Abstract
Background
This was a retrospective study conducted at a rural referral center in East Java, Indonesia, to evaluate the association between the platelet-to-lymphocyte ratio (PLR) on hospital admission and the incidence of new symptomatic heart failure (HF) within 6 months in patients with acute coronary syndrome (ACS).
Material/Methods
The study population consisted of all ACS patients who were hospitalized between 1 January and 31 December 2018 at a non-percutaneous coronary intervention-capable secondary referral hospital and came for a routine follow-up until 6 months afterwards. The diagnosis of new symptomatic HF was based on International Classification of Diseases 10th revision code I50.9.
Results
From 126 hospitalized patients, 92 patients were included in the analysis. The incidence rate of new symptomatic HF at 6 months was 70.65%. High PLR upon initial admission was significantly associated with new symptomatic HF incidence (odds ratio=1.70, P<0.001). PLR was also able to discriminate new symptomatic HF incidence at 6 months with area under the curve of 0.83 (P=0.001). Multivariate Cox regression analysis showed that PLR was an independent predictor for new symptomatic HF incidence (hazard ratio=4.5, P=0.001).
Conclusions
In a rural center in Indonesia, the PLR was independently correlated with the onset of new symptomatic HF in patients with ACS 6 months after hospital admission. The PLR may be a supplementary biomarker for clinical outcomes in patients with ACS for use in resource-limited regions.
Keywords: Acute Coronary Syndrome, Biomarkers, Heart Failure
Background
Acute coronary syndrome (ACS) is a leading cause of mortality worldwide. It is a group of conditions caused by reduction of blood flow to the heart, including ST elevation myocardial infarction (STEMI), non-ST elevation myocardial infarction (NSTEMI), and unstable angina [1]. ACS can cause a number of complications, and one of the major complications is heart failure (HF) [2,3]. It is associated with high in-hospital mortality and poor long-term survival [4]. A population-based study in Saudi Arabian patients revealed that in-hospital mortality and cardiogenic shock were significantly higher if HF was presented [5]. A prospective cohort registry in the UK that evaluated the long-term prognosis of HF patients after ACS found that new-onset heart failure was associated with a 4-fold increased risk of death at 6 months [6]. Moreover, even in a stable condition, HF could lead to a dimnished quality of life [7]. Thus, it is very important to predict symptomatic HF occurrence in patients who survived ACS.
It has been proposed that inflammation plays an important role in both ACS and HF [8,9]. Various types of inflammatory biomarkers have been developed to predict the outcome of these diseases, such as N-terminal prohormone of brain natriuretic peptide (NT pro-BNP), high-sensitivity C-reactive protein (hs-CRP), lipoprotein-associated phospholipase A2, and homocysteine [10–12]. Unfortunately, these biomarkers are still scarce and are even unaffordable in areas with limited resources. Therefore, a biomarker that is cheap and widely available is needed.
Platelet-to-lymphocyte ratio (PLR) is a new inflammatory biomarker that can be used to predict morbidity and mortality. It can be easily calculated from complete blood count analysis. Recent studies showed that higher PLR levels reflected a more severe inflammation process and was associated with higher in-hospital and long-term mortality and major adverse cardiovascular events (MACE) in ACS patients [13–15]. Another study also found that higher PLR was corelated with worse 6-month survival rate in patients with acute HF and might be a novel marker in AHF management [16]. However, to the best of our knowledge, only a few studies have used PLR to predict HF incidence following ACS [17,18]. Therefore, this retrospective study was conducted at a rural referral center in East Java, Indonesia, to evaluate the association between the PLR on hospital admission and the incidence of HF at 6 months in patients with ACS
Material and Methods
This study was a retrospective cohort study that adhered to the principles of the Declaration of Helsinki and received ethics approval from the department of health of Tuban city (approval number: 070/152/414.107/2019). The requirement of written informed consent was waived because this was a retrospective study. This study was conducted at one of the non-percutaneous coronary intervention (PCI)-capable secondary referral government hospitals located at Tuban city, East Java region, Indonesia. Tuban city is considered as a rural area in this region. The study population was all ACS patients who were hospitalized between 1 January and 31 December 2018. ACS patients were defined as patients who presented with symptoms of ischemia in association with electrocardiographic or cardiac enzyme changes according to the ESC guideline, regardless of the presence of ST-segment elevation [19,20]. Patients were then followed up for 6 months after the ACS event. Inclusion criteria were patients with no symptoms or history of treatment for coronary artery disease or HF previously, valvular heart disease, chronic obstructive pulmonary disease, rhythm abnormalities, and no other inflammatory or infectious disease during hospitalization. Exclusion criteria were patients who had poor adherence to regular visits to the cardiovascular outpatient clinic after hospitalization and patients without complete data.
Data on demographic characteristics, clinical signs and symptoms, cardiovascular risk factors, medication, and initial admission laboratory results from emergency room were retrieved from medical records. To measure the red blood cells, white blood cells, neutrophils, platelets, and lymphocyte counts, peripheral blood was collected and placed in ethylenediaminetetraacetic acid-coated tubes. Afterwards, the blood was analyzed using a Sysmex XN-1000 Hematology Analyzer (Syxmex America, Inc, Lincolnshire, Illinois). The PLR was then calculated by dividing the platelet counts and lymphocyte counts. Baseline 2-dimensional transthoracic echocardiography (TTE) (Vivid S60N, General Electric Healthcare, Wauwatosa, Wisconsin) was done in the first 48 hours of admission to determine the left ventricle ejection fraction (LVEF) and to exclude any valvular condition.
The primary outcome was new clinical symptomatic HF incidence within 6 months after ACS, based on the 2016 ESC guidelines of HF diagnosis and New York Heart Association (NYHA) functional class II–IV [21]. The definition of symptomatic HF was used because a previous study has shown that patients with symptomatic HF had higher morbidity and mortality compared to healthy subjects or patients with asymptomatic left ventricular (LV) systolic dysfunction [22]. The diagnosis of new clinical symptomatic HF was based on International Classification of Diseases 10th revision code I50.9 that was retrieved from the medical records [23]. Secondary outcomes in this study were the length of stay (LOS) during hospitalization, in-hospital complications (acute lung edema, cardiogenic shock, and life-threatening arrhythmia), and MACE (rehospitalization because of heart failure, cerebrovascular disease (CVD), or myocardial infarction) within 6 months after ACS onset. A secondary analysis was also done to assess the correlation between PLR and Global Registry of Acute Coronary Event (GRACE) mortality risk score or LVEF during the first hospitalization for ACS [24].
Statistical Analysis
For descriptive analysis, continuous data were given as mean±standard deviation (SD) or median [interquartile range (IQR)] depending on data distribution, while categorical data were given as n (%). For univariate analysis, the chi-square test was used for analyzing categorical data, and the independent t test or Mann-Whitney U test was used for continuous data, as appropriate. Variables with P value <0.25 from univariate analysis were then included in multivariate logistic regression analysis using the backward selection method [25]. Model calibration was tested using the Hosmer-Lemeshow test. Area under the curve (AUC) was used to determine whether PLR can be used to predict outcome incidence at 6 months or not. To determine the best PLR cut-off value, Youden Index was used [26]. Cox proportional multivariate hazards regression model was used to determine the hazard ratio of the PLR cut-off for outcome incidence at 6 months. P value <0.05 was considered as statistically significant [27]. All statistical analysis were done using SPSS for Windows version 21.0 (IBM Corp., Armonk, New York).
Results
There were 126 hospitalized ACS patients during the study period, and 92 patients were included in the analysis. The mean age was 56 years old, and 66.3% were male. On the ACS spectrum, 17 (18.5%) patients presented with unstable angina and 75 (81.5%) patients presented with myocardial infarction (47 patients were STEMI and 28 patients were NSTEMI). The median of pre-hospital delay after ACS onset was 7 hours. The incidence of ACS was higher on weekdays and at night.
Baseline Characteristic
Patients’ baseline characteristic is presented based on the low level (<108) and high level of PLR value (≥108) based on median PLR level in this population (Table 1). Upon ACS presentation, patients with high PLR level were older and more of them had a history of hypertension compared to patients with low PLR level (P=0.041 and 0.001, respectively). Patients in the high PLR group also had higher GRACE score on admission (119±28.21 vs 102±34.68, P<0.001). All patients were treated regularly with optimal medication for ACS, including statin, anti-platelet, beta-blocker, and ace inhibitor. Thrombolytic was performed in all patients with STEMI that fulfilled the indication criteria for this procedure (34 patients), and was successful in 15 (44%) patients. Baseline TTE showed that 95% patients had diastolic dysfunction, 93% patients had LV dysfunction (LVEF <55%), and 99% patients had regional wall motion abnormality.
Table 1.
Baseline characteristics of study population.
| Variables | Total n=92 |
High PLR (≥108) n=46 |
Low PLR (<108) n=46 |
P value |
|---|---|---|---|---|
| Age, mean (SD) | 55.91 (9.83) | 58 (9.85) | 54 (9.46) | 0.041 |
| Man gender, n (%) | 61 (66.3) | 34 (73.9) | 27 (58.69) | 0.123 |
| Myocardial infarction, n (%) | 75 (81.52) | 41 (89.1) | 34 (73.9) | 0.060 |
| Heart rate, mean±SD | 83.86±20.20 | 82.63±18.01 | 85.12±22.37 | 0.573 |
| GRACE score, mean±SD | 110.82±32.47 | 118.89±28.21 | 102.74±34.68 | 0.016 |
| BMI, mean±SD | 24.44±3.23 | 24.57±3.28 | 24.32±3.21 | 0.713 |
| % LVEF, mean±SD | 47.14±12.04 | 45.34±11.98 | 49.07±11.96 | 0.160 |
| Symptom onset hour, median [IQR] | 6.75 [4–15.75] | 6.5 [3.90–15.50] | 7.5 [4.50–23.50] | 0.443 |
| Circadian morning onset, n (%) | 50 (54.35) | 23 (50.0) | 19 (41.3) | 0.4 |
| LMR, mean±SD | 8.63±5.55 | 8.08±5.41 | 9.95±5.77 | 0.006 |
| NLR, median [IQR] | 3.9 [2.51–5.53] | 4.27 [2.82–5.63] | 2.36 [1.19–2.54] | 0.000 |
| CKMB, median [IQR] | 32 [24.75–52] | 35 [27.65–55.42] | 26 [17.50–45.45] | 0.374 |
| Blood glucose, median [IQR] | 151 [120–227] | 159 [129–236] | 143 [86–205] | 0.463 |
| Diabetes mellitus, n (%) | 27 (29.35) | 12 (26.1) | 15 (32.6) | 0.982 |
| Hypertension, n (%) | 33 (35.87) | 24 (52.2) | 9 (19.6) | 0.001 |
| Active smoker, n (%) | 50 (54.35) | 28 (60.9) | 22 (47.8) | 0.209 |
| Family history of CVD, n (%) | 17 (18.4) | 9 (19.6) | 8 (17.4) | 0.567 |
| Length of stay, median [IQR] | 4 [4–5] | 4 [4–5] | 4 [4–6] | 0.893 |
| Beta blocker, n (%) | 80 (86.9) | 41 (89.1) | 39 (84.8) | 0.536 |
| RAAS blocker, n (%) | 82 (89.1) | 41 (89.1) | 41 (89.1) | 0.765 |
| Statin, n (%) | 92 (100) | 46 (100) | 46 (100) | 1.000 |
| DAPT, n (%) | 92 (100) | 41 (100) | 41 (100) | 1.000 |
ACS – acute coronary syndrome; BMI – body mass index; CKMB – creatine kinase myocardial band; CVD – cardiovascular diseases; DAPT – dual anti-platelet therapy; GRACE score – Global Registry of Acute Coronary Events score; IQR – interquartile range; LMR – leucocyte-to-monocyte ratio; LVEF – left ventricular ejection fraction; NLR – neutrophil-to-lymphocyte ratio; PLR – platelet-to-lymphocyte ratio; SD – standard deviation; RAAS blocker – renin-angiotensin-aldosterone system blocker. P value <0.05 was considered statistically significant.
Study Outcomes
There were no in-hospital outcome parameters associated with PLR (all P>0.05). For long-term outcomes, the incidence of 6-month new clinical symptomatic HF was significantly higher in patients with high PLR (odds ratio (OR)=1.70, 95% confidence interval=1.33–2.18, P<0.001) (Table 2).
Table 2.
In-hospital and long-term outcomes of patients with acute coronary syndrome.
| Variables | Total N=92 |
PLR median group | OR (95% CI) | p-value | |
|---|---|---|---|---|---|
| High (≥108) n=46 |
Low (<108) n=46 |
||||
| In Hospital outcome | |||||
| Acute lung oedema, n (%) | 37 (40.2) | 23 (50.0) | 14 (30.4) | 1.39 (0.98–1.96) | 0.058 |
| Cardiogenic Shock, n (%) | 12 (13.04) | 6 (13.0) | 6 (13.0) | 1.00 (0.85–1.17) | 1.000 |
| Life threatening arrhythmia, n (%) | 14 (15.38) | 9 (19.6) | 5 (10.9) | 1.11 (0.93–1.32) | 0.384 |
| Length of stay, median [IQR] | 4 [4–6] | 4 [4–5] | 4 [4–6] | 1.00 (0.99–1.04) | 0.893 |
| Long term outcome | |||||
| First HF symptom during 6-month follow up (NYHA class II–IV) (%) | 65 (70.65) | 41 (89.13) | 24 (52.17) | 1.70 (1.33–2.18) | <0.001 |
| MACE during 6-month follow up | 10 (10.86) | 4 (8.69) | 6 (13.04) | 0.67 (0.24–1.41) | 0.738 |
HF – heart failure; IQR – interquartile range; PLR – platelet-to-lymphocyte ratio; MACE – major adverse cardiac events; OR – odds ratio; PLR – platelet-to-lymphocyte ratio; NYHA – New York Heart Association. P value <0.05 was considered statistically significant.
The incidence rate of new clinical symptomatic HF at 6 months after ACS was 70% (65 patients). Among them, 41 patients (63%) developed early-onset symptomatic HF during the hospitalization (<7 days after ACS onset), and 24 patients (37%) developed late-onset symptomatic HF after hospitalization (≥7 days after ACS onset). The proportion of patients with diabetes mellitus or lower LVEF who develop symptomatic HF during hospitalization was significantly higher than after hospitalization (Table 3). On the contrary, the proportion of patients with hypertension who developed symptomatic HF during hospitalization was significantly lower. The PLR was significantly lower in patients who did not develop symptomatic HF compared to patients who developed acute-onset or late-onset symptomatic HF (both P<0.001) (Figure 1).
Table 3.
Comparison of characteristics between acute coronary syndrome patients who develop heart failure during vs after hospitalization.
| Variables | Heart failure (N=65) | p-value | |
|---|---|---|---|
| Early-onset in-hospital HF (<7 days) N=41 (63%) |
Late-onset out-hospital HF (≥7 days) N=24 (37%) |
||
| Age, mean±SD | 57.95±9.98 | 58.29±9.08 | 0.891 |
| Man gender, n (%) | 28 (68.29) | 20 (83.33) | 0.183 |
| Myocardial Infarction, n (%) | 38 (92.6) | 22 (91.6) | 0.882 |
| BMI, mean±SD | 24.90±2.83 | 23.92±3.50 | 0.224 |
| % LVEF, mean±SD | 40.36±11.06 | 49.47±7.22 | 0.001 |
| Symptom onset hour, median [IQR] | 7 [5–16] | 7 [5–16] | 0.600 |
| Hypertension, n (%) | 8 (19.5) | 11 (45.8) | 0.049 |
| Diabetes, n (%) | 15 (36.58) | 3 (12.5) | 0.036 |
| Active smoker, n (%) | 23 (56.9) | 17 (70.83) | 0.239 |
| Family history of CVD | 11 (26.8) | 5 (20.8) | 0.588 |
BMI – body mass index; CVD – cardiovascular diseases; HF – heart failure; IQR – interquartile range; LVEF – left ventricular ejection fraction; SD – standard deviation. P value <0.05 was considered statistically significant.
Figure 1.
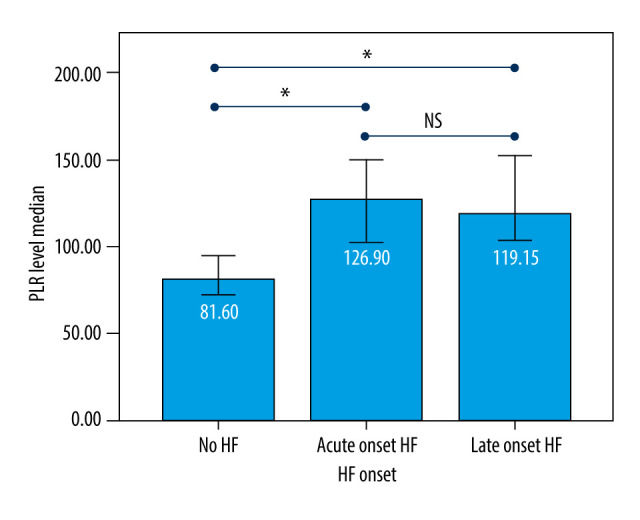
Platelet-to-lymphocyte ratio (PLR) difference based on heart failure (HF) onset. Acute-onset=HF occurred during the hospitalization and/or <7 days after acute coronary syndrome (ACS); Late-onset=HF occurred later after ACS hospitalization (≥7 days-6 months). P value <0.05 (*) was considered statistically significant, and P value >0.05 (NS) was considered not statistically significant.
In the sub-analysis among patients with myocardial infarction, there was no significant difference between STEMI and NSTEMI in symptomatic HF incidence at 6-month follow-up (hazard ratio (HR)=1.15, 95% CI=0.81–1.57, P=0.685). However, the failure of thrombolytic treatment in STEMI-eligible patients was a significant predictor for the occurrence of symptomatic HF at 6-month follow-up (HR=1.83, 95% CI=1.07–3.14, P=0.007). Furthermore, when compared with the non-eligible and failed thrombolytic STEMI patients, those with successful thrombolytic treatments were less likely to develop new symptomatic HF in the follow-up period (50% vs 87.9% incidence rate, HR=0.24, 95% CI=0.09–0.69, P=0.015).
Multivariate analysis of symptomatic HF incidence predictor
The association of different predictor variables with symptomatic HF incidence at 6 months was assessed in univariate and multivariate analyses (Table 4). In the multivariate logistic regression model for symptomatic HF incidence at 6 months after ACS, age, sex, myocardial infarction, in-hospital LV ejection fraction, circadian morning onset, admission PLR level, creatine kinase myocardial band (CKMB) level, hypertension status, smoking status, and family history of CVD were included. After adjusting for other factors, PLR level and LVEF remained significant (P<0.001). The Hosmer and Lemeshow test suggested the model had good fitness to the data (P=0.941). The model explained 65.9% (Nagelkerke R2) of the variance in symptomatic HF incidence and correctly classified 90.4% of the cases. As PLR level increase by 1 unit, the odds of having clinical symptomatic HF at 6 months also increased (adjusted OR=1.072, 95% CI=1.03–1.11, P<0.001). Meanwhile, higher baseline LVEF after ACS acted as a protective factor from symptomatic HF incidence at 6 months (adjusted OR=0.85, 95% CI=0.77–0.94, P=0.002). The final model showed that LVEF and PLR level had good discrimination for new symptomatic HF, with C statistic of 0.939 (P<0.001) (Figure 2).
Table 4.
Risk factors for developing heart failure at 6 months after acute coronary syndrome.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| COR | 95% CI | P value | AOR | 95% CI | P value | |
| Age | 1.052 | 0.879–1.25 | 0.582 | |||
| Man gender | 9.863 | 0.110–885.90 | 0.319 | |||
| Myocardial infarction | 4.858 | 0.337–69.93 | 0.245 | |||
| GRACE score | 1.008 | 0.958–1.06 | 0.751 | |||
| LVEF | 0.818 | 0.706–0.940 | 0.007 | 0.850 | 0.768–0.941 | 0.002 |
| Morning onset | 0.505 | 0.037–6.90 | 0.609 | |||
| PLR level | 1.121 | 1.033–1.210 | 0.006 | 1.072 | 1.031–1.114 | <0.001 |
| CKMB | 1.006 | 0.980–1.030 | 0.673 | |||
| Hypertension | 0.798 | 0.049–12.895 | 0.873 | |||
| Family history of CVD | 7.411 | 0.263–208.772 | 0.240 | |||
| Active smoker | 0.353 | 0.008–16.327 | 0.595 | |||
AOR – adjusted odds ratio; CKMB – creatine kinase myocardial band; COR – crude odds ratio; CVD – cardiovascular diseases; GRACE – global registry of acute coronary events; LVEF – left ventricular ejection fraction; PLR – platelet-to-lymphocyte ratio; 95% CI – 95% confidence interval. Variables with P value <0.25 from univariate analysis are included in multivariate analysis. P value <0.05 in multivariate analysis is considered statistically significant.
Figure 2.
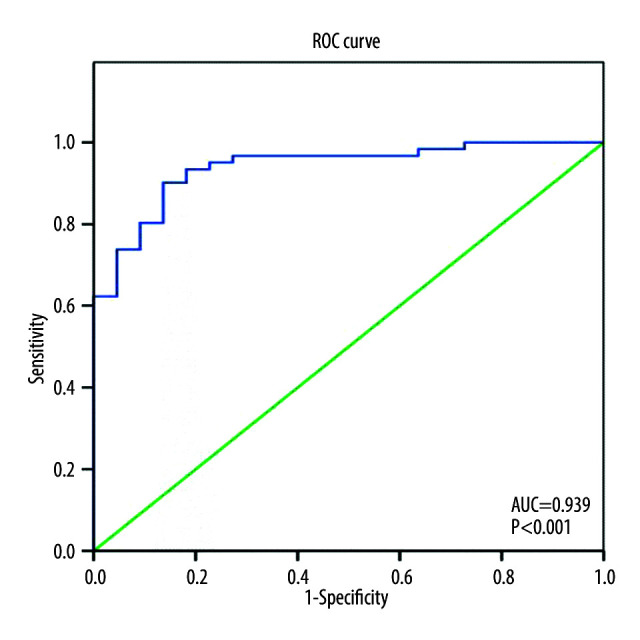
Receiver operating characteristic curve of the final model consist of left ventricular ejection fraction and platelet-to-lymphocyte ratio to predict heart failure incidence at 6 months after acute coronary syndrome. AUC – area under curve.
PLR as an Independent Predictor for Dymptomatic HF Incidence at 6 Months
PLR was able to positively discriminate new clinical symptomatic HF incidence at 6 months, with AUC of 0.83 (95% CI=0.75–0.92, P<0.001). The best cut-off for PLR level to predict new clinical symptomatic HF was 87 (sensitivity 0.89 [95% CI=0.82 to 0.94], specificity 0.63 [95% CI 0.53 to 0.72], positive likelihood ratio 2.41, positive predictive value 0.71 [95% CI=0.65 to 0.76], and negative predictive value 0.85 [95% CI=0.76 to 0.91]) (Figure 3). Using this cut-off, 58 out of 68 patients (85.3%) with PLR ≥87 had new symptomatic HF, whereas only 7 out of 24 (29.2%) with PLR <87 had new symptomatic HF (OR=2.92, 95% CI=1.55–5.50, P=0.001). Multivariate Cox regression was then used to calculate the hazard ratio for symptomatic HF incidence. After controlling for other confounding factors, PLR level ≥87 was shown to be an independent predictor of 6 months symptomatic HF incidence after ACS (HR=4.5, 95% CI=1.8–11, P=0.001) (Figure 4).
Figure 3.
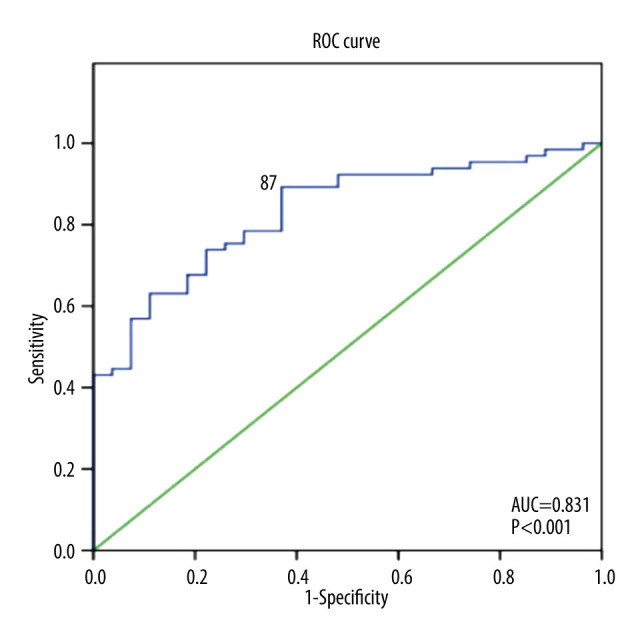
Receiver operating characteristic curve of platelet-to-lymphocyte ratio (PLR) as a predictor of heart failure (HF) incidence at 6 months. The best cut-off for PLR to discriminate 6-month HF is 87 (Sensitivity=89%, Specificity=63%, Odds ratio=2.92, 95% confidence interval 1.55–5.50, P<0.001). AUC – area under curve.
Figure 4.
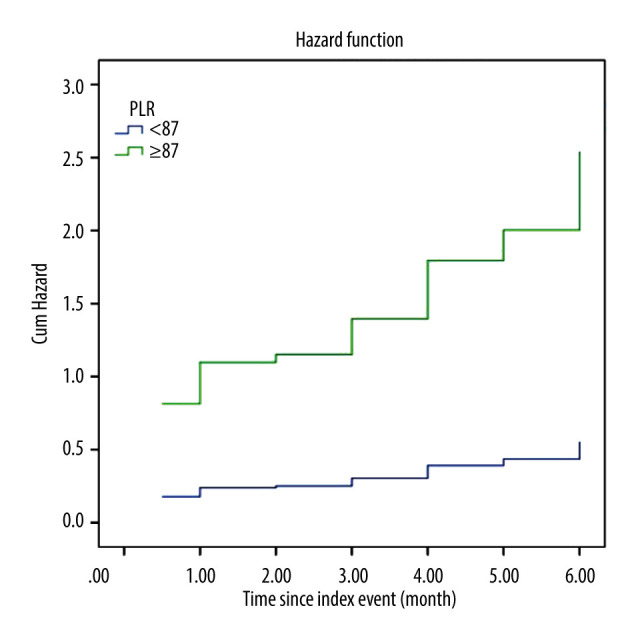
Multivariate Cox regression hazard function analysis showing differences between patients with high and low platelet-to-lymphocyte ratio (PLR) associated with the outcome of HF occurring within 6 months after hospital admission for acute coronary syndrome. The hazard ratio for high PLR (PLR ≥87) is 4.5 (95% confidence interval=1.8–11, P=0.001).
Secondary Analysis
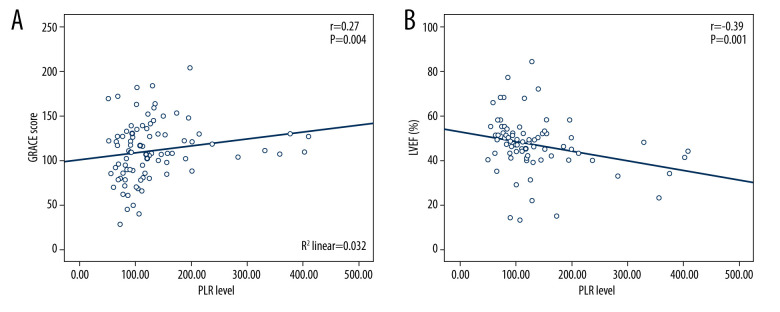
In secondary analysis using Pearson correlation, PLR was positively correlated with GRACE score (p=0.004, r=0.27) and negatively correlated with LVEF (p=0.001, r=−0.39) (Figure 5).
Figure 5.
Scatter plots showing the association between (A) platelet-to-lymphocyte ratio (PLR) and Global Registry of Acute Coronary Events score (GRACE) risk score, (B) PLR and left ventricular ejection fraction (LVEF).
Discussion
In this study, we found that 44.5% of the patients developed new symptomatic HF during admission. This incidence is higher than the previous study in Saudi Arabia that had 20% admission HF incidence [4]. This might be due to the long pre-hospital delay time (median 6.75 hours) in our study. Overall outcome at 6 months showed a 70% incidence rate of new clinical HF. This finding is higher than the result of the GRACE study with 17.6% and UK registry with 6.5% of new HF incidence rate at 6 months [6,28]. The high number of new HF cases in our study population might have been caused by a suboptimal revascularization therapy due to the absence of primary PCI strategy in our hospital. This hypothesis is also supported by the data from the GRACE study that showed patients with ACS who developed new HF were less likely to undergo cardiac catheterization [28].
From the onset of HF, our findings were similar to the population in the GRACE study [28], but with different risk factors. In our study, patients with early-onset HF were more likely to have diabetes and lower LVEF, and were less likely to have hypertension compared to the late-onset HF, whereas in the GRACE study, patients admitted with HF tended to be older and were more likely to have a history of coronary artery disease and atrial fibrillation [28].
From the univariate analysis, the risk factor predictor for developing new HF has some similarities to previous studies [6,28]. New findings in our study are the addition of GRACE score and biomarker from complete blood count and CKMB as a predictor of HF incidence. However, after multivariate logistic regression analysis, only LVEF and PLR remained significant as predictors of HF at 6 months after ACS. While previous studies only analyzed PLR either as an independent predictor for ACS [13–15] or acute HF outcome [16], our study is the first to show that PLR can also be used to predict symptomatic HF incidence at 6 months after ACS.
In regard to MACE outcome, many studies have analyzed PLR as an independent predictor for MACE and mortality in ACS or HF patient populations, including a meta-analysis of 8 studies involving 6627 participants [13,29–31]. Our study also showed that PLR had a weak correlation with GRACE mortality risk score at admission, which is a predictor of MACE within 6 months after ACS, similar to a previous study of STEMI patients [32]. However, the result of our study showed that MACE was not associated with higher PLR value. However, some studies reported that PLR value was a predictor of MACE in ACS patients [13,30]. These varied results might be caused by the difference in follow-up duration. While the observation period in our study was only 6 months, the follow-up duration in other studies were up to 5 years [13].
The cut-off points of PLR value for biomarker predictor risk group stratification varies across studies, depending on the disease, inflammation status, and population characteristics [13,33,34]. In our study, the cut-off PLR value for discriminating new symptomatic HF incidence at 6 months was lower than in previous studies. We hypothesized that patients who had an ACS event in a non-PCI facility or in a rural area were more prone to develop new HF at 6-month follow-up, despite optimal medication.
It is still not clear how the exact mechanism of PLR level is associated with adverse outcomes in ACS patients. Higher PLR value has been reported to be correlated with increased inflammatory activity and prothrombotic status due to relative thrombocytosis and megakaryocytic proliferation in ACS patients [29,35]. Furthermore, lower lymphocyte level due to increased cortisol in response to physiological stress can also be correlated with worse clinical outcomes in ACS patients [36]. Hence, PLR calculated from numbers of platelets and lymphocytes may be a novel indicator of inflammatory and prothrombotic status, and might be utilized to predict the outcome of ACS, including HF development. The mechanism involved in this includes the release of proteolytic enzymes and pro-inflammatory cytokines, secondary to the increased inflammatory state in ACS [37,38]. A previous study has shown that higher PLR is positively correlated with increased inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrotizing factor-α [39], which can damage the myocardium, resulting in reduced left ventricular function and HF [40]. High levels of pro-inflammatory cytokines might also lead to myocardial remodeling and cardiac arrhythmia, resulting in cardiac dysfunction [41]. Our secondary analysis also shows that PLR is negatively correlated with LVEF during hospitalization, which may affect the mechanism of HF development. This finding was in line with a previous study by Bekler et al (2015), which showed that high PLR was associated with left ventricular systolic dysfunction (LVEF <40%) in NSTEMI patients [17].
There were several limitations to this study. This study was a single-center study conducted in a rural area, which may be different than in an urban clinical setting. The relatively small number of samples compared to other studies might have caused several potential biases. The follow-up period was only 6 months, which may also have contributed to the non-significant result for MACE. Another limitation was that we had no comparable data on other biomarkers such as hs-CRP, IL-1, IL-6, or NT pro-BNP. Analysis of the correlation of PLR with these substances may have strengthened our study. However, despite the limitations, this is the first study to evaluate use of PLR as an independent predictor for new symptomatic HF after ACS.
Conclusions
In a rural center in Indonesia, PLR was independently correlated with the onset of symptomatic heart failure in patients with ACS 6 months after hospital admission. PLR may be a supplementary biomarker for clinical outcomes in patients with ACS for use in resource-limited regions. In patients with high PLR level, strict control of cardiovascular risk factors, routine follow-up visits, and close monitoring should be encouraged.
Footnotes
Conflict of interest: None declared
Department and Institution Where Work Was Done
Department of Cardiology and Vascular Medicine, Dr. Koesma General Hospital, Tuban, Indonesia.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
References
- 1.Singh A, Museedi AS, Grossman SA. Acute coronary syndrome. StatPearls: Treasure Island (FL); 2021. [PubMed] [Google Scholar]
- 2.Velazquez EJ, Pfeffer MA. Acute heart failure complicating acute coronary syndromes: A deadly intersection. Circulation. 2004;109(4):440–42. doi: 10.1161/01.CIR.0000113460.23813.50. [DOI] [PubMed] [Google Scholar]
- 3.Pattanayak JM, Gelfand EV. Complications of acute coronary syndrome. Management of Acute Coronary Syndromes. 2009:141–72. [Google Scholar]
- 4.AlFaleh H, Elasfar AA, Ullah A, et al. Acute heart failure with and without acute coronary syndrome: Clinical correlates and prognostic impact (from the HEARTS registry) BMC Cardiovasc Disord. 2016;16:98. doi: 10.1186/s12872-016-0267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albackr HB, Alhabib KF, Ullah A, et al. Prevalence and prognosis of congestive heart failure in Saudi patients admitted with acute coronary syndrome (from SPACE registry) Coron Artery Dis. 2013;24(7):596–601. doi: 10.1097/MCA.0b013e328364d98f. [DOI] [PubMed] [Google Scholar]
- 6.Shibata MC, Collinson J, Taneja AK, et al. Long term prognosis of heart failure after acute coronary syndromes without ST elevation. Postgrad Med J. 2006;82(963):55–59. doi: 10.1136/pgmj.2005.035766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juenger J, Schellberg D, Kraemer S, et al. Health related quality of life in patients with congestive heart failure: Comparison with other chronic diseases and relation to functional variables. Heart. 2002;87(3):235–41. doi: 10.1136/heart.87.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dick SA, Epelman S. Chronic heart failure and inflammation. Circ Res. 2016;119(1):159–76. doi: 10.1161/CIRCRESAHA.116.308030. [DOI] [PubMed] [Google Scholar]
- 9.Golia E, Limongelli G, Natale F, et al. Inflammation and cardiovascular disease: From pathogenesis to therapeutic target. Curr Atheroscler Rep. 2014;16(9):435. doi: 10.1007/s11883-014-0435-z. [DOI] [PubMed] [Google Scholar]
- 10.Carlquist JF, Muhlestein JB, Anderson JL. Lipoprotein-associated phospholipase A2: A new biomarker for cardiovascular risk assessment and potential therapeutic target. Expert Rev Mol Diagn. 2007;7(5):511–17. doi: 10.1586/14737159.7.5.511. [DOI] [PubMed] [Google Scholar]
- 11.Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14:6. doi: 10.1186/1475-2891-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richards AM. New biomarkers in heart failure: Applications in diagnosis, prognosis and guidance of therapy. Rev Esp Cardiol. 2010;63(6):635–39. doi: 10.1016/s1885-5857(10)70137-7. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Zhou Y, Ma Y, et al. The prognostic value of the platelet-to-lymphocyte ratio in acute coronary syndrome: A systematic review and meta-analysis. Kardiologia Polska. 2017:666–73. doi: 10.5603/KP.a2017.0068. [DOI] [PubMed] [Google Scholar]
- 14.Li X-T, Fang H, Li D, et al. Association of platelet to lymphocyte ratio with in-hospital major adverse cardiovascular events and the severity of coronary artery disease assessed by the Gensini score in patients with acute myocardial infarction. Chin Med J (Engl) 2020;133(4):415–23. doi: 10.1097/CM9.0000000000000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozcan Cetin EH, Cetin MS, Aras D, et al. Platelet to lymphocyte ratio as a prognostic marker of in-hospital and long-term major adverse cardiovascular events in ST-segment elevation myocardial infarction. Angiology. 2016;67(4):336–45. doi: 10.1177/0003319715591751. [DOI] [PubMed] [Google Scholar]
- 16.Ye GL, Chen Q, Chen X, et al. The prognostic role of platelet-to-lymphocyte ratio in patients with acute heart failure: A cohort study. Sci Rep. 2019;9(1):10639. doi: 10.1038/s41598-019-47143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bekler A, Gazi E, Yılmaz M, et al. Could elevated platelet-lymphocyte ratio predict left ventricular systolic dysfunction in patients with non-ST elevated acute coronary syndrome? Anat J Cardiol. 2015;15(5):385–90. doi: 10.5152/akd.2014.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W, Liu Q, Tang Y. Platelet to lymphocyte ratio in the prediction of adverse outcomes after acute coronary syndrome: A meta-analysis. Sci Rep. 2017;7:40426. doi: 10.1038/srep40426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39(2):119–77. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 20.Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37(3):267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 21.Dolgin Martin New York Heart Association Criteria Committee. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. Boston, Mass: Lippincott Williams and Wilkins; 1994. [Google Scholar]
- 22.Pandhi J, Gottdiener JS, Bartz TM, et al. Comparison of characteristics and outcomes of asymptomatic versus symptomatic left ventricular dysfunction in subjects 65 years old or older (from the Cardiovascular Health Study) Am J Cardiol. 2011;107(11):1667–74. doi: 10.1016/j.amjcard.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health O. ICD-10: International statistical classification of diseases and related health problems: tenth revision. 2nd ed. Geneva: World Health Organization; 2004. [Google Scholar]
- 24.Abu-Assi E, Garcia-Acuna JM, Pena-Gil C, Gonzalez-Juanatey JR. Validation of the GRACE risk score for predicting death within 6 months of follow-up in a contemporary cohort of patients with acute coronary syndrome. Rev Esp Cardiol. 2010;63(6):640–48. doi: 10.1016/s1885-5857(10)70138-9. [DOI] [PubMed] [Google Scholar]
- 25.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Di Leo G, Sardanelli F. Statistical significance: p value, 0.05 threshold, and applications to radiomics-reasons for a conservative approach. Eur Radiol Exp. 2020;4(1):18. doi: 10.1186/s41747-020-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steg PG, Dabbous OH, Feldman LJ, et al. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: Observations from the Global Registry of Acute Coronary Events (GRACE) Circulation. 2004;109(4):494–99. doi: 10.1161/01.CIR.0000109691.16944.DA. [DOI] [PubMed] [Google Scholar]
- 29.Fuentes QE, Fuentes QF, Andres V, et al. Role of platelets as mediators that link inflammation and thrombosis in atherosclerosis. Platelets. 2013;24(4):255–62. doi: 10.3109/09537104.2012.690113. [DOI] [PubMed] [Google Scholar]
- 30.Hudzik B, Szkodzinski J, Gorol J, et al. Platelet-to-lymphocyte ratio is a marker of poor prognosis in patients with diabetes mellitus and ST-elevation myocardial infarction. Biomark Med. 2015;9(3):199–207. doi: 10.2217/bmm.14.100. [DOI] [PubMed] [Google Scholar]
- 31.Temiz A, Gazi E, Güngör Ö, et al. Platelet/lymphocyte ratio and risk of in-hospital mortality in patients with ST-elevated myocardial infarction. Med Sci Monit. 2014;20:660–65. doi: 10.12659/MSM.890152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Acet H, Ertaş F, Akıl MA, et al. Relationship between hematologic indices and global registry of acute coronary events risk score in patients with ST-segment elevation myocardial infarction. Clin Appl Thromb Hemost. 2014;22(1):60–68. doi: 10.1177/1076029614533145. [DOI] [PubMed] [Google Scholar]
- 33.Fest J, Ruiter R, Ikram MA, et al. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: A population-based prospective cohort study. Sci Rep. 2018;8(1):10566. doi: 10.1038/s41598-018-28646-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou X, Du Y, Huang Z, et al. Prognostic value of PLR in various cancers: A meta-analysis. PLoS One. 2014;9(6):e101119. doi: 10.1371/journal.pone.0101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindemann S, Kramer B, Seizer P, Gawaz M. Platelets, inflammation and atherosclerosis. J Thromb Haemost. 2007;5(Suppl 1):203–11. doi: 10.1111/j.1538-7836.2007.02517.x. [DOI] [PubMed] [Google Scholar]
- 36.Kurtul A, Murat SN, Yarlioglues M, et al. Association of platelet-to-lymphocyte ratio with severity and complexity of coronary artery disease in patients with acute coronary syndromes. Am J Cardiol. 2014;114(7):972–78. doi: 10.1016/j.amjcard.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Fioranelli M, Bottaccioli AG, Bottaccioli F, et al. Stress and inflammation in coronary artery disease: A review psychoneuroendocrineimmunology-based. Front Immunol. 2018;9:2031. doi: 10.3389/fimmu.2018.02031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101(15):1767–72. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 39.Turkmen K, Erdur FM, Ozcicek F, et al. Platelet-to-lymphocyte ratio better predicts inflammation than neutrophil-to-lymphocyte ratio in end-stage renal disease patients. Hemodial Int. 2013;17(3):391–96. doi: 10.1111/hdi.12040. [DOI] [PubMed] [Google Scholar]
- 40.Durmus E, Kivrak T, Gerin F, et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio are predictors of heart failure. Arq Bras Cardiol. 2015;105(6):606–13. doi: 10.5935/abc.20150126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prabhu SD. Cytokine-induced modulation of cardiac function. Circ Res. 2004;95(12):1140–53. doi: 10.1161/01.RES.0000150734.79804.92. [DOI] [PubMed] [Google Scholar]