Abstract
Background
Body roundness index (BRI) is one of the obesity-related anthropometric indices. However, studies on the relationship between BRI and diabetes risk is limited. The purpose of this study was to explore the relationship between baseline BRI and incident type 2 diabetes mellitus (T2DM) in the Japanese population.
Methods
A retrospective longitudinal study of 15,310 participants in a physical examination program at Murakami Memorial Hospital in Japan from 2004 to 2015. The association between BRI levels and incident T2DM was analyzed by Cox proportional-hazards regression, smooth curve fitting, subgroup analyses, and a set of sensitivity analyses. Receiver operating characteristic curve analysis was used to assess the ability of BRI to predict diabetes.
Result
Baseline BRI levels were elevated in participants who developed T2DM. Baseline BRI levels were positively associated with incident T2DM after adjusting confounding variables (HR = 1.570, 95% CI 1.360–1.811). Additionally, we did a set of sensitivity analyses to ensure the robustness of the results. There was also a non-linear relationship between BRI and incident diabetes in both genders, and the inflection point of BRI was 4.137 in females and 3.146 in males. We found a strong positive correlation between BRI and the incidence of diabetes on the right of the inflection point (Male: HR = 1.827, 95% CI 1.449–2.303; Female: HR = 4.189, 95% CI 1.862–9.421). What’s more, among the anthropometric indices, BRI showed the optimal capability to predict T2DM (Male: AUC = 0.706, 95% CI 0.674–0.738; Female: AUC = 0.735, 95% CI 0.676–0.795).
Conclusion
An elevated BRI level in baseline was independently associated with incident T2DM. Baseline BRI improves the identification of patients at risk of T2DM and may enable early and optimized therapy to improve their outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12967-022-03321-x.
Keywords: Body roundness index, Incident type 2 diabetes mellitus, Non-linear relationship
Background
The global prevalence of diabetes among adults has been increasing in recent decades [1]. Diabetes brings enormous economic pressure on national health systems around the world, so prevention efforts are needed to reduce this burden [2]. Studies have shown that one of the main risk factors for type 2 diabetes (T2DM) is obesity. Elevated fatty acids in obesity and overweight impair insulin action, leading to insulin resistance and T2DM [3].
Body mass index (BMI) provides the convenient way to assess overweight and obesity, but it fails to distinguish between the accumulation of muscle and fat, leading muscular people to be misdiagnosed as overweight or obese [4]. Thomas et al. developed a anthropomorphic index body roundness index (BRI) to predictor body fat and visceral adipose tissue volume in 2013. It is calculated based on waist circumference (WC) and height [5]. Several cross-sectional studies have established the potential of BRI to identify T2DM. However, a large longitudinal study of BRI and T2DM in Japanese is limited. A non-linear relationship between T2DM and BRI has not been reported.
In the present study, we postulated that the baseline BRI level might serve as an early predictor of incident T2DM in Japan. To test this hypothesis, a retrospective longitudinal study was performed in participants who under physical examination program. The relationship between BRI levels and the risk of T2DM was explored in Japan. In addition, the relationship between BRI and incident T2DM was applied by generalized additive model (GAM). Our findings demonstrate that tracking the baseline BRI aids in the prediction of incident T2DM. This will help clinicians plan and initiate the management strategies early to improve outcomes for participants with prediabetes.
Methods
Study design
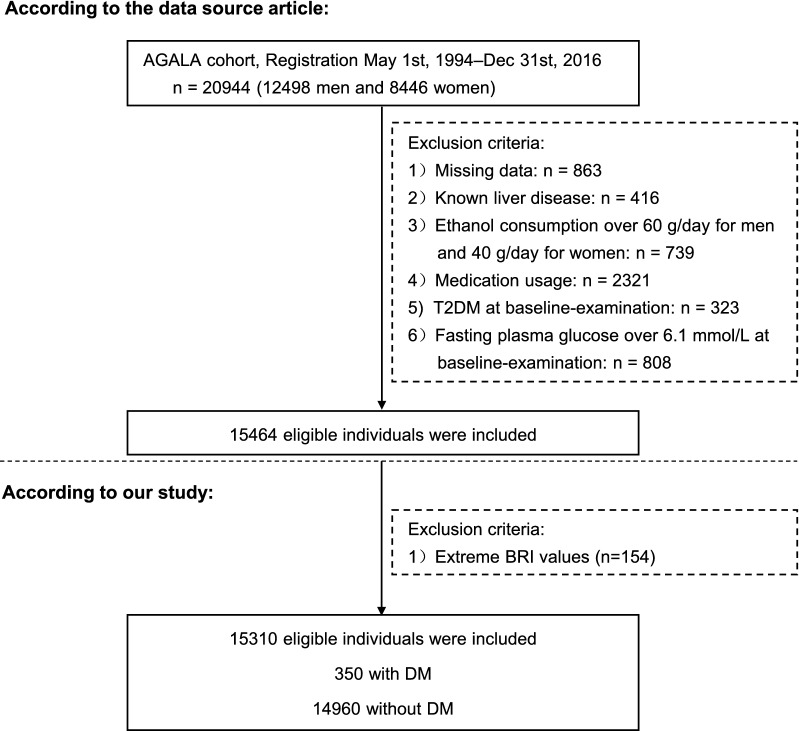
The study is a secondary retrospective study [6]. Data was obtained from the “DATADRYAD” (www. Datadryad.org) database. We quoted the Dryad data package on the basis of Dryad Terms of Service (Dryad data package: Takuro Okamura et al. (2018) Data from: Ectopic fat obesity presents the greatest risk for T2DM: a population-based longitudinal study. Dryad Digital Repository). Original data were obtained from NAGALA (NAfld in the Gifu Area, Longitudinal Analysis) database, which recruited 20,944 Japaneses who underwent a medical examination at Murakami Memorial Hospital from 2004 to 2015. Fifteen thousand four hundred and sixty-four participants in the analysis according to the following exclusion criteria: (1) participants diagnosed alcoholic fatty liver disease, viral hepatitis (n = 416), diabetes (n = 323) (2) anyone who took medication at baseline examination (n = 2321) (3) those with fasting plasma glucose ≥ 6.1 mmol/L (n = 808) (4) individuals who had a heavy drinking habit (ethanol consumption over 60 g/day for men and 40 g/day for women) (n = 739) (5) participants with missing covariate data were also excluded (n = 863). In addition, the anthropometric indices were calculated using the following equations: BMI = weight/height2, a body shape index (ABSI) = , . Individuals were excluded if the BRI value was an extreme value (the extreme value = mean ± 3SD) (n = 154) [7]. Finally, 15,310 subjects (8364 male and 6946 female) included in this study. Figure 1 described the study design and participant flow.
Fig. 1.
Study design and participant flow. NAGALA NAfld in Gifu Area, Longitudinal Analysis; T2DM type 2 diabetes mellitus
Data collection
Variables contained in the database file were as follows: sex, age, diastolic blood pressure (DBP), systolic blood pressure (SBP), WC, fasting plasma glucose (FPG), hemoglobinA1c (HbA1c), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), total cholesterol (TC), ethanol consumption, gamma-glutamyltransferase (GGT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), smoking status, fatty liver, exercise, days of follow-up and incident diabetes.
Lifestyle factors, including smoking and drinking habits, medical history, and physical activity, were obtained using questionnaires. Participants were divided into the following groups: no or minimal alcohol consumption: < 40 g/week; light alcohol consumption: 40–140 g/week; moderate alcohol consumption: 140–280 g/week; or heavy alcohol consumption: > 280 g/week [8]. The participants were categorized by smoking status: non-smokers were defined as never smokers, ex-smokers were past smokers who quit before the baseline visit, and current smokers were smokers at the baseline visit [6]. Regular exercisers are participants who regularly perform any type of exercise > 1 time/week [9]. Fatty liver was diagnosed by ultrasonography performed by trained technicians [10].
Follow-up and outcome definitions
Diabetes was defined as fasting plasma glucose ≥ 7 mmol/L, HbA1c ≥ 6.5%, or self-reported during the follow-up period [11]. During follow up, the outcome was the incident T2DM. The participants who lost to follow-up would still be analyzed in the study.
Statistical analysis
BRI was stratified into four groups: Q1 < 2.09; 2.09 ≤ Q2 < 2.63; 2.63 ≤ Q3 < 3.23; 3.23 ≤ Q4. Continuous variables with normal and skewed distributions were expressed as mean with standard deviation or median with interquartile range, and compared by one-way ANOVA or Kruskal–Wallis. Categorical variables were expressed as percentages of specific groups and compared by chi-square test. Cumulative incidence and person-year incidence were used to express incidence rate.
The Cox proportional hazards model was used to estimate the association between BRI and the incident T2DM with baseline BRI fitted as a continuous variable and categorical variables (stratified into 4 subgroups: Q1 < 2.09; 2.09 ≤ Q2 < 2.63; 2.63 ≤ Q3 < 3.23; 3.23 ≤ Q4).The lowest baseline BRI category was used as a common reference to compute the hazard ratios (HRs) and 95% confidence intervals (CIs) for the other baseline BRI strata. The results of unadjusted, minimally adjusted (age, gender, SBP, DBP, ethanol consumption, smoking status, and exercise) and fully adjusted (age, gender, SBP, DBP, FPG, ALT, AST, HbA1c, TC, HDL-C, ethanol consumption, smoking status, exercise, fatty liver) analyses are shown. The minimally adjusted was given as model I in Table 3 and the the fully adjusted was given as model II in Table 3. Survival estimates and time-to-event variables were computed using the Kaplan–Meier method. A log-rank test was used to compare the Kaplan–Meier probability of diabetes-free survival among BRI groups.
Table 3.
Relationship between BRI and the incident DM in different models
| Variable | Crude model | Model I | Model II | GAM |
|---|---|---|---|---|
| (HR, 95%CI, P) | (HR, 95% CI, P) | (HR, 95% CI, P) | (HR, 95% CI, P) | |
| BRI | 2.662 (2.377, 2.981) < 0.001 | 2.285 (2.013, 2.595) < 0.001 | 1.570 (1.360, 1.811) < 0.001 | 1.555 (1.339, 1.806) < 0.001 |
| BRI (quartile) | ||||
| Q1 | Ref. | Ref. | Ref. | Ref. |
| Q2 | 1.946 (1.191, 3.180) 0.007 | 1.569 (0.957, 2.572) 0.074 | 1.162 (0.704, 1.918) 0.557 | 1.251 (0.748, 2.089) 0.393 |
| Q3 | 3.192 (2.028, 5.023) < 0.001 | 2.144 (1.348, 3.412) 0.001 | 1.057 (0.652, 1.713) 0.822 | 1.070 (0.650, 1.762) 0.789 |
| Q4 | 9.385 (6.196, 14.216) < 0.001 | 5.421 (3.504, 8.387) < 0.001 | 1.892 (1.187, 3.017) 0.007 | 1.867 (1.150, 3.033) 0.011 |
| P for trend | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
Crude model: we did not adjust other covariants
Model I: we adjust age, gender, SBP, DBP, smoking status, ethanol consumption, habit of exercise
Model II: we adjust age, gender, SBP, DBP, smoking status, ethanol consumption, habit of exercise, ALT, AST, FBG, HbA1c, HDL-c, TC, TG
GAM: All covariates listed in Model II were adjusted. However, continuous covariates were adjusted as non-linearity
ALT alanine aminotransferase; AST aspartate aminotransferase; CI confidence interval; DBP diastolic blood pressure; FBG fasting blood glucose; HbA1c hemoglobin A1c; HDL-C high-density lipoprotein cholesterol; HR hazard ratio; Ref reference; SBP systolic blood pressure; TC total cholesterol; TG triglyceride
A series sensitivity analysis wer conducted to ensure the robustness of the relationship between BRI with the incident T2DM. The multivariable Cox proportional hazards model were repeated in the data set including participants without fatty liver or any alcohol consumption.
Applying smooth curve fitting and GAM to demonstrate the association of BRI with T2DM stratified by sex. The threshold effect of BRI on event T2DM was calculated using a two-piece linear regression model.
To assess the consistency of the association between BRI and the incident T2DM, we repeated the analyses in subgroups defined by sex, age, ethanol consumption, baseline SBP (< 140 mmHg, ≥ 140 mmHg), HDL-C (< 1 mmol/L, ≥ 1 mmol/L), ALT (< 40U/L, ≥ 40U/L), baseline DBP(< 90 mmHg, ≥ 90 mmHg), exercise, ethanol consumption, smoking status.
Receiver operating characteristic (ROC) curve was applied to estimate the ability of BRI, WC, ABSI and BMI to predict the risk of T2DM stratified by sex.
All of these analyses were performed with Empower-Stats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA) and the statistical software package R (http://www.R-project.org, The R Foundation). P values less than 0.05 (two-sided) were considered significant statistically.
Ethics approval and consent to participate
The author of the original study waived all copyright and related privilege of these data. This study was approved by the Ethics Committee of the Murakami Memorial Hospital, and informed consent was obtained from all subjects.
Result
Demographics and characteristics of the participants
The study included 15310 participants. The mean follow-up time was 5.39 years, of which 350 participants developed T2DM during the follow-up period, as shown in Fig. 1. The BRI value was normally distributed, ranging from 0.151 to 5.427 (Fig. 2). In age stratification by 10 intervals, male subjects had a higher BRI values than female subjects no matter what age group they were in (Fig. 3). We also found that the BRI values increased with age, both in male and female participants.
Fig. 2.
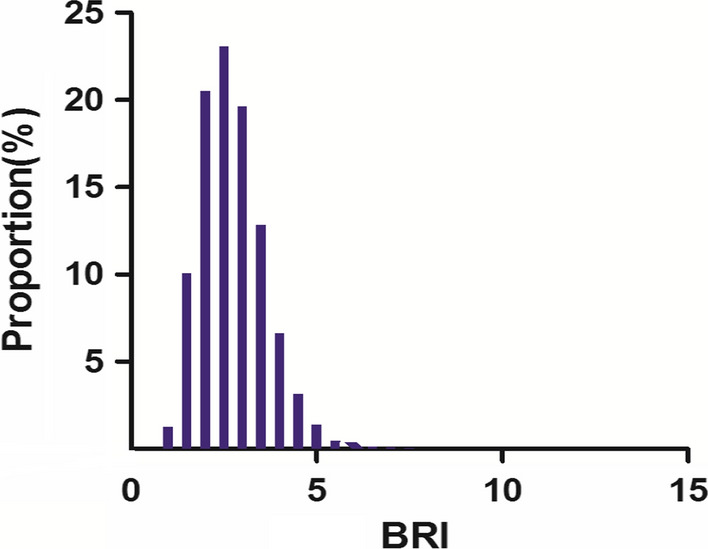
Distribution of BRI. It presented a skewed distribution while being in the range from 0.151 to 5.427
Fig. 3.
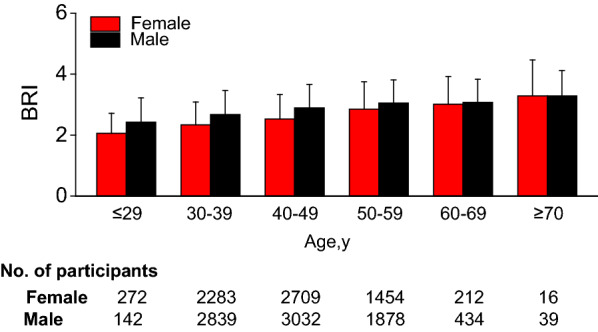
Bar graphs depicting mean BRI levels stratified according to age and gender categories
The characteristics of the participants were stratified by the quartiles of the BRI. About the anthropometric indices, participants with the highest quartiles of the BRI had the highest age, WC, BMI, ethanol consumption (P < 0.001; Table 1). What's more, participants with the highest quartiles of the BRI had the highest FBG, HbA1c, TC,TG, GGT, ALT, AST, SBP, DBP,WC and BMI values. Besides, HDL-C is high in participants with the lowest BRI range. (P < 0.001; Table 1).
Table 1.
The baseline characteristics of participants
| BRI | Q1 (< 2.09) | Q2 (2.09–< 2.63) | Q3 (2.63–< 3.23) | Q4 (≥ 3.23) | P value |
|---|---|---|---|---|---|
| Participants | 3828 | 3827 | 3827 | 3828 | |
| Age, years | 40.80 ± 8.39 | 42.67 ± 8.47 | 44.56 ± 8.54 | 46.75 ± 9.04 | < 0.001 |
| Gender—n (%) | < 0.001 | ||||
| Female | 2414 (63.062%) | 1790 (46.773%) | 1406 (36.739%) | 1336 (34.901%) | |
| Male | 1414 (36.938%) | 2037 (53.227%) | 2421 (63.261%) | 2492 (65.099%) | |
| Smoking status | < 0.001 | ||||
| Never-smoker | 2675 (69.88%) | 2284 (59.68%) | 2007 (52.44%) | 1962 (51.25%) | |
| Past-smoker | 451 (11.78%) | 697 (18.21%) | 878 (22.94%) | 912 (23.82%) | |
| Current-smoker | 702 (18.34%) | 846 (22.11%) | 942 (24.61%) | 954 (24.92%) | |
| Ethanol consumption g/wk | 31.38 ± 63.84 | 48.00 ± 80.30 | 55.92 ± 89.75 | 56.57 ± 90.76 | < 0.001 |
| Habit of exercise | 689 (18.00%) | 758 (19.81%) | 676 (17.66%) | 573 (14.97%) | < 0.001 |
| WC (m) | 0.66 ± 0.05 | 0.73 ± 0.04 | 0.79 ± 0.04 | 0.86 ± 0.06 | < 0.001 |
| BMI (kg/m2) | 19.02 ± 1.53 | 21.02 ± 1.52 | 22.69 ± 1.70 | 25.35 ± 2.45 | < 0.001 |
| FBG (mmol/L) | 4.99 ± 0.39 | 5.11 ± 0.39 | 5.22 ± 0.40 | 5.32 ± 0.39 | < 0.001 |
| HbA1c(%) | 5.104 ± 0.303 | 5.138 ± 0.308 | 5.177 ± 0.318 | 5.257 ± 0.334 | < 0.001 |
| TC (mmol/L) | 4.87 ± 0.81 | 5.02 ± 0.83 | 5.20 ± 0.85 | 5.40 ± 0.85 | < 0.001 |
| TG (mmol/L) | 0.53 (0.38–0.73) | 0.65 (0.46–0.96) | 0.84 (0.58–1.24) | 1.05 (0.70–1.55) | < 0.001 |
| HDL-C (mmol/L) | 1.66 ± 0.40 | 1.527 ± 0.392 | 1.386 ± 0.381 | 1.281 ± 0.346 | < 0.001 |
| GGT (U/L) | 14.41 ± 11.20 | 18.11 ± 15.27 | 21.98 ± 20.19 | 26.34 ± 21.24 | < 0.001 |
| ALT (U/L) | 14.00 (11.00–18.00) | 15.00 (12.00–20.00) | 18.00 (13.00–24.00) | 21.00 (16.00–31.00) | < 0.001 |
| AST (U/L) | 16.00 (13.00–19.00) | 17.00 (14.00–20.00) | 18.00 (14.00–21.00) | 19.00 (15.00–24.00) | < 0.001 |
| SBP (mmHg) | 106.89 ± 12.80 | 111.91 ± 13.06 | 116.32 ± 13.91 | 122.08 ± 15.14 | < 0.001 |
| DBP (mmHg) | 66.40 ± 8.82 | 69.62 ± 9.36 | 72.95 ± 10.05 | 76.88 ± 10.47 | < 0.001 |
Continuous data are expressed as mean ± SD or median (interquartile range). Categorical data are expressed as n (%)
One-way ANOVA, Kruskall Wallis test or Chi-Squared Test
ALT alanine aminotransferase; AST aspartate aminotransferase; BMI body mass index; BRI body roundness index; DBP diastolic blood pressure; FBG fasting blood glucose; GGT glutamyl transpeptidase; HbA1c hemoglobin A1c; HDL-C high-density lipoprotein cholesterol; SBP systolic blood pressure; TC total cholesterol; TG triglyceride; WC waist circumference
Relationship between BRI levels and incident NAFLD during follow-up
Univariate Cox proportional hazards analysis were performed to compare the role of BRI and other variables in predicting T2DM (Additional file 1: Table S1). Incident T2DM was positively associated with age, gender, BMI, BRI, ABSI, WC, ethanol consumption, current-smoke, AST, ALT, GGT, FPG, HbA1c, TG, TC, SBP, and DBP. On the contrary, exercise was irrelevant to T2DM. HDL-C was negatively associated with incident T2DM (Additional file 1: Table S1).
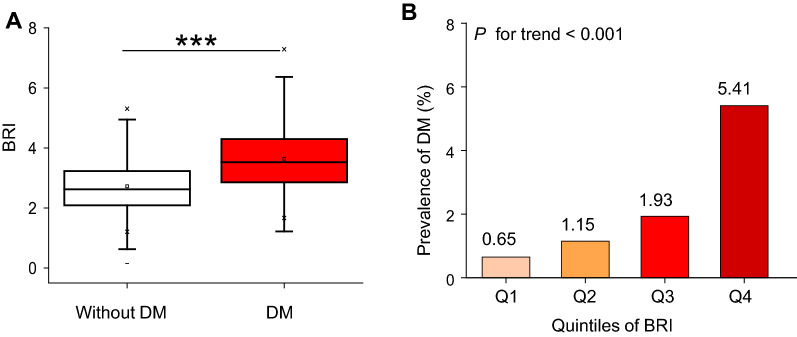
As shown in Fig. 4A, participants with T2DM had an elevated BRI level [median (interquartile range); 2.624 (2.088–3.231)], as compared with no T2DM [median (interquartile range); 3.526 (2.855–4.300)]. Compared with the lowest quartile in the BRI group, participants with higher quartiles had a higher cumulative incidence [Q1: 0.651(0.398–0.908), Q2: 1.112(0.792–1.452), Q3: 1.933(1.497–2.370), Q4: 5.407(4.690–6.124) P < 0.001 for trend] (Table 2 and Fig. 4B).
Fig. 4.
Elevation of BRI and its relationship with incident DM. A Levels of BRI in baseline were determined in patients with and without DM. Horizontal lines represent the median values and interquartile ranges. Kruskal–Wallis test. ***P < 0.001. B Prevalence of DM stratified according to BRI categories. Linear regression analysis, P for trend < 0.001
Table 2.
The incidence rate of diabetes
| BRI | Participants (n) | DM events (n) | Cumulative incidence (95% CI) (%) | Per 100,000 person-year |
|---|---|---|---|---|
| Total | 15,310 | 350 | 2.286 (2.049–2.523) | 377.304 |
| Q1 | 3828 | 25 | 0.651 (0.398–0.908) | 103.434 |
| Q2 | 3827 | 44 | 1.112 (0.792–1.452) | 193.004 |
| Q3 | 3827 | 74 | 1.933 (1.497–2.370) | 317.249 |
| Q4 | 3828 | 207 | 5.407 (4.690–6.124) | 921.214 |
| P for trend | < 0.001 |
BRI body roundness index; DM diabetes mellitus; n number; Q quarter
Kaplan–Meier survival curves for the probability of diabetes-free survival stratified by BRI groups were shown in Fig. 4. A higher BRI level at baseline was associated with a significantly lower probability of diabetes-free survival, indicating the top group with the highest diabetes risk.
The Cox proportional hazards regression model show the association between BRI and incident T2DM. In the crude model, we found that BRI was positively associated with incident T2DM (HR = 2.662, 95% CI 2.377–2.981). This association of BRI and T2DM persisted despite adjustment for sex, age, DBP, SBP, ethanol consumption, smoking status, exercise (HR = 2.285, 95% CI 2.013–2.595) (model I) or inclusion of the baseline mentioned above characteristics and ALT, AST, FBG, HbA1c, HDL-C, TC, TG (model II) (HR = 1.570, 95%CI 1.360–1.811) (Table 3).
Sensitivity analysis
The participants were stratified by the quartiles of the BRI. The trend of T2DM risk in BRI quartiles was significant (P for trend < 0.001) (Table 2). Compared with the bottom BRI quartile in the full model, the top quartile had a 89.2 percent increase in T2DM risk with the fully adjusted model (HR = 1.892, 95% CI 1.187–3.017, P < 0.001) (Table 3 and Fig. 5). GAM was used to insert the continuity covariate as a curve into the equation, which is basically consistent with Model II with the fully adjusted model (HR = 1.555, 95% CI 1.339–1.806, P < 0.001), which proves the robustness of the results (Table 3).
Fig. 5.
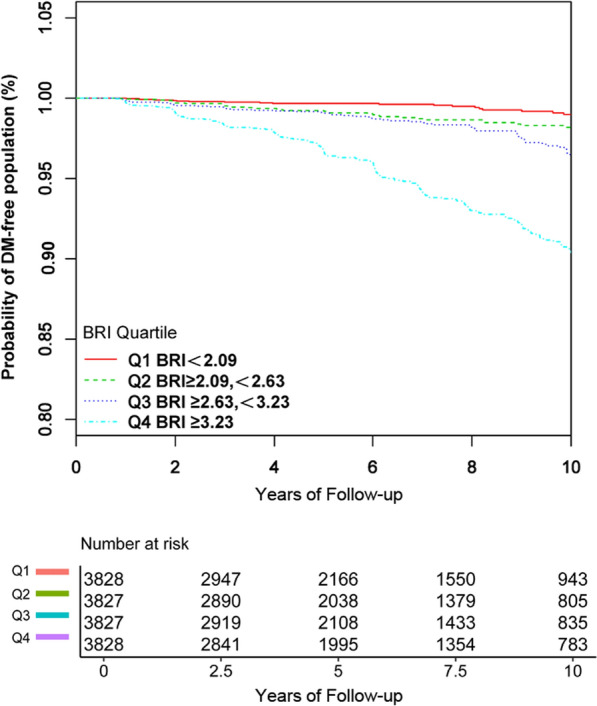
Kaplan Meier curves of overall survival stratified by the following baseline BRI categories
Furthermore, we excluded participants with fatty liver in other sensitivity analyses. The results suggested that BRI was also positively associated with incident T2DM with the fully adjusted model (HR = 1.102, 95% CI 0.867–1.401, P = 0.856). We also excluded any alcohol consumers for sensitivity analyses. The results suggested that BRI was still positively associated with incident T2DM with the fully adjusted model (HR = 1.716, 95% CI 1.452–2.027, P < 0.001) (Additional file 1: Table S2). The sensitivity analysis results showed that the relationship between BRI and the risk of T2DM was very robust.
The non-linear relationship between BRI levels and incident NAFLD
GAM and smooth curve fitting were used to study the relationship between BRI levels and incidence of T2DM. A non-linear relationship between the BRI level and the incidence of T2DM was detected after adjusting the confounding variables (age, gender, SBP, DBP, smoking status, ethanol consumption, habit of exercise, ALT, AST, GGT, FBG, HbA1c, HDL-C, TC, TG) (Fig. 6). A binary linear regression model and a recursive algorithm were used to calculate the inflection point of BRI (log likelihood ratio test P < 0.001). In male group, the relationship between BRI and T2DM was J-shaped. The inflection point of BRI were 3.147. On the right of inflection point, the effect size was 1.827 (95% CI 1.449–2.303; P < 0.001). However, on the left side of the inflection point, we did not observe a significant association between BRI and DM (HR = 0.903, 95% CI 0.606–1.346; P = 0.617). In female group, the relationship between BRI and diabetes is close to linear. The inflection point of BRI were 4.137. We found a strong positive correlation between BRI and the incidence of T2DM on the right of the inflection point (Female group: HR = 4.189, 95% CI 1.862–9.421, P < 0.001). While on the left side of the inflection point, their association tended to be weakened (Female group: HR = 1.418, 95% CI 0. 983–2.045, P = 0.062) (Table 4).
Fig. 6.
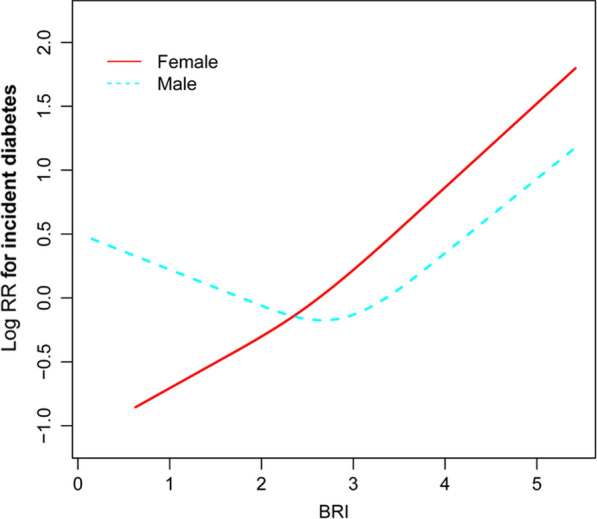
Association of BRI with the risk of DM stratified by sex. The non-linear relationship between BRI and incident of DM. A non-linear relationship between them was detected after adjusting for age, gender, BMI, SBP, DBP, TC, TG, HDL-C, ALT, AST, FBG,HbA1c%, smoking status, ethanol consumption, habit of exercise
Table 4.
The result of two-piecewise linear regression model stratified by sex
| Incident DM (male) | HR (95% CI) | P |
|---|---|---|
| Fitting model by standard linear regression | 1.475 (1.242, 1.753) | < 0.001 |
| Fitting model by two-piecewise linear regression | ||
| Inflection point of BRI | ||
| ≤ 3.146 | 0.903 (0.606, 1.346) | 0.617 |
| > 3.146 | 1.827 (1.449, 2.303) | < 0.001 |
| P for log likelihood ratio test | 0.011 | |
| Incident DM (female) | HR (95% CI) | P |
|---|---|---|
| Fitting model by standard linear regression | 1.828 (1.386, 2.412) | < 0.001 |
| Fitting model by two-piecewise linear regression | ||
| Inflection point of BRI | ||
| ≤ 4.137 | 1.418 (0.983, 2.045) | 0.062 |
| > 4.137 | 4.189 (1.862, 9.421) | < 0.001 |
| P for log likelihood ratio test | 0.045 | |
We adjusted age, gender, SBP, DBP, smoking status, ethanol consumption, habit of exercise, ALT, AST, GGT, FBG, HbA1c, HDL-c, TC, TG
ALT alanine aminotransferase; AST aspartate aminotransferase; CI confidence interval; DBP diastolic blood pressure; FBG fasting blood glucose; GGT glutamyl transpeptidase; HbA1c hemoglobin A1c; HDL-C high-density lipoprotein cholesterol; HR hazard ratio; Ref reference; SBP systolic blood pressure; TC total cholesterol; TG triglyceride
Relationship between BRI levels and incident T2DM in subgroup analyses
We performed sensitivity analyses to determine whether gender, SBP, DBP, smoking status, alcohol consumption, habit of exercise, HDL-C values, ALT values influenced the relationship between the BRI level and the incident T2DM. Table 5 showed that only alcohol consumption modify the relationship between BRI and incident T2DM (P for interaction = 0.0487). And a stronger association was observed in the population without alcohol consumption(HR 1.694; 95% CI 1.435–1.999). In contrast, population with alcohol consumption impared the relationship between BRI and incident T2DM. These results suggested that the relationship between BRI and incident T2DM was robust in most subgroups (Table 5).
Table 5.
Effect size of BRI on DM in prespecified and exploratory subgroups
| Characteristic | No. of participants | HR (95%CI) | P value | P for interacion |
|---|---|---|---|---|
| Gender | 0.1092 | |||
| Female | 6946 | 1.845 (1.461, 2.330) | < 0.001 | |
| Male | 8364 | 1.480 (1.254, 1.747) | < 0.001 | |
| SBP (mmHg) | 0.9664 | |||
| < 140 | 14,569 | 1.591 (1.375, 1.842) | < 0.001 | |
| ≥ 140 | 741 | 1.576 (1.024, 2.424) | 0.0386 | |
| DBP (mmHg) | 0.2317 | |||
| < 90 | 14,581 | 1.639 (1.411, 1.904) | < 0.001 | |
| ≥ 90 | 729 | 1.239 (0.801, 1.915) | 0.3357 | |
| Smoking status | 0.089 | |||
| Never-smoker | 8928 | 1.826 (1.478, 2.255) | < 0.001 | |
| Past-smoker | 2938 | 1.196 (0.863, 1.658) | 0.2821 | |
| Current-smoker | 3444 | 1.538 (1.234, 1.917) | 0.001 | |
| Alcohol consumption | 0.0634 | |||
| < 40 | 10,775 | 1.588 (1.318, 1.913) < 0.0001 | < 0.001 | |
| ≥ 40, < 140 | 2788 | 1.074 (0.744, 1.552) 0.7019 | 0.7019 | |
| ≥ 140, < 280 | 1360 | 0.879 (0.537, 1.439) 0.6072 | 0.6072 | |
| ≥ 280 | ||||
| ≥ 280 | 541 | 1.274 (0.670, 2.423) 0.4606 | 0.4606 | |
| Habit of exercise | 0.1853 | |||
| No | 12,614 | 1.529 (1.312, 1.781) | < 0.001 | |
| Yes | 2696 | 1.983 (1.387, 2.834) | 0.0002 | |
| HDL-c (mmol/L) | 0.1966 | |||
| < 1 | 1616 | 1.426 (1.109, 1.833) | 0.0056 | |
| ≥ 1 | 13,694 | 1.721 (1.467, 2.020) | < 0.0001 | |
| ALT (U/L) | 0.5719 | |||
| ≤ 40 | 14,499 | 1.621 (1.388, 1.893) | < 0.0001 | |
| > 40 | 811 | 1.464 (1.056, 2.029) | 0.0221 | |
Above model adjusted age, gender, SBP, DBP, smoking status, ethanol consumption, habit of exercise, ALT, AST, GGT, FBG, HbA1c, HDL-c, TC, TG
In each case, the model is not adjusted for the stratification variable
ALT alanine aminotransferase; AST aspartate aminotransferase; CI confidence interval; DBP diastolic blood pressure; FBG fasting blood glucose; GGT glutamyl transpeptidase; HbA1c hemoglobin A1c; HDL-C high-density lipoprotein cholesterol; HR hazard ratio; Ref reference; SBP systolic blood pressure; TC total cholesterol; TG triglyceride
BRI as a predictor for T2DM
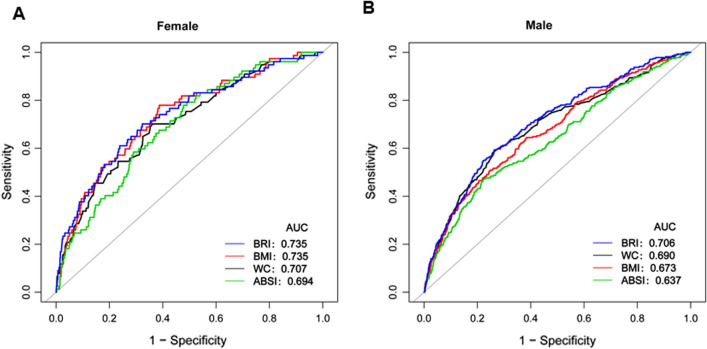
For predicting T2DM, the AUC of BRI were 0.7354 in female and 0.7061 in male (Additional file 1: Table S3). As shown in Fig. 7, the AUC of BRI for predicting T2DM was the greatest among the anthropometric indices, such as BMI, ABSI, and WC. Therefore, BRI value can be used to predicting the incident of T2DM during follow-up.
Fig. 7.
BRI for predicting DM in all participants by ROC analyses stratified by sex
Discussion
In this retrospective cohort of 15310 participants, we determined the association between elevated BRI levels and the occurrence of T2DM. The highest BRI level at baseline was associated with the incidence of T2DM after adjusting for age, gender, SBP, DBP, smoking status, alcohol consumption, exercise habits, GGT, AST, ALT, TC, TG, HDL-C, FBG, and HbA1c. In this study, the BRI level has a non-linear relationship with the incidence of T2DM stratified by sex. The inflection points of BRI were 3.146 in male and 4.137 in female. In addition, in male group, the relationship between BRI and T2DM was J-shaped. We found a strong positive correlation between BRI and the incidence of diabetes on the right of the inflection point (Male: HR = 1.827, 95% CI 1.449–2.303; Female: HR = 4.189, 95% CI 1.862–9.421).What’s more, the AUC of BRI for predicting T2DM was the greatest among the anthropometric indices, such as ABSI, WC, and BMI. These data indicate that BRI, as a new anthropometric index, can predict the occurrence of T2DM and provide important prognostic information.
The number of people with diabetes and impaired glucose tolerance has been increasing globally in recent decades [1]. Diabetes brings enormous economic pressure on national health systems around the world, so prevention efforts are needed to reduce this burden [2]. Obesity was considered an major risk factor for the development of T2DM [12]. BMI is an anthropometric measure for diagnosing obesity but it has limitations. It cannot distinguish between muscle-induced and fat-induced weight gain [13]. The concept of BRI provides a more accurate estimate of total percentage of fat and visceral fat tissue.
BRI is a obesity index, which was based on WC and height [5]. Compared with the other anthropometric indices such as WC, and BMI, BRI is a better predictor of body fat and visceral fat tissue volume [14, 15]. Previous studies have examined the association of the anthropometric indicators such as BMI, ABSI, WC, and BRI with T2DM risk and their performance in T2DM risk prediction [16–18]. In obese and overweight Chinese adults, BRI showed the optimal capability to identify IR in the cross-sectional data [14]. In a cross-sectional study, Chang et al. showed that BRI was superior to other anthropometric measures such as BMI, WC, and ABSI in predicting T2DM in Northeast China [16]. In a 15-year prospective study, the discriminatory power of BRI was superior to WC in southwest China [18]. However, the above studies were less in the Japanese population with a longitudinal study. In this longitudinal study of 15310 Japanese participants, a higher cumulative incidence of T2DM was observed at higher baseline BRI levels, which is supported by previous studies showing similar results. The results suggested a significant association between BRI and incident T2DM. BRI (increase by 1) has a significant association with T2DM after adjusting for confounding variables (HR = 1.570, 95% CI 1.360–1.811). The highest quartile of BRI was associated with increased risk for T2DM by 1.892-fold compared with the lowest quartile. Our research compares the AUC of these different anthropometric indicators in the ROC curve in both genders, ROC analysis shows that BRI is the strongest predictor of T2DM, compared with other anthropometric indicators, including BMI, WC, and ABSI in both sex categories.
Chronic ethanol consumption was established as risk factor for T2DM, which can affect glucose metabolism and insulin resistance by impairing pancreatic beta-cell mass and function [19]. In the present study we found alcohol consumption was the risk factors for T2DM. The relationship between ethanol consumption and BRI was linear and positively correlated (data not shown). Ethanol consumption may affect the relationship between BRI and T2DM. However, this study excluded participants with heavy drinking. AND the results showed BRI is an independent risk factor for T2DM. Meanwhile, the sensitivity analysis found that this relationship still exists among participants without alcohol intake.
Previous studies did not explore the possible curvilinear relationship between BRI and T2DM. In the present study, we analyzed the nonlinear relationship between BRI and T2DM for the first time. The result of the smooth curve showed that the relationship between BRI and T2DM was nonlinear in both genders after adjusting for confounders (age, gender, SBP, DBP, smoking status, ethanol consumption, habit of exercise, FBG, HbA1c, AST, GGT, ALT, TC, TG, HDL-C) (P < 0.0001). By using a two‐piecewise linear regression model, we calculated the inflection point of BRI. In male group, the relationship between BRI and diabetes is close to linear. When the BRI level was > 3.224, a 1 U/L increase in BRI level is accompanied by a 82.7% increase in HR for T2DM (HR 1.827; 95% CI 1.449–2.303), when the BRI level was ≤ 3.224, the BRI level was no correlation with incident T2DM (HR, 0.903; 95% CI 0.606–1.346) (Table 4). In female group, the relationship between BRI and T2DM was J-shaped. When the BRI level was > 4.137, a 1 U/L increase in BRI level is accompanied by a 318% increase in HR for T2DM (HR 4.189; 95% CI 1.862–9.421), when the BRI level was ≤ 4.137, the BRI level was no correlation with incident T2DM (HR, 1.418; 95% CI 0.983–2.045). Elevated BRI alerts the population at high risk of T2DM during follow-up, which will remind people to adjust their lifestyle habits earlier to improve outcomes.
Study strength and limitations
The present study has some advantages. First, compared with previous cross-sectional studies, this study was a longitudinal study, which conducted a relatively large sample of the Japanese physical examination population. Second, the non-linear relationship between BRI and T2DM was discovered in this study, and the infection point was calculated at the same time. Third, a series of sensitivity analyses were conducted to ensure the stable of the results, including converting BRI into categorical variables, using GAM to insert continuous covariates into the equation in the form of a curve, and analyze the association between BRI and incident T2DM after excluding alcohol consumers or fatty liver participants. Fourth, perform subgroup analysis to ensure that the relationship between BRI and T2DM is stable among different participants, and the results are stable, and the research results are expected to be successfully promoted in the Japanese physical examination population. In addition, we drew a ROC curve to measure the ability of BRI, BMI, WC, and ABSI to predict the risk of DM.
There are some limitations of the this study. First, our research only focuses on the Japanese population. Therefore, the results of this study cannot be applied to other regions and ethnic groups. Second, participants who had heavy drinking habits, viral hepatitis, or used any drugs at baseline were excluded, so the conclusions in this study may not be applicable to the general population. Third, this retrospective observational study provides the association between BRI and the incident T2DM. Therefore, the results need to be further verified by prospective studies.
Conclusions
This study demonstrates a positive and non-linear relationship between BRI and incident T2DM in the Japanese population. There was a threshold effect between the BRI level and incident T2DM. When BRI is higher than 4.137 in female and 3.146 in male, it is significant positive correlated with the incident T2DM. The results were expected to provide a reference for clinicians to control BRI. This study shows that BRI can be used as a predictor for early detection and prognosis of T2DM. Abnormal BRI levesl will help clinicians to further identify the high-risk population of T2DM in Japan, which may help clinicians develop management strategies in advance.
Supplementary Information
Additional file 1: Table S1. The results of univariate COX regression. Table S2. Relationship between BRI and incident diabetes in different sensitivity analyses. Table S3. AUC with the 95% CI of BMI, WC, ABSI and BRI for predicting DM stratified by sex.
Acknowledgements
None.
Abbreviations
- ABSI
A body shape index
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- AUC
Area under the curve
- BRI
Body roundness index
- BMI
Body mass index
- CI
Confidence interval
- DBP
Diastolic blood pressure
- FPG
Fasting plasma glucose
- GAM
Generalized additive models
- GGT
Gammaglutamyltransferase
- HbA1c
HemoglobinA1c
- HDL-C
High-density lipoprotein cholesterol
- ROC
Receiver operating characteristic
- SBP
Systolic blood pressure
- T2DM
Type 2 diabetes mellitus
- TC
Total cholesterol
- TG
Triglyceride
- WC
Waist circumference
Authors’ contributions
LLW contributed to the conception and design of the study. MZ and HLP was responsible for data analysis, LLW and MZ were responsible for data interpretation. HFH wrote the original draft and QJW verified the data. All authors were involved in the reviewing and editing of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by National Natural Science Foundation of China (Grant Number 82100710), Guangdong Basic and Applied Basic Research Foundation (Grant Number 2020A1515110398), and Shenzhen Key Medical Discipline Construction Fund (Grant Number SZXK009).
Availability of data and materials
Data can be downloaded from ‘DATADRYAD’ database (www.Datadryad.org).
Declarations
Ethics approval and consent to participate
In the previously published article [6], Takuro Okamura et al. has clearly stated that: the study was approved by the ethics committee of Murakami Memorial Hospital, and written informed consent for their data to be used was obtained from each participant.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Haofei Hu, Email: huhaofei0319@126.com.
Qijun Wan, Email: yiyuan2224@sina.com.
References
- 1.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Den Hartogh DJ, Vlavcheski F, Giacca A, MacPherson REK, Tsiani E. Carnosic acid attenuates the free fatty acid-induced insulin resistance in muscle cells and adipocytes. Cells. 2022;11(1):167. doi: 10.3390/cells11010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stefanescu A, Revilla L, Lopez T, Sanchez SE, Williams MA, Gelaye B. Using A Body Shape Index (ABSI) and Body Roundness Index (BRI) to predict risk of metabolic syndrome in Peruvian adults. J Int Med Res. 2020;48(1):300060519848854. doi: 10.1177/0300060519848854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas DM, Bredlau C, Bosy-Westphal A, Mueller M, Shen W, Gallagher D, Maeda Y, McDougall A, Peterson CM, Ravussin E, Heymsfield SB. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity. 2013;21(11):2264–2271. doi: 10.1002/oby.20408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: a population-based longitudinal study. Int J Obes. 2019;43(1):139–148. doi: 10.1038/s41366-018-0076-3. [DOI] [PubMed] [Google Scholar]
- 7.Zhang N, Hu X, Zhang Q, Bai P, Cai M, Zeng TS, Zhang JY, Tian SH, Min J, Huang HT, Zheng J, Peng MM, Li MJ, Chen LL. Non-high-density lipoprotein cholesterol: high-density lipoprotein cholesterol ratio is an independent risk factor for diabetes mellitus: Results from a population-based cohort study. J Diabetes. 2018;10(9):708–714. doi: 10.1111/1753-0407.12650. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto Y, Hamaguchi M, Kojima T, Ohshima Y, Ohbora A, Kato T, Nakamura N, Fukui M. Modest alcohol consumption reduces the incidence of fatty liver in men: a population-based large-scale cohort study. J Gastroenterol Hepatol. 2015;30(3):546–552. doi: 10.1111/jgh.12786. [DOI] [PubMed] [Google Scholar]
- 9.Ryu S, Chang Y, Kim DI, Kim WS, Suh BS. gamma-Glutamyltransferase as a predictor of chronic kidney disease in nonhypertensive and nondiabetic Korean men. Clin Chem. 2007;53(1):71–77. doi: 10.1373/clinchem.2006.078980. [DOI] [PubMed] [Google Scholar]
- 10.Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T, Kato T, Takeda N, Okuda J, Ida K, Kawahito Y, Yoshikawa T, Okanoue T. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102(12):2708–2715. doi: 10.1111/j.1572-0241.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 11.Basevi V, Di Mario S, Morciano C, Nonino F , Magrini N. Comment on: American diabetes association. Standards of medical care in diabetes–2011. Diabetes Care 2011;34(Suppl. 1):S11–S61. Diabetes care. 2011;34(5):e53. [DOI] [PMC free article] [PubMed]
- 12.Maggio CA, Pi-Sunyer FX. Obesity and type 2 diabetes. Endocrinol Metab Clin N Am. 2003;32(4):805–822. doi: 10.1016/S0889-8529(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 13.Gómez-Ambrosi J, Silva C, Galofré JC, Escalada J, Santos S, Millán D, Vila N, Ibañez P, Gil MJ, Valentí V, Rotellar F, Ramírez B, Salvador J, Frühbeck G. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int J Obes. 2012;36(2):286–294. doi: 10.1038/ijo.2011.100. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Wu HK, Wu XW, Cao Z, Tu YC, Ma Y, Li BN, Peng QY, Cheng J, Wu B, Zhou Z. The feasibility of two anthropometric indices to identify metabolic syndrome, insulin resistance and inflammatory factors in obese and overweight adults. Nutrition. 2019;57:194–201. doi: 10.1016/j.nut.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Q, Zhang K, Li Y, Zhen Q, Shi J, Yu Y, Tao Y, Cheng Y, Liu Y. Capacity of a body shape index and body roundness index to identify diabetes mellitus in Han Chinese people in Northeast China: a cross-sectional study. Diabetic Med. 2018;35(11):1580–1587. doi: 10.1111/dme.13787. [DOI] [PubMed] [Google Scholar]
- 16.Chang Y, Guo X, Chen Y, Guo L, Li Z, Yu S, Yang H, Sun Y. A body shape index and body roundness index: two new body indices to identify diabetes mellitus among rural populations in northeast China. BMC Public Health. 2015;15:794. doi: 10.1186/s12889-015-2150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Wang F, Wang J, Han X, Hu H, Yu C, Yuan J, Yao P, Miao X, Wei S, Wang Y, Chen W, Liang Y, Guo H, Zhang X, Zheng D, Tang Y, Yang H, He M. Using different anthropometric indices to assess prediction ability of type 2 diabetes in elderly population: a 5 year prospective study. BMC Geriatr. 2018;18(1):218. doi: 10.1186/s12877-018-0912-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, He S, Chen X. Capacity of different anthropometric measures to predict diabetes in a Chinese population in southwest China: a 15-year prospective study. Diabet Med. 2019;36(10):1261–1267. doi: 10.1111/dme.14055. [DOI] [PubMed] [Google Scholar]
- 19.Kim JY, Lee DY, Lee YJ, Park KJ, Kim KH, Kim JW, Kim WH. Chronic alcohol consumption potentiates the development of diabetes through pancreatic β-cell dysfunction. World J Biol Chem. 2015;6(1):1–15. doi: 10.4331/wjbc.v6.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. The results of univariate COX regression. Table S2. Relationship between BRI and incident diabetes in different sensitivity analyses. Table S3. AUC with the 95% CI of BMI, WC, ABSI and BRI for predicting DM stratified by sex.
Data Availability Statement
Data can be downloaded from ‘DATADRYAD’ database (www.Datadryad.org).