Abstract
Current efforts to engineer a clinically relevant tissue graft from human-induced pluripotent stem cells (hiPSCs) have relied on the addition or utilization of external scaffolding material. However, any imbalance in the interactions between embedded cells and their surroundings may hinder the success of the resulting tissue graft. Therefore, the goal of our study was to create scaffold-free, 3D-printed cardiac tissue grafts from hiPSC-derived cardiomyocytes (CMs), and to evaluate whether or not mechanical stimulation would result in improved graft maturation. To explore this, we used a 3D bioprinter to produce scaffold-free cardiac tissue grafts from hiPSC-derived CM cell spheroids. Static mechanical stretching of these grafts significantly increased sarcomere length compared to unstimulated free-floating tissues, as determined by immunofluorescent image analysis. Stretched tissue was found to have decreased elastic modulus, increased maximal contractile force, and increased alignment of formed extracellular matrix, as expected in a functionally maturing tissue graft. Additionally, stretched tissues had upregulated expression of cardiac-specific gene transcripts, consistent with increased cardiac-like cellular identity. Finally, analysis of extracellular matrix organization in stretched grafts suggests improved remodeling by embedded cardiac fibroblasts. Taken together, our results suggest that mechanical stretching stimulates hiPSC-derived CMs in a 3D-printed, scaffold-free tissue graft to develop mature cardiac material structuring and cellular fates. Our work highlights the critical role of mechanical conditioning as an important engineering strategy toward developing clinically applicable, scaffold-free human cardiac tissue grafts.
Keywords: engineered heart tissue, human-induced pluripotent stem cells (hiPSCs), maturation, mechanical microenvironment, tissue engineering
1 |. INTRODUCTION
The adult human heart is unable to naturally recover from severe traumatic, ischemic, or chronic damage, due to its limited regenerative potential (Urbanek et al., 2005; Xin et al., 2013). Although heart transplantation can address end-stage heart failure, organ shortages limit therapeutic availability. Therefore, human embryonic stem cells (hESCs) and human-induced pluripotent stem cells (hiPSCs) have been used to develop engineered heart tissues (EHTs), with the ultimate goal of using in vitro grown tissues to surgically repair injured human hearts (Bargehr et al., 2017; Gerbin & Murry, 2015; Tohyama & Fukuda, 2016; Tzahor & Poss, 2017; Uygur & Lee, 2016).
Recreating the material and cellular properties of adult cardiac tissue has proven to be a complex engineering challenge (Burnham et al., 2020; Patino-Guerrero et al., 2020). Many approaches to date focus on crafting a scaffolding material on to which cardiac myocytes are embedded and grown (Bian et al., 2017; Chen et al., 2008; Hansen et al., 2010; Hayashi et al., 2016; Legant et al., 2009; Liu et al., 2015; Ruan et al., 2016; Tulloch et al., 2011; Weinberger et al., 2016; Williams et al., 2015; Zimmermann et al., 2006). For example, cardiomyocytes (CMs) from neonatal rats can be mixed with collagen I and extracellular matrix factors, then mechanically conditioned, after which they adopt an adult-like phenotype (Zimmermann et al., 2002). CMs for human technology would ideally be derived from hESCs or hiPSCs (Burridge et al., 2012; Ioannis et al., 2015).
Despite initial successes, multifactorial interactions emerged between the embedded cells and their surroundings that highlight unsolved difficulties in tuning engineered scaffolds. In a simple example, excessive added extracellular matrix yields a stiff microenvironment, which can result in overexertion and subsequent failure of the embedded CMs (Engler et al., 2008). Conversely, insufficient extracellular matrix material yields a tissue that is too weak to be mechanically stressed, in turn causing CM dedifferentiation (Engler et al., 2008; Williams et al., 2015). Various spatiotemporal feedback effects present in the developmental context which affect cell identity must be accounted for, including but not limited to biochemical, electrical, mechanical, and shear stresses necessary for proper function (Andrés-Delgado & Mercader, 2016; Chan et al., 2013; Civitarese et al., 2016; McCain & Parker, 2011; Thrivikraman et al., 2018; Watson et al., 2020). Although some groups have achieved success in guiding hiPSC-derived CMs grown in scaffolding toward in vitro adult physiologic functionality, alternatively called a matured state, the scaffolding material itself may still be immunogenic, result in toxic degradation products, interfere with cell-to-cell connections, promote fibrous tissue formation during degradation, or be a mechanical mismatch with the recipient tissue microenvironment (Shimizu et al., 2006; Zimmermann et al., 2006).
Our 3D-printing approach to growing EHT, as described here, is designed to circumvent these complexities of a typical strategy which would otherwise use externally supplied scaffold or extracellular matrix as a major tissue-structuring substrate. Instead, we use a scaffold-free, 3D-printing process to position and grow hiPSC-derived CMs (hiPSC-CMs), then rely on the formation of biologically deposited and regulated extracellular matrix supplied by human fibroblasts intercalated within the engineered graft. We find that application of static stress to our 3D-printed EHT grafts promotes their maturation by regulating the formation of extracellular matrix, improving their super-cellular structuring and mechanical capabilities, and upregulating expected cardiac cell fate markers.
2 |. MATERIALS AND METHODS
2.1 |. Generation and use of hiPSCs
hiPSCs were derived from deidentified peripheral blood mononuclear cells of a Caucasian female donor. Informed consent from the donor was obtained permitting use of the tissue sample to generate and study hiPSCs. hiPSC reprogramming was performed by the Tomaselli group, and hiPSCs were used in accordance with university IRB regulations. All methods described were carried out in accordance with Johns Hopkins University regulations and guidelines, and approved by the university’s “Human Tissues, Body Fluids, and Cell lines Registration”, #BC1704170102. Differentiation into CMs was carried out by modulation of the Wnt signaling pathway using small molecules (CHIR99021, Tocris, R&D Systems, cat. #4423, and IWR-1, Sigma-Aldrich, cat. #I0161) as previously described (Lundy et al., 2014). hiPSC-CMs with beating verified by light microscopy gross examination were utilized between days 20 and 25 after differentiation.
2.2 |. Generation of engineered heart tissues
A cell suspension containing 70% hiPSC-CMs, 15% human cardiac fibroblasts (HFBs, Sciencell cat# 6330), and 15% human umbilical vein endothelial cells (HUVECS, Lonza, cat# CC-2935) was cultured in a 96-well plate. Once spontaneous spheroids had formed (72 h), a vacuum-regulated 3D bioprinter (Cyfuse 3D bioprinter, Amuza) was used to pick up and position each spheroid of cells in to an array 10 × 5 × 2 spheroids (4 mm × 2 mm × 0.8 mm) held in place by fine stainless steel needles. Following cell-spheroid fusion after an additional 72 h of culture, the coherent printed tissue was removed from the needle array then immediately used mechanical stress experiments. The procedure is summarized in Figure 1a. Complete, detailed cell culture procedures are described in the supplementary methods.
FIGURE 1.

Engineered heart tissue grafts are created using a scaffold-free, 3D-printing method, then stretched after mounting onto polydimethylsiloxane molds. (a) Human-induced pluripotent stem cell derived cardiomyocytes, cardiac fibroblasts, and human umbilical vein endothelial cells were combined in a ratio of 70%:15%:15% and incubated for 3 days to form cell spheroids. The spheroids are placed onto a stainless steel needle array using a vacuum operated 3D tissue printer, and allowed to culture for 3 days to allow for tissue fusion prior to removal from the stainless steel needle array. (b) Polydimethylsiloxane is cured around a plastic master to form a mold. After the mold is sterilized, stretched tissues are mounted onto the mold while unstretched tissues are allowed to culture free floating in media [Colour figure can be viewed at wileyonlinelibrary.com]
2.3 |. Application of mechanical stress
A 3D printer (MakerBot Replicator) was utilized to generate polylactic acid plastic masters. Dow Corning Sylgard 184 silicone (PDMS, Krayden, Cat# DC2065622) was mixed in a 10:1 ratio with catalyst 87-RC as recommended by the manufacturer, degassed, and poured over the master prior to curing for 48 h at 25°C. After the PDMS was cured, the plastic master was removed, resulting in a mold with two wells with defined dimensions of 2 mm (width) × 4.5 mm (length) × 3 mm (depth), with a central portion of the well without a PDMS bottom (2 mm wide) as shown in Figure 1b.
Excess PDMS was trimmed from the edges of the resultant mold and the mold was sterilized by washing with 70% ethanol for five minutes followed by air drying and further UV sterilization for 3 min. After UV sterilization, the PDMS molds were rinsed with 1× phosphate buffered saline three times for 5 min each. The bottoms of the wells were subsequently incubated with 10 μg/ml human recombinant laminin (Corning, cat# CB40221) for 30 min. The mold was rinsed with 1× phosphate buffered saline three times. For mechanical stimulation, EHT grafts were placed into the mold cavities and stretched to fill the PDMS well, corresponding to a 12.5% uniaxial stretch. The stretch was initially maintained with two anchor points at each end of the well where the tissue was affixed with stainless steel needles. The mold was maintained submerged in RPMI medium supplemented with B27 supplement which was changed every 48 h for the desired duration of culture.
2.4 |. Immunohistochemistry
PFA-fixed EHTs were cryosectioned, permeablized, and blocked before overnight incubation with primary antibodies: troponin T (Abcam, cat# AB8295 depending on host), collagen I (Abcam, cat#AB34710), collagen III (Abcam, cat#ab7778), or vimentin (Abcam, cat# ab92547). Confocal images were captured with a Zeiss LSM700 laser scanning confocal microscope and a Plan-Apochromat 40×/1.3 Oil DIC objective. Complete preparation procedures for sectioning and staining are described in the supplementary methods.
2.5 |. Quantification of immunofluorescence images
Immunofluorescence staining was performed for troponin T, collagen I, collagen III, vimentin, and DAPI. For each sample, between 30 and 40 individual sarcomere length measurements were taken and averaged from three randomly spaced images using ImageJ software. For quantification of area of expression for collagen I, collagen III, and vimentin staining, immunofluorescence signal exceeding a set threshold was calculated using ImageJ. Total number of cells for each image was also quantified. Areas of positive immunofluorescence staining exceeding the set threshold were normalized by the number of cells per image. Quantified data are represented as mean ± standard deviation.
Anisotropy has been previously defined (Boudaoud et al., 2014) as whether or not fibrils are ordered along a particular direction, with the dimensionless value 0 corresponding to fully disordered and value 1 corresponding to fully ordered fibrils. Using these definitions and the ImageJ software FibrilTool, the directional dependence or anisotropy of the extracellular matrix was assessed for stretched and unstretched tissues (Boudaoud et al., 2014).
2.6 |. Generation of spatiotemporal force maps
40 μm-diameter and FITC-labeled polystyrene particles coated with Protein G (PGFP-40052-5; Spherotech Inc.) were mixed with antifibronectin antibody (919801, Biolegend). After shaking for 1 h at room temperature, the antibody-conjugated particles were washed with PBS twice and stored at 4°C for subsequent use. The antibody-conjugated particles were used to decorate the stretched and unstretched tissues by incubating the particles and the tissues at 37°C and 5% carbon dioxide for 10 min. Finally, fluorescent images were acquired at 20 frames/second for 5 s using a 5× objective lens and a CCD camera.
For the creation of dynamic force maps, particle positions were tracked over time to obtain their displacement and velocity values using ImageJ-TrackMate. Lagrangian of Gaussian was applied to detect particles and Linear Assignment Problem was used to link particles. Based on the coordinates of particles, a triangular mesh was created with the Delaunay triangulation algorithm. The calculated particle velocity was then used to compute the local forces using the Stokes equation. Lastly, spatiotemporal force maps were created by applying linear interpolation between neighboring nodes. Additional details are described in (Park et al., 2018).
2.7 |. Measurement of tissue elastic modulus
To generate adequate magnetic forces, streptavidin Mag Sepharose particles with an average diameter of 80 μm (GE28-9857-38, GE Healthcare) were used. The Mag Sepharose particles were conjugated with antifibronectin antibody by first mixing the particles with protein G-biotin recombinant protein (29988, ThermoFisher) at a 1:50 ratio by volume based on their respective stock concentrations, and then by mixing the washed particles with antifibronectin antibody at a 1:100 ratio by volume. The antifibronectin antibody-conjugated Mag Sepharose particles were then added to the cardiac tissues to be incubated for 10 min, followed by gentle washes using the culture medium. The labeled tissues were then subjected to viscoelasticity measurements by magnetic tweezers.
An electric-coil-powered magnetic tweezers setup was used to generate magnetic forces exerted on the magnetic particles (Tim O’Brien et al., 2008). After positioning the pole tip of magnetic tweezers near the cardiac tissues using the 3D micrometer stage, the magnetic gradient field was created by applying 2-5 V for 3 s via PCI board (PCI-6713, National Instruments) and a MATLAB interface. Magnetic force driven by the magnetic field gradient then induced particle movement toward the pole tip. Time-lapse images were taken at 0.03 s time interval using a 10× objective lens.
Based on the acquired time-lapse images, the displacements of the particles (X) were quantified as described above. To calibrate the applied magnetic forces (F), unbound particles in the image were tracked over time, and the magnetic force overcoming the drag force was then calculated using the Stokes equation. The measured displacement of the bound particles and the forces experienced were nonlinearly fit to a Kelvin–Voigt four-element model to obtain the values of the mechanical property parameters:
where a is the particle radius, E0 and E1 are the effective elastic moduli, μ0 and μ1 are the effective viscous frictions, and τ is the relaxation time. Additional details are found in (Park et al., 2018).
2.8 |. Reverse-transcription quantitative PCR
Individual tissues used to prepare independent biological replicates were frozen in TRIzol directly from cell culture then homogenized. Phenol chloroform RNA extraction was used to prepare starting RNA. After subsequent cDNA synthesis, a master-mix qPCR reaction was prepared and measured on BioRad C1000/CFX96 thermal cycler. Fold expression change was normalized to the housekeeping gene GAPDH. Primer amplification efficiency validation, primer sequences, and complete RT-qPCR details are described in the supplementary methods.
2.9 |. Statistics
Statistical comparisons between groups were performed with Student’s unpaired t-test for continuous variables. Normality was assumed. For all analyses, significance was established at p-values less than 0.05. All statistical analyses were performed with Stata v.15.1 (StataCorp LP) statistical software.
3 |. RESULTS
3.1 |. Mechanical conditioning promotes growth of EHTs
We initially chose to pursue mechanical conditioning as a maturation strategy for EHT grafts in part because of its obvious macroscopic effects on tissue growth. 3D-printed EHT grafts were first observed to have a reproducible length, width, and thickness of 4, 2, and 1 mm, respectively, 3-day post-removal from the needle array, after undergoing spheroid fusion (Figure 2a). After culture for 4 weeks under differential static stress conditions, the tissues subjected to mechanical stretching were observed to maintain their original dimensions (Figure 2b), while the tissues not subject to mechanical stretching were reduced to 63% of their original volume, contracting to 2.5 mm × 2 mm × 1 mm (Figure 2c).
FIGURE 2.
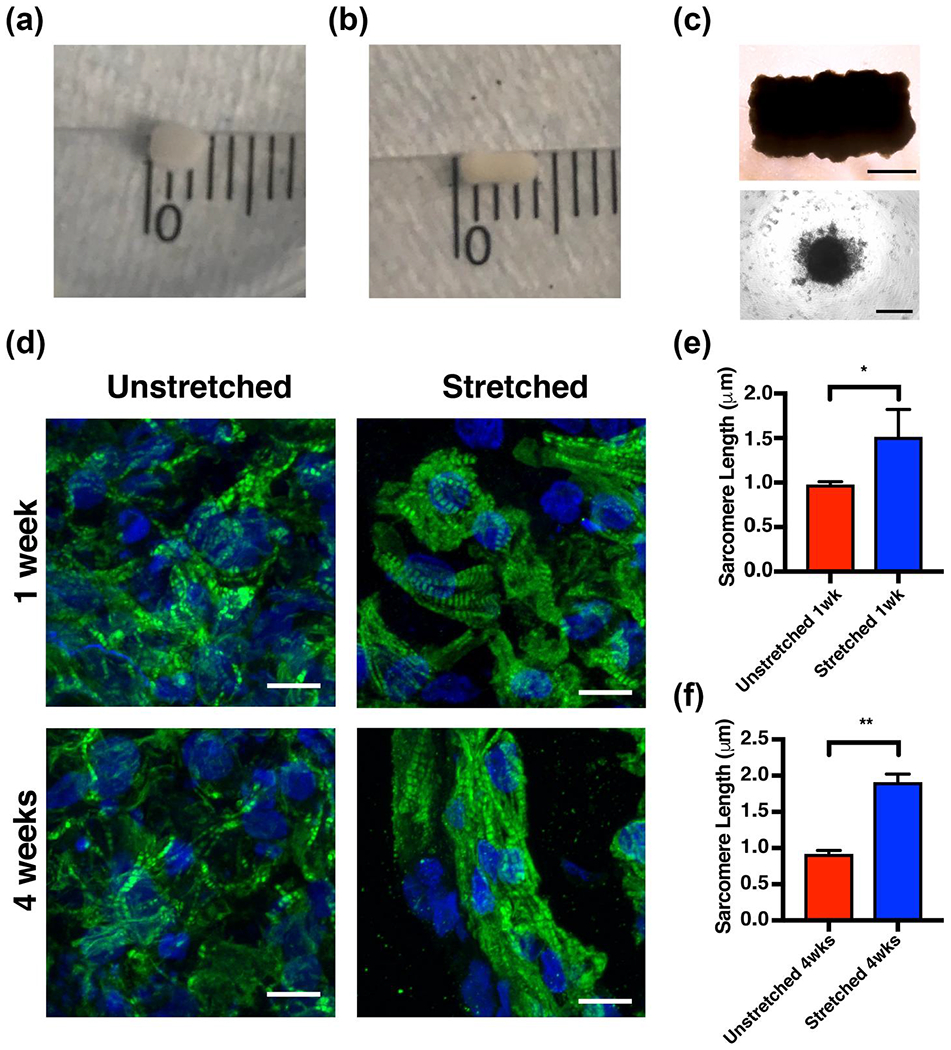
Mechanical stimulation of engineered heart tissue (EHT) grafts results in maintenance of gross tissue morphology and sarcomere elongation as early as 1 week after culture. (a) Light microscopy of an engineered heart tissue immediately after removal from the stainless steel needle array. The tissue (upper) is composed of individual spheroids (lower) of cells (scale bar = 1000 μm for the tissue and scale bar = 200 μm for the spheroid). Gross images of stretched (b) and unstretched (c) engineered heart tissues after 4 weeks of culture. Minor scale marks = 1 mm. (d) Immunohistochemical analysis of unstretched and stretched tissues at 1 and 4 weeks of culture (green = troponin T and blue = DAPI. Scale bar = 10 μm). Quantification of the average sarcomere length at 1 week (e) and 4 weeks (f) of culture; bars represent mean ± standard deviation, n = 3. *p < 0.05 and **p < 0.001 [Colour figure can be viewed at wileyonlinelibrary.com]
3.2 |. Mechanical conditioning promotes sarcomere elongation
We subsequently asked whether we could detect cellular or molecular differences between stretched and unstretched 3D-printed EHT grafts that could explain their differential growth. Consistent with the apparently strengthened stretched tissue grafts, immunohistochemistry revealed that, after 1 week of culture, tissues subjected to static mechanical stress had more elongated sarcomere structures compared to tissues that were unstretched. A measured 54% increase in average sarcomere length was statistically significant (1.51 ± 0.31 μm in stretched tissues; 0.98 ± 0.036 μm in unstretched tissues, n = 3) (Figure 2d,e). The magnitude of this effect grew when measured at 4 weeks of culture, when instead a 108% increase in mean sarcomere length was observed (1.90 ± 0.12 μm in stretched tissues; 0.91 ± 0.053 μm in unstretched tissues, n = 3) (Figure 2d,f).
3.3 |. Fibroblasts respond to mechanical conditioning by regulating deposited extracellular matrix
We then hypothesized that intercalated cardiac fibroblasts we included in our 3D-printed EHT grafts would respond to mechanical conditioning by regulating extracellular matrix deposited into the overall tissue graft material. To determine whether this was the case, immunohistochemistry was used to measure relative changes in collagen I and collagen III deposition as well as vimentin expression between stretched and unstretched tissues, after 4 weeks of culture. Significantly increased collagen I expression was observed in stretched compared to unstretched tissues (Figure 3a,b; 51.8 ± 19.9 μm2/cell stretched; 34.7 ± 8.1 μm2/cell unstretched; p < 0.05, n = 3). No statistically significant differences were found between stretched and unstretched tissues when interrogating collagen III (Figure 3a,c; 62.1 ± 22.9 μm2/cell vs. 46.2 ± 13.6 μm2/cell, n = 3) or vimentin expression (Figure 3a,d; 7.8 ± 8.3 μm2/cell vs. 8.3 ± 4.2 μm2/cell, n = 3).
FIGURE 3.
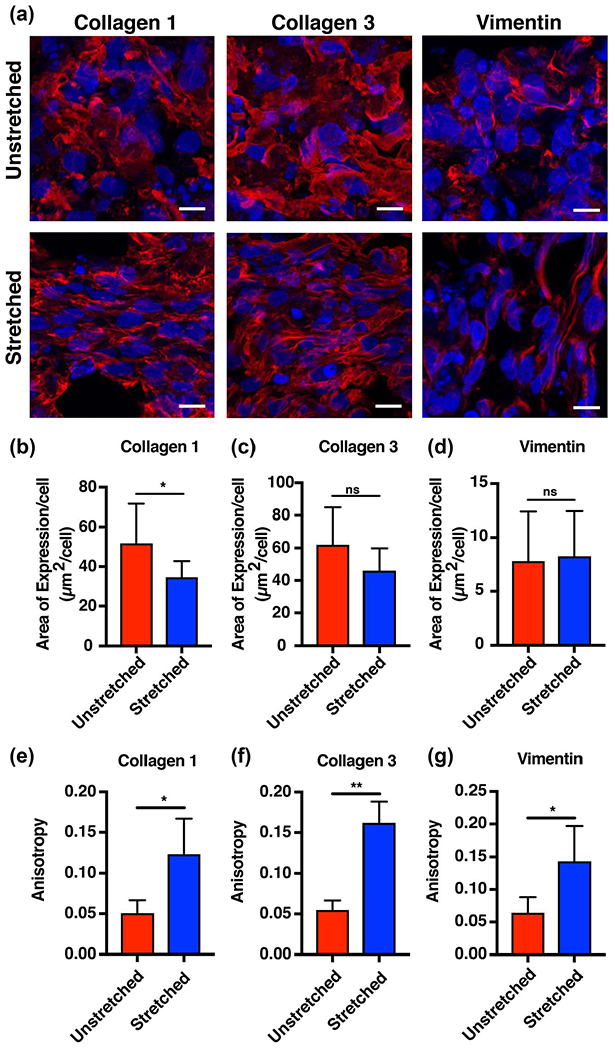
Mechanical conditioning of engineered heart tissue grafts results in directional deposition and organization of extracellular matrix. (a) Immunofluorescent staining for collagen I, collagen III, and vimentin revealed increased directionality of extracellular matrix proteins and intermediate filament proteins in stretched compared to unstretched tissues (red = collagen I, collagen III, or vimentin, blue = DAPI; scale bar = 10 μm). (b–d) Quantification reveals increased area of expression per cell for collagen I in the unstretched compared to the stretched tissues, and no significant difference in area of expression in collagen III and vimentin between the two groups. (e–g) Anisotropy increased for collagen I and collagen III fibers and vimentin intermediate filaments in the stretched compared to the unstretched tissues (bars represent mean ± standard deviation, *p < 0.05 and **p < 0.001, n = 3) [Colour figure can be viewed at wileyonlinelibrary.com]
To determine whether fibroblasts could not only deposit but also organize extracellular matrix in response to mechanical conditioning, we used FibrilTool to calculate the anisotropy (the magnitude of non-random directionality) of collagen I, collagen III, and vimentin fibers in stretched and unstretched tissues (Boudaoud et al., 2014). Collagen I and collagen III fibers were significantly more organized and aligned in stretched tissues compared to unstretched tissues (Figure 3a,e,f; 0.12 ± 0.04 vs. 0.050 ± 0.02 for collagen I and 0.16 ± 0.03 vs. 0.055 ± 0.01 for collagen III, *p < 0.05, **p < 0.001, n = 3). Likewise, statistically increased anisotropy of vimentin, an intermediate filament found in fibroblasts, was also measured in the stretched compared to the unstretched tissues (Figure 3a,g; 0.14 ± 0.05 vs. 0.064 ± 0.02, *p < 0.05, n = 3).
3.4 |. Mechanical conditioning improves contractile strength and tissue flexibility
If fibroblasts regulated extracellular matrix deposition in a functionally productive manner, we would expect two observable outcomes: first, that stretched tissues would be more flexible than unstretched tissues, consistent with associations between excess rigidity and heart failure (Bing et al., 1997). Second, that stretched tissues would have increased contractile force compared to unstretched tissues.
We therefore used live confocal immunofluorescent tracking of fibronectin-linked magnetic beads embedded within EHT grafts, which when pulled using an electromagnetic tweezer apparatus allowed us to calculate the elastic modulus and the maximal contractile force generated by the stretched and unstretched tissues (Figure 4a). Use of live confocal immunofluorescent bead tracking compared to other methods such as atomic force microscopy allowed us to evaluate both the elastic modulus of the tissue as well as the maximal contractile force generated by the stretched and unstretched tissues. Although not statistically significant, stretched tissues generated a greater maximal contractile force of 249 pN (standard error of 28.5) compared to 96 pN (standard error of 64.6) generated by unstretched tissues (Figure 4b, p = 0.075, n = 4). Furthermore, the elastic modulus of the tissues revealed a significantly lower normalized elastic modulus in the stretched tissue (0.37) compared to the unstretched tissues (1.00), or more simply, that the stretched tissue was a more flexible material than the unstretched tissue (Figure 4c, *p < 0.05, n = 4). The average elastic modulus of the unstretched tissues was used for normalization.
FIGURE 4.

Mechanical stimulation improves flexibility and increases maximal contractile force in engineered heart tissue grafts. (a) Schematic annotation of the electromagnetic tweezers apparatus used to pull on tissue-bound electromagnetic particles. This setup allows measurement of the elastic modulus of the tissue. (b) Maximal contractile force generated by stretched versus unstretched tissues at 4 weeks of culture. (c) Normalized elastic modulus for stretched and unstretched tissues at 4 weeks of culture (bars represent mean ± standard error, *p < 0.05, n = 4) [Colour figure can be viewed at wileyonlinelibrary.com]
3.5 |. Mechanical conditioning upregulates cardiac cell identity markers
Finally, we hypothesized that if static mechanical stretching conditioned EHT grafts toward maturity that cells inside a stretched graft would adopt a more cardiac-like cell fate. To this end, we queried the mRNA expression levels of cardiac-related genes using RT-qPCR. Targets included cardiac troponin T (TNNT2), connexin 43 (CX43), cardiac alpha-myosin heavy chain (MYH6), cardiac beta-myosin heavy chain (MYH7), calsequestrin 2 (CASQ2), and sarco/endoplasmic reticulum calcium-ATPase (SERCA2). Stretched tissues were found to have statistically significantly upregulated expression of MYH7 (1.7-fold increase), CASQ2 (2.3-fold increase), and SERCA2 (1.3-fold increase), consistent with the hypothesis that stretching promotes cardiac-like tissue identity at the cellular level (Figure 5, n = 6).
FIGURE 5.
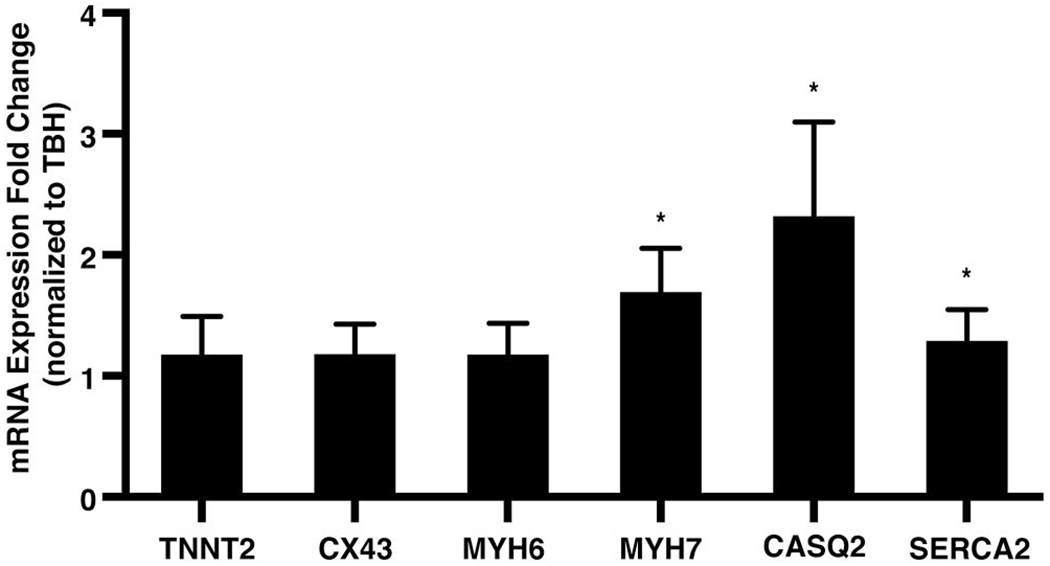
Mechanical stimulation upregulates cardiac-specific gene expression in engineered heart tissue (EHT) grafts. Statistically increased expression of MYH7, CASQ2, and SERCA2 was found in stretched compared to unstretched EHT grafts, as queried by RT-qPCR (bars represent mean ± 95% confidence interval, n = 6; *p < 0.05)
4 |. DISCUSSION
In this study, we used 3D-printing to produce an EHT graft that matures toward an adult-like functional state in response to static mechanical stress. In contrast to more conventional approaches to building a graft, our 3D-printing method notably omits the addition of scaffolding or extracellular matrix in the creation of the graft. Instead, extracellular matrix is biologically deposited by co-cultured cardiac fibroblasts, HUVECs, and hiPSC-CMs. We demonstrate that static mechanical stress can condition these 3D-printed EHT grafts to mature in at least several ways: by promoting growth, by regulating sarcomere and extracellular matrix synthesis and organization, by improving flexibility and beat contractile strength, and by increasing expression of cardiac cell identity markers. The resulting EHT graft is of a size immediately appropriate for small animal functional studies.
Since the landmark work of Zimmermann et al. (Zimmermann et al., 2002), application of mechanical stress has been thought to be a principal player in maturation of EHTs. However, the effect of mechanical conditioning has not been well-studied in engineered tissues devoid of extrinsic scaffolding material, Many previous groups (McCain & Parker, 2011; Ruan et al., 2016; Tulloch et al., 2011; Zhang et al., 2017) have demonstrated signs of maturation after application of mechanical stress but require addition of extrinsic extracellular matrix or scaffolding material. Our study reaffirms the integral role of mechanical conditioning and isolates its effects from any potential confounding from extrinsically added scaffolding material.
A variety of questions clearly remain that need to be addressed in larger-scale experiments, for which this work sets the stage. While mechanical conditioning as a maturation strategy is not without precedent, this study to our knowledge is the first to use it successfully on a scaffold-free, 3D-printed EHT graft. In studies utilizing external scaffolding material during the formation of the EHT graft (Pijnappels et al., 2008; Ruan et al., 2016; Tulloch et al., 2011; Zimmermann et al., 2002), it may be difficult to isolate the effect of external mechanical stimulus from the interaction between CMs and added extracellular matrix. Due to the absence of an externally supplied scaffold, we hypothesize that problems in integration, immunity, and inflammation which can otherwise be attributed to such scaffolds may also be mitigated.
There may be additional benefits of the biologically regulated and deposited extracellular structuring that our 3D-printing approach yields. Previous studies have identified a key role of fibroblasts in the maintenance and physiologic response by healthy myocardium to mechanical stretch (Baudino et al., 2006; Brilla et al., 1995; MacKenna et al., 2000; Souders et al., 2009; Stawowy et al., 2004). Our experiments indicate that EHT-embedded fibroblasts are able to respond to external mechanical cues by regulating extracellular matrix, as stretched EHTs were found to have increased organization of collagen fibers in the formed ECM. As a result, it may be possible to engineer fibroblasts or other 3D-printed cells to dynamically remodel extracellular matrix throughout growth, implantation, and integration to meet changing needs of the EHT graft or EHT-graft recipient interface. Thus, unlike the unchanging, homogenous material of a static chemical or biopolymer scaffold, these fibroblasts or other supporting co-cultured cells could act as control systems to tailor local biochemical, mechanical, and cellular microenvironments. Our 3D-printing approach may also allow architectural tailoring at the tissue scale, in which cell patterning can be designed to meet the needs of a particular cardiac injury without reengineering a chemical or biochemical process.
More immediately, our 3D-printing strategy appears to promote apparent EHT graft maturation without having to optimize the extracellular matrix mechanical microenvironment, which has been appreciated as a challenge in EHT graft development in the past (Engler et al., 2008). Rather than require an investigator pre-decide on the composition and properties of a scaffolding material, our EHT graft reaches a biologically generated natural equilibrium of extracellular matrix that when mechanically stretched is sufficient to support growth, organization of sarcomeres and that extracellular matrix, flexibility and contractile strength maturation, and upregulation of cardiac cell identity markers. Therefore, by exploiting the naturally homeostatic and responsive properties of biological systems, our work represents an incremental but foundational advance toward using such self-optimizing systems as an EHT graft design paradigm, enabling simpler design processes yet still sensitive grafts that are poised to adjust to their cardiac and culture environment.
ACKNOWLEDGMENTS
We thank Deborah DiSilvestre for cell culture assistance and differentiation of hiPSCs. This work was financially supported by the Irene Piccinini Investigator Research Fellowship (to C.L.), the National Institutes of Health T32 grant T32HL007227 (to C.L. via Johns Hopkins Department of Cardiology), and the Johns Hopkins Magic that Matters Fund (to N.H.).
Funding information
Irene Piccinini Investigator Research Fellowship; Johns Hopkins Magic that Matters Fund; National Institutes of Health, Grant/Award Number: T32HL007227
Footnotes
CONFLICT OF INTERESTS
The author(s) declare no competing interests.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Andrés-Delgado L, & Mercader N (2016). Interplay between cardiac function and heart development. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1863(7, Part B), 1707–1716. 10.1016/j.bbamcr.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargehr J, Hofsteen P, Bhandari S, Gambardella L, Ong L, Iyer D, Sampaziotis F, Weinberger F, Martinson M, Bernard W, Figg N, Bennett M, Murry C, Sinha S (2017). 5728Human embryonic stem cell derived epicardial cells advance cardiomyocyte-based heart regeneration. European Heart Journal, 38(suppl_1), ehx493.5728. 10.1093/eurheartj/ehx493.5728 [DOI] [Google Scholar]
- Baudino TA, Carver W, Giles W, & Borg TK (2006). Cardiac fibroblasts: Friend or foe? American Journal of Physiology - Heart and Circulatory Physiology, 291(3), H1015–H1026. 10.1152/ajpheart.00023.2006 [DOI] [PubMed] [Google Scholar]
- Bian W, Badie N, Iv HDH, & Bursac N (2017). Biomaterials Robust T-tubulation and maturation of cardiomyocytes using tissue- engineered epicardial mimetics Vector on EpicardialSurface. Biomaterials, 35(12), 3819–3828. 10.1016/j.biomaterials.2014.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing OHL, Ngo HQ, Humphries DE, Robinson KG, Lucey EC, Carver W, Brooks WW, Conrad CH, Hayes JA, & Goldstein RH (1997). Localization of α(I) collagen mRNA in myocardium from the spontaneously hypertensive rat during the transition from compensated hypertrophy to failure. Journal of Molecular and Cellular Cardiology, 29(9), 2335–2344. 10.1006/jmcc.1997.0465 [DOI] [PubMed] [Google Scholar]
- Brooks A, Burian A, Borowska-Wykręt D, Uyttewaal M, Wrzalik R, Kwiatkowska D, & Hamant O (2014). FibrilTool, an ImageJ plug-in to quantify fibrillar structures in raw microscopy images. Nature Protocols, 9(2), 457–463. 10.1038/nprot.2014.024 [DOI] [PubMed] [Google Scholar]
- Brilla CG, Zhou G, Rupp H, Maisch B, & Weber KT (1995). Role of angiotensin II and prostaglandin E2 in regulating cardiac fibroblast collagen turnover. The American Journal of Cardiology, 76(13), 8D–13D. [DOI] [PubMed] [Google Scholar]
- Burnham MP, Harvey R, Sargeant R, Fertig N, & Haddrick M (2020). A scalable approach reveals functional responses of iPSC cardiomyocyte 3D spheroids. SLAS DISCOVERY: Advancing the Science of Drug Discovery, 26(3), 352–363. 10.1177/2472555220975332 [DOI] [PubMed] [Google Scholar]
- Burridge PW, Keller G, Gold JD, & Wu JC (2012). Production of de novo cardiomyocytes: Human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell, 10(1), 16–28. 10.1016/j.stem.2011.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y-C, Ting S, Lee Y-K, Ng K-M, Zhang J, Chen Z, Siu C-W, Oh SKW, & Tse H-F (2013). Electrical stimulation promotes maturation of cardiomyocytes derived from human embryonic stem cells. Journal of Cardiovascular Translational Research, 6(6), 989–999. 10.1007/s12265-013-9510-z [DOI] [PubMed] [Google Scholar]
- Chen Q-Z, Bismarck A, Hansen U, Junaid S, Tran MQ, Harding SE, Ali NN, & Boccaccini AR (2008). Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials, 29(1), 47–57. 10.1016/j.biomaterials.2007.09.010 [DOI] [PubMed] [Google Scholar]
- Civitarese RA, Kapus A, McCulloch CA, & Connelly KA (2016). Role of integrins in mediating cardiac fibroblast–cardiomyocyte cross talk: A dynamic relationship in cardiac biology and pathophysiology. Basic Research in Cardiology, 112(1), 6. 10.1007/S00395-016-0598-6 [DOI] [PubMed] [Google Scholar]
- Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang H-Y, Speicher DW, & Discher DE (2008). Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: Scar-like rigidity inhibits beating. Journal of Cell Science, 121(Pt 22), 3794–3802. 10.1242/jcs.029678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbin KA, & Murry CE (2015). The winding road to regenerating the human heart. Cardiovascular Pathology, 24(3), 133–140. 10.1016/j.carpath.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen A, Eder A, Bönstrup M, Flato M, Mewe M, Schaaf S, Aksehirlioglu B, Schwörer A, Uebeler J, & Eschenhagen T (2010). Development of a drug screening platform based on engineered heart tissue. Circulation Research, 107(1), 35–44. 10.1161/CIRCRESAHA.109.211458 [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ochiai-shino H, Shiga T, Onodera S, Saito A, Shibahara T, & Azuma T (2016). Transplantation of human-induced pluripotent stem cells carried by self-assembling peptide nanofiber hydrogel improves bone regeneration in rat calvarial bone defects. BDJ Open, 2, 15007. 10.1038/bdjopen.2015.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannis K, Mohamed A, Vittavat T, & Wu JC (2015). Human induced pluripotent stem cell–derived cardiomyocytes. Circulation Research, 117(1), 80–88. 10.1161/CIRCRESAHA.117.305365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legant WR, Pathak A, Yang MT, Deshpande VS, McMeeking RM, & Chen CS (2009). Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues. Proceedings of the National Academy of Sciences, 106(25), 10097–10102. 10.1073/pnas.0900174106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Guan Y, & Zhang Y (2015). Chitosan as inter-cellular linker to accelerate multicellular spheroid generation in hydrogel scaffold. Polymer, 77, 366–376. 10.1016/j.polymer.2015.09.073 [DOI] [Google Scholar]
- Lundy SD, Gantz JA, Pagan CM, Filice D, & Laflamme MA (2014). Pluripotent stem cell derived cardiomyocytes for cardiac repair. Current Treatment Options in Cardiovascular Medicine, 16(7), 319. 10.1007/s11936-014-0319-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenna D, Summerour SR, & Villarreal FJ (2000). Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovascular Research, 46(2), 257–263. 10.1016/S0008-6363(00)00030-4 [DOI] [PubMed] [Google Scholar]
- McCain ML, & Parker KK (2011). Mechanotransduction: The role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflügers Archiv : European Journal of Physiology, 462(1), 89. 10.1007/s00424-011-0951-4 [DOI] [PubMed] [Google Scholar]
- Park S, Lui C, Jung WH, Maity D, Ong CS, Bush J, Maruthamuthu V, Hibino N, & Chen Y (2018). Mechanical characterization of hiPSC-derived cardiac tissues for quality control. Advanced Biosystems, 2(12), 1800251. 10.1002/adbi.201800251 [DOI] [Google Scholar]
- Patino-Guerrero A, Veldhuizen J, Zhu W, Migrino RQ, & Nikkhah M (2020). Three-dimensional scaffold-free microtissues engineered for cardiac repair. Journal of Materials Chemistry B, 8(34), 7571–7590. 10.1039/D0TB01528H [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnappels DA, Schalij MJ, Ramkisoensing AA, van Tuyn J, de Vries AAF, van der Laarse A, Ypey DL, & Atsma DE (2008). Forced alignment of mesenchymal stem cells undergoing cardiomyogenic differentiation affects functional integration with cardiomyocyte cultures. Circulation Research, 103(2), 167–176. 10.1161/ClRCRESAHA.108.176131 [DOI] [PubMed] [Google Scholar]
- Ruan J-L, Tulloch NL, Razumova MV, Saiget M, Muskheli V, Pabon L, Reinecke H, Regnier M, & Murry CE (2016). Mechanical stress conditioning and electrical stimulation promote contractility and force maturation of induced pluripotent stem cell-derived human cardiac tissue. Circulation, 134(20), 1557–1567. 10.1161/CIRCULATIONAHA.114.014998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Sekine H, Yang J, Isoi Y, Yamato M, Kikuchi A, Kobayashi E, & Okano T (2006). Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J, 20(6), 708–710. 10.1096/fj.05-4715fje [DOI] [PubMed] [Google Scholar]
- Souders CA, Bowers SLK, & Baudino TA (2009). Cardiac fibroblast. Circulation Research, 105(12), 1164–1176. 10.1161/CIRCRESAHA.109.209809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawowy P, Margeta C, Kallisch H, Seidah N, Chretien M, Fleck E, & Graf K (2004). Regulation of matrix metalloproteinase MT1-MMP/MMP-2 in cardiac fibroblasts by TGF-β1 involves furin-convertase. Cardiovascular Research, 63(1), 87–97. 10.1016/j.cardiores.2004.03.010 [DOI] [PubMed] [Google Scholar]
- Thrivikraman G, Boda SK, & Basu B (2018). Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: A tissue engineering perspective. Biomaterials, 150, 60–86. 10.1016/j.biomaterials.2017.10.003 [DOI] [PubMed] [Google Scholar]
- Tim O’Brien E, Cribb J, Marshburn D, Taylor RM, & Superfine R (2008). Chapter 16: Magnetic manipulation for force measurements in cell biology. In Correia John J., & Detrich William. Biophysical tools for biologists, Volume Two: In vivo techniques (Vol. 89, pp. 433–450). Burlington, MA: Academic Press. 10.1016/S0091-679X(08)00616-X [DOI] [PubMed] [Google Scholar]
- Tohyama S, & Fukuda K (2016). Future treatment of heart failure using human iPSC-derived cardiomyocytes. In Nakanishi T, Markwald RR, Baldwin HS, Keller BB, Srivastava D, & Yamagishi H (Eds.), Etiology and morphogenesis of congenital heart disease (pp. 25–31). Tokyo, Japan: Springer, 10.1007/978-4-431-54628-3_4 [DOI] [PubMed] [Google Scholar]
- Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, Pabon L, Reinecke H, & Murry CE (2011). Growth of engineered human myocardium with mechanical loading and vascular coculture. Circulation Research, 109(1), 47–59. 10.1161/CIRCRESAHA.110.237206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzahor E, & Poss KD (2017). Cardiac regeneration strategies: Staying young at heart. Science, 356(6342), 1035–1039. 10.1126/science.aam5894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, Bussani R, Beltrami AP, Quaini F, Bolli R, Leri A, Kajstura J, & Anversa P 2005). Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proceedings of the National Academy of Sciences, 102(24), 8692–8697. 10.1073/pnas.0500169102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uygur A, & Lee RT (2016). Mechanisms of cardiac regeneration. Developmental Cell, 36(4), 362–374. 10.1016/j.devcel.2016.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MC, Cherry-Kemmerling EM, & Black LD (2020). Cellmatrix interactions in cardiac development and disease. In Zhang Y (Ed.), Multi-scale extracellular matrix mechanics and mechanobiology (pp. 311–342). Springer International Publishing, 10.1007/978-3-030-20182-1_10 [DOI] [Google Scholar]
- Weinberger F, Breckwoldt K, Pecha S, Kelly A, Geertz B, Starbatty J, Yorgan T, Cheng K-H, Lessmann K, Stolen T, Scherrer-Crosbie M, Smith G, Reichenspurner H, Hansen A, & Eschenhagen T (2016). Cardiac repair in Guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Science Translational Medicine, 8(363), 363ra148. 10.1126/scitranslmed.aaf8781 [DOI] [PubMed] [Google Scholar]
- Williams C, Budina E, Stoppel WL, Sullivan KE, Emani S, Emani SM, & Black LD (2015). Cardiac extracellular matrix-fibrin hybrid scaffolds with tunable properties for cardiovascular tissue engineering. Acta Biomaterialia, 14, 84–95. 10.1016/j.actbio.2014.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Olson EN, & Bassel-Duby R (2013). Mending broken hearts: Cardiac development as a basis for adult heart regeneration and repair. Nature Reviews Molecular Cell Biology, 14, 529. 10.1038/nrm3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Kong CW, Tong MH, Chooi WH, Huang N, Li RA, & Chan BP (2017). Maturation of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) in 3D collagen matrix: Effects of niche cell supplementation and mechanical stimulation. Acta Biomaterialia, 49, 204–217. 10.1016/j.actbio.2016.ll.058 [DOI] [PubMed] [Google Scholar]
- Zimmermann W-H, Schneiderbanger K, Schubert P, Didié M, Münzel F, Heubach JF, Kostin S, Neuhuber WL, & Eschenhagen T (2002). Tissue engineering of a differentiated cardiac muscle construct. Circulation Research, 90(2), 223–230. [DOI] [PubMed] [Google Scholar]
- Zimmermann W-H, Melnychenko I, Wasmeier G, Didié M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, & Eschenhagen T (2006). Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nature Medicine, 12(4), 452–458. 10.1038/nm1394 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


