Abstract
Background
Steroid-resistant (SR) acute graft-versus-host disease (aGVHD) lacks standard second-line treatment. Mesenchymal stromal cells (MSCs) have potential efficacy in SR aGVHD. We aimed to assess the efficacy and safety of MSCs combined with basiliximab and calcineurin inhibitor as second-line therapy for SR aGVHD.
Methods
A randomized phase 3 trial involved 203 SR aGVHD patients at nine centers in China (September 2014–March 2019). Participants were randomized at a 1:1 ratio to receive second-line therapy with (n = 101) or without (n = 102) MSCs. The primary endpoint was the overall response (OR) at day 28. Secondary and safety endpoints included durable OR at day 56, failure-free survival, overall survival (OS), chronic GVHD (cGVHD), infection, hematological toxicity and relapse.
Results
Of 203 patients, 198 (97.5%; mean age, 30.1 years; 40.4% women) completed the study. The OR at day 28 was higher in the MSC group than the control group (82.8% [82 patients] vs. 70.7% [70]; odds ratio, 2.00; 95% confidence interval [CI], 1.01–3.94; P = 0.043). The durable OR at day 56 was also higher in the MSC group (78.8% [78 patients] vs. 64.6% [64]; odds ratio, 2.02; 95% CI, 1.08–3.83; P = 0.027). The median failure-free survival was longer in the MSC group compared with control (11.3 months vs. 6.0 months; hazard ratio (HR) 0.68; 95% CI, 0.48–0.95, P = 0.024). The 2-year cumulative incidence of cGVHD was 39.5% (95% CI, 29.3–49.4%) and 62.7% (51.4–72.1%) in the MSC and control groups (HR 0.55, 95% CI, 0.36–0.84; P = 0.005). Within 180 days after study treatments, the most common grade 3 and 4 adverse events were infections (65 [65.7%] in the MSC group vs. 78 [78.8%] in the control group) and hematological toxicity (37 [37.4%] vs. 53 [53.5%]). The 3-year cumulative incidence of tumor relapse was 10.1% (95% CI, 5.2–17.1) and 13.5% (7.5–21.2%) in the MSC and control groups, respectively (HR 0.75, 95% CI, 0.34–1.67, P = 0.610).
Conclusions
MSCs plus second-line treatments increase the efficacy of SR aGVHD, decrease drug toxicity of second-line drugs and cGVHD without increasing relapse, and are well-tolerated. MSCs could be recommended as a second-line treatment option for aGVHD patients.
Trial registration clinicaltrials.gov identifier: NCT02241018. Registration date: September 16, 2014, https://clinicaltrials.gov/ct2/show/NCT02241018.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-022-01240-4.
Keywords: Mesenchymal stromal cell, Steroid-resistant acute graft-versus-host disease, Second-line treatment, Allogeneic hematopoietic stem cell transplantation
Background
Acute graft-versus-host disease (aGVHD) remains one of the most frequent complications following allogeneic hematopoietic stem cell transplantation (allo-HSCT) with high mortality [1–5]. Corticosteroids are considered the first-line standard treatment for aGVHD; however, the response rate is only approximately 50%, and long-term survival is poor for those with steroid-resistant (SR) aGVHD [6–9]. Currently, standard second-line treatments for SR aGVHD have not been established [8, 10–13]. Available second-line therapy options include mycophenolate mofetil (MMF), anti-CD25 antibody, ruxolitinib, and so on [10, 14–20]. Deaths from SR aGVHD are only partly due to aGVHD itself, but are mostly due to the long-term influences of aGVHD, such as adverse effects of immunosuppressive agents such as infections and relapse, as well as chronic graft-versus-host disease (cGVHD) evolving from aGVHD. Therefore, new therapeutic agents are urgently needed for the management of SR aGVHD.
Mesenchymal stromal cells (MSCs) are multipotent progenitor cells that exist in various adult tissues, including bone marrow (BM) [21–24]. Based on their multipotency and immunomodulatory properties, they have been used successfully in the treatment of tissue repair and autoimmune diseases, including aGVHD [22, 25, 26]. Since 2004, Le Blanc et al. first reported that MSCs successfully rescued a pediatric patient experiencing refractory aGVHD, an increasing number of studies have been performed to investigate the effect of MSCs in aGVHD treatment [27–38]. Most studies, including our previous non-randomized study, suggested that MSCs were effective for SR aGVHD, but some studies showed that MSCs failed to improve the overall response (OR) of SR aGVHD, for example, a recent industrial MSC-led randomized controlled trial (RCT) [30]. Currently, although debates regarding MSCs as a treatment option for aGVHD are still ongoing, MSCs are recommended as evidence level A-II for aGVHD treatment [10]. However, not enough data from well-designed RCTs are available to verify the second-line treatment position of MSCs for aGVHD. In all previous prospective and retrospective studies, drug combinations exhibited considerable heterogeneity that had a strong impact on efficacy evaluation. In this study, we designed a phase 3 RCT to investigate the efficacy and safety of MSCs combined with second-line drugs for SR aGVHD, in which basiliximab and calcineurin inhibitor were “specified standardized second-line therapy.”
Methods
Study design and patients
This study was an open-label, multicenter, randomized, prospective, phase 3 trial conducted at nine hospitals in China between September 2014 and March 2019. Patients were eligible if they were aged 14 to 65 years and diagnosed with SR aGVHD [6, 16, 39]. Patients were excluded if aGVHD occurred due to tapering/discontinuing immunosuppressors or donor lymphocyte infusion (DLI) for prevention/treatment of primary disease relapse, received more than one previous treatment for SR aGVHD except for steroids before randomization, had uncontrolled infections, active visceral hemorrhage, or severe concomitant conditions not suitable for the trial. The diagnosis of aGVHD was according to the literature criteria established by the Mount Sinai Acute GVHD International consortium group [40]. SR aGVHD was defined as aGVHD worsening after 3 days of therapy onset with ≥ 2 mg/kg/day of methylprednisolone or equivalent, or failure to improve after 7 days of treatment initiation; or treatment failure during steroid taper (i.e., an increase in the methylprednisolone dose to ≥ 2 mg/kg/day or equivalent or an inability to taper the dose to < 0.5 mg/kg/day of methylprednisolone or equivalent for a minimum of 7 days) [6, 16, 39].
Approval was obtained from the institutional review board of each participating hospital, and all patients (or their guardians) provided written informed consent before enrollment. This study was performed in accordance with the Declaration of Helsinki.
Randomization and masking
Once evaluated as eligible, patients were randomly allocated to the MSC and control groups at a ratio of 1:1 according to the randomization principle after signing informed consent form. Randomization was performed with permuted blocks (block size four), and implemented through an interactive web-based response system. The statistical vendor generated the randomization codes, which were given to the interactive response system vendor to perform the randomization. Study site staff enrolled patients. The next assignment in the sequence remained concealed, as treatment was assigned remotely. Treatment allocations were not masked to the investigators or participants. The data analysis and assessments of outcomes were performed in a masked manner.
MSC preparation
MSCs were manufactured and provided by the Center for Stem Cell Biology and Tissue Engineering, Sun Yat-Sen University. MSCs were obtained from fresh BM of unrelated, HLA-mismatched, third-party donors after written informed consent. Isolation, culture and identification of MSCs were performed in accordance with our previous publication [31, 41–43]. Cells were harvested at passages 4 to 5, and fresh meeting release criteria MSCs were shipped to the clinical sites in 100 ml saline with a continuous temperature monitoring device at 4 °C (Additional file 1: Methods S1).
Interventions
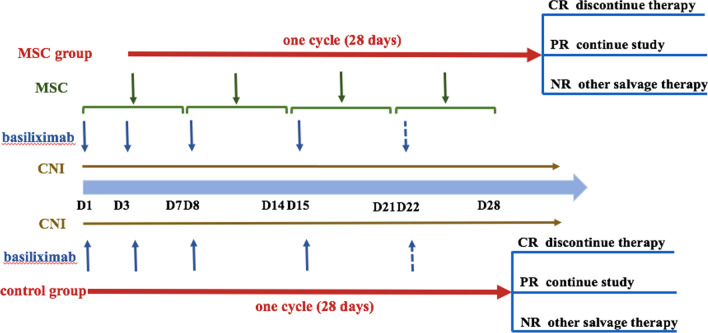
For patients assigned to the control group, basiliximab (20 mg per dose on day 1, 3, 8, and repeated weekly until aGVHD was reduced to grade < II) and calcineurin inhibitor (first choosing cyclosporine, if not tolerant, change to tacrolimus) considered as “specified standardized second-line therapy” were given in the first cycle (time from the initial treatments to continuous 28 days after that). Steroids were tapered after two doses of basiliximab and recommend tapering by 30% every 5 days and stopping within 4 weeks [44]. Other immunosuppressive agents, such as MMF, methotrexate (MTX), ruxolitinib and mammalian target of rapamycin (mTOR) inhibitor, were allowed after one cycle in NR patients by the attending physician. NR patients evaluated at day 28 in the control group could choose to receive MSCs treatment based on their voluntary principle (Fig. 1).
Fig. 1.
Treatment plan of SR aGVHD patients in the MSC and control group. CNI calcineurin inhibitor, CR complete response, PR partial response, NR no response
Patients assigned to MSC group also received “specified standardized second-line therapy” in the first cycle (time from the first dose of MSC infusion to continuous 28 days after that), and other immunosuppressive agents after one cycle in NR patients as the control group. MSCs were initiated within the following 7 days after the application of standardized second-line therapy. MSCs were given intravenously at a dose of 1 × 106 cells/kg once weekly for 4 consecutive weeks as a cycle. Further administration of MSCs was based on the response of MSCs evaluated at day 28. Complete response (CR) and no response (NR) patients discontinued MSCs treatment, while partial response (PR) patients continued to receive MSCs until aGVHD showed CR or MSCs had been infused for 8 doses (Fig. 1).
Patients visited every day from day 1 to day 7, weekly from day 8 to day 56, every month from day 56 to the third month and every 3 months thereafter to collect data on progression, survival, cGVHD and safety outcomes including relapse and infection.
Endpoints and assessments
The primary endpoint was the OR at day 28, which was defined as the proportion of patients who achieved CR and PR at day 28. The key secondary endpoint was the durable OR at day 56, which was defined as the proportion of patients who had response at day 28 and maintained until day 56. Other secondary endpoints included failure-free survival (time from randomization to relapse or progression of hematologic disease, non-relapse-related death, or the addition of new systemic therapy for aGVHD; the competing risk was the onset of cGVHD) [16], overall survival (OS), the incidence and severity of cGVHD, relapse and non-relapse mortality (NRM). The diagnosis of cGVHD was according to the NIH criteria [45].
Safety analyses were assessed by monitoring adverse events (AEs) and tumor relapse in all patients throughout the trial. AEs included infusion toxicity and infections, hematologic toxicity, et al., which were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Trial drug infusion-related safety was assessed by a physician investigator who remained at the patient’s bedside for the duration of the infusion and in intensive care unit for 6 h after the start of infusion to monitor for AEs. Follow-up care was monitored by physical examination and laboratory assessments, such as routine blood testing, liver, renal function and myocardial enzymes, BM assessment, CMV-DNA and EBV-DNA, et al. Grade 3 hematologic AEs were defined as ANC < 1.0–0.5 × 109/L or PLT < 30–20 × 109/L, and grade 4 hematologic AEs as ANC < 0.5 × 109/L or PLT < 20 × 109/L [46].
Statistical analysis
The sample size was calculated based on the primary endpoint, the OR rate of MSCs treatment for SR aGVHD, which was approximately 70% in a previous study [28]. To identify a 20% difference in OR rate of SR aGVHD with MSCs plus second-line drugs treatment, a minimum of 93 patients per group was required to provide the study with 80% power and a two-sided significance level of 0.05. Considering a dropout rate of 5%, sample size was increased to 98 patients for each group. The sample size calculation was conducted using PASS version 15 software.
Statistical analysis was performed using the intent-to-treat (ITT) population on June 30, 2020. ITT population was defined as all randomly assigned patients, which was the basis for the analysis of efficacy and safety endpoints. The incidence and severity of cGVHD were performed in the modified ITT (mITT) population, which excluded patients who received DLI as a prevention/treatment for relapse and MSCs as a salvage treatment for refractory aGVHD in the control group. All statistical analyses were performed using software SPSS 21.0 or R version 3.3.0. Patient data were compared using Fisher’s exact test for categorical variables and Mann–Whitney U tests for continuous variables. Kaplan–Meier curves for failure-free survival and OS were plotted, and the hazard ratios (HR) were calculated, along with the 95% confidence intervals (CI), with the use of a stratified Cox model. The cumulative incidence of cGVHD, relapse and NRM were calculated by accounting for competing risks. Competing risks for cGVHD included relapse and death without cGVHD. Relapse was a competing risk for NRM, and NRM was a competing risk for relapse. The comparison of the cumulative incidence in the presence of a competing risk was performed using the Fine and Gray method [47]. P < 0.05 for a two-sided text was considered statistically significant.
Results
Patients
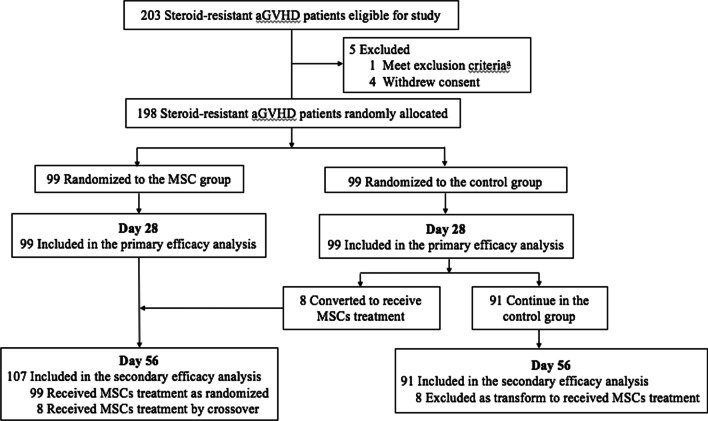
Between September 2014 and March 2019, a total of 203 patients with SR aGVHD were screened at enrollment, four of which withdrew informed consent and one met exclusion criteria. The remaining 198 patients were enrolled and randomly assigned to the MSC group (99 patients) or control group (99). The study flow diagram is shown in Fig. 2.
Fig. 2.
Flow of patient enrollment, randomization and follow-up
The baseline demographic, GVHD, transplantation-related and disease-related characteristics of patients in two groups are given in Table 1. Of 198 enrolled patients, the median age was 29 years (range, 14–59). A total of 73 patients (36.9%) had grade II aGVHD, 85 (42.9%) had grade III, and 40 (20.2%) had grade IV aGVHD. 19 (9.6%) patients developed upper gastrointestinal (GI) aGVHD, 156 (78.8%) developed lower GI aGVHD, 136 (68.7%) developed skin, and 89 (44.9%) developed liver aGVHD. The median time from transplantation to diagnosis of aGVHD was 30 days (14–132) in the MSC group and 28 days (16–124) in the control group. The two groups were balanced with respect to age, sex, primary disease and disease status at transplant, transplant modality and aGVHD characteristics.
Table 1.
Baseline, disease, transplantation and GVHD characteristics of patients with SR aGVHD in two groups
| Variable | MSC group No. (%) |
Control group No. (%) |
P |
|---|---|---|---|
| No. of patients | 99 | 99 | |
| Age, median (range), years | 28 (16–59) | 29 (16–57) | 0.680 |
| < 18 year | 14 | 17 | |
| ≥ 18 year | 85 | 82 | |
| Sex | 0.385 | ||
| Male | 62 (62.6%) | 56 (56.6%) | |
| Female | 37 (37.4%) | 43 (43.4%) | |
| Disease | 0.129 | ||
| AML | 39 (39.4%) | 49 (49.5%) | |
| ALL | 45 (45.5%) | 43 (43.4%) | |
| Others* | 15 (15.2%) | 7 (7.1%) | |
| MDS | 6 (6.1%) | 3 (3.0%) | |
| CML | 3 (3.0%) | 1 (1.0%) | |
| MM | 1 (1.0%) | 0 | |
| NHL | 0 | 1 (1.0%) | |
| Other acute leukemia | 5 (5.1%) | 2 (2.0%) | |
| Disease status at transplant | 0.524 | ||
| CR | 63 (63.6%) | 70 (70.7%) | |
| PR | 6 (6.1%) | 6 (6.1%) | |
| NR | 30 (30.3%) | 23 (23.2%) | |
| HLA typing | 1.000 | ||
| HLA matched | 51 (51.5%) | 51 (51.5%) | |
| HLA mismatched | 48 (48.5%) | 48 (48.5%) | |
| Conditioning | 0.200 | ||
| Bu-based | 51 (51.5%) | 42 (42.4%) | |
| TBI-based | 48 (48.5%) | 57 (57.6%) | |
| Donor source | 0.567 | ||
| PBSC | 53 (53.5%) | 57 (57.6%) | |
| PBSC + BM | 46 (46.5%) | 42 (42.4%) | |
| GVHD prevention | 0.886 | ||
| CsA + MTX or CsA + MTX + MMF | 42 (42.4%) | 43 (43.4%) | |
| CsA + MTX + MMF + ATG | 57 (57.6%) | 56 (56.6%) | |
| Grade of aGVHD | 0.771 | ||
| Grade 2 | 36 (36.4%) | 37 (37.4%) | |
| Grade 3 | 41 (41.4%) | 44 (44.4%) | |
| Grade 4 | 22 (22.2%) | 18 (18.2%) | |
| No. of aGVHD involved organs | 0.589 | ||
| 1 | 25 (25.3%) | 29 (29.3%) | |
| 2 | 46 (46.5%) | 48 (48.5%) | |
| 3 | 28 (28.3%) | 22 (22.2%) | |
| aGVHD involved organs | 0.860 | ||
| Skin | 73 (73.7%) | 63 (63.6%) | |
| Liver | 43 (43.4%) | 46 (46.5%) | |
| Upper GI | 9 (9.1%) | 10 (10.1%) | |
| Lower GI | 79 (79.8%) | 77 (77.8%) | |
| Onset of aGVHD median days (range) | 30 (14–132) | 28 (16–124) | 0.736 |
GVHD graft-versus-host disease, SR steroid-resistant, MSCs mesenchymal stromal cells, AML acute myeloid leukemia, ALL acute lymphocyte leukemia, CR complete response, PR partial response, NR no response, HLA human leukocyte antigen, Bu busulfan, TBI total body irradiation, PBSC peripheral blood stem cells, BM bone marrow, CsA cyclosporine, MTX methotrexate, MMF mycophenolate mofetil, ATG antithymocyte globulin, GI gastrointestinal
*Others included myelodysplastic syndrome (MDS), chronic myelogenous leukemia (CML), multiple myeloma (MM), non-hodgkin lymphoma (NHL) and other acute leukemia
Efficacy
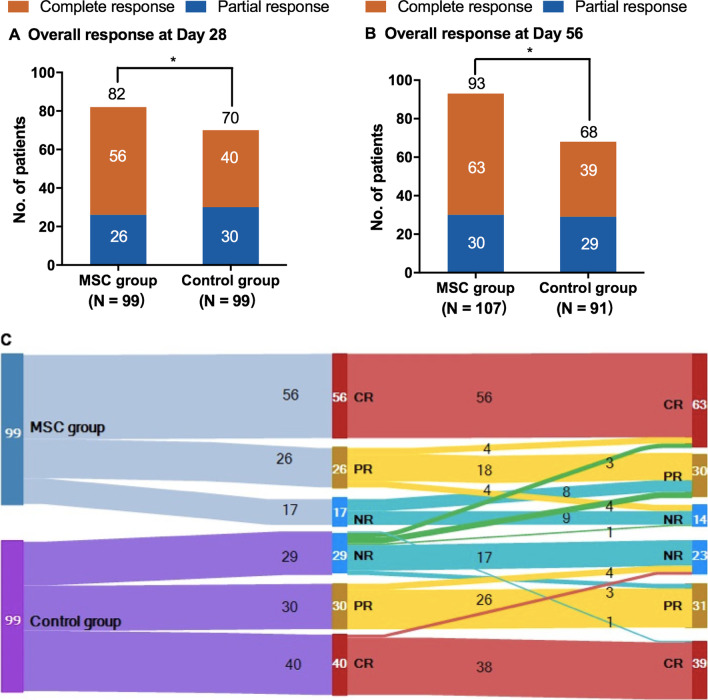
In the MSC group, the median number of MSC infusions for each patient was 5 (3–8). Median duration from the onset of aGVHD to the first MSC infusion was 10 days (6–17). For the primary efficacy evaluation at day 28, 56 of 99 patients (56.6%) achieved CR, 26 (26.3%) achieved PR, and 17 (17.2%) did not respond in the MSC group, while CR in 40 of 99 patients (40.4%), PR in 30 (30.3%) and NR in 29 (29.3%) in the control group. The OR rate at day 28 in the MSC group was significantly higher than that in the control group (82.8% [82 of 99 patients] vs. 70.7% [70 of 99]; odds ratio, 2.00; 95% CI, 1.01–3.94; P = 0.043). The proportions of patients with OR were the highest in patients with grade II aGVHD (97.2% [35 of 36 patients] in the MSC group vs. 91.9% [34 of 37] in the control group) and in those with grade III aGVHD (80.5% [33 of 41] vs. 68.2% [30 of 44]). However, the odds ratio for response in the MSC group as compared with control was the highest among patients with grade IV aGVHD (63.6% [14 of 22] vs. 33.3% [6 of 18]; odds ratio, 3.5; 95% CI, 0.95–12.97). The responses of patients with aGVHD in two groups are shown in Table 2 and Fig. 3A–C. The OR rate at day 56 was significantly higher in the MSC group than the control group (86.9% [93] vs. 74.7% [68]; odds ratio, 2.25; 95% CI, 1.08–4.68; P = 0.028; Fig. 3A–C). Durable OR at day 56 was also higher in the MSC group (78.8% [78] vs. 64.6% [64]; odds ratio, 2.03; 95% CI, 1.08–3.83; P = 0.027).
Table 2.
Treatment response of SR aGVHD between the two groups at day 28
| Outcomes | MSC group | Control group | P |
|---|---|---|---|
| No. of patients | 99 | 99 | |
| OR rate of aGVHD grade | |||
| II | 35 (97.2%) | 34 (91.9%) | 0.317 |
| III | 33 (80.5%) | 30 (68.2%) | 0.196 |
| IV | 14 (63.6%) | 6 (33.3%) | 0.057 |
| OR rate of aGVHD involved organs numbers | |||
| 1 | 24 (96.0%) | 26 (89.7%) | 0.375 |
| 》 2 | 58 (78.4%) | 44 (62.9%) | 0.002 |
| OR rate of aGVHD organs | |||
| Skin | 64 (87.7%) | 50 (79.4%) | 0.190 |
| Liver | 33 (76.7%) | 32 (69.6%) | 0.446 |
| Upper GI | 9 (100.0%) | 9 (90.0%) | 0.330 |
| Lower GI | 54 (68.4%) | 46 (59.7%) | 0.262 |
| OR rate of patients’ age | |||
| < 18 year | 12 (85.7%) | 11 (64.7%) | 0.183 |
| ≥ 18 year | 70 (82.4%) | 59 (71.9%) | 0.109 |
Fig. 3.
Assessment of response to acute graft-versus-host disease (aGVHD) treatments. A Overall response (OR) at day 28 after randomization, B OR at day 56 after randomization and C Sankey diagram of responses in the MSCs and control groups over time. Steroid-resistant (SR) aGVHD in the MSCs and control groups were shaded baby blue and ultramarine, respectively; the width of each bar represented their relative frequency with the study. Qualities of response at day 28 follow-up (second column from left) and at day 56 follow-up (third column from left) were depicted in red (CR), yellow (PR), and prussian blue (NR). The NR patients in the control group crossed over to receive MSCs treatment was depicted in green
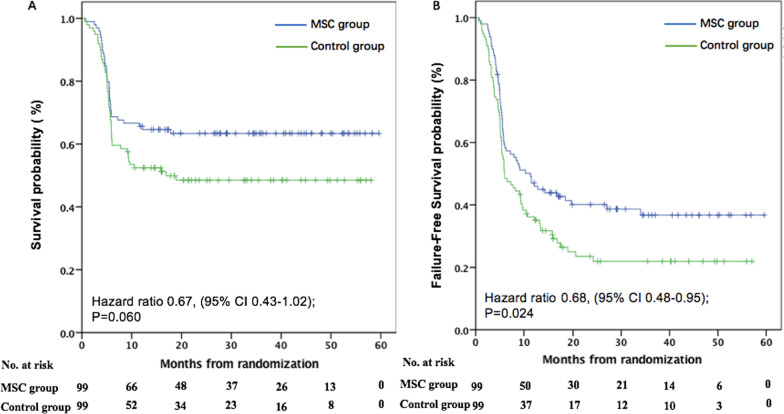
The median follow-up was 19.8 months (0.76–59.6) in the MSC group and 12.3 months (0.6–58.1) in the control group. In the MSCs group, 63 patients survived and 36 patients died, while 49 survived and 50 died in the control group. The causes of death in the MSC and control groups included primary disease relapse (n = 8 vs. 9), aGVHD (n = 9 vs. 14), cGVHD (n = 4 vs. 8), severe infections (n = 12 vs. 16), hemorrhagic disease (n = 3 vs. 2) and thrombotic microangiopathy (n = 0 vs. 1). The 6-month, 1-year and 3-year OS were 68.7% (95% CI, 64.0–73.4%), 67.1% (62.3–71.9%) and 63.4% (58.5–68.3%) in the MSC group versus 60.6% (55.7–65.5%), 54.8% (49.7–59.9%) and 48.5% (43.4–53.6%) in the control group, respectively (HR 0.76, 95% CI, 0.47–1.22; P = 0.248, HR 0.68, 95% CI, 0.43–1.07; P = 0.096, HR 0.67, 95% CI, 0.43–1.02; P = 0.060; Fig. 4A). The median failure-free survival was significantly longer in the MSC group than the control group (11.3 months vs. 6.0 months; HR 0.68; 95% CI, 0.48–0.95, P = 0.024) (Fig. 4B).
Fig. 4.
Overall survival (OS) and failure-free survival. A OS and B failure-free survival were stratified according to whether patients receiving MSCs post-randomization. And for these analysis, the eight patients in the control group who crossed over to receive MSCs are included in the control group. Failure-free survival was defined as time from randomization to relapse or progression of hematologic disease, non-relapse-related death or the addition of new systemic therapy for aGVHD, and the competing risk was the onset of chronic graft-versus-host disease (cGVHD). *P < 0.05, **P < 0.001
cGVHD
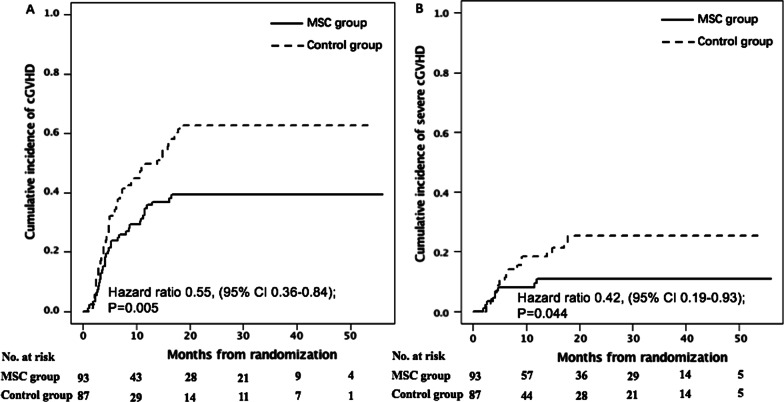
The 1-year and 2-year cumulative incidence of overall cGVHD was 35.0% (95% CI, 25.3–44.8%) versus 49.7% (38.7–59.8%) (HR 0.57, 95% CI, 0.36–0.91, P = 0.046) and 39.5% (29.3–49.4%) versus 62.7% (51.4–72.1%) (HR 0.55, 95% CI, 0.36–0.84, P = 0.005; Fig. 5A), and severe cGVHD was 9.4% (4.4–16.9%) versus 18.1% (10.4–27.6%) (HR 0.43, 95% CI, 0.17–1.06, P = 0.131) and 10.8% (5.2–18.6%) versus 25.3% (15.6–36.2%) (HR 0.42, 95% CI, 0.19–0.93, P = 0.044; Fig. 5B) in the MSC and control groups, respectively.
Fig. 5.
Cumulative incidence of overall chronic graft-versus-host disease (cGVHD) (A) and severe cGVHD (B). A, B Stratified according to whether patients receiving MSCs post-randomization. *P < 0.05, **P < 0.001
Safety
AEs from enrollment to 180 days after study treatments are shown in Table 3. Multiple infusions of MSCs were well-tolerated with no infusion-related AEs during infusion or within 6 h from the start of infusion. At least one type of grade 3–4 AE was reported for 83 (83.8%) of 99 patients in the MSC group and 85 (85.9%) of 99 in the control group. The most common grade 3–4 AEs for patients assigned to the MSC and control groups were infection and hematologic toxicity. Infection of any grade 3–4 occurred in 65 patients (65.7%) who received MSCs and in 78 (78.8%) who received control therapy (P = 0.039). Among patients with infection, the viral, bacterial and fungal infections in the MSC group, respectively, accounted for 69.2%, 38.5% and 10.8%, compared with 70.5%, 46.2% and 12.8% in the control group. Grade 3–4 hematologic toxicities occurred in 37 patients (37.4%) in the MSC group and 53 (53.5%) in the control group (P = 0.022).
Table 3.
Adverse events reported by interventional investigators
| Event | MSC group (N = 99) | Control group (N = 99) | ||
|---|---|---|---|---|
| Any grade | Grade ≧ 3 | Any grade | Grade ≧ 3 | |
| Any adverse event | 92 (92.9) | 83 (83.8%) | 95 (96.0%) | 85 (85.9%) |
| Hematologica | – | 37 (37.4%) | – | 53 (53.5%) |
| Platelets decreased | – | 21 (21.2%) | – | 30 (30.3%) |
| Neutrophils decreased | – | 16 (16.2%) | – | 23 (23.2%) |
| Skinb | 27 (27.3%) | 8 (8.1%) | 32 (32.3%) | 12 (12.1%) |
| Gastrointestinalb | 30 (30.3%) | 5 (5.1%) | 37 (37.4%) | 8 (8.1%) |
| Hepatobilinary or pancreaticb | 16 (16.2%) | 5 (5.1%) | 18 (18.2%) | 4 (4.0%) |
| Cardiac | 39 (39.4%) | 13 (13.1%) | 41 (41.4%) | 16 (16.2%) |
| Renal or genitourinary | 27 (27.3%) | 10 (10.1%) | 28 (28.3%) | 11 (11.1%) |
| Vascular | 18 (18.2%) | 7 (7.1%) | 19 (19.2%) | 10 (10.1%) |
| Infections | 75 (75.8%) | 65 (65.7%) | 81 (81.8%) | 78 (78.8%) |
| Secondary malignant disease | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
Table shows the adverse events that have an incidence of at least 10% in either group. The safety population included all patients who received at least one dose of trial therapy
aIncluded patients with decreases in platelet counts and neutrophil counts
bExcluded patients with aGVHD
Serious AEs (SAEs) occurred in 41 patients (41.4%) of MSC group and in 44 (44.4%) of control group (Table 4). Twenty-four patients in the MSC group and 34 in the control group died from SAE. Most deaths were attributed to serious aGVHD (nine patients [9.1%] in the MSC group and 14 [17.2%] in the control group). Other causes of death during the randomized treatment period were infections (8 vs. 12 patients in the MSC and control groups), relapse (5 vs. 6), and hemorrhagic disease (2 vs. 2). These deaths were not related to treatments.
Table 4.
Serious adverse effects
| Event | MSC group (N = 99) | Control group (N = 99) |
|---|---|---|
| Any adverse event | 41 (41.4%) | 44 (44.4%) |
| Hematologica | 9 (9.1%) | 13 (13.1%) |
| Platelets decreased | 6 (6.1%) | 8 (8.1%) |
| Platelet and neutrophil both decreased | 3 (3.0%) | 5 (5.1%) |
| Skinb | 0 (0%) | 1 (1.0%) |
| Gastrointestinalb | 4 (4.0%) | 5 (5.1%) |
| Hepatobilinary or pancreaticb | 2 (2.0%) | 2 (2.0%) |
| Cardiac (heart failure) | 4 (4.0%) | 6 (6.1%) |
| Renal or genitourinary (cystitis non-infective) | 4 (4.0%) | 5 (5.1%) |
| Vascular (thrombotic microangiopathy) | 0 (0%) | 1 (1.0%) |
| Infections | 12 (12.1%) | 16 (16.2%) |
| Secondary malignant disease | 0 (0%) | 0 (0%) |
aIncluded patients with decreases in platelet counts and neutrophil counts
bExcluded patients with aGVHD
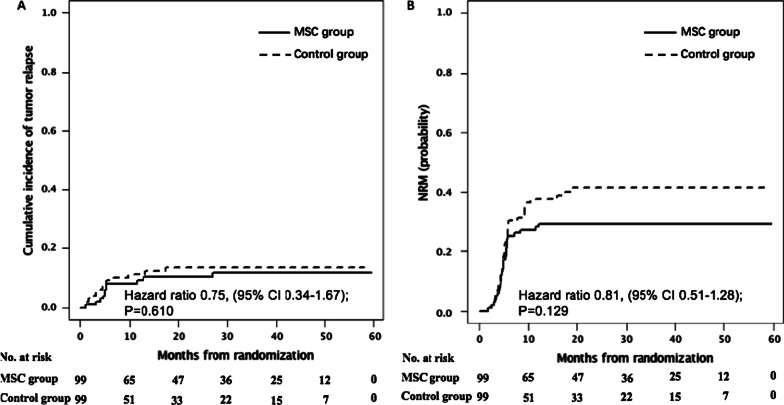
The 3-year cumulative incidence of relapse was 10.1% (95% CI, 5.2–17.1%) in the MSC group and 13.5% (7.5–21.2%) in the control group (HR 0.75, 95% CI, 0.34–1.67, P = 0.610, Fig. 6A). NRM at 3 years was 29.3% (20.6–38.5%) in the MSC group and 41.4% (31.3–51.1%) in the control group (HR 0.81, 95% CI, 0.51–1.28, P = 0.129, Fig. 6B).
Fig. 6.
Cumulative incidence of leukemia relapse (A) and non-relapse mortality (NRM) (B). A, B Stratified according to whether patients receiving MSCs post-randomization
Discussion
This open-label, randomized phase 3 trial shows that MSCs plus basiliximab and calcineurin inhibitor for SR aGVHD patients lead to a great improvement in efficacy, with a higher OR at day 28 and higher durable OR at day 56. MSC administration was also associated with prolonged failure-free survival than control. Moreover, we found that MSCs can reduce the side effects of second-line drugs, such as BM toxicity and infections. Distinguished from other studies, we adopted MSCs plus “specified standardized second-line therapy,” which minimized the confounding variable of heterogeneous second-line therapies, so that the results were more comparable.
Currently, we have a wide choice of second-line treatments that could be used to treat SR aGVHD, including ruxolitinib, monoclonal antibodies, MTX, mTOR inhibitor, etc. [10, 14–19]. However, little reliable information to determine which agents might be best for SR aGVHD patients. Therefore, no standard second-line treatments for SR aGVHD have been recommended. Ruxolitinib recently became the first drug approved for SR aGVHD treatment, with high response rates (55–62%) [16]. Anti-CD25 antibody as one of the most commonly used SR aGVHD treatments led to the response of 70.2% [15]. MSCs have been investigated in a large number of clinical trials as novel cellular therapy in GVHD [27–38]. In a phase II single-arm study involving 55 SR aGVHD patients with MSC treatment, OR rate was 70.9% [28]. In our preliminary non-randomized pilot study, we observed that MSCs led to a higher OR than control in aGVHD patients who failed second-line treatment [31]. In this RCT, we focused on SR aGVHD patients treated with MSCs plus basiliximab and calcineurin inhibitor as the “specified standardized second-line therapy”. The results showed that MSCs plus second-line drugs had a better response than treatment of single agent for SR aGVHD. But owing to unbalanced treatment cohorts and different definitions and timing of response assessments, the comparison needed to be caution. In contrast with these results, some studies documented that MSCs failed to improve the low response rate. Recent RCT based on the addition of industrial MSCs to heterogeneous second-line therapies in SR aGVHD patients failed to improve the durable CR at day 28 compared with the control [30]. Among all previous studies, drug combinations exhibited considerable heterogeneity that had a strong impact on efficacy evaluation. Besides, we surprisingly found that the NR SR aGVHD patients who received ruxolitinib in MSC group showed a higher efficacy than the control group (42.8% (3/7) vs. 11.1% (1/9), respectively). But the sample size is too small and large-scale clinical trials are needed.
What accounts for the opposite clinical outcomes concerning the efficiency of MSCs for aGVHD? The heterogeneity of MSC products partly explained the difference, which included MSCs source, manufacturing process, donors, culture passages, and the culture and expansion media [22, 48, 49]. Moreover, the heterogeneity of enrolled patients and treatment schedule also influenced the effects of MSC treatment [22, 28–31, 49]. The highlight of our RCT is that we standardized the second-line therapies for aGVHD in the MSC and control groups. To our knowledge, no RCT has been designed to eliminate the nonstandard influence of second-line drug combinations in MSC efficacy evaluation for aGVHD treatment.
Regarding safety, there remains debates over whether MSCs increase relapse, infection and BM suppression toxicity. Most studies have indicated that MSCs do not increase infection or relapse. However, Ning et al. reported that MSCs increased relapse in patients co-transplanted HSCs to prevent GVHD [50]. This study showed that relapse did not differ between the MSC and control groups. Of interest, we found that infection was improved by MSC treatment. The rational explanations are that MSCs promoting T-cell reconstitution and possessing antimicrobial ability by direct effects on pathogens or indirect effects through secreting soluble factors and enhancing anti-inflammatory function of immune cells [51–56]. Another interesting discovery is that MSCs improve BM toxicity, possibly because MSCs play a vital role in modulating BM microenvironment and supporting hematopoiesis [22, 25, 31, 57].
In addition, we found that the 2-year cumulative incidence of overall cGVHD and severe cGVHD was both decreased in the MSC group compared with controls, verifying our previous explore findings [31]. The mechanisms might be associated with MSCs alleviating thymus damage caused by aGVHD by improving the thymic negative selection, decreasing auto-reactive T-cell and inducing Treg production [31, 58–61].
A few highly relevant shortcomings of data presented here should be mentioned. First, this is a non-blinded and non-placebo controlled study, which may carry a higher risk of bias on the part of both the treating physician and the patient, usually in favor of the investigational arm. Moreover, SR aGVHD in our study was almost always diagnosed by clinical findings, which might influence the therapeutic evaluation of MSCs.
Conclusions
This trial shows that the addition of BM-derived third-party MSCs to second-line therapy leads to a higher therapeutic response and prolonged failure-free survival of SR aGVHD patients compared with controls. MSCs also decrease toxicity of second-line drugs and cGVHD without increasing relapse. MSCs could be recommended as a second-line treatment option for aGVHD patients.
Supplementary Information
Additional file 1: Method S1. Preparation of Mesenchymal Stromal Cells.
Acknowledgements
Not applicable.
Abbreviations
- aGVHD
Acute graft-versus-host disease
- allo-HSCT
Allogeneic hematopoietic stem cell transplantation
- SR
Steroid-resistant
- MMF
Mycophenolate mofetil
- cGVHD
Chronic graft-versus-host disease
- MSCs
Mesenchymal stromal cells
- BM
Bone marrow
- OR
Overall response
- RCT
Randomized controlled trial
- DLI
Donor lymphocyte infusion
- CR
Complete response
- NR
No response
- PR
Partial response
- MTX
Methotrexate
- mTOR
Mammalian target of rapamycin
- OS
Overall survival
- NRM
Non-relapse mortality
- AEs
Adverse events
- ITT
Intent-to-treat
- mITT
Modified ITT
- HR
Hazard ratios
- CI
Confidence intervals
- SAEs
Serious AEs
Authors' contributions
Drs. QL and PX had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. KZ, RL, ZF, XC and YW contributed equally to this work. Concept and design was done by KZ, RL, XZ, PX and QL. Acquisition, analysis, or interpretation of data was done by KZ, ZF, YW, FH, NX, XZ, SW, DL, LD, DN, JW, YL, XZ, YL and QL. Drafting of the manuscript was done by KZ, PX and QL. Critical revision of the manuscript for important intellectual content was done by KZ, RL, XC, YW, LX, XZ, XZ, PX and QL. Statistical analysis was carried out by KZ, RL, YW and LX.KZ and QL obtained funding. Administrative, technical or material support were given by XC, YW, XZ, SW, DL, LD, DN, JW, YL, XZ, YL and PX. Supervision was done by ZF, FH, NX, XZ, SW, JW, YL, XZ and PX. All authors read and approved the final manuscript.
Funding
The trial was supported by the National Key Research and Development Programme of China (2017YFA105500, 2017YFA0105504, 2017YFA0105503), the Research and Development Program in Key Areas of Guangdong Province (2019B020236004), Natural Science Foundation of Guangdong Province (2017A030310103), and the National Natural Science Foundation of China (81970161, 81700176 and 81770190).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in compliance with Declaration of Helsinki principles. All procedures involving human subjects were approved by the institutional review board of each participating hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ke Zhao, Ren Lin, Zhiping Fan, Xiaoyong Chen and Yu Wang contributed equally to this study
Contributor Information
A. P. Xiang, Email: xiangp@mail.sysu.edu.cn
Qifa Liu, Email: liuqifa628@163.com.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Nagler A, Labopin M, Houhou M, Aljurf M, Mousavi A, Hamladji RM, et al. Outcome of haploidentical versus matched sibling donors in hematopoietic stem cell transplantation for adult patients with acute lymphoblastic leukemia: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. J Hematol Oncol. 2021;14(1):53. doi: 10.1186/s13045-021-01065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanz J, Galimard JE, Labopin M, Afanasyev B, Sergeevich MI, Angelucci E, et al. Post-transplant cyclophosphamide containing regimens after matched sibling, matched unrelated and haploidentical donor transplants in patients with acute lymphoblastic leukemia in first complete remission, a comparative study of the ALWP of the EBMT. J Hematol Oncol. 2021;14(1):84. doi: 10.1186/s13045-021-01094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao XS, Huang XJ. Seeking biomarkers for acute graft-versus-host disease: where we are and where we are heading? Biomark Res. 2019;7:17. doi: 10.1186/s40364-019-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang YLQ, Lin R, Yang T, Xu YJ, Mo XD, Huang XJ. Optimizing antithymocyte globulin dosing in haploidentical hematopoietic cell transplantation: long-term follow-up of a muticenter, randomized controlled trial. Sci Bull. 2021;66(24):2498–2505. doi: 10.1016/j.scib.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Deeg HJ. How I treat refractory acute GVHD. Blood. 2007;109(10):4119–4126. doi: 10.1182/blood-2006-12-041889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18(8):1150–1163. doi: 10.1016/j.bbmt.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeiser R, Blazar BR. Acute graft-versus-host disease—biologic process, prevention, and therapy. N Engl J Med. 2017;377(22):2167–2179. doi: 10.1056/NEJMra1609337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brissot E, Labopin M, Moiseev I, Cornelissen JJ, Meijer E, Van Gorkom G, et al. Post-transplant cyclophosphamide versus antithymocyte globulin in patients with acute myeloid leukemia in first complete remission undergoing allogeneic stem cell transplantation from 10/10 HLA-matched unrelated donors. J Hematol Oncol. 2020;13(1):87. doi: 10.1186/s13045-020-00923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7(2):e157–e167. doi: 10.1016/S2352-3026(19)30256-X. [DOI] [PubMed] [Google Scholar]
- 11.Zhang XH, Chen J, Han MZ, Huang H, Jiang EL, Jiang M, et al. The consensus from The Chinese Society of Hematology on indications, conditioning regimens and donor selection for allogeneic hematopoietic stem cell transplantation: 2021 update. J Hematol Oncol. 2021;14(1):145. doi: 10.1186/s13045-021-01159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin PJ, Inamoto Y, Flowers ME, Carpenter PA. Secondary treatment of acute graft-versus-host disease: a critical review. Biol Blood Marrow Transplant. 2012;18(7):982–988. doi: 10.1016/j.bbmt.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dignan FL, Clark A, Amrolia P, Cornish J, Jackson G, Mahendra P, et al. Diagnosis and management of acute graft-versus-host disease. Br J Haematol. 2012;158(1):30–45. doi: 10.1111/j.1365-2141.2012.09129.x. [DOI] [PubMed] [Google Scholar]
- 14.Lin D, Hu B, Li P, Zhao Y, Xu Y, Wu D. Roles of the intestinal microbiota and microbial metabolites in acute GVHD. Exp Hematol Oncol. 2021;10(1):49. doi: 10.1186/s40164-021-00240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu SN, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. Prognostic factors and long-term follow-up of basiliximab for steroid-refractory acute graft-versus-host disease: updated experience from a large-scale study. Am J Hematol. 2020;95(8):927–936. doi: 10.1002/ajh.25839. [DOI] [PubMed] [Google Scholar]
- 16.Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 2020;382(19):1800–1810. doi: 10.1056/NEJMoa1917635. [DOI] [PubMed] [Google Scholar]
- 17.Busca A, Locatelli F, Marmont F, Ceretto C, Falda M. Recombinant human soluble tumor necrosis factor receptor fusion protein as treatment for steroid refractory graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2007;82(1):45–52. doi: 10.1002/ajh.20752. [DOI] [PubMed] [Google Scholar]
- 18.Kakihana K, Fujioka Y, Suda W, Najima Y, Kuwata G, Sasajima S, et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood. 2016;128(16):2083–2088. doi: 10.1182/blood-2016-05-717652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danylesko I, Bukauskas A, Paulson M, Peceliunas V, Gedde-Dahl DYT, Shimoni A, et al. Anti-alpha4beta7 integrin monoclonal antibody (vedolizumab) for the treatment of steroid-resistant severe intestinal acute graft-versus-host disease. Bone Marrow Transplant. 2019;54(7):987–993. doi: 10.1038/s41409-018-0364-5. [DOI] [PubMed] [Google Scholar]
- 20.Mankarious M, Matthews NC, Snowden JA, Alfred A. Extracorporeal photopheresis (ECP) and the potential of novel biomarkers in optimizing management of acute and chronic graft versus host disease (GvHD) Front Immunol. 2020;11:81. doi: 10.3389/fimmu.2020.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.da Silva ML, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(Pt 11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 22.Zhao K, Liu Q. The clinical application of mesenchymal stromal cells in hematopoietic stem cell transplantation. J Hematol Oncol. 2016;9(1):46. doi: 10.1186/s13045-016-0276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lan T, Luo M, Wei X. Mesenchymal stem/stromal cells in cancer therapy. J Hematol Oncol. 2021;14(1):195. doi: 10.1186/s13045-021-01208-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 25.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 26.Cai J, Wu Z, Xu X, Liao L, Chen J, Huang L, et al. Umbilical cord mesenchymal stromal cell with autologous bone marrow cell transplantation in established type 1 diabetes: a pilot randomized controlled open-label clinical study to assess safety and impact on insulin secretion. Diabetes Care. 2016;39(1):149–157. doi: 10.2337/dc15-0171. [DOI] [PubMed] [Google Scholar]
- 27.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 28.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 29.Bonig H, Kuci Z, Kuci S, Bakhtiar S, Basu O, Bug G, et al. Children and adults with refractory acute graft-versus-host disease respond to treatment with the mesenchymal stromal cell preparation "MSC-FFM"-outcome report of 92 patients. Cells. 2019;8(12):1577. doi: 10.3390/cells8121577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kebriaei P, Hayes J, Daly A, Uberti J, Marks DI, Soiffer R, et al. A phase 3 randomized study of remestemcel-L versus placebo added to second-line therapy in patients with steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2020;26(5):835–844. doi: 10.1016/j.bbmt.2019.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao K, Lou R, Huang F, Peng Y, Jiang Z, Huang K, et al. Immunomodulation effects of mesenchymal stromal cells on acute graft-versus-host disease after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21(1):97–104. doi: 10.1016/j.bbmt.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 32.Zhou T, Yuan Z, Weng J, Pei D, Du X, He C, et al. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 2021;14(1):24. doi: 10.1186/s13045-021-01037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Dalowski F, Kramer M, Wermke M, Wehner R, Rollig C, Alakel N, et al. Mesenchymal stromal cells for treatment of acute steroid-refractory graft versus host disease: clinical responses and long-term outcome. Stem Cells. 2016;34(2):357–366. doi: 10.1002/stem.2224. [DOI] [PubMed] [Google Scholar]
- 34.Kurtzberg J, Prockop S, Teira P, Bittencourt H, Lewis V, Chan KW, et al. Allogeneic human mesenchymal stem cell therapy (remestemcel-L, prochymal) as a rescue agent for severe refractory acute graft-versus-host disease in pediatric patients. Biol Blood Marrow Transplant. 2014;20(2):229–235. doi: 10.1016/j.bbmt.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Kebriaei P, Isola L, Bahceci E, Holland K, Rowley S, McGuirk J, et al. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15(7):804–811. doi: 10.1016/j.bbmt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Introna M, Lucchini G, Dander E, Galimberti S, Rovelli A, Balduzzi A, et al. Treatment of graft versus host disease with mesenchymal stromal cells: a phase I study on 40 adult and pediatric patients. Biol Blood Marrow Transplant. 2014;20(3):375–381. doi: 10.1016/j.bbmt.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 37.Dotoli GM, De Santis GC, Orellana MD, de Lima PK, Caruso SR, Fernandes TR, et al. Mesenchymal stromal cell infusion to treat steroid-refractory acute GvHD III/IV after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52(6):859–862. doi: 10.1038/bmt.2017.35. [DOI] [PubMed] [Google Scholar]
- 38.Ball LM, Bernardo ME, Roelofs H, van Tol MJ, Contoli B, Zwaginga JJ, et al. Multiple infusions of mesenchymal stromal cells induce sustained remission in children with steroid-refractory, grade III–IV acute graft-versus-host disease. Br J Haematol. 2013;163(4):501–509. doi: 10.1111/bjh.12545. [DOI] [PubMed] [Google Scholar]
- 39.Schoemans HM, Lee SJ, Ferrara JL, Wolff D, Levine JE, Schultz KR, et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology and guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 2018;53(11):1401–1415. doi: 10.1038/s41409-018-0204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016;22(1):4–10. doi: 10.1016/j.bbmt.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Wu M, Peng Y, Chen X, Sun J, Huang F, et al. Improvement in poor graft function after allogeneic hematopoietic stem cell transplantation upon administration of mesenchymal stem cells from third-party donors: a pilot prospective study. Cell Transplant. 2014;23(9):1087–1098. doi: 10.3727/096368912X661319. [DOI] [PubMed] [Google Scholar]
- 42.Xiong YY, Fan Q, Huang F, Zhang Y, Wang Y, Chen XY, et al. Mesenchymal stem cells versus mesenchymal stem cells combined with cord blood for engraftment failure after autologous hematopoietic stem cell transplantation: a pilot prospective, open-label, randomized trial. Biol Blood Marrow Transplant. 2014;20(2):236–242. doi: 10.1016/j.bbmt.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Chen S, Zhao K, Lin R, Wang S, Fan Z, Huang F, et al. The efficacy of mesenchymal stem cells in bronchiolitis obliterans syndrome after allogeneic HSCT: a multicenter prospective cohort study. EBioMedicine. 2019;49:213–222. doi: 10.1016/j.ebiom.2019.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang FF, Cheng YF, Xu LP, Zhang XH, Yan CH, Han W, et al. Basiliximab as treatment for steroid-refractory acute graft-versus-host disease in pediatric patients after haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2020;26(2):351–357. doi: 10.1016/j.bbmt.2019.10.031. [DOI] [PubMed] [Google Scholar]
- 45.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389–401.e381. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xuan L, Wang Y, Huang F, Fan Z, Xu Y, Sun J, et al. Sorafenib maintenance in patients with FLT3-ITD acute myeloid leukaemia undergoing allogeneic haematopoietic stem-cell transplantation: an open-label, multicentre, randomised phase 3 trial. Lancet Oncol. 2020;21(9):1201–1212. doi: 10.1016/S1470-2045(20)30455-1. [DOI] [PubMed] [Google Scholar]
- 47.Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36(27):4391–4400. doi: 10.1002/sim.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galipeau J. The mesenchymal stromal cells dilemma–does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy. 2013;15(1):2–8. doi: 10.1016/j.jcyt.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Locatelli F, Algeri M, Trevisan V, Bertaina A. Remestemcel-L for the treatment of graft versus host disease. Expert Rev Clin Immunol. 2017;13(1):43–56. doi: 10.1080/1744666X.2016.1208086. [DOI] [PubMed] [Google Scholar]
- 50.Ning H, Yang F, Jiang M, Hu L, Feng K, Zhang J, et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia. 2008;22(3):593–599. doi: 10.1038/sj.leu.2405090. [DOI] [PubMed] [Google Scholar]
- 51.Auletta JJ, Deans RJ, Bartholomew AM. Emerging roles for multipotent, bone marrow-derived stromal cells in host defense. Blood. 2012;119(8):1801–1809. doi: 10.1182/blood-2011-10-384354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qin A, Lai DH, Liu Q, Huang W, Wu YP, Chen X, et al. Guanylate-binding protein 1 (GBP1) contributes to the immunity of human mesenchymal stromal cells against Toxoplasma gondii. Proc Natl Acad Sci U S A. 2017;114(6):1365–1370. doi: 10.1073/pnas.1619665114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13(4):392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 54.Balan A, Lucchini G, Schmidt S, Schneider A, Tramsen L, Kuci S, et al. Mesenchymal stromal cells in the antimicrobial host response of hematopoietic stem cell recipients with graft-versus-host disease—friends or foes? Leukemia. 2014;28(10):1941–1948. doi: 10.1038/leu.2014.127. [DOI] [PubMed] [Google Scholar]
- 55.Peng Y, Chen X, Liu Q, Zhang X, Huang K, Liu L, et al. Mesenchymal stromal cells infusions improve refractory chronic graft versus host disease through an increase of CD5+ regulatory B cells producing interleukin 10. Leukemia. 2015;29(3):636–646. doi: 10.1038/leu.2014.225. [DOI] [PubMed] [Google Scholar]
- 56.Weng JY, Du X, Geng SX, Peng YW, Wang Z, Lu ZS, et al. Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transplant. 2010;45(12):1732–1740. doi: 10.1038/bmt.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meuleman N, Tondreau T, Ahmad I, Kwan J, Crokaert F, Delforge A, et al. Infusion of mesenchymal stromal cells can aid hematopoietic recovery following allogeneic hematopoietic stem cell myeloablative transplant: a pilot study. Stem Cells Dev. 2009;18(9):1247–1252. doi: 10.1089/scd.2009.0029. [DOI] [PubMed] [Google Scholar]
- 58.Lax S, Ross EA, White A, Marshall JL, Jenkinson WE, Isacke CM, et al. CD248 expression on mesenchymal stromal cells is required for post-natal and infection-dependent thymus remodelling and regeneration. FEBS Open Bio. 2012;2:187–190. doi: 10.1016/j.fob.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao L, Zhang Y, Hu B, Liu J, Kong P, Lou S, et al. Phase II multicenter, randomized, double-blind controlled study of efficacy and safety of umbilical cord-derived mesenchymal stromal cells in the prophylaxis of chronic graft-versus-host disease after HLA-haploidentical stem-cell transplantation. J Clin Oncol. 2016;34(24):2843–2850. doi: 10.1200/JCO.2015.65.3642. [DOI] [PubMed] [Google Scholar]
- 60.Zhan Y, Wang L, Liu G, Zhang X, Yang J, Pan Y, et al. The reparative effects of human adipose-derived mesenchymal stem cells in the chemotherapy-damaged thymus. Stem Cells Dev. 2019;28(3):186–195. doi: 10.1089/scd.2018.0142. [DOI] [PubMed] [Google Scholar]
- 61.Hu KX, Wang MH, Fan C, Wang L, Guo M, Ai HS. CM-DiI labeled mesenchymal stem cells homed to thymus inducing immune recovery of mice after haploidentical bone marrow transplantation. Int Immunopharmacol. 2011;11(9):1265–1270. doi: 10.1016/j.intimp.2011.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Method S1. Preparation of Mesenchymal Stromal Cells.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.