Abstract
The management of patients with liver metastases from colorectal cancer is still debated. Several therapeutic options and treatment strategies are available for an extremely heterogeneous clinical scenario. Adequate prediction of patients’ outcomes and of the effectiveness of chemotherapy and loco-regional treatments are crucial to reach a precision medicine approach. This has been an unmet need for a long time, but recent studies have opened new perspectives. New morphological biomarkers have been identified. The dynamic evaluation of the metastases across a time interval, with or without chemotherapy, provided a reliable assessment of the tumor biology. Genetics have been explored and, thanks to their strong association with prognosis, have the potential to drive treatment planning. The liver-tumor interface has been identified as one of the main determinants of tumor progression, and its components, in particular the immune infiltrate, are the focus of major research. Image mining and analyses provided new insights on tumor biology and are expected to have a relevant impact on clinical practice. Artificial intelligence is a further step forward. The present paper depicts the evolution of clinical decision-making for patients affected by colorectal liver metastases, facing modern biomarkers and innovative opportunities that will characterize the evolution of clinical research and practice in the next few years.
Keywords: Colorectal liver metastases, Biomarkers, Genetics, Immune infiltrate, Radiomics, Artificial Intelligence
Core Tip: The management of patients with colorectal liver metastases is challenging because the choice among different therapeutic options and strategies is not supported by strong evidence. A precision medicine approach has been an unmet need for a long time, but recent studies have opened new perspectives. In this paper, we will discuss new morphological approaches to assess tumor biology, the promising data from genetic analyses, the raising clinical relevance of the liver-tumor interface, and the potentialities of advanced imaging analysis and artificial intelligence. These are the keys to reach an effective personalized treatment in the near future.
INTRODUCTION
During the last decades, the surgeons and medical oncologists drove the multidisciplinary teams to the ambitious aim of curing patients with colorectal liver metastases[1]. Systemic therapy had a progressively increasing effectiveness[2,3]. To date, the median life expectancy of patients receiving state-of-the-art treatment exceeds 30 mo[1,2]. The new immunotherapies could further raise the bar. Liver surgery has been the game-changer: It rapidly became the standard thanks to its proven safety (mortality risk lower than 2%) and oncological effectiveness (actual 5- and 10-year survival rates of about 50% and 20%, respectively)[1,4-6]. All patients with technically resectable disease, sufficient future liver remnant volume, and disease control by chemotherapy are now considered for surgery[1,7]. The liver surgeons pursued aggressive indications and developed complex techniques to maximize the resectability rate, even considering liver transplantation in the most recent years[7-9]. However, this generated a paradox: We are now searching for criteria to identify patients that are technically resectable but do not benefit from surgery because of their unfavorable tumor biology (10%-15% of patients have an early recurrence and early cancer-related death after surgery)[10]. Finally, thermal ablation gained momentum. After having demonstrated its effectiveness in patients with hepatocellular carcinoma, radiofrequency and microwave ablation have been successfully applied to patients with colorectal liver metastases, achieving adequate disease control[11,12]. Percutaneous treatments are now even tested as alternative to surgery in randomized trials[13,14].
The management of such a complex scenario should rely on an adequate understanding of tumor biology and several decisions need for a precision medicine approach (e.g., the identification of the most appropriate schedule of systemic therapy, the selection of candidates to surgery, the indication to perioperative chemotherapy, the timing of colorectal and hepatic surgery in patients with synchronous metastases, and the choice between surgery and ablation). However, a recent study demonstrated that hepatobiliary surgeons have a huge heterogeneity in the treatment planning and surgical indications, the choice among different options being almost a throw of the dice[15]. Reliable biomarkers are urgently needed to drive a patient-tailored evidence-based approach.
In 2012, we depicted an evolving scenario with some preliminary evidence[16]. Where do we stand almost a decade later? In the present paper, we will provide a critical overview of traditional biomarkers, new proposals, and future perspectives (Figure 1 and Table 1).
Figure 1.
Available biomarkers for patients affected by colorectal liver metastases. A biomarker is defined as any parameter (molecular, cellular, clinical, imaging or identified by an artificial intelligence process) having a clinical role in narrowing or guiding treatment decisions and contributing to the estimation of the overall patient prognosis (prognostic biomarker), the clinical outcome after a treatment (predictive biomarker), or the properties of a clinical condition /disease (diagnostic biomarker).
Table 1.
Characteristics of different biomarkers of colorectal liver metastases.
|
Biomarker characteristics
|
||||||
|
Standardized
|
Reproducibility
|
Robustness (across series)
|
Early assessment
|
Reliability in prediction
|
(Potential) Clinical impact
|
|
| Morphology and clinical data | d | e | c | e | b | c |
| Dynamic evaluation | d | e | e | b | d | e |
| Genetics | c | d | d | e | e | e |
| Peritumoral tissue data | c | d | c | a | d | d |
| Radiomics | b | c | c | e | c | d |
| Artificial intelligence | a | a | b | d | d | e |
The performances of every biomarker are evaluated by a score, ranging from “a” if very low to “e” if very high.
MORPHOLOGY: AN OUTDATED BIOMARKER?
The tumor morphology is still the basis of several clinical decisions. The tumor burden defines the resectability of patients, and, in resectable ones, the need for perioperative chemotherapy[1,7]. The size of liver metastases determines the indication to thermal ablation (effective in nodules ≤ 30 mm)[17]. Several morphological parameters, including primary tumor data and tumor markers, have a prognostic value, and they have been combined into multiple scores to optimize their prognostic performance (Table 2)[18-23].
Table 2.
Some of the available scores for outcome prediction of patients with colorectal liver metastases candidates to surgery
|
Morphology-based scores
|
Morphology- and Genetics-based scores
|
||||||
|
|
Nordlinger et al[18], 1996
|
CRS, Fong et al[19], 1999
|
Iwatsuki et al[20], 1999
|
Rees et al[21], 2008
|
RAS Mutation CRS, Brudvik et al[60] 2017
|
GAME score, Margonis et al[61] 2018
|
Extended CRS, Lang et al[65], 2019
|
| Morphological parameters | |||||||
| Age | Yes (60 yr) | ||||||
| Primary tumor | |||||||
| Extension into the serosa | Yes | ||||||
| N status primary tumor | Yes | Yes | Yes | Yes | Yes | Yes | |
| Grading primary tumor | Yes | ||||||
| Liver metastases | |||||||
| Number | Yes (3) | Yes (1) | Yes (2) | Yes (3) | Yes (TBS) | ||
| Size | Yes (50 mm) | Yes (50 mm) | Yes (80 mm) | Yes (50 mm) | Yes (50 mm) | Yes (50 mm) | |
| Bilobar | Y | ||||||
| DFI | Yes (24 mo) | Yes (12 mo) | Yes (30 mo) | ||||
| Surgical margin | Yes (10 mm) | ||||||
| Extrahepatic disease | Yes | Yes | |||||
| CEA value | Yes (200 ng/mL) | Yes (60 ng/mL) | Yes (20 ng/mL) | ||||
| Genetic parameters | |||||||
| RAS | Yes | Yes1 | |||||
| RAS/RAF pathway | Yes | ||||||
| SMAD | Yes | ||||||
KRAS status.
DFI: Disease-free interval from primary to metastases; CEA: Carcinoembryonic antigen; CRS: Clinical risk score; GAME: Genetic and morphological evaluation; TBS: Tumor Burden Score.
Recent studies reaffirmed the role of tumor morphology as a biomarker and determinant of the treatment strategy. First, Sasaki et al[24] proposed to combine the number and size of metastases into a “Tumor Burden Score”, mimicking the Metroticket evidence for hepatocellular carcinoma[25]. They classified the patients into three groups and achieved a good stratification of survival, better than the stratification achieved by the size or the number of metastases when separately considered. Nevertheless, the Tumor Burden Score failed to select the candidates to surgery, the patients of the high-risk group (score ≥ 9) having an expected 5-year survival rate over 20%. Second, the primary tumor site has gained momentum. In comparison with patients having a left colonic tumor, those having a right colonic tumor are characterized by a lower response to chemotherapy, survival after surgery, and effectiveness of thermal ablation[26-29]. The embryological origin of the two parts of the colon (midgut for the right colon and hindgut for the left one) and the different genetic profiles of the tumors could explain such results. However, the impact of the primary tumor side on the treatment strategy is still to be defined, and, in this distinction (right vs left colonic cancer), the rectal cancers remain a blurred entity to elucidate. Third, a recent study based on the LiverMetSurvey data suggested that patients with synchronous multiple bilobar metastases should undergo a liver-first approach because this strategy achieves better survival than the alternative ones (i.e. the simultaneous and primary tumor-first approaches)[30]. This evidence could lead to a major change in current practice and definitively prioritizes the treatment of liver metastases in presence of a severe hepatic tumor burden. Fourth, in patients with liver and lung metastases, the pulmonary disease has shown a limited prognostic relevance[31]. Such data should be paired with those provided by Viganò et al[32], who demonstrated that the pathological response of colorectal metastases to systemic therapy changes according to the involved organ, being low in the lung and lymph nodes metastases, intermediate in the hepatic ones, and high in the peritoneal ones. The inhomogeneous prognostic relevance and chemosensitivity of the different tumor sites open new perspectives in treatment strategies and oncological research.
Despite its extensive adoption in current practice, tumor morphology is not a robust biomarker for several reasons. First, in patients undergoing systemic therapy, morphology gives a limited prediction of the response to treatment. Second, in resectable patients, it does not allow for an adequate selection of candidates. The number of colorectal metastases and the presence of extrahepatic disease are paradigmatic examples. Even if the number of nodules is a strong prognostic factor, there is not a numeric cut-off value beyond which resection is contraindicated, and some patients with numerous metastases may benefit from surgery[33-35]. Similarly, the presence of extrahepatic disease contraindicates surgery in a limited proportion of patients (unresectable lesions, distant lymph node metastases, and diffuse peritoneal disease combined with multiple hepatic metastases)[36-38]. Third, different morphological parameters have been reported by different studies, and none has been confirmed by all authors. Fourth, morphological criteria can be modified by chemotherapy (e.g., the tumor size), and it is unclear which value (before or after treatment) should be considered. Finally, tumor morphology offers a snapshot of the tumor and misses its evolution.
MOVING TOWARD A DYNAMIC VIEW
The tumor behavior is intuitively an effective surrogate biomarker of its biology. In the early 2000s, some authors proposed to adopt a time-test before surgery in patients with resectable colorectal liver metastases (i.e. an observation period to evaluate the tumor evolution)[39-41]. One-third to half of the patients developed additional lesions during the time-test and were excluded from resection. This policy has been early abandoned because of the advent of effective chemotherapy regimens, which combine observation and treatment. To date, neoadjuvant systemic therapy is a standard, and the tumor behavior during treatment is one of the most powerful prognostic factors. Since 2004, progression while on chemotherapy is even considered a contraindication to resection in resectable patients with few exceptions[42].
The prognostic role of the response to chemotherapy is indisputable, but three main limitations of this parameter should be highlighted: It excludes from surgery less than 10% of candidates[43]; the pathological evaluation of response has a poor agreement with the radiological one (about one-third of responders at imaging has no tumor regression at the pathology analysis)[44,45]; the no-progression during short chemotherapy (2-3 mo, the present standard) does not necessarily correspond to favorable biology and prognosis (about 15% of patients develop early recurrence after surgery)[10].
There is another time interval during which the tumor behavior can be analyzed. Patients must respect a 4-wk pause between the end of the systemic therapy and surgery (6 wk in case of anti-vascular endothelial growth factor treatment)[46,47]. We observed that about 15% of patients with tumor response or stabilization during chemotherapy have an early tumor progression in the interval between chemotherapy and surgery and an extremely poor outcome (0% survival at 2 years)[48]. Such a progression should contraindicate resection and dictates the need for restaging immediately before surgery.
Finally, percutaneous thermal ablation could contribute to the dynamic evaluation of colorectal liver metastases. It has been proposed as a time-test in patients with a synchronous disease or early recurrence after liver surgery with several benefits: Ablation provided a minimally invasive and effective treatment of the metastases, with high salvageability in case of local failure; avoided futile surgery in some cases; and spared chemotherapy for further disease progression[49,50].
Despite its effectiveness, the dynamic evaluation of colorectal metastases should be applied with caution. First, the time-test must be adequate. Progression during prolonged systemic therapy or after a long chemotherapy-surgery interval represents a loss of chance for resectable patients rather than a selection[48]. Even a disease progression in the interval between the two stages of a staged hepatectomy should not be considered tout-court an adequate selection of candidates[51]. Second, selected patients with a dimensional-only progression of the tumor and a limited hepatic tumor burden can be considered for surgery despite progression[52]. Finally, progression is not a definitive contraindication to resection, and surgery can be scheduled if the disease is controlled by a further line of chemotherapy[53,54].
GENETIC DATA: THE PANACEA FOR ALL THE UNCERTAINTIES?
Tumor genetics is the key to design a precision medicine approach. The sequencing of large series of metastases highlighted few high-frequency mutations, which have been extensively investigated for their association with the outcome. Tumor protein p53 (TP53) and APC gene mutations are the commonest ones (65%-75% and 45%-85% of patients, respectively)[55,56], but most studies focused on the RAS genes. KRAS and NRAS mutations are evident in one-third to half of the patients and have an established clinical impact: They preclude anti-epidermal growth factor receptor treatments and are associated with a lower response rate to chemotherapy, poorer survival, and higher risk of pulmonary metastases[57-59]. RAS status has been recently included in two prognostic scores for patients undergoing liver surgery (Table 2): The RAS Mutation Clinical Risk Score that considers the RAS status, metastases size, and N status of the primary tumor[60]; the Genetic And Morphological Evaluation (GAME) score that considers the KRAS status, carcinoembryonic antigen level, N status of the primary tumor, Tumor Burden Score, and presence of extrahepatic disease[61]. Both have been externally validated and outperformed the standard morphology-based scores. The patients with the highest scores had extremely poor outcome (0% recurrence-free survival at 2 years after surgery if RAS Mutation Clinical Risk Score = 3 or GAME score ≥ 6), but they were a marginal part of the cohort (14/564, 2.5%, and 18/1249, 1.4%, respectively).
The analysis of BRAF mutations generated a major interest despite their low frequency (4%-10%)[56,62]. The oncologists reported extremely poor survival of BRAF mutated patients, raising doubts about their candidacy to surgery[57,62]. Nevertheless, surgical series achieved an adequate outcome in selected BRAF mutated patients, suggesting that this genetic profile is a strong prognostic factor but should not be an absolute contraindication when the disease is adequately controlled by chemotherapy[63,64]. Additional mutations have been associated with prognosis, such as those of the TP53, PIK3CA, APC, and SMAD genes[65]. The Mainz group suggested that the performances of the aforementioned RAS score can be improved by replacing the RAS with the RAS-RAF pathway and adding the SMAD family (Table 2)[65]. The patients with all four negative prognostic factors (metastasis size > 50 mm, N+ primary tumors, and double mutation of the RAS-RAF pathway and SMAD family) had an extremely low median survival (1 year after surgery), but they were very few (only 5 out of 123, 4%). The MD Anderson Cancer Center group reported a cumulative negative prognostic impact of the mutations of TP53, RAS, and SMAD4: Survival progressively decreased with the increase in the number of the altered genes[66].
Those are the first steps of genetic-based precision medicine, but we have still to face some major challenges: Evidence is preliminary and needs robust validation to drive clinical practice; some criteria to select the candidates to surgery have been proposed, but they concern a minimal proportion of patients (< 5%)[60,61,65]; the discordance of the genetic profile between the primary tumor and metastases and their corresponding prognostic impact remain to be elucidated; tumor heterogeneity may lead to clonal populations with different mutations into a single metastasis, but their assessment is not yet standardized.
THE SOLUTION COULD BE OUTSIDE THE TUMOR
The liver-tumor interface could be the true battlefield where the interaction between the neoplastic cells and the “host” determines the prognosis. Several data are in favor of this hypothesis.
First, the pathology analysis of the peritumoral parenchyma highlighted the presence of the micrometastases (i.e. vascular and lymphatic tumoral emboli, perineural tissue infiltration, and satellite nodules)[44]. They are mainly localized within the first 2 mm of tissue surrounding the tumor, are reduced by chemotherapy, and negatively impact prognosis[44,67,68]. Micrometastases are the true determinants of the local recurrence risk after resection and thermal ablation.
Second, the profile of liver metastases has prognostic relevance. In 2009, Mentha et al[69] depicted the so-called “dangerous-halo” (i.e. a neoplastic regrowth at the tumor periphery due to an early reactivation of the metastases after the end of chemotherapy). This could represent the pathology counterpart of the radiological tumor progression that we observed in the interval chemotherapy-surgery. To date, the metastases’ profile has been named “tumor growth pattern” and has been distinguished into three types: Pushing, desmoplastic, and replacement[70]. The types correspond to different growing modalities: The metastases with a replacement pattern grow by co-opting the stroma and sinusoids; those with a pushing pattern have signs of active hypoxia-induced angiogenesis[71,72]. The replacement pattern is the most aggressive one and is associated with a lower response rate to chemotherapy, higher recurrence risk, and poorer survival[73-75]. In patients with a replacement pattern, we also observed an increased risk of local recurrence after surgery and the need for a wider surgical margin (unpublished data).
Third, a growing interest concerns the peri-tumoral immune infiltrate, especially after the introduction of modern immunotherapies. As for the primary colorectal cancers, an immunoscore, based on the presence of CD3+ and CD8+ cells in the core of liver metastases and at their invasion margin, achieved a good stratification of prognosis[76]. Additional cell populations have been investigated for their association with the outcome, such as the macrophages[77], but data are still preliminary.
Unfortunately, the biomarkers of the liver-tumor interface can be assessed only by the pathologist on the surgical specimen. The lack of an adequate non-invasive evaluation strongly reduces their clinical relevance. In addition, a comprehensive overview of the liver-tumor interface, merging the different pathology details, is still lacking, precluding a definitive understanding of the tumor-host interaction.
A further aspect deserves consideration; some features of the non-tumoral liver parenchyma could impact prognosis. Chemotherapy-associated sinusoidal injuries have been associated with the tumor response to chemotherapy; the more severe the sinusoidal dilatation the lower the response rate[44,78]. Nevertheless, the response to therapy and not the sinusoidal dilatation impacted survival[44]. In contrast, Viganò et al[44] depicted moderate/severe steatosis as a positive prognostic factor after surgery (5-year survival rate 53% vs 35%). These results have been confirmed by a subsequent analysis of the LiverMetSurvey database[79] and are in line with some studies reporting a favorable association between body mass index and prognosis[80,81]. We are still far from conclusive evidence and reliable explanation, but further investigations should be performed to potentially outline new therapeutic approaches.
RADIOMICS: IMAGING BEYOND THE VISIBLE DATA
Radiomics, or texture analysis, uses mathematical formulas to extract from medical imaging modalities invisible-to-the-eye patterns, which correlate with the biological properties of the analyzed tissue[82,83]. The complexity of analyses progressively increased, moving from histogram-based values to different types of matrices, filters, and transforms[84,85]. In patients with colorectal liver metastases, several potential applications of radiomics have been proposed[86]. First, it can predict the effectiveness of chemotherapy[87-95]. The decrease in entropy and increase in homogeneity of liver lesions after chemotherapy have been associated with the radiological tumor response. Some authors even reported the possibility to predict response to systemic therapy by analyzing the images at diagnosis before chemotherapy; higher entropy and lower homogeneity of liver metastases were associated with a subsequent higher response rate. When compared with the standard RECIST criteria, texture analysis achieved earlier and more accurate prediction. Second, radiomics have been associated with patients’ prognosis, metastases with higher entropy and lower homogeneity having a better survival[88,90,96]. The comparative analysis of the imaging modalities before and after chemotherapy further refined the prediction of the long-term outcome[89,91,92,94], and there is accumulating evidence that both radiomic scores and combined clinical-radiomic models outperform traditional predictors of survival[92]. Third, textural features of the tumor before thermal ablation can predict the risk of local recurrence[97]. Fourth, radiomics are associated with the pathology data (e.g., tumor grading, growth pattern, and regression grade after chemotherapy[88,98,99]). Finally, texture analysis has the potential to provide a non-invasive evaluation of the chemotherapy-associated liver injuries, which at present are poorly evaluated by standard imaging modalities[46,100].
The strength of radiomics relies on its capability to provide early prediction of the outcome and to reach a non-invasive estimation of the pathology details of colorectal metastases, anticipating data that are usually collected only after surgery. Further, the possibility to interpret the biological value of some radiomic features facilitates their implementation into clinical practice. For instance, entropy and heterogeneity, especially after contrast enhancement, clearly suggest the presence of active disease with heterogeneous clones, while homogeneity after chemotherapy reflects tumor necrosis due to a response to treatment. Finally, the development of technological tools to perform automatic segmentation of liver tumors will enable easier extraction of radiomic features, contributing to the spread of such data. However, the texture analysis suffers from some limitations: Some features, in particular the second-order ones, lack interpretability; radiomics has instability across different devices and acquisition protocols, especially for magnetic resonance images; studies differ in terms of software packages, analyzed phases, and reported features; and reliable cut-off values of radiomic parameters are lacking. Those issues have to be solved to speed up the application of radiomics into clinical practice.
ARTIFICIAL INTELLIGENCE: WHERE DO WE STAND?
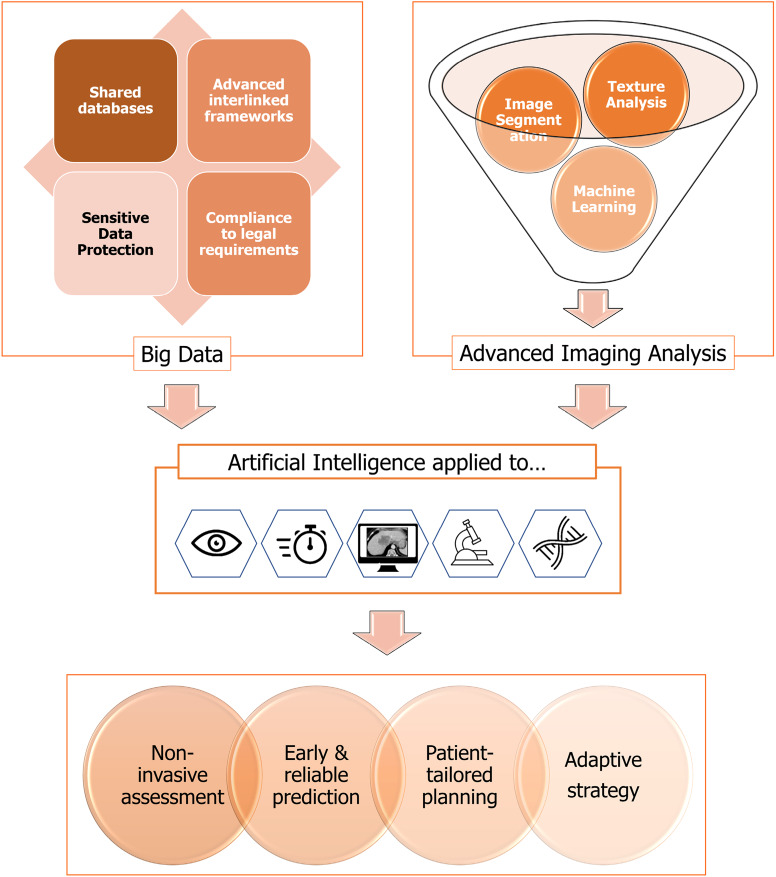
In the most recent years, the so-called “artificial intelligence” (AI) is the object of major interest and investments, with a consequent spike of AI-related publications[101]. Introduced in the 1950s, the term AI defines a computer program that, in a very specific setting, can “learn” and self-improve over time[102,103]. A demonstration of its potentialities took place in 1997, when a chess-playing AI, named Deep Blue, was able to beat the world champion Kasparov[104]. In medicine, AI is expected not only to optimize the prediction of an outcome by combining all available variables but also to update and improve continuously prediction according to the experienced results (Figure 2). AI can represent a major support to the decision-making processes, especially in the clinical scenarios with several therapeutic and strategical options and lack of consensus among experts, exactly as occurs for colorectal metastases[15]. In this sense, AI is not per se a biomarker but maximizes the profitability of all available data. However, AI may also have an additional role. It can be applied to medical imaging to identify new patterns that can contribute to diagnosis or prediction[105]. Such patterns, extractable from any type of imaging modality in a completely unbiased and unsupervised way, can be considered AI-derived biomarkers, subject to clinical validation[106]. Analogously, AI can identify biomarkers from any source of data, including clinical charts, medical reports, and images scan.
Figure 2.
Future developments in the treatment planning for patients with colorectal liver metastases based on radiomics, big data, and artificial intelligence.
A first attempt in using AI-based therapy guidance dates to 2005, when a decision matrix platform, named OncoSurge, was introduced to help clinicians deciding the best treatment of patients at first diagnosis of colorectal liver metastases (i.e. when the treatment planning has the greatest impact)[107]. This method was later validated against the multidisciplinary team meeting achieving an almost perfect agreement[108]. Since then, few studies have been published, but they outlined a progressive increase in AI performances[109-113]. The AI predicted the recurrence risk after surgery by taking into account clinical, pathology, and laboratory data[110,112]. The addition of radiomic features into the machine learning models further optimized and anticipated the prediction[109,111]. Wei et al[113] compared a clinical, radiomic, and AI-based model to predict response to first-line chemotherapy; the deep-learning model had the best results, outperforming not only the model based on clinical parameters but also the one including texture analysis.
So far, the AI implementation into everyday practice is a priority to fill the quantum leap toward personalized computer-assisted medicine and will probably become a standard for clinical decision-making in the near future. It will allow merging all biomarkers, from morphological criteria to radiomics and genetic ones, weighing their prognostic role. Nevertheless, some current limitations of AI should be kept in mind. First, it needs training on large datasets, as Deep Blue did analyzing data from millions of chess matches[104]. Big data are crucial, but their availability is still limited by legal constraints and privacy policies. Shared databases and advanced interlinked frameworks could be the starting point. Second, AI supports decisions and does not replace clinical judgment yet, but computer-derived recommendations could lead to some legal and insurance critical issues. Finally, several technical and technological obstacles currently relegate AI-based approaches to highly specialized centers into a research setting.
CONCLUSION
To date, we are still far from a solid precision medicine for patients affected by colorectal liver metastases because of the limited capability of the available biomarkers to predict survival, response to chemotherapy, and the effectiveness of loco-regional therapies. Nevertheless, major (r)evolutions are ongoing, and the clinical approach to patients with metastatic colorectal cancer is going to change in the near future. The genetic analyses will definitively unveil the tumor biology, becoming the consistent basis of treatment planning; new biomarkers, based on radiomics and liver-tumor interface characteristics, will further enrich our comprehension and prediction of the tumor evolution; AI will merge and balance all data to drive decision-making processes.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to disclose.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: June 22, 2021
First decision: July 14, 2021
Article in press: January 20, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tanabe S S-Editor: Chang KL L-Editor: Filipodia P-Editor: Chang KL
Contributor Information
Luca Viganò, Department of Hepatobiliary and General Surgery, IRCCS Humanitas Research Hospital, Rozzano 20089, MI, Italy; Department of Biomedical Sciences, Humanitas University, Pieve Emanuele 20072, MI, Italy. luca.vigano@hunimed.eu.
Visala S Jayakody Arachchige, Department of Hepatobiliary and General Surgery, IRCCS Humanitas Research Hospital, Rozzano 20089, MI, Italy; Department of Biomedical Sciences, Humanitas University, Pieve Emanuele 20072, MI, Italy.
Francesco Fiz, Nuclear Medicine, IRCCS Humanitas Research Hospital, Rozzano 20089, MI, Italy.
References
- 1.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 2.Tomasello G, Petrelli F, Ghidini M, Russo A, Passalacqua R, Barni S. FOLFOXIRI Plus Bevacizumab as Conversion Therapy for Patients With Initially Unresectable Metastatic Colorectal Cancer: A Systematic Review and Pooled Analysis. JAMA Oncol. 2017;3:e170278. doi: 10.1001/jamaoncol.2017.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrelli F, Barni S Anti-EGFR agents for liver metastases. Resectability and outcome with anti-EGFR agents in patients with KRAS wild-type colorectal liver-limited metastases: a meta-analysis. Int J Colorectal Dis. 2012;27:997–1004. doi: 10.1007/s00384-012-1438-2. [DOI] [PubMed] [Google Scholar]
- 4.Viganò L, Ferrero A, Lo Tesoriere R, Capussotti L. Liver surgery for colorectal metastases: results after 10 years of follow-up. Long-term survivors, late recurrences, and prognostic role of morbidity. Ann Surg Oncol. 2008;15:2458–2464. doi: 10.1245/s10434-008-9935-9. [DOI] [PubMed] [Google Scholar]
- 5.Creasy JM, Sadot E, Koerkamp BG, Chou JF, Gonen M, Kemeny NE, Balachandran VP, Kingham TP, DeMatteo RP, Allen PJ, Blumgart LH, Jarnagin WR, D'Angelica MI. Actual 10-year survival after hepatic resection of colorectal liver metastases: what factors preclude cure? Surgery. 2018;163:1238–1244. doi: 10.1016/j.surg.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torzilli G, Viganò L, Gatti A, Costa G, Cimino M, Procopio F, Donadon M, Del Fabbro D. Twelve-year experience of "radical but conservative" liver surgery for colorectal metastases: impact on surgical practice and oncologic efficacy. HPB (Oxford) 2017;19:775–784. doi: 10.1016/j.hpb.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Adam R, De Gramont A, Figueras J, Guthrie A, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L, Sobrero A, Tabernero J, Teh C, Van Cutsem E Jean-Nicolas Vauthey of the EGOSLIM (Expert Group on OncoSurgery management of LIver Metastases) group. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012;17:1225–1239. doi: 10.1634/theoncologist.2012-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torzilli G, Adam R, Viganò L, Imai K, Goransky J, Fontana A, Toso C, Majno P, de Santibañes E. Surgery of Colorectal Liver Metastases: Pushing the Limits. Liver Cancer. 2016;6:80–89. doi: 10.1159/000449495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Line PD, Ruffolo LI, Toso C, Dueland S, Nadalin S, Hernandez-Alejandro R. Liver transplantation for colorectal liver metastases: What do we need to know? Int J Surg. 2020;82S:87–92. doi: 10.1016/j.ijsu.2020.03.079. [DOI] [PubMed] [Google Scholar]
- 10.Viganò L, Gentile D, Galvanin J, Corleone P, Costa G, Cimino M, Procopio F, Torzilli G. Very Early Recurrence After Liver Resection for Colorectal Metastases: Incidence, Risk Factors, and Prognostic Impact. J Gastrointest Surg. 2021 doi: 10.1007/s11605-021-05123-w. [DOI] [PubMed] [Google Scholar]
- 11.Gillams A, Goldberg N, Ahmed M, Bale R, Breen D, Callstrom M, Chen MH, Choi BI, de Baere T, Dupuy D, Gangi A, Gervais D, Helmberger T, Jung EM, Lee F, Lencioni R, Liang P, Livraghi T, Lu D, Meloni F, Pereira P, Piscaglia F, Rhim H, Salem R, Sofocleous C, Solomon SB, Soulen M, Tanaka M, Vogl T, Wood B, Solbiati L. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontières meeting 2013. Eur Radiol. 2015;25:3438–3454. doi: 10.1007/s00330-015-3779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958–968. doi: 10.1148/radiol.12111851. [DOI] [PubMed] [Google Scholar]
- 13.Gurusamy K, Corrigan N, Croft J, Twiddy M, Morris S, Woodward N, Bandula S, Hochhauser D, Napp V, Pullan A, Jakowiw N, Prasad R, Damink SO, van Laarhoven CJHM, de Wilt JHW, Brown J, Davidson BR. Liver resection surgery versus thermal ablation for colorectal LiVer MetAstases (LAVA): study protocol for a randomised controlled trial. Trials. 2018;19:105. doi: 10.1186/s13063-018-2499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puijk RS, Ruarus AH, Vroomen LGPH, van Tilborg AAJM, Scheffer HJ, Nielsen K, de Jong MC, de Vries JJJ, Zonderhuis BM, Eker HH, Kazemier G, Verheul H, van der Meijs BB, van Dam L, Sorgedrager N, Coupé VMH, van den Tol PMP, Meijerink MR COLLISION Trial Group. Colorectal liver metastases: surgery versus thermal ablation (COLLISION) - a phase III single-blind prospective randomized controlled trial. BMC Cancer. 2018;18:821. doi: 10.1186/s12885-018-4716-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ignatavicius P, Oberkofler CE, Chapman WC, DeMatteo RP, Clary BM, D'Angelica MI, Tanabe KK, Hong JC, Aloia TA, Pawlik TM, Hernandez-Alejandro R, Shah SA, Vauthey JN, Torzilli G, Lang H, Line PD, Soubrane O, Pinto-Marques H, Robles-Campos R, Boudjema K, Lodge P, Adam R, Toso C, Serrablo A, Aldrighetti L, DeOliveira ML, Dutkowski P, Petrowsky H, Linecker M, Reiner CS, Braun J, Alikhanov R, Barauskas G, Chan ACY, Dong J, Kokudo N, Yamamoto M, Kang KJ, Fong Y, Rela M, De Aretxabala X, De Santibañes E, Mercado MÁ, Andriani OC, Torres OJM, Pinna AD, Clavien PA. Choices of Therapeutic Strategies for Colorectal Liver Metastases Among Expert Liver Surgeons: A Throw of the Dice? Ann Surg. 2020;272:715–722. doi: 10.1097/SLA.0000000000004331. [DOI] [PubMed] [Google Scholar]
- 16.Viganò L. Treatment strategy for colorectal cancer with resectable synchronous liver metastases: Is any evidence-based strategy possible? World J Hepatol. 2012;4:237–241. doi: 10.4254/wjh.v4.i8.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crocetti L, de Baére T, Pereira PL, Tarantino FP. CIRSE Standards of Practice on Thermal Ablation of Liver Tumours. Cardiovasc Intervent Radiol. 2020;43:951–962. doi: 10.1007/s00270-020-02471-z. [DOI] [PubMed] [Google Scholar]
- 18.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, Jaeck D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 19.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–18; discussion 318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, Geller DA, Gayowski TJ, Fung JJ, Starzl TE. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291–299. doi: 10.1016/s1072-7515(99)00089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 22.Nagashima I, Takada T, Matsuda K, Adachi M, Nagawa H, Muto T, Okinaga K. A new scoring system to classify patients with colorectal liver metastases: proposal of criteria to select candidates for hepatic resection. J Hepatobiliary Pancreat Surg. 2004;11:79–83. doi: 10.1007/s00534-002-0778-7. [DOI] [PubMed] [Google Scholar]
- 23.Konopke R, Kersting S, Distler M, Dietrich J, Gastmeier J, Heller A, Kulisch E, Saeger HD. Prognostic factors and evaluation of a clinical score for predicting survival after resection of colorectal liver metastases. Liver Int. 2009;29:89–102. doi: 10.1111/j.1478-3231.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki K, Morioka D, Conci S, Margonis GA, Sawada Y, Ruzzenente A, Kumamoto T, Iacono C, Andreatos N, Guglielmi A, Endo I, Pawlik TM. The Tumor Burden Score: A New "Metro-ticket" Prognostic Tool For Colorectal Liver Metastases Based on Tumor Size and Number of Tumors. Ann Surg. 2018;267:132–141. doi: 10.1097/SLA.0000000000002064. [DOI] [PubMed] [Google Scholar]
- 25.Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 26.Loupakis F, Yang D, Yau L, Feng S, Cremolini C, Zhang W, Maus MK, Antoniotti C, Langer C, Scherer SJ, Müller T, Hurwitz HI, Saltz L, Falcone A, Lenz HJ. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst. 2015:107. doi: 10.1093/jnci/dju427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moretto R, Cremolini C, Rossini D, Pietrantonio F, Battaglin F, Mennitto A, Bergamo F, Loupakis F, Marmorino F, Berenato R, Marsico VA, Caporale M, Antoniotti C, Masi G, Salvatore L, Borelli B, Fontanini G, Lonardi S, De Braud F, Falcone A. Location of Primary Tumor and Benefit From Anti-Epidermal Growth Factor Receptor Monoclonal Antibodies in Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer. Oncologist. 2016;21:988–994. doi: 10.1634/theoncologist.2016-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sagawa T, Sato Y, Hirakawa M, Hamaguchi K, Fukuya A, Okamoto K, Miyamoto H, Muguruma N, Fujikawa K, Takahashi Y, Takayama T. Clinical impact of primary tumour location, early tumour shrinkage, and depth of response in the treatment of metastatic colorectal cancer with first-line chemotherapy plus cetuximab or bevacizumab. Sci Rep. 2020;10:19815. doi: 10.1038/s41598-020-76756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou F, Yu X, Liang P, Han Z, Cheng Z, Yu J, Liu F, Hu Y. Does primary tumor location impact the prognosis of colorectal liver metastases patients after microwave ablation? Oncotarget. 2017;8:100791–100800. doi: 10.18632/oncotarget.18764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giuliante F, Viganò L, De Rose AM, Mirza DF, Lapointe R, Kaiser G, Barroso E, Ferrero A, Isoniemi H, Lopez-Ben S, Popescu I, Ouellet JF, Hubert C, Regimbeau JM, Lin JK, Skipenko OG, Ardito F, Adam R. Liver-First Approach for Synchronous Colorectal Metastases: Analysis of 7360 Patients from the LiverMetSurvey Registry. Ann Surg Oncol. 2021;28:8198–8208. doi: 10.1245/s10434-021-10220-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andres A, Mentha G, Adam R, Gerstel E, Skipenko OG, Barroso E, Lopez-Ben S, Hubert C, Majno PE, Toso C. Surgical management of patients with colorectal cancer and simultaneous liver and lung metastases. Br J Surg. 2015;102:691–699. doi: 10.1002/bjs.9783. [DOI] [PubMed] [Google Scholar]
- 32.Vigano L, Corleone P, Darwish SS, Turri N, Famularo S, Viggiani L, Rimassa L, Del Fabbro D, Di Tommaso L, Torzilli G. Hepatic and Extrahepatic Colorectal Metastases Have Discordant Responses to Systemic Therapy. Pathology Data from Patients Undergoing Simultaneous Resection of Multiple Tumor Sites. Cancers (Basel) 2021;13 doi: 10.3390/cancers13030464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viganò L, Capussotti L, Majno P, Toso C, Ferrero A, De Rosa G, Rubbia-Brandt L, Mentha G. Liver resection in patients with eight or more colorectal liver metastases. Br J Surg. 2015;102:92–101. doi: 10.1002/bjs.9680. [DOI] [PubMed] [Google Scholar]
- 34.Allard MA, Adam R, Giuliante F, Lapointe R, Hubert C, Ijzermans JNM, Mirza DF, Elias D, Laurent C, Gruenberger T, Poston G, Letoublon C, Isoniemi H, Lucidi V, Popescu I, Figueras J. Long-term outcomes of patients with 10 or more colorectal liver metastases. Br J Cancer. 2017;117:604–611. doi: 10.1038/bjc.2017.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vigano L, Di Tommaso L, Mimmo A, Sollai M, Cimino M, Donadon M, Roncalli M, Torzilli G. Prospective Evaluation of Intrahepatic Microscopic Occult Tumor Foci in Patients with Numerous Colorectal Liver Metastases. Dig Surg. 2019;36:340–347. doi: 10.1159/000489274. [DOI] [PubMed] [Google Scholar]
- 36.Elias D, Viganò L, Orsi F, Scorsetti M, Comito T, Lerut J, Cosola D, Torzilli G. New Perspectives in the Treatment of Colorectal Metastases. Liver Cancer. 2016;6:90–98. doi: 10.1159/000449492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasselgren K, Isaksson B, Ardnor B, Lindell G, Rizell M, Strömberg C, Loftås P, Björnsson B, Sandström P. Liver resection is beneficial for patients with colorectal liver metastases and extrahepatic disease. Ann Transl Med. 2020;8:109. doi: 10.21037/atm.2019.12.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adam R, de Haas RJ, Wicherts DA, Aloia TA, Delvart V, Azoulay D, Bismuth H, Castaing D. Is hepatic resection justified after chemotherapy in patients with colorectal liver metastases and lymph node involvement? J Clin Oncol. 2008;26:3672–3680. doi: 10.1200/JCO.2007.15.7297. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu Y, Yasui K, Sano T, Hirai T, Kanemitsu Y, Komori K, Kato T. Validity of observation interval for synchronous hepatic metastases of colorectal cancer: changes in hepatic and extrahepatic metastatic foci. Langenbecks Arch Surg. 2008;393:181–184. doi: 10.1007/s00423-007-0258-2. [DOI] [PubMed] [Google Scholar]
- 40.Lambert LA, Colacchio TA, Barth RJ Jr. Interval hepatic resection of colorectal metastases improves patient selection. Arch Surg. 2000;135:473–9; discussion 479. doi: 10.1001/archsurg.135.4.473. [DOI] [PubMed] [Google Scholar]
- 41.Yoshidome H, Kimura F, Shimizu H, Ohtsuka M, Kato A, Yoshitomi H, Furukawa K, Mitsuhashi N, Takeuchi D, Iida A, Miyazaki M. Interval period tumor progression: does delayed hepatectomy detect occult metastases in synchronous colorectal liver metastases? J Gastrointest Surg. 2008;12:1391–1398. doi: 10.1007/s11605-008-0540-9. [DOI] [PubMed] [Google Scholar]
- 42.Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, Levi F, Bismuth H. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–61; discussion 1061. doi: 10.1097/01.sla.0000145964.08365.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Collette L, Praet M, Bethe U, Van Cutsem E, Scheithauer W, Gruenberger T EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD) Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viganò L, Capussotti L, De Rosa G, De Saussure WO, Mentha G, Rubbia-Brandt L. Liver resection for colorectal metastases after chemotherapy: impact of chemotherapy-related liver injuries, pathological tumor response, and micrometastases on long-term survival. Ann Surg. 2013;258:731–40; discussion 741. doi: 10.1097/SLA.0b013e3182a6183e. [DOI] [PubMed] [Google Scholar]
- 45.Brouquet A, Blot C, Allard MA, Lazure T, Sebbagh M, Gayet M, Lewin M, Adam R, Penna C, Sa Cunha A, Benoist S. What is the Prognostic Value of a Discordant Radiologic and Pathologic Response in Patients Undergoing Resection of Colorectal Liver Metastases After Preoperative Chemotherapy? Ann Surg Oncol. 2020;27:2877–2885. doi: 10.1245/s10434-020-08284-1. [DOI] [PubMed] [Google Scholar]
- 46.Vigano L, Sollini M, Ieva F, Fiz F, Torzilli G. Chemotherapy-Associated Liver Injuries: Unmet Needs and New Insights for Surgical Oncologists. Ann Surg Oncol. 2021;28:4074–4079. doi: 10.1245/s10434-021-10069-z. [DOI] [PubMed] [Google Scholar]
- 47.Vigano L, De Rosa G, Toso C, Andres A, Ferrero A, Roth A, Sperti E, Majno P, Rubbia-Brandt L. Reversibility of chemotherapy-related liver injury. J Hepatol. 2017;67:84–91. doi: 10.1016/j.jhep.2017.02.031. [DOI] [PubMed] [Google Scholar]
- 48.Vigano L, Darwish SS, Rimassa L, Cimino M, Carnaghi C, Donadon M, Procopio F, Personeni N, Del Fabbro D, Santoro A, Torzilli G. Progression of Colorectal Liver Metastases from the End of Chemotherapy to Resection: A New Contraindication to Surgery? Ann Surg Oncol. 2018;25:1676–1685. doi: 10.1245/s10434-018-6387-8. [DOI] [PubMed] [Google Scholar]
- 49.Livraghi T, Solbiati L, Meloni F, Ierace T, Goldberg SN, Gazelle GS. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the "test-of-time approach". Cancer. 2003;97:3027–3035. doi: 10.1002/cncr.11426. [DOI] [PubMed] [Google Scholar]
- 50.Viganò L, Galvanin J, Poretti D, Del Fabbro D, Gentile D, Pedicini V, Solbiati L, Torzilli G. Percutaneous ablation of post-surgical solitary early recurrence of colorectal liver metastases is an effective "test-of-time" approach. Updates Surg. 2021;73:1349–1358. doi: 10.1007/s13304-021-01047-x. [DOI] [PubMed] [Google Scholar]
- 51.Viganò L, Torzilli G, Cimino M, Imai K, Vibert E, Donadon M, Castaing D, Adam R. Drop-out between the two liver resections of two-stage hepatectomy. Patient selection or loss of chance? Eur J Surg Oncol. 2016;42:1385–1393. doi: 10.1016/j.ejso.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 52.Viganò L, Capussotti L, Barroso E, Nuzzo G, Laurent C, Ijzermans JN, Gigot JF, Figueras J, Gruenberger T, Mirza DF, Elias D, Poston G, Letoublon C, Isoniemi H, Herrera J, Sousa FC, Pardo F, Lucidi V, Popescu I, Adam R. Progression while receiving preoperative chemotherapy should not be an absolute contraindication to liver resection for colorectal metastases. Ann Surg Oncol. 2012;19:2786–2796. doi: 10.1245/s10434-012-2382-7. [DOI] [PubMed] [Google Scholar]
- 53.Brouquet A, Overman MJ, Kopetz S, Maru DM, Loyer EM, Andreou A, Cooper A, Curley SA, Garrett CR, Abdalla EK, Vauthey JN. Is resection of colorectal liver metastases after a second-line chemotherapy regimen justified? Cancer. 2011;117:4484–4492. doi: 10.1002/cncr.26036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adam R, Yi B, Innominato PF, Barroso E, Laurent C, Giuliante F, Capussotti L, Lapointe R, Regimbeau JM, Lopez-Ben S, Isoniemi H, Hubert C, Lin JK, Gruenberger T, Elias D, Skipenko OG, Guglielmi A LiverMetSurvey International Contributing Centers. Resection of colorectal liver metastases after second-line chemotherapy: is it worthwhile? Eur J Cancer. 2017;78:7–15. doi: 10.1016/j.ejca.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Ishaque N, Abba ML, Hauser C, Patil N, Paramasivam N, Huebschmann D, Leupold JH, Balasubramanian GP, Kleinheinz K, Toprak UH, Hutter B, Benner A, Shavinskaya A, Zhou C, Gu Z, Kerssemakers J, Marx A, Moniuszko M, Kozlowski M, Reszec J, Niklinski J, Eils J, Schlesner M, Eils R, Brors B, Allgayer H. Whole genome sequencing puts forward hypotheses on metastasis evolution and therapy in colorectal cancer. Nat Commun. 2018;9:4782. doi: 10.1038/s41467-018-07041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330-337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tosi F, Magni E, Amatu A, Mauri G, Bencardino K, Truini M, Veronese S, De Carlis L, Ferrari G, Nichelatti M, Sartore-Bianchi A, Siena S. Effect of KRAS and BRAF Mutations on Survival of Metastatic Colorectal Cancer After Liver Resection: A Systematic Review and Meta-Analysis. Clin Colorectal Cancer. 2017;16:e153–e163. doi: 10.1016/j.clcc.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Tsilimigras DI, Ntanasis-Stathopoulos I, Bagante F, Moris D, Cloyd J, Spartalis E, Pawlik TM. Clinical significance and prognostic relevance of KRAS, BRAF, PI3K and TP53 genetic mutation analysis for resectable and unresectable colorectal liver metastases: A systematic review of the current evidence. Surg Oncol. 2018;27:280–288. doi: 10.1016/j.suronc.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 59.Vauthey JN, Zimmitti G, Kopetz SE, Shindoh J, Chen SS, Andreou A, Curley SA, Aloia TA, Maru DM. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. 2013;258:619–26; discussion 626. doi: 10.1097/SLA.0b013e3182a5025a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brudvik KW, Jones RP, Giuliante F, Shindoh J, Passot G, Chung MH, Song J, Li L, Dagenborg VJ, Fretland ÅA, Røsok B, De Rose AM, Ardito F, Edwin B, Panettieri E, Larocca LM, Yamashita S, Conrad C, Aloia TA, Poston GJ, Bjørnbeth BA, Vauthey JN. RAS Mutation Clinical Risk Score to Predict Survival After Resection of Colorectal Liver Metastases. Ann Surg. 2019;269:120–126. doi: 10.1097/SLA.0000000000002319. [DOI] [PubMed] [Google Scholar]
- 61.Margonis GA, Sasaki K, Gholami S, Kim Y, Andreatos N, Rezaee N, Deshwar A, Buettner S, Allen PJ, Kingham TP, Pawlik TM, He J, Cameron JL, Jarnagin WR, Wolfgang CL, D'Angelica MI, Weiss MJ. Genetic And Morphological Evaluation (GAME) score for patients with colorectal liver metastases. Br J Surg. 2018;105:1210–1220. doi: 10.1002/bjs.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yokota T, Ura T, Shibata N, Takahari D, Shitara K, Nomura M, Kondo C, Mizota A, Utsunomiya S, Muro K, Yatabe Y. BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br J Cancer. 2011;104:856–862. doi: 10.1038/bjc.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bachet JB, Moreno-Lopez N, Vigano L, Marchese U, Gelli M, Raoux L, Truant S, Laurent C, Herrero A, Le Roy B, Deguelte Lardiere S, Passot G, Hautefeuille V, De La Fouchardiere C, Artru P, Ameto T, Mabrut JY, Schwarz L, Rousseau B, Lepère C, Coriat R, Brouquet A, Sa Cunha A, Benoist S. BRAF mutation is not associated with an increased risk of recurrence in patients undergoing resection of colorectal liver metastases. Br J Surg. 2019;106:1237–1247. doi: 10.1002/bjs.11180. [DOI] [PubMed] [Google Scholar]
- 64.Margonis GA, Buettner S, Andreatos N, Kim Y, Wagner D, Sasaki K, Beer A, Schwarz C, Løes IM, Smolle M, Kamphues C, He J, Pawlik TM, Kaczirek K, Poultsides G, Lønning PE, Cameron JL, Burkhart RA, Gerger A, Aucejo FN, Kreis ME, Wolfgang CL, Weiss MJ. Association of BRAF Mutations With Survival and Recurrence in Surgically Treated Patients With Metastatic Colorectal Liver Cancer. JAMA Surg. 2018;153:e180996. doi: 10.1001/jamasurg.2018.0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lang H, Baumgart J, Heinrich S, Tripke V, Passalaqua M, Maderer A, Galle PR, Roth W, Kloth M, Moehler M. Extended Molecular Profiling Improves Stratification and Prediction of Survival After Resection of Colorectal Liver Metastases. Ann Surg. 2019;270:799–805. doi: 10.1097/SLA.0000000000003527. [DOI] [PubMed] [Google Scholar]
- 66.Kawaguchi Y, Kopetz S, Newhook TE, De Bellis M, Chun YS, Tzeng CD, Aloia TA, Vauthey JN. Mutation Status of RAS, TP53, and SMAD4 is Superior to Mutation Status of RAS Alone for Predicting Prognosis after Resection of Colorectal Liver Metastases. Clin Cancer Res. 2019;25:5843–5851. doi: 10.1158/1078-0432.CCR-19-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wakai T, Shirai Y, Sakata J, Kameyama H, Nogami H, Iiai T, Ajioka Y, Hatakeyama K. Histologic evaluation of intrahepatic micrometastases in patients treated with or without neoadjuvant chemotherapy for colorectal carcinoma liver metastasis. Int J Clin Exp Pathol. 2012;5:308–314. [PMC free article] [PubMed] [Google Scholar]
- 68.Kokudo N, Miki Y, Sugai S, Yanagisawa A, Kato Y, Sakamoto Y, Yamamoto J, Yamaguchi T, Muto T, Makuuchi M. Genetic and histological assessment of surgical margins in resected liver metastases from colorectal carcinoma: minimum surgical margins for successful resection. Arch Surg. 2002;137:833–840. doi: 10.1001/archsurg.137.7.833. [DOI] [PubMed] [Google Scholar]
- 69.Mentha G, Terraz S, Morel P, Andres A, Giostra E, Roth A, Rubbia-Brandt L, Majno P. Dangerous halo after neoadjuvant chemotherapy and two-step hepatectomy for colorectal liver metastases. Br J Surg. 2009;96:95–103. doi: 10.1002/bjs.6436. [DOI] [PubMed] [Google Scholar]
- 70.van Dam PJ, van der Stok EP, Teuwen LA, Van den Eynden GG, Illemann M, Frentzas S, Majeed AW, Eefsen RL, Coebergh van den Braak RRJ, Lazaris A, Fernandez MC, Galjart B, Laerum OD, Rayes R, Grünhagen DJ, Van de Paer M, Sucaet Y, Mudhar HS, Schvimer M, Nyström H, Kockx M, Bird NC, Vidal-Vanaclocha F, Metrakos P, Simoneau E, Verhoef C, Dirix LY, Van Laere S, Gao ZH, Brodt P, Reynolds AR, Vermeulen PB. International consensus guidelines for scoring the histopathological growth patterns of liver metastasis. Br J Cancer. 2017;117:1427–1441. doi: 10.1038/bjc.2017.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vermeulen PB, Colpaert C, Salgado R, Royers R, Hellemans H, Van Den Heuvel E, Goovaerts G, Dirix LY, Van Marck E. Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J Pathol. 2001;195:336–342. doi: 10.1002/path.966. [DOI] [PubMed] [Google Scholar]
- 72.Eefsen RL, Van den Eynden GG, Høyer-Hansen G, Brodt P, Laerum OD, Vermeulen PB, Christensen IJ, Wettergren A, Federspiel B, Willemoe GL, Vainer B, Osterlind K, Illemann M. Histopathological growth pattern, proteolysis and angiogenesis in chemonaive patients resected for multiple colorectal liver metastases. J Oncol. 2012;2012:907971. doi: 10.1155/2012/907971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fernández Moro C, Bozóky B, Gerling M. Growth patterns of colorectal cancer liver metastases and their impact on prognosis: a systematic review. BMJ Open Gastroenterol. 2018;5:e000217. doi: 10.1136/bmjgast-2018-000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eefsen RL, Vermeulen PB, Christensen IJ, Laerum OD, Mogensen MB, Rolff HC, Van den Eynden GG, Høyer-Hansen G, Osterlind K, Vainer B, Illemann M. Growth pattern of colorectal liver metastasis as a marker of recurrence risk. Clin Exp Metastasis. 2015;32:369–381. doi: 10.1007/s10585-015-9715-4. [DOI] [PubMed] [Google Scholar]
- 75.Frentzas S, Simoneau E, Bridgeman VL, Vermeulen PB, Foo S, Kostaras E, Nathan M, Wotherspoon A, Gao ZH, Shi Y, Van den Eynden G, Daley F, Peckitt C, Tan X, Salman A, Lazaris A, Gazinska P, Berg TJ, Eltahir Z, Ritsma L, Van Rheenen J, Khashper A, Brown G, Nystrom H, Sund M, Van Laere S, Loyer E, Dirix L, Cunningham D, Metrakos P, Reynolds AR. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med. 2016;22:1294–1302. doi: 10.1038/nm.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mlecnik B, Van den Eynde M, Bindea G, Church SE, Vasaturo A, Fredriksen T, Lafontaine L, Haicheur N, Marliot F, Debetancourt D, Pairet G, Jouret-Mourin A, Gigot JF, Hubert C, Danse E, Dragean C, Carrasco J, Humblet Y, Valge-Archer V, Berger A, Pagès F, Machiels JP, Galon J. Comprehensive Intrametastatic Immune Quantification and Major Impact of Immunoscore on Survival. J Natl Cancer Inst. 2018;110 doi: 10.1093/jnci/djx123. [DOI] [PubMed] [Google Scholar]
- 77.Donadon M, Torzilli G, Cortese N, Soldani C, Di Tommaso L, Franceschini B, Carriero R, Barbagallo M, Rigamonti A, Anselmo A, Colombo FS, Maggi G, Lleo A, Cibella J, Peano C, Kunderfranco P, Roncalli M, Mantovani A, Marchesi F. Macrophage morphology correlates with single-cell diversity and prognosis in colorectal liver metastasis. J Exp Med. 2020;217 doi: 10.1084/jem.20191847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao J, Sawo P, Rensen SS, Rouflart MMJ, Winstanley A, Vreuls CPH, Verheij J, van Mierlo KMC, Lodewick TM, van Woerden V, van Tiel FH, van Dam RM, Dejong CHC, Olde Damink SWM. Impact of chemotherapy-associated liver injury on tumour regression grade and survival in patients with colorectal liver metastases. HPB (Oxford) 2018;20:147–154. doi: 10.1016/j.hpb.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 79.Parkin E, O'Reilly DA, Adam R, Kaiser GM, Laurent C, Elias D, Capussotti L, Renehan AG LiverMetSurvey Centres. The effect of hepatic steatosis on survival following resection of colorectal liver metastases in patients without preoperative chemotherapy. HPB (Oxford) 2013;15:463–472. doi: 10.1111/hpb.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aparicio T, Ducreux M, Faroux R, Barbier E, Manfredi S, Lecomte T, Etienne PL, Bedenne L, Bennouna J, Phelip JM, François E, Michel P, Legoux JL, Gasmi M, Breysacher G, Rougier P, De Gramont A, Lepage C, Bouché O, Seitz JF for FFCD investigators. Overweight is associated to a better prognosis in metastatic colorectal cancer: A pooled analysis of FFCD trials. Eur J Cancer. 2018;98:1–9. doi: 10.1016/j.ejca.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 81.Renfro LA, Loupakis F, Adams RA, Seymour MT, Heinemann V, Schmoll HJ, Douillard JY, Hurwitz H, Fuchs CS, Diaz-Rubio E, Porschen R, Tournigand C, Chibaudel B, Falcone A, Tebbutt NC, Punt CJ, Hecht JR, Bokemeyer C, Van Cutsem E, Goldberg RM, Saltz LB, de Gramont A, Sargent DJ, Lenz HJ. Body Mass Index Is Prognostic in Metastatic Colorectal Cancer: Pooled Analysis of Patients From First-Line Clinical Trials in the ARCAD Database. J Clin Oncol. 2016;34:144–150. doi: 10.1200/JCO.2015.61.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, Sanduleanu S, Larue RTHM, Even AJG, Jochems A, van Wijk Y, Woodruff H, van Soest J, Lustberg T, Roelofs E, van Elmpt W, Dekker A, Mottaghy FM, Wildberger JE, Walsh S. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749–762. doi: 10.1038/nrclinonc.2017.141. [DOI] [PubMed] [Google Scholar]
- 83.Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, Zegers CM, Gillies R, Boellard R, Dekker A, Aerts HJ. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48:441–446. doi: 10.1016/j.ejca.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Timmeren JE, Cester D, Tanadini-Lang S, Alkadhi H, Baessler B. Radiomics in medical imaging-"how-to" guide and critical reflection. Insights Imaging. 2020;11:91. doi: 10.1186/s13244-020-00887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yip SS, Aerts HJ. Applications and limitations of radiomics. Phys Med Biol. 2016;61:R150–R166. doi: 10.1088/0031-9155/61/13/R150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fiz F, Viganò L, Gennaro N, Costa G, La Bella L, Boichuk A, Cavinato L, Sollini M, Politi LS, Chiti A, Torzilli G. Radiomics of Liver Metastases: A Systematic Review. Cancers (Basel) 2020;12 doi: 10.3390/cancers12102881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakanishi R, Oki E, Hasuda H, Sano E, Miyashita Y, Sakai A, Koga N, Kuriyama N, Nonaka K, Fujimoto Y, Jogo T, Hokonohara K, Hu Q, Hisamatsu Y, Ando K, Kimura Y, Yoshizumi T, Mori M. Radiomics Texture Analysis for the Identification of Colorectal Liver Metastases Sensitive to First-Line Oxaliplatin-Based Chemotherapy. Ann Surg Oncol. 2021;28:2975–2985. doi: 10.1245/s10434-020-09581-5. [DOI] [PubMed] [Google Scholar]
- 88.Lubner MG, Stabo N, Lubner SJ, del Rio AM, Song C, Halberg RB, Pickhardt PJ. CT textural analysis of hepatic metastatic colorectal cancer: pre-treatment tumor heterogeneity correlates with pathology and clinical outcomes. Abdom Imaging. 2015;40:2331–2337. doi: 10.1007/s00261-015-0438-4. [DOI] [PubMed] [Google Scholar]
- 89.Andersen IR, Thorup K, Andersen MB, Olesen R, Mortensen FV, Nielsen DT, Rasmussen F. Texture in the monitoring of regorafenib therapy in patients with colorectal liver metastases. Acta Radiol. 2019;60:1084–1093. doi: 10.1177/0284185118817940. [DOI] [PubMed] [Google Scholar]
- 90.Beckers RCJ, Trebeschi S, Maas M, Schnerr RS, Sijmons JML, Beets GL, Houwers JB, Beets-Tan RGH, Lambregts DMJ. CT texture analysis in colorectal liver metastases and the surrounding liver parenchyma and its potential as an imaging biomarker of disease aggressiveness, response and survival. Eur J Radiol. 2018;102:15–21. doi: 10.1016/j.ejrad.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 91.Dercle L, Lu L, Schwartz LH, Qian M, Tejpar S, Eggleton P, Zhao B, Piessevaux H. Radiomics Response Signature for Identification of Metastatic Colorectal Cancer Sensitive to Therapies Targeting EGFR Pathway. J Natl Cancer Inst. 2020;112:902–912. doi: 10.1093/jnci/djaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dohan A, Gallix B, Guiu B, Le Malicot K, Reinhold C, Soyer P, Bennouna J, Ghiringhelli F, Barbier E, Boige V, Taieb J, Bouché O, François E, Phelip JM, Borel C, Faroux R, Seitz JF, Jacquot S, Ben Abdelghani M, Khemissa-Akouz F, Genet D, Jouve JL, Rinaldi Y, Desseigne F, Texereau P, Suc E, Lepage C, Aparicio T, Hoeffel C PRODIGE 9 Investigators and PRODIGE 20 Investigators. Early evaluation using a radiomic signature of unresectable hepatic metastases to predict outcome in patients with colorectal cancer treated with FOLFIRI and bevacizumab. Gut. 2020;69:531–539. doi: 10.1136/gutjnl-2018-316407. [DOI] [PubMed] [Google Scholar]
- 93.Rahmim A, Bak-Fredslund KP, Ashrafinia S, Lu L, Schmidtlein CR, Subramaniam RM, Morsing A, Keiding S, Horsager J, Munk OL. Prognostic modeling for patients with colorectal liver metastases incorporating FDG PET radiomic features. Eur J Radiol. 2019;113:101–109. doi: 10.1016/j.ejrad.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ravanelli M, Agazzi GM, Tononcelli E, Roca E, Cabassa P, Baiocchi G, Berruti A, Maroldi R, Farina D. Texture features of colorectal liver metastases on pretreatment contrast-enhanced CT may predict response and prognosis in patients treated with bevacizumab-containing chemotherapy: a pilot study including comparison with standard chemotherapy. Radiol Med. 2019;124:877–886. doi: 10.1007/s11547-019-01046-4. [DOI] [PubMed] [Google Scholar]
- 95.Shur J, Orton M, Connor A, Fischer S, Moulton CA, Gallinger S, Koh DM, Jhaveri KS. A clinical-radiomic model for improved prognostication of surgical candidates with colorectal liver metastases. J Surg Oncol. 2019 doi: 10.1002/jso.25783. [DOI] [PubMed] [Google Scholar]
- 96.van Helden EJ, Vacher YJL, van Wieringen WN, van Velden FHP, Verheul HMW, Hoekstra OS, Boellaard R, Menke-van der Houven van Oordt CW. Radiomics analysis of pre-treatment [18F]FDG PET/CT for patients with metastatic colorectal cancer undergoing palliative systemic treatment. Eur J Nucl Med Mol Imaging. 2018;45:2307–2317. doi: 10.1007/s00259-018-4100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Taghavi M, Staal F, Gomez Munoz F, Imani F, Meek DB, Simões R, Klompenhouwer LG, van der Heide UA, Beets-Tan RGH, Maas M. CT-Based Radiomics Analysis Before Thermal Ablation to Predict Local Tumor Progression for Colorectal Liver Metastases. Cardiovasc Intervent Radiol. 2021;44:913–920. doi: 10.1007/s00270-020-02735-8. [DOI] [PubMed] [Google Scholar]
- 98.Cheng J, Wei J, Tong T, Sheng W, Zhang Y, Han Y, Gu D, Hong N, Ye Y, Tian J, Wang Y. Prediction of Histopathologic Growth Patterns of Colorectal Liver Metastases with a Noninvasive Imaging Method. Ann Surg Oncol. 2019;26:4587–4598. doi: 10.1245/s10434-019-07910-x. [DOI] [PubMed] [Google Scholar]
- 99.Rao SX, Lambregts DM, Schnerr RS, Beckers RC, Maas M, Albarello F, Riedl RG, Dejong CH, Martens MH, Heijnen LA, Backes WH, Beets GL, Zeng MS, Beets-Tan RG. CT texture analysis in colorectal liver metastases: A better way than size and volume measurements to assess response to chemotherapy? United European Gastroenterol J. 2016;4:257–263. doi: 10.1177/2050640615601603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Costa G, Cavinato L, Masci C, Fiz F, Sollini M, Politi LS, Chiti A, Balzarini L, Aghemo A, di Tommaso L, Ieva F, Torzilli G, Viganò L. Virtual Biopsy for Diagnosis of Chemotherapy-Associated Liver Injuries and Steatohepatitis: A Combined Radiomic and Clinical Model in Patients with Colorectal Liver Metastases. Cancers (Basel) 2021;13 doi: 10.3390/cancers13123077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kulkarni S, Seneviratne N, Baig MS, Khan AHA. Artificial Intelligence in Medicine: Where Are We Now? Acad Radiol. 2020;27:62–70. doi: 10.1016/j.acra.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 102.Wang P. On Defining Artificial Intelligence. JAGI. 2019;10:1–37. [Google Scholar]
- 103.Haenlein M, Kaplan A. A Brief History of Artificial Intelligence: On the Past, Present, and Future of Artificial Intelligence. Calif Manage Rev. 2019;61:5–14. [Google Scholar]
- 104.Hassabis D. Artificial Intelligence: Chess match of the century. Nature. 2017;544:413–414. [Google Scholar]
- 105.Erickson BJ, Korfiatis P, Akkus Z, Kline TL. Machine Learning for Medical Imaging. Radiographics. 2017;37:505–515. doi: 10.1148/rg.2017160130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Waldstein SM, Seeböck P, Donner R, Sadeghipour A, Bogunović H, Osborne A, Schmidt-Erfurth U. Unbiased identification of novel subclinical imaging biomarkers using unsupervised deep learning. Sci Rep. 2020;10:12954. doi: 10.1038/s41598-020-69814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Poston GJ, Adam R, Alberts S, Curley S, Figueras J, Haller D, Kunstlinger F, Mentha G, Nordlinger B, Patt Y, Primrose J, Roh M, Rougier P, Ruers T, Schmoll HJ, Valls C, Vauthey NJ, Cornelis M, Kahan JP. OncoSurge: a strategy for improving resectability with curative intent in metastatic colorectal cancer. J Clin Oncol. 2005;23:7125–7134. doi: 10.1200/JCO.2005.08.722. [DOI] [PubMed] [Google Scholar]
- 108.O'Reilly DA, Chaudhari M, Ballal M, Ghaneh P, Wu A, Poston GJ. The Oncosurge strategy for the management of colorectal liver metastases - an external validation study. Eur J Surg Oncol. 2008;34:538–540. doi: 10.1016/j.ejso.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 109.Giannini V, Rosati S, Defeudis A, Balestra G, Vassallo L, Cappello G, Mazzetti S, De Mattia C, Rizzetto F, Torresin A, Sartore-Bianchi A, Siena S, Vanzulli A, Leone F, Zagonel V, Marsoni S, Regge D. Radiomics predicts response of individual HER2-amplified colorectal cancer liver metastases in patients treated with HER2-targeted therapy. Int J Cancer. 2020;147:3215–3223. doi: 10.1002/ijc.33271. [DOI] [PubMed] [Google Scholar]
- 110.Paredes AZ, Hyer JM, Tsilimigras DI, Moro A, Bagante F, Guglielmi A, Ruzzenente A, Alexandrescu S, Makris EA, Poultsides GA, Sasaki K, Aucejo FN, Pawlik TM. A Novel Machine-Learning Approach to Predict Recurrence After Resection of Colorectal Liver Metastases. Ann Surg Oncol. 2020;27:5139–5147. doi: 10.1245/s10434-020-08991-9. [DOI] [PubMed] [Google Scholar]
- 111.Shu Z, Fang S, Ding Z, Mao D, Cai R, Chen Y, Pang P, Gong X. MRI-based Radiomics nomogram to detect primary rectal cancer with synchronous liver metastases. Sci Rep. 2019;9:3374. doi: 10.1038/s41598-019-39651-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Spelt L, Nilsson J, Andersson R, Andersson B. Artificial neural networks--a method for prediction of survival following liver resection for colorectal cancer metastases. Eur J Surg Oncol. 2013;39:648–654. doi: 10.1016/j.ejso.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 113.Wei J, Cheng J, Gu D, Chai F, Hong N, Wang Y, Tian J. Deep learning-based radiomics predicts response to chemotherapy in colorectal liver metastases. Med Phys. 2021;48:513–522. doi: 10.1002/mp.14563. [DOI] [PubMed] [Google Scholar]