In the last decade, the left atrium has been recognized as an independent factor involved in the pathophysiological process of multiple cardiovascular diseases.1, 2 Left atrial (LA) dilatation and impaired LA function are associated with an increased risk of hospitalization for heart failure (HF) and mortality in the general population as well as in patients with HF with preserved (HFpEF) and reduced ejection fraction (HFrEF).3–5 Although the left atrium had been commonly considered a buffer chamber between the pulmonary circulation and the left ventricle, increasing evidence suggests that LA ‘remodelling’ does not represent an innocent bystander, but rather plays an active role in the pathophysiology and development of HF.6 Indeed, the term ‘atrial failure’ has recently been proposed as a clinical entity, characterized by any anatomical, mechanical, or electrical dysfunction causing impaired heart performance and symptoms.7
Many clinical studies have shown the beneficial effect of pharmacological and device therapy on changes in left ventricular (LV) structure and function and demonstrated that reverse LV remodelling is associated with lower rates of subsequent cardiovascular events.8 Yet, similar evidence assessing the favourable effects of targeted medical therapy on LA structure and function and its prognostic implications is lacking.9 Therapeutic interventions may attenuate LA enlargement and dysfunction in patients both from the general population and those with prevalent HF. From this perspective, assessment of LA changes may represent a useful marker that captures the treatment effect on overall LA health (e.g. LA cardiomyocyte function, degree of LA fibrosis). Moreover, advances and development of echocardiographic measures aimed to assess myocardial fibre deformation, such as strain analysis, can further help in early identification of LA improvement after initiation of specific therapies.10, 11
Previous studies have shown some potential beneficial effect of specific treatments on improving LA structure and function across different HF stages. In patients with hypertension and atrial fibrillation, treatment with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers has been shown to reduce LA volume and improve LA function.9 It has been hypothesized that a direct anti-fibrotic effect may represent the main mechanism by which these drugs act. In patients with HFpEF, treatment with angiotensin receptor–neprilysin inhibitor resulted in a reduction of LA volume without an apparent effect on LV size or diastolic function.12 Interestingly, the effect was more pronounced among patients with lower plasma values of the soluble form of ST-2 and galectin-3, biomarkers that reflect collagen homeostasis. Similarly, in HFpEF patients, spironolactone improved measures of diastolic function only among those with lower myocardial collagen cross-linking (CCL) as estimated by the serum collagen type I C-terminal telopeptide to matrix metalloproteinase-1 ratio (CITP:MMP-1).13 It is possible that HFpEF patients with less severe myocardial fibrosis may be more responsive to treatment, which is further suggested by the fact that individuals with the lowest natriuretic peptides derived most benefit from spironolactone in the TOPCAT trial.14 From this perspective, targeting and preventing the fibrotic processes involving the left atrium may represent a goal to prevent progression of HF.
In this issue of the Journal, Ravassa et al.15 have evaluated the impact of treatment with spironolactone on reduction of LA volume according to the estimated burden of myocardial fibrosis in a patient population at increased risk of developing HF enrolled in the Heart ‘Omics’ in AGEing (HOMAGE) trial. The authors assessed CITP:MMP-1 as an indirect measure of myocardial CCL, and found that spironolactone reduced LA volume compared to placebo among patients with lower CCL (i.e. higher tertile of CITP:MMP-1). The effect appeared consistent after 1 month (mean difference compared to placebo: −1.77 [95% confidence interval, CI −2.94 to −0.59] ml/m2) and persisted to the end of the study at 9 months (−2.52 [95% CI −4.46 to −0.58] ml/m2), after adjustment for clinical confounders. It is worth noting that CCL did not modify the effect of spironolactone on other measures of LV diastolic function, raising the possibility that such findings are specific to the left atrium. A similar trend was also observed for the reduction of N-terminal pro B-type natriuretic peptide, supporting the hypothesis that among patients at risk for HFpEF, spironolactone may provide the most protective benefit to those with lower CCL.
Taken together, the results of the current analysis offer several clinical implications: (i) spironolactone may be most beneficial in preventing progression to overt HF among patients with less advanced fibrosis, as assessed by serum biomarkers; (ii) routine assessment of LA structure is an important measure to potentially capture the effect of therapeutic interventions aimed to improve cardiac function, independent of measures of LV function; and (iii) estimation of CCL may be feasible using plasma biomarkers, and CITP:MMP1 shows promise as a potential biomarker to guide certain HF preventive therapies through a precision medicine approach.
Given the post-hoc nature of the current study, these data should be interpreted as ‘hypothesis-generating’. The concept that mineralocorticoid receptor antagonist (MRA)-induced treatment response may vary according to the fibrotic bioprofile warrants prospective replication. Nonetheless, these interesting findings extend upon the group’s previous investigation among HFpEF patients to individuals at risk for HF, and reiterate the concept that collagen turnover is complex. Previous data from HFrEF and HFpEF populations have shown a beneficial effect of MRAs according to markers of collagen synthesis, such as the N-terminal propeptide of type III collagen (PIIINP) and procollagen type I C-peptide (PICP), supporting the concept that those with higher collagen content respond better to MRA therapy.13, 16 The current study suggests that independent of collagen formation, fibre quality (reflected by the degree of CCL) may decrease the effectiveness of MRAs on LA remodelling. Further investigations are required to understand if modification of CCL itself, as opposed to collagen synthesis, is a promising therapeutic target to prevent HF or treat those with overt disease. One may speculate that therapies that reduce collagen synthesis (i.e. MRAs) and those that decrease CCL (e.g. torsemide) may act synergistically to positively affect the LA myocardial fibrotic substrate (Figure 1). Additionally, the study by Ravassa et al. highlights the potential importance of using LA measures as a marker of treatment response. Given our understanding of the natural history and clinical implications of LA remodelling, it is logical to speculate that therapies aimed to reverse such process might improve clinical outcomes among high-risk patients. Further, prospective analyses are required to determine if LA reverse remodelling is a valid surrogate clinical endpoint for future HF clinical trials.
Figure 1.
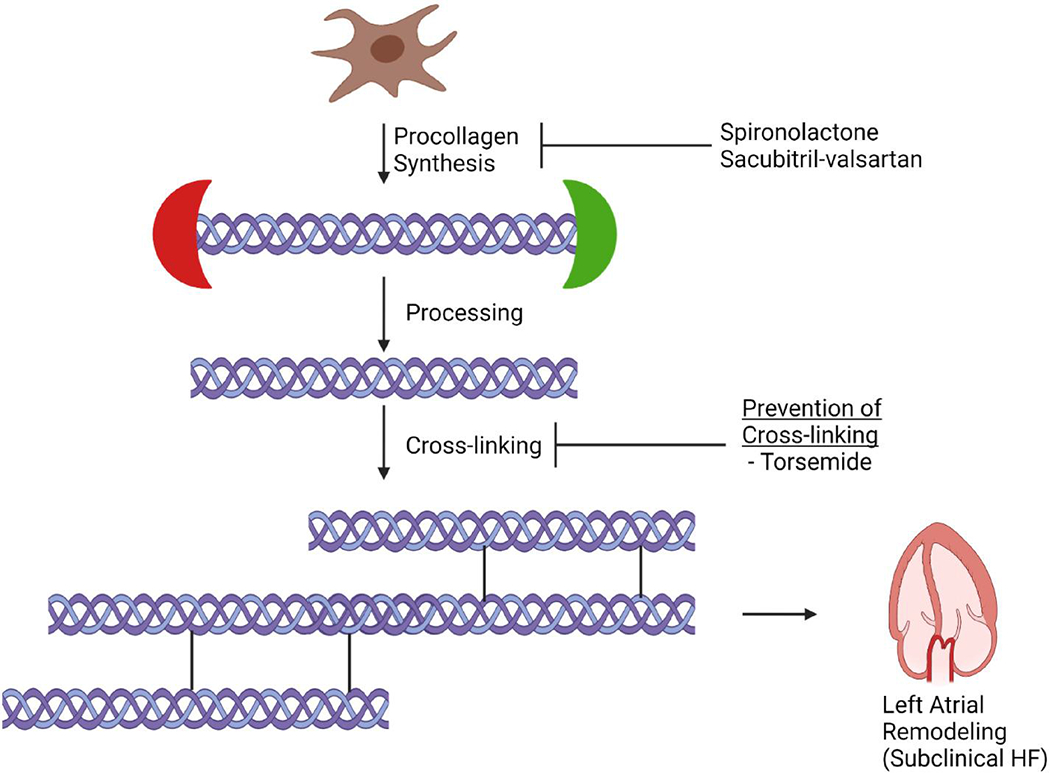
The role of collagen homeostasis in driving left atrial dysfunction: implications for treatment. Collagen metabolism involves synthesis of procollagen, processing to create collagen, cross-linking to create insoluble collagen, and subsequent degradation. Therapies aimed at decreasing collagen synthesis (e.g. mineralocorticoid receptor antagonists, angiotensin receptor-neprilysin inhibitor) may be insufficient in isolation in preventing left atrial remodelling in the setting of high rates of collagen cross-linking. As such, further investigation is required to understand if therapies that reduce collagen cross-linking (e.g., torsemide) or that degrade collagen itself may improve left atrial structure and function, and ultimately, prevent progression of subclinical heart failure (HF) to overt HF. This figure was created using BioRender.com
To date, limited advances have been made in the development of effective strategies to prevent HF. The current study highlights the importance of identifying markers of subclinical cardiac impairment, such as LA enlargement, to potentially adopt preventive treatment strategies prior to the development of clinically manifest disease. While further efforts are needed to assess the clinical implications of such strategies, attention should be paid to better personalize treatment according to clinical characteristics of patients. From this perspective, precision-based therapeutic approaches aimed to identify patients according to their fibrotic phenotype, who may benefit more from specific preventive interventions, are warranted.
Conflict of interest:
R.B.P. is supported by grant KL2TR001424 from the National Center for Advancing Translational Sciences. All other authors have nothing to disclose.
REFERENCES
- 1.Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014;63:493–505. [DOI] [PubMed] [Google Scholar]
- 2.Peigh G, Shah SJ, Patel RB. Left atrial myopathy in atrial fibrillation and heart failure: clinical implications, mechanisms, and therapeutic targets. Curr Heart Fail Rep. 2021;18:85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inciardi RM, Giugliano RP, Claggett B, Gupta DK, Chandra A, Ruff CT, et al. ENGAGE AF-TIMI 48 Investigators. Left atrial structure and function and the risk of death or heart failure in atrial fibrillation. Eur J Heart Fail. 2019;21:1571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin SH, Claggett B, Inciardi RM, Santos ABS, Shah SJ, Zile MR, et al. Prognostic value of minimal left atrial volume in heart failure with preserved ejection fraction. J Am Heart Assoc. 2021;10:e019545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pellicori P, Zhang J, Lukaschuk E, Joseph AC, Bourantas CV, Loh H, et al. Left atrial function measured by cardiac magnetic resonance imaging in patients with heart failure: clinical associations and prognostic value. Eur Heart J. 2015;36:733–42. [DOI] [PubMed] [Google Scholar]
- 6.Inciardi RM, Rossi A, Bergamini C, Benfari G, Maffeis C, Greco C, et al. Mitral regurgitation, left atrial structural and functional remodelling and the effect on pulmonary haemodynamics. Eur J Heart Fail. 2020;22:499–506. [DOI] [PubMed] [Google Scholar]
- 7.Triposkiadis F, Pieske B, Butler J, Parissis J, Giamouzis G, Skoularigis J. Global left atrial failure in heart failure. Eur J Heart Fail. 2016;18:1307–20. [DOI] [PubMed] [Google Scholar]
- 8.Kim GH, Uriel N, Burkhoff D. Reverse remodelling and myocardial recovery in heart failure. Nat Rev Cardiol. 2018;15:83–96. [DOI] [PubMed] [Google Scholar]
- 9.Thomas L, Abhayaratna WP. Left atrial reverse remodelling: mechanisms, evaluation, and clinical significance. JACC Cardiovasc Imaging. 2017;10:65–77. [DOI] [PubMed] [Google Scholar]
- 10.Inciardi RM, Galderisi M, Nistri S, Santoro C, Cicoira M, Rossi A. Echocardiographic advances in hypertrophic cardiomyopathy: three-dimensional and strain imaging echocardiography. Echocardiography. 2018;35:716–26. [DOI] [PubMed] [Google Scholar]
- 11.Minamisawa M, Inciardi RM, Claggett B, Cuddy SAM, Quarta CC, Shah AM, et al. Left atrial structure and function of the amyloidogenic V122I transthyretin variant in elderly African Americans. Eur J Heart Fail. 2021;23:1290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zile MR, Jhund PS, Baicu CF, Claggett BL, Pieske B, Voors AA, et al. ; Prospective Comparison of ARNI With ARB on Management of Heart Failure With Preserved Ejection Fraction (PARAMOUNT) Investigators. Plasma biomarkers reflecting profibrotic processes in heart failure with a preserved ejection fraction: data from the Prospective Comparison of ARNI With ARB on Management of Heart Failure with Preserved Ejection Fraction study. Circ Heart Fail. 2016;9:e002551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravassa S, Trippel T, Bach D, Bachran D, González A, López B, et al. Biomarker-based phenotyping of myocardial fibrosis identifies patients with heart failure with preserved ejection fraction resistant to the beneficial effects of spironolactone: results from the Aldo-DHF trial. Eur J Heart Fail. 2018;20:1290–9. [DOI] [PubMed] [Google Scholar]
- 14.Anand IS, Claggett B, Liu J, Shah AM, Rector TS, Shah SJ, et al. Interaction between spironolactone and natriuretic peptides in patients with heart failure and preserved ejection fraction: from the TOPCAT trial. JACC Heart Fail. 2017;5:241–52. [DOI] [PubMed] [Google Scholar]
- 15.Ravassa S, López B, Ferreira JP, Girerd N, Bozec E, Pellicori P, et al. ; HOMAGE Trial Committees and Investigators. Biomarker-based assessment of collagen cross-linking identifies patients at risk of heart failure more likely to benefit from spironolactone effects on left atrial remodelling. Insights from the HOMAGE clinical trial. Eur J Heart Fail. 2021. 10.1002/ejhf.2394. [DOI] [PubMed] [Google Scholar]
- 16.Zannad F, Alla F, Dousset B, Perez A, Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the Randomized Aldactone Evaluation Study (RALES). RALES Investigators. Circulation. 2000;102: 2700–6. [DOI] [PubMed] [Google Scholar]


