The title compound acts as a potential photosensitizer in photodynamic therapy against tumoral cells. It co-crystallizes with one solvent molecule of water. In the crystal, intermolecular π–π stacking interactions, C—H⋯π, C—H⋯F, and O—H⋯F and interactions are present.
Keywords: crystal structure, cobalt(III) complexes, bathophenanthroline, photodynamic therapy, photodynamic therapy
Abstract
The title compound, [Co(C72H48N6)](PF6)3·H2O, crystallizes with one tripositive complex molecule, three hexafluorophosphate anions and one solvent molecule of water in the asymmetric unit. The N6 coordination set around the central CoIII atom defines a distorted octahedral environment. Four fluorine atoms of one hexafluorophosphate anion are disordered over two sets of positions with site-occupancy factors of 0.697 (5) and 0.303 (5). In the crystal, intermolecular π–π stacking interactions, C—H⋯π, C—H⋯F and O—H⋯F and interactions are present.
Chemical context
Over the years, metal complexes with polypyridyl ligands have been investigated as photosensitizers in photodynamic therapy (PDT) against cancer. RuII remains undoubtedly the most studied metal for this purpose due to its tunable photophysical properties (Caspar et al., 2006 ▸; Howerton et al., 2012 ▸; Heinemann et al., 2017 ▸; Monro et al., 2019 ▸; McFarland et al., 2020 ▸).

Inspired by the exciting results reported with RuII, we were motivated to develop new metal-based complexes with similar structures. Among the transition metals, cobalt is commonly known for its potential to coordinate with chelate ligands like amino-acid compounds (Otter & Hartshorn, 2004 ▸) and polypyridyl derivative ligands. The resulting compounds were used in different fields of research. A series of CoIII complexes based on substituted 3-(pyridine-2-yl)-triazine ligands (Wang et al., 2004 ▸), or bis(1,10-phenanthroline), bis(2,2′-bipyridine) and derivatized imidazole-phenanthroline ligands were developed (Nagababu et al., 2008 ▸). These compounds were found to cleave calf thymus DNA (Zhang et al., 2001 ▸).
Cobalt complexes are not only used for biological purposes. For example, a series of substituted polypyridine ligands, acting in a bidentate or tridentate manner, coordinating to CoII were investigated as electron-transfer mediators in dye-sensitized solar cells (Sapp et al., 2002 ▸). Tris(2,2′-bipyridyl)-based ligands were also used to design redox stable CoII/III complexes for redox flow batteries (Yang et al., 2018 ▸).
Encouraged by these results, our team aimed at developing new cobalt complexes. Here we report on the synthesis and crystal structure of [tris(4,7-diphenyl-1,10-phenanthroline) cobalt(III)] tris (hexafluorophosphate) monohydrate, [CoIII(C72H48N6)]3+(PF6 −)3·H2O.
Structural commentary
The shape of the cobalt complex in the title compound is pseudooctahedral (Fig. 1 ▸). The cobalt(III) atom is coordinated by six nitrogen atoms from three dip ligands (dip = 4,7-diphenyl-1,10-phenanthroline). The Co—N bond lengths are in the range 1.934 (3)–1.954 (3) Å (Table 1 ▸) and correlate well with literature values observed for CoIII species. Indeed, the average Co—N bond length is 2.128 Å in CoI cations (three hits in the Cambridge Structural Database (CSD; Groom et al., 2016 ▸), 2.115 Å in CoII cations (106 hits), and 1.952 Å in CoIII cations (28 hits) in reported Co(phen)3 n+ (phen = phenanthroline) species. The bond angles between the axially bound ligand atoms are in the range 175.62 (13)–176.52 (13)° while the equatorial bond angles fall in the range 83.36 (12)–94.01 (13)°. The phenanthroline moieties (14 non-hydrogen atoms) of the dip ligands are almost planar according to the r.m.s. deviations calculated as 0.026 (N1^N2 moiety), 0.057 (N5^N6) and 0.106 (N3^N4) Å. As expected, the dihedral angles between the mean planes of the dip ligands are relatively close to 90° being 78.97 (5), 81.30 (4) and 86.09 (5)°. The phenyl rings substituting each phenanthroline ligand in para positions to the nitrogen atoms exhibit an intermediate orientation (45–60°) relative to the mean plane of the phenanthroline ring. The dihedral angles between the mean planes are 65.91 (13) and 46.44 (13)° within the N1^N2 ligand, 50.37 (12) and 60.35 (14)° within the N3^N4 ligand, and 54.66 (14) and 42.35 (14)° within the N5^N6 ligand.
Figure 1.
The molecular structure of the tris(4,7-diphenyl-1,10-phenanthroline)cobalt(III) cation of the title compound with displacement ellipsoids drawn at the 30% probability level. Hydrogen atoms are omitted for clarity.
Table 1. Selected geometric parameters (Å, °).
| Co1—N1 | 1.950 (3) | Co1—N4 | 1.942 (3) |
| Co1—N2 | 1.954 (3) | Co1—N5 | 1.941 (3) |
| Co1—N3 | 1.934 (3) | Co1—N6 | 1.940 (3) |
| Cg1⋯Cg2i | 3.707 (3) | ||
| N1—Co1—N2 | 83.72 (13) | N5—Co1—N1 | 88.67 (13) |
| N3—Co1—N1 | 175.62 (13) | N5—Co1—N2 | 93.26 (12) |
| N3—Co1—N2 | 92.66 (13) | N5—Co1—N4 | 176.52 (13) |
| N3—Co1—N4 | 84.01 (13) | N6—Co1—N1 | 93.44 (13) |
| N3—Co1—N5 | 94.01 (13) | N6—Co1—N2 | 175.65 (13) |
| N3—Co1—N6 | 90.31 (13) | N6—Co1—N4 | 93.77 (13) |
| N4—Co1—N1 | 93.48 (13) | N6—Co1—N5 | 83.36 (12) |
| N4—Co1—N2 | 89.70 (13) |
Symmetry code: (i)
 .
.
Supramolecular features
In the crystal, the complex cationic species interact with each other through π–π stacking interactions, forming chains extending perpendicular to the the b axis [Cg1⋯Cg2(1 + x,
 − y,
− y,
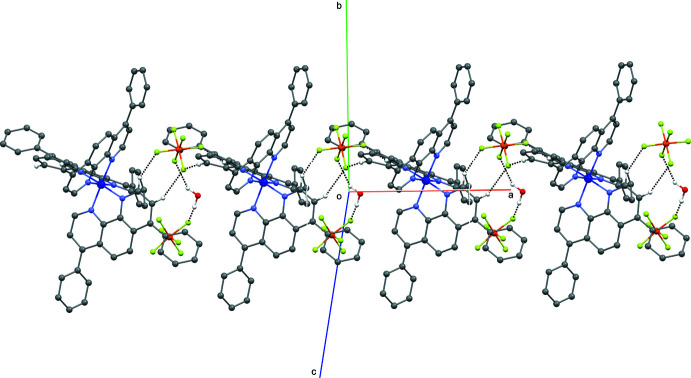
 + z) centroid-to-centroid distance of 3.707 (3) Å with Cg1 being the centroid of atoms C19–C24 and Cg2 the centroid of atoms C67–C72; Fig. 2 ▸, Table 2 ▸] and C—H⋯π interactions, forming layers parallel to the bc plane (Fig. 3 ▸, Table 2 ▸). Weak C—H⋯F and classical O—H⋯F intermolecular hydrogen bonds link the anionic hexafluorophosphate species (acceptors) to the tricationic molecules and to the solvent water molecules (donors). These interactions form chains along the a axis (Fig. 4 ▸). The most significant interactions for which C⋯F < 3.35 Å and C—H⋯F > 125°, and O⋯F < 3.00 Å and O–H⋯F > 125° are complied in Table 2 ▸.
+ z) centroid-to-centroid distance of 3.707 (3) Å with Cg1 being the centroid of atoms C19–C24 and Cg2 the centroid of atoms C67–C72; Fig. 2 ▸, Table 2 ▸] and C—H⋯π interactions, forming layers parallel to the bc plane (Fig. 3 ▸, Table 2 ▸). Weak C—H⋯F and classical O—H⋯F intermolecular hydrogen bonds link the anionic hexafluorophosphate species (acceptors) to the tricationic molecules and to the solvent water molecules (donors). These interactions form chains along the a axis (Fig. 4 ▸). The most significant interactions for which C⋯F < 3.35 Å and C—H⋯F > 125°, and O⋯F < 3.00 Å and O–H⋯F > 125° are complied in Table 2 ▸.
Figure 2.
A view of the crystal packing showing π–π stacking interactions forming chains extending perpendicular to the b axis.
Table 2. Hydrogen-bond geometry (Å, °).
Cg1, Cg2, Cg3 and Cg4 are the centroids of atoms C19–C24, C67–C72, C37–C42 and N5/C49–C53, respectively.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C9—H9⋯F7ii | 0.95 | 2.46 | 3.300 (5) | 148 |
| C10—H10⋯F9ii | 0.95 | 2.33 | 3.173 (5) | 148 |
| C25—H25⋯F1 | 0.95 | 2.45 | 3.204 (5) | 136 |
| C42—H42⋯F15B iii | 0.95 | 2.36 | 3.096 (7) | 134 |
| C48—H48⋯F7 | 0.95 | 2.39 | 3.328 (6) | 172 |
| C49—H49⋯F18A ii | 0.95 | 2.13 | 2.850 (9) | 132 |
| C58—H58⋯F12 | 0.95 | 2.26 | 2.963 (5) | 130 |
| O1—H1A⋯F17A | 0.87 (1) | 2.25 (7) | 2.965 (17) | 139 (9) |
| O1—H1A⋯F17B | 0.87 (1) | 2.19 (8) | 2.817 (10) | 128 (8) |
| O1—H1B⋯F11 | 0.87 (1) | 2.28 (7) | 2.977 (7) | 137 (8) |
| C17—H17⋯Cg3iv | 0.95 | 2.80 | 3.525 (6) | 134 |
| C46—H46⋯Cg4iii | 0.95 | 2.72 | 3.670 (6) | 177 |
| C63—H63⋯Cg5v | 0.95 | 2.59 | 3.466 (5) | 154 |
Symmetry codes: (ii)
 ; (iii)
; (iii)
 ; (iv)
; (iv)
 ; (v)
; (v)
 .
.
Figure 3.
A view of the crystal packing along the a axis. The C—H⋯π hydrogen bonds are shown as dashed lines.
Figure 4.
A view of the crystal packing showing C—H⋯F and O—H⋯F intermolecular hydrogen bonds forming chains along the a axis. For clarity, only the major occupancy component of the disordered PF6 − anion is shown.
Database survey
A search of the CSD (version 5.43, last updated November 2021; Groom et al., 2016 ▸) for similar M(dip)3 n+ compounds gave three hits: two compounds with RuII as the central metal cation (n = 2; CSD refcodes LAKCIN: Alatrash & Macdonnell, 2020 ▸; DOWREM: Goldstein et al., 1986 ▸) and one compound with NiII (n = 2; refcode EYAHUI: Hadadzadeh et al., 2011 ▸).
Synthesis and crystallization
[Tris(4,7-diphenyl-1,10-phenanthroline)cobalt(III)] tris(hexafluorophosphate) was obtained following the procedure previously described (McLaurin et al., 2009 ▸). The experimental protocol used for the synthesis has two steps: Firstly, the synthesis of the [bis(4,7-diphenyl-1,10-phenanthroline)cobalt(III) dichloride] chloride was carried out by the reaction of (4,7-diphenyl-1,10-phenanthroline) with cobalt(II) dichloride in methanol at reflux. The obtained compound was oxidized with chlorine gas made in situ to convert CoII to CoIII. Finally, the substitution of the dichloride group for the bidentate ligand (4,7-diphenyl-1,10-phenanthroline) was performed in ethylene glycol at reflux. After cooling to room temperature, ammonium hexafluorophosphate was added to obtain a dark-brown precipitate. The final complex was then isolated by filtration, washed with water and diethyl ether and dried under vacuum. Slow diffusion between methanol and diethyl ether of the acetonitrile solution of the obtained powder gave orange needles of the title compound suitable for X-ray diffraction.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 3 ▸. The C—H hydrogen atoms were positioned geometrically with C—H = 0.95 Å and refined with U iso(H) = 1.2U eq(C). The O—H hydrogen atoms were located in a difference-Fourier map, but their positional and isotropic displacement parameters were restrained with the SHELXL DFIX command and with U iso(H) = 1.5U eq(O), respectively. Four fluorine atoms of one hexafluorophosphate anion (P3 as the central atom) are disordered over two sets of positions with refined site-occupancy factors of 0.697 (5) and 0.303 (5). The corresponding P—F bond lengths and F—P—F bond angles were restrained with the DFIX and DANG commands while the displacement parameters were restrained with the SIMU command.
Table 3. Experimental details.
| Crystal data | |
| Chemical formula | [Co(C24H16N2)3](PF6)3·H2O |
| M r | 1509.02 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 160 |
| a, b, c (Å) | 11.23448 (10), 25.0698 (2), 23.3956 (2) |
| β (°) | 96.9903 (8) |
| V (Å3) | 6540.29 (10) |
| Z | 4 |
| Radiation type | Cu Kα |
| μ (mm−1) | 3.66 |
| Crystal size (mm) | 0.18 × 0.12 × 0.02 |
| Data collection | |
| Diffractometer | XtaLAB Synergy, Dualflex, Pilatus 200K |
| Absorption correction | Analytical [(CrysAlis PRO; Rigaku OD (2019 ▸) based on expressions derived by Clark & Reid, 1995 ▸] |
| T min, T max | 0.595, 0.929 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 71503, 13335, 11500 |
| R int | 0.040 |
| (sin θ/λ)max (Å−1) | 0.625 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.080, 0.243, 1.04 |
| No. of reflections | 13335 |
| No. of parameters | 953 |
| No. of restraints | 272 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 1.88, −1.05 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989022001359/wm5634sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989022001359/wm5634Isup2.hkl
CCDC reference: 2149884
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Crystal data
| [Co(C24H16N2)3](PF6)3·H2O | F(000) = 3064 |
| Mr = 1509.02 | Dx = 1.533 Mg m−3 |
| Monoclinic, P21/c | Cu Kα radiation, λ = 1.54184 Å |
| a = 11.23448 (10) Å | Cell parameters from 32214 reflections |
| b = 25.0698 (2) Å | θ = 2.6–78.7° |
| c = 23.3956 (2) Å | µ = 3.66 mm−1 |
| β = 96.9903 (8)° | T = 160 K |
| V = 6540.29 (10) Å3 | Plate, yellow |
| Z = 4 | 0.18 × 0.12 × 0.02 mm |
Data collection
| XtaLAB Synergy, Dualflex, Pilatus 200K diffractometer | 13335 independent reflections |
| Radiation source: micro-focus sealed X-ray tube, PhotonJet (Cu) X-ray Source | 11500 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.040 |
| Detector resolution: 5.8140 pixels mm-1 | θmax = 74.5°, θmin = 2.6° |
| ω scans | h = −14→13 |
| Absorption correction: analytical [(CrysAlisPro; Rigaku OD (2019) based on expressions derived by Clark & Reid, 1995] | k = −31→31 |
| Tmin = 0.595, Tmax = 0.929 | l = −29→26 |
| 71503 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: dual |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.080 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.243 | w = 1/[σ2(Fo2) + (0.1493P)2 + 10.0383P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.04 | (Δ/σ)max = 0.002 |
| 13335 reflections | Δρmax = 1.88 e Å−3 |
| 953 parameters | Δρmin = −1.05 e Å−3 |
| 272 restraints |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| C1 | 0.4968 (4) | 0.63319 (16) | 0.71887 (18) | 0.0419 (8) | |
| H1 | 0.436429 | 0.642234 | 0.688364 | 0.050* | |
| C2 | 0.4754 (4) | 0.59200 (17) | 0.75643 (18) | 0.0447 (9) | |
| H2 | 0.400029 | 0.574379 | 0.751734 | 0.054* | |
| C3 | 0.5613 (4) | 0.57648 (15) | 0.79997 (17) | 0.0401 (8) | |
| C4 | 0.6716 (3) | 0.60482 (14) | 0.80685 (16) | 0.0366 (7) | |
| C5 | 0.6857 (3) | 0.64682 (14) | 0.76893 (15) | 0.0337 (7) | |
| C6 | 0.7914 (3) | 0.67862 (14) | 0.77523 (15) | 0.0330 (7) | |
| C7 | 0.8836 (3) | 0.66881 (14) | 0.81965 (15) | 0.0344 (7) | |
| C8 | 0.9882 (3) | 0.70185 (15) | 0.82297 (16) | 0.0372 (8) | |
| C9 | 0.9894 (3) | 0.74058 (15) | 0.78113 (17) | 0.0392 (8) | |
| H9 | 1.057717 | 0.762959 | 0.781641 | 0.047* | |
| C10 | 0.8936 (3) | 0.74789 (15) | 0.73821 (16) | 0.0371 (8) | |
| H10 | 0.898217 | 0.775162 | 0.710376 | 0.044* | |
| C11 | 0.7676 (4) | 0.59493 (15) | 0.85110 (17) | 0.0420 (8) | |
| H11 | 0.760653 | 0.566427 | 0.877238 | 0.050* | |
| C12 | 0.8689 (4) | 0.62497 (15) | 0.85710 (17) | 0.0410 (8) | |
| H12 | 0.931331 | 0.616638 | 0.886880 | 0.049* | |
| C13 | 0.5409 (4) | 0.53348 (16) | 0.84122 (18) | 0.0430 (9) | |
| C14 | 0.6087 (4) | 0.48711 (17) | 0.84560 (19) | 0.0476 (9) | |
| H14 | 0.666919 | 0.481392 | 0.819985 | 0.057* | |
| C15 | 0.5927 (4) | 0.44903 (19) | 0.8869 (2) | 0.0558 (11) | |
| H15 | 0.638969 | 0.417219 | 0.889383 | 0.067* | |
| C16 | 0.5082 (5) | 0.4578 (2) | 0.9246 (2) | 0.0623 (13) | |
| H16 | 0.497500 | 0.432103 | 0.953341 | 0.075* | |
| C17 | 0.4402 (5) | 0.5034 (2) | 0.9205 (2) | 0.0612 (13) | |
| H17 | 0.383720 | 0.509189 | 0.946977 | 0.073* | |
| C18 | 0.4524 (4) | 0.54113 (19) | 0.8785 (2) | 0.0530 (10) | |
| H18 | 0.402162 | 0.571714 | 0.874737 | 0.064* | |
| C19 | 1.0915 (3) | 0.69433 (15) | 0.86768 (17) | 0.0398 (8) | |
| C20 | 1.2076 (4) | 0.69364 (18) | 0.8510 (2) | 0.0498 (10) | |
| H20 | 1.218902 | 0.698542 | 0.811734 | 0.060* | |
| C21 | 1.3058 (4) | 0.6857 (2) | 0.8924 (2) | 0.0597 (12) | |
| H21 | 1.384291 | 0.685222 | 0.881279 | 0.072* | |
| C22 | 1.2903 (5) | 0.67864 (19) | 0.9492 (2) | 0.0616 (13) | |
| H22 | 1.357999 | 0.672767 | 0.977012 | 0.074* | |
| C23 | 1.1762 (5) | 0.68000 (18) | 0.9662 (2) | 0.0552 (11) | |
| H23 | 1.166012 | 0.675357 | 1.005603 | 0.066* | |
| C24 | 1.0770 (4) | 0.68816 (16) | 0.92563 (18) | 0.0448 (9) | |
| H24 | 0.999071 | 0.689531 | 0.937390 | 0.054* | |
| C25 | 0.7630 (4) | 0.78075 (15) | 0.59100 (17) | 0.0393 (8) | |
| H25 | 0.776432 | 0.748309 | 0.571790 | 0.047* | |
| C26 | 0.8000 (4) | 0.82847 (17) | 0.56893 (17) | 0.0417 (8) | |
| H26 | 0.836290 | 0.827849 | 0.534316 | 0.050* | |
| C27 | 0.7860 (3) | 0.87685 (16) | 0.59549 (16) | 0.0376 (8) | |
| C28 | 0.7286 (3) | 0.87611 (15) | 0.64690 (15) | 0.0355 (7) | |
| C29 | 0.6894 (3) | 0.82666 (15) | 0.66521 (15) | 0.0336 (7) | |
| C30 | 0.6204 (3) | 0.82295 (14) | 0.71241 (15) | 0.0336 (7) | |
| C31 | 0.5873 (3) | 0.86886 (15) | 0.74036 (15) | 0.0353 (7) | |
| C32 | 0.5039 (3) | 0.86268 (16) | 0.78164 (16) | 0.0385 (8) | |
| C33 | 0.4640 (4) | 0.81197 (18) | 0.79079 (17) | 0.0448 (9) | |
| H33 | 0.406076 | 0.806748 | 0.816649 | 0.054* | |
| C34 | 0.5064 (4) | 0.76813 (17) | 0.76310 (17) | 0.0417 (8) | |
| H34 | 0.479814 | 0.733505 | 0.772105 | 0.050* | |
| C35 | 0.7016 (4) | 0.92207 (16) | 0.67916 (16) | 0.0411 (8) | |
| H35 | 0.732044 | 0.955821 | 0.669557 | 0.049* | |
| C36 | 0.6333 (4) | 0.91869 (15) | 0.72329 (16) | 0.0404 (8) | |
| H36 | 0.615865 | 0.950239 | 0.743172 | 0.048* | |
| C37 | 0.8235 (3) | 0.92742 (16) | 0.57065 (17) | 0.0406 (8) | |
| C38 | 0.7885 (4) | 0.93826 (17) | 0.51282 (18) | 0.0452 (9) | |
| H38 | 0.746110 | 0.912285 | 0.488768 | 0.054* | |
| C39 | 0.8163 (5) | 0.98768 (19) | 0.4904 (2) | 0.0546 (11) | |
| H39 | 0.791980 | 0.995228 | 0.450898 | 0.066* | |
| C40 | 0.8775 (5) | 1.0252 (2) | 0.5241 (2) | 0.0642 (13) | |
| H40 | 0.893511 | 1.059098 | 0.508530 | 0.077* | |
| C41 | 0.9166 (5) | 1.0136 (2) | 0.5815 (2) | 0.0694 (14) | |
| H41 | 0.961487 | 1.039373 | 0.604837 | 0.083* | |
| C42 | 0.8908 (4) | 0.9650 (2) | 0.6047 (2) | 0.0553 (11) | |
| H42 | 0.918661 | 0.957125 | 0.643728 | 0.066* | |
| C43 | 0.4570 (4) | 0.91008 (17) | 0.80923 (17) | 0.0438 (9) | |
| C44 | 0.5344 (4) | 0.94603 (19) | 0.84093 (18) | 0.0497 (10) | |
| H44 | 0.617986 | 0.938852 | 0.847674 | 0.060* | |
| C45 | 0.4885 (5) | 0.9920 (2) | 0.8623 (2) | 0.0669 (14) | |
| H45 | 0.540387 | 1.015941 | 0.884830 | 0.080* | |
| C46 | 0.3678 (6) | 1.0033 (2) | 0.8512 (3) | 0.0749 (16) | |
| H46 | 0.337699 | 1.035581 | 0.865191 | 0.090* | |
| C47 | 0.2904 (5) | 0.9684 (3) | 0.8202 (3) | 0.0775 (17) | |
| H47 | 0.207405 | 0.976544 | 0.812452 | 0.093* | |
| C48 | 0.3352 (4) | 0.9214 (2) | 0.8003 (2) | 0.0599 (12) | |
| H48 | 0.281748 | 0.896444 | 0.780217 | 0.072* | |
| C49 | 0.8241 (3) | 0.64466 (15) | 0.63394 (16) | 0.0369 (8) | |
| H49 | 0.883090 | 0.655760 | 0.664172 | 0.044* | |
| C50 | 0.8531 (3) | 0.60596 (15) | 0.59594 (16) | 0.0370 (8) | |
| H50 | 0.931940 | 0.591600 | 0.600143 | 0.044* | |
| C51 | 0.7700 (3) | 0.58771 (14) | 0.55201 (15) | 0.0328 (7) | |
| C52 | 0.6528 (3) | 0.61036 (14) | 0.54691 (15) | 0.0316 (7) | |
| C53 | 0.6319 (3) | 0.64999 (13) | 0.58581 (15) | 0.0308 (7) | |
| C54 | 0.5186 (3) | 0.67625 (14) | 0.58226 (15) | 0.0327 (7) | |
| C55 | 0.4255 (3) | 0.66234 (14) | 0.53978 (15) | 0.0329 (7) | |
| C56 | 0.3169 (3) | 0.69295 (15) | 0.53654 (17) | 0.0373 (8) | |
| C57 | 0.3126 (4) | 0.73284 (17) | 0.57683 (19) | 0.0442 (9) | |
| H57 | 0.242483 | 0.754196 | 0.575696 | 0.053* | |
| C58 | 0.4091 (3) | 0.74269 (16) | 0.61940 (18) | 0.0424 (9) | |
| H58 | 0.401972 | 0.770082 | 0.646843 | 0.051* | |
| C59 | 0.5533 (3) | 0.59306 (15) | 0.50671 (16) | 0.0357 (7) | |
| H59 | 0.563429 | 0.563724 | 0.482060 | 0.043* | |
| C60 | 0.4445 (3) | 0.61795 (15) | 0.50324 (16) | 0.0354 (7) | |
| H60 | 0.380459 | 0.605677 | 0.476192 | 0.042* | |
| C61 | 0.8004 (3) | 0.54451 (15) | 0.51347 (16) | 0.0358 (7) | |
| C62 | 0.8472 (3) | 0.49669 (16) | 0.53789 (18) | 0.0410 (8) | |
| H62 | 0.861949 | 0.493119 | 0.578564 | 0.049* | |
| C63 | 0.8717 (4) | 0.45475 (17) | 0.5028 (2) | 0.0485 (10) | |
| H63 | 0.902871 | 0.422302 | 0.519458 | 0.058* | |
| C64 | 0.8514 (4) | 0.45979 (19) | 0.4442 (2) | 0.0509 (10) | |
| H64 | 0.864681 | 0.430264 | 0.420356 | 0.061* | |
| C65 | 0.8114 (4) | 0.5080 (2) | 0.41955 (19) | 0.0542 (11) | |
| H65 | 0.802035 | 0.512000 | 0.378877 | 0.065* | |
| C66 | 0.7852 (4) | 0.55008 (18) | 0.45419 (17) | 0.0453 (9) | |
| H66 | 0.756653 | 0.582820 | 0.437249 | 0.054* | |
| C67 | 0.2124 (3) | 0.68412 (15) | 0.49285 (18) | 0.0393 (8) | |
| C68 | 0.2249 (4) | 0.67567 (16) | 0.43524 (18) | 0.0425 (8) | |
| H68 | 0.302622 | 0.674836 | 0.423196 | 0.051* | |
| C69 | 0.1243 (4) | 0.66842 (18) | 0.3950 (2) | 0.0515 (10) | |
| H69 | 0.133435 | 0.662434 | 0.355684 | 0.062* | |
| C70 | 0.0104 (4) | 0.66996 (19) | 0.4125 (2) | 0.0551 (11) | |
| H70 | −0.058344 | 0.664926 | 0.385150 | 0.066* | |
| C71 | −0.0030 (4) | 0.67879 (17) | 0.4697 (2) | 0.0536 (11) | |
| H71 | −0.081008 | 0.680011 | 0.481433 | 0.064* | |
| C72 | 0.0967 (3) | 0.68585 (16) | 0.5098 (2) | 0.0453 (9) | |
| H72 | 0.086903 | 0.691887 | 0.549084 | 0.054* | |
| Co1 | 0.65174 (5) | 0.71899 (2) | 0.67893 (2) | 0.03151 (17) | |
| N1 | 0.6003 (3) | 0.66029 (12) | 0.72480 (13) | 0.0348 (6) | |
| N2 | 0.7953 (3) | 0.71751 (11) | 0.73518 (13) | 0.0333 (6) | |
| N3 | 0.7088 (3) | 0.77952 (12) | 0.63882 (13) | 0.0343 (6) | |
| N4 | 0.5829 (3) | 0.77309 (12) | 0.72446 (13) | 0.0350 (6) | |
| N5 | 0.7153 (3) | 0.66689 (12) | 0.62928 (13) | 0.0317 (6) | |
| N6 | 0.5105 (3) | 0.71465 (12) | 0.62244 (14) | 0.0356 (6) | |
| F1 | 0.7023 (3) | 0.70768 (14) | 0.47885 (13) | 0.0702 (8) | |
| F2 | 0.5998 (3) | 0.78510 (16) | 0.47147 (16) | 0.0821 (10) | |
| F3 | 0.7786 (3) | 0.77811 (14) | 0.43789 (14) | 0.0705 (9) | |
| F4 | 0.6076 (3) | 0.78566 (14) | 0.37517 (14) | 0.0766 (10) | |
| F5 | 0.7095 (3) | 0.70887 (17) | 0.38305 (15) | 0.0810 (10) | |
| F6 | 0.5304 (3) | 0.71560 (14) | 0.41707 (13) | 0.0715 (9) | |
| P1 | 0.65426 (9) | 0.74674 (5) | 0.42702 (5) | 0.0482 (3) | |
| F7 | 0.1732 (3) | 0.82570 (15) | 0.72944 (19) | 0.0954 (12) | |
| F8 | 0.1590 (2) | 0.90313 (11) | 0.67956 (13) | 0.0646 (8) | |
| F9 | −0.0012 (2) | 0.84994 (12) | 0.68175 (14) | 0.0641 (7) | |
| F10 | 0.0829 (4) | 0.85907 (17) | 0.60049 (16) | 0.0907 (11) | |
| F11 | 0.0985 (3) | 0.78177 (13) | 0.6499 (2) | 0.0893 (12) | |
| F12 | 0.2617 (3) | 0.83455 (14) | 0.6485 (2) | 0.1071 (14) | |
| P2 | 0.12975 (10) | 0.84315 (5) | 0.66478 (6) | 0.0546 (3) | |
| F13 | 0.0178 (3) | 0.52703 (16) | 0.67741 (13) | 0.0883 (10) | |
| F14 | 0.1488 (5) | 0.5855 (2) | 0.7846 (2) | 0.1456 (16) | |
| F15A | −0.0113 (13) | 0.5394 (7) | 0.7751 (6) | 0.118 (3) | 0.303 (5) |
| F15B | 0.0464 (8) | 0.5084 (3) | 0.7714 (3) | 0.1140 (18) | 0.697 (5) |
| F16A | 0.1522 (16) | 0.5012 (5) | 0.7440 (7) | 0.121 (2) | 0.303 (5) |
| F16B | 0.2034 (5) | 0.5305 (3) | 0.7229 (3) | 0.1017 (16) | 0.697 (5) |
| F17A | 0.1890 (11) | 0.5803 (7) | 0.6993 (6) | 0.118 (3) | 0.303 (5) |
| F17B | 0.1091 (8) | 0.6030 (3) | 0.6892 (3) | 0.1148 (18) | 0.697 (5) |
| F18A | 0.0146 (12) | 0.6121 (4) | 0.7185 (5) | 0.113 (2) | 0.303 (5) |
| F18B | −0.0489 (6) | 0.5801 (3) | 0.7374 (3) | 0.1184 (18) | 0.697 (5) |
| P3 | 0.08027 (17) | 0.55560 (6) | 0.73172 (6) | 0.0755 (5) | |
| O1 | 0.2529 (5) | 0.6951 (3) | 0.7055 (3) | 0.1140 (19) | |
| H1A | 0.235 (8) | 0.666 (2) | 0.722 (4) | 0.171* | |
| H1B | 0.186 (5) | 0.705 (4) | 0.685 (4) | 0.171* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0364 (19) | 0.043 (2) | 0.047 (2) | −0.0070 (16) | 0.0041 (16) | −0.0024 (16) |
| C2 | 0.038 (2) | 0.045 (2) | 0.051 (2) | −0.0085 (17) | 0.0065 (16) | −0.0037 (18) |
| C3 | 0.047 (2) | 0.0334 (18) | 0.0419 (19) | −0.0061 (15) | 0.0133 (16) | −0.0050 (15) |
| C4 | 0.0432 (19) | 0.0307 (17) | 0.0366 (17) | −0.0028 (15) | 0.0080 (15) | −0.0044 (14) |
| C5 | 0.0342 (17) | 0.0308 (16) | 0.0370 (17) | 0.0017 (13) | 0.0077 (14) | −0.0049 (13) |
| C6 | 0.0337 (17) | 0.0295 (16) | 0.0360 (17) | 0.0021 (13) | 0.0057 (13) | −0.0055 (13) |
| C7 | 0.0355 (17) | 0.0304 (16) | 0.0374 (17) | 0.0010 (14) | 0.0051 (14) | −0.0056 (14) |
| C8 | 0.0363 (18) | 0.0357 (18) | 0.0390 (18) | 0.0025 (15) | 0.0019 (14) | −0.0074 (15) |
| C9 | 0.0357 (18) | 0.0365 (19) | 0.045 (2) | −0.0051 (15) | 0.0047 (15) | −0.0021 (15) |
| C10 | 0.0364 (18) | 0.0341 (18) | 0.0404 (19) | −0.0044 (14) | 0.0037 (15) | −0.0006 (14) |
| C11 | 0.052 (2) | 0.0317 (18) | 0.042 (2) | −0.0030 (16) | 0.0038 (17) | 0.0031 (15) |
| C12 | 0.047 (2) | 0.0338 (18) | 0.0404 (19) | −0.0001 (16) | −0.0003 (16) | 0.0009 (15) |
| C13 | 0.045 (2) | 0.0393 (19) | 0.045 (2) | −0.0108 (16) | 0.0089 (16) | −0.0042 (16) |
| C14 | 0.048 (2) | 0.044 (2) | 0.051 (2) | −0.0096 (18) | 0.0052 (18) | 0.0020 (18) |
| C15 | 0.056 (3) | 0.048 (2) | 0.062 (3) | −0.013 (2) | −0.002 (2) | 0.008 (2) |
| C16 | 0.069 (3) | 0.064 (3) | 0.052 (3) | −0.029 (3) | 0.000 (2) | 0.015 (2) |
| C17 | 0.071 (3) | 0.063 (3) | 0.053 (3) | −0.026 (3) | 0.021 (2) | −0.006 (2) |
| C18 | 0.059 (3) | 0.047 (2) | 0.056 (2) | −0.013 (2) | 0.020 (2) | −0.0057 (19) |
| C19 | 0.0374 (19) | 0.0333 (18) | 0.047 (2) | −0.0022 (15) | −0.0012 (16) | −0.0043 (15) |
| C20 | 0.039 (2) | 0.047 (2) | 0.063 (3) | 0.0004 (17) | 0.0015 (18) | −0.006 (2) |
| C21 | 0.040 (2) | 0.053 (3) | 0.082 (3) | 0.0034 (19) | −0.005 (2) | −0.014 (2) |
| C22 | 0.052 (3) | 0.045 (2) | 0.079 (3) | 0.004 (2) | −0.025 (2) | −0.005 (2) |
| C23 | 0.064 (3) | 0.044 (2) | 0.053 (2) | −0.004 (2) | −0.013 (2) | 0.0004 (19) |
| C24 | 0.045 (2) | 0.0359 (19) | 0.051 (2) | −0.0025 (16) | −0.0055 (17) | −0.0027 (16) |
| C25 | 0.041 (2) | 0.041 (2) | 0.0380 (19) | 0.0063 (15) | 0.0094 (15) | −0.0003 (15) |
| C26 | 0.043 (2) | 0.046 (2) | 0.0382 (19) | 0.0017 (16) | 0.0099 (15) | −0.0009 (16) |
| C27 | 0.0348 (18) | 0.0404 (19) | 0.0370 (18) | 0.0005 (15) | 0.0019 (14) | 0.0011 (15) |
| C28 | 0.0334 (17) | 0.0374 (18) | 0.0350 (17) | 0.0018 (14) | 0.0009 (14) | 0.0006 (14) |
| C29 | 0.0307 (16) | 0.0369 (18) | 0.0328 (16) | 0.0041 (14) | 0.0023 (13) | −0.0019 (14) |
| C30 | 0.0319 (17) | 0.0356 (18) | 0.0328 (16) | 0.0021 (14) | 0.0014 (13) | −0.0008 (14) |
| C31 | 0.0346 (17) | 0.0388 (19) | 0.0317 (16) | 0.0038 (14) | 0.0015 (13) | −0.0038 (14) |
| C32 | 0.0368 (18) | 0.044 (2) | 0.0345 (17) | 0.0033 (15) | 0.0017 (14) | −0.0065 (15) |
| C33 | 0.042 (2) | 0.054 (2) | 0.040 (2) | −0.0024 (18) | 0.0118 (16) | −0.0048 (17) |
| C34 | 0.042 (2) | 0.0412 (19) | 0.043 (2) | −0.0034 (16) | 0.0110 (16) | −0.0015 (16) |
| C35 | 0.048 (2) | 0.0366 (19) | 0.0388 (19) | 0.0003 (16) | 0.0033 (16) | −0.0009 (15) |
| C36 | 0.049 (2) | 0.0347 (18) | 0.0377 (18) | 0.0037 (16) | 0.0041 (16) | −0.0033 (15) |
| C37 | 0.0382 (19) | 0.041 (2) | 0.044 (2) | −0.0007 (15) | 0.0093 (15) | −0.0009 (16) |
| C38 | 0.051 (2) | 0.044 (2) | 0.043 (2) | 0.0030 (18) | 0.0130 (17) | 0.0009 (17) |
| C39 | 0.071 (3) | 0.048 (2) | 0.048 (2) | 0.005 (2) | 0.020 (2) | 0.0078 (19) |
| C40 | 0.085 (4) | 0.049 (3) | 0.064 (3) | −0.008 (2) | 0.027 (3) | 0.000 (2) |
| C41 | 0.079 (4) | 0.062 (3) | 0.070 (3) | −0.029 (3) | 0.019 (3) | −0.009 (3) |
| C42 | 0.057 (3) | 0.056 (3) | 0.053 (2) | −0.015 (2) | 0.010 (2) | −0.002 (2) |
| C43 | 0.045 (2) | 0.049 (2) | 0.0397 (19) | 0.0013 (17) | 0.0107 (16) | −0.0102 (17) |
| C44 | 0.050 (2) | 0.058 (3) | 0.041 (2) | 0.000 (2) | 0.0060 (17) | −0.0137 (19) |
| C45 | 0.072 (3) | 0.067 (3) | 0.065 (3) | −0.012 (3) | 0.022 (3) | −0.031 (3) |
| C46 | 0.076 (4) | 0.066 (3) | 0.087 (4) | 0.005 (3) | 0.028 (3) | −0.034 (3) |
| C47 | 0.057 (3) | 0.082 (4) | 0.096 (4) | 0.016 (3) | 0.022 (3) | −0.028 (3) |
| C48 | 0.046 (2) | 0.067 (3) | 0.068 (3) | 0.001 (2) | 0.014 (2) | −0.025 (2) |
| C49 | 0.0300 (17) | 0.0394 (19) | 0.0405 (18) | 0.0048 (14) | 0.0010 (14) | −0.0018 (15) |
| C50 | 0.0284 (16) | 0.0383 (18) | 0.0446 (19) | 0.0084 (14) | 0.0057 (14) | 0.0016 (15) |
| C51 | 0.0300 (16) | 0.0328 (17) | 0.0361 (17) | 0.0053 (13) | 0.0067 (13) | 0.0044 (14) |
| C52 | 0.0283 (16) | 0.0328 (16) | 0.0340 (16) | 0.0057 (13) | 0.0045 (13) | 0.0040 (13) |
| C53 | 0.0281 (16) | 0.0286 (16) | 0.0357 (17) | 0.0041 (13) | 0.0036 (13) | 0.0044 (13) |
| C54 | 0.0298 (16) | 0.0297 (16) | 0.0390 (17) | 0.0045 (13) | 0.0063 (13) | −0.0001 (13) |
| C55 | 0.0258 (15) | 0.0329 (17) | 0.0396 (18) | 0.0059 (13) | 0.0023 (13) | 0.0037 (14) |
| C56 | 0.0287 (17) | 0.0361 (18) | 0.047 (2) | 0.0056 (14) | 0.0031 (14) | 0.0035 (15) |
| C57 | 0.0332 (18) | 0.044 (2) | 0.054 (2) | 0.0123 (16) | −0.0005 (16) | −0.0041 (18) |
| C58 | 0.0354 (19) | 0.042 (2) | 0.049 (2) | 0.0125 (16) | 0.0021 (16) | −0.0072 (17) |
| C59 | 0.0330 (17) | 0.0353 (18) | 0.0380 (18) | 0.0062 (14) | 0.0013 (14) | −0.0015 (14) |
| C60 | 0.0279 (16) | 0.0373 (18) | 0.0398 (18) | 0.0039 (14) | −0.0007 (13) | −0.0002 (15) |
| C61 | 0.0280 (16) | 0.0392 (19) | 0.0404 (18) | 0.0073 (14) | 0.0050 (14) | −0.0025 (15) |
| C62 | 0.0371 (19) | 0.0384 (19) | 0.048 (2) | 0.0066 (15) | 0.0083 (16) | 0.0017 (16) |
| C63 | 0.041 (2) | 0.039 (2) | 0.067 (3) | 0.0078 (17) | 0.0133 (19) | −0.0022 (19) |
| C64 | 0.0326 (19) | 0.055 (2) | 0.066 (3) | 0.0083 (18) | 0.0083 (18) | −0.018 (2) |
| C65 | 0.043 (2) | 0.074 (3) | 0.045 (2) | 0.018 (2) | 0.0034 (17) | −0.012 (2) |
| C66 | 0.040 (2) | 0.054 (2) | 0.042 (2) | 0.0165 (18) | 0.0045 (16) | −0.0006 (17) |
| C67 | 0.0291 (17) | 0.0308 (17) | 0.056 (2) | 0.0043 (14) | −0.0011 (15) | 0.0028 (16) |
| C68 | 0.0334 (18) | 0.043 (2) | 0.049 (2) | 0.0037 (15) | −0.0016 (16) | 0.0079 (17) |
| C69 | 0.049 (2) | 0.048 (2) | 0.054 (2) | 0.0029 (19) | −0.0098 (19) | 0.0081 (19) |
| C70 | 0.039 (2) | 0.049 (2) | 0.072 (3) | −0.0013 (18) | −0.016 (2) | 0.013 (2) |
| C71 | 0.0287 (19) | 0.040 (2) | 0.089 (3) | 0.0037 (16) | −0.002 (2) | 0.006 (2) |
| C72 | 0.0306 (18) | 0.041 (2) | 0.064 (3) | 0.0064 (15) | 0.0042 (17) | −0.0010 (18) |
| Co1 | 0.0292 (3) | 0.0306 (3) | 0.0345 (3) | 0.0026 (2) | 0.0030 (2) | −0.0013 (2) |
| N1 | 0.0307 (14) | 0.0323 (15) | 0.0411 (16) | 0.0006 (12) | 0.0029 (12) | −0.0025 (12) |
| N2 | 0.0321 (15) | 0.0323 (15) | 0.0356 (15) | −0.0016 (11) | 0.0049 (12) | −0.0019 (11) |
| N3 | 0.0327 (15) | 0.0353 (16) | 0.0349 (15) | 0.0038 (12) | 0.0034 (12) | −0.0009 (12) |
| N4 | 0.0345 (15) | 0.0353 (15) | 0.0349 (15) | 0.0010 (12) | 0.0035 (12) | −0.0010 (12) |
| N5 | 0.0263 (13) | 0.0314 (14) | 0.0370 (15) | 0.0038 (11) | 0.0024 (11) | 0.0007 (12) |
| N6 | 0.0314 (15) | 0.0331 (15) | 0.0422 (16) | 0.0067 (12) | 0.0046 (12) | −0.0014 (12) |
| F1 | 0.0608 (17) | 0.083 (2) | 0.0634 (17) | −0.0028 (15) | −0.0056 (14) | 0.0286 (15) |
| F2 | 0.0568 (18) | 0.109 (3) | 0.078 (2) | 0.0160 (17) | −0.0011 (16) | −0.0121 (19) |
| F3 | 0.0454 (15) | 0.094 (2) | 0.0692 (18) | −0.0200 (14) | −0.0024 (13) | 0.0168 (16) |
| F4 | 0.0611 (18) | 0.095 (2) | 0.0685 (19) | −0.0068 (16) | −0.0116 (15) | 0.0352 (17) |
| F5 | 0.0593 (18) | 0.115 (3) | 0.0678 (19) | 0.0129 (18) | 0.0040 (15) | −0.0164 (18) |
| F6 | 0.0462 (15) | 0.102 (2) | 0.0642 (18) | −0.0228 (15) | 0.0000 (13) | 0.0158 (16) |
| P1 | 0.0321 (5) | 0.0658 (7) | 0.0457 (6) | −0.0021 (5) | 0.0007 (4) | 0.0134 (5) |
| F7 | 0.073 (2) | 0.074 (2) | 0.130 (3) | −0.0179 (17) | −0.029 (2) | 0.026 (2) |
| F8 | 0.0610 (17) | 0.0442 (15) | 0.090 (2) | −0.0024 (12) | 0.0151 (15) | −0.0051 (13) |
| F9 | 0.0478 (14) | 0.0667 (17) | 0.0801 (19) | 0.0022 (13) | 0.0170 (13) | 0.0018 (14) |
| F10 | 0.097 (3) | 0.106 (3) | 0.073 (2) | −0.022 (2) | 0.0282 (19) | −0.0089 (19) |
| F11 | 0.079 (2) | 0.0545 (18) | 0.140 (3) | −0.0101 (15) | 0.037 (2) | −0.0263 (19) |
| F12 | 0.0606 (19) | 0.0581 (18) | 0.212 (4) | 0.0066 (15) | 0.055 (2) | −0.008 (2) |
| P2 | 0.0403 (6) | 0.0423 (6) | 0.0830 (8) | 0.0009 (4) | 0.0152 (5) | −0.0074 (5) |
| F13 | 0.095 (2) | 0.111 (2) | 0.0591 (17) | 0.024 (2) | 0.0099 (16) | 0.0028 (17) |
| F14 | 0.194 (4) | 0.137 (3) | 0.098 (3) | −0.002 (3) | −0.015 (3) | −0.031 (3) |
| F15A | 0.143 (5) | 0.126 (5) | 0.085 (4) | 0.022 (5) | 0.019 (4) | 0.003 (4) |
| F15B | 0.144 (4) | 0.125 (4) | 0.073 (3) | 0.020 (3) | 0.013 (3) | 0.028 (3) |
| F16A | 0.142 (5) | 0.122 (5) | 0.095 (4) | 0.025 (4) | 0.001 (4) | 0.002 (4) |
| F16B | 0.092 (3) | 0.113 (4) | 0.096 (3) | 0.012 (3) | −0.004 (3) | −0.015 (3) |
| F17A | 0.133 (5) | 0.120 (5) | 0.093 (4) | −0.012 (5) | −0.020 (4) | 0.008 (4) |
| F17B | 0.146 (4) | 0.091 (3) | 0.110 (3) | −0.013 (3) | 0.025 (3) | −0.006 (3) |
| F18A | 0.138 (5) | 0.104 (4) | 0.098 (4) | 0.026 (4) | 0.017 (4) | 0.004 (4) |
| F18B | 0.147 (4) | 0.127 (4) | 0.085 (3) | 0.061 (3) | 0.031 (3) | −0.005 (3) |
| P3 | 0.1056 (12) | 0.0701 (9) | 0.0491 (7) | 0.0314 (8) | 0.0027 (7) | −0.0029 (6) |
| O1 | 0.097 (4) | 0.114 (4) | 0.140 (5) | 0.022 (3) | 0.053 (4) | 0.037 (4) |
Geometric parameters (Å, º)
| C1—H1 | 0.9500 | C44—C45 | 1.381 (7) |
| C1—C2 | 1.395 (6) | C45—H45 | 0.9500 |
| C1—N1 | 1.339 (5) | C45—C46 | 1.379 (8) |
| C2—H2 | 0.9500 | C46—H46 | 0.9500 |
| C2—C3 | 1.371 (6) | C46—C47 | 1.377 (9) |
| C3—C4 | 1.421 (5) | C47—H47 | 0.9500 |
| C3—C13 | 1.483 (5) | C47—C48 | 1.385 (7) |
| C4—C5 | 1.398 (5) | C48—H48 | 0.9500 |
| C4—C11 | 1.423 (6) | C49—H49 | 0.9500 |
| C5—C6 | 1.424 (5) | C49—C50 | 1.381 (5) |
| C5—N1 | 1.363 (5) | C49—N5 | 1.336 (5) |
| C6—C7 | 1.396 (5) | C50—H50 | 0.9500 |
| C6—N2 | 1.357 (5) | C50—C51 | 1.380 (5) |
| C7—C8 | 1.432 (5) | C51—C52 | 1.426 (5) |
| C7—C12 | 1.428 (5) | C51—C61 | 1.476 (5) |
| C8—C9 | 1.380 (6) | C52—C53 | 1.386 (5) |
| C8—C19 | 1.477 (5) | C52—C59 | 1.437 (5) |
| C9—H9 | 0.9500 | C53—C54 | 1.427 (5) |
| C9—C10 | 1.391 (5) | C53—N5 | 1.363 (4) |
| C10—H10 | 0.9500 | C54—C55 | 1.396 (5) |
| C10—N2 | 1.336 (5) | C54—N6 | 1.356 (5) |
| C11—H11 | 0.9500 | C55—C56 | 1.435 (5) |
| C11—C12 | 1.357 (6) | C55—C60 | 1.435 (5) |
| C12—H12 | 0.9500 | C56—C57 | 1.379 (6) |
| C13—C14 | 1.387 (6) | C56—C67 | 1.476 (5) |
| C13—C18 | 1.413 (6) | C57—H57 | 0.9500 |
| C14—H14 | 0.9500 | C57—C58 | 1.402 (6) |
| C14—C15 | 1.385 (6) | C58—H58 | 0.9500 |
| C15—H15 | 0.9500 | C58—N6 | 1.334 (5) |
| C15—C16 | 1.390 (8) | C59—H59 | 0.9500 |
| C16—H16 | 0.9500 | C59—C60 | 1.365 (5) |
| C16—C17 | 1.372 (8) | C60—H60 | 0.9500 |
| C17—H17 | 0.9500 | C61—C62 | 1.402 (5) |
| C17—C18 | 1.383 (7) | C61—C66 | 1.384 (5) |
| C18—H18 | 0.9500 | C62—H62 | 0.9500 |
| C19—C20 | 1.406 (6) | C62—C63 | 1.382 (6) |
| C19—C24 | 1.394 (6) | C63—H63 | 0.9500 |
| C20—H20 | 0.9500 | C63—C64 | 1.367 (7) |
| C20—C21 | 1.391 (7) | C64—H64 | 0.9500 |
| C21—H21 | 0.9500 | C64—C65 | 1.390 (7) |
| C21—C22 | 1.372 (8) | C65—H65 | 0.9500 |
| C22—H22 | 0.9500 | C65—C66 | 1.384 (6) |
| C22—C23 | 1.388 (8) | C66—H66 | 0.9500 |
| C23—H23 | 0.9500 | C67—C68 | 1.388 (6) |
| C23—C24 | 1.388 (6) | C67—C72 | 1.406 (5) |
| C24—H24 | 0.9500 | C68—H68 | 0.9500 |
| C25—H25 | 0.9500 | C68—C69 | 1.392 (6) |
| C25—C26 | 1.387 (6) | C69—H69 | 0.9500 |
| C25—N3 | 1.338 (5) | C69—C70 | 1.391 (7) |
| C26—H26 | 0.9500 | C70—H70 | 0.9500 |
| C26—C27 | 1.381 (6) | C70—C71 | 1.381 (8) |
| C27—C28 | 1.432 (5) | C71—H71 | 0.9500 |
| C27—C37 | 1.478 (5) | C71—C72 | 1.383 (6) |
| C28—C29 | 1.400 (5) | C72—H72 | 0.9500 |
| C28—C35 | 1.430 (5) | Co1—N1 | 1.950 (3) |
| C29—C30 | 1.428 (5) | Co1—N2 | 1.954 (3) |
| C29—N3 | 1.363 (5) | Co1—N3 | 1.934 (3) |
| C30—C31 | 1.396 (5) | Co1—N4 | 1.942 (3) |
| C30—N4 | 1.359 (5) | Co1—N5 | 1.941 (3) |
| C31—C32 | 1.434 (5) | Co1—N6 | 1.940 (3) |
| C31—C36 | 1.427 (5) | F1—P1 | 1.600 (3) |
| C32—C33 | 1.373 (6) | F2—P1 | 1.592 (4) |
| C32—C43 | 1.479 (5) | F3—P1 | 1.596 (3) |
| C33—H33 | 0.9500 | F4—P1 | 1.595 (3) |
| C33—C34 | 1.389 (6) | F5—P1 | 1.581 (4) |
| C34—H34 | 0.9500 | F6—P1 | 1.588 (3) |
| C34—N4 | 1.327 (5) | F7—P2 | 1.593 (4) |
| C35—H35 | 0.9500 | F8—P2 | 1.569 (3) |
| C35—C36 | 1.362 (6) | F9—P2 | 1.579 (3) |
| C36—H36 | 0.9500 | F10—P2 | 1.583 (4) |
| C37—C38 | 1.389 (6) | F11—P2 | 1.607 (3) |
| C37—C42 | 1.394 (6) | F12—P2 | 1.590 (3) |
| C38—H38 | 0.9500 | F13—P3 | 1.550 (4) |
| C38—C39 | 1.396 (6) | F14—P3 | 1.567 (4) |
| C39—H39 | 0.9500 | F15A—P3 | 1.583 (9) |
| C39—C40 | 1.359 (7) | F15B—P3 | 1.580 (6) |
| C40—H40 | 0.9500 | F16A—P3 | 1.593 (9) |
| C40—C41 | 1.392 (8) | F16B—P3 | 1.557 (6) |
| C41—H41 | 0.9500 | F17A—P3 | 1.636 (9) |
| C41—C42 | 1.380 (7) | F17B—P3 | 1.608 (6) |
| C42—H42 | 0.9500 | F18A—P3 | 1.610 (7) |
| C43—C44 | 1.400 (6) | F18B—P3 | 1.596 (5) |
| C43—C48 | 1.389 (6) | O1—H1A | 0.872 (5) |
| C44—H44 | 0.9500 | O1—H1B | 0.870 (5) |
| Cg1···Cg2i | 3.707 (3) | ||
| C2—C1—H1 | 119.2 | C53—C52—C59 | 117.8 (3) |
| N1—C1—H1 | 119.2 | C52—C53—C54 | 120.9 (3) |
| N1—C1—C2 | 121.6 (4) | N5—C53—C52 | 123.8 (3) |
| C1—C2—H2 | 119.4 | N5—C53—C54 | 115.3 (3) |
| C3—C2—C1 | 121.3 (4) | C55—C54—C53 | 120.8 (3) |
| C3—C2—H2 | 119.4 | N6—C54—C53 | 114.9 (3) |
| C2—C3—C4 | 117.8 (4) | N6—C54—C55 | 124.3 (3) |
| C2—C3—C13 | 122.7 (4) | C54—C55—C56 | 117.5 (3) |
| C4—C3—C13 | 119.4 (4) | C54—C55—C60 | 117.7 (3) |
| C3—C4—C11 | 124.7 (4) | C60—C55—C56 | 124.8 (3) |
| C5—C4—C3 | 118.0 (4) | C55—C56—C67 | 123.7 (3) |
| C5—C4—C11 | 117.3 (3) | C57—C56—C55 | 116.9 (3) |
| C4—C5—C6 | 120.9 (3) | C57—C56—C67 | 119.4 (3) |
| N1—C5—C4 | 122.9 (3) | C56—C57—H57 | 119.2 |
| N1—C5—C6 | 116.2 (3) | C56—C57—C58 | 121.7 (3) |
| C7—C6—C5 | 120.8 (3) | C58—C57—H57 | 119.2 |
| N2—C6—C5 | 115.3 (3) | C57—C58—H58 | 119.1 |
| N2—C6—C7 | 123.9 (3) | N6—C58—C57 | 121.8 (4) |
| C6—C7—C8 | 117.9 (3) | N6—C58—H58 | 119.1 |
| C6—C7—C12 | 117.5 (3) | C52—C59—H59 | 119.3 |
| C12—C7—C8 | 124.6 (3) | C60—C59—C52 | 121.3 (3) |
| C7—C8—C19 | 122.3 (3) | C60—C59—H59 | 119.3 |
| C9—C8—C7 | 116.6 (3) | C55—C60—H60 | 119.5 |
| C9—C8—C19 | 121.2 (4) | C59—C60—C55 | 121.1 (3) |
| C8—C9—H9 | 119.0 | C59—C60—H60 | 119.5 |
| C8—C9—C10 | 122.1 (4) | C62—C61—C51 | 118.8 (3) |
| C10—C9—H9 | 119.0 | C66—C61—C51 | 121.9 (3) |
| C9—C10—H10 | 119.2 | C66—C61—C62 | 119.3 (4) |
| N2—C10—C9 | 121.6 (3) | C61—C62—H62 | 120.0 |
| N2—C10—H10 | 119.2 | C63—C62—C61 | 120.0 (4) |
| C4—C11—H11 | 119.0 | C63—C62—H62 | 120.0 |
| C12—C11—C4 | 122.0 (4) | C62—C63—H63 | 119.9 |
| C12—C11—H11 | 119.0 | C64—C63—C62 | 120.2 (4) |
| C7—C12—H12 | 119.3 | C64—C63—H63 | 119.9 |
| C11—C12—C7 | 121.5 (4) | C63—C64—H64 | 119.9 |
| C11—C12—H12 | 119.3 | C63—C64—C65 | 120.2 (4) |
| C14—C13—C3 | 122.0 (4) | C65—C64—H64 | 119.9 |
| C14—C13—C18 | 119.3 (4) | C64—C65—H65 | 120.0 |
| C18—C13—C3 | 118.6 (4) | C66—C65—C64 | 120.0 (4) |
| C13—C14—H14 | 119.6 | C66—C65—H65 | 120.0 |
| C15—C14—C13 | 120.8 (4) | C61—C66—C65 | 120.0 (4) |
| C15—C14—H14 | 119.6 | C61—C66—H66 | 120.0 |
| C14—C15—H15 | 120.3 | C65—C66—H66 | 120.0 |
| C14—C15—C16 | 119.4 (5) | C68—C67—C56 | 122.0 (3) |
| C16—C15—H15 | 120.3 | C68—C67—C72 | 119.0 (4) |
| C15—C16—H16 | 119.8 | C72—C67—C56 | 119.0 (4) |
| C17—C16—C15 | 120.4 (4) | C67—C68—H68 | 119.8 |
| C17—C16—H16 | 119.8 | C67—C68—C69 | 120.5 (4) |
| C16—C17—H17 | 119.5 | C69—C68—H68 | 119.8 |
| C16—C17—C18 | 121.1 (5) | C68—C69—H69 | 120.1 |
| C18—C17—H17 | 119.5 | C70—C69—C68 | 119.8 (5) |
| C13—C18—H18 | 120.5 | C70—C69—H69 | 120.1 |
| C17—C18—C13 | 119.0 (5) | C69—C70—H70 | 119.9 |
| C17—C18—H18 | 120.5 | C71—C70—C69 | 120.2 (4) |
| C20—C19—C8 | 118.7 (4) | C71—C70—H70 | 119.9 |
| C24—C19—C8 | 121.9 (4) | C70—C71—H71 | 119.9 |
| C24—C19—C20 | 119.4 (4) | C70—C71—C72 | 120.2 (4) |
| C19—C20—H20 | 120.3 | C72—C71—H71 | 119.9 |
| C21—C20—C19 | 119.4 (5) | C67—C72—H72 | 119.8 |
| C21—C20—H20 | 120.3 | C71—C72—C67 | 120.3 (4) |
| C20—C21—H21 | 119.7 | C71—C72—H72 | 119.8 |
| C22—C21—C20 | 120.7 (5) | N1—Co1—N2 | 83.72 (13) |
| C22—C21—H21 | 119.7 | N3—Co1—N1 | 175.62 (13) |
| C21—C22—H22 | 119.9 | N3—Co1—N2 | 92.66 (13) |
| C21—C22—C23 | 120.3 (4) | N3—Co1—N4 | 84.01 (13) |
| C23—C22—H22 | 119.9 | N3—Co1—N5 | 94.01 (13) |
| C22—C23—H23 | 120.0 | N3—Co1—N6 | 90.31 (13) |
| C22—C23—C24 | 120.0 (5) | N4—Co1—N1 | 93.48 (13) |
| C24—C23—H23 | 120.0 | N4—Co1—N2 | 89.70 (13) |
| C19—C24—H24 | 119.9 | N5—Co1—N1 | 88.67 (13) |
| C23—C24—C19 | 120.1 (4) | N5—Co1—N2 | 93.26 (12) |
| C23—C24—H24 | 119.9 | N5—Co1—N4 | 176.52 (13) |
| C26—C25—H25 | 119.4 | N6—Co1—N1 | 93.44 (13) |
| N3—C25—H25 | 119.4 | N6—Co1—N2 | 175.65 (13) |
| N3—C25—C26 | 121.3 (3) | N6—Co1—N4 | 93.77 (13) |
| C25—C26—H26 | 118.8 | N6—Co1—N5 | 83.36 (12) |
| C27—C26—C25 | 122.3 (4) | C1—N1—C5 | 118.3 (3) |
| C27—C26—H26 | 118.8 | C1—N1—Co1 | 129.6 (3) |
| C26—C27—C28 | 117.0 (3) | C5—N1—Co1 | 112.1 (2) |
| C26—C27—C37 | 121.5 (3) | C6—N2—Co1 | 112.7 (2) |
| C28—C27—C37 | 121.4 (3) | C10—N2—C6 | 118.0 (3) |
| C29—C28—C27 | 117.3 (3) | C10—N2—Co1 | 129.4 (3) |
| C29—C28—C35 | 117.2 (3) | C25—N3—C29 | 118.2 (3) |
| C35—C28—C27 | 125.4 (3) | C25—N3—Co1 | 129.4 (3) |
| C28—C29—C30 | 121.0 (3) | C29—N3—Co1 | 112.3 (2) |
| N3—C29—C28 | 123.8 (3) | C30—N4—Co1 | 112.0 (2) |
| N3—C29—C30 | 115.2 (3) | C34—N4—C30 | 118.0 (3) |
| C31—C30—C29 | 120.6 (3) | C34—N4—Co1 | 130.0 (3) |
| N4—C30—C29 | 115.5 (3) | C49—N5—C53 | 118.0 (3) |
| N4—C30—C31 | 123.7 (3) | C49—N5—Co1 | 129.2 (3) |
| C30—C31—C32 | 117.3 (3) | C53—N5—Co1 | 112.7 (2) |
| C30—C31—C36 | 117.7 (3) | C54—N6—Co1 | 113.2 (2) |
| C36—C31—C32 | 124.9 (3) | C58—N6—C54 | 117.8 (3) |
| C31—C32—C43 | 120.2 (4) | C58—N6—Co1 | 129.0 (3) |
| C33—C32—C31 | 117.2 (3) | F2—P1—F1 | 89.8 (2) |
| C33—C32—C43 | 122.4 (4) | F2—P1—F3 | 89.8 (2) |
| C32—C33—H33 | 119.2 | F2—P1—F4 | 90.6 (2) |
| C32—C33—C34 | 121.5 (4) | F3—P1—F1 | 88.44 (17) |
| C34—C33—H33 | 119.2 | F4—P1—F1 | 179.46 (19) |
| C33—C34—H34 | 119.0 | F4—P1—F3 | 91.15 (17) |
| N4—C34—C33 | 122.1 (4) | F5—P1—F1 | 90.1 (2) |
| N4—C34—H34 | 119.0 | F5—P1—F2 | 179.5 (2) |
| C28—C35—H35 | 119.1 | F5—P1—F3 | 89.7 (2) |
| C36—C35—C28 | 121.7 (4) | F5—P1—F4 | 89.5 (2) |
| C36—C35—H35 | 119.1 | F5—P1—F6 | 90.9 (2) |
| C31—C36—H36 | 119.2 | F6—P1—F1 | 91.10 (17) |
| C35—C36—C31 | 121.5 (4) | F6—P1—F2 | 89.6 (2) |
| C35—C36—H36 | 119.2 | F6—P1—F3 | 179.26 (18) |
| C38—C37—C27 | 119.4 (4) | F6—P1—F4 | 89.32 (17) |
| C38—C37—C42 | 119.6 (4) | F7—P2—F11 | 88.6 (2) |
| C42—C37—C27 | 121.1 (4) | F8—P2—F7 | 91.27 (19) |
| C37—C38—H38 | 120.3 | F8—P2—F9 | 91.02 (16) |
| C37—C38—C39 | 119.3 (4) | F8—P2—F10 | 90.2 (2) |
| C39—C38—H38 | 120.3 | F8—P2—F11 | 179.4 (2) |
| C38—C39—H39 | 119.4 | F8—P2—F12 | 90.37 (18) |
| C40—C39—C38 | 121.1 (4) | F9—P2—F7 | 88.7 (2) |
| C40—C39—H39 | 119.4 | F9—P2—F10 | 90.0 (2) |
| C39—C40—H40 | 120.2 | F9—P2—F11 | 88.40 (18) |
| C39—C40—C41 | 119.6 (5) | F9—P2—F12 | 178.3 (2) |
| C41—C40—H40 | 120.2 | F10—P2—F7 | 178.0 (2) |
| C40—C41—H41 | 119.8 | F10—P2—F11 | 89.8 (2) |
| C42—C41—C40 | 120.5 (5) | F10—P2—F12 | 91.0 (3) |
| C42—C41—H41 | 119.8 | F12—P2—F7 | 90.2 (3) |
| C37—C42—H42 | 120.1 | F12—P2—F11 | 90.2 (2) |
| C41—C42—C37 | 119.8 (5) | F13—P3—F14 | 176.9 (3) |
| C41—C42—H42 | 120.1 | F13—P3—F15A | 98.2 (7) |
| C44—C43—C32 | 121.1 (4) | F13—P3—F15B | 91.1 (3) |
| C48—C43—C32 | 119.6 (4) | F13—P3—F16A | 85.5 (7) |
| C48—C43—C44 | 119.1 (4) | F13—P3—F16B | 91.4 (3) |
| C43—C44—H44 | 120.2 | F13—P3—F17A | 94.8 (6) |
| C45—C44—C43 | 119.6 (4) | F13—P3—F17B | 86.5 (3) |
| C45—C44—H44 | 120.2 | F13—P3—F18A | 95.6 (4) |
| C44—C45—H45 | 119.8 | F13—P3—F18B | 85.4 (3) |
| C46—C45—C44 | 120.4 (5) | F14—P3—F15A | 84.9 (7) |
| C46—C45—H45 | 119.8 | F14—P3—F15B | 91.4 (4) |
| C45—C46—H46 | 119.6 | F14—P3—F16A | 94.4 (7) |
| C47—C46—C45 | 120.8 (5) | F14—P3—F17A | 82.0 (6) |
| C47—C46—H46 | 119.6 | F14—P3—F17B | 91.1 (4) |
| C46—C47—H47 | 120.4 | F14—P3—F18A | 84.3 (4) |
| C46—C47—C48 | 119.1 (5) | F14—P3—F18B | 96.6 (3) |
| C48—C47—H47 | 120.4 | F15A—P3—F16A | 91.2 (6) |
| C43—C48—H48 | 119.5 | F15A—P3—F17A | 166.9 (9) |
| C47—C48—C43 | 121.0 (5) | F15A—P3—F18A | 91.7 (8) |
| C47—C48—H48 | 119.5 | F15B—P3—F17B | 177.3 (4) |
| C50—C49—H49 | 119.2 | F15B—P3—F18B | 87.3 (4) |
| N5—C49—H49 | 119.1 | F16A—P3—F17A | 91.0 (9) |
| N5—C49—C50 | 121.7 (3) | F16A—P3—F18A | 176.7 (9) |
| C49—C50—H50 | 119.3 | F16B—P3—F14 | 86.5 (3) |
| C51—C50—C49 | 121.4 (3) | F16B—P3—F15B | 93.3 (4) |
| C51—C50—H50 | 119.3 | F16B—P3—F17B | 88.1 (4) |
| C50—C51—C52 | 117.7 (3) | F16B—P3—F18B | 176.8 (3) |
| C50—C51—C61 | 120.8 (3) | F18A—P3—F17A | 85.9 (5) |
| C52—C51—C61 | 121.4 (3) | F18B—P3—F17B | 91.2 (4) |
| C51—C52—C59 | 124.8 (3) | H1A—O1—H1B | 104.3 (13) |
| C53—C52—C51 | 117.3 (3) | ||
| C1—C2—C3—C4 | 1.9 (6) | C33—C32—C43—C44 | 126.5 (5) |
| C1—C2—C3—C13 | 179.0 (4) | C33—C32—C43—C48 | −58.2 (6) |
| C2—C1—N1—C5 | −0.2 (6) | C33—C34—N4—C30 | 0.3 (6) |
| C2—C1—N1—Co1 | −179.7 (3) | C33—C34—N4—Co1 | −178.3 (3) |
| C2—C3—C4—C5 | 0.2 (5) | C35—C28—C29—C30 | 3.0 (5) |
| C2—C3—C4—C11 | 177.6 (4) | C35—C28—C29—N3 | −179.9 (3) |
| C2—C3—C13—C14 | 118.2 (5) | C36—C31—C32—C33 | 176.6 (4) |
| C2—C3—C13—C18 | −64.2 (5) | C36—C31—C32—C43 | 1.2 (6) |
| C3—C4—C5—C6 | 176.8 (3) | C37—C27—C28—C29 | 176.1 (3) |
| C3—C4—C5—N1 | −2.4 (5) | C37—C27—C28—C35 | 0.1 (6) |
| C3—C4—C11—C12 | −176.9 (4) | C37—C38—C39—C40 | 0.4 (7) |
| C3—C13—C14—C15 | 176.1 (4) | C38—C37—C42—C41 | 3.3 (7) |
| C3—C13—C18—C17 | −174.3 (4) | C38—C39—C40—C41 | 2.1 (8) |
| C4—C3—C13—C14 | −64.8 (5) | C39—C40—C41—C42 | −1.8 (9) |
| C4—C3—C13—C18 | 112.8 (5) | C40—C41—C42—C37 | −0.9 (8) |
| C4—C5—C6—C7 | −0.3 (5) | C42—C37—C38—C39 | −3.1 (6) |
| C4—C5—C6—N2 | 178.9 (3) | C43—C32—C33—C34 | 178.0 (4) |
| C4—C5—N1—C1 | 2.4 (5) | C43—C44—C45—C46 | −1.9 (8) |
| C4—C5—N1—Co1 | −177.9 (3) | C44—C43—C48—C47 | 2.7 (8) |
| C4—C11—C12—C7 | 1.0 (6) | C44—C45—C46—C47 | 1.9 (10) |
| C5—C4—C11—C12 | 0.5 (6) | C45—C46—C47—C48 | 0.4 (10) |
| C5—C6—C7—C8 | 179.1 (3) | C46—C47—C48—C43 | −2.7 (10) |
| C5—C6—C7—C12 | 1.8 (5) | C48—C43—C44—C45 | −0.4 (7) |
| C5—C6—N2—C10 | −178.6 (3) | C49—C50—C51—C52 | 0.3 (5) |
| C5—C6—N2—Co1 | 0.0 (4) | C49—C50—C51—C61 | −177.0 (3) |
| C6—C5—N1—C1 | −176.8 (3) | C50—C49—N5—C53 | 0.5 (5) |
| C6—C5—N1—Co1 | 2.9 (4) | C50—C49—N5—Co1 | 176.9 (3) |
| C6—C7—C8—C9 | −0.7 (5) | C50—C51—C52—C53 | 1.4 (5) |
| C6—C7—C8—C19 | −179.0 (3) | C50—C51—C52—C59 | −174.8 (3) |
| C6—C7—C12—C11 | −2.1 (6) | C50—C51—C61—C62 | 53.0 (5) |
| C7—C6—N2—C10 | 0.6 (5) | C50—C51—C61—C66 | −126.4 (4) |
| C7—C6—N2—Co1 | 179.2 (3) | C51—C52—C53—C54 | 177.5 (3) |
| C7—C8—C9—C10 | 0.8 (6) | C51—C52—C53—N5 | −2.3 (5) |
| C7—C8—C19—C20 | 132.8 (4) | C51—C52—C59—C60 | −177.9 (4) |
| C7—C8—C19—C24 | −47.5 (5) | C51—C61—C62—C63 | 177.3 (4) |
| C8—C7—C12—C11 | −179.3 (4) | C51—C61—C66—C65 | −178.0 (4) |
| C8—C9—C10—N2 | −0.3 (6) | C52—C51—C61—C62 | −124.1 (4) |
| C8—C19—C20—C21 | −178.9 (4) | C52—C51—C61—C66 | 56.4 (5) |
| C8—C19—C24—C23 | 178.6 (4) | C52—C53—C54—C55 | 0.5 (5) |
| C9—C8—C19—C20 | −45.5 (5) | C52—C53—C54—N6 | −179.2 (3) |
| C9—C8—C19—C24 | 134.3 (4) | C52—C53—N5—C49 | 1.4 (5) |
| C9—C10—N2—C6 | −0.5 (5) | C52—C53—N5—Co1 | −175.6 (3) |
| C9—C10—N2—Co1 | −178.8 (3) | C52—C59—C60—C55 | −0.1 (6) |
| C11—C4—C5—C6 | −0.9 (5) | C53—C52—C59—C60 | 5.8 (5) |
| C11—C4—C5—N1 | 180.0 (3) | C53—C54—C55—C56 | −175.9 (3) |
| C12—C7—C8—C9 | 176.5 (4) | C53—C54—C55—C60 | 5.2 (5) |
| C12—C7—C8—C19 | −1.9 (6) | C53—C54—N6—C58 | 176.3 (3) |
| C13—C3—C4—C5 | −177.0 (3) | C53—C54—N6—Co1 | −5.5 (4) |
| C13—C3—C4—C11 | 0.5 (6) | C54—C53—N5—C49 | −178.4 (3) |
| C13—C14—C15—C16 | −0.6 (7) | C54—C53—N5—Co1 | 4.6 (4) |
| C14—C13—C18—C17 | 3.4 (7) | C54—C55—C56—C57 | −1.6 (5) |
| C14—C15—C16—C17 | 0.9 (7) | C54—C55—C56—C67 | 177.9 (3) |
| C15—C16—C17—C18 | 1.1 (8) | C54—C55—C60—C59 | −5.4 (5) |
| C16—C17—C18—C13 | −3.2 (7) | C55—C54—N6—C58 | −3.4 (6) |
| C18—C13—C14—C15 | −1.6 (6) | C55—C54—N6—Co1 | 174.7 (3) |
| C19—C8—C9—C10 | 179.2 (4) | C55—C56—C57—C58 | −0.8 (6) |
| C19—C20—C21—C22 | 0.0 (7) | C55—C56—C67—C68 | −43.7 (6) |
| C20—C19—C24—C23 | −1.7 (6) | C55—C56—C67—C72 | 138.2 (4) |
| C20—C21—C22—C23 | −0.9 (7) | C56—C55—C60—C59 | 175.8 (4) |
| C21—C22—C23—C24 | 0.5 (7) | C56—C57—C58—N6 | 1.2 (7) |
| C22—C23—C24—C19 | 0.8 (6) | C56—C67—C68—C69 | −178.9 (4) |
| C24—C19—C20—C21 | 1.3 (6) | C56—C67—C72—C71 | 178.7 (4) |
| C25—C26—C27—C28 | −1.3 (6) | C57—C56—C67—C68 | 135.8 (4) |
| C25—C26—C27—C37 | −178.6 (4) | C57—C56—C67—C72 | −42.3 (5) |
| C26—C25—N3—C29 | 0.7 (6) | C57—C58—N6—C54 | 0.8 (6) |
| C26—C25—N3—Co1 | −178.7 (3) | C57—C58—N6—Co1 | −177.0 (3) |
| C26—C27—C28—C29 | −1.3 (5) | C59—C52—C53—C54 | −6.0 (5) |
| C26—C27—C28—C35 | −177.3 (4) | C59—C52—C53—N5 | 174.2 (3) |
| C26—C27—C37—C38 | 48.8 (5) | C60—C55—C56—C57 | 177.2 (4) |
| C26—C27—C37—C42 | −132.9 (4) | C60—C55—C56—C67 | −3.2 (6) |
| C27—C28—C29—C30 | −173.4 (3) | C61—C51—C52—C53 | 178.7 (3) |
| C27—C28—C29—N3 | 3.8 (5) | C61—C51—C52—C59 | 2.4 (5) |
| C27—C28—C35—C36 | 171.4 (4) | C61—C62—C63—C64 | 0.4 (6) |
| C27—C37—C38—C39 | 175.2 (4) | C62—C61—C66—C65 | 2.6 (6) |
| C27—C37—C42—C41 | −174.9 (5) | C62—C63—C64—C65 | 3.1 (7) |
| C28—C27—C37—C38 | −128.4 (4) | C63—C64—C65—C66 | −3.8 (7) |
| C28—C27—C37—C42 | 49.9 (6) | C64—C65—C66—C61 | 1.0 (7) |
| C28—C29—C30—C31 | 1.9 (5) | C66—C61—C62—C63 | −3.3 (6) |
| C28—C29—C30—N4 | 177.1 (3) | C67—C56—C57—C58 | 179.7 (4) |
| C28—C29—N3—C25 | −3.5 (5) | C67—C68—C69—C70 | 0.4 (6) |
| C28—C29—N3—Co1 | 176.0 (3) | C68—C67—C72—C71 | 0.5 (6) |
| C28—C35—C36—C31 | 1.3 (6) | C68—C69—C70—C71 | 0.2 (7) |
| C29—C28—C35—C36 | −4.6 (6) | C69—C70—C71—C72 | −0.4 (7) |
| C29—C30—C31—C32 | 171.2 (3) | C70—C71—C72—C67 | 0.0 (6) |
| C29—C30—C31—C36 | −5.1 (5) | C72—C67—C68—C69 | −0.7 (6) |
| C29—C30—N4—C34 | −171.9 (3) | N1—C1—C2—C3 | −2.0 (6) |
| C29—C30—N4—Co1 | 7.0 (4) | N1—C5—C6—C7 | 178.9 (3) |
| C30—C29—N3—C25 | 173.8 (3) | N1—C5—C6—N2 | −1.9 (4) |
| C30—C29—N3—Co1 | −6.6 (4) | N2—C6—C7—C8 | 0.0 (5) |
| C30—C31—C32—C33 | 0.6 (5) | N2—C6—C7—C12 | −177.4 (3) |
| C30—C31—C32—C43 | −174.8 (3) | N3—C25—C26—C27 | 1.7 (6) |
| C30—C31—C36—C35 | 3.6 (6) | N3—C29—C30—C31 | −175.5 (3) |
| C31—C30—N4—C34 | 3.2 (5) | N3—C29—C30—N4 | −0.3 (5) |
| C31—C30—N4—Co1 | −177.9 (3) | N4—C30—C31—C32 | −3.7 (5) |
| C31—C32—C33—C34 | 2.7 (6) | N4—C30—C31—C36 | −180.0 (3) |
| C31—C32—C43—C44 | −58.4 (5) | N5—C49—C50—C51 | −1.3 (6) |
| C31—C32—C43—C48 | 117.0 (5) | N5—C53—C54—C55 | −179.7 (3) |
| C32—C31—C36—C35 | −172.4 (4) | N5—C53—C54—N6 | 0.6 (5) |
| C32—C33—C34—N4 | −3.3 (6) | N6—C54—C55—C56 | 3.8 (5) |
| C32—C43—C44—C45 | 175.0 (4) | N6—C54—C55—C60 | −175.1 (3) |
| C32—C43—C48—C47 | −172.8 (5) |
Symmetry code: (i) x+1, −y+3/2, z+1/2.
Hydrogen-bond geometry (Å, º)
Cg1, Cg2, Cg3 and Cg4 are the centroids of atoms C19–C24, C67–C72, C37–C42 and N5/C49–C53, respectively.
| D—H···A | D—H | H···A | D···A | D—H···A |
| C1—H1···O1 | 0.95 | 2.52 | 3.132 (7) | 122 |
| C2—H2···F17A | 0.95 | 2.54 | 3.344 (14) | 143 |
| C9—H9···F7ii | 0.95 | 2.46 | 3.300 (5) | 148 |
| C10—H10···F9ii | 0.95 | 2.33 | 3.173 (5) | 148 |
| C10—H10···F11ii | 0.95 | 2.80 | 3.383 (5) | 120 |
| C25—H25···F1 | 0.95 | 2.45 | 3.204 (5) | 136 |
| C26—H26···F3 | 0.95 | 2.59 | 3.297 (5) | 132 |
| C42—H42···F15Biii | 0.95 | 2.36 | 3.096 (7) | 134 |
| C48—H48···F7 | 0.95 | 2.39 | 3.328 (6) | 172 |
| C48—H48···F8 | 0.95 | 2.59 | 3.278 (6) | 130 |
| C49—H49···F18Aii | 0.95 | 2.13 | 2.850 (9) | 132 |
| C50—H50···F13ii | 0.95 | 2.53 | 3.180 (5) | 126 |
| C50—H50···F17Bii | 0.95 | 2.72 | 3.392 (10) | 129 |
| C57—H57···F11 | 0.95 | 2.61 | 3.349 (5) | 135 |
| C58—H58···F12 | 0.95 | 2.26 | 2.963 (5) | 130 |
| O1—H1A···F17A | 0.87 (1) | 2.25 (7) | 2.965 (17) | 139 (9) |
| O1—H1A···F17B | 0.87 (1) | 2.19 (8) | 2.817 (10) | 128 (8) |
| O1—H1B···F11 | 0.87 (1) | 2.28 (7) | 2.977 (7) | 137 (8) |
| C17—H17···Cg3iv | 0.95 | 2.80 | 3.525 (6) | 134 |
| C46—H46···Cg4iii | 0.95 | 2.72 | 3.670 (6) | 177 |
| C63—H63···Cg5v | 0.95 | 2.59 | 3.466 (5) | 154 |
Symmetry codes: (ii) x+1, y, z; (iii) −x+1, y+1/2, −z+3/2; (iv) −x+1, y−1/2, −z+3/2; (v) −x+1, −y+1, −z+1.
Funding Statement
This work was funded by University of Carthage; Tunisian Ministry of Higher Education and Scientific Research.
References
- Alatrash, N. & Macdonnell, F. (2020). CSD Communication (refcode: LAKCIN). CCDC, Cambridge, England.
- Caspar, R., Cordier, C., Waern, J. B., Guyard-Duhayon, C., Gruselle, M., Le Floch, P. & Amouri, H. (2006). Inorg. Chem. 45, 4071–4078. [DOI] [PubMed]
- Clark, R. C. & Reid, J. S. (1995). Acta Cryst. A51, 887–897.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Goldstein, B. M., Barton, J. K. & Berman, H. M. (1986). Inorg. Chem. 25, 842–847.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Hadadzadeh, H., Mansouri, G., Rezvani, A., Khavasi, H. R., Skelton, B. W., Makha, M. & Charati, F. R. (2011). Polyhedron, 30, 2535–2543.
- Heinemann, F., Karges, J. & Gasser, G. (2017). Acc. Chem. Res. 50, 2727–2736. [DOI] [PubMed]
- Howerton, B. S., Heidary, D. K. & Glazer, E. C. (2012). J. Am. Chem. Soc. 134, 8324–8327. [DOI] [PubMed]
- McFarland, S. A., Mandel, A., Dumoulin-White, R. & Gasser, G. (2020). Curr. Opin. Chem. Biol. 56, 23–27. [DOI] [PMC free article] [PubMed]
- McLaurin, E. J., Greytak, A. B., Bawendi, M. G. & Nocera, D. G. (2009). J. Am. Chem. Soc. 131, 12994–13001. [DOI] [PMC free article] [PubMed]
- Monro, S., Colón, K. L., Yin, H., Roque, J., Konda, P., Gujar, S., Thummel, R. P., Lilge, L., Cameron, C. G. & McFarland, S. A. (2019). Chem. Rev. 119, 797–828. [DOI] [PMC free article] [PubMed]
- Nagababu, P., Shilpa, M., Satyanarayana, S., Latha, J. N. L., Karthikeyan, K. S. & Rajesh, M. (2008). Transition Met. Chem. 33, 1027–1033.
- Otter, C. A. & Hartshorn, R. M. (2004). Dalton Trans. pp. 150–156. [DOI] [PubMed]
- Rigaku OD (2019). CrysAlis PRO. Rigaku Oxford Diffraction, Yarnton, England.
- Sapp, S. A., Elliott, C. M., Contado, C., Caramori, S. & Bignozzi, C. A. (2002). J. Am. Chem. Soc. 124, 11215–11222. [DOI] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Wang, X.-L., Chao, H., Li, H., Hong, X.-L., Liu, Y.-J., Tan, L.-F. & Ji, L.-N. (2004). J. Inorg. Biochem. 98, 1143–1150. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Yang, C., Nikiforidis, G., Park, J. Y., Choi, J., Luo, Y., Zhang, L., Wang, S.-C., Chan, Y.-T., Lim, J., Hou, Z., Baik, M.-H., Lee, Y. & Byon, H. R. (2018). Adv. Energy Mater. 8, 1702897.
- Zhang, Q.-L., Liu, J.-G., Chao, H., Xue, G.-Q. & Ji, L.-N. (2001). J. Inorg. Biochem. 83, 49–55. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989022001359/wm5634sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989022001359/wm5634Isup2.hkl
CCDC reference: 2149884
Additional supporting information: crystallographic information; 3D view; checkCIF report