ABSTRACT
Objectives: N-nitrosodimethylamine (NDMA) is known to elicit carcinogenic activity in the liver and kidney of animals. There is a dearth of information of its effect in testis. This study evaluated the protective role of betulinic acid (BA) against NDMA-induced redox imbalance in testes of rats.
Methodology: Twenty-four male rats were assigned into four groups and treated with normal saline, BA, NDMA and [BA+NDMA]. BA (25 mg/kg) was given for 14 days, while NDMA (5 mg/kg) was given on days 7 and 12.
Results: Administration of NDMA significantly increased the weight and relative weight of testes by 51 and 71%, respectively, while treatment with BA attenuated the weight-gain. Furthermore, NDMA decreased the sperm count, motility and live–dead ratio by 57, 36 and 37%, respectively, and increased total sperm abnormality by 56%. However, BA attenuated the changes in the spermiogram of NDMA-treated rats. NDMA significantly decreased the activities of antioxidative enzymes, follicle-stimulating and luteinizing hormones, while testicular levels of thiobarbituric acid reactive substances and total cholesterol were increased. Also, NDMA increased the activities of aniline hydroxylase and aminopyrine-N-demethylase. Supplementation with BA attenuated NDMA-induced alteration in these biochemical indices.
Conclusion: BA protects against NDMA-induced redox imbalance via activation of antioxidative pathway.
KEYWORDS: Sperm quality, phase I and II enzymes, oxidative stress, antioxidant, toxicant
Introduction
Humans and animals are exposed to N-nitrosamines through chemical industries, processed foods, ground water and polluted environment, giving rise to toxicological implications [1]. The toxicity of NDMA has been associated with free radicals, reactive metabolites and inflammatory substances [2]. N-nitrosamines are activated by N-nitrosodimethylamine-N-demethylases I and II via α-hydroxylation [3]. The α-hydroxy nitrosamine formed is finally converted to an alkyl nitrenium or carbonium ion, which can alkylate DNA and other macromolecules [4]. It is also known that the carcinogenicity of nitrosamines is directly proportional to activities of the activating enzymes [5]. Recently, our studies on the hepatorenal toxicity of NDMA linked the toxicity of the toxicant to increase in DNA fragmentation, expression of Bcl-2, p53, Ki-67 and CD15 in rats [6,7]. However, there is a dearth of information on the effects of NDMA on male reproductive system.
Betulinic acid (BA) (3β-hydroxy-lup-20(29)-en-28-oic acid) is a pentacyclic lupane-type triterpene, first isolated from the bark of birch tree (Betula pendula Roth) [8]. This triterpene has also been found in other plants, including Aziziphus species, Syzygium species, Diospyros species and Orthosiphon stamineus [9–12]. BA possesses biological properties, including anti-inflammatory and apoptotic effects [13,14]. Some outstanding biological activities of BA include selective anticancer activity against metastatic over non-metastatic cancer cells, selectivity against cancerous cells over non-cancerous cells and exhibition of cytotoxicity against cancers that are resistant to conventional cancer drugs [15–18]. BA elicits antioxidant activity by reducing reactive oxygen species (ROS) production, and reversing superoxide anion-induced decrease in nitric oxide and endothelial nitric oxide synthase [19]. We hypothesized that BA, a polyphenolic compound, will readily lose electrons in vivo to terminate metabolites (mostly radicals) generated during NDMA metabolism, and thus ameliorates redox imbalance in testes of rats.
Materials and methods
Chemicals
NDMA and BA were purchased from Sigma Chemical Co. (Saint Louis, MO, U.S.A). Glutathione, hydrogen peroxide, 5,5′-dithios-bis-2-nitrobenzoic acid (DTNB) and epinephrine were purchased from Sigma Chemical Co., Saint Louis, MO, U.S.A. Trichloroacetic acid and thiobarbituric acid (TBA) were purchased from British Drug House (BDH) Chemical Ltd, Poole, UK. Other chemicals and reagents were of analytical grade and purest quality available.
Experimental animals
Male Wistar rats (135.7 ± 6.6 g; 128–142 g) were purchased from the Animal House of the Faculty of Basic Medical Sciences of the University of Ibadan, Nigeria. They were housed in plastic cages and fed on rats pellets and given drinking water ad libitum. The rats were acclimatized for 7 days before the experiment and subjected to 12-hour light/dark cycle and temperature of 29 ± 2°C. The study was approved by the Faculty of Basic Medical Sciences, University of Ibadan Animal Ethics Committee.
Study design
Twenty-four male Wistar rats were randomly assigned into four groups of six rats each. The first group (control) was given normal saline, second group (BA) was given BA alone (25 mg/kg body weight), third group (NDMA) was given NDMA alone (5 mg/kg body weight), while the fourth group (BA + NDMA) was given BA (25 mg/kg body weight) and NDMA (5 mg/kg body weight). The BA and NDMA were separately dissolved in normal saline. The choice of dosage and vehicle was based on a previous study [7]. BA was given by gavage for 14 consecutive days, while NDMA was given intraperitoneally on days 7 and 12 of the study. Administration of NDMA (days 7 and 12) was staggered purposively to produce the desired toxicity in the rats without mortality.
Collection of semen, blood and testes
The rats were fasted overnight after the last dose of BA on day 14. Blood was collected by ocular bleeding, allowed to clot and then centrifuged at 3000g for 10 minutes to obtain serum. Animals were euthanized by exsanguination, and semen was collected from epididymis immediately for analysis. The testes were excised, washed in ice-cold 1.15% potassium chloride solution to remove blood stains, dried and weighed. One portion of testicular tissue was homogenized using a Teflon homogenizer and centrifuged using a high speed refrigerated centrifuge (HITACHI) at 10,000g for 10 minutes to obtain postmitochondrial fraction. The other portion of the tissue was fixed in Bouin solution for histological analysis.
Sperm analysis
Sperm count and sperm motility were determined according to the method described by Oehninger et al. [20]. Sperm abnormalities were determined by assessing the morphological features, including sperm head, mid-piece and tail, as described by Duran et al. [21].
Biochemical analysis
Protein determination
Testicular protein levels were determined according to the method of Lowry et al. [22] using bovine serum albumin as the standard.
Hormonal assays
Serum testosterone was assayed by the enzyme-linked immunoabsorbent assay (ELISA) as described by Tietz [23] using Serozyme I Serono (Diagnostics, Freiburg, Germany). The testosterone concentration was obtained by correlating the absorbance of the test sample at 550 nm with the corresponding absorbance on the standard curve. The FSH, prolactin and LH concentrations were determined based on a solid-phase enzyme-linked immunoabsorbent assay, as described by Uotila et al. [24].
Determination of aniline hydroxylase activity
Aniline hydroxylase activity was determined based on the formation of p-aminophenol during the hydroxylation of aniline hydrochloride, as described by Ko et al. [25].
Determination of aminopyrine-N-demethylase activity
Activity of aminopyrine-N-demethylase was determined according to the method of Tu and Yang [26]. The assay is based on the N-demethylation of aminopyrine (4-dimethyl aminoantipyrine) to 4-aminoantipyrine with a stepwise formation of formaldehyde. The amount of formaldehyde formed was estimated by the method of Nash [27].
Determination of uridyl diphosphoglucuronsyl transferase activity
The uridyl diphosphoglucuronsyl transferase (UDPGT) activity was determined according to the method of Letelier et al. [28]. The activity is proportional to the rate of conjugation of p-nitrophenol with UDP-glucuronic acid.
Determination of total cholesterol, triglyceride and phospholipids levels
Testicular total cholesterol (TC), phospholipids (PL) and triglyceride (TG) levels were estimated according to the methods of Naito [29] and Buccolo and David [30].
Determination of thiobarbituric acid reactive substances level
Testicular lipid peroxidation (LPO) was estimated by determining the concentration of thiobarbituric acid reactive substance (TBARs), as described by Ohkawa et al. [31]. The method is based on the reactivity of an end product of LPO, malondialdehyde (MDA), with TBA to produce a pink adduct. The absorbance of the clear supernatant was read in a spectrophotometer against a reference blank at 532 nm. LPO was expressed in micromole MDA formed/mg protein using a molar extinction coefficient of 1.56 × 105/m/cm.
Determination of superoxide dismutase activity
Testicular superoxide dismutase (SOD) activity was determined by the method of Misra and Fridovich [32]. The method was based on the ability of SOD to inhibit the autoxidation of epinephrine (pH 10.2, 30°C). The increase in absorbance of assay reaction at 480 nm was monitored spectrophotometrically at 30 seconds intervals for 150 seconds. The specific activity of SOD was expressed in units/mg protein.
Determination of glutathione peroxidase, catalase and glutathione-S-transferase activities
Testicular glutathione peroxidase (GPx), catalase (CAT) and glutathione-S-transferase (GST) activities were determined by the methods of Andersen et al. [33], Aebi [34] and Habig et al. [35], respectively.
Assay of GPx
Assay is based on the reduction of organic peroxide in a reaction mixture and oxidation of reduced glutathione (GSH) to form disulfide glutathione (GSS). The GSSG is then reduced by glutathione reductase and NADPH in the reaction mixture forming NADP+, resulting in decreased absorbance at 412 nm. The decrease in absorbance at 412 nm is directly proportional to the activity of GPx. The GPx activity was expressed as mmol/mg protein.
Assay of CAT
The method is based on the ability of CAT to promote the decomposition of H2O2 in a reaction mixture. The change in absorbance during 3 minutes at 240 nm is a measure of CAT activity. CAT activity was expressed as units/mg protein.
Assay of GST
The method is based on the ability of GST to catalyze the conjugation of l-glutathione and CDNB to form a conjugate (GS-DNB), with an absorbance at 340 nm. Hence, the rate of increase in the absorption at 340 is directly proportional to GST activity in the sample. One unit of GST activity is defined as the amount of enzyme producing 1 mmol of GS-DNB conjugate per minute under the conditions of the assay. Specific activity of GST was expressed as micromoles of GS-DNB conjugate formed per minute per mg protein using an extinction coefficient of 9.61/mmol/cm.
Determination of GSH level
Testicular GSH level was determined using the method described by Mitchell et al. [36]. Briefly, the assay is involved in the oxidation of GSH by the sulfhydryl reagent DTNB to form a yellow derivative, 5′-thio-2-nitrobenzoic acid, with an absorbance at 412 nm. GSH level is proportional to absorbance at 412 nm. Values were expressed as µmol/g tissue.
Histology
A section of the testicular tissue fixed in Bouin solution was dehydrated in 95% ethanol and then cleared in xylene before embedded in paraffin. Microsections (3 μm) were prepared and stained with hematoxylin and eosin (H&E) dye, and were examined under a light microscope by a histopathologist who was ignorant of the treatment groups.
Statistical analysis
All values were expressed as the mean ± standard deviation of six animals per group. Data were analyzed using one-way analysis of variance followed by the post hoc Duncan multiple range test for the analysis of biochemical data using SPSS (10.0). Statistically significant values were taken at P < 0.05.
Results
Effects of BA on body-weight gain and reproductive indices in NDMA-treated rats
As shown in Table 1, NDMA caused a 44% decrease in body-weight gain of rats relative to controls. In the group treated with BA alone, body-weight gain was observed to increase insignificantly (P > 0.05) when compared with controls. However, in rats co-treated with NDMA and BA, the body-weight gain was found to increase by 52% relative to NDMA-treated animals. Furthermore, NDMA administration significantly (P < 0.05) increased the weight and relative weight of testes by 51 and 71%, respectively, when compared with controls. Upon supplementation with BA, both the weight and relative weight of testes were attenuated (Table 1). In addition, NDMA administration significantly (P < 0.05) decreased the sperm count, motility and live–dead ratio of rats by 36, 31 and 29%, and increased total sperm abnormality by 62%, respectively, relative to controls. However, supplementation with BA significantly (P < 0.05) attenuated the NDMA-induced alteration in spermiogram. Importantly, the sperm volume was insignificantly affected by NDMA intoxication (Table 2). Table 3 depicts the effect of BA on the levels of reproductive hormones in NDMA-treated rats. Administration of NDMA significantly (P < 0.05) decreased the serum concentrations of LH, FSH and testosterone by 30, 43 and 37%, respectively, when compared with controls. Supplementation with BA significantly (P < 0.05) increased the serum levels of LH, FSH and testosterone by 21, 57 and 39%, respectively, relative to NDMA-treated rats. The level of serum prolactin was insignificantly (P > 0.05) affected by treatment with NDMA and BA.
Table 1. Body weight and relative weight of testes of rats treated with NDMA and BA (alone and in combination).
| Grouping | Weights (g) | Weight of testes (g) | Relative weight of testes (% of body weight) |
||
|---|---|---|---|---|---|
| Initial | Final | Weight gain | |||
| Control | 136.67 ± 3.50 | 192.50 ± 5.73 | 55.83 ± 2.83 | 2.03 ± 0.13 | 1.06 ± 0.02 |
| BA | 131.00 ± 4.01 | 195.00 ± 2.25 | 64.17 ± 4.29a | 2.18 ± 0.19 | 1.12 ± 0.05b |
| NDMA | 131.67 ± 3.83 | 163.33 ± 6.69 | 31.67 ± 1.69c | 3.06 ± 0.16c | 1.86 ± 0.02c |
| BA + NDMA | 136.67 ± 5.16 | 184.17 ± 4.00 | 47.50 ± 4.05a | 2.10 ± 0.18a | 1.18 ± 0.05a |
Data expressed as mean ± S.D. (n = 6).
aStatistically different from NDMA (P < 0.05).
bStatistically similar to [BA + NDMA] (P > 0.05).
cStatistically different from control (P < 0.05).
BA: betulinic acid; NDMA: N-nitrosodimethylamine.
Table 2. Sperm qualities of rats treated with NDMA and BA (alone and in combination).
| Treatments groups | Motility (%) | Live–dead ratio | Sperm volume (ml) | Sperm count (×106/ml) | TSA (%) |
|---|---|---|---|---|---|
| Control | 87.50 ± 6.12 | 97.00 ± 1.55 | 5.18 ± 0.04 | 130.50 ± 8.76 | 7.48 ± 0.14 |
| BA | 83.83 ± 6.65a | 96.50 ± 1.64a | 5.17 ± 0.05a | 112.33 ± 9.24a | 7.80 ± 0.54a |
| NDMA | 60.11 ± 4.01b | 70.83 ± 9.70b | 5.18 ± 0.04 | 83.33 ± 8.14b | 12.14 ± 0.42b |
| BA + NDMA | 85.67 ± 6.06c | 91.83 ± 7.52c | 5.17 ± 0.05 | 107.33 ± 7.76c | 8.48 ± 0.72c |
Data expressed as mean ± S.D. (n = 6).
aStatistically similar to [BA + NDMA] (P > 0.05).
bStatistically different from control (P < 0.05).
cStatistically different from NDMA (P < 0.05).
TSA: total sperm abnormality; BA: betulinic acid; NDMA: N-nitrosodimethylamine.
Table 3. Follicle-stimulating hormone, testosterone and prolactin levels in the rats treated with NDMA and BA (alone and in combination).
| Treatments | FSH | LH | Testosterone | |
|---|---|---|---|---|
| (pg/ml) | Prolactin | |||
| Control | 9.25 ± 2.06 | 5.18 ± 0.94 | 2.43 ± 0.70 | 8.35 ± 2.08 |
| BA | 8.05 ± 1.41 | 5.02 ± 0.87a | 2.58 ± 0.73a,b | 8.50 ± 3.11a |
| NDMA | 5.25 ± 2.63c | 3.64 ± 0.58c | 1.53 ± 0.76c | 9.05 ± 6.95 |
| BA + NDMA | 8.25 ± 2.50b | 4.39 ± 0.30b | 2.13 ± 0.75b | 8.75 ± 2.22 |
Data expressed as mean ± S.D. (n = 6).
aStatistically similar to [BA+NDMA] (P > 0.05).
bStatistically different from NDMA (P < 0.05).
cStatistically different from control (P < 0.05).
BA: betulinic acid; NDMA: N-nitrosodimethylamine; FSH: follicle-stimulating hormone; LH: luteinizing hormone.
Effects of BA on lipid parameters and antioxidant status of NDMA-treated rats
Effects of NDMA on the levels of testicular TC, TG and PL are given in Table 4. Treatment with NDMA significantly (P < 0.05) increased TC (0.52 ± 0.18 mM/l) and TG (1.12 ± 0.39 mM/l) relative to controls (0.37 ± 0.09 and 0.45 ± 0.30 mM/l, respectively), while NDMA significantly (P < 0.05) decreased PL (1.02 ± 0.28 mM/l) relative to control (1.80 ± 0.37 mM/l). Also, NDMA increased TC/PL ratios from 0.17 (control) to 0.51 (NDMA-treated group). Interestingly, supplementation with BA significantly attenuated the NDMA-induced alteration in testicular lipid profile of the animals. Furthermore, treatment with NDMA increased the levels of TBARS by 94% (Figure 1), with a concomitant significant (P < 0.05) decrease in the activities of testicular SOD (29%), CAT (41%), GPx (48%), GST (37%) and level of GSH (35%) (Figures 2–5) when compared with their respective controls. However, supplementation with BA significantly (P < 0.05) attenuated the effects of TBARS, and the antioxidative parameters in the testes of rats.
Table 4. Testicular TC, TG and PL levels in the rats treated with NDMA and BA (alone and in combination).
| Treatments | TC (mM/l) | TG (mM/l) | PL (mM/l) | TC/PL |
|---|---|---|---|---|
| Control | 0.37 ± 0.09 | 0.45 ± 0.30 | 1.80 ± 0.37 | 0.17 |
| BA | 0.35 ± 0.12a | 0.66 ± 0.52a | 1.95 ± 0.43a | 0.18 |
| NDMA | 0.52 ± 0.18b | 1.12 ± 0.39b | 1.02 ± 0.28b | 0.51b |
| BA + NDMA | 0.41 ± 0.33c | 0.72 ± 0.12c | 1.75 ± 0.57 | 0.25c |
Data expressed as mean ± S.D. (n = 6).
aStatistically similar to [BA + NDMA] (P > 0.001).
bStatistically different from control (P < 0.05).
cStatistically different from NDMA (P < 0.05).
TC: total cholesterol; TG: triglycerides; PL: phospholipids; BA: betulinic acid; NDMA: N-nitrosodimethylamine.
Figure 1.
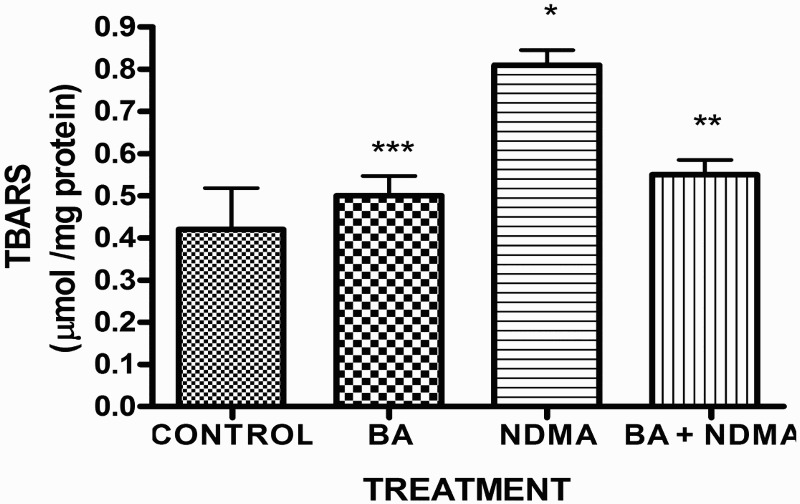
The levels of testicular TBARS in rats treated with NMDA and BA (alone and combination).
Figure 2.
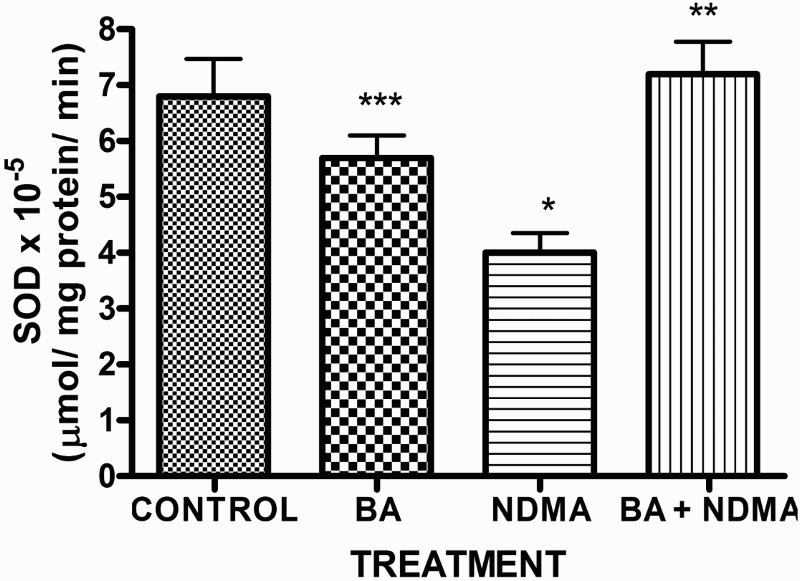
Activity of SOD in rats treated with NMDA and BA (alone and combination).
Figure 3.
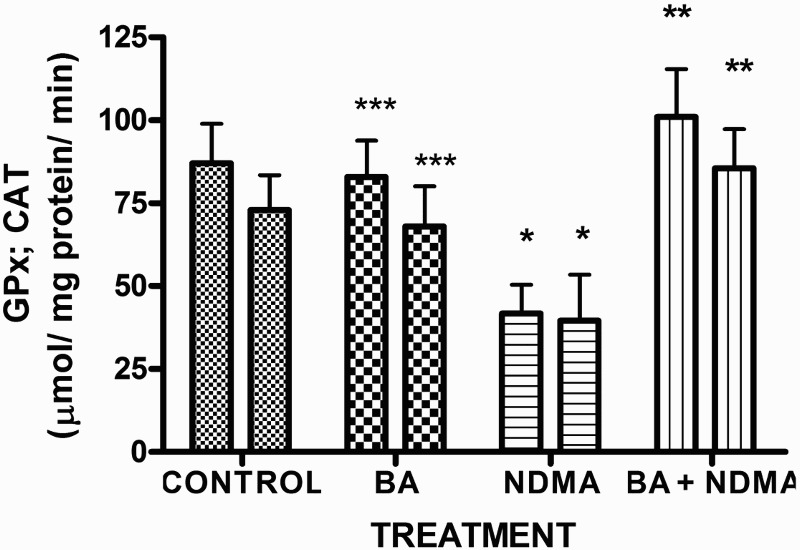
The activities of glutathione peroxidase and CAT in rats treated with NMDA and BA (alone and combination).
Figure 4.
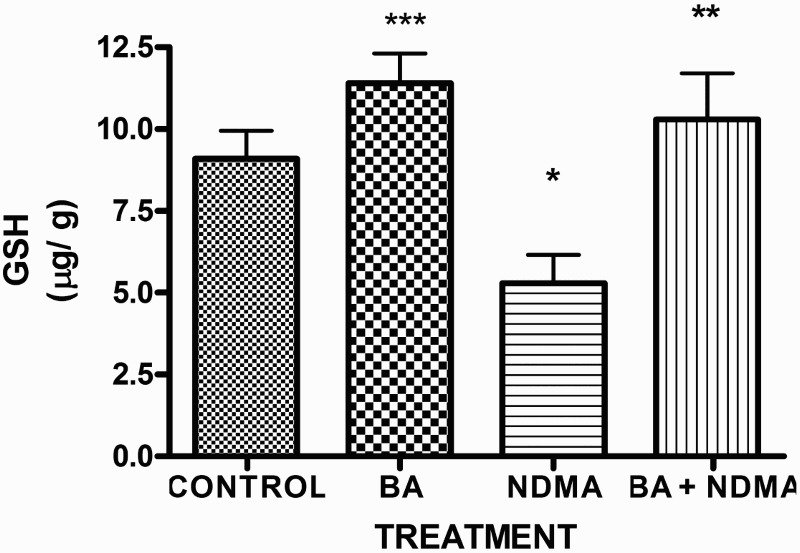
Levels of testicular GSH in rats treated with NMDA and BA (alone and combination).
Figure 5.
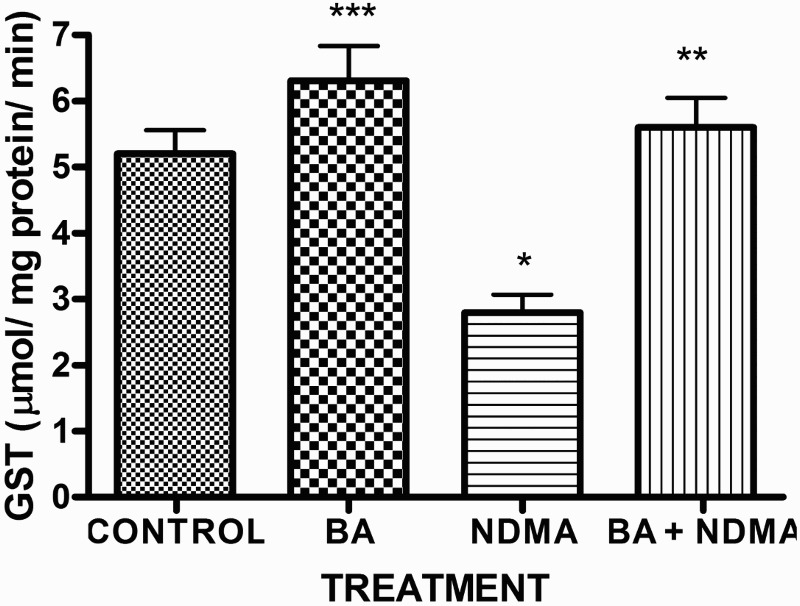
Activity of testicular GST in rats treated with NMDA and BA (alone and combination).
Effects of BA on drug-metabolizing enzymes and histomorphometry of testes in NDMA-treated rats
Effects of NDMA administration on phase I enzymes; aniline-4-hydroxylase (AnH) and aminopyrine-N-demethylase (AmD) and phase II enzyme; UDPGT are given in Table 5. NDMA administration significantly (P < 0.05) increased the activities of AnH and AmD in testes of rats by 326 and 59%, respectively, while UDPGT activity was lowered by 42% relative to controls. However, supplementation with BA reduced the alteration caused by NDMA on these biochemical parameters. Histological examination of the testicular tissues revealed that NDMA induced severe sub-capsular congestion, cyto-architectural changes and spermato-cellular necrosis in rats when compared with controls. Such cytological lesions were reduced in group supplemented with BA (Figure 6).
Table 5. Activities of phase 1 enzymes [aniline hydroxylase (AnH) and aminopyrine-N-demethylase (AmD)] and phase II enzyme (UDPGT) in the testis of rats treated with NDMA and BA (alone and in combination).
| Treatments groups | AnH | AmD | UDPGT |
|---|---|---|---|
| (μmol/mg protein) | (mmol/mg protein) | ||
| Control | 0.398 ± 0.59 | 0.530 ± 0.01 | 0.12 ± 0.02 |
| BA | 1.014 ± 098a | 0.485 ± 0.01a | 0.14 ± 0.03a |
| NDMA | 1.694 ± 0.86b | 0.840 ± 0.08b | 0.07 ± 0.007b |
| BA + NDMA | 0.910 ± 0.21c | 0.612 ± 0.03c | 0.10 ± 0.03c |
Data expressed as mean ± S.D. (n = 6).
aStatistically similar to [BA + NDMA] (P > 0.001).
bStatistically different from control (P < 0.05).
cStatistically different from NDMA (P < 0.05).
BA: betulinic acid; NDMA: N-nitrosodimethylamine.
Figure 6.
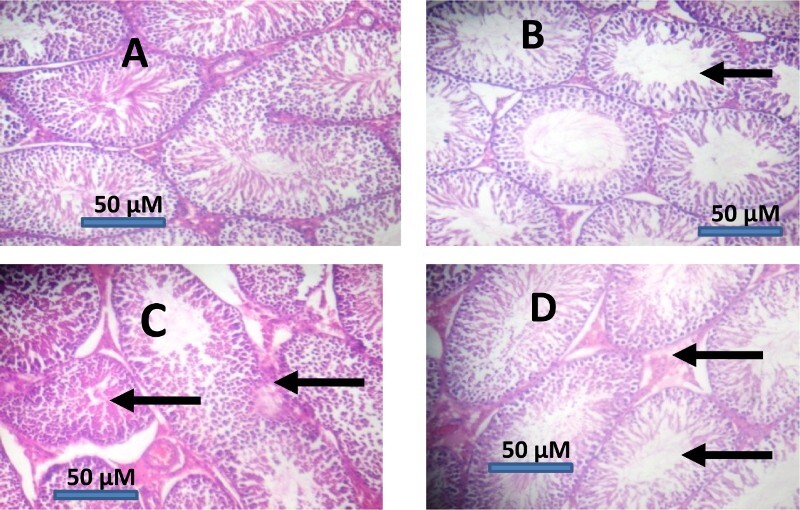
Representative photomicrographs of testes from control and NDMA-treated rats. Control testes (A) showed normal morphology. Treatment-related lesions such as mild congestion, degeneration of the seminiferous tubules and distortion in the testes of NDMA-treated rats (C) were reduced in [NDMA+BA]-treated rats (D), while photomicrographs from BA-treated rats (B) appeared similar to the control (A). BA: betulinic acid; NDMA: N-nitrosodimethylamine.
Discussion
The major findings from the NDMA administered rats were: decrease in body-weight gain, increase in weight and relative weight of testes, induction of phase I enzymes and inhibition of phase II enzyme, alteration in lipid, hormonal and antioxidant profiles as well as cyto-architectural changes in testes of rats. Notably, BA when given to NDMA-treated rats was able to mitigate against NDMA-induced adverse effects in these animals.
The observed increase in testicular weight seen in this study may have resulted from NDMA-induced testicular hypertrophy and necrotic degeneration of seminiferous tubules [37]. The ability of BA to reduce the weight and relative weight of testes of NDMA-treated indicates that this triterpenoid could protect against NDMA-induced testicular hypertrophy. NDMA is a toxicant found in processed foods and ground water, and its adverse impacts on organs, especially liver and kidney, have been studied [7]. However, there is a dearth of information on the effect of this toxicant on male reproductive system. In the present study, NDMA-intoxication increased the level of TBARS (index of LPO) in the testes of rats. LPO is simply the process of oxidation of polyunsaturated fatty acids by ROS to produce hydroperoxide and peroxyl radicals which can be converted to reactive aldehyde, such as MDA [38]. The elevated level of TBARS in the testes of NDMA-treated rats confirmed the induction of oxidative damage. Also, the observed decrease in testicular activities of SOD and CAT in NDMA-treated rats confirms enzyme inhibition probably due to the action of NDMA metabolites, which may enhance superoxide radical and H2O2 accumulation and thus exposed the testes to oxidative stress. SOD is involved in the control of decidual cell differentiation in rats and regulation of cell proliferation [39]. The decrease in SOD activity of NDMA-treated rats observed in this study is similar to the findings of Choi et al. [40], in which NDMA intoxication decreased hepatic SOD, CAT and GST activities of treated rats. In addition, administration of NDMA caused significant depletion of testicular glutathione and rapid loss of activities of enzymes of GSH pathway such as GPx and GST. GSH, a key cellular antioxidant, has been identified as the critical factor needed for spermatozoa maturation in aged animals [42]. Thus, the observed decrease in GSH is an indication of anti-fertility effect of NDMA. Furthermore, GPx and GST activities were significantly reduced in the testes of NDMA-treated rats. GPx protects against oxidative damage by reducing hydrogen peroxide to water [43]. The combined depletion of GSH, reduction of GPx and GST activities strongly suggest that NDMA may adversely affect glutathione metabolic pathway and promote oxidative damage in the testes. The observed effect of NDMA on markers of oxidative stress is consistent with previous studies [6,7,41]. However, supplementation with BA improved the antioxidant status (enzymatic and non-enzymatic) of NDMA-treated animals.
One of the mechanisms of anti-gonadal action of NDMA may be the inhibitory effect on some biochemical processes in tissues of animals. It has been suggested that reactive metabolites from NDMA may suppress gonadotropins release (LH and FSH) by the pituitary [44]. The normal production of FSH and LH by the pituitary is a factor required for spermatogenesis by seminiferous tubules [45]. LH is known to induce Leydig cell to secrete testosterone which regulates spermatogenesis via androgen receptors, while FSH regulates spermatogenesis by stimulating the production of Sertoli cell factors. In this study, NDMA-intoxication decreased the production of LH and FSH, thus may impair the endocrine regulation of spermatogenesis and consequently affects the reproductive function of the testes. The significant reduction in the level of testosterone observed in NDMA-treated rats confirms impaired steroidogenesis. Testosterone plays an important role in the regulation of spermatogenesis [46]. Thus, NDMA-administration decreased sperm count, sperm motility, live– dead ratio and increased total sperm abnormality in rats. The observed decline in sperm quality may be due to inadequate hormonal levels. The biochemical data from this study were also corroborated by the histology of testes, which showed that the NDMA-administration caused changes in cyto-architecture, congestion and degeneration of the seminiferous tubules. The histopathological result is consistent with the findings of Hard and Butler [37] who reported necrotic degeneration of seminiferous tubules following NDMA intoxication. Notably, BA significantly reduced the adverse effects of NDMA on the reproductive hormones, thereby improving the sperm quality of the animals.
Studies have shown that N-nitrosamines could generate high amounts of ROS that may promote the damage of endothelial region of arterial vessels, resulting in cardiovascular diseases [47]. The elevated levels of TC and TG, and decreased level of PL in NDMA-treated rats are indications that this toxicant could potentially impair lipid metabolism in the testes. Elevated levels of TC in testes may affect the hormonal response of this organ to the production of testosterone and may impair gonadal steroidogenesis. Furthermore, NDMA-intoxication in testes increased the activities of AnH and AmD, thus serving as inducers of these phase I enzymes. Induction of cytochrome P450-dependent monooxygenases will enhance xenobiotics metabolism, consequently forming more reactive metabolites. This may explain why NDMA metabolites could exert its toxic effect in the testes. This observation is in consistent with the findings of Sheweita and Mostafa [5] who reported that the carcinogenicity of N-nitrosamines increased with increase in the N-nitrosamines metabolites. Also, a study by Erkekoglu and Baydar [48] established that inhibition of N-Nitrosamines toxicity could be achieved via reduction in the activities of cytochrome P450-dependent enzymes. The UDPGT enzymes are located in the membrane of endoplasmic reticulum and catalyze the conjugation of xenobiotics with UDP-glucuronic acid to form polar conjugates that can be rapidly excreted [49]. Owing to its role in drug detoxification, impairment of UDPGT may constitute important determinants of toxicologic predisposition, with respect to chemical carcinogenesis and teratogenesis [50]. In this study, UDPGT activity was significantly reduced in NDMA-treated group, which may be an important factor for the observed toxicity of this nitrosamine. Interestingly, BA given to NDMA-treated rats restored the activities of phase I and II enzymes to near control values. This further confirms beneficial effect of BA in ameliorating NDMA-induced toxicity in rats.
In conclusion, our data showed that NDMA-induced redox imbalance in testes of rats led to endocrine disruption, dyslipidemia, lowered sperm quality and altered activities of drug-metabolizing enzymes which are amenable to BA supplementation.
Acknowledgments
This research was done without specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Notes on contributors
Dr Oluwatosin A. Adaramoye, B.Sc., M.Sc., Ph.D., is affiliated with the Drug Metabolism and Toxicology Laboratories, Department of Biochemistry, College of Medicine, University of Ibadan, Nigeria.
Dr Emmanual G. Adelekei, B.Sc., M.Sc., Ph.D., is affiliated with the Department of Biochemistry, Ladoke Akintola University of Technology, Ogbomoso, Nigeria.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Oluwatosin A. Adaramoye http://orcid.org/0000-0001-9960-6763
References
- 1.Herrmann SS, Deudahl-Olesen L, Granby K. Occurrence of volatile and non-volatile N-nitrosamines in processed meat products and role of heat treatment. Food Control. 2015;48:163–169. doi: 10.1016/j.foodcont.2014.05.030 [DOI] [Google Scholar]
- 2.Hebels DGAJ, Breide JJ, Khampnag R, et al. Radical mechanisms in nitrosamine- and nitrosamide-induced whole genome gene expression modulations in Caco-2 cells. Toxicol Sci. 2010;116:194–205. doi: 10.1093/toxsci/kfq121 [DOI] [PubMed] [Google Scholar]
- 3.Ortiz de Montellano PR, De Voss JJ. Cytochrome P450: structure, mechanism, and biochemistry. 3rd ed. Ortiz de Montellano PR, editor. New York (NY: ): Kluwer Academic/Plenum Publishers; 2005. p. 183–245. [Google Scholar]
- 4.Sheweita SA. Narcotic drugs change the expression of cytochrome P450 2E1 and 2C6 and other activities of carcinogen-metabolizing enzymes in the liver of male mice. Toxicology. 2003;191:133–142. doi: 10.1016/S0300-483X(03)00252-X [DOI] [PubMed] [Google Scholar]
- 5.Sheweita SA, Mostafa MH. N-nitrosamines and their effects on the level of glutathione, glutathione reductase and glutathione-S-transferase activities in the liver of male mice. Cancer Lett. 1996;99:29–34. doi: 10.1016/0304-3835(95)04034-X [DOI] [PubMed] [Google Scholar]
- 6.Adeleke GE, Adedosu OT, Adaramoye OA, et al. Hepatoprotective effect of methanol extract of Diospyros chloroxylon leaf in N-nitrosodimethylamine-induced hepatotoxicity in rats. Asian Pacific J Health Sci. 2016;3:142–152. [Google Scholar]
- 7.Adeleke GE, Adaramoye OA. Modulatory role of betulinic acid in N-nitrosodimethylamine-induced hepato-renal toxicity in male rats. Hum Expert Toxicol. 2016;13:1–10. [DOI] [PubMed] [Google Scholar]
- 8.Cichewicz RH, Kouzi SA. Chemistry, biological activity and chemotherapeutic potential of BA for the prevention and treatment of cancer and HIV infection. Med Res Rev. 2004;24:90–114. doi: 10.1002/med.10053 [DOI] [PubMed] [Google Scholar]
- 9.Singh P, Sharma S. Triterpenoid constituents of the seed of Diospyros melanozylon, Tecomella undulata and Terminalia bellirica. Indian Chem Soc. 1997;74:504–505. [Google Scholar]
- 10.Schuhly W, Heilmann J, Calis I, et al. New triterpenoids with antibacterial activity from zizyphusjoazeiro. Planta Med. 1999;65:740–743. doi: 10.1055/s-1999-14054 [DOI] [PubMed] [Google Scholar]
- 11.Chang CW, Wu TS, Hsieh YS, et al. Triterpenoids of Syzygiumformosanum. J Nat Prod. 1999;62:327–328. doi: 10.1021/np980313w [DOI] [PubMed] [Google Scholar]
- 12.Yam MF, Basir R, Asmawi MZ, et al. Antioxidant and hepatoprotective effects of Orthosiphon stamineus. Benth. Standardized extracts. Am J Chin Med. 2007;35:115–126. doi: 10.1142/S0192415X07004679 [DOI] [PubMed] [Google Scholar]
- 13.Kasperczyk H, La Ferla-Bruhl K, Westhoff MA, et al. Betulinic acid as a new activator of NF-Kappa B: molecular mechanisms and implications for cancer therapy. Oncogene. 2005;24:6945–6956. doi: 10.1038/sj.onc.1208842 [DOI] [PubMed] [Google Scholar]
- 14.Rabi T, Shukla S, Gupta S. Betulinic acid suppresses constitutive and TNF alpha-induced NF-kappa B activation and induces apoptosis in human prostate carcinoma PC-3 cells. Mol Carcinog. 2008;47:964–973. doi: 10.1002/mc.20447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rieber M, Srasberg-Rieber M. Induction of p53 without increase in p21 WAF 1 M betulinic acid mediated cell death is preferential for human metastatic melanoma. DNA Cell Biol. 1998;17:399–406. doi: 10.1089/dna.1998.17.399 [DOI] [PubMed] [Google Scholar]
- 16.Selzer E, Pimentel E, Wacheck V, et al. Effects of betulinic acid alone and in combination with irradiation in human melanoma cells. J Invest Dermatol. 2000;114:935–940. doi: 10.1046/j.1523-1747.2000.00972.x [DOI] [PubMed] [Google Scholar]
- 17.Zuco V, Supino R, Righetti SC, et al. Selective cytotoxicity of betulinic acid on tumor cell lines, but not normal cells. Cancer Lett. 2002;175:17–25. doi: 10.1016/S0304-3835(01)00718-2 [DOI] [PubMed] [Google Scholar]
- 18.Ehrhardt H, Fulda S, Fuhrer M, et al. Betulinic acid induced apoptosis in leukemia cells. Leukemia. 2004;18:1406–1412. doi: 10.1038/sj.leu.2403406 [DOI] [PubMed] [Google Scholar]
- 19.Qian L, Fu J, Cai X, et al. Betulinic acid inhibits superoxide anion-mediated impairment of endothelium-dependent relaxation in rats. Indian J Pharmacol. 2012;44:588–592. doi: 10.4103/0253-7613.100382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oehninger S, Acosta R, Morshedi M, et al. Relationship between morphology and motion characteristics of human spermatozoa in semen and in the swim-up sperm fractions. J Androl. 1990;11:446–452. [PubMed] [Google Scholar]
- 21.Duran EH, Morshedi M, Taylor S, et al. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod. 2002;17:3122–3128. doi: 10.1093/humrep/17.12.3122 [DOI] [PubMed] [Google Scholar]
- 22.Lowry OH, Rosbrough NJ, Farr AL, et al. Protein measurement with the Folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 23.Tietz NW. Clinical guide to laboratory tests. 3rd ed. Philadelphia (PA: ): W.B. Saunders Company; 1995. p. 215–223. [Google Scholar]
- 24.Uotila M, Ruoslahti E, Engvali EJ. Two-site sandwich enzyme immunoassay with monoclonal antibodies to human alpha-fetoprotein. J Immunol Methods. 1981;42:11–15. doi: 10.1016/0022-1759(81)90219-2 [DOI] [PubMed] [Google Scholar]
- 25.Ko IY, Park SS, Song BJ, et al. Monoclonal antibodies to ethanol-induced rat liver cytochrome p450 that metabolizes aniline and nitrosamines. Cancer Res. 1987;47:3101–3109. [PubMed] [Google Scholar]
- 26.Tu YY, Yang CS. High-affinity nitrosamine dealkylase system in rat liver microsome and its induction by fasting. Cancer Res. 1983;43:623–629. [PubMed] [Google Scholar]
- 27.Nash T. The colorimetric estimation of formaldehyde by means of Hantzsch reactions. Biochem J. 1953;55:416–421. doi: 10.1042/bj0550416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Letelier ME, Del Villar E, Sanchez E. Drug tolerance and detoxicating enzymes in Octodon degus and Wistar rats. A comparative study. Comp Biochem Physiol. 1985;80C:195–198. [DOI] [PubMed] [Google Scholar]
- 29.Naito HK. Cholesterol. Kaplan A, et al. Clin Chem. The C. V. Mosby Co. St Loius. Princeton: Toronto; 1984. p. 1194–11206.
- 30.Buccolo G, David H. Quantitative determination of serum triglycerides by use of enzymes. Clin Chem. 1973;19:476–482. [PubMed] [Google Scholar]
- 31.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxidesin animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3 [DOI] [PubMed] [Google Scholar]
- 32.Misra HP, Fridovich J. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1975;247:3170–3175. [PubMed] [Google Scholar]
- 33.Andersen HR, Nielsen JB, Nielsen F, et al. Antioxidative enzyme activities in human erythrocytes. Clin Chem. 1997;43:562–568. [PubMed] [Google Scholar]
- 34.Aebi H. Catalase in vitro. In: Packer L, editor. Methods in enzymology. Orlando (FL): Academic Press; 1984. p. 121–126. [DOI] [PubMed] [Google Scholar]
- 35.Habig W, Pabst M, Jakoby W. Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 36.Mitchell JR, Jollow DJ, Potter WZ, et al. Acetaminophen-induced hepatic necrosis I. Role of drug metabolism. J Pharmacol Expt Therap. 1973;187:185–194. [PubMed] [Google Scholar]
- 37.Hard GC, Butler WH. Toxicity of nitrosodimethylamine in the rat testis. J Pathol. 1970;102:201–207. doi: 10.1002/path.1711020403 [DOI] [PubMed] [Google Scholar]
- 38.Demir E, Kaya B, Soriano C, et al. Genotoxic analysis of four lipid-peroxidation products in the mouse lymphoma assay. Mutat Res. 2011;726:98–103. doi: 10.1016/j.mrgentox.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 39.Devasagayam TP, Sivabalan R, Tarachand U. Lipid peroxidation in the rat uterus during deciduoma-induced cell differentiation. Biochem Int. 1990;21:27–33. [PubMed] [Google Scholar]
- 40.Choi MJ, Zheng HM, Kim JM, et al. Protective effects of Centella asiatica leaf extract on dimethylnitrosamine-induced liver injury in rats. Mol Med Rep. 2016;14:4521–4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laloraya M, Kumar GP, Laloraya MM. Changes in the superoxide radical and superoxide dismutase levels in the uterus of Rattus norvegicus during the estrous cycle and a possible role for superoxide radical in uterine oedema and cell proliferation at proestrus. Biochem Cell Biol. 1991;69:313–316. doi: 10.1139/o91-048 [DOI] [PubMed] [Google Scholar]
- 42.Kopalli SR, Hwang SY, Won YJ, et al. Korean red ginseng extract rejuvenates testicular ineffectiveness and sperm maturation process in aged rats by regulating redox proteins and oxidative defense mechanisms. Exp Gerontol. 2015;69:94–102. doi: 10.1016/j.exger.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 43.Farombi EO, Adedara IA, Ebokaiwe AP, et al. Nigerian bonny light crude oil disrupts antioxidant systems in the testis and sperm of rats. Arch Environ Contam Toxicol. 2010;59:166–174. doi: 10.1007/s00244-009-9443-3 [DOI] [PubMed] [Google Scholar]
- 44.Kumar V, Kural MR, Pereira BMJ, et al. Spearmint induced hypothalamic oxidative stress and testicular anti-androgenicity in Male rats – altered levels of gene expression, enzymes and hormones. Food Chem Toxicol. 2008;46:3563–3570. doi: 10.1016/j.fct.2008.08.027 [DOI] [PubMed] [Google Scholar]
- 45.Sriraman V, Anbalagan M, Rao AJ. Hormonal regulation of Leydig cell proliferation and differentiation in rodent testis: a dynamic interplay between gonadotrophins and testicular factors. Reprod Biomed. 2005;11:507–518. doi: 10.1016/S1472-6483(10)61147-9 [DOI] [PubMed] [Google Scholar]
- 46.Yoshida S, Hiyoshi K, Ichinose T, et al. Effect of nanoparticles on the male reproductive system of mice. Int J Androl. 2009;32:337–342. doi: 10.1111/j.1365-2605.2007.00865.x [DOI] [PubMed] [Google Scholar]
- 47.Guengerich FP, Yun CH, Macdonald TL. Evidence for a 1-electron oxidation mechanism in N-dealkylation of N,N-dialkylanilines by cytochrome P450 2B1. Kinetic hydrogen isotope effects, linear free energy relationships, comparisons with horseradish peroxidase, and studies with oxygen surrogates. J Biol Chem. 1996;271:27321–27329. doi: 10.1074/jbc.271.44.27321 [DOI] [PubMed] [Google Scholar]
- 48.Erkekoglu P, Baydar T. Evaluation of the protective effect of ascorbic acid on nitrite- and nitrosamine-induced cytotoxicity and genotoxicity in human hepatoma line. Toxicol Mech Methods. 2010;20:45–52. doi: 10.3109/15376510903583711 [DOI] [PubMed] [Google Scholar]
- 49.Kato R, Estabrook RW, Cayen MN. Xenobiotic metabolism and disposition. Proceedings of the 2nd Int. ISSX Meeting; Kobe, Japan. Printed by London: Taylor and Francis Ltd; 1989. p. 81–88. [Google Scholar]
- 50.Wells PG, Mackenzie PI, Chowdhury JR, et al. Glucuronidation and the UDP-Glucuronosyl transferases in health and disease. Drug Metab Dispos. 2004;32:281–290. doi: 10.1124/dmd.32.3.281 [DOI] [PubMed] [Google Scholar]


