Abstract
Purpose of review
To review ocular manifestations and complications of Herpes Simplex Virus (HSV) and discuss recent advancements in diagnostic and treatment strategy.
Recent advancements
In vivo confocal microscopy has expanded our understanding of corneal nerve degeneration, corneal dendritic cell activity, and changes in biomechanical properties in HSV keratitis. Although currently available only as a research tool, metagenomic deep sequencing has the potential to improve diagnostic accuracy beyond the well-established polymerase chain reaction (PCR) technology, especially in atypical cases. Development of an HSV vaccine has shown some encouraging results in a murine model. New treatment options for neurotrophic cornea offer promise, specifically cenegermin nerve growth factor.
Summary
Ocular herpes simplex infection and its complications continue to cause significant visual burden and decreased quality of life. Familiarity with its clinical features, wider adoption of viral PCR diagnostic technology, and recognition of the need for long-term maintenance medications for recurrent or chronic cases form the basis for effective management. Metagenomic deep sequencing, the development of a herpes vaccine, and cenegermin nerve growth factor offer promise as diagnostic, preventive, and therapeutic options, respectively.
Keywords: herpes simplex virus, ocular herpes, herpes keratitis, HEDS
INTRODUCTION
Herpes Simplex Virus (HSV) is a human herpesvirus responsible for systemic infections such as orolabial herpes, HSV folliculitis, herpes gladiatorum, herpetic whitlow, herpes encephalitis, and eczema herpeticum. Ocular involvement can present as a primary infection or recurrence from latent disease, and spans all ocular tissues: blepharitis, conjunctivitis, epithelial keratitis, stromal keratitis, endotheliitis, iritis, trabeculitis and retinitis. Although ocular HSV infection is usually due to HSV-1, which establishes latency in the trigeminal ganglion, HSV-2 can also cause HSV keratitis.
EPIDEMIOLOGY
Ocular HSV infection is the most common cause of corneal blindness in developed countries [1] and significantly impairs quality of life among those affected [2]. Within the United States it is estimated that at least 50% of the population is infected with HSV-1 by the age of 30 and by the age of 60 nearly 100% of individuals harbor the infection in their trigeminal ganglion [3]. Despite this large public health burden, there is still active debate among experts regarding the optimal treatment of ocular HSV [4].
PATHOGENESIS
Visually significant complications associated with HSV keratitis, such as lipid keratopathy, corneal thinning, and neurotrophic cornea, arise from the complex interplay between viral activity and the host immune response. Confocal microscopy studies have shown that corneal dendritic cells continually evolve in density, distribution, and morphology during active herpetic endotheliitis [5,6]. Immune-mediated damage to the cornea stroma can affect the biomechanical properties of the cornea, resulting in reduced corneal hysteresis and decreased corneal resistance factor [7]. Cornea nerve degeneration often results in significantly reduced corneal sensation. In recent confocal microscopy studies of HSV keratitis, corneal nerves regenerated to a limited extent but not to a clinically significant level [8–10].
Diagnosis
Viral culture and PCR can detect HSV when there is active viral replication, such as with epithelial keratitis, endotheliitis, and retinitis. Viral PCR is preferable to viral culture due to more rapid results and higher sensitivity [11], and to Goldmann-Witmer coefficient analysis which is not widely available. Ongoing research using confocal microscopy has demonstrated diagnostic accuracy in identifying HSV keratitis, as well as measuring inflammatory response to treatment by identification of dendritic and inflammatory cells, [6,12] although this modality is highly dependent on operator and grader experience.
In primarily immune-mediated forms such as stromal keratitis, diagnosing HSV is primarily a clinical diagnosis based on appearance and response to empiric treatment. Tear film analyses may provide some clues as one study reported that the combined presence of HSV immunoglobulins and HSV-DNA in the tear film had a 98% positive predictive value for HSV stromal keratitis [13]. An even more promising technology that has the potential to not only improve diagnostic detection but also provide epidemiologic surveillance of ocular infections is metagenomic deep sequencing, which compares DNA or RNA extracted from minute intraocular samples to large, sequenced databases for very specific pathogen identification including HSV. [14,15]
HERPETIC BLEPHAROCONJUNCTIVITIS
Although primary ocular HSV is rare, when it does occur it is often as a self-limited blepharitis with vesicular lid lesions or follicular conjunctivitis, both of which may lead to subsequent keratitis. Accurate clinical diagnosis may be challenging as HSV blepharitis is uncommon and may be confused for the more ubiquitous meibomian gland dysfunction and follicular conjunctivitis can occur without lid lesions and mimic other causes of chronic conjunctivitis. Topical trifluridine, acyclovir ointment and in some cases, systemic antivirals are used to treat herpetic blepharoconjunctivitis (Table 1). Localized vesicular lesions in the setting of atopic dermatitis may represent eczema herpeticum or Kaposi varicelliform eruption, dermatological emergencies that can progress to Viral HSV encephalitis if not recognized and treated immediately [16].
Table 1.
Classification and Treatment of Herpes Simplex Virus Ocular Infection
| Classification | Alternate Terms | Treatment Recommendations |
|---|---|---|
| Epithelial Keratitis | Dendritic Ulcer Geographic Ulcer |
Therapeutic dose of oral or topical antiviral
Epithelial debridement |
| Stromal Keratitis without ulceration | Interstitial keratitis Immune/Wessely Ring |
|
| Stromal Keratitis with ulceration | Necrotizing stromal keratitis Ulcerating interstitial keratitis |
|
| Endotheliitis | Disciform keratitis Disciform endotheliitis Linear endotheliitis |
|
HERPES SIMPLEX KERATITIS
Presenting as epithelial keratitis, immune keratitis, and endotheliitis, HSV keratitis in adults usually represents recurrent HSV-1 rather than primary ocular HSV. Stress is a well-known trigger for reactivation and HSV keratitis has been reported more recently following intravitreal bevacizumab injection [17], rituximab [18], botulinum toxin injection [19,20], and after cataract surgery and cornea transplantation [21,22].
In neonates, primary HSV keratitis is due to HSV-2 and is acquired via antenatal, intrapartum, or postnatal exposure. Due to an immature immune system, HSV infection in the pediatric population may have a particularly severe and prolonged course and accurate identification and immediate treatment with antivirals is important to prevent amblyopia and poor visual outcomes [23–25].
Appropriate management of HSV keratitis requires an understanding that each of its various forms have both components of infection and immunologic response. While epithelial keratitis and endotheliitis is driven more by active viral replication, stromal keratitis is caused more by the immune response.
EPITHELIAL KERATITIS
Herpetic epithelial keratitis classically presents with corneal dendrites that are linear and branching with terminal bulbs (Figure 1). The dendrites are true corneal ulcers with fluorescein staining at the base and rose bengal staining of surrounding swollen epithelial cells. As the dendrites heal, a dendritic epitheliopathy and subepithelial haze or ghost dendrite may be seen, which can be distinguished from an active dendrite due to the lack of fluorescein staining. Other less common manifestations of HSV epithelial keratitis include larger geographic ulcers which heal more slowly and peripheral marginal ulcers which are accompanied by more inflammation due to their proximity to the limbus. These various manifestations of HSV epithelial keratitis can all lead to degeneration of corneal nerves, decreased corneal sensation, and stromal inflammation and scarring [26].
Figure 1.

Herpes Simplex epithelial keratitis with dendrite.
Epithelial keratitis usually self-resolves in 2 weeks, but early initiation of topical or systemic anti-viral medication reduces disease severity and course. (Table 1) The recent FDA approval of acyclovir 3% ointment adds a promising alternative to topical trifluridine which has significant surface toxicity. Epithelial debridement and placement of amniotic membrane may also accelerate healing and re-epithelialization [27].
Systemic agents are preferred over topical medications by many cornea specialists due to the high bioavailability, avoidance of surface toxicity, and ease of administration. Compared to oral acyclovir, valacyclovir has better penetration [28] and perhaps improved compliance due to decreased dose frequency. In cases refractory to oral acyclovir and valacyclovir, systemic valganciclovir has also been successfully used [29–31]. The many treatment options and lack of standardization has led to a push from the clinical community to standardize treatment [4,32].
STROMAL KERATITIS
HSV immune stromal keratitis may arise primarily or secondarily from infectious epithelial keratitis or endotheliitis. It is characterized by recurrent or chronic stromal inflammation due to retained viral antigens that trigger an antigen-antibody-complement cascade. Clinical manifestations include stromal infiltrates that may be unifocal, multifocal, or diffuse (Figure 2), stromal edema, an immune ring, cornea thinning, and sectoral or diffuse stromal neovascularization (Figure 3A and 3B). A posterior interstitial keratitis without endotheliitis has also been recently described [33]. With adequate treatment, these vessels can recede to ghost vessels [26], but without, lipid keratopathy can develop and cause visually significant stromal scarring. HSV stromal keratitis generally follows a chronic course with low-grade inflammation interspersed with periods of relative quiescence and intermittent flare-ups.
Figure 2.
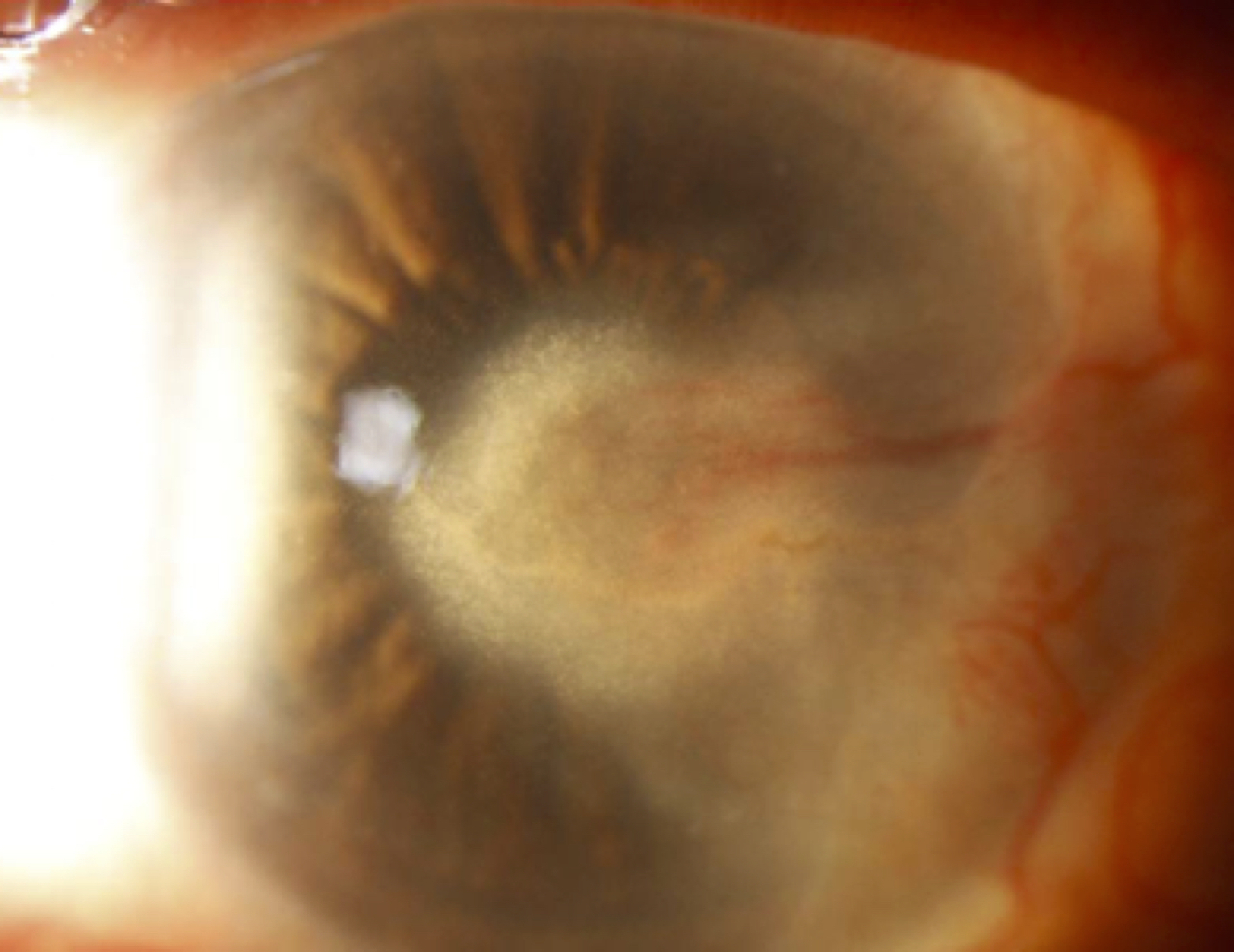
Herpes Simplex stromal keratitis with lipid keratopathy.
Figure 3.
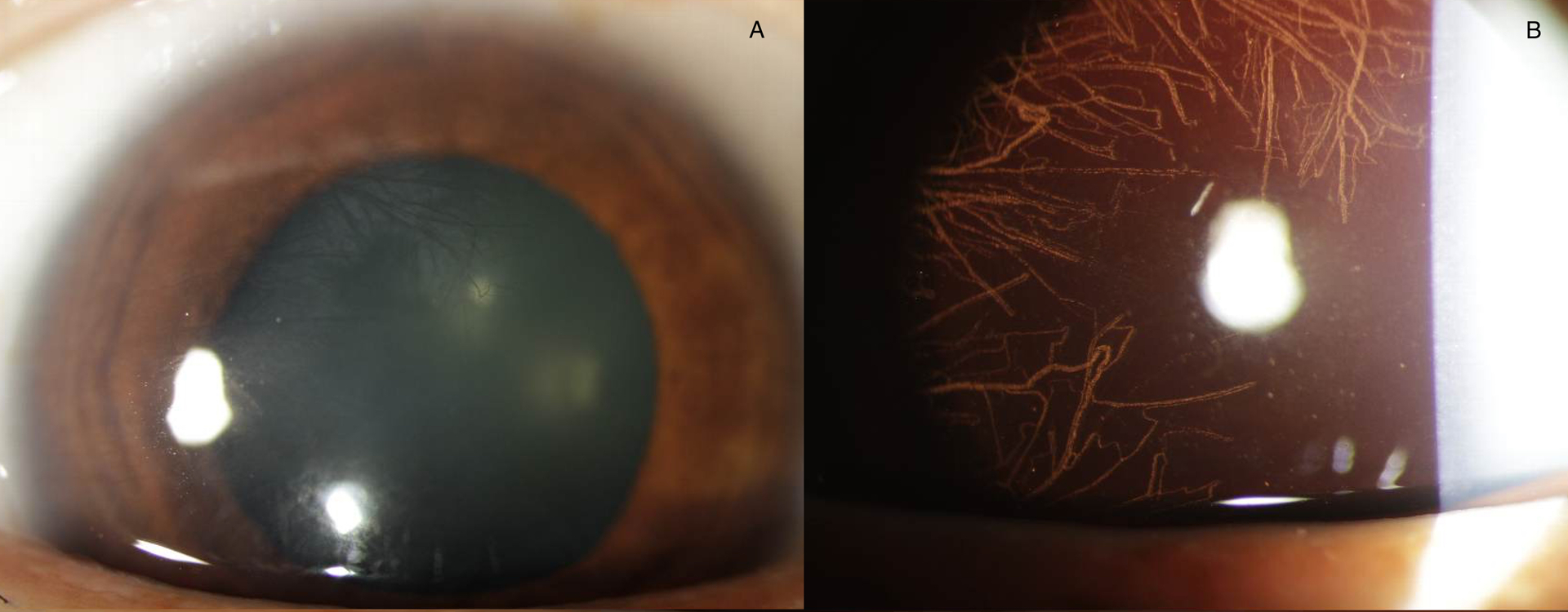
Herpes Simplex stromal keratitis with diffuse posterior stromal neovascularization (a), best seen with retroilluminaton (b).
When epithelial ulceration accompanies stromal keratitis, a more aggressive disease course that is less responsive to therapy ensues. At times referred to as necrotizing stromal keratitis, this presentation can see significant keratolysis and even corneal perforation. As it may resemble a fulminant bacterial or fungal infection, cultures should be taken to rule out other microbial agents. Full therapeutic dosing of oral antiviral medication is necessary (Table 1).
Developments in basic science research have deepened our understanding of immune related herpetic stromal keratitis. Inflammatory hypoxia and the discovery of elevated expression of hypoxia associated genes and regulation of subsets of T cells [34,35] are essential in the pathogenesis of HSV stromal keratitis and provide possible targets to more effectively control stromal keratitis [36].
The HEDS study established treatment strategy for immune stromal keratitis which included topical steroids to shorten the disease course and systemic antivirals to decrease the frequency of recurrence [37,38]. Long-term maintenance therapy with topical steroids is often required, with the goal to titrate to the minimal dosage required to suppress inflammation. In a recent randomized controlled trial of patients with HSV stromal keratitis, there was no statistically significant difference between topical cyclosporine-A 2% eyedrops and topical prednisolone 1% with regards to improvement in corneal densitometry and best corrected visual acuity [39], suggesting that cyclosporine is an effective alternative to steroids. Oral antiviral prophylaxis should accompany the use of topical steroids (Table 1).
ENDOTHELIITIS
Caused by active virus in the anterior chamber and characterized by keratic precipitates, iritis and stromal edema without infiltrate or neovascularization, HSV endotheliitis can be classified based on area of involvement into disciform, diffuse and linear. The most common of these, disciform endotheliitis presents with a central focal circular area of stromal edema with keratic precipitates and anterior uveitis. The uninvolved peripheral cornea is notably clear. Diffuse endotheliitis is less common and has keratic precipitates throughout the entire cornea and can even develop a retro-corneal plaque and hypopyon that resemble a fungal keratitis. In linear endotheliitis, a distinct linear or serpiginous distribution of keratic precipitates that progresses from the limbus demarcates involved edematous cornea and non-involved clear cornea. As linear endotheliitis in post-keratoplasty patients may mimic graft rejection, PCR analysis of aqueous humor can aid diagnosis [40,41].
As endotheliitis is due to active HSV infection in the anterior chamber, oral antiviral medications are generally initiated first, followed by topical steroids a few days later (Table 1). While disciform and diffuse endotheliitis are particularly responsive to topical steroids, linear endotheliitis can be more difficult to treat and require higher doses of oral antivirals and topical steroids.
Neurotrophic Keratopathy
Damage to cornea nerves from prior episodes of herpetic keratitis can lead to neurotrophic keratopathy, which ranges in severity from an irregular epithelial surface to an oval-shaped neurotrophic ulcer with a heaped-up epithelial border. Left untreated, stromal neovascularization, scarring, thinning, and even perforation may ensue.
Neurotrophic ulcers require vigilant care and judicious use of therapeutic options including lubrication, bandage contact lens, serum tears, tarsorrhaphy, and amniotic membrane placement. Systemic anti-viral medications should be maintained and in some cases topical steroids may help if inflammation is impairing epithelialization. Two newer therapeutic options have recently gained more interest. Cenegermin, a recombinant nerve growth factor targeting nerve pathology [42] has been shown to be effective in promoting corneal re-epithelialization following 8 weeks of treatment, although long term effect is still unknown [43]. The developing field of corneal neurotization has shown promise, with improvement in cornea sensation measured by Cochet-Bonnet aesthesiometry [44,45] as well as confocal microscopy demonstrating reinnervation at the subbasal nerve plexus [46].
INFECTIOUS HERPETIC ANTERIOR UVEITIS
Herpetic iridocyclitis is the most common cause of infectious anterior uveitis and usually, but not always, accompanies stromal keratitis or endotheliitis. Clinical features include diffuse keratic precipitates that may be fine, stellate, or granulomatous, elevated IOP, and iris atrophy [47]. The acute IOP elevation seen in herpetic trabeculitis responds very well to topical steroids, even without anti-hypertensive agents, but inadequate or lack of anti-viral treatment due to failure to diagnose HSV as the underlying cause can lead to recurrent hypertensive episodes, a major risk factor for the development of glaucoma [48].
Differentiating between HSV, VZV, and CMV anterior uveitis based on clinical findings alone can be challenging but a good review by Chan and Chee in 2019 identifies some distinguishing features [47]. Aqueous humor PCR analysis is often diagnostic but may require repeat anterior chamber taps as false positives can occur especially with concurrent anti-viral treatment.
HERPES RETINITIS
Acute Retinal Necrosis (ARN) and Progressive Outer Retinal Necrosis(PORN) are typically due to VZV, but HSV-1 and HSV-2 can cause ARN. A severe necrotizing retinitis, ARN presents with unilateral vision loss, photophobia, floaters and pain but approximately one third of patients develop bilateral disease [49]. Clinical features include one or more foci of necrotizing full thickness retinitis, intense intravitreal inflammation, occlusive vasculitis and arteriolar narrowing, and optic neuropathy [50]. Attempts to distinguish retinitis etiologies with optical coherence tomography are inconclusive [51] so PCR of a vitreous tap is still a favored approach.
Upon suspicion of ARN, intravenous acyclovir or oral valacyclovir must begin immediately. A retrospective study of 68 eyes showed that oral valacyclovir is an acceptable alternative to intravenous acyclovir [52]. In refractory cases, intravenous foscarnet or cidofovir may be considered [53]. Special attention should be paid to patients with a history of HSV encephalitis as HSV associated ARN has been reported in these patients many years after resolution of the encephalitis [54,55].
TREATMENT
Medical management forms the basis for treatment of ocular HSV infection and has been previously discussed and summarized in Table 1. Surgical treatment may be necessary to treat complications arising from HSV keratitis [56]. For example, application of cornea glue, tarsorrhaphy, tectonic keratoplasty, or conjunctival flap are options for progressive corneal thinning and corneal perforation. In countries with a shortage of donor corneas such as China, acellular porcine corneal stroma has been used as an urgent patch graft temporary solution [57] Visually significant corneal scarring can be addressed with deep anterior lamellar keratoplasty or penetrating keratoplasty, with a preference for the former due to reduced risk of immunogenic rejection. Corneal cross-linking for infectious keratitis shows promise but its efficacy for HSV keratitis is still uncertain [58,59].
Recent laboratory discoveries into how HSV gains entry via cell receptors, interacts with corneal dendritic cells, and impacts protein synthesis, VEGF pathways, and corneal nerve degeneration has deepened our understanding of HSV pathogenesis and opened doors for new therapeutic targets [5,31,60–62]. One exciting area focuses on a corneal dendritic cell based DNA vaccine that has shown promise in murine models [63,64]. In a recent study of mice previously exposed to a live-attenuated HSV candidate vaccine, when challenged with HSV-1, they did not develop any corneal pathology and had complete preservation of visual acuity [65]. If successfully translated into humans, an HSV vaccine would fundamentally change the landscape of management strategy.
CONCLUSION
Despite advances in our understanding of pathogenesis and management, HSV infection continues to be a leading cause of ocular morbidity. Early, accurate diagnosis is crucial to prevent many of the visually devastating complications of HSV infection. Recognition of the relationship between viral activity and host immune response underlies effective management strategy. Recent clinical advances include the newly approved acyclovir 3% ophthalmic ointment and nerve growth factor for neurotrophic cornea. Promising areas under investigation include diagnostic metagenomic deep sequencing, cornea neurotization for neurotrophic cornea, and development of an HSV vaccine.
KEY POINTS.
Polymerase Chain Reaction (PCR) analysis accurately detects many forms of ocular HSV, but metagenomic deep sequencing, currently only used for research purposes, has even greater potential, both for diagnosis and epidemiologic surveillance.
Recent laboratory discoveries and confocal microscopy analysis have provided greater insight into corneal dendritic cell activity and corneal nerve regeneration with Herpes Simplex Virus (HSV) infection
Balancing suppression of viral activity and control of immune response is a dynamic process requiring both antiviral medication and steroids, often for prolonged periods as maintenance therapy
Recombinant nerve growth factor and corneal neurotization offer promising options for neurotrophic cornea but require more investigation
Financial support and sponsorship
This work was supported by National Eye Institute core grant P30-026877 (Stanford) and Research to Prevent Blindness. The sponsors or funding organizations had no role in the design or conduct of this research.
Footnotes
Conflicts of interest: None of the authors have a proprietary/financial interest to disclose
REFERENCES AND RECOMMENDED READING
- 1.Farooq AV, Shah A, Shukla D. The role of herpesviruses in ocular infections [Internet]. Virus Adaptation and Treatment. 2010. [cited 2019 Jul 6]. Available from: https://www.dovepress.com/the-role-of-herpesviruses-in-ocular-infections-peer-reviewed-article-VAAT [Google Scholar]
- 2.Reynaud C, Rousseau A, Kaswin G, M’garrech M, Barreau E, Labetoulle M. Persistent Impairment of Quality of Life in Patients with Herpes Simplex Keratitis. Ophthalmology. 2017;124(2):160–9. [DOI] [PubMed] [Google Scholar]
- 3.Liesegang TJ. Herpes Simplex Virus Epidemiology and Ocular Importance: Cornea. 2001. Jan;20(1):1–13. [DOI] [PubMed] [Google Scholar]
- 4.Cabrera-Aguas M, Robaei D, McCluskey P, Watson S. Clinical translation of recommendations from randomized trials for management of herpes simplex virus keratitis. Clin Experiment Ophthalmol. 2018. Dec;46(9):1008–16. [DOI] [PubMed] [Google Scholar]
- 5.Kwon MS, Carnt NA, Truong NR, Pattamatta U, White AJ, Samarawickrama C, et al. Dendritic cells in the cornea during Herpes simplex viral infection and inflammation. Surv Ophthalmol. 2018. Aug;63(4):565–78. [DOI] [PubMed] [Google Scholar]
- *6.Wang T, Dong M, Jiang Y, Wang S, Shi W. Role of Dendritic Cells and Inflammatory Cells in Herpetic Endotheliitis: Analysis Using In Vivo Confocal Microscopy. Cornea. 2018. Jun;37(6):748–54. [DOI] [PubMed] [Google Scholar]; This study used confocal microscopy to examine 110 eyes with herpetic endotheliitis and characterized the changes in corneal dendritic cells throughout the healing process.
- 7.Marcos-Fernández MÁ, Tabernero SS, Herreras JM, Galarreta DJ. Impact of herpetic stromal immune keratitis in corneal biomechanics and innervation. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2018. Jan;256(1):155–61. [DOI] [PubMed] [Google Scholar]
- **8.Zemaitiene R, Rakauskiene M, Danileviciene V, Use V, Kriauciuniene L, Zaliuniene D. Corneal esthesiometry and sub-basal nerves morphological changes in herpes simplex virus keratitis/uveitis patients. Int J Ophthalmol. 2019;12(3):407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]; This prospective study of 30 patients with ocular HSV showed partial recovery of sub-basal nerve fibers using confocal microscopy as well as improvement in corneal sensation with esthesiometry at 6 months.
- 9.Moein H-R, Kheirkhah A, Muller RT, Cruzat AC, Pavan-Langston D, Hamrah P. Corneal nerve regeneration after herpes simplex keratitis: A longitudinal in vivo confocal microscopy study. Ocul Surf. 2018. Apr;16(2):218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danileviciene V, Zemaitiene R, Gintauskiene VM, Nedzelskiene I, Zaliuniene D. Corneal Sub-Basal Nerve Changes in Patients with Herpetic Keratitis During Acute Phase and after 6 Months. Med Kaunas Lith. 2019. May 27;55(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espy MJ, Ross TK, Teo R, Svien KA, Wold AD, Uhl JR, et al. Evaluation of LightCycler PCR for implementation of laboratory diagnosis of herpes simplex virus infections. J Clin Microbiol. 2000. Aug;38(8):3116–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *12.Wang YE, Tepelus TC, Vickers LA, Baghdasaryan E, Gui W, Huang P, et al. Role of in vivo confocal microscopy in the diagnosis of infectious keratitis. Int Ophthalmol. 2019. Jun 17; [DOI] [PubMed] [Google Scholar]; In this retrospective study of 46 patients, confocal microscopy accurately diagnosed bacterial, fungal, acanthamoeba, and viral keratitis.
- 13.Qiu J, Huang F, Wang Z, Xu J, Zhang C. The evaluation of diagnostic efficiency for stromal herpes simplex keratitis by the combination of tear HSV-sIgA and HSV-DNA. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2017. Jul;255(7):1409–15. [DOI] [PubMed] [Google Scholar]
- 14.Doan T, Pinsky BA. Current and future molecular diagnostics for ocular infectious diseases. Curr Opin Ophthalmol. 2016. Nov;27(6):561–7. [DOI] [PubMed] [Google Scholar]
- 15.Doan T, Wilson MR, Crawford ED, Chow ED, Khan LM, Knopp KA, et al. Illuminating uveitis: metagenomic deep sequencing identifies common and rare pathogens. Genome Med. 2016. 25;8(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finlow C, Thomas J. Disseminated herpes simplex virus: a case of eczema herpeticum causing viral encephalitis. J R Coll Physicians Edinb. 2018. Mar;48(1):36–9. [DOI] [PubMed] [Google Scholar]
- 17.Derham AM, Chen E, Bunya VY, OʼMalley RE. Bilateral Herpetic Keratitis After Bilateral Intravitreal Bevacizumab for Exudative Macular Degeneration. Cornea. 2017. Jul;36(7):878–9. [DOI] [PubMed] [Google Scholar]
- 18.Bernauer W, Schuler S, Borradori L. Rituximab and bilateral HSV epithelial keratitis in a patient with mucous membrane pemphigoid. J Ophthalmic Inflamm Infect. 2018. Aug 23;8(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narang P, Singh S, Mittal V. Bilateral herpes simplex keratitis reactivation after lacrimal gland botulinum toxin injection. Indian J Ophthalmol. 2018;66(5):697–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramappa M, Jiya PY, Chaurasia S, Naik M, Sharma S. Reactivation of herpes simplex viral keratitis following the botulinum toxin injection. Indian J Ophthalmol. 2018;66(2):306–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi X, Wang M, Li X, Jia Y, Li S, Shi W, et al. Characteristics of New Onset Herpes Simplex Keratitis after Keratoplasty. J Ophthalmol. 2018;2018:4351460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho YK, Kwon JW, Konda S, Ambati BK. Epithelial Keratitis After Cataract Surgery. Cornea. 2018. Jun;37(6):755–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vadoothker S, Andrews L, Jeng BH, Levin MR. Management of Herpes Simplex Virus Keratitis in the Pediatric Population. Pediatr Infect Dis J. 2018;37(9):949–51. [DOI] [PubMed] [Google Scholar]
- 24.Bodack MI. Case Series: Pediatric Herpes Simplex Keratitis. Optom Vis Sci Off Publ Am Acad Optom. 2019. Mar;96(3):221–6. [DOI] [PubMed] [Google Scholar]
- 25.Matos RJC, Pires JMS, Cortesão D. Management of Neonatal Herpes Simplex Infection: A Rare Case of Blepharoconjunctivitis and Concurrent Epithelial and Stromal Keratitis. Ocul Immunol Inflamm. 2018;26(4):625–7. [DOI] [PubMed] [Google Scholar]
- 26.Mannis MJ, Holland EJ, editors. Cornea. Fourth edition. Edinburgh ; N.Y: Elsevier; 2017. 2 p. [Google Scholar]
- 27.Cheng AMS, Tseng SCG. Self-Retained Amniotic Membrane Combined With Antiviral Therapy for Herpetic Epithelial Keratitis. Cornea. 2017. Nov;36(11):1383–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dias C, Nashed Y, Atluri H, Mitra A. Ocular penetration of acyclovir and its peptide prodrugs valacyclovir and val-valacyclovir following systemic administration in rabbits: An evaluation using ocular microdialysis and LC-MS. Curr Eye Res. 2002. Oct;25(4):243–52. [DOI] [PubMed] [Google Scholar]
- 29.Robinet-Perrin A, Tumiotto C, Cornut T, Santoni A, Touboul D, Goupil-Gouyette T, et al. Input of recombinant phenotyping for the characterization of a novel acyclovir-resistance mutation identified in a patient with recurrent herpetic keratitis. Antiviral Res. 2019. Jun 11;168:183–6. [DOI] [PubMed] [Google Scholar]
- 30.Koseoglu ND, Strauss BR, Hamrah P. Successful Management of Herpes Simplex Keratitis With Oral Valganciclovir in Patients Unresponsive or Allergic to Conventional Antiviral Therapy. Cornea. 2019. Jun;38(6):663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauer D, Keller J, Alt M, Schubert A, Aufderhorst UW, Palapys V, et al. Antibody-based immunotherapy of aciclovir resistant ocular herpes simplex virus infections. Virology. 2017;512:194–200. [DOI] [PubMed] [Google Scholar]
- 32.Conner E, Moore S. Why are we not prescribing more valaciclovir for herpes infections of the eye? Is it time for a change of practice in New Zealand? Clin Experiment Ophthalmol. 2018;46(4):446. [DOI] [PubMed] [Google Scholar]
- 33.Farooq AV, Paley GL, Lubniewski AJ, Gonzales JA, Margolis TP. Unilateral Posterior Interstitial Keratitis as a Clinical Presentation of Herpes Simplex Virus Disease. Cornea. 2018. Mar;37(3):375–8. [DOI] [PubMed] [Google Scholar]
- 34.Rao P, Suvas S. Development of Inflammatory Hypoxia and Prevalence of Glycolytic Metabolism in Progressing Herpes Stromal Keratitis Lesions. J Immunol Baltim Md 1950. 2019. Jan 15;202(2):514–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajasagi NK, Rouse BT. The Role of T Cells in Herpes Stromal Keratitis. Front Immunol. 2019;10:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajasagi NK, Rouse BT. Application of our understanding of pathogenesis of herpetic stromal keratitis for novel therapy. Microbes Infect. 2018. Nov;20(9–10):526–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barron BA, Gee L, Hauck WW, Kurinij N, Dawson CR, Jones DB, et al. Herpetic Eye Disease Study. A controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology. 1994. Dec;101(12):1871–82. [DOI] [PubMed] [Google Scholar]
- 38.Wilhelmus KR, Gee L, Hauck WW, Kurinij N, Dawson CR, Jones DB, et al. Herpetic Eye Disease Study. A controlled trial of topical corticosteroids for herpes simplex stromal keratitis. Ophthalmology. 1994. Dec;101(12):1883–95; discussion 1895–1896. [DOI] [PubMed] [Google Scholar]
- **39.Peyman A, Nayebzadeh M, Peyman M, Afshari NA, Pourazizi M. Topical cyclosporine-A versus prednisolone for herpetic stromal keratitis: a randomized controlled trial. Acta Ophthalmol (Copenh). 2019. Mar;97(2):e194–8. [DOI] [PubMed] [Google Scholar]; In this randomized controlled trial of 38 eyes with herpetic stromal keratitis, no significant differences in corneal densitometry and visual acuity were seen in the two treatments groups of topical prednisolone and oral acyclovir.
- 40.Shin J, Ra H, Rho CR. Herpes simplex virus linear endotheliitis in a post-keratoplasty patient: A case report. Medicine (Baltimore). 2019. Jan;98(3):e14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basak SK, Basak S. Recurrence of herpes simplex virus endotheliitis in a Descemet membrane endothelial keratoplasty graft: mimicking fungal interface infection. BMJ Case Rep. 2019. May 6;12(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dua HS, Said DG, Messmer EM, Rolando M, Benitez-del-Castillo JM, Hossain PN, et al. Neurotrophic keratopathy. Prog Retin Eye Res. 2018. Sep 1;66:107–31. [DOI] [PubMed] [Google Scholar]
- 43.Fleeman N, Mahon J, Nevitt S, Duarte R, Boland A, Kotas E, et al. Cenegermin for Treating Neurotrophic Keratitis: An Evidence Review Group Perspective of a NICE Single Technology Appraisal. PharmacoEconomics - Open. 2019. Jun 25; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Catapano J, Fung SSM, Halliday W, Jobst C, Cheyne D, Ho ES, et al. Treatment of neurotrophic keratopathy with minimally invasive corneal neurotisation: long-term clinical outcomes and evidence of corneal reinnervation. Br J Ophthalmol. 2019. Feb 15; [DOI] [PubMed] [Google Scholar]
- 45.Jowett N, Pineda Ii R. Corneal neurotisation by great auricular nerve transfer and scleral-corneal tunnel incisions for neurotrophic keratopathy. Br J Ophthalmol. 2018. Nov 23; [DOI] [PubMed] [Google Scholar]
- *46.Giannaccare G, Bolognesi F, Biglioli F, Marchetti C, Mariani S, Weiss JS, et al. In Vivo and Ex Vivo Comprehensive Evaluation of Corneal Reinnervation in Eyes Neurotized With Contralateral Supratrochlear and Supraorbital Nerves. Cornea. 2019. Jul 22; [DOI] [PubMed] [Google Scholar]; In this case series of 3 eyes, in vivo confocal microscopy and histopathological evaluation demonstrated successful reinnervation following direct corneal neurotization.
- **47.Chan NS-W, Chee S-P. Demystifying viral anterior uveitis: A review. Clin Experiment Ophthalmol. 2019;47(3):320–33. [DOI] [PubMed] [Google Scholar]; This review article describes in extensive detail the similarities and differences in clinical presentations of HSV, VZV, and CMV anterior uveitis.
- 48.Hoeksema L, Jansonius NM, Los LI. Risk Factors for Secondary Glaucoma in Herpetic Anterior Uveitis. Am J Ophthalmol. 2017. Sep;181:55–60. [DOI] [PubMed] [Google Scholar]
- 49.Lee JH, Agarwal A, Mahendradas P, Lee CS, Gupta V, Pavesio CE, et al. Viral posterior uveitis. Surv Ophthalmol. 2017. Aug;62(4):404–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koh YT, Ang BC-H, Ho SL, Beng Teoh SC, Agrawal R. Herpes Simplex Acute Retinal Necrosis Presenting as Unilateral Disc Swelling in Young Immunocompetent Patients. Ocul Immunol Inflamm. 2017. Dec;25(6):797–801. [DOI] [PubMed] [Google Scholar]
- 51.Invernizzi A, Agarwal AK, Ravera V, Mapelli C, Riva A, Staurenghi G, et al. Comparing optical coherence tomography findings in different aetiologies of infectious necrotising retinitis. Br J Ophthalmol. 2018;102(4):433–7. [DOI] [PubMed] [Google Scholar]
- 52.Baltinas J, Lightman S, Tomkins-Netzer O. Comparing Treatment of Acute Retinal Necrosis With Either Oral Valacyclovir or Intravenous Acyclovir. Am J Ophthalmol. 2018;188:173–80. [DOI] [PubMed] [Google Scholar]
- 53.Stryjewski TP, Scott NL, Barshak MB, Tobin EH, Mali JO, Young LH, et al. Treatment of Refractory Acute Retinal Necrosis with Intravenous Foscarnet or Cidofovir. Ocul Immunol Inflamm. 2018;26(2):199–203. [DOI] [PubMed] [Google Scholar]
- 54.Kobayashi T, Sekar P, Meier J, Streit J. Acute retinal necrosis in a patient with remote severe herpes simplex encephalitis. BMJ Case Rep. 2019. May 27;12(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Todokoro D, Kamei S, Goto H, Ikeda Y, Koyama H, Akiyama H. Acute retinal necrosis following herpes simplex encephalitis: a nationwide survey in Japan. Jpn J Ophthalmol. 2019. May 3; [DOI] [PubMed] [Google Scholar]
- 56.Tuli S, Gray M, Shah A. Surgical management of herpetic keratitis. Curr Opin Ophthalmol. 2018. Jul;29(4):347–54. [DOI] [PubMed] [Google Scholar]
- 57.Zheng J, Huang X, Zhang Y, Wang Y, Qin Q, Lin L, et al. Short-term results of acellular porcine corneal stroma keratoplasty for herpes simplex keratitis. Xenotransplantation. 2019. Apr 10;e12509. [DOI] [PubMed] [Google Scholar]
- 58.Abbouda A, Abicca I, Alió JL. Infectious Keratitis Following Corneal Crosslinking: A Systematic Review of Reported Cases: Management, Visual Outcome, and Treatment Proposed. Semin Ophthalmol. 2016;31(5):485–91. [DOI] [PubMed] [Google Scholar]
- 59.Khalili MR, Jahadi HR, Karimi M, Yasemi M. Corneal Collagen Cross-linking for Treatment of Bacterial and Herpetic Keratitis. J Clin Diagn Res JCDR. 2017. Jul;11(7):NC12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lobo A-M, Agelidis AM, Shukla D. Pathogenesis of herpes simplex keratitis: The host cell response and ocular surface sequelae to infection and inflammation. Ocul Surf. 2019. Jan;17(1):40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edwards RG, Longnecker R. Herpesvirus Entry Mediator and Ocular Herpesvirus Infection: More than Meets the Eye. J Virol. 2017. 01;91(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chucair-Elliott AJ, Gurung HR, Carr MM, Carr DJJ. Colony Stimulating Factor-1 Receptor Expressing Cells Infiltrating the Cornea Control Corneal Nerve Degeneration in Response to HSV-1 Infection. Invest Ophthalmol Vis Sci. 2017. 01;58(11):4670–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong L-L, Tang R, Zhai Y-J, Malla T, Hu K. DNA vaccine expressing herpes simplex virus 1 glycoprotein C and D protects mice against herpes simplex keratitis. Int J Ophthalmol. 2017;10(11):1633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang R, Zhai Y, Dong L, Malla T, Hu K. Immunization with dendritic cell-based DNA vaccine pRSC-NLDC145.gD-IL21 protects mice against herpes simplex virus keratitis. Immunotherapy. 2018;10(3):189–200. [DOI] [PubMed] [Google Scholar]
- **65.Royer DJ, Hendrix JF, Larabee CM, Reagan AM, Sjoelund VH, Robertson DM, et al. Vaccine-induced antibodies target sequestered viral antigens to prevent ocular HSV-1 pathogenesis, preserve vision, and preempt productive neuronal infection. Mucosal Immunol. 2019;12(3):827–39. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, a live-attenuated HSV-1 vaccine protected mice from subsequent HSV-1 challenges, permitting preservation of corneal clarity and vision.


