Abstract
The health benefits of physical activity (PA) have been widely recognized, yet traditional measures of PA, including questionnaires and category-based assessments of volume and intensity, provide only broad estimates of daily activities. Accelerometers have advanced epidemiologic research on PA by providing objective and continuous measurement of PA in free-living conditions. Wrist-worn accelerometers have become especially popular because of low participant burden. However, the validity and reliability of wrist-worn devices for adults have yet to be summarized. Moreover, accelerometer data provide rich information on how PA is accumulated throughout the day, but only a small portion of these rich data have been used by researchers. Last, new methodological developments are emerging that aim to overcome some of the limitations of accelerometers. In this review, we provide an overview of accelerometry research, with a special focus on wrist-worn accelerometers. We describe briefly how accelerometers work; summarize the validity and reliability of wrist-worn accelerometers; discuss the benefits of accelerometers, including measuring light-intensity PA; and discuss pattern metrics of daily PA recently introduced in the literature. A summary of large-scale cohort studies and randomized trials that implemented wrist-worn accelerometry is provided. We conclude the review by discussing new developments and directions of research using accelerometers, with a focus on wrist-worn accelerometers.
Keywords: accelerometry, bias, epidemiologic studies, exercise, sedentary behavior
Abbreviations:
- AC
activity count
- LPA
light-intensity physical activity
- MET
metabolic equivalent of task
- MPA
moderate-intensity physical activity
- MVPA
moderate to vigorous physical activity
- PA
physical activity
- PAEE
physical activity energy expenditure
- SB
sedentary behavior
- VPA
vigorous physical activity
The health benefits of engaging in physical activity (PA) have been widely recognized (1). Accurately quantifying trends in PA is vital to defining public health guidelines and gauging population-level risk for a wide range of diseases, chronic conditions, and functional outcomes. PA is defined as bodily movement that results in energy expenditure above a resting state (2). PA volume is “the total amount of activity accumulated over a specific period of time” (3, p. 354). According to the energy required for a given task, PA can be categorized into different intensity levels, from sedentary behavior (SB) to vigorous PA (VPA). SB refers to any activities with energy expenditure of not more than 1.5 metabolic equivalents (METs) while sitting, reclining, or lying down (1). Though the definition of SB excludes standing quietly, SB derived by wrist- or hip-worn accelerometers does not make the distinction between sitting or lying down and standing, because most devices do not provide information on body posture. Light PA (LPA), moderate-intensity PA (MPA), and VPA require 1.6–2.9, 3.0–5.9, and at least 6.0 METs, respectively (1).
The gold standard for measuring PA energy expenditure (PAEE) is doubly labeled water, a technique that quantifies total daily energy expenditure in free-living conditions (4). PAEE is estimated by subtracting dietary-induced thermogenesis and laboratory-measured resting metabolic rate from total energy expenditure (5). However, the technique is rarely used in large-scale epidemiologic studies, because of the expensive and complex procedures. PA questionnaires are instruments that can be administered to many participants at low cost. Although PA questionnaires provide context, such as the type of PA being performed and/or the location in which PA is performed (6), there are measurement and methodological challenges, including recall biases, variability in individual perception of activity intensity, and difficulty quantifying time spent in LPA (7–11).
The desire to overcome some of the limitations associated with self-report has led to the increasing use of wearable accelerometers over the past 2 decades. Accelerometers measure PA by quantifying movement, which is highly proportional to PAEE (12–15). Accelerometers are small and noninvasive, with sampling frequencies that can reach 100 observations/second (Hz) in habitual PA research, providing an objective assessment of movement-based PA across the entire intensity spectrum (from zero to maximal exertion). Large memory space and long battery life also facilitate continuous, sub-second measurement for weeks. When used in research, accelerometers are fitted to a specific body location, most commonly the hip, chest, thigh, lower back, and wrist. Wrist-worn devices have gained increasing popularity in recent years (Figure 1) in efforts to reduce participant burden and improve compliance (13, 16), and have been used to estimate PA in the National Health and Nutrition Examination Survey (NHANES), UK Biobank, and other large epidemiologic studies (16).
A few reviews have been published on accelerometers and their use in epidemiologic studies (5, 16–21); these give comprehensive overviews of many practical considerations when using accelerometers. However, new trends in accelerometry research warrant another review. First, wrist-worn accelerometers are becoming more prevalent in research (Figure 1), but previous reviews mainly focused on hip-worn accelerometers (5, 19, 21). Second, accelerometer use has generally centered on quantifying total amount of daily PA, classifying activity intensity, and estimating PAEE. Although these estimates are important for understanding the health benefits of PA and compliance with PA guidelines (1), they reduce the high-frequency time-series nature of accelerometer data to basic summary variables (e.g., active minutes/day). Thus, the enormous potential to understand the link between movement and health at a much deeper level using these rich data has yet to be fully realized (22). The recent development of novel accelerometry metrics that capture how and when PA is accumulated throughout the day have the potential to add new dimensions to PA epidemiologic research, beyond volume and intensity. Last, several methods have been developed in recent years to address several limitations of accelerometers, primarily related to recognizing certain body posture(s) and/or types of PA (e.g., sitting vs. lying or walking vs. climbing stairs) (9, 16, 20).
Figure 1.
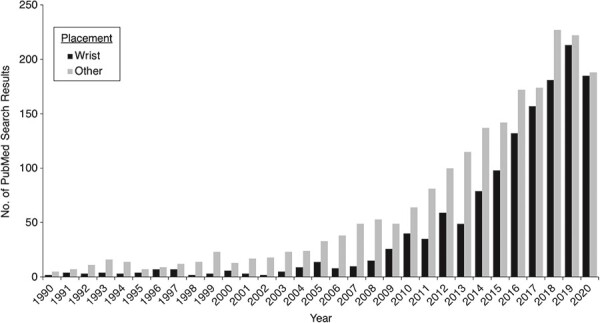
Number of PubMed search results for wrist accelerometers and accelerometers worn at other body locations.
In this review, first we describe how accelerometers work and what they measure. Second, we summarize studies examining validity and reliability of wrist-worn accelerometers. Third, we review innovative metrics of PA that go beyond volume, intensity, and energy expenditure, using the data on a wider scale. Next, we summarize epidemiologic studies in which wrist-worn accelerometers were implemented in their study protocols. Lastly, we discuss limitations of accelerometers and efforts of the scientific community to overcome them and then conclude with future directions. The scope of this review is limited to research conducted with adults.
ACCELEROMETER MEASUREMENT
As indicated by the name, accelerometers measure time-stamped accelerations of the movement of the body part to which they are attached in g's in 1 to 3 orthogonal planes (23). Because accelerations due to gravity and movement are recorded by the devices, the effects of gravity should to be removed when processing accelerometer data for PA measurement (24). Euclidian norm minus one and sum of vector magnitudes with gravity subtracted are 2 common acceleration metrics. Both Euclidian norm minus one and sum of vector magnitudes with gravity subtracted remove gravity from the vector magnitude, an aggregate of the accelerations from the 3 axes. Euclidian norm-minus-one rounds negative values of gravity-subtracted vector magnitude to zero and averages the values within a user-specified time window, or an epoch, whereas sum of vector magnitudes with gravity subtracted takes the absolute values and sums the values within an epoch.
The acceleration data from some accelerometer brands (e.g., ActiGraph, Pensacola, Florida), can also be processed into summary measures such as activity counts (ACs) within an epoch (e.g., ACs per minute), using the proprietary software of the device manufacturer (25). Compared with acceleration, ACs are less comparable across device brands but require less storage space on the device (26). This advantage is disappearing with the expansion of memory on the device that can store weeks of raw acceleration data. As a result, use of raw acceleration metrics is increasing.
Both accelerations and ACs are difficult to interpret clinically. Therefore, their values over a defined epoch are frequently classified into time spent in sedentary, light, moderate, or vigorous intensities, or used to estimate energy expenditure on a continuous scale. Steps per hour or day are another metrics that are widely understood by both researchers and the general public. However, using software algorithms to convert accelerometer data to steps may pose validity issues, potentially leading to over- or underestimation of steps per day (27–32). For example, differences in leg length and gait mechanics make step estimation using a single algorithm problematic (33). Also, the vibration from riding in a motorized vehicle may be incorrectly classified as steps (34).
Earlier versions of accelerometers tended to be worn on the trunk of the body and typically measured accelerations using vertical axis only to best capture the up-and-down movements consistent with ambulation. More recently, accelerometers have been upgraded to triaxial designs (vertical, medio-lateral, and anterior–posterior axes) to capture a wider range of movement. The primary benefit of the additional axes is believed to be better discrimination of LPA (35–37) and nonambulatory activities such as yoga (38, 39). Table 1 summarizes the number of axes, available acceleration data, and processing software for a few common research-grade accelerometer brands.
Table 1.
Common Research-Grade Wrist-Worn Accelerometers by Brand, Number of Axes, Data Type, Processing Software, and Company Website
| Brand | Current Models | Axis | Data Type | Processing Software | Company Website |
|---|---|---|---|---|---|
| ActiGraph | Centerpoint Insight Watch; GT9X; wGT3X-BT | Triaxial | ACs/g | Proprietary ActiLife software/open-source software | https://actigraphcorp.com/ |
| GENEActiv | Triaxial | g | Open-source software | https://www.activinsights.com/products/geneactiv/ | |
| Axivity | AX3; AX6 | Triaxial | g | Open-source software | https://axivity.com/ |
| Actiwatch | Actiwatch 2; Actiwatch Spectrum PRO; Actiwatch Spectrum Plus | Uniaxial | ACs | Proprietary Actiware software | https://www.usa.philips.com/healthcare/sites/actigraphy |
| Actical | Omnidirectional | ACs | Proprietary Actical software | Discontinued | |
| MotionWatch | MotionWatch 8; MotionWatch Rugged | Triaxial | ACs | Proprietary MotionWare software | https://www.camntech.com/motionwatch-8/ |
| Ambulatory Monitoring Actigraph | Uniaxial | ACs | Proprietary software | http://www.ambulatory-monitoring.com | |
| CSA | Uniaxial | ACs | Proprietary software | Discontinued |
Abbreviations: AC, activity count; CSA, Computer Science and Applications.
Wrist accelerometers facilitate 24-hour wear which includes sleep time. However, sleep periods may be misclassified as SB if not properly identified. Participant sleep diaries traditionally have been used to record sleep periods, but some devices, such as ActiGraph and Actiwatch (Philips Respironics, Bend, Oregon), have built-in sleep-detection features in their respective software programs. Open-source methods are also available to detect sleep periods, using raw acceleration data (40). Another threat to the accurate estimation of SB is nonwear time, periods when the accelerometer is collecting data but is not attached to the participant. Days on which nonwear time exceeds a threshold (e.g., 10% of 24 hours) are called nonvalid days and are excluded from analysis (41). On days with acceptable nonwear time, missing data can be imputed.
Though wrist-worn accelerometers can improve compliance in research and clinical settings (13, 16) and are the focus of this review, other placement locations may be more advantageous in terms of measurement accuracy, depending on the PA metric of interest. For example, ankle placement may be more accurate for step estimation (27), and thigh placement may better distinguish postures and activity types (42). Yet, evidence on the effect of placement for estimating energy expenditure is mixed. Some studies found the hip or thigh provided better estimates than the wrist (43–45), whereas others found the body locations were comparable (46) or the wrist to be more advantageous with certain algorithms for energy expenditure prediction (47). Different placements also may be differentially sensitive to intensities and types of PAs. Placement at the ankle and hip may be better for measuring moderate to vigorous physical activity (MVPA) (48), whereas wrist placement may be better for detecting daily tasks with more upper-body movement, such as household tasks (49). Therefore, careful consideration of placement and the desired outcome measure is warranted.
VALIDITY OF ACCELEROMETERS
Evaluation of validity and reliability of accelerometers should be specific to accelerometer output (uniaxial or multiaxial), placement, and the population in which they will be deployed (50). Models and algorithms add another layer of complexity to how accurately accelerometer data can be used to estimate energy expenditure and classify intensity and activity types. Finally, epoch length used for accelerometer data processing (e.g., 10 seconds, 30 seconds) can also affect intensity classification (51–54).
To review the validity of wrist-worn accelerometers, we searched PubMed using the search terms listed in Table 2. A total of 406 records were retrieved on July 6, 2020, and the titles and abstracts were screened for relevance. We limited inclusion to studies in which doubly labeled water and indirect calorimetry (the standard laboratory method for measuring energy expenditure) (55) were used as criterion methods. Studies of populations with specific diseases or disabilities were excluded. A total of 25 relevant papers were identified (13, 14, 43, 45–48, 56–73). These papers validated 2 basic uses of accelerometer data: total PA and intensity classification.
Table 2.
PubMed Search Terms Used in This Review
| Concept a | MeSH b | Key Words b |
|---|---|---|
| Figure | ||
| Physical activity | “Exercise” | “physical activity” (tiab) OR “activity” (tiab) OR “exercise” (tiab) |
| Accelerometer | “Acceleration” OR “Actigraphy” OR “Accelerometry” | “acceler*” (tiab) |
| Wrist worn | “wrist” | “wrist-worn” (tiab) OR “wrist*” (tiab) |
| Other placements | “Hip” OR “Thigh” | “hip-worn” (tiab) OR “hip” (tiab) OR chest-worn” (tiab) OR “chest” (tiab) OR “thigh-worn” (tiab) OR “thigh” (tiab) OR “lower back” (tiab) |
| Validity | ||
| Physical activity | “Exercise” | “physical activity” (tiab) OR “activity” (tiab) OR “exercise” (tiab) |
| Accelerometer | “Acceleration” OR “Actigraphy” OR “Accelerometry” | “acceler*” (tiab) |
| Validity | “doubly labeled water” (tiab) OR “indirect calorimetry” (tiab) OR valid* (tiab) OR “sensitivity” (tiab) OR “specificity” (tiab) OR “accuracy” (tiab) OR “precision” (tiab) | |
| Wrist worn | “wrist” | “wrist-worn” (tw) OR “wrist*” (tw) |
| Reliability | ||
| Physical activity | “Exercise” | “physical activity” (tiab) OR “activity” (tiab) OR “exercise” (tiab) |
| Accelerometer | “Acceleration” OR “Actigraphy” OR “Accelerometry” | “acceler*” (tiab) |
| Reliability | “reliab*” (tiab) | |
| Wrist worn | “wrist” | “wrist-worn” (tw) OR “wrist*” (tw) |
Abbreviations: MeSH, Medical Subject Headings; tiab, title and abstract; tw, text word.
a Concepts were combined by Boolean operator AND, except for wrist-worn and hip-worn concepts for Figure 1, which were not combined.
b MeSH terms and key words of the same concept were combined by Boolean operator OR.
Validity of estimating total PA
Of the 25 included papers, 17 discussed the validity of total PA measured by accelerometers against energy expenditure measured by doubly labeled water or indirect calorimetry (13, 14, 43, 45–48, 56–65). Another 3 papers were identified from other sources (44, 74, 75). The findings from these 20 papers are summarized in Table 3. Eleven studies examined ActiGraph and Computer Science and Applications devices, the predecessor of ActiGraph (19); 6 studies validated GENEA/GENEActiv (Activinsights Ltd., Cambridge, United Kingdom); 3 studies tested Actiwatch; and Actical (Philips Respironics), Axivity (Axivity, Newcastle upon Tyne, United Kingdom), and Ambulatory Monitoring Actigraph (Ambulatory Monitoring Inc., Ardsley, New York) were examined by 1 study each. Eight and 11 papers used ACs and acceleration metrics for validation, respectively. Only 3 studies were conducted under free-living conditions. Five studies deployed leave-one-out cross-validations for their energy-expenditure prediction models, and 3 studies validated previously published models.
Table 3.
Validity Studies Compared With Doubly Labeled Water and Indirect Calorimetry
| First Author, Year (Reference No.) | Device (Axis, Frequency, Epoch, Placement) | Study Population | Age Range, Years | Activity | Criterion | Algorithms | Findings |
|---|---|---|---|---|---|---|---|
| Neil-Sztramko, 2017 (59) | Actiwatch 2 (uniaxial, unspecified, 15 seconds, nondominant wrist) | 30 female participants | 40 (14.9)a | Treadmill walk/run at 2.0 mph, 3.0–3.5 mph, and fast self-selected speed; self-paced indoor walk at slow, medium, and fast speeds; stair ascend and descend; and a lift-and-carry task | METs calculated from Vo2 (mL/kg/minute) measured by indirect calorimetry | None | Correlation between ACs and METs: r = 0.69 |
| Lee, 2019 (57) | Actiwatch 2 (uniaxial, unspecified, 1 minute, both wrists) | 27 young adults | 18–26 | Treadmill run at 4, 6, 8, 10, and 12 km/h | METs calculated from Vo2 measured by indirect calorimetry | METs estimated by regression models including ACs and quadratic terms of ACs | Correlation between AC and METs: right wrist, r = 0.73; left wrist, r = 0.72. No significant biases between estimated and criterion METs |
| Chen, 2003 (63) | Actiwatch 64 (uniaxial, unspecified, 1 minute, dominant wrist) | 60 women | 20–52 | Structured exercise, including walking periods with average speeds of 0.6, 0.9, and 1.2 m/second and stepping periods with average speed of 12 steps/10 seconds, 18 steps/10 seconds, and 24 steps/minute. Laboratory-based spontaneous activities, including sitting, television viewing, deskwork, walking around the room, and voluntary exercises | EE (kcal) calculated from Vo2 and Vco2 measured by indirect calorimetry | EE estimated by nonlinear model including ACs | Correlation between AC and criterion EE: r = 0.646. Significant underestimation between estimated and criterion EE |
| Neil-Sztramko, 2017 (59) | ActiGraph GT3X+ (triaxial, unspecified, 15 seconds, nondominant wrist) | 30 female participants | 40 (14.9)a | Treadmill walk/run at 2.0 mph, 3.0–3.5 mph, and fast self-selected speed; self-paced indoor walk at slow, medium, and fast speeds; stair ascend and descend; and a lift-and-carry task | METs calculated from Vo2 (mL/kg/minute) measured by indirect calorimetry | None | Correlation between AC and METs: r = 0.69 |
| Lee, 2019 (57) | ActiGraph wGT3X (triaxial, unspecified, 1 minute, both wrists) | 27 young adults | 18–26 | Treadmill run at 4, 6, 8, 10, and 12 km/hour | METs calculated from Vo2 measured by indirect calorimetry | METs estimated by regression models including AC and quadratic terms of AC | Correlation between AC and METs: right wrist, r = 0.73; left wrist, r = 0.74 No significant biases between estimated and criterion METs |
| Ho, 2019 (58) | ActiGraph GT9X (triaxial, 30 Hz, 10-s, non-dominant wrist) | 90 adults | 22.9 (4.15)a | Treadmill walk/run at 4.8, 6.4, 8.0, 9.7, and 11.3 km/hour | EE (kcal/min) calculated from Vo2 (L/min) and Vco2 (L/min) measured by indirect calorimetry | 1) EE estimated by Freedson VM3 Combination (2011) available with ActiLife software 2) EE estimated by a linear model including AC, body weight, and heart rate | 1) The Freedson VM3 Combination model explained 38.4% of the variance in criterion EE and underestimated energy expenditure at each speed. 2) The linear model explained 80.2% of variance in criterion EE. Bias was not specified. |
| Ellingson, 2017 (61) | ActiGraph GT3X+ (triaxial, 100 Hz, 1 second and 15 seconds, right wrist) | 51 adults | 18–40 | Laboratory-based activities including supine resting, sitting reading a book, sitting typing, sitting fidgeting, standing reading a book, standing typing, standing fidgeting, climbing stairs, throwing/catching a ball, stationary biking, walking on a treadmill at 2 mph and 3 mph, walking at 3 mph typing, and running on a treadmill at 4.5 and 5.5 mph | METs calculated from Vo2 (mL/kg/minute) measured by indirect calorimetry | METs estimated from the Hildebrand linear method (43), Hildebrand nonlinear method, Staudenmayer linear method (47), and Staudenmayer random forest method (47) using acceleration | For the average across activities of all intensities, only the Hildebrand linear method was equivalent with the criterion METs. None of the methods was equivalent to the criterion METs within each intensity level. |
| Staudenmayer, 2015 (47) | ActiGraph GT3X+ (triaxial, 80 Hz, 15 seconds, dominant wrist) | 20 adults | 20–39 | Treadmill walk/run at 3.0 mph (5% grade), 4.0 mph (5% grade), and 5.5 mph laboratory-based free-living activities including stairs, tennis, shooting, fast walk, stacking boxes, raking, walking while carrying groceries, self-paced walk, golfing (swinging), slow walk, vacuuming, gardening, laundry, dusting, driving, office work | METs calculated from Vo2 (mL/kg/minute) measured by indirect calorimetry | METs estimated from linear model and machine-learning models (ANN, SVM, and RF) using acceleration | Cross-validation showed that linear, ANN, and RF models were unbiased and had good agreement with overall criterion METs. All models tended to overestimate METs when the actual METs were low and underestimated them when actual METs were high. |
| Stec, 2012 (44) | ActiGraph GT3X+ (triaxial, unspecified, 1-s, right wrist) | 30 adults | 21.7 (1.0)a | Resistance exercises, including machine bench press, machine shoulder press, machine squat, leg extension, leg curl, latissimus dorsi pull-down, triceps push down, and barbell biceps curl | EE (kcal) calculated from Vo2 measured by indirect calorimetry | None | Criterion EE only correlated with AC on the horizontal axis (r = −0.40), but not with AC on the other 2 axes or the sum of AC |
| Ellis, 2014 (46) | ActiGraph GT3X+ (triaxial, 30 Hz, 1 minute, nondominant wrist) | 40 adults | 35.8 (12.1)a | Laboratory-based household activities including laundry, window washing, dusting, dishes, and sweeping. Locomotion activities including stairs, slow walk, brisk walk, and jog | METs calculated from Vo2 (mL/kg/minute) measured by indirect calorimetry | METs estimated from RF machine-learning model | No significant biases between estimated and criterion METs |
| Strath, 2015 (62) | ActiGraph GT3X+ (triaxial, unspecified, 1 second, nondominant wrist) | 99 adults | ≥18 | Treadmill walk with speed from 40.2 to 107.2 m/minute in increments of 13.4 m/minute. Laboratory-based daily activities, including computer work, vacuuming, mopping/sweeping, carrying box of 3 weights, and walking with intermittent stair climbing | METs calculated from Vo2 (mL/kg/minute) measured by indirect calorimetry | METs estimated from TR machine-learning model using n-gram–based feature (unigrams) | No bias between estimated and criterion METs by leave-one-out validation. Nonsignificant increase in biases and overall error when applying model for 18–39 years age group to 40–64 years age group or ≥65 years age group |
| Hildebrand, 2014 (43) | ActiGraph GT3X+ (triaxial, 60 Hz, 1 second, nondominant wrist) | 30 adults | 18–65 | Laboratory-based activities, including lying supine, sitting, standing, taking off shoes, standing, moving 8 items on a bookshelf, writing a sentence, putting a paper in an envelope, sitting down, treadmill walk/run at 3, 5, and 8 km/hour, and stepping | Vo 2 (mL/kg/minute) measured by indirect calorimetry | Vo 2 estimated from linear regression model including the ENMO only | ENMO explained 75% of the variance in Vo2 |
| Melanson, 1995 (75) | CSA (uniaxial, unspecified, 5 seconds, nondominant wrist) | 28 adults | 21.0 (1.0)a for male participants; 21.0 (1.1)a for female participants | Treadmill walk/run at speeds of 4.8, 6.4, and 8.1 km/hour and 0%, 3%, and 6% grade | Vo 2 (mL/kg/minute) from indirect calorimetry, EE (kcal/min) calculated from Vo2 (L/min) | EE estimated using linear regression model including AC and body weight | Significant correlation between AC and Vo2 and EE: r = 0.89 and 0.81, respectively Linear regression model with R2 = 0.86; small mean difference between estimated and criterion EE by cross-validation |
| Swartz, 2000 (60) | CSA (uniaxial, unspecified, 60 seconds, dominant wrist) | 70 adults | 19–74 | Laboratory-based yard work, occupational, housework, family care, conditioning and recreational activities | METs calculated from Vo2 (mL/kg/minute) measured by indirect calorimetry | None | Significant correlation between AC and criterion METs: r = 0.181 |
| Hildebrand, 2014 (43) | GENEActiv (triaxial, 60 Hz, 1 second, nondominant wrist) | 30 adults | 18–65 | Laboratory-based activities, including lying supine, sitting, standing; taking off shoes; standing; moving 8 items on a bookshelf; writing a sentence; putting a paper in an envelope; sitting down; treadmill walk/run at 3, 5, and 8 km/hour; and stepping | Vo 2 (mL/kg/minute) measured by indirect calorimetry | Vo 2 estimated from linear regression model including the ENMO only | ENMO explained 76% of the variance in Vo2 |
| Duncan, 2020 (48) | GENEActiv (triaxial, 80 Hz, 1 second, both wrists) | 23 adults | 55–77 | Laboratory-based activities, including lying supine, seated reading, slow walking, medium walking, fast walking, folding laundry, sweeping the floor, and cycling | METs calculated from Vo2 (mL/kg/minute) measured by indirect calorimetry | None | Significant correlations between ENMO and criterion METs for nondominant wrist: r = 0.188 or 0.259 with cycling removed Significant correlations between ENMO and criterion METs for dominant wrist: r = 0.174 or 0.270 with cycling removed |
| Montoye, 2015 (45) | GENEActiv (triaxial, 20 Hz, 30 seconds, both wrists) | 39 adults | 22.1 (4.3)a | Laboratory-based free-living activities, including lying down, reading, playing a computer game, standing, laundry, sweeping, walking slowly, walking fast, jogging, cycling, stair use, biceps curls, squats | METs calculated from Vo2 (mL/kg/minute) measured by indirect calorimetry | METs estimated by ANN machine-learning model using 4 different feature sets | Significant correlation between estimated and criterion METs by leave-one-out cross-validation: r = 0.84 to 0.87 No statistically significant bias between estimated and criterion METs |
| Sirichana, 2017 (65) | GENEActiv (triaxial, 40 Hz, 60 seconds, both wrists) | 20 adults | 21 (1)a | Laboratory-based activities, including lying on back; sitting and reading a book; siting and doing computer work; washing dishes; sweeping; stacking and organizing chairs; treadmill walk at 1.5, 3, 4, and 6 mph and 0%, 3%, and 8% grade | METs calculated from Vo2 (mL/kg/minute) measured by indirect calorimetry | Piecewise linear regression model with SVMgs as dependent variable and METs as independent variable (linear spline at 6 METs) | Significant correlations between SVMgs and METs by regression model: nondominant wrist, R2 = 0.85; dominant wrist, R2 = 0.86 |
| van Hees, 2011 (13) | GENEA (triaxial, 40 Hz, 1 second, each wrist for half of the participants) | 30 pregnant and 65 nonpregnant women | 20–35 | Free-living conditions for 10 days | PAEE (MJ/day) derived from doubly labeled water | PAEE estimated from regression models using raw acceleration | Leave-one-out cross-validation showed acceleration explained 19% of the variance in criterion PAEE for nonpregnant women No statistically significant biases between estimated and criterion PAEE for nonpregnant women Nonsignificant correlation between acceleration and criterion PAEE for pregnant women |
| Esliger, 2011 (64) | GENEA (triaxial, 80 Hz, 1 minute, both wrists) | 60 adults | 40–65 | Laboratory-based activities, including lateral recumbent; seated computer work; standing; window washing; washing dishes; shelf stacking; sweeping; treadmill walk/run at 4, 5, 6, 8, 10, and 12 km/hour; stair ascent/descent at 80 steps/minute; and brisk and medium free-living walk | METs calculated from Vo2 (mL/kg/minute) measured by indirect calorimetry | None | Correlation between SVMgs and METs: left wrist, r = 0.86; right wrist, r = 0.83 |
| Correa, 2016 (74) | Actical (omnidirectional, 32 Hz, 1 minute, dominant wrist) | 70 adults | 42 (13)a | Free-living conditions for 1 week | PAEE (kcal/day) derived from doubly labeled water | PAEE estimates from Actical | Significantly overestimation between estimated and criterion PAEE |
| White, 2019 (14) | Axivity AX3 (triaxial, 100 Hz, 5 seconds, both wrists) | 193 adults | 40–66 | Free-living conditions for 9–14 days | PAEE (kJ/day/kg) derived from doubly labeled water | PAEE estimated from 4 models developed by White et al., 2016 (15) | Correlation between estimated and criterion PAEE: dominant wrist, r = 0.61 to 0.65; nondominant wrist, r = 0.63 to 0.68 No statistically significant biases between estimated and criterion PAEE |
| Patterson, 1993 (56) | Ambulatory monitoring actigraph (uniaxial, 10 Hz, unspecified, nondominant wrist) | 15 adults | 22–38 | Sedentary activities, including mental arithmetic task, self-paced reading, self-paced typing, and video game using a joy stick Graded activities, including treadmill walk/run at 5% grade and at 30%, 60%, 75%, and 90% of individual Vo2max; stepping at 20 and 36 steps/minute, and knee bends at 28 and 48 bends/minute |
Vo 2 (mL/kg/minute) from indirect calorimetry | None | Significant correlation between AC and Vo2: physical activity, r = 0.73; sedentary activity, r = 0.46 |
Abbreviations: AC, activity count; ANN, artificial neural network; CSA, Computer Science and Applications; EE, energy expenditure; ENMO, Euclidian norm minus one; MET, metabolic equivalent; PAEE, physical activity energy expenditure; RF, random forest; SVM, support vector machine; SVMgs, sum of vector magnitudes with gravity subtracted; TR, decision tree; Vo2, oxygen consumption.
a Values are expressed as mean (standard deviation).
All brands except Actical (74) had some validity in measuring total PA, especially when total PA was of interest, but a wide range of correlations with the criterion methods was reported (0.17 to 0.93) due to the wide range of AC and acceleration metrics used, differing energy-expenditure prediction models used, inclusion of other variables such as body weight, and the diverse ranges of activities performed. Results of 2 studies suggested that the validity differed by intensity of PA (47, 61). Stec and Rawson (44) showed that ACs from ActiGraph were not correlated with energy expenditure during resistance exercise (r =–0.01 to –0.40), suggesting limitations for this type of exercise. No evidence suggested the superiority of one brand over another. In 2 studies, authors found similar correlations with criterion energy expenditure between Actiwatch and ActiGraph (r = 0.69 to 0.74) (57, 59), and similar correlations were found in another study between ActiGraph and GENEActiv (R2 = 75% and 76%, respectively) (43).
It was unclear whether AC or acceleration was better for estimating total PA. Hildebrand et al. (43) reported a higher correlation with energy expenditure by indirect calorimetry, using Euclidian norm minus one in ActiGraph (r = 0.87), than did the correlations reported by Lee and Tse (r = 0.72 to 0.74) (57) and Neil-Sztramko e al. (r = 0.69) (59) using AC in ActiGraph.
The method used to compare AC and acceleration with criterion energy expenditure varied. In some studies, researchers used correlation or linear regression models with AC or accelerations alone, whereas others added body weight, heart rate, or nonlinear terms for acceleration in the regression models. Models including heart rate and linear splines for acceleration (58, 65, 75) reported higher R2 values (range, 0.80 to 0.86) than AC- or acceleration-only models (range, 0.03 to 0.76) (43, 48, 57, 59, 63). However, body weight and quadratic terms for accelerations explained little additional variance in criterion energy expenditure (13, 14, 75). Machine-learning models were used in 5 studies (45–47, 61, 62) to predict energy expenditure, using accelerometry features such as means, standard deviations, percentiles, and angles of acceleration. Good validity for machine-learning models was reported by 4 studies (45–47, 62), but bias was found for a published random forest method in 1 study (61). Two of these 5 studies also included linear models in their analyses and did not find that machine-learning models improved prediction (47, 61).
No included studies compared the performance of accelerometers in free-living environment and laboratory conditions. However, the correlations with criterion energy expenditure reported in studies in free-living conditions (r = 0.44 to 0.65) (13, 14) were comparable to the correlations reported by authors of several laboratory studies (r = 0.46 to 0.73) (56, 58, 59, 63).
Studies using leave-one-out cross-validation all demonstrated unbiased estimation of energy expenditure by accelerometers (13, 47, 62, 73, 75). However, validations of published energy-expenditure prediction models in different populations were less promising. Ellingson et al. (61) found good validity for the linear model developed by Hildebrand et al. (43) and but biased estimation for the models developed by Staudenmayer et al. (47). Ho et al. (58) applied the Freedson VM3 Combination (2011) model (76) available in the ActiGraph software and found underestimation of energy expenditure during treadmill walking. Four models previously developed using GENEActiv worn on the nondominant wrist (15) produced unbiased estimation of PAEE, using Axivity on the nondominant and dominant wrists in a different sample (14).
Validity of PA intensity classification
Eight of the 25 included papers established intensity cutpoints using indirect calorimetry as the criterion (43, 48, 57, 59, 64, 66–68). These papers are listed in Table 4. All the reported cutpoints were for absolute PA intensity, given that the criterion was energy expenditure (1). There was substantial variability in the intensity cutpoints, even within the same accelerometer brand and model. This may be due to a combination of factors, including methods used to identify cutpoints (i.e., receiver operating characteristic analysis vs. linear regression) (77), activities performed (78), and epoch length (51–54). None of the papers considered relative intensity, which is defined in reference to individual’s aerobic capacity (e.g., percent heart-rate reserve, percent maximal oxygen consumption, lactate thresholds, and/or Borg rate of perceived exertion) (1).
Table 4.
Studies Included in This Review and that Defined the Intensity Cutpoints
| First Author, Year (Reference No.) | Device (Axis, Frequency, Epoch, Placement) | Study Population | Age Range, Years | Method | Cutpoints | Sensitivity and Specificity | Discrimination AUC |
|---|---|---|---|---|---|---|---|
| Lee, 2019 (57) | Actiwatch 2 (uniaxial, unspecified, 1 minute, both wrists) | 27 young adults | 18–26 | Cutpoints were identified using linear regression models. | Right wrist SB–LPA: 61 cpm; LPA–MPA: 450 cpm; MPA–VPA: 1,322 cpm Left wrist SB–LPA: 58 cpm; LPA–MPA: 399 cpm; MPA–VPA: 1,404 cpm |
Right wrist SB: NA; LPA: 93.7% SE, 55.2% SP; MPA: 77.3% SE, 81.9% SP; VPA: 61.7% SE, 91.6% SP Left wrist SB: NA; LPA: 96.2% SE, 49.1% SP; MPA: 79.9% SE, 76.5% SP; VPA: 42.4% SE, 90.7% SP |
Right wrist: SB: NA; LPA: 86.5%; MPA: 86.4%; VPA: 84.5% Left wrist: SB: NA; LPA: 88.7%; MPA: 83.6%; VPA: 78.5% |
| Neil-Sztramko, 2017 (59) | Actiwatch 2 (uniaxial, unspecified, 15 seconds, nondominant wrist) | 30 female participants | 40 (14.9)a | Cutpoints were identified by maximizing SE and SP. | SB–LPA: 145 cpm; LPA–MPA: 274 cpm MPA–VPA: 597 cpm | SB: 100% SE, 92.2% SP; LPA: NA; MPA: 97.3% SE, 81.6% SP; VPA: 73.0% SE, 66.9% SP | SB: 0.96; LPA: NA; MPA: 0.92; VPA: 0.77 |
| Lee, 2019 (57) | ActiGraph wGT3X (triaxial, unspecified, 1 minute, both wrists) | 27 young adults | 18–26; | Cutpoints were identified using linear regression models. | Right wrist SB–LPA: 418 cpm, LPA–MPA: 4,793 cpm, MPA–VPA: 15,696 cpm Left wrist SB–LPA: 232 cpm, LPA–MPA: 4,515 cpm, MPA–VPA: 15,044 cpm |
Right wrist SB: NA; LPA: 92.4% SE, 48.9% SP; MPA: 76.4% SE, 88.0% SP; VPA: 61.1% SE, 90.9% SP Left wrist SB: NA; LPA: 95.2% SE, 39.3% SP; MPA: 74.5% SE, 88.1% SP; VPA: 60.9% SE, 89.7% SP |
Right wrist: SB: NA, LPA: 85.4%, MPA: 87.1%, VPA: 85.7% Left wrist: SB: NA, LPA: 84.7%, MPA: 86.5%, VPA: 85.3% |
| Neil-Sztramko, 2017 (59) | ActiGraph GT3X+ (triaxial, unspecified, 15 seconds, nondominant wrist) | 30 female participants | 40 (14.9)a | Cutpoints were identified by maximizing se and sp. | SB–LPA: 1,514 cpm LPA–MPA: 2,199 cpm MPA–VPA: 4,712 cpm | SB: 100% SE, 92.2% SP LPA: NA MPA: 94.5% SE, 83.8% SP VPA: 89.2% SE, 56.3% SP | SB: 0.96; LPA: NA; MPA: 0.95; VPA: 0.79 |
| Rhudy, 2020 (66) | ActiGraph GT9X Link (triaxial, unspecified, 1 minute, left wrists) | 44 adults | 26.1 (9.65)a | Cutpoints were identified by maximizing se and sp. | SB–LPA: NA; LPA–MPA: 4,836 cpm; MPA–VPA: 8,453 cpm | SB: NA; LPA: NA; MPA: 86.4% SE, 89.7% SP; VPA: 93.0% SE, 91.6% SP | SB: NA; LPA: NA; MPA: 0.93; VPA: 0.96 |
| Hildebrand, 2014 (43) | ActiGraph GT3X+ (triaxial, 60 Hz, 1 second, unspecified) | 30 adults | 34.2 (10.7)a | Cutpoints were identified using linear regression models. | SB–LPA: undefined; LPA–MPA: ENMO = 100.6 mg; MPA–VPA: ENMO = 428.8 mg | SB: NA; LPA: NA; MPA: 54%–59% classification accuracy; VPA: 89%–92% classification accuracy | Unspecified |
| Hildebrand, 2014 (43) | GENEActiv (triaxial, 60 Hz, 1 second, unspecified) | 30 adults | 34.2 (10.7)a | Cutpoints were identified using linear regression models | SB–LPA: undefined; LPA–MPA: ENMO = 93.2 mg; MPA–VPA: ENMO = 418.3 mg |
SB: NA; LPA: NA; MPA: 54%–59% classification accuracy; VPA: 89%–92% classification accuracy | Unspecified |
| Duncan, 2020 (48) | GENEActiv (triaxial, 80 Hz, 1 second, both wrists) | 23 adults | 55–77 | Cutpoints were identified by maximizing se and sp | Nondominant wrist: SB–LPA: ENMO = 17.5 mg and 19.2 mg (cycling activity removed); LPA–MPA: ENMO = 121.9 mg and 89.8 mg (cycling activity removed); MPA–VPA: NA Dominant wrist SB–LPA: ENMO = 10.1 mg; ENMO = 20.2 mg (cycling activity removed); LPA–MPA: ENMO = 18.2 g and 113.9 g (cycling activity removed); MPA–VPA: NA |
Nondominant wrist—SB: 78.1% SE, 78.9% SP; 73.8% SE, 80.7% SP (cycling activity removed); LPA: NA; MVPA: 86.6% SE, 64.6% SP 67.4% SE, 80.6% SP (cycling activity removed). Dominant wrist—SB: 88.2% SE, 83.8% SP 77.2% SE, 88.1% SP (cycling activity removed) LPA: NA MVPA: 79.0% SE, 60.3% SP 64.8% SE, 79.8% SP (cycling activity removed) |
Nondominant wrist SB: 0.821 and 0.814 (cycling activity removed); MVPA: 0.659 and 0.912 (cycling activity removed) Dominant wrist: SB: 0.910 and 0.977 (cycling activity removed); MVPA: 0.692 and 0.715 (cycling activity removed) |
| Esliger, 2011 (64) | GENEA (triaxial, 80 Hz, 1 minute, both wrists) | 60 adults | 40–65 | Cutpoints were identified by maximizing SE and SP | Right wrist SB–LPA: SVMgs = 386 g × min; LPA–MPA: SVMgs = 440 g × min; MPA–VPA: SVMgs = 2,098 g × min Left wrist SB–LPA: SVMgs = 217 g × min; LPA–MPA: SVMgs = 645 g × min; MPA–VPA: SVMgs = 1,810 g × min |
Right wrist SB: 99% SE, 96% SP; LPA: NA; MPA: 100% SE, 56% SP; VPA: 78% SE, 97% SP Left wrist SB: 97% SE, 95% SP; LPA: NA; MPA: 95% SE, 72% SP; VPA: 78% SE, 98% SP |
Right wrist: SB: 0.98; LPA: NA; MPA: 0.84; VPA: 0.89 Left wrist: SB: 0.98; LPA: NA; MPA: 0.91; VPA: 0.91 |
| Landry, 2015 (67) | MotionWatch 8 (triaxial, unspecified, 1 minute, nondominant wrist) | 23 adults | 57–80 | Cutpoints were identified by minimizing the distance to (0, 1) on ROC curve given sp > 0.90 for MVPA | SB–LPA: 178.5 cpm; LPA–MPA: 562.5 cpm; MPA–VPA: NA | SB: 78% SE, 70% SP; LPA: NA; MVPA: 34% SE, 90% SP | SB: 0.81; MVPA: 0.79 |
| Diaz, 2018 (68) | Actical (omnidirectional, 32 Hz, 1 minute, nondominant wrist) | 24 adults | 20–54 | Cutpoints were identified for 99%, 95%, 90%, 85%, 80%, and 75% of se and by maximizing SE and SP | Maximizing SE and SP: SB–LPA: NA; LPA–MPA: 1,031 cpm; MPA–VPA: 3,589 cpm | Maximizing SE and SP: SB: NA; LPA: NA; MPA: 85.6% SE, 87.5% SP; VPA: 88.0% SE, 98.7% SP | MPA: 0.93; VPA: 0.96 |
Abbreviations: AUC, area under the ROC curve; cpm, count per minute; ENMO, Euclidean norm minus one; LPA, light physical activity; MPA, moderate physical activity; MVPA, moderate to vigorous physical activity; NA, not applicable; ROC, receiver operating characteristic; SB, sedentary behavior; SE, sensitivity; SP, specificity; SVMgs, sum of vector magnitudes with gravity subtracted; VPA, vigorous physical activity.
a Values are expressed as mean (standard deviation).
Validation on published cutpoints was conducted by authors of 4 of the 25 studies (Table 5) (48, 69–71). Three of the published cutpoints (79–81) were not included in Table 3 because activPAL (PAL Technologies Ltd., Glasgow, United Kingdom), a thigh-worn accelerometer that detects sitting and lying-like behaviors versus standing than other body locations (82) was used as the criterion for SB. In general, both sensitivity and specificity were worse in the validation population than in the population from whom the cutpoints were developed.
Table 5.
Validation Studies on Published Cutpoints or Algorithm
| First Author, Year (Reference No.) for Validation Studies | First Author, Year (Reference No.) for Published Cutpoint or Algorithm | Age Range of Development Population, | Age Range of Validation Population, | Accuracy in Development Population | Accuracy in Validation Population |
|---|---|---|---|---|---|
| Welch, 2013 (69) | Esliger, 2011 (64) | 40–65 | 20–60 | Left wrist: SB: 97%, SE, 95% SP; LPA: unspecified; MPA: 95% SE, 72% SP; VPA: 78% SE, 98% SP | Left wrist: SB: 69.7% SE, 85.7% SP; LPA: 44.9% SE, 82.5% SP; MPA: 46.2% SE, 74.3% SP; VPA: 70.7% SE, 69.9% SP |
| Ellis, 2016 (71) | Hildebrand, 2014 (43) | 34.2 (10.7)a | 55.2 (15.3)a | Cutpoint of ENMO = 100 mg for MPA Classification accuracy: MPA: 54%–59%; VPA: 89%–92% |
The study validated classification of MVPA only: significant underestimation of mean minutes/day in MVPA |
| Duncan, 2020 (48) | Hildebrand, 2014 (43) | 34.2 (10.7)a | 63.2 (6.5)a | Cutpoint of ENMO = 100 mg for MPA Classification accuracy: MPA: 54%–59%; VPA: 89%–92% |
Nondominant wrist MVPA: 79% SE, 59% SP Dominant wrist MVPA: 76% SE, 61% SP |
| Duncan, 2020 (48) | Hildebrand, 2017 (81) | 34.2 (10.7)a | 63.2 (6.5)a | Cutpoint of ENMO = 44.8 mg for SB SB: 98% SE, 74% SP |
Nondominant wrist SB: 51% SE, 91% SP Dominant wrist SB: 51% SE, 93% SP |
| Duncan, 2020 (48) | Sanders, 2019 (79) | 60–86 | 55–77 | Cutpoint at ENMO = 6 mg for SB, 19 mg for MVPA; SB: 62% SE, 93% SP; LPA: unspecified; MVPA: 86% SE, 89% SP | Nondominant wrist SB: 71% SE, 80% SP; MVPA: 59% SE, 64% SP Dominant wrist SB: 79% SE, 89% SP; MVPA: 62% SE, 59% SP |
| Marcotte, 2020 (70) | Koster, 2016 (80) | 70–92 | 18–25 | Cutpoint at 1,853 cpm for SB SB: 81.5% SE, 76.6% SP Cutpoint at 376 counts/15 seconds for SB SB: 75.2% SE, 74.1% SP |
Cutpoint at 1,853 cpm: 72.8% SE, 84.5% SP Cutpoint at 376 counts/15 seconds: 66.0% SE, 81.2% SP Significant underestimation by both cutpoints of time spent in SB |
| Marcotte, 2020 (70) | Hildebrand, 2017 (81) | 34.2 (10.7)a | 20.4 (1.3)a | Cutpoint of ENMO = 44.8 mg for SB SB: 98% SE, 74% SP |
89.0% SE, 57.5% SP. Significant overestimation of time spent in SB |
Abbreviations: cpm, count per minute; ENMO, Euclidean norm minus one; LPA, light physical activity; MPA, moderate physical activity; MVPA, moderate to vigorous physical activity; SB, sedentary behavior; SE, sensitivity; SP, specificity; VPA, vigorous physical activity.
a Values are expressed as mean (standard deviation).
Ellingson et al. (61) validated another approach of classifying PA intensity. Accelerometer data were used to estimate METs using linear and nonlinear equations and grouped into intensity categories. Three equations were evaluated, and all were found to have excellent sensitivity and specificity for VPA (98%, 89%, and 98% sensitivity, respectively; and 99% specificity for all 3 equations) but not for SB (0%, 89%, and 39% sensitivity; 100%, 60%, and 90% specificity, respectively), LPA (67%, 21%, and 45% sensitivity; 37%, 90%, and 64% specificity, respectively), and moderate-intensity PA (40%, 37%, and 44% sensitivity; 84%, 86%, and 74% specificity, respectively) (61).
Table 6 summarizes common machine-learning approaches for intensity classification. In 5 of the 25 studies, 4 approaches were evaluated: artificial neural network, random forest, support vector machine, and decision tree (47, 61, 70, 72, 73). The collection of accelerometry features used as input for the machine-learning models varied from study to study. Moderate to good accuracy (classification accuracy 53.5–78.5%) was reported in most studies, but Montoye et al. (72) found very good accuracy for both wrists (classification accuracy, 95.9% and 84.1%, respectively). It is unclear whether these complex methods are better than absolute cutpoints. Staudenmayer et al. (47) suggested that machine-learning methods outperformed the cutpoint method when comparing wrist machine-learning models with hip data cutpoints. Marcotte et al. (70), however, found similar classification accuracies using cutpoints and machine-learning methods at the wrist.
Table 6.
Machine-Learning Approaches for Physical Activity Intensity Classification
| First Author, Year (Reference No.) | Features | Device | Findings |
|---|---|---|---|
| Artificial Neural Network | |||
| Staudenmayer, 2015 (47) |
VM statistics: mean, SD, percentage of the power in 0.6–2.5 Hz, dominant frequency, fraction of power at the dominant frequency Angle statistics: mean and SD of angle of acceleration relative to vertical on the device |
ActiGraph GT3X+ (80 Hz, 15-second epoch, dominant wrist) | Leave-one-out cross-validated classification accuracy for LPA, MPA, and VPA was approximately 72% (no values given in the article; approximation from figure) |
| Montoye, 2016 (72) | 10th, 25th, 50th, 75th, and 90th percentiles of raw acceleration | GENEActiv (20 Hz, 30-second epoch, both wrists) | Leave-one-out cross validation results as follows: Left wrist: SB: 97.5%, SE, 99.2% SP; LPA: 99.0%, se, 99.3% SP; MVPA: 90.8% SE, 95.4% SP Overall classification accuracy was 95.9% Significant overestimation of time spent in LPA (2.2 minutes) Significant underestimation of time spent in MVPA (1.9 minutes) Right wrist: SB: 93.1%, SE, 97.6% SP; LPA: 97.8% SE, 98.7% SP; MVPA: 65.7% SE, 84.1% SP SP Overall classification accuracy was 85.4% Significant overestimation of time spent in SB (1.2 minutes) and LPA (7.4 minutes) Significant underestimation of time spent in MVPA (8.6 minutes) |
| Montoye, 2018 (73) | Tested 6 feature sets. Feature that performed the best in out-of-sample validation: 3 sets of statistics for 3 axes: mean, SD, minimum, maximum, 10th, 25th, 50th, 75th, and 90th percentiles of acceleration signal |
GENEActiv (20 and 60 Hz, 30-second epoch, left wrist) | Two out-of-sample validated classification accuracies were 77.0% and 77.7%. |
| Random Forest | |||
| Staudenmayer, 2015 (47) |
VM statistics: mean, SD, percentage of the power in 0.6–2.5 Hz, dominant frequency, fraction of power at the dominant frequency Angle statistics: mean and SD of angle of acceleration relative to vertical on the device |
ActiGraph GT3X+ (80 Hz, 15-second epoch, dominant wrist) | Leave-one-out cross-validated classification accuracy for LPA, MPA, and VPA was 75% |
| Ellingson, 2017 (61) |
VM statistics: mean and SD Angle statistics: mean and SD of angle of acceleration Other: features derived from fast Fourier transform analysis of the signal |
ActiGraph GT3X+ (100 Hz, 15-second epoch, right wrist) | Validation of the model developed by Staudenmayer (47): SB: 43% SE, 93% SP; LPA: 48% SE, 60% SP; MPA: 48% SE, 81% SP; VPA: 99% SE, 99% SP Overall classification accuracy was 53.5% |
| Marcotte, 2020 (70) |
VM statistics: mean, SD, percentage of the power in 0.6–2.5 Hz, dominant frequency, fraction of power at the dominant frequency Angle statistics: mean and SD of angle of acceleration relative to the vertical axis on the device |
ActiGraph GT3X-BT (100 Hz, 15-second epoch, nondominant wrist) | The study validated classification of SB only using model developed by Staudenmayer (47): 61.3% SE and 84.2% SP; classification accuracy, 70.6%; significant underestimation of time spent in SB |
| Montoye, 2018 (73) | Tested 6 feature sets. Feature that performed the best in out-of-sample validation: 3 sets of statistics for 3 axes: mean, SD, minimum, maximum, 10th, 25th, 50th, 75th, and 90th percentiles of acceleration signal |
GENEActiv (20 and 60 Hz, 30-second epoch, left wrist) | Two out-of-sample validated classification accuracies were 77.3% and 78.5%. |
| Support Vector Machine | |||
| Staudenmayer, 2015 (47) |
VM statistics: mean, SD, percentage of the power in 0.6–2.5 Hz, dominant frequency, fraction of power at the dominant frequency Angle statistics: mean and SD of angle of acceleration relative to vertical on the device |
ActiGraph GT3X+ (80 Hz, 15-second epoch, dominant wrist) | Leave-one-out cross-validated classification accuracy for LPA, MPA, and VPA was approximately 73% (no values given in the article; approximation from figure) |
| Montoye, 2018 (73) | Tested 6 feature sets. Feature that performed the best in out-of-sample validation: 3 sets of statistics for 3 axes: mean, SD, minimum, maximum, 10th, 25th, 50th, 75th, and 90th percentiles of acceleration signal |
GENEActiv (20 and 60 Hz, 30-second epoch, left wrist) | Two out-of-sample validated classification accuracies were 76.1% and 70.9%. |
| Decision Tree | |||
| Staudenmayer, 2015 (47) |
VM statistics: mean, SD, percentage of the power in 0.6–2.5 Hz, dominant frequency, fraction of power at the dominant frequency Angle statistics: mean and SD of angle of acceleration relative to vertical on the device |
ActiGraph GT3X+ (80 Hz, 15-second epoch, dominant wrist) | Leave-one-out cross-validated classification accuracy for LPA, MPA, and VPA was 74% |
| Marcotte, 2020 (70) |
VM statistics: mean, SD, percentage of the power in 0.6–2.5 Hz, dominant frequency, fraction of power at the dominant frequency Angle statistics: mean and SD of angle of acceleration relative to the vertical axis on the device |
ActiGraph GT3X-BT (100 Hz, 15-second epoch, nondominant wrist) | The study validated classification of SB only using model developed by Staudenmayer (47): 70.0% Se and 81.7% Sp; classification accuracy, 74.7%; significant underestimation of time spent in SB |
| Montoye, 2018 (73) | Tested 6 feature sets. Features that performed the best in out-of-sample validation: 3 sets of statistics for 3 axes: mean, SD, minimum, maximum, 10th, 25th, 50th, 75th, and 90th percentiles of acceleration signal |
GENEActiv (20 and 60 Hz, 30-second epoch, left wrist) | Two out-of-sample validated classification accuracies were 76.4% and 75.7%. |
Abbreviations: LPA, light physical activity; MPA, moderate physical activity; MVPA, moderate to vigorous physical activity; SB, sedentary behavior; SD, standard deviation; SE, sensitivity; SP, specificity; VM, vector magnitude; VPA, vigorous physical activity.
In conclusion, wrist-worn accelerometers are valid instruments for measuring total PA and classifying PA intensities. However, caution should be taken when applying published intensity cutpoints. Validation in the population of interest may be needed to understand the change in sensitivity and specificity and the potential impact on analysis.
RELIABILITY OF ACCELEROMETERS
Using the search terms listed in Table 2, we performed a separate search of PubMed for articles reporting on assessment of the reliability of wrist-worn accelerometers. Seven articles were identified from 92 results retrieved on July 10, 2020 (56, 67, 83–87). Four of these articles addressed the number of valid days needed for reliable measurement of PA. Dillon et al. (85) found 6 days of monitoring were necessary to achieve reliability greater than 0.80 for VPA and 2–3 days for other intensities among middle-aged adults wearing GENEActive (100 Hz; 1-minute epoch). Ricardo et al. (87) found 5 days were needed to achieve reliable estimates of LPA and MVPA for young adults (aged 18 and 30 years) for GENEActive (85.7 Hz; 5-second epoch). Falck et al. (84) found that the MotionWatch 8 (CamNtech, Cambridge, United Kingdom) (3–11 Hz; 1-minute epoch) needed 1–2 wear-days to achieve reliability of 0.80 for SB, LPA, and MVPA among older adults. The 1 study in which ActiGraph was used was conducted with pregnant women; at least 3 days were required to measure overall PA reliably, but 7 days were needed for MVPA (83). Despite different device brands, sampling frequency, and epoch lengths, the findings from these studies are consistent: more days are needed if activities of higher intensities are of interest.
PA may vary by season or by month (88, 89). Though a reliable measurement of PA can be achieved with 3–7 valid days, the PA measured may not be representative of yearly habitual PA. However, longer measurement periods may reduce participant compliance. Seven consecutive wear-days is typical of accelerometer studies.
Authors of 3 publications examined different types of reliability for ActiGraph, MotionWatch 8, and the Ambulatory Monitoring Actigraph. Ozemek et al. (86) tested the interdevice reliability of 2 ActiGraph GT3X+ monitors (100 Hz; 1-minute epoch) worn on the dominant wrist while activities of daily living were performed. A strong intraclass correlation coefficient of 0.989 was reported for the vector magnitude counts (86). Landry et al. (67) also found good interdevice reliability (intraclass correlation coefficient = 0.979) for MotionWatch 8 devices (unspecified hertz; 1-minute epoch) worn on the nondominant wrist. Patterson et al. (56) examined the test–retest reliability of the Ambulatory Monitoring Actigraph (10 Hz; unspecified epoch) using 2 measurements within 1 week from 4 adults. They found high correlation (0.97–0.99) between the 2 measurements.
EXPANSION OF ACCELEROMETRY RESEARCH
Accelerometers generally are thought to be more accurate and reliable than self-report in measuring volume, intensity, and frequency of PA, especially for LPAs and nonexercise activities of daily living in free-living conditions (9, 16, 20). This is paramount to accurately time-stamping and quantifying daily PA in a detailed manner, because these activities encompass the majority of the activities in which people engage (90). Several observational studies and randomized control trials have shown health benefits associated with these types of activity, but these have mainly been limited to accelerometers placed on the hip. In the NHANES and studies in other cohorts, LPAs were reported to have many health benefits, including lower cardiovascular risk factors (91–94), larger hippocampal volume (95), better health and well-being (96, 97), and lower mortality risk (91, 98, 99). In another recent NHANES study, researchers found that greater step intensity was not associated with lower mortality risk after adjusting for total steps per day (100). Nonexercise activity has been linked to death, as well (101, 102). Findings from randomized control trials also suggested that lifestyle activities such as walking, taking stairs, and low-intensity supervised exercise were beneficial to weight loss, reduction of cardiovascular risk factors, and improvement in physical function (103, 104). Studies should replicate these finding using wrist accelerometers, which may better capture light-intensity upper body movements than do hip accelerometers (49).
Another advantage of accelerometers over questionnaires is the ability to measure patterns of daily PA (20). This feature allows research to go beyond traditional PA metrics, characterizing how and when PA is accumulated throughout the day. Understanding these patterns may be important in lower-activity populations who engage in little to no MVPA and may highlight novel opportunities for intervention. Pattern metrics include sedentary and active bouts, breaks in sedentary time, the active-to-sedentary transition probability, and diurnal patterns. These metrics have mostly been assessed using hip- or chest-worn accelerometers, but studies using wrist accelerometers are emerging (41).
Sedentary and active bouts are defined as the consecutive minutes during which activity intensity falls in the range of SB or an active state (105). Studies have linked more prolonged sedentary bouts per day with incident metabolic syndrome in adults (106), abdominal obesity (107), and death in older adults (108). Time spent in longer MVPA bouts (≥5 minutes and ≥10 minutes) decreased with age, beginning in early adulthood (109–111). Older adults who more frequently engaged in short active bouts (<5 minutes) had elevated mortality risk but not those who engaged in longer active bouts (≥5 minutes) (112). However, more time spent in MVPA was associated with higher odds of successful aging, whether it was spent in short (<10 minutes) or long bouts (≥10 minutes) (113).
Sedentary and active bouts have also been used in randomized clinical trials. For example, the Lifestyle Interventions and Independence for Elders study was a randomized clinical trial completed in 2014 that demonstrated an MPA intervention reduced the risk of major mobility disability compared with a health education program in older adults (114). At baseline, a larger percentage of wake time spent in active bouts of ≥2 minutes and ≥5 minutes, but not total active time, was associated with better performance on memory tests (115). Over 24 months, participants in the PA intervention spent less time in sedentary bouts of ≥10 minutes and ≥30 minutes (116), and spent more time in active bouts of ≥5 minutes and ≥10 minutes than did participants in the health education group (117).
Breaks in SB are defined as “a non-sedentary bout in between two sedentary bouts” by the Sedentary Behavior Research Network (105, p. 9). Operationally, studies often define breaks in SB as the number of interruptions of activity (≥1 minute) when in a sedentary state. More breaks in sedentary time were beneficially associated with cardiometabolic risk factors (118, 119) and lower likelihood of impairment in activities of daily living and instrumental activities of daily living (120, 121).
In contrast to sedentary breaks, the active-to-sedentary transition probability is the probability (range, 0–1) of transitioning from an active state to a sedentary state (122). It is calculated as the reciprocal of the average active-bout duration. Higher active-to-sedentary transition probability is conceptualized as a more fragmented pattern of daily PA. Higher active-to-sedentary transition probability has been associated with lower physical function (122), higher perceived fatigability and performance fatigability (122, 123), and greater risk of death (112, 124), even after adjusting for total volume of activity. Moreover, cancer survivors and patients with glaucoma with higher degrees of visual-field damage also had a more fragmented PA pattern (125, 126).
Diurnal patterns of PA measure PA within multiple time windows throughout the day. They can help assess changes in PA over time of day, understand rest-activity rhythms, and identify the ideal time of day to target for intervention. Several researchers have found more pronounced declines in PA in the afternoon than in the morning with higher age (127–132), though authors of 1 study found the decline in PA with age was similar through the day (133). Different patterns throughout working days were associated with various health measures, including body composition and cardiorespiratory fitness (134). Moreover, LPA in the morning and afternoon benefitted sleep, whereas PA of any intensity after 8:00 pm adversely affected sleep (135). A few factors, including falls (136), more severe visual damage (126), and higher level of fatigability (137), were linked to less PA during waking hours. Retirement delayed the time when activity was initiated and reduced the number of activity peaks during the day (138).
LARGE-SCALE WRIST-ACCELEROMETRY RESEARCH
Many large epidemiologic studies have implemented accelerometers, and major studies that used hip- or chest-worn accelerometers have been reviewed in a few review articles (9, 16). We complement these reviews by providing an overview of studies that adopted wrist-worn protocols (Table 7).
Table 7.
Cohort Studies and Randomized Trials That Implemented Accelerometers
| Study Name | Study Type | Device | Dates of Collection |
|---|---|---|---|
| NHANES | Population-based survey | ActiGraph GT3X+ | 2011–2014 |
| BLSA | Prospective cohort study | ActiGraph GT9X Link | 2015–ongoing |
| STURDY | Randomized control trial | ActiGraph GT9X Link | 2015–2019 |
| NSHAP | Population-based survey | Actiwatch Spectrum | 2010–2011 |
| UK Biobank | Prospective cohort study | Axivity AX3 | 2013–2015 |
| The Fenland Study | Prospective cohort study | GENEActiv | 2005–2015 |
| Whitehall II | Prospective cohort study | GENEActiv | 2012–2013 |
| FinHealth 2017 Survey | Population-based survey | ActiGraph GT9X Link | 2017 |
| FIREA | Prospective cohort study | ActiGraph wActiSleep-BT | 2014–ongoing |
| Pelotas (Brazil) birth cohorts | Birth cohorts | GENEActiv | 2010–2013 |
| ActiGraph wGT3X-BT | 2015–2017 |
Abbreviations: BLSA, Baltimore Longitudinal Study of Aging; FIREA, Finnish Retirement and Aging Study; NHANES, National Health and Nutrition Examination Survey; NSHAP, National Social Life, Health and Aging Project; STURDY, Study to Understand Fall Reduction and Vitamin D in You.
NHANES is a stratified, multistaged, probabilistic sample representative of the civilian, noninstitutionalized US population (139). NHANES participants wore the ActiGraph GT3X+ on the nondominant wrist for 7 days in the 2011–2012 and 2013–2014 cycles (6). NHANES wrist-accelerometer data were released in November 2020.
The Baltimore Longitudinal Study of Aging is a study of human aging, established in 1958. The procedures for participant enrollment and the inclusion criteria have been described elsewhere (140). A 7-day wear protocol of the ActiGraph GT9X Link on the nondominant wrist was implemented in 2015, replacing a previous protocol using a chest-worn monitor (Actiheart; CamNtech) (141). As of March 2020, data from 780 participants had been collected.
The National Social Life, Health and Aging Project is a longitudinal, population-based survey representative of older adults born between 1920 and 1947. The study aims to “understand the well-being of older, community-dwelling Americans by examining the interactions among physical health and illness, medication use, cognitive function, emotional health, sensory function, health behaviors, social connectedness, sexuality, and relationship quality.” (142) Participants were recruited in 2005–2006 (wave 1) and followed up in 2010–2011 (wave 2). A subset of wave 2 participants (n = 738) was asked to wear an Actiwatch Spectrum on their nondominant wrist for 3 days (143).
The Healthy Aging in Neighborhoods of Diversity Across the Life Span study is a cohort study of adults (White and Black) aged 30–64 years at enrollment. Participants are followed up every 4–5 years. A subset (n = 760) of wave 4 (2013–2017) participants wore the ActiGraph GT3X+ on their wrist for 1 week (144).
The Study to Understand Fall Reduction and Vitamin D in You is a recently completed randomized control trial of the use of vitamin D supplementation for fall prevention in older adults. The primary outcome of the trial was time to first fall or death (145). PA was 1 of the secondary outcomes and was measured using the ActiGraph GT9X Link on the nondominant wrist for 7 days at baseline, 3, 12, and 24 months (146). Data were collected on 664 participants.
Many other large US studies have implemented wrist accelerometry in their ongoing research protocols. For example, wrist accelerometry has been introduced in the Atherosclerosis Risk in Communities Study (147), the Peripheral Artery Disease Study of Study of Latinos, which is an ancillary study to the Hispanic Community Cohort Study/Study of Latinos (148), the Multicenter AIDS Cohort Study (149), the National Health and Aging Trends Study (150), and the Aging and Cognitive Health Evaluation in Elders Study (151) (J.A.S., personal communication). The protocol details are not yet published for these studies and therefore are excluded from Table 7.
Studies conducted in the United Kingdom also collected wrist-worn accelerometer data. The UK Biobank is a large, prospective cohort study with 500,000 participants residing in the United Kingdom. The goal of the study is to investigate genetic and nongenetic determinants of diseases in middle and old age. Researchers implemented wrist-worn Axivity AX3 for 7-day wear for 103,578 participants between 2013 and 2015 (132). Repeated measures are available for 2,500 participants (152). The Fenland Study is an ongoing study in the United Kingdom that recruited volunteers born between 1950 and 1975. The study is designed to investigate the interaction between genetic and lifestyle factors and obesity and related metabolic disorders. Phase 1 of the study was completed between 2005 and 2015. All participants were asked to wear a chest-mounted Actiheart, and a subsample of 2,100 participants also wore a GENEActiv accelerometer on the nondominant wrist simultaneously for 6 days (153). The Whitehall II Study is a cohort of UK civil servants established in the mid to late 1980s (1985–1988) that was designed to investigate health inequality between social classes. A total of 4,029 participants provided valid accelerometer data measured by the GENEActiv device worn on the nondominant wrist over 9 days during the 2012–2013 wave (154).
Two studies in Finland introduced wrist-worn accelerometry: the FinHealth 2017 Survey and the Finnish Retirement and Aging Study. The FinHealth 2017 Survey is a 2-stage cluster sample representative of adults older than 18 years in Finland; 933 participants in the cohort also participated in a “physical activity and sleep” substudy and wore a ActiGraph GT9X Link on their nondominant wrist for 7 days (131). The Finnish Retirement and Aging Study is a cohort study established in 2013 in which researchers aim to examine change in health behaviors during the retirement transition and the health and functional consequences of work and retirement (155). A subsample of the cohort was invited to participate in an activity substudy during which they were asked to wear the triaxial ActiGraph wActiSleep-BT for 7 days. Assessments before retirement were available for 903 participants (156), and repeated measurements were available for 527 participants as of March 2019 (157).
Several birth cohorts in Pelotas, Brazil, have also collected wrist-worn, accelerometer-assessed PA data. Participants of the 1982, 1993 and 2004 cohorts (n = 10,029) wore GENEActiv accelerometers on their nondominant wrist for 4–7 days, including at least 1 weekend day, between 2010 and 2013 (110). For the 2015 cohort, participants of the antenatal substudy during 16 to 24 weeks of gestation, fathers at 12-month follow-up, and mothers at 24-month follow-up after birth, entered a 7-day accelerometer protocol wearing the triaxial ActiGraph wGT3X-BT (158). Accelerometer data were collected from 2,620 pregnant women (109).
CHALLENGES WITH ACCELEROMETRY RESEARCH
Accelerometers, despite their many advantages and increasing popularity, also come with measurement and interpretation challenges. Some of these challenges are inherent limitations. For example, wrist-worn accelerometers may not adequately measure certain types of low-impact PAs such as cycling, yoga, and strength training (16) or capture the motion of certain activities, such as carrying groceries while walking or pushing a stroller (20).
Other challenges are methodological. Posture detection is currently difficult using wrist-worn accelerometers (9). Though devices worn on the thigh can better differentiate sitting or lying down and standing and stepping (42), they cannot differentiate sitting from lying down (159). The inclinometer built into some ActiGraph models can distinguish sitting and standing postures when worn on the thigh (85.7%–100% correct) (160), but the accuracy was not good on the waist or wrist (mean absolute percent error > 44%) (161). Several methods have been developed to address this limitation. A second activPAL fixed to the chest in addition to the thigh can perfectly discriminate sitting and lying (159). Machine-learning and threshold-based methods were also developed to improve posture classification. Clark et al. (162) reviewed such studies with various placement locations, including the wrist. The majority of these studies had very small sample sizes and were conducted in structured or semi-structured conditions, with mixed results; in some studies, researchers found inaccurate classification of lying down, sitting, and standing, even in semi-controlled conditions (163–166), but others reported good accuracy (71, 167–171).
Last, wrist-worn accelerometers do not provide straightforward information on the types of PA performed. Overcoming this challenge would greatly advance PA research by providing more details about PA as a behavior and providing new insights toward health surveillance and outcomes. Machine-learning models to classify activity types (162, 166, 169–173) have been proposed for wrist-worn accelerometers. However, most of the studies published to date have mainly focused on method validation. Applications in research to characterize free-living PA types and investigate associations with health outcomes are limited (174).
FUTURE DIRECTIONS
Over the past several years, PA research has expanded beyond volume- and intensity-based metrics to patterns, posture, and activity types to maximize the use of the rich information contained in high-frequency accelerometer data. Yet to date, posture and activity-type classification methods have generally been limited to small sample sizes and laboratory environments. Validation in free-living conditions with more representative samples are needed to further understand the utility of these novel methods in epidemiologic research, because they hold considerable promise for advancing the science of PA.
Accelerometers can also be used to measure biomechanics such as physical function in free-living conditions. For example, gait speed, an important predictor of disability and death (175–177), is commonly assessed in the laboratory using a usual paced 4-m or 6-m walk. However, standardized gait speed measured in the laboratory may not adequately reflect free-living gait speed or variability (178). Methods to extract gait parameters, such as cadence and stride, are emerging (179–181). Studies are needed to investigate whether free-living gait parameters provide more insights into health and functional status than do laboratory-based gait assessments.
We have reviewed the validity of several published, absolute intensity cutpoints, but it is debatable whether absolute or relative cutpoints are the best approach to classify activity intensity, because individuals vary greatly by cardiorespiratory fitness levels (182). Time spent in MVPA estimated using relative cutpoints differed markedly from the estimates generated using absolute cutpoints using hip- or chest-worn accelerometers in several studies (183–185). Yet to date, studies that apply relative cutpoints for wrist accelerometers are few (186).
Finally, accelerometers have been used in many large-scale epidemiologic studies around the world. Multicohort harmonization of data will facilitate global PA research. However, different device brands and data collection and processing protocols impose challenges in pooling studies (16). Several recommendations have been proposed for data harmonization (187). For previously collected data, it has been suggested that raw acceleration data (measured in g's) should be made available, and the types of monitors whose data can be pooled should be determined (187). Moreover, a new accelerometer data metric, Monitor-Independent Movement Summary, has been proposed to improve the pooling of the data from different accelerometer brands (188), but it has not be widely used. Work to process and harmonize raw data across device brands, placement, and data collection protocols is warranted.
CONCLUSION
Accelerometers have transformed PA epidemiologic research by making it possible to objectively and continuously measure PA in free-living conditions in large populations. In recent years, wrist-worn accelerometers have become increasingly popular due to better participant compliance and the ability to measure activity and sleep over a 24-hour protocol. To date, research suggests wrist accelerometers provide good validity and reliability, including quantifying total amount of PA, estimating energy expenditure, and classifying activity intensities. However, though clinically important, the traditional uses of accelerometer data reduce the rich information collected. Emerging pattern metrics of daily PA provide fairly simple ways to use data in a greater scope. Small studies have demonstrated promise in measuring posture, identifying activity types, and extracting free-living gait parameters. The potential utility of these measures to provide more sensitive information about health and functional status warrants additional investigation. Last, much data has been, and continues to be, collected in large-scale epidemiologic studies around the world. Because movement and health are intrinsically linked, data harmonization across these studies would greatly facilitate movement-based health research, providing a platform to refine and enhance public health recommendations and intervention efforts.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, United States (Fangyu Liu, Amal A. Wanigatunga, Jennifer A. Schrack); and Center on Aging and Health, Johns Hopkins University, Baltimore, Maryland, United States (Amal A. Wanigatunga, Jennifer A. Schrack).
This work was funded by National Institute on Aging (NIA) grant U01AG057545. A.A.W. also is supported by NIA grants R01AG061786 and P30AG059298; and J.A.S. also is supported by NIA grant R01AG061786.
Conflict of interest: none declared.
Data availability: Data sharing is not applicable to this article because no new data were created or analyzed in this study.
REFERENCES
- 1. 2018 Physical Activity Guidelines Advisory Committee . 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: US Department of Health and Human Services; 2018:779. https://health.gov/our-work/physical-activity/current-guidelines/scientific-report. Accessed July 13, 2020. [Google Scholar]
- 2. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- 3. Powell KE, Paluch AE, Blair SN. Physical activity for health: what kind? How much? How intense? On top of what? Annu Rev Public Health. 2011;32(1):349–365. [DOI] [PubMed] [Google Scholar]
- 4. Schoeller DA, Santen E. Measurement of energy expenditure in humans by doubly labeled water method. J Appl Physiol Respir Environ Exerc Physiol. 1982;53(4):955–959. [DOI] [PubMed] [Google Scholar]
- 5. Plasqui G, Westerterp KR. Physical activity assessment with accelerometers: an evaluation against doublylLabeled water. Obesity (Silver Spring). 2007;15(10):2371–2379. [DOI] [PubMed] [Google Scholar]
- 6. Troiano RP, McClain JJ, Brychta RJ, et al. Evolution of accelerometer methods for physical activity research. Br J Sports Med. 2014;48(13):1019–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(2 suppl):1–14. [DOI] [PubMed] [Google Scholar]
- 8. Matthews CE, Moore SC, George SM, et al. Improving self-reports of active and sedentary behaviors in large epidemiologic studies. Exerc Sport Sci Rev. 2012;40(3):118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee I-M, Shiroma EJ. Using accelerometers to measure physical activity in large-scale epidemiological studies: issues and challenges. Br J Sports Med. 2014;48(3):197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bonnefoy M, Normand S, Pachiaudi C, et al. Simultaneous validation of ten physical activity questionnaires in older men: a doubly labeled water study. J Am Geriatr Soc. 2001;49(1):28–35. [DOI] [PubMed] [Google Scholar]
- 11. Washburn RA. Assessment of physical activity in older adults. Res Q Exerc Sport. 2000;71(2 Suppl):S79–S88. [PubMed] [Google Scholar]
- 12. Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–781. [DOI] [PubMed] [Google Scholar]
- 13. Hees VT, Renström F, Wright A, et al. Estimation of daily energy expenditure in pregnant and non-pregnant women using a wrist-worn tri-axial accelerometer. PLoS One. 2011;6(7):e22922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White T, Westgate K, Hollidge S, et al. Estimating energy expenditure from wrist and thigh accelerometry in free-living adults: a doubly labelled water study. Int J Obes (Lond). 2019;43(11):2333–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. White T, Westgate K, Wareham NJ, et al. Estimation of physical activity energy expenditure during free-living from wrist accelerometry in UK adults. PLoS One. 2016;11(12):e0167472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schrack JA, Cooper R, Koster A, et al. Assessing daily physical activity in older adults: unraveling the complexity of monitors, measures, and methods. J Gerontol A Biol Sci Med Sci. 2016;71(8):1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shiroma EJ, Schrack JA, Harris TB. Accelerating accelerometer research in aging. J Gerontol A Biol Sci Med Sci. 2018;73(5):619–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Migueles JH, Cadenas-Sanchez C, Ekelund U, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med. 2017;47(9):1821–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Trost SG, Mciver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. 2005;37(11 suppl):S531–S543. [DOI] [PubMed] [Google Scholar]
- 20. Murphy SL. Review of physical activity measurement using accelerometers in older adults: considerations for research design and conduct. Prev Med. 2009;48(2):108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Plasqui G, Bonomi AG, Westerterp KR. Daily physical activity assessment with accelerometers: new insights and validation studies. Obes Rev. 2013;14(6):451–462. [DOI] [PubMed] [Google Scholar]
- 22. Rowlands AV. Moving forward with accelerometer-assessed physical activity: two strategies to ensure meaningful, interpretable and comparable measures. Pediatr Exerc Sci. 2018;30(4):450–456. [DOI] [PubMed] [Google Scholar]
- 23. Chen KY, Bassett DR. The technology of accelerometry-based activity monitors: current and future. Med Sci Sports Exerc. 2005;37(11 suppl):S490–S500. [DOI] [PubMed] [Google Scholar]
- 24. Hees VT, Gorzelniak L, León ECD, et al. Separating movement and gravity components in an acceleration signal and implications for the assessment of human daily physical activity. PLoS One. 2013;8(4):e61691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bai J, Di C, Xiao L, et al. An activity index for raw accelerometry data and its comparison with other activity metrics. PLoS One. 2016;11(8):e0160644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karas M, Bai J, Strączkiewicz M, et al. Accelerometry data in health research: challenges and opportunities. Stat Biosci. 2019;11(2):210–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Korpan SM, Schafer JL, Wilson KC, et al. Effect of ActiGraph GT3X+ position and algorithm choice on step count accuracy in older adults. J Aging Phys Act. 2015;23(3):377–382. [DOI] [PubMed] [Google Scholar]
- 28. Esliger DW, Probert A, Gorber SC, et al. Validity of the Actical Accelerometer step-count function. Med Sci Sports Exerc. 2007;39(7):1200–1204. [DOI] [PubMed] [Google Scholar]
- 29. Toth LP, Park S, Springer CM, et al. Bassett DR, Video-recorded validation of Wearable step counters under free-living conditions. Med Sci Sports Exerc. 2018;50(6):1315–1322. [DOI] [PubMed] [Google Scholar]
- 30. John D, Morton A, Arguello D, et al. “What is a step?” differences in how a step is detected among three popular activity monitors that have impacted physical activity research. Sensors. 2018;18(4):1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chow JJ, Thom JM, Wewege MA, et al. Accuracy of step count measured by physical activity monitors: the effect of gait speed and anatomical placement site. Gait Posture. 2017;57:199–203. [DOI] [PubMed] [Google Scholar]
- 32. Phillips LJ, Petroski GF, Markis NE. A comparison of accelerometer accuracy in older adults. Res Gerontol Nurs. 2015;8(5):213–219. [DOI] [PubMed] [Google Scholar]
- 33. Kuo P-L, Urbanek JK, Schrack JA. Age-related bias in total step count recorded by wearable devices. JAMA Intern Med. 2019;179(11):1602–1602. [DOI] [PubMed] [Google Scholar]
- 34. Le Masurier GC, Tudor-Locke C. Comparison of pedometer and accelerometer accuracy under controlled conditions. Med Sci Sports Exerc. 2003;35(5):867–871. [DOI] [PubMed] [Google Scholar]
- 35. Sagelv EH, Ekelund U, Pedersen S, et al. Physical activity levels in adults and elderly from triaxial and uniaxial accelerometry. The Tromsø Study. PLoS One. 2019;14(12):e0225670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Westerterp KR. Physical activity assessment with accelerometers. Int J Obes Relat Metab Disord. 1999;23(suppl 3):S45–S49. [DOI] [PubMed] [Google Scholar]
- 37. Yamada Y, Yokoyama K, Noriyasu R, et al. Light-intensity activities are important for estimating physical activity energy expenditure using uniaxial and triaxial accelerometers. Eur J Appl Physiol. 2009;105(1):141–152. [DOI] [PubMed] [Google Scholar]
- 38. Smith MP, Horsch A, Standl M, et al. Uni- and triaxial accelerometric signals agree during daily routine but show differences between sports. Sci Rep. 2018;8(1):15055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Corder K, Brage S, Ekelund U. Accelerometers and pedometers: methodology and clinical application. Curr Opin Clin Nutr Metab Care. 2007;10(5):597–603. [DOI] [PubMed] [Google Scholar]
- 40. Migueles JH, Rowlands AV, Huber F, et al. GGIR: a research community–driven open source R package for generating physical activity and sleep outcomes from multi-day raw accelerometer data. J Meas Phys Behav. 2019;2(3):188–196. [Google Scholar]
- 41. Wanigatunga AA, Wang H, An Y, et al. Association between brain volumes and patterns of physical activity in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2020;76(8):1504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Crowley P, Skotte J, Stamatakis E, et al. Comparison of physical behavior estimates from three different thigh-worn accelerometers brands: a proof-of-concept for the Prospective Physical Activity, Sitting, and Sleep Consortium (ProPASS). Int J Behav Nutr Phys Act. 2019;16(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hildebrand M, Van Hees VT, Hansen BH, et al. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc. 2014;46(9):1816–1824. [DOI] [PubMed] [Google Scholar]
- 44. Stec MJ, Rawson ES. Estimation of resistance exercise energy expenditure using triaxial accelerometry. J Strength Cond Res. 2012;26(5):1413–1422. [DOI] [PubMed] [Google Scholar]
- 45. Montoye AHK, Mudd LM, Biswas S, et al. Energy expenditure prediction using raw accelerometer data in simulated free living. Med Sci Sports Exerc. 2015;47(8):1735–1746. [DOI] [PubMed] [Google Scholar]
- 46. Ellis K, Kerr J, Godbole S, et al. A random forest classifier for the prediction of energy expenditure and type of physical activity from wrist and hip accelerometers. Physiol Meas. 2014;35(11):2191–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Staudenmayer J, He S, Hickey A, et al. Methods to estimate aspects of physical activity and sedentary behavior from high-frequency wrist accelerometer measurements. J Appl Physiol. 2015;119(4):396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Duncan MJ, Rowlands A, Lawson C, et al. Using accelerometry to classify physical activity intensity in older adults: what is the optimal wear-site? Eur J Sport Sci. 2020;20(8):1131–1139. [DOI] [PubMed] [Google Scholar]
- 49. He B, Bai J, Zipunnikov VV, et al. Predicting human movement with multiple accelerometers using Movelets. Med Sci Sports Exerc. 2014;46(9):1859–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Strath SJ, Pfeiffer KA, Whitt-Glover MC. Accelerometer use with children, older adults, and adults with functional limitations. Med Sci Sports Exerc. 2012;44(suppl 1):S77–S85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Orme M, Wijndaele K, Sharp SJ, et al. Combined influence of epoch length, cut-point and bout duration on accelerometry-derived physical activity. Int J Behav Nutr Phys Act. 2014;11(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fabre N, Lhuisset L, Bernal C, et al. Effect of epoch length on intensity classification and on accuracy of measurement under controlled conditions on treadmill: towards a better understanding of accelerometer measurement. PLoS One. 2020;15(1):e0227740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ayabe M, Kumahara H, Morimura K, et al. Epoch length and the physical activity bout analysis: an accelerometry research issue. BMC Res Notes. 2013;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hibbing PR, Bassett DR, Crouter SE. Modifying accelerometer cut-points affects criterion validity in simulated free-living for adolescents and adults. Res Q Exerc Sport. 2020;91(3):514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McArdle WD, Katch FI, Katch VL. Exercise Physiology: Nutrition, Energy, and Human Performance. Philadelphia, PA: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 56. Patterson SM, Krantz DS, Montgomery LC, et al. Automated physical activity monitoring: validation and comparison with physiological and self-report measures. Psychophysiology. 1993;30(3):296–305. [DOI] [PubMed] [Google Scholar]
- 57. Lee P, Tse CY. Calibration of wrist-worn ActiWatch 2 and ActiGraph wGT3X for assessment of physical activity in young adults. Gait Posture. 2019;68:141–149. [DOI] [PubMed] [Google Scholar]
- 58. Ho C-S, Chang C-H, Lin K-C, et al. Correction of estimation bias of predictive equations of energy expenditure based on wrist/waist-mounted accelerometers. PeerJ. 2019;7:e7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Neil-Sztramko SE, Rafn BS, Gotay CC, et al. Determining activity count cut-points for measurement of physical activity using the Actiwatch2 accelerometer. Physiol Behav. 2017;173:95–100. [DOI] [PubMed] [Google Scholar]
- 60. Swartz AM, Strath SJ, Bassett DR, et al. Estimation of energy expenditure using CSA accelerometers at hip and wrist sites. Med Sci Sports Exerc. 2000;32(9 suppl):S450–S456. [DOI] [PubMed] [Google Scholar]
- 61. Ellingson LD, Hibbing PR, Kim Y, et al. Lab-based validation of different data processing methods for wrist-worn ActiGraph accelerometers in young adults. Physiol Meas. 2017;38(6):1045–1060. [DOI] [PubMed] [Google Scholar]
- 62. Strath SJ, Kate RJ, Keenan KG, et al. Ngram time series model to predict activity type and energy cost from wrist, hip and ankle accelerometers: implications of age. Physiol Meas. 2015;36(11):2335–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chen KY, Acra SA, Majchrzak K, et al. Predicting energy expenditure of physical activity using hip- and wrist-worn accelerometers. Diabetes Technol Ther. 2003;5(6):1023–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Esliger DW, Rowlands AV, Hurst TL, et al. Validation of the GENEA accelerometer. Med Sci Sports Exerc. 2011;43(6):1085–1093. [DOI] [PubMed] [Google Scholar]
- 65. Sirichana W, Dolezal BA, Neufeld EV, et al. Wrist-worn triaxial accelerometry predicts the energy expenditure of non-vigorous daily physical activities. J Sci Med Sport. 2017;20(8):761–765. [DOI] [PubMed] [Google Scholar]
- 66. Rhudy MB, Dreisbach SB, Moran MD, et al. Cut points of the Actigraph GT9X for moderate and vigorous intensity physical activity at four different wear locations. J Sports Sci. 2020;38(5):503–510. [DOI] [PubMed] [Google Scholar]
- 67. Landry GJ, Falck RS, Beets MW, et al. Measuring physical activity in older adults: calibrating cut-points for the MotionWatch 8©. Front Aging Neurosci. 2015;7:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Diaz KM, Krupka DJ, Chang MJ, et al. Wrist-based cut-points for moderate- and vigorous-intensity physical activity for the Actical accelerometer in adults. J Sports Sci. 2018;36(2):206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Welch WA, Bassett DR, Thompson DL, et al. Classification accuracy of the wrist-worn gravity estimator of normal everyday activity accelerometer. Med Sci Sports Exerc. 2013;45(10):2012–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Marcotte RT, Petrucci GJ, Cox MF, et al. Estimating sedentary time from a hip- and wrist-worn accelerometer. Med Sci Sports Exerc. 2020;52(1):225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ellis K, Kerr J, Godbole S, et al. Hip and wrist accelerometer algorithms for free-living behavior classification. Med Sci Sports Exerc. 2016;48(5):933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Montoye AHK, Pivarnik JM, Mudd LM, et al. Validation and comparison of accelerometers worn on the hip, thigh, and wrists for measuring physical activity and sedentary behavior. AIMS Public Health. 2016;3(2):298–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Montoye AHK, Westgate BS, Fonley MR, et al. Cross-validation and out-of-sample testing of physical activity intensity predictions with a wrist-worn accelerometer. J Appl Physiol (1985). 2018;124(5):1284–1293. [DOI] [PubMed] [Google Scholar]
- 74. Correa JB, Apolzan JW, Shepard DN, et al. Evaluation of the ability of three physical activity monitors to predict weight change and estimate energy expenditure. Appl Physiol Nutr Metab. 2016;41(7):758–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Melanson EL, Freedson PS. Validity of the Computer Science and Applications, Inc. (CSA) activity monitor. Med Sci Sports Exerc. 1995;27(6):934–940. [PubMed] [Google Scholar]
- 76. ActiGraph . What is the difference among the energy expenditure algorithms? https://actigraphcorp.force.com/support/s/article/What-is-the-difference-among-the-Energy-Expenditure-Algorithms. Accessed July 24, 2020.
- 77. Jago R, Zakeri I, Baranowski T, et al. Decision boundaries and receiver operating characteristic curves: new methods for determining accelerometer cutpoints. J Sports Sci. 2007;25(8):937–944. [DOI] [PubMed] [Google Scholar]
- 78. Thiese MS, Hegmann KT, Behrens TK, et al. Important differences in accelerometer cut points for quantifying physical activity in a nested occupational cohort. J Exerc Sports Orthop. 2014;1(1). https://symbiosisonlinepublishing.com/exercise-sports-orthopedics/exercise-sports-orthopedics02.php. Accessed November 28, 2020. [Google Scholar]
- 79. Sanders GJ, Boddy LM, Sparks SA, et al. Evaluation of wrist and hip sedentary behaviour and moderate-to-vigorous physical activity raw acceleration cutpoints in older adults. J Sports Sci. 2019;37(11):1270–1279. [DOI] [PubMed] [Google Scholar]
- 80. Koster A, Shiroma EJ, Caserotti P, et al. Comparison of sedentary estimates between activPAL and hip- and wrist-worn ActiGraph. Med Sci Sports Exerc. 2016;48(8):1514–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hildebrand M, Hansen BH, van Hees VT, et al. Evaluation of raw acceleration sedentary thresholds in children and adults. Scand J Med Sci Sports. 2017;27(12):1814–1823. [DOI] [PubMed] [Google Scholar]
- 82. Kozey-Keadle S, Libertine A, Lyden K, et al. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc. 2011;43(8):1561–1567. [DOI] [PubMed] [Google Scholar]
- 83. Silva SG, Evenson KR, Ekelund U, et al. How many days are needed to estimate wrist-worn accelerometry-assessed physical activity during the second trimester in pregnancy? PLoS One. 2019;14(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Falck RS, Landry GJ, Brazendale K, et al. Measuring physical activity in older adults using MotionWatch 8 actigraphy: how many days are needed? J Aging Phys Act. 2017;25(1):51–57. [DOI] [PubMed] [Google Scholar]
- 85. Dillon CB, Fitzgerald AP, Kearney PM, et al. Number of days required to estimate habitual activity using wrist-worn GENEActiv accelerometer: a cross-sectional study. PLoS One. 2016;11(5):e0109913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ozemek C, Kirschner MM, Wilkerson BS, et al. Intermonitor reliability of the GT3X+ accelerometer at hip, wrist and ankle sites during activities of daily living. Physiol Meas. 2014;35(2):129–138. [DOI] [PubMed] [Google Scholar]
- 87. Ricardo LIC, Wendt A, Galliano LM, et al. Number of days required to estimate physical activity constructs objectively measured in different age groups: findings from three Brazilian (Pelotas) population-based birth cohorts. PLoS One. 2020;15(1):e0216017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tucker P, Gilliland J. The effect of season and weather on physical activity: a systematic review. Public Health. 2007;121(12):909–922. [DOI] [PubMed] [Google Scholar]
- 89. Kang M, Bassett DR, Barreira TV, et al. Measurement effects of seasonal and monthly variability on pedometer-determined data. J Phys Act Health. 2012;9(3):336–343. [DOI] [PubMed] [Google Scholar]
- 90. Owen N, Sparling PB, Healy GN, et al. Sedentary behavior: emerging evidence for a new health risk. Mayo Clin Proc. 2010;85(12):1138–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Füzéki E, Engeroff T, Banzer W. Health benefits of light-intensity physical activity: a systematic review of accelerometer data of the National Health and nutrition examination survey (NHANES). Sports Med. 2017;47(9):1769–1793. [DOI] [PubMed] [Google Scholar]
- 92. LaCroix AZ, Bellettiere J, Rillamas-Sun E, et al. Association of light physical activity measured by accelerometry and incidence of coronary heart disease and cardiovascular disease in older women. JAMA Netw Open. 2019;2(3):e190419-e190419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Khoja SS, Almeida GJ, Chester Wasko M, et al. Light intensity physical activity is associated with lower cardiovascular risk factor burden in rheumatoid arthritis. Arthritis Care Res. 2016;68(4):424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Healy GN, Dunstan DW, Salmon J, et al. Objectively measured light-intensity physical activity is independently associated with 2-h plasma glucose. Diabetes Care. 2007;30(6):1384–1389. [DOI] [PubMed] [Google Scholar]
- 95. Varma VR, Chuang Y-F, Harris GC, et al. Low-intensity daily walking activity is associated with hippocampal volume in older adults. Hippocampus. 2015;25(5):605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Varma VR, Tan EJ, Wang T, et al. Low-intensity walking activity is associated with better health. J Appl Gerontol. 2014;33(7):870–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Buman MP, Hekler EB, Haskell WL, et al. Objective light-intensity physical activity associations with rated health in older adults. Am J Epidemiol. 2010;172(10):1155–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rees-Punia E, Evans EM, Schmidt MD, et al. Mortality risk reductions for replacing sedentary time with physical activities. Am J Prev Med. 2019;56(5):736–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Fishman EI, Steeves JA, Zipunnikov V, et al. Association between objectively measured physical activity and mortality in NHANES. Med Sci Sports Exerc. 2016;48(7):1303–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Saint-Maurice PF, Troiano RP, Bassett DR, et al. Association of daily step count and step intensity with mortality among US adults. JAMA. 2020;323(12):1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hamer M, Oliveira C, Demakakos P. Non-exercise physical activity and survival: English longitudinal study of ageing. Am J Prev Med. 2014;47(4):452–460. [DOI] [PubMed] [Google Scholar]
- 102. Matthews CE, Jurj AL, Shu X-O, et al. Influence of exercise, walking, cycling, and overall nonexercise physical activity on mortality in Chinese women. Am J Epidemiol. 2007;165(12):1343–1350. [DOI] [PubMed] [Google Scholar]
- 103. Andersen RE, Wadden TA, Bartlett SJ, et al. Effects of lifestyle activity vs structured aerobic exercise in obese women: a randomized trial. JAMA. 1999;281(4):335–340. [DOI] [PubMed] [Google Scholar]
- 104. Brown M, Sinacore DR, Ehsani AA, et al. Low-intensity exercise as a modifier of physical frailty in older adults. Arch Phys Med Rehabil. 2000;81(7):960–965. [DOI] [PubMed] [Google Scholar]
- 105. Tremblay MS, Aubert S, Barnes JD, et al. Sedentary behavior research network (SBRN)–terminology consensus project process and outcome. Int J Behav Nutr Phys Act. 2017;14(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Honda T, Chen S, Yonemoto K, et al. Sedentary bout durations and metabolic syndrome among working adults: a prospective cohort study. BMC Public Health. 2016;16(1):888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Júdice PB, Silva AM, Sardinha LB. Sedentary bout durations are associated with abdominal obesity in older adults. J Nutr Health Aging. 2015;19(8):798–804. [DOI] [PubMed] [Google Scholar]
- 108. Diaz KM, Howard VJ, Hutto B, et al. Patterns of sedentary behavior and mortality in U.S. middle-aged and older adults. Ann Intern Med. 2017;167(7):465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Silva SG, Evenson KR, Silva ICM, et al. Correlates of accelerometer-assessed physical activity in pregnancy—the 2015 Pelotas (Brazil) Birth Cohort Study. Scand J Med Sci Sports. 2018;28(8):1934–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Silva IC, Hees VT, Ramires VV, et al. Physical activity levels in three Brazilian birth cohorts as assessed with raw triaxial wrist accelerometry. Int J Epidemiol. 2014;43(6):1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ramires VV, Wehrmeister FC, Böhm AW, et al. Physical activity levels objectively measured among older adults: a population-based study in a southern city of Brazil. Int J Behav Nutr Phys Act. 2017;14(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wanigatunga AA, Di J, Zipunnikov V, et al. Association of total daily physical activity and fragmented physical activity with mortality in older adults. JAMA Netw Open. 2019;2(10):e1912352-e1912352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Menai M, Hees VT, Elbaz A, et al. Accelerometer assessed moderate-to-vigorous physical activity and successful ageing: results from the Whitehall II Study. Sci Rep. 2017;8:45772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE Study randomized clinical trial. JAMA. 2014;311(23):2387–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wanigatunga AA, Manini TM, Cook DR, et al. Community-based activity and sedentary patterns are associated with cognitive performance in mobility-limited older adults. Front Aging Neurosci. 2018;10:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wanigatunga AA, Ambrosius WT, Rejeski WJ, et al. Association between structured physical activity and sedentary time in older adults. JAMA. 2017;318(3):297–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wanigatunga AA, Tudor-Locke C, Axtell RS, et al. Effects of a long-term physical activity program on activity patterns in older adults. Med Sci Sports Exerc. 2017;49(11):2167–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Healy GN, Dunstan DW, Salmon J, et al. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care. 2008;31(4):661–666. [DOI] [PubMed] [Google Scholar]
- 119. Healy GN, Matthews CE, Dunstan DW, et al. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003–06. Eur Heart J. 2011;32(5):590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Chen T, Narazaki K, Haeuchi Y, et al. Associations of sedentary time and breaks in sedentary time with disability in instrumental activities of daily living in community-dwelling older adults. J Phys Act Health. 2016;13(3):303–309. [DOI] [PubMed] [Google Scholar]
- 121. Sardinha LB, Ekelund U, Santos L, et al. Breaking-up sedentary time is associated with impairment in activities of daily living. Exp Gerontol. 2015;72:57–62. [DOI] [PubMed] [Google Scholar]
- 122. Schrack JA, Kuo P-L, Wanigatunga AA, et al. Active-to-sedentary behavior transitions, fatigability, and physical functioning in older adults. J Gerontol A Biol Sci Med Sci. 2019;74(4):560–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Palmberg L, Rantalainen T, Rantakokko M, et al. The associations of activity fragmentation with physical and mental fatigability among community-dwelling 75-, 80- and 85-year-old people. J Gerontol A Biol Sci Med Sci. 2020;75(9):e103–e110. [DOI] [PubMed] [Google Scholar]
- 124. Smirnova E, Leroux A, Cao Q, et al. The predictive performance of objective measures of physical activity derived from accelerometry data for 5-year all-cause mortality in older adults: National Health and Nutritional Examination Survey 2003–2006. J Gerontol A Biol Sci Med Sci. 2020;75(9):1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wanigatunga AA, Gresham GK, Kuo P-L, et al. Contrasting characteristics of daily physical activity in older adults by cancer history. Cancer. 2018;124(24):4692–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. E J-Y, Schrack JA, Mihailovic A, et al. Patterns of daily physical activity across the spectrum of visual field damage in glaucoma patients. Ophthalmology. 2020;128(1):70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Schrack JA, Zipunnikov V, Goldsmith J, et al. Assessing the “physical cliff”: detailed quantification of age-related differences in daily patterns of physical activity. J Gerontol A Biol Sci Med Sci. 2014;69(8):973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Davis MG, Fox KR. Physical activity patterns assessed by accelerometry in older people. Eur J Appl Physiol. 2007;100(5):581–589. [DOI] [PubMed] [Google Scholar]
- 129. Davis MG, Fox KR, Hillsdon M, et al. Objectively measured physical activity in a diverse sample of older urban UK adults. Med Sci Sports Exerc. 2011;43(4):647–654. [DOI] [PubMed] [Google Scholar]
- 130. Sartini C, Wannamethee SG, Iliffe S, et al. Diurnal patterns of objectively measured physical activity and sedentary behaviour in older men. BMC Public Health. 2015;15(1):609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wennman H, Pietilä A, Rissanen H, et al. Gender, age and socioeconomic variation in 24-hour physical activity by wrist-worn accelerometers: the FinHealth 2017 survey. Sci Rep. 2019;9(1):6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Doherty A, Jackson D, Hammerla N, et al. Large scale population assessment of physical activity using wrist worn accelerometers: the UK biobank study. PLoS One. 2017;12(2):e0169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Arnardottir NY, Koster A, Van Domelen DR, et al. Objective measurements of daily physical activity patterns and sedentary behaviour in older adults: age, gene/environment susceptibility-Reykjavik study. Age Ageing. 2013;42(2):222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Stenholm S, Pulakka A, Leskinen T, et al. Daily physical activity patterns and their association with health-related physical fitness among aging workers—the Finnish retirement and aging study. J Gerontol A Biol Sci Med Sci. 2021;76(7):1242–1250. [DOI] [PubMed] [Google Scholar]
- 135. Wendt A, Silva ICM, Gonçalves H, et al. Short-term effect of physical activity on sleep health: a population-based study using accelerometry. J Sport Health Sci. 2020;S2095-2546(20):30053–30063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Nastasi AJ, Ahuja A, Zipunnikov V, et al. Objectively measured physical activity and falls in well-functioning older adults: findings from the Baltimore Longitudinal Study of Aging. Am J Phys Med Rehabil. 2018;97(4):255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wanigatunga AA, Simonsick EM, Zipunnikov V, et al. Perceived fatigability and objective physical activity in mid- to late-life. J Gerontol A Biol Sci Med Sci. 2018;73(5):630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Pulakka A, Leskinen T, Suorsa K, et al. Physical activity across retirement transition by occupation and mode of commute. Med Sci Sports Exerc. 2020;52(9):1900–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Johnson CL, Paulose-Ram R, Ogden CL, et al. National Health and Nutrition Examination Survey: analytic guidelines, 1999-2010. Vital Health Stat. 2013;2(161):1–24. [PubMed] [Google Scholar]
- 140. Kuo P-L, Schrack JA, Shardell MD, et al. A roadmap to build a phenotypic metric of ageing: insights from the Baltimore Longitudinal Study of Aging. J Intern Med. 2020;287(4):373–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Adelnia F, Urbanek J, Osawa Y, et al. Moderate-to-vigorous physical activity is associated with higher muscle oxidative capacity in older adults. J Am Geriatr Soc. 2019;67(8):1695–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. National Opinion Research Center at University of Chicago . National Social Life, Health, and Aging Project (NSHAP). https://www.norc.org/Research/Projects/Pages/national-social-life-health-and-aging-project.aspx. Accessed July 16, 2020.
- 143. Huisingh-Scheetz MJ, Kocherginsky M, Magett E, et al. Relating wrist accelerometry measures to disability in older adults. Arch Gerontol Geriatr. 2016;62:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Claudel SE, Shiroma EJ, Harris TB, et al. Cross-sectional associations of neighborhood perception, physical activity, and sedentary time in community-dwelling socioeconomically diverse adults. Front Public Health. 2019;7:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Appel LJ, Michos ED, Mitchell CM, et al. The effects of four doses of vitamin D supplements on falls in older adults. Ann Intern Med. 2021;174(2):145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Michos ED, Mitchell CM, Miller ER, et al. Rationale and Design of the Study to Understand Fall Reduction and Vitamin D in You (STURDY): a randomized clinical trial of vitamin D supplement doses for the prevention of falls in older adults. Contemp Clin Trials. 2018;73:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. The ARIC investigators . The atherosclerosis risk in communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 148. Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20(8):629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kaslow RA, Ostrow DG, Detels R, et al. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126(2):310–318. [DOI] [PubMed] [Google Scholar]
- 150. Freedman VA, Kasper JD. Cohort profile: the National Health and Aging Trends Study (NHATS). Int J Epidemiol. 2019;48(4):1044–1045g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Deal JA, Goman AM, Albert MS, et al. Hearing treatment for reducing cognitive decline: design and methods of the aging and cognitive health evaluation in elders randomized controlled trial. Alzheimers Dement (N Y). 2018;4:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Bycroft C, Freeman C, Petkova D, et al. The UK biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Perez-Pozuelo I, White T, Westgate K, et al. Diurnal profiles of physical activity and postures derived from wrist-worn accelerometry in UK adults. J Meas Phys Behav. 2020;3(1):39–49. [Google Scholar]
- 154. Sabia S, Hees VT, Shipley MJ, et al. Association between questionnaire- and accelerometer-assessed physical activity: the role of sociodemographic factors. Am J Epidemiol. 2014;179(6):781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Univeristy of Turku . About Firea | Finnish Retirement and Aging Study (FIREA). https://sites.utu.fi/firea/en/about-firea/. Accessed July 25, 2020.
- 156. Pulakka A, Leskinen T, Koster A, et al. Daily physical activity patterns among aging workers: the Finnish Retirement and Aging Study (FIREA). Occup Environ Med. 2019;76(1):33–39. [DOI] [PubMed] [Google Scholar]
- 157. Suorsa K, Pulakka A, Leskinen T, et al. Objectively measured sedentary time before and after transition to retirement: the Finnish Retirement and Aging Study. J Gerontol A Biol Sci Med Sci. 2020, 75(9):1737–1743. [DOI] [PubMed] [Google Scholar]
- 158. Hallal PC, Bertoldi AD, Domingues MR, et al. Cohort profile: the 2015 Pelotas (Brazil) Birth Cohort Study. Int J Epidemiol. 2018;47(4):1048–1048h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Bassett DR, John D, Conger SA, et al. Detection of lying down, sitting, standing, and stepping using two activPAL monitors. Med Sci Sports Exerc. 2014;46(10):2025–2029. [DOI] [PubMed] [Google Scholar]
- 160. Steeves JA, Bowles HR, McClain JJ, et al. Ability of thigh-worn ActiGraph and activPAL monitors to classify posture and motion. Med Sci Sports Exerc. 2015;47(5):952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. An H-S, Kim Y, Lee J-M. Accuracy of inclinometer functions of the activPAL and ActiGraph GT3X+: a focus on physical activity. Gait Posture. 2017;51:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Clark CCT, Nobre GC, Fernandes JFT, et al. Physical activity characterization: does one site fit all? Physiol Meas. 2018;39(9):09TR02. [DOI] [PubMed] [Google Scholar]
- 163. De Vries SI, Garre FG, Engbers LH, et al. Evaluation of neural networks to identify types of activity using accelerometers. Med Sci Sports Exerc. 2011;43(1):101–107. [DOI] [PubMed] [Google Scholar]
- 164. Gyllensten IC, Bonomi AG. Identifying types of physical activity with a single accelerometer: evaluating laboratory-trained algorithms in daily life. IEEE Trans Biomed Eng. 2011;58(9):2656–2663. [DOI] [PubMed] [Google Scholar]
- 165. Rowlands AV, Yates T, Olds TS, et al. Wrist-worn accelerometer-brand independent posture classification. Med Sci Sports Exerc. 2016;48(4):748–754. [DOI] [PubMed] [Google Scholar]
- 166. Sasaki JE, Hickey AM, Staudenmayer JW, et al. Performance of activity classification algorithms in free-living older adults. Med Sci Sports Exerc. 2016;48(5):941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Bao L, Intille SS. Activity recognition from user-annotated acceleration data. In: Ferscha A, Mattern F, eds. Pervasive Computing. Lecture Notes in Computer Science. Berlin, Germany: Springer; 2004:1–17. [Google Scholar]
- 168. Trost SG, Zheng Y, Wong W-K. Machine learning for activity recognition: hip versus wrist data. Physiol Meas. 2014;35(11):2183–2189. [DOI] [PubMed] [Google Scholar]
- 169. Pavey TG, Gilson ND, Gomersall SR, et al. Field evaluation of a random forest activity classifier for wrist-worn accelerometer data. J Sci Med Sport. 2017;20(1):75–80. [DOI] [PubMed] [Google Scholar]
- 170. Kheirkhahan M, Mehta S, Nath M, et al. A bag-of-words approach for assessing activities of daily living using wrist accelerometer data. In: 2017 IEEE International Conference on Bioinformatics and Biomedicine (BIBM). 2017:678–685. [Google Scholar]
- 171. Kheirkhahan M, Chakraborty A, Wanigatunga AA, et al. Wrist accelerometer shape feature derivation methods for assessing activities of daily living. BMC Med Inform Decis Mak. 2018;18(suppl 4):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Dutta A, Ma O, Toledo M, et al. Identifying free-living physical activities using lab-based models with wearable accelerometers. Sensors (Basel). 2018;18(11):3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Mannini A, Intille SS, Rosenberger M, et al. Activity recognition using a single accelerometer placed at the wrist or ankle. Med Sci Sports Exerc. 2013;45(11):2193–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Willetts M, Hollowell S, Aslett L, et al. Statistical machine learning of sleep and physical activity phenotypes from sensor data in 96,220 UK biobank participants. Sci Rep. 2018;8(1):7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Perera S, Patel KV, Rosano C, et al. Gait speed predicts incident disability: a pooled analysis. J Gerontol A Biol Sci Med Sci. 2016;71(1):63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–M231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Takayanagi N, Sudo M, Yamashiro Y, et al. Relationship between daily and in-laboratory gait speed among healthy community-dwelling older adults. Sci Rep. 2019;9(1):3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Fasel B, Duc C, Dadashi F, et al. A wrist sensor and algorithm to determine instantaneous walking cadence and speed in daily life walking. Med Biol Eng Comput. 2017;55(10):1773–1785. [DOI] [PubMed] [Google Scholar]
- 180. Karas M, Stra Czkiewicz M, Fadel W, et al. Adaptive empirical pattern transformation (ADEPT) with application to walking stride segmentation. Biostatistics. 2021;22(2):331–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Urbanek JK, Harezlak J, Glynn NW, et al. Stride variability measures derived from wrist- and hip-worn accelerometers. Gait Posture. 2017;52:217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Gil-Rey E, Maldonado-Martín S, Gorostiaga EM. Individualized accelerometer activity cut-points for the measurement of relative physical activity intensity levels. Res Q Exerc Sport. 2019;90(3):327–335. [DOI] [PubMed] [Google Scholar]
- 183. Schrack JA, Leroux A, Fleg JL, et al. Using heart rate and accelerometry to define quantity and intensity of physical activity in older adults. J Gerontol A Biol Sci Med Sci. 2018;73(5):668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Siddique J, Aaby D, Montag SE, et al. Individualized relative-intensity physical activity accelerometer cut points. Med Sci Sports Exerc. 2020;52(2):398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Rejeski WJ, Marsh AP, Brubaker PH, et al. Analysis and interpretation of accelerometry data in older adults: the LIFE Study. J Gerontol A Biol Sci Med Sci. 2016;71(4):521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Hernando C, Hernando C, Collado EJ, et al. Establishing cut-points for physical activity classification using triaxial accelerometer in middle-aged recreational marathoners. PLoS One. 2018;13(8):e0202815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Wijndaele K, Westgate K, Stephens SK, et al. Utilization and harmonization of adult Accelerometry data: review and expert consensus. Med Sci Sports Exerc. 2015;47(10):2129–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. John D, Tang Q, Albinali F, et al. An open-source monitor-independent movement summary for Accelerometer data processing. J Meas Phys Behav. 2019;2(4):268–281. [DOI] [PMC free article] [PubMed] [Google Scholar]


