Abstract
Background
Salix matsudana (Koidz.) is a widely planted ornamental allotetraploid tree species. Genetic engineering can be used to enhance the tolerance of this species to soil salinization, endowing varieties with the ability to grow along coastlines, thereby mitigating afforestation and protecting the environment. The AP2/ERF family of transcription factors (TFs) plays multidimensional roles in plant biotic/abiotic stress tolerance and plant development. In this study, we cloned the SmAP2-17 gene and performed functional analysis of its role in salt tolerance. This study aims to identify key genes for future breeding of stress-resistant varieties of Salix matsudana.
Results
SmAP2-17 was predicted to be a homolog of AP2-like ethylene-responsive transcription factor ANT isoform X2 from Arabidopsis, with a predicted ORF of 2058 bp encoding an estimated protein of 685 amino acids containing two conserved AP2 domains (PF00847.20). SmAP2-17 had a constitutive expression pattern and was localized to the nucleus. The overexpression of the native SmAP2-17 CDS sequence in Arabidopsis did not increase salt tolerance because of the reduced expression level of ectopic SmAP2-17, potentially caused by salt-induced RNAi. Transgenic lines with high expression of optimized SmAP2-17 CDS under salt stress showed enhanced tolerance to salt. Moreover, the expression of general stress marker genes and important salt stress signaling genes, including RD29A, ABI5, SOS3, AtHKT1, and RBohF, were upregulated in SmAP2-17-overexpressed lines, with expression levels consistent with that of SmAP2-17 or optimized SmAP2-17. Promoter activity analysis using dual luciferase analysis showed that SmAP2-17 could bind the promoters of SOS3 and ABI5 to activate their expression, which plays a key role in regulating salt tolerance.
Conclusions
The SmAP2-17 gene isolated from Salix matsudana (Koidz.) is a positive regulator that improves the resistance of transgenic plants to salt stress by upregulating SOS3 and ABI5 genes. This study provides a potential functional gene resource for future generation of salt-resistant Salix lines by genetic engineering.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-022-03487-y.
Keywords: Salix matsudana, AP2/ERF, Salt tolerance, Arabidopsis, Overexpression
Background
The APETALA2/ethylene responsive factor (AP2/ERF) family transcription factors (TFs) is a plant-specific gene family with many members, which are classified into APETALA2(AP2), RELATED TO ABSCISIC ACID INSENSITIVE3/VIVIPAROUS 1 (RAV), DEHYDRATION-RESPONSIVE ELEMENT BINDING proteins (DREBs), ETHYLENE RESPONSIVE FACTORS (ERFs), and Soloist subfamilies, according to the number or structure of AP2 and other conserved domains [1, 2]. AP2/ERF family TFs play important and multidimensional roles in plant biotic/abiotic stress tolerance and plant growth, differentiation, and development [3–8]. DREB members extensively participate in the response and regulation of abiotic stress tolerance, such as cold, drought, heat, and salt tolerance, by directly regulating the expression of stress-responsive genes [5, 9]. In addition to genes from Arabidopsis, many studies have illustrated the function of DREB genes from other plant species, such as maize, wheat, and soybean, by overexpressing these genes in model plants [10–16]. Members of the ERF subfamily are also involved in abiotic stress responses, and members of the ERF-VII subfamily from Arabidopsis and rice have been verified to play major roles in flooding and submergence tolerance (hypoxia stress) [17–19]. Arabidopsis AP2s play a central role in developmental processes, including axillary floral meristem development, gynoecium development, shoot regeneration, brace root development, internode elongation, and trichome formation [20–25]. Members of the AP2 subfamilies have also been reported to mediate diverse abiotic stress responses. AINTEGUMENTA (ANT) is an example of this. In addition to controlling organ cell number and size, ANT also negatively regulates salt tolerance by repressing SOS3-LIKE CALCIUM BINDING PROTEIN 8 (SCABP8/CBL10), a putative Ca2+ sensor that protects Arabidopsis shoots against salt stress and maintains ion homeostasis [26].
Salix matsudana Koidz., an allotetraploid member of Salicaceae, is an important and widely planted ornamental tree species with strong and wide adaptability to diverse environmental stresses, including salinity [27, 28]. The genome of S. matsudana was recently sequenced, and the family members and classification of the SmAP2/ERF gene family were identified and their expression patterns under salt stress were analyzed [28, 29]. From the sequencing results, 364 SmAP2/ERF members were identified and named from the S. matsudana genome and classified into four major subfamilies: AP2 (55 members), ERF (166 members), DREB (135 members), and RAV (6 members) [29]. The expression levels of 4 genes from the AP2 subgroup, 10 genes from the DREB subgroup, and 13 genes from the ERF subgroup were strongly induced by salt stress [29]. Considering the important roles of AP2/ERF family TFs in stress tolerance, there is an urgent need to uncover the function of the SmAP2/ERF genes, especially those genes with induced expression patterns under stress. These researches will promote the application of these genes in molecular assisted breeding of Salix plants. However, because of the difficulties in genetic transformation and the unavailability of genomic sequences, the functional characterization of SmAP2/ERF members remains largely unexplored in Salix and other woody plant species. In the present study, a putative AP2 family gene, SmAP2-17, whose expression was induced under salt stress, was isolated and cloned from Salix matsudana. To determine the function of SmAP2-17, this gene was transformed into Arabidopsis via Agrobacterium-mediated transformation to evaluate its role in tolerance to salt stress and uncover its downstream genes.
Results
Isolation and bioinformatics analysis of SmAP2-17 and its homolog proteins
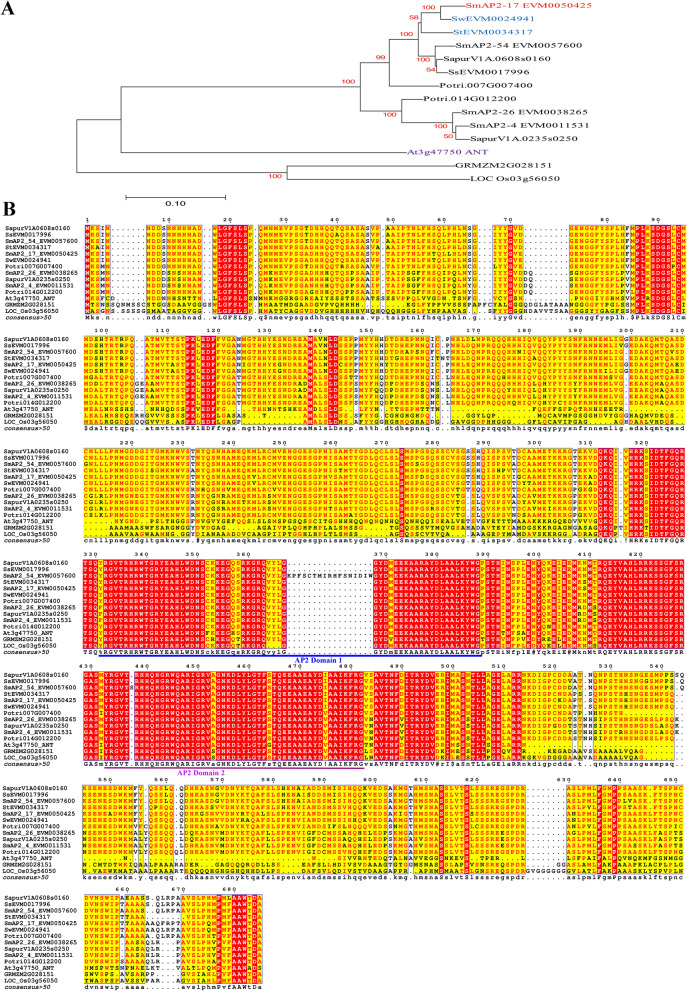
The coding sequence of SmAP2-17 was 2058 bp in length and encoded a protein comprised of 685 amino acids with a molecular mass of 76.3 kDa and an isoelectric point of 6.45. In the genome annotation, SmAP2-17 was predicted to be a homolog of AP2-like ethylene-responsive transcription factor ANT isoform X2. SmAP2-17 shares an identity of 44.65% with Arabidopsis ANT protein. Phylogenetic analysis showed that SmAP2-17 was closely related to proteins from Salix and Populus, such as SwEVM0024941.1 (96% identity), SapurV1A.0608s0160.1 (92.81% identity), and Potri.007G007400.1 (87.01% identity) from Salix wilsonii, Salix purpurea, and Populus trichocarpa, respectively (Fig. 1a). Other homologs shared a lower identity, in particular GRMZM2G028151_T01 and LOC_Os03g56050.1 from maize and rice, respectively. Multiple sequence alignment analysis showed that these proteins shared two conserved AP2/ERF domains (Fig. 1b). The protein sequences of SmAP2-17 and its homologs are listed in File S1.
Fig. 1.
Phylogenetic analysis and sequence alignment of SmAP2-17 and its homologs. a Phylogenetic analysis of the SmAP2-17 protein with its orthologous genes in other plant species. The phylogenetic tree was constructed using the neighbor-joining method with 1000 bootstrap replicates in MEGA 7.0 software. b Alignment of the SmAP2-17 protein with its orthologous genes in Salix, Populus, maize and Arabidopsis. There are two AP2 domains in the proteins, which were labeled with blue and pink lines, respectively. ANT is an Arabidopsis protein, whose gene locus is At3g47750. SapurV1A.0608s0160.1 and Potri.007G007400.1 come from Salix purpurea and Populus trichocarpa, respectively. GRMZM2G028151_T01 and LOC_Os03g56050.1 are proteins from maize and rice. Proteins with Sw, Sm, St, and Ss denote proteins that belong to Salix wilsonii, Salix matsudana, Salix integra and Salix suchowensis, respectively
Analysis of promoter elements of SmAP2-17 and its homolog genes
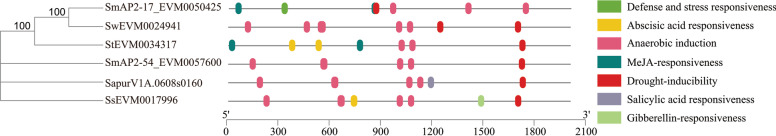
The 2000-bp promoter sequences of SmAP2-17 and its five homologs in Salix were isolated (File S2) and cis-element motif analysis was performed using the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). The anaerobic induction motif had the highest number and was present in the promoter region of all genes. The drought-inducibility motif also appeared in all genes, but most genes had only one (the only exception being SwEVM0024941, having two). Apart from the anaerobic induction motif and drought-inducibility motif, the abscisic acid responsiveness motif, defense and stress responsiveness motif, and MeJA responsiveness are also present in the promoter region of SmAP2-17 (the position of the abscisic acid responsiveness motif and MeJA-responsiveness motif almost overlapped, Table S1). The abscisic acid responsiveness motif was also found in StEVM0034317 and SsEVM0017996. The salicylic acid responsiveness motif and gibberellin-responsiveness motif were only found in SapurV1A.0608s0160 and SsEVM0017996, respectively (Fig. 2).
Fig. 2.
Cis-acting regulatory elements related to the stress and hormone response in the promoter regions of SmAP2-17 and its homolog genes from Salix. The cis-acting elements in the promoter of SmAP2-17 and its five homolog genes from Salix were predicted using the online PlantCARE tool and illustrated using TBtools software. Detailed information is provided in Table S1
Expression pattern and subcellular location
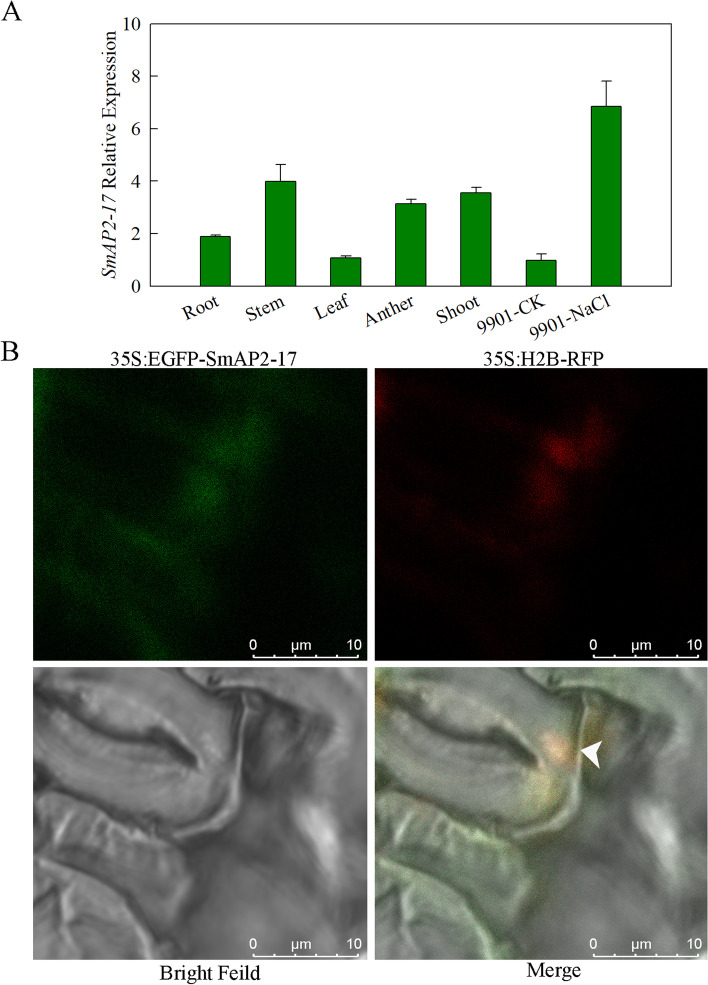
Gene expression patterns can provide useful clues to gene function. To study the expression profiles of SmAP2-17, the transcripts of SmAP2-17 across different tissues and under salt stress were examined using qRT-PCR. In different tissues (root, stem, leaf, shoot, and anther) of S. matsudana, the highest accumulation of SmAP2-17 was observed in the stems and shoots, followed by the roots and anther, while low expression levels were detected in the leaves. Salt stress induced the expression of SmAP2-17, with an expression level that was eightfold higher than normal conditions (Fig. 3a).
Fig. 3.
Expression pattern and subcellular localization of SmAP2-17 protein. a Expression patterns of SmAP2-17 in the roots, stems, leaves, anthers, and shoots of S. matsudana and under salt stress were measured using qRT-PCR. b Subcellular localization of the SmAP2-17 protein. The 35S:EGFP-SmAP2-17 fusion construct and the nucleus localization marker 35S:H2B-RFP construct were co-transformed into tobacco epidermal leaves. The arrowhead indicates the merged signal (yellow) with EGFP (green) and RFP (red) co-located in the nucleus. Scale bar, 10 μm
The subcellular localization of SmAP2-17 was examined to gain useful insights into its molecular function. WoLF PSORT (http://www.genscript.com/wolf-psort.html) predicted that SmAP2-17 resides in the nucleus. To confirm this prediction, the coding sequence of SmAP2-17 was fused to the C-terminus of green fluorescent protein (GFP) driven by the cauliflower mosaic virus 35S promoter, and the construct was transiently expressed in tobacco leaf epidermal cells. The expression of the red fluorescent protein (RFP) fusion histone protein, H2B-RFP, was used as a nuclear localization marker. As shown in Fig. 3b, a yellow fluorescence signal from the co-localization of SmAP2-17-GFP construct (green) and H2B-RFP (red) was observed in the nucleus of leaf stomata guard cells. This finding indicates that SmAP2-17 was localized in the nucleus (Fig. 3b).
SmAP2-17 did not confer transgenic Arabidopsis tolerance to salt stress due to salt-induced gene silencing
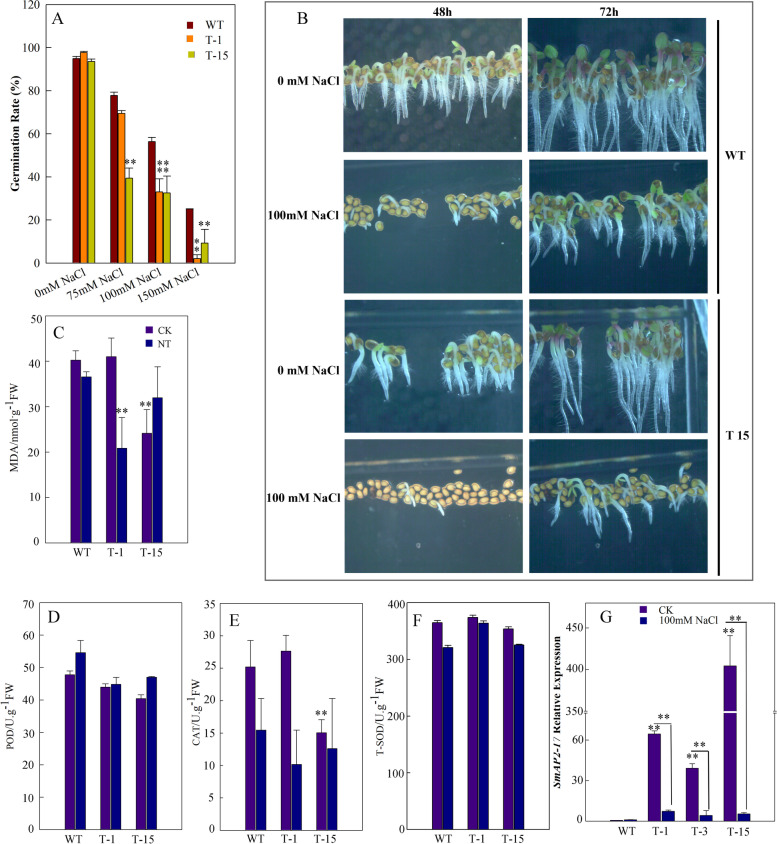
To characterize the role of SmAP2-17 in salt tolerance, the construct containing the SmAP2-17 coding sequence (CDS) driven by the 35S enhancer was transformed into Arabidopsis. After screening and molecular identification, two transgenic lines were used to perform different treatments of NaCl on 1/2 MS medium to determine the germination rates of Arabidopsis. Upon salt treatment using 75 mM NaCl, 100 mM NaCl, and 150 mM NaCl, the germination rates of the T1 and T15 transgenic lines at 72 h were significantly lower than those of the wild-type (WT) (Fig. 4a). The delayed germination phenotype could be observed clearly in photographs (Fig. 4b), which showed germination and growth phenotypes of WT and T15 at 48 h and 72 h after planting the seeds on 1/2 MS medium or 1/2 MS medium supplemented with 100 mM NaCl (Fig. 4b).
Fig. 4.
SmAP2-17-overexpression transgenic lines did not show increased tolerance to salt stress. a SmAP2-17-overexpression transgenic line T-15 had a delayed germination phenotype under salt stress compared to the WT (wild-type) at 48 h and 72 h after sowing. b Statistical analysis of the germination rate of WT and transgenic lines T-15 and T-1 at 72 h after sowing under different concentrations of NaCl (75 mM NaCl, 100 mM NaCl, and 150 mM NaCl). c qRT-PCR analysis of the expression levels of SmAP2-17 in WT and transgenic lines T-1 and T-15 under normal conditions and after treatment with 100 mM NaCl. c-f Physiological indices of transgenic and WT Arabidopsis seedlings under normal and salt stress conditions. The MDA content (c) and antioxidant enzyme activities, including peroxidase (d), catalase (e), and superoxide dismutase (f), were measured in WT and transgenic lines T-15 and T-1 under normal and salt stress conditions. Two-week-old seedlings were exposed to 200 mM NaCl for 4 d before the measurements. g qRT-PCR analysis of the expression levels of SmAP2-17 in WT and transgenic lines T-1 T-3, and T-15 under normal and salt stress (100 mM) conditions. Data represent the mean of three replicates, and the error bars indicate the SD; *P < 0.05 and **P < 0.01 by Student’s t-test
Salt stress can induce oxidative stress through the generation and accumulation of reactive oxygen species (ROS). Antioxidant enzymes, such as peroxidase (POD), superoxide dismutase (SOD), and catalase (CAT), play key roles in scavenging ROS and protecting plants from oxidative damage [30]. The malondialdehyde (MDA) content reflects the degree of lipid peroxidation and indirectly reflects the degree of cell damage [30]. The activities of these antioxidant enzymes and MDA content were also measured in the SmAP2-17-overexpression and WT plants. The results showed that there was no substantial difference in the activities of POD and SOD under normal conditions and after salt stress treatment. The MDA content was lower in T-15 under normal conditions and in T-1 under salt treatment. CAT activity was lower in T-15 than in WT and T-1, but no marked difference was observed under salt stress (Fig. 4c-f).
The data from lower CAT enzyme activity and higher MDA content were consistent with the salt sensitive phenotype of the overexpression plants, which did not exhibit increased salt tolerance. To determine the role of SmAP2-17, we detected the expression level of SmAP2-17 in overexpression plants after salt stress, and found that the expression level of SmAP2-17 decreased sharply in all lines (Fig. 4g). Considering that the higher level of SmAP2-17 expression in the SmAP2-17-overexpression was silenced after salt stress, we proposed that SmAP2-17 mRNA may be the target of a putative salt-induced miRNA molecule. Recently, a large number of abiotic stress-induced miRNAs, including the salt-induced miRNA gene ath-MIR167d, have been reported [31]. The secondary complementary structure of SmAP2-17 mRNA and a salt-induced miRNA ath-MIR167d suggest that SmAP2-17 mRNA may be digested through ath-MIR167d mediated RNAi process (Fig. S1).
Phenotypic and physiology analysis of codon-optimized SmAP2-17 gene overexpression transgenic lines
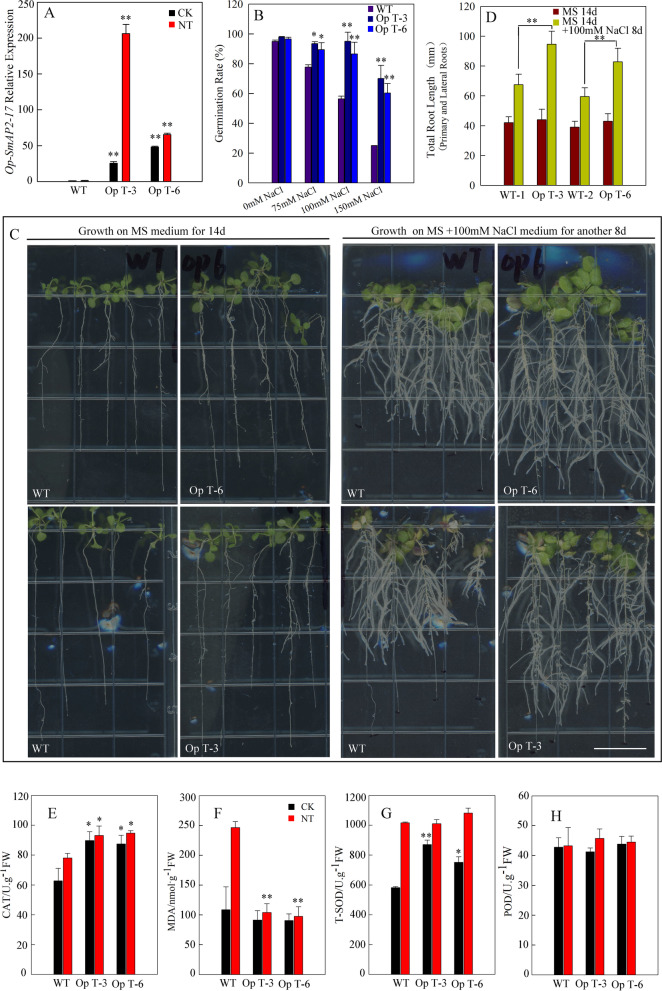
Based on our assumption, we optimized the SmAP2-17 mRNA codons of the ath-MIR167d binding site and placed the optimized CDS behind the 35S enhancer in the plant binary vector. The new SmAP2-17-overexpression construct was named Op- SmAP2-17 and transformed into Arabidopsis via the floral dip method. The Op- SmAP2-17-overexpression transgenic lines were screened and verified following the same protocol as the native SmAP2-17 gene. First, the expression level of Op- SmAP2-17 was detected in the WT and Op-SmAP2-17-overexpression transgenic lines Op T-3 and Op T-6 in 1/2 MS medium and medium containing 100 mM NaCl. The qRT-PCR results showed that salt stress did not decrease the expression level of Op-SmAP2-17 mRNA in Op T-3 and Op T-6 lines. In contrast, the expression level in Op T-3 increased by approximately tenfold when compared with that in WT control (Fig. 5a).
Fig. 5.
Effects of codon-optimized SmAP2-17-overexpression on salt stress tolerance in transgenic Arabidopsis. a qRT-PCR analysis of the expression levels of codon-optimized SmAP2-17-overexpression in WT and transgenic lines Op T-3 and Op T-6 under normal and salt stress (100 mM) conditions. b Statistical analysis of the germination rate of the WT and transgenic lines Op T-3 and Op T-6 at 72 h after sowing under different concentrations of NaCl (75 mM NaCl, 100 mM NaCl, and 150 mM NaCl). c Transgenic lines Op T-3 and Op T-6 had more and longer lateral roots than the WT. d Statistical comparison of the total root length (primary root + [lateral root number × average length of lateral roots]) between WT and transgenic lines Op T-3 and Op T-6. e–h Physiological indices of transgenic and WT Arabidopsis seedlings under normal and stress conditions. The MDA content (e) and antioxidant enzyme activities, including peroxidase (f), catalase (g), and superoxide dismutase (h), were measured in the WT and transgenic lines Op T-3 and Op T-6 under normal and salt stress conditions. Two-week-old seedlings were exposed to 200 mM NaCl for 4 d before the measurements. Data represent the mean of three replicates, and the error bars indicate the SD; *P < 0.05 and **P < 0.01 by Student’s t-test
The Op-SmAP2-17-overexpression transgenic lines Op T-3 and Op T-6 were subjected to 72 h of germination. The results showed that, contrary to the germination rate of native SmAP2-17 transgenic lines, Op T-3 and Op T-6 had higher germination rates than the WT when treated with 75 mM NaCl, 100 mM NaCl, and 150 mM NaCl (Fig. 5b). A higher difference was found after treatment with 150 mM NaCl, the germination rate of WT decreased to about 20%, while about 60% of the Op T-3 and Op T-6 seeds still could germinate (Fig. 5b). To further evaluate the salt tolerance phenotype of Op T-3 and Op T-6, the growth of these plants was assessed on MS medium containing 100 mM NaCl. After 14 days of growth on normal MS medium, the seedlings of the WT, Op T-3, and Op T-6 lines were transplanted to MS medium containing 100 mM NaCl and grown for another eight days. The results showed that after 8 days of treatment with 100 mM NaCl, the seedlings of Op T-3 and Op T-6 grew more and longer lateral roots than the WT (Fig. 5c). The number and length of the primary and lateral roots were counted, and the total root length was calculated (primary root + [lateral root number × average length of lateral roots]) for comparison (Fig. 5d). The total root length of Op T-3 and Op T-6 was found to increase by 40–50% compared to that of the WT (Fig. 5d).
With regards to the four physiological indexes, the CAT enzyme content in the two Op lines was higher than that in the WT after treatment with 200 mM NaCl (Fig. 5e). By contrast, the MDA content was lower than in the WT under the same conditions (Fig. 5f). The SOD enzyme content in the two Op lines was higher than that in the WT under normal conditions, whereas no difference was found in these lines under salt stress (Fig. 5g). There was no difference in the POD content under both the normal and salt stress conditions (Fig. 5h).
Analysis of gene expression pattern of general stress marker genes and salt stress-related genes in SmAP2-17-overexpression transgenic lines
Plants can activate many transcriptional and physiological processes in order to grow and survive under conditions of salt and abiotic stress. Several genes have regulatory roles and positive effects on abiotic stress tolerance, including stress-responsive marker genes (DREB2A, RD20, ABI5, RD29A, ATGolS2, ABF3, AtMYB2, and RD29B) and salinity-responsive genes (AtNXH1, AtNXH2, AtHKT1, RBohF, and AtSOS3), which play important roles in helping plants to cope with and survive under conditions of salt stress [32–36].
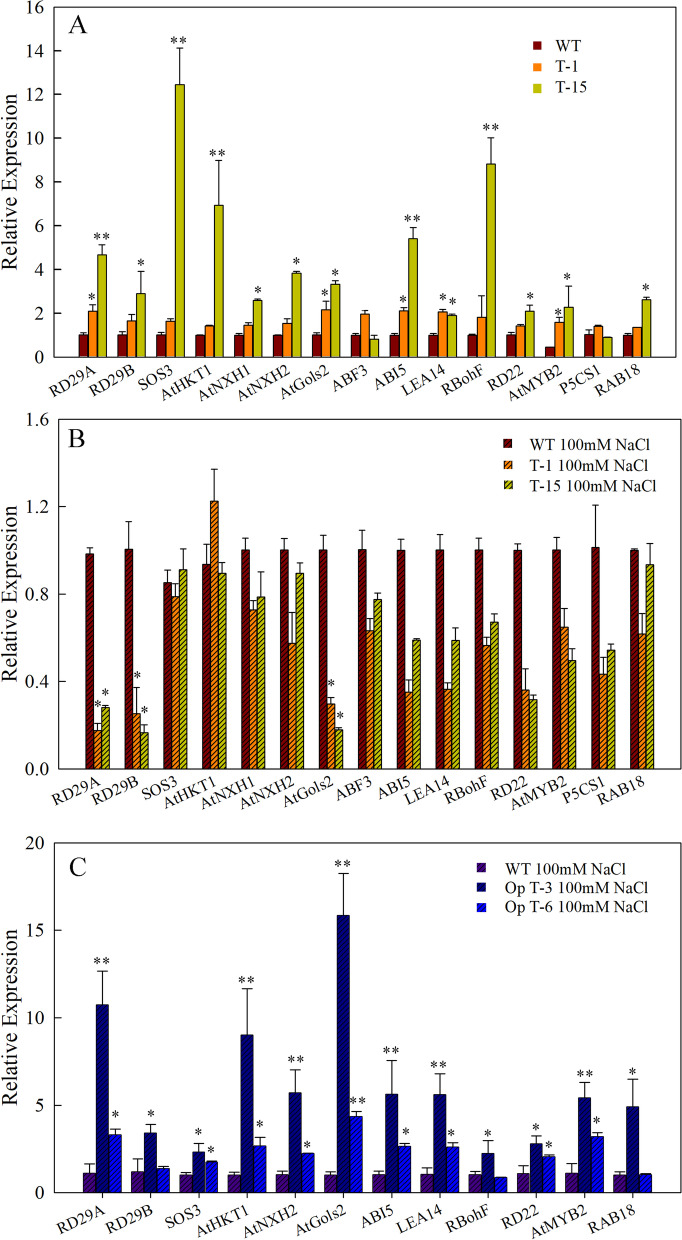
To further explore the downstream genes regulated by SmAP2-17 under salt stress, the expression patterns of stress/salt-responsive genes were evaluated by qRT-PCR. In the native SmAP2-17-overexpression transgenic lines, under normal conditions, many genes were found to be upregulated, especially in T-15, which expressed SmAP2-17 at extremely high levels. The expression levels of 13 out of 15 genes we detected were upregulated by over two-fold as compared to those in the WT (Fig. 6a). The top five genes with the highest increase in expression were RD29A, ABI5, SOS3, AtHKT1, and RBohF. In the transgenic line T-15, the expression level of SOS3 was over 12-fold higher than that in the WT (Fig. 6a). Interestingly, these five genes were induced with much higher expression levels than those in T-1, and the expression level of these genes was consistent with the higher expression of SmAP2-17 in T-15 (Fig. 6a).
Fig. 6.
Relative expression levels of stress responsive marker genes and salinity responsive genes in WT and transgenic lines under normal conditions and after treatment with NaCl. a Relative expression levels of 15 genes in the WT and transgenic lines T-1 and T-15 under normal conditions. b Relative expression levels of 15 genes in the WT and transgenic lines T-1 and T-15 treated with 200 mM NaCl for 24 h. c Relative expression levels of 12 genes in the WT and transgenic lines Op T-3 and Op T-6 treated with 200 mM NaCl for 24 h. Data represent the mean ± SD of three biological replicates. *P < 0.05 and **P < 0.01 by Student’s t-test
After treatment with 100 mM NaCl, the expression patterns of these genes in T-1 and T-15 were also detected by qRT-PCR. Following the gene silencing of SmAP2-17 after salt stress, the expression of all genes detected was inhibited with expression levels that were lower or the same under the salt stress treatment (Fig. 6b).
In the Op T-3 and Op T-6 overexpression transgenic lines with optimized codons of SmAP2-17 CDS, the expression patterns of 12 genes were examined after salt stress. The results showed that these genes in T-1 and T-15 were not inhibited in the Op T-3 and Op T-6 lines. All of the genes had a higher expression level than that in the WT, and the expression level of these genes was consistent with the higher expression level of Op-SmAP2-17 in Op T-3 (Fig. 6c).
Promoter motif analysis on putative downstream gene of SmAP2-17
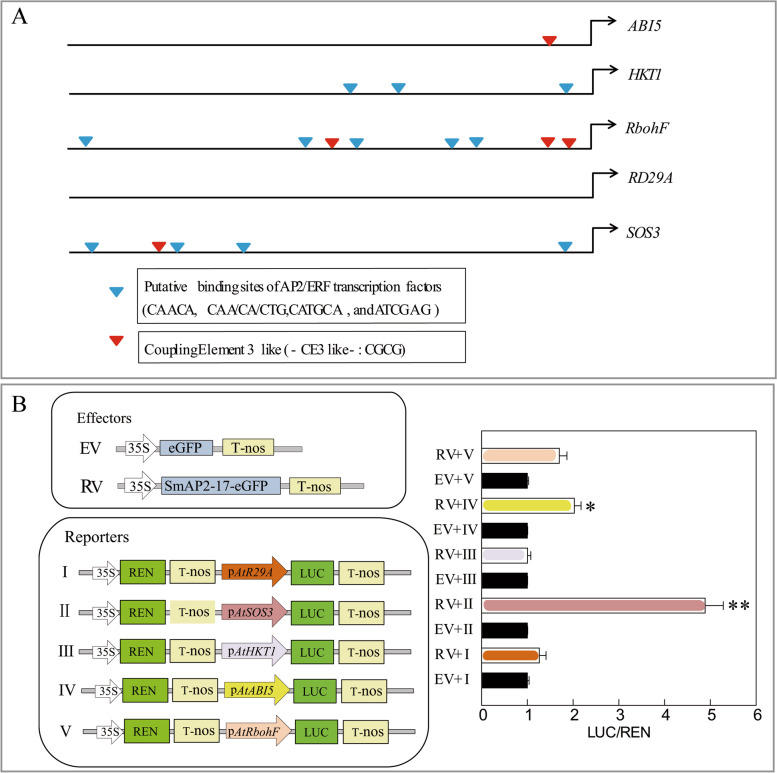
Based on the expression pattern of these genes, consistent with the expression level of SmAP2-17 in T-15 and Op-SmAP2-17 in OpT-3, we selected five genes as putative downstream genes of SmAP2-17: ABI5, HKT1, RbohF, RD29A, and SOS3. We downloaded the 2000-bp promoter sequence of the five genes and performed motif analysis using the PlantCARE platform. The putative binding site motifs of AP2/ERF transcription factors (including CAACA, CAA/CA/CTG, CATGCA, and ATCGAG) and coupling element 3-like (CE3-like) (CGCG) were identified in the promoters of these five genes (Fig. 7a). No AP2 binding and CE3-like motifs were found in the RD29A gene promoter, while only one CE3-like motif was identified in the ABI5 gene promoter and three AP2 binding motifs were present on the HKT1 gene promoter. RbohF had the highest number of these motifs, including five AP2 binding motifs and three CE3-like motifs. The last one gene, SOS3, had four AP2 binding motifs and one CE3-like motif (Fig. 7a).
Fig. 7.
SOS3 and ABI5 expression is activated in the presence of SmAP2-17 as measured by the luciferase reporter assays. a Schematic diagrams of the putative AP2/ERF binding elements in the 2000-bp promoter region of the five candidate downstream genes. b Left panel: Schematic diagrams of effector and reporter plasmids used in transient transactivation assays: top, schematic diagrams of control (EV, empty vector) and effector (RV, recombination vector); bottom, schematic diagrams of reporter constructs. The promoter sequences from five candidate downstream genes were cloned upstream of the LUC reporter gene. The REN located downstream of the 35S promoter was used as an internal control. Right panel: The results showed that five candidate downstream genes, SOS3 and ABI5, were regulated by SmAP2-17. Relative activity was calculated as LUC/REN, but was normalized to the reporter only. Data represent the mean ± SD of three biological replicates. *P < 0.05 and **P < 0.01 by Student’s t-test
SmAP2-17 binds the promoter region of SOS3 and ABI5 and drives the expression of the Luc gene
Next, a dual luciferase reporter assay was used to determine whether these five genes were the direct downstream genes of SmAP2-17. The 2000-bp promoter sequences of ABI5, HKT1, RbohF, RD29A, and SOS3 were cloned upstream of the LUC gene in the pGreenII 0800-LUC vector. REN was used as an internal control, and a SmAP2-17-overexpression vector was also constructed. The SmAP2-17-overexpression vector construct and each gene promoter::LUC construct were co-transformed into tobacco leaf cells via agrobacteria-mediated transformation. The signal intensities of LUC and REN were detected, and the LUC/REN ratios were calculated. The results showed that from the five genes analyzed, SmAP2-17 could bind to the promoter region of SOS3 and ABI5 and activate the expression of the downstream LUC gene (Fig. 7b). In the ectopic system, these results showed that SmAP2-17 may directly bind to the upstream regulatory region and upregulate the expression levels of SOS3 and ABI5, thereby enhancing the salt tolerance of the SmAP2-17-overexpression transgenic lines.
Discussion
The APETALA2/ethylene response factor (AP2/ERF) transcription factor (TF) is a superfamily of plant species that plays multiple roles in plant growth and development, stress tolerance, and the integration of phytohormonal signals [3]. Since the first AP2/ERF TF AP2 was identified in Arabidopsis as a controller in flower and seed development, several genes of the DREB and ERF subfamily have been explored for their molecular roles and mechanisms, particularly in biotic and abiotic stresses [9, 11, 14, 37–39]. Previous studies on the function of AP2/ERF have mainly focused on model plants and crops, and some studies have explored the AP2/ERF genes of trees, mainly from Populus and Broussonetia papyrifera [40–43]. Salix matsudana (Koidz.) is an important and widely cultivated ornamental tree species [28]. As a result of the genome-wide analysis of AP2/ERF family members of Salix matsudana (Koidz.), the differential expression patterns of some SmAP2/ERF genes under salt stress have been investigated [29]. However, little is known about the functional roles of SmAP2/ERF and their mechanisms in Salix. Transferring a single gene encoding stress-tolerance transcription factors into plants could improve plant drought, salt, and freezing tolerance. Therefore, uncovering their roles in Salix salt stress tolerance is important for the creation of new germplasms and the provision of environmental protection in the future [44].
SmAP2-17 may serve as a TF and play roles in salt stress tolerance
In this study, SmAP2-17 encoding a TF protein of the SmAP2/ERF family was cloned from S. matsudana to determine its potential role in Salix resistance to salt stress. The phylogenetic tree analysis of SmAP2-17 and other AP2 subfamily proteins from maize, rice, Arabidopsis, and other Salix species showed that the SmAP2-17 protein was more similar to members of the Salix genus, especially SwEVM0024941 and SiEVM0034317 from S. wilsonii and S. integra, respectively (Fig. 1a). All of the members of the Salix genus showed 40% identity to Arabidopsis ANT protein, which plays roles both in plant development and abiotic stress tolerance [26, 45]. Furthermore, the multiple alignment of amino acid sequences indicated that SmAP2-17 protein had two conserved AP2 domains in the central region with characteristics similar to those of other SmAP2 proteins and Arabidopsis ANT (Fig. 1b). SmAP2-17 was a ubiquitously expressed gene detected in all tissues examined, whose expression could be highly induced by salt stress (Fig. 3a). Colocalization with the nuclear marker histone H2B showed that the SmAP2-17 protein was localized to the nucleus (Fig. 3b). Promoter motif analysis revealed TC-rich repeats and ABRE elements, which are likely to function in defense, stress responsiveness, and abscisic acid responsiveness, respectively. Among the six homolog genes from Salix, SmAP2-17 was the only gene with TC-rich repeat elements (Fig. 2). These results suggest that SmAP2-17 may serve as a TF and play an important role in salt stress tolerance.
Analysis of salt resistance in SmAP2-17-overexpression transgenic Arabidopsis
To determine whether SmAP2-17 is involved in regulating the salt stress response, overexpression transgenic lines were obtained and their salt tolerance abilities were studied. The results of phenotype analysis and physiological index showed that, under salt stress, the overexpression transgenic lines did not possess higher salt tolerance ability. These results implied that SmAP2-17 was not a positive regulator in the salt stress response. However, the expression level of SmAP2-17 in T-1 and T-15 after salt stress did not support this conclusion (Fig. 4). The qRT-PCR results showed that, after salt treatment, the high levels of SmAP2-17 expression in T-1 and T-15 fell sharply; therefore, SmAP2-17 could not work properly after salt stress (Fig. 4g). The reduced expression level of SmAP2-17 may not be the consequence of post-transcriptional silencing because of its higher expression level, since under normal conditions, silence phenomena did not occur. Thus, we proposed that SmAP2-17 may be the target of a salt-induced microRNA, with studies previously reporting several salt-induced microRNA genes in Arabidopsis [31]. Through the analysis of RNA secondary structures, we found that ath-MIR167d can bind to SmAP2-17 mRNA to initiate mRNA degradation (Fig. S1).
To provide support for our proposition and to further clarify the function of SmAP2-17, another version of SmAP2-17 with an optimized codon at the binding site was introduced into Arabidopsis, revealing two new overexpression transgenic lines, Op T-3 and Op T-6. Phenotype analysis and physiological index measurement provided evidence that SmAP2-17 played a positive role in salt stress tolerance and that the overexpression of SmAP2-17 in Arabidopsis could lead to a higher tolerance to conditions of salt stress (Fig. 5).
To obtain the overexpression transgenic lines, the cauliflower mosaic virus (CaMV) 35S promoter was used. The 35S promoter is the most frequently used promoter, with a strong and constitutive expression pattern [46]. Although it functions as a constitutive expression pattern, the 35S promoter has variable expression patterns in different organs of Arabidopsis thaliana and in response to abiotic stress [46, 47]. However, the phenomena reported in our study on transgenic lines, namely that salt stress induced a sharp miRNA-mediated reduction in the expression of SmAP2-17, has not been previously reported.
With regards to the increased sensitivity of the T-1 and T-15 transgenic lines to salt stress, although we were unable to provide a reasonable explanation, the data of four physiological indexes and the downregulation of stress marker genes were all in line with the salt-sensitive phenotype (Fig. 4, Fig. 6b).
SmAP2-17 may directly regulate the expression of SOS3 and ABI5
Some studies have demonstrated that several AP2 TF genes enhance tolerance to salt stress through the regulation of stress/ABA-responsive genes and genes from the salt stress signaling regulatory network [11, 26, 41, 48]. To gain insights into the molecular functions of SmAP2-17 in salt stress tolerance, we detected several well-characterized stress-responsive marker genes and salt stress in transgenic lines (Fig. 6). The qRT-PCR results of gene expression showed that 13 out of the 15 genes detected were upregulated in transgenic lines. Five genes, including RD29A, ABI5, SOS3, AtHKT1, and RBohF, were significantly upregulated, and the expression levels of these genes were consistent with the expression levels of SmAP2-17 in different transgenic lines and lines treated with salt stress (Fig. 6a, Fig. 4g). In T-15, which had the highest SmAP2-17 expression level, the expression levels of five genes were also the highest. After salt stress in T15, the expression of SmAP2-17 decreased sharply, followed by that of the marker genes mentioned above (Fig. 6b). In the Op T-3 and Op T-6 plants, salt treatment did not influence the expression level of SmAP2-17, and the expression of RD29A, ABI5, SOS3, AtHKT1, and RBohF remained at a higher level (Fig. 6c).
The expression levels of these genes were consistent with those of SmAP2-17, which suggested that SmAP2-17 may regulate the expression of these genes directly or indirectly. In the promoter/regulatory sequence of representative target genes of the AP2/ERF proteins, many conserved binding motifs, including GCC-box (AGCCGCC), DRE/CRT elements, and coupling elements 3-like (CE3-like) (CGCG) exist [48–50]. We detected the cis-elements in the promoter region of five genes and found that, except for the RD29A gene promoter, at least one or several motifs were found in the regulatory region of four other genes (Fig. 7a). The results of a dual luciferase reporter assay showed that SmAP2-17 binds to the promoter region of SOS3 and ABI5 and activates the expression of the downstream LUC gene (Fig. 7b). These results indicate that SOS3 and ABI5 are the direct downstream genes of SmAP2-17 in transgenic Arabidopsis. SOS3 is a component of the salt-overly sensitive (SOS) pathway; it could physically interact with the Thr/Ser protein kinase SOS2. Then SOS2 phosphorylated SOS1 after being recruited to the plasma membrane (PM). SOS1 is a PM Na+/H+ antiporter responsible for excluding Na+ from the cytosol to the apoplast and the soil solution to mitigate salt stress [51, 52]. ABI5 encodes a member of the basic leucine zipper transcription factor family. It involves in ABA signaling during seed maturation and germination and also plays a positive role in drought and high-salinity conditions [53, 54]. By upregulating the expression levels of SOS3 and ABI5, the overexpression of SmAP2-17 in Arabidopsis improves the tolerance of transgenic plants to salt stress by inducing ABA signaling and regulating salt stress-related genes.
Conclusions
In this study, SmAP2-17 was cloned from Salix matsudana, and its function in salt tolerance was determined. By upregulating SOS3 and ABI5, SmAP2-17 was found to play a positive role in salt stress tolerance, and the overexpression of SmAP2-17 in Arabidopsis could increase the tolerance of Arabidopsis. Our results provide a basis for the further functional characterization of SmAP2-17 in Salix and the potential use of this salt-resistant gene in the creation of new Salix germplasms.
Methods
Plant materials, growth conditions, and treatments
Arabidopsis thaliana ecotype Col-0 was obtained from Arabidopsis Biological Resource Center with permission and used for the genetic transformation of the SmAP2-17 gene. Sterilized Arabidopsis seeds of the Col-0 (WT) and transgenic lines were sown on the agar medium of 1/2 Murashige and Skoog (MS) agar medium, and vernalized in the dark for 2 days at 4 °C. The seedlings were then cultured under long-day conditions (16 h light/8 h dark cycle) at 22 °C. For transgenic line screening, 25 mg/L hygromycin was added to the medium; for salt stress treatment with different concentrations of NaCl, different amounts of NaCl were dissolved into the medium. After growing for 2 weeks on medium, the seedlings were transplanted into soil and cultured in a greenhouse at 22 °C under long-day conditions. The 5-week-old plants were watered with 200 mM NaCl to mimic the salt stress treatment. After treatment for 72 h, leaf samples of Arabidopsis were collected and used to measure the levels of antioxidant enzyme activity and the MDA content.
The salix matsudana cultivar ‘9901’ was used in this study. ‘9901’ willow, a fine and widely planted variety of willow, was selected by many willow experts from Shandong Academy of Forestry. ‘9901’ willow was introduced and cultivated in the botany Garden of Nantong University and authorized for only scientific research purpose. One-year-old, 10-cm long cuttings of Salix matsudana ‘9901’clone were planted in Hoagland solution for 20 days to induce shoots and roots. The cuttings with shoots and roots were transferred to a solution of 150 mM NaCl and cultured for 24 h. Willow samples from different organs and root samples after salt stress were collected and used for RNA extraction.
Cloning and sequence analysis of SmAP2-17
The coding sequence of SmAP2-17 was amplified from the cDNA of S. matsudana using gene-specific primers (Table S2) and ligated into the pGEM-T vector through T-A cloning. Lasergene software was used to deduce the amino acid sequence of SmAP2-17. Arabidopsis ANT protein and other homologous proteins from Salix purpurea, Populus trichocarpa, maize, rice and other Salix genus were obtained by blasting against proteins datasets downloaded from the Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html) and NCBI (https://www.ncbi.nlm.nih.gov/) websites. The sequence alignment of these proteins was performed using the ESPript website (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_clustalw.html). MEGA 6.0 was used to construct a phylogenetic tree of SmAP2-17 and other plant ANT-Like proteins using the neighbor-joining (NJ) method [55]. The cis-acting elements in the promoter of SmAP2-17 and its five homolog genes from the Salix genus were predicted using the online PlantCARE tool (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) and illustrated using TBtools software [56, 57]. The coding sequences and promoter sequences of SmAP2-17 and its homologs are provided in File S1 and S2.
Plasmid construction and transformation of SmAP2-17 in Arabidopsis
The full-length coding sequence of SmAP2-17 (File S1) amplified from pGEM-T-SmAP2-17 using specific primers listed in Table S2 were inserted into the pWM101 vector using Hieff Clone™ Plus One Step Cloning recombination Kit (Yeasen, Shanghai). The SmAP2-17 cDNA was cloned under the control of the cauliflower mosaic virus 35S (CaMV 35S) promoter, the construct was named pWM101-35S:SmAP2-17. The optimized codon of the SmAP2-17 CDS sequence (File S1) was synthesized by General Biology Company (Hefei, China), and the same protocol was used to obtain the pWM101-35S:Op-SmAP2-17 construct.
The pWM101-35S:SmAP2-17 and pWM101-35S:Op-SmAP2-17 plasmids were then transformed into Agrobacterium tumefaciens GV3101 competent cells by electroporation, and Arabidopsis genetic transformation was performed using the floral dip method [58]. All SmAP2-17/Op-SmAP2-17-overexpression transgenic seedlings were screened using 25 mg/L hygromycin to obtain positive plants. These plants were then further verified by genotyping and expression level detection.
Subcellular localization of SmAP2-17
Using the one-step cloning method, the complete coding sequence of SmAP2-17 amplified by PCR using specific primers was inserted into the pCAMBIA2300-35S:NEGFP vector under the control of the CaMV 35S promoter to generate an in-frame fusion construct, 35S:EGFP-SmAP2-17. As a positive nuclear marker, the cDNA of a previously characterized nuclear histone protein, H2B, was fused to the mCherry gene to generate 35S:H2B-mCherry. The constructs of 35S:H2B-mCherry and 35S:EGFP-SmAP2-17 were co-transformed into Agrobacterium tumefaciens strain GV3101 (pSoup-19) using the heat shock method. Agroinfiltration was performed as previously reported [59]. Recombination agrobacteria were suspended in 10 mM MgCl2 and 150 mg/l acetosyringone. The cells were allowed to stand in this medium for 3 h, and then infiltrated into the abaxial air spaces of young leaves of 2- to 4-week-old N. benthamiana plants using a syringe with the needle removed. The agroinfiltrated tobacco leaves after 48 h agro-infiltration were photographed using a confocal laser scanning microscope (Leica, Germany).
RNA isolation and real-time qRT-PCR analysis
Total RNA was extracted from different organs of S. matsudana or Arabidopsis seedlings using the TaKaRa MiniBEST Plant RNA Extraction Kit (Takara, Beijing, China). Reverse transcription for the first strand cDNA synthesis was performed with 3 µg of total RNA using PrimeScript™ RT reagent Kit with gDNA Eraser (Perfect Real Time) (Takara, Beijing, China). The qRT-PCR experiments were performed on an ABI 7500 Real-Time PCR system (Applied Biosystems, USA) with TB Green™ Premix Ex Taq™ II (Tli RNaseH Plus) (Takara, Beijing, China). The actin genes AtActin2 (AT3G18780) and SpActin1 (SapurV1A.0655s0050.1) from Arabidopsis and S. matsudana were used as internal reference genes to normalize the data. The expression level differences between different tissues, samples, and treatments were evaluated using the 2−ΔΔCt method, as previously described [60]. Three independent biological repeats were performed to ensure an accurate statistical analysis. The specific primers used are listed in Table S2.
Dual luciferase reporter assays
To verify the interaction between SmAP2-17 and the putative AP2/ERF binding sites in the promoter sequences of five genes, including RD29A, ABI5, SOS3, AtHKT1, and RBohF, luciferase reporter assays were conducted in the model plant species tobacco via transient expression as previously reported [61, 62]. The PCR-amplified 2000-bp promoter sequences from five genes were cloned into pGreenII 0800-LUC vectors to obtain reporter plasmids. The cloning procedure was the same as the SmAP2-17 CDS cloning procedure, that is, through T-A cloning and one-step recombination cloning. The construct pCAMBIA2300-35S:SmAP2-17-NEGFP was used as an effector construct, while the pCAMBIA2300-35S:NEGFP construct was used as a negative effector control. The effector/negative effector control and reporter constructs were subsequently transferred into Agrobacterium tumefaciens strain GV3101 (P19). The recombinant A. tumefaciens strains were cultured to an OD600 of 1.0, and strains with effector/negative effector control and reporter constructs were mixed in a 1:1 proportion. After sedimentation, the confluent bacterium was re-suspended in 10 ml of infiltration media (10 mM MgCl2, 0.5 M acetosyringone) to an OD600 of 0.2, and incubated at room temperature without shaking for 2 h. The tobacco leaves of 2- to 4-week-old plants were then infiltrated using the virus genes activated GV3101 (P19) using a needle-free syringe. After infiltration, the tobacco plants were incubated in an illuminated chamber for 48–72 h at 25 °C, a Dual-Luciferase Reporter Assay System (Promega, USA) was used to determine LUC and REN luciferase activities, according to the manufacturer’s instructions.
Analysis of salt tolerance
Sterilized seeds from the WT, SmAP2-17-overexpression, and codon-optimized SmAP2-17-overexpression plants were sown on 1/2 MS solid medium (control) or 1/2 MS medium containing different concentrations of NaCl (75 mM, 100 mM, 150 mM) to measure the germination rate after 72 h of culture. For the root length phenotype, the seeds of the WT and codon-optimized SmAP2-17-overexpression plants were sown on 1/2 MS solid medium and cultured for 14 days. Then, seedlings with the same length were transferred to 1/2 MS solid medium containing 100 Mm NaCl. After 8 days, the lengths of the roots of the WT and transgenic seedlings were compared. Two-week-old seedlings of the WT, SmAP2-17-overexpression, and codon-optimized SmAP2-17-overexpression lines were transplanted into soil from 1/2 MS medium and watered for two weeks before the stress treatment experiments. For salt stress, the seedlings were irrigated with 30 mL of 200 mM NaCl solution once every two days, or with pure water as a control. After 96 h, the physiological parameters, including MDA content and levels of antioxidant enzyme activity (e.g. peroxidase (POD), superoxide dismutase (SOD), and catalase (CAT)) were measured using the corresponding kits, according to the manufacturer’s instructions (Solarbio, Beijing, CN). All experiments included three biological replicates.
Supplementary Information
Additional file 1: Table S1. Cis-element motifs in the promoter region of SmAP2-17 and its homologous genes.
Additional file 2: File S1. Protein sequences of SmAP2-17 and its homolog genes.
Additional file 3: File S2. Promoter sequences of SmAP2-17 and its homolog genes.
Additional file 4: Table S2. List of primers used.
Additional file 5: Fig. S1. The secondary complementary structure of SmAP2-17 mRNA and salt-induced miRNA ath-MIR167d.
Acknowledgements
We thank editing service from Editage Company (https://www.editage.cn) for professional scientific editing the English text of a draft of this manuscript.
Abbreviations
- AP2/ERF
APETALA2/ethylene responsive factor
- ORF
Open reading frame
- CDS
Coding sequence
- TF
Transcription factor
- GFP
Green fluorescent protein
- RFP
Red fluorescent protein
- SOD
Superoxide dismutase
- POD
Peroxidase
- MDA
Malondialdehyde
- CAT
Catalase
- WT
Wild-type
- qRT-PCR
Quantitative reverse transcription polymerase chain reaction
- LUC
Luciferase
- REN
Renilla
- CaMV
Cauliflower mosaic virus
- MS
Murashige and Skoog
Authors’ contributions
Y.C. and J.Z. designed the study. Y.C., Y.D., Y.L., J.Y., Y.J., G.L., C.Y., F.Z., and B.L. performed the experiments. Y.C. generated all the constructs used in the Arabidopsis studies. Y.D. and Y.J. participated in generating and screening all the transgenic materials. Y.D. and Y.J. participated in phenotype and physiological analyses; Y.D. participated in the expression pattern analysis and subcellular localization. G.L. and C.Y performed sequence analysis, alignment, and motif prediction; F.Z. performed the qRT-PCR experiments; Y.C. and B.L. generated all constructs used in promoter activity analysis; Y.J. participated in the luciferase reporter assays. Y.L. carried out tissue culture and willow transformation; J.Y. performed genotype identification and expression pattern analysis on transgenic calli. Y.C. and J.Z. wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported financially by the National Natural Science Foundation of China (31971681). The supporters had no role in study design, data collection, data analysis, data interpretation, the writing of the manuscript or decision to publish.
Availability of data and materials
Proteins and nucleotides sequences in this study were downloaded from the Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html) and NCBI (https://www.ncbi.nlm.nih.gov/) websites and analyzed using ESPript (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_clustalw.html) and PlantCARE tool (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) websites. Sequence data of proteins and promoters described in this article are available using the accession numbers listed in Supplementary File S1 and S2 respectively. The datasets supporting the conclusions of this article are included within the article and its additional files, and available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
There are no ethical issues to report.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
The original online version of this article was revised: it was noted that due to a typesetting error Figure 3 and Figure 6 were captured incorrectly.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanhong Chen and Yuanhao Dai contributed equally to this work.
Change history
4/11/2022
A Correction to this paper has been published: 10.1186/s12870-022-03552-6
References
- 1.Xie Z, Nolan TM, Jiang H, Yin Y. AP2/ERF Transcription Factor Regulatory Networks in Hormone and Abiotic Stress Responses in Arabidopsis. Front Plant Sci. 2019;10:228. doi: 10.3389/fpls.2019.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakano T, Suzuki K, Fujimura T, Shinshi H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 2006;140(2):411–432. doi: 10.1104/pp.105.073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu C, Guo ZH, Hao PP, Wang GM, Jin ZM, Zhang SL. Multiple regulatory roles of AP2/ERF transcription factor in angiosperm. Bot Stud. 2017;58(1):6. doi: 10.1186/s40529-016-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutterson N, Reuber TL. Regulation of disease resistance pathways by AP2/ERF transcription factors. Curr Opin Plant Biol. 2004;7(4):465–471. doi: 10.1016/j.pbi.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal PK, Agarwal P, Reddy MK, Sopory SK. Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep. 2006;25(12):1263–1274. doi: 10.1007/s00299-006-0204-8. [DOI] [PubMed] [Google Scholar]
- 6.Harrop TWR, Mantegazza O, Luong AM, Béthune K, Lorieux M, Jouannic S, Adam H. A set of AP2-like genes is associated with inflorescence branching and architecture in domesticated rice. J Exp Bot. 2019;70(20):5617–5629. doi: 10.1093/jxb/erz340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akhtar M, Jaiswal A, Taj G, Jaiswal JP, Qureshi MI, Singh NK. DREB1/CBF transcription factors: their structure, function and role in abiotic stress tolerance in plants. J Genet. 2012;91(3):385–395. doi: 10.1007/s12041-012-0201-3. [DOI] [PubMed] [Google Scholar]
- 8.Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim Biophys Acta. 2012;1819(2):86–96. doi: 10.1016/j.bbagrm.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal PK, Gupta K, Lopato S. Agarwal P Dehydration responsive element binding transcription factors and their applications for the engineering of stress tolerance. J Exp Bot. 2017;68(9):2135–2148. doi: 10.1093/jxb/erx118. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Y, Chen M, Guo J, Wang Y, Min D, Jiang Q, Ji H, Huang C, Wei W, Xu H, et al. Overexpression of soybean DREB1 enhances drought stress tolerance of transgenic wheat in the field. J Exp Bot. 2020;71(6):1842–1857. doi: 10.1093/jxb/erz569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu J, Zhu C, Wang C, Liu L, Shen Q, Xu D, Wang Q. Maize transcription factor ZmEREB20 enhanced salt tolerance in transgenic Arabidopsis. Plant Physiol Biochem. 2021;159:257–267. doi: 10.1016/j.plaphy.2020.12.027. [DOI] [PubMed] [Google Scholar]
- 12.Zhang XX, Tang YJ, Ma QB, Yang CY, Mu YH, Suo HC, Luo LH, Nian H. OsDREB2A, a rice transcription factor, significantly affects salt tolerance in transgenic soybean. PLoS One. 2013;8(12):e83011. doi: 10.1371/journal.pone.0083011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donde R, Gupta MK, Gouda G, Kumar J, Vadde R, Sahoo KK, Dash SK, Behera L. Computational characterization of structural and functional roles of DREB1A, DREB1B and DREB1C in enhancing cold tolerance in rice plant. Amino Acids. 2019;51(5):839–853. doi: 10.1007/s00726-019-02727-0. [DOI] [PubMed] [Google Scholar]
- 14.Mao L, Deng M, Jiang S, Zhu H, Yang Z, Yue Y, Zhao K. Characterization of the DREBA4-Type Transcription Factor (SlDREBA4), Which Contributes to Heat Tolerance in Tomatoes. Front Plant Sci. 2020;11:554520. doi: 10.3389/fpls.2020.554520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang Y, Liu K, Zhang J, Li X, Xu K, Zhang Y, Qi J, Yu D, Wang J, Li C. JcDREB2, a Physic Nut AP2/ERF Gene, Alters Plant Growth and Salinity Stress Responses in Transgenic Rice. Front Plant Sci. 2017;8:306. doi: 10.3389/fpls.2017.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang Y, Li X, Zhang D, Gao B, Yang H, Wang Y, Guan K, Wood AJ. ScDREB8, a novel A-5 type of DREB gene in the desert moss Syntrichia caninervis, confers salt tolerance to Arabidopsis. Plant Physiol Biochem. 2017;120:242–251. doi: 10.1016/j.plaphy.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Hattori Y, Nagai K, Furukawa S, Song XJ, Kawano R, Sakakibara H, Wu J, Matsumoto T, Yoshimura A, Kitano H, et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature. 2009;460(7258):1026–1030. doi: 10.1038/nature08258. [DOI] [PubMed] [Google Scholar]
- 18.Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature. 2006;442(7103):705–708. doi: 10.1038/nature04920. [DOI] [PubMed] [Google Scholar]
- 19.Hinz M, Wilson IW, Yang J, Buerstenbinder K, Llewellyn D, Dennis ES, Sauter M, Dolferus R. Arabidopsis RAP2.2: an ethylene response transcription factor that is important for hypoxia survival. Plant Physiol. 2010;153(2):757–72. doi: 10.1104/pp.110.155077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwase A, Mitsuda N, Koyama T, Hiratsu K, Kojima M, Arai T, Inoue Y, Seki M, Sakakibara H, Sugimoto K, et al. The AP2/ERF transcription factor WIND1 controls cell dedifferentiation in Arabidopsis. Curr Biol. 2011;21(6):508–514. doi: 10.1016/j.cub.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Patil V, McDermott HI, McAllister T, Cummins M, Silva JC, Mollison E, Meikle R, Morris J, Hedley PE, Waugh R, et al. APETALA2 control of barley internode elongation. Development. 2019;146(11):dev170373. doi: 10.1242/dev.170373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duran-Medina Y, Serwatowska J, Reyes-Olalde JI, de Folter S, Marsch-Martinez N. The AP2/ERF Transcription Factor DRNL Modulates Gynoecium Development and Affects Its Response to Cytokinin. Front Plant Sci. 2017;8:1841. doi: 10.3389/fpls.2017.01841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Chen F, Li Y, Li P, Wang Y, Mi G, Yuan L. ZmRAP2.7, an AP2 transcription factor, is involved in maize brace roots development. Front Plant Sci. 2019;10:820. doi: 10.3389/fpls.2019.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun W, Gao D, Xiong Y, Tang X, Xiao X, Wang C, Yu S. Hairy Leaf 6, an AP2/ERF Transcription Factor, Interacts with OsWOX3B and Regulates Trichome Formation in Rice. Mol Plant. 2017;10(11):1417–1433. doi: 10.1016/j.molp.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Debernardi JM, Greenwood JR, Jean Finnegan E, Jernstedt J, Dubcovsky J. APETALA 2-like genes AP2L2 and Q specify lemma identity and axillary floral meristem development in wheat. Plant J. 2020;101(1):171–187. doi: 10.1111/tpj.14528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng LS, Wang YB, Yao SQ, Liu A. Arabidopsis AINTEGUMENTA mediates salt tolerance by trans-repressing SCABP8. J Cell Sci. 2015;128(15):2919–2927. doi: 10.1242/jcs.172072. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Yuan H, Yang Q, Li M, Wang Y, Li Y, Ma X, Tan F, Wu R. The genetic architecture of growth traits in Salix matsudana under salt stress. Hortic Res. 2017;4:17024. doi: 10.1038/hortres.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Yuan H, Li Y, Chen Y, Liu G, Ye M, Yu C, Lian B, Zhong F, Jiang Y, et al. Genome sequencing and phylogenetic analysis of allotetraploid Salix matsudana Koidz. Hortic Res. 2020;7(1):201. doi: 10.1038/s41438-020-00424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Shi SZ, Jiang Y, Zhong F, Liu G, Yu C, Lian B, Chen Y. Genome-wide investigation of the AP2/ERF superfamily and their expression under salt stress in Chinese willow (Salix matsudana) Peer J. 2021;9:e11076. doi: 10.7717/peerj.11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choudhury S, Panda P, Sahoo L, Panda SK. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav. 2013;8(4):e23681. doi: 10.4161/psb.23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma R, Upadhyay S, Bhat B, Singh G, Bhattacharya S, Singh A. Abiotic stress induced miRNA-TF-gene regulatory network: A structural perspective. Genomics. 2020;112(1):412–422. doi: 10.1016/j.ygeno.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Chen YH, Cao YY, Wang LJ, Li LM, Yang J, Zou MX. Identification of MYB transcription factor genes and their expression during abiotic stresses in maize. Biol Plantarum. 2018;62(2):222–230. doi: 10.1007/s10535-017-0756-1. [DOI] [Google Scholar]
- 33.Park HJ, Kim WY, Yun DJ. A New Insight of Salt Stress Signaling in Plant. Mol Cells. 2016;39(6):447–459. doi: 10.14348/molcells.2016.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ullah A, Sun H, Hakim. Yang X, Zhang X. A novel cotton WRKY gene, GhWRKY6-like, improves salt tolerance by activating the ABA signaling pathway and scavenging of reactive oxygen species. Physiol Plant. 2018;162(4):439–54. doi: 10.1111/ppl.12651. [DOI] [PubMed] [Google Scholar]
- 35.He L, Wu YH, Zhao Q, Wang B, Liu QL, Zhang L. Chrysanthemum DgWRKY2 Gene Enhances Tolerance to Salt Stress in Transgenic Chrysanthemum. Int J Mol Sci. 2018;19(7):2062. doi: 10.3390/ijms19072062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshida T, Mogami J, Yamaguchi-Shinozaki K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr Opin Plant Biol. 2014;21:133–139. doi: 10.1016/j.pbi.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Jofuku KD, den Boer BG, Van Montagu M, Okamuro JK. Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell. 1994;6(9):1211–1225. doi: 10.1105/tpc.6.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Debbarma J, Sarki YN, Saikia B, Boruah HPD, Singha DL, Chikkaputtaiah C. Ethylene Response Factor (ERF) Family Proteins in Abiotic Stresses and CRISPR-Cas9 Genome Editing of ERFs for Multiple Abiotic Stress Tolerance in Crop Plants: A Review. Mol Biotechnol. 2019;61(2):153–172. doi: 10.1007/s12033-018-0144-x. [DOI] [PubMed] [Google Scholar]
- 39.Lv K, Li J, Zhao K, Chen S, Nie J, Zhang W, Liu G, Wei H. Overexpression of an AP2/ERF family gene, BpERF13, in birch enhances cold tolerance through upregulating CBF genes and mitigating reactive oxygen species. Plant Sci. 2020;292:110375. doi: 10.1016/j.plantsci.2019.110375. [DOI] [PubMed] [Google Scholar]
- 40.Sun J, Peng X, Fan W, Tang M, Liu J, Shen S. Functional analysis of BpDREB2 gene involved in salt and drought response from a woody plant Broussonetia papyrifera. Gene. 2014;535(2):140–149. doi: 10.1016/j.gene.2013.11.047. [DOI] [PubMed] [Google Scholar]
- 41.Chen N, Tong S, Tang H, Zhang Z, Liu B, Lou S, Liu J, Liu H, Ma T, Jiang Y. The PalERF109 transcription factor positively regulates salt tolerance via PalHKT1;2 in Populus alba var. pyramidalis. Tree Physiol. 2020;40(6):717–30. doi: 10.1093/treephys/tpaa018. [DOI] [PubMed] [Google Scholar]
- 42.Chen J, Xia X, Yin W. A poplar DRE-binding protein gene, PeDREB2L, is involved in regulation of defense response against abiotic stress. Gene. 2011;483(1–2):36–42. doi: 10.1016/j.gene.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 43.Chen J, Xia X, Yin W. Expression profiling and functional characterization of a DREB2-type gene from Populus euphratica. Biochem Biophys Res Commun. 2009;378(3):483–487. doi: 10.1016/j.bbrc.2008.11.071. [DOI] [PubMed] [Google Scholar]
- 44.Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol. 1999;17(3):287–291. doi: 10.1038/7036. [DOI] [PubMed] [Google Scholar]
- 45.Krizek BA, Blakley IC, Ho YY, Freese N, Loraine AE. The Arabidopsis transcription factor AINTEGUMENTA orchestrates patterning genes and auxin signaling in the establishment of floral growth and form. Plant J. 2020;103(2):752–768. doi: 10.1111/tpj.14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyko A, Molinier J, Chatter W, Laroche A, Kovalchuk I. Acute but not chronic exposure to abiotic stress results in transient reduction of expression levels of the transgene driven by the 35S promoter. New Biotech. 2010;27(1):70–77. doi: 10.1016/j.nbt.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Kiselev KV, Aleynova OA, Ogneva ZV, Suprun AR, Dubrovina AS. 35S promoter-driven transgenes are variably expressed in different organs of Arabidopsis thaliana and in response to abiotic stress. Mol Biol Rep. 2021;48(3):2235–2241. doi: 10.1007/s11033-021-06235-x. [DOI] [PubMed] [Google Scholar]
- 48.Qu Y, Nong Q, Jian S, Lu H, Zhang M, Xia K. An AP2/ERF Gene, HuERF1, from Pitaya (Hylocereus undatus) Positively Regulates Salt Tolerance. Int J Mol Sci. 2020;21(13):4586. doi: 10.3390/ijms21134586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mishra S, Phukan UJ, Tripathi V, Singh DK, Luqman S, Shukla RK. PsAP2 an AP2/ERF family transcription factor from Papaver somniferum enhances abiotic and biotic stress tolerance in transgenic tobacco. Plant Mol Biol. 2015;89(1–2):173–186. doi: 10.1007/s11103-015-0361-7. [DOI] [PubMed] [Google Scholar]
- 50.Cheng MC, Liao PM, Kuo WW, Lin TP. The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol. 2013;162(3):1566–1582. doi: 10.1104/pp.113.221911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lou L, Yu F, Tian M, Liu G, Wu Y, Wu Y, Xia R, Pardo JM, Guo Y, Xie Q. ESCRT-I Component VPS23A Sustains Salt Tolerance by Strengthening the SOS Module in Arabidopsis. Mol Plant. 2020;13(8):1134–1148. doi: 10.1016/j.molp.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 52.Rolly NK, Imran QM, Lee IJ, Yun BW. Salinity Stress-Mediated Suppression of Expression of Salt Overly Sensitive Signaling Pathway Genes Suggests Negative Regulation by AtbZIP62 Transcription Factor in Arabidopsis thaliana. Int J Mol Sci. 2020;21(5):1726. doi: 10.3390/ijms21051726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang HC, Tsai MC, Wu SS, Chang IF. Regulation of ABI5 expression by ABF3 during salt stress responses in Arabidopsis thaliana. Bot Stud. 2019;60(1):16. doi: 10.1186/s40529-019-0264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc Natl Acad Sci U S A. 2000;97(21):11632–11637. doi: 10.1073/pnas.190309197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang HG, Kim J, Kim B, Jeong H, Choi SH, Kim EK, Lee HY, Lim PO. Overexpression of FTL1/DDF1, an AP2 transcription factor, enhances tolerance to cold, drought, and heat stresses in Arabidopsis thaliana. Plant Sci. 2011;180(4):634–641. doi: 10.1016/j.plantsci.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol Plant. 2020;13(8):1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 57.Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 59.Voinnet O, Rivas S, Mestre P, Baulcombe D. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 2003;33(5):949–956. doi: 10.1046/j.1365-313X.2003.01676.x. [DOI] [PubMed] [Google Scholar]
- 60.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nature Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 61.Sun S, Hu C, Qi X, Chen J, Zhong Y, Muhammad A, Lin M, Fang J. The AaCBF4-AaBAM3.1 module enhances freezing tolerance of kiwifruit (Actinidia arguta) Hortic Res. 2021;8(1):97. doi: 10.1038/s41438-021-00530-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hellens RP, Allan AC, Friel EN, Bolitho K, Grafton K, Templeton MD, Karunairetnam S, Gleave AP, Laing WA. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods. 2005;1:13. doi: 10.1186/1746-4811-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Cis-element motifs in the promoter region of SmAP2-17 and its homologous genes.
Additional file 2: File S1. Protein sequences of SmAP2-17 and its homolog genes.
Additional file 3: File S2. Promoter sequences of SmAP2-17 and its homolog genes.
Additional file 4: Table S2. List of primers used.
Additional file 5: Fig. S1. The secondary complementary structure of SmAP2-17 mRNA and salt-induced miRNA ath-MIR167d.
Data Availability Statement
Proteins and nucleotides sequences in this study were downloaded from the Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html) and NCBI (https://www.ncbi.nlm.nih.gov/) websites and analyzed using ESPript (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_clustalw.html) and PlantCARE tool (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) websites. Sequence data of proteins and promoters described in this article are available using the accession numbers listed in Supplementary File S1 and S2 respectively. The datasets supporting the conclusions of this article are included within the article and its additional files, and available from the corresponding author on reasonable request.