Abstract
Vertebrate rod and cone photoreceptors detect light via a specialized organelle called the outer segment. This structure is packed with light-sensitive molecules known as visual pigments that consist of a G-protein-coupled, seven-transmembrane protein known as opsin, and a chromophore prosthetic group, either 11-cis retinal (‘A1’) or 11-cis 3,4-didehydroretinal (‘A2’). The enzyme cyp27c1 converts A1 into A2 in the retinal pigment epithelium. Replacing A1 with A2 in a visual pigment red-shifts its spectral sensitivity and broadens its bandwidth of absorption at the expense of decreased photosensitivity and increased thermal noise. The use of vitamin A2-based visual pigments is strongly associated with the occupation of turbid aquatic habitats in which the ambient light is red-shifted. By modulating the A1/A2 ratio in the retina, an organism can dynamically tune the spectral sensitivity of the visual system to match the predominant wavelengths of light in its environment. As many as a quarter of all vertebrate species utilize A2, during part of their life cycle or under certain environmental conditions. A2 utilization therefore represents an important and widespread mechanism of sensory plasticity. This review provides an up-to-date account of the A1/A2 chromophore exchange system.
Graphical Abstract
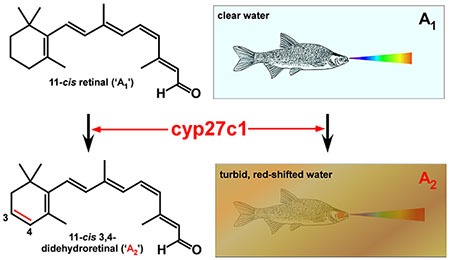
Introduction
Vertebrate rod and cone photoreceptors mediate vision in dim- and bright-light environments, respectively (Fig 1A,B). The light-sensitive molecule of a photoreceptor cell, known as the visual pigment, consists of two components: a G-protein-coupled, seven-transmembrane apo-protein known as opsin, and a chromophore prosthetic group bound via a Schiff base linkage to the side chain of a lysine within the opsin’s chromophore binding cleft (Fig. 1B). In vertebrates, two different chromophores are found: 11-cis retinal (derived from vitamin A1 and henceforth referred to as ‘A1’) and 11-cis 3,4-didehydroretinal (derived from vitamin A2 and henceforth referred to as ‘A2’)11,12. The only difference between the two chromophores is the presence of an additional double bond within the β-ionone ring of A2 (Fig. 1C). Replacing A1 with A2 in a visual pigment has four main effects: (1) it red-shifts the spectral absorption curve of the visual pigment; (2) it broadens the spectral bandwidth of absorption; (3) it decreases the pigment’s photosensitivity; and (4) it increases thermal noise (Fig. 1D–F)12,14. The wavelength of maximal sensitivity of a visual pigment (referred to as λmax) can be tuned toward shorter or longer wavelengths via two primary mechanisms: changes in the amino acids of the opsin; or exchange of one chromophore for the other. Opsin tuning via amino acid replacement has become an important model system for the study of molecular evolution in recent years15–21. In contrast, nearly a half century has elapsed since publication of the most recent comprehensive review of the vitamin A1/A2 chromophore exchange system12. The present review therefore aims to provide an up-to-date account of our understanding of the A1/A2 system and how it impacts spectral tuning and visual plasticity.
Figure 1. The vitamin A1/A2 chromophore exchange system.
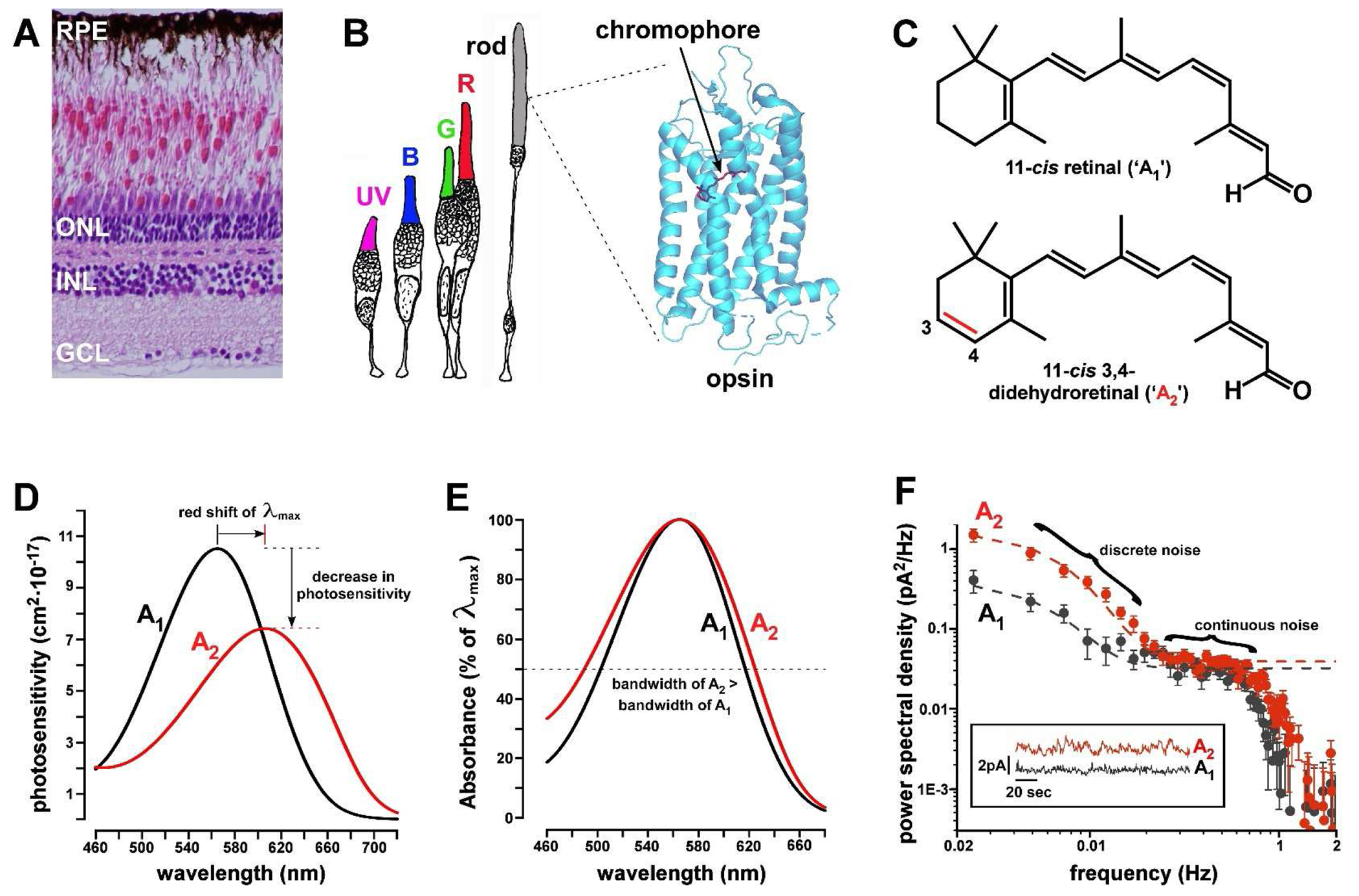
(A) H&E-stained histologic section of adult zebrafish retina. The photoreceptor cell bodies reside in the outer nuclear layer (ONL). INL = inner nuclear layer; GCL = ganglion cell layer; RPE = retinal pigment epithelium. (B) Drawing of rod and cone photoreceptor subtypes of adult zebrafish: UV = ultraviolet cone; B = blue cone; G = green cone; and R = red cone. Also shown is the crystal structure of bovine rhodopsin (RH1) with the 11-cis retinal (A1) chromophore in red. PDB code = 1F881. (C) Chemical structure of 11-cis retinal (A1) and 11-cis 3,4-didehydroretinal (A2). Note the position of the additional double bond (in red) within the terminal β-ionone ring of A2. (D) Photosensitivity curves of a typical LWS visual pigment either with A1 (λmax = 565 nm) or A2 (λmax = 606 nm). Curves are based on templates in Govardovskii et al.6 Note that the λmax of the A2 pigment is red-shifted by 41 nm relative to the A1 form in accordance with the formula in Fig. 3A. In addition, the photosensitivity of the A2 pigment is only ~70% that of the A1 form. (E) A2-based visual pigments have a wider bandwidth than A1 forms as shown here by superimposing the absorbance curves of A1 and A2 pigments with identical λmax = 565 nm. Dotted line indicates half-maximal absorbance. (F) This figure shows the noise power spectral density from electrical recordings of the light-sensitive current of individual larval tiger salamander (Ambystoma tigrina) rods either in their native A2-predominant form (red trace; A1:A2 ratio is ~0.26:0.74) or after regeneration with A1 (black trace; A1:A2 ratio is ~0.91:0.09)10. Note that the A2-based visual pigment is noisier than the A1 form. Photoreceptor ‘dark noise’ has discrete (low frequency) and continuous (high frequency) components. Discrete noise results from thermal isomerization events which occur with greater frequency in A2-based visual pigments. Continuous noise arises in components of the phototransduction cascade downstream of the visual pigment and occurs at similar rates in A1- and A2-based pigments. The inset shows recordings of a rod in the ‘A2’ state (red trace) and the ‘A1’ state (black trace). Note the large, lower-frequency deviations in the A2 trace that are absent from the A1 recording. Panel F is adapted, with permission from the author, from reference10.
The discovery of vitamin A1/A2 chromophore exchange
Scientists first studied purified extracts of vertebrate visual pigments toward the end of the 19th century. They found that solutions of visual pigments obtained from aerial and terrestrial species had a striking rose-pink color which rapidly bleached upon exposure to light. This visual pigment was named ‘rhodopsin’ based on the ancient Greek words, ῥóδoν, rose, and ὄψις, vision22. In 1880, Kühne and Sewall first noticed that visual pigment extracts from some species of fish were not rose-colored but purple25. This new visual pigment was subsequently named ‘porphyropsin’, based on the Greek term for the gastropod mollusks used in antiquity to produce ‘royal purple’ dye (πορφύρα, purple-fish, and ὄψις, vision)11,26,27. In 1896, Köttgen and Abelsdorff showed that the absorption spectra of visual pigments derived from reptiles, birds, and mammals peaked around 500 nm, whereas those of freshwater fish were red-shifted, peaking around 540 nm28. George Wald later demonstrated that ‘rhodopsins’ contain an A1 chromophore whereas ‘porphyropsins’ contain A211. He also showed that replacement of A1 with A2 in the same opsin produces a red shift in the λmax of the visual pigment11. While the terms ‘rhodopsin’ and ‘porphyropsin’ were once used to refer to any visual pigment containing A1 and A2, respectively, the subsequent discovery of cone opsins made this terminological distinction obsolete. The term ‘rhodopsin’ is now only used to refer to ‘rod opsins’ (i.e., RH1 opsins), whereas ‘porphyropsin’ is rarely used.
Species distribution and uses of vitamin A1/A2 chromophore exchange
The use of vitamin A2-based visual pigments is strongly associated with the occupation of turbid aquatic habitats, particularly ones with red-shifted or highly variable ambient light12. While A2-based visual pigments have never been identified in any species of bird, mammal, or fully terrestrial reptile (with the exception of two lizards, Anolis carolinensis and Podarcis siculus29), these pigments are widely distributed among fishes, amphibians, aquatic reptiles, and lamprey12. Indeed, data suggest that the vast majority of freshwater fishes utilize A2-based visual pigments30. While early studies showed A2 to be common in diadromous species (i.e., salmon, trout, eels, and lamprey) during the freshwater phase of their life cycle12,26,31–34, they found the use of A2 to be rare among fully marine species. Subsequent studies, however, have demonstrated the use of A2-based visual pigments in marine fishes from multiple families30,33,35–42, especially nearshore species inhabiting spectrally variable environments37. It is therefore unwise to assume a priori that a marine species does not utilize A2-based visual pigments. In total, it is likely that more than a quarter of all vertebrate species utilize A2-based visual pigments, at least during a part of their life cycle or under certain environmental conditions.
The vitamin A1/A2 chromophore exchange system is sometimes referred to as a ‘switch’, implying that organisms toggle between the use of one chromophore or the other in an all-or-none fashion. In fact, many species utilize both chromophores simultaneously, adjusting the A1/A2 ratio in response to physiological or environmental cues in a continuous manner12. The presence of both A1- and A2-based visual pigments in a single outer segment endows a photoreceptor with a broad, unimodal spectral response curve with a λmax intermediate between those of the pure A1- and A2-based pigments12. Thus, by adjusting the A1/A2 ratio, the organism can continuously tune λmax on a physiological time scale. It appears that some species (e.g., the ninespine stickleback, Pungitius pungitius) can independently tune the A1/A2 ratio in rods and cones and even within cone subtypes in the same retina43. Interestingly, A1/A2 ratio can vary among individual fish of a single species caught at the same time and place32,44,45. One author suggested that this phenomenon is particularly notable in schooling species and proposed that the broadening of spectral sensitivity of the school as a whole by individually variable A1/A2 ratios might confer a selective advantage in detecting predators12,44.
Fishes inhabiting clear waters tend to utilize A1-based visual pigments, while those found in more turbid environments tend to have a high proportion of A2 in their eyes. However, classifying fish species as either ‘A1’ or ‘A2’ is overly simplistic, because most published reports describe fish collected at a single location at only one time of the year, and thus ignore potential temporal dynamics of chromophore usage. For example, most published studies (with one exception46) indicate that zebrafish (Danio rerio) almost exclusively utilize A1-based pigments under standard laboratory conditions47,48. Yet, the zebrafish’s native streams and ponds in India and Bangladesh are likely subject to conditions of widely varying turbidity, particularly during the monsoon season49–51. It is possible, and even likely, that zebrafish utilize A2-based visual pigments in the wild when they encounter highly turbid environments. Indeed, laboratory studies have demonstrated that application of thyroid hormone (TH) to the water of zebrafish can induce a complete switch to A2-based visual pigments47,48, revealing a latent capacity to synthesize A2. Clearly, one cannot rule out the use of A2-based visual pigments by a given species based on examination of individuals caught at one time or in a single locale.
Species utilize A1/A2 exchange to fine-tune spectral sensitivity in accordance with season, migration status, and developmental stage12,52. Longitudinal studies have shown that A2 levels tend to be highest during the winter months and lowest during the summer, even at a single location32,53–55. Some have speculated that the increase in A2 during the winter may be a response to red-shifting of the ambient light spectrum caused by decreased solar elevation12,32. Alternatively, colder water temperatures may play a role since lower temperatures are known to increase A2 levels in several species34,56–63. Colder habitats might also favor A2 usage since the lower temperature mitigates the increased thermal noise associated with A2-based pigments (see below)64. As mentioned above, migratory species (salmon, trout, eels, and lamprey) alternate between A1 and A2-based pigments, shifting to A1 upon entering clear marine environments, and favoring A2 upon entering more turbid, inland waterways12,26,31–34,65. Some species switch between A1 and A2 according to developmental stage42,66–68. For example, the northern leopard frog (Lithobates pipiens) utilizes A2-based pigments during the aquatic tadpole stage and then switches to A1 upon metamorphosis into a semiterrestrial adult66. In contrast, amphibians that remain aquatic as adults, such as the African clawed toad (Xenopus laevis), appear to have A2-predominant retinas throughout life12. One study reported an increasing proportion of A2 in the eyes of older individuals of the common rudd (Scardinius erythrophthalmus) under controlled lighting conditions69, but the relationship between A2 levels and age has not been examined in other species.
Several species use A2-based pigments for specialized purposes. For example, unlike most anurans, the American bullfrog (Lithobates catesbeianus) retains A2-based pigments in the dorsal third of its retina, even as an adult70. Bullfrogs spend considerable time with their eyes positioned right above the surface of the water71. Thus, the A2-rich dorsal retina enables downward vision into the turbid, red-shifted aquatic environment, while the A1-rich ventral retina scans the aerial milieu. Similarly, the four-eyed fish (Anableps anableps) inhabits the surface of turbid waterways, viewing the aquatic and aerial environments with its dorsal and ventral retinas, respectively72,73. Despite marked morphologic differences between the dorsal and ventral retina and the expression of red-sensitive LWS opsin exclusively in the dorsal retina73, the distribution of A2 in the four-eyed fish’s retina is currently unknown74. One might speculate that the LWS opsin expressed in the dorsal retina is likely to be paired with an A2 chromophore to enhance aquatic vision. In contrast, some other fishes have been reported to have a higher proportion of A2 in the ventral retina, indicating that intraretinal distribution of A2 is species-specific75,76.
A specialized optical adaptation in certain shallow-water, nearshore fishes favors the use of A2. More than 100 species of fish can reversibly pigment their corneas upon exposure to bright light77. Corneal pigmentation is mediated by the movement of yellow and red carotenoid-containing organelles within specialized chromatophores whose processes extend across the pupil77. While the adaptive function of corneal pigmentation is debated and might differ between species78,79, in the masked greenling (Hexagrammos octogrammus), pigment density can be so high that the cornea acts as a long-pass spectral filter, precluding the transmission of light less than 520 nm79. This species has adopted the use of A2-based visual pigments to red-shift their medium- and long-wavelength-sensitive opsins and thereby enhance their ability to detect the longer wavelengths that are passed by the corneal filter, even in summertime when A2 levels in most species are low39,79.
Another interesting optical adaptation found in three genera of deep-sea dragonfish (Stomiidae) involves the use of A2-based visual pigments. In addition to the blue-green (450-500 nm) bioluminescent signals typically emitted by deep-sea fishes80, these dragonfishes emit a far-red (>700 nm) signal from periorbital photophores81–83. Since most deep-sea fishes are blind to long-wavelength light, far-red emission endows dragonfishes with the potential for covert prey illumination or ‘private’ signaling between individuals84. To detect this signal, dragonfishes have evolved rod opsins (RH1) with red-shifted λmax85. These fishes couple their red-shifted opsin with an A2 chromophore in a sub-population of rods, thereby enhancing sensitivity to far-red signals85–88. Remarkably, the dragonfish Malacosteus niger red-shifts sensitivity even further by using derivatives of bacteriochlorophyll as photosensitizers84,89,90.
Environmental factors controlling the A1/A2 ratio
What accounts for the widespread utilization of A1/A2 chromophore exchange among aquatic organisms and its rarity among terrestrial species? The most likely answer is the variable and labile quality of light in aquatic, and especially freshwater, habitats (Fig. 2). Light transmission in water can be affected by both biotic and abiotic factors (e.g., chlorophyll-containing plankton, suspended inorganic particles, and dissolved compounds). These factors modify both the amount and spectral distribution of light available for vision (Fig. 2B). Opsin switching (i.e., changes in the expression of opsin genes) and A1/A2 chromophore exchange are the primary mechanisms whereby species modify their spectral sensitivity in response to changes in their photic environment15. It is therefore not surprising that evolution has favored the emergence of both A1/A2 chromophore exchange and highly diverse opsin gene repertoires in fishes12,15,91. Despite the established role of water temperature in controlling A1/A2 ratio in some fishes34,56–62, there is broad consensus that light intensity, duration (i.e., day length), and wavelength are the most important environmental variables determining A1/A2 ratio in the majority of species12. Environments with less light of shorter duration and longer wavelengths tend to favor an increase in A2 levels12. Indeed, emerging evidence from the evolutionarily diverse cichlid fishes (Cichlidae) indicates that selection for higher A2 levels has likely played a key role in adaptation to turbid, red-shifted environments (Fig. 2B,C)13,24,92–95.
Figure 2. Light in aquatic habitats is highly variable.
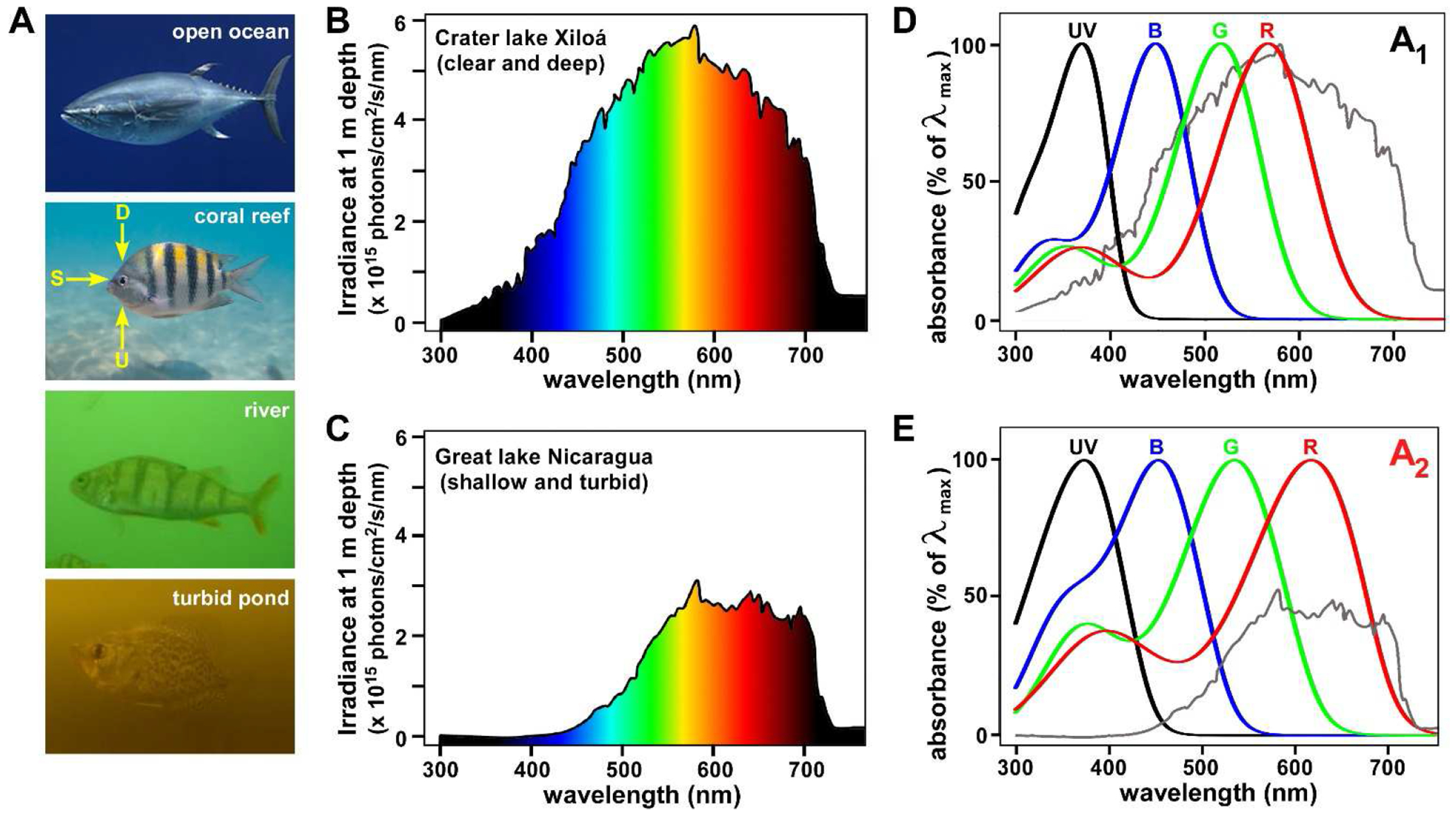
(A) Aquatic species experience widely varying and labile photic environments in which the transmission of light is modulated by both suspended and dissolved matter in the water column. Light variability was likely a major impetus for the evolution of the A1/A2 chromophore system. D = downwelling light; S = sidewelling light (i.e., the horizontal visual field); U = upwelling light. Photo credits2–5. (B, C) Spectral irradiance in two Central American cichlid habitats with marked differences in water clarity. Turbidity decreases the amount of light available for vision and preferentially absorbs shorter wavelengths, effectively red-shifting the spectral distribution. A recent study showed that expression of cyp27c1 (the enzymes that converts A1 into A2) in the eyes of cichlids correlates with the spectral distribution of light in these habitats13. (D, E) The spectral absorbance curves of the A1 (D) and A2 (E) forms of the four cone visual pigments of the goldfish, a typical tetrachromatic teleost. The curves are based on templates in Govardovskii et al.6, using the following values for λmax from reference23: A1 forms (370.1, 447.2, 515.9, 565.9 nm); A2 forms (381.9, 454.1, 534.9, 617.5 nm). The irradiance curves from panels B and C (gray) are superimposed on the spectral absorbance curves in D and E, respectively. Note how the λmax of the A1 and A2 forms of the red cone (LWS) pigment are well-positioned to capture the predominant wavelengths of light in clear and turbid habitats, respectively. This figure demonstrates how switching between A1 and A2 allows an organism to tune its spectral sensitivity to match the predominant wavelengths in its environment. The spectral irradiance curves in panels B and C are adapted, with permission of the author, from reference24.
Given the outsized importance of light quality and quantity in determining opsin and A1/A2 usage, it is surprising that so few studies of fish vision have included ambient light measurements. In studies that do incorporate such measures, the data are often limited to quantification of up- or downwelling light at varying depths13,36,43,96,97. While certainly laudable, such studies do not typically measure the light that actually reaches the animal’s eye. Yet, in surfperches (Embiotocidae) photoreceptor spectral tuning most strongly correlates with sidewelling irradiance (i.e., the horizontal visual field), not up-or downwelling light (Fig. 2A)37. Since changes in spectral tuning via opsin or chromophore exchange occur over days to weeks12,32, these changes likely reflect cumulative light exposure at the retinal surface. Ideally, studies of spectral sensitivity should include a ‘fish eye’ view of light98, summated over time via a miniature head-mounted camera or spectrophotometer, perhaps with special weighting of visual features critical to organismal fitness (e.g., the reflectance spectra of predators or potential mates99). Such an approach would likely reveal much stronger correlations between spectral tuning and light exposure than have heretofore been observed using more indirect measures of ambient light.
The enzymatic mechanism and transcriptional control of vitamin A1-to-A2 conversion
The existence of an enzyme that converts vitamin A1 into A2 was proposed more than a half century ago12. Early studies showed a strong correlation between A2 levels in retina and in retinal pigment epithelium (RPE)63,70,100, suggesting that A2 might be synthesized in the RPE and then passed to the retina during the visual cycle101. To identify the enzyme mediating A2 synthesis, my lab used RNA-seq to compare the transcriptomes of RPE from TH-treated zebrafish vs. untreated controls as well as the transcriptomes of dorsal vs. ventral bullfrog RPE47. We identified a single gene that was both upregulated in TH-treated zebrafish RPE and enriched in the dorsal bullfrog RPE, the cytochrome P450 family member, cyp27c147. P450 enzymes are involved in the metabolism of a wide range of xenobiotic compounds and endogenous small molecules, including retinoids102. Thus, cyp27c1 was an excellent candidate for the long-hypothesized ‘vitamin A1 3,4-dehydrogenase’. Subsequent analysis demonstrated that this enzyme is localized to the RPE in zebrafish and American bullfrog, and that its expression correlates with the presence of A247. We also showed that cyp27c1 is sufficient to convert vitamin A1 and its congeners into their corresponding A2 forms47,103. Lastly, we engineered zebrafish with mutations in cyp27c1 and showed that the gene is required for endogenous synthesis of A247. Knock-out of cyp27c1 also eliminates the zebrafish’s ability to red-shift its photoreceptor spectral sensitivity in response to TH treatment and reduces its ability to see and respond to near-infrared light of 770 nm47. Some fishes display differences in the A1/A2 ratio between photoreceptor classes, suggesting that cyp27c1 might be differentially expressed in individual photoreceptor subtypes in these species, rather than exclusively in the RPE43,86. Differences in the A1/A2 ratio between rods and cones might also be accounted for by expression of cyp27c1 in Müller glia which support cone, but not rod, pigment regeneration104. Interestingly, there does not appear to be an enzyme that converts A2 into A1. Instead, a switch from A2 to A1 likely occurs via progressive turnover of the retinoid pool in the RPE.
Despite the apparent absence of A2-based visual pigments in some groups (i.e., birds and mammals), orthologs of cyp27c1 are found in all vertebrate classes. Expression of cyp27c1 strongly correlates with the presence of A2 in the retina of the sea lamprey (Petromyzon marinus)105, an agnathan that diverged from jawed vertebrates during the Cambrian period ~500 million years ago106. The sea lamprey switches between A1- and A2-predominance at different stages of its migratory life cycle31, suggesting that the capacity for A2 production may have facilitated the initial invasion of turbid inland waterways by early vertebrates105,107. As expected, cyp27c1 orthologs are nearly ubiquitous among the sequenced genomes of fishes, amphibians, and reptiles. More surprisingly, nearly all sequenced bird genomes also retain an intact copy of cyp27c1. The role of this enzyme in birds is currently unknown, but studies suggest potential functions outside of the eye. For example, 3,4-didehydroretinoic acid (a derivative of vitamin A2), is the predominant form of ‘retinoic acid’ found in the developing chicken (Gallus gallus) embryo108,109.
Orthologs of cyp27c1 are present in most mammalian species including humans, but the gene appears to have been lost in three groups. BLAST searches using human CYP27C1 as a query revealed loss of cyp27c1 in bats (Chiroptera), rodents (Rodentia) with the exception of squirrel-related clades (Sciuromorpha), and Afrotheria (with the possible exception of manatees). The retention of cyp27c1 orthologs among squirrel-like clades suggests early evolutionary branching of Sciuromorpha prior to cyp27c1 loss, a finding consistent with recent phylogenetic studies110,111. Interestingly, cyp27c1 orthologs appear to be absent from all sequenced Afrotheria genomes, with the exception of that of the West Indian manatee (Trichechus manatus) which retains a gene encoding a protein with ~68% amino acid identity to human CYP27C1 and with shared synteny (BIN1 — CYP27C1 — ERCC3). The retention of a possible ortholog of cyp27c1 in West Indian manatee is intriguing, because this species inhabits turbid coastal waters, estuaries, and rivers and, along with the three other species of sea cow (Sirenia), represents the only fully aquatic sub-clade within Afrotheria112. This finding raises the possibility that sea cows (and perhaps other aquatic mammals inhabiting turbid water such as river dolphins) might use A2-based visual pigments. The presence of cyp27c1 orthologs in terrestrial mammals is more puzzling, but the enrichment of both CYP27C1 transcripts and 3,4-didhydroretinoids in human skin113–116, and the ability of human CYP27C1 to convert vitamin A1 into A2103,113, suggest a role for this enzyme in the integument.
Organisms dynamically modulate A1/A2 ratio in response to both physiological changes and environmental variables. In some species, such as salmon preparing to migrate, the initiation of the A1-to-A2 switch precedes the fish’s entry into the new photic environment12,32, suggesting that chromophore exchange is part of a suite of anticipatory physiologic changes and is therefore likely under systemic hormonal control. In other species, the A1/A2 ratio can be modulated locally within the eye. Bridges and Yoshikami showed that when held in constant darkness the common rudd converts nearly all chromophore to A269,100. Upon re-exposure to light the fish then reverts to A1. This reversion can be prevented by placing an opaque plastic cap over one eye, while the uncapped eye reverts normally69,100. Thus, in the rudd, changes in the photic environment can be sensed locally within a single eye and transduced into changes in the A1/A2 ratio independent of the other eye.
How do animals sense changes in their internal state or external milieu and transduce this signal into changes in cyp27c1 expression? The answer to this question is currently unknown, but TH signaling appears to play a role, at least in some species. Studies in salmon, trout, zebrafish, goldfish (Carassius auratus), and shiners (Richardsonius balteatus, Notemigonus crysoleucas, and Laxilus cornutus) indicate that application of TH increases the percentage of A2 in the eye34,117–122, while in sunfish (Lepomis sp.) and American bullfrog it has the opposite effect67,123–125. To identify the transcription factors that mediate induction of cyp27c1 in response to TH, my lab assayed zebrafish with mutations in the three known TH nuclear receptors (thraa, thrab, and thrb). We found that no single TH nuclear receptor is required for TH-mediated induction of cyp27c1 but that deletion of all three completely eliminates cyp27c1 expression and the resulting conversion of A1 to A2126. Despite this knowledge, we still do not understand the mechanism whereby some species flip the polarity of the response, reducing A2 levels upon exposure to TH67,123–125. We also do not know how changes in the light environment are sensed and transduced into changes in TH signaling in the RPE. These are important problems for future work.
In addition to controlling cyp27c1 expression, TH signaling is required for red-sensitive LWS opsin expression in many vertebrates126–129. In zebrafish, mutations in thrb cause LWS cone precursors to be transfated into UV cones126. TH signaling also appears to play a role in controlling expression of paralogous opsin genes, possibly in response to changes in the photic environment. Temple and colleagues showed that TH treatment of coho salmon (Oncorhynchus kisutch) can induce increased expression of a RH2 paralog with red-shifted λmax130. Another study demonstrated that TH treatment of zebrafish induces a shift in expression toward red-shifted RH2 and LWS paralogs131. Taken together, these findings suggest that TH signaling coordinates a multi-level response to changes in long-wavelength light in the environment. One might speculate that in early vertebrate evolution both A1/A2 exchange and the expression of red-shifted opsins came under the control of TH signaling as a mechanism of coordinating physiologic changes, perhaps in a jawless ancestor undergoing metamorphosis or in one preparing to migrate into fresh water.
The extent of red shift upon switching from A1 to A2 is correlated with λmax
One of the most remarkable features of the A1/A2 system is that the longer the λmax-A1, the greater the red shift upon switching to A2 (Fig. 3A,B)12,66,132,133. For example, in an early study of the northern leopard frog using microspectrophotometry (MSP)66, the authors found that the LWS pigment (λmax-A1 = 575 nm) underwent a red-shift of 45 nm upon switching from A1 to A2, whereas the RH1 pigment (λmax-A1 = 502 nm) underwent a red shift of 25 nm and the SWS2 pigment (λmax-A1 = 432 nm) a red shift of only 6 nm. The authors noted that these data points fall exactly on a straight line when graphed against λmax-A1. Multiple subsequent studies have confirmed this linear relationship23,43,48,134, at least for λmax-A1 > 407 nm (Fig. 3A,B). The functional consequence of this relationship is that switching from A1 to A2 results in a large extension of visual sensitivity into the far-red region without much of a corresponding loss of sensitivity at the short-wavelength end of the spectrum (Fig. 2D,E).
Figure 3. The relationship between λmax-A1 and λmax-A2.
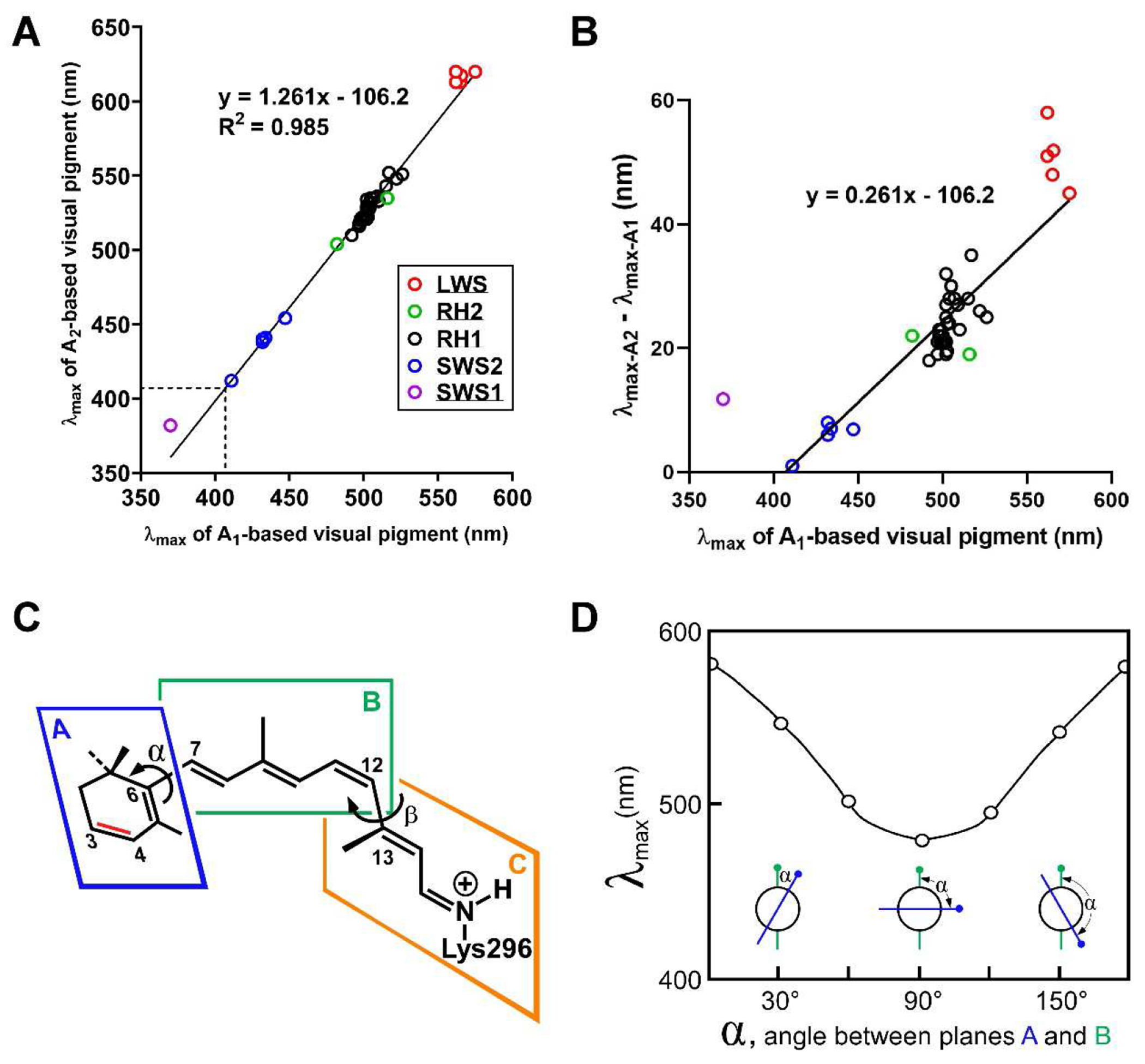
(A) This graph shows the relationship between λmax-A1 and λmax-A2 for 57 different rod (RH1) and cone (SWS1, SWS2, RH2, LWS) visual pigment pairs from numerous species (primary data and references in Supplemental Table S1). The relationship is well described by a straight line (R2 = 0.985; equation for the fitted line is shown). The dotted lines indicate the point at which λmax-A1 and λmax-A2 are equal (~407 nm). (B) The same data as in panel A but with λmax-A1 plotted against λmax-A2 - λmax-A1 to highlight how the red shift increases with increasing λmax-A1. The marked deviation of the one SWS1 pigment (purple) is discussed in the main text. Values for LWS pigments (red) also appear to deviate somewhat from the fitted line. It is not currently known whether this deviation is real or attributable to measurement errors. (C) The retinal chromophore consists of three planes (A, B, C) which can rotate relative to each other. Rotation about the C6-C7 bond (dihedral angle α) alters the degree of co-planarity between planes A and B, thereby modifying the extent of electron delocalization into the β-ionone ring. Blatz and Liebman have proposed that the relationship between λmax-A1 and λmax-A2 might be explained by differences in α across visual pigments4,5,7. (D) A theoretical modeling analysis suggested that λmax can be tuned over a range of >100 nm by changing α, the dihedral angle between planes A and B8,9. Panel D is adapted from reference8.
A notable implication of the linear relationship between λmax-A1 and λmax-A2 shown in Fig. 3A is that the red shift caused by an A1-to-A2 switch is predicted to be zero when the λmax-A1 equals ~407 nm. Two published datasets are consistent with this prediction. First, an MSP study of zebrafish (Danio rerio) found no statistically significant red shift of the λmax of the SWS2 pigment (λmax-A1 = 411 nm) upon switching to A2 (λmax-A2 = 412 nm)48. The authors did, however, observe that the half-bandwidth of the absorption curve of the A2-based SWS2 pigment was broader than that of the A1-based, indicating that chromophore exchange had indeed occurred (Fig. 1E). In a second study, Makino and colleagues measured the λmax of the SWS2 visual pigment of the tiger salamander (Ambystoma tigrinum) reconstituted with three different 9-cis retinals: 9-cis 5,6-dihydroretinal, which lacks double bonds in the β-ionone ring and is referred to by the authors as A0, 9-cis retinal (referred to as A1), and 9-cis 3,4-didehydroretinal (referred to as A2)135. As expected from the equation in Fig. 1A, the authors observed only very small red shifts of the SWS2 pigment upon addition of one or two double bonds to the β-ionone ring of the chromophore (λmax-A0 = 415 nm; λmax-A1 = 418 nm; λmax-A2 = 422 nm). Although more data are needed to precisely define the λmax-A1 value at which the red shift equals zero, the published data strongly suggest that such a point exists near 407 nm.
A counterintuitive prediction of the equation in Fig. 3A is that at λmax-A1 < 407 nm, A2-based pigments should absorb at shorter wavelengths than their A1 counterparts! For example, an SWS1 pigment with λmax-A1 = 370.1 nm would be predicted to have λmax-A2 = 360.5 nm. Instead, measurements of the goldfish SWS1 pigment (λmax-A1 = 370.1 nm) indicate a red shift of nearly 12 nm upon switching to A2 (λmax-A2 = 381.9 nm)23. This apparent deviation from linearity has led some authors to suggest that A1/A2 data would be better fit by a non-linear (e.g., parabolic) function23,136. Yet, careful measurement of multiple A1/A2 pairs within individual labs have almost always found a nearly perfect linear relationship between λmax-A1 and λmax-A2 (at least for λmax > 407 nm)23,48,66, suggesting that deviations from linearity are more likely due to differences in technique between laboratories or experimental error. How can we reconcile these conflicting viewpoints?
I propose that deviations from linearity at very short λmax might be due to fundamental differences in the mechanisms of spectral tuning used by visual pigments with an unprotonated Schiff base and those with a protonated Schiff base136–138. It has long been known that all SWS2, RH1, RH2, and LWS visual pigments contain a protonated Schiff base linkage8,139,140. Furthermore, the presence of this positive charge on the chromophore and the distance of the counterion within the binding cleft play a major role in tuning the λmax of the visual pigment8,141–144. In contrast, SWS1-based visual pigments fall into two distinct spectral classes: those with λmax-A1 < 400 nm (i.e., ultraviolet-sensitive) and those with λmax-A1 > 400 nm (violet-sensitive)145–148. Visual pigments in the former class contain an unprotonated Schiff base, whereas those in the latter class have a protonated Schiff base17,136. Given the fundamental role played by protonation in defining the electronic state of the chromophore and consequently its spectral tuning, it is reasonable to conclude that a visual pigment with an unprotonated Schiff base might be tuned differently137.
I therefore suggest that the relationship between λmax-A1 and λmax-A2 is linear for all visual pigments with a protonated Schiff base, and that a different, and currently unknown equation describes the relationship between λmax-A1 and λmax-A2 for visual pigments with an unprotonated Schiff base. At the present time, there are very few high-confidence measurements of A1/A2 pairs of visual pigments with an unprotonated Schiff base. In fact, the above-mentioned study of the goldfish SWS1 pigment is the only high-quality data point I have found in this range23. Another study purporting to analyze A1/A2 pairs of ultraviolet pigments relied on paired A1 and A2 values derived from different species of fish (under the unproven assumption that the opsins were identical) or from the absorption spectra of pure A1 and A2 chromophores dissolved in ethanol136. Clearly, to define the relationship between λmax-A1 and λmax-A2 in the ultraviolet region, more high-quality measurements of A1/A2 pairs are needed, especially in the range λmax-A1 = 350-385 nm.
Why is the extent of red shift upon switching from A1 to A2 linearly correlated with λmax in visual pigments with a protonated Schiff base? A definitive answer to this question is not yet available and will depend on a deeper understanding of the physical mechanisms of visual pigment spectral tuning. Spectral tuning largely depends on the presence/absence of a protonated Schiff base and the extent of π-electron delocalization along the polyene chain and into the β-ionone ring of the chromophore141–144,149. A greater number of conjugated double bonds (as in A2-based visual pigments) and a greater extent of π-electron delocalization result in greater red shifts in λmax7,8,138,150. Charge delocalization is modulated by both electrostatic interactions between the chromophore and the amino acid side chains within the opsin, as well as by steric interactions that distort the geometry of the chromophore, in turn, affecting its electronic state8,138,141–144.
Nearly fifty years ago, Blatz and Liebman proposed a simple mechanism to account for the relationship between λmax-A1 and λmax-A27. They suggested that the extent of π-electron delocalization into the β-ionone ring is modulated by the degree of co-planarity between the plane of the β-ionone ring (plane A in Fig. 3C) and that of the polyene chain (plane B in Fig. 3C). When the two planes are entirely co-planar (i.e, when α, the dihedral angle between planes A and B, is equal to 0°), maximal π-electron delocalization into the β-ionone ring occurs, and a maximal red shift is achieved8,9,137,142–144. In contrast, when planes A and B are at a right angle to each other (α = 90° in Fig. 3C,D), π-electron delocalization cannot extend into the β-ionone ring, and a maximal blue shift results. Values of α between 0° and 90° would produce intermediate λmax values.
This tuning mechanism could explain the observed relationship between λmax-A1 and λmax-A2 because the full effect of the additional double bond of the A2 chromophore would be accessible for conjugation at α = 0°, while neither of the ring double bonds would be accessible for conjugation at α = 90°. Several modeling studies are consistent with a role for Blatz and Liebman’s mechanism in spectral tuning8,137,142, and the crystal structure of the medium-wavelength-sensitive bovine rhodopsin (an RH1 opsin) demonstrates an ‘intermediate’ value of α as would be predicted by this model151. Nonetheless, a number of experiments using ‘locked’ chromophores134 (in which a chemical bridge prevents changes in α) or 5,6-dihydroretinals135 (which lack ring double bonds) indicate that changes in α alone cannot account for the full-range of spectral tuning observed in naturally occurring visual pigments. In conclusion, the Blatz and Liebman mechanism may play a role in spectral tuning, particularly in the ‘violet-blue’ region of the spectrum, but further modeling and experiments are required to evaluate this idea.
The disadvantages of vitamin A2-based visual pigments
The advantages of A2-based pigments (red-shifted and broadened spectral sensitivity and the potential for continuous tuning of λmax) are counterbalanced by two notable disadvantages: they have lower thermal stability and lower intrinsic photosensitivity than A1-based pigments. Cis-to-trans isomerization of a visual pigment and consequent activation of the phototransduction cascade can be caused either by absorption of a photon (light) or by random thermal fluctuations (heat)10,14,152. Thermal isomerization is sometimes referred to as ‘dark noise’ or ‘dark light’ because the resultant activation of the phototransduction cascade is indistinguishable from that caused by light-induced isomerization14. Dark noise sets a fundamental limit to an organism’s ability to detect photons at very low light levels, because it is impossible to distinguish between photoisomerization and thermal isomerization of the visual pigment153,154. Thus, visual detection in dim light is a signal-to-noise discrimination task: a switch from A1 to A2 might increase signal by more precisely matching λmax to the predominant wavelengths of transmitted light, but this increase is offset by an increase in thermal noise10. Ala-Laurila and colleagues have estimated that replacing A1 with A2 in tiger salamander RH1 results in a red shift of 26 nm and a 36-fold increase in dark noise10. These antagonistic effects pose a conundrum for organisms in turbid environments in which the predominant wavelengths of light are red-shifted (favoring the use of A2-based pigments) while the amount of transmitted light is simultaneously reduced (favoring less noisy A1-based pigments).
The potential advantages of a switch to A2 are further offset by a second factor: the lower photosensitivity of A2-based visual pigments compared to those with A110,155. Photosensitivity (αγ) is a measure of the efficiency with which absorption of light by a visual pigment (or other molecule) induces a specific change in that pigment155–157. It is the product of two terms: α, the absorption coefficient, which is a measure of the efficiency of light absorption, and γ, the quantum efficiency, which is a measure of the efficiency with which the absorbed light causes isomerization155,157. Dartnall found that the average photosensitivity of A1-based RH1 visual pigments was 10.5 (cm2 × 10−17 per chromophore), while the average photosensitivity of A2-based RH1 pigments was 7.4157. Thus, the photosensitivity of A2-based visual pigments is only ~70% that of A1-based pigments (Fig. 1D). Both increased noise and decreased photosensitivity counterbalance the advantages of A2-based visual pigments in low light, but these disadvantages are likely of little consequence in bright light. It is therefore possible that the main selective advantage of A2 is the large red shift (>40 nm) it confers on long-wavelength-sensitive cone opsins (e.g., Fig. 2E). However, Donner has noted that some A2-utilizing species appear to have evolved RH1 opsins with greater thermal stability than species that do not use A214,158. The implication is that thermally stabilizing RH1 mutations act to counter the increased noise of the A2 chromophore. Furthermore, the presence of such mutations implies that the species in question must use A2 under low-light conditions where thermal noise would be selectively relevant. Overall, the nearly ubiquitous utilization of A2 in turbid aquatic environments suggests that its advantages outweigh the disadvantages, irrespective of whether natural selection is acting primarily on the photopic or scotopic visual system.
Unsolved problems related to the A1/A2 chromophore system
In this section I recap what I consider to be the most interesting outstanding questions related to the A1/A2 system, listed in the order in which they arise in the main text. (1) Why are A2-based visual pigments so rare among fully terrestrial vertebrates, and what is their role in the two species (Anolis carolinensis and Podarcis siculus) known to possess them? (2) What factors determine the distribution of A2 usage among fully marine fishes? (3) What are the mechanisms that control differential A2 utilization in different parts of the retina (e.g., in the American bullfrog, the four-eyed fish, etc.)? (4) What features of the environment (temperature; salinity; turbidity; light intensity, duration, and wavelength etc.) play the biggest role in determining A1/A2 ratio, and what is the best way to measure them? (5) What are the molecular mechanisms whereby environmental signals are sensed, transduced, and integrated into changes in A1/A2 ratio in the eye? (6) Are A2-based visual pigments used by any birds or mammals? (7) What is the function of cyp27c1 in species that do not use A2 in the eye? (8) What are the mechanisms that permit some species (e.g., deep-sea dragonfishes and ninespine sticklebacks) to differentially tune the A1/A2 ratio in individual photoreceptor subtypes? (9) Are there enzymes other than cyp27c1 that mediate production of A2 in vertebrates? (10) Is TH signaling always involved in the control of cyp27c1 expression or do TH-independent mechanisms exist? (11) Is the coordinate regulation of A1/A2 exchange and the expression of red-shifted opsins by TH signaling fortuitous or does it have a deeper physiologic or evolutionary significance? (12) What is the equation (or equations) that describes the relationship between λmax-A1 and λmax-A2, especially at wavelengths <400 nm, and what are the mechanisms of spectral tuning that underlie this relationship? (13) To what extent is visual pigment spectral tuning mediated by the degree of co-planarity between the β-ionone ring and the polyene chain of the chromophore? (14) To what degree does the lower photosensitivity and increased noise of A2-based visual pigments limit their utility? (15) Is the selective advantage of A2 utilization mainly attributable to its effects on rod or cone vision? (16) What is the magnitude of the selective advantage that A2 utilization confers? (17) Can we identify mutations that independently control thermal stability and spectral tuning of opsins? (18) Are thermally stabilizing opsin mutations a necessary accompaniment of A2 utilization?
Supplementary Material
Acknowledgements
This review is dedicated to the memory of Victor Govardovskii. I thank P. Ala-Laurila and J. Torres-Dowdall for permission to adapt figures from their published works. I thank L. Volkov for providing the image used in Fig. 1A. I also thank M. Toomey for providing R scripts for drawing spectral absorbance curves. Lastly, I wish to express my gratitude to the following individuals who kindly read the manuscript and provided insightful feedback: P. Ala-Laurila, V. Kefalov, D. Murphy, Y. Ogawa, J. Rusch, M. Toomey, and L.Volkov. The author’s research program is supported by funding from the National Institutes of Health (EY026672, EY025196, and EY030075).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palczewski K et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science (New York, N.Y.) 289, 739–745, doi: 10.1126/science.289.5480.739 (2000). [DOI] [PubMed] [Google Scholar]
- 2. https://www.montereybayaquarium.org/stories/chefs-worldwide-speak-out-to-save-pacific-bluefin-tuna.
- 3. https://unsplash.com/photos/mjPMBpsxICY.
- 4. https://commons.wikimedia.org/wiki/File:Tauchen_Straussee_-_Gr%C3%BCnstich.jpg.
- 5. https://www.reelchase.com/wp-content/uploads/2017/03/Learn-the-Best-Tips-on-How-to-Catch-Crappie-in-Muddy-Water.jpg.
- 6.Govardovskii VI, Fyhrquist N, Reuter T, Kuzmin DG & Donner K In search of the visual pigment template. Visual neuroscience 17, 509–528 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Blatz PE & Liebman PA Wavelength regulation in visual pigments. Exp. Eye Res 17, 573–580 (1973). [DOI] [PubMed] [Google Scholar]
- 8.Honig B, Greenberg AD, Dinur U & Ebrey TG Visual-pigment spectra: implications of the protonation of the retinal Schiff base. Biochemistry 15, 4593–4599, doi: 10.1021/bi00666a008 (1976). [DOI] [PubMed] [Google Scholar]
- 9.Nakanishi K Why 11-cis-retinal? Amer. Zoologist 31, 479–489 (1991). [Google Scholar]
- 10.Ala-Laurila P, Donner K, Crouch RK & Cornwall MC Chromophore switch from 11-cis-dehydroretinal (A2) to 11-cis-retinal (A1) decreases dark noise in salamander red rods. The Journal of physiology 585, 57–74, doi: 10.1113/jphysiol.2007.142935 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wald G The porphyropsin visual system. The Journal of general physiology 22, 775–794 (1939). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bridges CDB in Handbook of Sensory Physiology VII/1 (ed Dartnall HHA) 417–480 (Springer-Verlag, 1972). [Google Scholar]
- 13.Härer A, Meyer A & Torres-Dowdall J Convergent phenotypic evolution of the visual system via different molecular routes: How Neotropical cichlid fishes adapt to novel light environments. Evolution letters 2, 341–354, doi: 10.1002/evl3.71 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donner K Spectral and thermal properties of rhodopsins: closely related but not tightly coupled. Russian Journal of Physiology 106, 421–435 (2020). [Google Scholar]
- 15.Carleton KL, Escobar-Camacho D, Stieb SM, Cortesi F & Marshall NJ Seeing the rainbow: mechanisms underlying spectral sensitivity in teleost fishes. The Journal of experimental biology 223, doi: 10.1242/jeb.193334 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoyama S Molecular evolution of vertebrate visual pigments. Progress in retinal and eye research 19, 385419, doi: 10.1016/S1350-9462(00)00002-1 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Altun A, Morokuma K & Yokoyama S H-bond network around retinal regulates the evolution of ultraviolet and violet vision. ACS chemical biology 6, 775–780, doi: 10.1021/cb200100f (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt DM, Carvalho LS, Cowing JA & Davies WL Evolution and spectral tuning of visual pigments in birds and mammals. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 364, 2941–2955 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart NS & Hunt DM Avian visual pigments: characteristics, spectral tuning, and evolution. The American naturalist 169 Suppl 1, S7–26, doi: 10.1086/510141 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Osorio D & Vorobyev M Photoreceptor spectral sensitivities in terrestrial animals: adaptations for luminance and colour vision. Proc R Soc Lond B 272, 1745–1752 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenaley CP, Devaney SC & Fjeran TT The complex evolutionary history of seeing red: molecular phylogeny and the evolution of an adaptive visual system in deep-sea dragonfishes (Stomiiformes: Stomiidae). Evolution; international journal of organic evolution 68, 996–1013, doi: 10.1111/evo.12322 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Ewald E & Kühne W Untersuchungen über den Sehpurpur. Unters. physiol. Inst. Heidelberg 1, 181 (1878). [Google Scholar]
- 23.Parry JW & Bowmaker JK Visual pigment reconstitution in intact goldfish retina using synthetic retinaldehyde isomers. Vision research 40, 2241–2247, doi: 10.1016/s0042-6989(00)00101-2 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Torres-Dowdall J et al. Rapid and Parallel Adaptive Evolution of the Visual System of Neotropical Midas Cichlid Fishes. Molecular biology and evolution 34, 2469–2485, doi: 10.1093/molbev/msx143 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Kühne W,S,H Zur Physiologie des Sehepithels, inbesondere der Fische. Untersuch. Physiol. Inst. Univ. Heidelberg 3, 221 (1880). [Google Scholar]
- 26.Wald G Visual purple system in fresh-water fishes. Nature 139, 1017 (1937). [Google Scholar]
- 27.Ziderman II Purple dye made from shellfish in antiquity. Review of Progress in Coloration 16, 46–52 (1986). [Google Scholar]
- 28.Köttgen E, Abelsdorff G Absorption and Zersetzung des Sehpurpurs bei den Wirbeltieren. Z. Psychol. Physiol. Sinnesorg 12, 161–184 (1896). [Google Scholar]
- 29.Provencio I, Loew ER & Foster RG Vitamin A2-based visual pigments in fully terrestrial vertebrates. Vision research 31, 2201–2208 (1992). [DOI] [PubMed] [Google Scholar]
- 30.Toyama M et al. Presence of rhodopsin and porphyropsin in the eyes of 164 fishes, representing marine, diadromous, coastal and freshwater species--a qualitative and comparative study. Photochemistry and photobiology 84, 996–1002, doi: 10.1111/j.1751-1097.2008.00344.x (2008). [DOI] [PubMed] [Google Scholar]
- 31.Wald G The metamorphosis of visual systems in the sea lamprey. The Journal of general physiology 40, 901–914 (1957). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beatty DD A study of the succession of visual pigments in Pacific salmon (Oncorhynchus). Canadian journal of zoology 44, 429–455 (1966). [DOI] [PubMed] [Google Scholar]
- 33.Wald G The visual systems of euryhaline fishes. The Journal of general physiology 25, 235–245, doi: 10.1085/jgp.25.2.235 (1941). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beatty DD Visual pigments and the labile scotopic visual system of fish. Vision research 24, 1563–1573, doi: 10.1016/0042-6989(84)90314-6 (1984). [DOI] [PubMed] [Google Scholar]
- 35.Lythgoe JN in Handbook of Sensory Physiology, VII/1: Photochemistry of Vision (ed Dartnall HJA) 602–624 (Springer-Verlag, 1972). [Google Scholar]
- 36.Munz FW, McFarland WN in The Visual System in Vertebrates(ed Crescitelli F) 193–274 (Springer, 1977). [Google Scholar]
- 37.Cummings ME & Partridge JC Visual pigments and optical habitats of surfperch (Embiotocidae) in the California kelp forest. Journal of comparative physiology. A, Neuroethology, sensory, neural, and behavioral physiology 187, 875–889, doi: 10.1007/s00359-001-0258-6 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Ali MA & Heumann WR Distribution of vitamins A1 and A2 in the retinas of some marine fishes from the Gulf of California. Vision research 10, 1307–1310, doi: 10.1016/0042-6989(70)90043-x (1970). [DOI] [PubMed] [Google Scholar]
- 39.Kondrashev SL & Lamash NE Unusual A1/A2-visual pigment conversion during light/dark adaptation in marine fish. Comparative biochemistry and physiology. Part A, Molecular & integrative physiology 238, 110560, doi: 10.1016/j.cbpa.2019.110560 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Munz FW The photosensitive retinal pigments of fishes from relatively turbid coastal waters. The Journal of general physiology 42, 445–459, doi: 10.1085/jgp.42.2.445 (1958). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White EM, Goncalves DM, Partridge JC & Oliveira RF Vision and visual variation in the peacock blenny. Journal of Fish Biology 65, 227–250 (2004). [Google Scholar]
- 42.Cohen JL, Hueter RE & Organisciak DT The presence of a porphyropsin-based visual pigment in the juvenile lemon shark (Negaprion brevirostris). Vision research 30, 1949–1953, doi: 10.1016/0042-6989(90)90014-c (1990). [DOI] [PubMed] [Google Scholar]
- 43.Saarinen P et al. Spectral tuning by selective chromophore uptake in rods and cones of eight populations of nine-spined stickleback (Pungitius pungitius). The Journal of experimental biology 215, 2760–2773, doi: 10.1242/jeb.068122 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Bridges CD Variability and relationships of fish visual pigments. Vision research 5, 239–251, doi: 10.1016/0042-6989(65)90002-7 (1966). [DOI] [PubMed] [Google Scholar]
- 45.Bridges CD Variation of visual pigment amongst individuals of an American minnow, Notemigonus crysolecuas boscii. Vision research 4, 233–239, doi: 10.1016/0042-6989(64)90005-7 (1964). [DOI] [PubMed] [Google Scholar]
- 46.Endeman D, Klaassen LJ & Kamermans M Action spectra of zebrafish cone photoreceptors. PloS one 8, e68540, doi: 10.1371/journal.pone.0068540 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enright JM et al. Cyp27c1 Red-Shifts the Spectral Sensitivity of Photoreceptors by Converting Vitamin A1 into A2. Current biology : CB 25, 3048–3057, doi: 10.1016/j.cub.2015.10.018 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allison WT, Haimberger TJ, Hawryshyn CW & Temple SE Visual pigment composition in zebrafish: Evidence for a rhodopsin-porphyropsin interchange system. Visual neuroscience 21, 945–952, doi: 10.1017/s0952523804216145 (2004). [DOI] [PubMed] [Google Scholar]
- 49.Spence R, Fatema MK, Reichard M, Huq KA & Wahab MA The distribution and habitat preferences of the zebrafish in Bangladesh. Journal of Fish Biology 69, 1435–1448 (2006). [Google Scholar]
- 50.Arunachalam M, Raja M, Vijayakumar C, Malaiammal P & Mayden RL Natural history of zebrafish (Danio rerio) in India. Zebrafish 10, 1–14, doi: 10.1089/zeb.2012.0803 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Parichy DM Advancing biology through a deeper understanding of zebrafish ecology and evolution. Elife 4, doi: 10.7554/eLife.05635 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Temple SE et al. Seasonal cycle in vitamin A1/A2-based visual pigment composition during the life history of coho salmon (Oncorhynchus kisutch). Journal of comparative physiology. A, Neuroethology, sensory, neural, and behavioral physiology 192, 301–313, doi: 10.1007/s00359-005-0068-3 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Dartnall HJA, Lander MR & Munz FW in Progess in Photobiology (eds Christensen BC & Buchmann B) (Elsevier, 1961). [Google Scholar]
- 54.Bridges CD Effect of season and environment on the retinal pigments of two fishes. Nature 203, 191–192, doi: 10.1038/203191a0 (1964). [DOI] [PubMed] [Google Scholar]
- 55.Makino M, Nagai K & Suzuki T Seasonal variation of the vitamin A2-based visual pigment in the retina of adult bullfrog, Rana catesbeiana. Vision research 23, 199–204, doi: 10.1016/0042-6989(83)90143-8 (1983). [DOI] [PubMed] [Google Scholar]
- 56.Allen DM & McFarland WN The effect of temperature on rhodopsin-porphyropsin ratios in a fish. Vision research 13, 1303–1309, doi: 10.1016/0042-6989(73)90206-x (1973). [DOI] [PubMed] [Google Scholar]
- 57.Cristy M Effects of temperature and light intensity on the visual pigments of rainbow trout. Vision research 16, 1225–1228, doi: 10.1016/0042-6989(76)90045-6 (1976). [DOI] [PubMed] [Google Scholar]
- 58.McFarland WN & Allen DM The effect of extrinsic factors on two distinctive rhodopsin-porphyropsin systems. Canadian journal of zoology 55, 1000–1009, doi: 10.1139/z77-126 (1977). [DOI] [PubMed] [Google Scholar]
- 59.Tsin AT Steady-state visual pigment composition in rainbow trout. Vision research 19, 1269–1271, doi: 10.1016/0042-6989(79)90194-9 (1979). [DOI] [PubMed] [Google Scholar]
- 60.Tsin AT The visual pigment composition of rainbow trout. Vision research 19, 131–135, doi: 10.1016/0042-6989(79)90042-7 (1979). [DOI] [PubMed] [Google Scholar]
- 61.Tsin AT & Beatty DD Goldfish rhodopsin: P499. Vision research 18, 1453–1455, doi: 10.1016/0042-6989(78)90243-2 (1978). [DOI] [PubMed] [Google Scholar]
- 62.Tsin AT & Beatty DD Visual pigment changes in rainbow trout in response to temperature. Science (New York, N.Y.) 195, 1358–1360, doi: 10.1126/science.841335 (1977). [DOI] [PubMed] [Google Scholar]
- 63.Tsin AT & Beatty DD Visual pigments and vitamins A in the adult bullfrog. Exp Eye Res 30, 143–153, doi: 10.1016/0014-4835(80)90108-6 (1980). [DOI] [PubMed] [Google Scholar]
- 64.Aho AC, Donner K, Hydén C, Larsen LO & Reuter T Low retinal noise in animals with low body temperature allows high visual sensitivity. Nature 334, 348–350, doi: 10.1038/334348a0 (1988). [DOI] [PubMed] [Google Scholar]
- 65.Beatty DD Visual pigments of the american eel Anguilla rostrata. Vision research 15, 771–776, doi: 10.1016/0042-6989(75)90254-0 (1975). [DOI] [PubMed] [Google Scholar]
- 66.Liebman PA & Entine G Visual pigments of frog and tadpole (Rana pipiens). Vision research 8, 761–775 (1968). [DOI] [PubMed] [Google Scholar]
- 67.Wilt FH The differentiation of visual pigments in metamorphosing larvae of Rana catesbeiana. Dev. Biol 1, 199–233 (1959). [Google Scholar]
- 68.Crescitelli F The natural history of visual pigments. Annals of the New York Academy of Sciences 74, 230–255 (1959). [DOI] [PubMed] [Google Scholar]
- 69.Bridges CD & Yoshikami S The rhodopsin-porphyropsin system in freshwater fishes. 1. Effects of age and photic environment. Vision research 10, 1315–1332, doi: 10.1016/0042-6989(70)90084-2 (1970). [DOI] [PubMed] [Google Scholar]
- 70.Reuter TE, White RH & Wald G Rhodopsin and porphyropsin fields in the adult bullfrog retina. The Journal of general physiology 58, 351–371 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Surface HA First report on the economic features of the amphibians of Pennsylvania. Zool. Bull. Div. Zool. Pa. Dept. Agr 3, 65 (1913). [Google Scholar]
- 72.Miller RR Ecology, habits and relationships of the middle american cuatro ojos, Anableps dowi (pisces: Anablepidae) Copeia 1, 82–91 (1979). [Google Scholar]
- 73.Owens GL, Rennison DJ, Allison WT & Taylor JS In the four-eyed fish (Anableps anableps), the regions of the retina exposed to aquatic and aerial light do not express the same set of opsin genes. Biology letters 8, 86–89, doi: 10.1098/rsbl.2011.0582 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bridges CD Porphyropsin in retina of four-eyed fish, Anableps anableps. Nature 300, 384, doi: 10.1038/300384a0 (1982). [DOI] [PubMed] [Google Scholar]
- 75.Denton EJ, Muntz WR & Northmore DP The distribution of visual pigment within the retina in two teleosts. J. Mar. Biolog. Assoc. UK 51, 905–915 (1971). [Google Scholar]
- 76.Muntz WR & Northmore DP Visual pigments from different parts of the retina in rudd and trout. Vision research 11, 551–561, doi: 10.1016/0042-6989(71)90076-9 (1971). [DOI] [PubMed] [Google Scholar]
- 77.Orlov OY & Kondrashev SL Changeable coloration of cornea in fishes and its distribution. Yugoslav. Physiol. Pharmacol. Acta 34, 359–369 (1998). [Google Scholar]
- 78.Kondrashev SL The spectral sensitivity of photoreceptors and the screening function of the cornea of greenlings (Hexagrammidae). Russian Journal of Marine Biology 45, 22–30 (2019). [Google Scholar]
- 79.Kondrashev SL Long-wave sensitivity in the masked greenling (Hexagrammos octogrammus), a shallow-water marine fish. Vision research 48, 2269–2274, doi: 10.1016/j.visres.2008.07.004 (2008). [DOI] [PubMed] [Google Scholar]
- 80.Herring PJ The spectral characteristics of luminous marine organisms. Proc. R. Soc. Lond. B 220, 183–217 (1983). [Google Scholar]
- 81.Widder EA, Latz MI, Herring PJ & Case JF Far red bioluminescence from two deep-sea fishes. Science (New York, N.Y.) 225, 512–514 (1984). [DOI] [PubMed] [Google Scholar]
- 82.Herring P & Cope C Red bioluminescence in fishes: on the suborbital photophores of Malacosteus, Pachystomias and Aristostomias. Mar. Biol 148, 383–394 (2005). [Google Scholar]
- 83.de Busserolles F, Fogg L, Cortesi F & Marshall J The exceptional diversity of visual adaptations in deep-sea teleost fishes. Seminars in cell & developmental biology 106, 20–30, doi: 10.1016/j.semcdb.2020.05.027 (2020). [DOI] [PubMed] [Google Scholar]
- 84.Douglas RH, Genner MJ, Hudson AG, Partridge JC & Wagner HJ Localisation and origin of the bacteriochlorophyll-derived photosensitizer in the retina of the deep-sea dragon fish Malacosteus niger. Scientific reports 6, 39395, doi: 10.1038/srep39395 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Douglas RH, Partridge JC & Marshall NJ The eyes of deep-sea fish. I: Lens pigmentation, tapeta and visual pigments. Progress in retinal and eye research 17, 597–636, doi: 10.1016/s1350-9462(98)00002-0 (1998). [DOI] [PubMed] [Google Scholar]
- 86.Bowmaker JK, Dartnall HJA, Herring PJ Longwave-sensitive visual pigments in some deep-sea fishes: segregation of ‘paired’ rhodopsins and porphyropsins. J. Comp. Physiol. A 163, 685–698 (1988). [Google Scholar]
- 87.Partridge JC & Douglas RH Far-red sensitivity of dragon fish. Nature 375, 21–22(1995). [Google Scholar]
- 88.Partridge JC, Shand J, Archer SN, Lythgoe JN & van Groningen-Luyben WA Interspecific variation in the visual pigments of deep-sea fishes. Journal of comparative physiology. A, Sensory, neural, and behavioral physiology 164, 513–529, doi: 10.1007/bf00610445 (1989). [DOI] [PubMed] [Google Scholar]
- 89.Douglas RH et al. Enhanced retinal longwave sensitivity using a chlorophyll-derived photosensitiser in Malacosteus niger, a deep-sea dragon fish with far red bioluminescence. Vision research 39, 2817–2832, doi: 10.1016/s0042-6989(98)00332-0 (1999). [DOI] [PubMed] [Google Scholar]
- 90.Douglas RH et al. Dragon fish see using chlorophyll. Nature 393, 423–424 (1998). [Google Scholar]
- 91.Lin JJ, Wang FY, Li WH & Wang TY The rises and falls of opsin genes in 59 ray-finned fish genomes and their implications for environmental adaptation. Scientific reports 7, 15568, doi: 10.1038/s41598-017-15868-7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Terai Y et al. Visual adaptation in Lake Victoria cichlid fishes: depth-related variation of color and scotopic opsins in species from sand/mud bottoms. BMC evolutionary biology 17, 200, doi: 10.1186/s12862-017-1040-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Terai Y et al. Divergent selection on opsins drives incipient speciation in Lake Victoria cichlids. PLoS biology 4, e433, doi: 10.1371/journal.pbio.0040433 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carleton KL & Yourick MR Axes of visual adaptation in the ecologically diverse family Cichlidae. Seminars in cell & developmental biology 106, 43–52, doi: 10.1016/j.semcdb.2020.04.015 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Escobar-Camacho D et al. Variable vision in variable environments: the visual system of an invasive cichlid (Cichla monoculus) in Lake Gatun, Panama. The Journal of experimental biology 222, doi: 10.1242/jeb.188300 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McFarland WN & Munz FW Part II: The photic environment of clear tropical seas during the day. Vision research 15, 1063–1070, doi: 10.1016/0042-6989(75)90002-4 (1975). [DOI] [PubMed] [Google Scholar]
- 97.Munz FW & McFarland WN Part I: Presumptive cone pigments extracted from tropical marine fishes. Vision research 15, 1045–1062, doi: 10.1016/0042-6989(75)90001-2 (1975). [DOI] [PubMed] [Google Scholar]
- 98.Zimmermann MJY et al. Zebrafish Differentially Process Color across Visual Space to Match Natural Scenes. Current biology : CB 28, 2018–2032.e2015, doi: 10.1016/j.cub.2018.04.075 (2018). [DOI] [PubMed] [Google Scholar]
- 99.Schneider RF, Rometsch SJ, Torres-Dowdall J & Meyer A Habitat light sets the boundaries for the rapid evolution of cichlid fish vision, while sexual selection can tune it within those limits. Mol Ecol 29, 1476–1493, doi: 10.1111/mec.15416 (2020). [DOI] [PubMed] [Google Scholar]
- 100.Bridges CD & Yoshikami S The rhodopsin-porphyropsin system in freshwater fishes. 2. Turnover and interconversion of visual pigment prosthetic groups in light and darkness: role of the pigment epithelium. Vision research 10, 1333–1345, doi: 10.1016/0042-6989(70)90085-4 (1970). [DOI] [PubMed] [Google Scholar]
- 101.Palczewski K & Kiser PD Shedding new light on the generation of the visual chromophore. Proc Natl Acad Sci U S A 117, 19629–19638, doi: 10.1073/pnas.2008211117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Coon MJ Cytochrome P450: nature’s most versatile biological catalyst. Annual review of pharmacology and toxicology 45, 1–25, doi: 10.1146/annurev.pharmtox.45.120403.100030 (2005). [DOI] [PubMed] [Google Scholar]
- 103.Kramlinger VM et al. Human cytochrome P450 27C1 catalyzes 3,4-desaturation of retinoids. FEBS letters 590, 1304–1312, doi: 10.1002/1873-3468.12167 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang JS & Kefalov VJ The cone-specific visual cycle. Progress in retinal and eye research 30, 115–128, doi: 10.1016/j.preteyeres.2010.11.001 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morshedian A et al. Cambrian origin of the CYP27C1-mediated vitamin A1-to-A2 switch, a key mechanism of vertebrate sensory plasticity. Royal Society open science 4, 170362, doi: 10.1098/rsos.170362 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kuraku S & Kuratani S Time scale for cyclostome evolution inferred with a phylogenetic diagnosis of hagfish and lamprey cDNA sequences. Zoological science 23, 1053–1064, doi: 10.2108/zsj.23.1053 (2006). [DOI] [PubMed] [Google Scholar]
- 107.Halstead LB The vertebrate invasion of fresh water. Phil. Trans. R. Soc. Lond. B 309, 243–258 (1985). [Google Scholar]
- 108.Maden M, Sonneveld E, van der Saag PT & Gale E The distribution of endogenous retinoic acid in the chick embryo: implications for developmental mechanisms. Development (Cambridge, England) 125, 4133–4144 (1998). [DOI] [PubMed] [Google Scholar]
- 109.Thaller C & Eichele G Isolation of 3,4-didehydroretinoic acid, a novel morphogenetic signal in the chick wing bud. Nature 345, 815–819, doi: 10.1038/345815a0 (1990). [DOI] [PubMed] [Google Scholar]
- 110.Churakov G et al. Rodent evolution: back to the root. Molecular biology and evolution 27, 1315–1326, doi: 10.1093/molbev/msq019 (2010). [DOI] [PubMed] [Google Scholar]
- 111.Asher RJ, Smith MR, Rankin A & Emry RJ Congruence, fossils and the evolutionary tree of rodents and lagomorphs. Royal Society open science 6, 190387, doi: 10.1098/rsos.190387 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gaspard JC 3rd et al. Detection of hydrodynamic stimuli by the Florida manatee (Trichechus manatus latirostris). Journal of comparative physiology. A, Neuroethology, sensory, neural, and behavioral physiology 199, 441–450, doi: 10.1007/s00359-013-0822-x (2013). [DOI] [PubMed] [Google Scholar]
- 113.Johnson KM, Phan TTN, Albertolle ME & Guengerich FP Human mitochondrial cytochrome P450 27C1 is localized in skin and preferentially desaturates trans-retinol to 3,4-dehydroretinol. The Journal of biological chemistry 292, 13672–13687, doi: 10.1074/jbc.M116.773937 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rollman O & Vahlquist A Vitamin A in skin and serum--studies of acne vulgaris, atopic dermatitis, ichthyosis vulgaris and lichen planus. The British journal of dermatology 113, 405–413 (1985). [DOI] [PubMed] [Google Scholar]
- 115.Torma H & Vahlquist A Biosynthesis of 3-dehydroretinol (vitamin A2) from all-trans-retinol (vitamin A1) in human epidermis. The Journal of investigative dermatology 85, 498–500 (1985). [DOI] [PubMed] [Google Scholar]
- 116.Vahlquist A The identification of dehydroretinol (vitamin A2) in human skin. Experientia 36, 317–318 (1980). [DOI] [PubMed] [Google Scholar]
- 117.munz f. W., Swanson RT Thyroxine-induced changes in the proportions of visual pigments. Amer. Zoologist 5, 683 (1965). [Google Scholar]
- 118.Jacquest WL & Beatty DD Visual pigment changes in the rainbow trout, Salmo gairdneri. Canadian journal of zoology 50, 1117–1126, doi: 10.1139/z72-149 (1972). [DOI] [PubMed] [Google Scholar]
- 119.Cristy M Effects of prolactin and thyroxine on the visual pigments of trout, Salmo gairdneri. General and comparative endocrinology 23, 58–62, doi: 10.1016/0016-6480(74)90053-7 (1974). [DOI] [PubMed] [Google Scholar]
- 120.Allen DM Measurements of serum thyroxine and the proportions of rhodopsin and porphyropsin in rainbow trout. Canadian journal of zoology 55, 836–842, doi: 10.1139/z77-108 (1977). [DOI] [PubMed] [Google Scholar]
- 121.Allen DM Photic control of the proportions of two visual pigments in a fish. Vision research 11, 1077–1112, doi: 10.1016/0042-6989(71)90114-3 (1971). [DOI] [PubMed] [Google Scholar]
- 122.Beatty DD Visual pigment changes in juvenile kokanee salmon in response to thyroid hormones. Vision research 9, 855–864 (1969). [DOI] [PubMed] [Google Scholar]
- 123.Naito K & Wilt FH The conversion of vitamin A1 to retinene in a fresh-water fish. The Journal of biological chemistry 237, 3060–3064 (1962). [PubMed] [Google Scholar]
- 124.Ohtsu K, Naito K & Wilt FH Metabolic basis of visual pigment conversion in metamorphosing Rana catesbeiana. Dev Biol 10, 216–232, doi: 10.1016/0012-1606(64)90042-9 (1964). [DOI] [PubMed] [Google Scholar]
- 125.Wilt FH The organ specific action of thyroxin in visual pigment differentiation. Journal of embryology and experimental morphology 7, 56–63 (1959). [PubMed] [Google Scholar]
- 126.Volkov LI et al. Thyroid hormone receptors mediate two distinct mechanisms of long-wavelength vision. Proc Natl Acad Sci U S A 117, 15262–15269, doi: 10.1073/pnas.1920086117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ng L et al. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nature genetics 27, 94–98 (2001). [DOI] [PubMed] [Google Scholar]
- 128.Eldred KC et al. Thyroid hormone signaling specifies cone subtypes in human retinal organoids. Science (New York, N.Y.) 362, doi: 10.1126/science.aau6348 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Suzuki SC et al. Cone photoreceptor types in zebrafish are generated by symmetric terminal divisions of dedicated precursors. Proc Natl Acad Sci U S A 110, 15109–15114, doi: 10.1073/pnas.1303551110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Temple SE et al. Effects of exogenous thyroid hormones on visual pigment composition in coho salmon (Oncorhynchus kisutch). The Journal of experimental biology 211, 2134–2143, doi: 10.1242/jeb.009365 (2008). [DOI] [PubMed] [Google Scholar]
- 131.Mackin RD et al. Endocrine regulation of multichromatic color vision. Proc Natl Acad Sci U S A 116, 16882–16891, doi: 10.1073/pnas.1904783116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dartnall HJA & Lythgoe JN in Ciba Foundation Symposium - Colour Vision: Physiology and Experimental Psychology (A. Churchill Ltd., 1965). [Google Scholar]
- 133.Munz FW & Schwanzara SA A nomogram for retinene-2-based visual pigments. Vision research 7, 111–120, doi: 10.1016/0042-6989(67)90078-8 (1967). [DOI] [PubMed] [Google Scholar]
- 134.Makino CL et al. Effects of modified chromophores on the spectral sensitivity of salamander, squirrel and macaque cones. The Journal of physiology 424, 545–560, doi: 10.1113/jphysiol.1990.sp018082 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Makino CL, Groesbeek M, Lugtenburg J & Baylor DA Spectral tuning in salamander visual pigments studied with dihydroretinal chromophores. Biophysical journal 77,1024–1035, doi: 10.1016/s0006-3495(99)76953-5 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Harosi FI An analysis of two spectral properties of vertebrate visual pigments. Vision research 34, 1359–1367 (1994). [DOI] [PubMed] [Google Scholar]
- 137.Zhu S, Brown MF & Feller SE Retinal conformation governs pKa of protonated Schiff base in rhodopsin activation. Journal of the American Chemical Society 135, 9391–9398, doi: 10.1021/ja4002986 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Altun A, Yokoyama S & Morokuma K Spectral tuning in visual pigments: an ONIOM(QM:MM) study on bovine rhodopsin and its mutants. The journal of physical chemistry. B 112, 6814–6827, doi: 10.1021/jp709730b (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Morton RA & Pitt GA Studies on rhodopsin. IX. pH and the hydrolysis of indicator yellow. The Biochemical journal 59, 128–134, doi: 10.1042/bj0590128 (1955). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Honig B & Ebrey TG The structure and spectra of the chromophore of the visual pigments. Annual review of biophysics and bioengineering 3, 151–177, doi: 10.1146/annurev.bb.03.060174.001055 (1974). [DOI] [PubMed] [Google Scholar]
- 141.Kochendoerfer GG, Lin SW, Sakmar TP & Mathies RA How color visual pigments are tuned. Trends in biochemical sciences 24, 300–305, doi: 10.1016/s0968-0004(99)01432-2 (1999). [DOI] [PubMed] [Google Scholar]
- 142.Sekharan S, Katayama K, Kandori H & Morokuma K Color vision: “OH-site” rule for seeing red and green. Journal of the American Chemical Society 134, 10706–10712, doi: 10.1021/ja304820p (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Collette F, Renger T, Müh F & Schmidt Am Busch M Red/Green Color Tuning of Visual Rhodopsins: Electrostatic Theory Provides a Quantitative Explanation. The journal of physical chemistry. B 122, 4828–4837, doi: 10.1021/acs.jpcb.8b02702 (2018). [DOI] [PubMed] [Google Scholar]
- 144.Ernst OP et al. Microbial and animal rhodopsins: structures, functions, and molecular mechanisms. Chemical reviews 114, 126–163, doi: 10.1021/cr4003769 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Odeen A & Hastad O The phylogenetic distribution of ultraviolet sensitivity in birds. BMC evolutionary biology 13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Odeen A & Hastad O Complex distribution of avian color vision systems revealed by sequencing the SWS1 opsin from total DNA. Molecular biology and evolution 20, 855–861, doi: 10.1093/molbev/msg108 (2003). [DOI] [PubMed] [Google Scholar]
- 147.Shi Y, Radlwimmer FB & Yokoyama S Molecular genetics and the evolution of ultraviolet vision in vertebrates. Proc Natl Acad Sci U S A 98, 11731–11736, doi: 10.1073/pnas.201257398 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cuthill IC et al. Ultraviolet vision in birds. Advances in the Study of Behavior 29, 159–214 (2000). [Google Scholar]
- 149.Honig B et al. An external point-charge model for wavelength regulation in visual pigments. J. Am. Chem. Soc 101, 7084–7086 (1979). [Google Scholar]
- 150.Rajamani R, Lin YL & Gao J The opsin shift and mechanism of spectral tuning in rhodopsin. Journal of computational chemistry 32, 854–865, doi: 10.1002/jcc.21663 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Okada T et al. The retinal conformation and its environment in rhodopsin in light of a new 2.2 A crystal structure. Journal of molecular biology 342, 571–583, doi: 10.1016/j.jmb.2004.07.044 (2004). [DOI] [PubMed] [Google Scholar]
- 152.Luo DG, Yue WW, Ala-Laurila P & Yau KW Activation of visual pigments by light and heat. Science (New York, N.Y.) 332, 1307–1312, doi: 10.1126/science.1200172 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Barlow HB Retinal noise and absolute threshold. J Opt Soc Am 46, 634–639, doi: 10.1364/josa.46.000634 (1956). [DOI] [PubMed] [Google Scholar]
- 154.Donner K Noise and the absolute thresholds of cone and rod vision. Vision research 32, 853–866, doi: 10.1016/0042-6989(92)90028-h (1992). [DOI] [PubMed] [Google Scholar]
- 155.Dartnall HJA in Handbook of Sensory Physiology, VII/1: Photochemistry of Vision (ed Dartnall HJA) 122–145 (Springer-Verlag, 1972). [Google Scholar]
- 156.Goodeve CF & Wood LJ The photosensitivity of diphenylamine p-diazonium sulphate by the method of photometric curves. Proc. Roy. Soc. A 166, 342–353 (1938). [Google Scholar]
- 157.Dartnall HJ The photosensitivities of visual pigments in the presence of hydroxylamine. Vision research 8, 339–358, doi: 10.1016/0042-6989(68)90104-1 (1968). [DOI] [PubMed] [Google Scholar]
- 158.Donner K, Firsov ML & Govardovskii VI The frequency of isomerization-like ‘dark’ events in rhodopsin and porphyropsin rods of the bull-frog retina. The Journal of physiology 428, 673–692,doi: 10.1113/jphysiol.1990.sp018234 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


