Abstract
Cognitive and behavioural comorbidities are prevalent in childhood and adult epilepsies and impose a substantial human and economic burden. Over the past century, the classic approach to understanding the aetiology and course of these comorbidities has been through the prism of the medical taxonomy of epilepsy, including its causes, course, characteristics and syndromes. Although this ‘lesion model’ has long served as the organizing paradigm for the field, substantial challenges to this model have accumulated from diverse sources, including neuroimaging, neuropathology, neuropsychology and network science. Advances in patient stratification and phenotyping point towards a new taxonomy for the cognitive and behavioural comorbidities of epilepsy, which reflects the heterogeneity of their clinical presentation and raises the possibility of a precision medicine approach. As we discuss in this Review, these advances are informing the development of a revised aetiological paradigm that incorporates sophisticated neurobiological measures, genomics, comorbid disease, diversity and adversity, and resilience factors. We describe modifiable risk factors that could guide early identification, treatment and, ultimately, prevention of cognitive and broader neurobehavioural comorbidities in epilepsy and propose a roadmap to guide future research.
ToC blurb:
This Review offers a novel theoretical perspective on the neurobehavioural comorbidities of adult and childhood epilepsy, involving new analytical approaches, derivation of new taxonomies, and consideration of the diverse forces that influence cognition and behaviour in individuals with epilepsy.
Introduction
Epilepsy is a costly and complicated international public health problem1,2 In addition to recurrent seizures, epilepsy is associated with abnormalities in cognition, psychiatric status and social–adaptive behaviors — complications that are referred to collectively as the neurobehavioural comorbidities of the epilepsies (BOX 1). These comorbidities represent substantial life burdens for which the aetiology and most effective treatments continue to be sought.
Box 1 |. Neurobehavioural comorbidities of epilepsy.
Cognition
Higher neuropsychological abilities assessed by objective tests involving intelligence, academic skills, language, visuoperceptual–spatial, memory, executive, attention–working memory and sensorimotor functions.
Emotional–behavioural
Diverse aspects of behaviour, personality and psychiatric status assessed by standardized patient or proxy-completed questionnaires or structured psychiatric interviews, including evaluation of depression, anxiety, neurodevelopmental disorders (for example, autism spectrum disorder, attention-deficit/hyperactivity disorder and specific learning disabilities) and social cognition.
Social–adaptive
Performance in diverse areas of functional status (for example, social cognition) assessed by structured assessment audit of day-to-day abilities including employment, independent living, social network, marital status and quality of life.
This Review offers a novel theoretical perspective on the neurobehavioural comorbidities of adult and childhood epilepsy, involving new analytical approaches, derivation of new taxonomies, and consideration of the diverse forces that influence cognition and behaviour in individuals with epilepsy. In several respects, this approach is consistent with the concept of precision medicine, which, according to the International Consortium for Personalized Medicine1,3, relies on “characterization of individuals’ phenotypes and genotypes (for example, molecular profiling, medical imaging, lifestyle data) for tailoring the right therapeutic strategy for the right person at the right time, and/or to determine the predisposition to disease or deliver timely and targeted prevention.” Precision medicine is having a substantial impact on many medical specialties4,5, including neurology4,5 and important subspecialty areas such as epilepsy6–9, but has been extended minimally to the comorbidities of epilepsy10.
We begin by reviewing the classic paradigm that has dominated neuropsychological and behavioural research in epilepsy, highlighting the many striking inconsistencies of this paradigm. Next, we discuss an emerging taxonomy that harnesses the inherent heterogeneity of the neurobehavioural comorbidities of epilepsy. Finally, we propose a reformulated paradigm that encompasses a broader range of important aetiologies of cognitive and behavioural phenotypes. We believe that this new taxonomy will accelerate efficient identification, intervention and prevention efforts for individual patients with epilepsy. Our focus is on evidence from the broad spectrum of focal (including lesional) and genetic generalized epilepsies, and we do not address the severe childhood epilepsies associated with developmental delays and markedly abnormal EEG backgrounds, such West and Lennox–Gastaut syndromes, which have been addressed elsewhere11.
The classic paradigm
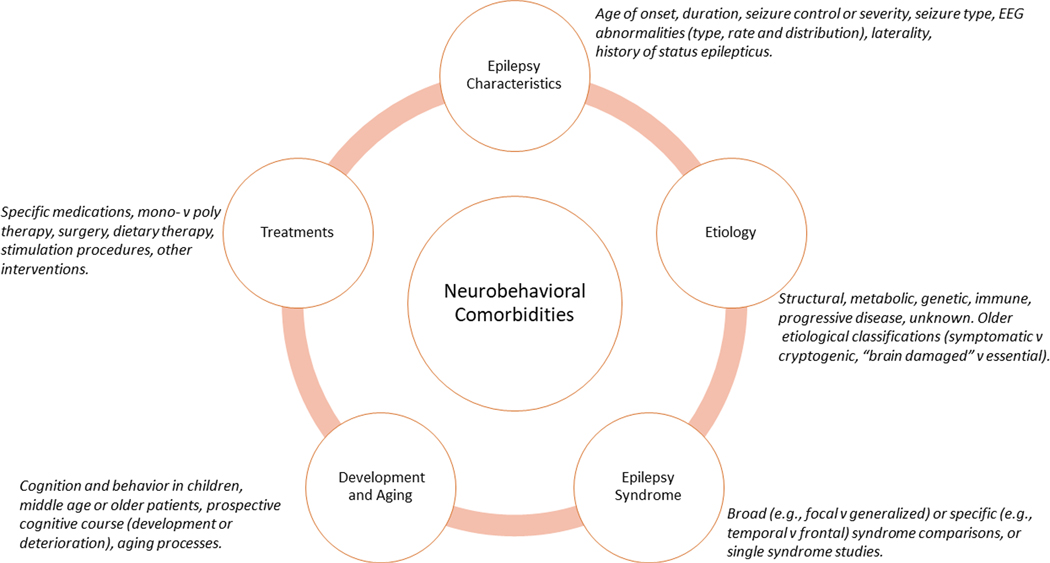
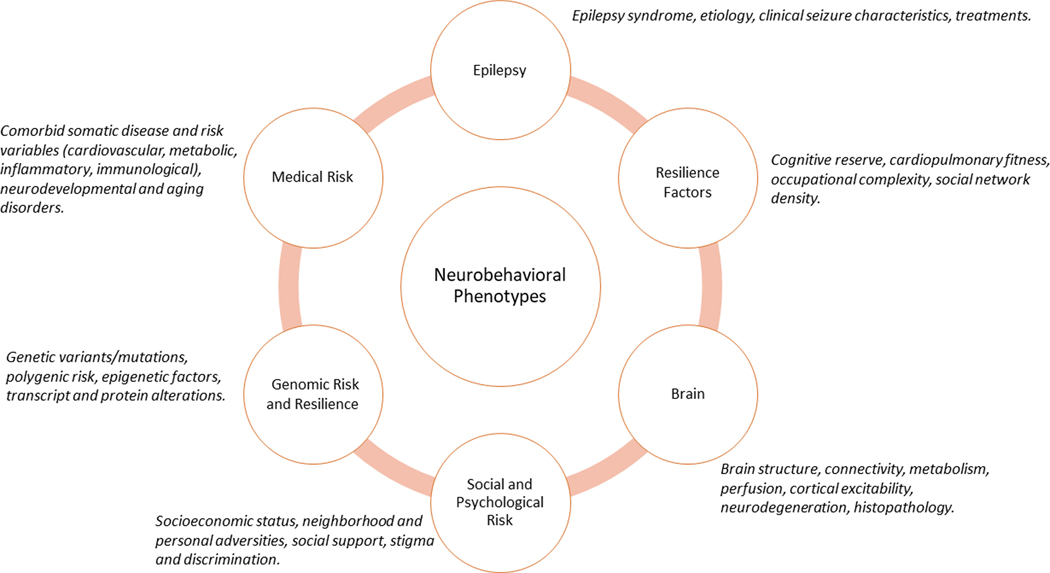
Epilepsy can be accompanied by a broad range of somatic, psychiatric and neuropsychological comorbidities, and studies to gain a better understanding of the aetiology of these comorbidities and their course across the lifespan have been ongoing for >100 years12. A primary focus of these studies has been the association between cognitive and behavioural complications and the fundamental medical taxonomy of epilepsy, that is, those factors related to its aetiology, course, characteristics and treatment in young, mature and ageing patients (FIG. 1).
Fig. 1 |.
The classic paradigm of neurobehavioural comorbidities of epilepsy. The outer ring depicts five major factors (and associated exemplars) that have long been considered, alone or in combination, to exert direct and/or indirect influences on the causes and course of neurobehavioural comorbidities in epilepsy.
The classic paradigm that has driven much of the research into the neurobehavioural comorbidities of epilepsy emerged from an interest in the effects of epilepsy on cognition. Early studies identified objective cognitive impairments associated with epilepsy13–15, initially focusing on global cognitive ability (that is, intelligence) and later exploring specific cognitive domains as the understanding of human cognition and its assessment evolved16. The existence of substantial heterogeneity in cognition among patients with epilepsy rapidly became evident, prompting efforts to identify clinical correlates of cognitive dysfunction, such as age of onset and seizure frequency. The earliest empirical examples of this approach date back to the 1920s14 and persist to the present day17,18. Since the 1940s, efforts to characterize the relationship between disease-related factors and cognition have been reflected in narrative19–27 as well as systematic and meta-analytic reviews28–32. Factors that have been linked to an increased risk of cognitive impairment include earlier age of onset, increasing duration of epilepsy, poorer seizure control, symptomatic epilepsies, number of lifetime generalized epilepsies and episodes of status epilepticus, number and type of medications, and the type, frequency and severity of EEG abnormalities. However, owing to variability in the reliability and reproducibility of the findings, these relationships have undergone continual re-evaluation.
Efforts to classify seizures according to their aetiology and underlying pathophysiology were especially influential in this research. Early attempts were limited by an imprecise understanding of the epilepsies and were driven largely by clinical theorizing (for example, ‘predisposing’ versus ‘exciting’ aetiologies)33 but were subsequently advanced by the application of EEG and the development and evolution of the International League Against Epilepsy (ILAE) Classification of the Epilepsies34–37. Neuropsychological and behavioural research paralleled the evolution of this taxonomy, and efforts to link specific cognitive abnormalities with distinct epilepsy syndromes followed, aptly referred to as the ‘lesion’ or ‘localization’ model38. This model has provided an important organizing influence to explore the neuropsychology of epilepsy, as well as the behavioural complications associated with focal39, 40 and generalized epilepsies28. Exemplars of the lesion model include memory impairment in temporal lobe epilepsy (TLE)41, dysexecutive function in frontal lobe epilepsy (FLE)42, disrupted attention in absence epilepsy43, abnormalities in aspects of language in Rolandic epilepsy44, visuoperceptual and spatial impairments in occipital epilepsy45, and abnormal primary memory and behaviour in juvenile myoclonic epilepsy (JME)46,47.
Challenges to the classic paradigm
Substantial challenges to the classic model have accumulated from several sources, including cognitive, neuroimaging, neuropathology and clinical research. These studies indicate that the neurobehavioural comorbidities of epilepsy are more variable and extensive than would be predicted by lesion location alone. In a prescient view, Jokeit and Schacher48 argued that because the taxonomy of the epilepsies was constructed independently of neuropsychological concepts, specific associations between cognitive deficits and epilepsy type and aetiology could represent exceptions rather than the rule. Furthermore, neurobehavioural comorbidities often predate seizure onset, posing an additional challenge to the classic paradigm.
Cognitive research
Neuropsychological impairments associated with epilepsy do not always respect the hypothesized boundaries of the classic lesion model. In both children49–54 and adults55–61 with temporal or frontal lobe epilepsies, cognitive anomalies are often more widespread and generalized than would be anticipated on the basis of the lesion location. This pattern of generalized cognitive impairment has also been observed in less well-investigated focal epilepsies involving posterior regions (occipital and/or parietal) in children45,62,63 and adults64. Furthermore, meta-analyses in Rolandic epilepsy and genetic generalized epilepsies27,31 have reported widespread cognitive abnormalities29.
Conversely, abnormalities in specific prototypical cognitive domains have been reported across diverse epilepsy syndromes. For instance, executive dysfunction has been reported in TLE51,65,66, FLE54,66, JME67, absence epilepsy68 and Rolandic epilepsy69,70. Similarly, language impairments have been reported in absence epilepsy71,72, FLE30,52, Rolandic epilepsy73,74 and JME72,75,76, as well as in TLE, even when seizures arise from the non-dominant hemisphere77,78. Thus, empirical links between purported domain-specific cognitive impairments and specific epilepsy syndromes are more complex than predicted by the classic model.
Direct multi-syndrome comparisons have demonstrated considerable overlap of cognitive abnormalities in both new-onset72,79 and established epilepsies. For example, surprisingly few substantial differences were observed between children with Rolandic epilepsy, absence epilepsy and FLE on measures of intelligence (different on only two of 54 syndrome comparisons)80 and memory (two of 21 syndrome comparisons)81. Nolan et al.82 demonstrated reduced intellectual performance across children with diverse epilepsy syndromes, with any differences being primarily in magnitude rather than type of impairment. Similarly, memory performance was reduced across all groups of individuals with TLE, FLE or absence epilepsies83, again varying primarily in magnitude.
Despite the clear clinical distinction between TLE and FLE, cognitive patterns can be similar in these syndromes owing to extensive frontotemporal connectivity59–61. For example, in TLE, the presence of executive dysfunction — a domain impairment long considered to be a hallmark of FLE — has been linked to neurobiological influences exerted directly by the frontal lobe and/or indirectly through broader network connectivity. These influences (BOX 2) have been demonstrated across metabolic (18F-FDG PET)84, 85, EEG86, morphometric (atrophy)87–90, diffusion-weighted imaging91,92, resting-state93 and task-activated functional MRI (fMRI)94,95, and functional connectivity analyses96. Similarly, psychiatric complications such as depression in TLE have been linked to co-occurring frontal lobe hypometabolism97, 98 and structural abnormalities in the frontal lobes99,100.
Box 2 |. Executive dysfunction and network changes in TLE.
Executive dysfunction in temporal lobe epilepsy (TLE), as identified through neuropsychological assessment, has been related to abnormal findings in frontal, frontostriatal and midline parietal networks. The diverse sets of findings supporting this perspective are listed below.
EEG
Increased rate of interictal epileptiform discharges to the frontal lobe86.
Metabolism
18F-FDG PET hypometabolism extending to the prefrontal lobe85.
Brain volume and diffusion
Altered activation patterns
Altered resting-state connectivity
We do not mean to imply that all attempts to link specific comorbidities to specific features of the lesion model have failed, but the non-supportive and contradictory findings, as reviewed above, are striking. Sophisticated methodologies such as machine learning and other advanced analytics have been shown to discriminate both between syndromes (for example, FLE versus TLE or TLE versus Rolandic epilepsy)66,101,102 and within syndromes (for example, left versus right TLE)58,102–104. Such technologies could enhance the value of syndrome or lesion approaches, but even here they are prone to inconsistent findings, and standardized measures of diagnostic accuracy will be required before they can be routinely adopted in the clinic. Despite the aid of technological innovations for improving syndrome discrimination, the fundamental heterogeneity in cognitive and behavioural presentations, within and across epilepsy syndromes and clinical seizure features, must still be considered and embraced in any competing model. In our proposed taxonomy, a lesion is but one of several neuropathological considerations for understanding cognitive comorbidities in individual patients.
Neuropathology
Histopathological and neuroimaging investigations can assist in identifying abnormalities that might explain unanticipated syndrome-specific cognitive and behavioural findings. An early study of a series of 26 autopsied patients with TLE revealed multifocal abnormalities affecting the hippocampus (85% of cases), cerebellum (46%), amygdala (42%), thalamus (34%) and cortex (23%), with only 3% of individuals showing no appreciable pathology105. More recent neuropathological investigations have identified distributed cortical anomalies106, as well as the presence of neurodegenerative features and proteinopathies107–110, in the brains of people with focal epilepsies. Among the few neuropathological studies that have been conducted in patients with primary generalized epilepsies, microdysgenesis with variable regional distribution has been reported in some111,112 but not all113. In a relevant nonhuman primate (baboon) model of JME114, untreated animals with spontaneous seizures exhibited a reduced number of cortical neurons overall, with the greatest reductions being observed in primary somatosensory and primary motor cortices and the smallest reductions in visual regions115.
Neuroimaging
Quantitative neuroimaging studies provide perhaps the clearest understanding of why cognitive anomalies do not always adhere to the classic lesion model or follow syndrome-specific patterns. Three primary lines of evidence are problematic for the classic lesion model.
First, imaging abnormalities often extend substantially beyond the primary areas of electrophysiological abnormality. Distributed neuroimaging abnormalities in TLE include widespread volume loss116, cortical thinning117–119, and alterations in gyral and sulcal curvature and total cortical surface area120,121. In primary generalized epilepsies, a meta-analysis has revealed widespread cortical and subcortical volume loss extending beyond the thalamocortical networks that are postulated to be the primary seizure generators122. Similarly, distributed anomalies in white matter microstructure were reported in a meta-analysis of 1,122 healthy controls and 1,027 people with epilepsy123. Decreased fractional anisotropy and increased mean diffusivity were observed in commissural, association and projection white matter fibres in TLE and FLE, with less impact in generalized epilepsy.
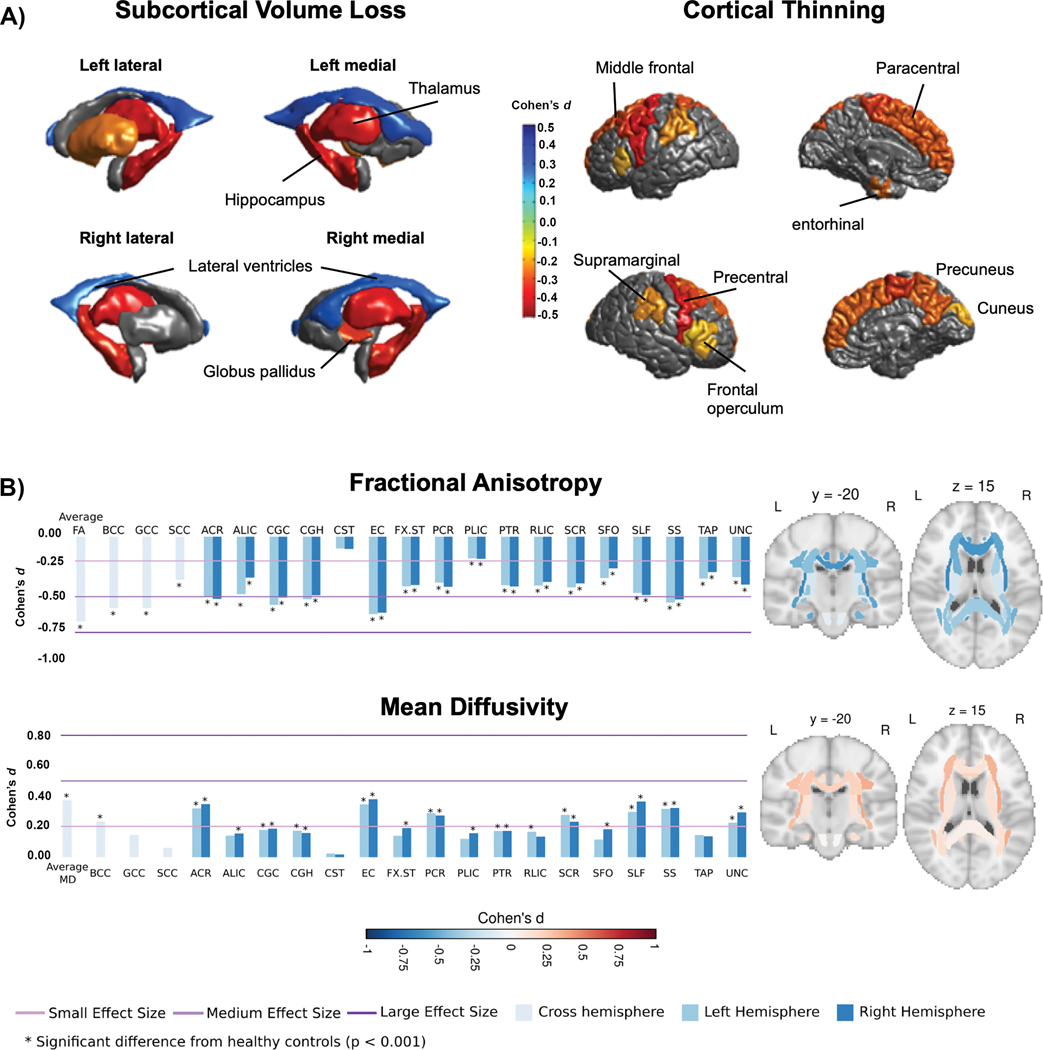
Second, across common epilepsy syndromes, evidence is emerging that structural abnormalities are more likely to be shared than syndrome-specific, as reflected in the Enhancing NeuroImaging and Genetics through Meta-Analysis (ENIGMA-Epilepsy) project, an international database that includes 1,727 healthy controls and 2,149 patients with common epilepsy syndromes124. Despite the presence of some prototypic syndrome-specific findings, such as ipsilateral hippocampal volume loss in TLE, all patient groups demonstrated reduced thalamic, hippocampal and right pallidal volumes, as well as bilateral increases in the volume of the lateral ventricles (FIG. 2a, left). In addition, widespread cortical thinning, involving the precentral, paracentral, supramarginal, precuneus and cuneus, left entorhinal and multiple prefrontal regions, was observed across all epilepsy syndromes (FIG. 2a, right)124. Apart from medial temporal lobe abnormalities in patients with left TLE, syndrome-specific findings were rare.
Fig. 2 |.
Subcortical, cortical and diffusion findings in ENIGMA-Epilepsy. a
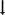 Subcortical volume (left) and cortical thickness (right) abnormalities shared across all epilepsy syndromes in the ENIGMA-Epilepsy meta-analysis124. Coloured bar represents Cohen’s d effect size estimates for case–control differences in each subcortical or cortical region. Red and yellow shading depicts regions with greater volume loss or thinning in patients relative to controls, whereas blue shading represents regions with higher volume relative to controls. Patients with epilepsy had lower volumes of the bilateral thalami and hippocampi and right pallidum relative to controls, and increased volume of the lateral ventricles. The patients also showed cortical thinning in the precentral and paracentral gyri bilaterally and in the left prefrontal, superior parietal and cuneus. b
Subcortical volume (left) and cortical thickness (right) abnormalities shared across all epilepsy syndromes in the ENIGMA-Epilepsy meta-analysis124. Coloured bar represents Cohen’s d effect size estimates for case–control differences in each subcortical or cortical region. Red and yellow shading depicts regions with greater volume loss or thinning in patients relative to controls, whereas blue shading represents regions with higher volume relative to controls. Patients with epilepsy had lower volumes of the bilateral thalami and hippocampi and right pallidum relative to controls, and increased volume of the lateral ventricles. The patients also showed cortical thinning in the precentral and paracentral gyri bilaterally and in the left prefrontal, superior parietal and cuneus. b
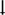 White matter microstructural differences across 38 fibre tracts for the ‘all epilepsies’ cohort compared with controls125. All values represent Cohen’s d effect size estimates for differences in fractional anisotropy and mean diffusivity between each patient group and healthy controls. Positive effect sizes reflect diffusion values greater than controls and negative effect sizes represent values lower than controls. The y and z values represent the slice number for the coronal and axial planes, respectively. Across all epilepsies, the greatest effects on fractional anisotropy were observed in the body and genu of the corpus callosum, external capsule, cingulum and corona radiata. The greatest effects on mean diffusivity were observed in the external capsule, anterior corona radiata and superior longitudinal fasciculus. Part a reprinted with permission from ref.124. Part b adapted with permission from ref.125.
White matter microstructural differences across 38 fibre tracts for the ‘all epilepsies’ cohort compared with controls125. All values represent Cohen’s d effect size estimates for differences in fractional anisotropy and mean diffusivity between each patient group and healthy controls. Positive effect sizes reflect diffusion values greater than controls and negative effect sizes represent values lower than controls. The y and z values represent the slice number for the coronal and axial planes, respectively. Across all epilepsies, the greatest effects on fractional anisotropy were observed in the body and genu of the corpus callosum, external capsule, cingulum and corona radiata. The greatest effects on mean diffusivity were observed in the external capsule, anterior corona radiata and superior longitudinal fasciculus. Part a reprinted with permission from ref.124. Part b adapted with permission from ref.125.
In a subsequent diffusion MRI study from ENIGMA-Epilepsy, white matter microstructural alterations were observed across all epilepsy syndromes in 36 of 38 association, commissural and projection fibres125. Across patient groups, reductions in fractional anisotropy and increases in mean diffusivity were greatest in the genu and body of the corpus callosum, cingulum and external capsule (FIG. 2b). Although the severity of the alterations varied across epilepsy syndromes and was most pronounced in mesial TLE125, bilateral alterations in many anterior midline fibres were uniform across groups. These broad patterns of structural and microstructural alterations, shared across epilepsy syndromes, could help to explain the distributed nature of cognitive impairments and the variable ability to identify syndrome-specific impairments.
Last, analyses of macroscale and mesoscale connectivity patterns have demonstrated widespread network-level differences between controls and patients with either focal126 or generalized127,128 epilepsies. These analyses included correlation or covariance matrices derived from fMRI and single-photon emission CT129, 18F-FDG PET130, scalp EEG and magnetoencephalography131,132, intracranial EEG and electrocorticography133, and structural MRI134. Connectivity analysis has been used to identify the epileptic network in individual patients135,136 and to identify disease-specific patterns of abnormal connectivity at the group level. Common findings include abnormal cortical–subcortical connectivity137–139, increased connectivity within the putative primary epileptic network133,135,140,141 and downstream network dysfunction on a more global or multi-network scale142,143. Widespread downstream network abnormalities have been reported across epilepsy syndromes, including paediatric focal epilepsy144, FLE142, TLE145, childhood absence epilepsy146 and JME128. Taken together, these findings suggest that epilepsy can cause disruption of networks far beyond the one that is responsible for primary seizure generation.
Important questions that emerge from this research include how different imaging features (or atrophy patterns) lead to the development of cognitive impairment in epilepsy, and what factors drive these changes if they are not syndrome-specific. Some evidence suggests that in patients with drug-resistant epilepsy, early-onset seizures disrupt white matter development, especially in late-myelinating frontotemporal association tracts147,148. Microstructural damage to these long-range tracts could lead to impairments in attention and executive functioning as a result of cortico-cortical disconnection. Microstructural damage to short-range, U-shaped fibres directly beneath the cortex might also contribute to cognitive impairment in patients with epilepsy by disrupting communication between neighbouring cortical regions. In addition, longitudinal studies have shown that in patients with focal epilepsy syndromes such as TLE, long disease durations can lead to widespread age-accelerated cortical thinning, thereby exacerbating global cognitive, memory, and processing speed impairments149,150,151.
Clinical research
A range of cognitive, behavioural and brain abnormalities are known to be present in both children152,153 and adults154,155 at the time of diagnosis of epilepsy, long before any potential impact of recurrent seizures, psychosocial consequences or antiseizure medications is evident. Furthermore, neurobehavioural anomalies have been reported to occur well before the first recognized seizure152,156. These neurobehavioural comorbidities at or before epilepsy onset are inconsistent with the classic paradigm, which assumes that neurobehavioural risk accrues over the disease course, and they highlight the need to explore other potential common aetiological pathways157, including genetic aetiologies, which are addressed below.
From lesions to networks
The fundamental view of the nature of epilepsy has evolved from the conceptualization of a discrete area, the epileptogenic zone158, to the suggestion that ‘focal epilepsy’ affects networks far beyond this zone. The latter assertion has been supported by evidence from invasive stereotactic EEG159–161 and from connectivity analysis using scalp EEG162,163, structural MRI164,165 and fMRI166,167. Even the concept of an epileptogenic zone might be flawed, as some patients have several nodes within a broader epileptic network that are capable of independent seizure generation168.
Neuropsychological research in the epilepsy field is also moving beyond the lesion model to focus more on disrupted networks37,38,169–172. Specifically advocated is a search for cognitive, behavioural and imaging phenotypes within and/or across epilepsy syndromes that are not restricted or constrained by the disease taxonomy54,169,170,173 — an endeavour that is entirely consistent with precision medicine. However, despite the shift in the conceptualization of epilepsy, along with considerable evidence that challenges the classic lesion model, this model is likely to persist until a satisfactory alternative paradigm can be found that assimilates both contradictory and contemporary findings174.
Moving towards a new model and taxonomy
Cognitive phenotypes
Recent research has demonstrated the utility of a phenotypic approach to the neurobehavioural comorbidities of epilepsy93,175,176,177,178,179–181,182–189,190 and (BOX 3, Supplementary Table 1). To date, 17 taxonomic investigations have characterized phenotypes of objective or subjective cognition and, where available, their related neuroimaging correlates (Supplementary Table 1).
Box 3 |. Phenotypic investigations of neurobehavioral status in epilepsy.
This box lists the range of cognitive and behavioral phenotype investigations that have been conducted in paediatric and adult epilepsy cohorts (see Supplementary Table 1 for details).
Cognitive phenotypes
Academic achievement in temporal lobe epilepsy175.
Neuropsychological and imaging status in temporal lobe epilepsy93,176,177,180,181,182,183,184,185,187,188.
Neuropsychological status in frontal lobe epilepsy186.
Patterns of activation on language task-based functional MRI in children with focal epilepsy178
Neuropsychological status in children with diverse epilepsy syndromes179.
Parent-rated executive function189.
Behavioural phenotypes
The landscape of cognitive phenotypes.
The studies listed in Supplementary Table 1 investigated phenotypes relating to academic skills (word reading, spelling and arithmetic)175, objectively assessed cognition93,176,179–182,184,187,191 and parent-reported executive function189. In addition, six studies characterized neuroimaging correlates of the identified cognitive phenotypes, using structural MRI93,177,179,180,183, diffusion MRI180,182,183,188, activation fMRI (language)178, resting-state fMRI93,183 and/or advanced network analytics (for example, graph theory) on structural MRI, diffusion MRI or resting-state fMRI data93,182,183,188,185. These studies focused predominantly on adults with TLE178,179,192. Characterization of cognitive phenotypes in syndrome groups other than TLE has been undertaken, albeit on a limited scale. Among children (aged 8–18 years) with recent-onset focal or generalized epilepsies, three cognitive phenotypes were identified that cut across different epilepsy syndromes: average and comparable to controls; mild impairment across multiple cognitive domains; and impairment across all domains with severe attentional impairment179. Among adults with FLE, four cognitive phenotypes were identified: intact, generalized, single domain (language) impaired and multiple domain (language and executive function) impaired186. When children with drug-related epilepsy treated medically or surgically were followed up for 4–11 years, two prospective cognitive phenotype groups — average cognition (55% of sample) and impaired cognition (45%) — with different trajectories were identified regardless of treatment intervention (Supplementary Table 1)190.
These findings indicate that the classic lesion-based cognitive and behavioural profiles are imprecise and fail to reflect the substantial heterogeneity in clinical presentation. Further research in a range of epilepsy syndromes will be needed to further explore the possibility of a phenotype-based profiling approach that could inform a revised taxonomy.
Phenotype distributions across investigations.
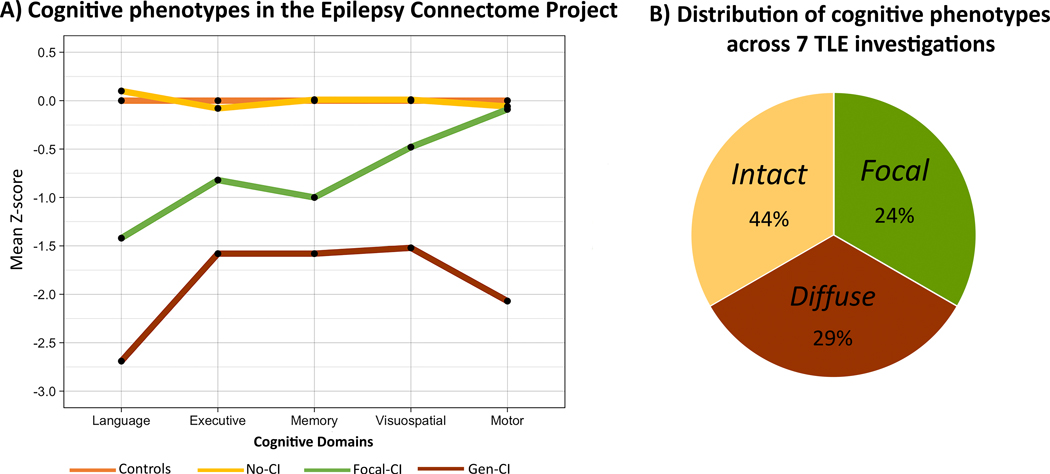
Of the cognitive phenotypes reported among adults with TLE, three are particularly prevalent (FIG. 3): an ‘intact’ or minimally impaired subgroup, largely comparable to healthy controls; a generalized impaired subgroup with abnormal scores across all administered cognitive metrics; and a subgroup93 (or occasionally two subgroups181), exhibiting the expected pattern of cognitive anomalies for TLE, predominantly affecting memory, language and/or executive function (FIG. 3a)181. As FIG. 3b illustrates, the proportion of patients with TLE in the intact subgroup is surprisingly large, ranging from 27–54% across investigations (mean 44%). This patient group is infrequently discussed in the epilepsy literature and was arguably unanticipated among individuals with medication-resistant epilepsy presenting as surgical candidates181,184. The proportion with generalized cognitive impairment, another unexpected phenotype for a focal epilepsy, ranged from 15–44% (mean 29%), and the remaining individuals exhibited more focal cognitive patterns, involving reduced executive function and/or speed, and memory and/or language impairments181,182. A number of clinical and demographic variables have been associated with these phenotypes, albeit with some variability in findings (Supplementary Table 1).
Fig. 3 |.
Cognitive phenotypes and their distribution. a | The Epilepsy Connectome Project identified three cognitive phenotypes in patients with temporal lobe epilepsy (TLE): intact or minimally impaired, comparable to healthy controls; generalized impairment, with abnormal scores across all administered cognitive metrics; and focal impairment, predominantly affecting memory, language and/or executive function93. The z-scores represent performance of the epilepsy groups compared with controls, with negative values indicating worse performance. b | Distribution of cognitive phenotypes across seven investigations in individuals with TLE93,176,180,181,182,184,187.
Neuroimaging correlates of cognitive phenotypes.
Relationships have been detected between cognitive phenotypes and both the degree and distribution of neuroimaging abnormality (Supplementary Table 1). Typically, no or minimal structural, diffusion and resting-state differences are evident between minimally impaired phenotypes and control groups, whereas marked differences in these imaging measures are observed between generalized impairment phenotypes and controls93,177,180,182. Overall, neuroimaging differences are more prominent and consistent when network metrics, based on diffusion MRI, resting-state fMRI or network analyses of imaging data, are examined93,180,182,188.
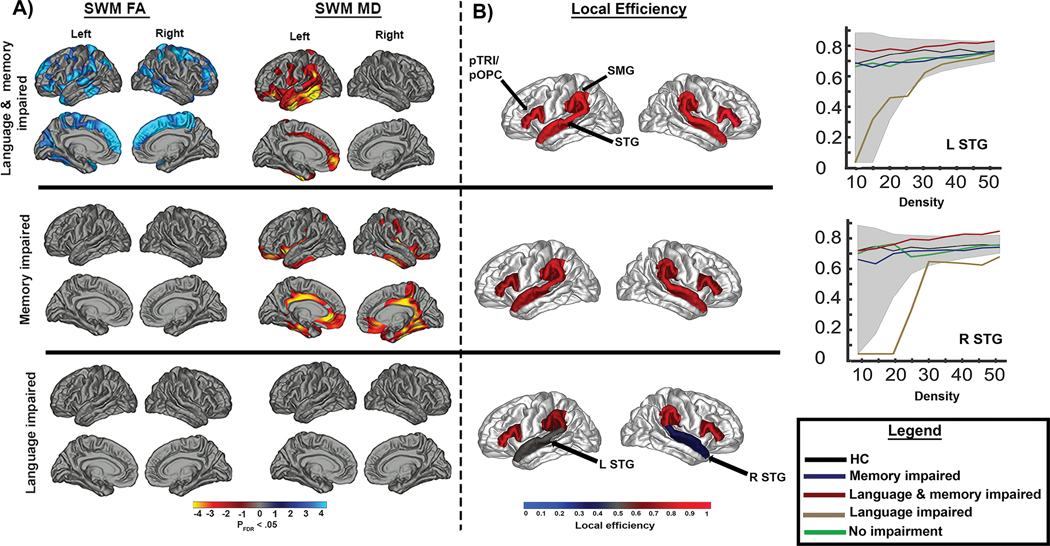
Neuroimaging of cognitive phenotypes carries implications for traditional comorbidity research, in which a common approach is to administer a comprehensive cognitive battery to examine a single cognitive metric (for example, memory) in relation to clinical or imaging metrics (for example, connectivity), with little attention being paid to the impact of other co-occurring cognitive impairments. However, clear differences in white matter signatures can be appreciated when white matter network pathology is examined in patients with pure memory impaired versus mixed memory and language phenotypes (FIG. 4), highlighting the importance of distinguishing single-domain from multidomain impairments182. Brain network changes that give rise to multidomain impairments might not be additive and could be synergistic. Addressing how different patterns of network pathology lead to multidomain impairments is essential to understand the full cognitive burden experienced by any single patient.
Fig. 4 |.
Diffusion and network findings across discrete cognitive phenotypes of TLE. a | Differences in superficial white matter (SWM) fractional anisotropy and mean diffusivity across cognitive phenotypes in individuals with temporal lobe epilepsy (TLE) relative to healthy controls. Blue and cyan represent lower values and red and yellow represent higher values than controls. b | Local efficiency differences between healthy controls and each cognitive phenotype within perisylvian regions (depicted in red), including the pars triangularis (pTRI)/pars opercularis (pOPC), superior temporal gyrus (STG) and supramarginal gyrus (SMG). Significant differences between patients with TLE and healthy controls are depicted in grey and blue. The line graphs demonstrate differences in local efficiency within the left and right STG between healthy controls and each cognitive phenotype across different network densities. Shaded areas represent the upper and lower boundaries of local efficiency for healthy controls. In both panels, patients with single-domain memory or language impairments demonstrate findings distinct from patients with multiple domain impairments. Patients with both language and memory impairment showed widespread SWM abnormalities, whereas patients with memory impairments alone showed SWM abnormalities predominantly in the bilateral temporal lobes and cingulum. Patients with language impairments alone showed distinct abnormalities in perisylvian network structure that were not apparent at the regional SWM level. Adapted with permission from ref.182.
Examination of global and local (temporal lobe) functional connectivity in TLE has demonstrated increased connectivity in the temporal lobe epileptogenic region that is not associated with the distribution of cognitive phenotypes185. Instead, global connectivity metrics — namely, clustering coefficient and rich club proportion — were predictive of the cognitive phenotype. These findings suggest that focal hyperconnectivity in the epileptogenic region contributes to the broader global network disorganization that is most closely linked to cognitive phenotypes.
Intracranial EEG provides a potential complementary technique to investigate cognitive phenotypes and cognitive processing193, in particular, the oscillations involved in mediating the large-scale neural networks194,195 that address cognitive adequacy in people with epilepsy. Memory deficits in TLE have been linked to pathological hippocampal oscillatory activity, with implications for alterations in large-scale neuronal synchronization196. Intermittent pathological high-frequency oscillations in non-lesional epilepsy have been linked to disrupted encoding of stimuli197. The combination of these observational approaches with parallel lines of research that use electrophysiological stimulation to identify pathological networks198,199 or map cognitive function200 provides potential avenues to better understand the underlying neurobiological differences that lead to distinct cognitive phenotypes. Further research is needed to explore the relationships between pathological electrophysiological activity, altered resting state metrics and disruptions to cognitive networks in epilepsy.
Approaches to cognitive phenotyping.
The cognitive phenotypes in TLE are relatively stable and reproducible, even when different methodological approaches are used for their assessment. Empirically driven methods, such as unsupervised cluster analysis, have been the most common approaches. However, diagnostic neuropsychological approaches (that is, actuarial methods), in which groups are determined on the basis of pattern of impairment (>1–2 SD below a normative sample) across cognitive domains, have also been used. A recent head-to-head comparison between cluster analysis and a diagnostic approach187 yielded a concordance rate of 82.6% with good agreement (κ = 0.716) and, importantly, both approaches identified the same three broad phenotypes described above. This study demonstrates the validity of a diagnostic approach to characterize phenotypic patterns of impairment at the individual patient level in a clinical setting — a crucial requirement for a precision-based therapeutic approach.
Behavioural phenotypes
BOX 3 indicates the phenotypic approaches to behavioural, developmental and psychosocial complications of the epilepsies that support broad application of this approach.
Depressive symptoms.
In a 2016 study171, Rayner et al. performed cluster analysis of nine depressive symptoms (as recognized by the Diagnostic and Statistical Manual of Mental Disorders) in adult patients with focal epilepsies (n = 91) and controls (n = 77). This analysis identified three phenotypes and associated features: a ‘cognitive’ depression phenotype (17% of epilepsy participants) characterized by self-critical thoughts and dysphoria with associated memory deficit; a ‘somatic’ depression phenotype (7%), characterized by vegetative symptoms and anhedonia, with greater anxiety compared with the other phenotype and controls; and a non-depressed epilepsy phenotype (76%).
Developmental trajectories.
In a study published in 2012, Wilson et al.201 examined prospective developmental trajectories — operationally defined academic achievement, occupational achievement, peer social competence, relationship status and independence — in patients with childhood-onset TLE (n = 54). Three cluster trajectories were identified: normal development (52% of participants); altered and achieving some but not all developmental tasks (37%); and delayed and achieving few developmental tasks (11%). The normal group outperformed the altered and delayed groups across a range of cognitive measures, and additional analyses demonstrated that the phenotypes were independently related to chronicity of seizures, cognitive status, surgically remediable epilepsy and gender.
Child behavioural problems.
Assessment of children with new-onset epilepsies (n = 183) and normally developing controls (n = 107), using the Child Behavior Checklist, identified three behavioural phenotypes: a ‘normal’ group that was comparable to controls across all behaviour problem scales (67% of participants); a subset with abnormal scores across all scales (22%); and a specific non-externalizing behaviour disorder group (11%)192. The phenotypes correlated with diverse cognitive, familial, developmental and neuroimaging (cortical thickness) factors.
Adult behavioural problems.
In a study published in 2021, adults with TLE (n = 96) and healthy controls (n = 82) were assessed with the Symptom Checklist 90-Revised (SCL-90-R) and unsupervised machine-learning techniques were used to identify latent TLE groups186. As a group, the patients with TLE patients exhibited significantly higher (more abnormal) scores across all nine SCL-90-R scales compared with controls. However, cluster analysis identified three latent groups: unimpaired with no scale elevations compared with controls (Cluster 1, 42% of the patients with TLE); mild-to-moderate symptomatology characterized by significant elevations across several SCL-90-R scales compared with controls (Cluster 2, 35%); and marked symptomatology with significant elevations across all scales compared with controls and the other TLE phenotype groups (Cluster 3, 23%). Significant associations were observed between cluster membership and demographic (education), clinical epilepsy (perceived seizure severity and bitemporal lobe seizure onset), and neuropsychological status (intelligence, memory and executive function), but structural neuroimaging correlates were minimal. Concurrent validity of the behavioural phenotype grouping was demonstrated through association with psychiatric (current and lifetime-to-date DSM IV Axis 1 disorders and current treatment) and quality-of-life variables.
Psychosocial profiles.
To study psychosocial profiles, Josephson et al.10 examined patient-reported outcome data from 462 individuals with epilepsy. Cluster analysis revealed three groups, who were deemed to have high (46% of participants), intermediate (33%) or low (21%) psychosocial health. The clusters were differentiated by the degree of seizure control, need for partially or completely subsidized income support, inability to drive, and history of a psychiatric disorder.
Health-related quality of life.
Sajobi et al. (2017)202 assessed and tracked health-related quality of life (HRQoL) for 2 years in 373 children with newly diagnosed epilepsy. Multi-trajectory modelling characterized three longitudinal HRQoL trajectory groups: high HRQoL (44.7% of participants), intermediate HRQoL (37.0%) and low HRQoL (18.3%). Predictors of HRQoL trajectories included less severe epilepsy, absence of cognitive and behavioural problems, lower parental depression scores, better family functioning and fewer family demands. Additional longitudinal (2-year) investigations of young people (aged 2–12 years at study entry) with new-onset epilepsies have similarly identified a spectrum of HRQoL trajectories203,204, ranging from individuals with stable, intact and even superior HRQoL to individuals at increased risk and concern for poor HRQoL.
Summary.
Although attempts to identify broadly defined behavioural phenotypes are progressing, they are complicated by the need for more diversity in the comorbidities addressed and the dependent measures utilized compared with the cognitive studies. Therefore, we do not yet have broad taxonomic agreement or the ability to identify the phenotypic status of individual patients. However, these investigations have been more inclusive than the cognitive studies with regard to representation of diverse focal and generalized syndromes among children and adults (Supplementary Table 1).
Broadening the scope of risk and resilience factors
An unfortunate byproduct of the classic paradigm has been the underappreciation of potentially relevant risk and resilience factors for cognitive and behavioural comorbidities in epilepsy. This situation is not unique to epilepsy: a bibliometric analysis of trends in the precision medicine literature revealed that of 5,552 articles published from 2012 to 2018, mostly in medical specialty journals (particularly oncology), only 1.6% included terms related to social and environmental determinants of health, health disparities or health equities in their abstract and/or title5. Most articles used definitions of precision medicine related to tailored, individualized or personalized treatment and genetics and/or biology, with less than one-third including environment and lifestyle.
A precision approach requires an alternative framework for the cognitive and behavioural risk and resilience factors in epilepsy (FIG. 5) that more broadly addresses the aetiology of common comorbidities and targets potential treatments and prevention efforts. Conventional risks, including the epilepsy itself and neuroimaging abnormalities, as well as ever-present concerns regarding medication effects205,206, will remain embedded in this framework. In addition, incorporation of other factors related to cognition and behaviour, such as genomic, medical, social and lifestyle factors, will offer a more comprehensive and contemporary approach.
Fig. 5 |.
A next-generation paradigm for neurobehavioural phenotypes of epilepsy. The outer ring depicts six major factors (and associated exemplars) that alone or in combination are proposed to exert direct and/or indirect influences on the causes and course of neurobehavioural phenotypes in epilepsy.
Genomic risk.
Identification of genomic risk and resilience factors for neurobehavioural comorbidities in epilepsy is essential to a precision medicine approach. However, although genomic research related to the development of epilepsy and related neuropathologies has burgeoned in recent years207–211, research into the role of genomic factors in neurobehavioural comorbidities of epilepsy is still in its infancy212. Most neurobehavioural studies to date have been conducted in genetic epilepsy syndromes, such as tuberous sclerosis complex or Dravet syndrome212. Genomic contributions to comorbidities in non-syndromic, idiopathic epilepsies are largely unexplored and might have their own genetic causes or shared genetic risk factors with epilepsy (Supplementary Figure 1). Environmental factors (the exposome) are also likely to have an important role and to interact with genomic factors to influence cognitive and behavioural phenotypes. The genetic liability model for common diseases posits that the additive effects of many genetic and environmental factors contribute to an individual’s liability to develop a disease or disorder, and this model is also likely to apply to common comorbidities including memory impairment and depression213,214.
An emerging literature examining cognitive, behavioural and imaging abnormalities in unaffected family members (in particular, siblings or parents) of patients with epilepsy has raised interest in the potential genetic contributions to the neurobehavioural comorbidities of epilepsy. These far-ranging findings include effects on cortical and subcortical structures, including hippocampal and white matter volumes in relatives of individuals with TLE syndromes215–219; cognition, imaging and cortical excitability in relatives of individuals with JME220–226; reading problems, difficulties with speech sound discrimination and cognitive dysfunction in relatives of individuals with Rolandic epilepsy227–229; and behavioural problems in relatives of children with epilepsy230,231.
Cognition and behaviour are affected to varying degrees in epilepsy, even among patients with the same type of epilepsy and pathological substrate (for example, mesial TLE with hippocampal sclerosis)176. Genomic factors are likely to account for some of this variability or to serve as key modifiers in epilepsy comorbidities, and might provide important insights to aid the future development of therapeutic approaches for these conditions. The studies to date (TABLE 1) have focused largely on genetic variants and have identified associations with memory impairment232–235, executive dysfunction236,237, working memory impairment236, slowed processing speed234, depression238 and anxiety238, among other comorbidities. The potential role of epigenomic, transcriptomic and proteomic changes in epilepsy comorbidities is now beginning to be explored in humans239–241, and we recently identified >1,000 transcripts that were differentially expressed between TLE patients with and without memory impairment242. This study revealed overrepresentation of genes in pathways pertaining to brain-related neurological dysfunction and neurodegenerative diseases, such as apolipoprotein E (APOE), amyloid precursor protein (APP), microtubule-associated protein tau (MAPT) and serine/threonine-protein kinase PINK1, mitochondrial (PINK1). Importantly, several microRNAs were also differentially expressed and were predicted to target a large subset of the identified transcripts, suggesting such upstream processes could serve as biomarkers and potential treatment targets for memory impairment in TLE242.
Table 1 |.
Genetic factors associated with neurobehavioural comorbidities in epilepsy
| Comorbidity | Gene (polymorphism) | Cohort |
|---|---|---|
| Memory impairment | APOE (rs7412, rs429358) | Temporal lobe epilepsy232,233 |
| BDNF (rs6265) | Mesial temporal lobe epilepsy235 | |
| BDNF (rs1491850, rs2030324, rs11030094, rs12273363) | Newly diagnosed epilepsy234 | |
| REST (rs1105434, rs2227902) | Newly diagnosed epilepsy234 | |
| Executive dysfunction | BDNF (rs6265) | Temporal lobe epilepsy234 |
| COMT (rs4680) | Temporal lobe epilepsy and paediatric epilepsy (mixed types)236 | |
| MTHFR (rs1801133) | Paediatric epilepsy (mixed types)236 | |
| Impaired working memory | MTHFR (rs1801133) | Paediatric epilepsy (mixed types)236 |
| COMT (rs4680) | Paediatric epilepsy (mixed types)236 | |
| Reduced processing speed | REST (rs3796529) | Newly diagnosed epilepsy234 |
| Depression | BDNF (rs6265) | Refractory epilepsy238 |
| Anxiety | COMT (rs4680) | Refractory epilepsy238 |
APOE, apolipoprotein E; BDNF, brain-derived neurotrophic factor; COMT, catechol-O-methyltransferase; MTHFR, methylenetetrahydrofolate reductase; REST, RE1-silencing transcription factor.
Although much work remains to be done, a great deal has already been learned about the potential genetic contributions to epilepsy comorbidities through research on epilepsy syndromes associated with single-gene mutations243. Furthermore, animal studies and human research in epilepsy and other CNS disorders have highlighted the utility of genomic strategies in elucidating the biological underpinnings of common comorbidities associated with these conditions. We believe that incorporation of such methods into neuropsychological research in epilepsy will be essential to understand the observed phenotypic variability and to develop a precision medicine approach to the neuropsychology of epilepsy.
Social and psychological risk.
Epilepsy in adults is known to be more prevalent in lower (disadvantaged) socioeconomic groups, and is independent of social drift244 and other established risk factors, such as head injury and stroke245. Population-based investigations have demonstrated that adults with epilepsy have an increased likelihood of living in households with the lowest annual incomes246. These individuals are also sevenfold more likely to report experiencing discrimination due to health problems and have greater odds of experiencing domestic violence and sexual abuse compared with the general population247.
Although disadvantage, food insecurity, reduced personal safety and other hardships, including stigma and discrimination, are well known and documented in adults and children with epilepsy248–250, their relationship to neurobehavioural comorbidities has been vastly understudied, with just a few reports demonstrating their relevance to behaviour251, cognition252, 253 and quality of life254. In the Epilepsy Connectome Project, variables reflective of disadvantage, such as lower parental education, increased parental unemployment and increased racial diversity, were associated with the cognitive phenotypes and their underlying biological alterations (for example, anomalies on resting-state fMRI), underscoring the utility of a more comprehensive approach93. These risk factors can also have clinical consequences. The use of epilepsy treatments such as surgery is disproportionately low among ethnic minorities in the USA — a phenomenon that has been attributed to a host of factors, including access to care, fear, education, mistrust in the health-care system and physician bias255. Furthermore, epilepsy mortality rates are higher among non-Hispanic Black patients than in their non-Hispanic white counterparts256. Lack of access to specialized epilepsy care leads to poorer seizure control, which could exacerbate related issues, including cognitive impairment, psychiatric and behavioural comorbidities, and poor quality of life.
Direct and easily accessible markers of neighbourhood adversity257 are available that can inform the social determinants of health258. In the USA, the Neighborhood Adversity Index, an indicator of socioeconomic status disadvantage within a given region, has demonstrated applicability to brain disorders including Alzheimer disease259, in which disadvantage has been shown to be linked to imaging abnormalities (decreased hippocampal and cortical volumes)260 and underlying neuropathology261. This type of approach could also be informative for epilepsy.
Medical risk.
Given the prevalence and co-occurrence of somatic comorbidities in people with epilepsy262,263, interest in their relationships with cognitive and behavioural phenotypes is expected to grow. Direct characterization of comorbid disease and specific metabolic, vascular, inflammatory, immunological and other risks to cognition and behaviour in epilepsy is underway. The available evidence indicates that many medical risk factors, including obesity, diabetes and inflammatory markers, are overrepresented among individuals with epilepsy are and related to cognition264–267. Other important factors, such as atherosclerosis268, are widely documented in population-based epilepsy research, but have yet to be examined in relation to brain neuroimaging metrics, behaviour and cognition in people with epilepsy.
Resilience and reserve.
The identification of resilience factors — especially those that are modifiable — is crucial for epilepsy intervention and prevention efforts. These factors are of intense interest in other fields, such as ageing and preclinical and clinical neurodegenerative disorders269–271, and are of particular relevance in epilepsy in light of growing concern about brain and cognitive ageing processes in epilepsy150,272,273. Problematic lifestyle practices that have been documented in individuals with epilepsy include decreased physical fitness, activity and mental activities, smoking and social isolation246,274–276. Interventions targeted at improving health and lifestyle practices in patients with epilepsy have yet to be widely implemented in clinical practice. However, improvements in mood277, memory278 and executive function279 were reported in initial exercise intervention trials, and alterations in resting-state functional connectivity were linked to cognitive improvement278.
Resilience factors, which have been shown to be important in other areas of inquiry280, have not been extensively studied in epilepsy to date. However, consistent with the wider literature, protective effects of higher global ability level (intelligence)281,282, bilingualism283 and years of education56,284–286 on neurobehavioural status have been reported in people with epilepsy. These studies were all cross-sectional in nature, and a causal modelling approach will be needed to explore the roles of these factors in shaping cognitive and behavioural phenotypes. As noted above, certain genotypes have been linked to cognitive functioning in people with epilepsy (TABLE 1). However, genetic research in epilepsy comorbidities is in its infancy, and much work remains to be done to identify the factors that are most important for cognitive and behavioural resilience.
Self-efficacy beliefs lead to better adoption of the health habits and coping skills that are needed to manage chronic conditions such as epilepsy. In the context of epilepsy, self-management approaches have tended to focus on medication management rather than broader lifestyle modifications that are intended to improve overall health287,288. Self-management behaviours can be improved via interventions such as education, focused interventions and psychosocial therapy (for example, cognitive behavioural therapy289). However, many epilepsy self-management studies have excluded patients with cognitive impairment289. Furthermore, low health literacy and comorbid mental illnesses such as depression reduce the effectiveness of self-management interventions. The phenotypic approach that we advocate could enable self-management training to be tailored to the patient’s level of cognitive functioning and health-care literacy while also addressing mood-related issues such as depression and anxiety. Importantly, this proposed approach might help clinicians to implement individualized health-enhancing lifestyle behaviours that address health-related risk factors such as hypertension, obesity and diabetes, which are overrepresented among people with epilepsy.
Applications and benefits of the proposed paradigm
By placing the focus on the individual patient, a proposed new paradigm (FIG. 5) has direct clinical utility. Identification of a patient’s phenotype, as determined by empirical or actuarial methods, places them in a clinically meaningful category. A broader consideration of potential risk factors for neurobehavioural comorbidities could inform improvements to routine patient assessment and history taking. Validation of factors that predict adverse phenotype membership would lead to a better understanding of modifiable and non-modifiable treatment targets and, importantly, their relative predictive power, which would indicate where the most clinical impact might result. More generally, application of this paradigm would accelerate a better understanding of the relative predictive power of classic epilepsy-related versus non-epilepsy-related risk factors.
The relationship of phenotypes to longer-term cognitive and behavioural outcomes would yield valuable prognostic information to guide timely interventions in people with epilepsy. For example, factors that are likely to be linked to the generalized cognitive impairment phenotype include long-duration epilepsy (non-modifiable), elevated vascular risk (possibly modifiable), untreated depression and sleep apnoea (modifiable), and older age (non-modifiable). Understanding how this combination of risk factors manifests at the individual level in the phenotype of interest could help guide behavioural interventions and predict the risk of future decline or improvement (see ref.290 for a precision-based case example). However, a realistic view of the strengths and limitations of an approach of this type deserves careful consideration for future clinical and research efforts, as reviewed previously9.
Conclusions
In this Review, we have proposed a ‘next-generation’ precision approach to the neurobehavioural comorbidities of epilepsy, which offers to advance our understanding by identifying phenotypes that are applicable to individual patients, along with their correlates, course and, ultimately, their underlying genotypes. This revised paradigm embraces findings that are problematic for the classic model while retaining components of that model, including neuroimaging findings, which are consistent with the network view of epilepsy and now, as we have shown in this article, its comorbidities. This revised model integrates established aetiologies but expands them considerably with new directions for clinical research designed to improve patient care and quality of life, enhance biomarker discovery and inspire possible therapeutic strategies, with a focus on modifiable lifestyle factors. This phenotypic approach has the potential to alter the perspective of epilepsy from a condition characterized as ‘debilitating’ to one reflecting its actual underlying heterogeneity, whereby substantial proportions of adults and children with epilepsy demonstrate intact cognition, behaviour and quality of life. This change of emphasis should help us to focus on those most in need, moving to a multidimensional approach to care that will be crucial for improving neurobehavioural outcomes and quality of life.
Supplementary Material
Key points.
The cognitive and behavioural complications of the epilepsies have traditionally been examined in relation to the core characteristics of the disorder, such as the epilepsy syndrome, its aetiology, the frequency and severity of seizures, and treatments.
This ‘lesion model’ has been the predominant paradigm for over 100 years; however, substantial evidence of patient heterogeneity from cognitive, behavioural, neuroimaging, neuropathological, network science and clinical studies is inconsistent with this model.
A precision approach to epilepsy neurobehavioural comorbidities requires an understanding of this natural heterogeneity, which could be aided by a new taxonomy based on cognitive and behavioural phenotyping.
This Review surveys the literature that has identified cognitive and behavioural phenotypes in children and adults with epilepsy and provides a synopsis of the evolving taxonomy.
A new and expanded paradigm is proposed, which includes sophisticated neurobiological measures, genomics, comorbid medical disease, diversity and adversity, and resilience factors.
Acknowledgements
The authors acknowledge funding from NIH KL2 TR000440 (R. M. B.), NIH R01 NS097719 (R. M. B.), NIH R01 NS065838 (C. R. M.), NIH R21 NS107739 (C. R. M.), NIH T32 MH018399 (E. K.), NIH R01 NS111022 (A. F. S. and B. P. H.) and NIH F31 NS111883-01 (A. R.). The authors thank Mary Lou Smith for reading of a pre-submission draft.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.England MJ, Liverman CT, Schultz AM & Strawbridge LM Epilepsy across the spectrum: promoting health and understanding. A summary of the Institute of Medicine report. Epilepsy Behav 25, 266–276 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Epilepsy: A public health imperative. World Health Organization, https://www.who.int/publications-detail-redirect/epilepsy-a-public-health-imperative (2019). [Google Scholar]
- 3.Venne J. et al. International consortium for personalized medicine: an international survey about the future of personalized medicine. Per Med 17, 89–100 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Jameson JL & Longo DL Precision medicine--personalized, problematic, and promising. N Engl J Med 372, 2229–2234 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Williams JR et al. Current applications of precision medicine: a bibliometric analysis. Per Med 16, 351–359 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strzelczyk A, Klein KM & von Podewils F. Editorial: Burden of illness in people with epilepsy: from population-based studies to precision medicine. Front Neurol 9, 1164 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kearney H, Byrne S, Cavalleri GL & Delanty N. Tackling epilepsy with high-definition precision medicine: a review. JAMA Neurol 76, 1109–1116 (2019). [DOI] [PubMed] [Google Scholar]
- 8.EpiPM Consortium. A roadmap for precision medicine in the epilepsies. Lancet Neurol 14, 1219–1228 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Josephson CB & Wiebe S. Precision medicine: academic dreaming or clinical reality? Epilepsia 62 (Suppl. 2), S78–S89 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Josephson CB. et al. Psychosocial profiles and their predictors using patient-reported outcomes and machine learning. Epilepsia 61, 1201–1210 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Nickels KC, Zaccariello MJ, Hamiwka LD & Wirrell EC Cognitive and neurodevelopmental comorbidities in paediatric epilepsy. Nat Rev Neurol 12, 465–76 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Loring DW History of neuropsychology through epilepsy eyes. Arch Clin Neuropsychol 25, 25973 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallin JEW Eight months of psycho-clinical research at the New Jersey State Village for Epileptics, with some results from the Binet-Simon testing. Epilepsia A3, 366–380 (1912). [Google Scholar]
- 14.Fox JT The response of epileptic children to mental and educational tests. . British Journal of Medical Psychology 4, 235–248 (1924). [Google Scholar]
- 15.Collins AL, Atwell CR, Moore M. Stanford-Binet response patterns in epileptics. Amer J Orthopsychiatry 8, 51–63 (1938). [Google Scholar]
- 16.Hermann B. Intelligence and epilepsy: The early era. Epilepsy Behav 101, 106597 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Jarčušková D, Palušná M, Gazda J, Feketeová E. & Gdovinová Z. Which clinical and neuropsychological factors are responsible for cognitive impairment in patients with epilepsy? Int J Public Health 65, 947–956 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Gavrilovic A. et al. Impact of epilepsy duration, seizure control and EEG abnormalities on cognitive impairment in drug-resistant epilepsy patients. Acta Neurol Belg 119, 403–410 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Harrower-Erickson M. in Epilepsy and Cerebral Localization: a Study of the Mechanism, Treatment and Prevention of Epileptic Seizures (eds Penfield W. & Erickson TC) 546–574. (Charles C Thomas, Springfield IL, 1941). [Google Scholar]
- 20.Collins AL Epileptic intelligence. J Consult Psychol 15, 392–9 (1951). [DOI] [PubMed] [Google Scholar]
- 21.Lennox W. in Epilepsy and Related Disorders (eds Lennox WG & Lennox-Buchthal MA) 659–699 (Little, Brown and Company, Boston MA, 1960). [Google Scholar]
- 22.Tarter RE Intellectual and adaptive functioning in epilepsy. a review of 50 years of research. Dis Nerv Syst 33, 763–70 (1972). [PubMed] [Google Scholar]
- 23.Brown SW & Reynolds EH in Epilepsy and Psychiatry (eds Reynolds EH & Trimble MR) 147–164 (Churchill Livingston, Edinburgh, 1981). [Google Scholar]
- 24.Dodrill CB & Matthews CG The role of neuropsychology in the assessment and treatment of persons with epilepsy. Am Psychol 47, 1139–1142 (1992). [DOI] [PubMed] [Google Scholar]
- 25.Elger CE, Helmstaedter C. & Kurthen M. Chronic epilepsy and cognition. Lancet Neurol 3, 663–672 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Baxendale S. & Thompson P. The new approach to epilepsy classification: Cognition and behavior in adult epilepsy syndromes. Epilepsy Behav 64, 253–256 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Ratcliffe C. et al. Cognitive function in genetic generalized epilepsies: insights from neuropsychology and neuroimaging. Front Neurol 11, 144 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loughman A, Bendrups NA & D’Souza WJ A systematic review of psychiatric and psychosocial comorbidities of genetic generalised epilepsies (GGE). Neuropsychol Rev 26, 364–375 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Wickens S, Bowden SC & D’Souza W. Cognitive functioning in children with self-limited epilepsy with centrotemporal spikes: A systematic review and meta-analysis. Epilepsia 58, 1673–1685 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Verche E, San Luis C. & Hernández S. Neuropsychology of frontal lobe epilepsy in children and adults: Systematic review and meta-analysis. Epilepsy Behav 88, 15–20 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Fonseca Wald ELA et al. Towards a better understanding of cognitive deficits in absence epilepsy: a systematic review and meta-analysis. Neuropsychol Rev 29, 421–449 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith A, Syvertsen M. & Pal DK Meta-analysis of response inhibition in juvenile myoclonic epilepsy. Epilepsy Behav 106, 107038 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Shorvon SD The causes of epilepsy: changing concepts of etiology of epilepsy over the past 150 years. Epilepsia 52, 1033–1044 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Gastaut H. Clinical and electroencephalographical classification of epileptic seizures. Epilepsia 10 (Suppl.), 2–13 (1969). [PubMed] [Google Scholar]
- 35.Wolf P. Basic principles of the ILAE syndrome classification. Epilepsy Res 70 (Suppl. 1), S20–S26 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Wolf P. History of epilepsy: nosological concepts and classification. Epileptic Disord 16, 261–269 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Scheffer IE et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 58, 512–521 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baxendale S. & Thompson P. Beyond localization: the role of traditional neuropsychological tests in an age of imaging. Epilepsia 51, 2225–2230 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Gold JA, Sher Y. & Maldonado JR Frontal lobe epilepsy: a primer for psychiatrists and a systematic review of psychiatric manifestations. Psychosomatics 57, 445–464 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Foran A, Bowden S, Bardenhagen F, Cook M. & Meade C. Specificity of psychopathology in temporal lobe epilepsy. Epilepsy Behav 27, 193–199 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Gloor P, Jasper H. & Milner B. Higher functions of the nervous system. Annu Rev Physiol 18, 359–386 (1956). [DOI] [PubMed] [Google Scholar]
- 42.Milner B. Psychological aspects of focal epilepsy and its neurosurgical management. Adv Neurol 8, 299–321 (1975). [PubMed] [Google Scholar]
- 43.Mirsky AF, Primac DW, Marsan CA, Rosvold HE & Stevens JR A comparison of the psychological test performance of atients with focal and nonfocal epilepsy. Exp Neurol 2, 75–89 (1960). [DOI] [PubMed] [Google Scholar]
- 44.Staden U, Isaacs E, Boyd SG, Brandl U. & Neville BG Language dysfunction in children with Rolandic epilepsy. Neuropediatrics 29, 242–248 (1998). [DOI] [PubMed] [Google Scholar]
- 45.Germanò E. et al. Benign childhood epilepsy with occipital paroxysms: neuropsychological findings. Epilepsy Res 64, 137–150 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Swartz BE, Halgren E, Simpkins F, Syndulko K. Primary memory in patients with frontal and primary generalized epilepsy. Journal of Epilepsy 7, 232–241 (1994). [Google Scholar]
- 47.Janz D, Christian W. Impulsiv-petit mal. Dtsch Z Nervenheilk 176, 346–386 (1957). [Google Scholar]
- 48.Jokeit H. & Schacher M. Neuropsychological aspects of type of epilepsy and etiological factors in adults. Epilepsy Behav 5 (Suppl. 1), S14–S20 (2004). [DOI] [PubMed] [Google Scholar]
- 49.Schoenfeld J. et al. Neuropsychological and behavioral status of children with complex partial seizures. Dev Med Child Neurol 41, 724–731 (1999). [DOI] [PubMed] [Google Scholar]
- 50.Guimarães CA et al. Temporal lobe epilepsy in childhood: comprehensive neuropsychological assessment. J Child Neurol 22, 836–840 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Rzezak P. et al. Frontal lobe dysfunction in children with temporal lobe epilepsy. Pediatr Neurol 37, 176–185 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Braakman HM et al. Cognitive and behavioral complications of frontal lobe epilepsy in children: a review of the literature. Epilepsia 52, 849–856 (2011). [DOI] [PubMed] [Google Scholar]
- 53.Braakman HM et al. Cognitive and behavioural findings in children with frontal lobe epilepsy. Eur J Paediatr Neurol 16, 707–715 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Smith ML Rethinking cognition and behavior in the new classification for childhood epilepsy: Examples from frontal lobe and temporal lobe epilepsies. Epilepsy Behav 64, 313–317 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Hermann BP, Seidenberg M, Schoenfeld J. & Davies K. Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Arch Neurol 54, 369–376 (1997). [DOI] [PubMed] [Google Scholar]
- 56.Oyegbile TO et al. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology 62, 1736–1742 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Wang WH et al. Neuropsychological performance and seizure-related risk factors in patients with temporal lobe epilepsy: a retrospective cross-sectional study. Epilepsy Behav 22, 728–734 (2011). [DOI] [PubMed] [Google Scholar]
- 58.Hwang G. et al. Cognitive slowing and its underlying neurobiology in temporal lobe epilepsy. Cortex 117, 41–52 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patrikelis P. et al. Preoperative neuropsychological presentation of patients with refractory frontal lobe epilepsy. Acta Neurochir (Wien) 158, 1139–1150 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Patrikelis P, Angelakis E. & Gatzonis S. Neurocognitive and behavioral functioning in frontal lobe epilepsy: a review. Epilepsy Behav 14, 19–26 (2009). [DOI] [PubMed] [Google Scholar]
- 61.Exner C. et al. Neuropsychological performance in frontal lobe epilepsy. Seizure 11, 20–32 (2002). [DOI] [PubMed] [Google Scholar]
- 62.Gülgönen S, Demirbilek V, Korkmaz B, Dervent A. & Townes BD Neuropsychological functions in idiopathic occipital lobe epilepsy. Epilepsia 41, 405–411 (2000). [DOI] [PubMed] [Google Scholar]
- 63.Gleissner U, Kuczaty S, Clusmann H, Elger CE & Helmstaedter C. Neuropsychological results in pediatric patients with epilepsy surgery in the parietal cortex. Epilepsia 49, 700–704 (2008). [DOI] [PubMed] [Google Scholar]
- 64.Traianou A, Patrikelis P, Kosmidis MH, Kimiskidis V. & Gatzonis S. The neuropsychological profile of parietal and occipital lobe epilepsy. Epilepsy Behav 94, 137–143 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Stretton J. & Thompson PJ Frontal lobe function in temporal lobe epilepsy. Epilepsy Res 98, 113 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Helmstaedter C, Kemper B. & Elger CE Neuropsychological aspects of frontal lobe epilepsy. Neuropsychologia 34, 399–406 (1996). [DOI] [PubMed] [Google Scholar]
- 67.Wandschneider B, Thompson PJ, Vollmar C. & Koepp MJ Frontal lobe function and structure in juvenile myoclonic epilepsy: a comprehensive review of neuropsychological and imaging data. Epilepsia 53, 2091–2098 (2012). [DOI] [PubMed] [Google Scholar]
- 68.Conant LL, Wilfong A, Inglese C. & Schwarte A. Dysfunction of executive and related processes in childhood absence epilepsy. Epilepsy Behav 18, 414–423 (2010). [DOI] [PubMed] [Google Scholar]
- 69.Filippini M. et al. Neuropsychological profile in new-onset benign epilepsy with centrotemporal spikes (BECTS): Focusing on executive functions. Epilepsy Behav 54, 71–79 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Neri ML et al. Neuropsychological assessment of children with rolandic epilepsy: executive functions. Epilepsy Behav 24, 403–407 (2012). [DOI] [PubMed] [Google Scholar]
- 71.Caplan R. et al. Language in pediatric epilepsy. Epilepsia 50, 2397–2407 (2009). [DOI] [PubMed] [Google Scholar]
- 72.Jackson DC et al. The neuropsychological and academic substrate of new/recent-onset epilepsies. J Pediatr 162, 1047–1053 e1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verly M. et al. Evaluation of the language profile in children with rolandic epilepsy and developmental dysphasia: Evidence for distinct strengths and weaknesses. Brain Lang 170, 18–28 (2017). [DOI] [PubMed] [Google Scholar]
- 74.Smith AB, Bajomo O. & Pal DK A meta-analysis of literacy and language in children with rolandic epilepsy. Dev Med Child Neurol 57, 1019–1026 (2015). [DOI] [PubMed] [Google Scholar]
- 75.Carvalho KC et al. Cognitive performance in juvenile myoclonic epilepsy patients with specific endophenotypes. Seizure 40, 33–41 (2016). [DOI] [PubMed] [Google Scholar]
- 76.Sonmez F, Atakli D, Sari H, Atay T. & Arpaci B. Cognitive function in juvenile myoclonic epilepsy. Epilepsy Behav 5, 329–336 (2004). [DOI] [PubMed] [Google Scholar]
- 77.Hamberger MJ & Cole J. Language organization and reorganization in epilepsy. Neuropsychol Rev 21, 240–251 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bartha-Doering L. & Trinka E. The interictal language profile in adult epilepsy. Epilepsia 55, 1512–1525 (2014). [DOI] [PubMed] [Google Scholar]
- 79.Fastenau PS et al. Neuropsychological status at seizure onset in children: risk factors for early cognitive deficits. Neurology 73, 526–534 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lopes AF et al. Intellectual functioning in children with epilepsy: frontal lobe epilepsy, childhood absence epilepsy and benign epilepsy with centro-temporal spikes. Seizure 22, 886–892 (2013). [DOI] [PubMed] [Google Scholar]
- 81.Lopes AF, Monteiro JP, Fonseca MJ, Robalo C. & Simões MR Memory functioning in children with epilepsy: frontal lobe epilepsy, childhood absence epilepsy, and benign epilepsy with centrotemporal spikes. Behav Neurol 2014, 218637 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nolan MA et al. Intelligence in childhood epilepsy syndromes. Epilepsy Res 53, 139–150 (2003). [DOI] [PubMed] [Google Scholar]
- 83.Nolan MA et al. Memory function in childhood epilepsy syndromes. J Paediatr Child Health 40, 20–27 (2004). [DOI] [PubMed] [Google Scholar]
- 84.Laurent A. et al. Metabolic correlates of cognitive impairment in mesial temporal lobe epilepsy. Epilepsy Behav 105, 106948 (2020). [DOI] [PubMed] [Google Scholar]
- 85.Jokeit H. et al. Prefrontal asymmetric interictal glucose hypometabolism and cognitive impairment in patients with temporal lobe epilepsy. Brain 120, 2283–2294 (1997). [DOI] [PubMed] [Google Scholar]
- 86.Dinkelacker V, Xin X, Baulac M, Samson S. & Dupont S. Interictal epileptic discharge correlates with global and frontal cognitive dysfunction in temporal lobe epilepsy. Epilepsy Behav 62, 197–203 (2016). [DOI] [PubMed] [Google Scholar]
- 87.Keller SS, Baker G, Downes JJ & Roberts N. Quantitative MRI of the prefrontal cortex and executive function in patients with temporal lobe epilepsy. Epilepsy Behav 15, 186–195 (2009). [DOI] [PubMed] [Google Scholar]
- 88.Riley JD, Moore S, Cramer SC & Lin JJ Caudate atrophy and impaired frontostriatal connections are linked to executive dysfunction in temporal lobe epilepsy. Epilepsy Behav 21, 80–87 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hermann B, Hansen R, Seidenberg M, Magnotta V. & O’Leary D. Neurodevelopmental vulnerability of the corpus callosum to childhood onset localization-related epilepsy. Neuroimage 18, 284–292 (2003). [DOI] [PubMed] [Google Scholar]
- 90.Tuchscherer V. et al. Extrahippocampal integrity in temporal lobe epilepsy and cognition: thalamus and executive functioning. Epilepsy Behav 17, 478–482 (2010). [DOI] [PubMed] [Google Scholar]
- 91.Reyes A. et al. Decreased neurite density within frontostriatal networks is associated with executive dysfunction in temporal lobe epilepsy. Epilepsy Behav 78, 187–193 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kucukboyaci NE et al. Role of frontotemporal fiber tract integrity in task-switching performance of healthy controls and patients with temporal lobe epilepsy. J Int Neuropsychol Soc 18, 57–67 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hermann B. et al. Network, clinical and sociodemographic features of cognitive phenotypes in temporal lobe epilepsy. Neuroimage Clin 27, 102341 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oyegbile TO et al. Executive dysfunction is associated with an altered executive control network in pediatric temporal lobe epilepsy. Epilepsy Behav 86, 145–152 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oyegbile TO et al. Default mode network deactivation in pediatric temporal lobe epilepsy: Relationship to a working memory task and executive function tests. Epilepsy Behav 94, 124–130 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang C. et al. Characteristics of resting-state functional connectivity in intractable unilateral temporal lobe epilepsy patients with impaired executive control function. Front Hum Neurosci 11, 609 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bromfield EB et al. Cerebral metabolism and depression in patients with complex partial seizures. Arch Neurol 49, 617–623 (1992). [DOI] [PubMed] [Google Scholar]
- 98.Salzberg M. et al. Depression in temporal lobe epilepsy surgery patients: an FDG-PET study. Epilepsia 47, 2125–2130 (2006). [DOI] [PubMed] [Google Scholar]
- 99.Butler T. et al. Cortical thickness abnormalities associated with depressive symptoms in temporal lobe epilepsy. Epilepsy Behav 23, 64–67 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nogueira MH et al. Major depressive disorder associated with reduced cortical thickness in women with temporal lobe epilepsy. Front Neurol 10, 1398 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Conradi N. et al. Factorial validity of a neuropsychological test battery and its ability to discern temporal lobe epilepsy from frontal lobe epilepsy — a retrospective study. Seizure 74, 81–88 (2020). [DOI] [PubMed] [Google Scholar]
- 102.Lima EM et al. The executive profile of children with benign epilepsy of childhood with centrotemporal spikes and temporal lobe epilepsy. Epilepsy Behav 72, 173–177 (2017). [DOI] [PubMed] [Google Scholar]
- 103.Frank B. et al. Machine learning as a new paradigm for characterizing localization and lateralization of neuropsychological test data in temporal lobe epilepsy. Epilepsy Behav 86, 58–65 (2018). [DOI] [PubMed] [Google Scholar]
- 104.Roger E. et al. A machine learning approach to explore cognitive signatures in patients with temporo-mesial epilepsy. Neuropsychologia 142, 107455 (2020). [DOI] [PubMed] [Google Scholar]
- 105.Margerison JH & Corsellis JA Epilepsy and the temporal lobes. A clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain 89, 499–530 (1966). [DOI] [PubMed] [Google Scholar]
- 106.Blanc F. et al. Investigation of widespread neocortical pathology associated with hippocampal sclerosis in epilepsy: a postmortem study. Epilepsia 52, 10–21 (2011). [DOI] [PubMed] [Google Scholar]
- 107.Tai XY et al. Review: neurodegenerative processes in temporal lobe epilepsy with hippocampal sclerosis: Clinical, pathological and neuroimaging evidence. Neuropathol Appl Neurobiol 44, 70–90 (2018). [DOI] [PubMed] [Google Scholar]
- 108.Gourmaud S. et al. Alzheimer-like amyloid and tau alterations associated with cognitive deficit in temporal lobe epilepsy. Brain 143, 191–209 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mackenzie IR & Miller LA Senile plaques in temporal lobe epilepsy. Acta Neuropathol 87, 504–510 (1994). [DOI] [PubMed] [Google Scholar]
- 110.Smith KM et al. Tau deposition in young adults with drug-resistant focal epilepsy. Epilepsia 60, 2398–2403 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meencke HJ, Janz D. & Cervos-Navarro J. Neuropathology of primary generalized epilepsies with awakening grand mal. Acta Neuropathol Suppl 7, 378–380 (1981). [DOI] [PubMed] [Google Scholar]
- 112.Meencke HJ & Janz D. Neuropathological findings in primary generalized epilepsy: a study of eight cases. Epilepsia 25, 8–21 (1984). [DOI] [PubMed] [Google Scholar]
- 113.Opeskin K, Kalnins RM, Halliday G, Cartwright H. & Berkovic SF Idiopathic generalized epilepsy: lack of significant microdysgenesis. Neurology 55, 1101–1106 (2000). [DOI] [PubMed] [Google Scholar]
- 114.Croll L, Szabo CA, Abou-Madi N. & Devinsky O. Epilepsy in nonhuman primates. Epilepsia 60, 1526–1538 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Young NA et al. Epileptic baboons have lower numbers of neurons in specific areas of cortex. Proc Natl Acad Sci U S A 110, 19107–19112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Keller SS & Roberts N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia 49, 741–757 (2008). [DOI] [PubMed] [Google Scholar]
- 117.Lin JJ et al. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cereb Cortex 17, 2007–2018 (2007). [DOI] [PubMed] [Google Scholar]
- 118.Keller SS et al. Morphometric MRI alterations and postoperative seizure control in refractory temporal lobe epilepsy. Hum Brain Mapp 36, 1637–1647 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McDonald CR et al. Regional neocortical thinning in mesial temporal lobe epilepsy. Epilepsia 49, 794–803 (2008). [DOI] [PubMed] [Google Scholar]
- 120.Oyegbile T. et al. Quantitative measurement of cortical surface features in localization-related temporal lobe epilepsy. Neuropsychology 18, 729–737 (2004). [DOI] [PubMed] [Google Scholar]
- 121.Ronan L. et al. Cortical curvature analysis in MRI-negative temporal lobe epilepsy: a surrogate marker for malformations of cortical development. Epilepsia 52, 28–34 (2011). [DOI] [PubMed] [Google Scholar]
- 122.Nuyts S, D’Souza W, Bowden SC & Vogrin SJ Structural brain abnormalities in genetic generalized epilepsies: A systematic review and meta-analysis. Epilepsia 58, 2025–2037 (2017). [DOI] [PubMed] [Google Scholar]
- 123.Slinger G, Sinke MR, Braun KP & Otte WM White matter abnormalities at a regional and voxel level in focal and generalized epilepsy: A systematic review and meta-analysis. Neuroimage Clin 12, 902–909 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Whelan CD et al. Structural brain abnormalities in the common epilepsies assessed in a worldwide ENIGMA study. Brain 141, 391–408 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hatton SN et al. White matter abnormalities across different epilepsy syndromes in adults: an ENIGMA-Epilepsy study. Brain 143, 2454–2473 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Caciagli L, Bernhardt BC, Hong SJ, Bernasconi A. & Bernasconi N. Functional network alterations and their structural substrate in drug-resistant epilepsy. Front Neurosci 8, 411 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carney PW & Jackson GD Insights into the mechanisms of absence seizure generation provided by EEG with functional MRI. Front Neurol 5, 162 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vollmar C. et al. Motor system hyperconnectivity in juvenile myoclonic epilepsy: a cognitive functional magnetic resonance imaging study. Brain 134, 1710–1719 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Blumenfeld H. et al. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain 132, 999–1012 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang KL et al. Metabolic covariance networks combining graph theory measuring aberrant topological patterns in mesial temporal lobe epilepsy. CNS Neurosci Ther 25, 396–408 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Englot DJ et al. Global and regional functional connectivity maps of neural oscillations in focal epilepsy. Brain 138, 2249–2262 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.van Mierlo P, Höller Y, Focke NK & Vulliemoz S. Network Perspectives on Epilepsy Using EEG/MEG Source Connectivity. Front Neurol 10, 721 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lagarde S. et al. Interictal stereotactic-EEG functional connectivity in refractory focal epilepsies. Brain 141, 2966–2980 (2018). [DOI] [PubMed] [Google Scholar]
- 134.Morgan VL, Conrad BN, Abou-Khalil B, Rogers BP & Kang H. Increasing structural atrophy and functional isolation of the temporal lobe with duration of disease in temporal lobe epilepsy. Epilepsy Res 110, 171–178 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee HW et al. Altered functional connectivity in seizure onset zones revealed by fMRI intrinsic connectivity. Neurology 83, 2269–2277 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dansereau CL et al. Detection of abnormal resting-state networks in individual patients suffering from focal epilepsy: an initial step toward individual connectivity assessment. Front Neurosci 8, 419 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dinkelacker V. et al. Hippocampal-thalamic wiring in medial temporal lobe epilepsy: Enhanced connectivity per hippocampal voxel. Epilepsia 56, 1217–1226 (2015). [DOI] [PubMed] [Google Scholar]
- 138.Pedersen M, Curwood EK, Vaughan DN, Omidvarnia AH & Jackson GD Abnormal Brain Areas Common to the Focal Epilepsies: Multivariate Pattern Analysis of fMRI. Brain Connect 6, 208–215 (2016). [DOI] [PubMed] [Google Scholar]
- 139.He X. et al. Disrupted basal ganglia-thalamocortical loops in focal to bilateral tonic-clonic seizures. Brain 143, 175–190 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Haneef Z. et al. Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia 55, 137–145 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liao W. et al. Altered functional connectivity and small-world in mesial temporal lobe epilepsy. PLoS One 5, e8525 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Luo C, An D, Yao D. & Gotman J. Patient-specific connectivity pattern of epileptic network in frontal lobe epilepsy. Neuroimage Clin 4, 668–675 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nedic S. et al. Using network dynamic fMRI for detection of epileptogenic foci. BMC Neurol 15, 262 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Widjaja E. et al. Disrupted Global and Regional Structural Networks and Subnetworks in Children with Localization-Related Epilepsy. AJNR Am J Neuroradiol 36, 1362–1368 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Haneef Z, Lenartowicz A, Yeh HJ, Engel J Jr. & Stern JM Effect of lateralized temporal lobe epilepsy on the default mode network. Epilepsy Behav 25, 350–357 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bai X. et al. Resting functional connectivity between the hemispheres in childhood absence epilepsy. Neurology 76, 1960–1967 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kemmotsu N. et al. MRI analysis in temporal lobe epilepsy: cortical thinning and white matter disruptions are related to side of seizure onset. Epilepsia 52, 2257–2266 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee CY et al. Microstructural integrity of early- versus late-myelinating white matter tracts in medial temporal lobe epilepsy. Epilepsia 54, 1801–1809 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Thompson PJ & Duncan JS Cognitive decline in severe intractable epilepsy. Epilepsia 46, 1780–1787 (2005). [DOI] [PubMed] [Google Scholar]
- 150.Galovic M. et al. Progressive cortical thinning in patients with focal epilepsy. JAMA Neurol 76, 1230–1239 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hermann BP et al. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol 60, 80–87 (2006). [DOI] [PubMed] [Google Scholar]
- 152.Austin JK et al. Behavior problems in children before first recognized seizures. Pediatrics 107, 115–122 (2001). [DOI] [PubMed] [Google Scholar]
- 153.Hermann BP, Jones JE, Jackson DC & Seidenberg M. Starting at the beginning: the neuropsychological status of children with new-onset epilepsies. Epileptic Disord 14, 12–21 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Taylor J. et al. Patients with epilepsy: cognitively compromised before the start of antiepileptic drug treatment? Epilepsia 51, 48–56 (2010). [DOI] [PubMed] [Google Scholar]
- 155.Witt JA & Helmstaedter C. Cognition in the early stages of adult epilepsy. Seizure 26, 65–68 (2015). [DOI] [PubMed] [Google Scholar]
- 156.Oostrom KJ, Smeets-Schouten A, Kruitwagen CL, Peters AC & Jennekens-Schinkel A. Not only a matter of epilepsy: early problems of cognition and behavior in children with “epilepsy only”--a prospective, longitudinal, controlled study starting at diagnosis. Pediatrics 112, 1338–1344 (2003). [DOI] [PubMed] [Google Scholar]
- 157.Keezer MR, Sisodiya SM & Sander JW Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol 15, 106–115 (2016). [DOI] [PubMed] [Google Scholar]
- 158.Lüders HO, Najm I, Nair D, Widdess-Walsh P. & Bingman W. The epileptogenic zone: general principles. Epileptic Disord 8 (Suppl. 2), S1–S9 (2006). [PubMed] [Google Scholar]
- 159.Goodale SE et al. Resting-state SEEG may help localize epileptogenic brain regions. Neurosurgery 86, 792–801 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li YH et al. Localization of epileptogenic zone based on graph analysis of stereo-EEG. Epilepsy Res 128, 149–157 (2016). [DOI] [PubMed] [Google Scholar]
- 161.Mao JW et al. Dynamic Network Connectivity analysis to identify epileptogenic zones based on stereo-electroencephalography. Front Comput Neurosci 10, 113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Storti SF, Galazzo IB, Khan S, Manganotti P. & Menegaz G. Exploring the Epileptic Brain Network Using Time-Variant Effective Connectivity and Graph Theory. IEEE J Biomed Health Inform 21, 1411–1421 (2017). [DOI] [PubMed] [Google Scholar]
- 163.van Mierlo P. et al. Accurate epileptogenic focus localization through time-variant functional connectivity analysis of intracranial electroencephalographic signals. Neuroimage 56, 1122–1133 (2011). [DOI] [PubMed] [Google Scholar]
- 164.Bernhardt BC, Hong S, Bernasconi A. & Bernasconi N. Imaging structural and functional brain networks in temporal lobe epilepsy. Front Hum Neurosci 7, 624 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Focke NK et al. Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Neuroimage 40, 728–737 (2008). [DOI] [PubMed] [Google Scholar]
- 166.Larivière S. et al. Microstructure-informed connectomics: enriching large-scale descriptions of healthy and diseased brains. Brain Connect 9, 113–127 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Morgan VL, Chang C, Englot DJ & Rogers BP Temporal lobe epilepsy alters spatiot-emporal dynamics of the hippocampal functional network. Neuroimage Clin 26, 102254 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jehi L. Outcomes of Epilepsy Surgery for Epileptic Networks. Epilepsy Curr 17, 160–162 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rayner G, Tailby C, Jackson G. & Wilson S. Looking beyond lesions for causes of neuropsychological impairment in epilepsy. Neurology 92, e680–e689 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wilson SJ & Baxendale S. The new approach to classification: rethinking cognition and behavior in epilepsy. Epilepsy Behav 41, 307–310 (2014). [DOI] [PubMed] [Google Scholar]
- 171.Rayner G, Jackson GD & Wilson SJ Two distinct symptom-based phenotypes of depression in epilepsy yield specific clinical and etiological insights. Epilepsy Behav 64, 336–344 (2016). [DOI] [PubMed] [Google Scholar]
- 172.Gonzalez LM & Wrennall JA A neuropsychological model for the pre-surgical evaluation of children with focal-onset epilepsy: An integrated approach. Seizure 77, 29–39 (2020). [DOI] [PubMed] [Google Scholar]
- 173.Rayner G. & Tailby C. Current concepts of memory disorder in epilepsy: edging towards a network account. Curr Neurol Neurosci Rep 17, 55 (2017). [DOI] [PubMed] [Google Scholar]
- 174.Parker D. Kuhnian revolutions in neuroscience: the role of tool development. Biol Philos 33, 17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Paradiso S, Hermann BP & Somes G. Patterns of academic competence in adults with epilepsy: a cluster analytic study. Epilepsy Res 19, 253–261 (1994). [DOI] [PubMed] [Google Scholar]
- 176.Hermann B, Seidenberg M, Lee EJ, Chan F. & Rutecki P. Cognitive phenotypes in temporal lobe epilepsy. J Int Neuropsychol Soc 13, 12–20 (2007). [DOI] [PubMed] [Google Scholar]
- 177.Dabbs K, Jones J, Seidenberg M. & Hermann B. Neuroanatomical correlates of cognitive phenotypes in temporal lobe epilepsy. Epilepsy Behav 15, 445–451 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Berl MM et al. Characterization of atypical language activation patterns in focal epilepsy. Ann Neurol 75, 33–42 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hermann BP et al. Cognitive phenotypes in childhood idiopathic epilepsies. Epilepsy Behav 61, 269–274 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rodríguez-Cruces R. et al. Association of white matter diffusion characteristics and cognitive deficits in temporal lobe epilepsy. Epilepsy Behav 79, 138–145 (2018). [DOI] [PubMed] [Google Scholar]
- 181.Elverman KH et al. Temporal lobe epilepsy is associated with distinct cognitive phenotypes. Epilepsy Behav 96, 61–68 (2019). [DOI] [PubMed] [Google Scholar]
- 182.Reyes A. et al. Cognitive phenotypes in temporal lobe epilepsy are associated with distinct patterns of white matter network abnormalities. Neurology 92, e1957–e1968 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kaestner E. et al. Identifying the neural basis of a language-impaired phenotype of temporal lobe epilepsy. Epilepsia 60, 1627–1638 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Baxendale S. & Thompson P. The association of cognitive phenotypes with postoperative outcomes after epilepsy surgery in patients with temporal lobe epilepsy. Epilepsy Behav 112, 107386 (2020). [DOI] [PubMed] [Google Scholar]
- 185.Struck AF et al. Regional and global resting-state functional MR connectivity in temporal lobe epilepsy: results from the Epilepsy Connectome Project. Epilepsy Behav 117, 107841 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hermann BP, Struck AF, Dabbs K, Seidenberg M. & Jones JE Behavioral phenotypes of temporal lobe epilepsy. Epilepsia Open 6, 369–380 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Reyes A. et al. Cognitive phenotypes in temporal lobe epilepsy utilizing data- and clinically driven approaches: moving toward a new taxonomy. Epilepsia 61, 1211–1220 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rodríguez-Cruces R, Bernhardt BC & Concha L. Multidimensional associations between cognition and connectome organization in temporal lobe epilepsy. Neuroimage 213, 116706 (2020). [DOI] [PubMed] [Google Scholar]
- 189.Modi AC et al. Executive functioning phenotypes in youth with epilepsy. Epilepsy Behav 90, 112–118 (2019). [DOI] [PubMed] [Google Scholar]
- 190.Puka K. & Smith ML Long-term outcomes of children with drug-resistant epilepsy across multiple cognitive domains. Dev Med Child Neurol 63, 690–696 (2021). [DOI] [PubMed] [Google Scholar]
- 191.Puka K. & Smith ML Long-term outcomes of children with drug-resistant epilepsy across multiple cognitive domains. Dev Med Child Neurol 63, 690–696 (2021). [DOI] [PubMed] [Google Scholar]
- 192.Hermann BP et al. Behavioral phenotypes of childhood idiopathic epilepsies. Epilepsia 61, 1427–1437 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Parvizi J. & Kastner S. Promises and limitations of human intracranial electroencephalography. Nat Neurosci 21, 474–483 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Helfrich RF & Knight RT Oscillatory dynamics of prefrontal cognitive control. Trends Cogn Sci 20, 916–930 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Le Van Quyen M. et al. High-frequency oscillations in human and monkey neocortex during the wake-sleep cycle. Proc Natl Acad Sci U S A 113, 9363–9368 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shuman T, Amendolara B. & Golshani P. Theta rhythmopathy as a cause of cognitive disability in TLE. Epilepsy Curr 17, 107–111 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liu S. & Parvizi J. Cognitive refractory state caused by spontaneous epileptic high-frequency oscillations in the human brain. Sci Transl Med 11, eaax7830 (2019). [DOI] [PubMed] [Google Scholar]
- 198.Matsumoto R, Kunieda T. & Nair D. Single pulse electrical stimulation to probe functional and pathological connectivity in epilepsy. Seizure 44, 27–36 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Keller CJ et al. Mapping human brain networks with cortico-cortical evoked potentials. Philos Trans R Soc Lond B Biol Sci 369, 20130528 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Alonso F, Sweet J. & Miller J. Speech mapping using depth electrodes: the “electric Wada”. Clin Neurol Neurosurg 144, 88–90 (2016). [DOI] [PubMed] [Google Scholar]
- 201.Wilson SJ et al. Developmental outcomes of childhood-onset temporal lobe epilepsy: a community-based study. Epilepsia 53, 1587–1596 (2012). [DOI] [PubMed] [Google Scholar]
- 202.Sajobi TT et al. Multivariate trajectories across multiple domains of health-related quality of life in children with new-onset epilepsy. Epilepsy Behav 75, 72–78 (2017). [DOI] [PubMed] [Google Scholar]
- 203.Loiselle KA, Ramsey RR, Rausch JR & Modi AC Trajectories of health-related quality of life among children with newly diagnosed epilepsy. J Pediatr Psychol 41, 1011–1021 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ramsey RR, Loiselle K, Rausch JR, Harrison J. & Modi AC Predictors of trajectories of epilepsy-specific quality of life among children newly diagnosed with epilepsy. Epilepsy Behav 57, 202–210 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Witt JA, Elger CE & Helmstaedter C. Adverse cognitive effects of antiepileptic pharmacotherapy: each additional drug matters. Eur Neuropsychopharmacol 25, 1954–1959 (2015). [DOI] [PubMed] [Google Scholar]
- 206.Witt JA & Helmstaedter C. Monitoring the cognitive effects of antiepileptic pharmacotherapy-approaching the individual patient. Epilepsy Behav 26, 450–456 (2013). [DOI] [PubMed] [Google Scholar]
- 207.Ye Z. et al. Somatic mutation: The hidden genetics of brain malformations and focal epilepsies. Epilepsy Res 155, 106161 (2019). [DOI] [PubMed] [Google Scholar]
- 208.Myers KA, Johnstone DL & Dyment DA Epilepsy genetics: Current knowledge, applications, and future directions. Clin Genet 95, 95–111 (2019). [DOI] [PubMed] [Google Scholar]
- 209.Hebbar M. & Mefford HC Recent advances in epilepsy genomics and genetic testing. F1000Res 9, 185 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kobow K. & Blümcke I. Epigenetics in epilepsy. Neurosci Lett 667, 40–46 (2018). [DOI] [PubMed] [Google Scholar]
- 211.Leu C. et al. Polygenic burden in focal and generalized epilepsies. Brain 142, 3473–3481 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Busch RM, Najm I, Hermann BP & Eng C. Genetics of cognition in epilepsy. Epilepsy Behav 41, 297–306 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kendler KS et al. Stressful life events, genetic liability, and onset of an episode of major depression in women. Am J Psychiatry 152, 833–842 (1995). [DOI] [PubMed] [Google Scholar]
- 214.Kendler KS & Karkowski-Shuman L. Stressful life events and genetic liability to major depression: genetic control of exposure to the environment? Psychol Med 27, 539–547 (1997). [DOI] [PubMed] [Google Scholar]
- 215.Alhusaini S. et al. Heritability of subcortical volumetric traits in mesial temporal lobe epilepsy. PLoS One 8, e61880 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Alhusaini S. et al. Temporal cortex morphology in mesial temporal lobe epilepsy patients and their asymptomatic siblings. Cereb Cortex 26, 1234–1241 (2016). [DOI] [PubMed] [Google Scholar]
- 217.Whelan CD et al. White matter alterations in patients with MRI-negative temporal lobe epilepsy and their asymptomatic siblings. Epilepsia 56, 1551–1561 (2015). [DOI] [PubMed] [Google Scholar]
- 218.Scanlon C. et al. MRI-based brain structure volumes in temporal lobe epilepsy patients and their unaffected siblings: a preliminary study. J Neuroimaging 23, 64–70 (2013). [DOI] [PubMed] [Google Scholar]
- 219.Long L. et al. Shared hippocampal abnormalities in sporadic temporal lobe epilepsy patients and their siblings. Epilepsia 61, 735–746 (2020). [DOI] [PubMed] [Google Scholar]
- 220.Iqbal N. et al. Neuropsychological profiles of patients with juvenile myoclonic epilepsy and their siblings: An extended study. Epilepsia 56, 1301–1308 (2015). [DOI] [PubMed] [Google Scholar]
- 221.Chowdhury FA et al. Impaired cognitive function in idiopathic generalized epilepsy and unaffected family members: an epilepsy endophenotype. Epilepsia 55, 835–840 (2014). [DOI] [PubMed] [Google Scholar]
- 222.Badawy RA, Vogrin SJ, Lai A. & Cook MJ Capturing the epileptic trait: cortical excitability measures in patients and their unaffected siblings. Brain 136, 1177–1191 (2013). [DOI] [PubMed] [Google Scholar]
- 223.Caciagli L. et al. Abnormal hippocampal structure and function in juvenile myoclonic epilepsy and unaffected siblings. Brain 142, 2670–2687 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wandschneider B. et al. Prospective memory in patients with juvenile myoclonic epilepsy and their healthy siblings. Neurology 75, 2161–2167 (2010). [DOI] [PubMed] [Google Scholar]
- 225.Wandschneider B. et al. Motor co-activation in siblings of patients with juvenile myoclonic epilepsy: an imaging endophenotype? Brain 137, 2469–2479 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wandschneider B. et al. Developmental MRI markers cosegregate juvenile patients with myoclonic epilepsy and their healthy siblings. Neurology 93, e1272–e1280 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Clarke T. et al. High risk of reading disability and speech sound disorder in rolandic epilepsy families: case-control study. Epilepsia 48, 2258–2265 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Smith AB et al. A neurocognitive endophenotype associated with rolandic epilepsy. Epilepsia 53, 705–711 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Verrotti A. et al. Neuropsychological impairment in children with Rolandic epilepsy and in their siblings. Epilepsy Behav 28, 108–112 (2013). [DOI] [PubMed] [Google Scholar]
- 230.Hesdorffer DC, Caplan R. & Berg AT Familial clustering of epilepsy and behavioral disorders: evidence for a shared genetic basis. Epilepsia 53, 301–307 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Almane DN et al. Contribution of Family Relatedness to neurobehavioral comorbidities in idiopathic childhood epilepsies. J Int Neuropsychol Soc 24, 653–661 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Busch RM et al. ApoE-epsilon4 is associated with reduced memory in long-standing intractable temporal lobe epilepsy. Neurology 68, 409–414 (2007). [DOI] [PubMed] [Google Scholar]
- 233.Gambardella A. et al. ApoE epsilon4 allele and disease duration affect verbal learning in mild temporal lobe epilepsy. Epilepsia 46, 110–117 (2005). [DOI] [PubMed] [Google Scholar]
- 234.Warburton A. et al. NRSF and BDNF polymorphisms as biomarkers of cognitive dysfunction in adults with newly diagnosed epilepsy. Epilepsy Behav 54, 117–127 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sidhu MK et al. The impact of brain-derived neurotrophic factor Val66Met polymorphism on cognition and functional brain networks in patients with intractable partial epilepsy. CNS Neurosci Ther 25, 223–232 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Colliva C. et al. Executive functioning in children with epilepsy: Genes matter. Epilepsy Behav 95, 137–147 (2019). [DOI] [PubMed] [Google Scholar]
- 237.Doherty C. et al. The role of genetic polymorphisms in executive functioning performance in temporal lobe epilepsy. Epilepsy Behav 121, 108088 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Doherty C. et al. BDNF and COMT, but not APOE, alleles are associated with psychiatric symptoms in refractory epilepsy. Epilepsy Behav 94, 131–136 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Busch RM et al. Verbal memory dysfunction is associated with alterations in brain transcriptome in dominant temporal lobe epilepsy. Epilepsia 61, 2203–2213 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bungenberg J. et al. Gene expression variance in hippocampal tissue of temporal lobe epilepsy patients corresponds to differential memory performance. Neurobiol Dis 86, 121–130 (2016). [DOI] [PubMed] [Google Scholar]
- 241.Tai XY et al. Hyperphosphorylated tau in patients with refractory epilepsy correlates with cognitive decline: a study of temporal lobe resections. Brain 139, 2441–2455 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Busch RM et al. Verbal memory dysfunction is associated with alterations in brain transcriptome in dominant temporal lobe epilepsy. Epilepsia 61, 2203–2213 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Noebels JL Single-gene determinants of epilepsy comorbidity. Cold Spring Harb Perspect Med 5, a022756 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Pickrell WO et al. Epilepsy and deprivation, a data linkage study. Epilepsia 56, 585–591 (2015). [DOI] [PubMed] [Google Scholar]
- 245.Hesdorffer DC et al. Socioeconomic status is a risk factor for epilepsy in Icelandic adults but not in children. Epilepsia 46, 1297–303 (2005). [DOI] [PubMed] [Google Scholar]
- 246.Kobau R. et al. Epilepsy surveillance among adults — 19 States, Behavioral Risk Factor Surveillance System, 2005. MMWR Surveill Summ 57, 1–20 (2008). [PubMed] [Google Scholar]
- 247.Nimmo-Smith V, Brugha TS, Kerr MP, McManus S. & Rai D. Discrimination, domestic violence, abuse, and other adverse life events in people with epilepsy: Population-based study to assess the burden of these events and their contribution to psychopathology. Epilepsia 57, 1870–1878 (2016). [DOI] [PubMed] [Google Scholar]
- 248.Gordon KE & Dooley JM Food insecurity and epilepsy in a nationally representative sample. Epilepsy Behav 43, 139–142 (2015). [DOI] [PubMed] [Google Scholar]
- 249.O’Malley JA, Klett BM, Klein MD, Inman N. & Beck AF Revealing the prevalence and consequences of food insecurity in children with epilepsy. J Community Health 42, 1213–1219 (2017). [DOI] [PubMed] [Google Scholar]
- 250.Fiest KM, Birbeck GL, Jacoby A. & Jette N. Stigma in epilepsy. Curr Neurol Neurosci Rep 14, 444 (2014). [DOI] [PubMed] [Google Scholar]
- 251.Carson J, Weir A, Chin RF & McLellan A. Socioeconomic deprivation is an independent risk factor for behavioral problems in children with epilepsy. Epilepsy Behav 45, 105–109 (2015). [DOI] [PubMed] [Google Scholar]
- 252.Baxendale S. & Heaney D. Socioeconomic status, cognition, and hippocampal sclerosis. Epilepsy Behav 20, 64–67 (2011). [DOI] [PubMed] [Google Scholar]
- 253.Singhi PD, Bansal U, Singhi S. & Pershad D. Determinants of IQ profile in children with idiopathic generalized epilepsy. Epilepsia 33, 1106–1114 (1992). [DOI] [PubMed] [Google Scholar]
- 254.Taylor J. et al. Factors predictive of resilience and vulnerability in new-onset epilepsy. Epilepsia 52, 610–618 (2011). [DOI] [PubMed] [Google Scholar]
- 255.Nathan CL & Gutierrez C. FACETS of health disparities in epilepsy surgery and gaps that need to be addressed. Neurol Clin Pract 8, 340–345 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Greenlund SF, Croft JB & Kobau R. Epilepsy by the Numbers: epilepsy deaths by age, race/ethnicity, and gender in the United States significantly increased from 2005 to 2014. Epilepsy Behav 69, 28–30 (2017). [DOI] [PubMed] [Google Scholar]
- 257.Kind AJH & Golden RN Social determinants of health: fundamental drivers of health inequity. WMJ 117, 231–232 (2018). [PubMed] [Google Scholar]
- 258.Kind AJH & Buckingham WR Making neighborhood-disadvantage metrics accessible — the Neighborhood Atlas. N Engl J Med 378, 2456–2458 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zuelsdorff M. et al. The Area Deprivation Index: a novel tool for harmonizable risk assessment in Alzheimer’s disease research. Alzheimers Dement (N Y) 6, e12039 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hunt JFV et al. Association of neighborhood-level disadvantage with cerebral and hippocampal volume. JAMA Neurol 77, 451–460 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Powell WR et al. Association of neighborhood-level disadvantage with Alzheimer disease neuropathology. JAMA Netw Open 3, e207559 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Téllez-Zenteno JF, Matijevic S. & Wiebe S. Somatic comorbidity of epilepsy in the general population in Canada. Epilepsia 46, 1955–1962 (2005). [DOI] [PubMed] [Google Scholar]
- 263.Gaitatzis A, Sisodiya SM & Sander JW The somatic comorbidity of epilepsy: a weighty but often unrecognized burden. Epilepsia 53, 1282–1293 (2012). [DOI] [PubMed] [Google Scholar]
- 264.Baxendale S. et al. The role of obesity in cognitive dysfunction in people with epilepsy. Epilepsy Behav 45, 187–190 (2015). [DOI] [PubMed] [Google Scholar]
- 265.Hermann BP, Sager MA, Koscik RL, Young K. & Nakamura K. Vascular, inflammatory, and metabolic factors associated with cognition in aging persons with chronic epilepsy. Epilepsia 58, e152–e156 (2017). [DOI] [PubMed] [Google Scholar]
- 266.Arend J. et al. Depressive, inflammatory, and metabolic factors associated with cognitive impairment in patients with epilepsy. Epilepsy Behav 86, 49–57 (2018). [DOI] [PubMed] [Google Scholar]
- 267.Reyes A. et al. The impact of cerebrovascular risk factors on postoperative memory decline in patients with left temporal lobe epilepsy. Epilepsy Behav 102, 106558 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Hamed SA Atherosclerosis in epilepsy: its causes and implications. Epilepsy Behav 41, 290–296 (2014). [DOI] [PubMed] [Google Scholar]
- 269.Livingston G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.[No authors listed] 2020 Alzheimer’s disease facts and figures. Alzheimers Dement 16, 391–460 (2020). [Google Scholar]
- 271.Beydoun MA et al. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health 14, 643 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Hermann B. et al. Growing old with epilepsy: the neglected issue of cognitive and brain health in aging and elder persons with chronic epilepsy. Epilepsia 49, 731–740 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sen A, Capelli V. & Husain M. Cognition and dementia in older patients with epilepsy. Brain 141, 1592–1608 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Vancampfort D, Ward PB & Stubbs B. Physical activity and sedentary levels among people living with epilepsy: A systematic review and meta-analysis. Epilepsy Behav 99, 106390 (2019). [DOI] [PubMed] [Google Scholar]
- 275.Vancampfort D, Ward PB & Stubbs B. Physical fitness levels and moderators in people with epilepsy: A systematic review and meta-analysis. Epilepsy Behav 99, 106448 (2019). [DOI] [PubMed] [Google Scholar]
- 276.Johnson EC, Helen Cross J. & Reilly C. Physical activity in people with epilepsy: a systematic review. Epilepsia 61, 1062–1081 (2020). [DOI] [PubMed] [Google Scholar]
- 277.Roth DL, Goode KT, Williams VL & Faught E. Physical exercise, stressful life experience, and depression in adults with epilepsy. Epilepsia 35, 1248–1255 (1994). [DOI] [PubMed] [Google Scholar]
- 278.Allendorfer JB et al. A pilot study of combined endurance and resistance exercise rehabilitation for verbal memory and functional connectivity improvement in epilepsy. Epilepsy Behav 96, 44–56 (2019). [DOI] [PubMed] [Google Scholar]
- 279.Feter N, Alt R, Häfele CA, da Silva MC & Rombaldi AJ Effect of combined physical training on cognitive function in people with epilepsy: results from a randomized controlled trial. Epilepsia 61, 1649–1658 (2020). [DOI] [PubMed] [Google Scholar]
- 280.Stern Y, Barnes CA, Grady C, Jones RN & Raz N. Brain reserve, cognitive reserve, compensation, and maintenance: operationalization, validity, and mechanisms of cognitive resilience. Neurobiol Aging 83, 124–129 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Rzezak P. et al. Higher IQ in juvenile myoclonic epilepsy: Dodging cognitive obstacles and “masking” impairments. Epilepsy Behav 86, 124–130 (2018). [DOI] [PubMed] [Google Scholar]
- 282.Rzezak P, Guimarães CA, Guerreiro MM & Valente KD The impact of intelligence on memory and executive functions of children with temporal lobe epilepsy: Methodological concerns with clinical relevance. Eur J Paediatr Neurol 21, 500–506 (2017). [DOI] [PubMed] [Google Scholar]
- 283.Reyes A. et al. Does bilingualism increase brain or cognitive reserve in patients with temporal lobe epilepsy? Epilepsia 59, 1037–1047 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Jokeit H. & Ebner A. Long term effects of refractory temporal lobe epilepsy on cognitive abilities: a cross sectional study. J Neurol Neurosurg Psychiatry 67, 44–50 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Witt JA & Helmstaedter C. Should cognition be screened in new-onset epilepsies? A study in 247 untreated patients. J Neurol 259, 1727–1731 (2012). [DOI] [PubMed] [Google Scholar]
- 286.Helmstaedter C. & Witt JA Multifactorial etiology of interictal behavior in frontal and temporal lobe epilepsy. Epilepsia 53, 1765–1773 (2012). [DOI] [PubMed] [Google Scholar]
- 287.Kobau R. & DiIorio C. Epilepsy self-management: a comparison of self-efficacy and outcome expectancy for medication adherence and lifestyle behaviors among people with epilepsy. Epilepsy Behav 4, 217–225 (2003). [DOI] [PubMed] [Google Scholar]
- 288.Dilorio C. & Henry M. Self-management in persons with epilepsy. J Neurosci Nurs 27, 338–343 (1995). [DOI] [PubMed] [Google Scholar]
- 289.Luedke MW et al. Self-management of epilepsy: a systematic review. Ann Intern Med 171, 117–126 (2019). [DOI] [PubMed] [Google Scholar]
- 290.Josephson CB et al. Prediction tools for psychiatric adverse effects after levetiracetam prescription. JAMA Neurol 76, 440–446 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Zhang C. et al. Impaired prefrontal cortex-thalamus pathway in intractable temporal lobe epilepsy with aberrant executive control function: MRI evidence. Clin Neurophysiol 130, 484–490 (2019). [DOI] [PubMed] [Google Scholar]
- 292.Diao L. et al. Abnormalities of the uncinate fasciculus correlate with executive dysfunction in patients with left temporal lobe epilepsy. Magn Reson Imaging 33, 544–550 (2015). [DOI] [PubMed] [Google Scholar]
- 293.Hermann BP, Struck AF, Dabbs K, Seidenberg M. & Jones JE Behavioral phenotypes of temporal lobe epilepsy. Epilepsia Open 6, 369–380 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.