Recent studies have suggested that chimeric antigen receptor (CAR) T cells be moved to second-line therapy for some patients with relapsed diffuse large B-cell lymphoma (DLBCL). Shadman et al retrospectively compared outcomes of autologous hematopoietic cell transplantation (auto-HCT) and CAR T-cell therapy for patients in partial remission after salvage chemotherapy in the Center for International Blood and Marrow Transplant Research database. They found a lower rate of relapse and superior overall survival for patients undergoing auto-HCT. The data suggest that auto-HCT remains the preferred therapy for subgroups of patients with relapsed DLBCL.
Key Points
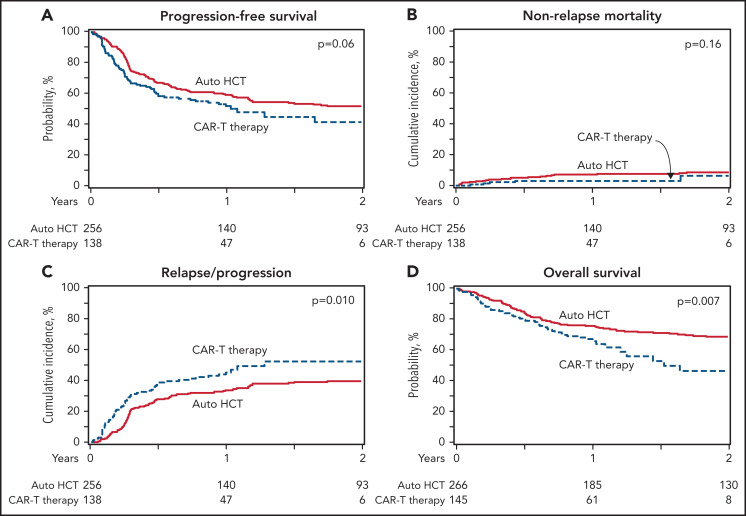
In patients with DLBCL in PR postsalvage, auto-HCT and CAR-T gave 2-year progression-free survival (PFS) of 52% vs 42% and OS of 69% vs 47%.
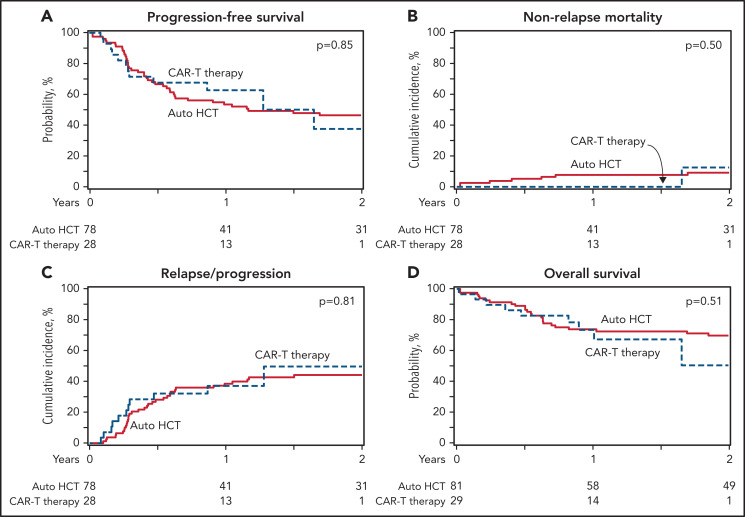
In patients with ≤2 prior lines of therapy, there was no difference in PFS or OS between the 2 groups.
Visual Abstract
Abstract
The relative efficacy of autologous hematopoietic cell transplant (auto-HCT) vs chimeric antigen receptor T-cell (CAR-T) therapy in patients with diffuse large B-cell lymphoma (DLBCL) who achieve a partial remission (PR) after salvage chemotherapy is not known. Using the Center for International Blood & Marrow Transplant Research registry database, we identified adult patients with DLBCL who received either an auto-HCT (2013-2019) or CAR-T treatment with axicabtagene ciloleucel (2018-2019) while in a PR by computed tomography or positron emission tomography scan. We compared the clinical outcomes between the 2 cohorts using univariable and multivariable regression models after adjustment for relevant baseline and clinical factors. In the univariable analysis, the 2-year progression-free survival (52% vs 42%; P = .1) and the rate of 100-day nonrelapse mortality (4% vs 2%; P = .3) were not different between the 2 cohorts, but consolidation with auto-HCT was associated with a lower rate of relapse/progression (40% vs 53%; P = .05) and a superior overall survival (OS) (69% vs 47%; P = .004) at 2 years. In the multivariable regression analysis, treatment with auto-HCT was associated with a significantly lower risk of relapse/progression rate (hazard ratio = 1.49; P = .01) and a superior OS (hazard ratio = 1.63; P = .008). In patients with DLBCL in a PR after salvage therapy, treatment with auto-HCT was associated with a lower incidence of relapse and a superior OS compared with CAR-T. These data support the role of auto-HCT as the standard of care in transplant-eligible patients with relapsed DLBCL in PR after salvage therapy.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a curable disease, and ∼60% of patients do not require further treatment after an initial anthracycline-based and rituximab-containing regimen. However, outcomes of patients who are not cured with first-line treatment are poor, and defining the optimal treatment strategy remains to be an unmet clinical need.1 The current standard of care for fit patients with relapsed/refractory DLBCL, including for patients with early therapy failure (defined as relapse or progression in ≤1 year from original diagnosis), comprises treatment with an alternative salvage regimen(s) followed by high-dose chemotherapy and autologous hematopoietic cell transplant (auto-HCT) consolidation in those who achieve either complete remission (CR) or partial remission (PR).2-4 Although there seems to be a universal agreement in this approach for patients who achieve a CR, the practice is less uniform in the case of PR patients because they are also potential candidates for chimeric antigen receptor T-cell (CAR-T) therapy.5-7
Recently, the Center for International Blood and Marrow Transplant Research (CIBMTR) reported high clinical efficacy of auto-HCT in patients with DLBCL who received an auto-HCT while in a positron emission tomography (PET)-positive PR, with a 5-year progression-free survival (PFS) rate of ∼40%.8 Efficacy of auto-HCT vs CAR-T cell therapy for patients with DLBCL achieving a PR as the best response to therapy has not been compared in prospective trials. Ongoing randomized trials in the subset of patients with DLBCL with early treatment failure are comparing salvage therapy followed by auto-HCT consolidation in responding patients vs proceeding directly with CAR-T treatment without attempting salvage therapy (NCT03570892, NCT03575351, and NCT03391466). However, these trials are not designed to address the management of patients with DLBCL already achieving a PR in response to salvage therapies. This question is highly clinically relevant because patients with relapsed DLBCL are often referred to transplant or cell therapy programs only after starting salvage therapy.
Using the CIBMTR database, we compared the outcomes of patients with DLBCL who achieved a PR as the best response to therapy and received either auto-HCT or CAR-T treatment.
Methods
Data source
For details of the data source, please refer to the supplemental material available on the Blood Web site. This study is approved by the Medical College of Wisconsin and the National Marrow Donor Program institutional review boards.
Patients
Adult patients (age ≥18 years) with DLBCL, high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements or primary mediastinal large B-cell lymphoma who achieved a PR per international working group criteria9,10 and underwent either an auto-HCT between 2013 and 2019 or CAR-T treatment with axicabtagene ciloleucel (axi-cel) between 2018 and 2019 were included in this analysis. Pretransplant or pre-CAR-T imaging with either PET or computed tomography scans were acceptable, but patients with an available negative PET scan (Deauville 1-3) were excluded from the study because they would not meet the PR criteria per the 2014 Lugano definition.9 Patients in the CAR-T cohort with a prior auto-HCT were excluded from the analysis.
Definitions and endpoints
Disease status before auto-HCT or CAR-T and determination of the PR status was defined per Lugano working group classification and determined by local radiologic assessments at transplant or immunotherapy centers.9 The primary endpoint of the study was PFS, defined as the time from either auto-HCT or CAR-T to relapse or death from any cause. Secondary endpoints: overall survival (OS) defined as the time from treatment to death of any cause, the cumulative incidence of nonrelapse mortality (NRM) defined as death without preceding disease progression, the cumulative incidence of relapse/progression defined as the time from treatment to relapse or disease progression and hematopoietic recovery. For CAR-T patients, the cumulative incidence of cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS) were calculated. CRS and ICANS were graded using the American Society of Transplant and Cellular Therapy grading criteria.11 Subgroups analyses were performed for (1) patients with an available PET scan before auto-HCT or CAR-T and (2) patients who received ≤2 or >2 prior lines of treatment.
Statistical analysis
We compared the baseline characteristics between the auto-HCT and CAR-T cohorts using the Kruskal-Wallis test for continuous variables and the Pearson χ2 test for categorical variables after ignoring the missing data. The Kaplan-Meier estimator and the log-rank test were used to compare the OS and PFS of the auto-HCT and CAR-T cohorts. The cumulative incidence function with Gray’s test was used to compare the hematopoietic recovery, NRM, and relapse/progression rates in the 2 cohorts to account for competing events. We used the Cox proportional hazard model for PFS and OS; and the proportional cause-specific hazards model for NRM and relapse/progression to compare the 2 cohorts. The variables included in the regression model were: age (continuous and by decade), Karnofsky performance status (≥90 vs <90 vs missing), refractoriness to first-line treatment, number of lines of prior therapies, the interval between diagnosis and auto-HCT or CAR-T (≥1 year vs <1 year vs missing), size of the largest residual node at auto-HCT or CAR-T (<3 cm vs 3-5 cm vs >5 cm vs unknown). The forward stepwise selection was used to identify significant variables at a significance level of 0.05. Interactions between variables in the final regression model were also checked. Proportional hazards assumption was examined by testing covariates’ time-varying effects. To confirm the regression analysis results further, we conducted propensity score matching. To calculate propensity scores, we used multiple imputations using R package smcfcs and Rubin’s rule to handle missing covariates.12-14 Using calculated propensity scores, we matched the auto-HCT and CAR-T cohorts using R package MatchIt with a 1:2 matching ratio for CAR-T and auto-HCT.15 The marginal model was used to handle correlation within matched pairs.16 All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and R version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
We identified 411 patients with DLBCL who received either an auto-HCT (n = 266) or CAR-T therapy (n = 145) while in a PR by computed tomography or PET scan during the study period. Baseline characteristics are summarized in Table 1. There was no significant difference in age (median 58 vs 60 years; P = .07), performance status (Karnofsky ≥90: 51% vs 39%; P = .09), and the proportion of patients who had a pretreatment PET scan (83% vs 87%; P = .36) for disease assessment. Auto-HCT patients had received fewer median lines of prior therapies (median 2 vs 3; P < .001). Although not statistically significant, fewer patients in the auto-HCT group had the largest pretreatment residual node measuring >5 cm (29% vs 41%; P = .05). Twenty-three patients (16%) received bridging therapy between leukapheresis and lymphodepletion in the CAR-T cohort. Disease response was reassessed in 15 of them following bridging, and none converted their PR to CR following bridging. Fourteen patients received CAR-T therapy after auto-HCT relapse.
Table 1.
Baseline characteristics of DLBCL patients who received auto-HCT or CAR-T while in a PR
| Auto-HCT | CAR-T | P value* | |
|---|---|---|---|
| Number of patients† | 266 | 145 | |
| Number of centers | 88 | 40 | |
| Demographics | |||
| Patient age | |||
| Median, y (range) | 58 (18-80) | 60 (24-91) | .07‡ |
| ≥60 y (%) | 118 (44) | 73 (50) | .25§ |
| Male sex | 167 (63) | 89 (61) | .78§ |
| Race | <.001§ | ||
| Caucasian | 166 (62) | 123 (83) | |
| African American | 58 (22) | 10 (7) | |
| Other‖ | 11 (4) | 1 (1) | |
| Missing | 11(4) | 7 (5) | |
| At diagnosis | |||
| Stage at diagnosis | .99§ | ||
| III-IV, no. (%) | 163 (61) | 80 (55) | |
| Missing | 42 (16) | 35 (24) | |
| High-grade B-cell lymphoma, with MYC and BCL2 and/or BCL6 rearrangements, no. (%) | 10 (16) | 25 (17) | .84§ |
| Missing (not collected before 2018) | 204 | ||
| LDH elevated at diagnosis | 74 (28) | 37 (26) | .53§ |
| Missing | 152 (57) | 92 (63) | |
| Extranodal involvement at diagnosis, no. (%) | 136 (51) | 72 (50) | .51§ |
| Missing | 42 (16) | 19 (13) | |
| Prior treatments | |||
| Refractory to first-line therapy, no. (%) | 160 (60) | 79 (55) | .61§ |
| Missing | 6 (2) | 22 (15) | |
| Time from diagnosis to auto-HCT or CAR-T, mo, no. (%) | .30§ | ||
| ≤12 mo | 103 (39) | 64 (44) | |
| >12 mo | 162 (61) | 81 (56) | |
| Missing | 1 (0) | 0 | |
| Lines of therapy before auto-HCT or CAR-T | |||
| Median (range) | 2 (1-6) | 3 (2-11) | <.001‡ |
| More than 2 lines, no. % | 89 (33) | 97 (67) | <.001§0 |
| Number of prior treatment lines | |||
| 1 | 48 (18) | 0 | |
| 2 | 124 (47) | 42 (29) | |
| 3 | 55 (21) | 52 (36) | |
| 4 | 22 (8) | 23 (16) | |
| 5 or more | 12 (4) | 22 (15) | |
| Missing | 5 (2) | 6 (4) | |
| Pre auto-HCT or CAR-T | |||
| KPS | .09§ | ||
| ≥90 | 136 (51) | 56 (39) | |
| Missing | 2 (3) | 13 (9) | |
| Largest node before auto-HCT or CAR-T, no. (%) | .05§ | ||
| <3 cm | 41 (15) | 21 (15) | |
| 3-5 cm | 65 (24) | 26 (18) | |
| >5 cm | 76 (29) | 60 (41) | |
| Missing | 84 (32) | 38 (26) | |
| Imaging before auto-HCT or CAR-T, no. (%) | .36§ | ||
| PET or PET/CT | 222 (83) | 126 (87) | |
| CT | 44 (17) | 19 (13) | |
| Conditioning regimen, no. (%) | |||
| BEAM | 203 (76) | N/A | |
| Bu/Cy | 15 (6) | N/A | |
| CBV | 43 (16) | N/A | |
| Other | 43 (16) | N/A | |
| Lymphodepletion regimen, no. (%) | |||
| Flu/Cy | N/A | 145 (100) | |
| Year of auto-HCT or CAR-T, no. (%) | <.001§ | ||
| 2018 and after | 66 (20) | 145 (100) | |
| Follow-up, median, mo (range) | 38 (3-79) | 12 (3-26) |
BEAM, carmustine, etoposide, cytarabine and melphalan; Bu/Cy, busulfan/cyclophosphamide; CBV, cy, carmustine, etoposide; CT, computed tomography; Flu/Cy, fludarabine, cyclophosphamide; KPS, Karnofsky performance score; LDH, lactate dehydrogenase.
P values were calculated ignoring the missing values.
This includes 11 patients with primary mediastinal large B-cell lymphomas in the auto-HCT group and 4 in the CAR-T therapy group.
Hypothesis testing Kruskal-Wallis test.
Hypothesis testing Pearson χ2 test.
Patient race – other: auto-HCT: 1 native Hawaiian or other Pacific islander, 5 American Indian or Alaska Native, 5 more than 1 race. CAR-T: 1 more than 1 race.
Univariable analysis
The 2-year PFS was 52% (95% confidence interval [CI], 46-58) in the auto-HCT group and 42% (95% CI, 30-53) in the CAR-T group (P = .1) (Figure 1A; Table 2). NRM rates were not different between the 2 cohorts with a 100-day cumulative incidence of NRM of 4% (95% CI, 2-7) vs 2% (95% CI, 0-5) (P = .3) in auto-HCT and CAR-T patients, respectively (Figure 1B). The cumulative incidence of relapse/progression was lower with auto-HCT at 1 year (34% [95% CI, 28-40] vs 45% [95% CI, 37-54]; P = .03) and 2 years (40% [95% CI, 33-46] vs 52% [95% CI, 41-63]; P = .05]) (Figure 1C). The 2-year OS rate was higher in the auto-HCT group compared with the CAR-T (69% [95% CI, 63-74] vs 47% [95% CI, 33-60]; P = .004) (Figure 1D).
Figure 1.
Auto-HCT vs CAR-T in patients with DLBCL in PR (all patients). (A) Progression-free survival. (B) Nonrelapse mortality. (C) Progression/relapse. (D) Overall survival.
Table 2.
Univariable analysis of outcomes in patients treated with auto-HCT or CAR-T while in a PR
| Auto-HCT (N = 266) | CAR-T (N = 145) | ||||
|---|---|---|---|---|---|
| Outcomes | N eval | Prob (95% CI) | N eval | Prob (95% CI) | P value |
| Nonrelapse mortality | 256 | 138 | .2* | ||
| 100 d | 4 (2-7) | 2 (0-5) | .3† | ||
| 1 y | 7 (4-11) | 3 (1-6) | .05† | ||
| 2 y | 9 (5-13) | 6 (1-16) | .6† | ||
| Progression/relapse | 256 | 138 | .01* | ||
| 1 y | 34% (28-40) | 45% (37-54) | .03† | ||
| 2 y | 40% (33-46) | 52% (41-63) | .05† | ||
| Progression-free survival | 256 | 138 | .1* | ||
| 1 y | 59% (53-65) | 52% (43-61) | .2† | ||
| 2 y | 52% (46-58) | 42% (30-53) | .1† | ||
| Overall survival | 266 | 145 | .01* | ||
| 1 y | 76% (70-81) | 67% (59-75) | .1† | ||
| 2 y | 69% (63-74) | 47% (33-60) | .004† | ||
N eval, number evaluated; Prob, probability.
Overall P values from the log-rank test (PFS and OS) and Gray’s test (NRM and progression/relapse).
P values from the Wald test given time.
Information about probabilities of hematopoietic recovery in both cohorts and CRS and ICANS in the CAR-T cohort are summarized in supplemental Table 1.
Subgroup analyses
In a subgroup analysis of patients with an available PET scan before auto-HCT (n = 222) or CAR-T (n = 126), there was no difference the 2-year PFS rate (54% [95% CI, 47-61] vs 43% [95% CI, 32-55]; P = .1) but consolidation with auto-HCT was associated with a lower 2-year cumulative incidence of relapse/progression (39% [95% CI, 32-46] vs 54% [95% CI, 42-66]; P = .03) and an improved 2-year OS rate (71% [95% CI, 65-77] vs 49% [95% CI, 34-63]; P = .006). There was no difference in the 100-day NRM (3% [95% CI, 1-5] vs 2% [95% CI, 0-5]; P = .5) (Table 3). When the subgroup analysis only included patients with ≤2 prior lines of therapy, there was no difference in the 1-year PFS (59% [95% CI, 52-67] vs 65% [95% CI, 49-79]; P = .5), cumulative incidence of relapse/progression (33% [95% CI, 26-41] vs 35% [95% CI, 21-51]; P = .8) or OS (76% [95% CI, 69-82] vs 77% [95% CI, 62-89]; P = .9) but auto-HCT (n = 172) was associated with a higher 100-day NRM compared with CAR-T (n = 42) (4% vs 0; P = .01) (Figure 2; supplemental Table 2). Focusing on patients with more than 2 prior lines of treatment, auto-HCT group (n = 89) had a superior OS at 2 years (63% [95% CI, 53-73] vs 44% [95% CI, 28-60]; P = .04) but there was no difference in the other endpoints (supplemental Tables 3 and 4). When analysis was limited to patients with early treatment failure (primary refractory disease or relapse within 12 months of diagnosis), auto-HCT (n = 186) vs CAR-T (n = 110) cohorts had no significant difference in 2-year PFS (53% [95% CI, 46-60] vs 40% [95% CI, 30-51]; P = .05), but auto-HCT group had lower relapse/progression rate (38% [95% CI, 31-45] vs 56% [95% CI, 45-67]; P = .006) and superior OS (66% [95% CI, 59-73] vs 40% [95% CI, 26-56]; P = .003) compared with the CAR-T group at 2 years.
Table 3.
Subgroup univariable analysis of outcomes in patients treated with auto-HCT or CAR-T while in a PR in patients with known positive PET scan before treatment
| Auto-HCT (N = 222) | CAR-T (N = 126) | ||||
|---|---|---|---|---|---|
| Outcomes | N eval | Prob (95% CI) | N eval | Prob (95% CI) | P value |
| Nonrelapse mortality | 215 | 119 | .2* | ||
| 100 d | 3% (1-5) | 2% (0-5) | .5† | ||
| 1 y | 6% (3-9) | 3% (1-6) | .1† | ||
| 2 y | 7% (4-11) | 3% (1-6) | .04† | ||
| Progression/relapse | 215 | 119 | .007* | ||
| 1 y | 33% (27-39) | 46% (36-55) | .03† | ||
| 2 y | 39% (32-46) | 54% (42-66) | .03† | ||
| Progression-free survival | 215 | 119 | .04* | ||
| 1 y | 61% (55-68) | 52% (42-61) | .1† | ||
| 2 y | 54% (47-61) | 43% (32-55) | .1† | ||
| Overall survival | 222 | 126 | .005* | ||
| 1 y | 79% (73-84) | 69% (60-77) | .06† | ||
| 2 y | 71% (65-77) | 49% (34-63) | .006† | ||
N eval, number evaluated; Prob, probability.
Overall P values from the log-rank test (PFS and OS) and Gray’s test (NRM and Progression/relapse).
P values from the Wald-test given time.
Figure 2.
Auto-HCT vs CAR-T in patients with DLBCL in PR (patients with ≤2 prior lines of treatment). (A) Progression-free survival. (B) Nonrelapse mortality. (C) Progression/relapse. (D) Overall survival.
Multivariable regression analysis
In the multivariable regression analysis of outcomes, there was no significant difference between the 2 cohorts in terms of PFS (hazard ratio [HR] =1.33 [95% CI, 0.98-1.80]; P = .06) and NRM (HR = 0.49 (95% CI, 0.18-1.32]; P = .16) but treatment with CAR-T was associated with a significantly higher risk of relapse/progression (HR 1.49 [95% CI, 1.08-2.05]; P = .01) and mortality (HR = 1.63 [95% CI, 1.14-2.33]; P = .008) (Table 4). We performed additional analysis after propensity score matching of the 2 cohorts (supplemental Table 5), and the HRs of primary and secondary endpoints were directionally consistent with the overall multivariate analysis, although the associations were no longer statistically significant (supplemental Table 6).
Table 4.
Multivariable regression analysis of outcomes in patients treated with auto-HCT or CAR-T while in a PR
| Outcomes | N | HR | 95% CI lower limit | 95% CI lower limit | P value |
|---|---|---|---|---|---|
| Nonrelapse mortality | |||||
| Age, y | |||||
| <60 | 212 | 1.00 | |||
| ≥60 | 182 | 2.92 | 1.31 | 6.50 | .009 |
| Main effect | |||||
| Auto-HCT | 256 | 1.00 | |||
| CAR-T | 138 | 0.49 | 0.18 | 1.32 | .16 |
| Progression/relapse | |||||
| Main effect | |||||
| Auto-HCT | 256 | 1.00 | |||
| CAR-T | 138 | 1.49 | 1.08 | 2.05 | .01 |
| Progression-free survival | |||||
| Main effect | |||||
| Auto-HCT | 256 | 1.00 | |||
| CAR-T | 138 | 1.33 | 0.98 | 1.80 | .06 |
| Overall survival | |||||
| Main effect | |||||
| Auto-HCT | 266 | 1.00 | |||
| CAR-T | 145 | 1.63 | 1.14 | 2.33 | .008 |
Variables included in the multivariable analysis: age (continuous by decade), Karnofsky performance score (≥90 vs <90 vs missing), refractoriness to first line, lines of prior treatment, interval between diagnosis and auto-HCT or CAR-T (≥1 y vs <1 y vs missing), largest node at auto-HCT or CAR-T (<3 cm vs 3-5 cm vs >5 cm vs unknown).
Cause of death
During the follow-up, 91 patients (34%) from the auto-HCT group and 52 (36%) from the CAR-T group died. The primary disease was the most common cause of mortality in both groups accounting for 74% and 75% of deaths in the auto-HCT and CAR-T groups, respectively. Other common causes were infections (6%) and organ failure (4%) in the auto-HCT group and infections (4%), cytokine release syndrome (4%), organ failure (4%), and malignancies (4%) in the CAR-T group.
Discussion
In this retrospective analysis using the CIBMTR registry, we compared the outcomes of patients with DLBCL treated with auto-HCT or CAR-T therapy while in a PR after the last therapy line. Our results indicate similar PFS but a lower risk of relapse and a superior OS with auto-HCT. These data support the role of auto-HCT as standard of care in transplant-eligible patients with relapsed DLBCL that achieve at least a PR as the best response to salvage therapy but more data are needed to answer this question in patients with fewer lines of salvage therapy.
The introduction of CAR-T therapy as a therapeutic option for patients with relapsed DLBCL has been a major advancement in lymphoma treatment. Long-term remissions are achieved in 30% to 40% of patients receiving CAR-T therapy according to the clinical trial data and in the real-world setting.17-19 This outstanding efficacy in a patient population with historically poor outcomes has led to increased utilization of CAR-T treatment, including in DLBCL patients who achieve only a PR after salvage attempt, for whom 1 recommended treatment strategy continues to be an auto-HCT per published guidelines.20 In fact, the efficacy of auto-HCT has been recently confirmed in another CIBMTR analysis showing a 41% 5-year PFS in patients in PET-positive PR receiving an auto-HCT regardless of the timing of initial relapse/refractoriness consistent with the previous reports.8,21
Three ongoing randomized controlled trials (A Study to Compare the Efficacy and Safety of JCAR017 to Standard of Care in Adult Subjects With High-risk, Transplant-eligible Relapsed or Refractory Aggressive B-cell Non-Hodgkin Lymphomas [TRANSFORM] NCT03575351; Efficacy of Axicabtagene Ciloleucel Compared to Standard of Care Therapy in Subjects With Relapsed/Refractory Diffuse Large B Cell Lymphoma [ZUMA-7] NCT03391466; and Tisagenlecleucel in Adult Patients With Aggressive B-cell Non-Hodgkin Lymphoma [NCT03570892]) are comparing the efficacy and safety of each of the 3 approved CAR-T products (Lisocabtagene Maraleucel, axi-cel, and tisa-cel) with the standard of care (ie, salvage chemotherapy followed by auto-HCT) in the relapsed/refractory setting. All 3 trials only included patients with primary refractory or early relapsed (within 12 months) disease and excluded those who received any second-line systemic treatment. From the 3 studies, the available information is from press releases of the TRANSFORM and ZUMA-7 studies in June 2021, which indicated an event-free survival benefit with liso-cel or axi-cel over standard of care in patients with DLBCL with early treatment failure.22,23 However, although informative, these studies will not address the clinical question that we aimed to answer with this analysis because this study specifically included patients in a PR after salvage treatment. In addition, the result of these trials may not be applicable to patients with DLBCL not fulfilling early relapse criteria.
Rather than identifying a “winner” approach, our findings help determine the optimal treatment sequence for this patient population. Given the established clinical efficacy of auto-HCT in this setting and with the complexity and the financial burden of using CAR-T therapy, especially in the absence of data suggesting its superiority when compared with the standard of care, the logical treatment sequence will include using auto-HCT first, knowing the proven efficacy of CAR-T in the posttransplant failure setting. Because auto-HCT remains to be a major treatment modality for DLBCL, continued support for ongoing and planned studies to further improve the efficacy of this effective treatment is necessary. Such studies would incorporate novel targeted agents to the auto-HCT either by augmenting the conditioning regimen or as a maintenance strategy.24
Our analysis has inherent limitations of a retrospective study. Using the registry data, the cohorts are not entirely balanced, and we were limited in assessing the clinical decision process behind selecting each treatment modality. Another important limitation relates to the subjectivity of PR definition, especially in the nonclinical trial setting. With the current definition of a PR based on the standard response assessment guidelines, a wide range of patients with various tumor bulk and different levels of chemo-responsiveness could have potentially been included in this category. By including the pretreatment largest node size in our models, we attempted to some degree control for this limitation, but the possibility of residual confounder effect cannot be ruled out especially given the higher numerical percentage of patients with >5 cm lymph node.25-27 We observed consistent results in our subgroup analysis focusing on patients with an available PET scan before treatment mitigating the risk effect modification by the diagnostic modality.21 Also, there was no independent centralized reading of the scans to confirm the reported PR response by the transplant center. Patients in the auto-HCT cohort were included from a longer period (2013-2019) compared with the CAR-T patients (2018-2019). This approach is justified given the comparable auto-HCT outcomes before and after 2018 (data not shown). Comparison between CAR-T and auto-HCT was made using propensity score matching as well. Although the effect on disease relapse/progression and mortality was directionality consistent with the primary analysis, it was not statistically significant. This subset analysis reduced the sample size, which likely affected the power to detect statistical significance. The observed difference was mainly observed in patients with ≥2 prior lines of treatment. This cutoff was used for subgroup analysis, given the implications in clinical practice. Although lack of statistical significance in patients with <2 prior lines of treatment warrants further attention and ideally prospective trials in that group, the small number of patients in this subgroup analysis limits our ability for making a strong conclusion, particularly because we did not observe an interaction (effect modification) between the number of prior treatment lines and treatment modality and outcomes. Patients in the CAR-T group were more heavily pretreated and it is possible that the CAR-T may show improved efficacy in patients with fewer lines of prior treatment Last, we only included patients treated with axi-cel to reduce the heterogeneity given the small number of patients treated with tisa-cel in the registry, fulfilling eligibility criteria for this study at the time of the analysis.
In summary, for patients with DLBCL who achieve a PR after salvage chemotherapy, treatment with auto-HCT was associated with a similar PFS rate, but led to a lower incidence of relapse and an improved OS compared with CAR-T in this analysis. We anxiously await full results of randomized phase 3 data to inform the optimal second-line therapy. However, regardless of the outcome of those studies, our findings will have clinical implications. We anticipate that even if CAR-T cell therapy is widely viewed as the superior treatment option (based on the press release TRANSFORM and ZUMA-7 studies), there will still be patients who will receive chemotherapy after relapse for many reasons: (1) not meeting the eligibility criteria; (2) lack of immediate access to CAR-T therapy; or (3) simply based on patient or physician preferences. These data will inform decisions if those patients achieve a PR. Last, these data highlight the need for a prospective randomized trial for patients with chemosensitive disease and in a PR after salvage therapy comparing CAR-T to the standard of care.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The CIBMTR is supported primarily by grants from the National Institutes of Health National Cancer Institute (NCI) Public Health Service (U24CA076518), the National Heart, Lung and Blood Institute, and the National Institute of Allergy and Infectious Diseases; NCI (U24CA233032), Health Resources and Services Administration (HHSH250201700006C), the Office of Naval Research (N00014-20-1-2705 and N00014-20-1-2832). Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie, Accenture, Actinium Pharmaceuticals Inc., Adaptive Biotechnologies Corporation, Adienne SA, Allovir Inc., Amgen Inc., Astellas Pharma US, bluebird bio inc., Bristol Myers Squibb Co., CareDx, CSL Behring, CytoSen Therapeutics Inc., Daiichi Sankyo Co. Ltd., Eurofins Viracor, ExcellThera, Fate Therapeutics, Gamida-Cell Ltd., Genentech Inc, Gilead, GlaxoSmithKline, Incyte Corporation, Janssen/Johnson & Johnson, Jasper Therapeutics, Jazz Pharmaceuticals Inc., Karyopharm Therapeutics, Kiadis Pharma, Kite, a Gilead Company, Kyowa Kirin, Magenta Therapeutics, Medac GmbH, Merck & Co., Millennium, the Takeda Oncology Co., Miltenyi Biotec Inc., MorphoSys, Novartis Pharmaceuticals Corporation, Omeros Corporation, Oncopeptides Inc., Orca Biosystems Inc., Pfizer Inc., Pharmacyclics LLC, Sanofi Genzyme, Seagen Inc., Stemcyte, Takeda Pharmaceuticals, Tscan, Vertex, Vor Biopharma, and Xenikos BV.
Footnotes
CIBMTR supports accessibility of research in accord with the National Institutes of Health Data Sharing Policy and the National Cancer Institute Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases deidentified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.S. and M.H. conceived and designed the study; Y.C. and M.H. collected and assembled data; Y.C., M.H., and K.W.A. provided data analysis; all authors interpreted the data; M.S. and M.H. prepared the first draft prepared; and all authors helped revised the manuscript.
Conflict-of-interest disclosure: M.S. reports consulting, advisory Boards, steering committees or data safety monitoring committees: Abbvie, Genentech, AstraZeneca, Sound Biologics, Pharmacyclics, Beigene, Bristol Myers Squibb, Morphosys, TG Therapeutics, Innate Pharma, Kite Pharma, Adaptive Biotechnologies, Epizyme, Eli Lilly, Adaptimmune, and Atara Biotherapeutics; research funding: Mustang Bio, Celgene, Bristol Myers Squibb, Pharmacyclics, Gilead, Genentech, Abbvie, TG Therapeutics, Beigene, AstraZeneca, Sunesis, Atara Biotherapeutics, and GenMab. M.A.K.-D. reports other from Daiichi Sankyo, outside the submitted work. C.S.S. reports grants from Juno Therapeutics, Celgene, Bristol-Myers Squibb, Precision Biosciences, and Sanofi Genzyme; personal fees from Precision Biosciences, Sanofi Genzyme, Juno Therapeutics, Spectrum Pharmaceuticals, Novartis, Genmab, Kite (a Gilead Company), Celgene, Gamida Cell, Karyopharm Therapeutics, and GlaxoSmithKline, outside the submitted work. A.F.H. reports research funding from BMS, Merck, Genentech, Inc./F. Hoffmann-La Roche Ltd, Gilead Sciences, Seattle Genetics, AstraZeneca, and ADC Therapeutics; consultancy for BMS, Merck, Genentech, Inc./F. Hoffmann-La Roche Ltd, Gilead Sciences, Seattle Genetics, and Karyopharm; travel, accommodations, and expenses from BMS. M.H. reports research support/funding: Takeda Pharmaceutical Company, Spectrum Pharmaceuticals, and Astellas Pharma; consultancy: Janssen, Incyte Corporation, ADC Therapeutics, Celgene Corporation, Omeros, Verastem, and MorphoSys; speaker’s bureau: Sanofi Genzyme, AstraZeneca, and BeiGene. F.L.L. reports scientific advisory role: Allogene, Amgen, Bluebird Bio, BMS/Celgene, Calibr, Cellular Biomedicine Group, GammaDelta Therapeutics, Iovance, Kite Pharma, Janssen, Legend Biotech, Novartis, Wugen, and Umoja; research funding: Kite Pharma (institutional), Allogene (institutional), Novartis (institutional); and patents, royalties, and other intellectual property: several patents held by the institution in my name (unlicensed) in the field of cellular immunotherapy. C.J.T. reports research funding: Juno Therapeutics/BMS, Nektar Therapeutics, and AstraZeneca; scientific sdvisory boards: Precision Biosciences, Eureka Therapeutics, Caribou Biosciences, T-CURX, Myeloid Therapeutics, ArsenalBio, and Century Therapeutics; ad hoc advisory boards (past 12 months): Allogene, Amgen, and AsherBio; stock/options: Precision Biosciences, Eureka Therapeutics, Caribou Biosciences, Myeloid Therapeutics, and ArsenalBio; patents: has the right to receive royalties from Fred Hutchinson as an inventor on patents related to CAR T-cell therapy that are licensed to Juno Therapeutics/BMS.
Correspondence: Mehdi Hamadani, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, 9200 W. Wisconsin Ave, Suite C5500, Milwaukee, WI 53226; e-mail: mhamadani@mcw.edu.
REFERENCES
- 1.Sehn LH, Salles G. Diffuse large B-cell lymphoma. N Engl J Med. 2021;384(9):842-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanate AS, Majhail NS, Savani BN, et al. Indications for hematopoietic cell transplantation and immune effector cell therapy: guidelines from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2020;26(7):1247-1256. [DOI] [PubMed] [Google Scholar]
- 3.Hamadani M, Hari PN, Zhang Y, et al. Early failure of frontline rituximab-containing chemo-immunotherapy in diffuse large B cell lymphoma does not predict futility of autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20(11):1729-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333(23):1540-1545. [DOI] [PubMed] [Google Scholar]
- 5.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene Maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020; 396(10254):839-852. [DOI] [PubMed] [Google Scholar]
- 6.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-cell therapy in refractory large B-Cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377(26):2545-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah NN, Ahn KW, Litovich C, et al. Is autologous transplant in relapsed DLBCL patients achieving only a PET+ PR appropriate in the CAR T-cell era? [published correction appears in Blood. 2021;137(20):2854-2855] Blood. 2021; 137(10):1416-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheson BD, Fisher RI, Barrington SF, et al. ; United Kingdom National Cancer Research Institute . Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheson BD, Pfistner B, Juweid ME, et al. ; International Harmonization Project on Lymphoma . Revised response criteria for malignant lymphoma. J Clin Oncol. 2007; 25(5):579-586. [DOI] [PubMed] [Google Scholar]
- 11.Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019;25(4):625-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartlett JWK. R. Multiple imputation of covariates by substantive model compatible fully conditional specification 2020. [R package version 1.4.2]. Available from: https://cran.r-project.org/web/packages/smcfcs/. [DOI] [PMC free article] [PubMed]
- 13.Bartlett JW, Seaman SR, White IR, Carpenter JR; Alzheimer’s Disease Neuroimaging Initiative . Multiple imputation of covariates by fully conditional specification: accommodating the substantive model. Stat Methods Med Res. 2015;24(4):462-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubin DR. Multiple Imputation for Nonresponse in Surveys. Indianapolis, IN: John Wiley & Sons, Inc.; 1987 [Google Scholar]
- 15.Ho D, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42(8):28. [Google Scholar]
- 16.Lee EW, Wei LJ, Amato DA, Leurgans S. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. In: Goel PKPKG, ed. In Survival Analysis: State of the Art. Dordrecht, Netherlands: Kluwer Academic; 1992: 237-247 [Google Scholar]
- 17.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobson CA, Hunter BD, Redd R, et al. Axicabtagene Ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J Clin Oncol. 2020;38(27):3095-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chong EA, Ruella M, Schuster SJ; Lymphoma Program Investigators at the University of Pennsylvania . Five-year outcomes for refractory B-cell lymphomas with CAR T-cell therapy. N Engl J Med. 2021;384(7):673-674. [DOI] [PubMed] [Google Scholar]
- 20.Zelenetz AD, Gordon LI, Abramson JS, et al. NCCN Guidelines Insights: B-cell lymphomas, version 3.2019. J Natl Compr Canc Netw. 2019;17(6):650-661. [DOI] [PubMed] [Google Scholar]
- 21.Sauter CS, Matasar MJ, Meikle J, et al. Prognostic value of FDG-PET prior to autologous stem cell transplantation for relapsed and refractory diffuse large B-cell lymphoma. Blood. 2015;125(16):2579-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bristol Myers Squibb Announces Positive Topline Results from Phase 3 TRANSFORM Trial Evaluating Breyanzi (Lisocabtagene Maraleucel) Versus Chemotherapy Followed by Stem Cell Transplant in Second-line Relapsed or Refractory Large B-cell Lymphoma 2021. Available from: https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Announces-Positive-Topline-Results-from-Phase-3-TRANSFORM-Trial-Evaluating-Breyanzi-lisocabtagene-maraleucel-Versus-Chemotherapy-Followed-by-Stem-Cell-Transplant-in-Second-line-Relapsed-or-Refractory-Large-B-cell-Lymphoma/default.aspx.
- 23.Kite announces Yescarta® CAR T-cell therapy improved event-free survival by 60% over chemotherapy plus stem cell transplant in second-line relapsed or refractory large B-cell lymphoma 2021. Available from: https://www.kitepharma.com/news/press-releases/2021/6/kite-announces-yescarta-car-tcell-therapy-improved-eventfree-survival-by-60-over-chemotherapy-plus-stem-cell-transplant-in-secondline- relapsed-or.
- 24.Kanate AS, Kumar A, Dreger P, et al. Maintenance therapies for Hodgkin and non-Hodgkin lymphomas after autologous transplantation: a consensus project of ASBMT, CIBMTR, and the Lymphoma Working Party of EBMT. JAMA Oncol. 2019;5(5):715-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locke FL, Rossi JM, Neelapu SS, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(19): 4898-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dean EA, Mhaskar RS, Lu H, et al. High metabolic tumor volume is associated with decreased efficacy of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(14):3268-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain MD, Zhao H, Wang X, et al. Tumor interferon signaling and suppressive myeloid cells are associated with CAR T-cell failure in large B-cell lymphoma. Blood. 2021;137(19):2621-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.