Abstract
Background & Aims:
Limitations of endoscopic sampling may result in missed dysplasia at the diagnosis of Barrett’s esophagus (BE). However, the role of close follow-up endoscopy is unclear. The aim was to evaluate the proportion of patients diagnosed with ‘missed’ dysplasia within 18 months of their index non-dysplastic BE (NDBE) diagnosis.
Methods:
This was a retrospective analysis of a cohort of BE patients diagnosed during 1990-2019 at the Houston VA. Patients with BE on index esophagogastroduodenoscopy (EGD) were classified as NDBE, indefinite dysplasia, or dysplastic (low- or high-grade dysplasia) based on initial biopsies. We identified NDBE patients who had follow-up EGD within 3-18 months after index EGD. We used logistic regression models to estimate odds ratios (OR) and 95% confidence intervals (95%CI) for risk factors of dysplasia on follow-up EGD.
Results:
We identified 614 patients who had BE on index EGD. Among those with NDBE and follow-up EGD within 3-18 months (n=271), 4.1% had definite dysplasia on follow-up, and an additional 14.0% had indefinite dysplasia. Proportions of definite or indefinite dysplasia at follow-up within 3-18 months significantly decreased from 32.6% among patients with index EGD before 2009 to 11.7% among patients with index EGD after 2013 (p-for-trend = 0.068). Those with any indefinite or definite dysplastic BE at follow-up within 3-18 months after index EGD (n=49) were more likely to have BE length ≥3 cm on index EGD (OR, 3.39; 95%CI, 1.63-7.08) than those with persistent NDBE or no BE on follow-up.
Conclusion:
The occurrence of missed dysplasia on an index EGD has decreased over time. However, those with long segment BE were more than three times as likely to have missed dysplasia, and this group could benefit from dysplasia surveillance within 18 months of BE diagnosis.
Keywords: Barrett’s esophagus, esophageal adenocarcinoma, missed dysplasia, dysplasia rate, incidence, risk factors
INTRODUCTION
The incidence of esophageal adenocarcinoma (EAC) has increased over the past 5 decades in Western countries.1 Barrett’s esophagus (BE) is the only known precursor to EAC,2 and carries a 30- to 125-fold greater risk of EAC compared to the general population.3 Among patients with BE, the strongest predictor of progression to EAC is the presence and degree of dysplasia. While the annual risk of progression to EAC in nondysplastic BE (NDBE) is approximately 0.2%,4, 5 the risk is significantly higher for BE patients who are indefinite for dysplasia (0.6% per year)6 or have low grade dysplasia (LGD; 2.5% per year)7, 8 or high grade dysplasia (HGD; 7-19% per year).9
In clinical practice, dysplasia or EAC in BE can be missed due to endoscopic sampling variations and errors compounded by low interobserver agreement among pathologists grading dysplasia on tissue from BE biopsy.10 Missed dysplasia on initial BE-diagnosing endoscopy would have implications for the management of BE with regard to intensity of surveillance, early detection of cancer, and endoscopic therapy. Due to the high risk of progression to EAC from dysplastic BE and potential for missed dysplasia/EAC on initial endoscopy, it was previously recommended by the American College of Gastroenterology (ACG) to repeat surveillance within 1 year of BE diagnosis to rule in or out dysplasia.11 However, these guidelines changed in 2016 such that secondary assessment of dysplasia status within 1 year of index BE diagnosis is no longer recommended when sufficient numbers of biopsies are obtained during the initial endoscopy (i.e., 4 biopsies every 2 cm or at least 8 biopsies if BE length is <2 cm).12 This recommendation is consistent with the current guidelines from the American Society for Gastrointestinal Endoscopy (ASGE) and American Gastroenterological Association (AGA).13,14
Few studies have examined the extent or predictors of missed dysplasia. A population-based cohort study of 162 patients with initial NDBE from Olmsted County, Minnesota found that 2 HGD/EAC (1.2%) and 13 LGD (8.0%) on repeat endoscopy within 24 months of their NDBE-diagnosing endoscopy (combined 9.3%).15 Adherence to Seattle biopsy protocol (biopsies to every 2 cm of BE) was not a predictor of missed dysplasia/EAC in that study. A second population-based cohort study of 314 patients from Olmsted County with NDBE reported a missed dysplasia rate of 13%, however 53 of these cases were diagnosed at HGD/EAC at initial endoscopy, and so the true rate may be lower.16 A meta-analysis of 15 studies published through 2015 reporting outcomes for patients with baseline NDBE found that 19.0% of all HGD/EAC were detected within 1 year of initial negative endoscopy, suggesting prevalent or missed dysplasia/EAC.17 A population-based cohort study by van Putten et al. published in 2018 of the Northern Ireland Barrett’s register found that, of 267 patients with newly diagnosed HGD/EAC, 12.7% were “missed” on index endoscopy with diagnosis within 3-12 months after BE diagnosis (9% in NDBE and 25% in BE with LGD).18 However, the missed proportion of LGD in those with baseline NDBE was not evaluated in these studies. Therefore, it is unclear if guidelines by the ACG, AGA, and ASGE are supported by consistent, high level evidence.
The aim of this study was to evaluate the prevalence and predictors of dysplasia detected on a follow-up endoscopy performed within 3-18 months after initial diagnosis of NDBE in a cohort of United States veterans under BE surveillance.
METHODS
This was a retrospective cohort study at the Michael E. DeBakey Veteran Affairs Medical Center (MEDVAMC) in Houston, Texas. We included patients diagnosed with BE on esophagogastroduodenoscopy (EGD; BE was defined as specialized intestinal epithelium in the histopathological examination of at least one biopsy obtained from endoscopically suspected BE areas) from 11/1990-1/2019. The index EGD was defined as the first endoscopy with at least one esophageal biopsy demonstrating BE. BE segment length was prospectively recorded according to the Prague C and M criteria. For this analysis, BE length was simplified as the highest value for circumference length (C) or the maximum (M) extent of the endoscopically visualized BE segment.19 Patients were followed through January 2020; follow-up EGD was defined as the first repeat endoscopy with esophageal biopsy after index EGD within 3-18 months or 19-60 months. We chose a cutoff of ≤ 18 months to account for any scheduling delays that would not allow patients to return for secondary assessment exactly 12 months after their index BE-diagnosing endoscopy. We chose the second cutoff point to be 60 months as current practice guidelines recommend endoscopic surveillance of NDBE at 3-5 years (36-60 months).12, 14 Patients were excluded if 1) there was no intestinal metaplasia on histology from the index EGD suspecting BE, 2) follow-up EGD was <3 months of index EGD as there may have been a confounding indication for repeat EGD such as a nodular lesion or peptic ulcer disease, 3) endoscopic treatment of BE (defined as radiofrequency ablation, argon plasma coagulation, gold probe cautery, or endoscopic mucosal resection) was performed during the follow-up EGD, or 4) there was no follow-up EGD with esophageal biopsy. Dysplasia status in all cases of LGD or HGD was assessed by two board certified pathologists (DR and LG) as part of routine practice (i.e., not study-related).
This research was approved by the Institutional Review Boards for Human Subjects Research for Baylor College of Medicine and the VA Research and Development Committee of the MEDVAMC.
We performed structured manual reviews of the VA Computerized Patient Record System for information on age at index EGD and follow-up EGD, race and ethnicity (non-Hispanic White, African-American, Hispanic, other), height, weight at each endoscopy, smoking history (never, former, current), alcohol history (never, former, current), hiatal hernia at index EGD (absent, present), and use of proton pump inhibitors (PPI).
Statistical Analysis
We calculated the prevalence and 95% confidence intervals (95%CI) of definite dysplasia (LGD/HGD) or any indefinite or definite dysplasia (defined as indefinite for dysplasia [IND], LGD, or HGD) on repeat endoscopy within 3-18 months and 19-60 months for those with NDBE on index EGD. We compared those with any indefinite or definite dysplastic BE on follow-up EGD to those with no BE or persistent NDBE on follow-up with respect to several sociodemographic and clinical factors. Socio-demographic factors included age at index EGD, sex, race/ethnicity, smoking status, alcohol status, and body mass index (BMI; normal <25, overweight 25-29.9, obese ≥30 kg/m2). Clinical factors included hiatus hernia, BE length, and PPI use. Pearson’s chi-square test was used for categorical variables if expected values were >5, and Fisher’s exact test was used if expected values were ≤5. Student’s t-test was used for continuous variables.
We calculated the proportion of any indefinite or definite dysplasia as well as definite dysplasia only on follow-up by date of index EGD categorized by quartiles (11/1/1990-3/12/2009, 3/25/2009-4/1/2011, 4/7/2011-6/6/2013, and 6/13/2013-1/11/2019) and stratified by repeat endoscopy within 3-18 months and 19-60 months after index EGD. We calculated the proportion of indefinite or definite dysplasia (as well as definite dysplasia only) on follow-up intervals: 3-12 months, 13-24 months, 25-36 months, 36-60 months (combined into one category as this is the current recommended surveillance interval for NDBE), and >60 months. We also examined proportions of definite dysplasia at these intervals stratified by BE length <3cm and ≥3cm at index EGD.
We used logistic regression models to estimates odds ratios (OR) and 95%CI for associations with dysplastic BE on repeat EGD. Age, sex, hiatal hernia, and variables that were statistically significantly associated with risk of dysplastic BE on repeat EGD in univariate analysis were included in the multivariable model
Sensitivity Analyses
We performed several sensitivity analyses to test the robustness of the results to the years during which index EGD was done. We adjusted for the time period by quartiles in which index EGD was performed in the logistic regression model. We also performed univariate and multivariate analyses after excluding 108 patients whose index EGD was performed between 11/1/1990-3/12/2009 (the first quartile of patients).
All analyses were performed using Stata version 15.1 (StataCorp, College Station, TX), and a 2-tailed p-value of <0.05 was considered statistically significant.
RESULTS
Study Population
We identified 614 patients with any confirmed BE on index EGD; 439 patients (71.5%) had a follow-up EGD with biopsy within 18 months and 131 patients (28.5%) had a follow-up EGD 19-60 months after the index EGD (Figure 1). The mean follow-up duration from index to follow-up EGD was 19.2 months (standard deviation, SD, 21.2 months); 9.2 months (SD 4.5 months) among those with follow-up within 18 months and 34.7 months (SD, 11.8 months) in those with follow-up 19-60 months. Overall, 95.9% were male, and the mean age at index EGD was 61.0 years (SD, 8.7 years). Most (79.9%, 95%CI, 76.4-82.8%) were non-Hispanic white, 10.3% (8.1-12.9%) were African-American, and 9.8% (7.7-12.4%) were Hispanic.
Figure 1.
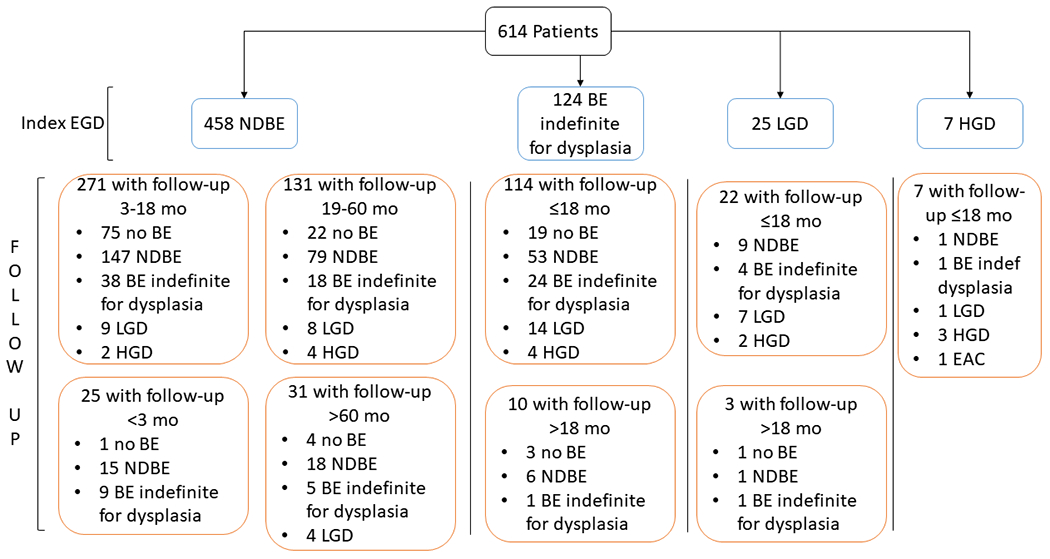
Flow diagram of 614 patients from index EGD to follow-up EGD over the study period (1990-2019).
Follow-Up of NDBE
We identified 458 patients with NDBE on index EGD and excluded 25 patients with follow-up EGD ≤ 3 months after index EGD and 31 patients with follow-up EGD > 60 months after index EGD. Among those with NDBE on their index EGD and follow-up > 3 months but ≤ 18 months from index EGD (n=271), 18.1% had any indefinite or definite dysplasia on follow-up EGD (38 IND, 9 LGD, 2 HGD), 4.1% had definite dysplasia (LGD or HGD), and none had EAC (Table 1). Among those with NDBE on their index EGD and follow-up EGD > 18 months but ≤ 60 months after index EGD (n=131), 22.9% had indefinite or definite dysplasia or EAC on follow-up (18 IND, 8 LGD, 3 HGD, and 1 EAC) and 9.2% had LGD/HGD or EAC.
Table 1.
Esophageal biopsy results on initial (index) upper endoscopy and follow-up upper endoscopy for 530 patients. Eleven patients with dysplasia on index EGD, 52 patients with follow-up EGD within 3 months of index EGD, and 32 patients with follow-up EGD > 60 months after index EGD were excluded.
| Index EGD | ||
|---|---|---|
| Nondysplastic BE (n=271) | BE Indefinite for Dysplasia (n=87) | |
| Follow-Up EGD ≤ 18 months (n=439) | ||
| No BE | 75 (27.7%) | 18 (20.7%) |
| Nondysplastic BE | 147 (54.2%) | 41 (47.1%) |
| BE Indefinite for Dysplasia | 38 (14.0%) | 16 (18.4%) |
| Dysplastic BE | 11 (4.1%) | 12 (13.8%) |
| Nondysplastic BE (n=131) | BE Indefinite for Dysplasia (n=9) | |
| Follow-Up EGD 19-60 mo (n=140) | ||
| No BE | 22 (16.8%) | 3 (33.3%) |
| Nondysplastic BE | 79 (60.3%) | 5 (55.6%) |
| BE Indefinite for Dysplasia | 18 (13.7%) | 1 (11.1%) |
| Dysplastic BE | 12 (9.2%) | 0 (0.0%) |
Among those with follow-up of NDBE within 3-18 months, the proportion with any indefinite or definite dysplasia on follow-up varied by race/ethnicity and BE length on index EGD (Table 2). Patients with any indefinite or definite dysplasia on follow-up were more likely to be non-Hispanic white (77.6% vs. 73.4%; p=0.042) and have BE length ≥3 cm on index EGD (38.8% vs. 15.3%; p-value<0.001) than those with no BE or persistent NDBE on follow-up. The proportion with any indefinite or definite dysplasia on follow-up EGD within 18 months was 35.8% among those with NDBE and BE length ≥ 3 cm on initial EGD compared to 13.8% among those with BE length < 3 cm (p<0.001). Likewise, for patients with follow-up of NDBE 19-60 months after index EGD, the proportion with any indefinite or definite dysplasia on follow-up was higher for those with BE length ≥3 cm on index EGD compared to BE length < 3 cm (33.9% vs. 13.0%; p-value=0.005).
Table 2.
Clinical and demographic characteristics of those with no BE or persistent NDBE and those who progressed from NDBE to BE indefinite for dysplasia or dysplastic BE (low grade dysplasia or high grade dysplasia) on follow-up EGD.
| Follow-Up of NDBE 3-18 mo (n=271) | Follow-Up of NDBE 19-60mo (n=131) | |||||
|---|---|---|---|---|---|---|
| No BE or Persistent NDBE (n=222) | Any Indefinite or Definite Dysplastic BE (n=49) | P-value | No BE or Persistent NDBE (n=101) | Any Indefinite or Definite Dysplastic BE (n=30) | P-value* | |
| Age (yrs) at index EGD, mean (SD) | 60.8 (8.4 years) | 60.9 (8.7 years) | 0.910 | 60.7 (8.9 years) | 60.5 (8.9 years) | 0.903 |
| Sex | ||||||
| Male | 211 (95.1%) | 47 (95.9%) | 1.000 | 95 (94.1%) | 30 (100.0%) | 0.336 |
| Female | 11 (4.9%) | 2 (4.1%) | 6 (5.9%) | 0 (0.0%) | ||
| Race | ||||||
| Non-Hispanic White | 163 (73.4%) | 38 (77.6%) | 0.042 | 85 (84.2%) | 25 (90.0%) | 0.749 |
| African-American | 35 (15.8%) | 2 (4.1%) | 9 (8.9%) | 1 (3.3%) | ||
| Hispanic | 24 (10.8%) | 9 (18.4%) | 7 (6.9%) | 2 (6.7%) | ||
| BMI | ||||||
| <25 | 45 (20.3%) | 11 (22.5%) | 0.738 | 19 (18.8%) | 1 (3.3%) | 0.081 |
| 25 to </=30 | 81 (36.5%) | 15 (30.6%) | 44 (43.6%) | 17 (56.7%) | ||
| 30+ | 96 (43.2%) | 23 (46.9%) | 38 (37.6%) | 12 (40.0%) | ||
| Smoking History | ||||||
| Never Smoker | 56 (25.2%) | 11 (22.5%) | 0.084 | 29 (28.7%) | 9 (30.0%) | 0.659 |
| Current Smoker | 69 (29.7%) | 8 (16.3%) | 20 (19.8%) | 8 (26.7%) | ||
| Former Smoker | 100 (45.1%) | 30 (61.2%) | 52 (51.5%) | 13 (43.3%) | ||
| Alcohol History | ||||||
| Never Drinker | 99 (44.6%) | 15 (30.6%) | 0.104 | 47 (46.5%) | 8 (26.7%) | 0.043 |
| Current Drinker | 81 (36.5%) | 19 (38.8%) | 34 (33.7%) | 18 (60.0%) | ||
| Former Drinker | 42 (18.9%) | 15 (30.6%) | 20 (19.8%) | 4 (13.3%) | ||
| PPI use | ||||||
| No | 71 (32.0%) | 16 (32.7%) | 0.927 | 21 (20.8%) | 8 (26.7%) | 0.496 |
| Yes | 151 (68.0%) | 33 (67.3%) | 80 (79.2.0%) | 22 (73.3%) | ||
| Mean BE Length at index EGD (cm, SD) | 1.69 (2.05 cm) | 2.80 (2.03 cm) | 0.002 | 2.89 (2.8 cm) | 5.18 (4.0 cm) | <0.001 |
| BE Length | ||||||
| < 3 cm | 188 (84.7%) | 30 (61.2%) | <0.001 | 60 (59.4%) | 9 (30.0%) | 0.005 |
| ≥ 3 cm | 34 (15.3%) | 19 (38.8%) | 41 (40.6%) | 21 (70.0%) | ||
| Hiatal Hernia | ||||||
| < 3cm | 112 (50.5%) | 21 (42.9%) | 0.138 | 45 (44.6%) | 12 (40.0%) | 0.958 |
| ≥ 3 cm | 82 (36.9%) | 25 (51.0%) | 43 (42.6%) | 14 (46.7%) | ||
| Missing | 28 (12.6%) | 3 (6.1%) | 13 (12.9%) | 4 (13.3%) | ||
| Year of Index EGD (in quartiles) | ||||||
| 1st Quartile (11/1/1990-3/26/2009) | 19 (8.6%) | 9 (18.4%) | 0.096 | 41 (40.6%) | 13 (43.3%) | 0.723 |
| 2nd Quartile (3/31/2009-4/7/2011) | 60 (27.0%) | 16 (32.7%) | 22 (21.8%) | 7 (23.3%) | ||
| 3rd Quartile (4/15/2011-5/29/2013) | 70 (31.5%) | 14 (28.6%) | 19 (18.8%) | 3 (10.0%) | ||
| 4th Quartile (6/4/2013-11/27/2018) | 73 (32.9%) | 10 (20.4%) | 19 (18.8%) | 7 (23.3%) | ||
Abbreviations: BE, Barrett’s esophagus; NDBE, nondysplastic Barrett’s esophagus; EGD, esophagogastroduodenoscopy; yrs, years; SD, standard deviation; BMI, body mass index; PPI, proton pump inhibitor; cm, centimeter
For categorical variables, p-value calculated from Chi-square test if expected frequency > 5, Fisher’s exact test if expected frequency ≤ 5.
In the multivariable model, BE length on index EGD was the only factor statistically significantly associated with indefinite or definite dysplasia on follow-up (Table 3). NDBE patients with BE length ≥ 3 cm on index EGD had over 3-fold higher risk (OR, 3.39; 95%CI, 1.63-7.08) of missed dysplasia on follow-up EGD within 3-18 months compared to NDBE patients with BE length < 3 cm on their index EGD.
Table 3.
Logistic regression model predicting change from NDBE at index EGD to indefinite or definite dysplasia on follow-up EGD 3-18 months after index EGD (n=49) and 19-60 months after index EGD (n=30). The full model contained all the variables listed in the table.
| Follow-Up of NDBE 3-18 mo (n=271) | Follow-Up of NDBE 19-60 mo (n=131) | |
|---|---|---|
| Adjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Age at index EGD (ref<60 years) | 1.46 (0.72-2.98) | 1.27 (0.52-3.14) |
| Male sex (ref: female) | 1.27 (0.25-6.38) | -- |
| NHW race (ref: other) | 1.09 (0.50-2.35) | 1.48 (0.38-5.72) |
| BE length ≥ 3 cm (ref < 3 cm) | 3.39 (1.63-7.08) | 3.50 (1.40-8.77) |
| Hiatal hernia ≥ 3 cm (ref: < 3cm) | 1.23 (0.61-2.47) | 0.81 (0.31-2.12) |
Abbreviations: BE, Barrett’s esophagus; NDBE, nondysplastic Barrett’s esophagus; EGD, esophagogastroduodenoscopy; OR: odds ratio; CI: confidence interval; NHW: non-Hispanic white; cm, centimeter
Time Trends of Performing Follow-up EGD
The proportion of BE patients with follow-up EGD within 18 months was low (43.0%) between 3/17/1992 and 8/18/2009 (the first quartile of patients) but increased and remained consistent throughout the rest of the study duration (the last three quartiles of patients) at 69.3% (8/28/2009-6/3/2011), 81.0% (6/7/2011-6/19/2013), and 76.2% (6/25/2013-1/27/2018).
The proportion of patients with NDBE on index EGD who had any indefinite or definite dysplasia at follow-up within 3-18 months of index EGD decreased over the study period (32.6% in the first quartile, 3/17/1992-8/18/2009; 18.6% in the second quartile, 8/28/2009-6/3/2011; 16.1% in the third quartile, 6/7/2011-6/19/2013; and 11.7% in the last quartile, 6/25/2013-1/27/2018; p-for-trend = 0.068; Figure 2). The proportion with definite dysplasia at follow-up within 3-18 months of NDBE on index EGD also decreased from 11.6% in the first quartile to 4.3% in the second quartile, 3.7% in the third quartile, and 0.0% in the last quartile (p-for-trend=0.059). On the other hand, the proportion with any indefinite or definite dysplasia on follow-up 19-60 months after index EGD did not change over the study period (p-for-trend = 0.776), nor did the proportion with definite dysplasia (1st quartile: 7.0%, 2nd quartile: 6.5%, 3rd quartile: 5.3%, 4th quartile: 20.8%; p-for-trend=0.289).
Figure 2.
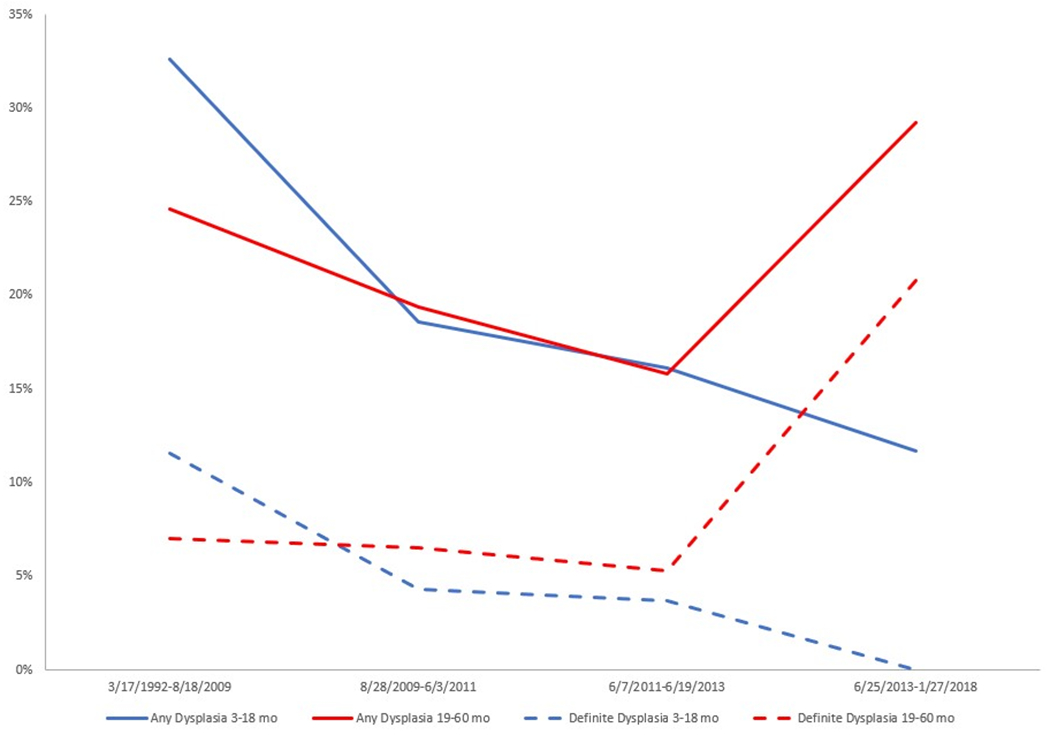
Proportions of any dysplasia (indefinite for dysplasia, low grade dysplasia, high grade dysplasia, and esophageal adenocarcinoma) and proportions of definite dysplasia (low and high grade dysplasia and esophageal adenocarcinoma) at follow-up EGD over the study period (in quartiles) stratified by follow-up within 3-18 months and 19-60 months after index EGD.
The proportion of patients with long segment BE ≥ 3 cm at index EGD decreased over the study period from 53.0% in the first quartile to 21.2% in the fourth quartile (Supplementary Table 1). The proportion of with any indefinite or definite dysplasia on follow-up EGD in patients with BE length ≥ 3 cm on index EGD did not change over the study period regardless of timing of follow-up endoscopy.
The proportion with any dysplasia increased from 17.2% at 3-12 months to 31.4% at 25-36 months and then remained consistent during the longer follow-up intervals (p-for-trend 0.180; Supplementary Figure 1). The proportion of definite dysplasia, however, steadily increased with longer follow-up intervals from 2.7% at 3-12 months, 6.7% at 13-24 months, 8.6% at 25-36 months, and 12.9% at >60 months (p-for-trend 0.002). When stratified by BE length, the proportion of definite dysplasia for BE length ≥3cm is high at follow-up 13-24 months after index BE diagnosis at 20.59% then dropped to 13.3% at >60 months (Supplementary Figure 2). Conversely, the proportion of definite dysplasia for BE length <3cm was low at shorter follow-up intervals (ranging 0-3.25% at follow-up <36 months) and was higher (12.5%) at >60 months of follow-up.
Possible Missed HGD/EAC
There were 17 cases of HGD or EAC detected during follow-up endoscopy any time during the study period, 8 of which developed within 18 months of follow-up (2 from NDBE on index EGD, 4 from IND, and 2 from LGD). The two cases of HGD detected in NDBE within 18 months of follow-up had BE length < 3cm and were male; one was Hispanic, and the other was non-Hispanic white. Three cases of HGD and 1 EAC were diagnosed on follow-up of NDBE > 18 months after index EGD; all were in non-Hispanic white males with BE length ≥ 3cm. These 4 cases were diagnosed on a range of 35-59 months after diagnosis of NDBE.
Sensitivity Analyses
We adjusted for the time period in which index EGD was performed in quartiles of patients in the logistic regression model, and also found that BE length at index EGD was associated with any indefinite or definite dysplasia on follow-up EGD within 3-18 months (adjusted OR, 3.00; 95%CI, 1.38-6.50) and 19-60 months (adjusted OR, 3.41; 95%CI, 1.31-8.87; Supplementary Table 2).
After excluding all patients whose index EGD was performed between 11/1/1990 and 8/18/2009 (first quartile of patients), we found that non-Hispanic white race and BE length were associated with any indefinite or definite dysplasia on follow-up EGD within 3-18 months on univariate analyses (Supplementary Table 3). In the multivariate model, BE length at index EGD was the only significant predictor of any indefinite or definite dysplasia on follow-up EGD within 3-18 months (adjusted OR, 3.11; 95%CI, 1.25-7.76) and > 18 months (adjusted OR, 5.11; 95%CI, 1.33-19.7; Supplementary Table 4).
DISCUSSION
We observed that among patients with NDBE on a BE-diagnosing index EGD, the proportions of indefinite or definite dysplasia of any grade found on follow-up EGD within 18 months of the index EGD decreased from 32.1% in 1990-2009 to 12.0% in 2013-2019. The proportion of missed definite dysplasia (defined as LGD or HGD on follow-up EGD within 18 months of the BE-diagnosing index EGD) decreased from 17.9% in 1990-2009 to 0% in 2013-2019. BE length ≥ 3 cm at index EGD was the only consistently significant predictor of indefinite or definite dysplasia on follow-up EGD. These findings have implications for the practice of routine short-term follow-up EGD among patients with newly diagnosed BE.
The proportion of missed definite dysplasia (LGD or HGD) within 18 months of initial BE-diagnosing endoscopy in this study was overall low (4.1%), and virtually disappeared in the latter part of the study period. A population-based cohort study from Olmsted County, Minnesota found a 9.3% miss proportion of LGD/HGD/EAC after NDBE diagnosis on index EGD.15 However, this study was of findings on follow-up through 24 months of index EGD, and BE cases initially identified from 1976 to 2011, although the majority of cases were from 1998-2011 (73.8%). The decline in the proportion of possible missed dysplasia during recent years may have to do with increased recognition, training, and adherence to systematic biopsy protocol of suspected BE, use of high definition scopes, increased use of PPI and therefore low likelihood of concomitant erosive esophagitis or stricture,20 but in large part is attributed to the decline in the proportions of long BE segments observed in this and other studies.16, 21–23
A study by Abrams et al. of 10,958 endoscopies with BE from a multi-center United States pathology database from 2002-2007 reported only 51.2% of cases were adherent to biopsy guidelines.24 Another study of 53,541 endoscopies with BE from 2012 -2017 by Wani et al. from the GI Quality Improvement Consortium registry (a voluntary quality improvement registry in the United States) found that adherence to biopsy protocol ranged from 81.1% to 84.8% over the study period,25 much higher than the reported proportion of 51.2% in 2002-2007 by Abrams et al. An increase in adherence to biopsy protocol over time may possibly account for the decrease in missed dysplasia observed over time in our study. However, we were unable to examine this factor due to inconsistent reporting of number of biopsies.
The additional potential benefits of a follow-up EGD within a relatively short time of the index EGD is finding BE indefinite for dysplasia, especially in short BE segments. In our study, the proportion of indefinite dysplasia on follow-up EGD was 14.1% overall and 11.0% in the last quartile. It is important that this diagnosis is not missed, as IND BE carries a higher risk for progression to neoplasia compared to NDBE with an incidence rate of 1.5 per 100 person-years.6, 26 Similarly, our study found a higher proportion of definite dysplastic BE on follow-up of BE indefinite for dysplasia at 12.4% compared to follow-up of NDBE (6.2%). Additionally, the proportion of patients with NDBE on index EGD whose follow-up EGD did not confirm BE was 23.3% overall and 22.9% in the last quartile. We previously reported in a subset of the current study cohort that most with suspected endoscopic only BE remain negative for BE at follow-up and suggests that this 23.3% of patients with no BE on follow-up EGD do not need further surveillance.27
We found that BE length on index EGD was the only predictor of missed dysplasia (IND, LGD, or HGD). Patients with long segment BE (≥ 3cm) had almost 3.5-fold higher risk of missed dysplasia compared to patients with segment length < 3 cm. This is consistent with the previous study from Olmstead County found that long segment BE was significantly associated with missed dysplasia.15 This may be due to a decrease in adherence to systematic biopsy protocols with longer segment BE on index EGD. The study of >10,000 endoscopies with BE by Abrams et al. found that adherence to biopsy guidelines was significantly inversely associated with increased BE length (p-for-trend=0.03).24 This is also consistent with the study by Wani et al. of >50,000 endoscopies with BE which reported a strong inverse association between BE length (categorized as ≤ 4 cm, > 4 to 6cm, > 6 cm to 8 cm, and > 8 cm) and adherence to Seattle biopsy protocol.28 Even with adherence to biopsy protocol, only 5-10% of BE surface mucosa is sampled, especially in longer segments, which may lead to increased risk for missed dysplasia.29 Novel methods to augment BE surface area sampling and improve dysplasia detection, however further studies are needed.13, 30
Our findings and the available literature suggest a possible benefit to the practice of a follow-up endoscopy within 18 months after the initial diagnosis of NDBE in those with long segment BE. Thus if 18-month follow-up is reserved for the high-risk group with a BE length of at least 3cm, potentially 4 of the 6 cases with HGD or cancer would have been detected. Gastroenterology practices should also consider individually monitoring their own dysplasia miss proportions over a 12-18 month horizon and make informed, data-driven decisions about further continuing the practice of short-term follow-up endoscopy
There were several limitations to this study. It was conducted in a veteran population of mainly non-Hispanic white males, limiting generalizability of the results to other populations. Adherence to biopsy protocol was not reliably recorded and could not be accounted as a confounding factor for missed dysplasia. Despite a strict inclusion definition, approximately 27.7% with NDBE on index EGD had no BE on follow-up within 18 months. Likewise, 16% with NDBE had no BE on follow-up after 18 months. We suspect that these were cases with short or very short segment BE, especially those with hiatal hernias where sampling is known to be difficult and inconsistent. Lastly, the sample size may have been too small to detect differences between those with dysplastic BE at follow-up compared to those with no BE or persistent NDBE.
Strengths of our study included using a consistent and strict definition to diagnose BE and excluding all those with BE on endoscopy only (i.e., without intestinal metaplasia on biopsy). Additionally, the medical records of all patients were manually reviewed to confirm diagnosis of endoscopic and histologic BE in order to avoid misclassification. Lastly, this was a prospectively maintained cohort of patients with BE.
In conclusion, we report a high proportion (18.1%) of missed indefinite or definite dysplasia within 18 months of the initial diagnosis of NDBE, especially in those with long segment BE (35.8%). We recommend considering repeat endoscopic examination around 12-18 months in those with BE length ≥ 3 cm.
Supplementary Material
Acknowledgements:
We would like to acknowledge and thank our two gastrointestinal pathologists, Daniel Rosen and Linda K. Green, for their assistance.
Financial Support:
This work is funded in part by National Institutes of Health grant NCI R01 116845, and the Texas Digestive Disease Center NIH DK58338. Dr. El-Serag is also supported by NIDDK K24-04-107. This research was supported in part with resources at the VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (#CIN 13-413), at the Michael E. DeBakey VA Medical Center, Houston, TX. The opinions expressed reflect those of the authors and not necessarily those of the Department of Veterans Affairs, the US government or Baylor College of Medicine.
Footnotes
Potential Competing Interests: The authors report no competing interests for this publication.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worlwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Spechler SJ. Barrett’s esophagus and esophageal adenocarcinoma: pathogenesis, diagnosis, and therapy. Med Clin North Am 2002;86:1423–45. [DOI] [PubMed] [Google Scholar]
- 3.Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med 2011;365:1375–83. [DOI] [PubMed] [Google Scholar]
- 4.Desai TK, Krishnan K, Samala N, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett’s oesophagus: a meta-analysis. Gut 2012;61:970–6. [DOI] [PubMed] [Google Scholar]
- 5.Peters Y, Honing J, Kievit W, et al. Incidence of progression of persistent nondysplastic Barrett’s esophagus to malignancy. Clin Gastroenterol Hepatol 2019;17:869–77. [DOI] [PubMed] [Google Scholar]
- 6.Krishnamoorthi R, Mohan BP, Jayaraj M, et al. Risk of progression in Barrett’s esophagus indefinite for dysplasia: a systematic review and meta-analysis. Gastrointest Endosc 2020;91:3–10. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Manickam P, Amin AV, et al. Incidence of esophageal adenocarcinoma in Barrett’s esohagus with low-grade dysplasia: a systematic review and meta-analysis. Gastrointest Endosc 2014;79:897–909. [DOI] [PubMed] [Google Scholar]
- 8.Kestens C, Offerhaus GJA, van Baal JWPM, et al. Patients with Barrett’s esophagus and persistent low-grade dysplasia have an increased risk for high-grade dysplasia and cancer. Clin Gastroenterol Hepatol 2016;14:956–62. [DOI] [PubMed] [Google Scholar]
- 9.Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc 2008;67:394–8. [DOI] [PubMed] [Google Scholar]
- 10.van der Wel MJ, Coleman HG, Bergman JJ, et al. Histopathologist features predictive of diagnostic concordance at expert level among a large international sample of pathologists diagnosing Barrett’s dysplasia using digital pathology. Gut 2019. [DOI] [PubMed] [Google Scholar]
- 11.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol 2008;103:788–97. [DOI] [PubMed] [Google Scholar]
- 12.Shaheen NJ, Falk GW, Iyer PG, et al. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016;111:30–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qumseya B, Sultan S, Bain P, et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest Endosc 2019;90:335–9. [DOI] [PubMed] [Google Scholar]
- 14.Association AG, Spechler SJ, Sharma P, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011;140:1084–91. [DOI] [PubMed] [Google Scholar]
- 15.Visrodia K, Iyer PG, Schleck CD, et al. Yield of repeat endoscopy in Barrett’s esophagus with no dysplasia and low-grade dysplasia: a population-based study. Dig Dis Sci 2016;61:158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhaliwal L, Codipilly DC, Gandhi P, et al. Neoplasia detection rate in Barrett’s esophagus and its impact on missed dysplasia: results from a large population-based database. Clin Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visrodia K, Singh S, Krishnamoorthi R, et al. Magnitude of missed esophageal adenocarcinoma after Barrett’s esophagus diagnosis: a systematic review and meta-analysis. Gastroenterology 2016;150:599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Putten M, Johnston BT, Murray LJ, et al. “Missed” oesophageal adenocarcinoma and high-grade dysplasia in Barrett’s oesophagus patients: a large population-based study. United European Gastroenterol J 2018;6:519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M Criteria. Gastroenterology 2006;131:1392–9. [DOI] [PubMed] [Google Scholar]
- 20.El-Serag H, Lau M. Temporal trends in new and recurrent oesophageal strictures in a Medicare population. Aliment Pharmacol Ther 2007;25:1223–9. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen T, Alsarraj A, El-Serag H. Brief report: the length of newly diagnosed Barrett’s esophagus may be decreasing. Dis Esophagus 2015;28:418–21. [DOI] [PubMed] [Google Scholar]
- 22.El-Serag HB, Aguirre T, Kuebeler M, et al. Is the length of newly diagnosed Barrett’s esophagus decreasing? The experience of a VA Health Care System. Clin Gastroenterol Hepatol 2004;2:296–300. [DOI] [PubMed] [Google Scholar]
- 23.El-Serag HB, Aguirre T, Kuebeler M, et al. The length of newly diagnosed Barrett’s oesophagus and prior use of acid suppressive therapy. Aliment Pharmacol Ther 2004;19:1255–60. [DOI] [PubMed] [Google Scholar]
- 24.Abrams JA, Kapel RC, Lindberg GM, et al. Adherence to biopsy guidelines for Barrett’s esophagus surveillance in the community setting in the United States. Clin Gastroenterol Hepatol 2009;7:736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wani S, Williams JL, Komanduri S, et al. Time trends in adherence to surveillance intervals and biopsy protocol among patients with Barrett’s esophagus. Gastroenterology 2019. [DOI] [PubMed] [Google Scholar]
- 26.Sinh P, Anaparthy R, Young PE, et al. Clinical outcomes in patients with a diagnosis of “indefinite for dysplasia” in Barrett’s esophagus: a multicenter cohort study. Endoscopy 2015;47:669–74. [DOI] [PubMed] [Google Scholar]
- 27.Khandwalla HE, Graham DY, Kramer JR, et al. Barrett’s esophagus suspected at endoscopy but no specialized intestinal metaplasia on biopsy, what’s next? Am J Gastroenterol 2014;109:178–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wani S, Williams JL, Komanduri S, et al. Endoscopists systematically undersample patients with long-segment Barrett’s esophagus: an analysis of biopsy sampling practices from a quality improvement registry. Gastrointest Endosc 2019;90:732–41. [DOI] [PubMed] [Google Scholar]
- 29.Iyer PG. Dysplasia detection in Barrett’s esophagus: Is the glass half full or half empty? Gastrointest Endosc 2019;90:742–44. [DOI] [PubMed] [Google Scholar]
- 30.Alshelleh M, Inamdar S, McKinley M, et al. Incremental yield of dysplasia detection in Barrett’s esophagus using volumetric laser endomicroscopy with and without laser marking compared with a standardized random biopsy protocol. Gastrointest Endosc 2018;88:35–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


