Abstract
Ross River virus (RRV) is a fascinating, important arbovirus that is endemic and enzootic in Australia and Papua New Guinea and was epidemic in the South Pacific in 1979 and 1980. Infection with RRV may cause disease in humans, typically presenting as peripheral polyarthralgia or arthritis, sometimes with fever and rash. RRV disease notifications in Australia average 5,000 per year. The first well-described outbreak occurred in 1928. During World War II there were more outbreaks, and the name epidemic polyarthritis was applied. During a 1956 outbreak, epidemic polyarthritis was linked serologically to a group A arbovirus (Alphavirus). The virus was subsequently isolated from Aedes vigilax mosquitoes in 1963 and then from epidemic polyarthritis patients. We review the literature on the evolutionary biology of RRV, immune response to infection, pathogenesis, serologic diagnosis, disease manifestations, the extraordinary variety of vertebrate hosts, mosquito vectors, and transmission cycles, antibody prevalence, epidemiology of asymptomatic and symptomatic human infection, infection risks, and public health impact. RRV arthritis is due to joint infection, and treatment is currently based on empirical anti-inflammatory regimens. Further research on pathogenesis may improve understanding of the natural history of this disease and lead to new treatment strategies. The burden of morbidity is considerable, and the virus could spread to other countries. To justify and design preventive programs, we need accurate data on economic costs and better understanding of transmission and behavioral and environmental risks.
Ross River virus (RRV) is a mosquito-transmitted Alphavirus that is endemic and enzootic in Australia and Papua New Guinea (59, 85, 92, 167, 179). It caused a large epidemic in 1979 and 1980 involving Fiji, New Caledonia, Samoa, and the Cook Islands (4, 56, 95, 123, 130, 162, 197). RRV causes the most common arboviral disease in Australia, a characteristic syndrome of constitutional effects, rash, and rheumatic manifestations (60, 66, 122). Joint pain and disability may persist for several months (78). The number of reported cases in Australia during 1991 to 2000 averaged 4,745 per year, peaking at 7,823 in 1996 (Communicable Diseases Network—Australia New Zealand, http://www.health.gov.au/pubhlth /cdi/nndss/year002.htm).
The syndrome caused by RRV was initially referred to as epidemic polyarthritis (53, 95). Subsequently, the term Ross River fever has appeared and is commonly used by the public (130). More recently, the term RRV disease has come into use (59). The latter term was proposed by Marshall and Miles (130), then supported by Fraser (60), and is justified because not all people with symptomatic infection present with fever or joint pain (59): 20 to 60% of patients have the former, while 83 to 98% have the latter (33, 60, 82, 144, 181, 194, 218). An additional problem is that epidemic polyarthritis includes disease caused by both RRV and Barmah Forest virus (BFV) (124). Here we use RRV disease to refer to symptomatic infections and RRV infection to include both symptomatic and asymptomatic infections.
This article discusses all aspects of the biology of RRV, with an emphasis on human infection and disease. First, the biology of arboviruses is reviewed. Historical aspects of RRV research, and the structure of the virus and its relation to other alphaviruses are then discussed. Then immune response, pathogenesis, and laboratory diagnosis are discussed, followed by a section on RRV disease. Knowledge of vertebrate reservoir hosts and mosquito vectors is then considered, followed by sections dealing with the epidemiology and public health significance of RRV disease. The final section synthesizes the information to draw conclusions regarding the significance of RRV disease and directions for future research.
ARBOVIRUSES—IMPORTANT CONCEPTS
Definition
Arboviruses are “maintained in nature principally, or to an important extent, through biological transmission between susceptible vertebrate hosts by haematophagous arthropods; they multiply and produce viraemia in the vertebrates, multiply in the tissues of the arthropods, and are passed on to new vertebrates by the bites of arthropods after a period of extrinsic incubation” (226).
Vertebrate Reservoir Hosts
Most medically important arboviruses are transmitted to humans from other vertebrate species. To be an important reservoir for human infection, an animal must fulfill the criteria set forth in reference 178: “The host is present in large numbers and is readily accessible to vectors in time and space. The host is attractive to arthropod vectors and allows vectors to feed upon it. The host is susceptible to virus infection, experiences low mortality from infection, and becomes viremic with a titer of sufficient magnitude and duration to infect susceptible blood-feeding vectors. The life history of the host proceeds in such a way that immunologically susceptible individuals enter the population at times of active transmission. Host herd immunity remains low.”
To establish that a vertebrate is a reservoir host, the best evidence is frequent virus isolation from wild vertebrates. This is seldom possible because viremia is usually short-lived. Consequently, antibody prevalence is frequently used as an indicator of the prevalence of past infection. However, laboratory studies of infection are needed to confirm the role of a vertebrate as a reservoir host, because this is the only means of establishing the level and duration of viremia (178).
Vector Competence
Four criteria establish the vector competence of a mosquito species (193): (i) isolation of the disease-producing agent from wild-caught specimens, (ii) demonstration of its ability to become infected by feeding upon a viremic host, (iii) demonstration of its ability to transmit by bite, and (iv) field evidence confirming association of the infected arthropod with the vertebrate population in which the infection is occurring.
The overall risk for arbovirus disease in human populations is determined first by the presence of virus and vectors and second by the vector competence of the virus-vector system. Therefore, vector competence is central to the epidemiology of human arbovirus diseases.
EARLY OUTBREAKS AND DISCOVERY OF RRV
In 1928 there were two reports of an “unusual epidemic” in Narrandera and Hay in New South Wales (54, 148) (Fig. 1). These are now thought to have been due to RRV. In Hay, Nimmo (148) described a syndrome of “pain, skin eruption and general manifestations” in which “painful swelling of the joints” predominated. Fifteen years later, during the Second World War, several outbreaks of arthralgia and arthritis were described in the Northern Territory, Queensland, and the Schouten Islands off the northern coast of Papua New Guinea (53, 72, 76, 80, 186, 214) (Fig. 1). In 1946, Dowling (53) applied the name epidemic polyarthritis to the syndrome.
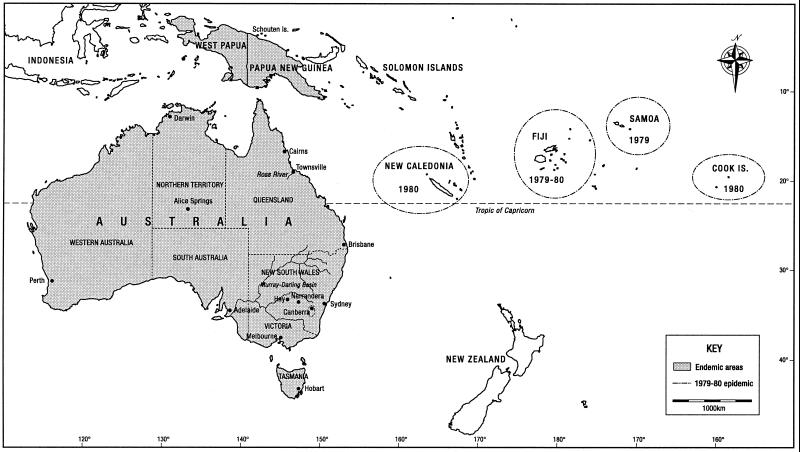
FIG. 1.
Map showing cities, towns, and geographical features discussed in the text, areas where RRV is endemic, and the 1979 to 1980 South Pacific epidemic of RRV disease.
An epidemic occurred in the Murray Valley (Fig. 1) in 1956, with a rash, usually maculopapular, being common (36 of 36 [10], 15 of 16 [70], and 45 of 45 [222]) and joint pains less so (23 of 35 had joint or muscle pains [10], 12 of 16 had joint and limb pains [70], and slightly more than one-third of 45 complained of joint pains [222]). Shope and Anderson (184) noted the similarity of the outbreak to an outbreak of “acute virus polyarthritis” caused by Chikungunya virus in Tanganyika in 1952 (159, 163). Differences were also noted, particularly the sudden onset of severe arthritis and fever followed by rash in the African outbreak, contrasted with the gradual onset of mild symptoms in the Australian outbreak, with fever rarely noted and mild (<38.3°C) when present (184). Because Chikungunya was known to be a group A arbovirus (now the genus Alphavirus), Shope and Anderson (184) sought serologic evidence that a group A arbovirus might be the cause of epidemic polyarthritis in Australia. Sixteen pairs of acute and convalescent sera were tested, and six showed antibody to one or more of 10 group A arboviruses. On hemagglutination inhibition (HI) testing, two patient's sera showed diagnostic rises in titer to Semliki Forest virus (SF), one to AMM2354 (now Bebaru) virus, and one to both viruses. Neutralization (N) testing for AMM2354 and AMM2021 (now Getah) viruses gave negative results for all acute-phase sera, but four convalescent-phase sera showed definite evidence of antibodies against one or both viruses. Complement fixation (CF) testing of two pairs of sera was negative for all viruses except Getah virus, against which convalescent sera fixed complement. On the basis of this evidence, Shope and Anderson (184) suggested that the cause of epidemic polyarthritis was an unknown group A arbovirus.
Next, Doherty et al. (51) isolated a group A arbovirus from Aedes vigilax mosquitoes trapped beside Ross River in Townsville, North Queensland (Fig. 1), in 1959, using intracerebral (IC) inoculation of 1- to 2-day-old mice. They tested sera from 20 patients with epidemic polyarthritis for antibodies against the virus, designated T48 (T for Townsville and 48 for the mosquito pool number [R. L. Doherty, personal communication, 2000]) as well as three other group A arboviruses (Sindbis, Getah, and Bebaru). All 20 patients had significant changes in titer of HI and/or CF antibodies to T48. Antibody titer to T48 was at least equal to that against the other viruses, and in most cases was higher. Seven patients had no significant antibody response to Getah or Bebaru virus. It was hypothesized that the virus causing epidemic polyarthritis was related to, or was, T48 (51). The link between T48 and epidemic polyarthritis was further strengthened when Doherty et al. (47) demonstrated serologic evidence of T48 infection among epidemic polyarthritis patients in Queensland. Subsequently, the name Ross River virus was applied, and T48 became the type strain (42).
Finally in 1972, RRV was isolated using IC inoculation of human serum into 1-to 2-day-old mice and subsequent serial passage after IC inoculation of brain suspension from any mice found sick or dead (45). The serum used was from a 7-year-old aboriginal boy from Edward River, North Queensland, who had a headache and fever but no arthralgia or arthritis. The child's nonspecific illness meant that the association between infection with RRV and the clinical entity known as epidemic polyarthritis remained conjectural (45).
In 1979 RRV spread to the South Pacific, causing large epidemics in Fiji, the Cook Islands, and Samoa (4, 56, 95, 123, 130, 162, 197) (Fig. 1). Aaskov et al. (4), Fauran et al. (57), Rosen et al. (162), and Tesh et al. (197) isolated RRV from 1, 42, 51, and 1 patient, respectively, all with arthralgia. The Pacific epidemic was the first occasion on which virus had been isolated from patients with symptoms of epidemic polyarthritis. Then, in 1985, Aaskov et al. (7) isolated RRV from an Australian patient with symptoms of epidemic polyarthritis. This was the first isolation of RRV from a patient with typical symptoms of polyarthritis in Australia.
STRUCTURE AND PHYLOGENETIC RELATIONSHIPS
Structure of the Virus
The genus Alphavirus contains small, enveloped viruses. The genome is single-stranded positive-sense RNA (90). The genome of the T48 strain of RRV is 11,853 nucleotides in length and codes for four nonstructural proteins (nsP1 to nsP4), a capsid protein, and three envelope glycoproteins (E1 to E3) (55). The E3 glycoprotein is not incorporated in the virion (26). The capsid protein and the genome form the nucleocapsid, which is about 400 Å in diameter, contains 240 copies of the capsid protein, and has T = 4 icosahedral symmetry (26, 191). T is the triangulation number, giving a multiplier of 60 that gives the number of subunits in the virus structure (81). The E1 and E2 viral glycoproteins are embedded in the lipid bilayer to form the envelope (191). Single E1 and E2 molecules associate to form heterodimers. The E1-E2 heterodimers form one-to-one contacts between E2 and nucleocapsid monomers (26).
Phylogenetic Relationships and Evolution
RRV belongs to the family Togaviridae, which contains the genera Alphavirus and Rubivirus. Alphavirus contains 24 registered members, all of which are arboviruses (211), and a 25th member, Trocara virus, has recently been added (199). The genus Rubivirus contains only rubella virus (177).
On the basis of the nucleotide sequence of the nonstructural proteins, the genus Alphavirus segregates into New World (American) and Old World (Eurasian-African-Australasian) viruses (212). An estimate of the time of divergence of the Old and New World alphaviruses was made on the basis of studies of the Venezuelan equine encephalitis virus (VEE) complex. Limited portions of the nsP4, E1, and 3′ untranslated regions of the genome were sequenced and phylogenetic trees were constructed (210). Assuming an evolutionary rate of 5 × 10−4 substitutions per nucleotide per year, the New World eastern equine encephalitis virus (EEE) and VEE complexes diverged only about 1,400 years ago (213). In a later study, phylogenetic analysis of the nsP1, nsP2, and nsP4 proteins was carried out. On the basis of the phylogeny and the estimate for the time of divergence of the New World EEE and VEE complexes, the Old and New World alphaviruses diverged as recently as 2,000 to 3,000 years ago (212).
On serologic criteria, RRV is classified in the SF antigenic complex of the genus Alphavirus, one of seven serologic complexes in the genus (22). The other complexes are EEE, Middleberg, Ndumu, VEE, western equine encephalitis virus (WEE), and BFV (22). The alphaviruses can be grouped into three larger groups on the basis of genome sequence, the VEE-EEE group, the SF virus (SFV) group, and the Sindbis virus (SIN) group (191). The SF group contains RRV, Sagiyama virus (SAGV) (211), SFV, Middleburg, Chikungunya, BFV, Getah, Bebaru, Mayaro, Una, and O'nyong-nyong (ONNV) viruses (191). Of these, complete nucleotide sequences have been published for RRV, SFV, ONNV, and SAGV, with RRV and SFV more closely related to each other than to ONNV (112, 183, 191). Analysis of the nsP4 and E2 genomic RNAs suggest that SAGV is most closely related to RRV (183), and it is now classified as a subtype of RRV (211). Given that RRV and SFV are thought to have diverged between 1,000 and 2,000 years ago (192), RRV and SAGV presumably diverged more recently. The relatively recent divergence of these three Old World alphaviruses from an ancestral virus in Australia, Africa, or Japan makes dispersal by birds seem likely.
IMMUNE RESPONSE, PATHOGENESIS, AND LABORATORY DIAGNOSIS
Immune Response to Infection and Pathogenesis of Arthritis
Following the bite of an infected mosquito, RRV particles are inoculated and attach to a cell surface receptor, possibly an integrin (105). The virus then penetrates and is uncoated within the cell (161). Primary replication occurs in skeletal muscle (73, 89, 146, 147). RRV then enters the blood, where clearance is mediated principally by neutralizing antibodies and type 1 interferon (86, 106, 229). At the time that symptoms commence, virus usually cannot be cultured from peripheral blood (124), so presumably it has been cleared by the mechanisms discussed above but has infected other tissues. The most prominent and disabling symptom of RRV disease is arthralgia. Therefore, the following discussion is focused on joint infection and the pathogenesis of arthralgia and arthritis.
Immune complexes are involved in the pathogenesis of some viral arthritides (65, 225), but Fraser et al. (65) could not find immune complexes in the serum or synovial fluid of RRV disease patients. Immune complexes deplete complement and attract neutrophils, but Fraser et al. (65) and Clarris et al. (28) found normal C3 and C4 in serum of RRV disease arthritis patients. Synovial effusions in acute RRV disease consist mainly of monocytes and activated macrophages (28, 64, 84), which are attracted and activated by monocyte chemoattractant protein 1 (MCP-1), which is produced by RRV-infected synovial fibroblasts in vitro (134). Thus, direct and indirect evidence argues against immune complexes as the cause of RRV arthralgia and arthritis.
Viable RRV has not been isolated from the joints of patients with chronic joint symptoms following RRV infection (28, 60). Viral antigen had been demonstrated in leukocytes from joint effusions, and viral RNA has been demonstrated by PCR testing of synovial biopsy specimens (64, 188). Human synovial cells can be productively infected with RRV in vitro (34, 91). La Linn and coworkers (105, 107) showed that macrophages could be persistently and productively infected with RRV and suggested this might play a role in persistence through the phagocytosis of dying cells by other macrophages. Neutralizing antibody cannot clear RRV from infected macrophages (106, 107). Patients with RRV disease have a marked virus-specific T-lymphocyte proliferative response (3), and cytotoxic T lymphocytes can clear RRV-infected macrophages (106, 107). La Linn et al. (106) suggested that the development of chronic arthralgia and/or arthritis could be explained through the inability of some individuals to generate RRV-specific cytotoxic T lymphocytes and hence clear infection. This hypothesis fits well with earlier observations of Fraser and his group (62, 67). They found that CD8+ lymphocytes (usually cytotoxic T lymphocytes [160]) predominate in the skin rashes of patients who have made rapid recoveries from RRV disease (67). CD4+ lymphocytes (usually helper T lymphocytes [160]) predominate in the mononuclear synovial effusions of patients with chronic RRV disease symptoms (62). Also, human leukocyte antigen DR7 is more common among RRV disease patients than among controls (68). Human leukocyte antigen restriction of the ability to generate specific cytotoxic T lymphocytes and consequent persistent infection of joint macrophages or synovium may offer a mechanism for the persistence of symptoms (105, 188). La Linn et al. (107) demonstrated that RRV-infected macrophages secrete nitric oxide, an inflammation mediator. Interferon gamma, a cytokine produced by T lymphocytes, is elevated in synovial effusions of patients with RRV disease (105). One or both of these substances may mediate inflammation in RRV-induced synovitis. Synovial fibroblasts infected with RRV in vitro produce MCP-1, which has been implicated in the pathogenesis of some other viral arthritides and rheumatoid arthritis (134). RRV arthritis is probably an infectious arthritis, possible inflammatory mediators have been identified, and an immunological mechanism for chronic symptoms occurring in some people has been suggested.
Laboratory Diagnosis
Virus isolation from humans is rarely achieved, probably because RRV does not persist beyond the early stages of disease. Diagnosis is usually made on serologic grounds (124).
Because of its production early in the course of Alphavirus infection and the fact that it usually does not persist at high titer (23, 25) the detection of immunoglobulin M (IgM) in an acute-phase specimen provides a presumptive diagnosis of recent infection (121). Sucrose gradient ultracentrifugation is required to separate IgM for HI testing, and only reference laboratories use the technique (D. Phillips, WHO Collaborating Centre for Arbovirus Reference and Research, Brisbane, Queensland, Australia, personal communication, 1999). In most laboratories HI cannot distinguish IgM and IgG. Enzyme-linked immunosorbent assay (ELISA) allows measurement of IgG and IgM separately, enabling presumptive diagnosis by a single IgM-positive specimen (121, 136). False-positive ELISA results may be caused by BFV, rubella (157), Q fever (150), and noninfectious causes such as rheumatoid factor, although the last is normally controlled for in the testing procedure.
A confirmed diagnosis cannot be made with a single serum specimen. Two sera obtained 10 to 14 days apart should be collected and tested in parallel by the same laboratory. The acute-phase serum should be collected within 7 days, and the convalescent-phase serum within 8 to 28 days of onset of illness (121). The standard for confirmed diagnosis of Alphavirus infections is a fourfold or greater increase or decrease in antibody titer determined by HI, CF, or N testing (23, 121).
Until 1999, most testing for RRV infection in Australia was done using indirect ELISA with a kit manufactured by PanBio; a second kit manufactured by Biocene is also now available (J. Kapeleris, PanBio, personal communication, 2001). Flexman et al. (59) suggest that IgG seroconversion, that is, an IgG-negative acute and IgG-positive convalescent specimen on ELISA testing, confirms the diagnosis. Based on ELISA testing of 124 HI-positive patients infected in South Australia from 1996 to 1997, the sensitivity of the PanBio ELISA kit is 98.5 and 84.6% and the specificity is 96.5 and 97.6% for IgM and IgG, respectively (120; M. Qiao, personal communication, 2001).
RRV DISEASE
Incubation Period
The incubation period for most arboviruses in 5 to 15 days (185). RRV usually incubates for 7 to 9 days, based on the experience of 20 patients who lived in areas of no risk, visited transmission zones, and developed symptoms with diagnosis confirmed by HI testing (63). Incubation may be as long as 21 days or as short as 3 days. The first estimate is based on a questionnaire-survey of notified cases (143) and the latter on a single case report (162).
Clinical Manifestations
RRV disease causes a characteristic syndrome, including constitutional effects, rash, and rheumatic manifestations (60). Several less common presentations have also been described.
Fraser (60) described the clinical features of a series of 43 patients. At first he actively sought cases receiving medical or surgical care in the Murray Valley town of Echuca, Victoria, for any symptom that could be attributed to RRV or other viral infections. Later, cases were referred from the casualty department of the Royal Melbourne Hospital, rheumatologists, family practitioners, and infectious disease and other consultant physicians (J. R. E. Fraser, personal communication, 1997). Patients referred from rheumatologists and other consultant physicians might have been unusual. However, this description is still the best account of RRV disease and is especially useful because it was done by a single clinician with appropriate skills. Unless otherwise specified, the description that follows is based on Fraser's observations.
Joint manifestations.
Joint symptoms and signs are usually symmetrical and acute in onset, ranging from tenderness with minor restriction of movement to extreme redness and swelling. Effusions are common and peripheral joints are predominantly involved. Fraser (60) does not report the frequency of involvement of specific joints, but this information is noted in five other less direct studies of RRV disease (Table 1). These studies were carried out between 1971 and 1992 in Western Australia (33), New South Wales (218), South Australia (180), the whole of Australia (144), and Fiji (4). The methods for case ascertainment differ, but the results clearly show that ankles, fingers, wrists, and knees are the joints most commonly affected. The metacarpophalangeal joints are also very frequently affected by pain (78).
TABLE 1.
Joints affected by RRV disease
| Joint | Frequency of joint involvement (% of cases)
|
||||
|---|---|---|---|---|---|
| Seglenieks and Moore (180) (n = 115) | Aaskov et al.a (4) (n = 36) | Mudge and Aaskov (144) (n = 400) | Condon and Rouse (33) (n = 189) | Westley-wise et al. (218) (n = 80) | |
| Fingers | 50c | 80 | 81 | 63 | |
| Hand | 45 | ||||
| Thumb | 53 | 58 | |||
| Wrist | 36 | 70 | 80 | 100 | 61 |
| Elbow | 17 | 40 | 44 | 71 | |
| Shoulder | 38 | 47 | 62 | ||
| Hip | 4 | 10 | 27 | ||
| Knee | 39 | 80 | 80 | 100 | 64 |
| Ankle | 50 | 78 | 88 | 97 | 64 |
| Feet | 49 | 36 | |||
| Toes | 47 | ||||
| Back | 14b | 36 | 37 | 56 | |
| Neck | 36 | 12 | 70 | ||
| Jaw | 10 | 15 | |||
Percentages taken from a bar graph.
Includes back or neck.
Includes fingers and hand.
Rash.
Rash affects about 50% of people with RRV disease, usually lasts 5 to 10 days, and may be the sole manifestation of infection. The rash appears mainly on the limbs and trunk, but may also occur on the palms, soles, digits, face, and even the scalp. Most commonly the skin eruption is maculopapular, but it may be vesicular or purpuric (60, 66).
Constitutional effects.
Fever affects from one third to one half of patients. Rash, fever, and arthralgia may occur in any sequence. Myalgia is common, reported by up to 58% of patients (144). Lymphadenopathy occurs quite often, sore throat and coryza less frequently, and diarrhea is rare (60, 66). In a small study, Bennett et al. (17) noted that fatigue and malaise rather than anxiety and depression accompanied RRV disease. Fatigue typically affects over 50% of patients.
Other presentations.
Other manifestations reported include splenomegaly, hematuria, and glomerulonephritis (11, 39, 60, 65, 222). Paresthesia may occur due to entrapment neuropathy. Headache, neck stiffness, and photophobia may occur. So far there are only three case reports suggesting meningitis or encephalitis caused by RRV infection, indicating that these must be rare. None of the three cases were proven to be caused by RRV, and more effort should be made to isolate RRV from cerebrospinal fluid if future cases occur. Because of the serious nature of such manifestations, these cases are reviewed in detail, although the evidence that RRV infection caused these cases is not strong.
The first report was of a 27-year-old man who died of encephalitis in Papua New Guinea during 1980 or 1981 (the date of onset is not stated in the paper) and had RRV antibody titers of 1:160 1 week after hospital admission. No other cause of encephalitis could be found, but he did not have arthralgia or arthritis, RRV was not isolated, and autopsy could not be performed, so the evidence that RRV caused his death is weak (179).
Lucas and Qiao (120) reported a 33-year-old male who was admitted to the intensive care unit of the Alice Springs (Fig. 1) hospital with encephalitis. There was no rash or evidence of arthritis. Cerebrospinal fluid Gram stain, latex agglutination for bacterial capsular antigens, cryptococcal antigen, and India ink stain tests were negative. There was no serologic evidence for other causes of acute and postinfectious meningoencephalitis. On admission, HI and ELISA testing for IgG and IgM to RRV were negative. IgM on ELISA was positive after 8 days and remained so for 34 days postadmission. IgG was negative on both of these occasions, but became positive 90 days postadmission. A diagnostic (≥4-fold) rise in total antibody titer was not observed using HI testing. The IgG seroconversion after 90 days was later than would be expected, even allowing for the slow seroconversion noted by Qiao (120; M. Qiao, personal communication, 1999).
Penna and Irving (J. E. Penna and L. G. Irving, letter, Med. J. Aust. 159:492–493, 1993) reported a possible case of meningitis caused by RRV. A 40-year-old male developed headache and neck stiffness 1 week after onset of fever and arthralgia. HI testing gave a result of 80 with RRV as the antigen and <20 with Murray Valley encephalitis virus as the antigen. RRV IgM was detected in serum. Serologic tests for a number of other infectious causes of meningitis were negative.
Illness duration and prognosis.
Recent estimates of the duration of symptoms with RRV disease have increased substantially compared with estimates made when the disease was first described. RRV disease has now become notorious in public discourse, with a reputation for inducing prolonged and disabling arthritis.
In the first described RRV outbreak, Nimmo (148) noted that “many individuals have been unable to carry on their employment for periods up to three weeks.” Subsequent reports focused on observations of soldiers in the second World War (53, 76, 186). Halliday and Horan (76) suggested that “joint enlargement subsides within a week, but painful limitation of joint movement persists for two or three weeks longer.” Dowling (53) found that joint swelling persisted for 4 to 7 days and pain usually disappeared a few days after swelling subsided, but 3 of 94 had joint pain persisting for 3 months after onset. Sibree (186) found that 17 of 28 soldiers were able to carry on normal army duties. Eleven others were admitted to hospital, but eight of these resumed duties within 23 days. Three remained in hospital for 60 to 70 days due to persistently painful joints.
From the 1980s, reports on the clinical features began to suggest that some patients developed chronic disease lasting for months to years. Fraser (60) reported that 50% had recovered after 6 months, 75% in just over 12 months, and 5% had not recovered after 4 years. In a large New South Wales outbreak, Hawkes et al. (82) found that 11% were incapacitated for longer than 12 weeks. Three recent studies suggested that an even longer duration of symptoms was typical. Condon and Rouse (33) reported that 24 to 42 months after onset 57% of RRV disease patients had intermittent or continuous joint pain. Selden and Cameron (181) found that 30 months after diagnosis, 64% had joint pain. Westley-Wise et al. (218) found that 12 months after onset, 52.5% had joint pain. The validity of the last three studies is doubtful. They involved mailed questionnaires sent to notified cases months or years after disease onset, and the calculations are based on the fraction who responded. In the study of Selden and Cameron, only 179 of 814 responded at 30 months (181). This created a serious selection bias, as it is much more likely that those who were ill would respond, boosting estimated rates. Also, these studies used nonstandardized and nonvalidated questionnaires, lacked clinical review, did not exclude alternative diagnoses, and had no control groups.
A priori, we would not expect RRV disease morbidity to vary much. Evolution of the virus is slow (21), and it remains genetically homogeneous over large geographic areas (119, 176). This stability suggests that temporal or spatial changes in virulence are unlikely to occur. Methodological differences among the morbidity reports and substantial self-reporting bias in the three mailed questionnaire studies of the 1990s (33, 181, 218), together with cultural inflation of morbidity (88) over time, account for these differing estimates. The so-called cultural inflation of morbidity (88) results from increasing health expectations on the part of the public and cultural pressures on health professionals to treat an ever-growing set of nonfatal diseases, leading to a paradoxical increase in demand for health services as mortality declines.
The lack of clinical review and exclusion of alternative diagnoses may be a threat to validity. An analogous situation exists with chronic Lyme disease in the United States and Canada and suggests that caution should be exercised in ascribing chronic symptoms to an infectious disease of which there is a high level of public awareness and concern. Both infections have been implicated as causes of chronic rheumatic disease. In the case of Lyme disease, chronic illness directly attributable to Borrellia burgdorferi infection is considerably less common than previously thought. Burdge and O'Hanlon (20), working in Canada, assessed 65 patients sent to a multidisciplinary referral center for Lyme disease. By applying strict criteria for diagnosis and intensively investigating patients, major alternative diagnoses were made for 77%, probable diagnoses of chronic fatigue syndrome and fibromyalgia were made for 17%, and 6% remained undiagnosed. Only two patients were judged to have probable Lyme disease. An American study of Lyme disease produced similar results (190). Some intimation that the same phenomenon might be occurring with RRV disease is provided by Keary (P. J. Keary, Abstr. 40th Annu. Sci. Conf. Aust. Rheumatol. Assoc., abstr. 16, 1997), who studied 103 patients with serologically confirmed RRV infection. Thirty either had preexisting or subsequently developed another rheumatic disease. The prevalence of rheumatic symptoms in the community is high (127, 206). It follows that prevalent symptoms not causally associated with RRV disease are likely to have been included in the results of the three 1990s RRV symptom studies (33, 181, 218), leading to an overestimate of symptom prevalence and duration. Experience with Lyme disease and the study by Keary both suggest that it is unwise to assume that the symptoms reported in these studies have been caused by RRV infection.
Until the natural history of RRV disease morbidity is better understood, the public health importance of this disease cannot be determined. This requires repeated clinical assessments of a community-based inception cohort of symptomatic persons with confirmed incident RRV disease, using standard measures of morbidity, and comparing results to expected levels of morbidity for people of that age and sex. One of us (D.H.) has completed such a study in North Queensland (78), and the information obtained will now be considered.
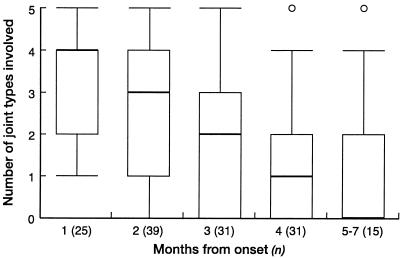
The inception cohort was a group of 47 family practice patients diagnosed with RRV disease in and around Cairns (Fig. 1) in 1998. One physician trained in rheumatological assessment (D. Harley) reviewed all patients three times in their homes from 7 to 210 days after diagnosis. The assessment included a clinical history, rheumatologic examination, and standard validated health questionnaires (Clinical Health Assessment Questionnaire [CLINHAQ] [69, 139, 223] and Short Form-36 [SF-36] [135]). The time course of symptoms and signs over approximately 7 months from onset is shown in the following table and graphs. There was a progressive diminution in symptom prevalence over time. The box plot (Fig. 2) quantifies the involvement, by pain within the week of review, of the five most commonly involved joint types (finger, metacarpophalangeal, wrist, knee, and ankle joints) in 141 questionnaire responses from 47 patients. The median number of painful joint groups decreased from four to one over the first 4 months and reached zero after 5 to 7 months. However, at least one joint remained abnormal on clinical examination for one quarter of the 15 examinations performed after 5 to 7 months (Table 2). Of the four individuals who had abnormal findings in months 5 to 7, one was a 54-year-old woman with severe preexisting osteoarthritis who had swelling of the ankle joint and proximal interphalangeal joints of the fingers and grade I tenderness (195) of her knee joint. These findings reflected her underlying disease rather than having been caused by RRV disease and therefore inflate the prevalence of joint abnormalities at this review.
FIG. 2.
Number of joint types that were painful over 7 months of postinfection follow-up for 47 cases of RRV disease, Cairns, 1998.
TABLE 2.
Prevalence of joint abnormality over 7 months of follow-up, RRV disease, Cairns, 1998
| Time postonset (mo) | No. of examinationsa | Prevalence (%) of abnormal exams of one or more jointsb |
|---|---|---|
| 1 | 25 | 60 |
| 2 | 39 | 62 |
| 3 | 31 | 39 |
| 4 | 31 | 32 |
| 5–7 | 15 | 27 |
Forty-seven individuals were examined, each on three occasions, giving 141 examinations.
Objective signs on joint examination—tenderness, effusion, enthesopathy, swelling, heat, or other abnormal findings.
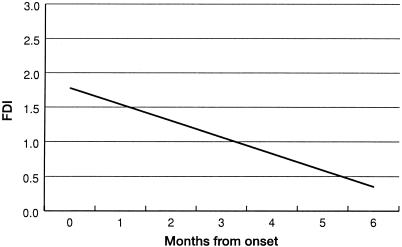
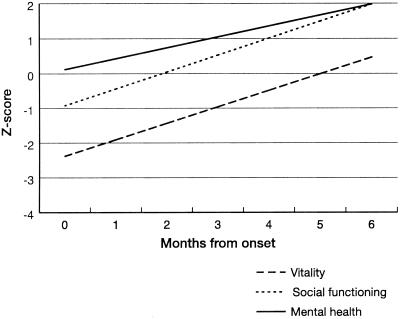
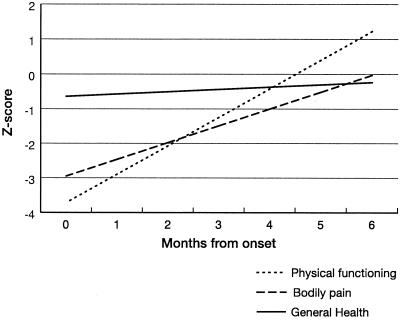
Figures 3 through 5 show the steady improvement in difficulties with activities of daily living (ADL), and physical, psychological, and social function over 6 months after infection. All analyses were linear regressions, with the study subject included as a random effect. Figure 3 shows the CLINHAQ Functional Disability Index (FDI) regressed against time since symptom onset. The FDI measures difficulties with ADL and can be interpreted as follows (187): 2.0 to 3.0, severely handicapped; 1.25 to 2.0, many major problems with ADL; 0.5 to 1.25, reasonably self-sufficient; and 0.0 to 0.5, self-sufficient. Figures 4 and 5 show physical and psychological dimensions of the SF-36 health status questionnaire, respectively. These are age- and sex-adjusted departures from the population standard for the Australian State of Queensland (207) in standard deviation units, regressed against time since symptom onset. It is clear that the group returns to normal physically over 4 to 6 months and psychologically over 2 to 5 months.
FIG. 3.
Clinical Health Assessment Questionnaire Functional Disability Index (FDI) regressed against time since symptom onset for RRV disease cases, Cairns, 1998.
FIG. 5.
Psychological dimensions of the SF-36, age and sex standardized to the Queensland population and regressed against time since symptom onset for RRV disease cases, Cairns, 1998.
FIG. 4.
Physical dimensions of the SF-36, age and sex standardized to the Queensland population and regressed against time since symptom onset for RRV disease cases, Cairns, 1998.
The results of this study indicate that RRV disease does cause pain and suffering for several months in a substantial proportion of patients, but progressive resolution indicates that treating doctors are justified in taking an optimistic outlook regarding complete recovery over some months, whilst acknowledging the appreciable temporary disability that may result.
Treatment
Recommendations on treatment for RRV disease are not based on clinical trials. After extensive clinical experience with RRV disease, Fraser (66) reported that nonsteroidal anti-inflammatory drugs (NSAIDs) can give dramatic symptomatic relief. Condon and Rouse (33) found that of 255 patients, 36.4% felt that NSAIDs provided the “best and most effective relief,” and 16.4% felt that aspirin or paracetamol was the most effective. Physical interventions (swimming, hydrotherapy, physiotherapy, or massage) were the most beneficial for 10.3% of patients, but for 24.1% rest was the only source of symptom relief. Some clinicians use oral corticosteroid therapy and have found that patients respond well (D. Harley, unpublished data), but it is doubtful whether steroids are needed, and the adverse effects should constrain their use. In theory, steroids could make RRV disease worse, but this has not been observed.
The available information is limited but suggests a stepwise approach would be best for treatment of RRV disease arthritis; first, a trial of rest and physical therapy, followed by simple analgesics, and then NSAIDs if necessary. Until more information is available, steroid treatment cannot be recommended.
ECOLOGY OF RRV
Vertebrate Reservoir Hosts
Theoretically, to establish the status of putative reservoir hosts, we require information on behavior, ecology, immunology, and reproductive cycles and the course of arbovirus infection. In practice, most knowledge of the likely vertebrate reservoir hosts for arboviruses has come from antibody prevalence surveys and experimental studies of infection. This is true for RRV.
Here we review knowledge of reservoir hosts for RRV, including the infrequent isolation of virus from vertebrates, extensive antibody prevalence surveys, the numerous experimental infection studies, and current thinking about reservoir hosts.
Seven RRV isolates have been reported from nonhuman vertebrates, remarkably few for such an important zoonotic infection (Table 3). The information base for antibody prevalence in verterbrates is much larger than for virus isolates (Table 4).
TABLE 3.
Isolates of RRV from nonhuman vertebrates
| Species | No. of isolates | No. of animals tested | Reference |
|---|---|---|---|
| Placental mammals | |||
| Horse (Equus caballus) | 1 | 8 | 149 |
| 1 | 24 | ||
| Marsupials | |||
| Agile wallaby (Macropus agilis) | 2 | 17 | 49 |
| Birds | 775 (104 species) | 221 | |
| Magpie lark (Grallina cyanoleuca) | 1 | ||
| Flycatcher (Microeca fascinans) | 1 | ||
| Masked finch (Poephila personata) | 1 |
Adapted from reference 208.
IgG ELISA.
TABLE 4.
RRV antibody prevalence surveys of nonhuman vertebratesa
| Species | Method and results
|
Reference | ||||||
|---|---|---|---|---|---|---|---|---|
| HI
|
N
|
HI
|
N
|
|||||
| % Positive | n | % Positive | n | % Positive | n | % Positive | n | |
| Placental mammals | ||||||||
| Flying fox (Pteropus sp.) | 0 | 7 | 0 | 8 | 48 | |||
| Blackish flying fox (Pteropus gouldii) | 50 | 6 | 100 | 3 | 49 | |||
| Little red flying fox (Pteropus scapulatus) | 5 | 21 | 71 | |||||
| 0 | 21 | 0 | 1 | 49 | ||||
| Gray-headed flying fox (Pteropus poliocephalus) | 25 | 261 | 71 | |||||
| Pseudo-rat (Pseudomys fumeus) | 0 | 1 | 132 | |||||
| House mouse (Mus musculus) | 0 | 4 | 71 | |||||
| 0 | 7 | 203 | ||||||
| New Holland mouse (Gyomys novaehollandiae) | 25 | 72 | 71 | |||||
| Rats (unidentified) | 3 | 33 | 197 | |||||
| 2 | 150 | 0 | 71 | 48 | ||||
| Black rat (Rattus rattus) | 0 | 18 | 71 | |||||
| 0 | 3 | 132 | ||||||
| Brown rat (Rattus norvegicus) | 0 | 1 | 71 | |||||
| Eastern water rat (Hydromys chrysogaster) | 12 | 26 | 100 | 1 | 49 | |||
| Sombre Downs rat (Rattus sordidus) | 7 | 14 | 49 | |||||
| Swamp rat (Rattus lutreolus) | 9 | 11 | 71 | |||||
| 0 | 72 | 203 | ||||||
| Bush rat (Rattus fuscipes) | 0 | 124 | 203 | |||||
| 0 | 7 | 132 | ||||||
| Rabbit (Oryctolagus cuniculus) | 0 | 1 | 132 | |||||
| Dog (Canis familiaris) | 20 | 45 | 197 | |||||
| 8 | 24 | 11 | 36 | 48 | ||||
| 53 | 30 | 40 | 5 | 49 | ||||
| 33 | 33 | 50 | 34 | 162 | ||||
| Domestic cat (Felis catus) | 0 | 16 | 197 | |||||
| Feral pig (Sus scrofa) | 50 | 4 | 100 | 2 | 49 | |||
| Domestic pig (Sus scrofa) | 15 | 27 | 197 | |||||
| 43 | 70 | 77 | 47 | 162 | ||||
| Goat (Capra hircus) | 0 | 2 | 204 | |||||
| 0 | 8 | 38 | 8 | 48 | ||||
| 17 | 6 | 203 | ||||||
| Sheep (Ovis aries) | 11 | 36 | 24 | 17 | 48 | |||
| 0 | 4 | 203 | ||||||
| Cow (Bos taurus) | 20 | 40 | 31 | |||||
| 19 | 187 | 203 | ||||||
| 54 | 41 | 62 | 21 | 48 | ||||
| 33 | 12 | 100 | 11 | 162 | ||||
| 6 | 102 | 137 | ||||||
| 67 | 66 | 100 | 21 | 49 | ||||
| Horse (Equus caballus) | 65 | 23 | 31 | |||||
| 62 | 120 | 203 | ||||||
| 86 | 36 | 89 | 37 | 48 | ||||
| ∼60 | 1,300b | 14 | ||||||
| Marsupials | ||||||||
| Agile wallaby (Macropus agilis) | 85 | 169 | 19 | 12 | 49 | |||
| Brush wallaby (Macropus rufogriseus) | 67 | 21 | 100 | 5 | 48 | |||
| 86 | 7 | 203 | ||||||
| 0 | 2 | 204 | ||||||
| 67 | 33 | 137 | ||||||
| Black-striped wallaby (Macropus dorsalis) | 27 | 11 | 100 | 1 | 48 | |||
| Southern whip-tail wallaby (Macropus parryi) | 66 | 3 | 100 | 1 | 48 | |||
| Red-necked pademelon (Thylogale thetis) | 0 | 3 | 48 | |||||
| Tasmanian pademelon (Thylogale billardierii) | 56 | 50 | 137 | |||||
| Swamp wallaby (Wallabia bicolor) | 83 | 6 | 203 | |||||
| 50 | 2 | 204 | ||||||
| Gray kangaroo (Macropus giganteus) | 58 | 36 | 100 | 13 | 48 | |||
| 50 | 2 | 204 | ||||||
| 50 | 30 | 203 | ||||||
| 40 | 10 | 137 | ||||||
| 89 | 28 | 100 | 5 | 49 | ||||
| 74 | 39 | 168 | ||||||
| Red kangaroo (Macropus rufus) | 52 | 25 | 100 | 10 | 48 | |||
| 0 | 2 | 204 | ||||||
| Northern nail-tail wallaby (Onychogalea unguifera) | 100 | 1 | 49 | |||||
| Barred bandicoot (Perameles gunnii) | 100 | 2 | 137 | |||||
| Brindled bandicoot (Isoodon macrourus) | 1 | 64 | 50 | 2 | 48 | |||
| Brown bandicoot (Isoodon obesulus) | 25 | 4 | 203 | |||||
| 100 | 7 | 137 | ||||||
| Long-nosed bandicoot (Perameles nasuta) | 25 | 8 | 203 | |||||
| 0 | 40 | 48 | ||||||
| Bilby (Macrotis lagotis) | 0 | 1 | 48 | |||||
| Ringtail possum (Pseudocheirus peregrinus) | 0 | 1 | 137 | |||||
| 66 | 3 | 71 | ||||||
| Common brush tail possum (Trichosurus vulpecula) | 100 | 1 | 71 | |||||
| 50 | 2 | 49 | ||||||
| 77 | 13 | 137 | ||||||
| 4 | 243 | 14 | ||||||
| Common wombat (Vombatus ursinus) | 43 | 7 | 137 | |||||
| Potoroo (Potorous apicalis) | 100 | 2 | 137 | |||||
| Bettong (Bettongia gaimardi) | 100 | 1 | 137 | |||||
| Tasmanian devil (Sarcophilus harrisii) | 100 | 4 | 137 | |||||
| Sugar glider (Petaurus breviceps) | 50 | 2 | 71 | |||||
| Koala (Phascolarctos cinereus) | 16 | 93 | 168 | |||||
| White-footed dunnart (Sminthopsis leucopus) | 0 | 3 | 203 | |||||
| Monotremes | ||||||||
| Short-beaked echidna (Tachyglossus aculeatus) | 0 | 6 | 203 | |||||
| Birds | ||||||||
| Birds (mixed species) | 1 | 145 | 6 | 16 | 48 | |||
| 50 | 48 | 63 | 8 | 49 | ||||
| Frogmouth owl (Podargus strigoides) | 17 | 6 | 71 | |||||
| Azure kingfisher (Alcyone azurea) | 0 | 1 | 71 | |||||
| Raven (Corvus coronoides) | 0 | 1 | 71 | |||||
| Kookaburra (Dacelo gigas) | 0 | 1 | 71 | |||||
| Yellow-faced honeyeater (Melithaga chrysops) | 0 | 4 | 71 | |||||
| Fowl | 5 | 20 | 197 | |||||
| 9 | 147 | 52 | ||||||
| Reptiles | ||||||||
| Unidentified | 0 | 15 | 49 | |||||
The results of experimental infection studies conducted on over 20 species are shown in Table 5. Largely on the basis of the data in Table 5, Kay and Aaskov (95) concluded that “marsupials are more competent hosts than placental mammals which, in turn, are more effective amplifiers than birds.” However, this is only true if level and duration of experimental viremia are equated with reservoir competence. Even then, with only a small number of experimental infection studies performed on a small number of animals, it seems premature to rank vertebrate hosts for their importance as reservoirs. As outlined in the introduction to this section, many factors other than level and duration of viremia must be taken into account to determine reservoir status.
TABLE 5.
Results of experimental infection of nonhuman vertebrates with RRVa
| Species | Maximum titer of viremia (log10/LD50b or PFU/ml) | Duration of viremia (days) | No. tested | Reference |
|---|---|---|---|---|
| Placental mammals | ||||
| Adult laboratory mouse (Mus musculus) | 5.4b | 1–3 | 130 | |
| Adult wild mouse (Mus musculus) | 0 | 130 | ||
| New Holland mouse (Pseudomys novaehollandiae) | 7.6 | 1–5 | 130 | |
| Black rat (Rattus rattus) | 4.6 | 3–6 | 130 | |
| Brown rat (Rattus norvegicus) | 7.4 | 1–3 | 4 | 220 |
| Swamp rat (Rattus lutreolus) | 0 | 130 | ||
| Gray-headed flying fox (Pteropus poliocephalus) | 0–1 | 130 | ||
| Red flying fox (Pteropus scapulatus) | 0 | 130 | ||
| Rabbit (Oryctolagus cuniculus) | 4.7b | 0–3 | 4 | 220 |
| Horse (Equus caballus) | 6.3 | 0–5 | 11 | 95 |
| Cow (Bos taurus) | 0 | 95 | ||
| Sheep (Ovis aries) | 5.0b | 2–5 | 95 | |
| Juvenile domestic pig (Sus scrofa) | 4.2 | 1–3 | 130 | |
| Juvenile feral pig (Sus scrofa) | 4.8b | 0–5 | 95 | |
| Marsupials | ||||
| Bandicoot (Isoodon macrourus) | 7.2b | 1–6 | 4 | 220 |
| Marsupial mouse (Antechinus stuartii) | 4.4 | 1–4 | 130 | |
| Marsupial mouse (Antechinus sp.) | 8.0b | 1–6 | 6 | 220 |
| Tammar wallaby (Macropus eugenii) | 5.3 | 2–3 | 130 | |
| Agile wallaby (Macropus agilis) | 7.4b | 0–5 | 95 | |
| Gray kangaroo (Macropus giganteus) | 6.1b | 6 | 95 | |
| Birds | ||||
| Adult domestic fowl | 0 | 4 | 220 | |
| Day-old domestic fowl | 5.0b | 1–5 | 12 | 220 |
| Rufous night heron (Nycticorax caledonicus) | 0 | 130 | ||
| Australian magpie lark (Grallina cyanoleuca) | 0 | 130 | ||
| Pigeon (Columba livia) | 0 | 5 | 220 | |
| Black duck (Anas superciliosa) | 2.3b | 0–5 | 95 | |
| Little corella (Cacatua sanguinea) | <2.3b | 0–1 | 95 |
From references 95, 130, and 220. Only Whitehead (220) gives detailed methods. The T48 strain of RRV was used as a 20% suspension of suckling mouse brain in rabbit serum-saline. Experimental hosts were inoculated subcutaneously with virus diluted 10−4.5. To determine viremia, blood from experimental hosts was diluted 1:10. Blood was titrated by intraperitoneal inoculation of suckling mice. Virus titers were expressed as the minimum dose sufficient to kill 50% of inoculated mice (LD50) per gram of undiluted blood. Marshall and Miles (130) and Kay and Aaskov (95) reproduce results from Whitehead (220) as well as their own unpublished data, but do not provide detailed methods.
Assayed in suckling mice. LD50, lethal dose 50% endpoint.
The antibody prevalence and laboratory infection and transmission data suggest significant roles for macropods as reservoir hosts. Macropus agilis, the agile wallaby, is one of the few vertebrates from which RRV has been isolated (Table 3), and antibody prevalence surveys also suggest high rates of infection (Table 4). In 1971, in a paper on arbovirus infections of mosquitoes and mammals at Mitchell River mission (now Kowanyama) on Cape York Peninsula in remote North Queensland, Doherty et al. (49) concluded that “accumulating evidence suggests that the association of large marsupials, especially wallabies, with Ross River and Kokobera viruses is ecologically significant, and that the basic epidemiology of the human disease epidemic polyarthritis may therefore involve cycles of transmission between these large macropods and mosquitoes.”
Later, Doherty (43) qualified this opinion for urban areas, saying “it is difficult to see what mammal hosts could maintain epidemics in, for example, suburbs of Brisbane where wallabies, kangaroos and rodents are not in high population.” Russell (167, 168) considers that the natural vertebrate hosts of RRV are native mammals such as kangaroos. Mackenzie et al. (122) and Mackenzie and Smith (124) consider that macropods are the major vertebrate hosts. Before the white invasion of Australia, macropods may have been the reservoir for natural enzootic RRV transmission cycles, but it does not follow that they are the major reservoir for the large numbers of human infections that occur in Australia. Macropods are rare in urban Australia, and they cannot be the usual source of metropolitan human infections, the main component of reported RRV disease. Other animals must be involved in urban areas.
Antibody to RRV has been detected in brush tail possums, a common urban marsupial, albeit in very few animals. Three studies using HI testing have tested between 1 and 13 animals and found antibody prevalences of between 50 and 100% (49, 71, 137). A larger study of 243 animals used N testing but detected antibody in only 4% (14) (Table 4). Given the large numbers of possums in many Australian cities, these animals warrant further investigation as potential reservoir hosts; indeed, recent experimental work at the Queensland Institute of Medical Research (QIMR) suggests they could be important reservoir hosts (A. Boyd, personal communication, 2000).
Outbreaks in major metropolitan centers (9, 113, 122, 152) have led Mackenzie et al. (122) to speculate that horses may act as amplifying hosts. RRV has been isolated from horses on two occasions (24, 149). Prevalence of IgG was 60% in a survey of 1,300 horses in Victoria during 1995 to 1996 (14). Horses can experimentally infect Culex annulirostris (a major vector of RRV) (103). They also develop relatively high-titered viremias (6.3 PFU/ml) which may last up to 5 days (Table 5). These data indicate that horses may play a role as amplifying hosts when present in appreciable numbers.
Given their close association with humans and their reported high RRV antibody prevalences in the case of dogs (Table 4), domestic dogs and cats should be studied further. Unpublished work at QIMR suggests that dogs and cats may not be important reservoir hosts (A. Boyd, personal communication, 2000).
RRV is relatively homogeneous over large geographical areas, suggesting that humans or livestock transported by air or possibly flying foxes are able to transport the virus long distances (119, 123, 125, 176). If birds act as reservoir hosts, they could also account for these observations.
Serologic surveys and virus isolations from mosquitoes trapped near flying fox camps suggest that flying foxes might be reservoir hosts (49, 71, 79, 158). However, experimentally infected gray-headed (Pteropus poliocephalus) and red (Pteropus scapulatus) flying foxes failed to develop detectable viremias (130). Also, Ryan et al. (175) concluded that the gray-headed flying fox, Pteropus poliocephalus, was not an important reservoir host because only 10 of 510 (2%) A. vigilax mosquitoes fed on infected flying foxes were infected with RRV after an extrinsic incubation period, and because RRV could not be detected in any of 122 blood-fed A. vigilax immediately after feeding on infected P. poliocephalus. RRV has been isolated frequently from mosquitoes collected at flying fox camps in Brisbane and Cairns (79, 158). Harley et al. (79) obtained 26 (79%) of 33 isolates of RRV in Cairns during 1996 to 1998 from within 1 km of a spectacled flying fox (Pteropus conspicillatus) camp. The number of positive pools from within 1 km of the camp was significantly greater than for mosquitoes trapped elsewhere, for all species trapped, and for the important vector species C. annulirostris. The spectacled flying fox, P. conspicillatus, is limited in its geographical range, but occurs in large colonies close to human habitation in Cairns, a city with a high incidence of RRV disease (75, 152). No studies of antibody prevalence or experimental infection exist for this species. The role of flying foxes as RRV reservoir hosts remains unclear.
It has been suggested that birds do not play a role as reservoir hosts, largely based on experimental studies of infection (Table 5) (95). But birds are ubiquitous in Australia and relatively few species have been studied (95). RRV antibody prevalences in birds have generally been low (Table 4). One exception to this is from Doherty et al. (49), who found that 50% of 48 and 63% of 8 birds of mixed species collected at Mitchell River mission (now Kowanyama) on Cape York Peninsula from December 1967 to April 1969 had HI and N antibodies, respectively. There is serologic evidence for infection of various species of birds (48–50, 71, 197, 221). In 1972, Doherty (43) suggested that despite the variable and often low antibody prevalence found in birds, their role as reservoir hosts should receive critical attention. He noted that antibodies might be short-lived, not accurately reflecting past infection. There is some evidence for this. Kay et al. (103) found that 14% of C. annulirostris mosquitoes that fed on little corellas (a species of parrot) with viremias of maximum titer 2.3 suckling mouse infectious dose LD50/ml subsequently became infected. However, the birds did not show serologic evidence of infection on HI testing. The first isolates of RRV from vertebrates were from the heart muscle of three birds (Table 3), but the only birds in which experimental viremia has been induced are day-old chicks (130). The genetic homogeneity of RRV isolates from geographically widespread areas (119, 176) suggests that the role of birds as reservoir hosts should be reevaluated. The close relationship between RRV in Australia, its subtype SAGV in Japan, and SFV in Africa (112, 183) makes it likely that a recent ancestral virus used birds as a reservoir host.
Overall, the search for reservoir hosts of RRV has placed too much reliance on the results of experimental infection studies. Data do not exist linking infection in vertebrates with human disease. This information is technically and logistically difficult to obtain and needs to measure exposure to infected suburban horses, cattle, dogs, cats, possums, and birds. There are good grounds for reappraising the role of birds as RRV reservoir hosts. Also, there is evidence from epidemics in the South Pacific and Western Australia that humans can act as reservoir hosts for RRV. This is discussed more fully in Transmission Cycles.
Mosquito Vectors
This section examines information on mosquito vectors of RRV infection. We consider the prevailing opinion that the main vectors of human RRV infection in Australia are A. vigilax, Aedes camptorhynchus, and C. annulirostris (95, 123, 125, 167–169). This opinion arose because (i) there have been more RRV isolates from these species than from others; (ii) these species are infected with and transmit RRV in laboratory studies; and (iii) these species have been found to be abundant, often with RRV isolated from them, in temporal and spatial association with outbreaks of RRV disease.
This evidence is tabulated and discussed below. Other species that might be vectors are also considered. RRV has been isolated from over 30 mosquito species in Australia (27, 79, 114, 158, 167, 173). The vector status of most of these is unknown, because data on experimental infection and transmission and association with human infection, which are requisites to establish vector status, are lacking (123, 193).
Table 6 shows isolates of RRV from mosquitoes. Data for male and blood-fed female mosquitoes have been excluded from the table. Male mosquitoes do not blood feed (30) and therefore cannot transmit RRV to humans. Isolation of virus from males implies transovarial transmission (200). While this is likely to be important for maintenance of RRV in nature, it has no direct impact on transmission of RRV to humans, the primary focus of this review. Virus isolation from blood-fed females is not strong evidence for vector competence. It means only that they have taken a blood meal with virus in it; barriers to transmission, the mesonteronal infection, dissemination, and salivary gland escape barriers may prevent such mosquitoes from acting as vectors (77).
TABLE 6.
RRV isolates from mosquitoes
| Genus and speciesa | No. of mosquitoes examined | No. of RRV isolates | State, territory, or countryb | Reference(s) |
|---|---|---|---|---|
| Aedes | ||||
| A. alboannulatus | 2 | WA | 114 | |
| A. alternans | 3,321 | 1 | NSW | 170 |
| 60 | 3 | WA | 118 | |
| 1 | 1 | QLD | 158 | |
| A. bancroftianus | VIC | 129 | ||
| A. camptorhynchus | 4 | VIC | Aldred et al., 1990c | |
| 1,848 | 1 | TAS | 138 | |
| NSW | 168 | |||
| 4 | WA | 117 | ||
| 79 | WA | 116 | ||
| 2 | NSW | 27 | ||
| 36 | WA | 114 | ||
| A. carmenti | 6,146 | 14 | QLD | 79 |
| A. clelandi | 1 | WA | 116 | |
| A. daliensis | 1,106 | 1 | WA | 118 |
| A. flavifrons | 248 | 2 | TAS | 137 |
| A. funereus | 2 | NSW | 27 | |
| 2,943 | 12 | QLD | 158 | |
| 1 | QLD | 171 | ||
| 11,094 | 1 | QLD | 173 | |
| A. imprimens | 99 | 1 | QLD | 79 |
| A. kochi | 99 | 1 | QLD | 173 |
| 11,405 | 2 | QLD | 79 | |
| A. lineatus | 4,644 | 1 | QLD | 79 |
| A. multiplex | 2,531 | 1 | QLD | 173 |
| A. normanensis | 13,305 | 8 | QLD | 46,93 |
| ≅40,000 | 15 | NT | 219 | |
| 28,909 | 6 | WA | 19 | |
| A. notoscriptus | 1 | NT | 219 | |
| 1 | NSW | 27 | ||
| 7,087 | 13 | QLD | 158 | |
| 637 | 1 | QLD | 79 | |
| A. phaecasiatus | 1 | NT | 219 | |
| A. polynesiensis | 267 | 6 | Cook Islands | 162 |
| A. procax | 2 | NSW | 27 | |
| 4,266 | 4 | QLD | 158 | |
| 1 | QLD | 171 | ||
| 1,478 | 1 | QLD | 173 | |
| A. ratcliffei | 1 | WA | 116 | |
| A. sagax | VIC | 129 | ||
| 2 | WA | 116 | ||
| A. theobaldi | VIC | 129 | ||
| A. tremulus | 1 | WA | 116 | |
| A. vigilax | 88 | 1 | QLD | 51 |
| 34,843 | 9 | NSW | 71 | |
| 1 | NSW | 202 | ||
| ≅40,000 | 2 | NT | 219 | |
| 119,817 | 77 | NSW | 170 | |
| 30 | WA | 116 | ||
| 13,325 | 40 | WA | 118 | |
| 5,517 | 9 | QLD | 158 | |
| 23 | NSW | 27 | ||
| 5 | WA | 114 | ||
| 1 | QLD | 171 | ||
| 2,077 | 1 | QLD | 173 | |
| 3,308 | 1 | QLD | 79 | |
| E. N. Marks's species 85 | 268 | 1 | WA | 118 |
| Anopheles | ||||
| A. amictus s.l. | 1,205 | 1 | QLD | 46,93 |
| QLD & NSW | 130 | |||
| A. annulipes s.l. | VIC | 129 | ||
| 4 | NSW | 27 | ||
| 1 | WA | 116 | ||
| A. farauti s.l. | 30,551 | 1 | Western Province, PNG | 87 |
| Coquilletidia | ||||
| C. linealis | 1 | NSW | 32 | |
| 202 | 1 | NSW | 71 | |
| 17,119 | 1 | NSW | 170 | |
| 3 | WA | 116 | ||
| 4 | NSW | 27 | ||
| Culex | ||||
| C. annulirostris | 3 | QLD | 49 | |
| 8 | NSW | 224 | ||
| 6,153 | 1 | NSW | 71 | |
| VIC | 131 | |||
| 136,077 | 16 | NSW & VIC | 133 | |
| 84,010 | 9 | QLD | 46,93 | |
| 2,847 | 13 | WA | 228 | |
| VIC | 128 | |||
| ≅190,000 | 23 | NT | 219 | |
| 47 | NSW | 27 | ||
| 9,475 | 23 | QLD | 158 | |
| 2 | QLD | 171 | ||
| 2,751 | 1 | QLD | 173 | |
| 30,541 | 9 | QLD | 79 | |
| C. australicus | VIC | 129 | ||
| 759 | 2 | WA | 118 | |
| C. gelidus | 257 | 1 | QLD | 79 |
| C. globocoxitus | 1 | WA | 114 | |
| C. quinquefasciatus | 2,608 | 7 | New Caledonia, Vanuatu, Wallis, and Horne Islands | 58 |
| 1,572 | 2 | WA | 118 | |
| C. sitiens | 1,207 | 1 | WA | 118 |
| 97 | 1 | QLD | 158 | |
| Mansonia | ||||
| M. uniformis | 1 | NSW | 224 | |
| 898 | 1 | QLD | 173 | |
| M. septempunctata | 913 | 3 | QLD | 79 |
| Tripteroides sp. | 1 | WA | 116 |
The subgenera Verrallina and Ochlerotatus of the genus Aedes have recently been elevated to genus rank (155, 156). We have used the older nomenclature to avoid confusion with the existing literature.
Australian states and territories: NSW, New South Wales; NT, Northern Territory; QLD, Queensland; TAS, Tasmania; VIC, Victoria; WA, Western Australia. PNG, Papua New Guinea.
J. Aldred, J. Campbell, G. Davis, N. Lehmann, and J. Wolstenholme, letter, Med. J. Aust. 153:434, 1990.
Sudia et al. (193), in a paper on VEEV in Dade County, Florida, listed four criteria for establishing the vector status of a mosquito species (see earlier section on Vector Competence). These criteria will be used below to assess the evidence on mosquito vectors of RRV.
Research to isolate RRV from wild-caught mosquitoes, the first criterion to incriminate a vector, has been extensive (Table 6). Most of this work has been done in the last 10 years, reflecting the growing realization of the remarkable complexity of RRV ecology and epidemiology and growing public concern about RRV disease. Attempts to show that mosquito species can be infected by viremic hosts and transmit by biting others— the second and third criteria for incriminating vectors—have also required extensive work. The evidence spans 20 years and involves experiments with five genera and 20 species conducted in Australian and American laboratories (Table 7). To weigh the evidence on putative vector status, we use the first three criteria as well as the fourth criterion of field evidence linking infected mosquitoes with infected vertebrates, humans in the current context. We consider the major human vectors first, noting their distribution in Australia.
TABLE 7.
Experimental infection with and transmission of RRV by mosquitoesa
| Genus and species | Location | Infection or infectious doseb | Transmission | Reference |
|---|---|---|---|---|
| Aedes | ||||
| A. aegypti | Fiji | 4.2 PFU | + | 141 |
| Townsville, QLD | 4.9 LD50 | + | 99 | |
| Jakarta | + | + | 74 | |
| Townsville, QLD | 3.8 PFU | + | 16 | |
| A. alboannulatus | Southen NSW | + | 16 | |
| A. albopictus | Houston | + | + | 142 |
| Beijing | 2.5 PFU | + | 141 | |
| Hawaii | 2.6 PFU | + | 141 | |
| Shanghai | 2.4 PFU | − | 141 | |
| Singapore | 4.0 PFU | + | 141 | |
| Sri Lanka | 4.1 PFU | + | 141 | |
| A. alternans | Batemans Bay, NSW | + | − | 217 |
| A. australis | New Zealand | + | + | 126 |
| A. camptorhynchus | Batemans Bay, NSW | 2.4 PFU | + | 15 |
| Southern NSW | 4.5 PFU | 16 | ||
| A. funereus | Brisbane, QLD | 4.2 CCID50 V | + | 175 |
| Redcliffe, QLD | + | 100 | ||
| Maroochydore, QLD | 4.5 CCID50 V | + | 173 | |
| A. multiplex | Maroochydore, QLD | 4.9 CCID50 V | − | 173 |
| A. notoscriptus | New Zealand | + | − | 126 |
| Southern NSW | 5.1 PFU | 16 | ||
| Maroochydore, QLD | + | − | 174 | |
| Sydney, NSW | + | + | 41 | |
| Brisbane, QLD | 3.2 CCID50 V | + | 209 | |
| Maroochydore, QLD | 4.0 CCID50 V | − | 173 | |
| A. oceanicus | Tonga | + | 104 | |
| A. polynesiensis | Raratonga | + | + | 74 |
| American Samoa | + | + | 74 | |
| Fiji | <1.2 PFU | − | 141 | |
| Rarotonga | <1.2 PFU | + | 141 | |
| Samoa | <1.2 PFU | + | 141 | |
| Tahiti | <1.2 PFU | + | 141 | |
| A. procax | Maroochydore, QLD | 3.4 CCID50 V | + | 173 |
| A. pseudoscutellaris | Fiji | 2.0 PFU | + | 141 |
| A. rubrithorax | Southern NSW | + | 16 | |
| A. tongae tabu | Tonga | + | + | 104 |
| A. vexans | Tonga | + | + | 104 |
| A. vigilax | Batemans Bay, NSW | + | 216 | |
| Maroochydore, QLD | + | + | 174 | |
| Redcliffe, QLD | 4.5 LD50 | + | 94 | |
| Batemans Bay, NSW | 3.4 CCID50 B | 201 | ||
| Brisbane, QLD | + | 98 | ||
| Maroochydore, QLD | 3.8 CCID50 V | + | 173 | |
| Coquilletidia xanthogaster | Brisbane, QLD | + | 98 | |
| Culex | ||||
| C. annulirostris | Brisbane, QLD | + | 98 | |
| Brisbane, QLD | + | + | 102 | |
| Maroochydore, QLD | + | + | 173 | |
| Griffith, NSW | + | 216 | ||
| C. australicus | Maroochydore, QLD | 4.5 CCID50 V | + | 173 |
| C. quinquefasciatus | Florida | − | − | 74 |
| Brisbane, QLD | − | 101 | ||
| Charleville, QLD | + | 101 | ||
| Kowanyama, QLD | + | 101 | ||
| Cairns, QLD | − | 101 | ||
| Mildura, VIC | + | 101 | ||
| Darwin, NT | + | 101 | ||
| C. sitiens | Maroochydore, QLD | + | 173 | |
| Tonga | + | 104 | ||
| Mansonia uniformis | Brisbane, QLD | + | 98 | |
| Maroochydore, QLD | + | + | 173 |
Infection is indicated as + (positive virus infection or transmission to suckling mice) or −(negative virus infection or transmission to sucking mice). PFU, virus dose in log10 Vero PFU per mosquito required to infect 50% (Vero cells are an African green monkey kidney fibroblast cell line). LD50, virus dose in log10 suckling mouse LD50 per mosquito required to infect 50%. CCID50, virus dose in log10 CCID50 per mosquito required to infect 50% in BHK (baby hamster kidney) cells, (B) or Vero cells (V).
A. vigilax is absent from Tasmania and the Victorian coastline, but is otherwise continuously present along the Australian coastline and will disperse tens of kilometers from breeding sites, having been recorded as far as 320 km west from the Queensland coast (110). It appears intermittently in plague proportions in urban areas around much of the Australian coastline (110). Most commonly, this mosquito breeds in temporary bodies of saline to brackish water (110). It is mainly an evening biter and, in Australia, is exophilic, with indoor biting being exceptional (110). A. vigilax will feed on mammals and also on birds and reptiles when available (30, 110, 166). A. vigilax has yielded about 200 RRV isolates from a variety of areas (Table 6). RRV isolation rates from A. vigilax have been as high as 1 in 43 during epidemics in the Pilbara region in Western Australia; the timing of isolates is consistent with this species' being a vector for humans (118, 123). An outbreak of RRV and BFV infection occurred in Nhulunbuy, Northern Territory, in February 1992, and almost all the mosquitoes trapped were A. vigilax (140).
A. camptorhynchus occurs in New South Wales, Victoria, Tasmania, South Australia, and Western Australia and is a common salt marsh mosquito in coastal areas in the southern part of its range. Adults bite humans, domestic animals, and birds during the day and particularly after sunset (110). Over 100 isolates of RRV have been made from A. camptorhynchus in Western Australia, Victoria, New South Wales, and Tasmania (Table 6). Two studies have shown that A. camptorhynchus can be infected with RRV and that it can transmit the virus to suckling mice (Table 7). In 1991, an RRV isolate came from A. camptorhynchus trapped on the east coast of Tasmania, where at least six human RRV infections had occurred at around the same time, a relatively large number of cases in Tasmania (138). Campbell et al. (24) trapped mosquitoes in southeast Gippsland, Victoria, during a large outbreak. A. camptorhynchus was the most common species trapped, constituting 96% of the entire catch, and yielded four isolates of RRV. A. camptorhynchus infection rates as high as 1:30 have been recorded before and during outbreaks in coastal southwest Western Australia (123). During the course of an outbreak in Perth and the southwest of Western Australia in 1991 to 1992, Lindsay et al. (113) obtained 10 of 13 isolates from A. camptorhynchus. During an outbreak in southwest Western Australia in 1995 to 1996, 36 of 44 isolates came from A. camptorhynchus (114).
Culex annulirostris is widely distributed in all Australian states except Tasmania. This mosquito breeds in temporary or semipermanent ground waters and effluent run-off and is a crepuscular feeder both in- and outdoors (108). C. annulirostris feeds upon humans, other mammals including marsupials, and (rarely) on birds (97, 108, 145, 164, 165, 205). About 140 RRV isolates have come from C. annulirostris (Table 6). Four studies have shown that this species can be infected with RRV, while two demonstrated that it could transmit RRV to suckling mice (Table 7). Marhall and Miles (130) suggest that C. annulirostris may be more important to maintain primary cycles than as an epidemic vector. Russell (167), however, notes that C. annulirostris was probably an epidemic vector in a 1983 to 1984 outbreak in New South Wales. During that outbreak, C. annulirostris numbers were well above average during the summer months (167), when most cases occurred (82). Marshall and Miles (130) and Russell (167) agree that C. annulirostris is predominantly an inland rural vector. However, RRV isolates have been obtained from C. annulirostris trapped in Queensland coastal areas, and it undoubtedly also plays a vector role in these areas (79, 158, 173).
Interest in Aedes notoscriptus as a possible vector of RRV was aroused when 13 isolates were recovered from mosquitoes trapped in Brisbane during an outbreak (158), although two isolates had been obtained from this species in the Northern Territory and New South Wales previously (Table 6). Ritchie et al. (158) also speculated that A. notoscriptus might play a role in RRV transmission to humans during winter, as they obtained an RRV isolate in June 1994. A. notoscriptus uses peridomestic larval habitats, readily bites humans, and has been observed feeding on poultry, marsupials, horses, and cattle (111). It feeds at all times during the day and night, but with peaks of activity close to sunset and sunrise (111). Four studies have shown that this mosquito can be infected with RRV and one that it can transmit the virus (Table 7).
Rosen et al. (162) obtained six isolates of RRV from A. polynesiensis collected on the island of Rarotonga, Cook Islands, in 1980 at the time of an outbreak. These are the only RRV isolates from this species. A. polynesiensis has been shown to transmit RRV in laboratory experiments (74, 141). It occurs on most islands in the eastern Pacific but not in Australia and breeds in natural and artificial containers (109, 162). A. polynesiensis commonly feeds on humans (109). While only six isolates have been obtained from A. polynesiensis, these were obtained at the time of an outbreak, and isolates were not obtained from C. annulirostris, a known vector. It is probable that A. polynesiensis was a vector in human-mosquito-human RRV transmission in the Cook Islands and probably other parts of the Pacific in 1979 and 1980.
RRV has never been isolated from wild-caught A. aegypti (Table 6), but four studies have demonstrated that this species can be infected with and transmit the virus (16, 74, 99, 141) (Table 7). Harley et al. (79) did not isolate RRV from 121 adult female A. aegypti collected in Cairns, Queensland, during 1997. A. aegypti is adapted to breeding around human habitation and will oviposit in artificial containers and cavities in trees (109). It feeds primarily on humans, is endophilic, and has peaks of biting activity in the early morning and late afternoon (182). The distribution of A. aegypti in Australia was reviewed in detail by Lee et al. (109). For Queensland from 1981 to 1983, Kay et al. (96) reported A. aegypti along the Pacific coast around Cairns and Townsville, along the northern coast at Normanton and Karumba on the Gulf of Carpentaria, and in the Torres Strait Islands, and inland in northwestern zones around Cloncurry, Winton, Dirranbandi, and Roma.
The foregoing outlines descriptive evidence linking mosquito species to human RRV disease. Three recent studies have moved beyond this by testing causal hypotheses regarding the location or abundance of mosquito breeding and the measured risk for human disease. Using a cross-sectional design in the Peel region of Western Australia, Lindsay et al. (115) found that 99% of RRV cases occurred within 3 km of the breeding sites for A. camptorhynchus and A. vigilax. Dale and coworkers (37, 38; P. E. R. Dale, A. Muhar, L. Thalib, and E. Arito, Abstr. 3rd Nat. Conf. Mosq. Control Assoc. Aust., 1998) used remote sensing and Geographic Information Systems in the analysis of risk for RRV infection by mapping mosquito breeding sites and disease notifications. They showed that RRV notifications, age standardized by suburb, correlated with particular geographical features (wetlands, bushland, littoral vegetation, and riparian vegetation). Three of these vegetation types (wetlands, littoral, and riparian vegetation) are known to be suitable for mosquito breeding. Bushland would be expected to contain macropod reservoir hosts. While these studies cannot incriminate specific vector species, they do represent significant moves toward correlating human disease with environment. Ryan et al. (172) went further and related abundance of mosquito species trapped to standardized morbidity ratios for RRV notifications in one census district of Maroochy Shire in southeast Queensland from 1991 to 1996. They found a positive correlation between C. annulirostris and Aedes funereus numbers and square-root-transformed standardized morbidity ratios. This was not the case for A. vigilax or Aedes procax. Their study provides evidence linking the former two mosquito species to RRV human transmission in Maroochy Shire.
Taken together, the evidence regarding mosquito vectors for RRV points to the ecological heterogeneity of transmission settings and indicates that several vectors are involved in Australia. There is sufficient evidence to assume that A. vigilax, A. camptorhynchus, and C. annulirostris are vectors of human RRV infection. Other mosquito species, A. notoscriptus in particular, may be also be vectors in Australia. A. polynesiensis is a potential vector in the South Pacific.
EPIDEMIOLOGY OF INFECTION AND DISEASE
Antibody Prevalence Surveys
The 11 published antibody prevalence surveys include data from all Australian regions (except Tasmania and West Australia) and Southeast Asia and the Pacific Islands (Table 8); interpretation is complex because of different sampling methods and patchy geographical representation.
TABLE 8.
RRV antibody prevalence surveys in human populations
| Study period | Population and sampling schemea | Methodb | No. tested | Antibody prevalence (%) | Reference |
|---|---|---|---|---|---|
| 1957–1964 | Aboriginal communities in north and other parts of QLD, including Brisbane and the Torres Strait, sampling scheme not specified | HI, N | 636c | 50d | 48 |
| 1966–1971 | Aboriginal communities in north QLD & NT, sampling scheme not specified; symptomatic patients from eastern QLD | HI | 2,148 | 47e | 44 |
| 55f | |||||
| 42g | |||||
| 1958–1974 | Surveys for meliodosis, arboviruses, parasites, and sera taken for other reasons in SE Asia and Pacific Islands | N | 3,178 | 15h | 196 |
| 1974 | Random sampling of households in Echuca, VIC | HI | 739 | 15 | 61 |
| 1974–1975 | Children under 11 years from north central and southeast QLD and Darwin, NT, sampling scheme not specified | HI | 2,730 | 8 | 189 |
| 1978–1979 | Residents of Yeppoon, Townsville, and Innisfail, QLD, sampling scheme not specified | HI | 345 | 22d | 6 |
| 1981–1982 | Sera from blood donors to NSW and VIC blood transfusion services, RFDS Broken Hill, hospital, commonwealth, and private pathology laboratories in NSW | HI | 16,842 | 13 | 18 |
| 1988 | Sera from residents of Raymond Island and Loch Sport, Gippsland, VIC, sampling scheme not specified | ELISA | 523 | 25 | 24 |
| 1989 | Sera referred to QLD Health Laboratory of Microbiology and Pathology from patients with suspected viral or arboviral infections | HI, ELISA | 2,010 | 32 | 151 |
| 1991–1992 | Sera from permanent residents of western NSW and northern VIC from selected districts and with equal representation of age groups 0–9, 10–19, 20–29, 30–39, 40–49, 50–59, and ≥60 yr | HI | 2,635i | 39 | 83 |
| 1992 | Donors to the SA Red Cross Blood Transfusion Service | ELISA | 2,952 | 8 | 215 |
Abbreviations: QLD, Queensland; NT, Northern Territory; SE Asia, Southeast Asia; VIC, Victoria; RFDS, Royal Flying Doctor Service; NSW, New South Wales; SA, South Australia.
HI, hemagglutination inhibition; N, neutralization; ELISA, enzyme-linked immunosorbent assay.
Number tested for HI antibody; 308 of these were tested for N antibodies.
Prevalence on HI testing; 45% of 308 sera showed antibodies on N testing.
Result on HI testing of 507 sera from north Queensland aboriginal communities.
Result on HI testing of 178 sera from Northern Territory aboriginal communities.
Result of HI testing of 1,463 sera from eastern Queensland submitted from patients with “encephalitis, pyrexia, polyarthritis, hepatitis and occasionally other conditions” (44).
Antibody prevalence given for entire survey of sera from Taiwan, Malaysia, Phillipines, Indonesia, Papua New Guinea, Solomon Islands, New Hebrides, New Caledonia, Samoa, and Caroline Islands. Antibody prevalence as high as 64% in two Sepik villages in Papua New Guinea.
2,873 sera included in study, but results only given for 2,635 sera tested for RRV antibodies.
Only one survey used a formal population-based sampling scheme (61). The others used convenience samples, such as blood donors or patients with suspected virus or arbovirus infection (151, 215). Blood donor studies underrepresent children and may overestimate population antibody prevalence. Even in the one published study in which random, population-based sampling was used, children were underrepresented (61). Passive surveillance of submitted diagnostic sera, used in two of the surveys (44, 151), probably overestimated antibody prevalence, but this would depend on the symptomatology being investigated and the prevalence of other infections in the population sampled. These sources of bias must be considered when interpreting the published antibody studies.
Reported antibody prevalence differs among geographical areas. Doherty et al. (48) found an antibody prevalence of 19% in 78 sera from Brisbane, southeast Queensland, 64% for 258 sera from residents of Mitchell River Mission (now Kowanyama), Western Cape York Peninsula, Queensland, and 45% among 121 sera from aboriginal communities in northeast Queensland. Phillips et al. (151) found an antibody prevalence of 31.6% in 2,010 sera submitted for suspected viral or arboviral infections in Queensland. Boughton et al. (18) found an antibody prevalence of 12.8% in residents of New South Wales and Victoria. Weinstein et al. (215) found an antibody prevalence of 8.4% in South Australian blood donors. Among these studies there is a gradient of decreasing antibody prevalence from north to south. An exception was the study by Campbell et al. (24), showing relatively high antibody prevalence (25%) in residents of Gippsland, Victoria, for sera taken after an outbreak.
An increase in antibody prevalence across the 1980s is apparent when comparing the studies of Boughton et al. (18) and Hawkes et al. (83). The population comparisons involved specific towns and areas using data from the first paper in the second. Overall, antibody prevalences rose from 13 to 39%, suggesting that infection may be becoming more common in parts of New South Wales.
Most of the studies demonstrated increasing antibody prevalence with increasing age. Exceptions are the reports by Weinstein et al. (215) and Tesh et al. (196). The prevalence of antibodies was consistently a little higher among males than females when this was reported (18, 44, 83, 151, 189, 215) (Table 9).
TABLE 9.
Sex distribution of antibodies to RRV
| % Positive (no. tested)
|
Male-female ratioa | Reference | |
|---|---|---|---|
| Male | Female | ||
| 45 (727) | 39 (736) | 1.2 | 44 |
| 8 (1,532) | 7 (1,198) | 1.1 | 189 |
| 34 (1,046) | 29 (964) | 1.2 | 151 |
| 17 (—) | 11 (—) | 1.6 | 18 |
| 45 (871)b | 36 (1,274)b | 1.3 | 83 |
| 10 (1,447) | 7 (1,505) | 1.4 | 215 |
Overall mean male-female ratio, 1.3.
These do not add to the total 2,873 sera tested because no data on sex-specific antibody prevalences are given for two districts.
The studies above show that risk of infection differs geographically and the prevalence of past infection is generally higher in the north than the south of Australia. With two exceptions, the studies show that antibody prevalence increases with age. Unless this is an age cohort effect, the age pattern indicates a relatively constant age-specific risk for infection. This contrasts with risk for disease, which has generally been shown to be highest for young adults. Behavioral and environmental risks for disease would presumably be similar to those for infection, but host characters may modify the age-specific risk for disease relative to that for infection. If so, this would account for the different age distributions of infection and disease. Antibody prevalence surveys show that prevalence of past infection is slightly higher in males. Male and female risks may vary due to differing behavior.
Asymptomatic to Symptomatic Case Ratio
The first estimate of the asymptomatic to symptomatic case ratio, 50:1, comes from north and central Queensland in 1978 to 1979 (6). The second, 3.0:1, is from an epidemic in Fiji in 1979 (4). The third estimate, 0.3:1, is from a study by Hawkes et al. (82) of an RRV epidemic in New South Wales during 1983 to 1984. The fourth estimate, 1.2:1, comes from the experience of Marines in a joint U.S. and Australian military exercise at Shoalwater Bay, Queensland, during March 1997 (171). Only the second (3.0:1) and last (1.2:1) estimates are reliable.
The Queensland estimate by Aaskov et al. (6) was based on incidences of 0.37 and 15 to 35 per 1,000 per annum calculated for symptomatic and asymptomatic infection, respectively. The ratio was therefore between 41:1 (15/0.37) and 95:1 (35/0.37); it was originally given as 50:1 but more recently has been reported as 80:1 (95). Neither figure is justified. Incidence of symptomatic infection was calculated by dividing the number of cases recruited via family physicians by the estimated population of the study area. To determine the incidence of asymptomatic infection, 171 people were studied serologically twice over 1 year to detect fourfold rises in RRV HI titer. Sixty-one percent of the asymptomatic subjects were soldiers from Townsville; they were not living within the area where cases were reported. Given their occupation, they were not likely to have resided in Townsville for a long period. So the populations from which symptomatic and asymptomatic infections originated were not comparable. Six of 171 (35 of 1,000) showed a fourfold rise in HI titer. With only six asymptomatic infections to estimate incidence, the confidence intervals would be wide. An incidence range of 15 to 35 asymptomatic infections per 1,000 per annum is reported, without stating how the lower estimate was obtained. The ratios estimated are likely to be too high because symptomatic cases were probably undercounted.
An RRV epidemic began in April 1979 on the Island of Viti Levu in Fiji, particularly around Nadi. In the nearby village of Nawaka, 97 of 416 people (23.3%) reported joint pain. Overall, 386 villagers had HI antibodies to RRV after the epidemic (4). Assuming that antibodies reflect infections during the epidemic and that all symptomatic cases were counted, the asymptomatic-symptomatic ratio was 289 to 97 or 3.0:1. If the preepidemic prevalence of antibodies was 13%, as suggested by Aaskov et al. (4), the estimate becomes 2.5:1. However, other Fijian research suggests that preepidemic antibody prevalence would have been zero (12, 130), making 3.0:1 the correct estimate. This estimate is based on clinical and serologic assessment of a single population. The population is well defined, so this is likely to be an accurate estimate of the asymptomatic to symptomatic case ratio, at least for symptomatic disease with arthralgia in Fijians.
Hawkes et al. (82) estimate that 257 of an estimated 340 infections in Griffith Shire, New South Wales, during a 1983 to 1984 epidemic were symptomatic, an asymptomatic-symptomatic ratio of 0.3:1. The probable number of infections, 340, was extrapolated from the appearance of antibody in 2 of 94 blood donor sera after the epidemic. It was assumed that all cases of RRV disease were counted in Griffith Shire. No case definition for RRV disease was given. The small number of seroconversions in blood donors and other methodological problems invalidate this estimate.
In March 1997, 1,300 U.S. Marines were involved in Operation Tandem Thrust at Shoalwater Bay, Queensland. There were nine cases of RRV disease. On the basis of serologic study of 938 Marines, the infection rate was 1.5% (171). Therefore, there would have been 20 infections expected among the 1,300 Marines, making the asymptomatic-symptomatic ratio 11 to 9, or 1.2:1.
Superficially, there appears to be considerable variation in asymptomatic-symptomatic ratios. Indeed, Kay and Aaskov (95), noting the enormous difference between the estimates of Aaskov et al. (6) and Hawkes et al. (82), suggest that the asymptomatic-symptomatic ratio differs in endemic and epidemic transmission, but those two estimates were too unreliable to permit such conclusions. The only reliable estimates are those based on the Fijian data of Aaskov et al. (4), giving an asymptomatic-symptomatic ratio of 3.0:1 during epidemic transmission in Fiji in 1979, and the experience of visiting U.S. Marines in a high-risk area of Queensland in 1997, giving a ratio of 1.2:1 (171). The ratios in other epidemiological circumstances or among other human populations are unknown.
Notifications and Incidence
There have been no population-based studies of the incidence of infection or disease; notifications collected by state and federal health authorities in Australia are the only source of information. Notification to state health departments, the National Notifiable Diseases Surveillance System, and the Federal Department of Human Services and Health through the LabVISE system (a sentinel scheme collecting data on viruses and other infectious agents from virology and serology laboratories) requires at least a single positive test for IgM, a presumptive diagnosis (121). The majority of notifications in Australia are presumptive diagnoses (35, 36).
The average annual number of notified cases in Australia from 1991 to 2000 was 4,745, peaking at 7,823 in 1996 (Communicable Diseases Network—Australia New Zealand, http://www.health.gov.au/pubhlth/cdi/nndss/year002.htm). During 1992 there was a reported national incidence of 36.4 per 100,000 per annum (227). The reported incidence in Queensland was nearly four times higher, 139.6 per 100,000 per annum— the highest incidence for any Australian state (227). The zone of highest incidence within Queensland, 278.4 per 100,000 per annum, was in the Northern Statistical Division (local government areas of Townsville, Bowen, Burdekin, Charters Towers, and Dalrymple in North Queensland) (227). During 1992 the reported incidence in Cairns was 148.1, in Townsville was 321.3, and in Brisbane was 95.7 per 100,000 per annum (152). This figure was probably atypical for Brisbane—from 1989 to 1991, the highest incidence per 100,000 per annum was 28.7 (152). But for Cairns and Townsville, the incidence was always above 100, an order of magnitude greater than for Brisbane, reflecting the increased RRV risk for coastal areas in tropical compared with subtropical zones (152). In 1998 the national incidence was 16.5 per 100,000, and 63% of notifications came from the state of Queensland (198). As in 1992, the highest incidence occurred in the Northern Statistical Division, though the central Queensland Fitzroy Statistical Division had a similarly high incidence (over 150 per 100,000 per annum) (198).
Overall, over recent years the likelihood of a person's becoming infected has varied markedly in the temperate zone states and has been stable in the entirely tropical areas of Queensland and the Northern Territory (Table 10). High incidences of human infection must relate to high numbers of vector mosquitoes and immunologically susceptible human and other vertebrate hosts, with temperature, rainfall, and other environmental factors also playing a role. Season certainly is important, with far more cases occurring in summer and autumn (Table 11).
TABLE 10.
Australian RRV notifications per 100,000 per annuma by state or territory of notification, 1991 to 2000a
| Year | No. of notificationsb
|
|||||||
|---|---|---|---|---|---|---|---|---|
| ACT | NSW | NT | QLD | SA | TAS | VIC | WA | |
| 1991 | 0 | 7 | 281 | 72 | 0 | 0 | 9 | 9 |
| 1992 | 1 | 5 | 134 | 137 | 5 | 0 | 3 | 44 |
| 1993 | 1 | 10 | 138 | 77 | 57 | 0 | 28 | 11 |
| 1994 | 0 | 5 | 171 | 99 | 2 | 0 | 1 | 6 |
| 1995 | 1 | 4 | 208 | 52 | 2 | 6 | 1 | 12 |
| 1996 | 0 | 17 | 69 | 149 | 2 | 16 | 3 | 86 |
| 1997 | 3 | 27 | 116 | 70 | 46 | 3 | 24 | 40 |
| 1998 | 2 | 8 | 62 | 56 | 4 | 2 | 3 | 19 |
| 1999 | 2 | 17 | 71 | 64 | 3 | 14 | 6 | 30 |
| 2000 | 5 | 12 | 76 | 41 | 26 | 2 | 7 | 67 |
Population denominators from Australian Bureau of Statistics, Census of Population and Housing, 1996, which includes 1991 and 1996 census data. Populations for intercensus and postcensus years calculated using simple linear interpolation. Numbers of notifications from Communicable Diseases Network, Australia New Zealand, (http://www.health.gov.au/pubhlth/cdi/nndss/year002.htm, updated 15/2/2001).
ACT, Australian Capital Territory; NSW, New South Wales; NT, Northern Territory; QLD, Queensland; SA, South Australia; TAS, Tasmania; VIC, Victoria; WA, Western Australia.
TABLE 11.
Australian RRV notifications by month of onset, 1991 to 2000a
| Year | No. of notifications
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | Total | |
| 1991 | 128 | 605 | 800 | 885 | 501 | 201 | 119 | 74 | 44 | 64 | 46 | 65 | 3,532 |
| 1992 | 163 | 608 | 1,537 | 1,232 | 642 | 536 | 168 | 85 | 213 | 131 | 144 | 171 | 5,630 |
| 1993 | 254 | 820 | 1,809 | 827 | 539 | 230 | 299 | 123 | 124 | 118 | 140 | 145 | 5,428 |
| 1994 | 383 | 825 | 858 | 725 | 504 | 252 | 97 | 57 | 47 | 77 | 57 | 78 | 3,960 |
| 1995 | 182 | 226 | 261 | 439 | 631 | 433 | 99 | 59 | 63 | 60 | 96 | 53 | 2,602 |
| 1996 | 239 | 1,511 | 2,699 | 1,554 | 805 | 332 | 205 | 87 | 52 | 72 | 103 | 164 | 7,823 |
| 1997 | 498 | 1,116 | 1,263 | 1625 | 1,107 | 463 | 195 | 107 | 59 | 63 | 74 | 116 | 6,686 |
| 1998 | 234 | 270 | 625 | 507 | 467 | 126 | 79 | 67 | 71 | 56 | 198 | 396 | 3,094 |
| 1999 | 390 | 650 | 871 | 935 | 617 | 277 | 100 | 110 | 72 | 94 | 112 | 179 | 4,407 |
| 2000 | 454 | 700 | 753 | 613 | 770 | 323 | 120 | 69 | 74 | 110 | 144 | 158 | 4,288 |
Source: Communicable Diseases Network, Australia New Zealand (http://www.health.gov.au/pubhlth/cdi/nndss/year002.htm, updated 15/2/2001).
Age and Sex of Cases
RRV disease occurs most commonly among adults aged between 25 and 39, with the age groups most commonly affected being 25 to 39 (144), 30 to 39 (33), 30 to 44 (181), 30 to 39 (82), and 50 to 59 (218) years. There is no clear sex effect. The male-to-female ratio has been reported as 0.6:1 (144), 1.4:1 (33), 0.8:1 (82), 1.7:1 (218), and between 0.7:1 and 1.3:1 according to age (181). The sex ratios given above for notified cases and those reported for antibody prevalence surveys (Table 9; male-female ratio, 1.3:1) are very similar, suggesting that determinants of RRV infection and disease do not differ much by sex. As noted in the section on antibody prevalence surveys, RRV antibody prevalence usually increases with age, even though the preponderance of RRV disease cases occur in young adults. Age-specific risks for RRV infection and disease must differ, presumably because of age-related differences in the immune response to infection.
Transmission Cycles
Nonhuman vertebrates are usually involved in the maintenance of arboviruses (178). By analogy with the New World members of the genus Alphavirus (213), and on the basis of the evidence presented above, RRV is maintained as an enzootic infection and is transmitted to humans during epizootic and epidemic periods.
In certain areas, vertical transmission in mosquito vectors occurs. Field and laboratory data suggest that vertical transmission occurs in A. vigilax (94, 118), Aedes tremulus (118), and A. camptorhynchus (40). Dessication-resistant Aedes eggs can survive for several months away from water (29), so these could provide an important means of virus maintenance.
Rosen et al. (162) provided the first strong evidence for human-mosquito-human transmission by isolating RRV from 51 patients in the Cook Islands, demonstrating viremias high enough to contribute to the transmission cycle. Marshall and Miles (130) consider it a “virtual certainty” that human-mosquito-human transmission was maintained during the outbreak in the South Pacific in 1979 and 1980. They point to the low seroprevalence in nonhuman vertebrates, the rapid spread of the epidemic, and the demonstration of high-titered viremia in humans (162, 197). Tesh et al. (197) found a neutralizing antibody prevalence of 43.8% in 393 human sera collected early in the epidemic. Among animals tested for neutralizing antibodies, the highest prevalence was for dogs, with 20% of 45 being positive; pigs, chickens, and rats had lower prevalences, and no antibody was detected in banded rails (a flightless bird) or cats (197). It is implausible that such a high human seroprevalence, so soon after the onset of the epidemic, could have been associated with zoonotic infection without higher seroprevalence among animals, unless the animal responsible for enzootic transmission was not studied.
Although there is some contention, RRV was probably not present in Fiji before the epidemic and appears not to have become established enzootically after the epidemic (4, 12, 130). This would fit most plausibly with the virus infecting humans largely or exclusively, and with the absence of suitable nonhuman vertebrate reservoirs. In the South Pacific, virus was isolated from humans with RRV disease symptoms by four groups, a feat not so easily achieved in Australia, suggesting higher viremias for the Pacific outbreaks (4, 57, 162, 197). Lindsay et al. (116) suggest that human-mosquito-human transmission was likely in an outbreak in suburban Perth from 1991 to 1992 because the first reported infection in several suburbs appeared to have been acquired outside Perth, but all subsequent infections were locally acquired. Overall, it seems that under appropriate circumstances RRV may be maintained for some time in a human-mosquito-human cycle, but that maintenance involves enzootic cycles and, in some environments, vertical transmission in mosquitoes.
Infection by nonmosquito transmission is probably unimportant. The evidence on human transplacental transmission is conflicting (5, 8). Transmission in blood transfusions has been suggested but has not been investigated (P. Weinstein, S. R. Weinstein, and R. J. Rowe, letter, Med. J. Aust. 163:276, 1995).
PUBLIC HEALTH ASPECTS
Costs of RRV Disease
A conservative cost estimate for RRV disease in Australia can be made on the basis of the average number of notified cases from 1991 to 2000 (4,745 per year). Most notifications would have been made on the basis of a positive IgM test (35, 36), although generally IgG and IgM testing would be performed together. The cost of the testing is A$23.80 (one Australian dollar is worth approximately US$0.50) per test for IgG and IgM ELISA, plus a collection fee of A$14.60, giving a total of A$38.40. At times of low and high disease incidence, the proportion of tests that are positive varies between less than 5 and 25%, respectively, for a pathology laboratory doing a large amount of the testing in Queensland (I. Gardner, personal communication, 1999). Assuming that 5 to 25% of tests are positive for the whole of Australia, the number of tests ordered to diagnose 4,745 cases will range from 18,980 to 94,900. The cost for testing will therefore be A$728,832 to A$3,644,160 in an average year, and this excludes testing performed to exclude other causes of arthralgia. Serologic testing for RRV clearly represents a considerable expenditure from the Australian health budget, and testing to exclude important differential diagnoses costs even more.
There are also costs for medical care. A consultation with a family physician costs A$22.95. If each of 4,745 RRV disease cases attends a family physician twice, once to have the testing arranged and once for counseling and to arrange treatment, the cost will be A$217,796 per annum, not including the cost of medication.
Westley-Wise et al. (218), Condon and Rouse (33), and Selden and Cameron (181) found that 62, 49, and 40%, respectively, of cases took at least 1 week off from work as a result of RRV disease. The average Australian weekly earning before tax for full-time work as of May 1999 was A$749.40 (13). Therefore, lost earnings, if half of the cases of RRV disease in an average year take 1 week off work, will be A$1,777,952.
The calculations above reveal that testing for RRV disease and treating it, and lost earnings as a result of it, are costing Australia between A$2.7 and A$5.6 million in an average year, without counting the substantial cost of other investigations, treatment, or decreased work output extending beyond 1 week. To calculate the full costs, one would also have to take into account the intangible costs of pain and suffering.
Potential for Epidemics and Dispersal
The factors determining human risk for RRV disease are complicated and poorly understood. Conditions necessary include adequate populations of reservoir hosts (potentially humans in some settings), vector mosquitoes, and appropriate climatic conditions for transmission. Within Australia, these conditions occur every year in tropical areas and less frequently in southern parts of the continent. To spread to other countries, these conditions must prevail and the virus must be introduced. This probably involved a viremic human in the South Pacific epidemic of 1979 and 1980. In late April 1979, eight cases of polyarthritis and rash, now known to have been RRV disease, were notified from a medical practice near Nadi International Airport in Fiji. For about 10 days reported cases were only from this area. Subsequently, the disease spread rapidly throughout the Fiji Islands, causing an estimated 50,000 clinical cases. In Fiji the epidemic occurred mainly from April to June 1979, with few cases being reported by September 1979, though laboratory-proven cases were reported until September 1980 (4, 130). In August 1979 the disease spread to American Samoa, where it was estimated 13,500 people were infected (130, 197). Then, between February and March and between January and June 1980, RRV disease cases occurred in the Cook Islands and New Caledonia, respectively (56, 130, 162). The number of cases in the Cook Islands is unknown; 32 cases were serologically confirmed in New Caledonia, but there were undoubtedly a larger number of infections (56, 130, 162). It is probable that a viremic traveler introduced RRV to Fiji, but introduction by a viremic animal is also theoretically possible, as may have occurred, for example, in the case of West Nile virus in New York (154).
The South Pacific epidemic suggests that RRV can be transported via viremic humans. However, the fact that RRV epidemics have not occurred elsewhere suggests either that suitable vectors and reservoir hosts are not present or that arrival of viremic humans from Australia and Papua New Guinea in receptive areas is an uncommon event. The history of the South Pacific epidemic suggests that even if an epidemic can be sustained for some time, endemicity will not be established unless reservoir hosts are present. While RRV disease cases present and are diagnosed in areas outside the endemic countries (153), the likelihood of arrival of a viremic person is small.
The arbovirus extrinsic incubation period is inversely related to temperature (200), so the risk for outbreaks is lower in colder countries. Medical practitioners in countries where RRV is not endemic should be aware of RRV, and if a case is diagnosed, this should be discussed with local public health authorities. Isolation of the patient from mosquitoes is appropriate. If enzootic cycles involving marsupials are necessary to maintain the virus, there is little likelihood of endemicity developing in countries without marsupials. However, the virus's ability to infect multiple mosquito and vertebrate species indicates its potential to become established in new tropical and subtropical areas of the world.
Population Health Interventions
Aaskov et al. (2) advocate blood donor surveillance for RRV, but there are no population health measures of proven effect to activate when increasing numbers of infections are detected. Mosquito control by spraying may decrease transmission and community concern. A candidate vaccine for RRV exists (1, 229), but the cost of vaccine trials is hard to justify for a disease that occurs only in Australia and Papua New Guinea and is never fatal.
A recent North Queensland case-control study suggested that individual efforts to decrease risk were effective, particularly the use of repellents, citronella candles, mosquito coils, and light-colored clothing (78). It also showed a significantly increased risk associated with camping in that setting, consistent with enzootic transmission involving macropods. There is evidence that A. notoscriptus is a vector of RRV (41, 209). This mosquito, as discussed in the section on mosquito vectors, breeds in containers around dwellings. In coastal parts of north Queensland, bromeliads (an ornamental plant that holds water in which mosquitoes breed) were found to increase risk for RRV disease (78). This observation is consistent with a vector role for A. notoscriptus. Therefore, reduction of mosquito breeding around dwellings should decrease risk. Health education regarding decreasing individual risk, particularly when in the bush in areas of high endemicity or during epidemics, should be given greater emphasis in campaigns to control RRV disease.
CONCLUSIONS
RRV causes at least 4,745 disease cases in Australia per annum, on average. Conservatively estimated, the yearly cost is $2.8 to A$5.7 million. The infection is also endemic in Papua New Guinea, but the number of cases there is not known and other health problems are more important. RRV can spread to other countries; already it has caused large epidemics in several nonendemic South Pacific countries, but fortunately did not become established there permanently.
An enormous literature exists on the mosquito and vertebrate associations of RRV, but little is known about the human risks for infection and disease. Research should continue on the vectors and vertebrate hosts posing RRV risks for humans, especially in tropical Australia where the risk is high. We also need much more research on the environmental and behavioral determinants of infection. Such work could yield evidence for public health measures to reduce incidence by planning to avoid mosquito breeding or vertebrate hosts in suburban areas, or through behavioral measures to reduce the chances of being bitten by vector mosquitoes.
RRV disease causes considerable morbidity, and the limited data available on natural history suggest that many patients are unwell for weeks to months but then recover. Basic research on immunology and pathology of RRV disease may lead to better therapy in place of the current ad hoc approach to treatment. To enhance the prospects of generating funds for the research needed, more accurate information is needed on RRV morbidity along with better estimates of the overall economic burden.
ACKNOWLEDGMENTS
This review arose from a Ph.D. completed by David Harley at the Australian Centre for International and Tropical Health and Nutrition of the University of Queensland. An Australian National Health and Medical Research Council Dora Lush Biomedical Scholarship and the Royal Australian College of General Practitioners provided financial support.
David Bossingham trained D. Harley in rheumatologic examination. Di James is thanked for drawing the map (Fig. 1) and the graphs (Fig. 2 to 5). Comments from two anonymous reviewers were also greatly appreciated.
ADDENDUM IN PROOF
Tong et al. (S. Tong, P. Bi, J. Hayes, K. Donald, and J. MacKenzie, Am. J. Trop. Med. Hyg. 65:171–176, 2001) have recently shown that the number of localities in Queensland giving notice of RRV cases increased significantly during the years 1985 to 1996. This fits with the pattern of increasing prevalence of past RRV infection noted in antibody prevalence surveys in New South Wales during the 1980s (C. R. Boughton, R. A. Hawkes, H. M. Naim, J. Wild, and B. Chapman, Med. J. Aust. 141:700–704, 1984; R. A. Hawkes, J. Pamplin, C. R. Boughton, and H. M. Naim, Med. J. Aust. 159:159–162, 1993) and suggests that both the incidence and the geographic distribution of RRV are increasing. Another significant discovery is that of a new species of Alphavirus in sub-Antarctic elephant seal populations, transmitted by seal lice (M. La Linn, M. J. Gardner, D. Warrilow, G. A. Darnell, C. R. McMahon, I. Field, A. D. Hyatt, R. W. Slade, and A. Suhrbier, J. Virol. 75:4103–4109, 2001), further evidence of the extraordinary diversity and adaptability of this genus of arbovirus.
REFERENCES
- 1.Aaskov J, Williams L, Yu S. A candidate Ross River virus vaccine: preclinical evaluation. Vaccine. 1997;15:1396–1404. doi: 10.1016/s0264-410x(97)00051-0. [DOI] [PubMed] [Google Scholar]
- 2.Aaskov J G, Chen J-Y, Hanh N T H, Dennington P M. Surveillance for Ross River virus infection using blood donors. Am J Trop Med Hyg. 1998;58:726–730. doi: 10.4269/ajtmh.1998.58.726. [DOI] [PubMed] [Google Scholar]
- 3.Aaskov J G, Fraser J R E, Dalglish D A. Specific and non-specific immunological changes in epidemic polyarthritis patients. Aust J Exp Biol Med Sci. 1981;59:599–608. doi: 10.1038/icb.1981.52. [DOI] [PubMed] [Google Scholar]
- 4.Aaskov J G, Mataika J U, Lawrence G W, Rabukawaqa V, Tucker M M, Miles J A R, Dalglish D A. An epidemic of Ross River virus infection in Fiji, 1979. Am J Trop Med Hyg. 1981;30:1053–1059. doi: 10.4269/ajtmh.1981.30.1053. [DOI] [PubMed] [Google Scholar]
- 5.Aaskov J G, Nair K, Lawrence G W, Dalglish D A, Tucker M. Evidence of transplacental transmission of Ross River virus in humans. Med J Aust. 1981;2:20–21. [PubMed] [Google Scholar]
- 6.Aaskov J G, Ross P, Davies C E A, Innis M D, Guard R W, Stallman N D, Tucker M. Epidemic polyarthritis in northeastern Australia, 1978–1979. Med J Aust. 1981;2:17–19. [PubMed] [Google Scholar]
- 7.Aaskov J G, Ross P V, Harper J J, Donaldson M D. Isolation of Ross River virus from epidemic polyarthritis patients in Australia. Aust J Exp Biol Med Sci. 1985;63:587–597. doi: 10.1038/icb.1985.62. [DOI] [PubMed] [Google Scholar]
- 8.Aleck K A, Rosen L, Pettitt D J, Boveington C, Bennett P H. Absence of intrauterine infection following Ross River virus infection during pregnancy. Am J Trop Med Hyg. 1983;32:618–620. doi: 10.4269/ajtmh.1983.32.618. [DOI] [PubMed] [Google Scholar]
- 9.Amin J, Hueston L, Dwyer D E, Capon A. Ross River virus infection in the north-west outskirts of the Sydney basin. Commun Dis Intell. 1997;22:101–102. doi: 10.33321/cdi.1998.22.18. [DOI] [PubMed] [Google Scholar]
- 10.Anderson S G, French E L. An epidemic exanthem associated with polyarthritis in the Murray Valley. Med J Aust. 1957;2:113–117. doi: 10.5694/j.1326-5377.1957.tb57732.x. [DOI] [PubMed] [Google Scholar]
- 11.Anstey N, Currie B, Tai K S. Case report: Ross River virus disease presenting with hematuria. Southeast Asian J Trop Med Public Health. 1991;22:281–283. [PubMed] [Google Scholar]
- 12.Austin F J, Maguire T. Ross River virus in the southwest Pacific. In: Mackenzie J S, editor. Viral diseases in South-East Asia and the Western Pacific. Sydney, Canberra, Australia: Academic Press; 1982. pp. 528–531. [Google Scholar]
- 13.Australian Bureau of Statistics. Average weekly earnings: states and Australia (catalogue number 6302.0). Canberra, Australia: Australian Bureau of Statistics; 1999. [Google Scholar]
- 14.Azuolas J K. Arboviral diseases of horses and possums. Arbovirus Res Aust. 1997;7:5–7. [Google Scholar]
- 15.Ballard J W O, Marshall I D. An investigation of the potential of Aedes camptorhynchus (Thom.) as a vector of Ross River virus. Aust J Exp Biol Med Sci. 1986;64:197–200. doi: 10.1038/icb.1986.21. [DOI] [PubMed] [Google Scholar]
- 16.Ballard W. The Ross River virus (RRV) vector competence of four mosquito species collected on the south coast of New South Wales. B.Sc. (Hons.) thesis. Canberra, Australia: Australian National University; 1982. [Google Scholar]
- 17.Bennett B K, Hickie I B, Vollmer-Conna U S, Quigley B, Brennan C M, Wakefield D, Douglas M P, Hansen G R, Tahmindjis A J, Lloyd A R. The relationship between fatigue, psychological and immunological variables in acute infectious illness. Aust N Z J Psychiatry. 1998;32:180–186. doi: 10.3109/00048679809062727. [DOI] [PubMed] [Google Scholar]
- 18.Boughton C R, Hawkes R A, Naim H M, Wild J, Chapman B. Arbovirus infections in humans in New South Wales: seroepidemiology of the alphavirus group of togaviruses. Med J Aust. 1984;141:700–704. [PubMed] [Google Scholar]
- 19.Broom A K, Wright A E, Mackenzie J S, Lindsay M D, Robinson D. Isolation of Murray Valley encephalitis and Ross River viruses from Aedes normanensis (Diptera: Culicidae) in Western Australia. J Med Entomol. 1989;26:100–103. doi: 10.1093/jmedent/26.2.100. [DOI] [PubMed] [Google Scholar]
- 20.Burdge D R, O'Hanlon D P. Experience at a referral center for patients with suspected Lyme disease in an area of nonendemicity: first 65 patients. Clin Infect Dis. 1993;16:558–560. doi: 10.1093/clind/16.4.558. [DOI] [PubMed] [Google Scholar]
- 21.Burness A T H, Pardoe I, Faragher S G, Vrati S, Dalgano L. Genetic stability of Ross River virus during epidemic spread in nonimmune humans. Virology. 1988;167:639–643. [PubMed] [Google Scholar]
- 22.Calisher C H, Karabatsos N. Arbovirus serogroups: Definition and geographic distribution. In: Monath T P, editor. The arboviruses: epidemiology and ecology. Vol. 1. Boca Raton, Fla: CRC Press; 1988. pp. 19–57. [Google Scholar]
- 23.Calisher C H, Monath T P. Togaviridae and Flaviviridae: the alphaviruses and flaviviruses. In: Lennette E H, Halonen P, Murphy F A, editors. Laboratory diagnosis of infectious diseases: principles and practice. Vol. 2. New York, N.Y: Springer-Verlag; 1988. pp. 414–434. [Google Scholar]
- 24.Campbell J, Aldred J, Davis G. Some aspects of the natural history of Ross River virus in South East Gippsland, Victoria. Arbovirus Res Aust. 1989;5:24–28. [Google Scholar]
- 25.Carter I W J, Smythe L D, Fraser J R E, Stallman N D, Cloonan M J. Detection of Ross River virus immunoglobulin M antibodies by enzyme-linked immunosorbent assay using antibody class capture and comparison with other methods. Pathology. 1985;17:503–508. doi: 10.3109/00313028509105510. [DOI] [PubMed] [Google Scholar]
- 26.Cheng R H, Kuhn R J, Olson N H, Rossmann M G, Choi H K, Smith T J, Baker T S. Nucleocapsid and glycoprotein organization in an enveloped virus. Cell. 1995;80:621–630. doi: 10.1016/0092-8674(95)90516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clancy J G, Russell R C. The ‘other’ mosquitoes of New South Wales. Arbovirus Res Aust. 1997;7:41–46. [Google Scholar]
- 28.Clarris B J, Doherty R L, Fraser J R E, French E L, Muirden K D. Epidemic polyarthritis: a cytological, virological and immunochemical study. Aust N Z J Med. 1975;5:450–457. doi: 10.1111/j.1445-5994.1975.tb03056.x. [DOI] [PubMed] [Google Scholar]
- 29.Clements A N. The biology of mosquitoes. Vol. 1. London, England: Chapman and Hall; 1992. [Google Scholar]
- 30.Clements A N. The biology of mosquitoes. Vol. 2. New York, N.Y.: CABI Publishing; 1999. [Google Scholar]
- 31.Cloonan M J, O'Neill B J, Vale T G, Carter I W, Williams J E. Ross River virus activity along the south coast of New South Wales. Aust J Exp Biol Med Sci. 1982;60:701–706. doi: 10.1038/icb.1982.71. [DOI] [PubMed] [Google Scholar]
- 32.Cloonan M J, Russell R C. Ross River virus activity on the south coast of New South Wales: virological and entomological investigations. Arbovirus Res Aust. 1986;4:124–126. [Google Scholar]
- 33.Condon R J, Rouse I L. Acute symptoms and sequelae of Ross River virus infection in South-Western Australia: a follow-up study. Clin Diagn Virol. 1995;3:273–284. doi: 10.1016/s0928-0197(94)00043-3. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham A L, Fraser J R E. Ross River virus infection of human synovial cells in vitro. Aust J Exp Biol Med Sci. 1985;63:197–204. [PubMed] [Google Scholar]
- 35.Curran M. Annual report of the CDI virology and serology reporting scheme, 1993. Commun Dis Intell. 1994;18:570–596. [Google Scholar]
- 36.Curran M. Annual report of the CDI virology and serology laboratory reporting scheme, 1995. Commun Dis Intell. 1996;20:507–524. [Google Scholar]
- 37.Dale P E, Ritchie S A, Territo B M, Morris C D, Muhar A, Kay B H. An overview of remote sensing and GIS for surveillance of mosquito vector habitats and risk assessment. J Vector Ecol. 1998;23:54–61. [PubMed] [Google Scholar]
- 38.Dale P E R, Morris C D. Culex annulirostris breeding sites in urban areas: Using remote sensing and digital image analysis to develop a rapid predictor of potential breeding areas. J Am Mosq Control Assoc. 1996;12:316–320. [PubMed] [Google Scholar]
- 39.Davies D J, Moran J E, Niall J F, Bryan G B. Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? BMJ. 1982;285:606. doi: 10.1136/bmj.285.6342.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhileepan K, Azoulas J K, Gibson C A. Evidence of vertical transmission of Ross River and Sindbis viruses (Togaviridae: Alphavirus) by mosquitoes (Diptera: Culicidae) in southeastern Australia. J Med Entomol. 1996;33:180–182. doi: 10.1093/jmedent/33.1.180. [DOI] [PubMed] [Google Scholar]
- 41.Doggett S L, Russell R C. Aedes notoscriptus can transmit inland and coastal isolates of Ross River and Barmah Forest viruses from New South Wales! Arbovirus Res Aust. 1997;7:79–81. [Google Scholar]
- 42.Doherty R L. Ross River virus. In: Taylor R M, editor. Catalogue of arthropod-borne viruses of the world. Vol. 1760. Washington D.C.: U.S. Department of Health, Education, and Welfare; 1967. p. 421. [Google Scholar]
- 43.Doherty R L. Arboviruses of Australia. Aust Vet J. 1972;48:172–180. doi: 10.1111/j.1751-0813.1972.tb09267.x. [DOI] [PubMed] [Google Scholar]
- 44.Doherty R L. Surveys of haemagglutination-inhibiting antibody to arboviruses in aborigines and other population groups in northern and eastern Australia, 1966–1971. Trans R Soc Trop Med Hyg. 1973;67:197–205. doi: 10.1016/0035-9203(73)90144-2. [DOI] [PubMed] [Google Scholar]
- 45.Doherty R L, Carley J G, Best J C. Isolation of Ross River virus from man. Med J Aust. 1972;1:1083–1084. doi: 10.5694/j.1326-5377.1972.tb116646.x. [DOI] [PubMed] [Google Scholar]
- 46.Doherty R L, Carley J G, Kay B H, Filippich C, Marks E N, Frazier C L. Isolation of virus strains from mosquitoes collected in Queensland, 1972–1976. Aust J Exp Biol Med Sci. 1979;57:509–520. doi: 10.1038/icb.1979.52. [DOI] [PubMed] [Google Scholar]
- 47.Doherty R L, Gorman B M, Whitehead R H, Carley J G. Studies of epidemic polyarthritis: the significance of three group A arboviruses isolated from mosquitoes in Queensland. Aust Ann Med. 1964;13:322–327. doi: 10.1111/imj.1964.13.4.322. [DOI] [PubMed] [Google Scholar]
- 48.Doherty R L, Gorman B M, Whitehead R H, Carley J G. Studies of arthropod-borne virus infections in Queensland. V. Survey of antibodies to group A arboviruses in man and other animals. Aust J Exp Biol Med Sci. 1966;44:365–378. doi: 10.1038/icb.1966.35. [DOI] [PubMed] [Google Scholar]
- 49.Doherty R L, Standfast H A, Domrow R, Wetters E J, Whithead R H, Carley J G. Studies of the epidemiology of arthropod-borne virus infections at Mitchell River Mission, Cape York Peninsula, North Queensland. IV. Arbovirus infections of mosquitoes and mammals, 1967–1969. Trans R Soc Trop Med Hyg. 1971;65:504–513. doi: 10.1016/0035-9203(71)90161-1. [DOI] [PubMed] [Google Scholar]
- 50.Doherty R L, Standfast H A, Wetters E J, Whithead R H, Barrow G J, Gorman B M. Virus isolation and serological studies of arthropod-borne virus infections in a high rainfall area of north Queensland. Trans R Soc Trop Med Hyg. 1968;62:862–873. doi: 10.1016/0035-9203(68)90014-x. [DOI] [PubMed] [Google Scholar]
- 51.Doherty R L, Whitehead R H, Gorman B M, O'Gower A K. The isolation of a third group A arbovirus in Australia with preliminary observations on its relationship to epidemic polyarthritis. Aust J Sci. 1963;26:183–184. [Google Scholar]
- 52.Doherty R L, Whitehead R H, Wetters E J, Gorman B M. Studies of the epidemiology of arthropod-borne virus infections at Mitchell River mission, Cape York Peninsula, North Queensland. II. Arbovirus infections of mosquitoes, man and domestic fowls, 1963–1966. Trans R Soc Trop Med Hyg. 1968;62:430–438. doi: 10.1016/0035-9203(68)90095-3. [DOI] [PubMed] [Google Scholar]
- 53.Dowling P G. Epidemic polyarthritis. Med J Aust. 1946;2:245–246. doi: 10.5694/j.1326-5377.1974.tb70990.x. [DOI] [PubMed] [Google Scholar]
- 54.Edwards A M. An unusual epidemic. Med J Aust. 1928;1:664–665. [Google Scholar]
- 55.Faragher S G, Meek A D J, Rice C M, Dalgarno L. Genome sequences of a mouse-avirulent and a mouse-virulent strain of Ross River virus. Virology. 1988;163:509–526. doi: 10.1016/0042-6822(88)90292-9. [DOI] [PubMed] [Google Scholar]
- 56.Fauran P, Donaldson M, Harper J, Oseni R A, Aaskov J G. Characterization of Ross River viruses isolated from patients with polyarthritis in New Caledonia and Wallis and Futuna Islands. Am J Trop Med Hyg. 1984;33:1228–1231. doi: 10.4269/ajtmh.1984.33.1228. [DOI] [PubMed] [Google Scholar]
- 57.Fauran P, Le Gonidec G, Panon G. Etude comparative de deux methodes pour l'isolement du Ross River dans les infections humaines. Ann Virol (Inst Pasteur) 1983;134E:181–189. [Google Scholar]
- 58.Fauran P, Le Gonidec G, Rodhain F. Recherche sur l'infection virale des moustiques du Pacifique sud dans les conditions naturelles. Bull Soc Pathol Exot Filiales. 1984;77:628–636. [PubMed] [Google Scholar]
- 59.Flexman J P, Smith D W, Mackenzie J S, Fraser J R E, Bass S P, Hueston L, Lindsay M D A, Cunningham A L. A comparison of the diseases caused by Ross River virus and Barmah Forest virus. Med J Aust. 1998;169:159–163. doi: 10.5694/j.1326-5377.1998.tb116019.x. [DOI] [PubMed] [Google Scholar]
- 60.Fraser J R E. Epidemic polyarthritis and Ross River virus disease. Clin Rheum Dis. 1986;12:369–388. [PubMed] [Google Scholar]
- 61.Fraser J R E, Ahern A P, Alexander J, Jones J M, Lung D Y L, Moore F M, Moysey C D, Phillips P J, Pryor I, Leach R, Macaulay E D, Christie D, Gust I D, White J. Arbovirus infection in a Murray Valley community I. Prevalence of antibodies, December, 1974. Med J Aust. 1976;1:257–259. [PubMed] [Google Scholar]
- 62.Fraser J R E, Becker G J. Mononuclear cell types in chronic synovial effusions of Ross River virus disease. Aust N Z J Med. 1984;14:505–506. doi: 10.1111/j.1445-5994.1984.tb03629.x. [DOI] [PubMed] [Google Scholar]
- 63.Fraser J R E, Cunningham A L. Incubation time of epidemic polyarthritis. Med J Aust. 1980;1:550–551. doi: 10.5694/j.1326-5377.1980.tb135109.x. [DOI] [PubMed] [Google Scholar]
- 64.Fraser J R E, Cunningham A L, Clarris B J, Aaskov J G, Leach R. Cytology of synovial effusions in epidemic polyarthritis. Aust N Z J Med. 1981;11:168–173. doi: 10.1111/j.1445-5994.1981.tb04226.x. [DOI] [PubMed] [Google Scholar]
- 65.Fraser J R E, Cunningham A L, Matthews J D, Riglar A. Immune complexes and Ross River virus disease (epidemic polyarthritis) Rheumatol Int. 1988;8:113–117. doi: 10.1007/BF00272432. [DOI] [PubMed] [Google Scholar]
- 66.Fraser J R E, Marshall I D. Epidemic polyarthritis (Ross River virus disease) handbook. Canberra, Australia: Commonwealth Department of Community Services and Health; 1989. [Google Scholar]
- 67.Fraser J R E, Ratnamohan V M, Dowling J P G, Becker G J, Varigos G A. The exanthem of Ross River virus infection: histology, location of virus antigen and nature of inflammatory infiltrate. J Clin Pathol. 1983;36:1256–1263. doi: 10.1136/jcp.36.11.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fraser J R E, Tait B, Aaskov J G, Cunningham A L. Possible genetic determinants in epidemic polyarthritis caused by Ross River virus infection. Aust N Z J Med. 1980;10:597–603. doi: 10.1111/j.1445-5994.1980.tb04238.x. [DOI] [PubMed] [Google Scholar]
- 69.Fries J P, Spitz P W, Young D Y. The dimensions of health outcomes: The Health Assessment Questionnaire, disability and pain scales. J Rheumatol. 1982;9:789–793. [PubMed] [Google Scholar]
- 70.Fuller C O, Warner P. Some epidemiological and laboratory observations on an epidemic rash and polyarthritis occurring in the upper Murray region of South Australia. Med J Aust. 1957;2:117–120. doi: 10.5694/j.1326-5377.1957.tb57733.x. [DOI] [PubMed] [Google Scholar]
- 71.Gard G, Marshall I D, Woodroofe G M. Annually recurrent epidemic polyarthritis and Ross River virus activity in a coastal area of New South Wales. II. Mosquitoes, viruses and wildlife. Am J Trop Med Hyg. 1973;22:551–560. doi: 10.4269/ajtmh.1973.22.551. [DOI] [PubMed] [Google Scholar]
- 72.Goswell G. A further report of an epidemic of acute polyarthritis. Med J Aust. 1946;2:861–862. [PubMed] [Google Scholar]
- 73.Grimley P, Friedman R. Arboviral infection of voluntary striated muscles. J Infect Dis. 1970;122:45–52. doi: 10.1093/infdis/122.1-2.45. [DOI] [PubMed] [Google Scholar]
- 74.Gubler D J. Transmission of Ross River virus by Aedes polynesiensis and Aedes aegypti. Am J Trop Med Hyg. 1981;30:1303–1306. doi: 10.4269/ajtmh.1981.30.1303. [DOI] [PubMed] [Google Scholar]
- 75.Hall L S. Identification, distribution and taxonomy of Australian flying-foxes (Chiroptera: Pteropodidae) Aust Mammal. 1987;10:75–79. [Google Scholar]
- 76.Halliday J H, Horan J P. An epidemic of polyarthritis in the Northern Territory. Med J Aust. 1943;2:293–295. [Google Scholar]
- 77.Hardy J L, Houk E J, Kramer L D, Reeves W C. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu Rev Entomol. 1983;28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- 78.Harley D. Ross River virus: ecology, natural history of disease and epidemiology in tropical Queensland. Ph.D. thesis. Brisbane, Queensland, Australia: Australian Centre for International and Tropical Health and Nutrition, The University of Queensland; 2000. [Google Scholar]
- 79.Harley D, Ritchie S, Phillips D, van den Hurk A. Mosquito isolates of Ross River virus from Cairns, Queensland, Australia. Am J Trop Med Hyg. 2000;62:561–565. doi: 10.4269/ajtmh.2000.62.561. [DOI] [PubMed] [Google Scholar]
- 80.Harris L. Polyarthritis in the Northern Territory. Med J Aust. 1944;1:546. [Google Scholar]
- 81.Harrison S C, Skehel J J, Wiley D C. Virus structure. In: Fields B N, Knipe D L, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Field's virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 59–99. [Google Scholar]
- 82.Hawkes R A, Boughton C R, Naim H M, Stallman N D. A major outbreak of epidemic polyarthritis in New South Wales during the summer of 1983/1984. Med J Aust. 1985;143:330–333. doi: 10.5694/j.1326-5377.1985.tb123054.x. [DOI] [PubMed] [Google Scholar]
- 83.Hawkes R A, Pamplin J, Boughton C R, Naim H M. Arbovirus infections in humans in high-risk areas of south-eastern Australia: a continuing study. Med J Aust. 1993;159:159–162. doi: 10.5694/j.1326-5377.1993.tb137778.x. [DOI] [PubMed] [Google Scholar]
- 84.Hazleton R A, Hughes C, Aaskov J G. The inflammatory response in the synovium of a patient with Ross River arbovirus infection. Aust N Z J Med. 1985;15:336–339. doi: 10.1111/j.1445-5994.1985.tb04048.x. [DOI] [PubMed] [Google Scholar]
- 85.Hii J, Dyke T, Dagoro H, Sanders R C. Health impact assessments of malaria and Ross River virus infection in the Southern Highlands Province of Papua New Guinea. P N G Med J. 1997;40:14–25. [PubMed] [Google Scholar]
- 86.Hwang S Y, Hertzog P J, Holland K A, Sumarsono S H, Tymms M J, Hamilton J A, Whitty G, Bertoncello I, Kola I. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proc Natl Acad Sci USA. 1995;92:11284–11288. doi: 10.1073/pnas.92.24.11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johansen C A, van den Hurk A F, Ritchie S A, Zborowski P, Nisbet D J, Paru R, Bockarie M J, MacDonald J, Drew A C, Khromykh T I, MacKenzie J S. Isolation of Japanese encephalitis virus from mosquitoes (Diptera: Culicidae) collected in the Western Province of Papua New Guinea, 1997–1998. Am J Trop Med Hyg. 2000;62:631–638. doi: 10.4269/ajtmh.2000.62.631. [DOI] [PubMed] [Google Scholar]
- 88.Johansson S R. The Health Transition: the cultural inflation of morbidity during the decline of mortality. Health Transit Rev. 1991;1:39–67. [PubMed] [Google Scholar]
- 89.Johnson R T. Virus invasion of the central nervous system: a study of Sindbis virus infection in the mouse using flourescent antibody. Am J Pathol. 1965;46:929–943. [PMC free article] [PubMed] [Google Scholar]
- 90.Johnston R E, Peters C J. Alphaviruses. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 843–898. [Google Scholar]
- 91.Journeaux S F, Brown W G, Aaskov J G. Prolonged infection of human synovial cells with Ross River virus. J Gen Virol. 1987;68:3165–3169. doi: 10.1099/0022-1317-68-12-3165. [DOI] [PubMed] [Google Scholar]
- 92.Karabatsos N. International catalogue of arboviruses. 3rd ed. San Antonio, Tex: American Society of Tropical Medicine and Hygiene; 1985. [Google Scholar]
- 93.Kay B H. Seasonal abundance of Culex annulirostris and other mosquitoes at Kowanyama, North Queensland, and Charleville, South West Queensland. Aust J Exp Biol Med Sci. 1979;57:497–508. doi: 10.1038/icb.1979.51. [DOI] [PubMed] [Google Scholar]
- 94.Kay B H. Three modes of transmission of Ross River virus by Aedes vigilax (Skuse) Aust J Exp Biol Med Sci. 1982;60:339–344. doi: 10.1038/icb.1982.37. [DOI] [PubMed] [Google Scholar]
- 95.Kay B H, Aaskov J G. Ross River virus (Epidemic polyarthritis) In: Monath T P, editor. The arboviruses: epidemiology and ecology. Vol. 4. Boca Raton, Fla: CRC Press; 1989. pp. 93–112. [Google Scholar]
- 96.Kay B H, Barker-Hudson P, Stallman N D, Wiemers M A, Marks E N, Holt P J, Muscio M, Gorman B M. Dengue fever: Reappearance in northern Queensland after 26 years. Med J Aust. 1984;140:264–268. doi: 10.5694/j.1326-5377.1984.tb104033.x. [DOI] [PubMed] [Google Scholar]
- 97.Kay B H, Boreham P F L, Williams G M. Host preferences and feeding patterns of mosquitoes (Diptera: Culicidae) at Kowanyama, Cape York Peninsula, northern Queensland. Bull Entomol Res. 1979;69:441–457. [Google Scholar]
- 98.Kay B H, Carley J G, Barrow G J, Walker P J, Fanning I D. Experimental innoculation of arthropods. Annu Rep Queensland Inst Med. 1972;27:8–9. [Google Scholar]
- 99.Kay B H, Carley J G, Fanning I D, Filippich C. Quantitative studies of the vector competence of Aedes aegypti, Culex annulirostris and other mosquitoes (Diptera:Culicidae) with Murray Valley encephalitis and other Queensland arboviruses. J Med Entomol. 1979;16:59–66. doi: 10.1093/jmedent/16.1.59. [DOI] [PubMed] [Google Scholar]
- 100.Kay B H, Carley J G, Filippich C. The multiplication of Queensland and New Guinean arboviruses in Aedes funereus (Theobald) (Diptera: Culicidae) J Med Entomol. 1977;13:451–453. doi: 10.1093/jmedent/13.4-5.451. [DOI] [PubMed] [Google Scholar]
- 101.Kay B H, Fanning I D, Carley J G. Vector competence of Culex pipiens quinquefasciatus for Murray Valley encephalitis, Kunjin, and Ross River viruses from Australia. Am J Trop Med Hyg. 1982;31:844–848. doi: 10.4269/ajtmh.1982.31.844. [DOI] [PubMed] [Google Scholar]
- 102.Kay B H, Fanning I D, Wilson M, Tichon M, Smith D. Mosquito ecology and mosquito-virus relationships. Annu Rep Queensland Inst Med Res. 1976;31:11–16. [Google Scholar]
- 103.Kay B H, Hall R A, Fanning I D, Mottram P, Young P L, Pollitt C C. Experimental infection of vertebrates with Murray Valley encephalitis and Ross River viruses. Arbovirus Res Aust. 1986;4:71–75. [Google Scholar]
- 104.Kay B H, Miles J A R, Gubler D J, Mitchell C J. Vectors of Ross River virus: an overview. In: Mackenzie J S, editor. Viral diseases in South-east Asia and the Western Pacific. Sydney, Australia: Academic Press; 1982. pp. 532–536. [Google Scholar]
- 105.La Linn M, Aaskov J G, Suhrbier A. Antibody-dependent enhancement and persitence in macrophages of an arbovirus associated with arthritis. J Gen Virol. 1996;77:407–411. doi: 10.1099/0022-1317-77-3-407. [DOI] [PubMed] [Google Scholar]
- 106.La Linn M, Mateo L, Gardner J, Suhbier A. Alphavirus-specific cytotoxic T lymphocytes recognize a cross-reactive epitope from the capsid protein and can eliminate virus from persistently infected macrophages. J Virol. 1998;72:5146–5153. doi: 10.1128/jvi.72.6.5146-5153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.La Linn M, Suhbier A. Persistence of Ross River virus in macrophages. Arbovirus Res Aust. 1997;7:153–159. [Google Scholar]
- 108.Lee D J, Hicks M M, Debenham M L, Griffiths M, Marks E N, Bryan J H, Russell R C. The Culicidae of the Australasian region. Vol. 7. Canberra, Australian Capital Territory, Australia: Australian Government Publishing Service; 1989. [Google Scholar]
- 109.Lee D J, Hicks M M, Griffiths M, Debenham M L, Bryan J H, Russell R C, Geary M, Marks E N. The Culicidae of the Australasian region. Vol. 4. Canberra, Australian Capital Territory, Australia: Australian Government Publishing Service; 1987. [Google Scholar]
- 110.Lee D J, Hicks M M, Griffiths M, Marks E N. The Culicidae of the Australasian region. Vol. 3. Canberra, Australian Capital Territory, Australia: Australian Government Publishing Service; 1984. [Google Scholar]
- 111.Lee D J, Hicks M M, Griffiths M, Russell R C, Marks E N. The Culicidae of the Australasian region. Vol. 2. Canberra, Australian Capital Territory, Australia: Australian Government Publishing Service; 1982. [Google Scholar]
- 112.Levinson R S, Strauss J H, Strauss E G. Complete sequence of the genomic RNA of O'nyong-nyong virus and its use in the construction of alphavirus phylogenetic trees. Virology. 1990;175:110–123. doi: 10.1016/0042-6822(90)90191-s. [DOI] [PubMed] [Google Scholar]
- 113.Lindsay M, Condon R, Mackenzie J, Johansen C, D'Ercole M, Smith D. A major outbreak of Ross River virus infection in the south-west of Western Australia and the Perth metropolitan area. Commun Dis Intell. 1992;16:290–294. [Google Scholar]
- 114.Lindsay M, Oliveira N, Jasinka E, Johansen C, Harrington S, Wright T, Smith D, Shellam G. Western Australia's largest recorded outbreak of Ross River virus disease. Arbovirus Res Aust. 1997;7:147–152. [Google Scholar]
- 115.Lindsay M D, Broom A K, Oliveira N, Jasinska E, van Heuzen B, Caulfield S, McMinn P, Smith D W, Shellam G R. Western Australian Arbovirus Surveillance and Research Program, annual report, 1997– 1998. Perth, Western Australia, Australia: The University of Western Australia; 1999. [Google Scholar]
- 116.Lindsay M D, Johansen C, Broom A K, D'Ercole M, Wright A E, Condon R, Smith D W, Mackenzie J S. The epidemiology of outbreaks of Ross River virus infection in Western Australia in 1991–1992. Arbovirus Res Aust. 1992;6:72–76. [Google Scholar]
- 117.Lindsay M D, Latchford J A, Wright E A, Mackenzie J S. Studies on the ecology of Ross River virus in the south-west of Western Australia. Arbovirus Res Aust. 1989;5:28–34. [Google Scholar]
- 118.Lindsay M D A, Broom A K, Wright A E, Johansen C A, Mackenzie J S. Ross River virus isolations from mosquitoes in arid regions of Western Australia: implication of vertical transmission as a means of persistence of the virus. Am J Trop Med Hyg. 1993;49:686–696. doi: 10.4269/ajtmh.1993.49.686. [DOI] [PubMed] [Google Scholar]
- 119.Lindsay M D A, Coelen R J, Mackenzie J S. Genetic heterogeneity among isolates of Ross River virus from different geographical regions. J Virol. 1993;67:3576–3585. doi: 10.1128/jvi.67.6.3576-3585.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lucas R E, Qiao M. A case of encephalitis in central Australia due to Ross River virus? Aust N Z J Med. 1999;29:268–270. doi: 10.1111/j.1445-5994.1999.tb00695.x. [DOI] [PubMed] [Google Scholar]
- 121.Mackenzie J S, Broom A K, Calisher C H, Cloonan M J, Cunningham A L, Gibson C A, Hueston L, Lindsay M D, Marshall I D, Phillips D A, Russell R C, Sheridan J, Smith D W, Smythe L, Vitarana T, Worswick D. Diagnosis and reporting of arbovirus infections in Australia. Arbovirus Res Aust. 1993;6:89–93. [Google Scholar]
- 122.Mackenzie J S, Broom A K, Hall R A, Johansen C A, Lindsay M D, Phillips D A, Ritchie S A, Russell R C, Smith D W. Arboviruses in the Australian region, 1990 to 1998. Commun Dis Intell. 1998;22:93–100. doi: 10.33321/cdi.1998.22.17. [DOI] [PubMed] [Google Scholar]
- 123.Mackenzie J S, Lindsay M D, Coelen R J, Broom A K, Hall R A, Smith D W. Arboviruses causing human disease in the Australasian zoogeographic region. Arch Virol. 1994;136:447–467. doi: 10.1007/BF01321074. [DOI] [PubMed] [Google Scholar]
- 124.Mackenzie J S, Smith D W. Mosquito-borne viruses and epidemic polyarthritis. Med J Aust. 1996;164:90–93. doi: 10.5694/j.1326-5377.1996.tb101357.x. [DOI] [PubMed] [Google Scholar]
- 125.Mackenzie J S, Smith D W, Ellis T M, Lindsay M D, Broom A K, Coelen R J, Hall R A. Human and animal arboviral diseases in Australia. In: Gilbert G L, editor. Recent advances in microbiology. Vol. 2. Melbourne, Victoria, Australia: Australian Society of Microbiology; 1994. pp. 1–91. [Google Scholar]
- 126.Maguire T. Do Ross River and dengue viruses pose a threat to New Zealand? N Z Med J. 1994;107:448–450. [PubMed] [Google Scholar]
- 127.March L M, Brnabic A J M, Skinner J C, Schwarz J M, Finnegan T, Druce J, Brooks P M. Musculoskeletal disability among elderly people in the community. Med J Aust. 1998;168:439–442. doi: 10.5694/j.1326-5377.1998.tb139023.x. [DOI] [PubMed] [Google Scholar]
- 128.Marshall I D. John Curtin School of Medical Research Annual Report. Canberra, Australian Capital Territory, Australia: Australian National University; 1984. Epidemiology of arboviruses; pp. 116–117. [Google Scholar]
- 129.Marshall I D. John Curtin School of Medical Research Annual Report. Canberra, Australian Capital Territory, Australia: Australian National University; 1985. Epidemiology of arboviruses: Barmah Forest project; pp. 132–133. [Google Scholar]
- 130.Marshall I D, Miles J A R. Ross River virus and epidemic polyarthritis. In: Harris K F, editor. Current topics in vector research. Vol. 2. New York, N.Y: Praeger Publishers; 1984. pp. 31–56. [Google Scholar]
- 131.Marshall I D, Woodroofe G M. John Curtin School of Medical Research Annual Report. Canberra, Australian Capital Territory, Australia: Australian National University; 1973. Viral epidemiology: arboviruses; pp. 88–89. [Google Scholar]
- 132.Marshall I D, Woodroofe G M, Gard G P. Arboviruses of coastal south eastern Australia. Aust J Exp Biol Med Sci. 1980;58:91–102. doi: 10.1038/icb.1980.9. [DOI] [PubMed] [Google Scholar]
- 133.Marshall I D, Woodroofe G M, Hirsch S. Viruses recovered from mosquitoes and wildlife serum collected in the Murray Valley of South-Eastern Australia, February 1974, during an epidemic of encephalitis. Aust J Exp Biol Med Sci. 1982;60:457–470. doi: 10.1038/icb.1982.51. [DOI] [PubMed] [Google Scholar]
- 134.Mateo L, La Linn M, McColl S R, Cross S, Gardner J, Suhrbier A. An arthrogenic alphavirus induces monocyte chemoattractant protein-1 and interleukin-8. Intervirology. 2000;43:55–60. doi: 10.1159/000025023. [DOI] [PubMed] [Google Scholar]
- 135.McDowell I, Newell C. The Short-Form-36 Health Survey. In: McDowell I, Newell C, editors. Measuring health: a guide to rating scales and questionnaires. New York, N.Y: Oxford University Press; 1996. pp. 446–456. [Google Scholar]
- 136.McIntosh K. Diagnostic virology. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 401–430. [Google Scholar]
- 137.McManus T J, Marshall I D. The epidemiology of Ross River virus in Tasmania. Arbovirus Res Aust. 1986;4:127–131. [Google Scholar]
- 138.McManus T J, Russell R C, Wells P J, Clancy J G, Fennell M, Cloonan M J. Further studies on the epidemiology and effects of Ross River virus in Tasmania. Arbovirus Res Aust. 1992;6:68–72. [Google Scholar]
- 139.Meenan R F, Mason J H, Anderson J J, Guccione A A, Kazis L E. AIMS2: the content and properties of a revised and expanded Arthritis Impact Measurement Scales Health Status Questionnaire. Arthritis Rheum. 1992;35:1–10. doi: 10.1002/art.1780350102. [DOI] [PubMed] [Google Scholar]
- 140.Merianos A, Farland A M, Patel M, Currie B, Whelan P, Dentith H, Smith D. A concurrent outbreak of Barmah Forest and Ross River virus disease in Nhulunbuy, Northern Territory. Commun Dis Intell. 1992;16:110–111. [Google Scholar]
- 141.Mitchell C J, Gubler D J. Vector competence of geographic strains of Aedes albopictus and Aedes polynesiensis and certain other Aedes (Stegomyia) mosquitoes for Ross River virus. J Am Mosq Control Assoc. 1987;3:142–147. [PubMed] [Google Scholar]
- 142.Mitchell C J, Miller B R, Gubler D J. Vector competence of Aedes albopictus from Houston, Texas, for dengue serotypes 1 to 4, yellow fever and Ross River viruses. J Am Mosq Control Assoc. 1987;3:460–465. [PubMed] [Google Scholar]
- 143.Mudge P R. A survey of epidemic polyarthritis in the Riverland area, 1976. Med J Aust. 1977;1:649–651. doi: 10.5694/j.1326-5377.1977.tb130990.x. [DOI] [PubMed] [Google Scholar]
- 144.Mudge P R, Aaskov J G. Epidemic polyarthritis in Australia, 1980–1981. Med J Aust. 1983;2:269–273. doi: 10.5694/j.1326-5377.1983.tb122461.x. [DOI] [PubMed] [Google Scholar]
- 145.Muller M J, Murray M D, Edwards J A. Blood-sucking midges and mosquitoes feeding on mammals at Beatrice Hill, N.T. Aust J Zool. 1981;29:573–588. [Google Scholar]
- 146.Murphy F A, Taylor W, Mims C, Marshall I D. Pathogenesis of Ross River virus infection in mice. II. Muscle, heart, and brown fat lesions. J Infect Dis. 1973;127:129–138. doi: 10.1093/infdis/127.2.129. [DOI] [PubMed] [Google Scholar]
- 147.Nathanson N. Pathogenesis. In: Monath T P, editor. St. Louis encephalitis. Washington, D.C.: American Public Health Association; 1980. pp. 201–236. [Google Scholar]
- 148.Nimmo J R. An unusual epidemic. Med J Aust. 1928;1:549–550. [Google Scholar]
- 149.Pascoe R R, St. George T D, Cybinski D H. The isolation of a Ross River virus from a horse. Aust Vet J. 1978;54:600. doi: 10.1111/j.1751-0813.1978.tb02435.x. [DOI] [PubMed] [Google Scholar]
- 150.Phillips D A, Aaskov J G, Taylor C, Atkin C, Wiemers M A. Diagnostic procedures for arboviral infections. Arbovirus Res Aust. 1992;6:307–310. [Google Scholar]
- 151.Phillips D A, Murray J R, Aaskov J G, Weimers M A. Clinical and subclinical Barmah Forest virus infection in Queensland. Med J Aust. 1990;152:463–466. doi: 10.5694/j.1326-5377.1990.tb125304.x. [DOI] [PubMed] [Google Scholar]
- 152.Phillips D A, Sheridan J, Aaskov J G, Murray J, Wiemers M A. Epidemiology of arbovirus infection in Queensland, 1989–1992. Arbovirus Res Aust. 1993;6:245–248. [Google Scholar]
- 153.Proll S, Dobler G, Pfeffer M, Jelinek T, Nothdurft H D, Loscher T. Persistent arthralgias in Ross River virus disease after travel to the South Pacific. Dtsch Med Wochenschr. 1999;124:759–762. doi: 10.1055/s-2007-1024409. [DOI] [PubMed] [Google Scholar]
- 154.Rappole J H, Derrikson S R, Hubalek Z. Migratory birds and spread of West Nile virus in the Western Hemisphere. Emerg Infect Dis. 2000;6:319–328. doi: 10.3201/eid0604.000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Reinert J F. Restoration of Verallina to generic rank in tribe Aedini (Diptera:Culicidae) and descriptions of the genus and three included subgenera. Contrib Am Entomol Inst. 1999;31:1–79. [Google Scholar]
- 156.Reinert J F. New classification for the composite genus Aedes (Diptera: Culicidae: Aedini), elevation of subgenus Ochlerotatus to generic rank, reclassification of the other subgenera, and notes on certain subgenera and species. J Am Mosq Control Assoc. 2000;16:175–188. [PubMed] [Google Scholar]
- 157.Rich G, McKechnie J, McPhan I, Richards B. Laboratory diagnosis of Ross River virus infection. Commun Dis Intell. 1993;17:208–209. [Google Scholar]
- 158.Ritchie S A, Fanning I D, Phillips D A, Standfast H A, McGinn D, Kay B H. Ross River virus in mosquitoes (Diptera: Culicidae) during the 1994 epidemic around Brisbane, Australia. J Med Entomol. 1997;34:156–159. doi: 10.1093/jmedent/34.2.156. [DOI] [PubMed] [Google Scholar]
- 159.Robinson M C. An epidemic of virus disease in southern province, Tanganyika territory, in 1952–53. Trans R Soc Trop Med Hyg. 1955;49:28–32. doi: 10.1016/0035-9203(55)90080-8. [DOI] [PubMed] [Google Scholar]
- 160.Roitt I. Roitt's essential immunology 9th ed. Oxford, England: Blackwell Science; 1997. [Google Scholar]
- 161.Roizman B, Palese P. Multiplication of viruses: an overview. In: Fields B N, Knipe D L, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Field's virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 101–111. [Google Scholar]
- 162.Rosen L, Gubler D J, Bennett P H. Epidemic polyarthritis (Ross River) virus infection in the Cook Islands. Am J Trop Med Hyg. 1981;30:1294–1302. doi: 10.4269/ajtmh.1981.30.1294. [DOI] [PubMed] [Google Scholar]
- 163.Ross R W. The Newala epidemic. III. The virus: isolation, pathogenic properties and relationship to the epidemic. J Hyg. 1956;54:177–191. doi: 10.1017/s0022172400044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Russell R C. Culex annulirostris Skuse (Diptera:Culicidae) at Appin, N.S.W.—bionomics and behaviour. J Aust Entomol Soc. 1986;25:103–109. [Google Scholar]
- 165.Russell R C. Host-attraction studies of Culex annulirostris Skuse (Diptera:Culicidae) near Echuca, in the Murray Valley of Victoria. J Aust Entomol Soc. 1987;26:375–378. [Google Scholar]
- 166.Russell R C. The mosquito fauna of Conjola state forest on the south coast of New South Wales. 2. Female feeding behaviour and flight activity. Gen Appl Entomol. 1987;19:17–24. [Google Scholar]
- 167.Russell R C. Ross River virus: disease trends and vector ecology in Australia. Bull Soc Vector Ecol. 1994;19:73–81. [Google Scholar]
- 168.Russell R C. Arboviruses and their vectors in Australia: an update on the ecology and epidemiology of some mosquito-borne arboviruses. Rev Med Vet Entomol. 1995;83:141–158. [Google Scholar]
- 169.Russell R C. Vectors vs. humans in Australia: who is on top down under? An update on vector-borne disease and research on vectors in Australia. J Vector Ecol. 1998;23:1–46. [PubMed] [Google Scholar]
- 170.Russell R C, Cloonan M J, Wells P J, Vale T G. Mosquito (Diptera: Culicidae) and arbovirus activity on the South Coast of New South Wales, Australia, in 1985–1988. J Med Entomol. 1991;28:796–804. doi: 10.1093/jmedent/28.6.796. [DOI] [PubMed] [Google Scholar]
- 171.Russell R C, Cope S E, Yund A J, Hueston L. Combatting the enemy—mosquitoes and Ross River virus in a joint military exercise in tropical Australia. Am J Trop Med Hyg. 1998;39:S307. [Google Scholar]
- 172.Ryan P A, Do K A, Kay B H. Spatial and temporal analysis of Ross River virus disease patterns at Maroochy Shire, Australia: association between human morbidity and mosquito (Diptera: Culicidae) abundance. J Med Entomol. 1999;36:515–521. doi: 10.1093/jmedent/36.4.515. [DOI] [PubMed] [Google Scholar]
- 173.Ryan P A, Do K-A, Kay B H. Definition of Ross River virus vectors at Maroochy shire, Australia. J Med Entomol. 2000;37:146–152. doi: 10.1603/0022-2585-37.1.146. [DOI] [PubMed] [Google Scholar]
- 174.Ryan P A, Kay B H. Ross River virus ecology is complex. Arbovirus Res Aust. 1997;7:247–251. [Google Scholar]
- 175.Ryan P A, Martin L, Mackenzie J S, Kay B H. Investigation of gray-headed flying foxes (Pteropus poliocephalus) (Megachiroptera: Pteropodididae) and mosquitoes in the ecology of Ross River virus in Australia. Am J Trop Med Hyg. 1997;57:476–482. doi: 10.4269/ajtmh.1997.57.476. [DOI] [PubMed] [Google Scholar]
- 176.Sammels L M, Coelen R J, Lindsay M D, Mackenzie J S. Geographic distribution and evolution of Ross River virus in Australia and the Pacific Islands. Virology. 1995;212:20–29. doi: 10.1006/viro.1995.1449. [DOI] [PubMed] [Google Scholar]
- 177.Schlesinger S, Schlesinger M J. Togaviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 825–841. [Google Scholar]
- 178.Scott T W. Vertebrate host ecology. In: Monath T P, editor. The arboviruses: epidemiology and ecology. Vol. 1. Boca Raton, Fla: CRC Press; 1988. pp. 257–280. [Google Scholar]
- 179.Scrimgeour E M, Aaskov J G, Matz L R. Ross River virus arthritis in Papua New Guinea. Trans R Soc Trop Med Hyg. 1987;81:833–834. doi: 10.1016/0035-9203(87)90045-9. [DOI] [PubMed] [Google Scholar]
- 180.Seglenieks Z, Moore B W. Epidemic polyarthritis in South Australia: report of an outbreak in 1971. Med J Aust. 1974;2:552–556. doi: 10.5694/j.1326-5377.1974.tb70993.x. [DOI] [PubMed] [Google Scholar]
- 181.Selden S M, Cameron A S. Changing epidemiology of Ross River virus disease in South Australia. Med J Aust. 1996;165:313–317. doi: 10.5694/j.1326-5377.1996.tb124989.x. [DOI] [PubMed] [Google Scholar]
- 182.Service M W. Review: importance of ecology in Aedes aegypti control. Southeast Asian J Trop Med Public Health. 1992;23:681–690. [PubMed] [Google Scholar]
- 183.Shirako Y, Yamaguchi Y. Genome structure of Sagiyama virus and its relatedness to other alphaviruses. J Gen Virol. 2000;81:1353–1360. doi: 10.1099/0022-1317-81-5-1353. [DOI] [PubMed] [Google Scholar]
- 184.Shope R E, Anderson S G. The virus aetiology of epidemic exanthem and polyarthritis. Med J Aust. 1960;1:156–158. doi: 10.5694/j.1326-5377.1960.tb105259.x. [DOI] [PubMed] [Google Scholar]
- 185.Shope R E, Meegan J M. Arboviruses. In: Evans A S, Kaslow R A, editors. Viral infections of humans: epidemiology and control. 4th ed. New York, N.Y: Plenum Medical Book Company; 1997. pp. 151–183. [Google Scholar]
- 186.Sibree E W. Acute polyarthritis in Queensland. Med J Aust. 1944;2:565–567. [Google Scholar]
- 187.Siegert C E H, Vleming L-J, Vandenbroucke J P, Cats A. Measurement of disability in Dutch rheumatoid arthritis patients. Clin Rheumatol. 1984;3:305–309. doi: 10.1007/BF02032335. [DOI] [PubMed] [Google Scholar]
- 188.Soden M, Vasudevan H, Roberts B, Coelen R, Hamlin G, Vasudevan S, La Brooy J. Detection of viral ribonucleic acid and histologic analysis of inflamed synovium in Ross River virus infection. Arthritis Rheum. 2000;43:365–369. doi: 10.1002/1529-0131(200002)43:2<365::AID-ANR16>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 189.Stallman N D, Wiemers M A, Bourke A T C. Serology in diagnosis and surveys of man. Arbovirus Res Aust. 1976;1:46–64. [Google Scholar]
- 190.Steere A C, Taylor E, McHugh G L, Logigian E L. The overdiagnosis of Lyme disease. JAMA. 1993;269:1812–1816. [PubMed] [Google Scholar]
- 191.Strauss J H, Strauss E G. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Strauss J H, Strauss E G. Genetic recombination as a molecular basis for the evolution of arboviruses. In: Saluzzo J F, Dodet B, editors. Factors in the emergence of arbovirus diseases. Amsterdam, The Netherlands: Elsevier; 1997. pp. 37–50. [Google Scholar]
- 193.Sudia W D, Newhouse V F, Chappell W A. Venezuelan equine encephalitis virus-vector studies following a human case in Dade County, Florida, 1968. Mosq News. 1969;29:596–600. [Google Scholar]
- 194.Tai K S, Whelan P I, Patel M S, Currie B S. An outbreak of epidemic polyarthritis (Ross River virus disease) in the Northern Territory during the 1990–1991 wet season. Med J Aust. 1993;158:522–525. doi: 10.5694/j.1326-5377.1993.tb121866.x. [DOI] [PubMed] [Google Scholar]
- 195.Talley N J, O'Connor S O. Clinical examination. Sydney, Australia: MacLennan and Petty; 1992. [Google Scholar]
- 196.Tesh R B, Gajdusek D C, Carruto R M, Cross J H, Rosen L. The distribution and prevalence of arbovirus neutralizing antibodies among human populations in southeast Asia and the Pacific islands. Am J Trop Med Hyg. 1975;24:664–675. doi: 10.4269/ajtmh.1975.24.664. [DOI] [PubMed] [Google Scholar]
- 197.Tesh R B, McLean R G, Shroyer D A, Calisher C H, Rosen L. Ross River virus (Togaviridae: Alphavirus) infection (epidemic polyarthrits) in American Samoa. Trans R Soc Trop Med Hyg. 1981;75:426–431. doi: 10.1016/0035-9203(81)90112-7. [DOI] [PubMed] [Google Scholar]
- 198.Thomson J, Lin M, Halliday L, Preston G, McIntyre P, Gidding H, Amin J, Roberts L, Higgins K, Brooke F, Milton A, O'Brien E, Witteveen D, Crerar S. Australia's notifiable diseases status, 1998: annual report of the National Notifiable Diseases Surveillance System. Commun Dis Intell. 1999;23:277–305. doi: 10.33321/cdi.1999.23.40. [DOI] [PubMed] [Google Scholar]
- 199.Travassos da Rosa A P A, Turell M, Watts D M, Powers A M, Vasconcelos P F C, Jones J, Klein T A, Dohm D, Shope R E, Degallier N, Popov V L, Russell K L, Weaver S C, Guzman H, Calampa C, Brault A C, Lemon A P, Tesh R B. Trocara virus: A newly recognized Alphavirus (Togaviridae) isolated from mosquitoes in the Amazon basin. Am J Trop Med Hyg, 2001;64:93–97. doi: 10.4269/ajtmh.2001.64.93. [DOI] [PubMed] [Google Scholar]
- 200.Turrell M J. Horizontal and vertical transmission of viruses by insect and tick vectors. In: Monath T P, editor. The arboviruses: epidemiology and ecology. Vol. 1. Boca Raton, Fla: CRC Press; 1988. pp. 127–152. [Google Scholar]
- 201.Vale T G, Dowling M L, Cloonan M J. Infection and isolation of Ross River virus in the mosquito vector Aedes vigilax (Skuse) Aust J Zool. 1992;40:35–41. [Google Scholar]
- 202.Vale T G, McPhie K A, Carter I W, James G S, Cloonan M J. Arbovirus activity along the south coast of New South Wales (1981–1984) Commun Dis Intell. 1985;85:8–9. [Google Scholar]
- 203.Vale T G, Spratt D M, Cloonan M J. Serological evidence of arbovirus infection in native and domesticated mammals on the south coast of New South Wales. Aust J Zool. 1991;39:1–7. [Google Scholar]
- 204.van Buynder P, Sam G, Russell R, Murphy J, Cunningham A, Hueston L, Aaskov J, Coolahan L, Matthews W. Barmah Forest virus epidemic on the south coast of New South Wales. Commun Dis Intell. 1995;19:188–191. [Google Scholar]
- 205.van den Hurk A, Johansen C A, Nisbet D, Zborowsky P, Ritchie S A, Mackenzie J S. Unraveling the enigma of Japanese encephalitis in north Queensland from 1995 to the present. Arbovirus Res Aust. 2000;8:379–386. [Google Scholar]
- 206.Verbrugge L M, Ascione F J. Exploring the iceberg: common symptoms and how people care for them. Med Care. 1987;25:539–569. [PubMed] [Google Scholar]
- 207.Watson E K, Firman D W, Baade P D, Ring I. Telephone administration of the SF-36 health survey: validation studies and population norms for adults in Queensland. Aust N Z J Public Health. 1996;20:359–363. doi: 10.1111/j.1467-842x.1996.tb01046.x. [DOI] [PubMed] [Google Scholar]
- 208.Watson T M. Ecology and behaviour of Aedes notoscriptus (Skuse): Implications for arbovirus transmission in southeast Queensland. Ph.D. thesis. The University of QueenslandBrisbane, Queensland, Australia: Australian Centre for International and Tropical Health and Nutrition; 1998. [Google Scholar]
- 209.Watson T M, Kay B H. Vector competence of Aedes notoscriptus (Diptera: Culicidae) for Ross River virus in Queensland, Australia. J Med Entomol. 1998;35:104–106. doi: 10.1093/jmedent/35.2.104. [DOI] [PubMed] [Google Scholar]
- 210.Weaver S C, Bellew L A, Rico-Hesse R. Phylogenetic analysis of alphaviruses in the Venezuelan equine encephalitis complex and identification of the source of epizootic viruses. Virology. 1992;191:282–290. doi: 10.1016/0042-6822(92)90190-z. [DOI] [PubMed] [Google Scholar]
- 211.Weaver S C, Dalgarno L, Frey T K, Huang H V, Kinney R M, Rice C M, Roehrig J T, Shope R E, Strauss E G. Family Togaviridae. In: van Regenmortel M H V, Fauquet C M, Bishop D H L, Carstens E B, Estes M K, Lemon S M, Maniloff J, Mayo M A, McGeogh D J, Pringle C R, Wickner R B, editors. Virus taxonomy: the seventh report of the International Committee on Taxonomy of Viruses. San Diego, Calif: Academic Press; 2000. pp. 886–887. [Google Scholar]
- 212.Weaver S C, Hagenbaugh A, Bellew L A, Netesov S V, Volchkov V E, Chang G J, Clarke D K, Goussett L, Scott T W, Trent D W, Holland J J. A comparison of the nucleotide sequences of eastern and western equine encephalomyelitis viruses with those of other alphaviruses and related RNA viruses. Virology. 1993;197:375–390. doi: 10.1006/viro.1993.1599. [DOI] [PubMed] [Google Scholar]
- 213.Weaver S C, Rico-Hesse R, Scott T W. Genetic diversity and slow rates of evolution in New World alphaviruses. Curr Top Microbiol Immunol. 1992;176:99–117. doi: 10.1007/978-3-642-77011-1_7. [DOI] [PubMed] [Google Scholar]
- 214.Weber F C, Oppel T W, Raymond R W. A mild exanthematous disease seen in the Schouten Islands. Am J Trop Med. 1946;26:489–492. doi: 10.4269/ajtmh.1946.s1-26.489. [DOI] [PubMed] [Google Scholar]
- 215.Weinstein P, Cameron S, Worswick D, Mcintyre A. Human sentinels for arbovirus surveillance and regional risk classification in South Australia. Med J Aust. 1994;160:494–499. [PubMed] [Google Scholar]
- 216.Wells P J, Russell R C, Cloonan M J. Investigating vector competence of Culex annulirostris and Aedes vigilax for Ross River virus and other alpha- and bunyaviruses. Arbovirus Res Aust. 1993;6:10–14. [Google Scholar]
- 217.Wells P J, Russell R C, Cloonan M J, Hueston L, Geary M J. Virus infection and vector competence of Aedes alternans (Westwood) (Diptera: Culicidae) for Ross River virus. J Aust Entomol Soc. 1994;33:373–375. [Google Scholar]
- 218.Westley-Wise V J, Beard J R, Sladden T J, Dunn T M, Simpson J. Ross River virus infection on the North Coast of New South Wales. Aust N Z J Public Health. 1996;20:87–92. doi: 10.1111/j.1467-842x.1996.tb01343.x. [DOI] [PubMed] [Google Scholar]
- 219.Whelan P I, Weir R P. The isolation of alpha and flaviviruses from mosquitoes in the Northern Territory, 1982–1992. Arbovirus Res Aust. 1992;6:270–277. [Google Scholar]
- 220.Whitehead R H. Experimental infection of vertebrates with Ross River and Sindbis viruses, two group A arboviruses isolated in Australia. Aust J Exp Biol Med Sci. 1969;47:11–15. doi: 10.1038/icb.1969.2. [DOI] [PubMed] [Google Scholar]
- 221.Whitehead R H, Doherty R L, Domrow R, Standfast H A, Wetters E J. Studies of the epidemiology of arthropod-borne virus infections at Mitchell River mission, Cape York Peninsula, North Queensland. III. Virus studies of wild birds, 1964–1967. Trans R Soc Trop Med Hyg. 1968;62:439–445. doi: 10.1016/0035-9203(68)90096-5. [DOI] [PubMed] [Google Scholar]
- 222.Wilson J G. The Murray Valley rash. Med J Aust. 1957;2:120–122. doi: 10.5694/j.1326-5377.1957.tb57734.x. [DOI] [PubMed] [Google Scholar]
- 223.Wolfe F. Practical issues in psychosocial measures. J Rheumatol. 1997;24:990–993. [PubMed] [Google Scholar]
- 224.Woodroofe G M. Isolation and identification of arboviruses from arthropods and vertebrates: techniques, problems and roles in surveillance. Arbovirus Res Aust. 1976;1:67–76. [Google Scholar]
- 225.Woolf A D. Viral infections and chronic arthritis. In: Calabro J J, Dick W C, editors. Infections and arthritis. Dordrecht, The Netherlands: Kluwer Academic; 1989. pp. 53–83. [Google Scholar]
- 226.World Health Organisation. Arboviruses and human disease. Geneva, Switzerland: World Health Organisation; 1967. [Google Scholar]
- 227.World Health Organisation. Ross River virus infection. World Health Org Wkly Epidemiol Rec. 1994;69:98–99. [PubMed] [Google Scholar]
- 228.Wright A E, Anderson S, Stanley N F, Liehne P F S, Britten D K. A preliminary investigation of the ecology of arboviruses in the Derby area of the Kimberley region, Western Australia. Aust J Exp Biol Med Sci. 1981;59:357–367. doi: 10.1038/icb.1981.30. [DOI] [PubMed] [Google Scholar]
- 229.Yu S, Aaskov J G. Development of a candidate vaccine against Ross River virus infection. Vaccine. 1994;12:1118–1124. doi: 10.1016/0264-410x(94)90182-1. [DOI] [PubMed] [Google Scholar]