Abstract
Objectives:
The induced membrane technique (IMT) is a 2-stage surgical approach that has become increasingly popular to manage bone defects. Preclinical investigations have been conducted to better understand and define several aspects of this technique. This review summarizes the literature regarding the IMT performed in animal models and identifies potential future directions.
Data Sources:
Biosis Citation Index, Ovid Embase, and Ovid MEDLINE databases were searched from inception up to June 23, 2021 for articles related to the IMT.
Study Selection:
Animal studies involving the use of the IMT for segmental defects in long bones were selected. Only full-length original research articles published in English or French were included.
Data Extraction:
Two authors extracted the data from the selected studies and a third author verified the accuracy of the information.
Data Synthesis:
Information concerning the animal model, the surgical procedures, and the outcome measures were recorded for each study and compiled.
Conclusions:
Forty-seven studies were included in this review. Twenty-nine studies (62%) performed both stages of the technique, but only 8 (17%) reported on radiographic union rates explicitly and 5 (11%) included biomechanical testing. A large proportion of the preclinical literature on the IMT has failed to report on radiographic union as an outcome. While studies reporting membrane properties are valuable, they may not provide information that translates into clinical practice or further clinical research if the ultimate outcome of bony healing is not considered. Future animal studies of the IMT should consider this in their study design.
Keywords: bone defect, bone regeneration, induced membrane technique, Masquelet technique, nonunion
1. Introduction
The treatment of segmental bone defects and fracture nonunion represents a major clinical challenge for orthopedic surgeons. Segmental bone defects can occur as a result of trauma, infection, or tumor resection. On average, nonunion occurs in 5% of fractures, with rates reaching over 10% for femoral or tibial fractures.[1] Nonunion leads to additional surgical procedures, higher use of medication and healthcare resources, and a decrease in the patient's quality of life.[2,3]
The induced membrane technique (IMT), also known as the Masquelet technique, is a 2-stage surgical procedure that has gained popularity for the management of fracture nonunion and large bone defects. It is less complicated to perform than other techniques (such as vascularized grafting or bone transport), and healing time is theoretically not affected by the defect size.[4] Traditionally, the first stage consists of implanting a polymethylmethacrylate (PMMA) spacer into the defect site. Over subsequent weeks, the spacer induces the formation of a membrane surrounding the defect due to the foreign body immune response. The second stage involves removing the PMMA spacer through an incision in the membrane, followed by autologous bone grafting into the preserved space.
Despite the increased clinical application of the IMT, several aspects of the technique are controversial and the subject of ongoing debate, and many of these issues are difficult to investigate clinically. As a result, several animal models have been developed to characterize the IMT and test the effect of parameter variations on membrane formation and subsequent bone healing.
The objective of this study was to systematically review the literature related to the IMT performed in animal models in order to summarize the current state of knowledge and identify future directions for preclinical research.
2. Methods
2.1. Literature search strategy
BIOSIS Citation Index (Web of Science), Embase (Ovid), and MEDLINE (Ovid) databases were searched, from inception up to June 23, 2021, for studies investigating the IMT in animal models.
2.2. Inclusion and exclusion criteria
Original research articles fulfilling the following criteria were included: (1) written in English or French; (2) conducted in animal models; (3) studied segmental defects in long bones; (4) performed at least the first stage of the IMT (insertion of spacer). Our exclusion criteria were, in order: (1) duplicate; (2) unavailable full text; (3) not in selected languages (English or French); (4) abstract only; (5) not an animal study; (6) not an original research article (eg, review and editorial); (7) not IMT; (8) not a segmental defect in a long bone.
2.3. Selection of studies
Screening was performed using Covidence systematic review software (Veritas Health Innovation; Melbourne, Australia). All publications were independently screened by 2 reviewers based on their title and abstract, followed by a full-length assessment to determine final inclusion. Disagreements between 2 reviewers were resolved by a third reviewer.
2.4. Data extraction
Basic information (author, year, title) was recorded for all included studies. Data pertaining to the following elements was extracted, if available: animal model (species, strain, age, weight, and sex), sample size and groups, surgical technique (bone, size of defect, fixation technique, spacer material, duration of spacer implantation, graft material at the second stage, time to euthanasia after second stage), investigatory techniques, and outcomes. We also categorized the studies as having analyzed the induced membrane, bone formation/healing, or both.
We recorded if studies completed only the first stage surgery or both stages. Of the studies that completed a second stage surgery, we noted if they performed radiographic assessment (eg, plain X-rays and micro-computed tomography) and, in such cases, if bone union rates were explicitly reported for each group based on this assessment. We also recorded if bones were subjected to biomechanical testing.
3. Results
3.1. Search results
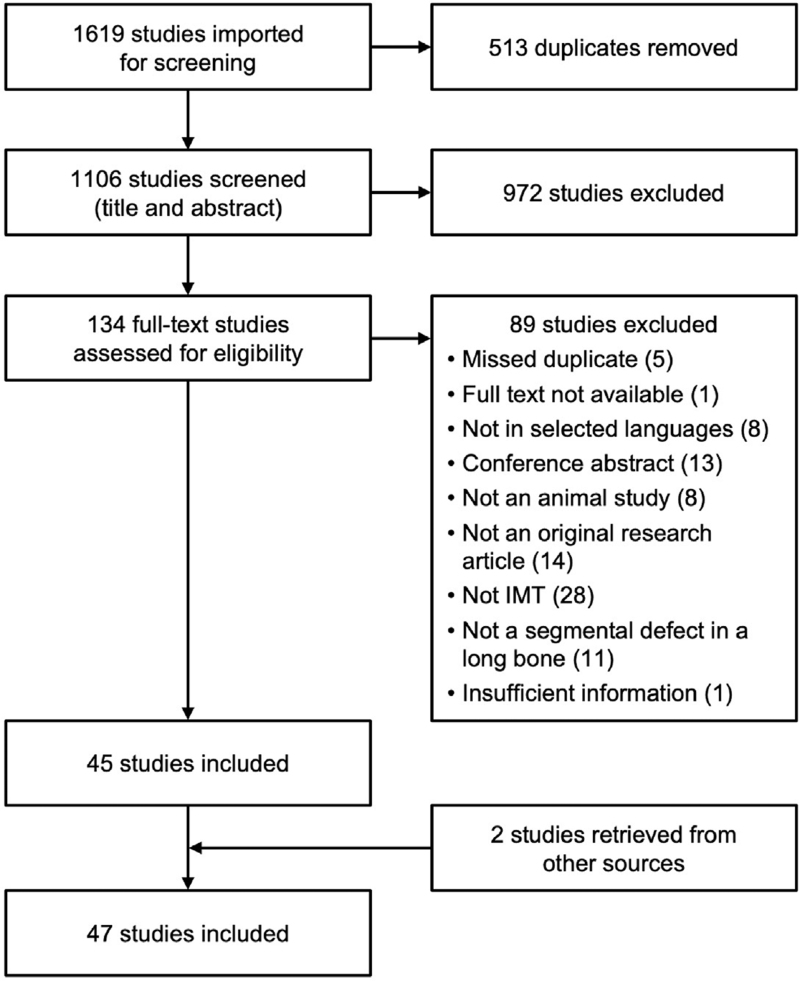
The search strategies from BIOSIS Citation Index, Embase, and MEDLINE retrieved 1619 articles (Fig. 1). After automatic removal of duplicates and following screening based on title and abstract, 134 full-text articles were assessed for eligibility according to the inclusion and exclusion criteria. Following exclusions, 45 studies remained. Two additional articles were identified from screening reference lists, leading to a total of 47 studies included in this systematic review.
Figure 1.
Flow diagram illustrating the process of study screening and selection. IMT = induced membrane technique.
3.2. Animal models
Six different animal species were used in the included studies (Table 1). Rats were most common (n = 28 studies; 60%),[5–32] followed by rabbits (n = 11; 23%),[33–43] and sheep (n = 5; 11%).[44–48] Chickens,[49] goats,[50] and mice[51] were each used in only 1 study (2%). Thirty studies (64%) used male animals and 9 (19%) used female animals; the information was not provided for 8 studies (17%). The vast majority of studies were performed in healthy animals under normal conditions. Only 3 studies examined the IMT in a context of compromised healing (infection [n = 2][23,25] or radiation exposure [n = 1])[24]. In each case, the study was conducted in a rat model and only the first stage of the IMT was performed.
Table 1.
Summary of study characteristics.
| Species | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Rat 28 (59.6%) | Rabbit 11 (23.4%) | Sheep 5 (10.6%) | Chicken 1 (2.1%) | Goat 1 (2.1%) | Mouse 1 (2.1%) | All species 47 (100%) | |
| Bone | |||||||
| Femur | 26 (92.9%) | 2 (18.2%) | 1 (20.0%) | 1 (100%) | 30 (63.8%) | ||
| Metatarsus | 3 (60.0%) | 3 (6.4%) | |||||
| Radius | 7 (63.6%) | 1 (100%) | 8 (17.0%) | ||||
| Tibia | 2 (7.1%) | 1 (20.0%) | 1 (100%) | 4 (8.5%) | |||
| Ulna | 2 (18.2%) | 2 (4.3%) | |||||
| Stages | |||||||
| Stage 1 only | 11 (39.3%) | 5 (45.5%) | 1 (100%) | 1 (100%) | 18 (38.3%) | ||
| Stage 1 + 2 | 17 (60.7%) | 6 (54.5%) | 5 (100%) | 1 (100%) | 29 (61.7%) | ||
| Primary outcome∗ | |||||||
| Membrane analysis | 11 (39.3%) | 5 (45.5%) | 1 (100%) | 1 (100%) | 18 (38.3%) | ||
| Bone healing | 7 (25.0%) | 4 (36.4%) | 3 (60.0%) | 1 (100%) | 15 (31.9%) | ||
| Both | 9 (32.1%) | 2 (18.2%) | 2 (40.0%) | 13 (27.7%) | |||
| Stage 2 analyses | |||||||
| Radiographic assessment after second stage | 16 (57.1%) | 5 (45.5%) | 5 (100%) | 1 (100%) | 27 (57.4%) | ||
| Reported radiographic union rate† | 5 (17.9%) | 1 (9.1%) | 2 (40.0%) | 8 (17.0%) | |||
| Biomechanical testing | 4 (14.3%) | 1 (9.1%)‡ | 5 (10.6%) | ||||
3.3. Bone defects and methods of fixation
Thirty studies (64%) performed surgery on the femur, 8 (17%) on the radius, 4 (9%) on the tibia, 3 (6%) on the metatarsus, and 2 (4%) on the ulna (Table 1). Defect sizes varied by animal and anatomic location. In rats, the median defect size was 7 mm in the femur (range: 0.75–10; n = 26) and 6 mm in the tibia (n = 1). In rabbits, the median defect size was 15 mm in the radius (9–15; n = 7), 25 mm in the ulna (15–35; n = 2), and 12.5 mm in the femur (10–15; n = 2). In sheep, the median defect size was 25 mm in the metatarsus (25–25; n = 3), 30 mm in the femur (n = 1), and 50 mm in the tibia (n = 1). In chickens, goats, and mice, the defect size was 15 mm (radius), 50 mm (tibia), and 3 mm (femur), respectively (n = 1). Only 1 study investigated different defect sizes (4, 6, and 8 mm, created in the rat tibia).[26]
Regarding the methods of fixation, 29 studies (62%) used plate and screws, 5 (11%) used an intramedullary nail, 4 (9%) used an external fixator, 1 (2%) used a K-wire, 2 (4%) used a splint only, and 7 (15%) used no fixation at all. Among these, 1 study compared fixation with plate and screws to no fixation, after creating a defect in the radius of rabbits.[42]
3.4. Spacers: duration of implantation and materials
The median number of weeks the spacer remained within the bone defect following the first stage surgery was 4 weeks in rats (range: 3–16; n = 24), 4 weeks in rabbits (4–6; n = 6), 6 weeks in sheep (4–6; n = 5), and 4 weeks in goats (n = 1). Fifteen studies investigated multiple time points: 7 in rats (range: 0.1–16 weeks), 6 in rabbits (2–8 weeks), 1 in chickens (2.1–4.3 weeks), and 1 in mice (2–8 weeks). Four studies used multiple time points in 1 experimental arm (for animals undergoing the first stage only) and a single time point for another arm (for animals undergoing both stages). No study compared the effect of variable duration of spacer implantation on eventual bone healing after the second surgical stage.
A PMMA spacer (antibiotic-free) was used in 36/47 studies, as the only type of spacer used or in comparison with other spacer materials. In a few cases, PMMA-based spacers contained additional components (other than antibiotics), such as calcium carbonate,[21] zirconium oxide,[49] or poly(d,l-lactic-co-glycolic acid) microparticles and carboxymethylcellulose.[25] Thirteen studies (28%) used PMMA spacers impregnated with antibiotics. Other spacer types included epoxy (n = 1), polyvinyl alcohol (n = 1), silicone (n = 1), stainless steel (n = 1), and titanium (n = 3). Only 1 study did not use any form of PMMA spacer, using epoxy instead.[6] Different spacer materials were compared in 8 studies, each of which compared alternatives to PMMA. In addition, only 3 studies compared PMMA-based spacers that were either impregnated with antibiotics or not.[21,23,25] However, none of these studies performed the second stage of the IMT; their analyses focused on infection clearance and/or membrane properties. Finally, some authors compared smooth spacers to those with a roughened[9,30] or textured surface.[50]
3.5. Graft materials, cells or growth factors, and time of euthanasia after the second surgical stage
Twenty-nine studies (62%) performed the second stage surgery in an animal model (Table 1). Autologous bone graft was used in 18 studies. In rats, the caudal tail vertebrae were most commonly used (n = 6); 3 studies did not specify the source of autologous bone graft. Graft material was most often taken from the iliac crest of the same animal in rabbits (n = 4) and sheep (n = 3). Other sites of autologous graft harvest included the humerus in sheep (n = 1) and sternum in goats (n = 1). In 4 studies, graft was obtained from donor animals (Sprague Dawley rats), and the use of allograft was reported in only 2 studies (1 in rabbits and 1 in sheep). Bone substitutes served as graft material in 12 studies. Various ceramics (eg, β-tricalcium phosphate, calcium carbonate, calcium sulfate, and/or hydroxyapatite) were used alone or in combination (as a commercial or customized preparation), or mixed with other types of materials (eg, polycaprolactone and polylactic acid). Coral was also used in a study in sheep.[48]
Cells (eg, mesenchymal stem cells [MSC], bone marrow-derived mononuclear cells, endothelial progenitor cells) have been implanted in conjunction with graft material in 6 studies (3 in rats, 1 in rabbits, and 2 in sheep). A technique mimicking the collection of bone graft and MSC with a reamer-irrigator-aspirator has been used in rabbits.[38] In an additional study, MSC overexpressing stromal cell-derived factor 1 were delivered in the bone marrow of Wistar rats at the first surgery.[32] Moreover, growth factors (eg, bone morphogenetic protein [BMP]-2, BMP-7, epidermal growth factor) or platelet-rich plasma have also been used, either at the second surgical stage in conjunction with graft material (3 studies; 2 in rats, 1 in rabbits), at the first surgery or between stages (3 studies; 1 in rats, 2 in rabbits).
Following the second surgical stage, animals were euthanized for further analysis at the following median time points: 8 weeks in rats (range: 6–12; n = 15), 8 weeks in rabbits (6–12; n = 3), 21 weeks in sheep (12–26; n = 4), 12 weeks in goats (n = 1). Additionally, some studies in rats (n = 2), rabbits (n = 3), and sheep (n = 1) had multiple endpoints, ranging from 0.1 to 10 weeks, 2 to 12 weeks, and 2 to 8 weeks, respectively.
3.6. Outcomes
The primary outcome was analysis of the induced membrane in 18 studies (38%), bone healing in 15 studies (32%), and both in 13 studies (28%) (Table 1). One study focused on eradication of infection and did not report on membrane properties or bone healing.[23] Studies investigating the induced membrane targeted different aspects, including: thickness, cell content, gene expression, protein concentration (eg, growth factors), extracellular matrix composition and/or vascularity. In addition, Gaio et al tested the barrier, tensile, and shrinkage properties of the induced membrane.[9]
While 27 studies (57%) used radiographic imaging methods after the second stage, only 8 (17%) reported rates of bone union explicitly for each of the study groups (Tables 1, S1). Finally, 5 studies (11%) performed biomechanical testing after grafting (Table 1), but only 2 of them also reported union rates.[31,35]
A summary of the studies that performed the second stage surgery and used radiographic imaging is presented in Table S1.
4. Discussion
The IMT has become a popular technique used for treatment of fracture nonunion. However, there remains ongoing debate regarding how the technique is best applied in a clinical setting. Animal models have been used to characterize the IMT and determine the impact of multiple variations in surgical technique. In this article, we systematically reviewed the literature and analyzed 47 studies investigating the IMT in animal models, to summarize the current knowledge and suggest future research directions.
Out of the 47 studies, 29 (62%) completed both stages of the IMT and 27 (57%) reported using radiographic imaging methods after the second stage. However, only 8 studies (17%) provided radiographic healing rates in a clear and precise manner for every study group. Some authors used radiographic scales to emphasize specific aspects of bone healing (eg, defect filling and bone remodeling). However, many of these scoring systems cannot be directly translated into a union rate. Other articles do mention that union was achieved in a group but do not further specify an exact rate. Therefore, it would be more clinically meaningful for future studies to include an objective assessment of the union status and report it explicitly.
The second stage of the IMT was not performed at all in 38% of studies (18/47). Accordingly, these investigations aimed primarily at analyzing the induced membrane. Such work has produced valuable knowledge about the membrane characteristics, observed under various conditions related to the first surgical stage.[52,53] However, this approach limits our understanding of how these conditions affect the ultimate outcome of bony healing after the second stage surgery. The induced membrane is believed to play an essential role in the success of the technique by supporting healing in different ways, such as providing mechanical stability to the graft, and acting as a barrier to prevent soft tissue invasion.[54] The membrane is also vascularized and contains stem cells and growth factors (eg, BMP-2 and vascular endothelial growth factor),[52,53] which could contribute to graft viability and further promote healing. However, these presumed roles and contributions still need to be investigated thoroughly, in relation to bone healing outcomes. In a recent study, samples of induced membranes were collected from patients at the second surgical stage for analysis.[55] Six months later, based on clinical outcomes, the patients were defined as responders (8 cases) and nonresponders (3 cases) to the IMT. In comparison to the responder group, the induced membranes of the nonresponders had an inner layer that was either absent or thinner, a lower cell density, altered extracellular matrix remodeling, and no MSC obtained from explants in vitro. While the cohort was small and heterogeneous, this article is presumed to be the first reporting a link between clinical healing outcomes and membrane characteristics, emphasizing the need for further research.
Among the included studies, some attempts were made to assess the membrane's roles, notably by including groups where the membrane was removed during the second stage. Luangphakdy et al examined the influence of using a textured spacer, which doubled the surface area of the membrane, as well as scraping the membrane's inner layer before grafting on bone healing.[50] However, it remains uncertain which aspects of the induced membrane have the greatest impact on bone healing. For example, is there a threshold below or above which membrane thickness would affect the outcome? Is there an optimal range of growth factor concentrations or vascularity? Without answers to such questions, results based solely on the membrane analysis are difficult to interpret and do little to guide clinical treatment or even future clinical studies.
Several aspects of the IMT have only been investigated in a limited manner in the preclinical literature and, therefore, remain to be explored in further detail. For example, different durations of spacer implantation have only been tested in studies that did not perform the second surgical stage; as a result, the influence of this factor on ultimate bone healing remains unclear. This aspect is of particular interest for orthopedic surgeons as it could influence the surgical timing for their patients. Another debated question concerns the routine use of antibiotic-impregnated spacers at the first surgical stage. This practice has become relatively commonplace in the clinical literature,[56] despite concerns expressed regarding the development of antibiotic resistance or the impact on the induced membrane. Yet, among the studies that compared spacers with and without antibiotics, none of them performed the second stage surgery, once again leaving uncertainty regarding the impact on bone healing.
While bone healing differs between sexes, only a small proportion of the included studies reported using female animals, and no study directly compared males and females. Similarly, the effect of animal age has not been investigated, and methods of fixation have also not been directly compared. As previously mentioned, the success of the IMT is theoretically not affected by defect size; however, this concept has not been thoroughly investigated. Only 1 study has explored multiple defect sizes.[26]
Another clinically relevant aspect that has received limited attention in research is the use of models of compromised healing. Indeed, the vast majority of the studies in our review rely on healthy animal models. Only 2 studies modeled infection at the surgical site but their measured outcomes were related to infection clearance and/or membrane analysis, as they did not perform the second stage surgery. Nevertheless, patients with open fractures, infected nonunions, and osteomyelitis represent a substantial proportion of the patients treated with the IMT.[56] It would also be relevant to test the IMT in the presence of comorbidities known for their deleterious effect on bone healing, such as diabetes, and for which animal models are available.
Finally, while graft material harvested from the iliac crest is 1 of the most common clinical approaches, only 7 studies obtained autologous graft from the iliac crest. Whether due to anatomical differences or technical limitations, bone graft was often collected from other sources that may not translate to clinical practice. In general, descriptions of bone grafting protocols are limited, which further complicates the evaluation of graft material quality. Considering that the overall success of the IMT cannot be isolated from the quality of bone graft, it is critical to optimize the grafting protocol before making comparisons with other techniques.
5. Conclusions
The growing clinical use and popularity of the IMT seems to have resulted in an acceleration of animal research on the topic. However, as this review demonstrates, various gaps and limitations exist in the preclinical literature with respect to assessment approaches, reporting, or targets of investigation. To provide more meaningful conclusions from a clinical standpoint, future studies should aim to report union rates explicitly, based on radiographic evaluation, and functional assessment of bones should be encouraged. Studies including both stages of the techniques should also be favored. Nevertheless, a better understanding of how the membrane characteristics influence healing outcomes remains crucial. Many aspects of clinical importance still need to be investigated, such as the impact on bone healing of antibiotic-impregnated spacers, time between stages, sex or comorbidities. Finally, models of the IMT should be described in detail, particularly in relation to the grafting protocol.
Supplementary Material
Acknowledgments
We would like to thank Christian Hegner for his help with the development of the search strategies.
References
- 1. Zura R, Xiong Z, Einhorn T, et al. Epidemiology of fracture nonunion in 18 human bones. JAMA Surg. 2016;151:e162775. [DOI] [PubMed] [Google Scholar]
- 2. Antonova E, Le TK, Burge R, et al. Tibia shaft fractures: costly burden of nonunions. BMC Musculoskelet Disord. 2013;14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brinker MR, Trivedi A, O’Connor DP. Debilitating effects of femoral nonunion on health-related quality of life. J Orthop Trauma. 2017;31:e37–e42. [DOI] [PubMed] [Google Scholar]
- 4. Mauffrey C, Barlow BT, Smith W. Management of segmental bone defects. J Am Acad Orthop Surg. 2015;23:143–153. [DOI] [PubMed] [Google Scholar]
- 5. Bilal Ö, Topak D, Kinaş M, et al. Epidermal growth factor or platelet-rich plasma combined with induced membrane technique in the treatment of segmental femur defects: an experimental study. J Orthop Surg Res. 2020;15:601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bosemark P, Perdikouri C, Pelkonen M, et al. The Masquelet induced membrane technique with BMP and a synthetic scaffold can heal a rat femoral critical size defect. J Orthop Res. 2015;33:488–495. [DOI] [PubMed] [Google Scholar]
- 7. DeBaun MR, Stahl AM, Daoud AI, et al. Preclinical induced membrane model to evaluate synthetic implants for healing critical bone defects without autograft. J Orthop Res. 2019;37:60–68. [DOI] [PubMed] [Google Scholar]
- 8. Fenelon M, Etchebarne M, Siadous R, et al. Comparison of amniotic membrane versus the induced membrane for bone regeneration in long bone segmental defects using calcium phosphate cement loaded with BMP-2. Mater Sci Eng C Mater Biol Appl. 2021;124:112032. [DOI] [PubMed] [Google Scholar]
- 9. Gaio N, Martino A, Toth Z, et al. Masquelet technique: the effect of altering implant material and topography on membrane matrix composition, mechanical and barrier properties in a rat defect model. J Biomech. 2018;72:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gohel N, Senos R, Goldstein SA, et al. Evaluation of global gene expression in regenerate tissues during Masquelet treatment. J Orthop Res. 2020;38:2120–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gouron R, Petit L, Boudot C, et al. Osteoclasts and their precursors are present in the induced-membrane during bone reconstruction using the Masquelet technique. J Tissue Eng Regen Med. 2017;11:382–389. [DOI] [PubMed] [Google Scholar]
- 12. Gruber HE, Riley FE, Hoelscher GL, et al. Osteogenic and chondrogenic potential of biomembrane cells from the PMMA-segmental defect rat model. J Orthop Res. 2012;30:1198–1212. [DOI] [PubMed] [Google Scholar]
- 13. Gruber HE, Gettys FK, Montijo HE, et al. Genomewide molecular and biologic characterization of biomembrane formation adjacent to a methacrylate spacer in the rat femoral segmental defect model. J Orthop Trauma. 2013;27:290–297. [DOI] [PubMed] [Google Scholar]
- 14. Henrich D, Seebach C, Nau C, et al. Establishment and characterization of the Masquelet induced membrane technique in a rat femur critical-sized defect model. J Tissue Eng Regen Med. 2016;10:E382–E396. [DOI] [PubMed] [Google Scholar]
- 15. Leiblein M, Koch E, Winkenbach A, et al. Size matters: effect of granule size of the bone graft substitute (Herafill® on bone healing using Masquelet's induced membrane in a critical size defect model in the rat's femur. J Biomed Mater Res B Appl Biomater. 2020;108:1469–1482. [DOI] [PubMed] [Google Scholar]
- 16. Leiblein M, Kolb T, Christian L, et al. Introduction of a new surgical method to improve bone healing in a large bone defect by replacement of the induced membrane by a human decellularized dermis repopulated with bone marrow mononuclear cells in rat. Materials (Basel). 2020;13:2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leiblein M, Winkenbach A, Koch E, et al. Impact of scaffold granule size use in Masquelet technique on periosteal reaction: a study in rat femur critical size bone defect model. Eur J Trauma Emerg Surg. 2020;Published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li S, Zhou H, Hu C, et al. Total flavonoids of rhizoma drynariae promotes differentiation of osteoblasts and growth of bone graft in induced membrane partly by activating Wnt/beta-catenin signaling pathway. Front Pharmacol. 2021;12:675470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ma YF, Jiang N, Zhang X, et al. Calcium sulfate induced versus PMMA-induced membrane in a critical-sized femoral defect in a rat model. Sci Rep. 2018;8:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McBride-Gagyi S, Toth Z, Kim D, et al. Altering spacer material affects bone regeneration in the Masquelet technique in a rat femoral defect. J Orthop Res. 2018;36:2228–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nau C, Seebach C, Trumm A, et al. Alteration of Masquelet's induced membrane characteristics by different kinds of antibiotic enriched bone cement in a critical size defect model in the rat's femur. Injury. 2016;47:325–334. [DOI] [PubMed] [Google Scholar]
- 22. Nau C, Simon S, Schaible A, et al. Influence of the induced membrane filled with syngeneic bone and regenerative cells on bone healing in a critical size defect model of the rat's femur. Injury. 2018;49:1721–1731. [DOI] [PubMed] [Google Scholar]
- 23. Roukoz S, El Khoury G, Saghbini E, et al. Does the induced membrane have antibacterial properties? An experimental rat model of a chronic infected nonunion. Int Orthop. 2020;44:391–398. [DOI] [PubMed] [Google Scholar]
- 24. Sagardoy T, Ehret C, Bareille R, et al. Influence of external beam radiotherapy on the properties of polymethyl methacrylate-versus silicone-induced membranes in a bilateral segmental bone defect in rats. Tissue Eng Part A. 2018;24:703–710. [DOI] [PubMed] [Google Scholar]
- 25. Shah SR, Smith BT, Tatara AM, et al. Effects of local antibiotic delivery from porous space maintainers on infection clearance and induction of an osteogenic membrane in an infected bone defect. Tissue Eng Part A. 2017;23:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shen Z, Lin H, Chen G, et al. Comparison between the induced membrane technique and distraction osteogenesis in treating segmental bone defects: an experimental study in a rat model. PLoS One. 2019;14:e0226839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shen Z, Chen Z, Shi X, et al. Comparison between tonifying kidney yang and yin in treating segmental bone defects based on the induced membrane technique: an experimental study in a rat model. Evid Based Complement Alternat Med. 2020;2020:6575127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tang Q, Jin H, Tong M, et al. Inhibition of Dll4/Notch1 pathway promotes angiogenesis of Masquelet's induced membrane in rats. Exp Mol Med. 2018;50:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tang Q, Tong M, Zheng G, et al. Masquelet's induced membrane promotes the osteogenic differentiation of bone marrow mesenchymal stem cells by activating the Smad and MAPK pathways. Am J Transl Res. 2018;10:1211–1219. [PMC free article] [PubMed] [Google Scholar]
- 30. Toth Z, Roi M, Evans E, et al. Masquelet technique: effects of spacer material and micro-topography on factor expression and bone regeneration. Ann Biomed Eng. 2019;47:174–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Verboket RD, Leiblein M, Janko M, et al. From two stages to one: acceleration of the induced membrane (Masquelet) technique using human acellular dermis for the treatment of non-infectious large bone defects. Eur J Trauma Emerg Surg. 2020;46:317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang H, Li X, Li J, et al. SDF-1 mediates mesenchymal stem cell recruitment and migration via the SDF-1/CXCR4 axis in bone defect. J Bone Miner Metab. 2021;39:126–138. [DOI] [PubMed] [Google Scholar]
- 33. Arıcan G, Özmeriç A, Fırat A, et al. Micro-CT findings of concentrated growth factors (CGF) on bone healing in Masquelet's technique-an experimental study in rabbits. Arch Orthop Trauma Surg. 2022;142:83–90. [DOI] [PubMed] [Google Scholar]
- 34. Bethel M, McDowell S, Chitteti B, et al. The properties of inducible membranes in animals and humans. Int J Med Eng Inform. 2017;9:189–200. [Google Scholar]
- 35. Cho JW, Kim BS, Yeo DH, et al. 3D-printed, bioactive ceramic scaffold with rhBMP-2 in treating critical femoral bone defects in rabbits using the induced membrane technique. J Orthop Res. 2021;39:2671–2680. [DOI] [PubMed] [Google Scholar]
- 36. Eriksson E, Björkenheim R, Strömberg G, et al. S53P4 bioactive glass scaffolds induce BMP expression and integrative bone formation in a critical-sized diaphysis defect treated with a single-staged induced membrane technique. Acta Biomater. 2021;126:463–476. [DOI] [PubMed] [Google Scholar]
- 37. Liu H, Hu G, Shang P, et al. Histological characteristics of induced membranes in subcutaneous, intramuscular sites and bone defect. Orthop Traumatol Surg Res. 2013;99:959–964. [DOI] [PubMed] [Google Scholar]
- 38. Liu Z, Ge Y, Zhang L, et al. The effect of induced membranes combined with enhanced bone marrow and 3D PLA-HA on repairing long bone defects in vivo. J Tissue Eng Regen Med. 2020;14:1403–1414. [DOI] [PubMed] [Google Scholar]
- 39. Meng ZL, Wu ZQ, Shen BX, et al. Reconstruction of large segmental bone defects in rabbit using the Masquelet technique with alpha-calcium sulfate hemihydrate. J Orthop Surg Res. 2019;14:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tarchala M, Engel V, Barralet J, et al. A pilot study: alternative biomaterials in critical sized bone defect treatment. Injury. 2018;49:523–531. [DOI] [PubMed] [Google Scholar]
- 41. Wang X, Wei F, Luo F, et al. Induction of granulation tissue for the secretion of growth factors and the promotion of bone defect repair. J Orthop Surg Res. 2015;10:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xie J, Liu D, Wang H, et al. Effects of topical mechanical stability on the formation of Masquelet membrane in a rabbit radial defect model. Sci Rep. 2020;10:18939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yılmaz O, Özmeriç A, Alemdaroğlu KB, et al. Effects of concentrated growth factors (CGF) on the quality of the induced membrane in Masquelet's technique – an experimental study in rabbits. Injury. 2018;49:1497–1503. [DOI] [PubMed] [Google Scholar]
- 44. Christou C, Oliver RA, Yu Y, et al. The Masquelet technique for membrane induction and the healing of ovine critical sized segmental defects. PLoS One. 2014;9:e114122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cordonnier T, Sohier J, Sensébé L, et al. Healing of long-bone defects in sheep metatarsals using bioceramics and mesenchymal stem cells. Curr Orthop Pract. 2012;23:369–376. [Google Scholar]
- 46. Klaue K, Knothe U, Anton C, et al. Bone regeneration in long-bone defects: tissue compartmentalisation? In vivo study on bone defects in sheep. Injury. 2009;40:S95–S102. [DOI] [PubMed] [Google Scholar]
- 47. Viateau V, Guillemin G, Calando Y, et al. Induction of a barrier membrane to facilitate reconstruction of massive segmental diaphyseal bone defects: an ovine model. Vet Surg. 2006;35:445–452. [DOI] [PubMed] [Google Scholar]
- 48. Viateau V, Guillemin G, Bousson V, et al. Long-bone critical-size defects treated with tissue-engineered grafts: a study on sheep. J Orthop Res. 2007;25:741–749. [DOI] [PubMed] [Google Scholar]
- 49. Cueva LOB, Rahal SC, Fonseca-Alves CE, et al. Masquelet-induced membrane characteristics in chicken radii bone defects. J Avian Med Surg. 2021;35:51–59. [DOI] [PubMed] [Google Scholar]
- 50. Luangphakdy V, Pluhar GE, Piuzzi NS, et al. The effect of surgical technique and spacer texture on bone regeneration: a caprine study using the Masquelet technique. Clin Orthop Relat Res. 2017;475:2575–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang W, Zuo R, Long H, et al. Advances in the Masquelet technique: myeloid-derived suppressor cells promote angiogenesis in PMMA-induced membranes. Acta Biomater. 2020;108:223–236. [DOI] [PubMed] [Google Scholar]
- 52. Klein C, Monet M, Barbier V, et al. The Masquelet technique: current concepts, animal models, and perspectives. J Tissue Eng Regen Med. 2020;14:1349–1359. [DOI] [PubMed] [Google Scholar]
- 53. Alford AI, Nicolaou D, Hake M, et al. Masquelet's induced membrane technique: review of current concepts and future directions. J Orthop Res. 2021;39:707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Masquelet AC, Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am. 2010;41:27–37. [DOI] [PubMed] [Google Scholar]
- 55. Durand M, Barbier L, Mathieu L, et al. Towards understanding therapeutic failures in Masquelet surgery: first evidence that defective induced membrane properties are associated with clinical failures. J Clin Med. 2020;9:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fung B, Hoit G, Schemitsch E, et al. The induced membrane technique for the management of long bone defects. Bone Joint J. 2020;102-B:1723–1734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.