+ T-cell levels and HIV-1 load (248, 298), adding support to the hypothesis that immune activation is instrumental rather than simply a consequence of HIV-1 pathogenesis. Indeed, increased immune activation, as determined by lymphocyte phenotype or measurement of soluble immune markers, correlates with shortened survival in HIV-1-infected persons (97, 98, 123, 214, 372). Moreover, in advanced disease, survival correlates more strongly with T-lymphocyte expression of CD38 than with the plasma virus burden or virus chemokine receptor usage (120).
TNF-α pathway.
Chronic activation of the TNF-α-signaling pathway plays a pivotal role in HIV-1 pathogenesis (reviewed in reference 227), leading to increased HIV-1 transcription (discussed above), induction of mononuclear cell apoptosis, and suppression hematopoiesis (discussed below). The HIV-1 envelope glycoprotein gp120 directly induces TNF-α secretion by peripheral blood mononuclear cells (169, 230, 286) and directly upregulates viral replication in an autocrine and paracrine manner in chronically infected cell lines (42, 67, 274) as well as in peripheral blood mononuclear cells (338). Thus, following the initial establishment of host infection, ongoing propagation of HIV-1 may be promoted by continual immunological activation and induction of TNF-α secretion by the virus itself. The importance of chronic induction of the HIV-1 LTR by TNF-α in HIV-1 pathogenesis may, in part, explain why HIV-1 is more pathogenic than HIV-2. The HIV-2 enhancer responds more weakly to TNF-α and NF-κB than does the HIV-1 enhancer (142). Indeed, the plasma virus load is much lower in individuals infected with HIV-2 than in those infected with HIV-1 (278), and this difference has been confirmed to be due to reduced viral transcription rather than differential susceptibility of mononuclear cells to the two virus types (277).
Data indicate that increased TNF-α secretion may represent a primary disturbance in the immune system, which leads to more generalized secondary manifestations of immune activation (197, 210). TNF-α, together with these secondary immunological effects, also plays an important role in the depletion of the CD4+ lymphocyte population, as discussed below.
Immune activation and CD4+ lymphocyte depletion.
The major part of the chronic phase of HIV-1 infection is clinically asymptomatic. However, the advent of HAART permitted new studies of viral and cellular kinetics, which revealed HIV-1 infection to be a highly dynamic process (158, 346). There is massive covert HIV-1 replication in the lymphoid tissues at all stages of HIV-1 infection (92), and this is accompanied by the continual destruction and regeneration of CD4+ lymphocytes (158, 346). It is estimated that productively HIV-1-infected cells and plasma virions have average life spans of only 2.2 and 0.3 days, respectively, and the average total HIV-1 production is estimated to be 10.3 × 109 virions per day (265). The development of the characteristic T lymphocytopenia with disease progression in HIV-1-infected persons is the result of a complex pattern of gradual changes in T-cell subset composition with depletion of both CD4+ and CD8+ naive (CD45RA+) and CD4+ memory (CD45RO+) subsets (reviewed in reference 63). Several mechanisms have been proposed to explain this lymphocytopenia, including virus-induced cell death, immune destruction of infected cells, apoptosis, and impaired lymphocyte regeneration.
In view of the very small proportion of CD4+ cells that are actually infected with HIV-1 (143), direct HIV-mediated cytopathic effects and elimination by immune mechanisms such as cytotoxic T-lymphocyte killing and antibody-dependent cellular cytotoxicity would not be sufficient to account for the rapid CD4+ cell turnover that occurs in HIV-infected persons (5, 265). Apoptosis, resulting from inappropriate induction of activation-induced cell death, represents a more important mechanism that leads to depletion of both CD4+ and CD8+ lymphocytes in HIV-1-infected persons (3, 138, 231). During the chronic phase of HIV-1 infection, the generalized immune activation that is associated with HIV-1 increases signaling through the proapoptotic pathways of T lymphocytes, and a high proportion of the cells subsequently undergoing apoptosis are activated memory cells expressing HLA-DR, CD38, CD45RO and Fas (127). TNF-α, as well as promoting HIV-1 transcription (as discussed above), plays a key role in activation of the proapoptotic mononuclear cell pathways, and differential signaling through type I and type II TNF receptors regulates these different effects (194).
Importantly, induction of apoptotic cell death occurs not only in virus-infected cells but also in noninfected bystander cells, which are the predominant cells lost (102). The susceptibility of mononuclear cells to apoptosis in HIV-infected persons correlates with the degree of lymphocyte activation in peripheral blood (127) and in lymphoid tissue (244). Modeling analysis also suggests that the degree of systemic immune activation correlates with CD4+ T-cell losses (197). However, studies analyzing the correlation of the frequency of apoptosis and HIV-1 disease progression have yielded contradictory results (127, 232, 244), possibly reflecting the fact that apoptosis does not represent the single dominant mode of T-cell depletion.
More recent experimental evidence using a mathematical interpretation of telomere length in CD4+ cells subsets suggests that the rate of CD4+ cell production is only moderately increased in HIV-1-infected persons (360), decreasing the likelihood that hematopoietic exhaustion represents the major cause of lymphocytopenia in AIDS. Quantification of thymic output by measuring the excisional DNA products of T-cell receptor gene rearrangement indicates that HIV-1 infection impairs thymic maturation of naive (CD45RA+) lymphocytes (90). Furthermore, there is also evidence of impairment of hematopoiesis within the bone marrow. Both HIV-1 envelope glycoproteins and proinflammatory cytokines (including TNF-α) suppress bone marrow progenitor cells (114, 212). Thus, it is now clear that CD4+ lymphocyte depletion is the result of a combination of specific virus-induced cell death, widespread activation-induced loss of the memory (CD45RO+) pool, and impaired renewal of the naive (CD45RA+) cell pool.
In addition to the generalized loss of CD4+ cells, immune activation may lead to the clonal deletion of CD4+ cells during the process of major histocompatibility complex class II-restricted antigen presentation (216, 220, 331), as discussed above. This process might contribute to the progressive loss of T-cell responses to common recall antigens observed during HIV-1 disease progression (65). Selective loss of cells with specific V-beta regions leads to restriction of the T-cell receptor repertoire and may also result from immune dysregulation (126, 163, 284). While it was previously hypothesized that such cellular loss may be the result of an HIV-encoded superantigen leading to selective activation and subsequent deletion of these cells, there is little evidence to support this. The functional impact of this phenomenon is also not known but persists despite the commencement of HAART (71, 195).
EXOGENOUS IMMUNE-ACTIVATING STIMULI AND HIV-1 REPLICATION
The plasma HIV-1 load is relatively stable during the clinically asymptomatic phase of chronic HIV infection, and the level probably represents a balance between activation-driven viral replication and the host antiviral response. However, although minor self-reported illnesses have little or no impact on virus load (53), many clinically significant, exogenous inflammatory stimuli are associated with significant increases in the systemic HIV-1 load. This is presumably due to the acceleration of virus production in actively replicating cells as well as to the induction of latent virus in immunologically quiescent cells (40, 58).
Immunizations
Serial measurement of the plasma HIV-1 load in HIV-infected persons receiving immunizations has enabled the impact of clearly defined immune-activating stimuli on HIV-1 replication in vivo to be determined. Administration of tetanus toxoid leads to increases in the plasma HIV-1 load (250, 319), which typically rises threefold above baseline and peaks 2 to 4 weeks postimmunization; thereafter, the load declines to baseline levels by approximately 5 weeks (250, 319). The ability to detect proviral DNA in blood and lymph nodes (319) and to isolate HIV-1 from blood (258) is also enhanced following tetanus toxoid immunization.
Increases in the plasma HIV-1 load have been observed in the majority of patients following administration of oral cholera toxin (a 2- to 60-fold increase has been detected) (255) and pneumococcal vaccine (a 1- to 586-fold increase has been detected) (38) but not hepatitis B immunization (53, 55). Moreover, studies of the impact of influenza immunization have yielded contrasting results, with patients having either large increases (up to 369-fold) (157, 290, 320) or no increases (38, 109, 122, 283, 368) in their virus load.
The contrasting findings in these studies may result from clinical differences in the study populations, differences in the use of antiretroviral agents, and variations in the immunogenicity of the vaccine preparations. Another factor that may be important in determining whether immunizations induce a rise in the HIV-1 load is the stage of disease. For example, in a study of influenza immunization in HIV-infected persons, Staprans et al. found an approximately 10-fold mean rise in HIV-1 load in persons with CD4+ counts of <200 × 106/liter compared to a mean increase of >50-fold in persons with CD4+ counts of >500 × 106/liter (320). In contrast, no increase in plasma HIV-1 load was detectable in recent HIV-1 seroconverters following pneumococcal, Haemophilus influenzae type b, or diphtheria toxoid immunization (165).
Viral Infections
As discussed above, much interest has focused on certain viruses that encode transcription factors, which directly transactivate the HIV-1 LTR and thereby increase HIV-1 replication in vitro (Table 1). However, there are relatively few data on the effects of these viral coinfections on the HIV-1 load in vivo.
Acute reactivation of HSV-1 and HSV-2 frequently causes oral and genital lesions in HIV-infected persons, and such acute episodes are associated with a 3.4-fold median increase in the plasma HIV-1 load (234). Furthermore, of great importance with respect to HIV-1 transmission is the finding that HIV-1 can frequently be detected in genital ulcers caused by HSV-2 in men (299). The levels of CMV and HIV-1 in plasma appear to be independent of one another (35), and the CMV load and HIV-1 proviral load in autopsy tissues were found to be discordant (94). Although shedding of HIV-1 in semen is positively associated with shedding of CMV, the two processes appear to have independent immunological controls (181).
Among persons with advanced immunosuppression, HHV-6 causes is a common disseminated infection and is regarded as an important potential cofactor in HIV-1 replication and CD4+ T-cell loss (reviewed in reference 208). Depending on the cell type and conditions, HHV-6 may either enhance (112, 207, 208) or suppress (13) HIV-1 replication in vitro. However, a postmortem tissue study found that levels of HHV-6, but not HHV-7 or CMV, were associated with the HIV-1 proviral DNA load, suggesting that replication of the two viruses is linked in vivo (94).
Human T-cell leukemia virus types 1 and 2 (HTLV-1 and HTLV-2), hepatitis C virus (HCV), and hepatitis B virus (HBV) are common coinfections in HIV-1-infected intravenous drug users. Although HTLV-1 gene products enhance HIV-1 replication in vitro (34), coinfection with this virus is not associated with increased plasma HIV-1 load or other markers of disease progression in vivo (146, 300). HBV and HCV are able to increase (304) and suppress (318), respectively, HIV-1 replication in vitro. However, there are no data on the impact of HCV and HBV coinfections on HIV-1 load in vivo.
Bacterial Infections
Although bacteria are not known to encode gene products that directly transactivate the HIV-1 LTR, certain bacterial products are able to induce HIV-1 replication via NF-κ B-dependent mechanisms in latently infected, resting CD4+ cells (239) and cells of monocytic lineage (233, 276). Thus, by means of cellular activation, bacteria may enhance HIV-1 replication in vivo.
Many studies have shown that Mycobacterium tuberculosis and certain mycobacterial cell wall components induce HIV-1 replication in vitro (125, 196, 307, 374), enhance the viral infectivity of monocytes (329), and increase the transmission of HIV-1 from monocytes to lymphocytes (217). Although latent M. tuberculosis infection has no impact on the plasma HIV-1 RNA load (221), the development of active TB may lead to marked increases (up to 160-fold) in the plasma HIV-1 load (125, 328). Coinfected persons have higher HIV-1 RNA concentrations than do CD4-matched persons with HIV-infection but no opportunistic infections (177). Furthermore, in patients with pleural TB, the HIV-1 load is greater in pleural fluid compared than in plasma (Z. Toossi, unpublished data), indicating that viral replication is enhanced at the site of mycobacterial disease.
The potential for TB to increase the HIV-1 load in vivo may be greater than that of other common opportunistic infections because of the chronic clinical course of active TB, the critical role that TNF-α plays in the host response to mycobacterial disease (172), and the marked systemic immune activation that accompanies M. tuberculosis/HIV-1 coinfection (185, 336). Although resolution of TB with antituberculosis drug treatment in a small group of Western subjects was associated with a marked decline in the HIV-1 load (125), little or no reduction in virus load was seen in larger cohorts of African patients successfully treated for TB (192, 344) (L. Morris, D. J. Martin, L. Sacks, S. Pendle, L. Page-Shipp, H. Bredell, T. C. Quinn, and R. E. Chaisson. Abstr. 5th Conf. Retroviruses Opportunistic Dis., abstr. 259, 1998). In coinfected West Africans, persistent elevation of the plasma HIV-1 load was associated with sustained elevation of TNF-α concentrations in plasma, despite the resolution of other parameters of immune activation during TB treatment (192). In view of this, pentoxifylline and thalidomide, both inhibitors of TNF-α-mediated activation of the HIV-1 LTR, have been evaluated as adjuncts to antituberculosis drugs in coinfected persons. Use of these drugs was not associated with a substantial reduction in HIV-1 load (177, 344), and trials using more potent immunosuppressant therapy such as corticosteroids are currently in progress.
Although M. avium enhances HIV-1 replication in mononuclear cells in vitro (85, 117), prospective clinical studies have found that M. avium bacteremia is not associated with an increase in the plasma HIV-1 RNA concentration (140). Moreover, no reduction in plasma virus load has been observed following treatment of this opportunistic infection (140, 211). Since M. avium typically causes disease in individuals with advanced immunosuppression, it is conceivable that the rate of HIV-1 replication in such patients is either maximal or obtunded by antiretroviral drugs, which were received by the majority of patients in these studies.
Acute increases in plasma HIV-1 load have been observed in association with a wide variety of acute bacterial coinfections, including pneumonia and otitis media caused by Streptococcus pneumoniae and H. influenzae (41, 222), Pneumocystis carinii pneumonia (88, 326), and a variety of other infections (326). A median increase in the HIV-1 load of five- to eightfold from baseline was observed in these studies, and in the three studies with an adequate duration of follow-up the median virus load decreased to near baseline levels 2 months after treatment for the bacterial coinfection was initiated (41, 88, 222). Thus, there is consistent evidence that acute bacterial infections are associated with increases in the plasma HIV-1 load that are reversible following treatment of the infection.
Several bacteria that may be present in the genital tract have been demonstrated to upregulate HIV-1 replication through induction of NF-κB; these include lactobacilli (178), Gardnerella vaginalis (149), and Treponema pallidum (327). While the organisms that cause bacterial vaginosis lead to increases in the concentrations of in TNF-α and IL-1β in the genital secretions (324), the impact of these organisms on the HIV-1 load is not currently known. The impact of STDs on the biology of HIV-1 in the genital tract and on HIV-1 transmission is considered later in this review.
Parasitic Infections
A large proportion of the world's HIV-infected population live in developing countries, where coinfection with parasitic diseases is very common. Malaria infection induces a potent proinflammatory cytokine drive and is potentially an important cofactor for HIV-1 disease progression. Plasmodium falciparum induces HIV-1 replication by a TNF-α-dependent pathway in vitro (367). In one study, adults in Malawi with acute falciparum malaria coinfection had a sevenfold-higher median plasma HIV-1 load than did HIV-infected controls who did not have malaria but who were not matched for stage of HIV disease; 4 weeks after anti-malaria treatment, a small but significant decrease in plasma viremia accompanied the more substantial reduction in TNF-α concentrations (160). In contrast, falciparum malaria has not been found to adversely impact HIV-2 RNA or proviral load in infected persons in West Africa (11, 12). This may relate to differences in transcriptional activation of the two viruses, with the HIV-2 enhancer responding weakly to TNF-α and NF-κB in comparison to the HIV-1 enhancer (142).
Among other parasitic diseases that commonly coinfect HIV-infected persons in some developing countries, visceral leishmaniasis, an intracellular protozoal infection, has emerged as an important opportunistic infection (331a; reviewed in reference 358). Studies indicate that Leishmania donovani can enhance HIV-1 replication in monocytoid cells in vitro (25), and the HIV-1 plasma load is higher in leishmania-infected persons than in HIV-infected controls without coinfection (280). However, two small studies of the impact of antileishmania treatment on the plasma HIV-1 load yielded conflicting results (24, 44). Antigens of Toxoplasma gondi, another intracellular protozoon and a common cause of opportunistic infection in persons with AIDS, also increases HIV-1 replication in mononuclear cells in vitro (18), but in vivo data are lacking.
Schistosomiasis is also a common coinfection in HIV-infected persons in some countries. It has been hypothesized that the dominant TH2 lymphocyte responses that accompany S. mansoni infection may promote HIV-1 replication in vivo. However, a study of 30 HIV-1-infected patients with schistosomiasis in Kenya found that effective treatment of schistosomiasis was not accompanied by a reduction in the plasma HIV-1 load (189). There is a very high prevalence of gastrointestinal helminthic infections in people living in developing countries. However, there is only a single unpublished report suggesting that clearance of such parasites with anthelmintic drugs has a beneficial effect on the plasma HIV-1 load (D. Wolday, S. Maayan, Z. G. Mariam, S. Britton, A. Landay, and Z. Bentwich, Abstr. 7th Conf. Retroviruses Opportunistic Infect., abstr. 139, 2000).
Other Inflammatory Stimuli
Little is known about the effect of fungal infections on HIV-1 replication in vivo. However, Cryptococcus neoformans induces HIV-1 replication and enhances HIV infectivity in vitro (147, 267) and cryptococcal meningitis leads to an increased HIV-1 load in cerebrospinal fluid (241). It is also likely that inflammatory stimuli of any etiology, if of sufficient intensity, will upregulate HIV-1 replication in vivo. For example, evidence of increased HIV-1 replication has been found in blood and resected intestinal tissue from a patient with severe acute ulcerative colitis (309) and also in serous fluid obtained from the bullous skin lesions of a patient with Stevens-Johnson syndrome (81).
Thus, it is clear that many different immunological stimuli impact the plasma HIV-1 load in vivo, including immunizations as well as viral, bacterial, parasitic, and fungal infections and other inflammatory stimuli. In addition to increasing the plasma HIV-1 load, these stimuli may have other important effects on the biology of HIV-1 infection in vivo.
IMMUNE ACTIVATION AND BIOLOGY OF HIV-1 IN VIVO
Immune Activation and HIV-1 Load
In the previous section we reviewed the impact of a wide variety of different exogenous immune activating stimuli on the HIV-1 load in blood plasma. Due to the comparative ease of sampling and measuring the HIV-1 load in blood plasma rather than in tissue samples, a great majority of these data are derived from measurements of the HIV-1 RNA load in blood. However, although measurements of the plasma HIV-1 load are important predictors of disease progression (229), it must be recognized that lymphoid tissue is the major cellular reservoir (>98%) of viral replication in persons not receiving HAART (261). Moreover, during the clinically asymptomatic stages of disease, there is also dissociation between HIV-1 load in blood and lymphoid tissues (261). Thus, measurements of blood plasma HIV-1 load incompletely assess the impact of exogenous inflammatory stimuli on HIV-1 replication in vivo.
HIV-1 load in body tissues.
Proinflammatory cytokines are constitutively expressed at high levels in the lymphoid tissues of HIV-infected persons (131). However, using double-staining techniques for HIV-1 RNA or DNA and for TNF-α expression, studies of the colocalization of TNF-α expression and HIV-1 replication in tissue biopsy specimens have yielded contradictory results (200, 249). Nevertheless, evidence suggests that exogenous inflammatory stimuli in vivo do impact HIV-1 replication in lymphoid tissues as well as in blood. Tetanus toxoid immunization increases the detection of HIV-1 RNA and proviral DNA in lymph nodes (319), mycobacterial and pneumocystis infections increase HIV-1 RNA detection in lymph nodes (240), and focal antigen presentation leading to intense immunological activity increases HIV-1 expression in the white pulps of the spleen (56, 130).
HIV-1 load in anatomical compartments.
While systemic immune activation may lead to increases in the HIV-1 RNA concentration in both blood and lymphoid tissues, enhancement of virus replication may be even greater at the primary anatomical site of inflammation and may be compartmentalized from that occurring in blood. Thus, the HIV-1 load is greater in bronchoalveolar lavage fluid than in plasma in patients with pulmonary TB and HIV-1 coinfection (245), and the virus load is also greater in pleural fluid than in plasma in those with pleural TB (Toossi, unpublished). Similarly, the HIV-1 load in cerebrospinal fluid is compartmentalized from blood (37, 62), and the virus load is higher in cerebrospinal fluid than in blood in persons with either cryptococcal (37) or tuberculous (241) meningitis.
Perhaps of greater importance, infections in the genital tract may not only disrupt the mucosal barrier but also increase the local HIV-1 load in genital secretions, promoting sexual and mother-to-child transmission of HIV-1. HIV-1 detection in semen is increased in men with urethritis and gonorrhea (68, 243), and treatment of these infections lowers the seminal plasma HIV-1 load (68). Similarly, detection of HIV-1 proviral DNA in the female genital tract is associated with cervical inflammation and increased vaginal discharge (64, 166, 180). More specifically, Neisseria gonorrhoeae and Chlamydia trachomatis infections enhance the detection of HIV-1 RNA in cervicovaginal secretions, an effect that decreases after successful treatment of the STDs (118). Also, a 200-fold mean increase in the HIV-1 load was observed in the genital secretions of women who developed ulceration and inflammation of the cervix (193, 364); this effect on the HIV-1 load was strikingly compartmentalized to the genital tract and correlated strongly with parallel increases in local concentrations of proinflammatory cytokines (193). Not only are these findings in the genital tract important with respect to HIV-1 transmission, but also mastitis in lactating HIV-infected women is associated with an increased virus load in breast milk and with increased vertical transmission of HIV-1 (303). Thus, irrespective of any impact on systemic viral burden, inflammatory lesions at specific anatomical sites may have a major impact on local HIV-1 replication, which may have important consequences for the transmission of HIV-1, as discussed later in this review.
Cellular compartments of HIV-1 replication.
The level of cell-free HIV-1 in blood is maintained by continuous rounds of de novo infection in short-lived lymphocytes (158), and the great majority of cell-free HIV-1 in plasma (>98%) is thought to be derived from lymphocytes rather than cells of the monocytic lineage (265). Nevertheless, APCs serve as important reservoirs of HIV-1 (220, 224, 331). Increasing concentrations of TNF-α (14, 15, 298), sCD14 (201), and neopterin (17) in serum suggest that activation of macrophages increases with HIV-1 disease progression, and this is further heightened in the presence of opportunistic infection (188, 190, 192). Macrophages may therefore constitute an increasingly productive source of HIV-1 in the latter stages of the disease course, especially in the presence of opportunistic infections (254, 342).
Data to support this hypothesis are limited and largely relate to the impact of opportunistic infections with mycobacteria, which are pathogens that reside within macrophages. Both macrophages (329) and lymphocytes (111) obtained from patients with pulmonary TB show enhanced susceptibility to productive infection with HIV-1 in vitro. However, using in situ hybridization, lymph node biopsiy specimens from HIV-infected persons with M. avium or P. carinii infection indicate that in addition to lymphocytes, tissue macrophages are highly productive sources of HIV-1 replication (254). Although these results differ from those of others (335), analysis of HIV-1 in plasma samples by using an immunomagnetic HIV-1 capture technique confirms that the macrophage compartment contributes significantly to the cell-free plasma HIV-1 load in patients with pulmonary TB (188). Moreover, detection of HIV-1 derived from CD14+ macrophages is enhanced in pleural fluid samples obtained from HIV-infected patients with pleural TB and in plasma of HIV-infected individuals with acute P. falciparum malaria (T. Pisell, Z. Toossi, I. Hoffman, S. T. Butera, and S. D. Lawn, Abstr. AIDS Pathog. Conf. abstr. 146, 2001).
Thus, evidence suggests that cells of the monocytic lineage are important sources of virus replication during opportunistic infections. These cells may also play an important role in the persistence and pathogenesis of HIV-1 infection (reviewed in reference 115), serving as long-lived viral reservoirs that are able to transmit virus to other cells (220). It is possible that increased seeding of HIV-1 provirus into these cells during opportunistic infections may contribute to the adverse long-term effects of opportunistic infections on the deterioration of immune function (51, 352).
Impact of HIV-1 Genotype
Typically, a homogeneous virus population proliferates in blood during primary HIV-1 infection (373) and subsequently undergoes rapid genotypic diversification following the development of HIV-1-specific cytotoxic T-lymphocyte responses (314). A markedly heterogeneous virus population is thus present in the blood of persons with chronic HIV-1 infection (136, 279), and these viruses are similar to those present in the lymph nodes, bone marrow, and spleen (337). However, there is genotypic compartmentalization of viruses present in the brain, lungs, and testes (337, 361) from those in blood.
Immune activation may lead to the differential expression of HIV-1 genotypes, both in the systemic circulation and at anatomical sites of inflammation. Studies of microdissected splenic white pulps revealed exquisite compartmentalization of HIV-1 genotypes, resulting from highly localized antigen stimulation (56). Tetanus toxoid immunization may result in transient expression of previously undetectable HIV-1 quasispecies in blood (256). Genotypic differences have been found to exist between the major HIV-1 species present in blood and in bronchoalveolar lavage fluid obtained from diseased lung segments in persons with pulmonary TB (245), and HIV-1-infected individuals with active pulmonary TB have greater viral genotypic diversity than do HIV-1-infected controls (69). Increased viral genotypic diversification driven by immune activation may increase the chance of the expression of more virulent quasispecies with the potential to affect disease progression. However, there are currently no data to support such a hypothesis.
Differential expression of HIV-1 quasispecies in the genital tract may also be important with respect to HIV-1 transmission. It is clear that despite the presence in of a genotypically diverse pool of HIV-1 in plasma of persons with chronic HIV-1 infection, only selected strains are transmitted by the sexual route (373) or from mother to child (359). Among the pool of latent proviruses present in the cellular reservoirs of the female genital tract, some may represent the transmissible virus strain that originally infected the host. It is possible that inflammation in the genital tract may lead to local expression and shedding of this transmissible virus. In support of this hypothesis, cervical ulceration has been shown to greatly increase the HIV-1 load in the female genital tract, and the majority of this virus was from locally increased replication (193, 364). HIV-1 present in semen and blood in males is also genotypically compartmentalized (72, 269, 376), with virus in semen arising from a distinct cellular reservoir (43, 170). The presence of STDs associated with increases in semen viral load (68, 230) may possibly lead to the differential expression of viral quasispecies that impact virus transmission.
Impact on HIV-1 Phenotype
Aspects of the HIV-1 phenotype that may be affected by immune-activating stimuli in vivo include the ability to induce syncytium formation, the finding of coreceptor usage, and the incorporation of host surface cell molecules into the viral envelope. Few data are available for the first two factors, although Ostrowski et al. reported that tetanus immunization in one patient who harbored both SI and non-syncytium-inducing (NSI) viruses resulted in the preferential enhancement of the NSI strain (256). With regard to coreceptor expression, it has been suggested that heightened immune activation arising from exogenous immune-activating stimuli in African individuals (289) may be responsible for the increased expression of HIV-1 strains that are dependent on the CCR5 coreceptor (66). This might be a result of viral selection due to the preferential expression of CCR5 in these individuals (167).
On budding from host cells, HIV-1 particles incorporate a variety of host molecules in the virion envelope (reviewed in reference 330) and thereby may acquire a surface phenotype that reflects that of the host cell (46). Immune-activating events result in the upregulation of many host cell surface molecules that may be incorporated in the envelope of budding viruses. Indeed, upregulation of intracellular cell adhesion molecule 1 (ICAM-1) and HLA-DR on the surface of mononuclear cells leads to increased incorporation of these molecules in the envelope of progeny HIV-1 particles in vitro (50, 108). Both HLA-DR and ICAM-1 serve as adhesion molecules and, when present in the envelope, enhance the infectivity of the virus in vitro (46, 50, 108), possibly by promoting virus-cell interactions.
These in vitro findings may be important for the pathogenesis and transmission of HIV-1 in vivo. Among HIV-infected persons, detection of HIV-1 bearing HLA-DR in the envelope is increased in the plasma of those with pulmonary TB compared those without TB (186). There is also evidence that viral incorporation of HLA-DR is increased at local anatomical sites of inflammation. In HIV-infected persons with pleural TB, detection of HIV-1 bearing HLA-DR is increased in pleural fluid compared to plasma (S. D. Lawn, T. Pisell, Z. Toossi, and S. T. Butera, ASM Conf. Abstr., Tuberculosis 2000: Past, Present, and Future, abstr. 66, 2000). Also, in HIV-infected women with cervical inflammation and ulceration, detection of HIV-1 bearing HLA-DR is increased in genital secretions compared to that in plasma (193). It is possible that by means of increased incorporation of HLA-DR in the HIV-1 envelope, opportunistic infections and other inflammatory stimuli may promote the propagation of HIV-1 in mononuclear cells in vivo and lead to increased transmissibility of virus locally expressed in the genital tract (193). However, there are no data that demonstrate whether this is a pathophysiologically significant mechanism in vivo.
In the HIV-1 envelope, HLA-DR is also functional in superantigen presentation (291), and thus increased viral incorporation of this molecule in the presence of opportunistic infections may lead to further enhancement of host cellular activation. Although other host cell molecules are also present in the HIV-1 envelope (188; reviewed in reference 330), their significance, if any, to the immunopathogenesis and transmission of HIV-1 is not known.
IMMUNE ACTIVATION AND HIV-1 TRANSMISSION
Sexual Transmission
Worldwide, heterosexual contact is the predominant mode of HIV-1 transmission, particularly in sub-Saharan Africa and increasingly so throughout Asia (332). As well as behavioral risk factors, a wide variety of biological risk factors are associated with the risk of heterosexual transmission (reviewed in reference 292). These relate both to the ability of the infected person to transmit infectious virus and to the susceptibility of the partner to infection with the virus on exposure.
The plasma viral load has been identified as the major predictor of the risk of sexual transmission of HIV-1 (253, 264, 282). Heterosexual transmission is uncommon in persons with a plasma viral load of <1,500 HIV-1 RNA copies/ml (282), and it is therefore possible that immune-activating stimuli that result in an increased systemic HIV-1 load may increase the risk of HIV-1 sexual transmission.
As discussed above, localized immune activation may have marked effects on HIV-1 replication within the genital tract, and aside from the virus load in the systematic circulation, undoubtedly the characteristics of the locally expressed virus in the genital tract are major determining factors for HIV-1 transmission risk. Indeed, in women transmitting HIV-1, the increased risk of transmission associated with an increasing plasma virus load may simply reflect the direct correlation between the HIV-1 loads in plasma and in the genital tract (148).
The direct impact of STDs on the local biology of HIV-1 in the male and female genital tracts may have important consequences for the risk of sexual transmission of HIV-1. There is a clear association between both ulcerative and non ulcerative STDs and increased risk of sexual transmission of HIV-1, even after adjustment for sexual behaviour (reviewed in references 292 and 345); the association with genital ulcer disease is particularly strong (45, 86, 152). This effect may, in part, be due to breaches in the genital epithelium, which is an effective barrier to HIV-1 (135). Development of mucosal ulceration would expose activated inflammatory mononuclear cells and render an uninfected recipient more susceptible to acquisition of HIV-1 (292). In addition, many studies have documented that the HIV-1 load in the genital tract is increased in the presence of STDs and genital tract inflammation (193; reviewed in references 292 and 345). Major increases in the concentrations of proinflammatory cytokines and immune activation markers were found to strongly correlate with major increases in the virus load in the genital secretions of HIV-infected women on development of genital ulceration (193, 365). The frequent detection of HIV-1 in genital ulcers caused by HSV-2 (299) may be the result of both the proinflammatory drive and the direct transactivation of the HIV-1 LTR by HSV-encoded gene products. Furthermore, of importance with regard to homosexual transmission of HIV-1, inflammation of the anorectal mucosa is an independent determinant of HIV-1 RNA shedding and HIV-1 DNA detection in the anorectal canal (176).
In addition to effects on the genital epithelium and local virus load, STDs may have other important local effects on the biology of HIV-1. As discussed above, local cellular activation may result in alterations in the HIV-1 envelope phenotype and genotypic repertoire and may lead to expression of viruses with greater transmissibility. Furthermore, genital secretions in the presence of genital tract inflammation contain not only increased HIV-1 load but also high concentrations of proinflammatory cytokines that theoretically may actually activate genital tract cells in uninfected sexual contacts, potentially enhancing their susceptibility to HIV infection (193).
Mother-to-Child Transmission
Mother-to-child transmission of HIV-1 may occur before, during, or after birth (reviewed in reference 246). Immune activation associated with local infections in the placenta, the maternal genital tract, and the breast (during lactation) may affect the biology of HIV-1 at those sites and increase the risk of virus transmission.
HIV-1 load in maternal blood and genital secretions.
The HIV-1 load in plasma is a major determinant of mother-to-child HIV-1 transmission as well as of heterosexual transmission (87, 100). An HIV-1 RNA concentration in blood greater than 100,000 copies/ml is associated with increased risk of mother-to-child transmission (100, 377). An increased plasma HIV-1 load may increase the transplacental transmission of virus to the fetus in utero as well as exposing the baby to increased virus concentrations in the female genital tract intrapartum. Maternal STDs (218, 219), Epstein-Barr, virus shedding (271), vaginal candidiasis, and cervical inflammation (262) are all associated with increased HIV-1 transmission, possibly due to the local impact on the biology of HIV-1 in the genital tract, as discussed above.
Chorioamnionitis.
Acute inflammation and chronic inflammation of the placental membranes, which are associated with prolonged rupture of the membranes and with preterm birth (124), are risk factors for mother-to-child transmission of HIV-1 (reviewed in references 124, 322, and 340). Massive secretion of proinflammatory cytokines by local inflammatory mononuclear cells in the amniotic fluid infected with bacteria (7) may increase local HIV-1 replication and account for the increased risk of mother-to-child transmission associated with chorioamnionitis. Placental malaria is also a common cause of placental inflammation in tropical countries, and some data suggest that this also increases the risk of mother-to-child transmission of HIV-1 (33), although ongoing studies have yet to confirm this.
Mastitis and lactation.
It is estimated that breastfeeding increases the rate of mother-to-child transmission of HIV-1 by 7 to 22% (reviewed in reference 246). Although the mechanisms and factors associated with transmission of HIV-1 through breast milk are not clearly defined, mastitis, an inflammatory process in the breast, is associated with an increased HIV-1 load in breast milk and increased mother-to-child transmission of HIV-1 (303). Mastitis may increase the HIV-1 load in breast milk by opening up the paracellular pathways between mammary alveolar cells, allowing inflammatory cells and extracellular fluid to enter the milk, or even by promoting HIV-1 replication in infected mononuclear cells present in the inflammatory breast tissue.
Immune activation in the neonate.
In addition to maternal factors, the level of immunological activation in the neonate may determine the risk of neonatal acquisition of HIV-1 from an infected mother. During the first months of life, especially in African infants, TNF-α concentrations in blood are higher than those present in adults and are able to stimulate higher levels of HIV-1 replication in HIV-infected cell lines (39). This may be an important factor in the acquisition of perinatal HIV-1 infection.
IMMUNE ACTIVATION AND HIV-1 DISEASE PROGRESSION
A multitude of viral and host factors may affect the progression of HIV infection in vivo: HIV-1 infection progresses more rapidly than HIV-2 infection (223); genetic factors affect cellular susceptibility to HIV-1 infection (356); specific viral and host gene deletions may both impair HIV-1 pathogenesis (76, 77, 162, 175); there are intersubject variations in specific anti viral mechanisms such as cytotoxic T-lymphocyte activity (144); and some individuals are able to prevent or contain HIV-1 infection by undetermined immunological mechanisms (47, 153). In addition to these viral and host factors, there is evidence that immune-activating stimuli may affect disease progression in HIV-1-infected persons (reviewed in reference 29).
In comparison to other host and viral factors, the relative contribution of immune-activating stimuli to disease progression is difficult to assess and remains incompletely defined. It is suggested that induction of successive increases in systemic HIV-1 load may lead to more rapid deterioration in the already impaired immune system and may also hasten the appearance of mutant viruses that may escape the established antiviral mechanisms. The plasma HIV-1 load is a major indicator of the prognosis (229), and there is abundant evidence that exogenous immune-activating stimuli cause increases in HIV-1 replication in blood (38, 41, 125, 234, 250, 255, 290, 319, 320, 326), in tissues (56, 249, 254, 319), and in anatomical compartments (37, 62, 193, 241, 245). However, the epidemiological evidence to support the hypothesis that immune-activating stimuli also accelerate HIV-1 disease progression in the longer term is less clear.
Retroviral Disease Progression in an Animal Model
Data from studies of an animal model of retroviral disease suggest that immune activation does indeed accelerate disease progression and reduce survival. In a study by Folks et al., uninfected and simian immunodeficiency virus (SIV) mac 251-infected rhesus macaques were subjected to pronounced immune activation at regular intervals by repeated immunization with a combined preparation of allogenic cells, keyhole limpet hemocyanin, and tetanus toxoid (107). Survival was shorter in SIV-infected monkeys receiving immunization than in control groups of monkeys subjected to either SIV infection or immunization alone. Reduced survival in this model did not correlate with increased viral load in blood, possibly due to a plateau effect in viral replication in these animals (F. Villinger and T. M. Folks, unpublished data).
Impact of Chronic Viral Coinfections
Many of the data regarding the impact of coinfections on progression and survival of HIV-infected persons come from studies of chronic viral coinfections. Among the herpesviruses, epidemiological evidence confirms that CMV coinfection is associated with reduced survival (294, 312, 315). Furthermore, a meta-analysis of studies of acyclovir prophylaxis of HSV infection indicates that prevention of HSV infection or reactivation of HSV latent infection leads to improved survival of persons with HIV-1 infection (164). Evidence for accelerated HIV diseases progression due to coinfection with HHV-6, though, is anecdotal (31).
Although HCV coinfection has had no impact on HIV-1 progression in several cohorts (89, 204, 281, 365), other studies have detected accelerated HIV-1 progression among a cohort of hemophiliacs (75) and among subgroups of HCV-coinfected persons, including those with HCV-1 coinfection (296) and those with early HIV-1 disease (270). In contrast, in studies published to date, coinfections with HTLV-1 or HTLV-2 (145, 155, 301) or with HBV (119, 311, 313) have shown no impact on HIV-1 disease progression, even though these viruses upregulate HIV-1 replication in vitro. Together, these studies show that some, but not all, viruses that induce HIV-1 replication in mononuclear cells in vitro are associated with an adverse clinical effect on HIV-1 disease progression in coinfected persons.
Impact of Other Opportunistic Infections
As discussed below, it is very difficult to determine epidemiologically whether opportunistic pathogens reduce patient survival through a direct effect on mortality alone or whether they also accelerate the loss of immune function and enhance disease progression. In light of this, many of the following data are limited in promoting our understanding of the effects of immune-activating events on HIV-1 disease progression.
P. carinii pneumonia, M. avium complex disease, Candida esophagitis, toxoplasmosis, cryptosporidiosis (51), and TB (198) are all associated with an increased risk of death in HIV-infected persons that is independent of the CD4+ lymphocyte count. However, a further important finding is that despite successful anti-TB treatment, HIV-infected individuals surviving TB coinfection have an increased incidence of new opportunistic infections, accelerated decline in immune function, and increased mortality (352). This indicates that TB has an adverse effect on HIV-1 disease progression and leads to reduced survival that is independent of TB-associated mortality. A meta-analysis of chemoprophylaxis against TB in HIV-infected persons in both developing and industrialized countries found increased survival in those who were tuberculin skin test positive (355). Similarly, the survival of Western subjects with AIDS is prolonged by chemoprophylaxis of P. carinii pneumonia (52) and M. avium complex (236). However, in contrast, P. falciparum malaria was not found to affect the rate of disease progression in infants with congenital HIV-1 infection (134), and Mycoplasma infections (hypothesized as playing an important cofactor role [235]) were not found to contribute to the rate of disease progression (2).
Limitations of the Epidemiological Data
Many of the studies of the effects of immune activation on the biology of HIV-1 infection in vitro and in vivo would suggest that exogenous immune-activating stimuli may have a substantial impact on disease progression in HIV-infected persons. However, the epidemiological data to support this hypothesis are limited. It is difficult to differentiate between increased mortality directly associated with opportunistic infection itself and that attributable to a cofactor effect accelerating HIV-1 progression. Similarly, it is not known whether increased survival in HIV-infected persons receiving antimicrobial chemoprophylaxis simply reflects decreased mortality directly attributable to fewer opportunistic infections or whether it also reflects the prevention of a cofactor effect on HIV-1 disease progression.
Studies of the comparative survival of HIV-infected persons following recovery from different opportunistic infections are largely unhelpful in determining the relative cofactor effect of different copathogens, since these organisms cause disease in persons with different levels of immunosuppression, giving rise to lead-time bias in the results. Indeed, some infections, such as TB, may occur during the clinically asymptomatic phase of HIV-1 infection, and there may be a long lag time before any adverse effect on clinical disease progression becomes apparent; many studies have an insufficient duration of follow-up to detect this. In persons in developing countries, synergy between multiple infectious agents may cause an adverse effect on HIV-1 progression; in this case, it is possible that elimination or prevention of only a single factor may not yield any apparent benefit on survival or disease progression, and multiple interventions may be required (189).
It has been suggested that opportunistic infections such as TB may serve as markers of immunosuppression that are independent of the blood CD4+ lymphocyte count, potentially confounding case-control studies in which groups are matched based on their blood CD4+ lymphocyte count (78). Indeed, matching cases and controls in such studies primarily by CD4+ lymphocyte count is likely to be inadequate, and more sophisticated matching of multiple variables, including the plasma HIV-1 load, may match more optimally for preexposure prognosis (30, 229). Such a matching process, however, would be far less easily applied to large epidemiological studies that are required to address this question.
Thus, there are many difficulties in designing epidemiological studies to determine whether exogenous immune-activating stimuli impact the natural history of HIV infection. Although many studies have attempted to examine the effect of TB on HIV-1 progression, an extensive review of the epidemiological data was unable to conclude whether TB impacts progression of immune deficiency (78).
Disease Progression in HIV-Infected Persons in Developing Countries
It is hypothesized that chronic immune activation due to TB, helminth infections, recurrent malaria, and waterborne pathogens may accelerate the progression of HIV-1 infection to AIDS, particularly in persons living in sub-Saharan Africa and other developing parts of the world (21, 22). Indeed, the level of systemic immune activation as assessed by cytokine production and levels of immune activation markers in serum is higher in African subjects than in matched subjects living in the West (288, 289). Furthermore, the plasma HIV-1 load was reported to be greater in men living in sub-Saharan Africa than in individuals living in Europe or the United States who were matched for blood CD4+ lymphocyte count (91).
If the effect of coinfections on HIV-1 progression were substantial, one would expect that disease progression would be faster and survival would be shorter in those living in Africa, where such events are far more frequent. Data from sub-Saharan Africa, albeit limited in several respects, suggest that survival may be shorter than in comparable populations of HIV-infected persons in the West (reviewed in references 129, 237, and 238). A more recent report of a seroconverter cohort in Haiti also support this (82). However, the differences in survival may simply indicate differences in the virulence of prevalent pathogens and disparities in access to health care between HIV-infected persons in the West and those in developing countries (reviewed in reference 129). There is clearly a great need for further data to determine the effect of these copathogens on disease progression among HIV-infected persons in these countries, since such knowledge would have important public health implications regarding approaches to addressing the HIV-1 epidemic in such parts of the world.
TREATMENT STRATEGIES
In this review, we have documented that immune activation in response to HIV-1 infection plays a central role in the immunopathogenesis of AIDS and that both systemic and local exogenous immune-activating stimuli may further affect the progression and transmission of this disease. Direct or indirect reduction of immune activation is therefore central to therapeutic strategies in HIV-infected persons.
Immunosuppressant Drugs in Treatment of HIV-1 Infection
Several factors provide a clear rationale for the use of immunosuppressive treatments in HIV-1 infection (151). Immune activation (i) promotes HIV-1 replication in vitro (58, 106, 252, 333, 369) and in vivo (125, 319, 326), (ii) increases during the clinical progression of HIV-1 infection (14, 197, 298), (iii) is an independent risk factor for death (120), and (iv) may contribute to CD4+ T-cell loss in HIV-infected persons through the induction of apoptosis (3, 138). In addition, autoimmunity has previously been hypothesized as possibly playing an important role in HIV-1 pathogenesis (reviewed in reference 260). Various immunosuppressive drugs have been used in HIV-infected persons to target the secretion of TNF-α by macrophages (thalidomide and pentoxifylline) or to reduce the activation status of lymphocytes (cyclosporin A and mycophenolic acid) or have been used because of more generalized immunosuppressive properties (prednisolone).
Pentoxifylline and thalidomide.
Both thalidomide and pentoxifylline decrease the production of TNF-α, resulting in reduced NF-κB-mediated HIV-1 replication in mononuclear cells in vitro (28, 101, 215). However, in clinical trials of pentoxifylline in HIV-infected persons, the viral load did not decrease despite diminished TNF-α production by stimulated peripheral blood mononuclear cells (83, 84). Similarly, thalidomide treatment has not been found to reduce either the HIV-1 load or the TNF-α concentration in serum in HIV-infected persons, despite leading to other improvements in immune function (150).
Cyclosporin A.
The nuclear factor of activated T cells (NF-ATc), a transcription-enhancing factor for IL-2 (95), is a cellular factor that induces a highly permissive state for HIV-1 replication in primary CD4+ cells (173). Cyclosporin A potently suppresses T-cell activation by forming a complex with the molecular chaperone cyclophilin A, which then inhibits the production of NF-ATc by blocking the phosphatase activity of calcineurin (95). There is also evidence that cyclosporin A has direct antiviral activity by inhibiting the normal interaction between Gag polyprotein and cyclophilin A (206) and also by preventing the incorporation of cyclophilin A into virions (323). In vitro, cyclosporin inhibits HIV-1 infection and replication within T-cell lines (343). However, initial results from studies of the effects of cyclosporin A in HIV-infected persons were contradictory (8, 268). Long-term follow-up of a group of such patients suggests that treatment may have a short-term beneficial effect on the CD4+ lymphocyte count but no effect on p24 antigenemia (199). No randomized, placebo-controlled clinical trials of the use of cyclosporin in HIV-infected persons have been published, although an early report from such a study suggests that low-dose cyclosporin has little effect on immune activation in HIV-infected individuals (L. H. Calabrese, et al., Abstr. 7th Conf. Retroviruses Opportunistic Infect., abstract 373, 2000).
Glucocorticoids.
Glucocorticoids have broad anti-inflammatory and immunoregulatory properties (reviewed in reference 74). They regulate gene expression by binding to glucocorticoid response elements, and the LTRs of many retroviruses, including HIV-1, contain sequences with homology to glucocorticoid receptor elements (168). Opposing effects on HIV-1 transcription have been found in vitro, depending on the cell line used (36, 184, 293). However, glucocorticoids also inhibit apoptosis in HIV-infected persons (205), leading to sustained increases in peripheral CD4+ T-cell counts (9). Although prednisolone therapy was found to decrease the activation of CD4+ cells and levels of immunoglobulins in a group of HIV-infected persons, no reduction in the level of HIV-1 RNA in plasma was observed (9). In a small study of four patients, short-term steroid treatment reduced serum neopterin and TNF-RII levels and was associated with a transient significant decrease in the plasma HIV-1 load (171). However, two other, larger trials of prednisone treatment in persons with either asymptomatic disease (9) or advanced HIV infection (228) found no beneficial reduction in the plasma HIV-1 load.
Other immunosuppressive agents.
Mycophenolic acid, in addition to inhibiting HIV-1 reverse transcription, was recently reported to deplete the activated pool of CD4+ T lymphocytes in vivo and warrants further investigation in controlled clinical trials (54). Numerous other immune-based therapeutic strategies for HIV infection have been previously reviewed (93, 321, 334). The drive to develop immunosuppressive agents was, at least temporarily, superseded in the mid-1990s when HAART was introduced.
HAART, Immune Activation, and Latency
HAART leads to dramatic decreases in the plasma HIV-1 load followed by gradual reconstitution of the immune system (141, 195). The profound changes in viral and cellular dynamics during HAART lead to a generalized decrease in the level of immune activation. Secretion of proinflammatory cytokines decreases (4, 6, 27, 96, 110, 121); CD8+ (27, 96, 121) and CD4+ (27) T cells and monocytes (4) in peripheral blood acquire more inactive cell surface phenotypes; and cellular expression of CCR5 and CXCR4 viral coreceptors (6) and soluble adhesion molecules (226) also decreases. Moreover, the numeric restoration of the peripheral CD4+ T-lymphocyte population is also accompanied by a reversal of the functional impairment of CD4 T cells with restitution of IL-2 secretion (349). Thus, by inhibiting HIV-1 replication, HAART substantially reverses the immunopathological processes that result from the infection.
However, HAART has now brought the issue of viral latency very much to the forefront, and this provides a huge new therapeutic challenge. Only a small proportion of the pool of HIV-infected mononuclear cells in vivo are transcriptionally active at any point in time (5), and much of the inactive virus exists in a transcriptionally incompetent, unintegrated form (59, 369). However, following the introduction of HAART, it has become clear that antiretroviral agents fail to eliminate HIV-1 from the body despite prolonged suppression of viremia. HIV-1 provirus can exist in a latent, integrated form in inactive circulating CD4+ T lymphocytes (59, 103, 362) and monocytes (183). These latent viral pools, together with sequestered sites that support ongoing low-level HIV-1 replication (61, 139), are major obstacles to a therapeutic cure of HIV-1 infection by HAART.
In the pre-HAART era, the rationale for the use of the majority of immunomodulating therapeutic strategies was to suppress activation-induced HIV-1 replication (151). Now, however, new immunomodulating strategies used in conjunction with antiretroviral agents are directed at activating, and thereby purging, the latently HIV-1-infected cellular pool (57). It has been found that cytokines that activate T cells are able to induce HIV-1 replication in vitro in latently infected CD4+ T cells obtained from infected persons receiving HAART (58). Following this observation, clinical trials of IL-2 in combination with HAART indicate that the size of the latent pool of resting CD4+ cells that contain replication-competent HIV-1 can be reduced (57) but not eliminated (60). At present, the complexity of the host-virus relationship seems to present an insuperable barrier to the elimination of HIV-1 from infected persons.
Prevention and Treatment of Coinfections
The widespread use of HAART in the treatment of HIV-infected persons in westernized countries has resulted in a phenomenal decrease in the incidence of opportunistic infections and has greatly increased survival. For these individuals, the antiretroviral drugs are the major determinant of prognosis and the potential cofactor effect of opportunistic infections is now a more minor consideration. However, the vast majority (>95%) of the world's HIV-infected people do not currently have access to antiretroviral drugs. Most of these people live in developing countries, where the quality and access to health care is often limited and where there is a high incidence of endemic infectious diseases such as malaria, TB, and infections by helminths and waterborne pathogens, which may adversely affect HIV-1 disease progression. Prevention or early treatment of these diseases may therefore represent an important strategy in addressing the HIV-1 epidemic in developing countries.
In addition to its use in prophylaxis of P. carinii infection in HIV-infected persons living in industrialized countries (104), trimethoprim-sulfamethoxazole prophylaxis reduces morbidity and mortality in HIV-infected patients with TB in Africa though the prevention of other opportunistic infections (354). Clearly, antibiotic prophylaxis represents an important strategy that should be further evaluated. The use of isoniazid as prophylaxis against TB in HIV-infected persons reduces the incidence of TB; however, overall cohort survival was improved in only in one of four community-based studies using this strategy (reviewed in reference 78), but survival was improved in a meta-analysis of purified protein derivative-positive patients (355). To date, no studies have examined the important question of whether recurrent treatment of gastrointestinal helminths or prophylaxis of malaria has a beneficial effect on HIV-1 disease progression in HIV-infected persons living in areas where these diseases are endemic. In addition to prophylactic measures, the rapid and effective treatment of opportunistic infections in HIV-infected persons is clearly important. Delays in the diagnosis and commencement of treatment of infections such as TB not only have acute consequences on morbidity and mortality but also may lead to escalation of any adverse affect on the HIV-1 burden (187, 191). In addition to addressing the effect of coinfections on HIV-infected persons, it is clear that reduction of the spread of HIV-1 infection is an extremely important goal. One of the most critical components of HIV-1 control measures in both westernized and developing countries is the prevention, diagnosis, and treatment of STDs (137), which are potent cofactors in the transmission of the virus within the community.
CONCLUSIONS
It is clear that the life cycle of HIV-1 is intimately linked to the activation state of infected mononuclear cells and that systemic immune activation is both the result of and a driving force behind the phenomenal viral and cellular kinetics in HIV-infected persons. Thus, immune activation is central to the immunopathogenesis of HIV-1 infection in vivo.
The induction of immune activation by HIV-1 envelope glycoproteins in vivo stimulates ongoing viral replication and activation-induced mononuclear cell apoptosis, thereby promoting the maintenance of infection and progression of disease. In addition, exogenous immune-activating stimuli such as opportunistic infections and immunizations enhance HIV-1 replication in vivo. This not only results in increases in virus load in the systemic circulation, body tissues, and anatomical compartments but also may induce change in the genotype and phenotype of expressed viruses. Epidemiological data, albeit limited, indicate that by these means, some but not all such immune-activating stimuli significantly contribute to long-term acceleration of disease progression in HIV-infected persons. In the genital tract, the marked effects of immune activation on the biology of HIV-1 provide plausible mechanisms that would explain, at least in part, the potent cofactor effect of genital tract inflammation on sexual and mother-to-child transmission of HIV-1. Prevention of STDs by both behavioral and biological means and treatment of STDs are clearly vital components of strategies to control the transmission of HIV-1.
The widespread use of HAART in the treatment of HIV-infected persons in westernized countries has resulted in a marked reduction in opportunistic infections and a great improvement in prognosis; the potential cofactor effect of coinfections in these individuals is now a more minor consideration. However, in countries where antiretroviral drugs are not yet widely available and where there is a high incidence of opportunistic and endemic infectious diseases, studies on the effect of such coinfections on HIV-1 pathogenesis are greatly needed. Research also should determine the effect of treatment and prevention of coinfections on HIV-1 disease progression. Moreover, the development of simple, nontoxic, immunomodulating treatments that reduce the systemic HIV-1 burden and slow the progression of disease may provide therapeutic options in addition to the use of antiretroviral drugs.
ACKNOWLEDGMENTS
The body of research performed by Stephen D. Lawn that led to the writing of this review was funded by the Wellcome Trust, London, United Kingdom, and subsequently by a Research Participation Program administered by the Oak Ridge Institute for Science and Education, Oak Ridge, Tenn.
REFERENCES
- 1.Actor J K, Shirai M, Kullberg M C, Buller R M L, Sher A. Helminth infection results in decreased virus-specific CD8+ cytotoxic T-cell responses as well as delayed virus clearance. Proc Natl Acad Sci USA. 1993;90:948–952. doi: 10.1073/pnas.90.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ainsworth J G, Hourshid S, Easterbrook P J, Gilroy C B, Weber J N, Taylor-Robinson D. Mycoplasma species in rapid and slow HIV progressors. Int J STD AIDS. 2000;11:76–79. doi: 10.1177/095646240001100202. [DOI] [PubMed] [Google Scholar]
- 3.Ameisen J C, Capron A. Cell dysfunction and depletion in AIDS: the programmed cell death hypothesis. Immunol Today. 1991;12:102–105. doi: 10.1016/0167-5699(91)90092-8. [DOI] [PubMed] [Google Scholar]
- 4.Amirayan-Chevillard N, Tissot-Dupont H, Capo C, Brunet C, Dignat-George F, Obadia Y, Gallais H, Mege J L. Impact of highly active anti-retroviral therapy (HAART) on cytokine production and monocyte subsets in HIV-infected patients. Clin Exp Immunol. 2000;120:107–112. doi: 10.1046/j.1365-2249.2000.01201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson R W, Ascher M S, Sheppard H W. Direct HIV cytopathicity cannot account for CD4 decline in AIDS in the presence of homeostasis: a worst-case dynamic analysis. J Acquired Immune Defic Syndr Hum Retrovirol. 1998;17:245–252. doi: 10.1097/00042560-199803010-00010. [DOI] [PubMed] [Google Scholar]
- 6.Andersson J, Fehniger T E, Patterson B K, Pottage J, Agnoli M, Jones P, Behbahani H, Landay A. Early reduction of immune activation in lymphoid tissue following highly active HIV therapy. AIDS. 1998;12:F123–129. doi: 10.1097/00002030-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Andrews W W, Goldenberg R L, Hauth J C. Preterm labor: emerging role of genital tract infections. Infect Agents Dis. 1995;4:196–211. [PubMed] [Google Scholar]
- 8.Andrieu J M, Even P, Venet A, Tourani J M, Stern M, Lowenstein W, Audroin C, Eme D, Masson D, Sors H, Israel-Biet D, Beldjord K. Effects of cyclosporin on T-cell subsets in human immunodeficiency virus disease. Clin Immunol Immunopathol. 1988;47:181–198. doi: 10.1016/0090-1229(88)90071-2. [DOI] [PubMed] [Google Scholar]
- 9.Andrieu J M, Lu W, Levy R. Sustained increases in CD4 cell counts in asymptomatic human immunodeficiency virus type 1-seropositive patients treated with prednisolone for 1 year. J Infect Dis. 1995;171:523–530. doi: 10.1093/infdis/171.3.523. [DOI] [PubMed] [Google Scholar]
- 10.Antoni B A, Stein S B, Rabson A B. Regulation of human immunodeficiency virus infection: implications for pathogenesis. Adv Virus Res. 1994;43:53–145. doi: 10.1016/s0065-3527(08)60047-0. [DOI] [PubMed] [Google Scholar]
- 11.Ariyoshi K, van der Loef M S, Berry N, Jaffar N, Whittle H. Plasma HIV viral load in relation to season and to Plasmodium falciparum parasitaemia. AIDS. 1999;13:1145–1146. doi: 10.1097/00002030-199906180-00023. [DOI] [PubMed] [Google Scholar]
- 12.Ariyoshi K, Berry N, Wilkins A, Ricard D, Aaby P, Naucler A, Nigom P T, Jobe O, Jaffar S, Dias F, Tedder R S, Whittle H. A community-based study of human immunodeficiency virus type 2 provirus load in a rural village in West Africa. J Infect Dis. 1996;173:245–248. doi: 10.1093/infdis/173.1.245. [DOI] [PubMed] [Google Scholar]
- 13.Asada H, Klaus-Kovtun V, Golding H, Katz S I, Blauvelt A. Human herpesvirus 6 infects dendritic cells and suppresses human immunodeficiency virus type 1 replication in coinfected cultures. J Virol. 1999;73:4019–4028. doi: 10.1128/jvi.73.5.4019-4028.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aukrust P, Liabakk N B, Muller F, Espevik T, Froland S S. Activation of tumor necrosis factor-alpha system in HIV-1 infection: association with markers of immune activation. Infection. 1995;23:9–15. doi: 10.1007/BF01710050. [DOI] [PubMed] [Google Scholar]
- 15.Aziz N, Nishanian P, Taylor J M, Mitsuyasu R T, Jacobson J M, Dezube B J, Lederman M M, Detels R, Fahey J L. Stability of plasma levels of cytokines and soluble activation markers in patients with human immunodeficiency virus infection. J Infect Dis. 1999;179:843–848. doi: 10.1086/314673. [DOI] [PubMed] [Google Scholar]
- 16.Baeuerle P A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991;1072:63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- 17.Baier-Bitterlich G, Wachter H, Fuchs D. Role of neopterin and 7,8-dihydroneopterin in human immunodeficiency virus infection: marker for disease progression and pathogenic link. J Acquired Immune Defic Syndr Hum Retrovirol. 1996;13:184–193. doi: 10.1097/00042560-199610010-00010. [DOI] [PubMed] [Google Scholar]
- 18.Bala S, England G, Kovacs J, Wahl L, Martin M, Sher A, Gazzinelli R T. Toxoplasma gondii soluble products induce cytokine secretion by macrophages and potentiate in vitro replication of a monotropic strain of HIV. J Eukaryot Microbiol. 1994;41:7S. [PubMed] [Google Scholar]
- 19.Barcellini W, Rizzardi G P, Poli G, Tambussi G, Velati C, Meroni P L, Dalgleish A G, Lazzarin A. Cytokines and soluble receptor changes in the transition from primary to early chronic HIV type 1 infection. AIDS Res Hum Retroviruses. 1996;12:325–331. doi: 10.1089/aid.1996.12.325. [DOI] [PubMed] [Google Scholar]
- 20.Bass H Z, Nishanian P, Hardy W D, Mitsuyasu R T, Esmail E, Cumberland W, Fahey J L. Immune changes in HIV-1 infection: significant correlations and differences in serum markers and lymphoid phenotypic antigens. Clin Immunol Immunopathol. 1992;64:63–70. doi: 10.1016/0090-1229(92)90060-2. [DOI] [PubMed] [Google Scholar]
- 21.Bentwich Z, Kalinkovich A, Weisman Z. Immune activation is a dominant factor in the pathogenesis of African AIDS. Immunol Today. 1995;16:187–191. doi: 10.1016/0167-5699(95)80119-7. [DOI] [PubMed] [Google Scholar]
- 22.Bentwich Z, Kalinkovich A, Weisman Z, Borkow G, Beyers B, Beyers A D. Can eradication of helminthic infections change the face of AIDS and tuberculosis? Immunol Today. 1999;11:485–487. doi: 10.1016/s0167-5699(99)01499-1. [DOI] [PubMed] [Google Scholar]
- 23.Bergamini A, Faggioli E, Bolacchi F, Gessani S, Cappannoli L, Ucella I, Demin F, Capozzi M, Cicconi R, Placido R, Vendetti S, Colizzi G M V, Rocchi G. Enhanced production of tumor necrosis factor-α and interleukin-6 due to prolonged response to lipopolysaccharide in human macrophages infected in vitro with human immunodeficiency virus type 1. J Infect Dis. 1999;179:832–842. doi: 10.1086/314662. [DOI] [PubMed] [Google Scholar]
- 24.Berhe N, Wolday D, Hailu A, Abraham Y, Ali A, Gebre-Michael T, Desjeux P, Sonnerborg A, Akuffo H, Britton S. HIV viral load and response to antileishmanial chemotherapy in co-infected patients. AIDS. 1999;13:1921–1925. doi: 10.1097/00002030-199910010-00015. [DOI] [PubMed] [Google Scholar]
- 25.Bernier R, Turco S J, Olivier M, Tremblay M. Activation of human immunodeficiency virus type 1 in monocytoid cells by the protozoan parasite Leishmania donovani. J Virol. 1995;69:7282–7285. doi: 10.1128/jvi.69.11.7282-7285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernstein M S, Tong-Starksen S E, Locksley R M. Activation of human monocyte-derived macrophages with lipopolysaccharide decreases human immunodeficiency virus replication in vitro at the level of gene expression. J Clin Investig. 1991;88:540–545. doi: 10.1172/JCI115337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bisset L R, Cone R W, Huber W, Battegay M, Vernazza P L, Weber R, Grob P J, Opravil M. Highly active antiretroviral therapy during early HIV infection reverses T-cell activation and maturation abnormalities. Swiss HIV Cohort Study. AIDS. 1998;12:2115–2123. doi: 10.1097/00002030-199816000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Biswas D K, Dezube B J, Ahlers C M, Pardee A B. Pentoxifylline inhibits HIV-1 LTR-driven gene expression by blocking NF-κB action. J Acquired Immun Defic Syndr. 1993;6:778–786. [PubMed] [Google Scholar]
- 29.Blanchard A, Montagnier L, Gougeon M-L. Influence of microbial infections on the progression of HIV disease. Trends Microbiol. 1997;5:326–331. doi: 10.1016/S0966-842X(97)01089-5. [DOI] [PubMed] [Google Scholar]
- 30.Blatt S P, McCarthy W F, Bucko-Krasnicka B, Melcher G P, Boswell R N, Dolan J, Freeman T M, Rusnak J M, Hensley R E, Ward W W, Barnes D, Hendrix C W. Multivariate models for predicting progression to AIDS and survival in human immunodeficiency virus-infected persons. J Infect Dis. 1995;171:837–844. doi: 10.1093/infdis/171.4.837. [DOI] [PubMed] [Google Scholar]
- 31.Blazquez M V, Madueno J A, Jurado R, Fernandez-Areas N, Munoz E. Human herpesvirus-6 and the course of human immunodeficiency virus infection. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;9:389–394. [PubMed] [Google Scholar]
- 32.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bloland P B, Wirima J J, Steketee R W, Chilima B, Hightower A, Berman J G. Maternal HIV infection and infant mortality in Malawi: evidence for increased mortality due to placental malaria infection. AIDS. 1995;9:721–726. doi: 10.1097/00002030-199507000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Bohnlein E, Siekevitz M, Ballard D W, Lowenthal J W, Rimsky L, Bogerd H, Hoffman J, Wano Y, Franza B R, Greene W C. Stimulation of the human immunodeficiency virus type 1 enhancer by the human T-cell leukemia virus type I tax gene product involves the action of inducible cellular proteins. J Virol. 1989;63:1578–1586. doi: 10.1128/jvi.63.4.1578-1586.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boriskin Y S, Sharland M, Dalton R, duMont G, Booth J C. Viral loads in dual infection with HIV-1 and cytomegalovirus. Arch Dis Child. 1999;80:132–136. doi: 10.1136/adc.80.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bressler P, Poli G, Justement J S, Biswas P, Fauci A S. Glucocorticoids synergize with tumor necrosis factor alpha in the induction of HIV expression from a chronically infected promonocytic cell line. AIDS Res Hum Retroviruses. 1993;9:547–551. doi: 10.1089/aid.1993.9.547. [DOI] [PubMed] [Google Scholar]
- 37.Brew B J, Pemberton L, Cunningham P, Law M G. Levels of human immunodeficiency virus type 1 RNA in cerebrospinal fluid correlate with AIDS dementia stage. J Infect Dis. 1997;175:963–966. doi: 10.1086/514001. [DOI] [PubMed] [Google Scholar]
- 38.Brichacek B, Swindells S, Janoff E N, Pirruccello S, Stevenson M. Increased plasma human immunodeficiency virus type 1 burden following antigenic challenge with pneumococcal vaccine. J Infect Dis. 1996;174:1191–1199. doi: 10.1093/infdis/174.6.1191. [DOI] [PubMed] [Google Scholar]
- 39.Brown C C, Poli G, Lubaki N, St. Louis M, Davachi F, Musey L, Manzila T, Kovacs A, Quinn T C, Fauci A S. Elevated levels of tumor necrosis factor-alpha in Zairian neonate plasmas: implications for perinatal infection with the human immunodeficiency virus. J Infect Dis. 1994;169:975–980. doi: 10.1093/infdis/169.5.975. [DOI] [PubMed] [Google Scholar]
- 40.Bukrinsky M I, Stanwick T L, Dempsey M P, Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991;254:423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bush C E, Donovan R M, Markowitz N P, Kvale P, Saravolatz L D. A study of HIV RNA viral load in AIDS patients with bacterial pneumonia. J Acquired Immune Defic Syndr Hum Retrovirol. 1996;13:23–26. doi: 10.1097/00042560-199609000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Butera S T, Roberts B D, Folks T M. Regulation of HIV-1 expression by cytokine networks in a CD4+ model of chronic infection. J Immunol. 1993;150:625–634. [PubMed] [Google Scholar]
- 43.Byrn R A, Zhang D, Eyre R, McGowan K, Kiessling A A. HIV-1 in semen: an isolated virus reservoir. Lancet. 1997;350:1141. doi: 10.1016/S0140-6736(97)24042-0. [DOI] [PubMed] [Google Scholar]
- 44.Cacopardo B, Nigro L, Preiser W, Fama A, Satariano M I, Braner J, Celesia B M, Weber B, Russo R, Doerr H W. Prolonged Th2 cell activation and increased viral replication in HIV-Leishmania co-infected patients despite treatment. Trans R Soc Trop Med Hyg. 1996;90:434–435. doi: 10.1016/s0035-9203(96)90538-6. [DOI] [PubMed] [Google Scholar]
- 45.Cameron D W, Simonsen J N, D'Costa J N, Ronald A R, Maitha G M, Gakinya M N, Cheang M, Ndinya-Achola J O, Piot P, Brunham R C, Plummer F A. Female to male transmission of human immunodeficiency virus type 1: risk factors for seroconversion in men. Lancet. 1989;ii:403–407. doi: 10.1016/s0140-6736(89)90589-8. [DOI] [PubMed] [Google Scholar]
- 46.Cantin R, Fortin J-F, Lamontagne G, Tremblay M. The acquistion of host-derived major histocompatibility complex class II glycoproteins by human immunodeficiency virus type 1 accelerates the process of virus entry and infection in human T-lymphoid cells. Blood. 1997;90:1091–1100. [PubMed] [Google Scholar]
- 47.Cao Y, Qin L, Zhang L, Safrit J, Ho D D. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 48.Capobianchi M R. Induction of lymphomonocyte activation by HIV-1 glycoprotein gp120. Possible role in AIDS pathogenesis. J Biol Regul Homeostatic Agents. 1996;10:83–91. [PubMed] [Google Scholar]
- 49.Carroll R G, Riley J L, Levine B L, Blair P J, St. Louis D C, June C H. The role of co-stimulation in regulation of chemokine receptor expression and HIV-1 infection in primary T lymphocytes. Semin Immunol. 1998;10:195–202. doi: 10.1006/smim.1998.0131. [DOI] [PubMed] [Google Scholar]
- 50.Castilletti C, Capobianchi M R, Fais S, Abbate I, Ficociello B, Ameglio F, Cordiali Fei P, Santini S M, Dianzani F. HIV type 1 grown on interferon γ-treated U937 cells shows selective increase in virion-associated intercellular adhesion molecule 1 and HLA-DR and enhanced infectivity for CD4-negative cells. AIDS Res Hum Retrovirus. 1995;11:547–553. doi: 10.1089/aid.1995.11.547. [DOI] [PubMed] [Google Scholar]
- 51.Chaisson R E, Gallant J E, Keruly J C, Moore R D. Impact of opportunistic disease on survival in patients with HIV infection. AIDS. 1998;12:29–33. doi: 10.1097/00002030-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Chaisson R E, Keruly J, Richman D D, Moore R D. Pneumocystis prophylaxis and survival in patients with advanced human immunodeficiency virus infection treated with zidovudine. Arch Intern Med. 1992;152:2009–2013. [PubMed] [Google Scholar]
- 53.Chapman L E, Green T A, Ahmed F, Parekh B S, Rimland D, Kaplan J E, Thompson M A, Folks T M. Effect of clinical events on plasma HIV-1 RNA levels in persons with CD4+ T-lymphocyte counts of more than 500 × 106 cells/l. AIDS. 2000;14:1135–1146. doi: 10.1097/00002030-200006160-00010. [DOI] [PubMed] [Google Scholar]
- 54.Chapuis A G, Paolo Rizzardi G, D'Agostino C, Attinger A, Knabenhans C, Fleury S, Acha-Orbea H, Pantaleo G. Effects of mycophenolic acid on human immunodeficiency virus infection in vitro and in vivo. Nat Med. 2000;6:762–768. doi: 10.1038/77489. [DOI] [PubMed] [Google Scholar]
- 55.Cheeseman S H, Davaro R E, Ellison R T., III Hepatitis B vaccination and plasma HIV-1 RNA. N Engl J Med. 1996;334:1272. doi: 10.1056/NEJM199605093341916. [DOI] [PubMed] [Google Scholar]
- 56.Cheynier R, Henrichwark S, Hadida F, Pelletier E, Oksenhendler E, Autran B, Wain-Hobson S. HIV and T cell expansion in splenic white pulps is accompanied by infiltration of HIV-specific cytotoxic T lymphocytes. Cell. 1994;78:373–387. doi: 10.1016/0092-8674(94)90417-0. [DOI] [PubMed] [Google Scholar]
- 57.Chun T W, Engel D, Mizell S B, Hallahan C W, Fischette M, Park S, Davey R T, Jr, Dybul M, Kovacs J A, Metcalf J A, Mican J M, Berrey M M, Corey L, Lane H C, Fauci A S. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med. 1999;5:651–655. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 58.Chun T W, Engel D, Mizell S B, Ehler L A, Fauci A S. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J Exp Med. 1998;188:83–91. doi: 10.1084/jem.188.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chun T W, Carruth L, Finzi D, Shen X, DiGiuseppe J A, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn T C, Kuo Y H, Brookmeyer R, Zeiger M A, Barditch-Crovo P, Siliciano R F. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 60.Chun T W, Davey R T, Jr, Engel D, Lane H C, Fauci A S. Re-emergence of HIV after stopping therapy. Nature. 1999;401:874–875. doi: 10.1038/44755. [DOI] [PubMed] [Google Scholar]
- 61.Chun T W, Davey Jr R T, Ostrowski M, Shawn Justement J, Engel D, Mullins J I, Fauci A S. Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat Med. 2000;6:757–761. doi: 10.1038/77481. [DOI] [PubMed] [Google Scholar]
- 62.Cinque P, Vago L, Ceresa D, Mainini F, Terreni M R, Vagani A, Torri W, Bossolasco S, Lazzarin A. Cerebrospinal fluid HIV-1 RNA levels: correlation with HIV encephalitis. AIDS. 1998;12:389–394. doi: 10.1097/00002030-199804000-00007. [DOI] [PubMed] [Google Scholar]
- 63.Clark D R, de Boer R J, Wolthers K C, Miedema F. T cell dynamics in HIV-1 infection. Adv Immunol. 1999;73:301–327. doi: 10.1016/s0065-2776(08)60789-0. [DOI] [PubMed] [Google Scholar]
- 64.Clemetson D B A, Moss G B, Willerford D M, Hensel M, Emonyi W, Holmes K K, Plummer F, Ndinya-Achola J, Roberts P L, Hillier S, Kreiss J K. Detection of HIV DNA in cervical and vaginal secretions. JAMA. 1993;269:2860–2864. [PubMed] [Google Scholar]
- 65.Clerici M, Shearer G M. A Th1 to Th2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;107:107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 66.Clerici M, Butto S, Lukwiya M, Saresella M, Declich S, Trabattoni D, Pastori C, Piconi S, Fracasso C, Fabiani M, Ferrante P, Rizzardini G, Lopalco L. Immune activation in Africa is environmentally-driven and is associated with upregulation of CCR5. Italian-Ugandan AIDS Project. AIDS. 2000;14:2083–2092. doi: 10.1097/00002030-200009290-00003. [DOI] [PubMed] [Google Scholar]
- 67.Clouse K A, Powell D, Washington I, Poli G, Strebel K, Farrar W, Barstad P, Kovacs J, Fauci A S, Folks T M. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol. 1989;142:431–438. [PubMed] [Google Scholar]
- 68.Cohen M S, Hoffman I F, Royce R A, Kazembe P, Dyer J R, Daly C C, Zimba D, Vernazza P L, Maida M, Fiscus S A, Eron J J., Jr Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 69.Collins K R, Mayanja-Kizza H, Sullivan B A, Quinones-Mateu M E, Toossi Z, Arts E J. Greater diversity of HIV-1 quasispecies in HIV-infected individuals with active tuberculosis. J Acquired Immune Defic Syndr. 2000;24:408–417. doi: 10.1097/00126334-200008150-00002. [DOI] [PubMed] [Google Scholar]
- 70.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Connors M, Kovacs J A, Krevat S, Gea-Banacloche J C, Sneller M C, Flanigan M, Metcalf J A, Walker R E, Falloon J, Baseler M, Feuerstein I, Masur H, Lane H C. HIV infection induces changes in CD4+ T-cell phenotype and depletions within the CD4+ T-cell repertoire that are not immediately restored by antiviral or immune-based therapies. Nat Med. 1997;3:533–540. doi: 10.1038/nm0597-533. [DOI] [PubMed] [Google Scholar]
- 72.Coombs R W, Speck C E, Hughes J P, Lee W, Sampoleo R, Ross S O, Dragavon J, Peterson G, Hooton T M, Collier A C, Corey L, Koutsky L, Krieger J N. Association between culturable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: evidence for compartmentalization of HIV-1 between semen and blood. J Infect Dis. 1998;177:320–330. doi: 10.1086/514213. [DOI] [PubMed] [Google Scholar]
- 73.Cossarizza A, Ortolani C, Mussini C, Borghi V, Guaraldi G, Mongiardo N, Bellesia E, Franceschini M G, De Rienzo B, Franceschi C. Massive activation of immune cells with an intact T cell repertoire in acute human immunodeficiency virus syndrome. J Infect Dis. 1995;172:105–112. doi: 10.1093/infdis/172.1.105. [DOI] [PubMed] [Google Scholar]
- 74.Cupps T R, Fauci A S. Corticosteroid-mediated immunoregulation in man. Immunol Rev. 1982;65:133–155. doi: 10.1111/j.1600-065x.1982.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 75.Daar E S, Lynn H, Donfield S, Gomperts E, O'Brien S J, Hilgartner M W, Hoots W K, Chernoff D, Arkin S, Wong W Y, Winkler C A. Hepatitis C virus load is associated with human immunodeficiency virus type 1 disease progression in hemophiliacs. J Infect Dis. 2001;183:589–595. doi: 10.1086/318539. [DOI] [PubMed] [Google Scholar]
- 76.Deacon N J, Tyskin A, Solomon A, Smith K, Ludford-Menting M, Hooker D J, McPhee D A, Greenway A L, Ellett A, Chatfield C, Lawson V A, Crowe S, Maerz A, Sonza S, Learmont J, Sullivan J S, Cunningham A, Dwyer D, Dowton D, Mills J. Genomic structure of an attenuated quasispecies of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 77.Dean M, Carrington M, Winkler C, Huttley G A, Smith M W, Allikmets R, Goedert J J, Buchbinder S P, Vittinghoff E, Gomperts E, Donfield S, Vlahov D, Kaslow R, Saah A, Rinaldo C, Detels R, O'Brien S J. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 78.Del Amo J, Malin A S, Pozniak A, De Cock K M. Does tuberculosis accelerate the progresson of HIV disease? Evidence from basic science and epidemiology. AIDS. 1999;13:1151–1158. doi: 10.1097/00002030-199907090-00002. [DOI] [PubMed] [Google Scholar]
- 79.de Roda Husman A M, Blaak H, Brouwer M, Schuitemaker H. CC chemokine receptor 5 cell-surface expression in relation to CC chemokine receptor 5 genotype and the clinical course of HIV-1 infection. J Immunol. 1999;163:4597–4603. [PubMed] [Google Scholar]
- 80.de Roda Husman A M, van Rij R P, Blaak H, Broersen S, Schuitemaker H. Adaptation to promiscuous usage of chemokine receptors is not a prerequisite for human immunodeficiency virus type 1 disease progression. J Infect Dis. 1999;180:1106–1115. doi: 10.1086/314987. [DOI] [PubMed] [Google Scholar]
- 81.Descamps V, Tattevin P, Descamps D, L'Heriteua F, Schortgen F, Regnier B. HIV-1 infected patients with toxic epidermal necrolysis: an occupational risk for healthcare workers. Lancet. 1999;353:1855–1856. doi: 10.1016/S0140-6736(99)91384-3. [DOI] [PubMed] [Google Scholar]
- 82.Deschamps M M, Fitzgerald D W, Pape J W, Johnson W D. HIV infection in Haiti: natural history and disease progression. AIDS. 2000;14:2515–2521. doi: 10.1097/00002030-200011100-00014. [DOI] [PubMed] [Google Scholar]
- 83.Dezube B J, Pardee A B, Chapman B, Beckett L A, Korvick J A, Novick W J, Chiurco J, Kasdan P, Ahlers C M, Ecto L T, Crumpacker C S. Pentoxifyllinc decreases tumor necrosis factor expression and serum triglycerides in people with AIDS. J Acquired Immun Defic Syndr. 1993;6:787–794. [PubMed] [Google Scholar]
- 84.Dezube B J, Lederman M M, Spritzler J G, Chapman B, Korvick J A, Flexner C, Dando S, Mattiacci M R, Ahlers C M, Zhang L, Novick W J, Jr, Kasdan P, Fahey J L, Pardee A B, Crumpacker C S the ACTG. High-dose pentoxifylline in patients with AIDS: inhibition of tumor necrosis factor production. National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group. J Infect Dis. 1995;171:1628–1632. doi: 10.1093/infdis/171.6.1628. [DOI] [PubMed] [Google Scholar]
- 85.Dezzutti C S, Swords W E, Guenthner P C, Sasso D R, Wahl L M, Drummond A H, Newman G W, King C H, Quinn F D, Lal R B. Involvement of matrix metalloproteinases in human immunodeficiency virus type 1-induced replication by clinical Mycobacterium avium isolates. J Infect Dis. 1999;180:1142–1152. doi: 10.1086/314992. [DOI] [PubMed] [Google Scholar]
- 86.Dickerson M C, Johnston J, Delea T E, White A, Andrews E. The causal role for genital ulcer disease as a risk factor for transmission of human immunodeficiency virus. Sex Transm Dis. 1996;23:429–440. doi: 10.1097/00007435-199609000-00015. [DOI] [PubMed] [Google Scholar]
- 87.Dickover R E, Garratty E M, Herman S A, Sim M S, Plaeger S, Boyer P J, Keller M, Deveikis A, Stiehm E R, Bryson Y J. Identification of levels of maternal HIV-1 RNA associated with risk of perinatal transmission. Effect of maternal zidovudine treatment on viral load. JAMA. 1996;275:599–605. [PubMed] [Google Scholar]
- 88.Donovan R M, Bush C E, Markowitz N P, Baxa D M, Saravoiatz L D. Changes in virus load markers during AIDS-Associated opportunistic diseases in human immunodeficiency virus-infected persons. J Infect Dis. 1996;174:401–403. doi: 10.1093/infdis/174.2.401. [DOI] [PubMed] [Google Scholar]
- 89.Dorrucci M, Pezzotti P, Phillips A N, Lepri A C, Rezza G. Coinfection of hepatitis C virus with human immunodeficiency virus and progression to AIDS. Italian Seroconversion Study. J Infect Dis. 1995;172:1503–1508. doi: 10.1093/infdis/172.6.1503. [DOI] [PubMed] [Google Scholar]
- 90.Douek D C, McFarland R D, Keiser P H, Gage E A, Massey J M, Haynes B F, Polis M A, Haase A T, Feinberg M B, Sullivan J L, Jamieson B D, Zack J A, Picker L J, Koup R A. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–669. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 91.Dyer J R, Kazembe P, Vernazza P L, Gilliam B L, Maida M, Zimba D, Hoffman I F, Royce R A, Schock J L, Fiscus S A, Cohen M S, Eron J J., Jr High levels of human immunodeficiency virus type 1 in blood and semen of seropositive men in sub-Saharan Africa. J Infect Dis. 1998;177:1742–1746. doi: 10.1086/517436. [DOI] [PubMed] [Google Scholar]
- 92.Embretson J, Zupancic M, Ribas J L, Burke A, Racz P, Tenner-Racz K, Haase A T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;362:359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 93.Emery S, Lane H C. Immune-based therapies in HIV infection: recent developments. AIDS. 1996;10(Suppl. A):S159–163. doi: 10.1097/00002030-199601001-00022. [DOI] [PubMed] [Google Scholar]
- 94.Emery V C, Atkins M C, Bowen E F, Clark D A, Johnson M A, Kidd I M, McLaughlin J E, Phillips A N, Strappe P M, Griffiths P D. Interactions between beta-herpesviruses and human immunodeficiency virus in vivo: evidence for increased human immunodeficiency viral load in the presence of human herpesvirus 6. J Med Virol. 1999;57:278–282. doi: 10.1002/(sici)1096-9071(199903)57:3<278::aid-jmv11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 95.Emmel E A, Verweij C L, Durand D B, Higgens K M, Lacy E, Crabtree G R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989;246:1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- 96.Evans T G, Bonnez W, Soucier H R, Fitzgerald T, Gibbons D C, Reichman R C. Highly active antiretroviral therapy results in a decrease in CD8+ T cell activation and preferential reconstitution of the peripheral CD4+ T cell population with memory rather than naive cells. Antiviral Res. 1998;39:163–173. doi: 10.1016/s0166-3542(98)00035-7. [DOI] [PubMed] [Google Scholar]
- 97.Fahey J L, Taylor J M, Manna B, Nishanian P, Aziz N, Giorgi J V, Detels R. Prognostic significance of plasma markers of immune activation, HIV viral load and CD4 T-cell measurements. AIDS. 1998;12:1581–1590. doi: 10.1097/00002030-199813000-00004. [DOI] [PubMed] [Google Scholar]
- 98.Fahey J L, Taylor J M, Detels R, Hofmann B, Melmed R, Nishanian P, Giorgi J V. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990;322:166–172. doi: 10.1056/NEJM199001183220305. [DOI] [PubMed] [Google Scholar]
- 99.Fakoya A, Matear P M, Filley E, Rook G A, Stanford J, Gilson R J, Beecham N, Weller I V, Vyakarnam A. HIV infection alters the production of both type 1 and 2 cytokines but does not induce a polarized type 1 or 2 state. AIDS. 1997;11:1445–1452. doi: 10.1097/00002030-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 100.Fang G, Burger H, Grimson R, Tropper P, Nachman S, Mayers D, Weislow O, Moore R, Reyelt C, Hutcheon N, Baker D, Weiser B. Maternal plasma human immunodeficiency virus type 1 RNA level: a determinant and projected threshold for mother-to-child transmission. Proc Natl Acad Sci USA. 1995;92:12100–12104. doi: 10.1073/pnas.92.26.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fazely F, Dezube B, Allen Ryan J, Pardee A B, Ruprecht R M. Pentoxifylline (Trental) decrease the replication of the human immunodeficency virus type 1 in human peripheral blood mononuclear cells and in cultured T cells. Blood. 1991;8:1653–1656. [PubMed] [Google Scholar]
- 102.Finkel T H, Tudor-Williams G, Banda N K, Cotton M F, Curiel T, Monks C, Baba T W, Ruprecht R M, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 103.Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho D D, Richman D D, Siliciano R F. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 104.Fischl M A, Dickinson G M, La Voie L. Safety and efficacy of sulfamethoxazole and trimethoprim chemoprophylaxis for Pneumocystis carinii pneumonia in AIDS. JAMA. 1988;259:1185–1189. doi: 10.1001/jama.259.8.1185. [DOI] [PubMed] [Google Scholar]
- 105.Folks T M, Justement J, Kinter A, Dinarello C A, Fauci A S. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987;238:800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- 106.Folks T M, Clouse K A, Justement J, Rabson A, Duh E, Kehrl J H, Fauci A S. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci USA. 1989;86:2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Folks T M, Rowe T, Villinger F, Parekh B, Mayne A, Anderson D, McClure H, Ansari A A. Immune stimulation may contribute to enhanced progression of SIV induced disease in rhesus macaques. J Med Primatol. 1997;26:181–189. doi: 10.1111/j.1600-0684.1997.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 108.Fortin J F, Cantin R, Lamontagne G, Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J Virol. 1997;71:3588–3596. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fowke K R, D'Amico R, Chernoff D N, Pottage J C, Jr, Benson C A, Sha B E, Kessler H A, Landay A L, Shearer G M. Immunologic and virologic evaluation after influenza vaccination on HIV-1 infected patients. AIDS. 1997;11:1013–1021. doi: 10.1097/00002030-199708000-00010. [DOI] [PubMed] [Google Scholar]
- 110.Franco J M, Rubio A, Rey C, Leal M, Macias J, Pineda J A, Sanchez B, Sanchez-Quijano A, Nunez-Roldan A, Lissen E. Reduction of immune system activation in HIV-1-infected patients undergoing highly active antiretroviral therapy. Eur J Clin Microbiol Infect Dis. 1999;18:733–736. doi: 10.1007/s100960050388. [DOI] [PubMed] [Google Scholar]
- 111.Garrait V, Cadranel J, Esvant H, Herry I, Morinet P, Mayaud C, Israel-Biet D. Tuberculosis generates a microenvironment enhancing the productive infection of local lymphocytes by HIV. J Immunol. 1997;159:2824–2830. [PubMed] [Google Scholar]
- 112.Garzino-Demo A, Chen M, Lusso P, Berneman Z, DiPaolo J A. Enhancement of TAT-induced transactivation of the HIV-1 LTR by two genomic fragments of HHV-6. J Med Virol. 1996;50:20–24. doi: 10.1002/(SICI)1096-9071(199609)50:1<20::AID-JMV5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 113.Gaynor R. Cellular transcription factors involved in the regulation of HIV-1 gene expression. AIDS. 1992;6:347–363. doi: 10.1097/00002030-199204000-00001. [DOI] [PubMed] [Google Scholar]
- 114.Geissler R G, Ottmann O G, Eder M, Kojouharoff G, Hoelzer D, Ganser A. Effect of recombinant human transforming growth factor beta and tumor necrosis factor alpha on bone marrow progenitor cells of HIV-infected persons. Ann Hematol. 1991;62:151–155. doi: 10.1007/BF01703139. [DOI] [PubMed] [Google Scholar]
- 115.Gendelman H E, Orenstein J M, Baca L M, Weiser B, Burger H, Kalter D C, Meltzer M S. The macrophage in the persistence and pathogenesis of HIV infection. AIDS. 1989;3:475–495. doi: 10.1097/00002030-198908000-00001. [DOI] [PubMed] [Google Scholar]
- 116.Gendelman H E, Phelps W, Feigenbaum L, Ostrove J M, Adachi A, Howley P M, Khoury G, Ginsberg H S, Martin M A. Trans-activation of the human immunodeficiency virus long terminal repeat sequence by DNA viruses. Proc Natl Acad Sci USA. 1986;83:9759–9763. doi: 10.1073/pnas.83.24.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ghassemi M, Asadi F K, Andersen B R, Novak R M. Mycobacterium avium induces HIV upregulation through mechanisms independent of cytokine induction. AIDS Res Hum Retroviruses. 2000;16:435–440. doi: 10.1089/088922200309098. [DOI] [PubMed] [Google Scholar]
- 118.Ghys P D, Fransen K, Diallo M O, Ettiegne-Traore V, Coulibaly I M, Yeboue K M, Kalish M L, Maurice C, Whitaker J P, Greenberg A E, Laga M. The associations between cervicovaginal HIV shedding, sexually transmitted diseases and immunosuppression in female sex workers in Abidjan, Cote D'Iviore. AIDS. 1997;11:F85–F93. doi: 10.1097/00002030-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 119.Gilson R J, Hawkins A E, Beecham M R, Ross E, Waite J, Briggs M, McNally T, Kelly G E, Tedder R S, Weller I V. Interactions between HIV and hepatitis B virus in homosexual men: effects on the natural history of infection. AIDS. 1997;11:597–606. doi: 10.1097/00002030-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 120.Giorgi J V, Hultin L E, McKeating J A, Johnson T D, Owens B, Jacobson L P, Shih R, Lewis J, Wiley D J, Phair J P, Wolinsky S M, Detels R. Shorter survival in advanced immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation then with plasma virus burden or virus chemokine receptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 121.Giorgi J V, Majchrowicz M A, Johnson T D, Hultin P, Matud J, Detels R. Immunologic effects of combined protease inhibitor and reverse transcriptase inhibitor therapy in previously treated chronic HIV-1 infection. AIDS. 1998;12:1833–1844. doi: 10.1097/00002030-199814000-00015. [DOI] [PubMed] [Google Scholar]
- 122.Glesby M J, Hoover D R, Farzadegan H, Margolick J B, Saah A J. The effect of influenza vaccinations on human immunodeficiency virus type 1 load: a randomized, double-blind, placebo-controlled study. J Infect Dis. 1996;174:1332–1336. doi: 10.1093/infdis/174.6.1332. [DOI] [PubMed] [Google Scholar]
- 123.Godfried M H, van der Poll T, Weverling G J, Mulder J W, Jansen J, van Deventer S J, Sauerwein H P. Soluble receptors for tumor necrosis factor as predictors of progression to AIDS in asymptomatic human immunodeficiency virus type 1 infection. J Infect Dis. 1994;169:739–745. doi: 10.1093/infdis/169.4.739. [DOI] [PubMed] [Google Scholar]
- 124.Goldenberg R L, Vermund S H, Goepfert A R, Andrews W W. Choriodecidual inflammation: a potentially preventable cause of perinatal HIV-1 transmission? Lancet. 1998;352:1927–1930. doi: 10.1016/S0140-6736(98)04453-5. [DOI] [PubMed] [Google Scholar]
- 125.Goletti D, Weissman D, Jackson R W, Graham N M H, Vlahov D, Klein R S, Munsiff S S, Ortona L, Cauda R, Fauci A S. Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J Immunol. 1996;157:1271–1278. [PubMed] [Google Scholar]
- 126.Gougeon M L, Dadaglio G, Garcia S, Muller-Alouf H, Roue R, Montagnier L. Is a dominant superantigen involved in AIDS pathogenesis? Lancet. 1993;342:50–51. doi: 10.1016/0140-6736(93)91914-8. [DOI] [PubMed] [Google Scholar]
- 127.Gougeon M L, Lecoeur H, Dulioust A, Enouf M G, Crouvoiser M, Goujard C, Debord T, Montagnier L. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J Immunol. 1996;156:3509–3520. [PubMed] [Google Scholar]
- 128.Granowitz E V, Saget B M, Wang M Z, Dinarello C A, Skolnik P R. Interleukin 1 induces HIV-1 expression in chronically infected U1 cells: blockade by interleukin 1 receptor antagonist and tumor necrosis factor binding protein type 1. Mol Med. 1995;1:667–677. [PMC free article] [PubMed] [Google Scholar]
- 129.Grant A D, Djomand G, De Cock K M. Natural history and spectrum of disease in adults with HIV/AIDS in Africa. AIDS. 1997;11(Suppl. B):S43–S54. [PubMed] [Google Scholar]
- 130.Gratton S, Cheynier R, Dumaurier M J, Oksenhendler E, Wain-Hobson S. Highly restricted spread of HIV-1 and multiply infected cells within splenic germinal centers. Proc Natl Acad Sci USA. 2000;97:14566–14571. doi: 10.1073/pnas.97.26.14566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Graziosi C, Pantaleo G, Fauci A S. Comparative analysis of constitutive cytokine expression in peripheral blood and lymph nodes of HIV-infected individuals. Res Immunol. 1994;145:602–605. doi: 10.1016/s0923-2494(05)80040-9. [DOI] [PubMed] [Google Scholar]
- 132.Graziosi C, Pantaleo G, Gantt K R, Fortin J P, Demarest J F, Cohen O J, Sekaly R P, Fauci A S. Lack of evidence for the dichotomy of Th1 and Th2 predominance in HIV-infected individuals. Science. 1994;265:248–252. doi: 10.1126/science.8023143. [DOI] [PubMed] [Google Scholar]
- 133.Graziosi C, Gantt K R, Vaccarezza M, Demarest J F, Daucher M, Saag M S, Shaw G M, Quinn T C, Cohen O J, Welbon C C, Pantaleo G, Fauci A S. Kinetics of cytokine expression during primary human immunodeficiency virus type 1 infection. Proc Natl Acad Sci USA. 1996;93:4386–4391. doi: 10.1073/pnas.93.9.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Greenberg A E, Nsa W, Ryder R W, Medi M, Nzeza M, Kitadi N, Baangi M, Malanda N, Davachi F, Hassig S E. Plasmodium falciparum malaria and perinatally acquired human immunodeficiency virus type 1 infection in Kinshasa, Zaire. A prospective, longitudinal cohort study of 587 children. N Engl J Med. 1991;325:105–109. doi: 10.1056/NEJM199107113250206. [DOI] [PubMed] [Google Scholar]
- 135.Greenhead P, Hayes P, Watts P S, Laing K G, Griffin G E, Shattock R J. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol. 2000;74:5577–5586. doi: 10.1128/jvi.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Groenink M, Fouchier R A, Broersen S, Baker C H, Koot M, van't Wout A B, Huisman H G, Miedema F, Tersmette M, Schuitemaker H. Relation of phenotype evolution of HIV-1 to envelope V2 configuration. Science. 1993;260:1513–1516. doi: 10.1126/science.8502996. [DOI] [PubMed] [Google Scholar]
- 137.Grosskurth H, Mosha F, Todd J, Mwijarubi E, Klokke A, Senkoro K, Mayaud P, Changalucha J, Nicoll A, ka-Gina G, Newell J, Mugeye K, Mabey D, Hayes R. Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: randomised controlled trial. Lancet. 1995;346:530–536. doi: 10.1016/s0140-6736(95)91380-7. [DOI] [PubMed] [Google Scholar]
- 138.Groux H, Torpier G, Monte D, Mouton Y, Capron A, Ameisen J C. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992;175:331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gunthard H F, Frost S D, Leigh-Brown A J, Ignacio C C, Kee K, Perelson A S, Spina C A, Havlir D V, Hezareh M, Looney D J, Richman D D, Wong J K. Evolution of envelope sequences of human immunodeficiency virus type 1 in cellular reservoirs in the setting of potent antiviral therapy. J Virol. 1999;73:9404–9412. doi: 10.1128/jvi.73.11.9404-9412.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Haas D W, Lederman M M, Clough L A, Wallis R S, Chernoff D, Crampton S L. Proinflammatory cytokine and human immunodeficiency virus RNA levels during early Mycobacterium avium complex bacteremia in advanced AIDS. J Infect Dis. 1998;177:1746–1749. doi: 10.1086/517437. [DOI] [PubMed] [Google Scholar]
- 141.Hammer S M, Squires K E, Hughes M D, Grimes J M, Demeter L M, Currier J S, Eron J J, Jr, Feinberg J E, Balfour H H, Jr, Deyton L R, Chodakewitz J A, Fischl M A. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 142.Hannibal M C, Markovitz D M, Clark N, Nabel G J. Differential activation of human immunodeficiency virus type 1 and 2 transcription by specific T-cell activation signals. J Virol. 1993;67:5035–5040. doi: 10.1128/jvi.67.8.5035-5040.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Harper M E, Marselle L M, Gallo R C, Wong-Staal F. Detection of lymphocytes expressing HTLV-III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci USA. 1986;83:772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Harrer T, Harrer E, Kalams S A, Elbeik T, Staprans S I, Feinberg M B, Cao Y, Ho D D, Yilma T, Caliendo A M, Johnson R P, Buchbinder S P, Walker B D. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS Res Hum Retroviruses. 1996;12:585–592. doi: 10.1089/aid.1996.12.585. [DOI] [PubMed] [Google Scholar]
- 145.Harrison L H, Schechter M. Human T cell lymphotropic virus type II and human immunodeficiency virus type 1 disease progression. J Infect Dis. 1997;176:308–309. doi: 10.1086/514035. [DOI] [PubMed] [Google Scholar]
- 146.Harrison L H, Quinn T C, Schechter M. Human T cell lymphotropic virus type I does not increase human immunodeficiency virus viral load in vivo. J Infect Dis. 1997;175:438–440. doi: 10.1093/infdis/175.2.438. [DOI] [PubMed] [Google Scholar]
- 147.Harrison T S, Nong S, Levitz S M. Induction of human immunodeficiency virus type 1 expression in monocytic cells by Cryptococcus neoformans and Candida albicans. J Infect Dis. 1997;6:485–491. doi: 10.1086/514068. [DOI] [PubMed] [Google Scholar]
- 148.Hart C E, Lennox J L, Pratt-Palmore M, Wright T C, Schinazi R F, Evans-Strickfaden T, Bush T J, Schnell C, Conley L J, Clancy K A, Ellerbrock T V. Correlation of human immunodeficiency virus type 1 RNA levels in blood and the female genital tract. J Infect Dis. 1999;179:871–882. doi: 10.1086/314656. [DOI] [PubMed] [Google Scholar]
- 149.Hashemi F B, Ghassemi M, Roebuck K A, Spear G T. Activation of human immunodeficiency virus type 1 expression by Gardnerella vaginalis. J Infect Dis. 1999;179:924–930. doi: 10.1086/314674. [DOI] [PubMed] [Google Scholar]
- 150.Haslett P A, Klausner J D, Makonkawkeyoon S, Moriera A, Metatratip P, Kunachiwa W, Maneekarn N, Vongchan P, Corral L G, Elbeik T, Shen Z, Kaplan G. Thalidomide stimulates T cell responses and interleukin 12 production in HIV-infected patients. AIDS Res Hum Retroviruses. 1999;15:1169–1179. doi: 10.1089/088922299310269. [DOI] [PubMed] [Google Scholar]
- 151.Hausen A, Dierich M P, Fuchs D, Hengster P, Reibnegger G, Schulz T, Werner E R, Wachter H. Immunosuppressants in patients with AIDS. Nature. 1986;320:114. doi: 10.1038/320114a0. [DOI] [PubMed] [Google Scholar]
- 152.Hayes R J, Schultz K F, Plummer F A. The cofactor effect of genital ulcers on the per-exposure risk of HIV transmission in sub-Saharan Africa. J Trop Med Hyg. 1995;98:1–8. [PubMed] [Google Scholar]
- 153.Heeney J L, Beverley P, McMichael A, Shearer G, Strominger J, Wahren B, Weber J, Gotch F. Immune correlates of protection from HIV and AIDS—more answers but yet more questions. Immunol Today. 1999;20:247–251. doi: 10.1016/s0167-5699(98)01437-6. [DOI] [PubMed] [Google Scholar]
- 154.Heng M C, Heng S Y, Allen S G. Co-infection and synergy of human immunodeficiency virus-1 and herpes simplex virus-1. Lancet. 1994;343:255–258. doi: 10.1016/s0140-6736(94)91110-x. [DOI] [PubMed] [Google Scholar]
- 155.Hershow R C, Galai N, Fukuda K, Graber J, Vlahov D, Rezza G, Klein R S, Des Jarlais D C, Vitek C, Khabbaz R, Freels S, Zuckerman R, Pezzotti P, Kaplan J E. An international collaborative study of the effects of coinfection with human T-lymphotropic virus type II on human immunodeficiency virus type 1 disease progression in injection drug users. J Infect Dis. 1996;174:309–317. doi: 10.1093/infdis/174.2.309. [DOI] [PubMed] [Google Scholar]
- 156.Hildreth J E, Orentas R J. Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncytium formation. Science. 1989;244:1075–1078. doi: 10.1126/science.2543075. [DOI] [PubMed] [Google Scholar]
- 157.Ho D. HIV-1 viraemia and influenza. Lancet. 1992;339:1549. doi: 10.1016/0140-6736(92)91321-x. [DOI] [PubMed] [Google Scholar]
- 158.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 159.Ho W Z, Harouse J M, Rando R F, Gonczol E, Srinivasan A, Plotkin S A. Reciprocal enhancement of gene expression and viral replication between human cytomegalovirus and human immunodeficiency virus type 1. J Gen Virol. 1990;71:97–103. doi: 10.1099/0022-1317-71-1-97. [DOI] [PubMed] [Google Scholar]
- 160.Hoffman I F, Jere C S, Taylor T E, Munthali P, Dyer J R, Wirima J J, Rogerson S J, Kumwenda N, Eron J J, Fiscus S A, Chakraborty H, Taha T E, Cohen M S, Molyneux M E. The effect of Plasmodium falciparum malaria on HIV-1 RNA blood plasma concentration. AIDS. 1999;13:487–494. doi: 10.1097/00002030-199903110-00007. [DOI] [PubMed] [Google Scholar]
- 161.Honda Y, Rogers L, Nakata K, Zhao B Y, Pine R, Nakai Y, Kurosu K, Rom W N, Weiden M. Type I interferon induces inhibitory 16-kD CCAAT/enhancer binding protein (C/EBP) beta, repressing the HIV-1 long terminal repeat in macrophages: pulmonary tuberculosis alters C/EBP expression, enhancing HIV-1 replication. J Exp Med. 1998;188:1255–1265. doi: 10.1084/jem.188.7.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Huang Y, Paxton W A, Wolinsky S M, Neumann A U, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbakhsh K, Kunstman K, Erickson D, Dragon E, Landau N R, Phair J, Ho D D, Koup R A. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 163.Imberti L, Sottini A, Bettinardi A, Puoti M, Primi D. Selective depletion in HIV infection of T cells that bear specific T cell receptor V beta sequences. Science. 1991;254:860–862. doi: 10.1126/science.1948066. [DOI] [PubMed] [Google Scholar]
- 164.Ioannidis J P, Collier A C, Cooper D A, Corey L, Fiddian A P, Gazzard B G, Griffiths P D, Contopoulos-Ioannidis D G, Lau J, Pavia A T, Saag M S, Spruance S L, Youle M S. Clinical efficacy of high-dose acyclovir in patients with human immunodeficiency virus infection: a meta-analysis of randomized individual patient data. J Infect Dis. 1998;178:349–359. doi: 10.1086/515621. [DOI] [PubMed] [Google Scholar]
- 165.Janoff E N, Tasker S A, Stevenson M, Rubins J B, O'Brien J, Utz G, Weiss P, Hall F W, Wallace M R. Immune activation and virologic response to immunization in recent HIV type 1 seroconverters. AIDS Res Hum Retroviruses. 1999;15:837–845. doi: 10.1089/088922299310746. [DOI] [PubMed] [Google Scholar]
- 166.John G C, Nduati R W, Mbori-Ngacha D, Overbaugh J, Welch M, Richardson B A, Ndinya-Achola J, Bwayo J, Krieger J, Onyango F, Kreiss J K. Genital shedding of human immunodeficiency virus type 1 DNA during pregnancy: association with immunosuppression, abnormal cervical or vaginal discharge, and severe vitamin A deficiency. J Infect Dis. 1997;175:57–62. doi: 10.1093/infdis/175.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kalinkovich A, Weisman Z, Leng Q, Borkow G, Stein M, Greenberg Z, Zlotnikov S, Eitan S, Bentwich Z. Increased CCR5 expression with decreased beta chemokine secretion in Ethiopians: relevance to AIDS in Africa. J Hum Virol. 1999;2:283–289. [PubMed] [Google Scholar]
- 168.Katsanakis C D, Sekeris C E, Spandidos D A. The human immunodeficiency virus long terminal repeat contains sequences showing partial homology to glucocorticoid responsive elements. Anticancer Res. 1991;11:381–383. [PubMed] [Google Scholar]
- 169.Khanna K V, Yu X F, Ford D H, Ratner L, Hildreth J K, Markham R B. Differences among HIV-1 variants in their ability to elicit secretion of TNF-alpha. J Immunol. 2000;164:1408–1415. doi: 10.4049/jimmunol.164.3.1408. [DOI] [PubMed] [Google Scholar]
- 170.Kiessling A A, Fitzgerald L M, Zhang D, Chhay H, Brettler D, Eyre R C, Steinberg J, McGowan K, Byrn R A. Human immunodeficiency virus in semen arises from a genetically distinct reservoir. AIDS Res Hum Retroviruses. 1998;14:S33–S41. [PubMed] [Google Scholar]
- 171.Kilby J M, Tabereaux P B, Mulanovich V, Shaw G M, Bucy R P, Saag M S. Effects of tapering doses of oral prednisone on viral load among HIV-infected patients with unexplained weight loss. AIDS Res Hum Retroviruses. 1997;13:1533–1537. doi: 10.1089/aid.1997.13.1533. [DOI] [PubMed] [Google Scholar]
- 172.Kindler V, Sappino A P, Grau G E, Pighuet P F, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 173.Kinoshita S, Chen B K, Kaneshima H, Nolan G P. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell. 1998;95:595–604. doi: 10.1016/s0092-8674(00)81630-x. [DOI] [PubMed] [Google Scholar]
- 174.Kinoshita S, Su L, Amano M, Timmerman L A, Kaneshima H, Nolan G P. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity. 1997;6:235–244. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- 175.Kirchoff F, Greenough T C, Brettler D B, Sullivan J L, Desrosiers R C. Brief report. Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 176.Kiviat N B, Critchlow C W, Hawes S E, Kuypers J, Surawicz C, Goldbaum G, van Burik J A, Lampinen T, Holmes K K. Determinants of human immunodeficiency virus DNA and RNA shedding in the anal-rectal canal of homosexual men. J Infect Dis. 1998;177:571–578. doi: 10.1086/514239. [DOI] [PubMed] [Google Scholar]
- 177.Klausner J D, Makonkawkeyoon S, Akarasewi P, Nakata K, Kasinrerk W, Corral L, Dewar R L, Lane H C, Freedman V H, Kaplan G. The effect of thalidomide on the pathogenesis of human immunodeficiency virus type 1 and M. tuberculosis infection. J Acquired Immune Defic Syndr Hum Retrovirol. 1996;11:247–257. doi: 10.1097/00042560-199603010-00005. [DOI] [PubMed] [Google Scholar]
- 178.Klebanoff S J, Watts D H, Meglin C, Headley C M. Lactobacilli and vaginal host defense: activation of the human immunodeficiency virus type 1 long terminal repeat, cytokine production, and NF-κB. J Infect Dis. 1999;179:653–660. doi: 10.1086/314644. [DOI] [PubMed] [Google Scholar]
- 179.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kreiss J, Willerford D M, Hensel M, Emonyi W, Plummer F, Ndinya-Achola J, Roberts P L, Hoskyn J, Hillier S, Kiviat N. Association between cervical inflammation and cervical shedding of human immunodeficiency virus DNA. J Infect Dis. 1994;170:1597–1601. doi: 10.1093/infdis/170.6.1597. [DOI] [PubMed] [Google Scholar]
- 181.Krieger J N, Coombs R W, Collier A C, Ross S O, Speck C, Corey L. Seminal shedding of human immunodeficiency virus type 1 and human cytomegalovirus: evidence for different immunologic controls. J Infect Dis. 1995;171:1018–1022. doi: 10.1093/infdis/171.4.1018. [DOI] [PubMed] [Google Scholar]
- 182.Kullberg M C, Pearce E J, Hieny S E, Sher A, Berzofsky J A. Infection with Schistosoma mansoni infection alters Th1/Th2 cytokine responses to a non-parasite antigen. J Immunol. 1992;148:3264–3270. [PubMed] [Google Scholar]
- 183.Lambotte O, Taoufik Y, de Goer M G, Wallon C, Goujard C, Delfraissy J F. Detection of infectious HIV in circulating monocytes from patients on prolonged highly active antiretroviral therapy. Acquired Immune Defic Syndr. 2000;23:114–119. doi: 10.1097/00126334-200002010-00002. [DOI] [PubMed] [Google Scholar]
- 184.Laurence J, Sellers M B, Sikder S K. Effect of glucocorticoids on chronic human immunodeficiency virus (HIV) infection and HIV promoter-mediated transcription. Blood. 1989;74:291–297. [PubMed] [Google Scholar]
- 185.Lawn S D, Rudolph D, Wiktor S, Coulibaly D, Ackah A, Lal R B. Tuberculosis and HIV infection are independently associated with elevated serum concentrations of tumour necrosis factor receptor type 1 and β2-microglobulin, respectively. Clin Exp Immunol. 2000;122:79–84. doi: 10.1046/j.1365-2249.2000.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lawn S D, Butera S T. Incorporation of HLA-DR into the HIV-1 envelope: correlation with stage of disease and the effect of opportunistic infection in vivo. J Virol. 2000;74:10256–10259. doi: 10.1128/jvi.74.21.10256-10259.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lawn S D, Griffin G E. An irreversible cost of delays in the diagnosis of tuberculosis in HIV-coinfected persons in sub-Sahran Africa. Int J Tuberc Lung Dis. 2001;5:200–201. [PubMed] [Google Scholar]
- 188.Lawn S D, Roberts B D, Griffin G E, Folks T M, Butera S T. Cellular compartments of HIV-1 replication: determination by virion-associated host proteins and the impact of opportunistic infection in vivo. J Virol. 2000;74:139–145. [PMC free article] [PubMed] [Google Scholar]
- 189.Lawn S D, Karanja D M S, Mwinzi P, Andove J, Colley D G, Folks T M, Secor W E. The effect of treatment of schistosomiasis on blood plasma HIV-1 RNA concentration in coinfected individuals. AIDS. 2000;14:2437–2443. doi: 10.1097/00002030-200011100-00004. [DOI] [PubMed] [Google Scholar]
- 190.Lawn S D, Labeta M O, Arias M, Acheampong J W, Griffin G E. Elevated serum concentrations of soluble CD14 in HIV-negative and HIV-positive patients with tuberculosis in Africa: prolonged elevation during antituberculosis treatment. Clin Exp Immunol. 2000;120:483–487. doi: 10.1046/j.1365-2249.2000.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lawn S D, Shattock R J, Griffin G E. Delay in the diagnosis of tuberculosis: a great new cost. Int J Tuberc Lung Dis. 1997;1:485–486. [PubMed] [Google Scholar]
- 192.Lawn S D, Shattock R J, Acheampong J W, Lal R B, Folks T M, Griffin G E, Butera S T. Sustained plasma TNF-α and HIV-1 load despite resolution of other immune activation parameters during treatment of tuberculosis in Africans. AIDS. 1999;13:2231–2237. doi: 10.1097/00002030-199911120-00005. [DOI] [PubMed] [Google Scholar]
- 193.Lawn S D, Subbarao S, Wright T C, Evans-Strickfaden T, Lennox J, Ellerbrock T, Butera S, Hart C E. Correlation between HIV-1 RNA levels in the female genital tract and immune activation resulting from genital ulceration of the cervix. J Infect Dis. 2000;181:1950–1956. doi: 10.1086/315514. [DOI] [PubMed] [Google Scholar]
- 194.Lazdins J K, Grell M, Walker M R, Woods-Cook K, Scheurich P, Pfizenmaier K. Membrane tumor necrosis factor (TNF) induced cooperative signaling of TNFR60 and TNFR80 favors induction of cell death rather than virus production in HIV-infected T cells. J Exp Med. 1997;185:81–90. doi: 10.1084/jem.185.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lederman M M, Connick E, Landay A, Kuritzkes D R, Spritzler J, St. Clair M, Kotzin B L, Fox L, Chiozzi M H, Leonard J M, Rousseau F, Wade M, Roe J D, Martinez A, Kessler H. Immunologic responses associated with 12 weeks of combination antiretroviral therapy consisting of zidovudine, lamivudine, and ritonavir: results of AIDS Clinical Trials Group Protocol 315. J Infect Dis. 1998;178:70–79. doi: 10.1086/515591. [DOI] [PubMed] [Google Scholar]
- 196.Lederman M M, Georges D L, Kusner D J, Mudido P, Giam C-Z, Toossi Z. Mycobacterium tuberculosis and its purified protein derivative activate expression of human immunodeficiencey virus. J Acquired Immune Defic Syndr. 1991;7:727–733. [PubMed] [Google Scholar]
- 197.Lederman M M, Kalish L A, Asmuth D, Fiebig E, Mileno M, Busch M P. “Modeling” relationships among HIV-1 replication, immune activation and CD4+ T-cell losses using adjusted correlative analyses. AIDS. 2000;14:951–958. doi: 10.1097/00002030-200005260-00006. [DOI] [PubMed] [Google Scholar]
- 198.Leroy V, Salmi L R, Dupon M, Sentilhes A, Texier-Maugein J, Dequae L, Dabis F, Salamon R. Progression of human immunodeficiency virus infection in patients with tuberculosis disease. A cohort study in Bordeaux, France, 1988–1994. The Groupe d'Epidemiologie Clinique du Sida en Aquitaine (GECSA) Am J Epidemiol. 1997;145:293–300. doi: 10.1093/oxfordjournals.aje.a009105. [DOI] [PubMed] [Google Scholar]
- 199.Levy R, Jais J P, Tourani J M, Even P, Andrieu J M. Long-term follow-up of HIV positive asymptomatic patients having received cyclosporin A. Adv Exp Med Biol. 1995;374:229–234. doi: 10.1007/978-1-4615-1995-9_20. [DOI] [PubMed] [Google Scholar]
- 200.Li Q, Gebhard K, Schaker T, Henry K, Haase A T. The relationship between tumor necrosis factor and human immunodeficiency virus gene expression in lymphoid tissue. J Virol. 1997;71:7080–7082. doi: 10.1128/jvi.71.9.7080-7082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lien E, Aukrust P, Sundan A, Muller F, Froland S S, Espevik T. Elevated levels of serum-soluble CD14 in human immunodeficiency virus type 1 infection: correlation to disease progression and clinical events. Blood. 1998;92:2084–2092. [PubMed] [Google Scholar]
- 202.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 203.Liu Z, Cumberland W G, Hultin L E, Kaplan A H, Detels R, Giorgi J V. CD8+ T-lymphocyte activation in HIV-1 disease reflects an aspect of pathogenesis distinct from viral burden and immunodeficiency. J Acquired Immune Defic Syndr Hum Retrovirol. 1998;18:332–340. doi: 10.1097/00042560-199808010-00004. [DOI] [PubMed] [Google Scholar]
- 204.Llibre J M, Garcia E, Aloy A, Valls J. Hepatitis C virus infection and progression of infection due to human immunodeficiency virus. Clin Infect Dis. 1993;16:182. doi: 10.1093/clinids/16.1.182. [DOI] [PubMed] [Google Scholar]
- 205.Lu W, Salerno-Goncalves R, Yuan J, Sylvie D, Han D S, Andrieu J M. Glucocorticoids rescue CD4+ T lymphocytes from activation-induced apoptosis triggered by HIV-1: implications for pathogenesis and therapy. AIDS. 1995;9:35–42. doi: 10.1097/00002030-199501000-00005. [DOI] [PubMed] [Google Scholar]
- 206.Luban J, Bossolt K L, Franke E K, Kalpana G V, Goff S P. Human immunodeficiency virus type 1 gag protein binds to cyclophilins A and B. Cell. 1993;73:1067–1078. doi: 10.1016/0092-8674(93)90637-6. [DOI] [PubMed] [Google Scholar]
- 207.Lusso P, Garzino-Demo A, Crowley R W, Malnati M S. Infection of gamma/delta T lymphocytes by human herpesvirus 6: transcriptional induction of CD4 and susceptibility to HIV infection. J Exp Med. 1995;181:1303–1310. doi: 10.1084/jem.181.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lusso P, Ensoli B, Markham P D, Ablashi D V, Salahuddin S Z, Tschachler E, Wong-Staal F, Gallo R C. Productive dual infection of human CD4+ T lymphocytes by HIV-1 and HHV-6. Nature. 1989;337:370–373. doi: 10.1038/337370a0. [DOI] [PubMed] [Google Scholar]
- 209.Lusso P, Malnati M S, Garzino-Demo A, Crowley R W, Long E O, Gallo R C. Infection of natural killer cells by human herpesvirus 6. Nature. 1993;362:458–462. doi: 10.1038/362458a0. [DOI] [PubMed] [Google Scholar]
- 210.Macchia D, Almerigogna F, Parronchi P, Ravina A, Maggi E, Romagnani S. Membrane tumour necrosis factor-alpha is involved in the polyclonal B-cell activation induced by HIV-infected human T cells. Nature. 1993;363:464–466. doi: 10.1038/363464a0. [DOI] [PubMed] [Google Scholar]
- 211.MacGregor R R, Dreyer K, Herman S, Hocknell P K, Nghiem L, Tevere V J, Williams A L. Use of PCR in detection of Mycobacterium avium complex (MAC) bacteremia: sensitivity of the assay and effect of treatment for MAC infection on concentrations of human immunodeficiency virus in plasma. J Clin Microbiol. 1999;37:90–94. doi: 10.1128/jcm.37.1.90-94.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Maciejewski J P, Weichold F F, Young N S. HIV-1 suppression of hematopoiesis in vitro mediated by envelope glycoprotein and TNF-alpha. J Immunol. 1994;153:4303–4310. [PubMed] [Google Scholar]
- 213.Maggi E, Mazzetti M, Ravina A, Annunziato F, de Carli M, Piccinni M P, Manetti R, Carbonari M, Pesce A M, del Prete G, Romagnani S. Ability of HIV to promote a Th1 to Th0 shift and to replicate preferentially in Th2 and Th0 cells. Science. 1994;265:244–248. doi: 10.1126/science.8023142. [DOI] [PubMed] [Google Scholar]
- 214.Mahalingam M, Peakman M, Davies E T, Pozniak A, McManus T J, Vergani D. T cell activation and disease severity in HIV infection. Clin Exp Immunol. 1993;93:337–343. doi: 10.1111/j.1365-2249.1993.tb08182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Makonkawkeyoon S, Limson-Pobre R N, Moreira A L, Schauf V, Kaplan G. Thalidomide inhibits the replication of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1993;90:5974–5978. doi: 10.1073/pnas.90.13.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Manca F, Habeshaw J A, Dalgleish A G. HIV envelope glycoprotein, antigen specific T-cell responses, and soluble CD4. Lancet. 1990;335:811–815. doi: 10.1016/0140-6736(90)90935-x. [DOI] [PubMed] [Google Scholar]
- 217.Mancino G, Placido R, Bach S, Mariani F, Montesano C, Ercoli L, Zembala M, Colizzi V. Infection of human monocytes with Mycobacterium tuberculosis enhances human immunodeficiency virus type 1 replication and transmission to T cells. J Infect Dis. 1997;175:1531–1535. doi: 10.1086/516494. [DOI] [PubMed] [Google Scholar]
- 218.Mandelbrot L, Le Chenadec J, Berrebi A, Bongain A, Benifla J L, Delfraissy J F, Blanche S, Mayaux M J. Perinatal HIV-1 transmission: interaction between zidovudine prophylaxis and mode of delivery in the French Perinatal Cohort. JAMA. 1998;280:55–60. doi: 10.1001/jama.280.1.55. [DOI] [PubMed] [Google Scholar]
- 219.Mandelbrot L, Mayaux M J, Bongain A, Berrebi A, Moudoub-Jeanpetit Y, Benifla J L, Ciraru-Vigneron N, Le Chenadec J, Blanche S, Delfraissy J F. Obstetric factors and mother-to-child transmission of human immunodeficiency virus type 1: the French perinatal cohorts. SEROGEST French Pediatric HIV Infection Study Group. Am J Obstet Gynecol. 1996;175:661–667. doi: 10.1053/ob.1996.v175.a75478. [DOI] [PubMed] [Google Scholar]
- 220.Mann D L, Gartner S, Le Sane F, Buchow H, Popovic M. HIV-1 transmission and function of virus-infected monocytes/macrophages. J Immunol. 1990;144:2152–2158. [PubMed] [Google Scholar]
- 221.Manoff S B, Farzadegan H, Munoz A, Astemborski J A, Vlahov D, Rizzo R T, Solomon L, Graham N M. The effect of latent Mycobacterium tuberculosis infection on human immunodeficiency virus (HIV) disease progression and HIV RNA load among injecting drug users. J Infect Dis. 1996;174:299–308. doi: 10.1093/infdis/174.2.299. [DOI] [PubMed] [Google Scholar]
- 222.Marchisio P, Esposito S, Zanchetta N, Tornaghi R, Gismondo M R, Principi N. Effect of superimposed infections on viral replication in human immunodeficiency virus type 1-infected children. Pediatr Infect Dis J. 1998;17:755–757. doi: 10.1097/00006454-199808000-00020. [DOI] [PubMed] [Google Scholar]
- 223.Marlink R, Kanki P, Thior I, Travers K, Eisen G, Siby T, Traore I, Hsieh C C, Dia M C, Gueye E H, Hellinger J, Gueye-Ndiaye A, Sankale J-K, Ndoye I, Mboup S, Essex M. Reduced rate of disease development after HIV-2 infection compared to HIV-1. Science. 1994;265:1587–1590. doi: 10.1126/science.7915856. [DOI] [PubMed] [Google Scholar]
- 224.Martin J C, Bandres J C. Cells of the monocyte-macrophage lineage and pathogenesis of HIV-1 infection. J Acquired Immun Defic Syndr. 2000;22:413–429. doi: 10.1097/00126334-199912150-00001. [DOI] [PubMed] [Google Scholar]
- 225.Martinez-Maza O, Crabb E, Mitsuyasu R T, Fahey J L, Giorgi J V. Infection with the human immunodeficiency virus (HIV) is associated with an in vivo increase in B lymphocyte activation and immaturity. J Immunol. 1987;138:3720–3724. [PubMed] [Google Scholar]
- 226.Mastroianni C M, Lichtner M, Mengoni F, D'Agostino C, d'Ettorre G, Forcina G, Santopadre P, Massetti A P, Vullo V. Changes in circulating levels of soluble cell adhesion molecules following highly active antiretroviral treatment of HIV-1-infected patients. Clin Immunol. 2000;95:212–217. doi: 10.1006/clim.2000.4865. [DOI] [PubMed] [Google Scholar]
- 227.Matsuyama T, Kobayashi N, Yamamoto N. Cytokines and HIV infection: is AIDS a tumor necrosis factor disease? AIDS. 1991;5:1405–1417. doi: 10.1097/00002030-199112000-00001. [DOI] [PubMed] [Google Scholar]
- 228.McComsey G A, Whalen C C, Mawhorter S D, Asaad R, Valdez H, Patki A H, Klaumunzer J, Gopalakrishna K V, Calabrese L H, Lederman M M. Placebo-controlled trial of prednisone in advanced HIV-1 infection. AIDS. 2001;15:321–327. doi: 10.1097/00002030-200102160-00004. [DOI] [PubMed] [Google Scholar]
- 229.Mellors J W, Rinaldo Jr C R, Gupta P, White R M, Todd J A, Kingsley L A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 230.Merrill J E, Koyanagi Y, Chen I S. Interleukin-1 and tumor necrosis factor alpha can be induced from mononuclear phagocytes by human immunodeficiency virus type 1 binding to the CD4 receptor. J Virol. 1989;63:4404–4408. doi: 10.1128/jvi.63.10.4404-4408.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Meyaard L, Otto S A, Keet I P, Roos M T, Miedema F. Programmed death of T cells in human immunodeficiency virus infection. No correlation with progression to disease. J Clin Investig. 1994;93:982–988. doi: 10.1172/JCI117105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Meyaard L, Otto S A, Jonker R R, Mijnster M J, Keet R P, Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992;257:217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 233.Mikovits J A, Lohrey N C, Schulof R, Courtless J, Ruscetti F W. Activation of infectious virus from latent human immunodeficiency virus infection of monocytes in vivo. J Clin Investig. 1992;90:1486–1491. doi: 10.1172/JCI116016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mole L, Ripich S, Margolis D, Holodniy M. The impact of active herpes simplex virus infection on human immunodeficiency virus load. J Infect Dis. 1997;176:766–770. doi: 10.1086/517297. [DOI] [PubMed] [Google Scholar]
- 235.Montagnier L, Blanchard A. Mycoplasmas as cofactors in infection due to the human immunodeficiency virus. Clin Infect Dis. 1993;17(Suppl. 1):S309–S315. [PubMed] [Google Scholar]
- 236.Moore D, Chaisson R E. Survival analysis of two controlled trials of rifabutin prophylaxis against Mycobacterium avium complex in AIDS. AIDS. 1995;9:1337–1342. doi: 10.1097/00002030-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 237.Morgan D, Maude G H, Malamba S S, Okongo M J, Wagner H U, Mulder D W, Whitworth J A. HIV-1 disease progression and AIDS-defining disorders in rural Uganda. Lancet. 1997;350:245–250. doi: 10.1016/S0140-6736(97)01474-8. [DOI] [PubMed] [Google Scholar]
- 238.Morgan D, Malamba S S, Maude G H, Okongo M J, Wagner H U, Mulder D W, Whitworth J A. An HIV-1 natural history cohort and survival times in rural Uganda. AIDS. 1997;11:633–640. doi: 10.1097/00002030-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 239.Moriuchi H, Moriuchi M, Mizell S B, Ehler L A, Fauci A S. In vitro reactivation of human immunodeficiency virus 1 from latently infected, resting CD4+ T cells after bacterial stimulation. J Infect Dis. 2000;181:2041–2044. doi: 10.1086/315496. [DOI] [PubMed] [Google Scholar]
- 240.Moriuchi M, Moriuchi H, Turner W, Fauci A S. Exposure to bacterial products renders macrophages highly susceptible to T-tropic HIV-1. J Clin Investig. 1998;102:1540–1550. doi: 10.1172/JCI4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Morris L, Silber E, Sonnenberg P, Eintracht S, Nyoka S, Lyons S F, Saffer D, Koornhof H, Martin D J. High human immunodeficiency virus type 1 RNA load in the cerebrospinal fluid from patients with lymphocytic meningitis. J Infect Dis. 1998;177:473–476. doi: 10.1086/517379. [DOI] [PubMed] [Google Scholar]
- 242.Mosca J D, Bednarik D P, Raj N B, Rosen C A, Sodroski J G, Haseltine W A, Hayward G S, Pitha P M. Activation of human immunodeficiency virus by herpesvirus infection: identification of a region within the long terminal repeat that responds to a trans-acting factor encoded by herpes simplex virus 1. Proc Natl Acad Sci USA. 1987;84:7408–7412. doi: 10.1073/pnas.84.21.7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Moss G B, Overbaugh J, Welch M, Reilly M, Bwayo J, Plummer F A, Ndinya-Achola J O, Malisa M A, Kreiss J K. Human immunodeficiency virus DNA in urethral secretions in men: association with gonococcal urethritis and CD4 cell depletion. J Infect Dis. 1995;172:1469–1474. doi: 10.1093/infdis/172.6.1469. [DOI] [PubMed] [Google Scholar]
- 244.Muro-Cacho C A, Pantaleo G, Fauci A S. Analysis of apoptosis in lymph nodes of HIV-infected persons. Intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J Immunol. 1995;154:5555–5566. [PubMed] [Google Scholar]
- 245.Nakata K, Rom W, Honda Y, Condos R, Kanegasaki S, Cao Y, Weiden M. M. tuberculosis enhances HIV-1 replication in the lung. Am J Respir Crit Care Med. 1997;155:996–1003. doi: 10.1164/ajrccm.155.3.9117038. [DOI] [PubMed] [Google Scholar]
- 246.Newell M L. Mechanisms and timing of mother-to-child transmission of HIV-1. AIDS. 1998;12:831–837. doi: 10.1097/00002030-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 247.Nishanian P, Hofmann B, Wang Y, Jackson A L, Detels R, Fahey J L. Serum soluble CD8 molecule is a marker of CD8 T-cell activation in HIV-1 disease. AIDS. 1991;5:805–812. doi: 10.1097/00002030-199107000-00003. [DOI] [PubMed] [Google Scholar]
- 248.Nishanian P, Taylor J M, Manna B, Aziz N, Grosser S, Giorgi J V, Detels R, Fahey J L. Accelerated changes (inflection points) in levels of serum immune activation markers and CD4+ and CD8+ T cells prior to AIDS onset. J Acquired Immune Defic Syndr Hum Retrovirol. 1998;18:162–170. doi: 10.1097/00042560-199806010-00008. [DOI] [PubMed] [Google Scholar]
- 249.Nuovo G J, Forde A, MacConnell P, Fahrenwald R. In situ detection of PCR-amplified HIV-1 nucleic acids and tumor necrosis factor cDNA in cervical tissues. Am J Pathol. 1993;143:40–48. [PMC free article] [PubMed] [Google Scholar]
- 250.O'Brien W A, Grovit-Ferbas K, Namazi A, Ovcak-Derzic S, Wang H J, Park J, Yeramian C, Mao S H, Zack J A. Human immunodeficiency virus-type 1 replication can be increased in peripheral blood of seropositive patients after influenza vaccination. Blood. 1995;86:1082–1089. [PubMed] [Google Scholar]
- 251.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 252.Okamoto T, Matsuyama T, Mori S, Hamamoto Y, Kobayashi N, Yamamoto N, Josephs S F, Wong-Staal F, Shimotohno K. Augmentation of human immunodeficiency virus type 1 gene expression by tumor necrosis factor alpha. AIDS Res Hum Retroviruses. 1989;5:131–138. doi: 10.1089/aid.1989.5.131. [DOI] [PubMed] [Google Scholar]
- 253.Operskalski E A, Stram D O, Busch M P, Huang W, Harris M, Dietrich S L, Schiff E R, Donegan E, Mosley J W. Role of viral load in heterosexual transmission of human immunodeficiency virus type 1 by blood transfusion recipients. Transfusion Safety Study Group. Am J Epidemiol. 1997;146:655–661. doi: 10.1093/oxfordjournals.aje.a009331. [DOI] [PubMed] [Google Scholar]
- 254.Orenstein J M, Fox C, Wahl S M. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 255.Ortiga-de-Sampaio M B, Shattock R J, Hayes P, Griffin G E, Linhares-de-Carvalho M I, Ponce de Leon A, Lewis D J, Castello-Branco L R. Increase in plasma viral load after oral cholera immunization of HIV-infected subjects. AIDS. 1998;12:F145–F150. doi: 10.1097/00002030-199814000-00001. [DOI] [PubMed] [Google Scholar]
- 256.Ostrowski M A, Krakauer D C, Li Y, Justement S J, Learn G, Ehler L A, Stanley S K, Nowak M, Fauci A S. Effect of immune activation on the dynamics of human immunodeficiency virus replication on the distribution of viral quasispecies. J Virol. 1998;72:7772–7784. doi: 10.1128/jvi.72.10.7772-7784.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ostrowski M A, Justement S J, Catanzaro A, Hallahan C A, Ehler L A, Mizell S B, Kumar P N, Mican J A, Chun T W, Fauci A S. Expression of chemokine receptors CXCR4 and CCR5 in HIV-1-infected and uninfected individuals. J Immunol. 1998;161:3195–3201. [PubMed] [Google Scholar]
- 258.Ostrowski M A, Stanley S K, Justement J S, Gantt K, Goletti D, Fauci A S. Increased in vitro tetanus-induced production of HIV type 1 following in vivo immunization of HIV type 1-infected individuals with tetanus toxoid. AIDS Res Hum Retroviruses. 1997;13:473–480. doi: 10.1089/aid.1997.13.473. [DOI] [PubMed] [Google Scholar]
- 259.Ott M, Emiliani S, Van Lint C, Herbein G, Lovett J, Chirmule N, McCloskey T, Pahwa S, Verdin E. Immune hyperactivation of HIV-1-infected T cells mediated by Tat and the CD28 pathway. Science. 1997;275:1481–1485. doi: 10.1126/science.275.5305.1481. [DOI] [PubMed] [Google Scholar]
- 260.Pantaleo G, Graziosi C, Fauci A S. New concepts in the immunopathogenesis of human immunodeficiency virus infection. N Engl J Med. 1993;328:327–335. doi: 10.1056/NEJM199302043280508. [DOI] [PubMed] [Google Scholar]
- 261.Pantaleo G, Cohen O J, Schacker T, Vaccarezza M, Graziosi C, Rizzardi G P, Kahn J, Fox C H, Schnittman S M, Schwartz D H, Corey L, Fauci A S. Evolutionary pattern of human immunodeficiency virus (HIV) replication and distribution in lymph nodes following primary infection: implications for antiviral therapy. Nat Med. 1998;4:341–345. doi: 10.1038/nm0398-341. [DOI] [PubMed] [Google Scholar]
- 262.Panther L A, Tucker L, Xu C, Tuomala R E, Mullins J I, Anderson D J. Genital tract human immunodeficiency virus type 1 (HIV-1) shedding and inflammation and HIV-1 env diversity in perinatal HIV-1 transmission. J Infect Dis. 2000;181:555–563. doi: 10.1086/315230. [DOI] [PubMed] [Google Scholar]
- 263.Pearce E J, Caspar P, Grzych J-M, Lewis F A, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Pedraza M A, del Romero J, Roldan F, Garcia S, Ayerbe M C, Noriega A R, Alcami J. Heterosexual transmission of HIV-1 is associated with high plasma viral load levels and a positive viral isolation in the infected partner. J Acquired Immune Defic Syndr. 1999;21:120–125. [PubMed] [Google Scholar]
- 265.Perelson A S, Neumann A, Markowitz M, Leonard J M, Ho D D. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 266.Perkins N D, Edwards N L, Duckett C S, Agranoff A B, Schmid R M, Nabel G J. A cooperative interaction between NF-kappa B and Sp1 is required for HIV-1 enhancer activation. EMBO J. 1993;12:3551–3558. doi: 10.1002/j.1460-2075.1993.tb06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Pettoello-Mantovani M, Casadevall A, Smarnworawong P, Goldstein H. Enhancement of HIV type 1 infectivity in vitro by capsular polysaccharide of Cryptococcus neoformans and Haemophilus influenzae. AIDS Res Hum Retroviruses. 1994;10:1079–1087. doi: 10.1089/aid.1994.10.1079. [DOI] [PubMed] [Google Scholar]
- 268.Phillips A, Wainberg M A, Coates R, Klein M, Rachlis A, Read S, Shepherd F, Vellend H, Walmsley S, Halloran P, et al. Cyclosporine-induced deterioration in patients with AIDS. Can Med Assoc J. 1989;140:1456–1460. [PMC free article] [PubMed] [Google Scholar]
- 269.Ping L H, Cohen M S, Hoffman I, Vernazza P, Seillier-Moiseiwitsch F, Chakraborty H, Kazembe P, Zimba D, Maida M, Fiscus S A, Eron J J, Swanstrom R, Nelson J A. Effects of genital tract inflammation on human immunodeficiency virus type 1 V3 populations in blood and semen. J Virol. 2000;74:8946–8952. doi: 10.1128/jvi.74.19.8946-8952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Piroth L, Duong M, Quantin C, Abrahamowicz M, Michardiere R, Aho L S, Grappin M, Buisson M, Waldner A, Portier H, Chavanet P. Does hepatitis C virus co-infection accelerate clinical and immunological evolution of HIV-infected patients? AIDS. 1998;12:381–388. doi: 10.1097/00002030-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 271.Pitt J, Schluchter M, Jenson H, Kovacs A, LaRussa P, McIntosh K, Boyer P, Cooper E, Goldfarb J, Hammill H, Hodes D, Peavy H, Sperling R, Tuomala R, Shearer W. Maternal and perinatal factors related to maternal-infant transmission of HIV-1 in the P2C2 HIV study: the role of EBV shedding. Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV-1 Infection (P2C2 HIV) Study Group. J Acquired Immune Defic Syndr Hum Retrovirol. 1998;19:462–470. doi: 10.1097/00042560-199812150-00004. [DOI] [PubMed] [Google Scholar]
- 272.Pleskoff O, Treboute C, Alizon M. The cytomegalovirus-encoded chemokine receptor US28 can enhance cell-cell fusion mediated by different viral proteins. J Virol. 1998;72:6389–6397. doi: 10.1128/jvi.72.8.6389-6397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Poli G, Fauci A S. The effect of cytokines and pharmacologic agents on chronic HIV infection. AIDS Res Hum Retroviruses. 1992;8:191–196. doi: 10.1089/aid.1992.8.191. [DOI] [PubMed] [Google Scholar]
- 274.Poli G, Kinter A, Justement J S, Kehrl J H, Bressler P, Stanley S, Fauci A S. Tumor necrosis factor alpha functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc Natl Acad Sci USA. 1990;87:782–785. doi: 10.1073/pnas.87.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Poli G, Bressler P, Kinter A, Duh E, Timmer W C, Rabson A, Justement J S, Stanley S, Fauci A S. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. J Exp Med. 1990;172:151–158. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Pomerantz R J, Feinberg M B, Trono D, Baltimore D. Lipopolysaccharide is a potent monocyte/macrophage-specific stimulator of human immunodeficiency virus type 1 expression. J Exp Med. 1990;172:253–261. doi: 10.1084/jem.172.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Popper S J, Sarr A D, Gueye-Ndiaye A, Mboup S, Essex M E, Kanki P J. Low plasma human immunodeficiency virus type 2 viral load is independent of proviral load: low virus production in vivo. J Virol. 2000;74:1554–1557. doi: 10.1128/jvi.74.3.1554-1557.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Popper S J, Sarr A D, Travers K U, Gueye-Ndiaye A, Mboup S, Essex M E, Kanki P J. Lower human immunodeficiency virus (HIV) type 2 viral load reflects the difference in pathogenicity of HIV-1 and HIV-2. J Infect Dis. 1999;180:1116–1121. doi: 10.1086/315010. [DOI] [PubMed] [Google Scholar]
- 279.Poss M, Martin H L, Kreiss J K, Granville L, Choman B, Nyange P, Mandiliya K, Overbaugh J. Diversity of virus population from genital secretions and peripheral blood from women recently infected with human immunodeficiency virus type 1. J Virol. 1995;69:8118–8122. doi: 10.1128/jvi.69.12.8118-8122.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Preiser W, Cacopardo B, Nigro L, Braner J, Nunnari A, Doerr H W, Weber B. Immunological findings in HIV-Leishmania coinfection. Intervirology. 1996;39:285–288. doi: 10.1159/000150531. [DOI] [PubMed] [Google Scholar]
- 281.Quan C M, Krajden M, Grigoriew G A, Salit I E. Hepatitis C virus infection in patients infected with the human immunodeficiency virus. Clin Infect Dis. 1993;17:117–119. doi: 10.1093/clinids/17.1.117. [DOI] [PubMed] [Google Scholar]
- 282.Quinn T C, Wawer M J, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan M O, Lutalo T, Gray R H. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 283.Ramilo O, Hicks P J, Borvak J, Gross L M, Zhong D, Squires J E, Vitetta E S. T cell activation and human immunodeficiency virus replication after influenza immunization of infected children. Pediatr Infect Dis J. 1996;15:197–203. doi: 10.1097/00006454-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 284.Rebai N, Pantaleo G, Demarest J F, Ciurli C, Soudeyns H, Adelsberger J W, Vaccarezza M, Walker R E, Sekaly R P, Fauci A S. Analysis of the T-cell receptor beta-chain variable-region (V beta) repertoire in monozygotic twins discordant for human immunodeficiency virus: evidence for perturbations of specific V beta segments in CD4+ T cells of the virus-positive twins. Proc Natl Acad Sci USA. 1994;91:1529–1533. doi: 10.1073/pnas.91.4.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Reynes J, Portales P, Segondy M, Baillat V, Andre P, Reant B, Avinens O, Couderc G, Benkirane M, Clot J, Eliaou J F, Corbeau P. CD4+ T cell surface CCR5 density as a determining factor of virus load in persons infected with human immunodeficiency virus type 1. J Infect Dis. 2000;181:927–932. doi: 10.1086/315315. [DOI] [PubMed] [Google Scholar]
- 286.Rieckmann P, Poli G, Fox C H, Kehrl J H, Fauci A S. Recombinant gp120 specifically enhances tumor necrosis factor-alpha production and Ig secretion in B lymphocytes from HIV-infected individuals but not from seronegative donors. J Immunol. 1991;147:2922–2927. [PubMed] [Google Scholar]
- 287.Rizzardi G P, Barcellini W, Tambussi G, Lillo F, Malnati M, Perrin L, Lazzarin A. Plasma levels of soluble CD30, tumour necrosis factor (TNF)-alpha and TNF receptors during primary HIV-1 infection: correlation with HIV-1 RNA and the clinical outcome. AIDS. 1996;10:F45–F50. doi: 10.1097/00002030-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 288.Rizzardini G, Trabattoni D, Saresella M, Piconi S, Lukwiya M, Declich S, Fabiani M, Ferrante P, Clerici M. Immune activation in HIV-infected African individuals. Italian-Ugandan AIDS cooperation program. AIDS. 1998;12:2387–2396. doi: 10.1097/00002030-199818000-00007. [DOI] [PubMed] [Google Scholar]
- 289.Rizzardini G, Piconi S, Ruzzante S, Fusi M L, Lukwiya M, Declich S, Tamburini M, Villa M L, Fabiani M, Milazzo F, Clerici M. Immunological activation markers in the serum of African and European HIV-positive and -seronegative individuals. AIDS. 1996;10:1535–1542. doi: 10.1097/00002030-199611000-00012. [DOI] [PubMed] [Google Scholar]
- 290.Rosok B, Voltersvik P, Bjerknes R, Axelsson M, Haaheim L R, Asjo B. Dynamics of HIV-1 replication following influenza vaccination of HIV+ individuals. Clin Exp Immunol. 1996;104:203–207. doi: 10.1046/j.1365-2249.1996.25732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Rossio J L, Bess J, Henderson L E, Cresswell P, Arthur L O. HLA class II on HIV particles is functional in superantigen presentation to human T-cells: implications for HIV pathogenesis. AIDS Res Hum Retroviruses. 1995;11:1433–1439. doi: 10.1089/aid.1995.11.1433. [DOI] [PubMed] [Google Scholar]
- 292.Royce R A, Sena A, Cates W, Cohen M S. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 293.Russo F O, Patel P C, Ventura A M, Pereira C A. HIV-1 long terminal repeat modulation by glucocorticoids in monocytic and lymphocytic cell lines. Virus Res. 1999;64:87–94. doi: 10.1016/s0168-1702(99)00082-9. [DOI] [PubMed] [Google Scholar]
- 294.Sabin C A, Phillips A N, Lee C A, Janossy G, Emery V, Griffiths P D. The effect of CMV infection on progression of human immunodeficiency virus disease is a cohort of haemophilic men followed for up to 13 years from seroconversion. Epidemiol Infect. 1995;114:361–372. doi: 10.1017/s095026880005799x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Sabin C A, Devereux H, Phillips A N, Hill A, Janossy G, Lee C A, Loveday C. Course of viral load throughout HIV-1 infection. J Acquired Immune Defic Syndr. 2000;23:172–177. doi: 10.1097/00126334-200002010-00009. [DOI] [PubMed] [Google Scholar]
- 296.Sabin C A, Telfer P, Phillips A N, Bhagani S, Lee C A. The association between hepatitis C virus genotype and human immunodeficiency virus disease progression in a cohort of hemophilic men. J Infect Dis. 1997;175:164–168. doi: 10.1093/infdis/175.1.164. [DOI] [PubMed] [Google Scholar]
- 297.Sabin E A, Araujo M I, Carvalho E M, Pearce E J. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J Infect Dis. 1996;173:269–272. doi: 10.1093/infdis/173.1.269. [DOI] [PubMed] [Google Scholar]
- 298.Salazar-Gonzalez J F, Martinez-Maza O, Nishanian P, Aziz N, Shen L-P, Grosser S, Taylor J, Detels R, Fahey J L. Increased immune activation precedes the inflection point of CD4 T cells and the increased serum virus load in human immunodeficiency virus infection. J Infect Dis. 1998;178:423–430. doi: 10.1086/515629. [DOI] [PubMed] [Google Scholar]
- 299.Schacker T, Ryncarz A J, Goddard J, Diem K, Shaughnessy M, Corey L. Frequent recovery of HIV-1 from genital herpes simplex virus lesions in HIV-1-infected men. JAMA. 1998;280:61–66. doi: 10.1001/jama.280.1.61. [DOI] [PubMed] [Google Scholar]
- 300.Schechter M, Harrison L H, Halsey N A, Trade G, Santino M, Moulton L H, Quinn T C. Coinfection with human T-cell lymphotropic virus type I and HIV in Brazil. Impact on markers of HIV disease progression. JAMA. 1994;271:353–357. [PubMed] [Google Scholar]
- 301.Schechter M, Moulton L H, Harrison L H. HIV viral load and CD4+ lymphocyte counts in subjects coinfected with HTLV-I and HIV-1. J Acquired Immune Defic Syndr Hum Retrovirol. 1997;15:308–311. doi: 10.1097/00042560-199708010-00010. [DOI] [PubMed] [Google Scholar]
- 302.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 303.Semba R D, Kumwenda N, Hoover D R, Taha T E, Quinn T C, Mtimavalye L, Biggar R J, Broadhead R, Miotti P G, Sokoll L J, van der Hoeven L, Chiphangwi J D. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 1999;180:93–98. doi: 10.1086/314854. [DOI] [PubMed] [Google Scholar]
- 304.Seto E, Yen T S, Peterlin B M, Ou J H. Trans-activation of the human immunodeficiency virus long terminal repeat by the hepatitis B virus X protein. Proc Natl Acad Sci USA. 1988;85:8286–8290. doi: 10.1073/pnas.85.21.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Shattock R J, Burger D, Dayer J M, Griffin G E. Enhanced HIV replication in monocytic cells following engagement of adhesion molecules and contact with stimulated T cells. Res Virol. 1996;147:171–179. doi: 10.1016/0923-2516(96)80232-9. [DOI] [PubMed] [Google Scholar]
- 306.Shattock R J, Rizzardi G P, Hayes P, Griffin G E. Engagement of adhesion molecules (CD18, CD11a, CD45, CD44, and CD58) enhances human immunodeficiency virus type 1 replication in monocytic cells through a tumor necrosis factor-modulated pathway. J Infect Dis. 1996;174:54–62. doi: 10.1093/infdis/174.1.54. [DOI] [PubMed] [Google Scholar]
- 307.Shattock R J, Friedland J S, Griffin G E. Phagocytosis of Mycobacterium tuberculosis modulates human immunodeficiency virus replication in human monocytic cells. Res Virol. 1994;75:849–856. doi: 10.1099/0022-1317-75-4-849. [DOI] [PubMed] [Google Scholar]
- 308.Siekevitz M, Josephs S F, Dukovich M, Peffer N, Wong-Staal F, Greene W C. Activation of the HIV-1 LTR by T cell mitogens and the trans-activator protein of HTLV-I. Science. 1987;238:1575–1578. doi: 10.1126/science.2825351. [DOI] [PubMed] [Google Scholar]
- 309.Silver S, Wahl S M, Orkin B A, Orenstein J M. Changes in circulating levels of HIV, CD4, and tissue expression of HIV in a patient with recent-onset ulcerative colitis treated by surgery. Case report. J Hum Virol. 1999;2:52–57. [PubMed] [Google Scholar]
- 310.Sinicco A, Biglino A, Sciandra M, Forno B, Pollono A M, Raiteri R, Gioannini P. Cytokine network and acute primary HIV-1 infection. AIDS. 1993;7:1167–1172. doi: 10.1097/00002030-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 311.Sinicco A, Raiteri R, Sciandra M, Dassio G, Bechis G, Maiello A. The influence of cytomegalovirus on the natural history of HIV infection: evidence of rapid course of HIV infection in HIV-positive patients infected with cytomegalovirus. Scand J Infect Dis. 1997;29:543–549. doi: 10.3109/00365549709035891. [DOI] [PubMed] [Google Scholar]
- 312.Sinicco A, Raiteri R, Sciandra M, Bertone C, Lingua A, Salassa B, Gioannini P. Coinfection and superinfection of hepatitis B virus in patients infected with human immunodeficiency virus: no evidence of faster progression to AIDS. Scand J Infect Dis. 1997;29:111–115. doi: 10.3109/00365549709035869. [DOI] [PubMed] [Google Scholar]
- 313.Solomon R E, VanRaden M, Kaslow R A, Lyter D, Visscher B, Farzadegan H, Phair J. Association of hepatitis B surface antigen and core antibody with acquisition and manifestations of human immunodeficiency virus type 1 (HIV-1) infection. Am J Public Health. 1990;80:1475–1478. doi: 10.2105/ajph.80.12.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Soudeyns H, Paolucci S, Chappey C, Daucher M B, Graziosi C, Vaccarezza M, Cohen O J, Fauci A S, Pantaleo G. Selective pressure exerted by immunodominant HIV-1-specific cytotoxic T lymphocyte responses during primary infection drives genetic variation restricted to the cognate epitope. Eur J Immunol. 1999;29:3629–3635. doi: 10.1002/(SICI)1521-4141(199911)29:11<3629::AID-IMMU3629>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 315.Spector S A, Wong R, Hsia K, Pilcher M, Stempien M J. Plasma cytomegalovirus (CMV) DNA load predicts CMV disease and survival in AIDS patients. J Clin Investig. 1998;101:497–502. doi: 10.1172/JCI1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Spina C A, Prince H E, Richman D D. Preferential replication of HIV-1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J Clin Investig. 1997;99:1774–1785. doi: 10.1172/JCI119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Srikanth P, Castillo R C, Sridharan G, John T J, Zachariah A, Mathai D, Schwartz D H. Increase in plasma IL-10 levels and rapid loss of CD4+ T cells among HIV-infected individuals in south India. Int J STD AIDS. 2000;11:49–51. doi: 10.1258/0956462001914904. [DOI] [PubMed] [Google Scholar]
- 318.Srinivas R V, Ray R B, Meyer K, Ray R. Hepatitis C virus core protein inhibits human immunodeficiency virus type 1 replication. Virus Res. 1996;45:87–92. doi: 10.1016/s0168-1702(96)01361-5. [DOI] [PubMed] [Google Scholar]
- 319.Stanley S K, Ostrowski M A, Justement J S, Gantt K, Hedayati S, Mannix M, Roche K, Schwartzentruber D J, Fox C H, Fauci A S. Effect of immunization with a common recall antigen on viral expression in patients infected with human immunodeficiency virus type 1. N Engl J Med. 1996;334:1222–1230. doi: 10.1056/NEJM199605093341903. [DOI] [PubMed] [Google Scholar]
- 320.Staprans S I, Hamilton B L, Follansbee S E, Elbeik T, Barbosa P, Grant R M, Feinberg M B. Activation of virus replication after vaccination of HIV-infected individuals. J Exp Med. 1995;182:1727–1737. doi: 10.1084/jem.182.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Stein D S, Timpone J G, Gradon J D, Kagan J M, Schnittman S M. Immune-based therapeutics: scientific rationale and the promising approaches to the treatment of the human immunodeficiency virus-infected individual. Clin Infect Dis. 1993;17:749–771. doi: 10.1093/clinids/17.4.749. [DOI] [PubMed] [Google Scholar]
- 322.St. Louis M E, Kamenga M, Brown C, Nelson A M, Manzila T, Batter V, Behets F, Kabagabo U, Ryder R W, Oxtoby M, Quinn T C, Heyward W L. Risk for perinatal HIV-1 transmission according to maternal immunologic, virologic, and placental factors. JAMA. 1993;269:2853–2859. [PubMed] [Google Scholar]
- 323.Streblow D N, Kitabwalla M, Malkovsky M, Pauza C D. Cyclophilin A modulates processing of human immunodeficiency virus type 1 p55Gag: mechanism for antiviral effects of cyclosporin A. Virology. 1998;245:197–202. doi: 10.1006/viro.1998.9155. [DOI] [PubMed] [Google Scholar]
- 324.Sturm-Ramirez K, Gaye-Diallo A, Eisen G, Mboup S, Kanki P J. High levels of tumor necrosis factor-alpha and interleukin-1 beta in bacterial vaginosis may increase susceptibility to human immunodeficiency virus. J Infect Dis. 2000;182:467–473. doi: 10.1086/315713. [DOI] [PubMed] [Google Scholar]
- 325.Stylianou E, Aukrust P, Kvale D, Muller F, Froland S S. IL-10 in HIV infection: increasing serum IL-10 levels with disease progression—down-regulatory effect of potent anti-retroviral therapy. Clin Exp Immunol. 1999;116:115–120. doi: 10.1046/j.1365-2249.1999.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sulkowski M S, Chaisson R E, Karp C L, Moore R D, Margolick J B, Quinn T C. The effect of acute infectious illnesses on plasma human immunodeficiency virus type I load and the expression of serologic markers of immune activation among HIV-infected adults. J Infect Dis. 1998;178:1642–1648. doi: 10.1086/314491. [DOI] [PubMed] [Google Scholar]
- 327.Theus S A, Harrich D A, Gaynor R, Radolf J D, Norgard M V. Treponema pallidum, lipoproteins, and synthetic lipoprotein analogues induce human immunodeficiency virus type 1 gene expression in monocytes via NF-kappaB activation. J Infect Dis. 1998;177:941–950. doi: 10.1086/515240. [DOI] [PubMed] [Google Scholar]
- 328.Toossi Z, Mayanja-Kizza H, Hirsch C S, Edmonds K L, Spahlinger T, Hom D L, Aung H, Mugyenyi P, Ellner J J, Whalen C W. Impact of tuberculosis (TB) on HIV-1 activity in dually infected patients. Clin Exp Immunol. 2001;123:233–238. doi: 10.1046/j.1365-2249.2001.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Toossi Z, Sierra-Madero J G, Blinkhorn R A, Mettler M A, Rich E A. Enhanced susceptibility of blood monocytes from patients with pulmonary tuberculosis to productive infection with human immunodeficiency virus type 1. J Exp Med. 1993;177:1511–1516. doi: 10.1084/jem.177.5.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Tremblay M J, Fortin J-F, Cantin R. The acquisition of host-encoded proteins by nascent HIV-1. Immunol Today. 1998;19:346–351. doi: 10.1016/s0167-5699(98)01286-9. [DOI] [PubMed] [Google Scholar]
- 331.Tsunetsugu-Yokota Y, Akagawa K, Kimoto H, Suzuki K, Iwasaki M, Yasuda S, Hausser G, Hultgren C, Meyerhans A, Takemori T. Monocyte-derived cultured dendritic cells are susceptible to human immunodeficiency virus infection and transmit virus to resting T cells in the process of nominal antigen presentation. J Virol. 1995;69:4544–4547. doi: 10.1128/jvi.69.7.4544-4547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331a.UNAIDS. Leishmania and HIV in gridlock. WHO/CTD/LEISH/98.9 Add. 1. UNAIDS/98.23. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 332.UNAIDS Joint United Nations Program on HIV/AIDS. AIDS epidemiology update. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]
- 333.Unutmaz D, KeewalRamani V N, Marmon S, Littman D R. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J Exp Med. 1999;11:1735–1746. doi: 10.1084/jem.189.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Valdez H J, Lederman M M. /98. Cytokines and cytokine therapies in HIV infection. AIDS Clin Rev. 1997;1997/98:187–228. [PubMed] [Google Scholar]
- 335.Van der Ende M E, Schutten M, Raschdsorff B, Grossschupff G, Racz P, Osterhaus A D, Tenner-Racz K. CD4 T cells remain the major source of HIV-1 during end stage disease. AIDS. 1999;13:1015–1019. doi: 10.1097/00002030-199906180-00002. [DOI] [PubMed] [Google Scholar]
- 336.Vanham G, Edmonds K, Qing L, Hom D, Toossi Z, Jones B, Daley C L, Huebner B, Kestens L, Gigase P, Ellner J J. Generalised immune activation in pulmonary tuberculosis: coactivation with HIV infection. Clin Exp Immunol. 1996;103:30–34. doi: 10.1046/j.1365-2249.1996.907600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Van't Wout A B, Ran L J, Kuiken C L, Kootstra N A, Pals S T, Schuitemaker H. Analysis of the temporal relationship between human immunodeficiency virus type 1 quasipsecies in sequential blood samples and various organs obtained at autopsy. J Virol. 1998;72:488–495. doi: 10.1128/jvi.72.1.488-496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Vyakarnam A, McKeating J, Meager A, Beverley P C. Tumour necrosis factors (alpha, beta) induced by HIV-1 in peripheral blood mononuclear cells potentiate virus replication. AIDS. 1990;4:21–27. doi: 10.1097/00002030-199001000-00003. [DOI] [PubMed] [Google Scholar]
- 339.Vyakarnam A, Matear P M, Martin S J, Wagstaff M. Th1 cells specific for HIV-1 gag p24 are less efficient than Th0 cells in supporting HIV replication, and inhibit virus replication in Th0 cells. Immunology. 1995;86:85–96. [PMC free article] [PubMed] [Google Scholar]
- 340.Wabwire-Mangen F, Gray R H, Mmiro F A, Ndugwa C, Abramowsky C, Wabinga H, Whalen C, Li C, Saah A J. Placental membrane inflammation and risks of maternal-to-child transmission of HIV-1 in Uganda. J Acquired Immune Defic Syndr. 1999;22:379–385. doi: 10.1097/00126334-199912010-00009. [DOI] [PubMed] [Google Scholar]
- 341.Wahl S M, Greenwell-Wild T, Peng G, Hale-Donze H, Orenstein J M. Co-infection with opportunistic pathogens promotes human immunodeficiency virus type 1 infection in macrophages. J Infect Dis. 1999;179:S457–S460. doi: 10.1086/314814. [DOI] [PubMed] [Google Scholar]
- 342.Wahl S M, Greenwell-Wild T, Peng G, Hale-Donze H, Doherty T M, Mizel D, Orenstein J M. Mycobacterium avium complex augments macrophage HIV-1 production and increases CCR5 expression. Proc Natl Acad Sci USA. 1998;95:12574–12579. doi: 10.1073/pnas.95.21.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Wainberg M A, Dascal A, Blain N, Fitz-Gibbon L, Boulerice F, Numazaki K, Tremblay M. The effect of cyclosporine A on infection of susceptible cells by human immunodeficiency virus type 1. Blood. 1988;72:1904–1910. [PubMed] [Google Scholar]
- 344.Wallis R S, Nsubuga P, Whalen C, Mugerwa R D, Okwera A, Oette D, Jackson J B, Johnson J L, Ellner J J. Pentoxifylline therapy in human immunodeficiency virus-seropositive persons with tuberculosis: a randomized, controlled trial. J Infect Dis. 1996;174:727–733. doi: 10.1093/infdis/174.4.727. [DOI] [PubMed] [Google Scholar]
- 345.Wasserheit J N. Epidemiological synergy: relationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19:61–77. [PubMed] [Google Scholar]
- 346.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, Saag M S, Shaw G M. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 347.Weiden M, Tanaka N, Qiao Y, Zhao B Y, Honda Y, Nakata K, Canova A, Levy D E, Rom W N, Pine R. Differentiation of monocytes to macrophages switches the Mycobacterium tuberculosis effect on HIV-1 replication from stimulation to inhibition: modulation of interferon response and CCAAT/Enhancer binding protein beta expression. J Immunol. 2000;165:2028–2039. doi: 10.4049/jimmunol.165.4.2028. [DOI] [PubMed] [Google Scholar]
- 348.Weisman Z, Kalinkovich A, Borkow G, Stein M, Greenberg Z, Bentwich Z. Infection by different HIV-1 subtypes (B and C) results in a similar immune activation profile despite distinct immune backgrounds. J Acquired Immune Defic Syndr. 1999;21:157–163. [PubMed] [Google Scholar]
- 349.Weiss L, Ancuta P, Girard P M, Bouhlal H, Roux A, Cavaillon N H, Kazatchkine M D. Restoration of normal interleukin-2 production by CD4+ T cells of human immunodeficiency virus-infected patients after 9 months of highly active antiretroviral therapy. J Infect Dis. 1999;180:1057–1063. doi: 10.1086/315025. [DOI] [PubMed] [Google Scholar]
- 350.Weissman D, Dybul M, Daucher M B, Davey R T, Walker R E, Kovacs J A. Interleukin-2 up-regulates expression of the human immunodeficiency virus fusion coreceptor CCR5 by CD4+ lymphocytes in vivo. J Infect Dis. 2000;181:933–938. doi: 10.1086/315303. [DOI] [PubMed] [Google Scholar]
- 351.Weissman D, Barker T D, Fauci A S. The efficiency of acute infection of CD4+ T cells is markedly enhanced in the setting of antigen-specific immune activation. J Exp Med. 1996;183:687–692. doi: 10.1084/jem.183.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Whalen C, Horsbrough C R, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of HIV infection after tuberculosis. Am J Respir Crit Care Med. 1995;151:129–135. doi: 10.1164/ajrccm.151.1.7812542. [DOI] [PubMed] [Google Scholar]
- 353.Reference deleted.
- 354.Wiktor S Z, Sassan-Morokro M, Grant A D, Abouya L, Karon J M, Maurice C, Djomand G, Ackah A, Domoua K, Kadio A, Yapi A, Combe P, Tossou O, Roels T H, Lackritz E M, Coulibaly D, De Cock K M, Coulibaly I M, Greenberg A E. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Cote d'Ivoire: a randomised controlled trial. Lancet. 1999;353:1469–1475. doi: 10.1016/s0140-6736(99)03465-0. [DOI] [PubMed] [Google Scholar]
- 355.Wilkinson D, Squire S B, Garner P. Effect of preventive treatment for tuberculosis in adults infected with HIV: systematic review of randomised placebo controlled trials. Br Med J. 1998;317:625–629. doi: 10.1136/bmj.317.7159.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Williams L M, Cloyd M W. Polymorphic human gene(s) determines differential susceptibility of CD4 lymphocytes to infection by certain HIV-1 isolates. Virology. 1991;184:723–728. doi: 10.1016/0042-6822(91)90442-e. [DOI] [PubMed] [Google Scholar]
- 357.Williams M E, Montenegro S, Domingues A L. Leukocytes of patients with Schistosoma mansoni respond with a Th2 pattern of cytokine production to mitogen or egg antigens but with a Th0 patters to worm antigens. J Infect Dis. 1994;170:946–954. doi: 10.1093/infdis/170.4.946. [DOI] [PubMed] [Google Scholar]
- 358.Wolday D, Berhe N, Akuffo H, Britton S. Leishmania-HIV interaction: immunopathogenic mechanisms. Parasitol Today. 1999;15:182–187. doi: 10.1016/s0169-4758(99)01431-3. [DOI] [PubMed] [Google Scholar]
- 359.Wolinsky S M, Wike C M, Korber B T, Hutto C, Parks W P, Rosenblum L L, Kunstman K J, Furtado M R, Munoz J L. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science. 1992;255:1134–1137. doi: 10.1126/science.1546316. [DOI] [PubMed] [Google Scholar]
- 360.Wolthers K C, Noest A J, Otto S A, Miedema F, De Boer R J. Normal telomere lengths in naive and memory CD4+ T cells in HIV type 1 infection: a mathematical interpretation. AIDS Res Hum Retroviruses. 1999;15:1053–1062. doi: 10.1089/088922299310340. [DOI] [PubMed] [Google Scholar]
- 361.Wong J, Ignacio C C, Torriani F, Havlir D, Fitch N J S, Richman D D. In vivo compartmentalization of human immunodeficiency virus: evidence from the examination of pol sequences from autopsy tissues. J Virol. 1997;71:2059–2071. doi: 10.1128/jvi.71.3.2059-2071.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Wong J K, Hezareh M, Gunthard H F, Havlir D V, Ignacio C C, Spina C A, Richman D D. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 363.Woods T C, Roberts B D, Butera S T, Folks T M. Loss of inducible virus in CD45RA naive cells after human immunodeficiency virus-1 entry accounts for preferential viral replication in CD45RO memory cells. Blood. 1997;89:1635–1641. [PubMed] [Google Scholar]
- 364.Wright T C, Subbarao S, Ellerbrock T V, Lennox J L, Evans-Strickfaden T, Smith D G, Hart C E. HIV-1 expression in the female genital tract associated with cervical inflammation and ulceration. Am J Obstet Gynecol. 2001;184:279–285. doi: 10.1067/mob.2001.108999. [DOI] [PubMed] [Google Scholar]
- 365.Wright T L, Hollander H, Pu X, Held M J, Lipson P, Quan S, Polito A, Thaler M M, Bacchetti P, Scharschmidt B F. Hepatitis C in HIV-infected patients with and without AIDS: prevalence and relationship to patient survival. Hepatology. 1994;20:1152–1155. [PubMed] [Google Scholar]
- 366.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, Mackay C R. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Xiao L H, Owen S M, Rudolph D L, Lal R B, Lal A A. Plasmodium falciparum antigen-induced human immunodeficiency virus type 1 replication is mediated through induction of tumor necrosis factor-alpha. J Infect Dis. 1998;177:437–445. doi: 10.1086/514212. [DOI] [PubMed] [Google Scholar]
- 368.Yerly S, Wunderli W, Wyler C A, Kaiser L, Hirschel B, Suter S, Perrin L H, Siegrist C A. Influenza immunization of HIV-1 infected patients does not increase HIV-1 viral load. AIDS. 1994;8:1503–1504. doi: 10.1097/00002030-199410000-00022. [DOI] [PubMed] [Google Scholar]
- 369.Zack J A, Haislip A M, Krogstad P, Chen I S. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992;66:1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 371.Zangerle R, Gallati H, Sarcletti M, Weiss G, Denz H, Wachter H, Fuchs D. Increased serum concentrations of soluble tumor necrosis factor receptors in HIV-infected individuals are associated with immune activation. J Acquired Immune Defic Syndr. 1994;7:79–85. [PubMed] [Google Scholar]
- 372.Zangerle R, Steinhuber S, Sarcletti M, Dierich M P, Wachter H, Fuchs D, Most J. Serum HIV-1 RNA levels compared to soluble markers of immune activation to predict disease progression in HIV-1-infected individuals. Int Arch Allergy Immunol. 1998;116:228–239. doi: 10.1159/000023949. [DOI] [PubMed] [Google Scholar]
- 373.Zhang L Q, MacKenzie P, Cleland A, Holmes E C, Brown A J, Simmonds P. Selection for specific sequences in the external envelope protein of human immunodeficiency virus type 1 upon primary infection. J Virol. 1993;67:3345–3356. doi: 10.1128/jvi.67.6.3345-3356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Zhang Y, Nakata K, Weiden M, Rom W. Mycobacterium tuberculosis enhances HIV-1 replication by transcriptional activation at the long terminal repeat. J Clin Investig. 1995;95:2324–2331. doi: 10.1172/JCI117924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus K A, Reimann K A, Reinhart T A, Rogan M, Cavert W, Miller C J, Veazey R S, Notermans D, Little S, Danner S A, Richman D D, Havlir D, Wong J, Jordan H L, Schacker T W, Racz P, Tenner-Racz K, Letvin N L, Wolinsky S, Haase A T. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 376.Zhu T, Wang N, Carr A, Nam D S, Moor-Jankowski R, Cooper D A, Ho D D. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J Virol. 1996;70:3098–3107. doi: 10.1128/jvi.70.5.3098-3107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Zollner B, Feucht H H, Helling-Giese G, Mattner U M, Schartl W, Polywka S, Laufs R. Threshold of HIV-1 copy numbers for vertical transmission. AIDS. 1997;11:542–543. [PubMed] [Google Scholar]