Abstract
Introduction
Due to the importance of lung cancer early treatment because of its severity and extent worldwide a systematic literature review was conducted about the impact of delays in waiting times on the disease prognosis.
Materials and Methods
We conducted a systematic search of observational studies (2010-2020) including adult patients diagnosed with lung cancer and reporting healthcare timelines and their clinical consequences.
Results
We included 38 articles containing data on waiting times and prognosis; only 31 articles linked this forecast to a specific waiting time. We identified 41 healthcare time intervals and found medians of 6-121 days from diagnosis to treatment and 4-19.5 days from primary care to specialist visit: 37.5% of the intervals indicated better prognosis with longer waiting times.
Conclusions
All articles emphasized that waiting times must be reduced to achieve good management and prognosis of lung cancer. Further prospective studies are needed on the relationship between waiting times and prognosis of lung cancer.
Keywords: Lung cancer, Timeliness of care, Delays, Prognosis, Survival, Systematic literature review
Introduction
Lung cancer, the leading cause of cancer death worldwide, is a health problem of the first order due to the morbidity and mortality caused, and the economic impact it has on health systems [1, 2].
Diagnostic suspicion of early-stage lung cancer may be difficult because the clinical presentation is silent in the early stages and the differential diagnosis may be confusing in advanced stages. Progressive improvements in local and remote diagnostic techniques (EBUS and PET-TAC) and therapeutic advances (targeted therapies, immunotherapies, etc.) in the last decade have improved the prognosis in patients with lung cancer in advanced countries, including Spain. Five-year survival is between 12 and 18% [3] and is directly related to the stage at presentation and the histology: the 5-year survival of patients with localized stages of the disease ranges between 27% and 63%, in regional stages between 16 and 35% and in disseminated stages between 3 and 7%, with the lowest survival rate corresponding to small cell lung cancer (SCLC) [4]. In 2020, 1,796,144 deaths worldwide and 22,930 deaths in Spain were due to lung cancer [3, 5].
The clinical management of lung cancer patients requires complex coordination by specialized medical and surgical services, health service administrators, care managers and social service providers. The traditional approach of referring patients to different specialist consultations sequentially often results in care that is perceived as slow, fragmented, and poorly coordinated. To reduce these delays, agreed standards have been established for maximum acceptable waiting times for lung cancer-specific referral, diagnosis, and treatment times based on expert clinical opinion [1, 6, 7].
In the United Kingdom, the National Optimal Lung Cancer Pathway guidelines propose care algorithms to be used in conjunction with the British Thoracic Society (BTS) and the National Institute for Health and Care Excellence (NICE) guidelines, with the aim of achieving maximum times of 14 days for diagnosis and 28 days for treatment. However, these standards are not always met and delays in lung cancer care persist [1].
It is essential to obtain optimal clinical results in patients with suspected lung cancer to speed up the diagnostic process and early treatment as much as possible. Delays in any part of the process, from the initial evaluation and referral to the definitive diagnosis, treatment and follow-up may have negative consequences [1, 6, 8, 9].
Considering the importance of an early approach in the diagnosis and treatment of lung cancer, we carried out a systematic literature review (SLR) to determine the evidence of the impact of delays in the times of diagnosis and initial treatment on the disease prognosis.
Materials and methods
The SLR was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses Statement (PRISMA).
We selected observational studies of patients aged ≥ 18 years diagnosed with or with a clinical suspicion of small cell or non-small cell lung cancer conducted in Europe, the United States, Canada, Japan, Australia, New Zealand, and China. The study had to evaluate ≥ 1 variable related to healthcare deadlines and their effect on clinical outcomes. Randomized clinical trials were not included.
Two search strategies were designed, one for MEDLINE (through PubMed) and one for EMBASE, in which terms related to lung cancer, healthcare deadlines (waiting times, delays, early diagnosis, etc.) and clinical outcomes (prognosis, survival, mortality, etc.) were used. The time horizon of the search was January 1, 2010-November 24, 2020, to include advances in the last decade in the diagnosis and treatment of lung cancer. The language of the publications was limited to English and Spanish.
The titles and abstracts resulting from the search, after duplicate articles were removed, were evaluated by three reviewers (AGC, IAF, and MCA), and those that did not meet the inclusion and exclusion criteria were ruled out, noting the specific reasons. If there was disagreement between reviewers regarding the inclusion of an article, the criterion of a fourth reviewer (FIO) was used. A complete reading of the articles was made by three reviewers (AGC, IAF, and MCA) independently, and the reasons for non-selection were recorded.
Data from the selected articles were tabulated by three reviewers (AGC, IAF, and MCA) on a form developed specifically for extraction and validated by a fourth reviewer. From each article selected, we extracted the study characteristics (type of study, design, country of study, sample size, study duration, follow-up time), patient characteristics (mean age, sex ratio, disease stage), healthcare deadlines (time intervals evaluated between symptoms, diagnosis, and treatment [including mean, standard deviation, median or interquartile range]) and clinical outcomes (survival, mortality). All waiting time intervals were analyzed in calendar days (if an article reported delays in weeks, these values were multiplied by 7; if it reported delays in months, they were multiplied by 30.41).
All results focused on the healthcare timelines of lung cancer patients and their clinical consequences were evaluated.
The times evaluated were expressed as: (a) time from the appearance of symptoms or clinical or radiological suspicion (first abnormal imaging test) to the therapeutic intervention, (b) partial times, considering: (b1) time from the appearance of symptoms, clinical or radiological suspicion to diagnosis (lung cancer study, staging), (b2) time from diagnosis to therapeutic decision, (b3) time from therapeutic decision to treatment initiation. Clinical outcomes related to the prognosis were progression-free survival, overall survival, time to relapse, and mortality.
Initially, given the variations in the times considered and to interpret the information in the most aggregated and homogeneous way possible, the time intervals were grouped sequentially following the timeline that goes from diagnosis to treatment. The groups of time intervals evaluated were described using absolute frequencies (n) and percentages with respect to the total number of articles selected.
To evaluate the relationship between the time intervals stated and their association with the prognosis, we made a qualitative analysis that categorized the possible relationship between the time intervals and the specific prognosis in relation to survival and mortality.
Results
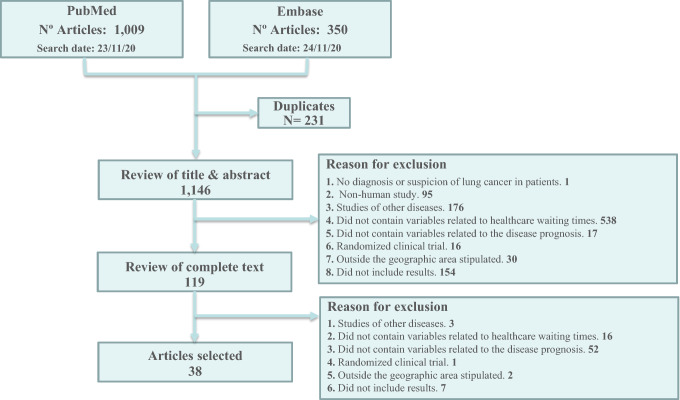
The search strategy and the decisions made during the selection of the articles included in the SLR are shown in the PRISMA flowchart (Fig. 1). The search identified 1359 articles for review, of which, after eliminating duplicate articles, 1146 were assessed for eligibility based on the title and abstract; of these, 1027 were excluded, mostly because they did not provide variables related to waiting times. The full text of the remaining 119 articles was evaluated, and 81 were excluded, mainly because they did not include variables related to the disease prognosis (n = 52) or waiting times (n = 16). Finally, 38 met the inclusion criteria.
Fig. 1.
PRISMA flowchart
Description of the studies
Thirty-four studies were retrospective observational studies, three were prospective observational studies, and one was a systematic literature review.
The studies included 1,225,328 patients, with a sample size ranging from 128 to 691,464. Twenty-one studies were conducted specifically in patients with non-small cell lung cancer (NSCLC), 13 in patients with any type of lung cancer, 3 in patients with SCLC, and one in patients with epidermoid NSCLC. Twenty-one studies investigated all disease stages, four included only patients with stage I–III, three included stage III and IV patients, three studies only included stage I patients, two studies included stage I and II patients, one study only included stage III patients, and another included only stage II and III, three studies did not specify this information.
There were wide variations and heterogeneity among the studies included. The quality was evaluated using the National Heart, Lung and Blood Institute tool. One of the most common shortcomings was the lack of justification of the number of patients needed to detect a relationship between the waiting time and the prognosis, and the statistical adjustment of the variables influencing the prognosis.
The characteristics of the studies selected are shown in Table 2.
Table 2.
Characteristics of the studies included
| Abbreviated reference and country | Study design, patients included | Study duration (follow-up time) | Type of cancer (stage) | Inclusion criteria | Interesting results |
|---|---|---|---|---|---|
|
Sheinson 2020 USA |
Retrospective, 442 patients | 7 years (NA) | SCLC (IIIB–IV) | ALK + SCLC, ALK + determination < 90 days and ALK inhibitor initiation < 90-days | HR, diagnosis to treatment time |
|
Bhandari 2020 USA |
Prospective, 64,491 patients | 4 years (death/last follow-up date) | SCLC (I–IV) | ≥ 18 years SCLC. Excluded if not received chemotherapy, initiation of chemotherapy > 180 days or if information was missing | Survival, diagnosis to initiation of chemotherapy time |
|
Rice 2020 USA |
Retrospective, 355 patients | 13 years (5 years) | NSCLC (III) | NSCLC, with curative intent with neoadjuvant chemo-radiotherapy ± surgery | OS and recurrence-free ratio at 5 years, diagnosis to treatment time |
|
Odell 2019 USA |
Retrospective, 141,723 patients | 15 years (2 years) | SCLC (I–IV) | NSCLC, with curative intent registered with the NCDB | HR, chemotherapy to adjuvant surgery time and surgery to adjuvant chemotherapy |
|
Crawford 2020 USA |
Retrospective, 3866 patients | 3 years (death/last follow-up date) | NSCLC (III–IV) | ≥ 18 years NSCLC, with first-line platinum-based chemotherapy | OS and relationship with dose delays and/or dose reduction, time to chemotherapy administration |
|
Bhandari 2019 USA |
Retrospective, 2992 patients | 3 years (7.5 months, 12 and 24 months) | SCLC (I–IV) | ≥ 18 years SCLC | Survival, diagnosis to treatment time |
|
Alanen 2019 Finland |
Retrospective, 221 patients | 1 year (1 year) | Lung cancer (I–IV) | Lung cancer | OS, symptoms to treatment time and setbacks: until medical visit, until diagnosis, until treatment |
|
Cushman 2020 USA |
Retrospective, 140,455 patients | 11 years (NA) | SCLC (I–III) | > 18 years NSCLC with curative intent registered in the NCDB | OS and time of initiation of treatment |
|
Kuroda 2018 Japan |
Retrospective, 293 patients | 5 years (5 years) | SCLC (IA) | NSCLC with surgery at a referral center CT diagnosis to surgery > 6 months | OS and DFS at 5 years after CT detection. Times since CT detection and surgery |
|
Malalasekera 2018 Australia |
Prospective, 128 patients | NA | Lung cancer (I–IV) | Adults NSCLC or SCLC | Diagnosis to treatment times, related survival |
|
Ha 2018 USA |
Retrospective, 177 patients | 7 years (NA) | Lung cancer (I–IIIA) | Lung cancer patients eligible for curative intent therapy | Diagnosis to treatment times, related survival |
|
Anggondowati 2020 USA |
Retrospective, 691,464 patients | 8 years (5 years) | SCLC (I–IV) | ≥ 18 NSCLC who underwent surgery, chemotherapy or radiation | Diagnosis to treatment times; OS and 5-year survival |
|
Hanaoka 2018 Japan |
Retrospective, 283 patients | 14 years (5 and 10 years) | NSCLC (I–IIIB) | NSCLC | diagnosis to treatment time, survival ratios |
|
Labbé 2017 Canada |
Retrospective, 1251 patients | 1.5 years (death/last follow-up date) | Lung cancer (I–IV) | Patients referred to the diagnostic evaluation program of the University Institute of Cardiology and Pulmonology of Quebec September 22, 2013–March 7, 2015 | Waiting times for research and treatment; PFS, RFS after primary surgical resection, and OS |
|
Vinod 2017 Australia |
Retrospective, 1926 patients | 6 years (NA) | SCLC (I–IV) | SCLC in the South Western Sydney Cancer Registry | Diagnosis to treatment time, mortality |
|
Jacobsen 2017 USA |
Systematic review (NA) | 11 years (NA) | All (I–IV) | PubMed studies 2007–2016, reporting waiting times | Waiting times and survival, resource utilization, costs and inequalities |
|
Kasymjanova 2017 Canada |
Retrospective, 721 patients | 5 years (1 year) | Lung cancer (I–IV) | Lung cancer | Multiple timepoints; survival |
|
Yang 2017 USA |
Retrospective, 4984 patients | 5 years (death/last follow-up date) | NSCLC with squamous cell carcinoma (I) | Squamous cell carcinoma in the NCDB treated with lobectomy without chemotherapy or radiation | Diagnosis to treatment time, survival ratios |
|
Salazar 2017 USA |
Retrospective, 31,474 patients | 9 years (5 years) | SCLC (I–III) | NSCLC underwent lobectomy or pneumonectomy treated with multi-agent chemotherapy, and who did not receive adjuvant chemotherapy | Resection to chemotherapy time; OS |
|
Jing 2016 China |
Prospective, 362 patients | 7 years (death/last follow-up date) | SCLC (II–IIIA) | NSMP undergoing lobectomy and adjuvant chemotherapy | OS and DFS, time from surgery to treatment |
|
Yennurajalingam 2017 USA |
Retrospective, 410 patients | 1 year (1 year) | SCLC (IIIB–IV) | NSDC in palliative care | OS, diagnosis to treatment time |
|
Coughlin 2015 Canada |
Retrospective, 222 patients | 1 year (1 year) | NSCLC (I–II) | NSCLC stages I-II, operated between 2010–2011 | OS, decision on surgery to surgery time |
|
Van 2015 Australia |
Retrospective, 713 patients | 2 years (death/last follow-up date) | Lung cancer (I–IV) | Lung cancer | Times between first abnormal diagnostic image, specialist consultation, biopsy, remission, oncology consultation and initiation of treatment, OS |
|
Gómez 2015 USA |
Retrospective, 28,732 patients | 3 years (1 year) | SCLC (I–IV) | ≥ 66 years NSCLC | Prevalence of delay in treatment, patient components, disease and medical supply that contributed to the delay in treatment, and effect of delay on survival, benchmarks on the timeliness of staging studies which could significantly reduce delays |
|
Redaniel 2015 UK |
Retrospective, 5737 patients | 11 years (5 years) | Lung cancer (NA) | ≥ 15 years breast, colorectal, lung, and prostate cancer | Time to diagnosis; related survival |
|
Samson 2015 USA |
Retrospective, 55,653 patients | 12 years (NA) | SCLC (I) | NSCLC undergoing pulmonary resection | Delays in time to surgery; survival |
|
Zicovic 2014 Montenegro |
Retrospective, 206 patients | 2 years (1 year) | Lung cancer (NA) | Lung carcinoma | Symptoms to primary care visit, primary care visit to diagnosis, visit to specialist to diagnosis; related survival |
|
Forrest 2015 UK |
Retrospective, 23,497 patients | 3 years (2 years) | Lung cancer (I–IV) | Lung cancer | Survival, diagnosis to treatment times |
|
Kanarek 2014 USA |
Retrospective, 174 patients | 6 years (1 year) | NSCLC (I–II) | Patients undergoing surgery for early-stage NSCLC at SKCCC | Diagnosis to first surgery time; related survival |
|
González-Barcala 2013 Spain |
Retrospective, 307 patients | 3 years (death) | Lung cancer (I–IV) | Lung cancer | Delays in diagnosis and treatment; related forecast |
|
Radzikowska 2013 Poland |
Retrospective, 3479 patients | 3 years (5 years) | SCLC (I–IV) | SCLC | Survival, delays caused to patients and inactivity of doctors, dates of first contact with a specialist doctor and first bronchoscopy |
|
Concannon 2020 USA |
Retrospective, 133 patients | 6 years (NA) | SCLC (I–IV) | NSCLC | OS, finding of suspected nodule by image until confirmation diagnosis (biopsy) and between diagnosis and first treatment times |
|
Booth 2013 Canada |
Substudy retrospective, 3354 patients | 2 years (4 years) | SCLC (I–IV) | NSCLC time to surgical resection ≤ 24 weeks of diagnosis | Surgery to treatment and diagnosis to treatment times, related survival |
|
Radzikowska 2012 Poland |
Retrospective, 10,386 patients | 3 years (death/last follow-up date) | SCLC (I–IV) | NSCLC | Dates of the first specialist visit and first bronchoscopy and associated survival |
|
Diaconescu 2011 Canada |
Retrospective, 665 patients | 2 years (3 years) | SCLC (NA) | NSCLC | Delay in treatment, associated survival |
|
Skaug 2011 Norway |
Retrospective, 271 patients | 6 years (1, 2, 3, 4, 5, 10 years) | Lung cancer (I–IV) | Lung cancer residents of the central area | OS, first symptom to diagnosis time |
|
González-Barcala 2010 Spain |
Retrospective, 481 patients | 3 years (NA) | Lung cancer (I–IV) | Lung cancer | Delay in the patient's consultation and the diagnostic and therapeutic process; associated survival |
|
Wah 2020 Australia |
Retrospective with spatial modeling, 3300 patients | 5 years (2 years) | SCLC (I–IV) | > 18 years NSCLC in the Victoria Lung Cancer Registry | Risk of any-cause death related to the time from diagnosis to treatment |
CT, computerized axial tomography; DFS, disease-free survival; HR, hazard ratio; NA, not available; NCDB, National Cancer Database; NSCLC, non-small cell lung cancer; OS, overall survival; PFS, progression-free survival; RFS, relapse-free survival; SCLC, small cell lung cancer
Description of times
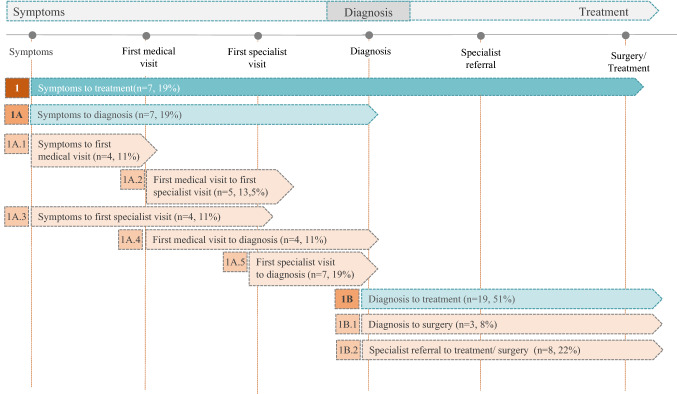
In the 38 selected articles, the results of 41 healthcare time intervals were originally described, which conditioned the type of analysis. The most common waiting times were from symptoms to treatment (7 articles, 19%), symptoms to diagnosis (7 articles, 19%), first specialist visit to diagnosis (7 articles, 19%), specialist referral to surgery or treatment (8 articles, 22%) and from diagnosis to treatment (19 articles, 51%) (Fig. 2). The median of the times studied was two time periods in the same study (IQR 1–4), with a maximum of 10. There were wide variations in how the results of the healthcare deadlines were summarized statistically including means, medians, minimum-maximums, and percentage of patients with delays in the time interval studied.
Fig. 2.
Time intervals according to the specifications of the articles selected
Description of healthcare time intervals
In articles that studied the time from symptoms to treatment in patients with lung cancer stages I–IV, the median (range) waiting time was 87.5 days (44–130.5). In patients with SCLC stages I–IV, one study reported the median waiting time was 78 days. In patients with NSCLC stages I–IV, the mean was 138.5 days and in patients without a definite stage the median was 62 days. Kuroda et al. [10] defined delay as a wait of > 6 months after diagnosis and until surgical treatment and found that, in patients with NSCLC stage IA, the mean waiting time was 411 days in patients treated in < 2 years, compared with 1669.9 days in patients whose waiting time was > 2 years.
Studies that directly assessed the time from symptoms to diagnosis reported mean or median waiting times of > 20 days. In patients with lung cancer stages I–IV, the median (range) waiting time was 33 days (23–66), and in patients with lung cancer where the stage was not specified, the median was 56 days. The median time was 69 days in patients with SCLC stages I–IV, and 75 days in patients with NSCLC. Concannon et al. [11] distinguished between patients with NSCLC stages I–II who were homeless (mean waiting time of 248 days) and those who had a home (mean waiting time of 116 days), and patients with NSCLC stages III–IV who were homeless (mean waiting time of 34.7 days) and those who had a home (mean waiting time of 46 days).
Several studies evaluated the means and medians of specific waiting times for different subintervals within the symptoms to diagnosis time, specifically:
For the symptoms to first specialist visit time, the median (range) waiting time was 33.25 days (8–53) for patients with lung cancer stages I–IV, and the mean of the means was 53.33 days.
For the symptoms to first medical visit time, the mean of the means of waiting time for patients with lung cancer without stage specification was 44.52 days, and for patients with lung cancer stages I–IV, one study reported a median of 58 days. For patients with SCLC stages I–IV, the median reported by one study was 30 days and a mean of 56.7 days in patients with stages I–IV NSCLC was found by another study.
For the first medical visit to first specialist visit, the median of the medians of waiting time for patients with lung cancer stages I–IV was 5 days. One study reported a mean of 14.49 days in lung cancer patients without specifying the stage. For patients with SCLC and stages I–IV NSCLC, the median was 19.5 and 17 days, respectively.
For the first medical visit to diagnosis, the mean waiting time for lung cancer patients in whom the stage was not specified was 29.54 days in the study by Zicovic et al. [12] and a median of 88 days in the study by Redaniel et al. [13]. In patients with SCLC and stages I–IV NSCLC, the median was 34 and 40 days, respectively.
For the first specialist visit to diagnosis, the median of the medians of waiting time in patients with lung cancer stages I–IV was 19.5 days, and the mean was 16.59 days in patients in whom the stage was not specified. In patients with SCLC stages I–IV, the median was 21 days and in patients with NSCLC stages I–IV the mean was 51.3 days.
For the diagnosis to treatment, the median of the medians of waiting time for patients with lung cancer stages I–IV was 31 days, and for patients in stage I–IIIA the mean was 35 days. Forrest et al. [14] indicated that 39.5% of patients had a delay in this time (defining delay as > 31 days from diagnosis to treatment). They also evaluated the time from referral to the specialist until treatment (defining delay as > 14 days) and found that 69.3% of patients had a delay. Samson et al. [15], defined a wait ≥ 8 weeks as a delay of treatment, and studied patients diagnosed with NSCLC stage I, finding that the median time of patients who waited less than 8 weeks from diagnosis to treatment was 29 days, and that of patients who waited 8 weeks or more was 77 days. In patients with NSCLC stage III, Rice et al. [16] distinguished between patients with private insurance, those with basic coverage and those without insurance. The mean waiting times were 25, 48 and 52 days, respectively, and a waiting time > 30 days was considered a delay. In patients with NSCLC stages I–II, the median of the medians of waiting time was 36.55 days. In patients with NSCLC stages I–III, the median wait between diagnosis and treatment was 28 days. In patients with NSCLC stages I–IIIB and IIIB–IV, the median was 121.6 days and 21 days, respectively. In patients with NSCLC stages I–IV, the median of medians was 33.5 days, in agreement with the study by Concannon et al. [11] in patients with NSCLC stages I–II who were homeless, in whom a median waiting time of 20 days and of 50 days in those with homes was reported. In patients with NSCLC stages III–IV without a home the mean was 49.9 days compared with 58.1 days in those with a home. Anggondowati et al. [17] distinguished according to disease progression, reporting median waiting times of 18 days for patients with metastases, 28 days for patients in the early stages and 27 days for patients in locally advanced stages. In patients with stages I–IV SCLC, the median of the medians of diagnosis to treatment was 7.5 days, while Bhandari et al. [18] found a mean of 18 days in these patients.
Three time periods that could not be grouped into any of the previously defined groups were identified: from the decision on surgery to the time of surgery, from diagnosis to contact with the specialist and from surgery to adjuvant treatment. The results were:
For the decision on surgery to surgery, the percentage of patients with stages I to II NSCLC whose waiting time was < 1 month was 24.8%, between 1 and 2 months 44.1%, between 2 and 3 months 19% and between 3 and 4 months 11.7%.
For the diagnosis to contact with the specialist, the median of medians in patients with lung cancer stages I–IV was 9 days, while Kanarek et al. [19] found the mean for NSCLC stages I–II patients was 61.2 days: in this study the surgeon was the specialist physician, after diagnosis by the oncologist.
For the surgery to systemic treatment or vice versa, the median waiting time in patients with NSCLC stages I–III was 48 days, and in patients with stages I–IV 56 days. Odell et al. [20] defined delay as > 120 days from chemotherapy to surgery and 180 days from surgery to chemotherapy: the percentage of patients with NSCLC stages I–IV with a delay, was 4% and 64%, respectively.
Relationship between healthcare waiting times and the prognosis
The 38 articles included reported, in addition to waiting times, the results related to the prognosis and 31 related the prognosis to a specific healthcare time evaluated. In general, there were wide variations in the results observed with respect to the prognosis in relation to the type of lung cancer studied, the stage and the time interval evaluated (Tables 1, 3).
Table 1.
Association between waiting times and survival
| Time intervals | Number of articles | Association | References |
|---|---|---|---|
| Symptoms to treatment | 2 | No association between delay and prognosis | [21, 22] |
| 3 | Longer waiting times improve the results forecast | [10, 23, 24] | |
| Symptoms to first specialist visit | 2 | No association between delay and prognosis | [23, 25] |
| Symptoms at first medical visit | 1 | Shorter waiting times improve the results forecast | [26] |
| 2 | No association between delay and prognosis | [21, 22] | |
| 1 | Longer waiting times improve the results forecast | [12] | |
| Symptoms to diagnosis | 1 | Longer waiting times improve the results forecast | [21] |
| 2 | No association between delay and prognosis | [11, 27] | |
| First medical visit to diagnosis | 1 | Longer waiting times improve the results forecast | [13] |
| 1 | Shorter waiting times improve the results forecast | [12] | |
| First specialist visit to diagnosis | 3 | No association between delay and prognosis | [12, 25, 28] |
| Diagnosis to treatment | 3 | No association between delay and prognosis | [11, 28, 29] |
| 9 | Longer waiting times improve the results forecast | [14, 18, 21–23, 25, 26, 30, 31] | |
| 9 | Shorter waiting times improve the results forecast | [15, 17, 19, 32–37] |
Table 3.
Waiting times and association with survival
| Abbreviated reference (author. year), and n of patients | Type of cancer | Stage | Time interval | Definition of delay according to article | Group | % patients | Mean (days) | SD | Median (days) | IQR | Minimum–maximum | Prognosis | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptoms to treatment |
Alanen 2019 221 patients |
Lung cancer | I–IV | First symptom to treatment | Time greater than the median | – | – | – | – | 130.5 | – | 20–488 | No association. In stage I survival improves if the time interval is shorter |
|
González-Barcala 2010 481 patients |
Lung cancer | I–IV | Symptoms to treatment | – | – | – | 123.6 | 141.6 | 87.5 | 53–139 | – | Improves survival with longer times | |
|
Radzikowska 2013 3479 patients |
SCLC | I–IV | Symptoms to treatment | – | – | – | 113.3 | 118.5 | 78 | 45–135 | – | No association | |
|
Diaconescu 2011 665 patients |
SCLC | Nd | First abnormal image to treatment | – | – | – | – | 62 | 30–108 | – | Improves survival with longer times for advanced disease, in the regional-local group survival is not related to waiting times | ||
|
Kuroda 2018 293 patients |
SCLC | AI | Suspected CP to surgery | > 24 months after CT detection | 882 | – | – | Improves survival with longer times | |||||
| Symptoms to first specialist visit |
González-Barcala 2013 307 patients |
Lung cancer | I–IV | Symptoms to first specialist visit | – | – | – | 53.6 | – | 53 | – | – | No association |
|
González-Barcala 2010 481 patients |
Lung cancer | I–IV | Symptoms to first specialist visit | – | – | – | 82.1 | 133.8 | 48.5 | 22–92.7 | – | No association | |
| Symptoms at first medical visit |
Radzikowska 2012 10,386 patients |
SCLC | I–IV | First symptom to first medical visit | – | – | 56.7 | – | – | – | – | Improves survival with shorter times | |
|
Alanen 2019 221 patients |
Lung cancer | I–IV | First symptom to first medical visit | Time greater than the median | – | – | – | – | 58 | – | 6–428 | No association in general. Survival in stage I improves if time interval is shorter | |
|
Radzikowska 2013 3479 patients |
SCLC | I–IV | Symptoms to first medical visit | – | – | – | 47.4 | 80.3 | 30 | 9–59 | – | No association | |
|
Zicovic 2014 206 patients |
Lung cancer | NA | Symptoms to primary care visit | – | – | – | 43.4 | – | 28 | – | 1.61–231 | Non-significantly survival improves with longer times | |
| S ymptoms to diagnosis |
Alanen 2019 221 patients |
I–IV | First medical visit to diagnosis | Time greater than the median | – | – | – | – | 33 | – | − 8 to 215 | Improves survival with longer times | |
|
Skaug 2011 271 patients |
Lung cancer | I–IV | First symptom to diagnosis | – | – | – | – | – | 66 | 32–113 | – | No association | |
|
Concannon 2020 133 patients |
SCLC | I–IV | Abnormal biopsy/diagnostic imaging | 50 days | Stages I–II homeless | 248 | – | – | – | – | No association | ||
| SCLC | I–IV | Abnormal biopsy/diagnostic imaging | 50 days | Stages I–II not homeless | 116 | – | – | – | – | No association | |||
| SCLC | I–IV | Abnormal biopsy/diagnostic imaging | 50 days | Stages III–IV homeless | 34.7 | – | – | – | – | No association | |||
| SCLC | I–IV | Abnormal biopsy/diagnostic imaging | 50 days | Stages III–IV not homeless | 46 | – | – | – | – | No association | |||
| First medical visit to diagnosis |
Redaniel 2015 5737 patients |
Lung cancer | NA (C34 ICD 10 (Malignant neoplasm of bronchus and lung.)) | Primary care visit to diagnosis | – | – | – | – | – | 88 | 34–210 | – | Improves survival with longer times, |
|
Zicovic 2014 206 patients |
Lung cancer | Nd | Primary care visit to diagnosis | – | – | – | 29.54 | – | – | – | 7–161 | No significantly improved survival with shorter times | |
| First specialist visit to diagnosis | Lung cancer | Nd | Specialist visit to diagnosis | – | – | – | 16.59 | – | – | – | – | No association | |
|
González-Barcala 2013 307 patients |
Lung cancer | I–IV | First specialist visit to diagnosis | – | – | – | 31.5 | – | 18 | – | – | No association | |
|
Ibbé. 2017 1251 patients |
Lung cancer | I–IV | Referral to specialist to diagnosis | – | – | – | – | – | 21 | 13–37 | – | No association | |
| Diagnosis to treatment |
Ha. 2018 177 patients |
Lung cancer | I–IIIA | Diagnosis to treatment | – | – | – | 35 | – | – | – | – | No association |
|
González-Barcala 2013 307 patients |
Lung cancer | I–IV | First specialist visit to treatment | – | – | – | 55.2 | – | 35 | – | – | Improves survival with longer times | |
|
González-Barcala 2010 481 patients |
Lung cancer | I–IV | First specialist visit to treatment | – | – | – | 41.1 | 52.9 | 27 | 17–42 | – | Improves survival with longer times | |
|
Labbé. 2017 1521 patients |
Lung cancer | I–IV | Referral to specialist to first treatment | – | – | – | – | 56 | 34–81 | – | No association | ||
| Lung cancer | I–IV | Referral to specialist to surgery | – | – | – | – | – | 70 | 51–91 | – | No association | ||
|
Forrest 2015 23,497 patients |
Lung cancer | I–IV | Referral to specialist to treatment | > 14 days | ≤ 14 days | 0.307 | – | – | – | – | – | Improves survival with longer times, in stages III and IV | |
| Lung cancer | I–IV | Referral to specialist to treatment | > 14 days | > 14 days | 0.693 | – | – | – | – | – | Improves survival with longer times, in stages III and IV | ||
|
Alanen 2019 221 patients |
Lung cancer | I–IV | Diagnosis to treatment | Time greater than the median | – | – | – | – | 27 | – | − 21 to 148 | Improves survival with longer times | |
|
Kasymjanova 2017 751 patients |
Lung cancer | I–IV | Diagnosis to treatment | – | treatment | – | – | – | 27 | 5–45 | – | Improves survival with shorter times | |
| Lung cancer | I–IV | Diagnosis to treatment | – | chemotherapy | – | – | – | 32 | 20–46 | – | Improves survival with shorter times | ||
| Lung cancer | I–IV | Diagnosis to treatment | – | radiotherapy | – | – | – | 35 | 19–55 | – | Improves survival with shorter times | ||
|
Forrest 2015 23,497 patients |
Lung cancer | I–IV | Diagnosis to treatment | > 31 days | ≤ 31 days | 0.605 | – | – | – | – | – | Improves survival with longer times, in stages III and IV | |
| Lung cancer | I–IV | Diagnosis to treatment | > 31 days | > 31 days | 0.395 | – | – | – | – | – | Improves survival with longer times, in stages III and IV | ||
|
González-Barcala 2013 307 patients |
Lung cancer | I–IV | Diagnosis to treatment | – | – | – | 23.5 | – | 14 | – | – | Improves survival with longer times | |
|
Samson 2015 55,653 patients |
SCLC | I | Diagnosis to surgery | < 8 weeks | – | – | – | – | 29 | 20–41 | – | Improves survival with shorter times | |
| SCLC | I | Diagnosis to surgery | 8 weeks or more | – | – | – | – | 77 | 64–102 | – | Improves survival with shorter times | ||
|
Kanarek 2014 174 patients |
SCLC | I–II | Diagnosis to Surgery | – | – | – | 67.2 | – | – | – | Improves survival with shorter times | ||
|
Sheinson 2020 442 patients |
SCLC | IIIB–IV | Diagnosis to treatment | 21 days | – | – | – | – | 21 | – | – | Shorter time less mortality | |
|
Cushman 2020 14,055 patients |
SCLC | I–III | Diagnosis to treatment | 45 days | – | – | – | – | 28 | – | 1–365 | Improves survival with shorter times | |
|
Concannon 2020 133 patients |
SCLC | I–IV | Biopsy to treatment | – | Stages I–II homeless | – | 20 | – | – | – | – | No association | |
| SCLC | I–IV | Biopsy to treatment | – | Stages I–II not homeless | – | 50 | – | – | – | – | No association | ||
| SCLC | I–IV | Biopsy to treatment | – | Stages III–IV homeless | – | 49.9 | – | – | – | – | No association | ||
| SCLC | I–IV | Biopsy to treatment | – | Stages III–IV not homeless | – | 58.1 | – | – | – | – | No association | ||
|
Wah 2020 3300 patients |
SCLC | I–IV | Diagnosis to treatment | > 14 days | > 14 days | 0.5 | – | – | – | – | Shorter time less mortality | ||
|
Anggondowati 2020 691,464 patients |
SCLC | I–IV | Diagnosis to treatment | – | Metastases | – | – | – | 18 | 11–36 | – | No association | |
| SCLC | I–IV | Diagnosis to treatment | – | Early stages | – | – | – | 28 | 2–51 | – | Shorter time less mortality | ||
| SCLC | I–IV | Diagnosis to treatment | – | Local disease | – | – | – | 27 | 13–46 | – | No association | ||
|
Vinod 2017 1926 patients |
SCLC | I–IV | Diagnosis to treatment | – | – | – | – | – | 32 | 15–18 | – | Improves survival with longer times, in stages III and IV | |
|
Gomez 2015 28,732 patients |
SCLC | I–IV | Diagnosis to treatment | > 35 days | Local disease | – | – | – | – | – | – | Improves survival with shorter times | |
| SCLC | I–IV | Diagnosis to treatment | > 35 days | Regional disease | – | – | – | – | – | – | No association | ||
|
Radzikowska 2012 10,386 patients |
SCLC | I–IV | Diagnosis to treatment | – | – | – | 32 | 0 | – | Improves survival with longer times | |||
|
Yang 2017 4984 patients |
NSCLC with squamous cell carcinoma | I | Diagnosis to treatment | – | – | – | – | – | 38 | 24–60 | – | Improves survival with shorter times | |
|
Bhandari 2020 64,491 patients |
SCLC | I–IV | Diagnosis to treatment | – | – | – | – | – | 18 | 9–31 | – | Improves survival with longer times, | |
|
Bhandari 2019 2992 patients |
SCLC | I–IV | Diagnosis to treatment | – | – | – | 18 | – | – | – | Improves survival with longer times, | ||
|
Radzikowska 2013 3479 patients |
SCLC | I–IV | Diagnosis to treatment | – | – | – | 28.9 | 64,4 | 6 | 6–21 | – | Improves survival with longer times | |
| Decision on surgery to surgery |
Coughlin 2015 222 patients |
SCLC | I–II | Decision on surgery to surgery | – | < 1 month | 0.248 | – | – | – | – | – | No association |
| SCLC | I–II | Decision on surgery to surgery | – | 1 < 2 months | 0.441 | – | – | – | – | – | No association | ||
| SCLC | I–II | Decision on surgery to surgery | – | 2 < 3 months | 0.19 | – | – | – | – | – | Improves survival with shorter times in stage II; No association for stage I | ||
| SCLC | I–II | Decision on surgery to surgery | – | 3 < 4 months | 0.117 | – | – | – | – | – | No association | ||
| Diagnosis to first specialist contact |
Kanarek 2014 174 patients |
SCLC | I–II | Diagnosis to first contact with the center | – | – | 61.2 | – | – | – | Improves survival with shorter times | ||
| Surgery to treatment/chemotherapy |
Odell 2019 141,723 patients |
SCLC | I–IV | Surgery to chemotherapy | > 180 days | – | – | 0.665 | – | NA | – | – | Improves survival with shorter times |
| SCLC | I–IV | Chemotherapy to surgery | > 120 days | – | – | 0.037 | – | – | – | – | Improves survival with shorter times | ||
|
Salazar 2017 31,474 patients |
SCLC | I–III | Surgery to treatment | – | – | – | – | – | 48 | 37–62 | – | No association | |
|
Jing 2016 362 patients |
SCLC | II–IIIA | Surgery to treatment | 8 weeks | – | – | – | – | 48 | – | 26–102 | Improves survival with shorter times | |
|
Booth 2013 3354 patients |
SCLC | I–IV | Surgery to treatment | – | – | – | – | – | 56 | – | 7–112 | No association |
NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; NA, not available; CT, computerized axial tomography
For the symptoms to treatment time, two studies reported no association between waiting time and survival or mortality, although Alanen [21] found improved survival when the waiting time was shorter in stage I patients. Three studies reported better patient survival when the waiting time was longer, although they justified these results by indicating that, in patients in earlier stages of the disease, the diagnostic study and assessment of staging may be more complex and require more tests, which could extend the times, compared with patients whose disease is more advanced.
For the symptoms to diagnosis time, two studies found no association with survival or mortality and one study reported improved survival when the waiting time was longer, associating this outcome with patients whose only possible treatment is palliative, since these patients are diagnosed faster due to the disease progression, while patients who opt for curative treatments undergo more tests to make a more accurate diagnosis, which lengthens waiting times [21].
For the diagnosis-to-treatment time, nine studies reported improved survival when the waiting time was shorter, three studies found no association between waiting time and survival or mortality, and nine studies reported improved survival when the waiting time was longer; in these studies the results obtained were justified by indicating that patients in more advanced stages, or who are older or with worse health are referred and treated more quickly than those in earlier stages, whose diagnosis may require more tests that delay the time to treatment, and in whom, despite being treated more quickly, due to the disease severity, the poor prognosis is not altered. In addition, the studies clarified that, despite these results, the timely treatment of patients with early-stage SCLC should be emphasized to prevent a worsening in staging, which has a large impact on survival [18, 22].
Discussion
We analyzed 38 articles on waiting times for the diagnosis and treatment of lung cancer published between 2010 and 2020 which related them to the prognosis. The studies selected were widely heterogeneous in terms of the design, the patient populations included, the structure of the health systems, the definition of the waiting time intervals evaluated, and the summary statistics used in the analysis of the results, which limits possible between-study comparisons.
In similar studies, Olsson 2019 [6] reported a range of medians for the diagnosis to treatment time of 12.5–52 days, and from primary care visit to specialist visit time of 1–12 days; Jacobsen et al. [1] found a median range of 6–45 days and 1–17 days, respectively, for the same time intervals. In our review, medians of 6–121 days were found for the diagnosis to treatment time and 4–19.5 days for the primary care visit to specialist visit time, suggesting that waiting times have not improved and efforts should be made to reach the recommended standard times of a median of 15 days between diagnosis and treatment and 7 days between the primary care visit and the specialist visit [7].
We found that 35% of the time intervals studied showed no relationship between mean or median waiting times and the disease prognosis. Paradoxically, in the rest of the times studied, 37.5% found a better prognosis with longer waiting times and 27.5% a better prognosis with shorter waiting times. Jacobsen et al. [1] and Olsson [6] also obtained disparate results in terms of the proportion of articles that related better patient prognosis with longer times, shorter times, or that the prognosis was not affected by the waiting times, although in these reviews the results were not related to the specific waiting times, but a general evaluation of the relationship was made.
Although the results show that a high proportion of studies associated prolonged waiting times with a better prognosis, all of them justify this association, arguing for the importance of early care and detection in more serious patients. This suggests that to achieve a good management and prognosis of lung cancer these waiting times must be reduced. Most articles which associated shorter waiting times with a worse prognosis justified this relationship by stating that patients in more advanced stages, or who were older or had comorbidities, are referred and treated more quickly than those who are in earlier stages; in these more advanced patients, despite being treated more quickly, the poor prognosis did not change, resulting in shorter survival times. The diagnosis of patients in early stages may require more testing or evaluation by hospital committees, which delays diagnostic and treatment times, but may improve the prognosis because treatment is more targeted and individualized. In addition, many patients will receive surgical treatment, and the time spent on the waiting list until surgery can help prolong these intervals.
Special attention should be paid to the psychological stress to which patients are subjected throughout the process from diagnosis to treatment. As shown by Labbe et al. and Kasymjanova et al. [28, 32], shorter waiting times have positive repercussions in terms of anxiety, mental health, quality of life and patient satisfaction, and lead to lower treatment costs.
The global situation, in which COVID-19 has impacted on cancer waiting times in general, and lung cancer in particular, should be considered. Gheorghe et al. [38], modeled the potentially avoidable deaths due to delays in cancer diagnosis in England in response to the pandemic and estimated the economic and quality of life lost. Nearly 3620 deaths due to breast, bowel, lung, and esophageal cancer could have been avoided in the next 5 years, representing a loss of 32,700 QALYs and €120. 83 million in productivity and, specifically in lung cancer, 10,900 QALYs and €4.45 million, compared with the 21,450 QALYs and €88.96 million lost due to deaths caused by COVID-19. Therefore, good coordination and early action in the management of lung cancer patients is essential to alleviate the delays and consequences derived from COVID-19.
One limitation of our study is the variation in the countries of the studies selected and the differences in health systems, which has a direct impact on waiting times and can cause confusion, as does the differing measures of waiting times, since each article defines these differently, which impacts on the comparability of the results and the complexity of the interpretation. However, we used PubMed and Embase to extract most available studies on the objective, thus providing an overview of the waiting times lung cancer patients are subject to and a detailed analysis of these times with the prognosis.
Generally, the waiting times usually include biases. The times are not accelerated if the patient is in the earlier disease stages, but they are in the advanced stages, due to the high mortality in this type of cancer, which results in contradictory results. As indicated by Adizie et al. [39], there are also more factors that skew waiting times, such as physician’s workloads and the organization of the treating center, which negatively affect the survival of lung cancer patients, the type of curative treatment administered and reductions in waiting times. Further prospective evidence is required to enable studies designed to provide more data on the relationship between waiting times and lung cancer prognosis.
In conclusion, patients value timely and effective care, and it is important to improve the diagnostic and therapeutic waiting times to which lung cancer patients are subjected, especially because these times influence the prognosis, with the aim of increasing the cure rate or, where appropriate, improving the quality of life and prolonging survival.
Acknowledgements
The authors acknowledge Oblikue Consulting S.L for the support given to write this manuscript.
Appendix A
Author contributions
MG, EFM, JAFV, ANM, and ASH contributed to the conceptualization, validation, analysis, data curation, writing—original draft preparation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded from consultancy fees received from AstraZeneca Farmacéutica España S.A.
Data availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Ethical approval and Informed consent
This study was a SLR based on a published studies consequently this research did not envolve humans and/or animals participants.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
María Guirado, Email: mariaspalux@hotmail.com.
Elena Fernández Martín, Email: efernandezmartin@salud.madrid.org.
Alberto Fernández Villar, Email: Jose.Alberto.Fernandez.Villar@sergas.es.
Arturo Navarro Martín, Email: anavarro@iconcologia.net.
Alfredo Sánchez-Hernández, Email: asanchezh@seom.org.
References
- 1.Jacobsen MM, Silverstein SC, Quinn M, Waterston LB, Thomas CA, Benneyan JC, et al. Timeliness of access to lung cancer diagnosis and treatment: a scoping literature review. Lung Cancer. 2017;112:156–164. doi: 10.1016/j.lungcan.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Vinas F, Ben Hassen I, Jabot L, Monnet I, Chouaid C. Delays for diagnosis and treatment of lung cancers: a systematic review. Clin Respir J. 2016;10:267–271. doi: 10.1111/crj.12217. [DOI] [PubMed] [Google Scholar]
- 3.Sociedad Española de Oncología Médica (SEOM). Las cifras del cáncer en España. 2021.
- 4.American Cancer Society. Tasas de supervivencia del cáncer de pulmón [Internet]. 2021. https://www.cancer.org/es/cancer/cancer-de-pulmon/deteccion-diagnostico-clasificacion-por-etapas/tasas-de-supervivencia.html. Cited 27 May 2021.
- 5.GLOBOCAN 2020 [Internet]. https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=1&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=1&include_nmsc_other=1. Cited 4 May 2021.
- 6.Olsson JK, Schultz EM, Gould MK. Timeliness of care in patients with lung cancer: a systematic review. Thorax. 2009;64:749–756. doi: 10.1136/thx.2008.109330. [DOI] [PubMed] [Google Scholar]
- 7.Ministerio de Sanidad. Estrategia en Cáncer del Sistema Nacional de Salud. 2021.
- 8.Malalasekera A, Nahm S, Blinman PL, Kao SC, Dhillon HM, Vardy JL. How long is too long? A scoping review of health system delays in lung cancer. Eur Respir Rev. 2018;27:180045. doi: 10.1183/16000617.0045-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasim F, Sabath BF, Eapen GA. Lung cancer. Medical clinics of North America. Philadelphia: W.B. Saunders; 2019. pp. 463–473. [DOI] [PubMed] [Google Scholar]
- 10.Kuroda H, Sugita Y, Ohya Y, Yoshida T, Arimura T, Sakakura N, et al. Importance of avoiding surgery delays after initial discovery of suspected non-small-cell lung cancer in clinical stage IA patients. Cancer Manag Res. 2018;11:107–115. doi: 10.2147/CMAR.S180757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Concannon KF, Thayer JH, Wu QV, Jenkins IC, Baik CS, Linden HM. Outcomes among homeless patients with non-small-cell lung cancer: a county hospital experience. JCO Oncol Pract. 2020;16:e1004–e1014. doi: 10.1200/JOP.19.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zivkovic D. Effect of delays on survival in patients with lung carcinoma in Montenegro—PubMed. Acta Clin Croat. 2014;53(4):390–398. [PubMed] [Google Scholar]
- 13.Redaniel MT, Martin RM, Ridd MJ, Wade J, Jeffreys M. Diagnostic intervals and its association with breast, prostate, lung and colorectal cancer survival in England: historical cohort study using the clinical practice research datalink. Metze K, editor. PLoS ONE. 2015;10:e0126608. doi: 10.1371/journal.pone.0126608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forrest LF, Adams J, Rubin G, White M. The role of receipt and timeliness of treatment in socioeconomic inequalities in lung cancer survival: population-based, data-linkage study. Thorax. 2015;70:138–145. doi: 10.1136/thoraxjnl-2014-205517. [DOI] [PubMed] [Google Scholar]
- 15.Samson P, Patel A, Garrett T, Crabtree T, Kreisel D, Krupnick AS, et al. Effects of delayed surgical resection on short-term and long-term outcomes in clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2015;99:1906–1913. doi: 10.1016/j.athoracsur.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rice SR, Vyfhuis MAL, Scilla KA, Burrows WM, Bhooshan N, Suntharalingam M, et al. Insurance status is an independent predictor of overall survival in patients with stage III non-small-cell lung cancer treated with curative intent. Clin Lung Cancer. 2020;21:e130–e141. doi: 10.1016/j.cllc.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Anggondowati T, Ganti AK, Islam KMM. Impact of time-to-treatment on overall survival of non-small cell lung cancer patients—an analysis of the national cancer database. Transl Lung Cancer Res. 2020;9:1202–1211. doi: 10.21037/tlcr-19-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhandari S, Pham D, Pinkston C, Oechsli M, Kloecker G. Timing of treatment in small-cell lung cancer. Med Oncol. 2019;36:47. doi: 10.1007/s12032-019-1271-3. [DOI] [PubMed] [Google Scholar]
- 19.Kanarek NF, Hooker CM, Mathieu L, Tsai H-L, Rudin CM, Herman JG, et al. Survival after community diagnosis of early-stage non-small cell lung cancer. Am J Med. 2014;127:443–449. doi: 10.1016/j.amjmed.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odell DD, Feinglass J, Engelhardt K, Papastefan S, Meyerson SL, Bharat A, et al. Evaluation of adherence to the commission on cancer lung cancer quality measures. J Thorac Cardiovasc Surg. 2019;157:1219–1235. doi: 10.1016/j.jtcvs.2018.09.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alanen V, Koivunen JP. Association of diagnostic delays to survival in lung cancer: single center experience. Acta Oncol. 2019;58:1056–1061. doi: 10.1080/0284186X.2019.1590635. [DOI] [PubMed] [Google Scholar]
- 22.Radzikowska E, Roszkowski-Sliz K, Chabowski M, Glaz P. Influence of delays in diagnosis and treatment on survival in small cell lung cancer patients. 2013:355–62. [DOI] [PubMed]
- 23.González-Barcala FJ, García-Prim JM, Álvarez-Dobaño JM, Moldes-Rodríguez M, García-Sanz MT, Pose-Reino A, et al. Effect of delays on survival in patients with lung cancer. Clin Transl Oncol. 2010;12:836–842. doi: 10.1007/s12094-010-0606-5. [DOI] [PubMed] [Google Scholar]
- 24.Diaconescu R, Lafond C, Whittom R. Treatment delays in non-small cell lung cancer and their prognostic implications. J Thorac Oncol. 2011;6:1254–1259. doi: 10.1097/JTO.0b013e318217b623. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Barcala FJ, Falagan JA, Garcia-Prim JM, Valdes L, Carreira JM, Puga A, et al. Timeliness of care and prognosis in patients with lung cancer. Ir J Med Sci (1971-). 2014;183:383–390. doi: 10.1007/s11845-013-1025-8. [DOI] [PubMed] [Google Scholar]
- 26.Radzikowska E, LastNameRoszkowski-Śliż K, Gła P. The impact of timeliness of care on survival in non-small cell lung cancer patients—PubMed. Pneumonol Alergol Pol. 2012;80(5):422–429. doi: 10.5603/ARM.27556. [DOI] [PubMed] [Google Scholar]
- 27.Skaug K, Eide GE, Gulsvik A. Predictors of long-term survival of lung cancer patients in a Norwegian community. Clin Respir J. 2011;5:50–58. doi: 10.1111/j.1752-699X.2010.00200.x. [DOI] [PubMed] [Google Scholar]
- 28.Labbé C, Anderson M, Simard S, Tremblay L, Laberge F, Vaillancourt R, et al. Wait times for diagnosis and treatment of lung cancer: a single-centre experience. Curr Oncol. 2017;24:367–373. doi: 10.3747/co.24.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ha D, Ries AL, Montgrain P, Vaida F, Sheinkman S, Fuster MM. Time to treatment and survival in veterans with lung cancer eligible for curative intent therapy. Respir Med. 2018;141:172–179. doi: 10.1016/j.rmed.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vinod SK, Chandra A, Berthelsen A, Descallar J. Does timeliness of care in non-small cell lung cancer impact on survival? Lung Cancer. 2017;112:16–24. doi: 10.1016/j.lungcan.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 31.Bhandari S, Kumar R, Pham D, Gaskins J, Kloecker G. Treatment timing in small cell lung cancer, a national cancer database analysis. Am J Clin Oncol. 2020;43:362–365. doi: 10.1097/COC.0000000000000676. [DOI] [PubMed] [Google Scholar]
- 32.Kasymjanova G, Small D, Cohen V, Jagoe RT, Batist G, Sateren W, et al. Lung cancer care trajectory at a Canadian centre: an evaluation of how wait times affect clinical outcomes. Curr Oncol. 2017;24:302–309. doi: 10.3747/co.24.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheinson D, Wong WB, Wu N, Mansfield AS. Impact of delaying initiation of anaplastic lymphoma kinase inhibitor treatment on survival in patients with advanced non-small-cell lung cancer. Lung Cancer. 2020;143:86–92. doi: 10.1016/j.lungcan.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Cushman TR, Jones B, Akhavan D, Rusthoven CG, Verma V, Salgia R, et al. The effects of time to treatment initiation for patients with non-small-cell lung cancer in the United States. Clin Lung Cancer. 2021;22:e84–97. doi: 10.1016/j.cllc.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Wah W, Stirling RG, Ahern S, Earnest A. Influence of timeliness and receipt of first treatment on geographic variation in non-small cell lung cancer mortality. Int J Cancer. 2021;148:1828–1838. doi: 10.1002/ijc.33343. [DOI] [PubMed] [Google Scholar]
- 36.Gomez DR, Liao K-P, Swisher SG, Blumenschein GR, Erasmus JJ, Buchholz TA, et al. Time to treatment as a quality metric in lung cancer: staging studies, time to treatment, and patient survival. Radiother Oncol. 2015;115:257–263. doi: 10.1016/j.radonc.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Yang C-FJ, Wang H, Kumar A, Wang X, Hartwig MG, Damico TA, et al. Impact of timing of lobectomy on survival for clinical stage IA lung squamous cell carcinoma. Chest. 2017;152:1239–1250. doi: 10.1016/j.chest.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 38.Gheorghe A, Maringe C, Spice J, Purushotham A, Chalkidou K, Rachet B, et al. Economic impact of avoidable cancer deaths caused by diagnostic delay during the COVID-19 pandemic: a national population-based modelling study in England, UK. Eur J Cancer. 2021. [DOI] [PMC free article] [PubMed]
- 39.Adizie JB, Khakwani A, Beckett P, Hubbard R, Navani N, Harden SV, et al. Impact of organisation and specialist service delivery on lung cancer outcomes. Thorax. 2019;74:546–550. doi: 10.1136/thoraxjnl-2018-212588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.