Abstract
Maintaining fluid balance is critical for life. The central components that control fluid intake are only partly understood. This contribution to the collection of papers highlighting work by members of the Society for the Study of Ingestive Behavior focuses on the role that dopamine has on fluid intake and describes the roles that various bioregulators can have on thirst and sodium appetite by influencing dopamine systems in the brain. The goal of the review is to highlight areas in need of more research and to propose a framework to guide that research. We hope that this framework will inspire researchers in the field to investigate these interesting questions in order to form a more complete understanding of how fluid intake is controlled.
Keywords: Sodium appetite, Water intake, Thirst, Angiotensin II, Mesolimbic, Sex Differences
1. Introduction
Thirst is an incredibly potent experience. Most of us know what it feels like to be thirsty and have experienced the hedonic value that comes with a thirst-quenching drink. This guiding sensation and salient experience is important for survival because water and the balance of water and electrolytes in the body is essential for life. Given the importance of water in biological systems, in health, and in our everyday experiences, it is not surprising that there is an extensive literature describing the neural and hormonal controls of drinking.
For decades, the study of thirst has been dominated by the double-depletion model that was first introduced by Epstein and Fitzsimons [1, 2]. This framework has been helpful in conceptualizing the two main fluid compartments (thus, “double”) and the separate, but overlapping, responses to depletion of fluid in either compartment. Indeed, water in the body can be separated into that existing inside our cells (intracellular) or outside our cells (extracellular). A reduction of water in the former generally occurs by an increase in the solutes in the extracellular space, creating osmotic pressure that draws water from the inside of cells into the surrounding interstitial space, thereby reducing the fluid inside cells without a physiologically relevant effect on the volume of the extracellular space. With respect to the control of fluid intake, this type of imbalance is detected by specialized cells called osmoreceptors that are found in various regions of the brain, including the organum vasculosum of the lamina terminalis (OVLT) and the subfornical organ (SFO) [3]. In contrast, loss of extracellular fluid, which includes both water and electrolytes, decreases volume without affecting osmolality. This requires a separate detection mechanism that occurs by changes in baroreceptor activity, which respond to altered blood pressure and volume in the periphery, and stimulation of the renin-angiotensin system [4]. Because of these separate but overlapping systems, the effects of fluid loss to either compartment can be detected and the behavior that results (drinking) can help restore fluid balance to the system. Indeed, aside from medical intervention, drinking is the only way that most mammals can restore whole body fluid balance, providing a strong motivation to drink (i.e., thirst) and to consume sodium (i.e., salt/sodium appetite). This appetitive phase of intake is, whenever possible, followed by a strong consummatory phase during which animals ingest copious amounts of water and, in the case of extracellular fluid loss, salt. Figure 1 presents a basic framework to describe how fluid intake occurs.
Figure 1:

The phases of fluid intake. In response to, or in anticipation of, a loss of fluid, thirst and fluid seeking behaviors are engaged to defend homeostasis. These appetitive phase behaviors lead to the consummatory phase of intake, during which fluids are consumed. This intake of fluid provides feedback through orosensory and postingestive mechanisms that are associated with respective differences in the number of times rats will lick in a burst of licks and the number of bursts of licks rats will make at a spout. These orosensory and postingestive signals feed back to the appetitive phase to either enhance or suppress thirst and fluid seeking.
Although research on the mechanisms underlying thirst has been important to understand the life-sustaining behavior of drinking, these studies also serve as a very useful model of motivated behavior in more general terms. Thirst, in many ways, is quintessential motivation. The word “thirst” itself is synonymous with desire and motivation. The first recognized written use of the Old English equivalent of thirst was in reference to a strong desire in a more general sense than a specific desire for water [5]. Thus, “thirst” was about motivation even before it was used to describe the longing for water. Although the control of fluid intake has been studied for decades, we still lack a complete understanding of the circuits involved and how they interact with systems traditionally associated with motivation and reinforcement. Accordingly, for our contribution to this year’s collection of SSIB papers, we highlight the current understanding of the neural and hormonal regulation of thirst and the relevant circuits that guide motivated behaviors. Specifically, we focus on midbrain dopamine signaling and the mesolimbic system, mostly in studies using rats and mice as subjects, as a likely mediator of this behavior. We, therefore, build upon the framework presented in Figure 1 throughout this review in an effort to provide a model that describes how various bioregulators can affect dopamine signaling to control fluid intake. We believe this is an area ripe for further study and hope this review creates a ‘thirst’ for knowledge that drives future research.
2. Neural Substrates of Motivated Behavior and Fluid Intake
The neural substrates underlying motivated behavior include a variety of nuclei distributed throughout the brain, but in particular, the mesolimbic dopamine system plays a critical role in reward processing and motivated behavior. The classic mesolimbic dopamine system involves projections from the ventral tegmental area (VTA) of the midbrain to the nucleus accumbens (NAc), including the core (NAcC) and shell (NAcSh) subregions of the NAc [6, 7]. Dopamine neurons in the VTA also project to other targets, including the prefrontal cortex [8], amygdala [9], and hippocampus [10], and release other neurotransmitters such as glutamate [11], although discussion of this is outside the scope of the current review. “Reward” is a complex construct that includes several separable components [12]. Activity of the VTA dopamine neurons can influence a variety of factors related to reward including learning [13, 14], impulsivity [15], and emotional valence [16]. Dopaminergic signaling within the VTA-to-NAc circuit is commonly studied for its role in governing reward and reinforcement for several salient targets, including food, drugs of abuse, and water and salt [17–24]. A variety of neural and hormonal inputs influence the activity of the VTA and NAc, which will be discussed in detail later in this review.
Although the mesolimbic dopamine pathway is frequently associated with motivation and reward, the VTA is not the sole midbrain source of dopamine. The adjacent substantia nigra (SN), in particular the SN pars compacta, contains a robust population of dopaminergic neurons [25, 26]. These dopaminergic SN cells project to dorsal striatal targets, including the caudate nucleus and putamen, comprising the nigrostriatal pathway. This dopaminergic system also has a role in reward processing, and additionally, has a key role in motor function and movement (recently reviewed in [27]). Thus, when conceptualizing the neural substrates underlying motivated behavior such as drinking, it is important to consider the roles of both of these dopaminergic systems, especially for disentangling the role of dopaminergic signaling in the appetitive versus consummatory aspects of drinking behavior.
To form a complete understanding of the interface between thirst and brain circuits responsible for motivation, it is important to first understand the circuits that detect and otherwise respond to fluid perturbations. This discussion must include what is meant by ‘thirst’ in this context. Indeed, ‘thirst’ is a subjective experience that is more easily probed in humans, who can be asked questions about their experiences. In laboratory animals, these questions only can be asked indirectly by measuring if the animal drinks or does not drink, and if it drinks, how much does it consume. Although the amount consumed may be a valid proxy for the level of thirst, it is difficult to know for sure. Indeed, humans drink without reporting thirst and can be encouraged or otherwise motivated to drink without any of the same subjective feeling that we call ‘thirst.’ A contest to consume more water in order to win a prize would be an example of high levels of intake, without any necessity for ‘thirst’ to be experienced. In this sense, those of us working with laboratory animals must take a leap of faith that the rat is experiencing something subjective, at least similar in some ways to what humans call thirst, when a stimulus drives drinking. In addition, we must consider the nature of the stimulus and the type of depletion that leads to drinking. Although the double-depletion model includes separate but overlapping structures, for the purpose here, the separation is less important because both forms of depletion lead to the motivation in question. It is also important to note that, at least in the laboratory, the study of thirst and sodium appetite are inextricably linked. This is likely because of the double-depletion model and its origins, but also because in the laboratory, most studies on sodium intake have provided sodium in fluid form. This is different from how many animals encounter sodium in the wild, where it is more often associated with food than with hydration. Sodium, however, plays a key role in the balance between intracellular and extracellular fluid, making it more physiologically relevant for fluid intake.
Irrespective of differences in the response to intracellular or extracellular depletions and thirst with and without sodium appetite, the key structures involved are the forebrain circumventricular organs (SFO and OVLT), which directly or indirectly detect changes in hydration and have reciprocal connections with the median preoptic nucleus (MnPO) [28–31]. An evaluation of the anatomical connections between the SFO, OVLT, and MnPO with the mesolimbic and nigrostriatal structures described above serves as an initial step. Although early tracing studies reported projections from the MnPO to the VTA [32], refined approaches that specifically identified inputs to dopamine cells did not recapitulate this finding and did not report direct connections from the thirst-relevant circumventricular structures to midbrain dopamine cells [33]. Midbrain dopamine cells, however, receive robust inputs from structures such as the paraventricular nucleus and lateral hypothalamus, which are well-studied targets of the SFO, OVLT, and MnPO [30, 34–42]. Accordingly, a two-step relay represents the shortest link between these structures and dopamine cells in the midbrain. The functional relevance of these projections and which relays are relevant for thirst control remain important open questions.
In addition to forebrain-midbrain connections that may form links between thirst-relevant structures and dopamine cells, thirst and sodium appetite are also influenced to varying degrees by hindbrain inputs. Work by Geerling and colleagues, for example, has identified aldosterone-sensitive neurons in the caudal brainstem, specifically within the nucleus of the solitary tract (NTS), that project to a variety of targets including the VTA [43, 44]. This represents a direct pathway through which aldosterone tone, which correlates with sodium need, can interface with the VTA to modulate fluid intake. Additional feedback related to consumed fluids appears to reach the brain via vagus nerve afferents from the gut [45, 46] that presumably engage NTS neurons. Given that the NTS contains neurons that project to the VTA, this represents another means by which fluid-relevant signals can influence the VTA and engage dopamine systems that may be important for fluid intake control. In addition to direct NTS-to-VTA projections, signals are relayed through structures such as the parabrachial nucleus, which is critical for proper sodium intake, receives robust projections from the NTS, and projects to the VTA [43, 47–52]. This represents another possible pathway through which peripheral signals can affect sodium intake. How these systems function, and whether the modulation of dopamine signaling is one mechanism by which the act of drinking can decrease appetitive or consummatory phases of thirst responses, remains an important topic of investigation. A better understanding of the feedback from the gut and mouth also may help reconcile what currently appears to be a discrepancy in the literature. Specifically, gastric feedback was found to have a very strong influence on fluid intake in the mouse [45, 46], but thirst responses were largely unaffected by gastric bypass surgery in the rat [53]. Moreover, fluid by mouth was shown to be more satiating than fluid infused directly into the gut of rats [54]. More research on how gut feedback influences intake in both rat and mouse models could help reconcile this apparent discrepancy and could reveal ways that intake affects and is affected by dopamine systems.
3. Dopamine and Fluid Intake
3.1. Dopamine and Water Intake
Anecdotal evidence from clinical populations suggests that disrupted dopamine signaling is associated with changes in water intake. For example, decreased water intake has been reported in patients with Parkinson’s disease [55], while copious intake is observed in patients with schizophrenia [56, 57]. Seminal studies establishing a role for dopamine in the control of fluid intake demonstrated that central injections of dopamine increased water intake in hydrated rats, while the dopamine antagonist haloperidol inhibited water intake stimulated by overnight deprivation [58]. This early work also showed that after overnight food restriction, blocking dopamine signaling with haloperidol decreased water intake with no change in food intake, and increasing dopamine signaling by giving L-dopa and a dopamine β-hydroxylase inhibitor increased the drinking response, again with no change in food intake [58]. These findings are important because they demonstrate a selective effect of dopamine on fluid intake, and not a secondary change in fluid intake due to prandial drinking. Twenty years later, it was demonstrated that knockout of dopamine from dopamine producing neurons generated a mouse that drank very little water, unless the mouse was given L-dopa to reverse the impact of the knockout. These mice, however, also had significant motor impairments which calls into question the selectivity of the drinking effects [59]. The “dopamine secretion interference” (DSI) mouse [60], a more recent model that avoids the gross motor impairments that confounded other transgenic dopamine manipulations, have reduced dopamine secretion in the striatum and NAc. These mice drink less after water deprivation, and the reduced intake is a function of fewer licking bursts. This distinction, that the effect was selective to licking bursts, is important because differences in burst number or burst size (number of licks per burst) are correlated with the nature of the change in intake. Specifically, changes in burst size indicate an effect on the hedonic value of the substance being consumed whereas changes in burst number relate to postingestive consequences (e.g., satiation; [61, 62]). Accordingly, the selective effect on burst number in the DSI mouse suggests that the transgenic manipulation altered postingestive feedback. This is, perhaps, surprising given the well-established role that dopamine plays in reward. This is further complicated by the finding that D1 receptor agonism increases intake, without selective effects on burst number or burst size. Treatment with a D2/D3 agonist, however, reduces drinking by decreasing both burst number and burst size, suggesting at least some effect on the orosensory response to the consumed water [60].
In addition to pharmacological and transgenic manipulations of dopamine, changes in endogenous mesolimbic dopamine related to the physiological state of the animal and the consumption of fluids have been observed. Specifically, the Roitman group has demonstrated changes in phasic dopamine signaling in the mesolimbic system during fluid intake. Intraoral infusions of water in water deprived rats increased phasic dopamine release in the NAcSh [23]. Their more recent work has also demonstrated that learned cues associated with water modify dopamine signaling. In water restricted rats trained to associate a cue with water delivery, presentation of a cue that predicted water access increased dopamine activity in the VTA and dopamine release in the NAc [24].
3.2. Dopamine and Sodium Intake
Sodium consumption is also associated with dopamine signaling. For example, intraoral infusions of sodium in sodium deprived rats increases phasic dopamine release in the NAcSh [23]. Despite this evidence demonstrating a role of dopamine signaling in the control of sodium intake, there are mixed reports on whether dopamine stimulates or inhibits intake. For instance, in sodium deplete rats, nonselective dopamine receptor antagonism and selective D2 receptor antagonism reduces sham, but not real, intake of sodium [63]. In more recent support for this finding, under both sodium replete and deplete conditions, antagonism of either D1 or D2 receptors decreased sodium intake, but did so differently. Specifically, D1 antagonism caused a reduction in burst number, whereas changes in intake after D2 antagonism occurred through reductions in both burst number and size [64]. This supports the idea that fluid intake changes mediated by D2 receptors occur through alterations in both postingestive feedback and orosensory feedback. In further support of these findings, treatment with a D1 or D2 antagonist decreased sodium intake by water deprived rats. The reduction in intake after the D1 antagonist occurred through a decrease in burst number, while the D2 antagonist decreased both burst number and burst size [65]. These studies suggest that dopamine signaling through both D1 and D2 receptors increases sodium intake during dehydration or sodium depletion. In contrast to these results, optogenetic and chemogenetic activation of VTA dopamine neurons has been reported to decrease sodium intake in sodium-deprived mice [66]. The apparent discrepancy could be a species difference, or could relate to other neurotransmitters that are co-released with dopamine from the VTA (for review see [67]) that could mask the intake effects observed after selective D1/D2 activation or inhibition. Adding to the lack of clarity about the role of dopamine, work from the McEwen lab suggests that sodium appetite may be more directly related to changes in opiate systems [68, 69] than it is to changes in dopamine, although some studies from the same group found changes in dopamine transporter and tyrosine hydroxylase in the striatum that were associated with sodium appetite [70, 71]. The latter studies are supported by work from a separate group that found sodium appetite-associated decreases in dopamine transporter activity in the NAc [72]. These studies highlight the role of dopamine in both the appetitive and consummatory aspects of water and sodium intake, but as various manipulations of dopamine signaling produce differential effects on burst size and burst number, future studies will be needed to clarify the potentially important details.
4. Intake-relevant peptides as bioregulators of the dopamine system
Although it is important to consider the effects of dopamine alone on fluid intake, dopamine is not acting alone, but rather acts in concert with peptide bioregulators. In this section, we focus on the roles of several intake-relevant peptides on fluid intake and describe how these peptides may act to modulate dopamine signaling.
4.1. Angiotensin II
One mechanism by which dopamine controls fluid intake is through interactions with the renin-angiotensin system. Indeed, the angiotensin type 1 receptor (AT1R) and the components to synthesize endogenous angiotensin II (AngII), including angiotensinogen and angiotensin converting enzyme, are expressed within the mesolimbic and nigrostriatal pathways [73–78]. In vitro studies in striatal slices demonstrate that AngII stimulates dopamine release [79] and dopamine metabolite levels are increased by AngII or decreased by AT1R antagonist treatment, respectively [80]. Importantly, in vivo studies also demonstrate an excitatory effect of AngII on dopamine activity. AngII increases levels of dopamine metabolites in the striatum [81] and increases extracellular dopamine [82, 83]. All of these effects are blocked by the AT1R antagonist losartan, demonstrating a causative role of AngII. In hydrated rats treated with AngII, cues associated with water also stimulate dopamine activity in the VTA, similar to that observed during dehydration [24]. Chronic antagonism of AT1R, however, also increases dopamine metabolite levels in the striatum which may suggest a compensatory mechanism of the dopamine system [80]. Nevertheless, these studies show that central dopamine levels are influenced by the renin-angiotensin system.
Given the ability of AngII to augment dopamine release in vitro and in vivo, it is not surprising that dopamine signaling is related to the fluid intake effects of AngII. Central antagonism of dopamine signaling with either haloperidol or spiroperidol blocks AngII-stimulated water intake [58]. In addition, destruction of central dopamine neurons by lateral ventricle injection of oxidopamine (6-OHDA) reduces AngII-stimulated water intake [58, 84, 85] and selective lesions of the nigrostriatal pathway attenuate AngII-induced drinking [85]. Finally, selective lesions of the dopamine neurons in the SN reduce AngII-stimulated water intake [86]. Given the critical role of motor function in drinking behavior, it is not surprising that signaling loss within the nigrostriatal pathway impairs intake. Whether dopamine loss in the mesolimbic pathway also inhibits AngII-stimulated intake is unclear. Studies of this nature will be critical to help disentangle how dopamine-AngII interactions influence the appetitive and/or consummatory aspects of fluid intake.
4.2. Glucagon-like Peptide-1
In addition to our understanding of fluid intake control that has been gained by studying AngII, research on some peptide systems that have been more studied for their roles in food intake has helped build upon our knowledge of how fluid intake is controlled. In this respect, a more complete understanding of how these and other bioregulators interact with dopamine to impact fluid intake could be especially fruitful. One such bioregulator is the peptide glucagon-like peptide-1 (GLP-1). GLP-1, produced by the intestine and in the brain [87–89], is likely best known for its role in glycemic control. Indeed, GLP-1-based pharmacotherapies are used for the treatment of type 2 diabetes mellitus [90, 91]. GLP-1 also has well-studied hypophagic effects [92–95] and drugs that target this system have received approval for the treatment of obesity or are under investigation for this potential use [96]. In addition to the well-studied effects on food intake, activation of the GLP-1 receptor (GLP-1R) decreases water and sodium intakes [94, 97–100]. Importantly, this intake suppression persists in the absence of food [98], demonstrating that it occurs independent of the hypophagic effect of GLP-1 (e.g., [101–104]). Central injections of GLP-1R agonists decrease fluid intake [94, 97–100] and changes in GLP-1-relevant gene expression suggest a central, rather than peripheral site of action [105]. Moreover, unlike the increase in circulating GLP-1 observed when rats were allowed to eat after fasting, changes in circulating GLP-1 were not observed in rats allowed to drink after deprivation, supporting a primary role for the central GLP-1 system in fluid intake control [105].
The precise site(s) of action of GLP-1 within the central nervous system remains a topic of investigation. GLP-1R is expressed in a variety of structures associated with fluid intake and recent studies found that GLP-1R-expressing GABAergic cells in the MnPO are rapidly activated by drinking and inhibit SFO neurons [106]. Whether or not this is a target for endogenous GLP-1 in the control of thirst remains unclear, especially because direct injections of GLP-1 agonist into the MnPO fail to inhibit drinking ([106] and unpublished data). Within the central nervous system, there are two primary sources of GLP-1, the NTS and the olfactory bulb [107–109], with evidence for widespread projections originating from the NTS [107, 108, 110–112]. In the present context, the most notable locations of these projections are in areas involved in the regulation of ingestive behavior and motivation [108, 113–115]. The presence of GLP-1 fibers in the SFO and OVLT, and the demonstration of direct projections from the NTS to these areas, make them likely targets of endogenous hindbrain GLP-1 [111, 116]. In addition to the interface between hindbrain GLP-1 and the SFO and OVLT, GLP-1 fibers and receptors are present in the VTA [108, 111, 117]. Selective activation of GLP-1R in the VTA suppresses a variety of motivated behaviors, including food intake and drug self-administration [118–121]. Interactions of GLP-1 and dopamine systems in the control of fluid intake have received far less attention. Preliminary unpublished studies, however, found that injection of the GLP-1R agonist exendin-4 (Ex-4) directly into the VTA reduced drinking after water deprivation in male rats (Figure 2), indicating that GLP-1 can affect fluid intake by impacting reward-relevant circuits. Indeed, it is tempting to speculate that hindbrain GLP-1 affects thirst through collaterals to forebrain thirst-relevant structures and by affecting structures involved more directly in motivation. Additional research is needed, however, to test these hypotheses and to evaluate the effects that GLP-1 may have on appetitive and consummatory phases of fluid intake. Nevertheless, there is clear evidence that GLP-1 affects fluid intake and motivation, making it an interesting potential link between thirst and regions responsible for motivation and reward.
Figure 2:

Injection of a GLP-1R agonist decreases water intake in the absence of food. Approximately 30 min before dark onset, food was removed and rats (n=14) were given intra-VTA injection of either vehicle (300 nl 0.9% saline) or Ex-4 (5 ng). Water intake was measured for 24 h. The injections were repeated at least 2 d later to complete a repeated measures design that was counter-balanced for order of injection. An asterisk is used to indicate p<0.05 using a paired t-test to analyze data from rats with accurately placed injections (n=10). Analysis of the 4 rats with injections that were outside of the VTA found no effect of Ex-4 (p=0.78).
4.3. Amylin
Amylin is another feeding-related peptide that may serve as a link between fluid intake and motivated behavior. Amylin is produced both in the pancreas [122], where it is co-secreted with insulin from beta cells [123, 124], and in the brain [125–127]. A robust body of literature shows that central or peripheral administration of amylin or amylin agonists reduces food intake by enhancing satiation, and in turn reduces body weight [128–133]. In contrast, data describing the effects of amylin on water intake and fluid balance are relatively scarce. A few experiments have investigated the effect of amylin or amylin receptor agonists on fluid intake in rodents. Intraperitoneal administration of amylin had no effect on water intake in water-deprived animals [128]. Other research demonstrated that subcutaneous injection of amylin stimulated drinking in rats that were not water-deprived, but did not have access to food during testing [134]; however, it is important to note that a high dose of amylin was used in this study compared to other studies on amylin-induced hypophagia [128, 129, 135]. In terms of effects of centrally administered amylin, injection of amylin into the hypothalamus reduces water intake when food is available [136], perhaps due to the hypophagic effects of this treatment and therefore concomitant reductions in prandial drinking. The differences in direction of effect in these studies may be due to experimental variables such as the hydration status of the animal. Mesolimbic amylin signaling clearly suppresses motivated behaviors such as self-administration of palatable food [137], and some data, described in more detail below, also suggest that mesolimbic amylin signaling may suppress stimulated water intake [138].
Anatomical studies of the neural targets of amylin and amylin receptor signaling support a link with fluid intake control. The amylin receptor comprises a G-protein coupled component, the calcitonin receptor (CTR), in association with one of three receptor activity modifying proteins (RAMPs) which increase amylin binding specificity [139–141], and these elements are expressed in thirst-related structures. For instance, both CTR and RAMP are expressed in the SFO [142]. It is important, however, to note that CTR as well as RAMPs have other roles outside amylin signaling (reviewed in [143–146]) and the expression of these components does not necessarily indicate the existence of a functional amylin receptor. The existence of functional amylin receptors in the SFO is suggested by in vitro studies demonstrating that amylin can activate SFO neurons [147, 148]. The particular behavioral consequences of amylin-induced SFO activation remain unclear, as the SFO is involved not only in fluid intake but also feeding [149] and blood pressure regulation [150, 151]. Importantly, SFO neurons that are activated by both amylin and AngII have been identified [134], highlighting a potential mechanism by which amylin may influence fluid balance.
Amylin is known to have important roles in motivated behaviors such as intake of palatable foods or alcohol [137, 138, 152–155] that are likely related to modulation of dopamine signaling. Several pieces of evidence suggest that amylin receptor activation has a suppressive effect on mesolimbic dopamine signaling. Amylin receptor components are expressed in VTA dopaminergic neurons [152] as well as in the NAcC and NAcSh [137, 156] providing potential substrates by which amylin receptor activation can directly impact mesolimbic signaling. Fast scan cyclic voltammetry studies in rats demonstrate that intra-VTA injection of the amylin receptor agonist salmon calcitonin (sCT) reduces food-evoked phasic dopamine signaling in the NAcC [152]. Importantly, the reduction in NAcC dopamine appears to be relevant for amylin-mediated reductions in food intake, as pharmacological activation of dopamine receptors in the NAcC can blunt the hypophagic effects of intra-VTA injection of sCT [152]. In mice, microdialysis studies have revealed that systemic or intra-VTA sCT can suppress alcohol-evoked dopamine in the NAcSh [154, 155]. Importantly, sCT can penetrate the blood-brain barrier and access the VTA in rodents [154], suggesting that direct mesolimbic amylin receptor activation could potentially explain the ability of peripherally administered sCT to suppress NAc dopamine. It also has been suggested that a pathway involving other key amylin responsive sites in the hindbrain is required for the ability of peripheral sCT to suppress dopamine in the accumbens [157]. The particular site(s) of action for systemically delivered amylin agonists to affect mesolimbic dopamine signaling, therefore, remain to be fully elucidated. Nevertheless, these findings collectively point toward a suppressive effect of amylin and amylin agonists on dopamine signaling in the brain, and in particular mesolimbic dopamine.
A few experiments have specifically examined the role of amylin receptor signaling in mesolimbic nuclei in drinking. Intra-VTA administration of sCT blunted the dipsogenic effects of lateral ventricle injections of AngII [138], suggesting that VTA amylin signaling may influence on stimulated water intake. On the other hand, VTA sCT reduced drinking in the presence of food, but had no effect on water intake in the absence of food [138]. This suggests that, under these conditions, VTA amylin signaling has a primary effect on food intake with an indirect effect on prandial drinking. Collectively, these data may indicate that VTA amylin signaling has more robust effects on fluid intake when animals are highly motivated to consume fluid. To date, however, examinations of the effects of intra-VTA amylin receptor activation on water intake have been limited to influences on drinking stimulated by water deprivation or AngII. Therefore, it will be important in future experiments to determine whether VTA amylin signaling may also modulate drinking induced by other dipsogenic stimuli.
The effects of amylin receptor activation on water intake also have been tested in the NAcC and NAcSh subnuclei. In the NAcSh, infusion of amylin reduced water intake in water-deprived rats, but this effect was not observed when the cannulae were angled to avoid traversing the ventricles [158]. Injection of amylin into the NAcC had no effect on drinking in water-deprived rats [158]. Although these data would seem to indicate that amylin signaling in the accumbens does not play a role in drinking behavior, the potential role of NAcC/NAcSh amylin signaling in modulating water intake as a result of different types of dehydration is not well-studied. This is an important consideration because accumbens amylin signaling has been shown to modulate the feeding effects of the opioid system [159]. Therefore, it seems important to consider the possibility that amylin, although not producing observable effects on its own, may interact with other signals to produce changes in fluid intake.
Drawing conclusions about the effects of amylin on drinking is further complicated by the lack of consistency in the approaches used. Specifically, some studies use overnight water deprivation prior to testing [128] whereas others use non-water-deprived animals [134]. Furthermore, differences in the properties of amylin and sCT (discussed in [160]) add more complexity when interpreting results of different studies. Whether reductions in mesolimbic dopamine signaling are relevant for amylin-mediated effects on water intake, whether different paradigms that stimulate water intake may be differentially affected by amylin, and whether appetitive and/or consummatory aspects of fluid intake are impacted by amylin receptor activation are all areas of investigation that warrant further study.
4.4. Ghrelin
Ghrelin, a peptide hormone produced by the stomach [161], is unique among feeding-related hormones due to its effect of stimulating, rather than suppressing, food intake. Various routes of administration of ghrelin in rodents and humans produce a rapid increase in feeding via activation of the growth hormone secretagogue receptor (GHSR) [162–164]. Ghrelin is another hormone frequently associated with energy balance control that also has potent effects on fluid balance and water intake. Like amylin, ghrelin activates SFO neurons [147], demonstrating a link between ghrelin signaling and circumventricular structures involved with the control of drinking. Central or peripheral administration of ghrelin reduces water intake in rats [165–168], but the experimental conditions by which water intake is stimulated reveal different effects of this hormone under different experimental approaches. For example, water intake stimulated by central administration of AngII is reliably suppressed by lateral ventricle injection of ghrelin in one-bottle tests [166, 167], as well as in two-bottle tests, when rats have a choice of consuming water or saline [168]. Studies examining the effect of ghrelin on intake stimulated by water deprivation have differed, however, with some reporting no effect of ghrelin [166] and others reporting a suppressive effect of ghrelin [165]. The fluid intake effects of ghrelin are not limited to water, but extend to saline intake stimulated by AngII [168, 169] and also to other natriorexigenic stimuli such as maintenance on a sodium deficient diet [169]. Thus, ghrelin clearly plays a role in the control of fluid intake, but the site(s) of action and mechanisms underlying these effects remain unresolved. Hindbrain nuclei are likely involved, because 4th ventricle administration of ghrelin blocks the stimulatory effects of AngII on fluid intake [168]. Exploration of this effect remains limited to specific dipsogenic and/or natriorexigenic stimuli, making it difficult to draw firm conclusions about the effects of ghrelin in the many ways that thirst and sodium appetite can be induced.
Like other feeding-relevant hormones discussed here, ghrelin also influences mesolimbic dopamine signaling, although this is frequently studied in the context of food reward rather than effects on other reinforcers such as water or salt. GHSR is expressed in VTA dopamine neurons [170, 171] and ghrelin administration reliably increases the activity of these neurons [170]. VTA ghrelin increases operant responding for a palatable food [172, 173], underscoring the role of ghrelin in this site on motivated food-directed behavior. Pharmacological antagonism of dopamine receptors in the NAc blocks the ability of VTA ghrelin to increase operant responding for a palatable food [173], thus indicating the relevance of the classical VTA-to-NAc dopaminergic pathway in mediating these effects. Both peripheral and central administration of ghrelin have been demonstrated to impact mesolimbic dopamine signaling, and consistent with the excitatory effect of ghrelin on VTA dopamine neurons, ghrelin administration generally increases mesolimbic dopamine signaling. For example, peripheral injection of ghrelin increased dopamine in the NAcSh but not NAcC [174]. Several studies have indicated that administration of ghrelin into the lateral ventricle or directly into the VTA increases dopamine levels in the NAc in response to food-related stimuli [175–177]. Investigations of the mesolimbic dopamine response to ghrelin in the context of water intake are, however, relatively scarce in the literature. In euhydrated rats, lateral ventricle administration of ghrelin had no effect on phasic dopamine signaling in the VTA evoked by a water-associated cue [24]. Because this study did not investigate the effect of ghrelin when fluid balance was disrupted, it did not rule out the possibility that ghrelin alters dopamine signaling relevant to the salient stimulus in a motivated animal. In other words, an effect of ghrelin on dopamine may only be apparent at times when motivation to consume fluid is high. Indeed, mesolimbic dopamine signaling increases in response to hypertonic saline in sodium-deprived animals and in response to water in water-deprived animals [23], underscoring a link between motivation for fluid and dopaminergic transmission.
4.5. Other intake-relevant peptides
Many additional peptides are known to influence fluid intake, but in many cases it is more difficult to draw connections between these peptides and dopamine systems for control of drinking and salt intake. Vasopressin, for instance, is critically important in maintaining body fluid homeostasis. Although there are data suggesting that dopamine systems affect vasopressin [178–181], there does not seem to be substantial evidence that vasopressin affects dopamine, and available evidence for a relationship in that direction is likely related to functions of vasopressin that are separate from its role in fluid homeostasis [182]. Likewise, relaxin has been shown to affect fluid intake [183], but without an established connection to midbrain dopamine systems.
Other peptides are linked with both feeding and drinking, but investigation of how these peptides may modulate dopamine to influence fluid intake remains limited. For example, oxytocin has well-established suppressive effects on feeding [184–187] and generally has suppressive effects on fluid intake [185, 187–190]. Oxytocin can modulate mesolimbic dopamine signaling, however, much of this literature comes from investigations of social behavior [191] and modulation of the effects of drugs [192, 193]. A recent paper suggests that central administration of oxytocin decreases the VTA dopamine neuronal response to a palatable food [194], but VTA oxytocin receptor-expressing neurons are largely glutamatergic rather than dopaminergic [195], hinting at a complex system via which oxytocin may modulate mesolimbic signaling relevant to motivated behavior and reinforcement. This underscores the need for further research in this area, particularly as it pertains to ingestive behaviors and fluid balance. Another signal associated with both food and fluid intake is neurotensin. Lateral hypothalamic neurotensin neurons promote drinking while ablations reduce daily water intake, and chemogenetic activation increases ab libitum and dehydration induced water intake [196, 197]. Lateral hypothalamic neurotensin neurons which are activated by dehydration do not, however, project to either the VTA or SNc [198].
Although several key feeding-relevant peptides and their roles in drinking and motivated behavior have been reviewed here, there are additional feeding signals that have not been widely investigated for a potential role in fluid balance but are known to impact motivated behaviors and mesolimbic dopamine signaling. For example, cholecystokinin (CCK), a peptide produced in the intestine and brain that reduces feeding and promotes satiation [199–201], has been shown to influence mesolimbic dopamine signaling (reviewed in [202]) and also is linked to control of fluid balance. Recent evidence suggests that CCK is produced in the SFO and acts to suppress drinking [203], and peripheral administration of CCK decreases lever pressing to obtain water in water-deprived rodents [204], indicating a direct link between this peptide and water-directed motivated behavior. Another example is the adipose-derived hormone leptin, which promotes negative energy balance (reviewed in [205–207]) and modulates mesolimbic dopamine responses to food stimuli [208]. Although systemic leptin does not alter drinking in water-deprived animals [209], it is not clear whether effects might be observed with a different route of administration of leptin and/or a different stimulus for water intake. Overall, despite the fact that food intake and water intake are usually associated quite closely, investigation of the roles of feeding-related peptides in water intake and fluid balance remains limited. Because elements of the mesolimbic dopamine system are key targets for the feeding effects of many of these peptides, and because the mesolimbic dopamine system is linked to fluid intake as described above, it is critical to develop and test hypotheses about the sites of actions of these peptides and how they may interact with the actions of dopamine to influence drinking behavior. Furthermore, it will be important to determine how these different bioregulators influence similar neural pathways to elicit selective effects on feeding or drinking, depending on the physiological state of the animal.
5. Sex differences and estrogenic modulation of dopamine and fluid intake
Both organizational (early life) and activational (adult) effects of gonadal hormones influence the neuronal circuits that control drinking behavior, resulting in sex differences in intake and fluctuations in intake as a function of estradiol (E2) levels. Generally, sex and estrogens affect fluid intake stimulated by extracellular, but not intracellular, dehydration, but these studies have focused exclusively on the consummatory aspect of intake. For example, water intake stimulated by hypertonic saline treatment is not different between males and females [210–212] and intake in females is not influenced by E2 treatment [213–216]. Various dipsogenic stimuli are used to produce or mimic some of the effects of extracellular dehydration, and sex differences and estrogenic effects in the drinking responses have been well reported. While there are some discrepancies in the literature (reviewed in [211, 217]), males generally drink more water than females after treatment with the diuretic furosemide, the β-adrenergic agonist isoproterenol, and after exogenous AngII [212, 214, 218, 219]. Stimulated and daily saline intake, however, is often enhanced in females compared to males [218, 220–222]. In females, endogenous and exogenous E2 decreases daily water and saline intake, as well as intake stimulated by AngII and isoproterenol [213–215, 218, 223–231]. While these activational effects of E2 likely contribute to the sex difference in intake after extracellular fluid loss, organizational effects of gonadal hormones also contribute to sex differences in intake. For example, a feminized brain is required for E2 to reduce AngII-stimulated intake [214], and a masculinized brain is required for the behavioral desensitization of water intake after repeated AngII-treatment [232]. In addition, organizational effects of gonadal hormones underlie sex differences in sodium intake in both rats [222, 233, 234] and mice [235]. Finally, water intake stimulated by fluid deprivation, which is a combination of extracellular and intracellular dehydration, is also influenced by sex and estrogens. Although E2 treatment decreases intake stimulated by water deprivation [215], females, perhaps surprisingly, consume more after deprivation than males [211, 236], More research, however, on how sex and E2 affect the appetitive aspects of fluid intake is necessary for a complete understanding of their role in drinking behavior. Furthermore, the precise mechanisms by which organizational and activational effects of gonadal hormones control fluid intake are unclear and also require more research, but sex differences within the dopaminergic system could be part of the answer.
Estrogen and androgen receptors along with the sex determining region Y protein (SRY) are expressed within the mesolimbic and nigrostriatal dopamine systems [237–243]. It is, therefore, not surprising that there are sex differences and activational effects of gonadal hormones on dopamine synthesis, release, and receptor activity. The effects of sex and gonadal hormones have been extensively reviewed elsewhere [244–250], but far less has been reviewed with direct relevance to effects on dopamine signaling outside of drug seeking behavior. Adult females have approximately 15-20% fewer tyrosine hydroxylase (TH)-expressing neurons in the SN compared to age-matched males [243, 251]. This sex difference can also be observed in mesencephalic tissue from E14.5 day old mice [252], suggesting that chromosomal sex may mediate this difference. Indeed, the SRY gene is expressed in TH neurons in the SN and VTA in adult male rats [253] and mice [243], and in vitro studies demonstrate that the SRY gene increases TH transcription [253]. The four core genotype model reveals that sex chromosome complement and not gonadal sex mediates this sex difference in embryonic mice [252]. Finally, SRY downregulation in the SN decreases TH expression [243]. In the VTA, however, females have a greater number of TH immunoreactive cells than males, although the mechanism driving this sex differences in unknown [251]. Activational effects of E2 do influence dopamine synthesis in adult females, and may contribute to this sex difference. For example, acute E2 increases TH mRNA levels in dopaminergic cell bodies in the VTA, but not SN, in adult OVX female rats [254]. Furthermore, E2 can increase dopamine biosynthesis in striatal catecholaminergic terminals by rapidly enhancing phosphorylation of TH [255]. In addition to sex differences in dopamine synthesis, sex differences in dopamine projections also exist. In males, about 30% of midbrain to prefrontal projections are dopaminergic, whereas the proportion is about 50% in females [242]. Whether there are sex difference in projections to the NAc and what mechanism underlies this sex difference is, however, unknown.
Most evidence supporting the role of sex and gonadal hormones controlling dopamine function comes from studies examining dopamine release, which have been extensively reviewed recently [250]. Within the striatum, release stimulated by either electrical stimulation of the medial forebrain bundle, cocaine, or haloperidol results in a greater increase in extracellular dopamine in females compared to males [256, 257]. While the results from these early studies demonstrated that these sex differences are independent of estrous cycle stage, more recent work from Calipari et al. has shown that VTA activity and DA release in the NAc is increased in estrous females, compared to males and diestrous females [258]. The discrepancies here could be species-specific, because rats were used in the earlier studies compared to mice in the report by Calipari, or due to differences between brain regions. There is, however, overwhelming evidence that activational effects of E2 increase dopamine release. Extracellular basal dopamine levels in the striatum are greater in proestrous and estrous females, compared to diestrous and OVX females [259, 260]. E2 treatment increases striatal dopamine levels in OVX females, but not males [259, 260], suggesting a role for activational and organizational effects mediating differences between the sexes. Furthermore, loss of E2 increases striatal dopamine turnover, which can be reversed by E2 treatment, while gonadectomy in males has no effect [261]. E2 also increases striatal dopamine release stimulated by electrical stimulation in the SN and KCl-stimulation [262, 263]. Similar effects are observed in the NAc, where local E2 treatment increases K+ stimulated dopamine release [264]. Furthermore, amphetamine, methamphetamine, and cocaine-stimulated dopamine release in the striatum have all been shown to be enhanced by E2 treatment in females [260, 263, 265–269], with no effect of E2 treatment in males [266–268]. This again, suggests that a feminized brain in combination with activational effects of E2 underlie sex differences in dopamine release. Finally, while E2 appears to act locally on presynaptic terminals to enhance dopamine release [255], estrogen receptors on medium spiny GABA neurons also attenuate GABA release, thereby enhancing dopamine release by a decrease in inhibition [270–273].
Finally, sex and gonadal hormones influence the expression of dopamine receptors, but again there are conflicting reports on the direction of these effects. Males have a greater D1 receptor density in the NAc [274]. Striatal D1 receptor density, however, has been reported to be greater in males than females [275], but also, along with D2 receptor density, greater in females compared to males [276]. Females have a higher density of and more cells expressing the D1-D2 heterodimer complexes than males in the NAcC and caudate putamen [274]. These sex differences are likely mediated, at least in part, by E2. Early in vitro studies with striatal neurons demonstrated that E2 reduced D2 receptor inhibition of adenylate cyclase activity and enhanced D1 receptor activation of adenylate cyclase [277]. In vivo studies show that OVX decreases striatal D1 receptor density [275] and that E2 treatment increases striatal D1 levels [278] and decreases D2 binding in the caudal striatum in OVX females but not castrated males [279]. Not all available research, however, supports the notion that E2 enhances dopamine signaling through receptor mediated mechanisms. For example, the density of striatal D1 receptors is highest on diestrus I and II, compared to proestrus [275]. Furthermore, in contrast to the study by Bazzett et al. [279], it has also been reported that OVX decreases D2 binding in the striatum and NAcC, which can be reversed by E2 treatment [280]. Regardless of these discrepancies, there is still ample evidence that E2 enhances dopamine receptor activity, providing an indirect mechanism by which E2 can influence motivated behaviors.
Despite some inconsistencies, which are inherent with any large body of research, we can draw the general conclusions that while males may have more TH expressing neurons [243, 251, 252], likely due to SRY gene activity [243, 252, 253], E2 enhances release of dopamine, and perhaps increases dopamine receptor expression in females [259, 260, 262–264]. Because modulation of dopamine signaling by sex and E2 occurs in both the mesolimbic and the nigrostriatal pathways, it is tempting to speculate that there are effects on both appetitive and consummatory aspects of fluid intake. Given that studies to date have focused almost entirely on the consummatory phase of fluid intake, studies of appetitive phase intake are critically needed to explore this possibility.
If dopamine is a primary dipsogen or acts in concert with other bioregulators to promote fluid intake, how might this system underlie sex differences or E2 effects on intake? In scenarios where intake is greater in males than in females, such as after AngII treatment [214, 218], the greater number of TH neurons in males, compared to females [243, 251, 252], may be an underlying mechanism. In scenarios where intake is greater in females than it is in males, such as after water deprivation [211, 236], enhanced dopamine release in females compared to males may be an underlying mechanism. The most well-characterized sex hormone-related effects on fluid intake, however, are the anti-dipsogenic and anti-natriorexigenic effects of E2. Given that E2 enhances dopamine signaling, and dopamine increases water intake, how can this be reconciled with the inhibitory effect of E2? The answer may be found by considering other bioregulators that are co-released with dopamine within the mesolimbic and nigrostriatal pathways that may provide an indirect way for E2 to influence dopamine signaling and, in turn, fluid intake. For instance, CCK is co-released with dopamine and has been shown to inhibit K+ stimulated dopamine release in the rostral NAc [281, 282]. It has been previously suggested that the anorexigenic effect of E2 involves a CCK-dopamine interaction [247]. Indeed, CCK also inhibits fluid intake [203, 283, 284], however, whether E2 potentiates its anti-dipsogenic effect is unknown and a ripe area for future research. As is unfortunately true with most neuronal functions, much more work is needed to understand how sex and gonadal hormones control fluid intake and if interactions with the dopamine system underlie these effects.
6. Conclusions
Dopamine plays a critical role in motivated behavior, but has not received nearly enough attention with respect to the neural controls of thirst and sodium appetite. Here we have reviewed available literature related to dopamine and fluid intake control, and present several suggestions for future research. Investigating bioregulators that influence fluid intake offers an excellent opportunity to provide a more complete understanding of how dopamine drives motivation for water and sodium and how thirst and sodium appetite are controlled. Specifically, we have highlighted the potential roles of AngII, GLP-1, amylin, ghrelin, sex, and E2 as important regulators of the dopaminergic system, and have discussed how interfacing with dopamine can ultimately affect fluid intake. This is not to say that these bioregulators cannot affect fluid intake independent of their effects on dopamine, but that interaction of these factors with dopamine offers a relatively unexplored mechanism for fluid intake control. In Figure 3, we build upon the framework illustrated in Figure 1 to propose a guide for future studies investigating the cooperative relationships between dopamine, bioregulators, and fluid intake. In particular, we believe it is important to focus on disentangling the contributions of mesolimbic and nigrostriatal dopamine signaling in the control of the appetitive versus consummatory aspects of fluid intake. We hope that the questions posed in this review will inspire the field to investigate this important yet under investigated area of research.
Figure 3:
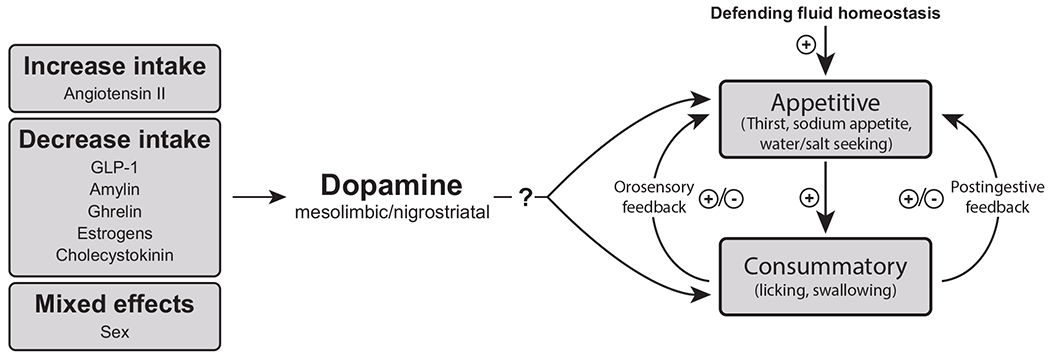
A proposed framework for conceptualizing the role of dopamine in thirst and sodium appetite. The model is not intended to be inclusive, but instead focuses on the influence that a variety of bioregulators (hormones, transmitters, and sex) have on dopamine and how that influence on dopamine systems can affect both appetitive and consummatory phases of intake. Research is needed to better understand and disentangle the roles of mesolimbic and nigrostriatal pathways and specifics related to the appetitive versus consummatory aspects of intake.
Acknowledgements
We thank Jessica Tabman for collecting the data used to generate Figure 2 and we are grateful that the editors invited us to contribute to this issue. This work was supported by a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation and a University at Buffalo Center for Ingestive Behavior Research Pilot Grant (EGM-B), NSF Grant 2019346 (JS), and NIH DK107500 (DD). EGM-B has received funding from Zealand Pharma and Boehringer-Ingelheim that was not used in support of this work. The order of appearance of the co-first authors was determined by a game of Buffalo themed trivia, in which Dr. Mietlicki-Baase decisively defeated Dr. Santollo by a score of 7-2.
References
- [1].Epstein AN Epilogue: Retrospect and prognosis. In: Epstein AN, Kissileff HR, Stellar E, ed. The Neuropsychology of Thirst: New Findings and Advances in Concepts. Washington, DC: V.H. Winston & Sons; 1973. p. 315–32. [Google Scholar]
- [2].Fitzsimons JT Some historical perspectives in the physiology of thirst. In: Epstein AN, Kissileff HR, Stellar E, ed. The Neuropsychology of Thirst: New Findings and Advances in Concepts. Washington, DC: V.H. Winston & Sons; 1973. p. 3–33. [Google Scholar]
- [3].Bourque CW, Oliet SH Osmoreceptors in the central nervous system. Annu Rev Physiol. 1997, 59:601–19. [DOI] [PubMed] [Google Scholar]
- [4].Daniels D Neurobehavioral Studies of Thirst. Reference Module in Neuroscience and Biobehavioral Psychology: Elsevier; 2020. [Google Scholar]
- [5].OED Online, “thirst, v.”. Oxford English Dictionary: Oxford University Press; 2020. www.oed.com/view/Entry/200874. Accessed 4 January 2021. [Google Scholar]
- [6].Fallon JH, Moore RY Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978, 180:545–80. [DOI] [PubMed] [Google Scholar]
- [7].Rodriguez-Lopez C, Clasca F, Prensa L The Mesoaccumbens Pathway: A Retrograde Labeling and Single-Cell Axon Tracing Analysis in the Mouse. Front Neuroanat. 2017, 11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Berger B, Thierry AM, Tassin JP, Moyne MA Dopaminergic innervation of the rat prefrontal cortex: a fluorescence histochemical study. Brain Res. 1976, 106:133–45 [DOI] [PubMed] [Google Scholar]
- [9].Fallon JH, Koziell DA, Moore RY Catecholamine innervation of the basal forebrain. II. Amygdala, suprarhinal cortex and entorhinal cortex. J Comp Neurol. 1978, 180:509–32. [DOI] [PubMed] [Google Scholar]
- [10].Scatton B, Simon H, Le Moal M, Bischoff S Origin of dopaminergic innervation of the rat hippocampal formation. Neurosci Lett. 1980, 18:125–31. [DOI] [PubMed] [Google Scholar]
- [11].Zhang S, Qi J, Li X, Wang HL, Britt JP, Hoffman AF, et al. Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nat Neurosci. 2015, 18:386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Berridge KC, Robinson TE Parsing reward. Trends Neurosci. 2003, 26:507–13. [DOI] [PubMed] [Google Scholar]
- [13].Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci. 2013, 16:966–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Salinas-Hernandez XI, Vogel P, Betz S, Kalisch R, Sigurdsson T, Duvarci S Dopamine neurons drive fear extinction learning by signaling the omission of expected aversive outcomes. Elife. 2018, 7:e38818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Winstanley CA, Zeeb FD, Bedard A, Fu K, Lai B, Steele C, et al. Dopaminergic modulation of the orbitofrontal cortex affects attention, motivation and impulsive responding in rats performing the five-choice serial reaction time task. Behav Brain Res. 2010, 210:263–72. [DOI] [PubMed] [Google Scholar]
- [16].Lammel S, Ion DI, Roeper J, Malenka RC Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011, 70:855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Brown HD, McCutcheon JE, Cone JJ, Ragozzino ME, Roitman MF Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur J Neurosci. 2011, 34:1997–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Meye FJ, Adan RA Feelings about food: the ventral tegmental area in food reward and emotional eating. Trends Pharmacol Sci. 2014, 35:31–40. [DOI] [PubMed] [Google Scholar]
- [19].Halbout B, Marshall AT, Azimi A, Liljeholm M, Mahler SV, Wassum KM, et al. Mesolimbic dopamine projections mediate cue-motivated reward seeking but not reward retrieval in rats. Elife. 2019, 8:e43551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ostlund SB, LeBlanc KH, Kosheleff AR, Wassum KM, Maidment NT Phasic mesolimbic dopamine signaling encodes the facilitation of incentive motivation produced by repeated cocaine exposure. Neuropsychopharmacology. 2014, 39:2441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu Y, Jean-Richard-Dit-Bressel P, Yau JO, Willing A, Prasad AA, Power JM, et al. The Mesolimbic Dopamine Activity Signatures of Relapse to Alcohol-Seeking. J Neurosci. 2020, 40:6409–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Thomas MJ, Kalivas PW, Shaham Y Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008, 154:327–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fortin SM, Roitman MF Challenges to Body Fluid Homeostasis Differentially Recruit Phasic Dopamine Signaling in a Taste-Selective Manner. J Neurosci. 2018, 38:6841–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hsu TM, Bazzino P, Hurh SJ, Konanur VR, Roitman JD, Roitman MF Thirst recruits phasic dopamine signaling through subfornical organ neurons. Proc Natl Acad Sci U S A. 2020, 117:30744–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Guyenet PG, Aghajanian GK Antidromic identification of dopaminergic and other output neurons of the rat substantia nigra. Brain Res. 1978, 150:69–84. [DOI] [PubMed] [Google Scholar]
- [26].Shimada S, Ishikawa M, Tanaka C Histochemical mapping of dopamine neurons and fiber pathways in dog mesencephalon. J Comp Neurol. 1976, 168:533–43. [DOI] [PubMed] [Google Scholar]
- [27].Sonne J, Reddy V, Beato MR Neuroanatomy, Substantia Nigra. StatPearls. Treasure Island (FL): StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- [28].Camacho A, Phillips MI Horseradish peroxidase study in rat of the neural connections of the organum vasculosum of the lamina terminalis. Neurosci Lett. 1981, 25:201–4. [DOI] [PubMed] [Google Scholar]
- [29].Lind RW, Van Hoesen GW, Johnson AK An HRP study of the connections of the subfornical organ of the rat. J Comp Neurol. 1982, 210:265–77. [DOI] [PubMed] [Google Scholar]
- [30].Miselis RR The efferent projections of the subfornical organ of the rat: a circumventricular organ within a neural network subserving water balance. Brain Res. 1981, 230:1–23. [DOI] [PubMed] [Google Scholar]
- [31].Saper CB, Levisohn D Afferent connections of the median preoptic nucleus in the rat: anatomical evidence for a cardiovascular integrative mechanism in the anteroventral third ventricular (AV3V) region. Brain Res. 1983, 288:21–31. [DOI] [PubMed] [Google Scholar]
- [32].Chiba T, Murata Y Afferent and efferent connections of the medial preoptic area in the rat: a WGA-HRP study. Brain Res Bull. 1985, 14:261–72. [DOI] [PubMed] [Google Scholar]
- [33].Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012, 74:858–73. [DOI] [PubMed] [Google Scholar]
- [34].McKinley MJ, Allen AM, May CN, McAllen RM, Oldfield BJ, Sly D, et al. Neural pathways from the lamina terminalis influencing cardiovascular and body fluid homeostasis. Clin Exp Pharmacol Physiol. 2001, 28:990–2. [DOI] [PubMed] [Google Scholar]
- [35].Anderson JW, Smith PM, Ferguson AV Subfornical organ neurons projecting to paraventricular nucleus: whole-cell properties. Brain Res. 2001, 921:78–85. [DOI] [PubMed] [Google Scholar]
- [36].Ferguson AV Angiotensinergic regulation of autonomic and neuroendocrine outputs: critical roles for the subfornical organ and paraventricular nucleus. Neuroendocrinology. 2009, 89:370–6. [DOI] [PubMed] [Google Scholar]
- [37].Uschakov A, Gong H, McGinty D, Szymusiak R Efferent projections from the median preoptic nucleus to sleep- and arousal-regulatory nuclei in the rat brain. Neuroscience. 2007, 150:104–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tanaka J, Nomura M, Kariya K, Nishimura J, Kimura F Median preoptic neurons projecting to the hypothalamic paraventricular nucleus are sensitive to blood pressure changes. Brain Res. 1993, 605:338–41. [DOI] [PubMed] [Google Scholar]
- [39].Li Z, Ferguson AV Subfornical organ efferents to paraventricular nucleus utilize angiotensin as a neurotransmitter. Am J Physiol. 1993, 265:R302–9. [DOI] [PubMed] [Google Scholar]
- [40].McKinley MJ, Bicknell RJ, Hards D, McAllen RM, Vivas L, Weisinger RS, et al. Efferent neural pathways of the lamina terminalis subserving osmoregulation. Prog Brain Res. 1992, 91:395–402. [DOI] [PubMed] [Google Scholar]
- [41].Tanaka J, Kaba H, Saito H, Seto K Subfornical organ efferents influence the activity of median preoptic neurons projecting to the hypothalamic paraventricular nucleus in the rat. Exp Neurol. 1986, 93:647–51. [DOI] [PubMed] [Google Scholar]
- [42].Miselis RR The subfornical organ’s neural connections and their role in water balance. Peptides. 1982, 3:501–2. [DOI] [PubMed] [Google Scholar]
- [43].Geerling JC, Loewy AD Aldosterone-sensitive neurons in the nucleus of the solitary: efferent projections. J Comp Neurol. 2006, 498:223–50. [PubMed] [Google Scholar]
- [44].Gasparini S, Resch JM, Narayan SV, Peltekian L, Iverson GN, Karthik S, et al. Aldosterone-sensitive HSD2 neurons in mice. Brain Struct Funct. 2019, 224:387–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kim DY, Heo G, Kim M, Kim H, Jin JA, Kim HK, et al. A neural circuit mechanism for mechanosensory feedback control of ingestion. Nature. 2020, 580:376–80. [DOI] [PubMed] [Google Scholar]
- [46].Zimmerman CA, Huey EL, Ahn JS, Beutler LR, Tan CL, Kosar S, et al. A gut-to-brain signal of fluid osmolarity controls thirst satiation. Nature. 2019, 568:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Herbert H, Moga MM, Saper CB Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990, 293:540–80. [DOI] [PubMed] [Google Scholar]
- [48].Tokita K, Inoue T, Boughter JD Jr. Afferent connections of the parabrachial nucleus in C57BL/6J mice. Neuroscience. 2009, 161:475–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Boughter JD Jr., Lu L, Saites LN, Tokita K Sweet and bitter taste stimuli activate VTA projection neurons in the parabrachial nucleus. Brain Res. 2019, 1714:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Qiao Y, Zhang CK, Li ZH, Niu ZH, Li J, Li JL Collateral Projections from the Lateral Parabrachial Nucleus to the Central Amygdaloid Nucleus and the Ventral Tegmental Area in the Rat. Anat Rec (Hoboken). 2019, 302:1178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Miller RL, Stein MK, Loewy AD Serotonergic inputs to FoxP2 neurons of the pre-locus coeruleus and parabrachial nuclei that project to the ventral tegmental area. Neuroscience. 2011, 193:229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Shin JW, Geerling JC, Stein MK, Miller RL, Loewy AD FoxP2 brainstem neurons project to sodium appetite regulatory sites. J Chem Neuroanat. 2011, 42:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Marshall A, Santollo J, Corteville C, Lutz TA, Daniels D Roux-en-Y gastric bypass does not affect daily water intake or the drinking response to dipsogenic stimuli in rats. Am J Physiol Regul Integr Comp Physiol. 2014, 307:R114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Miller NE, Sampliner RI, Woodrow P Thirst-reducing effects of water by stomach fistula vs. water by mouth measured by both a consummatory and an instrumental response. J Comp Physiol Psychol. 1957, 50:1–5. [DOI] [PubMed] [Google Scholar]
- [55].Ueki A, Otsuka M Life style risks of Parkinson’s disease: association between decreased water intake and constipation. J Neurol. 2004, 251 Suppl 7:vII18–23. [DOI] [PubMed] [Google Scholar]
- [56].Vieweg WV, David JJ, Rowe WT, Wampler GJ, Burns WJ, Spradlin WW Death from self-induced water intoxication among patients with schizophrenic disorders. J Nerv Ment Dis. 1985, 173:161–5. [DOI] [PubMed] [Google Scholar]
- [57].de Leon J, Verghese C, Tracy JI, Josiassen RC, Simpson GM Polydipsia and water intoxication in psychiatric patients: a review of the epidemiological literature. Biol Psychiatry. 1994, 35:408–19. [DOI] [PubMed] [Google Scholar]
- [58].Fitzsimons JT, Setler PE The relative importance of central nervous catecholaminergic and cholinergic mechanisms in drinking in response to antiotensin and other thirst stimuli. J Physiol. 1975, 250:613–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhou QY, Palmiter RD Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995, 83:1197–209. [DOI] [PubMed] [Google Scholar]
- [60].Kao KC, Hisatsune T Differential effects of dopamine D1-like and D2-like receptor agonists on water drinking behaviour under thirsty conditions in mice with reduced dopamine secretion. Eur J Neurosci. 2020, 51:584–97. [DOI] [PubMed] [Google Scholar]
- [61].Davis JD The microstructure of ingestive behavior. Ann N Y Acad Sci. 1989, 575:106–19; discussion 20-1. [DOI] [PubMed] [Google Scholar]
- [62].Davis JD, Smith GP, Singh B A microstructural analysis of the control of water and isotonic saline ingestion by postingestional stimulation. Physiol Behav. 1999, 66:543–8. [DOI] [PubMed] [Google Scholar]
- [63].Roitman MF, Schafe GE, Thiele TE, Bernstein IL Dopamine and sodium appetite: antagonists suppress sham drinking of NaCl solutions in the rat. Behav Neurosci. 1997, 111:606–11. [DOI] [PubMed] [Google Scholar]
- [64].D’Aquila PS, Rossi R, Rizzi A, Galistu A Possible role of dopamine D1-like and D2-like receptors in behavioural activation and “contingent” reward evaluation in sodium-replete and sodium-depleted rats licking for NaCl solutions. Pharmacol Biochem Behav. 2012, 101:99–106. [DOI] [PubMed] [Google Scholar]
- [65].Galistu A, D’Aquila PS Effect of the dopamine D1-like receptor antagonist SCH 23390 on the microstructure of ingestive behaviour in water-deprived rats licking for water and NaCl solutions. Physiol Behav. 2012, 105:230–3. [DOI] [PubMed] [Google Scholar]
- [66].Sandhu EC, Fernando ABP, Irvine EE, Tossell K, Kokkinou M, Glegola J, et al. Phasic Stimulation of Midbrain Dopamine Neuron Activity Reduces Salt Consumption. eNeuro. 2018, 5:ENEURO.0064-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Morales M, Margolis EB Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci. 2017, 18:73–85. [DOI] [PubMed] [Google Scholar]
- [68].Lucas LR, Pompei P, Ono J, McEwen BS Effects of adrenal steroids on basal ganglia neuropeptide mRNA and tyrosine hydroxylase radioimmunoreactive levels in the adrenalectomized rat. J Neurochem. 1998, 71:833–43. [DOI] [PubMed] [Google Scholar]
- [69].Lucas LR, Grillo CA, McEwen BS Salt appetite in sodium-depleted or sodium-replete conditions: possible role of opioid receptors. Neuroendocrinology. 2007, 85:139–47. [DOI] [PubMed] [Google Scholar]
- [70].Lucas LR, Grillo CA, McEwen BS Involvement of mesolimbic structures in short-term sodium depletion: in situ hybridization and ligand-binding analyses. Neuroendocrinology. 2003, 77:406–15. [DOI] [PubMed] [Google Scholar]
- [71].Lucas LR, Pompei P, McEwen BS Salt appetite in salt-replete rats: involvement of mesolimbic structures in deoxycorticosterone-induced salt craving behavior. Neuroendocrinology. 2000, 71:386–95. [DOI] [PubMed] [Google Scholar]
- [72].Roitman MF, Patterson TA, Sakai RR, Bernstein IL, Figlewicz DP Sodium depletion and aldosterone decrease dopamine transporter activity in nucleus accumbens but not striatum. Am J Physiol. 1999, 276:R1339–45. [DOI] [PubMed] [Google Scholar]
- [73].Lynch KR, Hawelu-Johnson CL, Guyenet PG Localization of brain angiotensinogen mRNA by hybridization histochemistry. Brain Res. 1987, 388:149–58. [DOI] [PubMed] [Google Scholar]
- [74].Thomas WG, Sernia C Immunocytochemical localization of angiotensinogen in the rat brain. Neuroscience. 1988, 25:319–41. [DOI] [PubMed] [Google Scholar]
- [75].Bunnemann B, Fuxe K, Metzger R, Bjelke B, Ganten D The semi-quantitative distribution and cellular localization of angiotensinogen mRNA in the rat brain. J Chem Neuroanat. 1992, 5:245–62. [DOI] [PubMed] [Google Scholar]
- [76].Paz MC, Marchese NA, Stroppa MM, Gerez de Burgos NM, Imboden H, Baiardi G, et al. Involvement of the brain renin-angiotensin system (RAS) in the neuroadaptive responses induced by amphetamine in a two-injection protocol. Behav Brain Res. 2014, 272:314–23. [DOI] [PubMed] [Google Scholar]
- [77].Chai SY, McKinley MJ, Mendelsohn FA Distribution of angiotensin converting enzyme in sheep hypothalamus and medulla oblongata visualized by in vitro autoradiography. Clin Exp Hypertens A. 1987, 9:449–60. [DOI] [PubMed] [Google Scholar]
- [78].Simonnet G, Giorguieff-Chesselet MF, Carayon A, Bioulac B, Cesselin F, Glowinski J, et al. [Angiotensin II and nigostriatal system (author’s transl)]. J Physiol (Paris). 1981, 77:71–9. [PubMed] [Google Scholar]
- [79].Simonnet G, Giorguieff-Chesselet MF Stimulating effect of angiotensin II on the spontaneous release of newly synthetized [3H]dopamine in rat striatal slices. Neurosci Lett. 1979, 15:153–8. [DOI] [PubMed] [Google Scholar]
- [80].Dwoskin LP, Jewell AL, Cassis LA DuP 753, a nonpeptide angiotensin II-1 receptor antagonist, alters dopaminergic function in rat striatum. Naunyn Schmiedebergs Arch Pharmacol. 1992, 345:153–9. [DOI] [PubMed] [Google Scholar]
- [81].Mendelsohn FA, Jenkins TA, Berkovic SF Effects of angiotensin II on dopamine and serotonin turnover in the striatum of conscious rats. Brain Res. 1993, 613:221–9. [DOI] [PubMed] [Google Scholar]
- [82].Brown DC, Steward LJ, Ge J, Barnes NM Ability of angiotensin II to modulate striatal dopamine release via the AT1 receptor in vitro and in vivo. Br J Pharmacol. 1996, 118:414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Braszko JJK,G; Witczuk B; Wisniewski K Angiotensin II, some of its fragments and salarasin affect dopamine in striatum but not in olfactory tubercle. Pharmacological Research. 1992, 25:9–10.1324492 [Google Scholar]
- [84].Gordon FJ, Brody MJ, Fink GD, Buggy J, Johnson AK Role of central catecholamines in the control of blood pressure and drinking behavior. Brain Res. 1979, 178:161–73. [DOI] [PubMed] [Google Scholar]
- [85].Sumners C, Woodruff GN, Poat JA Effects of specific dopamine lesions and dopamine receptor sensitivity on angiotensin II- and carbachol-induced thirst in rats. Psychopharmacology (Berl). 1981, 73:180–3. [DOI] [PubMed] [Google Scholar]
- [86].Gordon FJ, Brody MJ, Johnson AK Regional depletion of central nervous system catecholamines: effects on blood pressure and drinking behavior. Brain Res. 1985, 345:285–97. [DOI] [PubMed] [Google Scholar]
- [87].Holst JJ The physiology of glucagon-like peptide 1. Physiol Rev. 2007, 87:1409–39. [DOI] [PubMed] [Google Scholar]
- [88].Daniels D, Mietlicki-Baase EG Glucagon-Like Peptide 1 in the Brain: Where Is It Coming From, Where Is It Going? Diabetes. 2019, 68:15–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].McLean BA, Wong CK, Campbell JE, Hodson DJ, Trapp S, Drucker DJ Revisiting the complexity of GLP-1 action-from sites of synthesis to receptor activation. Endocr Rev. 2020,bnaa032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lovshin JA, Drucker DJ Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009, 5:262–9. [DOI] [PubMed] [Google Scholar]
- [91].Dahiya L, Kaur R, Kumar R, Kumar M, Palta K GLP-1 Receptor Agonists in Type 2 Diabetes Mellitus. Curr Diabetes Rev. 2020, 16:279–92. [DOI] [PubMed] [Google Scholar]
- [92].Gunn I, O’Shea D, Turton MD, Beak SA, Bloom SR Central glucagon-like peptide-I in the control of feeding. Biochem Soc Trans. 1996, 24:581–4. [DOI] [PubMed] [Google Scholar]
- [93].Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996, 379:69–72. [DOI] [PubMed] [Google Scholar]
- [94].Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M, et al. Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. Am J Physiol. 1996, 271:R848–56. [DOI] [PubMed] [Google Scholar]
- [95].Kanoski SE, Hayes MR, Skibicka KP GLP-1 and weight loss: unraveling the diverse neural circuitry. 2016, 310:R885–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Knudsen LB, Lau J The Discovery and Development of Liraglutide and Semaglutide. Front Endocrinol (Lausanne). 2019, 10:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Larsen PJ, Tang-Christensen M, Jessop DS Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997, 138:4445–55. [DOI] [PubMed] [Google Scholar]
- [98].McKay NJ, Kanoski SE, Hayes MR, Daniels D Glucagon-like peptide-1 receptor agonists suppress water intake independent of effects on food intake. Am J Physiol Regul Integr Comp Physiol. 2011, 301:R1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Navarro M, Rodriquez de Fonseca F, Alvarez E, Chowen JA, Zueco JA, Gomez R, et al. Colocalization of glucagon-like peptide-1 (GLP-1) receptors, glucose transporter GLUT-2, and glucokinase mRNAs in rat hypothalamic cells: evidence for a role of GLP-1 receptor agonists as an inhibitory signal for food and water intake. J Neurochem. 1996, 67:1982–91. [DOI] [PubMed] [Google Scholar]
- [100].Wang T, Edwards GL, Baile CA Glucagon-like peptide-1 (7-36) amide administered into the third cerebroventricle inhibits water intake in rats. Proc Soc Exp Biol Med. 1998, 219:85–91. [DOI] [PubMed] [Google Scholar]
- [101].Barrera JG, Sandoval DA, D’Alessio DA, Seeley RJ GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev Endocrinol. 2011, 7:507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ladenheim EE Gastrointestinal regulatory peptides and central nervous system mechanisms of weight control. Curr Opin Endocrinol Diabetes Obes. 2012, 19:13–8. [DOI] [PubMed] [Google Scholar]
- [103].Punjabi M, Arnold M, Geary N, Langhans W, Pacheco-Lopez G Peripheral glucagon-like peptide-1 (GLP-1) and satiation. Physiol Behav. 2011, 105:71–6. [DOI] [PubMed] [Google Scholar]
- [104].Tong J, Sandoval DA Is the GLP-1 system a viable therapeutic target for weight reduction? Rev Endocr Metab Disord. 2011, 12:187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].McKay NJ, Galante DL, Daniels D Endogenous glucagon-like peptide-1 reduces drinking behavior and is differentially engaged by water and food intakes in rats. J Neurosci. 2014, 34:16417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Augustine V, Gokce SK, Lee S, Wang B, Davidson TJ, Reimann F, et al. Hierarchical neural architecture underlying thirst regulation. Nature. 2018, 555:204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997, 77:257–70. [DOI] [PubMed] [Google Scholar]
- [108].Merchenthaler I, Lane M, Shughrue P Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999, 403:261–80. [DOI] [PubMed] [Google Scholar]
- [109].Mojsov S, Kopczynski MG, Habener JF Both amidated and nonamidated forms of glucagon-like peptide I are synthesized in the rat intestine and the pancreas. J Biol Chem. 1990, 265:8001–8. [PubMed] [Google Scholar]
- [110].Jin SL, Han VK, Simmons JG, Towle AC, Lauder JM, Lund PK Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: an immunocytochemical study. J Comp Neurol. 1988, 271:519–32. [DOI] [PubMed] [Google Scholar]
- [111].Rinaman L Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010, 1350:18–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Rinaman L Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol. 1999, 277:R582–90. [DOI] [PubMed] [Google Scholar]
- [113].Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995, 7:2294–300. [DOI] [PubMed] [Google Scholar]
- [114].Shughrue PJ, Lane MV, Merchenthaler I Glucagon-like peptide-1 receptor (GLP1-R) mRNA in the rat hypothalamus. Endocrinology. 1996, 137:5159–62. [DOI] [PubMed] [Google Scholar]
- [115].Uttenthal LO, Toledano A, Blazquez E Autoradiographic localization of receptors for glucagon-like peptide-1 (7-36) amide in rat brain. Neuropeptides. 1992, 21:143–6. [DOI] [PubMed] [Google Scholar]
- [116].Zardetto-Smith AM, Gray TS A direct neural projection from the nucleus of the solitary tract to the subfornical organ in the rat. Neurosci Lett. 1987, 80:163–6. [DOI] [PubMed] [Google Scholar]
- [117].Heppner KM, Kirigiti M, Secher A, Paulsen SJ, Buckingham R, Pyke C, et al. Expression and distribution of glucagon-like peptide-1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology. 2015, 156:255–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Alhadeff AL, Rupprecht LE, Hayes MR GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012, 153:647–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC, et al. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am J Physiol Endocrinol Metab. 2013, 305:E1367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Hernandez NS, Ige KY, Mietlicki-Baase EG, Molina-Castro GC, Turner CA, Hayes MR, et al. Glucagon-like peptide-1 receptor activation in the ventral tegmental area attenuates cocaine seeking in rats. Neuropsychopharmacology. 2018, 43:2000–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, et al. Glucagon-Like Peptide-1 Receptor Activation in the Ventral Tegmental Area Decreases the Reinforcing Efficacy of Cocaine. Neuropsychopharmacology. 2016, 41:1917–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Westermark P, Wernstedt C, O’Brien TD, Hayden DW, Johnson KH Islet amyloid in type 2 human diabetes mellitus and adult diabetic cats contains a novel putative polypeptide hormone. Am J Pathol. 1987, 127:414–7. [PMC free article] [PubMed] [Google Scholar]
- [123].Fehmann HC, Weber V, Goke R, Goke B, Arnold R Cosecretion of amylin and insulin from isolated rat pancreas. FEBS Lett. 1990, 262:279–81. [DOI] [PubMed] [Google Scholar]
- [124].Kahn SE, D’Alessio DA, Schwartz MW, Fujimoto WY, Ensinck JW, Taborsky GJ Jr., et al. Evidence of cosecretion of islet amyloid polypeptide and insulin by beta-cells. Diabetes. 1990, 39:634–8. [DOI] [PubMed] [Google Scholar]
- [125].Li Z, Kelly L, Heiman M, Greengard P, Friedman JM Hypothalamic Amylin Acts in Concert with Leptin to Regulate Food Intake. Cell Metab. 2015, 22:1059–67. [DOI] [PubMed] [Google Scholar]
- [126].Dobolyi A Central amylin expression and its induction in rat dams. J Neurochem. 2009, 111:1490–500. [DOI] [PubMed] [Google Scholar]
- [127].Szabo ER, Cservenak M, Dobolyi A Amylin is a novel neuropeptide with potential maternal functions in the rat. FASEB J. 2012, 26:272–81. [DOI] [PubMed] [Google Scholar]
- [128].Lutz TA, Del Prete E, Scharrer E Reduction of food intake in rats by intraperitoneal injection of low doses of amylin. Physiol Behav. 1994, 55:891–5. [DOI] [PubMed] [Google Scholar]
- [129].Lutz TA, Geary N, Szabady MM, Del Prete E, Scharrer E Amylin decreases meal size in rats. Physiol Behav. 1995, 58:1197–202. [DOI] [PubMed] [Google Scholar]
- [130].Mack CM, Soares CJ, Wilson JK, Athanacio JR, Turek VF, Trevaskis JL, et al. Davalintide (AC2307), a novel amylin-mimetic peptide: enhanced pharmacological properties over native amylin to reduce food intake and body weight. Int J Obes (Lond). 2010, 34:385–95. [DOI] [PubMed] [Google Scholar]
- [131].Rushing PA, Hagan MM, Seeley RJ, Lutz TA, Woods SC Amylin: a novel action in the brain to reduce body weight. Endocrinology. 2000, 141:850–3. [DOI] [PubMed] [Google Scholar]
- [132].Rushing PA, Seeley RJ, Air EL, Lutz TA, Woods SC Acute 3rd-ventricular amylin infusion potently reduces food intake but does not produce aversive consequences. Peptides. 2002, 23:985–8. [DOI] [PubMed] [Google Scholar]
- [133].Wielinga PY, Lowenstein C, Muff S, Munz M, Woods SC, Lutz TA Central amylin acts as an adiposity signal to control body weight and energy expenditure. Physiol Behav. 2010, 101:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Riediger T, Rauch M, Schmid HA Actions of amylin on subfornical organ neurons and on drinking behavior in rats. Am J Physiol. 1999, 276:R514–21. [DOI] [PubMed] [Google Scholar]
- [135].Lutz TA, Del Prete E, Szabady MM, Scharrer E Circadian anorectic effects of peripherally administered amylin in rats. Z Ernahrungswiss. 1995, 34:214–9. [DOI] [PubMed] [Google Scholar]
- [136].Chance WT, Balasubramaniam A, Chen X, Fischer JE Tests of adipsia and conditioned taste aversion following the intrahypothalamic injection of amylin. Peptides. 1992, 13:961–4. [DOI] [PubMed] [Google Scholar]
- [137].Mietlicki-Baase EG, Rupprecht LE, Olivos DR, Zimmer DJ, Alter MD, Pierce RC, et al. Amylin receptor signaling in the ventral tegmental area is physiologically relevant for the control of food intake. Neuropsychopharmacology. 2013, 38:1685–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Mietlicki-Baase EG, McGrath LE, Koch-Laskowski K, Krawczyk J, Reiner DJ, Pham T, et al. Amylin receptor activation in the ventral tegmental area reduces motivated ingestive behavior. Neuropharmacology. 2017, 123:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Christopoulos G, Perry KJ, Morfis M, Tilakaratne N, Gao Y, Fraser NJ, et al. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol Pharmacol. 1999, 56:235–42. [DOI] [PubMed] [Google Scholar]
- [140].Bailey RJ, Walker CS, Ferner AH, Loomes KM, Prijic G, Halim A, et al. Pharmacological characterization of rat amylin receptors: implications for the identification of amylin receptor subtypes. Br J Pharmacol. 2012, 166:151–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Zumpe ET, Tilakaratne N, Fraser NJ, Christopoulos G, Foord SM, Sexton PM Multiple ramp domains are required for generation of amylin receptor phenotype from the calcitonin receptor gene product. Biochem Biophys Res Commun. 2000, 267:368–72. [DOI] [PubMed] [Google Scholar]
- [142].Barth SW, Riediger T, Lutz TA, Rechkemmer G Peripheral amylin activates circumventricular organs expressing calcitonin receptor a/b subtypes and receptor-activity modifying proteins in the rat. Brain Res. 2004, 997:97–102. [DOI] [PubMed] [Google Scholar]
- [143].Masi L, Brandi ML Calcitonin and calcitonin receptors. Clin Cases Miner Bone Metab. 2007, 4:117–22. [PMC free article] [PubMed] [Google Scholar]
- [144].Pondel M Calcitonin and calcitonin receptors: bone and beyond. Int J Exp Pathol. 2000, 81:405–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Hay DL, Walker CS, Gingell JJ, Ladds G, Reynolds CA, Poyner DR Receptor activity-modifying proteins; multifunctional G protein-coupled receptor accessory proteins. Biochem Soc Trans. 2016, 44:568–73. [DOI] [PubMed] [Google Scholar]
- [146].Klein KR, Matson BC, Caron KM The expanding repertoire of receptor activity modifying protein (RAMP) function. Crit Rev Biochem Mol Biol. 2016, 51:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Pulman KJ, Fry WM, Cottrell GT, Ferguson AV The subfornical organ: a central target for circulating feeding signals. J Neurosci. 2006, 26:2022–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Smith PM, Chambers AP, Price CJ, Ho W, Hopf C, Sharkey KA, et al. The subfornical organ: a central nervous system site for actions of circulating leptin. Am J Physiol Regul Integr Comp Physiol. 2009, 296:R512–20. [DOI] [PubMed] [Google Scholar]
- [149].Smith PM, Rozanski G, Ferguson AV Acute electrical stimulation of the subfornical organ induces feeding in satiated rats. Physiol Behav. 2010, 99:534–7. [DOI] [PubMed] [Google Scholar]
- [150].Mangiapane ML, Simpson JB Subfornical organ: forebrain site of pressor and dipsogenic action of angiotensin II. Am J Physiol. 1980, 239:R382–9. [DOI] [PubMed] [Google Scholar]
- [151].Ishibashi S, Nicolaidis S Hypertension induced by electrical stimulation of the subfornical organ (SFO). Brain Res Bull. 1981, 6:135–9. [DOI] [PubMed] [Google Scholar]
- [152].Mietlicki-Baase EG, Reiner DJ, Cone JJ, Olivos DR, McGrath LE, Zimmer DJ, et al. Amylin modulates the mesolimbic dopamine system to control energy balance. Neuropsychopharmacology. 2015, 40:372–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Boyle CN, Lutz TA, Le Foll C Amylin - Its role in the homeostatic and hedonic control of eating and recent developments of amylin analogs to treat obesity. Mol Metab. 2018, 8:203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Kalafateli AL, Satir TM, Vallof D, Zetterberg H, Jerlhag E An amylin and calcitonin receptor agonist modulates alcohol behaviors by acting on reward-related areas in the brain. Prog Neurobiol. 2020:101969. [DOI] [PubMed] [Google Scholar]
- [155].Kalafateli AL, Vallof D, Jerlhag E Activation of amylin receptors attenuates alcohol-mediated behaviours in rodents. Addict Biol. 2019, 24:388–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Nashawi H, Gustafson TJ, Mietlicki-Baase EG Palatable food access impacts expression of amylin receptor components in the mesocorticolimbic system. Exp Physiol. 2020, 105:1012–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Whiting L, McCutcheon JE, Boyle CN, Roitman MF, Lutz TA The area postrema (AP) and the parabrachial nucleus (PBN) are important sites for salmon calcitonin (sCT) to decrease evoked phasic dopamine release in the nucleus accumbens (NAc). Physiol Behav. 2017, 176:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Baldo BA, Kelley AE Amylin infusion into rat nucleus accumbens potently depresses motor activity and ingestive behavior. Am J Physiol Regul Integr Comp Physiol. 2001, 281:R1232–42. [DOI] [PubMed] [Google Scholar]
- [159].Baisley SK, Baldo BA Amylin receptor signaling in the nucleus accumbens negatively modulates mu-opioid-driven feeding. Neuropsychopharmacology. 2014, 39:3009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Kern KA, Mietlicki-Baase EG Distributed amylin receptor signaling and its influence on motivated behavior. Physiol Behav. 2020, 222:112958. [DOI] [PubMed] [Google Scholar]
- [161].Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999, 402:65660. [DOI] [PubMed] [Google Scholar]
- [162].Asakawa A, Inui A, Kaga T, Katsuura G, Fujimiya M, Fujino MA, et al. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut. 2003, 52:947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001, 86:5992. [DOI] [PubMed] [Google Scholar]
- [164].Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000, 141:4325–8. [DOI] [PubMed] [Google Scholar]
- [165].Hashimoto H, Fujihara H, Kawasaki M, Saito T, Shibata M, Otsubo H, et al. Centrally and peripherally administered ghrelin potently inhibits water intake in rats. Endocrinology. 2007, 148:1638–47. [DOI] [PubMed] [Google Scholar]
- [166].Mietlicki EG, Nowak EL, Daniels D The effect of ghrelin on water intake during dipsogenic conditions. Physiol Behav. 2009, 96:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Hashimoto H, Otsubo H, Fujihara H, Suzuki H, Ohbuchi T, Yokoyama T, et al. Centrally administered ghrelin potently inhibits water intake induced by angiotensin II and hypovolemia in rats. J Physiol Sci. 2010, 60:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Plyler KS, Daniels D Fourth ventricle injection of ghrelin decreases angiotensin II-induced fluid intake and neuronal activation in the paraventricular nucleus of the hypothalamus. Physiol Behav. 2017, 178:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Mietlicki EG, Daniels D Ghrelin reduces hypertonic saline intake in a variety of natriorexigenic conditions. Exp Physiol. 2011, 96:1072–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006, 116:3229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006, 494:528–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011, 180:129–37. [DOI] [PubMed] [Google Scholar]
- [173].Skibicka KP, Shirazi RH, Rabasa-Papio C, Alvarez-Crespo M, Neuber C, Vogel H, et al. Divergent circuitry underlying food reward and intake effects of ghrelin: dopaminergic VTA-accumbens projection mediates ghrelin’s effect on food reward but not food intake. Neuropharmacology. 2013, 73:274–83. [DOI] [PubMed] [Google Scholar]
- [174].Quarta D, Di Francesco C, Melotto S, Mangiarini L, Heidbreder C, Hedou G Systemic administration of ghrelin increases extracellular dopamine in the shell but not the core subdivision of the nucleus accumbens. Neurochem Int. 2009, 54:89–94. [DOI] [PubMed] [Google Scholar]
- [175].Cone JJ, McCutcheon JE, Roitman MF Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J Neurosci. 2014, 34:490513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Cone JJ, Roitman JD, Roitman MF Ghrelin regulates phasic dopamine and nucleus accumbens signaling evoked by food-predictive stimuli. J Neurochem. 2015, 133:844–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Kawahara Y, Kawahara H, Kaneko F, Yamada M, Nishi Y, Tanaka E, et al. Peripherally administered ghrelin induces bimodal effects on the mesolimbic dopamine system depending on food-consumptive states. Neuroscience. 2009, 161:855–64. [DOI] [PubMed] [Google Scholar]
- [178].Badiani A, Vaccaro R, Burdino R, Casini A, Valeri P, Renda TG, et al. Dissociation in the effects of the D2/D3 dopaminergic agonist quinpirole on drinking and on vasopressin levels in the rat. Neurosci Lett. 2002, 325:79–82. [DOI] [PubMed] [Google Scholar]
- [179].Lightman SL, Iversen LL, Forsling ML Dopamine and [D-ALA2, D-Leu5]enkephalin inhibit the electrically stimulated neurohypophyseal release of vasopressin in vitro: evidence for calcium-dependent opiate action. J Neurosci. 1982, 2:78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].van den Buuse M Role of the mesolimbic dopamine system in cardiovascular homeostasis. Stimulation of the ventral tegmental area modulates the effect of vasopressin on blood pressure in conscious rats. Clin Exp Pharmacol Physiol. 1998, 25:661–8. [DOI] [PubMed] [Google Scholar]
- [181].Forsling ML, Williams H Central effects of dopamine on vasopressin release in the normally hydrated and water-loaded rat. J Physiol. 1984, 346:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Garate-Perez MF, Mendez A, Bahamondes C, Sanhueza C, Guzman F, Reyes-Parada M, et al. Vasopressin in the lateral septum decreases conditioned place preference to amphetamine and nucleus accumbens dopamine release. Addict Biol. 2021, 26:e12851. [DOI] [PubMed] [Google Scholar]
- [183].McKinley MJ, Denton DA, Ryan PJ, Yao ST, Stefanidis A, Oldfield BJ From sensory circumventricular organs to cerebral cortex: Neural pathways controlling thirst and hunger. J Neuroendocrinol. 2019, 31:e12689. [DOI] [PubMed] [Google Scholar]
- [184].Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides. 1991, 12:113–8. [DOI] [PubMed] [Google Scholar]
- [185].Arletti R, Benelli A, Bertolini A Oxytocin inhibits food and fluid intake in rats. Physiol Behav. 1990, 48:825–30. [DOI] [PubMed] [Google Scholar]
- [186].Morton GJ, Thatcher BS, Reidelberger RD, Ogimoto K, Wolden-Hanson T, Baskin DG, et al. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am J Physiol Endocrinol Metab. 2012, 302:E134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Verbalis JG, Blackburn RE, Hoffman GE, Stricker EM Establishing behavioral and physiological functions of central oxytocin: insights from studies of oxytocin and ingestive behaviors. Adv Exp Med Biol. 1995, 395:209–25. [PubMed] [Google Scholar]
- [188].McConn BR, Koskinen A, Denbow DM, Gilbert ER, Siegel PB, Cline MA Central injection of oxytocin reduces food intake and affects hypothalamic and adipose tissue gene expression in chickens. Domest Anim Endocrinol. 2019, 67:11–20. [DOI] [PubMed] [Google Scholar]
- [189].Blackburn RE, Samson WK, Fulton RJ, Stricker EM, Verbalis JG Central oxytocin inhibition of salt appetite in rats: evidence for differential sensing of plasma sodium and osmolality. Proc Natl Acad Sci U S A. 1993, 90:10380–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Puryear R, Rigatto KV, Amico JA, Morris M Enhanced salt intake in oxytocin deficient mice. Exp Neurol. 2001, 171:323–8. [DOI] [PubMed] [Google Scholar]
- [191].Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, et al. Gating of social reward by oxytocin in the ventral tegmental area. Science. 2017, 357:1406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Peters ST, Bowen MT, Bohrer K, McGregor IS, Neumann ID Oxytocin inhibits ethanol consumption and ethanol-induced dopamine release in the nucleus accumbens. Addict Biol. 2017, 22:702–11. [DOI] [PubMed] [Google Scholar]
- [193].Lee MR, Rohn MCH, Zanettini C, Coggiano MA, Leggio L, Tanda G Effect of systemically administered oxytocin on dose response for methylphenidate self-administration and mesolimbic dopamine levels. Ann N Y Acad Sci. 2019, 1455:173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Liu CM, Hsu TM, Suarez AN, Subramanian KS, Fatemi RA, Cortella AM, et al. Central oxytocin signaling inhibits food reward-motivated behaviors and VTA dopamine responses to food-predictive cues in male rats. Horm Behav. 2020, 126:104855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Peris J, MacFadyen K, Smith JA, de Kloet AD, Wang L, Krause EG Oxytocin receptors are expressed on dopamine and glutamate neurons in the mouse ventral tegmental area that project to nucleus accumbens and other mesolimbic targets. J Comp Neurol. 2017, 525:1094–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Brown J, Sagante A, Mayer T, Wright A, Bugescu R, Fuller PM, et al. Lateral Hypothalamic Area Neurotensin Neurons Are Required for Control of Orexin Neurons and Energy Balance. Endocrinology. 2018, 159:3158–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Kurt G, Woodworth HL, Fowler S, Bugescu R, Leinninger GM Activation of lateral hypothalamic area neurotensin-expressing neurons promotes drinking. Neuropharmacology. 2019, 154:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Brown JA, Wright A, Bugescu R, Christensen L, Olson DP, Leinninger GM Distinct Subsets of Lateral Hypothalamic Neurotensin Neurons are Activated by Leptin or Dehydration. Sci Rep. 2019, 9:1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Gibbs J, Young RC, Smith GP Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol. 1973, 84:488–95. [DOI] [PubMed] [Google Scholar]
- [200].Kraly FS, Carty WJ, Resnick S, Smith GP Effect of cholecystokinin on meal size and intermeal interval in the sham-feeding rat. J Comp Physiol Psychol. 1978, 92:697–707. [DOI] [PubMed] [Google Scholar]
- [201].Reidelberger RD Cholecystokinin and control of food intake. J Nutr. 1994, 124:1327S–33S. [DOI] [PubMed] [Google Scholar]
- [202].Ballaz S The unappreciated roles of the cholecystokinin receptor CCK(1) in brain functioning. Rev Neurosci. 2017, 28:573–85. [DOI] [PubMed] [Google Scholar]
- [203].Matsuda T, Hiyama TY, Kobayashi K, Kobayashi K, Noda M Distinct CCK-positive SFO neurons are involved in persistent or transient suppression of water intake. Nat Commun. 2020, 11:5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Ebenezer IS Systemic administration of cholecystokinin (CCK) inhibits operant water intake in rats: implications for the CCK-satiety hypothesis. Proc Biol Sci. 1996, 263:491–6. [DOI] [PubMed] [Google Scholar]
- [205].Friedman JM, Halaas JL Leptin and the regulation of body weight in mammals. Nature. 1998, 395:763–70. [DOI] [PubMed] [Google Scholar]
- [206].Ahima RS, Flier JS Leptin. Annu Rev Physiol. 2000, 62:413–37. [DOI] [PubMed] [Google Scholar]
- [207].Friedman JM Leptin and the endocrine control of energy balance. Nat Metab. 2019, 1:754–64. [DOI] [PubMed] [Google Scholar]
- [208].van der Plasse G, van Zessen R, Luijendijk MC, Erkan H, Stuber GD, Ramakers GM, et al. Modulation of cue-induced firing of ventral tegmental area dopamine neurons by leptin and ghrelin. Int J Obes (Lond). 2015, 39:1742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Patel JD, Ebenezer IS The effect of intraperitoneal administration of leptin on short-term food intake in rats. Eur J Pharmacol. 2008, 580:143–52. [DOI] [PubMed] [Google Scholar]
- [210].Begg DP, Sinclair AJ, Weisinger RS Reductions in water and sodium intake by aged male and female rats. Nutr Res. 2012, 32:865–72. [DOI] [PubMed] [Google Scholar]
- [211].Santollo J, Edwards AA How predictive is body weight on fluid intake in rats? It depends on sex. Physiol Behav. 2021, 229:113262. [DOI] [PubMed] [Google Scholar]
- [212].Santollo J, Myers KE, Rainer IL, Edwards AA Gonadal hormones in female rats protect against dehydration-induced memory impairments in the novel object recognition paradigm. Horm Behav. 2019, 114:104547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Findlay AL, Fitzsimons JT, Kucharczyk J Dependence of spontaneous and angiotensin-induced drinking in the rat upon the oestrous cycle and ovarian hormones. J Endocrinol. 1979, 82:215–25. [DOI] [PubMed] [Google Scholar]
- [214].Jonklaas J, Buggy J Angiotensin-estrogen interaction in female brain reduces drinking and pressor responses. Am J Physiol. 1984, 247:R167–72. [DOI] [PubMed] [Google Scholar]
- [215].Krause EG, Curtis KS, Davis LM, Stowe JR, Contreras RJ Estrogen influences stimulated water intake by ovariectomized female rats. Physiol Behav. 2003, 79:267–74. [DOI] [PubMed] [Google Scholar]
- [216].Mecawi AS, Lepletier A, Araujo IG, Fonseca FV, Reis LC Oestrogenic influence on brain AT1 receptor signalling on the thirst and sodium appetite in osmotically stimulated and sodium-depleted female rats. Exp Physiol. 2008, 93:1002–10. [DOI] [PubMed] [Google Scholar]
- [217].Santollo J Sex differences in angiotensin II-stimulated fluid intake. Exp Physiol. 2017, 102:1380–4. [DOI] [PubMed] [Google Scholar]
- [218].Santollo J, Torregrossa AM, Daniels D Sex differences in the drinking response to angiotensin II (AngII): Effect of body weight. Horm Behav. 2017, 93:128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Rowland NE, Morien A, Garcea M, Fregly MJ Aging and fluid homeostasis in rats. Am J Physiol. 1997, 273:R1441–50. [DOI] [PubMed] [Google Scholar]
- [220].Flynn FW, Schulkin J, Havens M Sex differences in salt preference and taste reactivity in rats. Brain Res Bull. 1993, 32:91–5. [DOI] [PubMed] [Google Scholar]
- [221].Krecek J Sex differences in salt taste: the effect of testosterone. Physiol Behav. 1973, 10:683–8. [DOI] [PubMed] [Google Scholar]
- [222].Chow SY, Sakai RR, Witcher JA, Adler NT, Epstein AN Sex and sodium intake in the rat. Behav Neurosci. 1992, 106:172–80. [DOI] [PubMed] [Google Scholar]
- [223].Santollo J, Daniels D Activation of G protein-coupled estrogen receptor 1 (GPER-1) decreases fluid intake in female rats. Horm Behav. 2015, 73:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Santollo J, Marshall A, Curtis KS, Speth RC, Clark SD, Daniels D Divergent effects of ERalpha and ERbeta on fluid intake by female rats are not dependent on concomitant changes in AT1R expression or body weight. Am J Physiol Regul Integr Comp Physiol. 2016, 311:R14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Krause EG, Curtis KS, Stincic TL, Markle JP, Contreras RJ Oestrogen and weight loss decrease isoproterenol-induced Fos immunoreactivity and angiotensin type 1 mRNA in the subfornical organ of female rats. J Physiol. 2006, 573:251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Jones AB, Curtis KS Differential effects of estradiol on drinking by ovariectomized rats in response to hypertonic NaCl or isoproterenol: Implications for hyper- vs. hypo-osmotic stimuli for water intake. Physiol Behav. 2009, 98:421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Kisley LR, Sakai RR, Ma LY, Fluharty SJ Ovarian steroid regulation of angiotensin II-induced water intake in the rat. Am J Physiol. 1999, 276:R90–6. [DOI] [PubMed] [Google Scholar]
- [228].Danielsen J, Buggy J Depression of ad lib and angiotensin-induced sodium intake at oestrus. Brain Res Bull. 1980, 5:501–4. [DOI] [PubMed] [Google Scholar]
- [229].Fregly MJ Attenuation of thirst in estrogen-treated rats. Fed Proc. 1978, 37:2694–8. [PubMed] [Google Scholar]
- [230].Fregly MJ, Rowland NE, Sumners C, Gordon DB Reduced dipsogenic responsiveness to intracerebroventricularly administered angiotensin II in estrogen-treated rats. Brain Res. 1985, 338:115–21. [DOI] [PubMed] [Google Scholar]
- [231].Fregly MJ, Thrasher TN Attenuation of angiotensin-induced water intake in estrogen-treated rats. Pharmacol Biochem Behav. 1978, 9:509–14. [DOI] [PubMed] [Google Scholar]
- [232].Santollo J, Volcko KL, Daniels D Sex Differences in the Behavioral Desensitization of Water Intake Observed After Repeated Central Injections of Angiotensin II. Endocrinology. 2018, 159:676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Leshem M, Epstein AN Ontogeny of renin-induced salt appetite in the rat pup. Dev Psychobiol. 1989, 22:437–45. [DOI] [PubMed] [Google Scholar]
- [234].Krecek J, Novakova V, Stibral K Sex differences in the taste preference for a salt solution in the rat. Physiol Behav. 1972, 8:183–8. [DOI] [PubMed] [Google Scholar]
- [235].Dadam FM, Caeiro XE, Cisternas CD, Macchione AF, Cambiasso MJ, Vivas L Effect of sex chromosome complement on sodium appetite and Fos-immunoreactivity induced by sodium depletion. Am J Physiol Regul Integr Comp Physiol. 2014, 306:R175–84. [DOI] [PubMed] [Google Scholar]
- [236].Quiros Cognuck S, Reis WL, Silva MS, Almeida-Pereira G, Debarba LK, Zorro SV, et al. Sex- and age-dependent differences in the hormone and drinking responses to water deprivation. Am J Physiol Regul Integr Comp Physiol. 2020, 318:R567–R78. [DOI] [PubMed] [Google Scholar]
- [237].Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol. 1998, 36:357–78. [DOI] [PubMed] [Google Scholar]
- [238].Creutz LM, Kritzer MF Estrogen receptor-beta immunoreactivity in the midbrain of adult rats: regional, subregional, and cellular localization in the A10, A9, and A8 dopamine cell groups. J Comp Neurol. 2002, 446:288–300. [DOI] [PubMed] [Google Scholar]
- [239].Kuppers E, Beyer C Expression of estrogen receptor-alpha and beta mRNA in the developing and adult mouse striatum. Neurosci Lett. 1999, 276:95–8. [DOI] [PubMed] [Google Scholar]
- [240].Shughrue PJ, Lane MV, Merchenthaler I Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997, 388:507–25. [DOI] [PubMed] [Google Scholar]
- [241].Kritzer MF Selective colocalization of immunoreactivity for intracellular gonadal hormone receptors and tyrosine hydroxylase in the ventral tegmental area, substantia nigra, and retrorubral fields in the rat. J Comp Neurol. 1997, 379:247–60. [DOI] [PubMed] [Google Scholar]
- [242].Kritzer MF, Creutz LM Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. J Neurosci. 2008, 28:9525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Dewing P, Chiang CW, Sinchak K, Sim H, Fernagut PO, Kelly S, et al. Direct regulation of adult brain function by the male-specific factor SRY. Curr Biol. 2006, 16:415–20. [DOI] [PubMed] [Google Scholar]
- [244].Becker JB Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999, 64:803–12. [DOI] [PubMed] [Google Scholar]
- [245].Becker JB Sexual differentiation of motivation: a novel mechanism? Horm Behav. 2009, 55:646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Becker JB, Chartoff E Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology. 2019, 44:166–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Yoest KE, Cummings JA, Becker JB Estradiol, dopamine and motivation. Cent Nerv Syst Agents Med Chem. 2014, 14:83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Morissette M, Le Saux M, D’Astous M, Jourdain S, Al Sweidi S, Morin N, et al. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Mol Biol. 2008, 108:327–38. [DOI] [PubMed] [Google Scholar]
- [249].Gillies GE, Virdee K, McArthur S, Dalley JW Sex-dependent diversity in ventral tegmental dopaminergic neurons and developmental programing: A molecular, cellular and behavioral analysis. Neuroscience. 2014, 282:69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Zachry JE, Nolan SO, Brady LJ, Kelly SJ, Siciliano CA, Calipari ES Sex differences in dopamine release regulation in the striatum. Neuropsychopharmacology. 2021, 46:491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].McArthur S, McHale E, Gillies GE The size and distribution of midbrain dopaminergic populations are permanently altered by perinatal glucocorticoid exposure in a sex-region- and time-specific manner. Neuropsychopharmacology. 2007, 32:146276. [DOI] [PubMed] [Google Scholar]
- [252].Carruth LL, Reisert I, Arnold AP Sex chromosome genes directly affect brain sexual differentiation. Nat Neurosci. 2002, 5:933–4. [DOI] [PubMed] [Google Scholar]
- [253].Milsted A, Serova L, Sabban EL, Dunphy G, Turner ME, Ely DL Regulation of tyrosine hydroxylase gene transcription by Sry. Neurosci Lett. 2004, 369:203–7. [DOI] [PubMed] [Google Scholar]
- [254].Serova LI, Maharjan S, Huang A, Sun D, Kaley G, Sabban EL Response of tyrosine hydroxylase and GTP cyclohydrolase I gene expression to estrogen in brain catecholaminergic regions varies with mode of administration. Brain Res. 2004, 1015:1–8. [DOI] [PubMed] [Google Scholar]
- [255].Pasqualini C, Olivier V, Guibert B, Frain O, Leviel V Acute stimulatory effect of estradiol on striatal dopamine synthesis. J Neurochem. 1995, 65:1651–7. [DOI] [PubMed] [Google Scholar]
- [256].Walker QD, Ray R, Kuhn CM Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology. 2006, 31:1193–202. [DOI] [PubMed] [Google Scholar]
- [257].Walker QD, Rooney MB, Wightman RM, Kuhn CM Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 2000, 95:1061–70. [DOI] [PubMed] [Google Scholar]
- [258].Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, et al. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun. 2017, 8:13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Xiao L, Becker JB Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neurosci Lett. 1994, 180:155–8. [DOI] [PubMed] [Google Scholar]
- [260].Ohtani H, Nomoto M, Douchi T Chronic estrogen treatment replaces striatal dopaminergic function in ovariectomized rats. Brain Res. 2001, 900:163–8. [DOI] [PubMed] [Google Scholar]
- [261].McArthur S, Murray HE, Dhankot A, Dexter DT, Gillies GE Striatal susceptibility to a dopaminergic neurotoxin is independent of sex hormone effects on cell survival and DAT expression but is exacerbated by central aromatase inhibition. J Neurochem. 2007, 100:678–92. [DOI] [PubMed] [Google Scholar]
- [262].Shams WM, Cossette MP, Shizgal P, Brake WG 17beta-estradiol locally increases phasic dopamine release in the dorsal striatum. Neurosci Lett. 2018, 665:2932. [DOI] [PubMed] [Google Scholar]
- [263].Becker JB Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synapse. 1990, 5:157–64. [DOI] [PubMed] [Google Scholar]
- [264].Thompson TL, Moss RL Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J Neurochem. 1994, 62:1750–6. [DOI] [PubMed] [Google Scholar]
- [265].Becker JB, Ramirez VD Sex differences in the amphetamine stimulated release of catecholamines from rat striatal tissue in vitro. Brain Res. 1981, 204:361–72. [DOI] [PubMed] [Google Scholar]
- [266].Becker JB, Ramirez VD Experimental studies on the development of sex differences in the release of dopamine from striatal tissue fragments in vitro. Neuroendocrinology. 1981, 32:168–73. [DOI] [PubMed] [Google Scholar]
- [267].Castner SA, Xiao L, Becker JB Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res. 1993, 610:127–34. [DOI] [PubMed] [Google Scholar]
- [268].Cummings JA, Jagannathan L, Jackson LR, Becker JB Sex differences in the effects of estradiol in the nucleus accumbens and striatum on the response to cocaine: neurochemistry and behavior. Drug Alcohol Depend. 2014, 135:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Shams WM, Sanio C, Quinlan MG, Brake WG 17beta-Estradiol infusions into the dorsal striatum rapidly increase dorsal striatal dopamine release in vivo. Neuroscience. 2016, 330:162–70. [DOI] [PubMed] [Google Scholar]
- [270].Grove-Strawser D, Boulware MI, Mermelstein PG Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010, 170:1045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Mermelstein PG Membrane-localised oestrogen receptor alpha and beta influence neuronal activity through activation of metabotropic glutamate receptors. J Neuroendocrinol. 2009, 21:257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Mermelstein PG, Becker JB, Surmeier DJ Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996, 16:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Schultz KN, von Esenwein SA, Hu M, Bennett AL, Kennedy RT, Musatov S, et al. Viral vector-mediated overexpression of estrogen receptor-alpha in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci. 2009, 29:1897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Hasbi A, Nguyen T, Rahal H, Manduca JD, Miksys S, Tyndale RF, et al. Sex difference in dopamine D1-D2 receptor complex expression and signaling affects depression- and anxiety-like behaviors. Biol Sex Differ. 2020, 11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Levesque D, Gagnon S, Di Paolo T Striatal D1 dopamine receptor density fluctuates during the rat estrous cycle. Neurosci Lett. 1989, 98:345–50. [DOI] [PubMed] [Google Scholar]
- [276].Orendain-Jaime EN, Ortega-Ibarra JM, Lopez-Perez SJ Evidence of sexual dimorphism in D1 and D2 dopaminergic receptors expression in frontal cortex and striatum of young rats. Neurochem Int. 2016, 100:62–6. [DOI] [PubMed] [Google Scholar]
- [277].Maus M, Bertrand P, Drouva S, Rasolonjanahary R, Kordon C, Glowinski J, et al. Differential modulation of D1 and D2 dopamine-sensitive adenylate cyclases by 17 beta-estradiol in cultured striatal neurons and anterior pituitary cells. J Neurochem. 1989, 52:410–8. [DOI] [PubMed] [Google Scholar]
- [278].Levesque D, Di Paolo T Chronic estradiol treatment increases ovariectomized rat striatal D-1 dopamine receptors. Life Sci. 1989, 45:1813–20. [DOI] [PubMed] [Google Scholar]
- [279].Bazzett TJ, Becker JB Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 1994, 637:163–72. [DOI] [PubMed] [Google Scholar]
- [280].Le Saux M, Di Paolo T Influence of oestrogenic compounds on monoamine transporters in rat striatum. J Neuroendocrinol. 2006, 18:25–32. [DOI] [PubMed] [Google Scholar]
- [281].Vaccarino FJ Nucleus accumbens dopamine-CCK interactions in psychostimulant reward and related behaviors. Neurosci Biobehav Rev. 1994, 18:207–14. [DOI] [PubMed] [Google Scholar]
- [282].Voigt MM, Wang RY In vivo release of dopamine in the nucleus accumbens of the rat: modulation by cholecystokinin. Brain Res. 1984, 296:189–93. [DOI] [PubMed] [Google Scholar]
- [283].Matsuda T, Hiyama TY, Niimura F, Matsusaka T, Fukamizu A, Kobayashi K, et al. Distinct neural mechanisms for the control of thirst and salt appetite in the subfornical organ. Nat Neurosci. 2017, 20:230–41. [DOI] [PubMed] [Google Scholar]
- [284].Menani JV, Johnson AK Cholecystokinin actions in the parabrachial nucleus: effects on thirst and salt appetite. Am J Physiol. 1998, 275:R1431–7. [DOI] [PubMed] [Google Scholar]


