Summary
Neurocognitive impairment is common in sickle cell disease (SCD) and is associated with significant functional limitations. In a cross-sectional analysis, we examined the association between hydroxyurea (HU) treatment and neurocognitive functioning from school-age to young adulthood in individuals with SCD. A total of 215 patients with HbSS/HbSβ0-thalassaemia (71% HU treated) and 149 patients with HbSC/HbSβ+-thalassaemia (20% HU treated) completed neurocognitive measures at one of four developmental stages: school-age (age 8–9 years), early adolescence (age 12–13 years), late adolescence (age 16–17 years) and young adulthood (ages 19–24 years). For participants with multiple assessments, only the most recent evaluation was included. In multivariable analysis adjusted for social vulnerability, HU treatment and sex, older age was associated with a reduction in overall intelligence quotient (IQ) of 0.55 points per year of life [standard error (SE) = 0·18, false discovery rate adjusted P value (PFDR) = 0.01] for patients with HbSS/HbSβ0-thalassaemia. Earlier initiation of HU (n = 152) in HbSS/HbSβ0-thalassaemia was associated with higher scores on neurocognitive measures across most domains, including IQ [estimate (SE) 0·77 (0·25)/year, PFDR = 0·01], after adjusting for social vulnerability, sex and treatment duration. These results support the early use of HU to limit the detrimental neurocognitive effects of SCD, while highlighting the need for additional measures to further mitigate neurocognitive deterioration.
Keywords: sickle cell disease, neurocognition, hydroxyurea, aging, fetal haemoglobin
Introduction
Patients with sickle cell disease (SCD) are at increased risk of numerous brain complications, including overt stroke, silent cerebral infarctions and chronic insufficiencies in oxygen and/or glucose delivery to the brain.1–3 Neurocognitive deficits are observed early in life.4 Over time, owing to accumulated micro-infarcts, chronic hypoxaemia and repeated tissue ischaemia,5 individuals with SCD tend to display slowed acquisition of neurocognitive skills.6
Among patients with SCD, there is significant heterogeneity in neurocognitive outcomes. In children, greater deficits in cognitive functioning are associated with more severe anaemia, lower total haemoglobin (Hb), lower fetal Hb (HbF), lower oxygen saturation, silent cerebral infarcts and overt stroke.7–9 While demographic factors, including reduced parent education and household income are also associated with poorer cognitive outcomes in childhood,7,8 risk factors for neurocognitive deficits are not well characterised across all developmental stages.
A single study, utilising the Cooperative Study of Sickle Cell Disease (CSSCD) cohort, examined longitudinal outcomes in SCD.6 Results indicated slowed cognitive growth compared to same age peers. However, that study did not examine a wide range of neurocognitive constructs such as memory or executive functioning, and the oldest participants were aged 17 years. Additionally, no participants in the CSSCD were treated with hydroxyurea (HU).7,10
Hydroxyurea increases HbF11 and is associated with less frequent pain episodes,12 reduced mortality12,13 and improved health-related quality of life.14 HU reduces overall transcranial Doppler ultrasonography velocities and stroke occurrence.15–17 Preliminary evidence suggests that HU treatment is positively associated with neurocognitive functioning.18–20 Prior work by our group demonstrated effects of HU use on measures of verbal reasoning, processing speed and working memory, yet most of these findings did not persist after adjustment for multiple comparisons.19 Prior studies assessing the relationship between HU treatment and neurocognition are limited by small samples and narrow age ranges.18–20 No studies have examined if age of HU initiation affects neurocognitive outcomes or mechanisms of action.
In the present study, we present a multi-age cross-sectional analysis to examine neurocognitive outcomes in individuals with SCD from school age through to young adulthood. The first objective was to examine the effect of age on neurocognitive functioning and to assess how age interacts with risk factors across the lifespan. We hypothesised that older age would be associated with worse performance, relative to same age peers. A secondary objective was to evaluate the association between HU use and neurocognitive outcomes. We hypothesised that current use of HU would be positively associated with neurocognitive functioning and that among those treated with HU, earlier treatment initiation would be positively correlated with neurocognition.
Methods
This research was approved by the Institutional Review Board (IRB) at St. Jude Children’s Research Hospital (St. Jude, USA). Written informed consent was obtained from all participants (or their legal guardian if a minor) and adolescents gave assent according to the requirements of the IRB.
Participants
All participants of the Sickle Cell Clinical Research Intervention Program (SCCRIP) study, aged 8–25 years, were eligible for this study. The design of SCCRIP has been described previously.21 Briefly, SCCRIP is a longitudinal lifetime cohort study that collects retrospective and prospective data on clinical, neurocognitive, psychosocial, geospatial and health outcomes of children, adolescents and adults with SCD. Neurocognitive assessments are performed approximately every 4 years between the ages of 8 and 24 years and every 6 years thereafter. These screening assessments are not clinical referrals, but a systematic surveillance, as patients were not selected for disease severity, prior central nervous system findings or existing cognitive concerns. Testing broadly occurred at one of four developmental stages: school age (age 8–9 years), early adolescence (age 12–13 years), late adolescence (age 16–17 years) and young adulthood (age 19–24 years). Of the 625 eligible participants in SCCRIP, 368 received a neurocognitive assessment. We excluded five patients with a history of stroke, resulting in a total sample of 363. A total of 59 participants received multiple neurocognitive assessments as part of longitudinal monitoring, and the most recent assessment was used for analyses. All data were collected between 2012 and 2018.
Demographic, medical and treatment variables
Demographic, medical and treatment variables were abstracted from the SCCRIP database. The Social Vulnerability Index (SVI)22,23 was used to classify individuals based on social vulnerabilities at the neighbourhood level (e.g. poverty, education and housing data)22; a higher percentile score indicates higher social vulnerability. Participants with HbSS/HbSβ0-thalassaemia received HU according to National Heart, Lung, and Blood Institute (NHLBI) guidelines.24 For participants with HbSC/HbSβ+-thalassaemia, HU initiation was guided by the frequency of acute disease complications.25 HU dosing began at 20 mg/kg/day and increased in 5 mg/kg increments every 8–12 weeks to the maximum tolerated dose, defined by an absolute neutrophil count of 2–4 × 109/l or the presence of haematological toxicity.26,27 All patients were kept at the maximum tolerated dose, as per our institution’s standard practice. Haematological indices including Hb, HbF, white blood cell (WBC) count and platelet count were performed at steady state on the day of neurocognitive testing or were the average value of measurements within 3 months prior to testing. Daytime Hb oxygen saturation was obtained on the day of the neurocognitive testing and >2 months from a blood transfusion.
Neurocognitive measures
Participants in SCCRIP completed a battery of neurocognitive tests. The administration of all measures was supervised by a licensed psychologist. The Wechsler Abbreviated Scale of Intelligence-Second Edition (WASI-II)28 provided an estimated Full-Scale Intelligence Quotient (IQ) (4-subtest; FSIQ), Verbal Comprehension Index and Perceptual Reasoning Index. Depending on the participant’s age, the Wechsler Adult Intelligence Scale, Fourth Edition (WAIS-IV)29 or Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV)30 Digit Span (working memory) and Coding (graphomotor processing speed) subtests were used. Delis–Kaplan Executive Function System31 subtests included Trail Making, Number Sequencing and Number/Letter Switching (executive task switching) and Category/Letter Fluency (verbal fluency). The Wide Range Assessment of Memory and Learning (WRAML), Second Edition,32 Story Memory subtest, measured verbal memory. Visuomotor integration was assessed using the Beery-Buktenica Visual Motor Integration (VMI) Test, Sixth Edition.33 Fine motor dexterity and speed were measured with the Grooved Pegboard Test.34 Academic achievement measures included Letter-Word Identification and Math Fluency from the Woodcock–Johnson Test of Achievement, Third Edition35.
Statistical analyses
Two-sample t-test or analysis of variance (ANOVA), Wilcoxon rank sum test or Kruskal–Wallis rank-sum test and Fisher’s exact test were used to evaluate two or more than two group differences on demographic and medical/treatment variables. The Shapiro–Wilk test was used to test for normality of the data.
For our first and second objectives, multivariate linear regression models were used to examine the effects of age and HU treatment on neurocognitive functioning after adjustment for SVI and sex. Due to differences in the pathophysiology of SCD based on genotype, we conducted all analyses separately for patients with HbSS/HbSβ0-thalassaemia and HbSC/HbSβ+-thalassaemia. We did not include laboratory values in our primary analyses, as they were considered potential treatment mechanisms that could mask the relationship between HU and neurocognitive functioning. To assess the effect of age at HU treatment initiation, duration of HU treatment was added to the model. Age was analysed both as a continuous and ordinal variable (i.e. 0 = school age, 1 = early adolescence, 2 = late adolescence and 3 = young adulthood).
Propensity score analyses were conducted to assess the HU effect on neurocognitive performance, controlling for covariates reflecting greater likelihood of receiving HU treatment.36,37 We estimated the propensity score by fitting a logistic regression model with HU treatment as the outcome and age, sex and SVI as covariates in the model. We used propensity score matching with a 1:1 ratio, without replacement, and nearest distance matching for age, sex and SVI using ‘matchit’ R software (R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria).38
As an exploratory objective, we assessed potential mechanisms of HU treatment on overall IQ. Laboratory measurements, including HbF, Hb, WBC count, platelet count and Hb oxygen saturation were examined individually with overall IQ in the linear regression models with adjustment for covariates described above. Covariates with significant associations at P < 0·10 were included in the multivariable analyses to obtain the final model using stepwise model selection. All the covariates were tested for multicollinearity before entering the model (a variance inflation factor <2). We then conducted mediation analyses to evaluate mediated effects of HU on IQ via laboratory measurements that were retained in the final model, after adjusting for potential confounding variables. Bootstrapping approach was further used to calculate the 95% confidence interval (CI) to confirm the mediation analyses results. The variation of IQ explained by individual covariates in the final model was calculated using the coefficient of multiple determination (R2).
For analyses involving multiple comparisons, false discovery rate (FDR) adjusted P values (PFDR) < 0.05 were used to account for multiple comparisons. Analyses were performed in R, version 3·5.3 and Statistical Analysis System (SAS) version 9·4 (SAS Institute Inc., Cary, NC, USA).
Results
Demographic and clinical characteristics
A total of 363 patients, aged 8–25 years, received neurocognitive testing and the mean (SD) age at evaluation was 14·1 (4·8) years. Those that received neurocognitive testing were statistically comparable to those who did not, except the latter were significantly younger (Table S1).
Age and neurocognitive performance
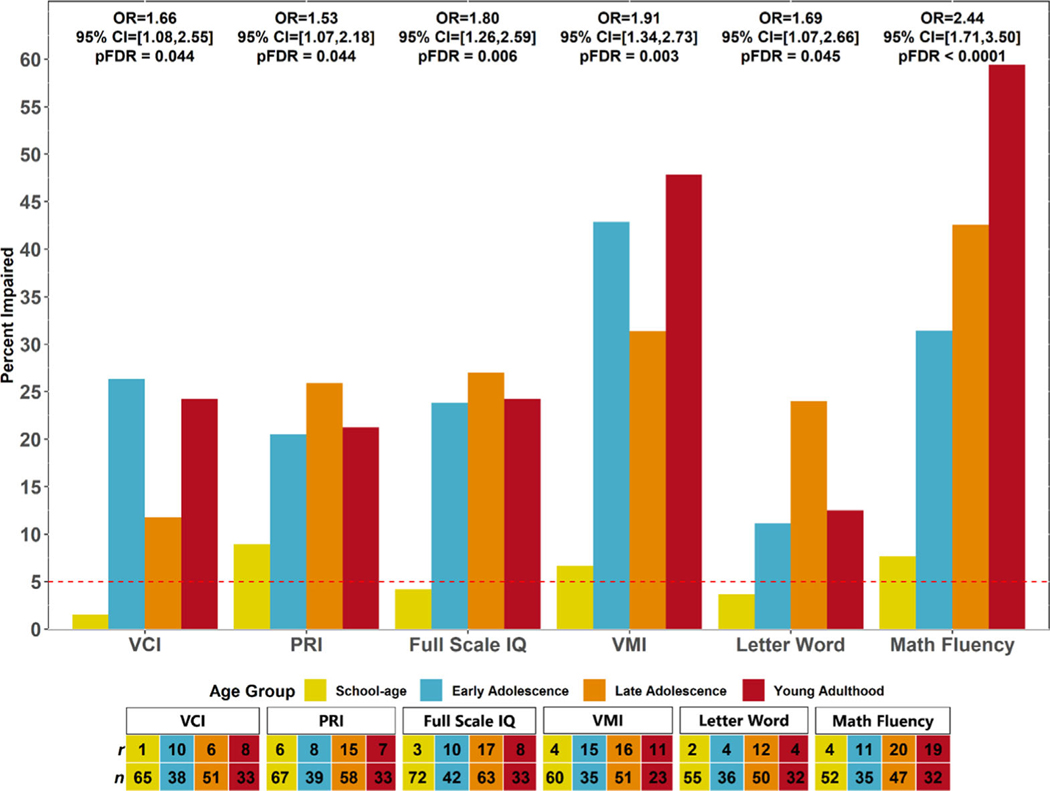
Older age was associated with poorer neurocognitive performance across most domains for patients with HbSS/HbSβ0-thalassaemia (PFDR < 0·05) after adjusting for covariates. For each year of life, there was a mean (SE) reduction of 1·24 (022), 1·79 (022) and 1·05 (0·23) points in word reading, math fluency and visuomotor integration respectively (all PFDR < 0·001). Overall IQ reduced by a mean (SE) of 0·55 (0·18) points/year of increased patient age (PFDR = 0·01). Older age was associated with poorer performance on a measure of word reading [PFDR = 0·02; mean (SE) estimate −1·05 (0·31)] for patients with HbSC/HbSβ+thalassaemia. No other measures were associated with age (PFDR > 0·05). Among patients with HbSS/HbSβ0-thalassaemia, the proportion of participants with clinically significant (<5th percentile) neurocognitive impairments increased across age time-points for most neurocognitive measures (PFDR < 0·05; Fig 1) after adjusting for covariates. Rates of impairment (<5th percentile) did not significantly increase across age time-points for any domain after adjustment for multiple comparisons (PFDR > 0·05; data not shown) among patients with HbSC/HbSβ+-thalassaemia.
Fig 1.
Frequency of neurocognitive impairment increases across age groups for patients with HbSS/HbSβ0-thalassaemia. FDR, false discovery rate adjustment for 23 neurocognitive outcomes; HU, hydroxyurea; OR, odds ratio; PRI, Perceptual Reasoning Index; VCI, Verbal Comprehension Index; VMI, Visual Motor Integration. Age groups include school-age (8–9 years), early adolescence (12–13 years), late adolescence (16–17 years), and young adulthood (19–24 years). ORs reflect the effect of a 1-unit increase in age group (where 0 = school age) at assessment on impairment (scoring at or below the 5th percentile) compared to no impairment after accounting for sex, social vulnerability, and current HU use. PFDR is FDR adjusted P value. The Verbal Comprehension Index, Perceptual Reasoning Index, and Full-Scale intelligence quotient (IQ) were measured with the Wechsler Abbreviated Scale of Intelligence, Second Edition. Visual Motor Integration was measured using the Beery Visual Motor Integration Test, Sixth Edition. Letter-Word and Math Fluency were measured with the Woodcock–Johnson Academic Achievement Test, Third Edition. Dashed line represents percentage of individuals expected to display clinically significant impairment (i.e. <5th percentile) in the normative population
Hydroxyurea and neurocognitive performance
Group differences on demographic and biological variables by HU treatment status separated by genotype are listed in Table I. Patients with HbSS/HbSβ0-thalassaemia currently treated with HU had higher levels of overall Hb (PFDR = 0·002), higher HbF (PFDR < 0·001) and a lower WBC count (PFDR < 0·001) than those not treated with HU. Patients with HbSC/HbSβ+-thalassaemia treated with HU were younger than those not treated (PFDR = 0·007).
Table I.
Participant characteristics by HU for the overall group and by genotype.
| HbSS/HbSβ0-thalassaemia | PFDR | HbSC/HbSβ+-thalassaemia | PFDR | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HU | No HU | HU | No HU | |||
| n = 152 | n = 63 | N = 215 | n = 29 | n = 119 | N = 148 | |
| N (%): | ||||||
| Sex | ||||||
| Female | 70 (46·05) | 34 (53·97) | 0·44 | 15 (51·72) | 63 (52·94) | 0·91 |
| Male | 82 (53·95) | 29 (46·03) | 14 (48·28) | 56 (47·06) | ||
| Race | ||||||
| African American | 151 (99·34) | 63 (100·00) | 1·00 | 29 (100·00) | 119 (100·00) | . |
| Other | 1 (0·66) | 0 (0·00) | . | |||
| Overall vulnerability summary ranking* | ||||||
| Very low (0·15-99) | 8 (5·26) | 4 (6·35) | >0.99 | 3 (10·34) | 6 (5·04) | 0·72 |
| Low (16·26-99) | 5 (3·29) | 3 (4·76) | 3 (10·34) | 7 (5·88) | ||
| Moderate (27·37-99) | 8 (5·26) | 3 (4·76) | 2 (6·90) | 7 (5·88) | ||
| High (38·48-99) | 12 (7·89) | 5 (7·94) | 2 (6·90) | 12 (10·08) | ||
| Very high (49·100) | 118 (77·63) | 48 (76·19) | 19 (65·52) | 87 (73·11) | ||
| Chronic transfusion at time of evaluation | 2 (1·32) | 7 (11·11) | 0·015 | . | ||
| HU treatment gap† | 27 (17·76) | . | 3 (10·34) | . | ||
| Transcranial Doppler‡ | ||||||
| Normal | 98 (67·12) | 39 (65·00) | 0·40 | . | ||
| Conditional | 35 (23·97) | 11 (18·33) | . | |||
| Abnormal | 13 (8·90) | 10 (16·67) | . | |||
| Age group | ||||||
| School age | 50 (32·89) | 24 (38·10) | 0·40 | 4 (13·79) | 45 (37·82) | 0·02 |
| Early adolescent | 27 (17·76) | 17 (26·98) | 5 (17·24) | 27 (22·69) | ||
| Late adolescent | 51 (33·55) | 13 (20·63) | 13 (44·83) | 39 (32·77) | ||
| Young adult | 24 (15·79) | 9 (14·29) | 7 (24·14) | 8 (6·72) | ||
|
| ||||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
|
| ||||||
| Age at evaluation, years | 14·34 (4·93) | 13·69 (4·95) | 0·54 | 16·60 (4·80) | 13·29 (4·31) | 0·007 |
| Age at HU initiation, years | 7·95 (4·73) | . | 12·90 (5·43) | . | ||
| Duration of HU treatment, years | 6·27 (4·27) | . | 3·74 (3·34) | . | ||
| HU dose, mg/kg | 25·66 (5·75) | . | 18·83 (5·45) | . | ||
| Haemoglobin, g/l | 93.7 (12.8) | 87.5 (10.3) | 0·002 | 114.2 (15.7) | 116.9 (10.8) | 0·72 |
| WBC count, x 109/l | 9·25 (3·98) | 11·95 (3·87) | <0.0001 | 7·21 (3·45) | 8·02 (3·29) | 0·17 |
| Fetal haemoglobin, % | 17·73 (9·11) | 10·98 (7·54) | <0.0001 | 5·57 (6·45) | 4·65 (6·07) | 0·72 |
| Platelet count, x 109/l | 389·81 (185·44) | 391·01 (123·38) | 0·36 | 252·45 (138·62) | 248·74 (98·89) | 0·72 |
| Haemoglobin oxygen saturation, % | 99·16 (1·25) | 98·72 (1·57) | 0·40 | 99·48 (0·77) | 99·64 (0·77) | 0·42 |
FDR, false discovery rate; HbF, fetal haemoglobin; HU, hydroxyurea; n, sample size; SD, standard deviation; WBC, white blood cell.
P values based on chi-square or Fisher’s Exact test for categorical variables and ANOVA or Kruskal–Wallis for continuous variables. FDR adjusted P (PFDR) < 0·05 were considered significant. Haematological indices were the average value of measurements collected within 3 months of or the one closest to neurocognitive assessment.
Classifies individuals based on social vulnerabilities at the neighbourhood level (e.g. poverty, education, housing data); a higher percentile score indicates higher social vulnerability.
Percentage of patients currently on HU that had a gap in treatment of ≥1 year at any time.
Only performed on patients with Hb SS/HbSβ0-thalassaemia. Transcranial Doppler mean flow velocity values for each artery: normal (<170 cm/s), conditional (170–199 cm/s), and abnormal (>200 cm/s).
Those treated with HU had higher scores on measures of verbal comprehension, perceptual reasoning and overall IQ for all genotypes (all P < 0·05; Table II and Table S2). However, after corrections for multiple comparisons, these effects only remained for patients with HbSS/HbSβ0-thalassaemia (all PFDR < 0·05 except for perceptual reasoning, PFDR = 0·062). Among patients with HbSS/HbSβ0thalassaemia, those taking HU had a mean (SE) 6·42 (1·96) point elevation in IQ compared to their untreated peers.
Table II.
Effect of current hydroxyurea treatment status on neurocognitive performance.
| HbSS/HbSβ0-thalassaemia | HbSC/HbSβ+-thalassaemia | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Domain | Measure | Score* | Estimate† | SE | Raw P | PFDR‡ | Estimate† | SE | Raw P | PFDR‡ |
| Reasoning | WASI-II | Verbal comprehension index | 6·50 | 2·18 | 0·003 | 0·019 | 6·99 | 2·75 | 0·012 | 0·093 |
| Perceptual reasoning index | 4·95 | 1·99 | 0·014 | 0·062 | 5·52 | 2·69 | 0·042 | 0·112 | ||
| Full-Scale IQ | 6·29 | 1·94 | 0·001 | 0·019 | 6·93 | 2·48 | 0·006 | 0·093 | ||
| Processing speed | WISC-IV or WAIS-IV | Coding | 0·56 | 0·34 | 0·104 | 0·239 | 0·8 | 0·59 | 0·182 | 0·262 |
| Working memory | WISC-IV or WAIS-IV | Digit span total | 0·75 | 0·38 | 0·051 | 0·166 | 0·86 | 0·6 | 0·154 | 0·236 |
| Memory | WRAML-2 | Story memory initial | 0·02 | 0·44 | 0·972 | 0·972 | 1·25 | 0·61 | 0·043 | 0·112 |
| Visuomotor | Beery VMI | Visual motor integration | 1·83 | 2·43 | 0·454 | 0·652 | 4·72 | 2·69 | 0·082 | 0·188 |
| Motor | Grooved pegboard | Fine motor dominant | 0·08 | 0·28 | 0·779 | 0·918 | 0·71 | 0·48 | 0·144 | 0·236 |
| Academic achievement | WJ Achievement-3 | Letter word | 2·97 | 2·46 | 0·23 | 0·441 | 4·44 | 3·52 | 0·21 | 0·262 |
| Math fluency | 2·67 | 2·54 | 0·295 | 0·521 | 1·16 | 3·66 | 0·752 | 0·752 | ||
FDR, false discovery rate; SE, standard error; WASI-II, The Wechsler Abbreviated Scale of Intelligence, Second Edition; WAIS-IV, Wechsler Adult Intelligence Scale, Fourth Edition; WISC-IV, Wechsler Intelligence Scales for Children, Fourth Edition; VMI, Beery–Buktenica Visual Motor Integration Test, Sixth Edition; WJ Achievement-3, Woodcock–Johnson Test of Achievement, Third Edition; WRAML-2, The Wide Range Assessment of Memory and Learning, Second Edition.
Groups represent HU treatment status at the time of neurocognitive evaluation.
Bold values statistically significant at P < 0.05.
Scaled scores with mean (SD) of 10 (3): Digit Span Total, Story Memory Initial; standard scores with a mean (SD) of 100 (15): Verbal Comprehension Index, Perceptual Reasoning Index, Full-Scale IQ, Visual Motor Integration, Letter Word, Math Fluency; z-scores with a mean (SD) of 0 (1): Fine Motor Dominant.
Estimates for effect of current use of HU on neurocognitive performance from linear regression model: neurocognitive measure = current use of HU + participant age at neurocognitive assessment + sex (ref = female) + social vulnerability.
FDR adjustment for 23 neurocognitive outcomes.
Using propensity score, we selected 62 HU-treated and 62 untreated patients with HbSS/HbSβ0-thalassaemia matched by age, SVI and sex. These two matched groups were comparable in all clinical features and propensity score (Table S3), except that HU-treated patients had higher HbF, Hb, lower WBC and platelet counts. The propensity score analysis confirmed the HU treatment effect on overall IQ (estimate 6·2, 95% CI 1·6–11·0; P = 0·009, PFDR = 0·14). Similarly, 29 HU-treated and 29 untreated patients with HbSC/HbSβ+-thalassaemia were matched by age, SVI and sex. The groups did not differ on any clinical or demographic feature. The analysis indicated an HU treatment effect on overall IQ (estimate 8·2, 95% CI 2·5–14; P = 0·007, PFDR = 0·064). However, due to limited power, none of the effects remained significant after adjusting for multiple comparisons (PFDR > 0·05).
Among patients with HbSS/HbSβ0-thalassaemia, longer duration of HU treatment was positively associated with neurocognitive performance on measures of perceptual reasoning and overall IQ, after adjusting for age at evaluation, SVI and sex, at PFDR < 0·05 (Table III and Table S4). In the group treated with HU, older age at initial HU treatment was associated with worse neurocognitive performance across most domains after adjusting for duration of treatment, sex and SVI at PFDR < 0·05 (Fig 2; Table III and Table S4). There was no effect of duration of HU treatment or age of treatment initiation on any neurocognitive outcome for patients with HbSC/HbSβ+-thalassaemia (PFDR > 0·05).
Table III.
Duration of hydroxyurea exposure and age at first exposure effect on neurocognitive performance.
| HbSS/HbSβ0-thalassaemia | HbSC/HbSβ+-thalassaemia | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| All patients | Patients on HU | All patients | Patients on HU | |||||||||||
|
|
|
|
|
|||||||||||
| Ml: adjusted effect of duration of HU treatment | M2: adjusted effect of age at initial HU treatment | Ml: adjusted effect of duration of HU treatment | M2: adjusted effect of age at initial HU treatment | |||||||||||
|
|
|
|
|
|||||||||||
| Domain | Measure | Score* | Estimate | SE | PFDR† | Estimate | SE | PFDR† | Estimate | SE | PFDR† | Estimate | SE | PFDR† |
| Reasoning | WASI-II | Verbal comprehension index | 0·59 | 0·24 | 0·07 | −0·77 | 0·27 | 0·01 | 1·01 | 0·49 | 0·16 | −0·66 | 0·49 | 0·56 |
| Perceptual reasoning index | 0·61 | 0·21 | 0·03 | −0·62 | 0·25 | 0·02 | 0·74 | 0·49 | 0·21 | 0·58 | 0·49 | 0·57 | ||
| Full-Scale IQ | 0·69 | 0·21 | 0·01 | −0·77 | 0·25 | 0·01 | 1·02 | 0·46 | 0·16 | −0·13 | 0·44 | 0·81 | ||
| Processing speed | WISC-IV /WAIS-IV | Coding | 0·04 | 0·04 | 0·34 | −0·13 | 0·05 | 0·01 | 0·07 | 0·11 | 0·51 | 0·16 | 0·09 | 0·52 |
| Working memory | WISC-IV /WAIS-IV | Digit span total | 0·05 | 0·04 | 0·36 | −015 | 0·05 | 0·01 | 0·19 | 0·11 | 0·18 | 0·06 | 0·08 | 0·65 |
| Memory | WRAML-2 | Story memory initial | 0·1 | 0·05 | 0·10 | −0·17 | 0·05 | 0·004 | 0·28 | 0·12 | 0·15 | −0·23 | 0·11 | 0·52 |
| Visuomotor | Beery VMI | Visual motor integration | 0·13 | 0·26 | 0·65 | −0·84 | 0·31 | 0·02 | 1·06 | 0·51 | 0·15 | 0·37 | 0·39 | 0·58 |
| Motor | Grooved pegboard | Fine motor dominant | −0·01 | 0·03 | 0·82 | −0·07 | 0·03 | 0·04 | 0·2 | 0·1 | 0·16 | 0·09 | 0·06 | 0·56 |
| Academic achievement | WJ Achievement-3 | Letter word | 0·56 | 0·25 | 0·09 | −1·56 | 0·3 | <0.001 | 0·76 | 0·62 | 0·31 | 0·12 | 0·4 | 0·81 |
| Math fluency | 0·43 | 0·27 | 0·22 | −2·4 | 0·31 | <0.001 | 0·46 | 0·66 | 0·51 | 0·37 | 0·56 | 0·67 | ||
FDR, false discovery rate; HU, hydroxyurea; SE, standard error; VMI, Beery-Buktenica Visual Motor Integration Test, Sixth Edition; WASI-II, The Wechsler Abbreviated Scale of Intelligence, Second Edition; WAIS-IV, Wechsler Adult Intelligence Scale, Fourth Edition; WISC-IV, Wechsler Intelligence Scales for Children, Fourth Edition; WJ Achievement-3, Woodcock·Johnson Test of Achievement, Third Edition; WRAML-2, The Wide Range Assessment of Memory and Learning, Second Edition.
Model 1 (M1): Score = duration of HU treatment + age at neurocognitive assessment + sex + social vulnerability. Model 2 (M2): Score = duration of HU treatment + age at initial HU treatment + sex + social vulnerability. For M1, if patients were not on HU treatment at the time of evaluation, then their duration of HU treatment is set as 0.
Bold values statistically significant at P < 0.05.
Scaled scores with mean (SD) of 10 (3): Digit Span Total, Story Memory Initial; standard scores with a mean (SD) of 100 (15): Verbal Comprehension Index, Perceptual Reasoning Index, Full-Scale IQ, Visual Motor Integration, Letter Word, Math Fluency; z-scores with a mean (SD) of 0 (1): Fine Motor Dominant.
FDR adjustment for 23 neurocognitive outcomes.
Fig 2.
Older age at treatment initiation is associated with poorer neurocognitive performance in patients with HbSS/HbSβ0-thalassaemia. FDR, false discovery rate adjustment for 23 neurocognitive outcomes; HU, hydroxyurea; PRI, Perceptual Reasoning Index; VCI, Verbal Comprehension Index; VMI, Visual Motor Integration. The PFDR reflects the effect of age at initial HU therapy on neurocognitive performance after accounting for duration of HU treatment, social vulnerability, and sex. All neurocognitive scores are presented as standard scores with a mean (SD) of 100 (15). The Verbal Comprehension Index, Perceptual Reasoning Index, and Full-Scale intelligence quotient (IQ) were measured with the Wechsler Abbreviated Scale of Intelligence, Second Edition. Visual Motor Integration was measured using the Beery Visual Motor Integration Test, Sixth Edition. Letter-Word and Math Fluency were measured with the Woodcock–Johnson Academic Achievement Test, Third Edition
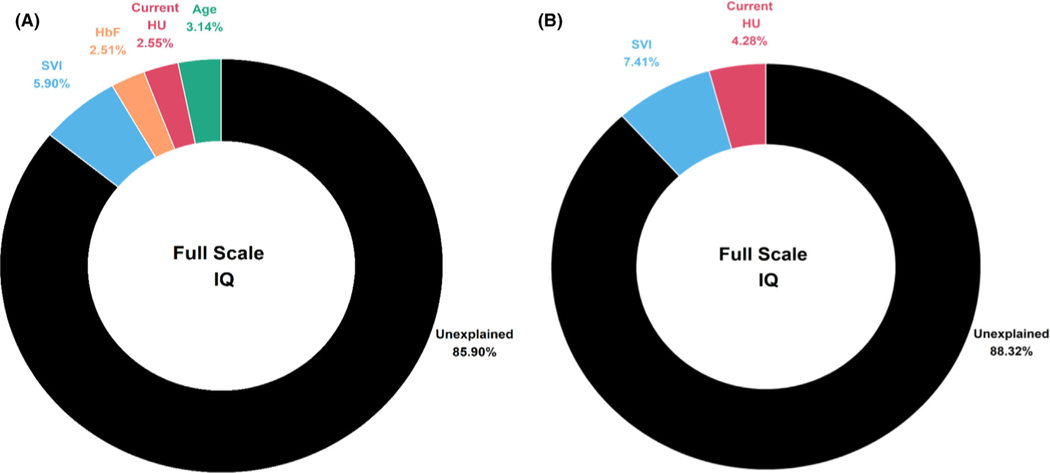
Variance in IQ explained
Variables from the first and second objectives were included alongside laboratory values that reflect disease severity and/or HU effect. Among patients with HbSS/HbSβ0-thalassaemia, four covariates: age, SVI, HbF and HU treatment status, remained in the final model (P < 0·05). These four factors combined accounted for 15·72% (95% CI 8·86–26·19) of the variance in overall IQ (Fig 3, Panel A). Of these predictors, SVI accounted for the greatest amount of variance (5·90%, 95% CI 1·26–13·37; P = 0·0004), followed by age at assessment (3·14%, 95% CI 0·19–9·33; P = 0·01), HU treatment (2·55%, 95%CI 0·08–8·36; P = 0·02) and HbF (2·51%, 95% CI 0·08–8·30; P = 0·02). For patients with HbSC/HbSβ+-thalassaemia, SVI accounted for the greatest amount of variance (7·41%, 95% CI 1·41–17·12; P = 0·001) followed by HU treatment status (4·28%, 95% CI 0·25–12·65; P = 0·01) (Fig 3, Panel B) in the final model.
Fig 3.
Variance in full-scale intelligence quotient (IQ) explained by biological, treatment, and demographic factors in patients with sickle cell disease separated by genotype. SVI, social vulnerability index; HbF, fetal haemoglobin; HU, current hydroxyurea treatment status. (A) Variance explained for patients with HbSS/HbSβ0-thalassaemia. (B) Variance explained for patients with HbSC/HbSβ+-thalassaemia. Separate models built for HbSS/HbSβ0-thalassaemia and HbSC/HbSβ+-thalassaemia. Age, SVI, sex, HU, HbF, total haemoglobin, platelet count, and haemoglobin oxygen saturation were included in the initial model. Covariates with significant associations at P < 0·10 were included in the multivariable analyses to obtain the final model using stepwise model selection with the final model only including variables reaching P< 0·05. Values are coefficient of determination (R2), calculated from linear regression model. The Full-Scale IQ was measured with the Wechsler Abbreviated Scale of Intelligence, Second Edition
Hydroxyurea mediation analyses of IQ
Among patients with HbSS/HbSβ0-thalassaemia, multivariate analyses revealed that HbF levels were positively associated with IQ (P = 0·02) after adjustment for age, SVI and HU treatment. In contrast, there was no effect of Hb, Hb oxygen saturation, WBC or platelet counts on IQ (P > 0·63). Therefore, we explored the effect of HU on IQ mediated by HbF. HbF mediated the effect of HU treatment on IQ (P = 0·03). There were also direct effects of HU on IQ (P = 0·03), after accounting for HbF. In contrast, no haematological indices were associated with IQ among patients with HbSC/HbSβ+-thalassaemia (all P > 0·35).
Discussion
In the present large cross-sectional analysis of patients with SCD, ranging from school age to young adulthood, worse neurocognitive performance was associated with increased social vulnerability and absence of HU treatment across genotypes. Patients with HbSS/HbSβ0-thalassaemia demonstrated worse performance with increasing age compared to normative expectations. In our present data, areas of neurocognitive weakness became most apparent in adolescence as patients prepare to transition to adulthood. Among those individuals treated with HU, the neurocognitive effects were reduced, but not eliminated.
Consistent with prior research,18,19 HU treatment is positively associated with neurocognitive performance in SCD. Across genotypes, those treated with HU at the time of their neurocognitive evaluation demonstrated an approximately 6-point elevation in IQ compared to their untreated peers. The effect of HU on neurocognition appeared to have different mechanisms that were dependent on the patient’s genotype. Among those with HbSS/HbSβ0-thalassaemia, the effect of HU was partially mediated by HbF, such that HU treatment was associated with increased HbF, which influenced neurocognitive functioning. Although HU reduces anaemia and improves oxygen delivery,15,39 our present data suggest an alternative pathway to impact cognition through increased HbF production without affecting the degree of anaemia. These results are consistent with prior studies that have observed a positive association between HbF and neurocognitive performance.9,40 In contrast, there was no relationship between HbF and IQ among patients with HbSC/HbSβ+-thalassaemia. Mechanisms to account for the relationship between HU treatment and neurocognitive functioning in HbSC/HbSβ+-thalassaemia require further research.
The present study is the first to demonstrate that the age of HU initiation is clinically meaningful. Patients with HbSS/HbSβ0-thalassaemia who started treatment at an older age displayed significantly poorer neurocognitive performance after adjustment for covariates, than those who initiated at an earlier age. Thus, beginning HU treatment earlier may mitigate some of the neurocognitive effects of SCD. HU displayed a protective effect across age groups and genotypes, offering support for improving access to HU treatment for all patients with SCD. Longitudinal research with a larger sample is needed to further explore these relationships.
Increased age was associated with poorer neurocognitive performance across most domains for patients with HbSS/HbSβ0-thalassaemia but not for patients with HbSC/HbSβ+-thalassaemia. The largest (negative) effects of age were seen on measures of academic knowledge and fluency. These findings likely reflect a combination of the neurological burden of SCD, large amounts of school absences and reduced academic resources. Children living in poverty, regardless of disease status, tend to show slowed development of academic skills and reduced academic attainment.41,42 Most of our present patients live within low-income households and attend schools that are underfunded and understaffed, placing them at risk of falling further behind academic expectations. The presence of chronic disease, such as haemophilia or cystic fibrosis, may increase risk of neurocognitive and academic difficulties; however, deficits generally do not significantly differ from normative expectations.43
The present study has several strengths including a large, representative, sample of patients that span school age to young adulthood. Extensive information on clinical characteristics was collected allowing for exploration of numerous predictors of neurocognitive functioning. The study included a wide range of neurocognitive outcomes that are sensitive to the effects of SCD. Yet, several limitations exist. Cross-sectional analyses limited the conclusions that could be drawn from our present data. Due to HU being standard of care, it was not possible/ethical to randomise patients to treatment approaches, so frequency matching was used to mitigate this limitation. Additionally, only patients with a clinical indication received neuroimaging. Based on previous research, it is likely that silent cerebral infarcts account for some of the variance in neurocognitive outcomes for these patients.44–46 The relationship between age, HU treatment and neurocognitive functioning may be altered after considering the presence of cerebral infarcts. Information on sociodemographic factors was also limited to the neighbourhood level, and we did not account for household factors such as family income or parental education, all potential predictors of neurocognitive functioning.7,47 Because we did not utilise a demographically matched or sibling-control group we cannot rule out the possibility that the age-related effects are primarily due to environmental factors rather than disease or treatment factors. In the future, we plan to collect more individualised sociodemographic data to better characterise the contribution of social determinants of health to neurocognitive outcomes in SCD.
To conclude, our present cross-sectional analyses suggest that patients with SCD demonstrate increasing neurocognitive deficits as they age, particularly among patients with HbSS/HbSβ0-thalassaemia. Early treatment with HU potentially provides some protection against the neurocognitive burden of SCD, although more research is needed to determine how the timing of this treatment affects outcomes. Despite assessing many relevant medical, treatment and demographic factors, we only captured a small amount of the variance in overall IQ. Accounting for neuroimaging and genetic factors is needed to better predict neurocognitive outcomes. Longitudinal studies that follow patients into adulthood are needed to fully understand neurocognitive functioning across the lifespan in SCD.
Supplementary Material
Table S1. Demographic comparison of participants with and without neurocognitive assessment.
Table S2. Effect of current hydroxyurea treatment status on neurocognitive performance.
Table S3. Characteristics of selected patients by hydroxyurea treatment in propensity score analysis.
Table S4. Duration of hydroxyurea exposure and age at first exposure effect on neurocognitive performance.
Acknowledgements
The authors thank Jason Hodges, PhD, Pei-Lin Chen, MPH, Courtney Mays, Erin MacArthur, MS, Madelene Wilson, Tiana Thomas, Ruth Johnson and Michelle Brignac, for support with data collection and regulatory matters.
Funding
Jane S. Hankins received funding from U01HL133996 during the conduct of this study. Allison A. King received funding from R01HL129241, K24HL148305, K12HL137942, U01HL143477 and 5U01HL133994 during the time of his study. Jerlym Porter was supported by K01HL125495 at the time of this project. This research was supported by the American Lebanese Syrian Associated Charities (ALSAC). Allison A. King and Jane S. Hankins receive research funding from Global Blood Therapeutics. Jane S. Hankins receives consultancy fees from Global Blood Therapeutics, Vindico Medical Education, UpToDate and bluebird bio. There is no other conflict of interest to report.
References
- 1.DeBaun MR, Armstrong FD, McKinstry RC, Ware RE, Vichinsky E, Kirkham FJ. Silent cerebral infarcts: a review on a prevalent and progressive cause of neurologic injury in sickle cell anemia. Blood. 2012;119:4587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gold JI, Johnson CB, Treadwell MJ, Hans N, Vichinsky E. Detection and assessment of stroke in patients with sickle cell disease: neuropsychological functioning and magnetic resonance imaging. Pediatr Hematol Oncol. 2008;25:409–21. [DOI] [PubMed] [Google Scholar]
- 3.Debaun MR, Derdeyn CP, McKinstry RC. Etiology of strokes in children with sickle cell anemia. Ment Retard Dev Disabil Res Rev. 2006;12:192–9. 10.1002/mrdd.20118. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong FD, Elkin TD, Brown RC, Glass P, Rana S, Casella JF, et al. Developmental function in toddlers with sickle cell anemia. Pediatrics. 2013;131:e406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown RT, Armstrong FD, Eckman JR. Neurocognitive aspects of pediatric sickle cell disease. J Learn Disabil. 1993;26:33–45. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Enos L, Gallagher D, Thompson R, Guarini L, Vichinsky E, et al. Neuropsychologic performance in school-aged children with sickle cell disease: a report from the Cooperative Study of Sickle Cell Disease. J Pediatr. 2001;139:391–7. [DOI] [PubMed] [Google Scholar]
- 7.King AA, Strouse JJ, Rodeghier MJ, Compas BE, Casella JF, McKinstry RC, et al. Parent education and biologic factors influence on cognition in sickle cell anemia. Am J Hematol. 2014;89:162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prussien KV, Siciliano RE, Ciriegio AE, Anderson AS, Sathanayagam R, DeBaun MR, et al. Correlates of cognitive function in sickle cell disease: a meta-analysis. J Pediatr Psychol. 2020;45:145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin S, Roderick MC, Abel C, Wolters P, Toledo-Tamula MA, Fitzhugh C, et al. Neurocognitive functioning in symptomatic adults with sickle cell disease: a description and comparison with unaffected siblings. Neuropsychol Rehabil. 2020;30:1666–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schatz J, Finke R, Roberts CW. Interactions of biomedical and environmental risk factors for cognitive development: a preliminary study of sickle cell disease. J Dev Behav Pediatr. 2004;25:303–10. [DOI] [PubMed] [Google Scholar]
- 11.Fitzhugh CD, Hsieh MM, Allen D, Coles WA, Seamon C, Ring M, et al. Hydroxyurea-increased fetal hemoglobin is associated with less organ damage and longer survival in adults with sickle cell anemia. PLoS One. 2015;10:e0141706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopes de Castro Lobo C, Pinto JF, Nascimento EM, Moura PG, Cardoso GP, Hankins JS. The effect of hydroxcarbamide therapy on survival of children with sickle cell disease. Br J Haematol. 2013;161:852–60. 10.1111/bjh.12323. [DOI] [PubMed] [Google Scholar]
- 13.Lê PQ, Gulbis B, Dedeken L, Dupont S, Vanderfaeillie A, Heijmans C, et al. Survival among children and adults with sickle cell disease in Belgium: benefit from hydroxyurea treatment. Pediatr Blood Cancer. 2015;62:1956–61. 10.1002/pbc.25608. [DOI] [PubMed] [Google Scholar]
- 14.Thornburg CD, Calatroni A, Panepinto JA. Differences in health related quality of life in children with sickle cell disease receiving hydroxyurea. J Pediatr Hematol Oncol. 2011;33:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmerman SA, Schultz WH, Burgett S, Mortier NA, Ware RE. Hydroxyurea therapy lowers transcranial Doppler flow velocities in children with sickle cell anemia. Blood. 2007;110:1043–7. 10.1182/blood2006-11-057893. [DOI] [PubMed] [Google Scholar]
- 16.Ware RE, Davis BR, Schultz WH, Brown RC, Aygun B, Sarnaik S, et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia—TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet. 2016;387:661–70. 10.1016/s0140-6736(15)01041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adegoke SA, Macedo-Campos RD, Braga JAP, Figueiredo MS, Silva GS. Changes in transcranial doppler flow velocities in children with sickle cell disease: the impact of hydroxyurea therapy. J Stroke Cerebrovasc Dis. 2018;27:425–31. 10.1016/j.jstrokecerebrovasdis.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Puffer E, Schatz J, Roberts CW. The association of oral hydroxyurea therapy with improved cognitive functioning in sickle cell disease. Child Neuropsychol. 2007;13:142–54. . [DOI] [PubMed] [Google Scholar]
- 19.Partanen M, Kang G, Wang WC, Krull K, King AA, Schreiber JE, et al. Association between hydroxycarbamide exposure and neurocognitive function in adolescents with sickle cell disease. Br J Haematol. 2020;189:1192–203. 10.1111/bjh.16519. [DOI] [PubMed] [Google Scholar]
- 20.Tarazi RA, Patrick KE, Iampietro M, Apollonsky N. Hydroxyurea use associated with nonverbal and executive skills in sickle cell anemia. J Pediatr Psychol. 2021. (Online ahead of print). 10.1093/jpepsy/jsab015 [DOI] [PubMed] [Google Scholar]
- 21.Hankins JS, Estepp JH, Hodges JR, Villavicencio MA, Robison LL, Weiss MJ, et al. Sickle cell clinical research and intervention program (SCCRIP): a lifespan cohort study for sickle cell disease progression from the pediatric stage into adulthood. Pediatr Blood Cancer. 2018;65:e27228. [DOI] [PubMed] [Google Scholar]
- 22.Cutter SL, Boruff BJ, Shirley WL. Social vulnerability to environmental hazards. Soc Sci Q. 2003;84:242–61. [Google Scholar]
- 23.Flanagan BE, Hallisey EJ, Adams E, Lavery A. Measuring community vulnerability to natural and anthropogenic hazards: the Centers for Disease Control and Prevention’s Social Vulnerability Index. J Environ Health. 2018;80:34. [PMC free article] [PubMed] [Google Scholar]
- 24.Yawn BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033–48. [DOI] [PubMed] [Google Scholar]
- 25.Luchtman-Jones L, Pressel S, Hilliard L, Brown RC, Smith MG, Thompson AA, et al. Effects of hydroxyurea treatment for patients with hemoglobin SC disease. Am J Hematol. 2016;91:238–42. [DOI] [PubMed] [Google Scholar]
- 26.Heeney MM, Ware RE. Hydroxyurea for children with sickle cell disease. Hematol Oncol Clin North Am. 2010;24:199–214. 10.1016/j.hoc.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estepp JH, Smeltzer MP, Kang G, Li C, Wang WC, Abrams C, et al. Aclinically meaningful fetal hemoglobin threshold for children with sickle cell anemia during hydroxyurea therapy. Am J Hematol. 2017;92:1333–9. 10.1002/ajh.24906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wechsler D. Wechsler abbreviated scale of intelligence. 2nd ed. Bloomington, MN: NCS Pearson; 2011. [Google Scholar]
- 29.Wechsler D. Wechsler adult intelligence scale–Fourth Edition (WAIS–IV). San Antonio, TX: The Psychological Corporation; 2014. [Google Scholar]
- 30.Wechsler D. Wechsler intelligence scale for children (WISC-IV). San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 31.Delis D. Delis-Kaplan executive function scale (D-KEFS). San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 32.Sheslow D, Adams W. Wide range assessment of memory and learning. 2nd ed. Lutz, FL: Psychological Assessment Resources; 2003. [Google Scholar]
- 33.Beery K, Buktenica N, Beery N. The Beery-Buktenica developmental test of visual-motor integration. 6th ed. Bloomington, MN: NCS Pearson; 2009. [Google Scholar]
- 34.Kløve H. Grooved pegboard test. Lafayette Instruments; 1963. [Google Scholar]
- 35.Woodcock R, McGrew K, Mather N. Woodcock-Johnson III tests of achievement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- 36.D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. [DOI] [PubMed] [Google Scholar]
- 37.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 38.Ho DE, Imai K, King G, Stuart EA. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal. 2007;15:199–236. 10.1093/pan/mpl013. [DOI] [Google Scholar]
- 39.Fields ME, Guilliams KP, Ragan D, Binkley MM, Mirro A, Fellah S, et al. Hydroxyurea reduces cerebral metabolic stress in patients with sickle cell anemia. Blood. 2019;133:2436–44. 10.1182/blood-2018-09-876318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruffieux N, Njamnshi AK, Wonkam A, Hauert CA, Chanal J, Verdon V, et al. Association between biological markers of sickle cell disease and cognitive functioning amongst Cameroonian children. Child Neuropsychol. 2013;19:143–60. [DOI] [PubMed] [Google Scholar]
- 41.Crosnoe R, Huston AC. Socioeconomic status, schooling, and the developmental trajectories of adolescents. Dev Psychol. 2007;43:1097. [DOI] [PubMed] [Google Scholar]
- 42.Duncan GJ, Magnuson KA. Can family socioeconomic resources account for racial and ethnic test score gaps? Future Child. 20·05;15:35–54. [DOI] [PubMed] [Google Scholar]
- 43.Moser JJ, Veale PM, McAllister DL, Archer DP. A systematic review and quantitative analysis of neurocognitive outcomes in children with four chronic illnesses. Pediatr Anesth. 2013;23:1084–96. 10.1111/pan.12255. [DOI] [PubMed] [Google Scholar]
- 44.Prussien KV, Jordan LC, DeBaun MR, Compas BE. Cognitive function in sickle cell disease across domains, cerebral infarct status, and the lifespan: a meta-analysis. J Pediatr Psychol. 2019;44:948–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schatz J, Brown R, Pascual J, Hsu L, DeBaun M. Poor school and cognitive functioning with silent cerebral infarcts and sickle cell disease. Neurology. 2001;56:1109–11. [DOI] [PubMed] [Google Scholar]
- 46.White DA, Moinuddin A, McKinstry RC, Noetzel M, Armstrong M, DeBaun M. Cognitive screening for silent cerebral infarction in children with sickle cell disease. J Pediatr Hematol Oncol. 2006;28:166–9. [DOI] [PubMed] [Google Scholar]
- 47.King AA, Rodeghier MJ, Panepinto JA, Strouse JJ, Casella JF, Quinn CT, et al. Silent cerebral infarction, income, and grade retention among students with sickle cell anemia. Am J Hematol. 2014;89:E188–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographic comparison of participants with and without neurocognitive assessment.
Table S2. Effect of current hydroxyurea treatment status on neurocognitive performance.
Table S3. Characteristics of selected patients by hydroxyurea treatment in propensity score analysis.
Table S4. Duration of hydroxyurea exposure and age at first exposure effect on neurocognitive performance.