INTRODUCTION
Viral infections are among the most common complications in children who have undergone hematopoietic cell transplantation (HCT) or solid organ transplantation (SOT), and are a significant source of morbidity and mortality. These infections include primary viral infections, most commonly caused by respiratory viruses, but also reactivation of prior infection, as is seen with herpesviruses.
Pediatric and adult recipients of HCT and SOT encounter similar viral infections and are treated with common antiviral agents. Paradigms for the prevention and treatment of viral infections differ between HCT and SOT recipients based on timing and depth of immune suppression around transplantation. Patients with lymphocyte dysfunction are at the highest risk for viral infection.1,2 In HCT recipients, lymphocyte depletion occurs in the pre-engraftment phase after chemotherapy conditioning and continues through ~100 days or more after transplant.2 After lymphocyte engraftment, patients who develop graft-versus-host disease (GVHD) require additional T-cell immunosuppression to minimize symptoms, placing them at continued risk for viral infection. Recipients of SOT are at the highest risk for viral infection or reactivation in the early and intermediate post-transplant period (up to 6 months) when immunosuppression is maximized.1 Subsequently, episodes of graft rejection are managed with additional lymphocyte suppressive medications, increasing the risk of viral infection.
During these periods of increased risk in both HCT and SOT recipients, antiviral agents are used for prophylaxis and treatment of infection (Table 1). However, few antiviral drugs with high-efficacy, low-toxicity profiles exist for use in immunocompromised children (Table 2). Published pediatric data are limited and many practice recommendations are extrapolated from studies in adult patients. Thus, the use of antiviral agents in children is often off-label. Here we review antiviral drugs used to prevent and treat common viral infections in immunocompromised children, including herpesviruses, human adenoviruses, and respiratory viruses (Fig. 1). We also discuss common practices with both approved and off-label indications for antiviral therapeutics in pediatric transplant recipients.
Table 1.
Antiviral drug indications and dosing
| Drug | Indication | Route | Age | Dose |
|---|---|---|---|---|
| Ganciclovir | Induction therapy for CMV syndrome or CMV disease | IV | All ages | 5 mg/kg every 12 h |
| Maintenance therapy for CMV syndrome or CMV disease | IV | All ages | 5 mg/kg every 24 h | |
| Prophylaxis against CMV infection | IV | All ages | Induction dosing for 7 d, followed by maintenance dosing | |
| Valganciclovir | CMV disease (mild to moderate) | Oral | All ages | 7 x BSA x CrCl (maximum dose 900 mg) |
| Prophylaxis against CMV infection | Oral | ≥4 mo to 16 y | 7 x BSA x CrCl (maximum dose 900 mg) | |
| Foscarnet | Induction therapy for CMV syndrome or CMV disease | IV | All ages | 60 mg/kg every 12 h |
| Maintenance therapy for CMV syndrome or CMV disease | IV | All ages | 90 mg/kg once daily | |
| Prophylaxis against CMV infection | IV | All ages | Induction dosing for 7 d, followed by maintenance dosing | |
| HSV infection, acyclovir-resistant | IV | All ages | 120 mg/kg/d divided every 8 or 12 h | |
| VZV infection, acyclovir-resistant | IV | All ages | 80–120 mg/kg/d divided every 8 or 12 h | |
| Cidofovir | Induction therapy for CMV syndrome or CMV disease | IV | All ages | 5 mg/kg every week for 2 consecutive weeks, with hyperhydration ± probenecid |
| Maintenance therapy for CMV syndrome or CMV disease | IV | All ages | 5 mg/kg every 2 wk, with hyperhydration ± probenecid | |
| Adenovirus infection | IV | All ages | 5 mg/kg every week, with hyperhydration ± probenecid | |
| HSV infection, acyclovir-resistant and foscarnet-resistant | IV | All ages | 5 mg/kg every week for 2 wk, then every 2 wk, with hyperhydration ± probenecid | |
| VZV infection, acyclovir-resistant | IV | All ages | 5 mg/kg every week, with hyperhydration ± probenecid | |
| Letermovir | Prophylaxis against CMV infection | IV, Oral | ≥18 y | 480 mg every 24 h |
| Acyclovir | HSV infection (severe or disseminated disease) | IV | All ages | 10 mg/kg every 8 h |
| HSV infection (mild disease) | Oral | All ages | 1000 mg/d, in 3–5 divided doses | |
| HSV encephalitis | IV | ≥4 mo | 500 mg/m2 every 8 h, or 10–15 mg/kg every 8 h | |
| Prophylaxis against HSV infection | IV | All ages | 5 mg/kg every 8 h | |
| Oral | ≥2 y | 600–1000 mg/d, in 3–5 divided doses | ||
| Varicella or varicella-zoster | IV | <2 y | 10 mg/kg every 8 h | |
| IV | ≥2 y | 500 mg/m2 every 8 h, or 10 mg/kg every 8 h | ||
| Valacyclovir | HSV infection (mild localized infection disease) | Oral | ≥2 y | 20 mg/kg every 12 h (maximum dose 1000 mg) |
| Prophylaxis against HSV infection | Oral | ≥2 y | <40 kg: 250 mg every 12 h ≥40 kg: 500 mg every 12–24 h |
|
| Varicella or varicella-zoster | Oral | ≥2 y | 20 mg/kg every 8 h (maximum dose 1000 mg) | |
| Prophylaxis against VZV infection | Oral | ≥2 y | <40 kg: 250 mg every 12 h ≥40 kg: 500 mg every 12–24 h |
|
| VZV postexposure prophylaxis | Oral | ≥2 y | <40 kg: 500 mg every 8 h ≥40 kg: 1000 mg every 8 h |
|
| Oseltamivir | Treatment of influenza A and B infection | Oral | 0–8 mo | 3 mg/kg every 12 h |
| Oral | 9–11 mo | 3.5 mg/kg every 12 h | ||
| Oral | 1–12 y | ≤15 kg: 30 mg every 12 h 15-≤23 kg: 45 mg every 12 h 23-≤40 kg: 60 mg every 12 h >40 kg: 75 mg every 12 h |
||
| Oral | ≥13 y | 75 mg every 12 h | ||
| Prophylaxis against influenza A and B infection | Oral | ≥3 mo | Same as treatment dose, except given once daily | |
| Oral | ||||
| Baloxavir | Treatment of influenza A and B infection | Oral | ≥12 y | 40–80 kg: 40 mg |
| Prophylaxis against influenza A and B infection | >80 kg: 80 mg | |||
| Peramivir | Treatment of influenza A and B infection | IV | ≥2 y | 12 mg/kg (maximum dose 600 mg) |
| Zanamivir | Treatment of influenza A and B infection | Inhaled | ≥7 y | 10 mg every 12 h |
| Prophylaxis against influenza A and B infection | Inhaled | ≥5 y | 10 mg every 24 h | |
| Ribavirin | Treatment of RSV infection | Inhaled | All ages | 2000 mg every 8 h |
| Oral | All ages | 20 mg/kg/d divided 3 times per day (maximum dose 600 mg) |
Data from the American Academy of Pediatrics. Non-HIV Antiviral Drugs. In: DW K, ed. Red Book: 2021–2024 Report of the Committee on Infectious Diseases: American Academy of Pediatrics; 2021:930–48; and Lexicomp Online. Pediatric and Neonatal Lexi-Drugs Online. July 30, 2021 ed. Waltham, MA: UpToDate, Inc; 2021
Table 2.
Key antiviral drugs, their mechanisms of action, and toxicities
| Drug | Mechanism of Action | Key Toxicities |
|---|---|---|
| Ganciclovir94 | Acyclic nucleoside analog of guanosine, undergoes triphosphorylation and then targets the viral DNA polymerase to inhibit viral DNA synthesis by competitively inhibiting incorporation of dGTP into DNA | Cytopenias Renal dysfunction |
| Valganciclovir95 | Prodrug of ganciclovir, rapidly converted to ganciclovir by intestinal and hepatic enzymes after oral administration | Cytopenias Renal dysfunction |
| Foscarnet | Pyrophosphate analog, directly inhibits CMV DNA replication by binding to the DNA polymerase | Renal dysfunction Electrolyte wasting |
| Cidofovir | Monophosphate nucleotide analog, phosphorylated to its diphosphate form and then inhibits the viral DNA polymerase by competitively inhibiting incorporation of dCTP into DNA | Renal dysfunction |
| Letermovir | Inhibits CMV viral terminase complex, preventing packaging of DNA before encapsidation of viral genome | Gastrointestinal symptoms |
| Maribavir | Inhibits phosphorylation of nuclear lamins by UL97 kinase, preventing formation of viral capsid and nuclear egress of viral particles; also impacts viral gene expression and DNA synthesis | Dysgeusia Nausea and vomiting |
| Acyclovir96 | Acyclic guanosine analog, undergoes triphosphorylation and then targets the viral DNA polymerase to inhibit viral DNA synthesis by competitively inhibiting incorporation of dGTP into DNA | Renal dysfunction Anemia Neutropenia Nausea and vomiting Rare neurologic toxicity |
| Valacyclovir97 | Prodrug of acyclovir; after oral administration, rapidly converted to acyclovir via first-pass intestinal and hepatic metabolism | Headache Nausea and vomiting Acute renal failure Thrombotic thrombocytopenic purpura/Hemolytic uremic syndrome |
| Oseltamivir | Competitive inhibitor of influenza neuraminidase, preventing release of new virions | Nausea and vomiting |
| Baloxavir | Cap-endonuclease inhibitor, inhibits endonuclease subdomain of the viral RNA polymerase, preventing transcription of viral mRNA | Diarrhea |
| Peramivir | Competitive inhibitor of influenza neuraminidase, preventing release of new virions | |
| Zanamivir | Competitive inhibitor of influenza neuraminidase, preventing release of new virions | Cough Sore throat |
| Ribavirin | Guanosine analog, multiple mechanisms of action have been proposed, including inhibition of the viral polymerase of RNA viruses | Hemolytic anemia |
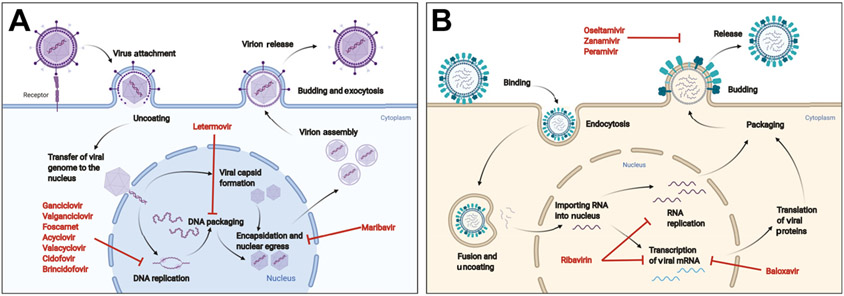
Fig. 1. Viral life cycles and mechanism of action of antiviral drugs.
(A) Herpesviruses: after initial infection, viral DNA enters the nucleus for replication and transcription. The nucleoside/nucleotide analogs block viral DNA replication by inhibiting the viral DNA polymerase. Letermovir acts to block the CMV terminase, preventing DNA packaging, whereas maribavir inhibits the viral UL97 kinase, preventing encapsidation of viral proteins. Letermovir and maribavir are only active against CMV. (B) Influenza viruses: after initial binding and endocytosis, the viral particles undergo fusion and uncoating, followed by importation of viral RNA into the nucleus. Viral RNA then undergoes replication and transcription into mRNA. Ribavirin may act to inhibit the viral RNA polymerase to prevent these processes. Baloxavir is a cap-endonuclease inhibitor that blocks mRNA transcription. After viral RNA and mRNA exit the nucleus, progeny virions are then formed and released. The neuraminidase inhibitors block the release of progeny virions. (Figures created with Biorender.com.)
HERPESVIRUS INFECTIONS
Herpesviruses are major pathogens in immunocompromised children and are particularly noteworthy because of their ability to establish latent infections. In transplant recipients, immunosuppressive agents can suppress cell-mediated immunity, enabling reactivation of latent herpesviruses. Herpesvirus reactivation may manifest as asymptomatic viremia or may present as symptomatic disease. The most clinically problematic herpesvirus in immunocompromised hosts is cytomegalovirus (CMV), though herpes simplex virus (HSV), varicella-zoster virus (VZV), or Epstein-Barr virus (EBV) also can cause significant disease.
CMV Infection
CMV is one of the most common opportunistic infections that affect HCT and SOT recipients. Approximately 60% to 80% of the population is infected with CMV by adulthood.3 Horizontal transmission through person-to-person contact is the most common route of acquisition for primary infection, though CMV can also be transmitted through blood products or organs from infected donors. Subsequent CMV reactivation after organ transplantation can then lead to asymptomatic viremia or tissue-invasive CMV disease. CMV has also been linked to graft rejection, GVHD, and increased risk of other opportunistic infections, making prevention of CMV a priority for improving transplant outcomes.4
Epidemiology and risk factors
The incidence of CMV infection and disease among transplant recipients has steadily declined as preventative strategies have evolved, with an associated improvement in mortality.5,6 HCT recipients at the highest risk for CMV infection are those with T-cell dysfunction, which is affected by conditioning regimen, transplant method, and immunosuppressive therapies used for GVHD. Conditioning regimens with strong lymphodepleting effects, such as those containing fludarabine or alemtuzumab, are associated with high rates of CMV infection.7 Cord blood and haploidentical transplant recipients, which are associated with delayed T-cell engraftment, are also at high risk for CMV reactivation.8
CMV infection after SOT can occur in both the early (<30 days) and the late (>6 months) post-transplant phases.7 In recipients of SOT, the type of transplanted organ impacts the risk of CMV reactivation and disease due to variation in immunosuppressive regimens used for different organs. Liver, heart, and kidney transplant recipients have a lower risk of CMV infection, whereas risk is higher in lung or small bowel transplant recipients.9
In the transplant setting, both donor and recipient serostatus strongly influence the risk of CMV infection or reactivation (Table 1). For that reason, universal screening of donor and recipient is recommended during the pretransplant evaluation for both SOT and HCT.4,10 Notably, children are less likely to have had prior CMV infection than adults, and thus are at higher risk for acquiring CMV from their donor.
Among adult HCT recipients, seropositivity is associated with increased mortality, and upward of 30% to 80% of seropositive recipients will have CMV reactivation post-transplant.7 The rate of CMV infection in seronegative patients is significantly lower, ranging from 0% to 12%. The high rates of CMV infection reflect the severe and prolonged immunosuppression associated with allogeneic HCT, as rates of CMV infection in seropositive recipients of autologous HCT are 0% to 33%.7
Seronegative SOT recipients (R−) who receive an organ from a seropositive donor (D+) comprise the highest risk donor-recipient combination for the development of primary infection and, ultimately, CMV disease.9 Seropositive recipients (R+) are at risk of CMV reactivation after initiation of immunosuppression. However, the risk is not limited solely to seropositive donors and recipients, and in one multicenter cohort of pediatric lung transplant recipients approximately 7% of D-/R-recipients developed primary CMV infection in the first 12 months after transplant.11
Clinical manifestations and diagnosis of CMV infection
Broadly speaking, CMV manifests in one of two forms: CMV infection or CMV disease. CMV infection is defined as the detection of viral antigens or nucleic acid in a body fluid or tissue specimen.12 Detection of CMV may represent primary infection, reactivation of prior infection, or reinfection. Detection of CMV in blood is also specifically defined as viremia (if detected by viral culture), antigenemia (if detected using a pp65 antigen assay), or DNAemia (if detected using nucleic acid amplification techniques).12 The latter is the most common contemporary method of CMV detection.
CMV disease is defined as the presence of clinical symptoms and is subdivided into 2 specific forms, CMV syndrome and end-organ disease. CMV syndrome involves the detection of CMV in the blood as well as 2 of the following symptoms12:
Fever for at least 2 days
New or increased malaise or fatigue
Leukopenia or neutropenia
Thrombocytopenia
Presence of ≥5% atypical lymphocytes
Elevation of alanine aminotransferase or aspartate aminotransferase
CMV syndrome can be difficult to distinguish from other transplant-related causes of fever, malaise, transaminitis, or cytopenias.
Tissue-invasive CMV disease can take many forms, depending on the affected organ system. The end-organ disease is diagnosed by the presence of CMV on histopathology, culture, virus isolation, immunohistochemistry, or DNA hybridization of biopsy tissue.12 Importantly, the presence of clinical symptoms and detectable CMV in blood or serum is not sufficient to definitively diagnose CMV disease–a tissue diagnosis is needed to prove the diagnosis. Definitive tissue diagnosis may not be able to be obtained in critically ill patients, in which case empirical treatment should not be delayed.
Prevention of CMV infection and disease
Prevention of CMV in the post-transplant period has generally taken one of two forms: prophylaxis with antiviral drugs or pre-emptive strategies (Table 1). In the former, antiviral drugs are given to at-risk patients after transplantation, usually for a prespecified period. With pre-emptive therapy, patients undergo serial screening for CMV infection, usually with quantitative polymerase chain reaction (PCR) assays. If there is evidence of CMV replication, identified by an increase in CMV copy number, treatment with antiviral therapy is then initiated.
Each strategy has positive and negative features.4,13 Universal prophylaxis decreases CMV infection and disease but is costly due to increased antiviral medication use and number of drug-related adverse events even in patients who may not have developed CMV infection or disease.14 Pre-emptive therapy enables stimulation of natural CMV immunity post-transplant and may allow for quicker immune reconstitution in HCT.13 However, logistical difficulties related to serial screening and the unknown indirect effects of early CMV DNAemia on the transplanted graft pose a challenge. In addition, the optimal threshold to trigger initiation of pre-emptive therapy has not been defined.4 It is not uncommon for a hybrid approach to be taken, wherein patients receive antiviral prophylaxis for a set period, followed by surveillance for the development of CMV DNAemia after discontinuation of prophylaxis.13 No clinical trials have yet evaluated the comparative efficacy of prophylaxis, pre-emptive therapy, or surveillance after prophylaxis strategies in children. Importantly, effective prevention of CMV infection has altered the epidemiology of CMV infection, to the point where many episodes of CMV infection and disease occur later after transplant, commonly after completion of CMV prophylaxis.15
Antiviral prophylaxis in HCT.
Historically, universal prophylaxis was used less frequently in HCT because of risks of myelosuppression with ganciclovir and valganciclovir, though it is used more frequently with the approval of new antiviral agents.13 Three recent systematic reviews and a corresponding meta-analysis have shown that antiviral prophylaxis is effective in reducing both CMV infection and CMV disease but not all-cause mortality.16-18
Ganciclovir and its oral prodrug, valganciclovir, provide strong anti-CMV activity, and have been shown to prevent CMV infection and disease in early trials in adult HCT.17 Pharmacokinetic studies have demonstrated that equal or higher drug concentrations can be achieved with valganciclovir compared with intravenous ganciclovir.19,20 However, the use of these agents is limited by adverse events such as neutropenia, which is reported in 25% to 62% of patients in clinical trials.16
Letermovir shows great promise as a drug for CMV prophylaxis, both in its ability to prevent CMV infection and its favorable side-effect profile. Placebo-controlled trials in adult HCT recipients showed that letermovir was effective at preventing CMV infection, and was associated with a decrease in all-cause mortality.21,22 Although this finding was not confirmed in a subsequent meta-analysis, studies are ongoing.17 The primary side effects of letermovir are gastrointestinal, with minimal hematologic or renal toxicity reported. Letermovir does not have antiviral activity against other herpesviruses, so patients receiving letermovir should also receive prophylaxis against HSV or VZV if indicated. A phase 2 trial evaluating the safety and tolerability of letermovir prophylaxis for CMV in pediatric HCT recipients is currently ongoing (NCT03940586).
Other drugs have been studied as potential prophylactic agents for CMV in HCT. Neither acyclovir nor valacyclovir significantly reduced CMV infection or disease compared with placebo,16,17 consistent with their lower level of activity against CMV in vitro.13 Maribavir, an oral benzimidazole riboside with potent anti-CMV activity in vitro, did not prevent CMV disease compared with placebo in phase 3 studies in both adult liver transplant and HCT recipients.23,24 Another oral agent, brincidofovir, did not improve rates of CMV infection at 24 weeks in adult HCT recipients compared with placebo and resulted in more significant adverse events.25,26 Foscarnet has not been studied as a prophylactic agent in controlled trials in HCT and its use is limited by renal toxicity.10
Antiviral prophylaxis in SOT.
Universal prophylaxis is frequently used in SOT, and prophylaxis after SOT has been shown to reduce CMV infection and disease, with positive outcomes regarding graft survival, incidence of opportunistic infections, and mortality.13,27 The prophylaxis regimen and duration of prophylaxis are determined by the transplanted organ and the serostatus of the donor and recipient (see Table 1).
Valganciclovir is the most frequently used prophylactic agent, though acyclovir, valacyclovir, and ganciclovir have also been studied for universal prophylaxis. Oral ganciclovir was more effective than acyclovir in preventing CMV infection and disease.28 Valganciclovir has been shown to be equivalent to ganciclovir in multiple studies.28,29 However, subgroup analyses in one study revealed that liver transplant recipients given valganciclovir prophylaxis had increased incidence of tissue invasive CMV,29 and valganciclovir has not been approved for prophylaxis in that population.
Pre-emptive therapy in HCT and SOT.
The basic principles of pre-emptive therapy are similar in HCT and SOT, though much of the data are derived from studies in HCT patients. Patients are monitored with serial (weekly) blood PCR testing. When a test is positive at a defined viral threshold (eg, 1000 copies per mL), antiviral therapy is initiated. Threshold levels are determined by each treatment team, given variability across PCR assays. Treatment is generally continued for a minimum of 2 weeks, with at least one negative CMV test required before discontinuation of therapy. If CMV is still detected after 2 weeks of therapy, then a transition to maintenance therapy can be executed. A prolonged duration of therapy may be required in patients with prolonged positivity or slow clearance of CMV.10 After discontinuation of therapy, patients resume surveillance testing. A second episode of CMV infection can be treated with the same drug initially, though alternative agents should be used if resistance is identified or suspected (see the following section “Management of resistant CMV infection and disease”).
Ganciclovir is the most commonly used initial treatment in pre-emptive therapy.10 Valganciclovir has also been used as pre-emptive therapy, though there have been few controlled trials in HCT or SOT.30 Foscarnet has also been shown to be effective as pre-emptive therapy in multiple small, randomized trials in adult HCT recipients.31,32 Maribavir is also under investigation as pre-emptive therapy in both HCT and SOT recipients.
Treatment of CMV Infection and Disease
Ganciclovir and valganciclovir are the first-line therapeutic agents for the treatment of CMV infection and disease (Table 1).4,10 For treatment of CMV disease, either ganciclovir or valganciclovir can be used as initial therapy, as valganciclovir was shown to be noninferior to ganciclovir in a randomized, controlled trial in adult SOT recipients.33 However, ganciclovir is preferred in cases of severe, life-threatening CMV disease, or when absorption of valganciclovir may be diminished (as in gastrointestinal CMV disease). After clinical improvement, patients can be transitioned to oral valganciclovir. Foscarnet can be used as an alternative first-line agent if ganciclovir cannot be given because of adverse effects.10,27
Antiviral therapy should be continued until resolution of clinical disease and clearance of CMV DNAemia. However, the absence of CMV DNA in the blood may not reflect clinical response in tissue-invasive disease. Longer courses of treatment may be needed in cases of gastrointestinal disease, pneumonitis, or CNS disease, based on clinical response.4
Management of resistant CMV infection and disease
Concern for antiviral resistance arises when a patient develops refractory CMV infection, which is defined as increasing or persistent CMV DNAemia after ≥2 weeks of antiviral therapy.34 Worsening symptoms or progression of organ-specific disease despite adequate therapy might also indicate the presence of resistant CMV.4,10,34 Resistant/refractory CMV infection can occur after prolonged exposure to antiviral agents.4,10 Subtherapeutic drug levels may also contribute to the development of resistance. In single-center studies in pediatric HCT, antiviral resistance was present in less than 10% of cases.35,36 Similarly, low levels of resistance have been reported in case series of CMV infection in SOT recipients.36
Molecular assays are used to identify specific mutations that confer resistance to each antiviral drug (Table 2). There are no clinical trial data to guide the management of resistant CMV infections, and the treatment principles for resistant infections are based on expert opinion.4,37 For alterations in UL97 that confer low-level ganciclovir resistance, such as the C592 G mutation, high-dose ganciclovir (10 mg/kg twice daily) may be effective.37 If high-level ganciclovir resistance is present, treatment-dose foscarnet can be used.38 If a mutation confers resistance to both ganciclovir and foscarnet, then cidofovir may be used, though efficacy is uncertain.37
Maribavir has shown promise in the treatment of resistant infections. In a phase 3 trial, adults treated with maribavir were more likely to have clearance of CMV DNAemia after 8 weeks compared to those treated with alternative therapies, including ganciclovir, valganciclovir, foscarnet, and cidofovir.39 There are limited data regarding the use of maribavir in children. There are no comparative data supporting the use of letermovir for the treatment of CMV infection.
Virus-specific T-lymphocytes (VSTs) have also been used to provide targeted therapy for refractory CMV infection.40 CMV-specific T-cells are selected from donors and then undergo ex vivo expansion before infusion into the infected patients. VSTs can be harvested from the patient’s HCT donor, or patients may be given “off-the-shelf” HLA-matched products from third-party VST banks. Several small-scale studies have reported that both donor VSTs and off-the-shelf products can effectively treat refractory CMV disease.41,42 However, data are limited and VSTs are not frequently used.
HSV Infection
HSV1 and HSV2 infections in transplant recipients are frequently caused by reactivation of prior infection, though primary infection is possible and typically more severe. HSV establishes latency in the dorsal root ganglia and can reactivate in states of physiologic stress or immune suppression. Donor-derived infections are rare because the virus is generally not present in the transplanted organ.43
Epidemiology and Risk Factors
The risk of HSV reactivation is related to a patient’s degree of immunosuppression.43 Infection frequently occurs in the first 4 weeks after HCT. HSV reactivation occurs less frequently in adult SOT, and in one large cohort of adult SOT recipients, 6.7% developed HSV infection within 1-year post-transplant, with a median onset of 66 days after SOT.44
Clinical Manifestations of HSV Infection
HSV infections in immunocompromised patients can present similarly to infection in immunocompetent hosts. Severe local infections, such as gingivostomatitis, esophagitis, or cutaneous disease, can occur.45 Highly immunocompromised patients such as HCT recipients are at risk for the development of disseminated disease with end-organ involvement and may be at risk for prolonged symptoms or a more severe disease course.43,46 Immunocompromised patients are also at risk for frequent HSV reactivations, and reactivation flares may be prolonged.45
Prevention of HSV Infection
HSV prophylaxis with acyclovir or valacyclovir was shown to be effective in reducing HSV reactivation and disease in one meta-analysis that included 22 randomized controlled trials of antiviral prophylaxis in adult HCT recipients.18 Antiviral prophylaxis against HSV is now the standard of care in seropositive HCT recipients.46 A meta-analysis of 12 randomized trials examining HSV reactivation following SOT in adults found that the use of acyclovir prophylaxis significantly reduced HSV disease.47 Owing to the relative protection against HSV conferred by anti-CMV agents, HSV prophylaxis is given to seropositive patients who are not receiving prophylaxis against CMV.
Treatment of HSV Infection
Intravenous acyclovir is the primary drug to treat HSV infection (Table 3). For more severe infections such as gingivostomatitis, meningoencephalitis, hepatitis, or pneumonitis, intravenous acyclovir is the preferred treatment. For mild disease or localized infections, oral valacyclovir is adequate.48 The duration of treatment is dependent on the severity of infection; for mild infections treatment of 7 to 10 days is likely sufficient, whereas severe infections are usually treated for 14 to 21 days. In immunocompromised patients, treatment is generally continued until immunosuppression resolves, though patients may be transitioned to a prophylactic regimen after the treatment course is completed.
Table 3.
CMV reactivation risk and prophylaxis regimens in transplant
| Serostatus | Organ | Risk of CMV Reactivation |
Recommendation |
|---|---|---|---|
| D−/R− | All Organs | Low | Prophylaxis not recommended |
| D+/R− | HCT | Intermediate | 100 d |
| Kidney, liver, or heart | High | 3–6 moa | |
| Lung | High | 6–12 mo | |
| D−/R+ | HCT | High | 100 d |
| Kidney, liver, or heart | Intermediate | 3–6 mo | |
| Lung | High | 6–12 mob | |
| D+/R+ | HCT | High | 100 d |
| Kidney, liver, or heart | Intermediate | 3–6 mo | |
| Lung | High | 6–12 mob |
Abbreviations: D+, donor seropositive; D−, donor seronegative; HCT, hematopoietic stem cell transplantation; R+, recipient seropositive; R−, recipient seronegative
Valganciclovir is not approved for use as prophylaxis in liver transplantation29
CMV immune globulin is sometimes given to high-risk lung transplant recipients, in addition to antiviral prophylaxis4
HSV that is resistant to acyclovir is rare; in one large study of adult HCT recipients, acyclovir-resistant HSV occurred in 0.4% of patients.49 Resistance testing is onerous and requires viral culture and phenotypic evaluation. However, isolates should be susceptible to foscarnet and cidofovir, both of which do not require the viral thymidine kinase for prodrug metabolism, thus are the preferred agents for acyclovir-resistant HSV.43,46
VZV Infection
Children who have not completed vaccination before transplant are at risk for developing primary infection. As with HSV, VZV develops latency in nerve ganglia and can reactivate with stress or immunosuppression. VZV reactivation is commonly symptomatic and can be severe and/or disseminated in immunocompromised hosts. Donor-derived infections are rare, but have been reported.50
Epidemiology and Risk Factors
The development of VZV infection post-transplant is associated with serostatus pretransplant and the patient’s overall level of immunosuppression. VZV infection has been reported to occur in 10% to 68% of pediatric and adult patients following HCT, particularly before T-cell engraftment and in patients who develop GVHD.46,51 In adult SOT, VZV is uncommon; 2.1% developing infection at 1 year after transplant and 4.0% overall in one cohort.44 Heart and lung transplant recipients have higher rates of VZV compared with other organ transplants. In children, the incidence is thought to be similar to that in adults.50
Clinical Manifestations
The clinical presentation of primary varicella and zoster in SOT and HCT recipients is similar to that of the general pediatric population, though immunocompromised children are at increased risk of severe disease.46,50 Primary VZV infection can present as a pruritic vesicular rash that evolves into crusted scabs or, less commonly, as hemorrhagic lesions.52 However, atypical infection without a notable rash can occur in immunocompromised children.46 Severe disease with dissemination to the liver, lungs, or other organs can occur in immunocompromised children. VZV reactivation, or zoster, manifests as itchy or painful groups of skin lesions that are conventionally localized to specific sensory dermatomes, though may present atypically in immunocompromised hosts including in a disseminated fashion similar to primary varicella.
Prevention and Treatment of VZV Infection
Antiviral prophylaxis with acyclovir or valacyclovir is effective in reducing the incidence of VZV disease in adult HCT and SOT recipients.46,47,50 Prophylaxis is generally given to seropositive HCT recipients for at least 1 year after transplant,46 though long-term use in SOT is uncommon.50 Antiviral prophylaxis against CMV also offers protection against VZV, and patients who receive CMV prophylaxis do not require additional VZV prophylaxis.18,46,47,50
When VZV infection occurs in immunocompromised children, early treatment is prudent to prevent dissemination and end-organ involvement. Intravenous acyclovir therapy is recommended for use in immunocompromised patients because of the risk of disseminated disease (Table 1).52 Oral acyclovir should not be used because of poor bioavailability, though some experts advocate for use of oral valacyclovir in patients deemed to be at low risk for dissemination.52 The duration of antiviral treatment is similar to that of HSV infection. Following the primary treatment course, patients generally receive antiviral prophylaxis until their immunocompromised state has resolved.
ADENOVIRUS INFECTIONS
Human adenoviruses (HAdVs) cause infections of the respiratory and gastrointestinal tracts, and conjunctivitis in healthy children. Infection in immunocompromised children is predominantly de novo infection, though HAdV infection from donor tissues has also been described in SOT.53
Epidemiology and Risk Factors
In pediatric HCT recipients, HAdVs range from 12% to 42%.53,54 Risk factors that have been identified in HCT include a T-cell-depleted graft, an unrelated donor, severe GVHD, or severe lymphopenia.54,55 The correlation between more profound immunosuppression and risk of infection is reflected in the variability of HAdV incidence in pediatric SOT, which ranges from 4% to 38% in liver transplant to greater than 50% in intestinal or heart, lung, or heart-lung transplant.56
Clinical Manifestations
In immunocompromised patients, HAdV infection can present with severe localized infection, including gastroenteritis/colitis, pneumonia, hepatitis, nephritis, or cystitis.53 HAdV can also cause disseminated disease characterized by severe sepsis and multi-organ-system involvement.57 Mortality can exceed 50% in disseminated disease.53,57
Prevention and Treatment of Adenovirus Infection
No evidence exists to support the use of antiviral drugs for prophylaxis of HAdV infection, and prophylaxis is not currently recommended for patients undergoing HCT or SOT.55,56
In HCT, pre-emptive treatment is recommended for patients at risk for HAdV infection and disease, and antiviral treatment may be initiated if HAdV is detected in the blood.55 For SOT recipients, asymptomatic infections are generally not treated.53,56 If symptomatic disease is present, antiviral therapy and reduction of immunosuppression are the first steps in management.56
Clinical trials or comparative effectiveness studies have not been conducted to evaluate antiviral treatment for HAdV infections resulting in no approved agents for the treatment of HAdV infections. Cidofovir, a nucleoside analog of cytosine, is the most frequently used antiviral drug to treat HAdV infection (Table 1).55,56 The optimal dose of cidofovir is unknown, although the most frequently used dosing is 5 mg/kg weekly. An alternative lower dose of 1 mg/kg 3 times per week has been used to mitigate toxicity.58 Owing to significant nephrotoxicity, hyperhydration is necessary and probenecid is given to decrease kidney tissue drug levels by competitively inhibiting cidofovir reuptake in the kidney. Toxicity and poor in vivo efficacy are major limiting factors for the use of cidofovir. Other drugs have been evaluated, including brincidofovir, ganciclovir, and ribavirin, but none are routinely used.
RESPIRATORY VIRUSES
Antiviral therapies are not available for the vast majority of respiratory viral infections that occur in pediatric transplant patients. Influenza virus and respiratory syncytial virus (RSV) are two exceptions. In this section, we discuss the presentation of influenza and RSV in pediatric transplant recipients as well as antiviral agents available to treat them.
Influenza
Influenza infection results in a spectrum of symptoms in pediatric transplant recipients from mild congestion to respiratory failure. Influenza has also been associated with graft rejection, particularly in lung transplant recipients, though current reports are conflicting and the pathophysiology behind this association is unclear.59,60
Epidemiology and Risk Factors
In transplant recipients, influenza virus infection mirrors the seasonality observed in immune-competent hosts with increased incidence in colder months. Symptomatic infection is more common in recipients of lung or heart transplants compared with liver and kidney recipients. Infection occurs more frequently in younger patients and during the first year after SOT, reflecting increased risks of infection during periods of substantial immunosuppression.61,62 Influenza infection is associated with increased hospitalization, need for mechanical ventilation, and death among adult SOT recipients,63 but does not appear to substantially impact mortality in pediatric SOT recipients.61,62
Clinical Manifestations
Influenza infection causes a similar clinical syndrome in transplant recipients compared with healthy children. Most patients present with fever and cough. Additional symptoms include rhinorrhea, headache, pharyngitis, myalgias, and gastrointestinal distress.63 In both SOT and HCT recipients, severe complications of influenza infection are more common than in the general population.64,65 Substantial complications observed in a recent large multicenter cohort of transplant recipients included lower respiratory tract infection (LRTI), hospitalization, and ICU admission,63 though lower rates of severe disease were observed in the small group of pediatric patients evaluated.
Prevention and Treatment of Influenza Infection
In contrast to herpesviruses, antiviral agents are not routinely used to prevent acquisition of influenza, rather rigorous infection prevention measures such as handwashing, masking, and avoidance of sick contacts are used. Inpatient infection prevention measures are particularly important given the risk of nosocomial infection in this population.
Pre-exposure prophylaxis.
Vaccination is an important preventative measure even among immunocompromised patients. Although serologic response to vaccination is variable among transplant recipients and lower than that in healthy controls, immunized transplant recipients have fewer complications of influenza infection than those who are unimmunized.66,67 Among adult and pediatric transplant recipients, those who received an annual influenza vaccine were less likely to present with a LRTI and less likely to require ICU admission.63
Antiviral agents.
Neuraminidase (NA) inhibitors are effective against influenza A and B and are approved for the treatment of influenza infection (Table 1). Oseltamivir is an oral agent with pharmacokinetic data available for all pediatric age groups. Zanamivir (inhaled) and peramivir (intravenous) are effective NA inhibitors that are less well studied in transplant recipients and children. Baloxavir is orally available and recently was approved for the treatment of uncomplicated influenza in patients older than 12 years.68 An international clinical trial investigating the utility of baloxavir to treat high-risk adolescent and adult outpatients (eg, with comorbid asthma or diabetes mellitus) with influenza infection showed similar efficacy of single-dose baloxavir to treatment courses of oseltamivir, though transplant recipients were excluded from this trial.69
Postexposure prophylaxis.
Although immunization is the most well-studied and effective form of preventing illness related to influenza infection, prophylaxis may be effective in select transplant recipients.70,71 A more well-defined preventative paradigm is that of postexposure prophylaxis in patients at high risk for complications of influenza infection. In numerous randomized, placebo-controlled trials, NA inhibitors were effective in preventing influenza illness in patients who had close contact (household or nosocomial) with an infected individual.72,73 Recommendations for SOT and HCT recipients include prophylaxis with oseltamivir or zanamivir within 48 hours of exposure.74,75
Treatment of influenza.
Treatment with oseltamivir or zanamivir is recommended for all pediatric transplant recipients with symptoms compatible with influenza infection, concurrent with diagnostic evaluation.74 Initiation of treatment within 48 hours of symptom onset is associated with decreased mortality and ICU admission.63,64 More data are available regarding the efficacy of oseltamivir in pediatric transplant recipients, although IV formulations (zanamivir or peramivir) may be considered in critically ill patients or those unable to tolerate oral medications. Although early treatment is associated with improved outcomes, symptomatic transplant recipients should receive antiviral therapy even if they present for care beyond the first 48 hours of illness.74 Treatment with antivirals should be at least 5 days and longer durations may be used in cases of prolonged viral shedding, which is more common in transplant recipients.76
Respiratory syncytial virus.
In transplant recipients, RSV can cause highly morbid lower respiratory tract disease. Substantial efforts have been made toward preventing the progression of RSV disease to LRTI in transplant recipients in order to mitigate morbidity and mortality.
Epidemiology and Risk Factors
Most children have been infected with RSV by age 2 years, though reinfection can occur at any point. The case fatality rate of immune-competent children with RSV is less than 0.5%, but has been reported to be up to 50% in pediatric HCT recipients.77,78 Risk factors for the development of LRTI or severe RSV disease among pediatric transplant recipients include age less than 2 years, daycare attendance, lung or heart/lung transplantation, underlying lung disease, and HCT.79
Clinical Manifestations and Diagnosis of RSV Infection
RSV may present as a nonspecific URI with rhinorrhea, cough, fever, and wheeze, and in healthy children is self-resolving within 1 to 2 weeks. Progression to LRTI manifests as bronchiolitis or pneumonia, which can progress to respiratory failure. In adult HCT recipients, up to 74% with RSV infection progress to LRTI though contemporary reports of pediatric HCT recipients suggest only ~20% progression to LRTI.80,81 Risk factors for progression to LRTI in HCT recipients are not well defined in pediatrics, but coinfections and pulmonary comorbidities are associated with progression to LRTI.80,82 Among SOT recipients, lung transplantation and lung disease are associated with increased severity of disease, although not consistently across all studies.74,83
PREVENTION AND TREATMENT OF RSV INFECTION
Prophylaxis.
RSV is transmitted through contact with secretions of an infected individual including through droplets and contaminated fomites. Contact precautions and isolation procedures should be used to prevent transmission of infection to transplant recipients. Palivizumab is an RSV-specific humanized monoclonal antibody, which targets the F glycoprotein of RSV, thereby inhibiting viral replication. Palivizumab is effective in preventing hospitalization for RSV infection and progression to LRTI in high-risk children less than 2 years of age, particularly those with lung disease of pre-maturity and congenital heart disease.84,85 Owing to substantial cost, the American Association of Pediatrics has generated guidelines for the use of RSV prophylaxis that suggest the use of palivizumab in immunosuppressed children under 2 years of age. Single-center studies have shown minimal benefit in reducing RSV infection in pediatric HCT recipients through the use of palivizumab.86 Very little data exist on the efficacy of palivizumab as prophylaxis in the pediatric SOT population. Current guidelines from AST suggest the use of palivizumab only in the youngest and most high-risk SOT recipients.74
Prevention and treatment of LRTI.
Few options are available for the treatment of RSV infection, but prevention of RSV progression to LRTI can mitigate severe disease and complications of RSV infection in pediatric transplant patients. Inhaled ribavirin has been studied both as a pre-emptive treatment in patients with URI and definitive treatment of established LRTI (Table 1). Early studies of inhaled ribavirin for the prevention of progression to LRTI in pediatric HCT recipients were promising: no treated patients in several single-center studies developed LRTI.87,88 More recent evaluations have shown a benefit in the prevention of LRTI progression as well as in mortality among those who received ribavirin.89 A large meta-analysis of HCT recipients treated with ribavirin suggested a trend toward decreased LRTI and decreased RSV-related mortality among patients treated with inhaled ribavirin.90 Ribavirin also is available as an oral formulation, and studies are mixed regarding a benefit of pre-emptive therapy in small studies of HCT and SOT recipients with RSV URI.91,92
Although institutional practice varies, treatment of HCT patients with ribavirin as a pre-emptive or treatment strategy is commonly used. The cost of inhaled ribavirin is significant and, given the modest benefit of pre-emptive treatment, contemporary practices vary in SOT recipients infected with RSV.93 The AST recommends preemptive treatment with inhaled ribavirin for RSV-infected pediatric lung transplant recipients, and treatment of other SOT recipients with LRTI should be considered depending on additional risk factors.74
SUMMARY
Viral infections are a major cause of morbidity and mortality in pediatric transplant patients. Antiviral drugs are used to prevent and treat these infections, but therapeutic options are limited. Future research is needed to further development of antiviral drugs and their use in children.
KEY POINTS.
Viral infections occur frequently in immunocompromised pediatric transplant recipients and can cause substantial morbidity and mortality.
Cytomegalovirus and other herpesviruses may cause primary infection, donor-derived infection, or reactivation of prior infection in transplant recipients; strategies for prevention of end-organ disease are essential to mitigate morbidity.
Few antiviral agents exist for the prophylaxis and treatment of viral infections.
Prophylactic, surveillance, and treatment strategies used for pediatric transplant recipients are frequently extrapolated from studies done primarily in adult patients.
Novel antiviral strategies, including drugs with unique mechanisms of action and adoptive T-cell therapy, are being investigated for use in transplant recipients.
CLINICS CARE POINTS.
Viral infections are common causes of morbidity and mortality in pediatric transplant patients.
Antiviral treatment options are limited, and preventative measures are essential.
Much of the antiviral treatment guidelines used in pediatric patients are extrapolated from data gathered in adult patients.
DISCLOSURE
This work was supported by funding from the National Institutes of Health (K08 CA212299), the Children’s Discovery Institute and the Washington University School of Medicine.
REFERENCES
- 1.Fishman JA. Infection in solid-organ transplant recipients. New Engl J Med 2007;357:2601–14. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasan A, Wang C, Srivastava DK, et al. Timeline, epidemiology, and risk factors for bacterial, fungal, and viral infections in children and adolescents after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2013;19:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Academy of Pediatrics. Cytomegalovirus infection. In: Kimberlin DW, Brady MT, Jackson MA, et al. , editors. Red book: 2021 report of the committee on infectious diseases. American Academy of Pediatrics; 2021. p. 310–7. [Google Scholar]
- 4.Kotton CN, Kumar D, Caliendo AM, et al. The Third International Consensus Guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2018;102:900–31. [DOI] [PubMed] [Google Scholar]
- 5.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med 2010;363:2091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicastro E, Giovannozzi S, Stroppa P, et al. Effectiveness of preemptive therapy for cytomegalovirus disease in pediatric liver transplantation. Transplantation 2017;101:804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Styczynski J Who Is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther 2018;7:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sauter C, Abboud M, Jia X, et al. Serious infection risk and immune recovery after double-unit cord blood transplantation without antithymocyte globulin. Biol Blood Marrow Transplant 2011;17:1460–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin JM, Danziger-Isakov LA. Cytomegalovirus risk, prevention, and management in pediatric solid organ transplantation. Pediatr Transplant 2011;15:229–36. [DOI] [PubMed] [Google Scholar]
- 10.Ljungman P, de la Camara R, Robin C, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis 2019;19:e260–72. [DOI] [PubMed] [Google Scholar]
- 11.Danziger-Isakov LA, Worley S, Michaels MG, et al. The risk, prevention, and outcome of cytomegalovirus after pediatric lung transplantation. Transplantation 2009;87:1541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis 2017;64:87–91. [DOI] [PubMed] [Google Scholar]
- 13.Haidar G, Boeckh M, Singh N. Cytomegalovirus infection in solid organ and hematopoietic cell transplantation: state of the evidence. J Infect Dis 2020;221:S23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Florescu DF, Qiu F, Schmidt CM, et al. A direct and indirect comparison meta-analysis on the efficacy of cytomegalovirus preventive strategies in solid organ transplant. Clin Infect Dis 2014;58:785–803. [DOI] [PubMed] [Google Scholar]
- 15.Limaye AP, Bakthavatsalam R, Kim HW, et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation 2006;81:1645–52. [DOI] [PubMed] [Google Scholar]
- 16.Chen K, Cheng MP, Hammond SP, et al. Antiviral prophylaxis for cytomegalovirus infection in allogeneic hematopoietic cell transplantation. Blood Adv 2018;2:2159–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gagelmann N, Ljungman P, Styczynski J, et al. Comparative efficacy and safety of different antiviral agents for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation: a systematic review and meta-analysis. Biol Blood Marrow Transplant 2018;24:2101–9. [DOI] [PubMed] [Google Scholar]
- 18.Beyar-Katz O, Bitterman R, Zuckerman T, et al. Anti-herpesvirus prophylaxis, pre-emptive treatment or no treatment in adults undergoing allogeneic transplant for haematological disease: systematic review and meta-analysis. Clin Microbiol Infect 2020;26:189–98. [DOI] [PubMed] [Google Scholar]
- 19.Winston DJ, Baden LR, Gabriel DA, et al. Pharmacokinetics of ganciclovir after oral valganciclovir versus intravenous ganciclovir in allogeneic stem cell transplant patients with graft-versus-host disease of the gastrointestinal tract. Biol Blood Marrow Transplant 2006;12:635–40. [DOI] [PubMed] [Google Scholar]
- 20.Einsele H, Reusser P, Bornhauser M, et al. Oral valganciclovir leads to higher exposure to ganciclovir than intravenous ganciclovir in patients following allogeneic stem cell transplantation. Blood 2006;107:3002–8. [DOI] [PubMed] [Google Scholar]
- 21.Chemaly RF, Ullmann AJ, Ehninger G. CMV prophylaxis in hematopoietic-cell transplantation. New Engl J Med 2014;371:576–7. [DOI] [PubMed] [Google Scholar]
- 22.Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med 2017;377:2433–44. [DOI] [PubMed] [Google Scholar]
- 23.Winston DJ, Saliba F, Blumberg E, et al. Efficacy and safety of maribavir dosed at 100 mg orally twice daily for the prevention of cytomegalovirus disease in liver transplant recipients: a randomized, double-blind, multicenter controlled trial. Am J Transplant 2012;12:3021–30. [DOI] [PubMed] [Google Scholar]
- 24.Marty FM, Ljungman P, Papanicolaou GA, et al. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis 2011;11:284–92. [DOI] [PubMed] [Google Scholar]
- 25.Marty FM, Winston DJ, Rowley SD, et al. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med 2013;369:1227–36. [DOI] [PubMed] [Google Scholar]
- 26.Marty FM, Winston DJ, Chemaly RF, et al. A randomized, double-blind, placebo-controlled phase 3 trial of oral brincidofovir for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2019;25:369–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients-Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019;33:e13512. [DOI] [PubMed] [Google Scholar]
- 28.Hodson EM, Ladhani M, Webster AC, et al. Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev 2013;(2):CD003774. [DOI] [PubMed] [Google Scholar]
- 29.Paya C, Humar A, Dominguez E, et al. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 2004;4:611–20. [DOI] [PubMed] [Google Scholar]
- 30.Chawla JS, Ghobadi A, Mosley J 3rd, et al. Oral valganciclovir versus ganciclovir as delayed pre-emptive therapy for patients after allogeneic hematopoietic stem cell transplant: a pilot trial (04-0274) and review of the literature. Transpl Infect Dis 2012;14:259–67. [DOI] [PubMed] [Google Scholar]
- 31.Reusser P, Einsele H, Lee J, et al. Randomized multicenter trial of foscarnet versus ganciclovir for preemptive therapy of cytomegalovirus infection after allogeneic stem cell transplantation. Blood 2002;99:1159–64. [DOI] [PubMed] [Google Scholar]
- 32.Moretti S, Zikos P, Van Lint MT, et al. Forscarnet vs ganciclovir for cytomegalovirus (CMV) antigenemia after allogeneic hemopoietic stem cell transplantation (HSCT): a randomised study. Bone Marrow Transplant 1998;22:175–80. [DOI] [PubMed] [Google Scholar]
- 33.Asberg A, Humar A, Rollag H, et al. Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 2007;7:2106–13. [DOI] [PubMed] [Google Scholar]
- 34.Chemaly RF, Chou S, Einsele H, et al. Definitions of resistant and refractory cytomegalovirus infection and disease in transplant recipients for use in clinical trials. Clin Infect Dis 2019;68:1420–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi SH, Hwang JY, Park KS, et al. The impact of drug-resistant cytomegalovirus in pediatric allogeneic hematopoietic cell transplant recipients: a prospective monitoring of UL97 and UL54 gene mutations. Transpl Infect Dis 2014;16:919–29. [DOI] [PubMed] [Google Scholar]
- 36.Kim YJ, Boeckh M, Cook L, et al. Cytomegalovirus infection and ganciclovir resistance caused by UL97 mutations in pediatric transplant recipients. Transpl Infect Dis 2012;14:611–7. [DOI] [PubMed] [Google Scholar]
- 37.El Chaer F, Shah DP, Chemaly RF. How I treat resistant cytomegalovirus infection in hematopoietic cell transplantation recipients. Blood 2016;128:2624–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avery RK, Arav-Boger R, Marr KA, et al. Outcomes in transplant recipients treated with foscarnet for ganciclovir-resistant or refractory cytomegalovirus infection. Transplantation 2016;100:e74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avery RK, Alain S, Alexander BD, Blumberg EA, Chemaly RF, Cordonnier C, Duarte RF, Florescu DF, Kamar N, Kumar D, Maertens J, Marty FM, Papanicolaou GA, Silveira FP, Witzke O, Wu J, Sundberg AK, Fournier M; SOLSTICE Trial Investigators. Maribavir for Refractory Cytomegalovirus Infections With or Without Resistance Post-Transplant: Results from a Phase 3 Randomized Clinical Trial. Clin Infect Dis. 2021. Dec 2:ciab988. doi: 10.1093/cid/ciab988. Epub ahead of print. PMID: 34864943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Houghtelin A, Bollard CM. Virus-specific T cells for the immunocompromised patient. Front Immunol 2017;8:1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feuchtinger T, Opherk K, Bethge WA, et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood 2010;116:4360–7. [DOI] [PubMed] [Google Scholar]
- 42.Prockop S, Doubrovina E, AN H, et al. Third party CMV-specific cytotoxic T cells for treatment of antiviral resistant CMV infection after hematopoietic stem cell transplant [abstract]. Blood 2016;128:61. [Google Scholar]
- 43.Lee DH, Zuckerman RA, Practice ASTIDCo. Herpes simplex virus infections in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Tansplant 2019;33:e13526. [DOI] [PubMed] [Google Scholar]
- 44.Martin-Gandul C, Stampf S, Hequet D, et al. Preventive strategies against cytomegalovirus and incidence of alpha-herpesvirus infections in solid organ transplant recipients: A Nationwide Cohort Study. Am J Transplant 2017;17:1813–22. [DOI] [PubMed] [Google Scholar]
- 45.American Academy of Pediatrics. Herpes Simplex. In: Kimberlin DW, MT Brady, Jackson MA, et al, editors. Red book: 2021 report of the committee on infectious diseases. American Academy of Pediatrics; 2021. p. 437–49. [Google Scholar]
- 46.Styczynski J, Reusser P, Einsele H, et al. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant 2009;43:757–70. [DOI] [PubMed] [Google Scholar]
- 47.Fiddian P, Sabin CA, Griffiths PD. Valacyclovir provides optimum acyclovir exposure for prevention of cytomegalovirus and related outcomes after organ transplantation. J Infect Dis 2002;186(Suppl 1):S110–5. [DOI] [PubMed] [Google Scholar]
- 48.Bomgaars L, Thompson P, Berg S, et al. Valacyclovir and acyclovir pharmacokinetics in immunocompromised children. Pediatr Blood Cancer 2008;51:504–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ariza-Heredia EJ, Chemaly RF, Shahani LR, et al. Delay of alternative antiviral therapy and poor outcomes of acyclovir-resistant herpes simplex virus infections in recipients of allogeneic stem cell transplant - a retrospective study. Transpl Int 2018;31:639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pergam SA, Limaye AP, Practice ASTIDCo. Varicella zoster virus in solid organ transplantation: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019;33:e13622. [DOI] [PubMed] [Google Scholar]
- 51.Vermont CL, Jol-van der Zijde EC, Hissink Muller P, et al. Varicella zoster reactivation after hematopoietic stem cell transplant in children is strongly correlated with leukemia treatment and suppression of host T-lymphocyte immunity. Transpl Infect Dis 2014;16:188–94. [DOI] [PubMed] [Google Scholar]
- 52.American Academy of Pediatrics. Varicella-Zoster Virus Infections. In: Kimberlin DW, Brady MT, Jackson MA, et al. , editors. Red book: 2021 report of the committee on infectious diseases . American Academy of Pediatrics; 2021. p. 869–83. [Google Scholar]
- 53.Lion T Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbiol Rev 2014;27:441–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fisher BT, Boge CLK, Petersen H, et al. Outcomes of human adenovirus infection and disease in a retrospective cohort of pediatric hematopoietic cell transplant recipients. J Pediatr Infect Dis Soc 2019;8:317–24. [DOI] [PubMed] [Google Scholar]
- 55.Matthes-Martin S, Feuchtinger T, Shaw PJ, et al. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: summary of ECIL-4 (2011). Transpl Infect Dis 2012;14:555–63. [DOI] [PubMed] [Google Scholar]
- 56.Florescu DF, Schaenman JM, Practice ASTIDCo. Adenovirus in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019;33:e13527. [DOI] [PubMed] [Google Scholar]
- 57.Munoz FM, Piedra PA, Demmler GJ. Disseminated adenovirus disease in immunocompromised and immunocompetent children. Clin Infect Dis 1998;27:1194–200. [DOI] [PubMed] [Google Scholar]
- 58.Ganapathi L, Arnold A, Jones S, et al. Use of cidofovir in pediatric patients with adenovirus infection. F1000Res 2016;5:758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin-Gandul C, Mueller NJ, Pascual M, et al. The Impact of infection on chronic allograft dysfunction and allograft survival after solid organ transplantation. Am J Transplant 2015;15:3024–40. [DOI] [PubMed] [Google Scholar]
- 60.Liu M, Mallory GB, Schecter MG, et al. Long-term impact of respiratory viral infection after pediatric lung transplantation. Pediatr Transplant 2010;14:431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Danziger-Isakov L, Steinbach WJ, Paulsen G, et al. A multicenter consortium to define the epidemiology and outcomes of pediatric solid organ transplant recipients with inpatient respiratory virus infection. J Pediatr Infect Dis Soc 2019;8:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu M, Worley S, Arrigain S, et al. Respiratory viral infections within one year after pediatric lung transplant. Transpl Infect Dis 2009;11:304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar D, Ferreira VH, Blumberg E, et al. A 5-Year Prospective Multicenter Evaluation of Influenza Infection in Transplant Recipients. Clin Infect Dis 2018;67:1322–9. [DOI] [PubMed] [Google Scholar]
- 64.Kumar D, Michaels MG, Morris MI, et al. Outcomes from pandemic influenza A H1N1 infection in recipients of solid-organ transplants: a multicentre cohort study. Lancet Infect Dis 2010;10:521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ljungman P, de la Camara R, Perez-Bercoff L, et al. Outcome of pandemic H1N1 infections in hematopoietic stem cell transplant recipients. Haematologica 2011;96:1231–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manuel O, Pascual M, Hoschler K, et al. Humoral response to the influenza A H1N1/09 monovalent AS03-adjuvanted vaccine in immunocompromised patients. Clin Infect Dis 2011;52:248–56. [DOI] [PubMed] [Google Scholar]
- 67.Ryan AL, Wadia UD, Jacoby P, et al. Immunogenicity of the inactivated influenza vaccine in children who have undergone allogeneic haematopoietic stem cell transplant. Bone Marrow Transplant 2020;55:773–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayden FG, Sugaya N, Hirotsu N, et al. Baloxavir Marboxil for Uncomplicated Influenza in Adults and Adolescents. N Engl J Med 2018;379:913–23. [DOI] [PubMed] [Google Scholar]
- 69.Ison MG, Portsmouth S, Yoshida Y, et al. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis 2020;20:1204–14. [DOI] [PubMed] [Google Scholar]
- 70.Jaiswal SR, Bhagwati G, Soni M, et al. Prophylactic oseltamivir during major seasonal influenza H1N1 outbreak might reduce both H1N1 and associated pulmonary aspergillosis in children undergoing haploidentical transplantation. Transpl Infect Dis 2020;22:e13309. [DOI] [PubMed] [Google Scholar]
- 71.Ison MG, Szakaly P, Shapira MY, et al. Efficacy and safety of oral oseltamivir for influenza prophylaxis in transplant recipients. Antivir Ther 2012;17:955–64. [DOI] [PubMed] [Google Scholar]
- 72.Hayden FG, Atmar RL, Schilling M, et al. Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N Engl J Med 1999;341:1336–43. [DOI] [PubMed] [Google Scholar]
- 73.Hayden FG, Gubareva LV, Monto AS, et al. Inhaled zanamivir for the prevention of influenza in families. Zanamivir Family Study Group. N Engl J Med 2000;343:1282–9. [DOI] [PubMed] [Google Scholar]
- 74.Manuel O, Estabrook M, American Society of Transplantation Infectious Diseases Community of P. RNA respiratory viral infections in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019;33:e13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009;15:1143–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khanna N, Steffen I, Studt JD, et al. Outcome of influenza infections in outpatients after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis 2009;11:100–5. [DOI] [PubMed] [Google Scholar]
- 77.Fisher BT, Alexander S, Dvorak CC, et al. Epidemiology and potential preventative measures for viral infections in children with malignancy and those undergoing hematopoietic cell transplantation. Pediatr Blood Cancer 2012;59:11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tam J, Papenburg J, Fanella S, et al. Pediatric Investigators Collaborative Network on Infections in Canada Study of Respiratory Syncytial Virus-associated Deaths in Pediatric Patients in Canada, 2003-2013. Clin Infect Dis 2019;68:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Science M, Akseer N, Asner S, et al. Risk stratification of immunocompromised children, including pediatric transplant recipients at risk of severe respiratory syncytial virus disease. Pediatr Transplant 2019;23:e13336. [DOI] [PubMed] [Google Scholar]
- 80.El-Bietar J, Nelson A, Wallace G, et al. RSV infection without ribavirin treatment in pediatric hematopoietic stem cell transplantation. Bone Marrow Transplant 2016;51:1382–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chemaly RF, Dadwal SS, Bergeron A, et al. A phase 2, randomized, double-blind, placebo-controlled trial of presatovir for the treatment of respiratory syncytial virus upper respiratory tract infection in hematopoietic-cell transplant recipients. Clin Infect Dis 2020;71:2777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chemaly RF, Ghantoji SS, Shah DP, et al. Respiratory syncytial virus infections in children with cancer. J Pediatr Hematol Oncol 2014;36:e376–81. [DOI] [PubMed] [Google Scholar]
- 83.Bridevaux PO, Aubert JD, Soccal PM, et al. Incidence and outcomes of respiratory viral infections in lung transplant recipients: a prospective study. Thorax 2014;69:32–8. [DOI] [PubMed] [Google Scholar]
- 84.Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics 1998;102:531–7. [PubMed] [Google Scholar]
- 85.Pignotti MS, Carmela Leo M, Pugi A, et al. Consensus conference on the appropriateness of palivizumab prophylaxis in respiratory syncytial virus disease. Pediatr Pulmonol 2016;51:1088–96. [DOI] [PubMed] [Google Scholar]
- 86.Teusink-Cross A, Davies SM, Danziger-Isakov L, et al. Restrictive palivizumab use does not lead to increased morbidity and mortality in pediatric hematopoietic stem cell transplantation patients. Biol Blood Marrow Transplant 2016;22:1904–6. [DOI] [PubMed] [Google Scholar]
- 87.Adams R, Christenson J, Petersen F, et al. Pre-emptive use of aerosolized ribavirin in the treatment of asymptomatic pediatric marrow transplant patients testing positive for RSV. Bone Marrow Transplant 1999;24:661–4. [DOI] [PubMed] [Google Scholar]
- 88.Adams RH. Preemptive treatment of pediatric bone marrow transplant patients with asymptomatic respiratory syncytial virus infection with aerosolized ribavirin. Biol Blood Marrow Transplant 2001;7(Suppl):16S–8S. [DOI] [PubMed] [Google Scholar]
- 89.Shah DP, Ghantoji SS, Shah JN, et al. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother 2013;68:1872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood 2011;117:2755–63. [DOI] [PubMed] [Google Scholar]
- 91.Gueller S, Duenzinger U, Wolf T, et al. Successful systemic high-dose ribavirin treatment of respiratory syncytial virus-induced infections occurring pre-engraftment in allogeneic hematopoietic stem cell transplant recipients. Transpl Infect Dis 2013;15:435–40. [DOI] [PubMed] [Google Scholar]
- 92.Trang TP, Whalen M, Hilts-Horeczko A, et al. Comparative effectiveness of aerosolized versus oral ribavirin for the treatment of respiratory syncytial virus infections: A single-center retrospective cohort study and review of the literature. Transpl Infect Dis 2018;20:e12844. [DOI] [PubMed] [Google Scholar]
- 93.Beaird OE, Freifeld A, Ison MG, et al. Current practices for treatment of respiratory syncytial virus and other non-influenza respiratory viruses in high-risk patient populations: a survey of institutions in the Midwestern Respiratory Virus Collaborative. Transpl Infect Dis 2016;18:210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ganciclovir injection. Lenoir (NC): Exela Pharma Sciences; 2017. [Google Scholar]
- 95.Valcyte (valganciclovir). San Francisco (CA): Genentech, Inc.; 2020. [Google Scholar]
- 96.Zovirax (acyclovir sodium). Brentford (England): GlaxoSmithKline; 2005. [Google Scholar]
- 97.Valtrex (valacyclovir hydrochloride) Caplets. (Brentford) England: GlaxoSmithKline; 2008. [Google Scholar]