Abstract
Context
The effects of the coronavirus disease 2019 (COVID-19) pandemic on the incident cases of pediatric type 1 diabetes (T1D) and type 2 diabetes (T2D) are not clear.
Objective
To identify trends in incidence and presentation of pediatric new-onset T1D and T2D during the COVID-19 pandemic.
Methods
A retrospective chart review was conducted. Demographics, anthropometrics, and initial laboratory results from patients ages 0 through 21 years who presented with new-onset diabetes to a pediatric tertiary care center were recorded.
Results
During the pandemic, incident cases of pediatric T1D increased from 31 in each of the prior 2 years to 46; an increase of 48%. Incident cases of pediatric T2D increased by 231% from 2019 to 2020. The number of incident cases of pediatric T2D increased significantly more than the number of incident cases of pediatric T1D (P = 0.009). Patients with T2D were more likely to present in diabetic ketoacidosis (DKA), though this was not statistically significant (P = 0.093). Severe DKA was higher compared with moderate DKA (P = 0.036) in incident cases of pediatric T2D. During the pandemic, for the first time, incident cases of T2D accounted for more than one-half of all newly diagnosed pediatric diabetes cases (53%).
Conclusions
There were more incident pediatric T1D and T2D cases as well as an increase in DKA severity in T2D at presentation during the COVID-19 pandemic. More importantly, incident T2D cases were higher than the incident T1D during the pandemic. This clearly suggests a disruption and change in the pediatric diabetes trends with profound individual and community health consequences.
Keywords: pediatric diabetes, type 1 diabetes, type 2 diabetes, COVID-19, diabetic ketoacidosis
The coronavirus disease 2019 (COVID-19) pandemic has introduced countless challenges to the medical field and has brought increased attention to children with preexisting disorders such as diabetes. More than 6 million children have tested positive for COVID-19 since the onset of the pandemic, a number that is projected to continue increasing [1]. Although children have lower rates of COVID-19 mortality, the presence of preexisting conditions such as diabetes can heighten the severity of their clinical presentations. Studies in adults have shown new-onset diabetes as well as expression of preexisting diabetes in a significant proportion of patients with COVID-19, both presenting with more severe infection [2]. Another study discussing the effect of the pandemic on adults with diabetes showed increases in diabetes-related stress, higher in young adults with type 2 diabetes (T2D) compared with older adults [3]. However, there are few studies evaluating the effects of COVID-19 on diabetes-related outcomes in children. Current data are not only limited but contradictory regarding the incidence and severity of presentation in childhood diabetes. Reports range from an increase in diabetic ketoacidosis rates in type 1 diabetes (T1D) and T2D in children to no change or even a decrease in the incidence of T1D during the pandemic [4-9].
There are several factors proposed to contribute to an increase in either the incidence or severity of pediatric diabetes. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19, infects and replicates in cells of the human endocrine and exocrine pancreas, and is suspected to downregulate the renin-angiotensin-aldosterone system, leading to eventual destruction of pancreatic beta cells [10-13]. Additionally, COVID-19 causes an hyperinflammatory state and may induce ketosis and ketoacidosis [13, 14]. Other factors include the immense behavioral and environmental changes since the onset of the pandemic. Across the globe, children were enrolled in school virtually, extracurricular activities were limited, and daily routines were adjusted to decrease the potential exposure to COVID-19. Consequences of this included increased screen time, unhealthy eating habits, decreased physical activity, and worsened sleep hygiene, which all have associations with increased body mass index (BMI) [15, 16].
The objectives of this retrospective study were to compare the rates of diagnosis of new-onset pediatric T1D and T2D cases and to compare the severity of clinical presentation among patients seen at a single academic medical center during the COVID-19 pandemic with those in the 2 years preceding the pandemic. These are discussed while considering demographic and anthropometric changes in the overall clinic patient cohort. We hypothesized that the number of incident cases of both T1D and T2D have increased during the pandemic and that the severity of clinical presentation increased.
Materials and Methods
Data Collection
This study was completed using retrospective review of the electronic medical record of all patients admitted to Duke University Hospital or seen as outpatients in the Duke Pediatric Endocrinology and Diabetes Clinics with a new diagnosis of diabetes over a 3-year period from April 1, 2018, until March 31, 2021. Time periods were separated as follows: April 1, 2018-March 31, 2019; April 1, 2019-March 31, 2020; and April 1, 2020-March 31, 2021. The final period represents the COVID-19 pandemic. Duke University Hospital is a 190-bed pediatric tertiary acute care hospital with 25 specialties. Our Diabetes Program, recognized by the American Diabetes Association for diabetes self-management education, offers the latest advances in T1D and T2D diabetes care, and provides clinical care to approximately 700 youth with T1D and 300 youth with T2D annually. All patients with new-onset T1D are admitted to the hospital, whereas patients with new-onset T2D are admitted only for management of severe hyperglycemia, ketosis, and/or challenging psychosocial circumstances.
The search protocol included any patient ages 0 to 21 at the time of initial diagnosis using International Classification of Diseases, 10th revision, diagnosis codes for T1D (E10*), T2D (E11*), and hyperglycemia (R73.9, R73.09). Each patient’s demographic and anthropometric data were collected at the time of initial diagnosis. We collected vital signs on presentation to the hospital or clinic, as well as hemoglobin A1c (HbA1c), venous pH, serum bicarbonate, beta hydroxybutyrate, c-peptide, sodium, initial point of care glucose, and anion gap. To assess renal and liver involvement, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, and blood urea nitrogen (BUN) levels were extracted. Given the effects of COVID-19 on inflammatory and autoimmune processes, we also collected white blood cell count, hemoglobin, platelet count, TSH, free thyroxine, and celiac panel including immunoglobulin A and tissue transglutaminase (TTG) antibodies at presentation. COVID test results were recorded at the time of presentation if tested, using Coronavirus (COVID-19) SARS-CoV-2 PCR or point of care Coronavirus (COVID-19) SARS-CoV-2 Rapid Test.
This protocol was approved and deemed exempt by the Duke University Health System institutional review board.
Definitions
This study’s criteria for new-onset diabetes aligns with guidelines of the American Diabetes Association, requiring the patient to have either a HbA1c ≥ 6.5%, a fasting plasma glucose of 126 mg/dL or higher, or a random glucose of 200 mg/dL or higher with symptoms of diabetes [17]. The diagnosis of T1D was based on presence of positive autoantibodies against GAD65, islet antigen 2, insulin, or zinc transporter 8 and a requirement for insulin. T2D was diagnosed in patients without autoantibodies and BMI percentile of at least 85% within 6 months of diagnosis. Those without autoantibody testing were categorized based on the impression of the pediatric endocrinologist with consideration of their initial presentation, weight trends, and insulin requirements.
Diabetic ketoacidosis (DKA) was defined as a serum pH ≤ 7.3 or bicarbonate ≤ 15 mmol/L, with ketonemia or ketonuria, and glucose of 200 mg/dL or greater. DKA was further categorized as severe (pH < 7.1), moderate (pH 7.1-7.19), or mild (pH 7.2-7.3) [18]. Plasma osmolarity was calculated by using the following formula (Posm = 2 [Na] + glucose (mg/dL)/18 + BUN (mg/dL)/2.8) unless it was measured directly [19]. For calculations, uncorrected Na levels were used. Celiac screening was considered positive if the TTG antibodies were ≥ 20 units.
Statistical analysis
Microsoft Excel (2016) was used to calculate descriptive statistics (eg, mean, SD) and perform statistical analysis of continuous variables (eg, ANOVA). Statistical analysis of categorical variables (eg, sex, race, ethnicity) was performed by χ 2 test or Fisher exact test using R studio version 3.5.2. Cochran Armitage trend tests (1-tailed, P value cut = 0.05) were performed to assess for increases in diabetes severity and T1D vs T2D proportion across the 3 years using R (version 1.4.1106) and the DescTools package [20, 21].
Results
Demographics
In total, 207 patients were diagnosed with incident diabetes from April 2018 through March 2021, 108 with T1D and 86 with T2D (Tables 1 and 2). Thirteen patients with incident diabetes did not meet the criteria for either type and were excluded from analysis. The race and ethnicity distributions for T1D and T2D patients were consistent without any statistical differences among the 3 years: 63% of patients with T1D were White, 27% black or African American, and 11% Hispanic or Latino on average across 3 years (Table 1). Among patients with T2D, 13% were White, 71% Black or African American, and 19% were Hispanic or Latino on average across 3 years (Table 2).
Table 1.
Type 1 diabetes demographic data
| 2018-2019 N = 31 |
2019-2020 N = 31 |
2020-2021 N = 46 |
P value | |
|---|---|---|---|---|
| Age, y (mean ± SD) | 9.87 ± 3.49 | 10.39 ± 4.89 | 10.46 ± 3.67 | 0.806 |
| Sex | ||||
| Male | 14 (45%) | 14 (45%) | 30 (65%) | 0.12 |
| Female | 17 (55%) | 17 (55%) | 16 (35%) | 0.12 |
| Race, N (%) | ||||
| White | 20 (65%) | 18 (58%) | 30 (65%) | 0.80 |
| Black or African American | 8 (26%) | 10 (33%) | 10 (22%) | 0.59 |
| More than 1 race | 1 (3%) | 0 (0%) | 0 (0%) | 0.57 |
| American Indian or Alaska Native | 0 (0%) | 1 (3%) | 0 (0%) | 0.57 |
| No response | 2 (6%) | 2 (6%) | 6 (13%) | 0.53 |
| Ethnicity, N (%) | ||||
| Hispanic or Latino | 3 (10%) | 3 (10%) | 6 (13%) | 0.86 |
| Non-Hispanic or Latino | 27 (87%) | 27 (87%) | 39 (85%) | 1 |
| No response | 1 (3%) | 1 (3%) | 1 (2%) | 1 |
| Insurance, N (%) | ||||
| Public | 9 (29%) | 15 (48%) | 19 (41%) | 0.29 |
| Private | 21 (68%) | 16 (52%) | 24 (52%) | 0.33 |
| Uninsured | 1 (3%) | 0 (0%) | 3 (7%) | 0.39 |
Age is represented in years indicated by group mean ± SD. Race, ethnicity, and insurance are indicated by number of participants (N) and percent of group total.
Table 2.
Type 2 diabetes demographic data
| 2018-2019 N = 17 |
2019-2020 N = 16 |
2020-2021 N = 53 |
P value | |
|---|---|---|---|---|
| Age, y (mean ± SD) | 14.00 ± 2.35 | 12.94 ± 2.64 | 13.81 ± 2.35 | 0.376 |
| Sex | ||||
| Male | 7 (41%) | 7 (44%) | 25 (47%) | 0.90 |
| Female | 10 (59%) | 9 (56%) | 28 (53%) | 0.90 |
| Race, N (%) | ||||
| White | 2 (12%) | 3 (19%) | 4 (8%) | 0.42 |
| Black or African American | 12 (70%) | 13 (81%) | 33 (62%) | 0.40 |
| More than 1 race | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| American Indian or Alaska Native | 0 (0%) | 0 (0%) | 2 (4%) | 1 |
| No response | 3 (18%) | 0 (0%) | 14 (26%) | 0.55 |
| Ethnicity, N (%) | ||||
| Hispanic or Latino | 3 (18%) | 0 (0%) | 11 (20%) | 0.13 |
| Non-Hispanic or Latino | 14 (82%) | 16 (100%) | 39 (74%) | 0.55 |
| No response | 0 (0%) | 0 (0%) | 3 (6%) | 1 |
| Insurance, N (%) | ||||
| Public | 12 (71%) | 12 (75%) | 39 (73%) | 1 |
| Private | 5 (29%) | 3 (19%) | 12 (23%) | 0.82 |
| Uninsured | 0 (0%) | 1 (6%) | 2 (4%) | 0.54 |
Age is represented in years indicated by group mean ± SD. Race, ethnicity, and insurance are indicated by number of participants (N) and percent of group total.
Changes in Incident Cases
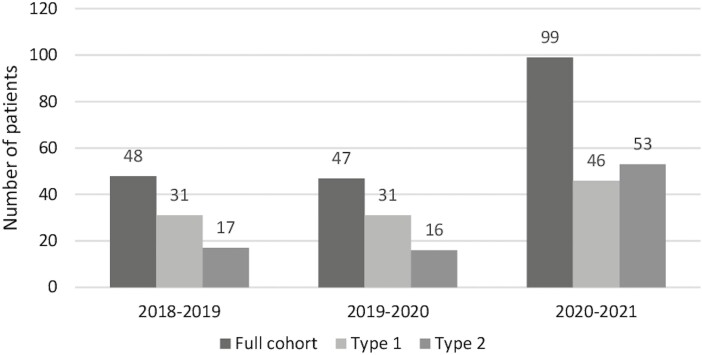
The number of patients diagnosed with T1D between April 1, 2020, and March 31, 2021 (n = 46), was 48% higher than the average number of patients with T1D (n = 31) from the previous 2 years (Fig. 1). There was also an increase in T2D, with 17 patients in 2018 and 16 in 2019, to 53 patients during the pandemic, a 231% increase from the year prior (Fig. 1). The number of incident pediatric T2D cases increased significantly more than the number of incident pediatric T1D cases from 2018 to 2020 (P = 0.009). The average age at initial diagnosis was 10 years for T1D and 13.5 years for T2D and did not differ significantly over the 3 years (Table 1). There were more females than males diagnosed with T1D with the exception of years 2020 and 2021, where 65% of patients were males, compared with 45% in prior years; however, this did not reach statistical significance (P = 0.12). No patients tested positive for COVID-19.
Figure 1.
Incident cases of pediatric type 1 diabetes and type 2 diabetes rose significantly during the COVID-19 pandemic compared with the prior 2 years. Exact number of cases are indicated on the top of each bar.
Anthropometric Trends and Clinical Severity at Presentation
T1D
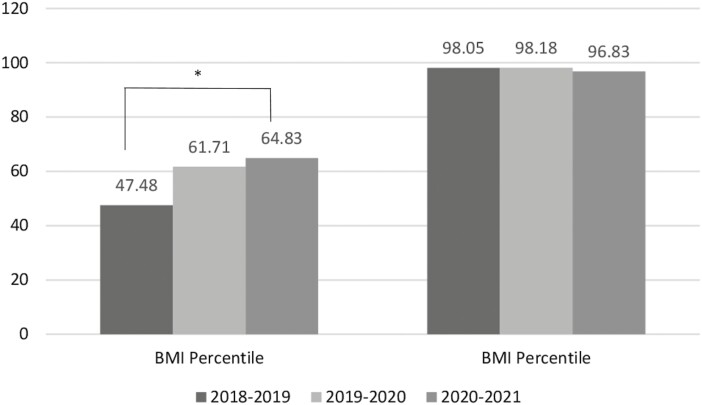
The BMI percentiles steadily increased in patients diagnosed with T1D, from the 47.48 percentile to the 64.83 percentile during 2021, nearing statistical significance (P = 0.064, Fig. 2). There were no changes in HbA1c at presentation (Table 3). Over the 3 years, 50% (n = 54) of patients presented in DKA, with 45% in 2018, 55% in 2019, and 50% in 2020. The number of T1D patients presenting with mild DKA in 2020 rose significantly compared with T1D patients without mild DKA over the 3 years (P = 0.027). Regarding secondary organ involvement, there were no statistical differences in the liver or kidney function tests (Table 4).
Figure 2.
BMI percentiles, T1D, and T2D. Average BMI percentile showed an increasing trend in patients with new-onset T1D from 2018 to 2021. *ANOVA P value < 0.1.
Table 3.
Type 1 diabetes initial laboratory results
| 2018-2019 | 2019-2020 | 2020-2021 | P value | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| HbA1c (%) | 12.28 ± 2.52 | 12.68 ± 2.06 | 12.09 ± 2.54 | 0.565 |
| Initial glucose (mg/dL) | 462.61 ± 151.14 | 522.84 ± 172.35 | 497.61 ± 257.95 | 0.522 |
| pH, venous | 7.22 ± 0.18 | 7.23 ± 0.16 | 7.25 ± 0.17 | 0.808 |
| Bicarbonate, venous (mmol/L) | 16.36 ± 8.53 | 15.51 ± 8.08 | 17.08 ± 8.05 | 0.719 |
| βOHB (mmol/L) | 5.68 ± 2.53 | 4.79 ± 2.72 | 4.89 ± 2.82 | 0.544 |
| Anion gap (mmol/L) | 17.71 ± 6.27 | 18.31 ± 5.47 | 17.72 ± 6.28 | 0.900 |
| Initial C-peptide (ng/mL) | 0.49 ± 0.44 | 0.53 ± 0.32 | 0.71 ± 1.01 | 0.396 |
| Plasma osmolarity (mOsm/kg) | 298.86 ± 11.50 | 299.08 ± 11.64 | 298.42 ± 12.96 | 0.971 |
P values indicated from ANOVA tests to identify significant changes throughout the 3 years.
Abbreviations: βOHB, beta hydroxybutyrate; HbA1c, hemoglobin A1c.
Table 4.
Type 1 diabetes secondary organ involvement and autoimmune laboratory results
| 2018-2019 | 2019-2020 | 2020-2021 | P value | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| ALT (U/L) | 19.69 ± 6.45 | 16.65 ± 3.89 | 23.46 ± 23.03 | 0.310 |
| AST (U/L) | 17.38 ± 4.79 | 17.26 ± 6.23 | 20.75 ± 7.97 | 0.162 |
| BUN (mg/dL) | 15.87 ± 11.5 | 13.55 ± 5.57 | 13.02 ± 4.67 | 0.251 |
| Creatinine (mg/dL) | 0.87 ± 0.63 | 0.83 ± 0.31 | 0.83 ± 0.35 | 0.904 |
| WBC (×109/L) | 11.92 ± 8.1 | 10.48 ± 10.2 | 10.20 ± 6.39 | 0.589 |
| Platelet (×109/L) | 335.00 ± 78.66 | 322.55 ± 10.65 | 310.38 ± 91.77 | 0.569 |
| Hgb (g/dL) | 14.66 ± 1.06 | 14.51 ± 1.32 | 14.81 ± 1.54 | 0.663 |
| FT4 (ng/dL) | 0.97 ± 0.23 | 0.93 ± 0.23 | 0.94 ± 0.2 | 0.779 |
| TSH (µIU/mL) | 2.56 ± 2.80 | 1.96 ± 1.46 | 2.21 ± 1.31 | 0.482 |
| IgA (mg/dL) | 169.46 ± 103.4 | 167.34 ± 100.6 | 176.21 ± 100.4 | 0.929 |
| TTG (Units) | 8.32 ± 10.96 | 12.69 ± 25.17 | 17.41 ± 36.53 | 0.412 |
P values indicated from ANOVA tests to identify significant changes throughout the 3 years.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; FT4, free thyroxine; Hgb, hemoglobin; Ig, immunoglobulin; TTG, tissue transglutaminase; WBC, white blood cell.
T2D
Anthropometric data for patients diagnosed with T2D did not differ significantly over the 3-year period. BMI percentile averaged 98.0 in 2018 and 2019, and 96.8 in 2020. At presentation, HbA1c averaged 9.0% in 2018, 9.6% in 2019, and 10.3% in 2020 (P = 0.223) (Table 5). There were no changes in liver or kidney function (Table 6).
Table 5.
Type 2 diabetes initial laboratory results
| 2018-2019 | 2019-2020 | 2020-2021 | P value | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| HbA1c (%) | 9.04 ± 2.62 | 9.56 ± 2.67 | 10.31 ± 2.85 | 0.223 |
| Initial glucose (mg/dL) | 248.06 ± 146.8 | 276.13 ± 145.16 | 358.64 ± 293.71 | 0.209 |
| pH, venous | 7.27 ± 0.11 | 7.27 ± 0.11 | 7.26 ± 0.13 | 0.986 |
| Bicarbonate, venous (mmol/L) | 21.36 ± 6.98 | 23.00 ± 4.81 | 20.82 ± 7.03 | 0.662 |
| βOHB (mmol/L) | 2.84 ± 3.51 | 2.39 ± 1.67 | 3.60 ± 0.769 | 0.769 |
| Anion gap (mmol/L) | 11.82 ± 4.42 | 12.10 ± 4.58 | 15.00 ± 0.254 | 0.254 |
| Initial C-peptide (ng/ml) | 3.68 ± 2.82 | 3.10 ± 2.24 | 2.84 ± 0.674 | 0.674 |
| Plasma osmolarity (mOsm/kg) | 292.51 ± 6.31 | 294.09 ± 9.89 | 299.08 ± 0.425 | 0.425 |
P values indicated from ANOVA tests to identify significant changes throughout the 3 years.
Abbreviations: βOHB, beta hydroxybutyrate; HbA1c, hemoglobin A1c.
Table 6.
Type 2 diabetes initial secondary organ involvement and autoimmune laboratory results
| 2018-2019 | 2019-2020 | 2020-2021 | P value | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| ALT (U/L) | 41.77 ± 45.36 | 53.10 ± 99.38 | 50.89 ± 53.89 | 0.881 |
| AST (U/L) | 32.38 ± 33.63 | 33.33 ± 46.48 | 38.49 ± 32.44 | 0.831 |
| BUN (mg/dL) | 9.61 ± 3.36 | 10.67 ± 3.89 | 10.11 ± 5.90 | 0.881 |
| Creatinine (mg/dL) | 0.72 ± 0.22 | 0.80 ± 0.34 | 0.90 ± 0.72 | 0.600 |
| WBC (×109/L) | 10.00 ± 9.46 | 9.46 ± 3.65 | 10.22 ± 4.67 | 0.913 |
| Platelet (×109/L) | 345.28 ± 73.35 | 322.43 ± 49.77 | 296.42 ± 69.9 | 0.171 |
| Hgb (g/dL) | 14.01 ± 1.72 | 14.39 ± 1.20 | 14.41 ± 1.83 | 0.858 |
| FT4 (ng/dL) | 2.21 ± 3.74 | 1.03 ± 0.18 | 0.94 ± 0.18 | 0.087 |
| TSH (µIU/mL) | 1.37 ± 0.79 | 7.91 ± 15.90 | 2.28 ± 1.15 | 0.022 |
| IgA (mg/dL) | 170.8 ± 65.09 | 143.0 ± 21.21 | 199.60 ± 93.1 | 0.586 |
| TTG (units) | 3.80 ± 1.64 | 3.50 ± 0.71 | 3.60 ± 2.08 | 0.975 |
P values indicated from ANOVA tests to identify significant changes throughout the 3 years.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; FT4, free thyroxine; Hgb, hemoglobin; Ig, immunoglobulin; TTG, tissue transglutaminase; WBC, white blood cell.
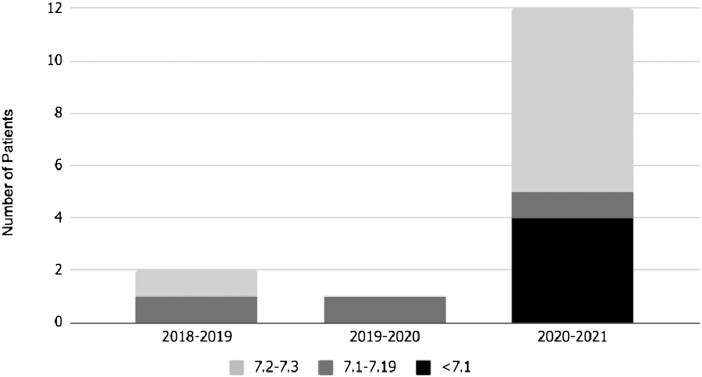
The rate of patients presenting in DKA increased overall, from 12% (n = 2) in 2018 and 6% (n = 1) in 2019, to 23% in 2020 (n = 12), an increase of 91.7% from 2018 to 2020 (Fig. 3). Patients with T2D were more likely to present in DKA, though this was not statistically significant (P = 0.093). When data were stratified by the severity of DKA, no patient presented in severe DKA until 2020 (n = 1), moderate DKA remained stable each year, and mild DKA increased from 6% (n = 1) in 2018 to 13% (n = 7) in 2020, an increase of 116% (Fig. 3). The number of T2D patients with severe DKA rose significantly more than the number of T2D patients with moderate DKA over the 3 years (P = 0.036). When comparing the race and ethnicity of the patients presenting in DKA, 100% of patients in 2018 and 2019 identified as Black or African American, and in 2020, 92% (n = 11) were Black or African American and 8% (n = 1) were Hispanic, showing the predominance of minority populations.
Figure 3.
DKA severity in type 2 diabetic patients increased during the COVID-19 pandemic (2020-2021) compared with prior years.
Discussion
There were more incident pediatric T1D and T2D cases as well as an increase in DKA at presentation during the initial year of the COVID-19 pandemic. Interestingly, incident T2D cases increased from 2018 to 2020 significantly more than T1D cases (P = 0.009). Furthermore, there was a higher number of incident T2D (n = 53) than incident T1D cases (n = 48) during the pandemic. This clearly suggests a disruption and change in the childhood diabetes trends during the COVID-19 pandemic with profound individual and community health consequences.
According to SEARCH, a population-based registry of diabetes, the incidence of diabetes in childhood and adolescence increased from 2002 to 2015 [22]. T1D incidence increased at an annual percent change of 1.9%, whereas the incidence of T2D increased at a rate of 4.8% per year [22]. However, the striking increases we have identified are far beyond the previously expected trajectories. A model using SEARCH data as well as the US Census projections estimated an increase of T1D by 23% and of T2D by 49% by the year 2050 [23-25]. Our data showed a 48% increase in T1DM and a 231% increase in T2D incident cases within just a year, a marked increase from the expected trends.
We also identified a significant increase in mild DKA at presentation for incident T1D and severe DKA in incident T2D cases consistent with the emerging literature [4, 7-9]. Data shared from the multisite registry Type 1 Diabetes Exchange reported a higher proportion of patients with T1D presenting in DKA when compared with previous years [5]. Likewise, in Poland, DKA presentation at the time of T1D diagnosis increased from 40.38% to 52.94% from 2019 to 2020, with a statistically significant number of DKA cases presenting in severe DKA (P = 0.0262) [26]. What is even more remarkable is the significant increase in severe DKA as the initial presentation of T2D in our pediatric population. In accord with our study, findings at Children’s Hospital LA reveal that their DKA prevalence rates increased from < 10% before the pandemic to 20% in 2020 (P = 0.029), and 2 patients diagnosed with T2D presented in severe DKA in 2020 compared with none prior [4]. Likewise, another study from Children’s National Hospital revealed an increase in DKA rates from 4% prepandemic to 23.4% during the first 12 months of the COVID-19 pandemic. In our study, the rate of DKA in T2D increased by 91.7%, with 2020 showing the only case of severe DKA and an increase of 116% in mild DKA. Importantly, these increases in DKA also mean that more patients are presenting as inpatient and require more extensive treatment. This confirms our hypothesis that patients are presenting with more severe illness during the pandemic.
There is reason to suspect that the COVID-19 virus downregulates angiotensin-converting enzyme 2 upon binding, leading to eventual destruction of pancreatic β cells [10-13]. Additionally, COVID-19 causes a hyperinflammatory state and may induce ketosis and ketoacidosis [13, 14]. Given that none of the patients tested positive for COVID, the hypothesis that SARS-CoV2 infection may destroy β cells may not apply to the outcome of this study.
Other potential explanations include patient delay in seeking care because of the pandemic lockdown or decreased clinic availability, and the immense behavioral and environmental changes since the onset of the pandemic. Around the world, children were enrolled in school virtually, extracurricular activities were limited, and daily routines were adjusted to decrease the potential exposure to COVID-19. Potential consequences include increased screen time, unhealthy eating habits, decreased physical activity, and worsened sleep hygiene, all of which have associations with increased BMI [15, 16]. A recent meta-analysis showed an increase in BMI in school-age children and an increase in obesity prevalence from 10.5% to 12.6% in high school and college students during the pandemic [27]. Another study completed by the Children’s Hospital of Philadelphia demonstrated an increased obesity prevalence from 13.7% to 15.4% in 2020 [28]. There were no significant changes in BMI in children and adolescents with T2D. Given that these patients had preexisting severe obesity, this might not be surprising. Although it did not reach statistical significance, there was a trend for an increase in BMI in patients diagnosed with T1D (P = 0.064). Consistent with our results, a study in Saudi Arabia demonstrated that although the BMI was still within the normal range, the average BMI was significantly higher in 2020 compared with the year prior [29]. Whether the increase in BMI may play a role in the rise in incident cases of T1D requires careful consideration. Independent of the COVID19 pandemic, previous studies have reported that an elevated BMI is associated with a higher risk of developing T1D [30]. For every 1-kg/m2 increase in BMI, there was a 6.3% increased relative risk in developing T1D [30]. It is proposed that dietary changes with an increase in adiposity can provide 1 link between rising BMI and incidence of T1D, causing pancreatic β-cell fragility and decline in β-cell mass [31]. Also, it is important to consider other possible etiologies of increasing T1D incidence such as the hygiene hypothesis, which connects our improved health hygiene to weaker innate defense mechanisms and increased susceptibility to autoimmune diseases including T1D [31].
Although there were no significant differences in the race or ethnicity of the patients presenting during the pandemic, it is important to highlight that the patients diagnosed with T2D who presented in DKA were all of minority race and ethnicity, specifically Black or African American (92%) or Hispanic or Latino (8%). One study in the United States demonstrated that non-Hispanic Black and Hispanic youth had worse metabolic control at the time of diagnosis of T2D, and non-Hispanic Blacks had 3 times the rate of DKA at presentation when compared with other racial or ethnic minorities [32].
Given the effects of COVID-19 on inflammatory and autoimmune processes, we collected data on white blood cell and platelet count [33, 34], TSH, free thyroxine, and celiac panel including immunoglobulin A and TTG antibodies at presentation. We did not observe any differences in white blood cell or platelet counts over the 3 years. Because of the autoimmune nature of T1D, children and adolescents are at elevated risk of developing additional autoimmune disorders, such as celiac or thyroid disease. Studies report that positive thyroid antibodies are present in 12% to 23% of T1D patients and celiac disease prevalence ranges from 1% to 16% [35]. Consistent with the literature, over the 3 years, 14.8% (n = 16) of our patients with T1D had positive celiac screening, with 12.9% (n = 4) in 2018, 12.9% (n = 4) in 2019, and 16.7% (n = 8) in 2020. Though not statistically significant, our data show a 24.9% increase in positive celiac screening during the pandemic (P = 0.282). None of the patients with T2D had positive celiac screening. There were no significant changes in thyroid dysfunction both in T1D and T2D. It will be interesting to monitor the rates of autoimmune disorders in patients with T1D and T2D in the upcoming years.
For disease severity at presentation, we investigated secondary organ involvement, including liver and kidney functions. Liver function was assessed through measurement of AST and ALT and kidney function through measurement of creatinine and BUN. The hemoglobin was also recorded to assess for signs of hemoconcentration, which can be seen in the setting of DKA resulting from hypovolemia. There were no statistically significant differences in liver or kidney function over the 3 years. Remarkably, the average ALT and AST levels for T1D were 20 U/L and 18.5 U/L, whereas the average AST and ALT levels for T2D were 48.6 U/L and 34.7 U/L, respectively. This supports the concept that patients with T2D are at higher risk of steatosis and developing nonalcoholic fatty liver disease [36]. Hemoglobin values remained consistent over the 3-year period.
Limitations of this study warrant consideration. No patients tested positive for COVID-19; thus, we cannot state that the trends observed were due to direct effects of COVID-19 infection. However, we did not measure SARS-CoV-2 antibodies and therefore do not know if any of the patients presenting after March 2020 had a prior COVID-19 infection that could have contributed to pancreatic damage [37]. There was no COVID-19 testing completed in the outpatient setting. Nevertheless, the findings suggest that social and economic factors exacerbated by COVID-19 contributed to the rising incidence of T1D and T2D in children. Because these data came from a single tertiary medical center, it may not be generalizable to a larger population. Lack of high-risk racial and ethnic minority groups such as Native Americans, Pacific Islanders, and Asian Americans in our T2D cohort should be highlighted. This points to the necessity of gathering data from multiple different sites globally. Last, we cannot derive the incidence data because of a lack of an appropriate denominator.
In conclusion, pediatric diabetes cases increased during the pandemic year and there was a significant increase in incident pediatric T2D cases compared with incident pediatric T1D cases from 2018 to 2020. Furthermore, there were more pediatric patients with mild DKA in T1D and severe DKA in T2D cases at presentation during the first year of the COVID-19 pandemic compared with prior years. Strikingly, for the first time, incident T2D cases accounted for a majority (53%) of all newly diagnosed pediatric diabetes cases in 2020. Given that T2D has a more aggressive course in youth than in adults, with early and rapid deterioration of β-cell function and rapid progression to insulin dependence (38-42), this raises great concern for long-term complications and comorbidities leading to profound individual and community health consequences. Thus, the social and economic factors associated with but not truly caused by COVID-19 should be targeted for achieving health equity and preventing lifelong health consequences.
Glossary
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- BUN
blood urea nitrogen
- COVID-19
coronavirus disease 2019
- DKA
diabetic ketoacidosis
- HbA1c
hemoglobin A1c
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- TTG
tissue transglutaminase
Financial Support
P.G.B. receives support from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under award number DK117067, Duke University Pediatric Departmental Support, Duke Strong Start Award Program, and Gall Family Support. R.M. receives support from Duke Pediatric Resident Scholars Award and Gall Family Support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
The authors have no conflicts of interest to declare.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in the References.
References
- 1. Home. Children and COVID-19: State-level data report. Accessed November 6, 2021. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/
- 2. Farag AA, Hassanin HM, Soliman HH, et al. Newly diagnosed diabetes in patients with COVID-19: different types and short-term outcomes. Trop Med Infect Dis. 2021;6(3):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fisher L, Polonsky W, Asuni A, Jolly Y, Hessler D. The early impact of the COVID-19 pandemic on adults with type 1 or type 2 diabetes: a national cohort study. J Diabetes Complicat. 2020;34(12):107748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chao LC, Vidmar AP, Georgia S. Spike in diabetic ketoacidosis rates in pediatric type 2 diabetes during the COVID-19 pandemic. Diabetes Care. 2021;44(6):1451-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43(8):e83-e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kamrath C, Rosenbauer J, Eckert AJ, et al. Incidence of COVID-19 and risk of diabetic ketoacidosis in new-onset type 1 diabetes. Pediatrics. 2021;148(3). doi: 10.1542/peds.2021-050856. [DOI] [PubMed] [Google Scholar]
- 7. Tittel SR, Rosenbauer J, Kamrath C, et al. Did the COVID-19 lockdown affect the incidence of pediatric type 1 diabetes in Germany? Yearbook of Paediatric Endocrinology. 2021. doi: 10.1530/ey.18.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rabbone I, Schiaffini R, Cherubini V, Maffeis C, Scaramuzza A. Has covid-19 delayed the diagnosis and worsened the presentation of type 1 diabetes in children? 2020. doi: 10.2337/figshare.12675089.v1. [DOI] [PubMed]
- 9. Marks BE, Khilnani A, Meyers A, Flokas ME, Gai J, Monaghan M, Streisand R, Estrada E. Increase in the diagnosis and severity of presentation of pediatric type 1 and type 2 diabetes during the COVID-19 pandemic. Horm Res Paediatr. 2021. doi: 10.1159/000519797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olivares-Reyes JA, Arellano-Plancarte A, Castillo-Hernandez JR. Angiotensin II and the development of insulin resistance: implications for diabetes. Mol Cell Endocrinol. 2009;302(2):128-139. [DOI] [PubMed] [Google Scholar]
- 11. Müller JA, Groß R, Conzelmann C, et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3(2):149-165. [DOI] [PubMed] [Google Scholar]
- 12. Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Modarelli R, Hendrix G, DeRusso M, Ozment C, Balikcioglu PG. The perfect storm: rapid progression of diabetic ketoacidosis in pediatric diabetes in the setting of covid-19. J Endocr Soc. 2021;5(Supplement_1):A402-A403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22(10):1935-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parekh N, Deierlein AL. Health behaviours during the coronavirus disease 2019 pandemic: implications for obesity. Public Health Nutr. 2020;23(17):3121-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guzmán V, Lissner L, Arvidsson L, et al. Associations of sleep duration and screen time with incidence of overweight in European children: the IDEFICS/i.family cohort. Obes Facts. 2021:1-7. doi: 10.1159/000519418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. American Diabetes A. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S15-S33. [DOI] [PubMed] [Google Scholar]
- 18. Wolfsdorf JI, Glaser N, Agus M, et al. ISPAD clinical practice consensus guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19(Suppl 27):155-177. [DOI] [PubMed] [Google Scholar]
- 19. Rasouli M. Basic concepts and practical equations on osmolality: biochemical approach. Clin Biochem. 2016;49(12):936-941. [DOI] [PubMed] [Google Scholar]
- 20. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2021. https://www.R-project.org/ [Google Scholar]
- 21. Andri Signorell et mult. al. DescTools: tools for descriptive statistics. R package version 0.99.43; 2021. [Google Scholar]
- 22. Divers J, Mayer-Davis EJ, Lawrence JM, et al. Trends in incidence of type 1 and type 2 diabetes among youths—selected counties and Indian Reservations, United States, 2002–2015. MMWR Morb Mortal Wkly Rep. 2020;69(6):161-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lawrence JM, Divers J, Isom S, et al. Trends in prevalence of type 1 and type 2 diabetes in children and adolescents in the US, 2001-2017. JAMA. 2021;326(8):717-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Imperatore G, Boyle JP, Thompson TJ, et al. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 2012;35(12):2515-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dabelea D. Diabetes in youth—looking backwards to inform the future: Kelly West Award Lecture 2017. Diabetes Care. 2018;41(2):233-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dżygało K, Nowaczyk J, Szwilling A, Kowalska A. Increased frequency of severe diabetic ketoacidosis at type 1 diabetes onset among children during COVID-19 pandemic lockdown: an observational cohort study. Pediat Endocrinol Diabetes Metab. 2020;26(4):167-175. [DOI] [PubMed] [Google Scholar]
- 27. Chang T-H, Chen Y-C, Chen W-Y, et al. Weight gain associated with covid-19 lockdown in children and adolescents: a systematic review and meta-analysis. Nutrients. 2021;13(10):3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jenssen BP, Kelly MK, Powell M, Bouchelle Z, Mayne SL, Fiks AG. Covid-19 and changes in child obesity. Pediatrics. 2021;147(5). doi 10.1542/peds.2021-050123. [DOI] [PubMed] [Google Scholar]
- 29. Alaqeel A, Aljuraibah F, Alsuhaibani M, et al. The impact of covid-19 pandemic lockdown on the incidence of new-onset type 1 diabetes and ketoacidosis among Saudi children. Front Endocrinol. 2021;12. doi: 10.3389/fendo.2021.669302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ferrara CT, Geyer SM, Liu Y-F, et al. Excess BMI in childhood: a modifiable risk factor for type 1 diabetes development? Diabetes Care. 2017;40(5):698-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liston A, Todd JA, Lagou V. Beta-cell fragility as a common underlying risk factor in type 1 and type 2 diabetes. Trends Mol Med. 2017;23(2):181-194. [DOI] [PubMed] [Google Scholar]
- 32. Bacha F, Cheng P, Gal RL, et al. Racial and ethnic disparities in comorbidities in youth with type 2 diabetes in the Pediatric Diabetes Consortium (PDC). 2021. doi: 10.2337/figshare.15174003. [DOI] [PubMed]
- 33. Smyth SS, McEver RP, Weyrich AS, et al. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7(11):1759-1766. [DOI] [PubMed] [Google Scholar]
- 34. Coates TD. Approach to the child with lymphocytosis or lymphocytopenia. UpToDate. Accessed November 12, 2021. https://www.uptodate.com/contents/approach-to-the-child-with-lymphocytosis-or-lymphocytopenia#!
- 35. Kakleas K, Soldatou A, Karachaliou F, Karavanaki K. Associated autoimmune diseases in children and adolescents with type 1 diabetes mellitus (T1DM). Autoimmun Rev. 2015;14(9): 781-797. [DOI] [PubMed] [Google Scholar]
- 36. Astudillo M, Tosur M, Castillo B, et al. Type 2 diabetes in prepubertal children. Pediatr Diabetes. 2021;22(7): 946-950. [DOI] [PubMed] [Google Scholar]
- 37. Geravandi S, Mahmoudi-aznaveh A, Azizi Z, Maedler K, Ardestani A. SARS-COV-2 and pancreas: a potential pathological interaction? Trends Endocrinol Metab. 2021;32(11):842-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Petitti DB, Klingensmith GJ, Bell RA, et al. SEARCH for diabetes in youth study group. glycemic control in youth with diabetes: the SEARCH for diabetes in Youth Study. J Pediatr. 2009;155:668-72.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zeitler P, Hirst K, Pyle L, et al. TODAY Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366:2247-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. TODAY Study Group. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and b-cell function in TODAY. Diabetes Care. 2013;36:1749-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. TODAY Study Group. Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36(6):1735-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Linder LB, Fradkin JE, Rodgers GP. The TODAY study: an NIH perspective on its implications for research. Diabetes Care. 2013;36(6):1775-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in the References.