ABSTRACT
Objectives: The objective of the present study was to evaluate oxidative/nitrative stress in the plasma of 50 patients suffering from the secondary progressive course of multiple sclerosis (MS), and to verify its correlation with physical and mental disability as assessed by the Expanded Disability Status Scale (EDSS), and the Beck Depression Inventory (BDI).
Methods: Oxidative and nitrative damage to proteins was determined by the level of carbonyl groups and 3-nitrotyrosine using ELISA test. Based on the reaction with Ellman’s reagent, we estimated the concentration of oxidized thiol groups. Additionally, we measured the level of lipid peroxidation.
Results: In plasma drawn from MS patients, we observed a significantly higher level of 3-NT (92%; P < 0.0003), carbonyl groups (29%; P < 0.0001) and thiobarbituric acid reactive substances (73%; P < 0.0001), as well as a lower concentration of thiol groups (33%; P < 0.0001), in comparison to healthy subjects. We noted positive correlations between the level of carbonyl groups or 3-NT and both diagnostic parameters, EDSS and BDI. Negative correlations were observed between concentration of -SH groups and EDSS and BDI.
Conclusion: Our results indicate that impaired red-ox balance can significantly promote neurodegeneration in secondary progressive MS.
KEYWORDS: Oxidative/nitrative stress, secondary progressive multiple sclerosis, EDSS, BDI
1. Introduction
Multiple sclerosis (MS) is a complex disease with several pathophysiological processes: inflammation, demyelination, oxidative stress, axonal damage and the repair mechanisms that occur in this disorder [1–3]. These processes are not uniformly represented in patient populations, but can selectively predominate in individual patients. Therefore, heterogeneity in phenotypic expression of MS has an effect on prognosis, and the therapeutic response of MS patients [4]. The early stage of MS is dominated by the inflammation, whereas the chronic phase of the disease is characterized by neurodegeneration and many other interacting processes [1]. The most common clinical form of MS is relapsing-remitting (RR MS), in which fluctuating relapses and remissions are observed. After about 10–20 years of the disease, RR MS (approximately 80% of cases) converts into another subtype of MS – secondary progressive disease (SP MS), which is characterized by irreversible progression of the disability [5–7]. Still, most researchers conduct their studies on the early stages of MS. Our study focused on a selected group of MS patients – only those in the secondary progressive stage of the disease.
Owing to the complexity of MS, it is difficult to predict the course of the disease and determine which pathological processes will dominate in a particular patient [4]. Immune cells play a central role in the initiation and propagation of this disease [6,7]. Their activation leads to accumulation of macrophages (microglia in the brain) and lymphocytes in the central nervous system (CNS), causing demyelination and destruction of axons, accompanied by generation of reactive oxygen species (ROS) [8–10]. Pro-inflammatory mechanisms in microglia can favour the disease’s progression [11]. However, some activated immune cells remain in the blood, and these are responsible for the respiratory burst and oxidative imbalance in plasma [11]. The concentrations of ROS can excessively increase under inflammatory conditions in patients with MS. There is growing evidence that oxidative stress is an important component in the pathogenesis of MS [12–15]. The imbalance between the cellular production of free radicals and cell antioxidant ability is a crucial mechanism, responsible for neuronal damage [16,17] and contributing to the clinical symptoms [16]. It should be considered that oxidative stress might be the primary mechanism in the pathogenesis of MS. Myelin proteins with structural damage could be targets for immune cells, which recognize themas foreign antigens.
The nervous system (brain, spinal cord, peripheral nerves) is rich in unsaturated fatty acids, iron and catecholamines (adrenaline, noradrenaline, dopamine), and this makes it susceptible to oxidative damage [18–20]. What is more, the brain is particularly sensitive to oxidation because of its high oxygen consumption and relatively low level of endogenous antioxidants [20,21].
So far, most proteomic studies of MS patients have focused on the composition of their cerebrospinal fluid (CSF) [21,22]. Using proteomic techniques on the CSF and serums of RR, PP, and SP MS patients, Teunissen et al. [23] found small proteins and peptides which seem to be promising biomarkers for the diagnosis and disease progression of MS.
In our opinion, there are several reasons that the markers of oxidative/nitrative stress in MS should be determined from plasma. We primarily measure the level of protein biomarkers of oxidative/nitrative damage to plasma, because it contains thousands of proteins and peptides in a total concentration range of >1015, while myelin contains approximately 80% lipids and about 20% proteins [24]. Therefore, protein biomarkers of oxidative stress achieve higher concentration in plasma than in CSF, even though MS is a disease that mainly affects the CNS [25,26]. The inflammatory cells (the reactive T-lymphocytes) mainly migrate across the blood–brain barrier (BBB) into the CNS, but a large number of immune cells remain in the blood and play a crucial role in the inflammatory processes [27,28]. Additionally, determination of circulating oxidative stress biomarkers in the plasma is a non-invasive method [29].
The aim of this study was to evaluate the level of oxidative-/nitrative-modified plasma proteins and lipids in the course of SP MS, and to establish a correlation between biomarkers of oxidative stress and two diagnostic scores. These are the Expanded Disability Status Scale (EDSS), a well-known method of quantifying disability in MS and monitoring changes in the level of disability over time that is widely used in clinical trials and in the assessment of patients with MS, and the Beck Depression Inventory (BDI), which is commonly used as a depression screening tool.
Understanding the molecular and biochemical basis of MS pathogenesis is crucial to the development of potential neuroprotective therapies for MS and other neurodegenerative diseases. Monitoring the levels of oxidative stress biomarkers might be useful in following disease progression, and also for assessing the efficacy of antioxidant treatments.
2. Material and methods
2.1. Demographic and clinical characteristics
Blood samples were collected from 50 patients suffering from SP MS, observed for a year beforehand and diagnosed according to the revised McDonald criteria [30]. The status of their MS as the secondary progressive course of the disease was ascertained according to Lubin and Reingold [31]. The initial relapsing-remitting phase of SP MS is followed by progression, with or without incidental relapses, remissions and plateaux. The patients were under Neurorehabilitation Ward control for 3 months, in which time they did not receive any immunostimulators, immunomodulators, hormones, minerals, vitamins or any other substitutions with an antioxidative effect. They were not treated with immunomodulating in this progressive stage of their MS for almost a year. These inclusion criteria allowed us to rule out interference with the effects of these drugs on oxidative stress parameters.
The clinical parameters of the patients (Table 1) were as follows: mean age 48.2 ± 15.2 years; EDSS score 5.5 ± 1.8; BDI score 9.6 ± 4.6, and mean disease duration of 14.3 ± 8.3 years. In our studies, we used BDI cut-off scores according to the Beck et al. [32], which are categorized as: normal (1–10); moderate (17–30); severe (31–40); extreme depression (over 40). Blood samples were taken at the Neurological Rehabilitation Division III General Hospital in Lodz, Poland.
Table 1. Baseline characteristics of SP MS patients.
| SP MS (n = 50) | |
|---|---|
| Age (years) | 48.2 ± 15.2 |
| % Female | 61% |
| MS duration (years) | 14.3 ± 8.3 |
| BMI (kg m−2) | 23.0 ± 3.5 |
| EDSS | 5.5 ± 1.8 |
| BDI | 9.6 ± 4.6 |
Control human blood samples were derived from 50 healthy volunteers, not taking any medication, who had never been diagnosed with MS or other chronic diseases and were without any neurological, hormonal illness or chronic inflammation. The control group and the MS group were matched by the age and sex.
The study protocol and all procedures were followed in accordance with the Helsinki Declaration and were approved by the Bioethics Committee of the Medical University of Lodz, Poland, with Resolution No. RNN/260/08/KB.
2.1.1. Isolation of plasma
The human blood samples were collected into citrate phosphate dextrose adenine-1, taken from a peripheral vein between 8 and 9 am and immediately centrifuged (1.500×g, 15 minutes, at 25°C) to get the plasma.
2.1.2. Measurement of carbonyl groups in human blood plasma
Carbonyl groups were detected using the ELISA method in plasma, and estimated as adducts of 2,4-dinitrophenylhydrazine (DNPH), according to the method described by Buss et al. [33] and modified by Alamdari et al. [34]. First, the microplates were incubated overnight at 4°C to allow the plasma proteins to be non-specifically adsorbed into the ELISA plates. Second, the wells were washed with 300 µL PBS and then human plasma proteins given to reaction with substrate DNPH (0.05 mM, 200 µl, pH 6.2). The plate was incubated for 45 minutes at room temperature. After incubation, all wells were washed five times with 300 μl PBS:ethanol (1:1, v/v), and for a last time with 300 μl PBS. The carbonyl groups were detected by the anti-DNP antibodies, and then by the antibodies conjugated with horseradish peroxidase. The oxidized albumin was used for the preparation of a standard curve, expressed as nmol of carbonyl groups/mg of the albumin that was required to confirm the linearity of the ELISA method. The level of carbonyl groups was determined spectrophotometrically (λ = 316 nm), according to Levine et al. [35].
2.1.3. Evaluation of lipid peroxidation level
Plasma samples were mixed with an equal volume of 15% (w/v) cold trichloroacetic acid in 0.25 M HCl, and with an equal volume of 0.37% (w/v) thiobarbituric acid in 0.25 M HCl. All samples were immersed in a boiling water bath for 10 minutes. After cooling, the samples were centrifuged and then the absorbance of thiobarbituric acid reactive substances (TBARS) was measured at λ = 535 nm, following the method described by Placer et al. [36]. Lipid peroxidation was calculated on the basis of a molar extinction coefficient of malondialdehyde, a reliable marker of lipid peroxidation (ε = 1.56 × 105 M−1 cm−1).
2.1.4. Determination of 3-nitrotyrosine in the plasma proteins
Detection of 3-NT in the plasma proteins was performed, according to Khan et al. [37], using a competitive ELISA test. Concentrations of nitrated plasma proteins was assessed based on a standard curve of 3-nitrotyrosine containing fibrinogen (3-NT-Fg). To receive the 3-NT-Fg, the human fibrinogen was treated with peroxynitrite in a final concentration of 1 mM. The amount of 3-NT in the fibrinogen was determined spectrophotometrically (λ = 430 nm; ε = 4.400 M−1 cm−1). After the spectrophotometric measurement was obtained, nitro-fibrinogen was used to prepare the standard curve, ranging from 10 to 1000 nM/l of 3-nitrotyrosine–fibrinogen equivalent.
2.1.5. Measurement of thiol groups
The total pot of sulfhydryl groups was measured using a method originally described by Ellman [38] and modified by Hu [39]. In this method, thiol compounds interact with the 5,5’dithiobis-(2-nitrobenzonic acid), forming the coloured anion 2-nitro-5-thiobenzoate (TNB−) [40]. TNB− ionizes to yellow TNB2− di-anion, which is quantified after 1 hour’s incubation in 37°C by measuring the absorbance at 412 nm. The concentration of sulfhydryl groups is expressed as µmol/l.
3. Statistical analysis
The statistical analysis was performed using the Stats Direct statistical software V. 2.7.2. All values in this study were expressed as mean ± SD. The results obtained were analysed for normality with a Shapiro–Wilk test. The significance of the differences between the values was determined by normality using an unpaired t-Student test (for data with normal distribution), or a Mann–Whitney U-test (for data with abnormal distribution).
Correlations between the parameters of oxidative stress biomarkers and the EDSS and BDI scores were made using Spearman’s rank correlation [41]. A level of P < 0.05 was accepted as statistically significant.
4. Results
In our study, we determined the changes of plasma oxidative/nitrative stress parameters in SP MS patients. We noted that all of the analysed biomarkers were significantly higher in SP MS patients than in healthy subjects. Our findings clearly demonstrate a statistically significant increase in the level of: carbonyl groups P < 0.0001 (Figure 1(A)); 3-NT P < 0.0003 (Figure 1(B)) in plasma proteins, and lipid peroxidation (expressed as TBARS) P < 0.0001 (Figure 1(D)). We also saw a statistically significant decrease in the level of thiol groups P < 0.0001 (Figure 1(C)) in plasma obtained from SP MS patients, in comparison to the control group. In addition, we evaluated the correlations between oxidative/nitrative biomarkers and the EDSS or BDI scores assessed in SP MS patients. We saw significant positive correlations between carbonyl group concentration and the EDSS score (Figure 2(A); Table 2), and BDI score (Figure 2(B); Table 1) and similarly between the concentration of 3-NT and the EDSS score (Figure 3(A); Table 2), and BDI score (Figure 3(B); Table 2). Significant negative correlations were observed between the level of –SH groups, and EDSS and BDI scores (Figure 4(A,B); Table 2).
Figure 1.
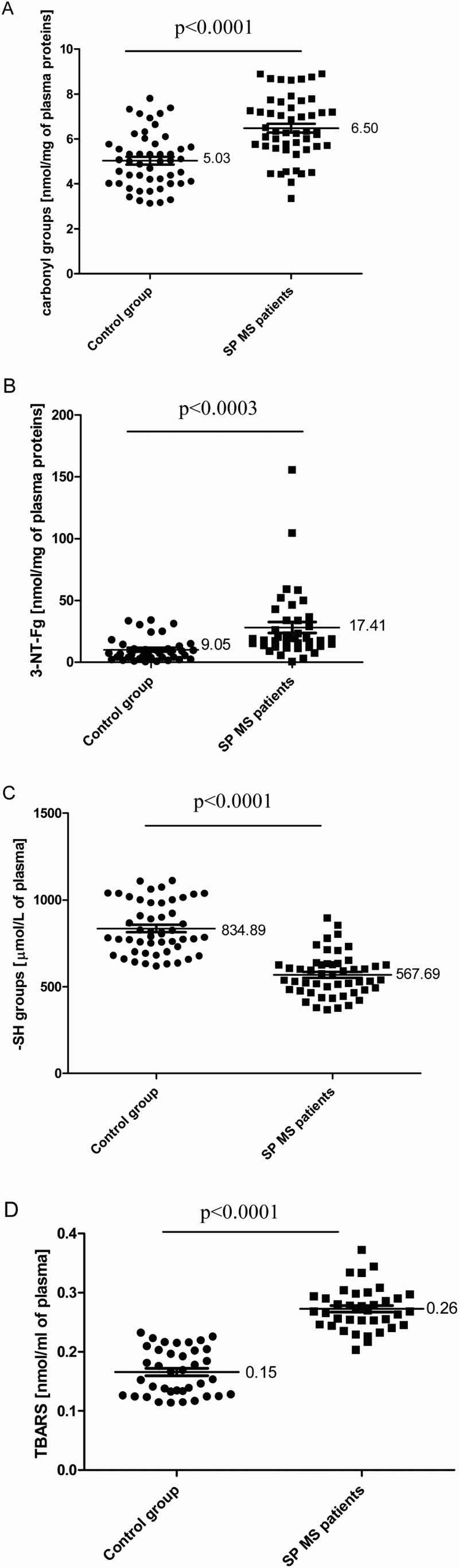
The level of carbonyl groups (n = 50, P < 0.0001) (A); 3-NT (n = 40, P < 0.0003) (B); –SH groups (n = 50, P < 0.0001) (C); TBARS (n = 35, P < 0.0001) (D) in plasma obtained from SP MS patients and control subjects.
Figure 2.
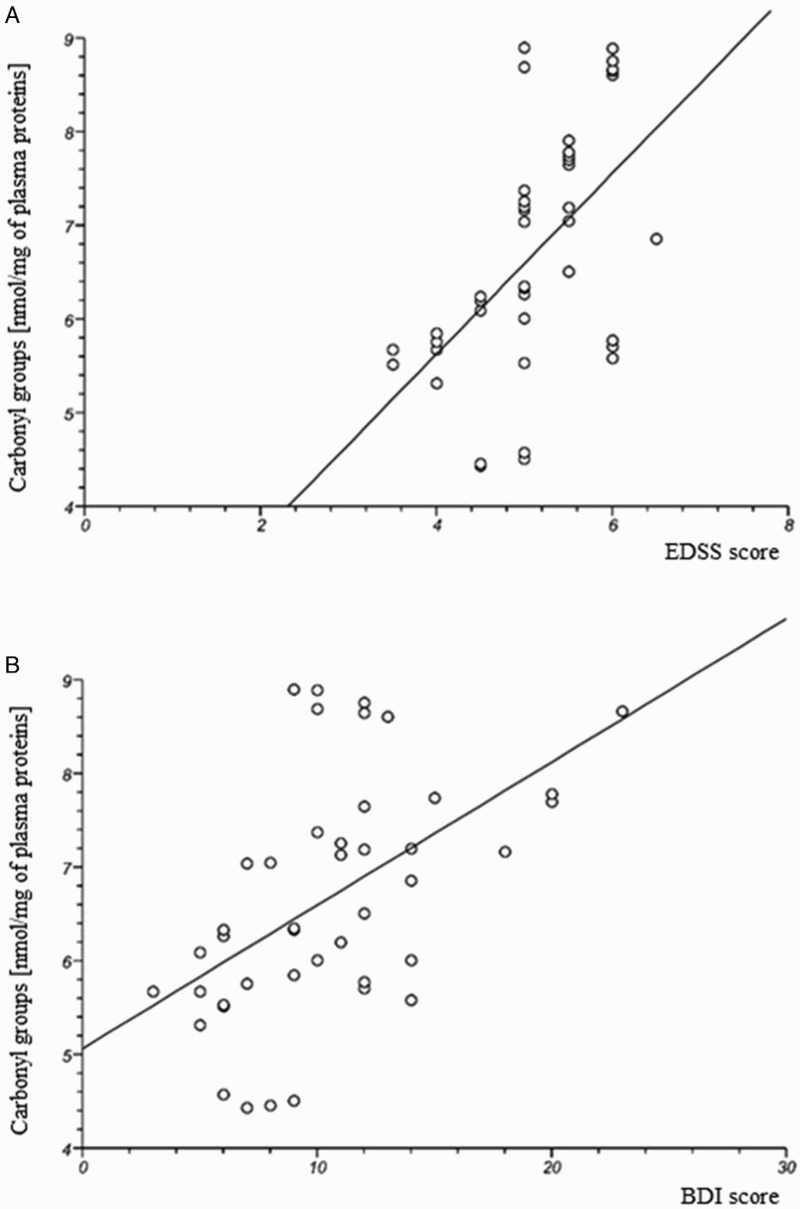
The positive correlation between carbonyl group level in plasma obtained from SP MS patients and clinical parameters: EDSS (A) and BDI (B) scores.
Table 2. Correlation coefficient values obtained for oxidative stress biomarkers and EDSS and BDI scores.
| Carbonyl groups | 3-Nitrotyrosine | −SH groups | TBARS | |
|---|---|---|---|---|
| EDSS | ||||
| Spearman’s rank correlation coefficient (Rho) | 0.567397 | 0.536638 | −0.634374 | 0.061674 |
| Probability of correlation | P < 0.0001 | P < 0.003 | P < 0.0001 | P < 0.3615 |
| BDI | ||||
| Spearman’s rank correlation coefficient (Rho) | 0.561886 | 0.422319 | −0.438609 | −0.007201 |
| Probability for correlation | P < 0.0001 | P < 0.0044 | P < 0.0018 | P < 0.4837 |
Notes: Correlations were analysed using Spearman’s rank correlation method. Table shows Spearman’s rank correlation coefficient (Rho) and probability of correlation (P).
Figure 3.
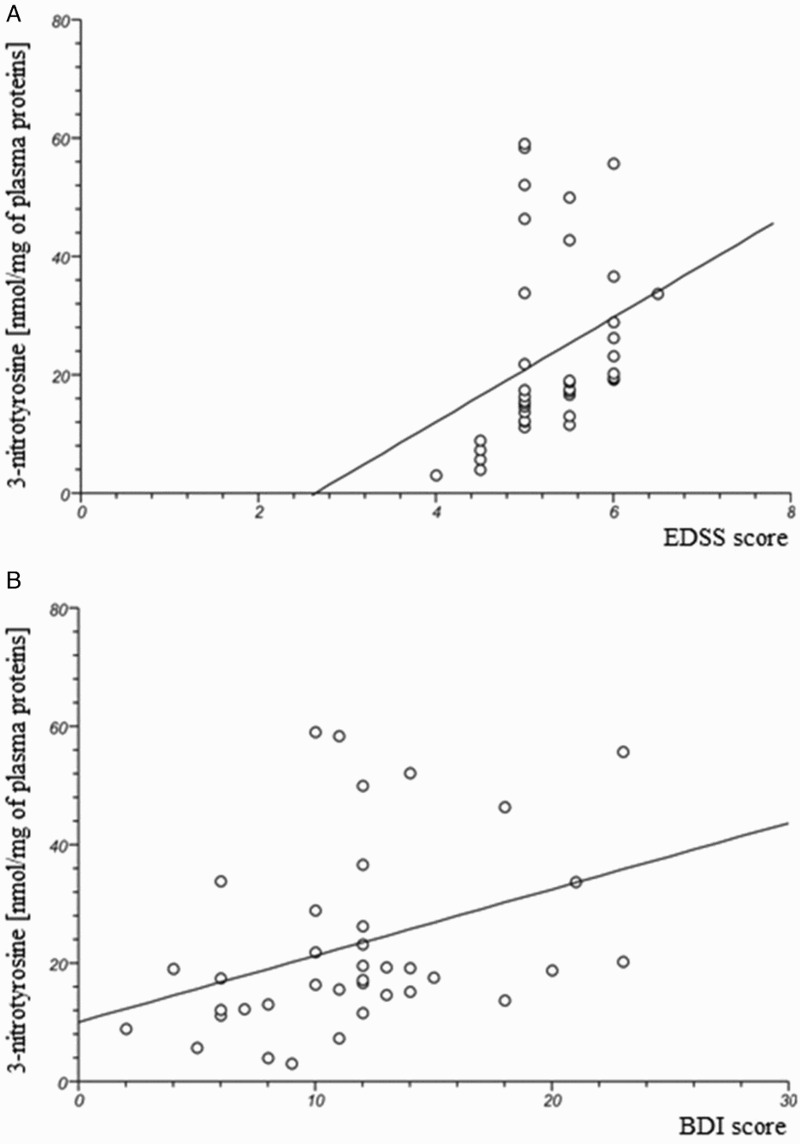
The positive correlation between 3-nitrotyrosine level in plasma obtained from SP MS patients and clinical parameters: EDSS (A) and BDI (B) scores.
Figure 4.
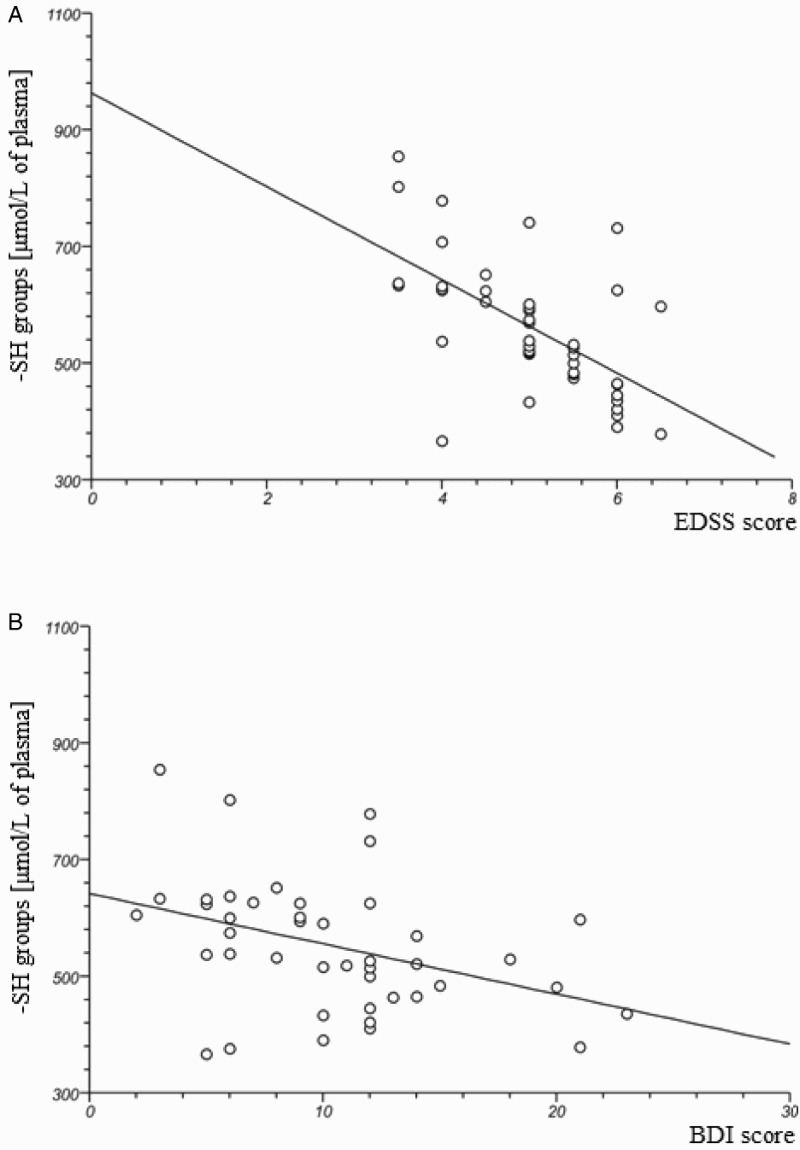
The negative correlation between-SH group level in plasma obtained from SP MS patients and clinical parameters: EDSS (A) and BDI (B) scores.
5. Discussion
Oxidative stress is part of both the inflammatory and neurodegenerative pathomechanisms of MS. Recently, the opinion has arisen that MS, as a complex disease, can be divided into inflammatory RR and degenerative SP phases, because in the majority of cases, oxidative stress dominates in this stage of MS [42]. The evidence for oxidative/nitrative stress in RR MS indicates both lipid peroxidation [3,26,43] and protein peroxide formation (carbonylation) [5,6,25], as well as nitrotyrosine formation (as a marker of peroxynitrite activity) [7]. This suggests the importance of the role of oxidative stress in the pathogenesis of MS. The tendency for increased presence of oxidative markers is mainly observed in cortical tissues and CSF [9,21,22], as the chemical composition of human CSF is considered to reflect brain metabolism [8,44]. However, recent reports indicate that plasma can be as a good source of oxidative stress markers as CSF. The increased level of ROS could be the result of considerably lower plasma concentrations of several key antioxidants and the reduced activity of antioxidant enzymes [16]. Lukáč et al. [45] confirmed the decreased level of total antioxidant status in the plasma of RR MS patients. To present the overall level of antioxidant capacity in SP MS patients, we evaluated total antioxidant status and showed it to be significantly reduced in the plasma of SP MS patients, in comparison to a healthy control group [46]. The decreased activity of low molecular weight antioxidants suggests their high consumption by free radicals. This could be related to their increased pro-oxidative status, due to persistent micro-inflammatory processes in the CNS, which are intensified by free radicals.
The results presented in here are a continuation of our previous research in this field of study – the involvement of oxidative stress in MS [47].
Lukáč et al. [45] demonstrated elevated concentrations in protein markers of oxidative/nitrative damages in the plasma of RR MS patients. The authors showed significantly increased concentrations of carbonyl groups and 3-nitrotyrosine in comparison to a healthy control group. However, Fiorini et al. [48] proved that oxidative damage mainly increases in the relapse phase of MS. They found higher total protein carbonyl levels in serum during the relapse phase of MS patients, in comparison to remission and healthy groups.
In the present study, we showed that protein oxidation can be crucial in the progression of SP MS. Mechanisms exist by which oxidative/nitrative products can accelerate progression in MS. The oxidative/nitrative products can inhibit several of the enzymes involved in respiration, thereby disturbing mitochondrial function and reducing ATP content, as demonstrated by neurons exposed to NO [17,49]. These products disrupt the transport of ATP along the axon and consequently contribute to neurodegeneration [4,16]. Furthermore, the oxidative/nitrative products in white matter plaques cause mitochondrial gene deletions, and the transport of defective mitochondria, in a retrograde manner, into the cell body of cortical neurons. With electron leakage from defective mitochondria, this process can further amplify oxidative injury [19]. It is believed that oxidation leads to the production of epitopes, which may provoke autoimmune responses [28]. Moreover, ROS/RNS are responsible for oxidation of fibrinogen – an abundant plasma coagulation factor – which favours enhanced risk of thrombotic diseases that reduce physical and mental performance in patients with MS, such as brain stroke and cardiac ischemia [50–54].
In our studies, oxidative damage to plasma proteins in SP MS patients has been expressed as an increased level of carbonyl groups (Figure 1(A)). Our studies are consistent with reports indicating an elevated level of carbonyl proteins in demyelinating diseases [15]. Oxidative stress accompanies pathological changes in some other neurodegenerative diseases, and is considered as a major upstream factor in the pathogenesis of these diseases. Several studies have demonstrated that in Alzheimer’s patients the level of protein carbonyl group and TBARS concentration in serum or plasma, are significantly higher than in healthy control groups [55–57]. The elevated level of carbonyl groups is also higher in plasma obtained from patients with Parkinson’s disease [58]. These findings show that the oxidative/nitrative biomarkers might be of particular utility of neurodegenerative process. However, for the first time, we demonstrated the statistically significant positive correlations between the intensity of the protein carbonylation process and the parameters of the EDSS score (Figure 2(A)) and BDI score (Figure 2(B)). This clearly indicates that the scale of the oxidative damage to proteins is referenced in the physical and mental functioning of the patients in SP MS. The positive correlation between carbonyl group concentration and disease severity as assessed by the EDSS score confirms that this parameter can be used as a useful qualitative and quantitative plasma biomarker of the progress of neurodegenerative complications. Depression is associated with increased disability and decreased cognitive impairment, and leads to delays in the rehabilitation process due to the deterioration of motivation and low mood. As demonstrated here, a significant positive correlation between the level of protein carbonylation and BDI scores indicates that carbonyl groups can serve as a significant prognostic parameter.
What is more, our findings show that the level of thiol groups in the plasma of SP MS patients was lower (approximately 30%) than in healthy controls (Figure 1(C)). These results are consistent with earlier studies that demonstrated the decreased level of thiol groups in plasma and CSF obtained from RR MS patients. Moreover, we noted a negative correlation between the thiol group level and EDSS and BDI scores (Figure 4(A,B)). We proved that the clinical parameters in MS of the level of disability and depression syndrome are associated with the extent of the oxidative damage of plasma proteins in vivo.
It is well known that peroxynitrite – a strong oxidant – is responsible for 3-NT formation in vivo [19,59] and that as a compound it is highly damaging to neurons in MS [15]. It is produced extremely quickly in the CNS, mainly by activated macrophages and microglia, which are responsible for axon disruption and the demyelination process [3]. Peroxynitrite plays a crucial role in the interruption of the BBB and promotion of the infiltration of inflammatory cells into the CNS [60]. So, peroxynitrite is an important pathogenetic factor in MS, which causes increased activity of the inflammatory processes [61]. High levels of reactive oxidative/nitrative species, such as nitric oxide, superoxide ions and peroxynitrite, have all been presented in CSF drawn from MS patients. Nitric oxide is generated in the CNS in response to the induction of inflammatory nitric oxide synthase [4]. Yuceyar et al. [62] showed increased serum and CSF levels of the stable end-products of nitric oxide (nitrite (NO2−) and nitrate (NO3−)) in MS patients, compared with control subjects. They observed that the levels of NO2− and NO3− in CSF and serum were significantly higher in both RR and SP subtypes of MS, in comparison to a healthy control with non-inflammatory neurological disease. However, the difference between concentrations of NO metabolites in RR and SP MS patients was not significant.
In our studies, we have shown the elevated level of 3-NT in the proteins of plasma derived from SP MS patients, in comparison to a healthy control group (Figure 1(B)). Moreover, we confirmed that an increased level of 3-NT correlates with the EDSS score, as well as with the BDI score, in SP MS patients. The significant positive correlations are indicated in Figure 3(A,B) and Table 2. For the first time then, our study revealed the possible association between not only oxidative but also nitrative damage of plasma proteins, and their participation in the development of depression and physical disability in SP MS patients.
Lipid peroxidation has been implicated in the pathogenesis of MS, and this free radical process is well known for its involvement in the breakdown of the myelin sheath [63,64]. The most widely used marker of lipid peroxidation is TBARS [36]. The studies described previously in this paper noted a significant increase (more than 80%) in the serum level of TBARS in RR MS patients, in comparison to the control groups [13,65]. Our results are in line with the studies by Karlík et al. [13], which proved the increased concentration of TBARS in plasma samples obtained from MS patients. They also demonstrated the significantly higher salivary level of TBARS in MS patients (about 50%), in comparison to healthy subjects [13]. Our findings demonstrate that the TBARS generation in plasma from SP MS patients is significantly higher (by 60%), than in healthy controls (Figure 1(D)).
Our data on SP MS patients are characterized by the irreversible progression of MS, and are surprisingly consistent with earlier research carried out on RR MS patients who had been in a period of recovery in at least the last three months prior to enrolment in the study [35]. The authors of that research demonstrated increased levels of oxidative stress biomarkers – lipid peroxidation products, carbonylated proteins – as well as a decreased level of sulfhydryl groups, in the plasma of patients with RR MS. They also presented the correlations between oxidative stress markers and disability, assessed by the EDSS score in RR MS patients [35]. In turn, our paper is the first report revealing the association between the level of oxidative/nitrative stress parameters and EDSS and BDI scores in insufficiently investigated types of SP MS. Although both our research and Oliveira et al.’s [66] are focused on two different subtypes of MS, a significant consistency in the results can be observed. However, it should be emphasized that we have not observed the relationship between plasma lipid peroxidation and the clinical parameters describing the progression of the disease. This might strongly suggest that it is primarily the level of protein oxidative/nitrative damages, not lipid peroxidation, that should be considered suitable plasma biomarkers. This could be due to a significantly higher protein concentration in the plasma, versus a large quantitative lipid predominance in nervous tissue. In our study, the concentrations of different investigated biochemical parameters (3-NT, carbonyl groups, thiol groups) correlate with the revised degrees of physical and mental disability determined on the basis of the EDSS and BDI scores. Therefore, the number of oxidative/nitrative protein damage markers could be used as a rating for non-invasive evaluation of oxidative stress in vivo, as well as of the progression of SP MS.
These findings make oxidative/nitrative biomarkers a specific marker of neurodegenerative process. Moreover, in the future these biological markers could determine the use of specific antioxidative/anti-nitrative therapies in the course of neurodegenerative diseases.
Notes on contributors
Agnieszka Morel is a PhD student in the Department of General Biochemistry of the University of Lodz. Her main research interests include the biological activity of blood platelets and platelet reactivity in multiple sclerosis.
Michał Bijak has obtained his PhD in biological sciences with biochemistry as a specialty. Since 2014, he has worked as an assistant professor at the Department of General Biochemistry of the University of Lodz. His main research interests include problems connected with thrombotic disorders.
Marta Niwald is a rehabilitation specialist in training from Poland (4/6 year, residency system) and also an assistant in Physical Medicine Department at Medical University in Lodz. Marta Niwald’s main interests are neurological rehabilitation and multiple Elżbieta Miller since 2012 has the degree of habilitated doctor in medical sciences, the discipline of medicine, as specialty medical rehabilitation. She is employed as an associate professor and the head of the Department of Physical Medicine, Medical University of Lodz. She is also the head of Neurorehabilitation Ward III General Hospital of Lodz, Poland. Her main fields of research include the markers of multiple sclerosis and the effect of additional therapies on biochemical parameters in neurodegeneration.
Joanna Saluk since 2012 has the degree of habilitated doctor in biological sciences, the discipline of biochemistry, as specialty biochemistry of hemostasis. She is employed as an associate professor in the Department of General Biochemistry, University of Lodz, Faculty of Biology and Environmental Protection. Her main research interests include the biological activity of blood platelets; functioning of homeostasis and platelet reactivity in autoimmune and neurodegenerative diseases.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Fitzner D, Simons M.. Chronic progressive multiple sclerosis – pathogenesis of neurodegeneration and therapeutic strategies. Curr Neuropharmacol. 2010;8(3):305–315. doi: 10.2174/157015910792246218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lassmann H, van Horssen J.. The molecular basis of neurodegeneration in multiple sclerosis. FEBS Lett. 2011;585:3715–3723. doi: 10.1016/j.febslet.2011.08.004 [DOI] [PubMed] [Google Scholar]
- 3.Miljković D, Spasojević I.. Multiple sclerosis: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2013;19(18):2286–2334. doi: 10.1089/ars.2012.5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller E. Multiple sclerosis. Adv Exp Med Biol. 2012;724:222–238. doi: 10.1007/978-1-4614-0653-2_17 [DOI] [PubMed] [Google Scholar]
- 5.Lublin FD. New multiple sclerosis phenotypic classification. Eur Neurol. 2014;72:1–5. doi: 10.1159/000367614 [DOI] [PubMed] [Google Scholar]
- 6.Miller E. Multiple sclerosis. In: Ahmad SI, editor. Neurodegenerative diseases. Springer Lands Bioscience; 2011. [Google Scholar]
- 7.Jongen PJ, Heerings M, Lemmens WA, et al. A prospective web-based patient-centred interactive study of long-term disabilities, disabilities perception and health-related quality of life in patients with multiple sclerosis in The Netherlands: the Dutch Multiple Sclerosis Study protocol. BMC Neurol. 2015;15:1. doi: 10.1186/s12883-015-0379-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi R, Laroni A, Weiner HL.. Role of the innate immune system in the pathogenesis of multiple sclerosis. J Neuroimmunol. 2010;221(1–2):7–14. doi: 10.1016/j.jneuroim.2009.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu GF, Alvarez E.. The immuno-pathophysiology of multiple sclerosis. Neurol Clin. 2011;2:257–278. doi: 10.1016/j.ncl.2010.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai RA, Davies AL, Tachrount M, et al. Cause and prevention of demyelination in a model multiple sclerosis lesion. Ann Neurol. 2016;79(4):591–604. doi: 10.1002/ana.24607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Najafi S, Mirshafiey A.. The effect of activated microglia in progression of multiple sclerosis. Int Trends Immun. 2015;4:96–104. [Google Scholar]
- 12.Gironi M, Borgiani B, Mariani E, et al. Oxidative stress is differentially present in multiple sclerosis courses, early evident, and unrelated to treatment. J Immunol Res. 2014;2014:1–9. doi: 10.1155/2014/961863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlík M, Valkovič P, Hančinová V, et al. Markers of oxidative stress in plasma and saliva in patients with multiple sclerosis. Clin Biochem. 2015;48(1–2):24–28. doi: 10.1016/j.clinbiochem.2014.09.023 [DOI] [PubMed] [Google Scholar]
- 14.Haider L, Fischer MT, Frischer JM, et al. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134(7):1914–1924. doi: 10.1093/brain/awr128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller E, Walczak A, Saluk J, et al. Oxidative modification of patient's plasma proteins and its role in pathogenesis of multiple sclerosis. Clin Biochem. 2012;45(1–2):26–30. doi: 10.1016/j.clinbiochem.2011.09.021 [DOI] [PubMed] [Google Scholar]
- 16.Miller E, Wachowicz B, Majsterek I.. Advances in antioxidative therapy of multiple sclerosis. Curr Med Chem. 2013;20(37):4720–4730. doi: 10.2174/09298673113209990156 [DOI] [PubMed] [Google Scholar]
- 17.Gilgun-Sherki Y, Melamed E, Offen D.. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol. 2004;3:261–268. [DOI] [PubMed] [Google Scholar]
- 18.Popa-Wagner A, Mitran S, Sivanesan S, et al. ROS and brain diseases: the good, the bad, and the ugly. Oxid Med Cell Longev. 2013;2013:1–14. doi: 10.1155/2013/963520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutowicz M. The influence of reactive oxygen species on the central nervous system. Postep Hig Med Dos. 2011;65:104–113. doi: 10.5604/17322693.933486 [DOI] [PubMed] [Google Scholar]
- 20.Singh N, Haldar S, Tripathi AK, et al. Brain iron homeostasis: from molecular mechanisms to clinical significance and therapeutic opportunities. Antioxid Redox Signal. 2014;20(8):1324–1363. doi: 10.1089/ars.2012.4931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang S, Wu J, Yang Y, et al. Proteomic analysis of the cerebrospinal fluid in multiple sclerosis and neuromyelitis optica patients. Mol Med Rep. 2012;5:1081–1086. [DOI] [PubMed] [Google Scholar]
- 22.Pavelek B, Vyšata O, Tambor V, et al. Proteomic analysis of cerebrospinal fluid for relapsing-remitting multiple sclerosis and clinically isolated syndrome. Biomed Rep. 2016;1:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teunissen CE, Koel-Simmelink MJ, Pham TV, et al. Identification of biomarkers for diagnosis and progression of MS by MALDI-TOF mass spectrometry. Mult Scler J. 2011;17(7):838–850. doi: 10.1177/1352458511399614 [DOI] [PubMed] [Google Scholar]
- 24.Podbielska M, Levery SB, Hogan EL.. The structural and functional role of myelin fast-migrating cerebrosides: pathological importance in multiple sclerosis. Clin Lipidol. 2011;6(2):159–179. doi: 10.2217/clp.11.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rommer PS, Greilberger J, Salhofer-Polanyi S, et al. Elevated levels of carbonyl proteins in cerebrospinal fluid of patients with neurodegenerative diseases. Tohoku J Exp Med. 2014;234(4):313–317. doi: 10.1620/tjem.234.313 [DOI] [PubMed] [Google Scholar]
- 26.Adamczyk B, Adamczyk-Sowa M.. New iinsights into the role of oxidative stress mechanisms in the pathophysiology and treatment of multiple sclerosis. Oxid Med Cell Longev. 2016;2016:1–18. doi: 10.1155/2016/1973834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer AM. Multiple sclerosis and the blood-central nervous system barrier. Cardiovasc Psychiatry Neurol. 2013;2013:1–10. doi: 10.1155/2013/530356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernández-Pedro NY, Espinosa-Ramirez G, de la Cruz VP, et al. Initial immunopathogenesis of multiple sclerosis: innate immune response. Clin Dev Immunol. 2013;2013:1–15. doi: 10.1155/2013/413465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frijhoff J, Winyard PG, Zarkovic N, et al. Clinical relevance of biomarkers of oxidative stress. Antioxid Redox Signal. 2015;23(14):1144–1170. doi: 10.1089/ars.2015.6317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lubin FD, Reingold SC.. Defining the clinical course of multiple sclerosis results of an international survey. National Multiple Sclerosis Society USA Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46(4):907–911. doi: 10.1212/WNL.46.4.907 [DOI] [PubMed] [Google Scholar]
- 32.Beck AT, Epstein N, Brown G, et al. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037/0022-006X.56.6.893 [DOI] [PubMed] [Google Scholar]
- 33.Buss H, Chan TP, Sluis KB, et al. Protein carbonyl measurement by a sensitive ELISA method. Free Rad Biol Med. 1997;23(3):361–366. doi: 10.1016/S0891-5849(97)00104-4 [DOI] [PubMed] [Google Scholar]
- 34.Alamdari DH, Kostidou E, Paletas K, et al. High sensitivity enzyme-linked immunosorbent assay (ELISA) method for measuring protein carbonyl in samples with low amounts of protein. Free Radic Biol Med. 2005;39(10):1362–1367. doi: 10.1016/j.freeradbiomed.2005.06.023 [DOI] [PubMed] [Google Scholar]
- 35.Levine RL, Garland D, Oliver CN, et al. Determination of carbonyl content in oxidatively modified proteins. Method Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-H [DOI] [PubMed] [Google Scholar]
- 36.Placer ZA, Cushman LL, Johnson BC.. Estimation of product of lipid peroxidation (malonyldialdehyde) in biochemical systems. Anal Biochem. 1966;16(2):359–364. doi: 10.1016/0003-2697(66)90167-9 [DOI] [PubMed] [Google Scholar]
- 37.Khan J, Brennan DM, Bradley N, et al. 3-nitrotyrosine in the proteins of human plasma determined by an Elisa method. Biochem J. 1998;330(2):795–801. doi: 10.1042/bj3300795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6 [DOI] [PubMed] [Google Scholar]
- 39.Hu ML. Measurement of protein thiol groups and glutathione in plasma. Method Enzymol. 1994;233:380–385. doi: 10.1016/S0076-6879(94)33044-1 [DOI] [PubMed] [Google Scholar]
- 40.Koster JF, Biemond P, Swaak AJ.. Intracellular and extracellular sulphydryl levels in rheumatoid arthritis. Ann Rheum Dis. 1986;45(1):44–46. doi: 10.1136/ard.45.1.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hauke J, Kossowski T.. Comparison of values of Pearson’s and Spearman’s correlation coefficients on the same sets of data. Quaest Geogr. 2011;2:87–93. [Google Scholar]
- 42.Trapp BD, Nave KA.. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313 [DOI] [PubMed] [Google Scholar]
- 43.Koch M, Ramsaransing GS, Arutjunyan AV, et al. Oxidative stress in serum and peripheral blood leukocytes in patients with different disease courses of multiple sclerosis. J Neurol. 2006;253(4):483–487. doi: 10.1007/s00415-005-0037-3 [DOI] [PubMed] [Google Scholar]
- 44.Stoop MP, Singh V, Dekker LJ, et al. Proteomics comparison of cerebrospinal fluid of relapsing remitting and primary progressive multiple sclerosis. PLoS One. 2010;5(8):e12442. doi: 10.1371/journal.pone.0012442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lukáč Š, Kalnovičová T, Muchová J.. Evaluation of oxidative and nitrosative stress in relapsing remitting multiple sclerosis. Health. 2013;05(11):1924–1928. doi: 10.4236/health.2013.511260 [DOI] [Google Scholar]
- 46.Miller E, Mrowicka M, Malinowska K, et al. Effects of whole-body cryotherapy on a total antioxidative status and activities of antioxidative enzymes in blood of depressive multiple sclerosis patients. World J Biol Psychiatry. 2011;12(3):223–227. doi: 10.3109/15622975.2010.518626 [DOI] [PubMed] [Google Scholar]
- 47.Miller E, Mrowicka M, Malinowska K, et al. The effects of whole-body cryotherapy and melatonin supplementation on total antioxidative status and some antioxidative enzymes in multiple sclerosis patients. Pol Merkur Lekarski. 2011;31(183):150–153. [PubMed] [Google Scholar]
- 48.Fiorini A, Koudriavtseva T, Bucaj E, et al. Involvement of oxidative stress in occurrence of relapses in multiple sclerosis: the spectrum of oxidatively modified serum proteins detected by proteomics and redox proteomics analysis. PLoS One. 2013;8(6):e65184. doi: 10.1371/journal.pone.0065184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mao P, Reddy PH.. Is multiple sclerosis a mitochondrial disease? Biochim Biophys Acta. 2010;1802(1):66–79. doi: 10.1016/j.bbadis.2009.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morel A, Bijak M, Miller E, et al. Relationship between the increased haemostatic properties of blood platelets and oxidative stress level in multiple sclerosis patients with the secondary progressive stage. Oxid Med Cell Longev. 2015;2015:1–10. doi: 10.1155/2015/240918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tseng CH, Huang WS, Lin CL, et al. Increased risk of ischaemic stroke among patients with multiple sclerosis. Eur J Neurol. 2015;22:500–506. doi: 10.1111/ene.12598 [DOI] [PubMed] [Google Scholar]
- 52.Liu S, Bai S, Qin Z, et al. Quantitative proteomic analysis of the cerebrospinal fluid of patients with multiple sclerosis. J Cell Mol Med. 2009;13:1586–1603. doi: 10.1111/j.1582-4934.2009.00850.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ottervald J, Franzén B, Nilsson K, et al. Multiple sclerosis: identification and clinical evaluation of novel CSF biomarkers. J Proteomics. 2010;73(6):1117–1132. doi: 10.1016/j.jprot.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 54.Mancall EL, Brock DG.. Clinical nauroanatomy. The anatomic basis for clinical neuroscience. Philadelphia; 2011: p. 1–11. [Google Scholar]
- 55.Cristalli DO, Arnal N, Marra FA, et al. Peripheral markers in neurodegenerative patients and their first-degree relatives. J Neurol Sci. 2012;314(1–2):48–56. doi: 10.1016/j.jns.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 56.Sinem F, Dildar K, Gökhan E, et al. The serum protein and lipid oxidation marker levels in Alzheimer’s disease and effects of cholinesterase inhibitors and antipsychotic drugs therapy. Curr Alzheimer Res. 2010;7(5):463–469. doi: 10.2174/156720510791383822 [DOI] [PubMed] [Google Scholar]
- 57.Torres LL, Quaglio NB, De Souza GT, et al. Peripheral oxidative stress biomarkers in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2011;26(1):59–68. [DOI] [PubMed] [Google Scholar]
- 58.Nikolova GD, Grigorov BG, Zheleva AM, et al. Influence of therapy on some important final products of oxidation of lipids, proteins and nucleic acids in patients with Parkinson’s Diseases. Biol Chem. 2014;4:253–260. [Google Scholar]
- 59.Olas B, Wachowicz B.. Role of reactive nitrogen species in blood platelet functions. Platelets. 2007;18(8):555–565. doi: 10.1080/09537100701504087 [DOI] [PubMed] [Google Scholar]
- 60.Bolton C, Wood EG, Scott GS, et al. A comparative evaluation of the response to peroxynitrite by a brain endothelial cell line and control of the effects by drug targeting. Cell Mol Neurobiol. 2009;29(5):707–717. doi: 10.1007/s10571-009-9391-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wachowicz B. Blood platelets as a peripheral cell in oxidative stress in psychiatric disorders. In: Dietrich-Muszalska A, Chauhan V, Grignon S, editors. Studies on psychiatric disorders. New York (NJ: ): 2015. p. 327–353. [Google Scholar]
- 62.Yuceyar N, Taşkiran D, Sağduyu A.. Serum and cerebrospinal fluid nitrite and nitrate levels in relapsing-remitting and secondary progressive multiple sclerosis patients. Clin Neurol Neurosurg. 2001;103(4):206–211. doi: 10.1016/S0303-8467(01)00144-5 [DOI] [PubMed] [Google Scholar]
- 63.Wang P, Xie K, Wang C, et al. Oxidative stress induced by lipid peroxidation is related with inflammation of demyelination and neurodegeneration in multiple sclerosis. Eur Neurol. 2014;72(3-4):249–254. doi: 10.1159/000363515 [DOI] [PubMed] [Google Scholar]
- 64.Koch M, Mostert J, Arutjunyan AV, et al. Plasma lipid peroxidation and progression of disability in multiple sclerosis. Eur J Neurol. 2007;14(5):529–533. doi: 10.1111/j.1468-1331.2007.01739.x [DOI] [PubMed] [Google Scholar]
- 65.Hadžović-Džuvo A, Lepara O, Valjevac A, et al. Serum total antioxidant capacity in patients with multiple sclerosis. Bosn J Basic Med Sci. 2011;11(1):33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oliveira SR, Kallaur AP, Simão AN, et al. Oxidative stress in multiple sclerosis patients in clinical remission: association with the expanded disability status scale. J Neurol Sci. 2012;321(1–2):49–53. doi: 10.1016/j.jns.2012.07.045 [DOI] [PubMed] [Google Scholar]


