In the crystal structure of the title compounds, discrete complexes with different Co coordinations are observed, which are linked by intermolecular hydrogen bonding into three-dimensional networks.
Keywords: crystal structure, cobalt thiocyanate, urotropine, hydrogen bonding, mixed ligand occupation
Abstract
The reaction of Co(NCS)2 with urotropine in ethanol leads to the formation of two different compounds, namely, bis(ethanol-κO)bis(hexamethylenetetramine-κN)bis(thiocyanato-κN)cobalt(II)–diaqua-κ 2O-bis(hexamethylenetetramine-κN)bis(thiocyanato-κN)cobalt(II)–ethanol–hexamethylenetetramine (1.2/0.8/1.6/4), [Co(NCS)2(C6H12N4)2(C2H6O)2]1.2·[Co(NCS)2(C6H12N4)2(H2O)2]0.8·1.6C2H6O·4C6H12N4, 1, and tris(ethanol-κO)(hexamethylenetetramine-κN)bis(thiocyanato-κN)cobalt(II), [Co(NCS)2(C6H12N4)(C2H6O)3], 2. In the crystal structure of compound 1, two crystallographically independent discrete complexes are observed that are located on centres of inversion. In one of them, the Co cation is octahedrally coordinated to two terminal N-bonded thiocyanate anions, two urotropine ligands and two ethanol molecules, whereas in the second complex 80% of the coordinating ethanol is exchanged by water. Formally, compound 1 is a mixture of two different complexes, i.e. diaquadithiocyanatobis(urotropine)cobalt(II) and diethanoldithiocyanatobis(urotropine)cobalt(II), that contain additional ethanol and urotropine solvate molecules leading to an overall composition of [Co(NCS)2(urotropine)2(ethanol)1.2(H2O)0.8·0.8ethanol·4urotropine. Both discrete complexes are linked by intermolecular O—H⋯O and O—H⋯N hydrogen bonding and additional urotropine solvate molecules into chains, which are further connected into layers. These layers combine into a three-dimensional network by pairs of centrosymmetric intermolecular C—H⋯S hydrogen bonds. In the crystal structure of compound 2, dithiocyanato(urotropine)triethanolcobalt(II), the cobalt cation is octahedrally coordinated to two terminal N-bonded thiocyanate anions, one urotropine ligand and three ethanol molecules into discrete complexes, which are located in general positions. These complexes are linked by intermolecular O—H⋯N hydrogen bonding into layers, which are further connected into a three-dimensional network by intermolecular C—H⋯S hydrogen bonding.
Chemical context
Recently, we reported the crystal structure of two new coordination compounds with the composition [Co(NCS)2(urotropine)2(ethanol)2] and [Co(NCS)2(ethanol)4](urotropine)2 (Krebs et al., 2022 ▸). Both compounds consist of discrete complexes, in which the cobalt cations are octahedrally coordinated by two terminal N-bonded thiocyanate anions and by four ethanol and two ethanol and two urotropine ligands, respectively. These investigations were performed to prepare precursors that on thermal decomposition transform into coordination polymers in which the cobalt cations are linked by μ-1,3 bridging thiocyanate anions into chains or layers (Näther et al., 2013 ▸). Several such compounds have been reported in the literature and they are of interest because they show ferromagnetic or antiferromagnetic ordering or a slow relaxation of the magnetization, which is indicative for single-chain magnetism (Böhme et al., 2020 ▸; Shi et al., 2006 ▸; Jin et al., 2007 ▸; Jochim et al., 2020 ▸; Prananto et al., 2017 ▸; Mautner et al., 2018 ▸; Rams et al., 2020 ▸; Ceglarska et al., 2021 ▸; Werner et al., 2014 ▸, 2015 ▸; Suckert et al., 2016 ▸; Wellm et al., 2020 ▸). In this context, urotropine as a coligand was of interest because this ligand is able to form networks (Czubacka et al., 2012 ▸; Li et al., 2012 ▸), is magnetically silent and one compound with cadmium had already been reported in which the metal cations are linked by the anionic ligands into chains (Bai et al., 2009 ▸).

However, for the preparation of the two compounds mentioned above, cobalt thiocyanate was reacted with urotropine in ethanol and X-ray powder measurements show that none of these compounds can be prepared as a pure crystalline phase. Either the desired compounds were obtained as the minor phase or the experimental powder patterns were completely different from the calculated one. These investigations indicate that additional compounds are present and that the desired compounds are not very stable and transform in solution. Therefore, additional crystallization experiments were performed, which lead to the formation of single crystals of two new compounds that were identified by single crystal X-ray diffraction. Even these compounds contain ethanol as a ligand but in one compound one coordination site is simultaneously occupied by ethanol and water, which might originate from some residual water in the solvent used in the synthesis, whereas in the second compound the cobalt cations are coordinated by only one urotropine and three ethanol ligands. All this indicates that, for this system, different species are in equilibrium in solution and some phase crystallizes, presumably by kinetic control, which means that the synthesis is difficult to control.
Structural commentary
The asymmetric unit of compound 1 consists of two crystallographically independent Co cations that are located on centres of inversion as well as two thiocyanate anions, four urotropine ligands, three ethanol and one water molecule that occupy general positions (Fig. 1 ▸). One of the cobalt cations (Co1) is sixfold coordinated to two terminal N-bonded thiocyanate anions, two urotropine ligands and two ethanol molecules into discrete complexes (Fig. 1 ▸, top left). The methyl carbon atom of these ethanol molecules is disordered in two positions and was refined using a split model. The second cobalt cation is also sixfold coordinated, forming discrete complexes, to two terminal N-bonded thiocyanate anions, two urotropine ligands and two oxygen atoms, but the latter positions are mixed occupied by water and ethanol in a ratio of 8:2, leading to an overall composition for 1 of [Co(NCS)2(urotropine)2(ethanol)1.2(H2O)0.8·1.6ethanol·4urotropine. In the case where it is occupied by water, an ethanol molecule is hydrogen bonded to this water molecule; if it is occupied by ethanol, this ethanol solvate molecule is not present (Fig. 1 ▸, top right). The position of the disordered O atoms of the water and ethanol molecule was resolved and all O—H H atoms were clearly located in the difference map and refined isotropically with reasonable displacement parameters, using restraints for the O—H distances (see Refinement). The Co—N bond lengths to the thiocanate anions are similar in both complexes, which is also valid for the bond length to the urotropine ligands (Table 1 ▸). In contrast, the Co—O bond length to the water molecule is shorter than those to the ethanol molecules (Table 1 ▸), even if there might be some uncertainty in the distances because of the disorder.
Figure 1.
Crystal structure of compound 1 with labelling and displacement ellipsoids drawn at the 50% probability level. Symmetry code for the generation of equivalent atoms: (i) −x + 1, −y + 1, −z + 2; (ii) −x + 2, −y + 1, −z + 1.
Table 1. Selected bond lengths (Å) for 1 .
| Co1—N1 | 2.0590 (16) | Co2—O2 | 2.029 (6) |
| Co1—O1 | 2.1388 (13) | Co2—O4 | 2.21 (3) |
| Co1—N11 | 2.2834 (15) | Co2—N21 | 2.2788 (16) |
| Co2—N2 | 2.0812 (16) |
The asymmetric unit of compound 2 consists of one crystallographically independent cobalt cation, one urotropine ligand and three ethanol molecules, all of them located in general positions (Fig. 2 ▸). In this compound the cobalt cations are sixfold coordinated to two terminal N-bonded thiocyanate anions, one urotropine ligand and three ethanol molecules. The Co—N and Co—O bond lengths are comparable to those in compound 1 and to similar ethanol complexes retrieved from the literature (Krebs et al., 2021a, Table 2 ▸). From the angles around the Co cations, it is obvious that in all compounds the octahedra are slightly distorted (see supporting information). It is noted that compound 2 completes the series of Co(NCS)2-urotropine compounds with ethanol as an additional ligand, because in this compound the cobalt cations are coordinated to one urotropine and three ethanol ligands, whereas in the other compounds reported recently the cobalt cations are either coordinated to two urotropine and two ethanol ligands or to four ethanol ligands (Krebs et al., 2021a).
Figure 2.
Crystal structure of compound 2 with labelling and displacement ellipsoids drawn at the 50% probability level.
Table 2. Selected bond lengths (Å) for 2 .
| Co1—N2 | 2.0615 (11) | Co1—O31 | 2.1157 (9) |
| Co1—N1 | 2.0624 (11) | Co1—O21 | 2.1314 (9) |
| Co1—O41 | 2.1021 (10) | Co1—N11 | 2.2489 (11) |
Supramolecular features
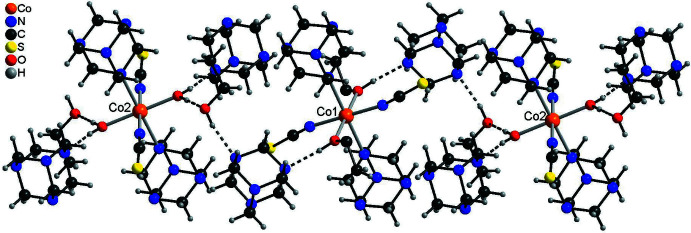
In the crystal structure of the title compound, extensive hydrogen bonding is observed (Table 3 ▸). The discrete complex around Co1 is linked to two urotropine solvate molecules via intermolecular O—H⋯N hydrogen bonding (Fig. 3 ▸ and Table 3 ▸). For the Co2 complex, two different surroundings are observed. In the case where this cation is coordinated to water, this water molecule is hydrogen bonded to two urotropine ligands and two ethanol molecules (Fig. 4 ▸, top and Table 3 ▸). There are two additional C—H⋯S hydrogen bonds, which are not shown for clarity. In the case where Co2 is coordinated to EtOH, the solvate ethanol molecule is not present and the surrounding is similar to that around Co1 with only hydrogen bonding to two urotropine ligands (compare Fig. 3 ▸ and Fig. 4 ▸, bottom). Both crystallographically independent complexes are linked into chains via intermolecular O—H⋯O and O—H⋯N hydrogen bonding (Fig. 5 ▸). The chains are further connected into layers by intermolecular C—H⋯O and C—H⋯N interactions. These layers are stacked onto each other and are linked by intermolecular centrosymmetric pairs of C—H⋯S hydrogen bonds, in which only the discrete complex built up of Co2 is involved (Fig. 6 ▸ and Table 3 ▸).
Table 3. Hydrogen-bond geometry (Å, °) for 1 .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1⋯N31 | 0.88 (2) | 1.92 (2) | 2.793 (2) | 170 (3) |
| C4′—H4′A⋯N43i | 0.96 | 2.50 | 3.243 (14) | 134 |
| C4′—H4′C⋯N44ii | 0.96 | 2.38 | 3.161 (10) | 138 |
| O2—H2A⋯N41 | 0.87 (2) | 1.88 (2) | 2.743 (7) | 173 (7) |
| O2—H2B⋯O3 | 0.87 (2) | 1.80 (2) | 2.665 (4) | 177 (5) |
| C5—H5A⋯S2iii | 0.97 | 3.02 | 3.925 (3) | 156 |
| O3—H3⋯N34 | 0.87 (2) | 1.97 (2) | 2.821 (3) | 167 (4) |
| O4—H4⋯N41 | 0.87 (2) | 1.94 (5) | 2.81 (3) | 170 (19) |
| C11—H11A⋯O1ii | 0.97 | 2.49 | 3.058 (2) | 117 |
| C11—H11B⋯N1 | 0.97 | 2.67 | 3.213 (2) | 116 |
| C12—H12B⋯N44 | 0.97 | 2.64 | 3.423 (3) | 138 |
| C13—H13A⋯N13iv | 0.97 | 2.70 | 3.563 (2) | 149 |
| C13—H13B⋯S2iii | 0.97 | 2.95 | 3.7150 (19) | 136 |
| C14—H14A⋯S2v | 0.97 | 2.93 | 3.840 (2) | 156 |
| C15—H15B⋯O1 | 0.97 | 2.61 | 3.118 (2) | 113 |
| C22—H22B⋯N12vi | 0.97 | 2.58 | 3.448 (2) | 149 |
| C25—H25A⋯O2vii | 0.97 | 2.50 | 3.026 (7) | 114 |
| C25—H25A⋯O4vii | 0.97 | 2.49 | 3.08 (3) | 119 |
| C25—H25B⋯N2 | 0.97 | 2.61 | 3.202 (3) | 119 |
| C26—H26A⋯S1ii | 0.97 | 2.98 | 3.655 (2) | 128 |
| C26—H26B⋯N22viii | 0.97 | 2.69 | 3.581 (3) | 152 |
| C33—H33A⋯N23 | 0.97 | 2.66 | 3.431 (3) | 137 |
| C45—H45A⋯S2vii | 0.97 | 3.01 | 3.959 (3) | 165 |
Symmetry codes: (i)
 ; (ii)
; (ii)
 ; (iii)
; (iii)
 ; (iv)
; (iv)
 ; (v)
; (v)
 ; (vi)
; (vi)
 ; (vii)
; (vii)
 ; (viii)
; (viii)
 .
.
Figure 3.
View of the discrete complex in compound 1 built up of Co1, which is connected to two urotropine solvate molecules via intermolecular O—H⋯N hydrogen bonding (shown as dashed lines).
Figure 4.
View of the two different coordinations of Co2 in compound 1 with H2O (top) and ethanol (bottom) with intermolecular hydrogen bonding shown as dashed lines.
Figure 5.
Part of the crystal structure of compound 1 showing the connection of the discrete complexes by the urotropine solvate molecules via intermolecular O—H⋯N hydrogen bonding (shown as dashed lines).
Figure 6.
Crystal structure of compound 1 with a view along the crystallographic b axis and intermolecular hydrogen bonding shown as dashed lines.
In the crystal structure of compound 2, the discrete complexes are linked by strong intermolecular O—H⋯N hydrogen bonding between two of the three O—H hydrogen atoms of the ethanol ligands and two urotropine N atoms into layers that are parallel to the bc plane (Fig. 7 ▸ and Table 4 ▸). These layers are further linked by intermolecular O—H⋯S and C—H⋯S hydrogen bonding into a three-dimensional network (Table 4 ▸). Some of the O—H⋯S and C—H⋯S angles are close to linearity, indicating that these are relatively strong interactions (Table 4 ▸).
Figure 7.
Crystal structure of compound 2 with a view along the crystallographic a axis and intermolecular O—H⋯N hydrogen bonding shown as dashed lines.
Table 4. Hydrogen-bond geometry (Å, °) for 2 .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C12—H12A⋯S2i | 0.99 | 2.87 | 3.6586 (13) | 137 |
| C12—H12B⋯S1ii | 0.99 | 2.92 | 3.8813 (13) | 164 |
| C15—H15A⋯S1iii | 0.99 | 2.99 | 3.9387 (13) | 161 |
| C15—H15B⋯S2iv | 0.99 | 2.94 | 3.7110 (13) | 135 |
| C16—H16A⋯O21 | 0.99 | 2.54 | 3.1009 (16) | 116 |
| C16—H16B⋯N1 | 0.99 | 2.47 | 3.1083 (17) | 122 |
| O21—H21⋯N13ii | 0.84 | 2.03 | 2.8424 (14) | 161 |
| C22—H22C⋯S1v | 0.98 | 3.02 | 3.9559 (16) | 161 |
| O31—H31⋯N12vi | 0.84 | 1.96 | 2.7969 (14) | 172 |
| O41—H41⋯S2vi | 0.84 | 2.37 | 3.2080 (10) | 174 |
Symmetry codes: (i)
 ; (ii)
; (ii)
 ; (iii)
; (iii)
 ; (iv)
; (iv)
 ; (v)
; (v)
 ; (vi)
; (vi)
 .
.
Database survey
In the Cambridge Structure Database (CSD version 5.42, last update November 2020; Groom et al., 2016 ▸) there are already several structures reported that contain cobalt thiocyanate and urotropine as a ligand, but only one of them contains additional ethanol (Krebs et al., 2021a). Most of them contain water as a ligand or solvate molecule. In [Co(NCS)2(H2O)4]·2urotropine (Refcode: XILXOG; Li et al., 2007 ▸), the cobalt cations are octahedrally coordinated by two thiocyanate anions and four water ligands with two additional urotropine ligands acting as solvate molecules. [Co(NCS)2(urotropine)2(H2O)2][Co(NCS)2(H2O)4]·2H2O (Refcode: MOTNIS; Liu et al., 2002 ▸, MOTNIS01; Zhang et al., 1999 ▸, MOTNIS02; Chakraborty et al., 2006 ▸, MOTNIS03; Lu et al., 2010 ▸) consists of two crystallographically independent discrete complexes in which the cobalt cations are coordinated by two terminal N-bonded thiocyanate anions and four water or two water and two urotropine ligands with additional water as solvate molecules. There is also one complex with water and methanol as ligands with the composition [Co(NCS)2(urotropine)(CH3OH)2(H2O)] (Refcode: POFGAT; Shang et al., 2008 ▸), in which the cobalt cations are octahedrally coordinated by the N atoms of two thiocyanate anions, two methanol, one water and one urotropine ligand. Moreover, a compound with the composition [Co(NCS)2(urotropine)2(CH3CN)2] that also consists of discrete complexes has been reported (Krebs et al., 2021 ▸). It is noted that even with other metal cations only discrete complexes are reported, such as, for example, with nickel (Refcode: XILROA; Bai et al., 2007 ▸, XILROA01; Lu et al., 2010 ▸), or zinc (Refcode: SIMXIY; Kruszynski et al., 2018). Finally, a crystal structure is reported with cadmium in which the Cd cations are linked by pairs of thiocyanate anions into chains, which are further linked by the urotropine ligand (Refcode: DOZZOI; Bai et al., 2009 ▸).
Synthesis and crystallization
Synthesis Co(NCS)2 and urotropine were purchased from Merck. All chemicals were used without further purification.
Crystals of compound 1 suitable for single-crystal X-ray diffraction were obtained after one day by the reaction of 0.15 mmol of Co(NCS)2 (26.3 mg) with 0.60 mmol of urotropine (84.1 mg) in 1.0 mL of ethanol at room temperature. The reaction of 0.15 mmol of Co(NCS)2 (26.3 mg) with 0.15 mmol of urotropine (21.0 mg) in 2.0 mL of ethanol at room temperature led to the formation of single crystals of compound 2.
The data collection for single-crystal structure analysis was performed using an XtaLAB Synergy, Dualflex, HyPix diffractometer from Rigaku with Cu Kα radiation.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 5 ▸. All non-hydrogen atoms were refined anisotropically. The C—H hydrogen atoms were located in the difference map but positioned with idealized geometry (methyl H atoms allowed to rotate but not to tip) and were refined isotropically with U iso(H) = 1.2U eq(C) (1.5 for methyl H atoms) using a riding model. The O—H hydrogen atoms were located in the difference map and were refined with restraints for the O—H distance (DFIX) and varying isotropic displacement parameters in compound 1, whereas in compound 2 they were positioned with idealized geometry allowed to rotate but not to tip and were refined isotropically with U iso(H) = 1.5U eq(O) using a riding model. In compound 1, the methyl group of the EtOH molecule coordinated to Co1 is disordered and was refined using a split model. In this compound, Co2 is either coordinated to water or to EtOH. In this case the O atoms occupy nearly the same crystallographic positions but finally both O atoms can be refined separately with anisotropic displacement parameters. In the case where Co2 is coordinated to water, it is hydrogen bonded to one EtOH solvate molecule. If Co2 is coordinated to EtOH, the position of the EtOH solvate molecule cannot be occupied. Therefore, the site occupation factor (sof) of the EtOH solvate molecule must be identical to that of the coordinated water molecule. In the beginning the sof was refined, leading to values close to 0.8 for the water and 0.2 for the coordinated EtOH molecule but in the final refinements it was fixed at 0.8 and 0.2. The H-atom positions of both, water and EtOH, were clearly located and were refined with restraints and varying isotropic displacement parameters. This leads to comparable and reasonable values for the O—H distances as well as for the isotropic displacement parameters of the O—H hydrogen atoms.
Table 5. Experimental details.
| 1 | 2 | |
|---|---|---|
| Crystal data | ||
| Chemical formula | [Co(NCS)2(C6H12N4)2(C2H6O)2]1.2·[Co(NCS)2(C6H12N4)2(H2O)2]0.8·1.6C2H6O·4C6H12N4 | [Co(NCS)2(C6H12N4)(C2H6O)3] |
| M r | 1684.84 | 453.49 |
| Crystal system, space group | Triclinic, P

|
Monoclinic, P21/n |
| Temperature (K) | 100 | 100 |
| a, b, c (Å) | 12.1536 (2), 12.9256 (3), 12.9374 (3) | 11.1463 (1), 15.7705 (1), 12.1824 (1) |
| α, β, γ (°) | 76.629 (2), 80.395 (2), 80.578 (2) | 90, 103.886 (1), 90 |
| V (Å3) | 1932.91 (7) | 2078.87 (3) |
| Z | 1 | 4 |
| Radiation type | Cu Kα | Cu Kα |
| μ (mm−1) | 4.97 | 8.58 |
| Crystal size (mm) | 0.16 × 0.12 × 0.08 | 0.2 × 0.18 × 0.03 |
| Data collection | ||
| Diffractometer | XtaLAB Synergy, Dualflex, HyPix | XtaLAB Synergy, Dualflex, HyPix |
| Absorption correction | Multi-scan (CrysAlis PRO; Rigaku OD, 2021 ▸) | Multi-scan (CrysAlis PRO; Rigaku OD, 2021 ▸) |
| T min, T max | 0.693, 1.000 | 0.427, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 25821, 8226, 7777 | 29441, 4431, 4373 |
| R int | 0.024 | 0.027 |
| (sin θ/λ)max (Å−1) | 0.639 | 0.635 |
| Refinement | ||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.040, 0.103, 1.09 | 0.025, 0.068, 1.08 |
| No. of reflections | 8226 | 4431 |
| No. of parameters | 545 | 242 |
| No. of restraints | 10 | 1 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.82, −0.69 | 0.32, −0.31 |
Supplementary Material
Crystal structure: contains datablock(s) 1, 2. DOI: 10.1107/S2056989022001037/tx2047sup1.cif
Structure factors: contains datablock(s) 1. DOI: 10.1107/S2056989022001037/tx20471sup2.hkl
Structure factors: contains datablock(s) 2. DOI: 10.1107/S2056989022001037/tx20472sup3.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
This project was supported by the State of Schleswig-Holstein and the Deutsche Forschungsgemeinschaft.
supplementary crystallographic information
Bis(ethanol-κO)bis(hexamethylenetetramine-κN)bis(thiocyanato-κN)cobalt(II)–diaqua-κ2O-bis(hexamethylenetetramine-κN)bis(thiocyanato-κN)cobalt(II)–ethanol–hexamethylenetetramine (1.2/0.8/1.6/4) (1) . Crystal data
| [Co(NCS)2(C6H12N4)2(C2H6O)2]1.2·[Co(NCS)2(C6H12N4)2(H2O)2]0.8·1.6C2H6O·4C6H12N4 | Z = 1 |
| Mr = 1684.84 | F(000) = 898 |
| Triclinic, P1 | Dx = 1.447 Mg m−3 |
| a = 12.1536 (2) Å | Cu Kα radiation, λ = 1.54178 Å |
| b = 12.9256 (3) Å | Cell parameters from 18138 reflections |
| c = 12.9374 (3) Å | θ = 3.7–79.3° |
| α = 76.629 (2)° | µ = 4.97 mm−1 |
| β = 80.395 (2)° | T = 100 K |
| γ = 80.578 (2)° | Plate, light colourless |
| V = 1932.91 (7) Å3 | 0.16 × 0.12 × 0.08 mm |
Bis(ethanol-κO)bis(hexamethylenetetramine-κN)bis(thiocyanato-κN)cobalt(II)–diaqua-κ2O-bis(hexamethylenetetramine-κN)bis(thiocyanato-κN)cobalt(II)–ethanol–hexamethylenetetramine (1.2/0.8/1.6/4) (1) . Data collection
| XtaLAB Synergy, Dualflex, HyPix diffractometer | 8226 independent reflections |
| Radiation source: micro-focus sealed X-ray tube, PhotonJet (Cu) X-ray Source | 7777 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.024 |
| Detector resolution: 10.0000 pixels mm-1 | θmax = 80.1°, θmin = 3.5° |
| ω scans | h = −15→15 |
| Absorption correction: multi-scan (CrysAlisPro; Rigaku OD, 2021) | k = −16→13 |
| Tmin = 0.693, Tmax = 1.000 | l = −16→16 |
| 25821 measured reflections |
Bis(ethanol-κO)bis(hexamethylenetetramine-κN)bis(thiocyanato-κN)cobalt(II)–diaqua-κ2O-bis(hexamethylenetetramine-κN)bis(thiocyanato-κN)cobalt(II)–ethanol–hexamethylenetetramine (1.2/0.8/1.6/4) (1) . Refinement
| Refinement on F2 | Hydrogen site location: mixed |
| Least-squares matrix: full | H atoms treated by a mixture of independent and constrained refinement |
| R[F2 > 2σ(F2)] = 0.040 | w = 1/[σ2(Fo2) + (0.0432P)2 + 1.7899P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.103 | (Δ/σ)max = 0.001 |
| S = 1.09 | Δρmax = 0.82 e Å−3 |
| 8226 reflections | Δρmin = −0.69 e Å−3 |
| 545 parameters | Extinction correction: SHELXL2016/6 (Sheldrick 2015b), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 10 restraints | Extinction coefficient: 0.00080 (10) |
| Primary atom site location: dual |
Bis(ethanol-κO)bis(hexamethylenetetramine-κN)bis(thiocyanato-κN)cobalt(II)–diaqua-κ2O-bis(hexamethylenetetramine-κN)bis(thiocyanato-κN)cobalt(II)–ethanol–hexamethylenetetramine (1.2/0.8/1.6/4) (1) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Bis(ethanol-κO)bis(hexamethylenetetramine-κN)bis(thiocyanato-κN)cobalt(II)–diaqua-κ2O-bis(hexamethylenetetramine-κN)bis(thiocyanato-κN)cobalt(II)–ethanol–hexamethylenetetramine (1.2/0.8/1.6/4) (1) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Co1 | 0.500000 | 0.000000 | 1.000000 | 0.01667 (10) | |
| Co2 | 1.000000 | 0.500000 | 0.500000 | 0.01929 (11) | |
| N1 | 0.38177 (14) | −0.10448 (13) | 1.03166 (13) | 0.0209 (3) | |
| C1 | 0.31474 (16) | −0.15722 (15) | 1.02515 (15) | 0.0219 (4) | |
| S1 | 0.22329 (5) | −0.23250 (5) | 1.01546 (5) | 0.03988 (15) | |
| N2 | 0.85107 (14) | 0.59836 (13) | 0.46671 (13) | 0.0238 (3) | |
| C2 | 0.76974 (17) | 0.65684 (15) | 0.45319 (15) | 0.0226 (4) | |
| S2 | 0.65758 (5) | 0.74450 (5) | 0.43091 (5) | 0.03506 (14) | |
| O1 | 0.37719 (11) | 0.13161 (11) | 0.94616 (11) | 0.0216 (3) | |
| H1 | 0.392 (3) | 0.1982 (16) | 0.927 (3) | 0.060 (10)* | |
| C3 | 0.26483 (18) | 0.13151 (18) | 0.91942 (18) | 0.0294 (4) | |
| H3AA | 0.250342 | 0.057923 | 0.930034 | 0.044* | 0.8 |
| H3AB | 0.262932 | 0.164417 | 0.844216 | 0.044* | 0.8 |
| H3BC | 0.250431 | 0.196444 | 0.865692 | 0.044* | 0.2 |
| H3BD | 0.271029 | 0.072225 | 0.883622 | 0.044* | 0.2 |
| C4 | 0.1725 (2) | 0.1909 (2) | 0.98618 (19) | 0.0200 (4) | 0.8 |
| H4A | 0.100686 | 0.185071 | 0.968034 | 0.030* | 0.8 |
| H4B | 0.183149 | 0.265097 | 0.971950 | 0.030* | 0.8 |
| H4C | 0.175324 | 0.160103 | 1.060858 | 0.030* | 0.8 |
| C4' | 0.1630 (6) | 0.1249 (14) | 0.9957 (8) | 0.047 (3) | 0.2 |
| H4'A | 0.104113 | 0.109715 | 0.962611 | 0.071* | 0.2 |
| H4'B | 0.140682 | 0.191965 | 1.018112 | 0.071* | 0.2 |
| H4'C | 0.176793 | 0.068708 | 1.056935 | 0.071* | 0.2 |
| O2 | 0.9172 (3) | 0.3693 (6) | 0.5467 (6) | 0.0215 (6) | 0.8 |
| H2A | 0.939 (5) | 0.308 (3) | 0.588 (5) | 0.07 (3)* | 0.8 |
| H2B | 0.8447 (17) | 0.372 (4) | 0.548 (4) | 0.077 (18)* | 0.8 |
| O3 | 0.69673 (15) | 0.37997 (14) | 0.54414 (15) | 0.0275 (4) | 0.8 |
| C5 | 0.6645 (2) | 0.2810 (2) | 0.5350 (2) | 0.0267 (5) | 0.8 |
| H5A | 0.586260 | 0.291950 | 0.523215 | 0.032* | 0.8 |
| H5B | 0.672275 | 0.228069 | 0.600949 | 0.032* | 0.8 |
| C6 | 0.7380 (2) | 0.2411 (2) | 0.4427 (2) | 0.0298 (5) | 0.8 |
| H6A | 0.731836 | 0.294723 | 0.378049 | 0.045* | 0.8 |
| H6B | 0.714164 | 0.176471 | 0.434837 | 0.045* | 0.8 |
| H6C | 0.814805 | 0.226809 | 0.456517 | 0.045* | 0.8 |
| H3 | 0.656 (3) | 0.406 (3) | 0.597 (2) | 0.054 (11)* | 0.8 |
| O4 | 0.8970 (16) | 0.367 (3) | 0.543 (3) | 0.0215 (6) | 0.2 |
| C7 | 0.7799 (8) | 0.3562 (8) | 0.5603 (9) | 0.026 (2) | 0.2 |
| H7A | 0.736856 | 0.426993 | 0.546472 | 0.032* | 0.2 |
| H7B | 0.759133 | 0.323060 | 0.634838 | 0.032* | 0.2 |
| C8 | 0.7500 (9) | 0.2899 (8) | 0.4899 (8) | 0.030 (2) | 0.2 |
| H8A | 0.762768 | 0.326382 | 0.416213 | 0.046* | 0.2 |
| H8B | 0.672194 | 0.279629 | 0.508926 | 0.046* | 0.2 |
| H8C | 0.796095 | 0.221486 | 0.499688 | 0.046* | 0.2 |
| H4 | 0.930 (14) | 0.306 (9) | 0.574 (17) | 0.02 (5)* | 0.2 |
| N11 | 0.53970 (13) | −0.04050 (12) | 0.83394 (12) | 0.0169 (3) | |
| N12 | 0.59878 (13) | −0.18558 (13) | 0.73316 (12) | 0.0199 (3) | |
| N13 | 0.46941 (13) | −0.02933 (13) | 0.66262 (12) | 0.0201 (3) | |
| N14 | 0.66683 (13) | −0.01109 (12) | 0.66326 (12) | 0.0189 (3) | |
| C11 | 0.57045 (16) | −0.15859 (14) | 0.83915 (14) | 0.0193 (4) | |
| H11A | 0.634285 | −0.184309 | 0.878516 | 0.023* | |
| H11B | 0.507809 | −0.195364 | 0.878363 | 0.023* | |
| C12 | 0.63690 (15) | 0.01248 (15) | 0.77084 (14) | 0.0189 (4) | |
| H12A | 0.618761 | 0.089443 | 0.764292 | 0.023* | |
| H12B | 0.701533 | −0.011012 | 0.809414 | 0.023* | |
| C13 | 0.56767 (16) | 0.02352 (15) | 0.60617 (15) | 0.0213 (4) | |
| H13A | 0.586413 | 0.007641 | 0.535122 | 0.026* | |
| H13B | 0.548205 | 0.100566 | 0.597793 | 0.026* | |
| C14 | 0.50120 (16) | −0.14575 (16) | 0.67457 (15) | 0.0220 (4) | |
| H14A | 0.518813 | −0.163034 | 0.604076 | 0.026* | |
| H14B | 0.437746 | −0.181983 | 0.712554 | 0.026* | |
| C15 | 0.44349 (16) | −0.00438 (15) | 0.76983 (15) | 0.0205 (4) | |
| H15A | 0.378951 | −0.038674 | 0.808307 | 0.025* | |
| H15B | 0.423192 | 0.072471 | 0.762594 | 0.025* | |
| C16 | 0.69351 (16) | −0.12818 (15) | 0.67461 (15) | 0.0208 (4) | |
| H16A | 0.758527 | −0.153184 | 0.712455 | 0.025* | |
| H16B | 0.713010 | −0.144866 | 0.603934 | 0.025* | |
| N21 | 0.96389 (13) | 0.53277 (12) | 0.66888 (12) | 0.0185 (3) | |
| N22 | 1.03238 (14) | 0.51130 (13) | 0.84249 (13) | 0.0209 (3) | |
| N23 | 0.83260 (14) | 0.50888 (13) | 0.83676 (13) | 0.0211 (3) | |
| N24 | 0.91408 (14) | 0.67678 (13) | 0.77182 (13) | 0.0207 (3) | |
| C21 | 0.86158 (16) | 0.48569 (15) | 0.72915 (15) | 0.0206 (4) | |
| H21A | 0.798587 | 0.514069 | 0.689148 | 0.025* | |
| H21B | 0.874416 | 0.408596 | 0.735116 | 0.025* | |
| C22 | 0.81436 (16) | 0.62564 (15) | 0.82683 (16) | 0.0221 (4) | |
| H22A | 0.794956 | 0.641618 | 0.897807 | 0.027* | |
| H22B | 0.751325 | 0.655797 | 0.787314 | 0.027* | |
| C23 | 1.00907 (16) | 0.62886 (15) | 0.83182 (16) | 0.0226 (4) | |
| H23A | 0.992555 | 0.645241 | 0.902716 | 0.027* | |
| H23B | 1.075775 | 0.660737 | 0.795445 | 0.027* | |
| C24 | 1.05778 (16) | 0.48863 (15) | 0.73384 (15) | 0.0201 (4) | |
| H24A | 1.073004 | 0.411640 | 0.739378 | 0.024* | |
| H24B | 1.125119 | 0.519334 | 0.697337 | 0.024* | |
| C25 | 0.94127 (16) | 0.65112 (14) | 0.66518 (15) | 0.0205 (4) | |
| H25A | 1.007066 | 0.683951 | 0.627731 | 0.025* | |
| H25B | 0.879088 | 0.681840 | 0.624686 | 0.025* | |
| C26 | 0.92924 (16) | 0.46570 (15) | 0.89559 (15) | 0.0217 (4) | |
| H26A | 0.942690 | 0.388452 | 0.902783 | 0.026* | |
| H26B | 0.911220 | 0.480513 | 0.967089 | 0.026* | |
| N31 | 0.41819 (13) | 0.34324 (13) | 0.86223 (13) | 0.0213 (3) | |
| N32 | 0.34067 (15) | 0.51211 (14) | 0.75097 (14) | 0.0266 (4) | |
| N33 | 0.47210 (17) | 0.51353 (15) | 0.87450 (15) | 0.0325 (4) | |
| N34 | 0.53923 (15) | 0.44041 (15) | 0.71314 (14) | 0.0289 (4) | |
| C31 | 0.31950 (17) | 0.40495 (16) | 0.81169 (17) | 0.0266 (4) | |
| H31A | 0.299473 | 0.365307 | 0.763932 | 0.032* | |
| H31B | 0.255972 | 0.411911 | 0.867126 | 0.032* | |
| C32 | 0.3720 (2) | 0.56912 (17) | 0.82560 (18) | 0.0329 (5) | |
| H32A | 0.386349 | 0.640567 | 0.787339 | 0.040* | |
| H32B | 0.309371 | 0.576543 | 0.881808 | 0.040* | |
| C33 | 0.56536 (19) | 0.50044 (19) | 0.78861 (19) | 0.0349 (5) | |
| H33A | 0.631573 | 0.462773 | 0.820177 | 0.042* | |
| H33B | 0.582728 | 0.570673 | 0.749263 | 0.042* | |
| C34 | 0.51269 (18) | 0.33500 (16) | 0.77563 (16) | 0.0272 (4) | |
| H34A | 0.578777 | 0.295933 | 0.806522 | 0.033* | |
| H34B | 0.494052 | 0.294447 | 0.727982 | 0.033* | |
| C35 | 0.43694 (18) | 0.49921 (17) | 0.66807 (17) | 0.0291 (4) | |
| H35A | 0.452907 | 0.569471 | 0.627554 | 0.035* | |
| H35B | 0.417450 | 0.460785 | 0.618972 | 0.035* | |
| C36 | 0.44831 (19) | 0.40664 (17) | 0.93320 (16) | 0.0275 (4) | |
| H36A | 0.386711 | 0.413627 | 0.990439 | 0.033* | |
| H36B | 0.514013 | 0.368715 | 0.965490 | 0.033* | |
| N41 | 0.98222 (14) | 0.16814 (13) | 0.66156 (13) | 0.0236 (3) | |
| N42 | 1.07891 (16) | −0.00576 (15) | 0.62924 (17) | 0.0350 (4) | |
| N43 | 1.09861 (17) | 0.05352 (15) | 0.79123 (17) | 0.0379 (5) | |
| N44 | 0.92004 (15) | 0.00149 (14) | 0.77178 (14) | 0.0276 (4) | |
| C41 | 1.04120 (19) | 0.10589 (18) | 0.58097 (18) | 0.0316 (5) | |
| H41A | 0.990963 | 0.107214 | 0.529629 | 0.038* | |
| H41B | 1.105888 | 0.139850 | 0.542356 | 0.038* | |
| C42 | 1.15409 (19) | −0.00508 (19) | 0.7069 (2) | 0.0420 (6) | |
| H42A | 1.219661 | 0.028026 | 0.669308 | 0.050* | |
| H42B | 1.179667 | −0.078443 | 0.740249 | 0.050* | |
| C43 | 0.9988 (2) | 0.00220 (19) | 0.84575 (19) | 0.0358 (5) | |
| H43A | 1.022883 | −0.071033 | 0.880625 | 0.043* | |
| H43B | 0.960481 | 0.040129 | 0.900804 | 0.043* | |
| C44 | 0.88530 (17) | 0.11291 (16) | 0.71995 (17) | 0.0260 (4) | |
| H44A | 0.845718 | 0.151596 | 0.773986 | 0.031* | |
| H44B | 0.833555 | 0.113901 | 0.670081 | 0.031* | |
| C45 | 1.0595 (2) | 0.16406 (17) | 0.7394 (2) | 0.0348 (5) | |
| H45A | 1.124033 | 0.199417 | 0.702869 | 0.042* | |
| H45B | 1.021402 | 0.202857 | 0.793862 | 0.042* | |
| C46 | 0.98007 (19) | −0.05437 (17) | 0.68850 (19) | 0.0326 (5) | |
| H46A | 0.928905 | −0.054578 | 0.638482 | 0.039* | |
| H46B | 1.003704 | −0.128358 | 0.721415 | 0.039* |
Bis(ethanol-κO)bis(hexamethylenetetramine-κN)bis(thiocyanato-κN)cobalt(II)–diaqua-κ2O-bis(hexamethylenetetramine-κN)bis(thiocyanato-κN)cobalt(II)–ethanol–hexamethylenetetramine (1.2/0.8/1.6/4) (1) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Co1 | 0.0194 (2) | 0.0162 (2) | 0.0148 (2) | −0.00317 (15) | −0.00286 (15) | −0.00303 (15) |
| Co2 | 0.0223 (2) | 0.0144 (2) | 0.0185 (2) | 0.00176 (16) | −0.00397 (16) | −0.00022 (16) |
| N1 | 0.0246 (8) | 0.0200 (7) | 0.0189 (7) | −0.0060 (6) | −0.0018 (6) | −0.0045 (6) |
| C1 | 0.0258 (9) | 0.0193 (9) | 0.0184 (9) | −0.0004 (7) | −0.0006 (7) | −0.0029 (7) |
| S1 | 0.0345 (3) | 0.0400 (3) | 0.0532 (4) | −0.0172 (2) | 0.0012 (3) | −0.0224 (3) |
| N2 | 0.0247 (8) | 0.0206 (8) | 0.0232 (8) | 0.0042 (6) | −0.0050 (6) | −0.0025 (6) |
| C2 | 0.0267 (10) | 0.0226 (9) | 0.0199 (9) | −0.0029 (8) | −0.0031 (7) | −0.0075 (7) |
| S2 | 0.0283 (3) | 0.0407 (3) | 0.0403 (3) | 0.0136 (2) | −0.0156 (2) | −0.0217 (2) |
| O1 | 0.0244 (7) | 0.0192 (6) | 0.0222 (6) | −0.0027 (5) | −0.0082 (5) | −0.0030 (5) |
| C3 | 0.0272 (10) | 0.0309 (11) | 0.0322 (11) | −0.0013 (8) | −0.0083 (8) | −0.0091 (9) |
| C4 | 0.0190 (11) | 0.0222 (12) | 0.0184 (11) | −0.0034 (10) | −0.0006 (8) | −0.0044 (10) |
| C4' | 0.042 (7) | 0.059 (10) | 0.040 (7) | −0.022 (7) | −0.012 (6) | 0.006 (7) |
| O2 | 0.0187 (17) | 0.0192 (8) | 0.0251 (10) | 0.0002 (16) | −0.0061 (15) | −0.0012 (7) |
| O3 | 0.0245 (10) | 0.0260 (9) | 0.0324 (10) | −0.0041 (7) | −0.0003 (7) | −0.0087 (7) |
| C5 | 0.0244 (13) | 0.0246 (12) | 0.0298 (13) | −0.0041 (10) | −0.0060 (10) | −0.0004 (10) |
| C6 | 0.0291 (13) | 0.0293 (13) | 0.0308 (14) | −0.0040 (11) | −0.0050 (11) | −0.0052 (11) |
| O4 | 0.0187 (17) | 0.0192 (8) | 0.0251 (10) | 0.0002 (16) | −0.0061 (15) | −0.0012 (7) |
| C7 | 0.013 (5) | 0.028 (5) | 0.035 (5) | 0.003 (4) | 0.002 (4) | −0.008 (4) |
| C8 | 0.032 (6) | 0.024 (5) | 0.028 (5) | −0.001 (4) | −0.006 (4) | 0.007 (4) |
| N11 | 0.0173 (7) | 0.0173 (7) | 0.0149 (7) | −0.0003 (6) | −0.0035 (5) | −0.0016 (6) |
| N12 | 0.0233 (8) | 0.0198 (7) | 0.0168 (7) | 0.0004 (6) | −0.0046 (6) | −0.0051 (6) |
| N13 | 0.0207 (8) | 0.0226 (8) | 0.0175 (7) | 0.0011 (6) | −0.0057 (6) | −0.0060 (6) |
| N14 | 0.0203 (7) | 0.0204 (8) | 0.0154 (7) | −0.0004 (6) | −0.0030 (6) | −0.0041 (6) |
| C11 | 0.0247 (9) | 0.0165 (8) | 0.0163 (8) | −0.0009 (7) | −0.0039 (7) | −0.0033 (7) |
| C12 | 0.0204 (9) | 0.0208 (9) | 0.0152 (8) | −0.0024 (7) | −0.0023 (7) | −0.0033 (7) |
| C13 | 0.0233 (9) | 0.0225 (9) | 0.0165 (8) | 0.0017 (7) | −0.0057 (7) | −0.0022 (7) |
| C14 | 0.0227 (9) | 0.0242 (9) | 0.0214 (9) | −0.0016 (7) | −0.0064 (7) | −0.0076 (7) |
| C15 | 0.0193 (9) | 0.0237 (9) | 0.0188 (9) | 0.0012 (7) | −0.0044 (7) | −0.0067 (7) |
| C16 | 0.0194 (9) | 0.0222 (9) | 0.0199 (9) | 0.0026 (7) | −0.0042 (7) | −0.0053 (7) |
| N21 | 0.0185 (7) | 0.0153 (7) | 0.0200 (7) | −0.0003 (6) | −0.0041 (6) | −0.0004 (6) |
| N22 | 0.0226 (8) | 0.0189 (7) | 0.0209 (8) | 0.0001 (6) | −0.0060 (6) | −0.0031 (6) |
| N23 | 0.0215 (8) | 0.0212 (8) | 0.0197 (8) | −0.0026 (6) | −0.0032 (6) | −0.0024 (6) |
| N24 | 0.0226 (8) | 0.0186 (7) | 0.0198 (8) | −0.0002 (6) | −0.0033 (6) | −0.0032 (6) |
| C21 | 0.0208 (9) | 0.0192 (9) | 0.0210 (9) | −0.0024 (7) | −0.0036 (7) | −0.0023 (7) |
| C22 | 0.0197 (9) | 0.0217 (9) | 0.0232 (9) | 0.0012 (7) | −0.0032 (7) | −0.0041 (7) |
| C23 | 0.0232 (9) | 0.0202 (9) | 0.0251 (9) | −0.0020 (7) | −0.0073 (7) | −0.0041 (7) |
| C24 | 0.0192 (8) | 0.0188 (8) | 0.0210 (9) | 0.0008 (7) | −0.0044 (7) | −0.0028 (7) |
| C25 | 0.0246 (9) | 0.0154 (8) | 0.0197 (9) | −0.0004 (7) | −0.0031 (7) | −0.0014 (7) |
| C26 | 0.0239 (9) | 0.0197 (9) | 0.0196 (9) | −0.0017 (7) | −0.0049 (7) | 0.0003 (7) |
| N31 | 0.0206 (8) | 0.0206 (8) | 0.0227 (8) | −0.0029 (6) | −0.0045 (6) | −0.0032 (6) |
| N32 | 0.0251 (8) | 0.0217 (8) | 0.0312 (9) | −0.0001 (7) | −0.0056 (7) | −0.0025 (7) |
| N33 | 0.0441 (11) | 0.0273 (9) | 0.0303 (9) | −0.0127 (8) | −0.0096 (8) | −0.0056 (7) |
| N34 | 0.0246 (8) | 0.0307 (9) | 0.0258 (9) | −0.0009 (7) | −0.0019 (7) | 0.0022 (7) |
| C31 | 0.0219 (9) | 0.0241 (10) | 0.0332 (11) | −0.0022 (8) | −0.0071 (8) | −0.0027 (8) |
| C32 | 0.0431 (13) | 0.0203 (10) | 0.0345 (11) | −0.0020 (9) | −0.0035 (9) | −0.0068 (8) |
| C33 | 0.0317 (11) | 0.0337 (11) | 0.0385 (12) | −0.0148 (9) | −0.0111 (9) | 0.0056 (9) |
| C34 | 0.0277 (10) | 0.0249 (10) | 0.0251 (10) | 0.0040 (8) | −0.0026 (8) | −0.0031 (8) |
| C35 | 0.0299 (11) | 0.0288 (10) | 0.0247 (10) | 0.0005 (8) | −0.0057 (8) | 0.0007 (8) |
| C36 | 0.0343 (11) | 0.0281 (10) | 0.0221 (9) | −0.0087 (8) | −0.0045 (8) | −0.0054 (8) |
| N41 | 0.0239 (8) | 0.0223 (8) | 0.0236 (8) | −0.0054 (6) | −0.0049 (6) | 0.0000 (6) |
| N42 | 0.0301 (10) | 0.0273 (9) | 0.0417 (11) | 0.0018 (7) | 0.0013 (8) | −0.0037 (8) |
| N43 | 0.0397 (11) | 0.0287 (10) | 0.0452 (11) | −0.0112 (8) | −0.0241 (9) | 0.0100 (8) |
| N44 | 0.0273 (9) | 0.0219 (8) | 0.0310 (9) | −0.0063 (7) | −0.0025 (7) | 0.0009 (7) |
| C41 | 0.0304 (11) | 0.0307 (11) | 0.0281 (10) | −0.0011 (9) | 0.0019 (8) | −0.0010 (9) |
| C42 | 0.0240 (11) | 0.0309 (12) | 0.0623 (17) | −0.0016 (9) | −0.0088 (10) | 0.0084 (11) |
| C43 | 0.0447 (13) | 0.0295 (11) | 0.0302 (11) | −0.0087 (10) | −0.0126 (10) | 0.0075 (9) |
| C44 | 0.0246 (10) | 0.0231 (9) | 0.0276 (10) | −0.0028 (8) | −0.0034 (8) | −0.0004 (8) |
| C45 | 0.0420 (13) | 0.0243 (10) | 0.0404 (12) | −0.0122 (9) | −0.0219 (10) | 0.0056 (9) |
| C46 | 0.0343 (11) | 0.0199 (10) | 0.0434 (13) | −0.0050 (8) | −0.0042 (10) | −0.0059 (9) |
Bis(ethanol-κO)bis(hexamethylenetetramine-κN)bis(thiocyanato-κN)cobalt(II)–diaqua-κ2O-bis(hexamethylenetetramine-κN)bis(thiocyanato-κN)cobalt(II)–ethanol–hexamethylenetetramine (1.2/0.8/1.6/4) (1) . Geometric parameters (Å, º)
| Co1—N1i | 2.0590 (16) | C16—H16B | 0.9700 |
| Co1—N1 | 2.0590 (16) | N21—C21 | 1.493 (2) |
| Co1—O1i | 2.1388 (13) | N21—C24 | 1.490 (2) |
| Co1—O1 | 2.1388 (13) | N21—C25 | 1.500 (2) |
| Co1—N11i | 2.2834 (15) | N22—C23 | 1.478 (2) |
| Co1—N11 | 2.2834 (15) | N22—C24 | 1.474 (2) |
| Co2—N2ii | 2.0812 (16) | N22—C26 | 1.469 (2) |
| Co2—N2 | 2.0812 (16) | N23—C21 | 1.465 (2) |
| Co2—O2 | 2.029 (6) | N23—C22 | 1.468 (2) |
| Co2—O2ii | 2.029 (6) | N23—C26 | 1.469 (2) |
| Co2—O4ii | 2.21 (3) | N24—C22 | 1.473 (2) |
| Co2—O4 | 2.21 (3) | N24—C23 | 1.470 (2) |
| Co2—N21 | 2.2788 (16) | N24—C25 | 1.465 (2) |
| Co2—N21ii | 2.2788 (16) | C21—H21A | 0.9700 |
| N1—C1 | 1.169 (3) | C21—H21B | 0.9700 |
| C1—S1 | 1.629 (2) | C22—H22A | 0.9700 |
| N2—C2 | 1.154 (3) | C22—H22B | 0.9700 |
| C2—S2 | 1.643 (2) | C23—H23A | 0.9700 |
| O1—H1 | 0.880 (18) | C23—H23B | 0.9700 |
| O1—C3 | 1.464 (2) | C24—H24A | 0.9700 |
| C3—H3AA | 0.9700 | C24—H24B | 0.9700 |
| C3—H3AB | 0.9700 | C25—H25A | 0.9700 |
| C3—H3BC | 0.9700 | C25—H25B | 0.9700 |
| C3—H3BD | 0.9700 | C26—H26A | 0.9700 |
| C3—C4 | 1.515 (3) | C26—H26B | 0.9700 |
| C3—C4' | 1.4495 (10) | N31—C31 | 1.486 (2) |
| C4—H4A | 0.9600 | N31—C34 | 1.474 (3) |
| C4—H4B | 0.9600 | N31—C36 | 1.486 (2) |
| C4—H4C | 0.9600 | N32—C31 | 1.466 (3) |
| C4'—H4'A | 0.9600 | N32—C32 | 1.471 (3) |
| C4'—H4'B | 0.9600 | N32—C35 | 1.465 (3) |
| C4'—H4'C | 0.9600 | N33—C32 | 1.469 (3) |
| O2—H2A | 0.872 (19) | N33—C33 | 1.467 (3) |
| O2—H2B | 0.871 (19) | N33—C36 | 1.463 (3) |
| O3—C5 | 1.433 (3) | N34—C33 | 1.480 (3) |
| O3—H3 | 0.871 (19) | N34—C34 | 1.470 (3) |
| C5—H5A | 0.9700 | N34—C35 | 1.480 (3) |
| C5—H5B | 0.9700 | C31—H31A | 0.9700 |
| C5—C6 | 1.505 (4) | C31—H31B | 0.9700 |
| C6—H6A | 0.9600 | C32—H32A | 0.9700 |
| C6—H6B | 0.9600 | C32—H32B | 0.9700 |
| C6—H6C | 0.9600 | C33—H33A | 0.9700 |
| O4—C7 | 1.427 (17) | C33—H33B | 0.9700 |
| O4—H4 | 0.87 (2) | C34—H34A | 0.9700 |
| C7—H7A | 0.9700 | C34—H34B | 0.9700 |
| C7—H7B | 0.9700 | C35—H35A | 0.9700 |
| C7—C8 | 1.507 (13) | C35—H35B | 0.9700 |
| C8—H8A | 0.9600 | C36—H36A | 0.9700 |
| C8—H8B | 0.9600 | C36—H36B | 0.9700 |
| C8—H8C | 0.9600 | N41—C41 | 1.483 (3) |
| N11—C11 | 1.499 (2) | N41—C44 | 1.482 (2) |
| N11—C12 | 1.488 (2) | N41—C45 | 1.475 (3) |
| N11—C15 | 1.496 (2) | N42—C41 | 1.465 (3) |
| N12—C11 | 1.465 (2) | N42—C42 | 1.469 (3) |
| N12—C14 | 1.472 (2) | N42—C46 | 1.464 (3) |
| N12—C16 | 1.476 (2) | N43—C42 | 1.479 (4) |
| N13—C13 | 1.474 (2) | N43—C43 | 1.473 (3) |
| N13—C14 | 1.470 (2) | N43—C45 | 1.469 (3) |
| N13—C15 | 1.468 (2) | N44—C43 | 1.465 (3) |
| N14—C12 | 1.467 (2) | N44—C44 | 1.467 (3) |
| N14—C13 | 1.472 (2) | N44—C46 | 1.463 (3) |
| N14—C16 | 1.473 (2) | C41—H41A | 0.9700 |
| C11—H11A | 0.9700 | C41—H41B | 0.9700 |
| C11—H11B | 0.9700 | C42—H42A | 0.9700 |
| C12—H12A | 0.9700 | C42—H42B | 0.9700 |
| C12—H12B | 0.9700 | C43—H43A | 0.9700 |
| C13—H13A | 0.9700 | C43—H43B | 0.9700 |
| C13—H13B | 0.9700 | C44—H44A | 0.9700 |
| C14—H14A | 0.9700 | C44—H44B | 0.9700 |
| C14—H14B | 0.9700 | C45—H45A | 0.9700 |
| C15—H15A | 0.9700 | C45—H45B | 0.9700 |
| C15—H15B | 0.9700 | C46—H46A | 0.9700 |
| C16—H16A | 0.9700 | C46—H46B | 0.9700 |
| N1i—Co1—N1 | 180.0 | N14—C16—H16A | 109.1 |
| N1—Co1—O1i | 89.16 (6) | N14—C16—H16B | 109.1 |
| N1i—Co1—O1 | 89.16 (6) | H16A—C16—H16B | 107.9 |
| N1—Co1—O1 | 90.84 (6) | C21—N21—Co2 | 110.79 (11) |
| N1i—Co1—O1i | 90.84 (6) | C21—N21—C25 | 106.97 (14) |
| N1i—Co1—N11 | 93.90 (6) | C24—N21—Co2 | 113.66 (11) |
| N1—Co1—N11 | 86.10 (6) | C24—N21—C21 | 107.30 (14) |
| N1—Co1—N11i | 93.90 (6) | C24—N21—C25 | 107.16 (14) |
| N1i—Co1—N11i | 86.10 (6) | C25—N21—Co2 | 110.66 (11) |
| O1—Co1—O1i | 180.00 (7) | C24—N22—C23 | 108.01 (14) |
| O1i—Co1—N11i | 91.57 (5) | C26—N22—C23 | 107.79 (15) |
| O1—Co1—N11 | 91.57 (5) | C26—N22—C24 | 108.16 (15) |
| O1—Co1—N11i | 88.43 (5) | C21—N23—C22 | 108.62 (14) |
| O1i—Co1—N11 | 88.43 (5) | C21—N23—C26 | 108.13 (15) |
| N11—Co1—N11i | 180.0 | C22—N23—C26 | 107.86 (15) |
| N2—Co2—N2ii | 180.0 | C23—N24—C22 | 108.30 (14) |
| N2—Co2—O4 | 85.7 (7) | C25—N24—C22 | 107.77 (15) |
| N2ii—Co2—O4ii | 85.7 (7) | C25—N24—C23 | 107.98 (15) |
| N2—Co2—O4ii | 94.3 (7) | N21—C21—H21A | 109.1 |
| N2ii—Co2—O4 | 94.3 (7) | N21—C21—H21B | 109.1 |
| N2—Co2—N21ii | 91.78 (6) | N23—C21—N21 | 112.54 (15) |
| N2ii—Co2—N21ii | 88.22 (6) | N23—C21—H21A | 109.1 |
| N2ii—Co2—N21 | 91.78 (6) | N23—C21—H21B | 109.1 |
| N2—Co2—N21 | 88.22 (6) | H21A—C21—H21B | 107.8 |
| O2ii—Co2—N2 | 89.20 (16) | N23—C22—N24 | 112.53 (15) |
| O2—Co2—N2ii | 89.20 (16) | N23—C22—H22A | 109.1 |
| O2ii—Co2—N2ii | 90.80 (16) | N23—C22—H22B | 109.1 |
| O2—Co2—N2 | 90.80 (16) | N24—C22—H22A | 109.1 |
| O2ii—Co2—O2 | 180.0 | N24—C22—H22B | 109.1 |
| O2—Co2—O4ii | 174.6 (9) | H22A—C22—H22B | 107.8 |
| O2ii—Co2—O4ii | 5.4 (9) | N22—C23—H23A | 109.1 |
| O2—Co2—N21 | 91.3 (2) | N22—C23—H23B | 109.1 |
| O2—Co2—N21ii | 88.7 (2) | N24—C23—N22 | 112.47 (15) |
| O2ii—Co2—N21ii | 91.3 (2) | N24—C23—H23A | 109.1 |
| O2ii—Co2—N21 | 88.7 (2) | N24—C23—H23B | 109.1 |
| O4ii—Co2—O4 | 180.0 | H23A—C23—H23B | 107.8 |
| O4ii—Co2—N21ii | 92.9 (9) | N21—C24—H24A | 109.1 |
| O4—Co2—N21 | 92.9 (9) | N21—C24—H24B | 109.1 |
| O4ii—Co2—N21 | 87.1 (9) | N22—C24—N21 | 112.52 (15) |
| O4—Co2—N21ii | 87.1 (9) | N22—C24—H24A | 109.1 |
| N21ii—Co2—N21 | 180.0 | N22—C24—H24B | 109.1 |
| C1—N1—Co1 | 164.85 (15) | H24A—C24—H24B | 107.8 |
| N1—C1—S1 | 178.89 (18) | N21—C25—H25A | 109.0 |
| C2—N2—Co2 | 175.29 (16) | N21—C25—H25B | 109.0 |
| N2—C2—S2 | 177.31 (19) | N24—C25—N21 | 112.98 (14) |
| Co1—O1—H1 | 122 (2) | N24—C25—H25A | 109.0 |
| C3—O1—Co1 | 129.84 (12) | N24—C25—H25B | 109.0 |
| C3—O1—H1 | 107 (2) | H25A—C25—H25B | 107.8 |
| O1—C3—H3AA | 109.0 | N22—C26—H26A | 109.0 |
| O1—C3—H3AB | 109.0 | N22—C26—H26B | 109.0 |
| O1—C3—H3BC | 106.0 | N23—C26—N22 | 112.80 (15) |
| O1—C3—H3BD | 106.0 | N23—C26—H26A | 109.0 |
| O1—C3—C4 | 113.01 (18) | N23—C26—H26B | 109.0 |
| H3AA—C3—H3AB | 107.8 | H26A—C26—H26B | 107.8 |
| H3BC—C3—H3BD | 106.3 | C34—N31—C31 | 107.28 (15) |
| C4—C3—H3AA | 109.0 | C34—N31—C36 | 107.97 (16) |
| C4—C3—H3AB | 109.0 | C36—N31—C31 | 107.86 (16) |
| C4'—C3—O1 | 125.1 (6) | C31—N32—C32 | 107.40 (17) |
| C4'—C3—H3BC | 106.0 | C35—N32—C31 | 107.85 (16) |
| C4'—C3—H3BD | 106.0 | C35—N32—C32 | 108.44 (17) |
| C3—C4—H4A | 109.5 | C33—N33—C32 | 108.52 (17) |
| C3—C4—H4B | 109.5 | C36—N33—C32 | 108.32 (17) |
| C3—C4—H4C | 109.5 | C36—N33—C33 | 108.01 (18) |
| H4A—C4—H4B | 109.5 | C34—N34—C33 | 107.50 (16) |
| H4A—C4—H4C | 109.5 | C34—N34—C35 | 108.18 (16) |
| H4B—C4—H4C | 109.5 | C35—N34—C33 | 107.51 (17) |
| C3—C4'—H4'A | 109.5 | N31—C31—H31A | 109.0 |
| C3—C4'—H4'B | 109.5 | N31—C31—H31B | 109.0 |
| C3—C4'—H4'C | 109.5 | N32—C31—N31 | 112.86 (16) |
| H4'A—C4'—H4'B | 109.5 | N32—C31—H31A | 109.0 |
| H4'A—C4'—H4'C | 109.5 | N32—C31—H31B | 109.0 |
| H4'B—C4'—H4'C | 109.5 | H31A—C31—H31B | 107.8 |
| Co2—O2—H2A | 127 (4) | N32—C32—H32A | 109.1 |
| Co2—O2—H2B | 123 (3) | N32—C32—H32B | 109.1 |
| H2A—O2—H2B | 106 (4) | N33—C32—N32 | 112.59 (17) |
| C5—O3—H3 | 112 (3) | N33—C32—H32A | 109.1 |
| O3—C5—H5A | 109.8 | N33—C32—H32B | 109.1 |
| O3—C5—H5B | 109.8 | H32A—C32—H32B | 107.8 |
| O3—C5—C6 | 109.6 (2) | N33—C33—N34 | 112.55 (17) |
| H5A—C5—H5B | 108.2 | N33—C33—H33A | 109.1 |
| C6—C5—H5A | 109.8 | N33—C33—H33B | 109.1 |
| C6—C5—H5B | 109.8 | N34—C33—H33A | 109.1 |
| C5—C6—H6A | 109.5 | N34—C33—H33B | 109.1 |
| C5—C6—H6B | 109.5 | H33A—C33—H33B | 107.8 |
| C5—C6—H6C | 109.5 | N31—C34—H34A | 109.1 |
| H6A—C6—H6B | 109.5 | N31—C34—H34B | 109.1 |
| H6A—C6—H6C | 109.5 | N34—C34—N31 | 112.62 (16) |
| H6B—C6—H6C | 109.5 | N34—C34—H34A | 109.1 |
| Co2—O4—H4 | 115 (10) | N34—C34—H34B | 109.1 |
| C7—O4—Co2 | 137 (2) | H34A—C34—H34B | 107.8 |
| C7—O4—H4 | 106 (10) | N32—C35—N34 | 112.61 (16) |
| O4—C7—H7A | 109.1 | N32—C35—H35A | 109.1 |
| O4—C7—H7B | 109.1 | N32—C35—H35B | 109.1 |
| O4—C7—C8 | 112.4 (15) | N34—C35—H35A | 109.1 |
| H7A—C7—H7B | 107.9 | N34—C35—H35B | 109.1 |
| C8—C7—H7A | 109.1 | H35A—C35—H35B | 107.8 |
| C8—C7—H7B | 109.1 | N31—C36—H36A | 109.2 |
| C7—C8—H8A | 109.5 | N31—C36—H36B | 109.2 |
| C7—C8—H8B | 109.5 | N33—C36—N31 | 111.87 (16) |
| C7—C8—H8C | 109.5 | N33—C36—H36A | 109.2 |
| H8A—C8—H8B | 109.5 | N33—C36—H36B | 109.2 |
| H8A—C8—H8C | 109.5 | H36A—C36—H36B | 107.9 |
| H8B—C8—H8C | 109.5 | C44—N41—C41 | 107.46 (16) |
| C11—N11—Co1 | 112.38 (10) | C45—N41—C41 | 108.17 (18) |
| C12—N11—Co1 | 110.45 (11) | C45—N41—C44 | 107.65 (16) |
| C12—N11—C11 | 106.96 (14) | C41—N42—C42 | 107.76 (19) |
| C12—N11—C15 | 106.95 (13) | C46—N42—C41 | 107.89 (17) |
| C15—N11—Co1 | 112.93 (11) | C46—N42—C42 | 107.80 (19) |
| C15—N11—C11 | 106.83 (14) | C43—N43—C42 | 107.73 (19) |
| C11—N12—C14 | 108.22 (14) | C45—N43—C42 | 108.42 (19) |
| C11—N12—C16 | 108.09 (14) | C45—N43—C43 | 107.79 (19) |
| C14—N12—C16 | 108.03 (14) | C43—N44—C44 | 108.03 (17) |
| C14—N13—C13 | 107.97 (15) | C46—N44—C43 | 108.19 (18) |
| C15—N13—C13 | 107.82 (14) | C46—N44—C44 | 107.81 (16) |
| C15—N13—C14 | 108.50 (14) | N41—C41—H41A | 109.1 |
| C12—N14—C13 | 108.45 (14) | N41—C41—H41B | 109.1 |
| C12—N14—C16 | 108.30 (14) | N42—C41—N41 | 112.64 (17) |
| C13—N14—C16 | 107.44 (14) | N42—C41—H41A | 109.1 |
| N11—C11—H11A | 109.0 | N42—C41—H41B | 109.1 |
| N11—C11—H11B | 109.0 | H41A—C41—H41B | 107.8 |
| N12—C11—N11 | 113.01 (14) | N42—C42—N43 | 112.48 (18) |
| N12—C11—H11A | 109.0 | N42—C42—H42A | 109.1 |
| N12—C11—H11B | 109.0 | N42—C42—H42B | 109.1 |
| H11A—C11—H11B | 107.8 | N43—C42—H42A | 109.1 |
| N11—C12—H12A | 109.0 | N43—C42—H42B | 109.1 |
| N11—C12—H12B | 109.0 | H42A—C42—H42B | 107.8 |
| N14—C12—N11 | 112.97 (15) | N43—C43—H43A | 109.1 |
| N14—C12—H12A | 109.0 | N43—C43—H43B | 109.1 |
| N14—C12—H12B | 109.0 | N44—C43—N43 | 112.46 (18) |
| H12A—C12—H12B | 107.8 | N44—C43—H43A | 109.1 |
| N13—C13—H13A | 109.1 | N44—C43—H43B | 109.1 |
| N13—C13—H13B | 109.1 | H43A—C43—H43B | 107.8 |
| N14—C13—N13 | 112.61 (14) | N41—C44—H44A | 109.1 |
| N14—C13—H13A | 109.1 | N41—C44—H44B | 109.1 |
| N14—C13—H13B | 109.1 | N44—C44—N41 | 112.30 (16) |
| H13A—C13—H13B | 107.8 | N44—C44—H44A | 109.1 |
| N12—C14—H14A | 109.1 | N44—C44—H44B | 109.1 |
| N12—C14—H14B | 109.1 | H44A—C44—H44B | 107.9 |
| N13—C14—N12 | 112.35 (15) | N41—C45—H45A | 109.2 |
| N13—C14—H14A | 109.1 | N41—C45—H45B | 109.2 |
| N13—C14—H14B | 109.1 | N43—C45—N41 | 112.17 (17) |
| H14A—C14—H14B | 107.9 | N43—C45—H45A | 109.2 |
| N11—C15—H15A | 109.0 | N43—C45—H45B | 109.2 |
| N11—C15—H15B | 109.0 | H45A—C45—H45B | 107.9 |
| N13—C15—N11 | 112.95 (14) | N42—C46—H46A | 108.9 |
| N13—C15—H15A | 109.0 | N42—C46—H46B | 108.9 |
| N13—C15—H15B | 109.0 | N44—C46—N42 | 113.21 (17) |
| H15A—C15—H15B | 107.8 | N44—C46—H46A | 108.9 |
| N12—C16—H16A | 109.1 | N44—C46—H46B | 108.9 |
| N12—C16—H16B | 109.1 | H46A—C46—H46B | 107.7 |
| N14—C16—N12 | 112.42 (15) | ||
| Co1—O1—C3—C4 | 121.56 (18) | C26—N22—C23—N24 | −57.6 (2) |
| Co1—O1—C3—C4' | 85.5 (9) | C26—N22—C24—N21 | 57.87 (19) |
| Co1—N11—C11—N12 | −178.62 (11) | C26—N23—C21—N21 | −58.52 (19) |
| Co1—N11—C12—N14 | 179.71 (11) | C26—N23—C22—N24 | 58.1 (2) |
| Co1—N11—C15—N13 | 179.36 (12) | C31—N31—C34—N34 | 57.9 (2) |
| Co2—O4—C7—C8 | 125 (2) | C31—N31—C36—N33 | −57.3 (2) |
| Co2—N21—C21—N23 | −177.58 (11) | C31—N32—C32—N33 | 58.9 (2) |
| Co2—N21—C24—N22 | 179.79 (11) | C31—N32—C35—N34 | −58.0 (2) |
| Co2—N21—C25—N24 | 178.24 (12) | C32—N32—C31—N31 | −58.1 (2) |
| C11—N11—C12—N14 | 57.12 (18) | C32—N32—C35—N34 | 58.0 (2) |
| C11—N11—C15—N13 | −56.60 (19) | C32—N33—C33—N34 | −57.9 (2) |
| C11—N12—C14—N13 | 58.77 (19) | C32—N33—C36—N31 | 58.5 (2) |
| C11—N12—C16—N14 | −58.43 (18) | C33—N33—C32—N32 | 57.4 (2) |
| C12—N11—C11—N12 | −57.24 (19) | C33—N33—C36—N31 | −58.9 (2) |
| C12—N11—C15—N13 | 57.65 (19) | C33—N34—C34—N31 | 57.9 (2) |
| C12—N14—C13—N13 | −58.23 (19) | C33—N34—C35—N32 | −58.0 (2) |
| C12—N14—C16—N12 | 58.46 (19) | C34—N31—C31—N32 | −58.4 (2) |
| C13—N13—C14—N12 | 58.01 (19) | C34—N31—C36—N33 | 58.3 (2) |
| C13—N13—C15—N11 | −58.61 (19) | C34—N34—C33—N33 | −58.4 (2) |
| C13—N14—C12—N11 | 57.91 (19) | C34—N34—C35—N32 | 57.8 (2) |
| C13—N14—C16—N12 | −58.50 (19) | C35—N32—C31—N31 | 58.6 (2) |
| C14—N12—C11—N11 | −58.54 (19) | C35—N32—C32—N33 | −57.4 (2) |
| C14—N12—C16—N14 | 58.45 (19) | C35—N34—C33—N33 | 57.9 (2) |
| C14—N13—C13—N14 | −58.58 (19) | C35—N34—C34—N31 | −58.0 (2) |
| C14—N13—C15—N11 | 58.09 (19) | C36—N31—C31—N32 | 57.7 (2) |
| C15—N11—C11—N12 | 57.00 (19) | C36—N31—C34—N34 | −58.1 (2) |
| C15—N11—C12—N14 | −57.03 (18) | C36—N33—C32—N32 | −59.6 (2) |
| C15—N13—C13—N14 | 58.46 (19) | C36—N33—C33—N34 | 59.3 (2) |
| C15—N13—C14—N12 | −58.6 (2) | C41—N41—C44—N44 | 58.1 (2) |
| C16—N12—C11—N11 | 58.22 (19) | C41—N41—C45—N43 | −57.3 (2) |
| C16—N12—C14—N13 | −58.04 (19) | C41—N42—C42—N43 | 58.4 (2) |
| C16—N14—C12—N11 | −58.39 (19) | C41—N42—C46—N44 | −58.3 (2) |
| C16—N14—C13—N13 | 58.63 (19) | C42—N42—C41—N41 | −58.4 (2) |
| C21—N21—C24—N22 | −57.37 (19) | C42—N42—C46—N44 | 57.9 (2) |
| C21—N21—C25—N24 | 57.47 (19) | C42—N43—C43—N44 | −58.0 (2) |
| C21—N23—C22—N24 | −58.9 (2) | C42—N43—C45—N41 | 57.5 (2) |
| C21—N23—C26—N22 | 58.7 (2) | C43—N43—C42—N42 | 58.1 (2) |
| C22—N23—C21—N21 | 58.28 (19) | C43—N43—C45—N41 | −58.8 (3) |
| C22—N23—C26—N22 | −58.6 (2) | C43—N44—C44—N41 | 58.3 (2) |
| C22—N24—C23—N22 | 57.5 (2) | C43—N44—C46—N42 | −57.9 (2) |
| C22—N24—C25—N21 | −58.47 (19) | C44—N41—C41—N42 | −57.8 (2) |
| C23—N22—C24—N21 | −58.54 (19) | C44—N41—C45—N43 | 58.5 (2) |
| C23—N22—C26—N23 | 58.2 (2) | C44—N44—C43—N43 | −58.6 (2) |
| C23—N24—C22—N23 | −57.9 (2) | C44—N44—C46—N42 | 58.7 (2) |
| C23—N24—C25—N21 | 58.31 (19) | C45—N41—C41—N42 | 58.1 (2) |
| C24—N21—C21—N23 | 57.83 (19) | C45—N41—C44—N44 | −58.2 (2) |
| C24—N21—C25—N24 | −57.34 (19) | C45—N43—C42—N42 | −58.3 (2) |
| C24—N22—C23—N24 | 59.1 (2) | C45—N43—C43—N44 | 58.8 (3) |
| C24—N22—C26—N23 | −58.31 (19) | C46—N42—C41—N41 | 57.7 (2) |
| C25—N21—C21—N23 | −56.89 (18) | C46—N42—C42—N43 | −57.8 (2) |
| C25—N21—C24—N22 | 57.21 (19) | C46—N44—C43—N43 | 57.8 (2) |
| C25—N24—C22—N23 | 58.72 (19) | C46—N44—C44—N41 | −58.4 (2) |
| C25—N24—C23—N22 | −58.9 (2) |
Symmetry codes: (i) −x+1, −y, −z+2; (ii) −x+2, −y+1, −z+1.
Bis(ethanol-κO)bis(hexamethylenetetramine-κN)bis(thiocyanato-κN)cobalt(II)–diaqua-κ2O-bis(hexamethylenetetramine-κN)bis(thiocyanato-κN)cobalt(II)–ethanol–hexamethylenetetramine (1.2/0.8/1.6/4) (1) . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1···N31 | 0.88 (2) | 1.92 (2) | 2.793 (2) | 170 (3) |
| C4′—H4′A···N43iii | 0.96 | 2.50 | 3.243 (14) | 134 |
| C4′—H4′C···N44i | 0.96 | 2.38 | 3.161 (10) | 138 |
| O2—H2A···N41 | 0.87 (2) | 1.88 (2) | 2.743 (7) | 173 (7) |
| O2—H2B···O3 | 0.87 (2) | 1.80 (2) | 2.665 (4) | 177 (5) |
| C5—H5A···S2iv | 0.97 | 3.02 | 3.925 (3) | 156 |
| O3—H3···N34 | 0.87 (2) | 1.97 (2) | 2.821 (3) | 167 (4) |
| O4—H4···N41 | 0.87 (2) | 1.94 (5) | 2.81 (3) | 170 (19) |
| C11—H11A···O1i | 0.97 | 2.49 | 3.058 (2) | 117 |
| C11—H11B···N1 | 0.97 | 2.67 | 3.213 (2) | 116 |
| C12—H12B···N44 | 0.97 | 2.64 | 3.423 (3) | 138 |
| C13—H13A···N13v | 0.97 | 2.70 | 3.563 (2) | 149 |
| C13—H13B···S2iv | 0.97 | 2.95 | 3.7150 (19) | 136 |
| C14—H14A···S2vi | 0.97 | 2.93 | 3.840 (2) | 156 |
| C15—H15B···O1 | 0.97 | 2.61 | 3.118 (2) | 113 |
| C22—H22B···N12vii | 0.97 | 2.58 | 3.448 (2) | 149 |
| C25—H25A···O2ii | 0.97 | 2.50 | 3.026 (7) | 114 |
| C25—H25A···O4ii | 0.97 | 2.49 | 3.08 (3) | 119 |
| C25—H25B···N2 | 0.97 | 2.61 | 3.202 (3) | 119 |
| C26—H26A···S1i | 0.97 | 2.98 | 3.655 (2) | 128 |
| C26—H26B···N22viii | 0.97 | 2.69 | 3.581 (3) | 152 |
| C33—H33A···N23 | 0.97 | 2.66 | 3.431 (3) | 137 |
| C45—H45A···S2ii | 0.97 | 3.01 | 3.959 (3) | 165 |
Symmetry codes: (i) −x+1, −y, −z+2; (ii) −x+2, −y+1, −z+1; (iii) x−1, y, z; (iv) −x+1, −y+1, −z+1; (v) −x+1, −y, −z+1; (vi) x, y−1, z; (vii) x, y+1, z; (viii) −x+2, −y+1, −z+2.
\ Tris(ethanol-κO)(hexamethylenetetramine-κN)bis(thiocyanato-\ κN)cobalt(II) (2) . Crystal data
| [Co(NCS)2(C6H12N4)(C2H6O)3] | F(000) = 956 |
| Mr = 453.49 | Dx = 1.449 Mg m−3 |
| Monoclinic, P21/n | Cu Kα radiation, λ = 1.54184 Å |
| a = 11.1463 (1) Å | Cell parameters from 23697 reflections |
| b = 15.7705 (1) Å | θ = 4.7–78.0° |
| c = 12.1824 (1) Å | µ = 8.57 mm−1 |
| β = 103.886 (1)° | T = 100 K |
| V = 2078.87 (3) Å3 | Block, intense orange |
| Z = 4 | 0.2 × 0.18 × 0.03 mm |
\ Tris(ethanol-κO)(hexamethylenetetramine-κN)bis(thiocyanato-\ κN)cobalt(II) (2) . Data collection
| XtaLAB Synergy, Dualflex, HyPix diffractometer | 4431 independent reflections |
| Radiation source: micro-focus sealed X-ray tube, PhotonJet (Cu) X-ray Source | 4373 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.027 |
| Detector resolution: 10.0000 pixels mm-1 | θmax = 78.2°, θmin = 4.7° |
| ω scans | h = −14→13 |
| Absorption correction: multi-scan (CrysAlisPro; Rigaku OD, 2021) | k = −20→18 |
| Tmin = 0.427, Tmax = 1.000 | l = −14→15 |
| 29441 measured reflections |
\ Tris(ethanol-κO)(hexamethylenetetramine-κN)bis(thiocyanato-\ κN)cobalt(II) (2) . Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.025 | w = 1/[σ2(Fo2) + (0.0403P)2 + 0.8765P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.068 | (Δ/σ)max = 0.002 |
| S = 1.08 | Δρmax = 0.32 e Å−3 |
| 4431 reflections | Δρmin = −0.31 e Å−3 |
| 242 parameters | Extinction correction: SHELXL2016/6 (Sheldrick 2015b), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 1 restraint | Extinction coefficient: 0.00065 (9) |
| Primary atom site location: dual |
\ Tris(ethanol-κO)(hexamethylenetetramine-κN)bis(thiocyanato-\ κN)cobalt(II) (2) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
\ Tris(ethanol-κO)(hexamethylenetetramine-κN)bis(thiocyanato-\ κN)cobalt(II) (2) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Co1 | 0.51335 (2) | 0.27266 (2) | 0.57458 (2) | 0.01059 (7) | |
| N1 | 0.67247 (10) | 0.34371 (7) | 0.62465 (9) | 0.0153 (2) | |
| C1 | 0.76466 (12) | 0.38035 (8) | 0.65618 (11) | 0.0141 (2) | |
| S1 | 0.89474 (3) | 0.43141 (2) | 0.70092 (3) | 0.02407 (10) | |
| N2 | 0.35462 (10) | 0.20093 (7) | 0.52823 (9) | 0.0153 (2) | |
| C2 | 0.25674 (12) | 0.17255 (8) | 0.49267 (11) | 0.0134 (2) | |
| S2 | 0.11817 (3) | 0.13335 (2) | 0.44031 (3) | 0.01552 (8) | |
| N11 | 0.48544 (9) | 0.32562 (7) | 0.39886 (9) | 0.0110 (2) | |
| N12 | 0.35142 (10) | 0.34740 (7) | 0.20867 (9) | 0.0131 (2) | |
| N13 | 0.49385 (10) | 0.45980 (7) | 0.29661 (9) | 0.0127 (2) | |
| N14 | 0.57416 (10) | 0.32908 (7) | 0.23263 (9) | 0.0137 (2) | |
| C11 | 0.36212 (11) | 0.30856 (8) | 0.32095 (10) | 0.0123 (2) | |
| H11A | 0.349734 | 0.246546 | 0.312045 | 0.015* | |
| H11B | 0.296401 | 0.331440 | 0.354686 | 0.015* | |
| C12 | 0.37105 (12) | 0.44001 (8) | 0.22303 (11) | 0.0140 (2) | |
| H12A | 0.363639 | 0.466531 | 0.148044 | 0.017* | |
| H12B | 0.306101 | 0.464431 | 0.256487 | 0.017* | |
| C13 | 0.58915 (12) | 0.42110 (8) | 0.24547 (11) | 0.0147 (2) | |
| H13A | 0.583835 | 0.446900 | 0.170375 | 0.018* | |
| H13B | 0.672136 | 0.433681 | 0.293783 | 0.018* | |
| C14 | 0.58124 (11) | 0.29098 (8) | 0.34359 (11) | 0.0130 (2) | |
| H14A | 0.664260 | 0.301634 | 0.393063 | 0.016* | |
| H14B | 0.570316 | 0.228850 | 0.334757 | 0.016* | |
| C15 | 0.45097 (12) | 0.31188 (8) | 0.15994 (11) | 0.0147 (2) | |
| H15A | 0.439358 | 0.249853 | 0.150305 | 0.018* | |
| H15B | 0.445047 | 0.337046 | 0.084374 | 0.018* | |
| C16 | 0.50169 (12) | 0.41931 (8) | 0.40684 (11) | 0.0126 (2) | |
| H16A | 0.437357 | 0.443759 | 0.441062 | 0.015* | |
| H16B | 0.583193 | 0.432292 | 0.457605 | 0.015* | |
| O21 | 0.41718 (8) | 0.37678 (6) | 0.62519 (8) | 0.01481 (19) | |
| H21 | 0.458750 | 0.419051 | 0.654234 | 0.022* | |
| C21 | 0.31119 (12) | 0.36740 (9) | 0.67299 (12) | 0.0174 (3) | |
| H21A | 0.320943 | 0.404600 | 0.740012 | 0.021* | |
| H21B | 0.306126 | 0.308048 | 0.697966 | 0.021* | |
| C22 | 0.19389 (14) | 0.39014 (12) | 0.58774 (14) | 0.0295 (3) | |
| H22A | 0.181805 | 0.351233 | 0.523285 | 0.044* | |
| H22B | 0.199742 | 0.448405 | 0.561561 | 0.044* | |
| H22C | 0.123733 | 0.385578 | 0.622869 | 0.044* | |
| O31 | 0.62924 (8) | 0.17312 (6) | 0.54561 (8) | 0.01390 (18) | |
| H31 | 0.695985 | 0.172350 | 0.595461 | 0.021* | |
| C31 | 0.59743 (13) | 0.08809 (8) | 0.50586 (12) | 0.0172 (3) | |
| H31A | 0.508810 | 0.085995 | 0.466374 | 0.021* | |
| H31B | 0.610588 | 0.049076 | 0.571334 | 0.021* | |
| C32 | 0.67444 (14) | 0.05882 (9) | 0.42600 (12) | 0.0210 (3) | |
| H32A | 0.658634 | 0.095774 | 0.359399 | 0.032* | |
| H32B | 0.652206 | 0.000353 | 0.402320 | 0.032* | |
| H32C | 0.762264 | 0.061398 | 0.464640 | 0.032* | |
| O41 | 0.54084 (10) | 0.22913 (6) | 0.74187 (8) | 0.0173 (2) | |
| H41 | 0.564436 | 0.261997 | 0.797094 | 0.026* | |
| C41 | 0.51429 (13) | 0.14737 (9) | 0.78184 (12) | 0.0197 (3) | |
| H41A | 0.591114 | 0.123102 | 0.829704 | 0.024* | |
| H41B | 0.485447 | 0.109128 | 0.716372 | 0.024* | |
| C42 | 0.41750 (15) | 0.15144 (12) | 0.84905 (16) | 0.0344 (4) | |
| H42A | 0.339647 | 0.171959 | 0.800591 | 0.052* | |
| H42B | 0.444763 | 0.190262 | 0.912993 | 0.052* | |
| H42C | 0.405042 | 0.094752 | 0.877347 | 0.052* |
\ Tris(ethanol-κO)(hexamethylenetetramine-κN)bis(thiocyanato-\ κN)cobalt(II) (2) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Co1 | 0.00865 (11) | 0.01120 (12) | 0.01104 (12) | −0.00058 (7) | 0.00060 (8) | −0.00032 (7) |
| N1 | 0.0121 (5) | 0.0164 (5) | 0.0155 (5) | −0.0011 (4) | −0.0003 (4) | −0.0007 (4) |
| C1 | 0.0149 (6) | 0.0116 (6) | 0.0154 (6) | 0.0034 (5) | 0.0029 (5) | 0.0009 (5) |
| S1 | 0.01192 (16) | 0.01850 (17) | 0.0400 (2) | −0.00438 (12) | 0.00274 (14) | −0.00662 (14) |
| N2 | 0.0143 (5) | 0.0149 (5) | 0.0157 (5) | −0.0015 (4) | 0.0016 (4) | 0.0008 (4) |
| C2 | 0.0157 (6) | 0.0123 (6) | 0.0122 (5) | 0.0019 (5) | 0.0035 (5) | 0.0015 (4) |
| S2 | 0.01174 (15) | 0.01751 (16) | 0.01595 (15) | −0.00302 (11) | 0.00064 (11) | −0.00024 (11) |
| N11 | 0.0099 (5) | 0.0110 (5) | 0.0114 (5) | −0.0007 (4) | 0.0014 (4) | −0.0008 (4) |
| N12 | 0.0133 (5) | 0.0133 (5) | 0.0117 (5) | −0.0004 (4) | 0.0015 (4) | 0.0004 (4) |
| N13 | 0.0117 (5) | 0.0125 (5) | 0.0132 (5) | −0.0006 (4) | 0.0018 (4) | 0.0005 (4) |
| N14 | 0.0135 (5) | 0.0143 (5) | 0.0137 (5) | −0.0003 (4) | 0.0038 (4) | 0.0003 (4) |
| C11 | 0.0101 (5) | 0.0146 (6) | 0.0111 (6) | −0.0015 (4) | 0.0006 (4) | 0.0010 (4) |
| C12 | 0.0117 (6) | 0.0128 (6) | 0.0156 (6) | 0.0005 (4) | −0.0003 (5) | 0.0017 (5) |
| C13 | 0.0126 (6) | 0.0150 (6) | 0.0169 (6) | −0.0014 (5) | 0.0044 (5) | 0.0002 (5) |
| C14 | 0.0116 (6) | 0.0145 (6) | 0.0134 (6) | 0.0029 (5) | 0.0037 (5) | 0.0006 (5) |
| C15 | 0.0157 (6) | 0.0157 (6) | 0.0127 (6) | −0.0009 (5) | 0.0037 (5) | −0.0018 (5) |
| C16 | 0.0131 (6) | 0.0115 (6) | 0.0124 (5) | −0.0006 (4) | 0.0017 (5) | −0.0010 (4) |
| O21 | 0.0123 (4) | 0.0142 (4) | 0.0191 (5) | −0.0019 (3) | 0.0062 (4) | −0.0027 (3) |
| C21 | 0.0156 (6) | 0.0202 (7) | 0.0183 (6) | −0.0013 (5) | 0.0078 (5) | −0.0025 (5) |
| C22 | 0.0155 (7) | 0.0410 (9) | 0.0313 (8) | 0.0045 (6) | 0.0044 (6) | −0.0042 (7) |
| O31 | 0.0114 (4) | 0.0129 (4) | 0.0148 (4) | 0.0013 (3) | −0.0020 (3) | −0.0017 (3) |
| C31 | 0.0169 (6) | 0.0139 (6) | 0.0190 (6) | −0.0001 (5) | 0.0007 (5) | −0.0031 (5) |
| C32 | 0.0215 (7) | 0.0214 (7) | 0.0175 (6) | 0.0063 (5) | −0.0008 (5) | −0.0041 (5) |
| O41 | 0.0230 (5) | 0.0156 (5) | 0.0122 (4) | −0.0048 (3) | 0.0022 (4) | −0.0001 (3) |
| C41 | 0.0209 (7) | 0.0159 (6) | 0.0192 (6) | −0.0026 (5) | −0.0011 (5) | 0.0040 (5) |
| C42 | 0.0219 (8) | 0.0392 (9) | 0.0434 (10) | −0.0028 (7) | 0.0103 (7) | 0.0176 (8) |
\ Tris(ethanol-κO)(hexamethylenetetramine-κN)bis(thiocyanato-\ κN)cobalt(II) (2) . Geometric parameters (Å, º)
| Co1—N2 | 2.0615 (11) | C14—H14B | 0.9900 |
| Co1—N1 | 2.0624 (11) | C15—H15A | 0.9900 |
| Co1—O41 | 2.1021 (10) | C15—H15B | 0.9900 |
| Co1—O31 | 2.1157 (9) | C16—H16A | 0.9900 |
| Co1—O21 | 2.1314 (9) | C16—H16B | 0.9900 |
| Co1—N11 | 2.2489 (11) | O21—C21 | 1.4446 (15) |
| N1—C1 | 1.1610 (18) | O21—H21 | 0.8400 |
| C1—S1 | 1.6335 (13) | C21—C22 | 1.505 (2) |
| N2—C2 | 1.1625 (18) | C21—H21A | 0.9900 |
| C2—S2 | 1.6437 (13) | C21—H21B | 0.9900 |
| N11—C16 | 1.4889 (16) | C22—H22A | 0.9800 |
| N11—C11 | 1.4955 (15) | C22—H22B | 0.9800 |
| N11—C14 | 1.4957 (15) | C22—H22C | 0.9800 |
| N12—C11 | 1.4771 (15) | O31—C31 | 1.4409 (16) |
| N12—C12 | 1.4810 (16) | O31—H31 | 0.8400 |
| N12—C15 | 1.4878 (16) | C31—C32 | 1.5157 (19) |
| N13—C16 | 1.4705 (16) | C31—H31A | 0.9900 |
| N13—C12 | 1.4783 (16) | C31—H31B | 0.9900 |
| N13—C13 | 1.4855 (16) | C32—H32A | 0.9800 |
| N14—C14 | 1.4640 (16) | C32—H32B | 0.9800 |
| N14—C13 | 1.4648 (17) | C32—H32C | 0.9800 |
| N14—C15 | 1.4695 (16) | O41—C41 | 1.4339 (16) |
| C11—H11A | 0.9900 | O41—H41 | 0.8400 |
| C11—H11B | 0.9900 | C41—C42 | 1.504 (2) |
| C12—H12A | 0.9900 | C41—H41A | 0.9900 |
| C12—H12B | 0.9900 | C41—H41B | 0.9900 |
| C13—H13A | 0.9900 | C42—H42A | 0.9800 |
| C13—H13B | 0.9900 | C42—H42B | 0.9800 |
| C14—H14A | 0.9900 | C42—H42C | 0.9800 |
| N2—Co1—N1 | 178.73 (4) | N14—C15—N12 | 111.62 (10) |
| N2—Co1—O41 | 90.12 (4) | N14—C15—H15A | 109.3 |
| N1—Co1—O41 | 88.61 (4) | N12—C15—H15A | 109.3 |
| N2—Co1—O31 | 93.75 (4) | N14—C15—H15B | 109.3 |
| N1—Co1—O31 | 86.35 (4) | N12—C15—H15B | 109.3 |
| O41—Co1—O31 | 88.09 (4) | H15A—C15—H15B | 108.0 |
| N2—Co1—O21 | 92.51 (4) | N13—C16—N11 | 113.06 (10) |
| N1—Co1—O21 | 87.27 (4) | N13—C16—H16A | 109.0 |
| O41—Co1—O21 | 86.42 (4) | N11—C16—H16A | 109.0 |
| O31—Co1—O21 | 171.68 (4) | N13—C16—H16B | 109.0 |
| N2—Co1—N11 | 91.69 (4) | N11—C16—H16B | 109.0 |
| N1—Co1—N11 | 89.57 (4) | H16A—C16—H16B | 107.8 |
| O41—Co1—N11 | 177.25 (4) | C21—O21—Co1 | 123.67 (8) |
| O31—Co1—N11 | 93.85 (4) | C21—O21—H21 | 109.5 |
| O21—Co1—N11 | 91.43 (4) | Co1—O21—H21 | 117.9 |
| C1—N1—Co1 | 176.60 (11) | O21—C21—C22 | 110.89 (12) |
| N1—C1—S1 | 179.67 (14) | O21—C21—H21A | 109.5 |
| C2—N2—Co1 | 168.63 (11) | C22—C21—H21A | 109.5 |
| N2—C2—S2 | 179.00 (12) | O21—C21—H21B | 109.5 |
| C16—N11—C11 | 107.39 (9) | C22—C21—H21B | 109.5 |
| C16—N11—C14 | 107.64 (10) | H21A—C21—H21B | 108.0 |
| C11—N11—C14 | 107.18 (10) | C21—C22—H22A | 109.5 |
| C16—N11—Co1 | 108.63 (7) | C21—C22—H22B | 109.5 |
| C11—N11—Co1 | 115.68 (7) | H22A—C22—H22B | 109.5 |
| C14—N11—Co1 | 110.02 (7) | C21—C22—H22C | 109.5 |
| C11—N12—C12 | 108.83 (10) | H22A—C22—H22C | 109.5 |
| C11—N12—C15 | 108.14 (10) | H22B—C22—H22C | 109.5 |
| C12—N12—C15 | 108.38 (10) | C31—O31—Co1 | 129.48 (8) |
| C16—N13—C12 | 107.78 (10) | C31—O31—H31 | 109.5 |
| C16—N13—C13 | 108.23 (10) | Co1—O31—H31 | 111.1 |
| C12—N13—C13 | 108.07 (10) | O31—C31—C32 | 111.56 (11) |
| C14—N14—C13 | 109.17 (10) | O31—C31—H31A | 109.3 |
| C14—N14—C15 | 108.43 (10) | C32—C31—H31A | 109.3 |
| C13—N14—C15 | 108.26 (10) | O31—C31—H31B | 109.3 |
| N12—C11—N11 | 111.76 (10) | C32—C31—H31B | 109.3 |
| N12—C11—H11A | 109.3 | H31A—C31—H31B | 108.0 |
| N11—C11—H11A | 109.3 | C31—C32—H32A | 109.5 |
| N12—C11—H11B | 109.3 | C31—C32—H32B | 109.5 |
| N11—C11—H11B | 109.3 | H32A—C32—H32B | 109.5 |
| H11A—C11—H11B | 107.9 | C31—C32—H32C | 109.5 |
| N13—C12—N12 | 111.64 (10) | H32A—C32—H32C | 109.5 |
| N13—C12—H12A | 109.3 | H32B—C32—H32C | 109.5 |
| N12—C12—H12A | 109.3 | C41—O41—Co1 | 128.97 (8) |
| N13—C12—H12B | 109.3 | C41—O41—H41 | 109.5 |
| N12—C12—H12B | 109.3 | Co1—O41—H41 | 121.3 |
| H12A—C12—H12B | 108.0 | O41—C41—C42 | 112.33 (13) |
| N14—C13—N13 | 112.16 (10) | O41—C41—H41A | 109.1 |
| N14—C13—H13A | 109.2 | C42—C41—H41A | 109.1 |
| N13—C13—H13A | 109.2 | O41—C41—H41B | 109.1 |
| N14—C13—H13B | 109.2 | C42—C41—H41B | 109.1 |
| N13—C13—H13B | 109.2 | H41A—C41—H41B | 107.9 |
| H13A—C13—H13B | 107.9 | C41—C42—H42A | 109.5 |
| N14—C14—N11 | 112.31 (10) | C41—C42—H42B | 109.5 |
| N14—C14—H14A | 109.1 | H42A—C42—H42B | 109.5 |
| N11—C14—H14A | 109.1 | C41—C42—H42C | 109.5 |
| N14—C14—H14B | 109.1 | H42A—C42—H42C | 109.5 |
| N11—C14—H14B | 109.1 | H42B—C42—H42C | 109.5 |
| H14A—C14—H14B | 107.9 |
\ Tris(ethanol-κO)(hexamethylenetetramine-κN)bis(thiocyanato-\ κN)cobalt(II) (2) . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C12—H12A···S2i | 0.99 | 2.87 | 3.6586 (13) | 137 |
| C12—H12B···S1ii | 0.99 | 2.92 | 3.8813 (13) | 164 |
| C15—H15A···S1iii | 0.99 | 2.99 | 3.9387 (13) | 161 |
| C15—H15B···S2iv | 0.99 | 2.94 | 3.7110 (13) | 135 |
| C16—H16A···O21 | 0.99 | 2.54 | 3.1009 (16) | 116 |
| C16—H16B···N1 | 0.99 | 2.47 | 3.1083 (17) | 122 |
| O21—H21···N13ii | 0.84 | 2.03 | 2.8424 (14) | 161 |
| C22—H22C···S1v | 0.98 | 3.02 | 3.9559 (16) | 161 |
| O31—H31···N12vi | 0.84 | 1.96 | 2.7969 (14) | 172 |
| O41—H41···S2vi | 0.84 | 2.37 | 3.2080 (10) | 174 |
Symmetry codes: (i) −x+1/2, y+1/2, −z+1/2; (ii) −x+1, −y+1, −z+1; (iii) x−1/2, −y+1/2, z−1/2; (iv) x+1/2, −y+1/2, z−1/2; (v) x−1, y, z; (vi) x+1/2, −y+1/2, z+1/2.
Funding Statement
This work was funded by Deutsche Forschungsgemeinschaft grant NA720/5-2.
References
- Bai, Y., Shang, W.-L., Dang, D.-B., Sun, J.-D. & Gao, H. (2009). Spectrochim. Acta Part A, 72, 407–411. [DOI] [PubMed]
- Bai, Y., Shang, W.-L., Zhang, F.-L., Pan, X.-J. & Niu, X.-F. (2007). Acta Cryst. E63, m2628.
- Böhme, M., Jochim, A., Rams, M., Lohmiller, T., Suckert, S., Schnegg, A., Plass, W. & Näther, C. (2020). Inorg. Chem. 59, 5325–5338. [DOI] [PubMed]
- Brandenburg, K. & Putz, H. (1999). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Ceglarska, M., Böhme, M., Neumann, T., Plass, W., Näther, C. & Rams, M. (2021). Phys. Chem. Chem. Phys. 23, 10281–10289. [DOI] [PubMed]
- Chakraborty, J., Samanta, B., Rosair, G., Gramlich, V., Salah El Fallah, M., Ribas, J., Matsushita, T. & Mitra, S. (2006). Polyhedron, 25, 3006–3016.
- Czubacka, E., Kruszynski, R. & Sieranski, T. (2012). Struct. Chem. 23, 451–459.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Jin, Y., Che, Y. X. & Zheng, J. M. (2007). J. Coord. Chem. 60, 2067–2074.
- Jochim, A., Rams, M., Böhme, M., Ceglarska, M., Plass, W. & Näther, C. (2020). Dalton Trans. 49, 15310–15322. [DOI] [PubMed]
- Krebs, C., Jess, I., Ceglarska, M. & Näther, C. (2022). Acta Cryst. E78, 66–70. [DOI] [PMC free article] [PubMed]
- Krebs, C., Jess, I. & Näther, C. (2021). Acta Cryst. E77, 1120–1125. [DOI] [PMC free article] [PubMed]
- Li, J., Meng, S., Zhang, J., Song, Y., Huang, Z., Zhao, H., Wei, H., Huang, W., Cifuentes, M. P., Humphrey, M. G. & Zhang, C. (2012). CrystEngComm, 14, 2787–2796.
- Li, X.-L., Niu, D.-Z. & Lu, Z.-S. (2007). Acta Cryst. E63, m2478.
- Liu, Q., Xi, H.-T., Sun, X.-Q., Zhu, J.-F. & Yu, K.-B. (2002). Chin. J. Struct. Chem. 21, 355–359.
- Lu, J., Liu, H.-T., Zhang, X.-X., Wang, D.-Q. & Niu, M.-J. (2010). Z. Anorg. Allg. Chem. 636, 641–647.
- Mautner, F. A., Traber, M., Fischer, R. C., Torvisco, A., Reichmann, K., Speed, S., Vicente, R. & Massoud, S. S. (2018). Polyhedron, 154, 436–442.
- Näther, C., Wöhlert, S., Boeckmann, J., Wriedt, M. & Jess, I. (2013). Z. Anorg. Allg. Chem. 639, 2696–2714.
- Prananto, Y. P., Urbatsch, A., Moubaraki, B., Murray, K. S., Turner, D. R., Deacon, G. B. & Batten, S. R. (2017). Aust. J. Chem. 70, 516–528.
- Rams, M., Jochim, A., Böhme, M., Lohmiller, T., Ceglarska, M., Rams, M. M., Schnegg, A., Plass, W. & Näther, C. (2020). Chem. Eur. J. 26, 2837–2851. [DOI] [PMC free article] [PubMed]
- Rigaku OD (2021). CrysAlis PRO. Rigaku Oxford Diffraction.
- Shang, W.-L., Bai, Y., Ma, C.-Z. & Li, Z.-M. (2008). Acta Cryst. E64, m1184–m1185. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Shi, J.-M., Chen, J.-N. & Liu, L.-D. (2006). Pol. J. Chem. 80, 1909–1913.
- Suckert, S., Rams, M., Böhme, M., Germann, L. S., Dinnebier, R. E., Plass, W., Werner, J. & Näther, C. (2016). Dalton Trans. 45, 18190–18201. [DOI] [PubMed]
- Wellm, C., Majcher-Fitas, A., Rams, M. & Näther, C. (2020). Dalton Trans. 49, 16707–16714. [DOI] [PubMed]
- Werner, J., Rams, M., Tomkowicz, Z. & Näther, C. (2014). Dalton Trans. 43, 17333–17342. [DOI] [PubMed]
- Werner, J., Tomkowicz, Z., Rams, M., Ebbinghaus, S. G., Neumann, T. & Näther, C. (2015). Dalton Trans. 44, 14149–14158. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Zhang, Y., Li, J., Xu, H., Hou, H., Nishiura, M. & Imamoto, T. (1999). J. Mol. Struct. 510, 191–196.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) 1, 2. DOI: 10.1107/S2056989022001037/tx2047sup1.cif
Structure factors: contains datablock(s) 1. DOI: 10.1107/S2056989022001037/tx20471sup2.hkl
Structure factors: contains datablock(s) 2. DOI: 10.1107/S2056989022001037/tx20472sup3.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report