Abstract
Introduction
The human orbitofrontal cortex (OFC) is involved in automatic response inhibition and conflict processing, but the mechanism of frequency-specific power changes that control these functions is unknown. Theta and gamma activity have been independently observed in the OFC during conflict processing, while theta-gamma interactions in other brain areas have been noted primarily in studies of memory. Within the OFC, it is possible that theta-gamma phase amplitude coupling (PAC) drives conflict processing.
Objective
This study aims to characterize the coupled relationship between theta and gamma frequency bands in the OFC during conflict processing using a modified Stroop task.
Methods
Eight epilepsy patients implanted with OFC stereotactic electroencephalography (SEEG) electrodes participated in a color-word modified Stroop task. PAC between theta phase and gamma amplitude was assessed to determine the timing and magnitude of neural oscillatory changes. Group analysis was conducted using a non-parametric cluster-permutation t-test on coherence values.
Results
Theta-low gamma (LG) PAC significantly increased in five out of eight patients during successful trials of the incongruent condition compared with the congruent condition. Significant increases in theta-LG PAC were most prominent during cue processing 200-800ms after cue presentation. On group analysis, trial-averaged mean theta-LG PAC was statistically significantly greater in the incongruent condition compared to the congruent condition (p < 0.001, Cohen’s d=0.51).
Conclusion
For the first time, we report that OFC theta phase and LG amplitude coupling increases during conflict resolution. Given the delayed onset after cue presentation, OFC theta-LG PAC may contribute to conflict processing after conflict detection and before motor response. This explanation follows the hypothesis that global theta waves modulate local gamma signals. Understanding this relationship within the OFC will help further elucidate the neural mechanisms of human conflict resolution.
Keywords: Orbitofrontal Cortex, Human, Conflict processing, Local Field Potential, Phase-amplitude coupling
Introduction
The orbitofrontal cortex (OFC) modulates appropriate performance of outcome selection behaviors such as decision-making and impulse control (1-3). Due to these functions, the OFC has been the subject of several studies in addictive behaviors, including online gaming addiction (4), disinhibited eating (5), and gambling disorders (6). Decreased OFC volumes have been associated with poor performance in conflict scenarios such as color-word Stroop tasks, suggesting its involvement with the inhibition of responses to stimuli and the resolution of conflicting scenarios (4, 5). While it is generally agreed upon that the OFC plays a part in outcome-oriented behavior and automatic response inhibition, it is not yet known exactly how power changes in oscillation activity control these processes.
In rodents, increases in OFC theta power (4-7 Hz) (7) and theta/low beta coherence (4-20 Hz) (8) are associated with choosing a preferred reward, and in macaques, theta oscillations are involved in reward-based learning (9). Under this role in value assignment, human gambling studies using functional magnetic resonance imaging (fMRI), magnetoencephalography (MEG), and standardized low-resolution electromagnetic tomography (sLORETA) have shown that OFC theta activity increases with winning scenarios and “near misses” (10) and decreases with losses (11). In conflict processing, theta activity has also been investigated in other regions, such as the anterior cingulate cortex (ACC), due to its perceived role in modulating outcome performance. An electroencephalogram (EEG) study with source localization analysis showed increased theta oscillations from the ACC in the incongruent condition of a conflict resolution task (12). While there is substantial evidence regarding the influence of theta activity on outcome selection, the role of low-gamma activity (30-100 Hz) in this process is not as well studied. In a study of the rodent OFC, low-gamma power was found alongside theta activity and was associated with reward identification during an odor go/no-go discrimination task (13). As for conflict scenarios, low-gamma activity has also been noted in the period of 500ms to 200ms prior to verbal responses for incongruent events in Stroop tasks in several regions of the brain, including the dorsolateral prefrontal, dorsolateral premotor, and supplementary motor areas (14). Increased theta and gamma activity have both been observed in the OFC and, in separate studies, during Stroop tasks (8-14). However, interactions between the two bands have not been studied in the OFC during a modified Stroop task.
There is evidence that theta oscillations are involved in long-term communication between brain areas and may be entrained by external stimulus input and internal neural activities (15). Gamma oscillations are thought to reflect the processing activities of local areas (16). Cross-frequency coupling (CFC), in which the phase or amplitude of one frequency range modulates the phase or amplitude of another, is believed to be the coordination mechanism between large-scale brain networks operating at behavioral timescales and local networks operating at computational and synaptic timescales (17). A commonly observed instance of CFC is phase-amplitude coupling (PAC), where the phase of low frequency oscillations modifies the amplitude of high frequency oscillations (18), which has been observed during successful memory retrieval tasks in prefrontal (19), frontotemporal (20-23), and hippocampal (22, 24, 25) brain areas. PAC between theta and low-gamma ranges increased during recall success in those various memory tasks. The prevailing understanding is that theta waves throughout the brain coordinate local gamma amplitude modulation to facilitate memory representation and mnemonic formation in cortical and hippocampal networks. However, understanding of theta-gamma PAC outside of the field of memory remains limited. To understand the neural communication of three sub-processes of conflict processing (conflict detection, resolution, and adaption) proposed by early studies (26, 27), Oehrn et al. used intracranial electroencephalography (iEEG) to measure theta-gamma PAC changes during an auditory Stroop task not only within dorsomedial prefrontal cortex (DMPFC) but also inter-regionally between the DMPFC and dorsolateral prefrontal cortex (DLPFC) (28). Increased theta-gamma PAC within the DLPFC after incongruent cue presentation is thought to correspond with the sub-process of conflict detection (26, 27), and DMPFC theta phase modulation of DLPFC gamma power increases after verbal response in the same incongruent condition. Increased theta-gamma PAC within the DLPFC after incongruent cue presentation was predictive of correct task performance, and DMPFC theta phase modulation of DLPFC gamma power increased after verbal response in the same incongruent condition. While this compelling relationship is well documented in the PFC, its role is less clear for other brain structures such as the OFC. In a rodent study by van Wingerden et al., OFC theta-gamma PAC during an olfactory decision-making task increased while sampling an odor, with the largest effect occurring when rats made correct decisions based on odor (2). The authors hypothesized that increased OFC theta-gamma PAC has a role both in stimulus evaluation and subsequent decision-making.
Given the known role of theta-gamma PAC in human conflict resolution (28), and the involvement of the OFC in stimulus evaluation and the Stroop task (2, 13), this study aims to characterize the role of theta-gamma PAC changes during the performance of a modified Stroop task in the human OFC using stereotactic electroencephalography (SEEG). In line with findings in other brain areas and rodent models, we hypothesize that increased coupling between theta phase and gamma amplitude will be observed within the OFC during conflict processing in the Stroop task.
Methods
Participants
Eight patients with epilepsy (age 20-62; three females) were included in this study. Patients with implanted SEEG electrodes were monitored in the epilepsy monitoring unit (EMU) at the Keck Hospital (University of Southern California) for approximately one week for clinically indicated seizure monitoring and localization. Team members included epileptologists, neurosurgeons, and approved study researchers. The brain regions and number of SEEG electrodes implanted were based only on clinical indications and never for solely research. All study activities were performed secondarily to clinical activities. Before SEEG implantation, the patients were invited to participate in this study approved by the University of Southern California Health Science Campus Institutional Review Board (HS-17-00554). Authorized research personnel discussed the study activities, risks, and benefits with patients to ensure full informed consent. All study participants were instructed that they could terminate their participation in the study at any time. A study session of the modified Stroop task lasted for 30 minutes, including instruction, practice, and actual performance. Participants were given a 10-minute break after finishing a single session and could then choose whether to perform a second session. No more than 2 sessions were performed per day. Patient enrollment occurred over 18 months (March 2019 through August 2020). Patient characteristics, including seizure onset zone (SOZ), are presented in Table 1.
Table 1.
Patient profiles
| ID | Gender | Age | Handedness | Wada test (Hemisphere) |
Seizure onset zone (SOZ) |
|---|---|---|---|---|---|
| 1 | F | 26 | Right | NA | Right Hippocampus |
| 2 | F | 21 | Right | NA | Right Hippocampus |
| 3 | M | 23 | Left | Left dominant | Right Amygdala |
| 4 | M | 31 | Right | Left dominant | Right Orbitofrontal Right Hippocampus |
| 5 | M | 36 | Right | NA | Right Anterior Cingulate Right Superior Temporal Right Premotor Cortex RPC13,RAR4,RPR4 |
| 6 | M | 32 | Right | NA | Right Hippocampus Right Anterior Insula |
| 7 | M | 40 | Right | NA | Left Mesial Temporal Left Hippocampus Left Amygdala |
| 8 | M | 27 | Right | NA | Left Hippocampus |
Study participant characteristics, including seizure onset zone and Wada test results (when applicable).
Electrodes and Recording Equipment
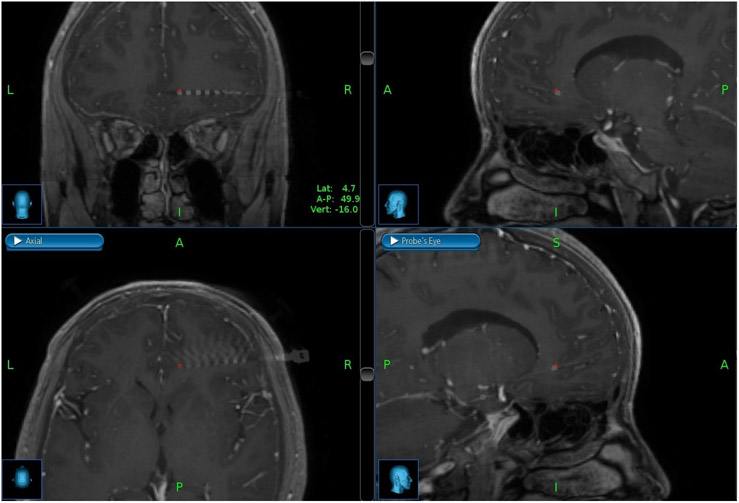
AD-TECH (Oak Creek, WI) depth electrodes, each with 8-10 macro ring-style contacts having a diameter of 1.1mm, length of 1.57mm, and center-to-center pitch 10mm, were implanted in each patient. Implanted electrode locations were confirmed with post-surgical CT imaging merged with pre-operative MRIs. Figure 1 shows merged CT/MRI images for a representative patient (2) in the sagittal, coronal, transverse, and probe’s-eye planes in the OFC. The NeuroPort™ Neural Signal Processor (Blackrock Microsystems, Salt Lake City, UT) was used to record the local field potentials (LFP). LFP data was digitally sampled at 2,000 samples/sec with 16 bits and 250nV resolution from the macro contacts in the gray matter of the OFC. Recording contacts were referenced to a quiet white matter contact identified by a study epileptologist during neural signal acquisition. Table 2 details characteristics of the implanted electrodes in each patient, including the number of electrodes in the OFC.
Figure 1.
Merged CT/MRI images for a representative patient in coronal, sagittal, axial, and probe’s eye planes within the orbitofrontal cortex.
Table 2.
Information of implanted leads in the orbitofrontal cortex (OFC)
| ID | # of implanted in OFC | Number of contacts in target gray matter |
Total number of contacts per lead |
|
|---|---|---|---|---|
| 1 | Right | 1 (Horizontal) | 2 | 10 |
| 2 | Right | 1 (Horizontal) | 3 | 8 |
| 3 | Right | 1 (Horizontal) | 2 | 10 |
| 4 | Right | 3 (Vertical) | 3 | 10 |
| Left | 2 (Horizontal & Vertical) | 4 | 10 | |
| 5 | Right | 1 (Horizontal) | 2 | 8 |
| 6 | Right | 2 (Horizontal & Vertical) | 1 | 8 |
| Left | 1 (Horizontal) | 2 | 8 | |
| 7 | Left | 1 (Vertical) | 2 | 10 |
| 8 | Left | 1 (Horizontal) | 4 | 10 |
Characteristics of implanted electrodes in each patient in the orbitofrontal cortex.
Experimental Paradigm
The modified Stroop task was implemented in MATLAB® (2018b, The MathWorks, Inc., United States) with the Psychophysics toolbox (29, 30). This modified task utilized eight colors: “Pink”, “Purple”, “Brown”, and “White” were added to the classical version with original four colors: “Red”, “Yellow”, “Green”, and “Blue”. The task was presented on a touch-screen monitor set up approximately 60cm away from the patient during the performance of the tasks. Participants were instructed to respond verbally into a microphone as soon as possible after each cue.
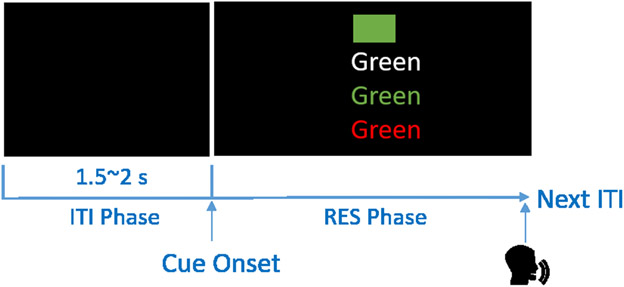
The task included four conditions (Figure 2). The first task condition was aimed at familiarizing participants with the colors. Participants were asked to name the color of a 15cm x 10cm solid color block shown with a black background on the monitor. In the second task condition, participants read the text on the monitor in white-colored font (e.g., the word “Green”). In the third condition, participants were required to name the color of the font which was congruent with the text (e.g., the word “Green” printed on the screen in green-colored font). In the last condition, the presented text was incongruent with the font color (e.g., the word “Green” printed on the screen in red-colored font). Participants were instructed to name the color of the font. A single task session was composed of 96 trials: 16 trials each for the first and second conditions (2 repetitions of each color) and 32 trials each for the third and fourth conditions (4 repetitions of each color). Congruent and incongruent trials were pseudo-randomly interleaved throughout the third and fourth conditions. One to two seconds (randomly selected with uniform probability) of black screen separated each trial (inter-trial interval; ITI). Participants were asked to restrict any movement during the task.
Figure 2.
The four conditions of the modified Stroop Task are presented with the correct response in parenthesis: 1) Name the color of the rectangle (“GREEN”); 2) read the text when the word is presented in white (“GREEN”); 3) name the text color when the written text matches the color (“GREEN”); 4) name the text color when the written text does not match the color (“RED”).
Artifact trial rejection and line noise removal
For artifact rejection, multi-taper spectrums (time-bandwidth product: 5; number of leading tapers: 9; frequency range of interest: 0.1 to 300Hz) were generated for each trial and channel using the Chronux software package (31, 32) in MATLAB® (2018b, The MathWorks, Inc., United States). Trials with power greater than 1,000μV2 between 0.1-1Hz were excluded from the analysis. Moreover, trials containing interictal spikes in the raw data, as identified by the manual review, were discarded. The number of artifact-free trials and total trials for the congruent and incongruent trial conditions for each patient is shown in Table 3. Line noise removal was processed for the remaining artifact-free trials by applying the “rmlinesmovingwinc” function in the Chronux software package. This function removed significant sine waves from continuous data by computing the F-statistic for the sine wave in overlapping moving windows. The remaining trials were trimmed to 3.5 second stimulus-locked segments (1.5s pre-stimulus, 2s post-stimulus) and 3.5 second response-locked segments (1.5s pre-response, 2s post-response) for further analysis. The period before cue onset in stimulus-locked segments was used as a baseline, while the period after cue onset is recognized as the cue-processing period. The pre-response period was defined as the period before the response in the response-locked segments.
Table 3.
Summary of the number of contacts showing significant phase-amplitude coupling (PAC) Change compared to ITI period
| Stimulus-locked segments | ||||
|---|---|---|---|---|
| ID | Number of contact shows active theta-LG PAC in incongruent condition |
Number of artifact-free trials/Number of total trials | ||
| Congruent | Incongruent | |||
| 1 | R | 0/2 | 62/64 | 60/64 |
| 2 | R | 1/3 | 62/64 | 59/64 |
| 3 | R | 1/2 | 61/64 | 57/64 |
| 4 | R | 0/3 | 60/64 | 61/64 |
| L | 0/4 | 60/64 | 61/64 | |
| 5 | R | 1/2 | 61/64 | 57/64 |
| 6 | R | 0/1 | 59/64 | 57/64 |
| L | 0/2 | 59/64 | 57/64 | |
| 7 | L | 2/2 | 24/32 | 23/32 (TRmin) |
| 8 | L | 2/4 | 55/64 | 47/64 |
| Response-locked segment | ||||
| ID | Number of contact shows active theta PAC |
Number of artifact-free trials/Number of total trials | ||
| Congruent | Incongruent | |||
| 1 | R | 0/2 | Same as the numbers in stimulus-locked information | |
| 2 | R | 1/3 | ||
| 3 | R | 0/3 | ||
| 4 | R | 0/3 | ||
| L | 0/4 | |||
| 5 | R | 0/2 | ||
| 6 | R | 0/1 | ||
| L | 0/2 | |||
| 7 | L | 0/2 | ||
| 8 | L | 0/4 | ||
Summary of contact in OFC for each patient. The total number of contacts and the number of contacts showing active theta-LG OAC changes are shown. Information on the number of trials that persisted for analysis after artifact removal is also displayed.
Phase-amplitude coupling (PAC) calculation
Statistical analyses and PAC calculations were performed at the level of individual contacts. For each gray matter contact in the OFC, PAC between phase in the low frequency range (2-45Hz) and amplitude in the high frequency range (30-300Hz) was calculated for each trial. Table 2 shows contact information, including the number of contacts in OFC gray matter for each patient. All patients except two (#4 and #6) had unilateral implantation in the OFC. We used the Kullback–Liebler (KL)-based modulation index (MI) method to evaluate PAC in the OFC during color-word conflict and non-conflict conditions. MI is a measure of distance from a uniform distribution to an observed statistical dependence between two frequency-range signals based on the concept of Kullback–Leibler distance (33-36). The first application of MI as a PAC measure was presented in the work by Tort and his colleagues, in which theta-gamma PAC during the learning of item-context associations was analyzed (37, 38). Here, we used the same protocol of calculating PAC (37-39).
We constructed a phase-amplitude comodulogram plot for presenting the level of coupling among multiple frequency pairs for each stimulus-locked and response-locked segment. The color represented in a given coordinate (x, y) of the comodulogram indicates how much the phase of the x frequency modulates the amplitude of the y frequency. The comodulogram was obtained by scanning multiple narrow-filtered frequency band pairs and calculating MI in each. Narrow filtering used two-way least-squares FIR filtering implemented in MATLAB from the EEGLAB toolbox (38). The bandwidths and steps used during scanning multiple narrowed-filtered frequency band pairs are as follows: 4Hz bandwidths with 2Hz steps for the phase-modulated frequency bands and 2Hz bandwidths with 0.5Hz for the amplitude-modulated frequency. The sizes of bandwidths and steps were chosen in accordance with previous literature outlining optimal frequency resolutions to generate comodulograms (40). Next, a Hilbert transform was used to extract the instantaneous phase and the instantaneous amplitude envelope from each segmented section in low and high frequency-filtered signals, respectively. Next, the instantaneous amplitude envelope distribution was computed for every 20° interval of the instantaneous phase calculated from the previous Hilbert transform. The coupling degree, which is the MI value, between the phase of low-frequency rhythm and the high-frequency amplitude was then determined by computing the entropy values of this distribution and normalizing by the maximum entropy value.
Within each subject, MI values were calculated in stimulus-locked segments and response-locked segments depending on the purpose of the comparison. The first comparison was conducted within one trial condition to compare MI values at baseline (ITI period) with values during the cue-processing period or pre-response period. MI values at baseline (ITI period) were determined. Then, for the first comparison conducted for stimulus-locked segments, the cue-processing period (length: 600ms) was defined as the period between 200ms and 800ms after cue presentation. MI values during the cue-processing period were compared to the values during baseline. For the response-locked segments, the pre-response period relevant for analysis was defined to be −700ms to −100ms (length: 600ms) prior to verbal response, and the MI values during this period were compared to baseline values.
The second comparison was conducted between two trial conditions to compare MI values at multiple time windows during the cue-processing and pre-response periods, respectively. For the comparison conducted during the cue-processing period, four investigating windows starting 200ms after cue onset (length: 600ms; step: 200ms) were extracted in stimulus-locked segments.
Statistical Analysis
A non-parametric cluster-permutation t-test was used to identify data points with statistically significant data points in the theta-frequency phase and LG frequency amplitude. This statistical method was chosen because it has been previously used to control for family-wise Type I error without making assumptions on the underlying sample distribution in multi-channel time-frequency neural data (41). First, for the comparison of MI values between the baseline and cue-processing period, the −800ms to −200ms period prior to the cue and the 200ms to 800ms period following the cue were labelled as the “resting phase” and “cue-processing phase,” respectively (2). Next, for each gray matter contact, the label of these two phases was randomly shuffled 1000 times across all trials. For each shuffle, a t-value and corresponding p-value, with a null hypothesis that there is a difference in MI between baseline and cue-processing phases, were calculated for each MI data point. MI points were only included and clustered if their p-value was smaller than 0.05. Using an uncorrected alpha value is appropriate to control for family-wise Type I error because a whole grid of MI data points was compared using the same statistical test rather than testing for an individual MI point. Only the largest cluster containing the most significant data points was labelled, and the sum of all t-values in this cluster was calculated. The shuffling process was repeated 1,000 times to generate a representative null distribution of summed t-values for the cue-processing phase for each gray matter contact. The 95th percentile value of this distribution was the threshold to determine significant clusters from unshuffled data in further single-sided testing. All non-parametric statistical testing was conducted with single-sided hypothesis testing when labeling polygons showing statistically significant differences in comodulograms. Single-sided testing was chosen rather than doublesided testing because, in this study, we focused on positive PAC changes which are represented on the right side of the null distribution of summed t-values. Finally, t- and p-values for each MI data point were calculated from unshuffled data, and the largest cluster of significant MI data points was identified as described above. The summed t-value from the largest cluster was compared to the 95th percentile value of the previously generated null distribution from shuffled data. The same protocol was used to compare MI values during the baseline and pre-response period.
The second comparison of MI values between congruent and incongruent trial conditions during cue-processing and pre-response periods were assessed using a similar statistical protocol. The singular difference is that an adjusted alpha level with Bonferroni correction of 0.0125 (0.05/4=0.0125) was applied because we performed the same analysis within each of the four windows separately.
Group Analysis
Group analysis was conducted to compare the calculated mean theta-LG MI values during the cue-processing period in both the incongruent and congruent trial conditions across all the participants. For both congruent and incongruent trial conditions, mean theta-LG MI values were calculated from 200 to 800ms after the cue (cue-processing period) for each stimulus-locked segment from one gray matter contact in each patient. A single gray matter contact was chosen to preserve equal weighting between patients included in group analysis. For patients who demonstrated multiple gray matter contacts showing significant PAC increases from the ITI period to cue-processing period, a random gray matter contact was selected to calculate mean theta-LG values. For the remainder of patients of no available gray matter contacts showing significant PAC increase, the deepest gray matter contact was selected to calculate mean theta-LG values. The smallest number of trials (TRmin=23, from 7 in the congruent trial condition) was applied using the following random sampling process to maintain the same weighting from each patient in the group analysis. For each patient, we drew TRmin samples with replacement from calculated mean theta-LG MI values and further calculated the mean of these samples. This process was repeated 1000 times for each patient in both task conditions. These trial-averaged mean theta-LG MI values were combined across all participants, and a paired t-test was applied to compare the two distributions of trial-averaged mean theta-LG MI values for congruent versus incongruent trial conditions.
The above-mentioned process was also performed for response-locked segments to further compare the mean theta-LG MI values between stimulus-locked and response-locked segments. For response-locked segments, mean theta-LG MI values were calculated from −700 to −100ms before verbal response (pre-response period) in both congruent and incongruent trial conditions. The delta values (incongruent mean theta-LG MI values minus congruent mean theta-LG MI values) were calculated for these two types of segments, and a paired t-test was used to investigate statistical significance between stimulus-locked and response-locked signals.
Behavioral analysis
Verbal responses recorded by microphone synchronously with neural activity were available in all participants. Response times measured from the audio recording of both congruent and incongruent trials were z-scored to the median and the median absolute deviation values of verbal reaction time for each patient. Trials with absolute z-score values greater than three were labeled as outliers and excluded from behavior analysis. To evaluate the sequential trial effect for the different congruency conditions, we compared verbal response times in congruent versus incongruent conditions stratified by the congruency status of the previous condition (42, 43). Two-way ANOVA was used to investigate response time among four combinations: two trial conditions of the previous trial by two trial conditions of the current one (Figure 6). A partial eta square value (η2) was applied to evaluate effect size based on the following standards: 0.01 to 0.06 indicates a small effect; 0.06 to 0.14 indicates a medium effect; larger than 0.14 indicates a large effect (43).
Figure 6.
Verbal response times (VRT) with 95% confidence interval for the four combinations of sequential congruency conditions (CC, CI, IC and II). The red line depicts trial-averaged VRT for current incongruent trial condition (Median values of these two combinations: CI: 1.03s, II: 1.02s), the blue line depicts VRT for the current congruent trial condition (Median values of these two combinations: CC: 0.92; IC: 0.90).
Results
Theta-LG PAC Increases
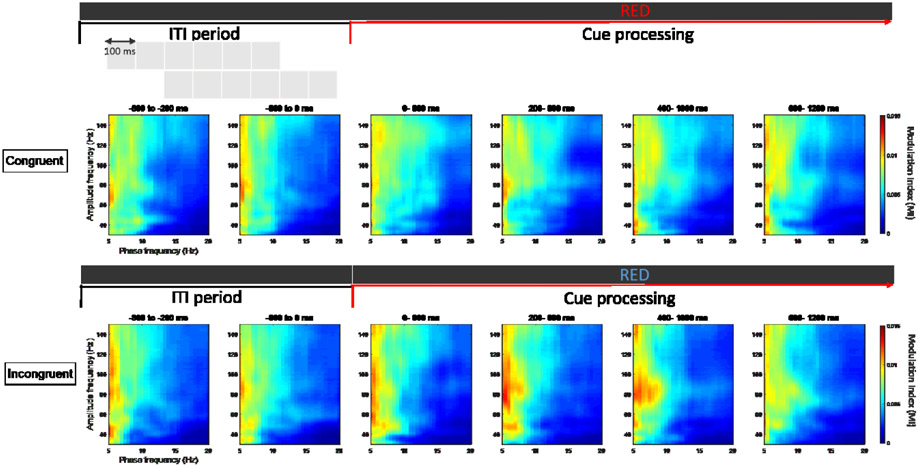
We analyzed PAC changes between baseline and cue presentation in stimulus-locked segments (200-800ms after cue onset (2, 44)) in both congruent and incongruent conditions for each patient. We found that five out of eight patients (the identical patients who showed significant increased theta-LG PAC values from baseline in the OFC during successful trials of the incongruent condition) showed increased theta-LG PAC values from baseline in the OFC during successful trials of the incongruent condition (cluster-based permutation t-test, p<0.05). Table 3 lists the number of gray matter contacts showing active theta-LG PAC changes in the incongruent trial condition for each patient. Figure 3 shows the difference between cue-processing and baseline comodulograms of one gray matter contact for each patient. Significant differences in PAC were only found in the incongruent condition (white polygon, Figure 3).
Figure 3.
Trial-averaged comodulograms for difference between cue-processing period and baseline. Each column depicts different comodulograms (cue-processing period - baseline) of both congruent and incongruent conditions of one gray matter contact for each patient. The upper row shows difference plots for congruent conditions, while the bottom row shows incongruent conditions. The overlaid white polygon encapsulates MI points of significant PAC changes compared to baseline.
Similarly, we examined theta-LG PAC changes between the baseline and the pre-response period in the response-locked segments for both congruent and incongruent conditions within each patient. Only one patient (#5) demonstrated significant theta-LG PAC changes during the pre-response period compared to the baseline (cluster-based permutation t-test, p<0.05). Figure 4 shows the difference between pre-response and baseline comodulograms of one gray matter contact for each patient. Significant differences of PAC found in patient #5 for the incongruent condition were also labeled by overlaid polygon (white polygon, Figure 4).
Figure 4.
Trial-averaged comodulograms for difference between pre-response period and baseline. Each column depicts difference comodulograms (pre-response period - baseline) of both congruent and incongruent conditions of one gray matter contact for each patient. The upper row shows difference plots for congruent conditions, while the bottom row shows incongruent conditions. Only for patient 5, the overlaid white polygon encapsulates MI points of significant PAC changes compared to baseline.
We analyzed multiple time windows in both cue-processing and pre-response periods to characterize the relevant time frame in detecting PAC pattern changes. Five out of eight patients showed significant differences in theta-LG PAC values between the congruent and incongruent conditions in the 200 to 800ms time window during the cue-processing period (Figure 5). One of these five patients (patient #5) demonstrated significant differences in two consecutive analysis windows, 200 to 800ms and 400 to 1000ms. Next, we investigated the multiple sliding windows during the pre-response period. No participant demonstrated significant pre-response PAC differences between congruent and incongruent conditions.
Figure 5.
Comparison of PAC between congruent and incongruent trial conditions at multiple time windows during the cue-processing period, contributed from one gray matter contact of one patient (P05). The upper row depicts trial-averaged comodulogram in 600 ms windows, time-locked to the cue onset for congruent condition, while bottom row indicates plots for incongruent condition. Colors indicate a magnitude scale from low (blue) to high (red) MI magnitude. Theta-low gamma PAC increases only for the incongruent trial condition and shows significant differences between trial conditions at two consecutive time windows, 200-800 and 400-1000 ms.
Group Analysis
Group analysis was conducted to compare trial-averaged mean theta-LG MI values during the cue-processing period for congruent versus incongruent conditions across patients. A paired sample t-test was conducted for this comparison, and the results showed a statistically significant difference (equal variances not assumed, t(7999)=37.12, p<0.001, Cohen’s d=0.51). To further investigate the difference between theta-LG MI values between stimulus-locked and response-locked segments, an analysis was conducted to compare delta trial-averaged mean theta-LG MI values when aligned to cue and those values when aligned to the verbal response. A paired sample t-test was conducted for this comparison, and the results showed a statistically significant difference (equal variances not assumed, t(7999)=24.80, p<0.001, Cohen’s d=0.38). The mean of the stimulus-locked distribution is 3.80*10−4, which is higher than the mean of the response-locked distribution (1.17*10−4).
Behavioral analysis
We found that verbal response time was affected by the congruency of the current trial (F(1, 405)=38.22, p<0.001, η2=0.05), while no effect was demonstrated from the trial condition of the previous trial (F(1, 405)=0.65, p=0.42, η2<0.01). However, no interaction effect was found between previous trial types and current trial types (F(1, 401)=0.18, p=0.67, η2<0.01). Figure 6 displays the median verbal response values with 95% confidence intervals for four sub-conditions from all trials across all participants.
Discussion
In this study, we compared PAC patterns in the OFC between successful color-word conflict trials and successful non-conflict trials from a modified Stroop task. We found increased PAC between theta phase and low-gamma (LG) amplitude during cue processing of incongruent trials with individual and group analysis. In addition, significant increases in theta-LG PAC were most prominent in the 200-800ms time window following cue presentation, during the cue-processing period. This observation could indicate that increased theta-LG PAC serves a cognitive role in evaluating a stimulus more than in response inhibition.
Besides our investigation within the OFC, PAC between theta and gamma frequency bands has been investigated in other brain areas. Oehrn and colleagues analyzed intracranial recordings from the human dorsal lateral prefrontal cortex (DLPFC) using an auditory version of the Stroop task (28). Their cross-frequency coupling (CFC) findings revealed increased PAC between theta phase and gamma amplitude within the DLPFC in an early time window (10-300ms after the stimulus) in successful incongruent trials. This activity is hypothesized to reflect successful conflict processing, based on previous studies suggesting that DLPFC activity influences accuracy and reaction times in Stroop task paradigms (27, 45, 46). In the present study, increased OFC theta-LG PAC patterns were found in the 200-800ms period after cue presentation, which is both later in onset and longer in duration than the corresponding DLPFC findings in Oehrn et al. Based on the proposed three sub-processes of conflict processing (conflict detection, resolution, and adaption) (26, 47), it is possible that, while the DLPFC may be involved in early conflict detection, the OFC demonstrates PAC changes more consistent with the subsequent resolution stage of conflict processing. In our study, theta-LG PAC was not observed during the pre-response period, suggesting a potentially diminished involvement of OFC theta-LG PAC in the activation (or inhibition) of the motor response.
Although theta-LG PAC is becoming increasingly recognized, the underlying reason behind the observed mechanism during conflict processing remains an open question. It has been hypothesized that different neural frequency bands may reflect varying spatiotemporal processing scales within the brain (48). For example, the prior investigation has suggested that low-frequency oscillations, such as the theta band, may reflect modulation over large spatial regions in long temporal windows (28) while high-frequency gamma bands may instead modulate smaller spatial regions over shorter temporal windows (15). Wulff and colleagues presented one possible mechanism of theta-gamma interactions, who used genetically modified mice to demonstrate that theta-gamma interactions depend on a particular class of GABAergic interneurons (49). PAC between theta phase and gamma amplitude appears to have a role in the integration of local neural activity into large-scale cerebral networks (17). Given this evidence, the observed theta-LG PAC pattern may be not only influential locally in the OFC, but also may integrate with others in brain areas. For example, in a previous study from our group, we found decreased theta coherence between the hippocampus and OFC during the period 100-400ms right after cue onset only for successful incongruent trials (50). This desynchronized theta activity may reflect modulation between the hippocampus and OFC to encode conflict information. Given the later onset of local PAC changes noted in this study, we hypothesize that the observed PAC pattern reflects the local computational response to inter-brain communication as seen in the prior study on coherence.
Several limitations should be noted in this investigation. First, given the binary nature of the Stroop task, we cannot correlate the magnitude of theta-LG PAC change with the strength of the conflict scenario. In another previous study of learning tasks, a strong correlation between the learning performance and the magnitude of hippocampal CFC was found in rodents, which showed that hippocampal CFC increased over time as learning performance improved (38). Future studies with varying degrees of conflict will be invaluable in determining if more difficult conflict correlates with magnitude of PAC change. For example, increasing task difficulty by variably constraining the response period may help give insight into this potential relationship.
An additional limitation of this study is that all participants had medically refractory epilepsy, which was the clinical indication for SEEG placement. Thus, underlying epileptic neurophysiology could theoretically influence recordings from these brain areas. Although this limitation is universal to SEEG studies, the intentional removal of trials containing interictal spikes or active seizure activity minimizes the probability that active epilepsy is unduly affecting the results of our investigation. Additionally, because electrodes are only placed for clinical necessity in the surveillance of refractory epilepsy, there is a natural heterogeneity among our patients in electrode number, precise contact site, and demographic characteristics. This limits our ability to isolate specific regions within the hippocampus and OFC.
Conclusion
In this study, we report a theta phase and low-gamma amplitude coupling pattern within the OFC that increases in the setting of conflict resolution. Given the 200-800ms onset of this finding after cue presentation, the theta-gamma relationship within the OFC may reflect the OFC’s role in processing conflict scenarios after conflict detection and before motor response. Understanding this relationship within the OFC will help further elucidate the underlying neural mechanisms of conflict resolution throughout the human brain.
Funding
This work was supported by the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health (KL2TR001854), Tianqiao and Chrissy Chen Brain-Machine Interface Center at Caltech, the Meira and Shaul G. Massry Foundation, and the Taiwan-USC Postdoctoral Fellowship Program.
References
- 1.Ouellet J, McGirr A, Van den Eynde F, Jollant F, Lepage M, Berlim MT. Enhancing decision-making and cognitive impulse control with transcranial direct current stimulation (tDCS) applied over the orbitofrontal cortex (OFC): A randomized and sham-controlled exploratory study. J Psychiatr Res. 2015;69:27–34. [DOI] [PubMed] [Google Scholar]
- 2.van Wingerden M, van der Meij R, Kalenscher T, Maris E, Pennartz CM. Phase-amplitude coupling in rat orbitofrontal cortex discriminates between correct and incorrect decisions during associative learning. J Neurosci. 2014;34(2):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schoenbaum G, Takahashi Y, Liu TL, McDannald MA. Does the orbitofrontal cortex signal value? Annals of the New York Academy of Sciences. 2011;1239(l):87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan K, Cheng P, Dong T, Bi Y, Xing L, Yu D, et al. Cortical thickness abnormalities in late adolescence with online gaming addiction. PLoS One. 2013;8(1):e53055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maayan L, Hoogendoorn C, Sweat V, Convit A. Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity (Silver Spring). 2011;19(7):1382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Wang Z, Boileau I, Dreher JC, Gelskov S, Genauck A, et al. Altered orbitofrontal sulcogyral patterns in gambling disorder: a multicenter study. Transl Psychiatry. 2019;9(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guillem K, Ahmed SH. Reorganization of theta phase-locking in the orbitofrontal cortex drives cocaine choice under the influence. Scientific reports. 2020;10(1):8041-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fatahi Z, Haghparast A, Khani A, Kermani M. Functional connectivity between anterior cingulate cortex and orbitofrontal cortex during value-based decision making. Neurobiol Learn Mem. 2018;147:74–8. [DOI] [PubMed] [Google Scholar]
- 9.Knudsen EB, Wallis JD. Closed-Loop Theta Stimulation in the Orbitofrontal Cortex Prevents Reward-Based Learning. Neuron (Cambridge, Mass). 2020;106(3):537–47.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dymond S, Lawrence NS, Dunkley BT, Yuen KS, Hinton EC, Dixon MR, et al. Almost winning: induced MEG theta power in insula and orbitofrontal cortex increases during gambling near-misses and is associated with BOLD signal and gambling severity. Neuroimage. 2014;91:210–9. [DOI] [PubMed] [Google Scholar]
- 11.Andreou C, Kleinert J, Steinmann S, Fuger U, Leicht G, Mulert C. Oscillatory responses to reward processing in borderline personality disorder. World J Biol Psychiatry. 2015;16(8):575–86. [DOI] [PubMed] [Google Scholar]
- 12.Hanslmayr S, Pastötter B, Bäuml K-H, Gruber S, Wimber M, Klimesch W. The Electrophysiological Dynamics of Interference during the Stroop Task. Journal of cognitive neuroscience. 2008;20(2):215–25. [DOI] [PubMed] [Google Scholar]
- 13.van Wingerden M, Vinck M, Lankelma JV, Pennartz CMA. Learning-associated gamma-band phase-locking of action-outcome selective neurons in orbitofrontal cortex. The Journal of neuroscience. 2010;30(30):10025–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koga S, Rothermel R, Juhász C, Nagasawa T, Sood S, Asano E. Electrocorticographic correlates of cognitive control in a stroop task—intracranial recording in epileptic patients. Human brain mapping. 2011;32(10):1580–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol. 2000;38(3):301–13. [DOI] [PubMed] [Google Scholar]
- 16.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–9. [DOI] [PubMed] [Google Scholar]
- 17.Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14(11):506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buzsaki G, Wang X-J. Mechanisms of Gamma Oscillations. Annual review of neuroscience. 2012;35(1):203–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan R, Bush D, Bonnefond M, Bandettini PA, Barnes GR, Doeller CF, et al. Medial prefrontal theta phase coupling during spatial memory retrieval. Hippocampus. 2014;24(6):656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Köster M, Finger H, Graetz S, Kater M, Gruber T. Theta-gamma coupling binds visual perceptual features in an associative memory task. Sci Rep. 2018;8(1):17688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Köster M, Friese U, Schöne B, Trujillo-Barreto N, Gruber T. Theta-gamma coupling during episodic retrieval in the human EEG. Brain Res. 2014;1577:57–68. [DOI] [PubMed] [Google Scholar]
- 22.Wang DX, Schmitt K, Seger S, Davila CE, Lega BC. Cross-regional phase amplitude coupling supports the encoding of episodic memories. Hippocampus. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griesmayr B, Gruber WR, Klimesch W, Sauseng P. Human frontal midline theta and its synchronization to gamma during a verbal delayed match to sample task. Neurobiology of learning and memory. 2010;93(2):208–15. [DOI] [PubMed] [Google Scholar]
- 24.Vivekananda U, Bush D, Bisby JA, Baxendale S, Rodionov R, Diehl B, et al. Theta power and theta-gamma coupling support long-term spatial memory retrieval. Hippocampus. 2021;31(2):213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, et al. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462(7271):353–7. [DOI] [PubMed] [Google Scholar]
- 26.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–52. [DOI] [PubMed] [Google Scholar]
- 27.Stern ER, Mangels JA. An electrophysiological investigation of preparatory attentional control in a spatial Stroop task. J CognNeurosci. 2006;18(6):1004–17. [DOI] [PubMed] [Google Scholar]
- 28.Oehrn CR, Hanslmayr S, Fell J, Deuker L, Kremers NA, Do Lam AT, et al. Neural communication patterns underlying conflict detection, resolution, and adaptation. J Neurosci. 2014;34(31):10438–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–6. [PubMed] [Google Scholar]
- 30.Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision. 1997;10:437–42. [PubMed] [Google Scholar]
- 31.Mitra P Mitra P, Observed brain dynamics. 2007: Oxford University Press. . [Google Scholar]
- 32.Townsend BR, Subasi E, Scherberger H. Grasp Movement Decoding from Premotor and Parietal Cortex. The Journal of Neuroscience. 2011;31(40):14386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.S C. A mathematical theory of communication. Bell Syst Tech J 27: 623–656379–423, 1948. [Google Scholar]
- 34.Frey SH, Vinton D, Norlund R, Grafton ST. Cortical topography of human anterior intraparietal cortex active during visually guided grasping. Brain Res Cogn Brain Res. 2005;23(2-3):397–405. [DOI] [PubMed] [Google Scholar]
- 35.S. K. The Kullback–Leibler distance. J Am Statist Assoc 41: 340–341, 1987. [Google Scholar]
- 36.Kullback S R L. On information and sufficiency. Ann Math Stat 22: 79–86, 1951. [Google Scholar]
- 37.Tort AB, Kramer MA, Thorn C, Gibson DJ, Kubota Y, Graybiel AM, et al. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc Natl Acad Sci U S A. 2008;105(51):20517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tort AB, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H. Theta-gamma coupling increases during the learning of item-context associations. Proc Natl Acad Sci U S A. 2009;106(49):20942–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tort AB, Komorowski R, Eichenbaum H, Kopell N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol. 2010;104(2):1195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Hemptinne C, Ryapolova-Webb ES, Air EL, Garcia PA, Miller KJ, Ojemann JG, et al. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci U S A. 2013;110(12):4780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164(1):177–90. [DOI] [PubMed] [Google Scholar]
- 42.Lorist MM, Jolij J. Trial History Effects in Stroop Task Performance Are Independent of Top-Down Control. PLOS ONE. 2012;7(6):e39802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen J Statistical Power Analysis for the Behavioral Sciences. New York: Routledge Academic; 1988. [Google Scholar]
- 44.Del Campo-Vera RM, Gogia AS, Chen KH, Sebastian R, Kramer DR, Lee MB, et al. Beta-band power modulation in the human hippocampus during a reaching task. J Neural Eng. 2020;17(3):036022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coste CP, Sadaghiani S, Friston KJ, Kleinschmidt A. Ongoing brain activity fluctuations directly account for intertrial and indirectly for intersubject variability in Stroop task performance. Cereb Cortex. 2011;21(11):2612–9. [DOI] [PubMed] [Google Scholar]
- 46.MacDonald AW 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–8. [DOI] [PubMed] [Google Scholar]
- 47.Mansouri FA, Tanaka K, Buckley MJ. Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nat Rev Neurosci. 2009;10(2):141–52. [DOI] [PubMed] [Google Scholar]
- 48.Dickson CT, Biella G, de Curtis M. Evidence for spatial modules mediated by temporal synchronization of carbachol-induced gamma rhythm in medial entorhinal cortex. J Neurosci. 2000;20(20):7846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wulff P, Ponomarenko AA, Bartos M, Korotkova TM, Fuchs EC, Bähner F, et al. Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2009;106(9):3561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang AM, Chen KH, Del Campo-Vera RM, Sebastian R, Gogia AS, Nune G, et al. Hippocampal and orbitofrontal theta band coherence diminishes during conflict resolution. World Neurosurg. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]