KEY POINTS
Prasugrel and ticagrelor are P2Y12 inhibitors that are more effective than clopidogrel at preventing ischemic events in patients presenting with acute coronary syndrome, although they cause more bleeding.
Dual antiplatelet therapy (acetylsalicylic acid and a P2Y12 inhibitor) is indicated for patients with acute coronary syndrome, usually for at least 1 year.
The type and duration of dual antiplatelet therapy is determined by a patient’s risk of recurrent ischemia and major bleeding.
Most patients with atrial fibrillation and acute coronary syndrome should be treated with a direct oral anticoagulant and clopidogrel for 12 months; acetylsalicylic acid should be added for up to 1 month after percutaneous coronary intervention.
After an acute coronary syndrome, as many as 1 in 5 patients will have a second ischemic event within 5 years.1,2 Residual risk is related to several factors that may be mitigated by pharmacologic and nonpharmacologic interventions.2 Antiplatelet therapy is a cornerstone in the management of acute coronary syndrome.1,3 Acetylsalicylic acid (ASA) — a cyclooxygenase-1 inhibitor — was introduced as an effective treatment for myocardial infarction almost 5 decades ago and remains the most widely used antiplatelet therapy.4,5 Although ASA is effective in reducing mortality rates,6 combining ASA with a second antiplatelet agent, a P2Y12 receptor inhibitor (known as dual antiplatelet therapy [DAPT]) provides additional benefit and is now the preferred initial strategy for acute coronary syndromes over ASA alone.7
We review emerging evidence regarding the use of antiplatelet therapy in acute coronary syndromes, as well as updates to the Canadian and European Society of Cardiology guidelines that highlight adjustments in the choice and duration of antiplatelet therapy, in addition to ASA (Box 1). We particularly focus on strategies to reduce bleeding risk after percutaneous coronary intervention (PCI).3,8
Box 1:
Literature search
We conducted a targeted, nonsystematic MEDLINE search from its inception until August 2021 using the terms “antiplatelet,” “clopidogrel,” “prasugrel,” “ticagrelor,” “acute coronary syndrome,” “myocardial infarction” or “bleeding.” We limited the search to articles in English and focused on randomized clinical trials or systematic reviews, although we did not apply any restriction on study type.
What are the options for oral antiplatelet therapy?
Clopidogrel is a second-generation thienopyridine that has a better safety profile than ticlopidine, a first-generation thienopyridine.9 Clopidogrel reduces ischemic events by almost 20% when added to ASA for patients presenting with acute coronary syndromes, with or without ST-segment elevation.7,10,11 It irreversibly antagonizes the receptor for platelet adenosine diphosphate (ADP)-P2Y12. The use of clopidogrel may be associated with gastrointestinal symptoms and skin rashes.
Prasugrel and ticagrelor are more potent P2Y12 inhibitors than clopidogrel. Prasugrel is a third-generation thienopyridine that exerts its antiplatelet properties by irreversibly antagonizing the ADP-P2Y12 receptor; similar to clopidogrel, it requires hepatic conversion to its active metabolites. Ticagrelor is part of the cyclopentyltriazolopyrimidine family and does not require hepatic conversion to its active metabolites before reversibly inhibiting the ADP-P2Y12 receptor. Ticagrelor may cause shortness of breath or increased levels of uric acid, which leads to gout.
Both prasugrel and ticagrelor provide faster and more consistent inhibition of platelet aggregation and are associated with a further 15%–20% relative risk reduction of ischemic events compared with clopidogrel.12,13 Ticagrelor also reduces cardiovascular and all-cause mortality rates.12 Although ticagrelor and prasugrel are associated with greater bleeding risk than clopidogrel, both are recommended over clopidogrel in patients with low bleeding risk. Recently, the ISAR-REACT-5 (Intracoronary Stenting and Antithrombotic Regimen 5) study reported fewer ischemic events associated with prasugrel, with no difference in the incidence of major bleeding, when compared with ticagrelor.14 However, a number of uncertainties preclude definitively recommending one drug over the other.15 Prasugrel is not recommended in patients older than 75 years of age and in those with a body weight less than 60 kg because of an increased risk of fatal and intracranial bleeding.13 A recent meta-analysis summarized the relative differences in ischemic and bleeding risks among the 3 different P2Y12 inhibitors (Table 1).16
Table 1:
| Drugs compared | No. of studies (no. of patients) | Ischemic risk | Bleeding risk |
|---|---|---|---|
| Ticagrelor v. clopidogrel | 6 RCTs (21 828)† | Ticagrelor associated with 18% reduction in cardiovascular mortality, 28% reduction in stent thrombosis and no difference in MI | Ticagrelor associated with 27% increase in major bleeding |
| Prasugrel v. clopidogrel | 4 RCTs (25 740) | Prasugrel associated with 10% reduction in cardiovascular mortality (95% CI 0.80–1.01), 50% reduction in stent thrombosis and 19% reduction in MI | Prasugrel associated with 26% increase in major bleeding |
| Prasugrel v. ticagrelor | 2 RCTs (5248) | Prasugrel associated with 32% reduction in stent thrombosis and no difference in cardiovascular mortality or MI | No difference in major bleeding |
Note: CI = confidence interval, RCT = randomized controlled trial, MI = myocardial infarction.
The reported ischemic and bleeding risks were all statistically significant except for the reduction of cardiovascular mortality of prasugrel compared with clopidogrel.
PLATO trial provided 85% of patients to total number.
Clopidogrel is currently recommended for patients with acute coronary syndromes who are at high bleeding risk or those who cannot take a potent P2Y12 inhibitor because of adverse effects or cost.3,8 Guidelines recommend treatment with clopidogrel as the initial antiplatelet drug for patients with ST-segment elevation myocardial infarction who are treated with fibrinolytic therapy. However, results of the TREAT (Ticagrelor in Patients With ST-Elevation Myocardial Infarction Treated With Pharmacological Thrombolysis) study indicated that switching from clopidogrel to ticagrelor within 24 hours did not lead to an increase in major bleeding in the first 30 days postlysis, compared with continuing clopidogrel.17
Current guidelines recommend the use of DAPT after an acute coronary syndrome, irrespective of the revascularization strategy, including for medically managed patients and those who undergo coronary artery bypass grafts.3,18 A subgroup analysis of 7985 patients from the CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Events) trial who did not undergo revascularization after randomization showed that adding clopidogrel to ASA reduced ischemic events by an absolute 1.9% after 12 months of follow-up, compared with placebo and ASA.7 Ticagrelor provides consistent benefit over clopidogrel in reducing ischemic events, irrespective of revascularization strategy.12,19
For patients with non-ST segment elevation acute coronary syndrome, (NSTACS) the timing of coronary angiography is a factor in the choice of antiplatelet treatment. For patients scheduled for coronary angiography within 24 hours of presentation, there is debate regarding whether patients should be preloaded with a potent P2Y12 inhibitor. Preloading with prasugrel or ticagrelor did not reduce ischemic events in patients with NSTACS scheduled for early coronary angiography.20,21 Thus, the European guidelines discourage routine preloading of P2Y12 inhibitors in patients with NSTACS who are planned for an early coronary angiogram and suggest starting DAPT once the need for angioplasty or stents is confirmed.3 This does not apply for patients presenting with ST-segment elevation myocardial infarction in whom preloading with DAPT is recommended. Preloading should also be considered in non-PCI centres because coronary angiography may be delayed. Further, given that, in Canada, most patients with NSTACS do not routinely undergo angiography within the first 24 hours of presentation, preloading with DAPT in patients with moderate-to-high risk of ischemia is reasonable.8 If there is suspicion of left main disease or possible aortic dissection, DAPT should not be given.3
How long should dual antiplatelet therapy be continued?
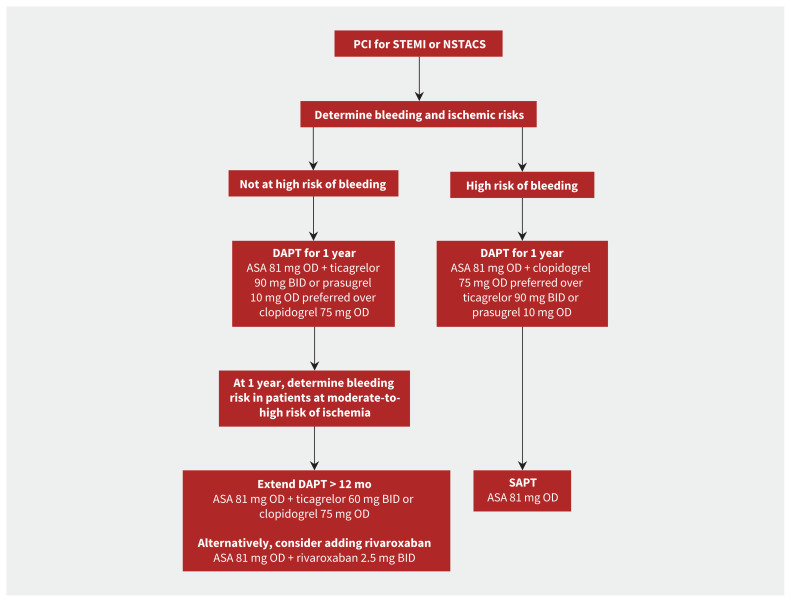
Current guidelines recommend DAPT for 1 year after an acute coronary syndrome, particularly in patients with heightened ischemic risk (Box 2 and Figure 1).3,8 However, patients at high risk of bleeding may be considered for shorter treatment duration. Two large randomized controlled trials evaluated extending the duration of DAPT beyond 12 months, using ticagrelor in patients with a history of myocardial infarction in the PEGASUS-TIMI 54 (Prevention with Ticagrelor of Secondary Thrombotic Events in High-Risk Patients with Prior Acute Coronary Syndrome – Thrombolysis In Myocardial Infarction 54) trial, and using clopidogrel or prasugrel in stable patients and patients with acute coronary syndromes who received a PCI in the Dual Antiplatelet Therapy trial.22,23 Both trials reported significant relative reductions in ischemic events of 15%–30%, but these benefits were offset by a significant increase in major bleeding.22,23 A recent meta-analysis confirmed these findings; DAPT extension beyond 12 months was associated with a 32% reduction in myocardial infarction and a 63% increase in major bleeding among patients who received a PCI.24
Box 2:
Features of patients at high risk of ischemic events
Previous stent thrombosis on adequate antiplatelet therapy
Stenting of the last remaining patent coronary artery
Diffuse multivessel disease, especially in patients with diabetes
Chronic kidney disease (i.e., creatinine clearance < 60 mL/min)
At least 3 stents implanted
At least 3 lesions treated
Bifurcation with 2 stents implanted
Total stented length greater than 60 mm
Treatment of a chronic total occlusion
History of ST-segment elevation myocardial infarction
Figure 1:
Antiplatelet recommendations in patients with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention (PCI). Note: ASA = acetylsalicylic acid, BID = twice daily, DAPT = dual antiplatelet therapy, NSTACS = non-ST segment elevation acute coronary syndrome, OD = once daily, SAPT = single antiplatelet therapy, STEMI = ST-segment elevation myocardial infarction.
Improvements in stent design and increased recognition of the importance of preventing bleeding led researchers to evaluate shorter durations of DAPT; the findings of noninferiority studies of stable patients and patients with acute coronary syndrome after PCI have supported this approach.25 Meta-analyses found comparable incidences of stent thrombosis and adverse ischemic events; however, myocardial infarction was more frequent in the shortened DAPT group.25 Recently, the MASTER-DAPT (Management of High Bleeding Risk Patients Post Bioresorbable Polymer Coated Stent Implantation With an Abbreviated Versus Prolonged DAPT Regimen) study randomized 4434 patients at high risk of bleeding who were free of cardiovascular events at 1 month after PCI to receive 1 month of DAPT or standard DAPT of at least 3 months after PCI.26 The measure of adverse clinical events (defined as a composite of death from any cause, myocardial infarction, stroke or major bleeding) was comparable between the 2 groups.26 Importantly, the incidence of major bleeding or clinically relevant, nonmajor bleeding was significantly lower in the shorter treatment group (6.5% v. 9.4%, p < 0.001).26
The use of a single antiplatelet agent was recently tested in an open-label, multicentre randomized study of 5438 patients who had completed 6–18 months of DAPT without any clinical events, compared with ASA alone. Over a mean follow-up of 24 months, clopidogrel reduced the composite outcome of all-cause death, myocardial infarction, stroke, readmission because of an acute coronary syndrome and major bleeding events (5.7% v. 7.7%; hazard ratio [HR] 0.73, 95% confidence interval [CI] 0.59–0.90).27 Both ischemic (3.7% v. 5.5%; HR 0.68, 95% CI 0.52–0.87) and any bleeding events (2.3% v. 3.3%; HR 0.70, 95% CI 0.51–0.98) were reduced with the use of clopidogrel.
Other strategies for extended secondary prevention include the use of a low dose of oral anticoagulant in combination with ASA. This has been used in the COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial for patients with stable atherosclerotic cardiovascular disease, including previous myocardial infarction, cerebrovascular and peripheral artery disease. 28 Compared with ASA alone, rivaroxaban (2.5 mg twice daily) and ASA reduced ischemic events by an absolute 1.3%, but was associated with more major bleeding (3.1% v. 1.9%).28
How is bleeding risk assessed and managed?
Models have been developed to quantify bleeding risk;1,29 they include clinical and biomarker variables and have moderate-to-good accuracy (Table 2). The DAPT, PRECISE-DAPT and PRAISE models are accessible online. In a meta-analysis of 88 563 patients, the DAPT score consistently identified patients at high risk of bleeding and ischemia in different cohorts of patients.39 Similarly, the PRECISE-DAPT model effectively identified patients who were not suitable for extended DAPT and were likely to be at risk of bleeding without a decrease in ischemic events.35 The PRECISE-DAPT model was also validated in cohorts of patients with acute coronary syndromes who underwent PCI and were treated with potent P2Y12 inhibitors, and showed moderate accuracy in predicting future bleeding risk.40
Table 2:
Models to estimate bleeding risk
| Model | Derived population | Score variables | Score description | Limitations |
|---|---|---|---|---|
| CRUSADE30 | 71 277 community-treated patients with NSTEMI | Hematocrit, creatinine clearance, baseline heart rate, baseline systolic blood pressure, female sex, signs of CHF on presentation, previous vascular disease and diabetes mellitus | Each independent variable was assigned weighted integers according to its coefficient value in the regression model. The sum of the weighted integers (range 1 to 100 points) estimates the risk of in-hospital major bleeding, with a curvilinear relation between CRUSADE bleeding score and predicted probabilities of major bleeding | Patients who died within 48 hours were excluded and early bleeding events may be underestimated. Patients on oral anticoagulation were excluded; similarly those with previous bleeding events or bleeding disorders were not included. CRUSADE is designed to predict in-hospital bleeding events |
| ACUITY31 | 17 421 patients with ACS (UA, NSTEMI and STEMI) | Age, female sex, serum creatinine, white blood cell count, anemia, NSTEMI, STEMI and the use of heparin plus glycoprotein IIb/IIIa inhibitor (rather than bivalirudin alone) | Each independent variable was assigned weighted integers according to its coefficient value in the regression model. The sum of the weighted integers (range 1 to 52 points) estimates the risk of 30-day non-CABG major bleeding, with curvilinear relation between ACUITY bleeding score and predicted probabilities of bleeding | Posthoc analysis of patients included in 2 RCTs. Potential variables of interest were not available to be incorporated in the model. Potent P2Y12 inhibitors were not studied |
| REACH32 | 64 589 at risk of CAD or with stable CAD | Age, peripheral arterial disease, CHF, diabetes, hypertension, smoking, antiplatelets, oral anticoagulants, hypercholesterolemia | Each factor was assigned a single point, except for CHF, hypertension, smoking and non-ASA antiplatelet therapy, which were assigned 2 points. Oral anticoagulation or DAPT were assigned 4 points. A score > 10 was associated with 6-fold increase in risk of serious bleeding over 2 years | The definition of serious bleeding used for the analyses was either a hemorrhagic stroke or bleeding leading to both hospitalization and transfusion. This may underestimate the rate of major bleeding events. Data regarding potent P2Y12 inhibitors were limited. The exposure to oral anticoagulation was extrapolated and did not account for potential changes over study follow-up |
| DAPT33* | 11 648 patients who tolerated DAPT for 1 year without ischemic or bleeding events | Age, cigarette smoking, diabetes mellitus, MI at presentation, previous PCI or previous MI, paclitaxel-eluting stent, stent diameter < 3 mm, CHF or LVEF < 30%, and vein graft stent | Each variable was assigned a single point except for age (65 to < 75 yr and ≥ 75 yr, for which patients were assigned −1 or −2, respectively). Those with CHF, LVEF or vein graft stent were assigned 2 points. Total scores ranged from −2 to 10, and those with scores ≥ 2 were considered high risk and extended DAPT was recommended. Patients with low scores (< 2) were considered low risk and extended DAPT was not recommended | DAPT score showed moderate accuracy in the derivation and validation cohort. It is designed to inform the duration of DAPT rather than predicting future bleeding events |
| PARIS34 | 4190 patients treated with DES (almost 60% had stable presentation) | Age, body mass index, triple therapy at discharge, anemia, current smoking and renal dysfunction | An integer-based risk score was developed for major bleeding (and ischemic events) at 2 years by assigning each variable a score of 2, except for anemia (score of 3) and age (higher score proportional to older patients). The score ranges from 0 to14 and ≥ 8 is considered high bleeding risk | Most patients were treated with clopidogrel, which limits generalizability to potent P2Y12 inhibitors. Duration of DAPT was not randomized and decision to stop antiplatelet was according to the clinician’s discretion |
| PRECISE-DAPT35* | 14 963 patients treated with DAPT after PCI were pooled from 8 RCTs with independent adjudication of events | Age, creatinine clearance, hemoglobin, white blood cell count and previous spontaneous bleeding. | Independent predictors of bleeding events that were identified in the multivariate regression model were assigned points based on the magnitude of association of each predictor with bleeding. A score ≥ 25 is considered HBR, and extended DAPT has been associated with increased bleeding in this group, unlike patients with low scores. PRECISE-DAPT showed improved integrated discrimination and reclassification performance compared with the PARIS score | The accuracy of the model in the validation cohort ranged from moderate to good. Frailty was not included as part of the risk model. Most patients were treated with clopidogrel and those on oral anticoagulation were excluded. Prediction of bleeding events in patients on prasugrel was poor |
| ARC-HBR trade-off model36,37 | 6641 patients who underwent PCI and were identified as HBR were pooled from 6 studies | Age, hemoglobin, renal dysfunction, liver disease, cancer, planned major surgery, COPD, current smoker, complex PCI procedure, oral anticoagulation at discharge | Variables were classified as a major or minor criterion for HBR. Major criterion is considered to confer risk of major bleeding of ≥ 4% or ≥ 1% intracranial hemorrhage at 1 year. This score outperformed PARIS and PRECISE-DAPT (alternative model without white blood cell count) | The criteria used were modified from those proposed in the ARC consensus to allow the use of available data. Infrequent, but wellrecognized, predictors were pooled as a single variable in the model (i.e., liver disease, cancer and planned surgery) |
| PRAISE38* | 19 826 patients presenting with ACS from 2 registries | Age, sex, diabetes, hypertension, hyperlipidemia, PAD, eGFR, previous MI, previous PCI, previous CABG, previous stroke, previous bleeding, malignancy, STEMI, LVEF, multivessel disease, complete revascularization, vascular access, DES and treatment with BBs, ACE inhibitors, ARBs, statins, oral anticoagulation and PPIs | Four machine learning models that used different classifiers were developed to predict occurrence of all-cause death, recurrent MI and major bleeding 1 year after discharge. Each model’s performance was assessed using a range of learning metrics and the best performing model was selected. When calculated, PRAISE provides 3 outcomes of the calculated score for death, MI, and major bleeding. | It was not possible to fully compare PRAISE with PARIS or PRECISE-DAPT given the insufficient clinical data. Although PRAISE has been prospectively validated in external cohorts, it has not been used in RCTs to aid decision-making of DAPT duration |
Note: ACE = angiotensin-converting enzyme, ACS = acute coronary syndrome, ACUITY = Acute Catheterization and Urgent Intervention Triage Strategy, ARB = angiotensin-receptor blocker, ARC = Academic Research Consortium, ASA = acetylsalicylic acid, BB = beta-blocker, CABG = coronary artery bypass graft, CAD = coronary artery disease, CHF = congestive heart failure, COPD = chronic obstructive pulmonary disease, CRUSADE = Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the American College of Cardiology/American Heart Association Guidelines, DAPT = dual antiplatelet therapy, DES = drug-eluting stent, HBR = high bleeding risk, eGFR = estimated glomerular filtration rate, LVEF = left ventricle ejection fraction, MI = myocardial infarction, NSTEMI = non-ST segment elevation myocardial infarction, PAD = periphral artery disease, PARIS = Patterns of Nonadherence to Antiplatelet Regimen In Stented Patients, PCI = percutaneous coronary intervention, PPI = proton pump inhibitor, PRAISE = Predicting with Artificial Intelligence Risk after Acute Coronary Syndromes, PRECISE-DAPT = Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy, RCT = randomized controlled trial, REACH = Reduction of Atherothrombosis for Continued Health Registry, STEMI = ST-segment elevation myocardial infarction, UA = unstable angina.
DAPT score is available at https://tools.acc.org/daptriskapp/#!/content/calculator/, PRECISE-DAPT is available at http://precisedaptscore.com/predapt/ and PRAISE is available at https://praise.hpc4ai.it/.
The PRAISE model used machine learning to predict bleeding, ischemic risk and all-cause deaths.38 The model was derived from 2 cohorts of patients with acute coronary syndromes who were treated with clopidogrel or more potent P2Y12 inhibitors. It accurately predicted major bleeding, as well as acute MI and all-cause mortality. Importantly, machine learning risk-scoring models need to be tested in randomized controlled trials to assess their impact on clinical outcomes.
Strategies to reduce bleeding risk at the time of PCI include the use of radial access (preferable to accessing a more central artery), using fluoroscopy or ultrasonography guidance to access the common femoral artery41 and selecting the right stent platform that is indicated for patients with high bleeding risk or is considered safe for early discontinuation of DAPT.26,42 Bleeding risk can also be reduced with the use of clopidogrel rather than prasugrel or ticagrelor in patients who are at high risk.41 After hospital discharge, several strategies should be used to reduce bleeding risk (Box 3).
Box 3:
Strategies to reduce bleeding risk following hospital discharge
Shorter duration of dual antiplatelet therapy
Use of clopidogrel rather than ticagrelor or prasugrel
Avoidance of nonsteroidal anti-inflammatory drugs
Optimal blood pressure management
Abstinence from alcohol
Use of mobility aids, when appropriate
Use of proton pump inhibitor for all patients after percutaneous coronary intervention, as recommended by current guidelines.18
Screening and eradication of Helicobacter pylori (a large randomized controlled trial is underway to assess this strategy in patients with acute myocardial infarction)43
Correction of anemia to reduce the impact of bleeding
The management of acute bleeding events is discussed elsewhere. 44 Current guidelines categorize patients according to the type of bleeding event and recommend management according to severity (Table 3).18 Reversal agents are currently available for some oral anticoagulants, and antidotes to P2Y12 inhibitors are being developed.45 A ticagrelor reversal agent, bentracimab (PB2452), is a human monoclonal antibody that provides immediate and sustained reversal of the antiplatelet effects of ticagrelor in healthy volunteers.45 It is currently being studied in the REVERSE-IT (Rapid and Sustained Reversal of Ticagrelor–Intervention Trial) trial (NCT04286438). Management of bleeding events in patients on DAPT can be challenging and involvement of specialists should be considered (Box 4).
Table 3:
Management of bleeding events in patients with acute coronary syndrome receiving antithrombotic therapy
| Bleeding event | Event description | Antithrombotic treatment modification | |
|---|---|---|---|
| Original treatment | Modification | ||
| Trivial bleeding | A bleeding event not requiring medical attention or further evaluation (e.g., skin bruising, self-resolving epistaxis, minimal conjunctival bleeding) | DAPT |
|
| Concomitant OAC* |
|
||
| Mild bleeding | A bleeding event requiring medical attention without need for hospital admission (e.g., major epistaxis, moderate conjunctival bleeding, genitourinary or gastrointestinal bleeding without substantial blood loss, mild hemoptysis) | DAPT |
|
| Concomitant OAC* |
|
||
| Moderate bleeding | A bleeding event requiring hospital admission or associated with substantial blood loss (≥ 3 mmol/L hemoglobin) without hemodynamic instability (e.g., genitourinary, respiratory, upper or lower gastrointestinal bleeding with substantial blood loss or requiring transfusion) | DAPT |
|
| Concomitant OAC* |
|
||
| Severe bleeding | A bleeding event associated with severe blood loss (≥ 5 mmol/L hemoglobin) in a hemodynamically unstable patient requiring hospital admission (e.g., severe genitourinary, respiratory or gastrointestinal bleeding, bleeding into critical spaces such as pericardium, retroperitoneum, intraocular spinal or intracranial spaces) | DAPT |
|
| Concomitant OAC* |
|
||
| Life-threatening bleeding | Any severe active bleeding that poses a threat to a patient’s life (e.g., massive genitourinary, respiratory or gastrointestinal bleeding, active intracranial, spinal or intraocular hemorrhage, any bleeding causing hemodynamic instability) | DAPT |
|
| Concomitant OAC* |
|
||
Note: 4F-PCC = 4-factor prothrombin complex concentrate, ACS = acute coronary syndrome, CHA2DS2-VASc = score that evaluates risk of ischemic stroke, DAPT = dual antiplatelet therapy, DOAC = direct oral anticoagulant, FFP = fresh frozen plasma, GI = gastrointestinal, INR = international normalized ratio, OAC = oral anticoagulant, SAPT = single antiplatelet therapy, TT = triple therapy, VKA = vitamin K antagonist.
Concomitant OAC with antiplatelet therapy, including both SAPT and DAPT.
Box 4:
Indications for referral to cardiologist and other specialist regarding antiplatelet treatment
Bleeding events in patients within 1 year of acute coronary syndrome; advice from a cardiologist about resumption or discontinuation of a second antiplatelet agent should be sought early, and consultation with a gastroenterologist should also be considered, if indicated
New onset atrial fibrillation
Patients with planned noncardiac surgery (particularly major surgeries)
Patients with chronic bleeding diatheses, such as hemophilia or severe liver disease; consultation with a hematologist should be considered
Patients who develop thrombocytopenia; consultation with a hematologist should be considered
Patients who develop a cerebrovascular event; consultation with a neurologist should be considered
Can treatment with acetylsalicylic acid be stopped early?
Early stopping of ASA while maintaining P2Y12 inhibition has recently been tested, based on experimental data that suggested the synergistic effect of inhibiting cyclooxygenase-1 with ASA and P2Y12 inhibitors is less relevant in the presence of potent P2Y12 inhibitors.46 A recent meta-analysis of data from 16 898 patients with acute coronary syndromes showed that P2Y12 inhibitor monotherapy after 1–3 months of DAPT reduced bleeding events by 50% with no significant increase in ischemic events, compared with 12 months of DAPT.24,47 A recent meta-analysis included individual data from 24 096 patients enrolled in 6 randomized trials and highlighted the superiority of P2Y12 inhibitor monotherapy over DAPT in reducing ischemic events in women, and the reduction of bleeding events when a potent P2Y12 inhibitor was part of the DAPT regime.48 Nonetheless, P2Y12 inhibitor monotherapy is still not widely used in the management of patients after PCI.
What are the indications for dual antiplatelet therapy in patients with atrial fibrillation?
Dual antiplatelet therapy does not prevent stroke and systemic thromboembolism in patients with atrial fibrillation as effectively as oral anticoagulation.49 Conversely, an oral anticoagulant does not reduce coronary ischemic events, including stent thrombosis, as effectively as DAPT. Thus, triple therapy combining DAPT and OAC is recommended in patients with acute coronary syndromes, either medically managed or after PCI, who also have atrial fibrillation. Direct oral anticoagulants are associated with a lower rate of bleeding events than vitamin K antagonists, and are therefore preferred in patients with atrial fibrillation,50 except in those with mechanical heart valves, moderate-to-severe mitral stenosis or advanced renal disease. Clopidogrel is the P2Y12 of choice in patients receiving triple therapy, rather than ticagrelor or prasugrel, because of the lower risk of bleeding.3
The duration of triple therapy remains a matter of debate, given the increased risk of major bleeding over time. The AUGUSTUS (Open-label, 2 × 2 Factorial, Randomized Controlled, Clinical Trial to Evaluate the Safety of Apixaban vs Vitamin K Antagonist and Aspirin vs. Aspirin Placebo in Patients With Atrial Fibrillation and Acute Coronary Syndrome or Percutaneous Coronary Intervention) trial showed that, beyond the first 30 days (the period of highest risk for stent thrombosis), ASA and oral anticoagulation increased bleeding events without significantly reducing ischemic events when compared with placebo and oral anticoagulation in patients receiving P2Y12 inhibitors.51,52
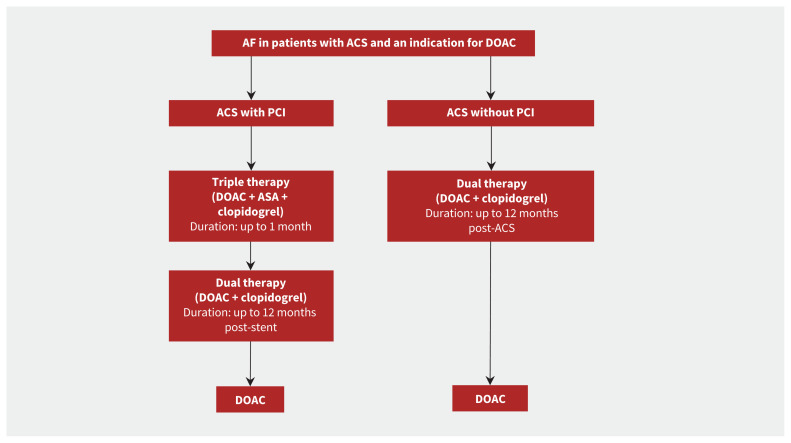
Numerous studies, including a recent meta-analysis, found that combining oral anticoagulation and single antiplatelet therapy reduced bleeding risk compared with triple therapy.53 Importantly, these studies were not powered to detect potential differences in ischemic events, and the meta-analysis found an increased risk of stent thrombosis associated with the combination therapy.53 A small study of consecutive patients suggested that the ischemic risk was greater in patients who underwent complex PCI procedures without the use of ASA immediately after a PCI procedure.54 Therefore, triple therapy should be considered in patients at high risk of ischemia and of stent thrombosis and low risk of bleeding for up to 1 month after PCI (Figure 2). After 1 month, ASA should be stopped, and oral anticoagulation and a P2Y12 inhibitor (preferably clopidogrel, given its lower bleeding risk) should be continued up to 12 months in patients after acute coronary syndrome. At 1 year, oral anticoagulation monotherapy should be used for secondary prevention of stroke.55
Figure 2:
Antiplatelet management in patients with acute coronary syndrome (ACS) and atrial fibrillation (AF). Direct oral anticoagulation (DOAC) is preferred over warfarin; however, if warfarin is to be used the recommended international normalized ratio target is 2.0–2.5. The timing of when to discontinue acetylsalicyclic acid (ASA) will depend on the individual patient’s ischemic and bleeding risk. Note: PCI = percutaneous coronary intervention.
How should antiplatelet agents be switched?
De-escalation from a more potent P2Y12 inhibitor to clopidogrel occurs in up to 28% of patients with acute coronary syndromes, most often because of bleeding or high risk of bleeding.56 Similarly, switching between potent P2Y12 inhibitors may be required if specific adverse effects such as shortness of breath or gout develop in patients receiving ticagrelor. An international consensus document and Canadian guidelines provide guidance to physicians when switching between P2Y12 inhibitors (Box 5).30,57
Box 5:
Switching between oral P2Y12 inhibitors
Ticagrelor to clopidogrel
Ticagrelor has a relatively fast offset of action. Clopidogrel should be administered 24 hours after the last dose of ticagrelor. A 600 mg loading dose of clopidogrel should be considered unless the patient had a recent bleeding event, in which case clopidogrel 75 mg should be considered.
Prasugrel to clopidogrel
The prolonged offset of prasugrel means that the usual clopidogrel maintenance dose of 75 mg daily should be started 24 hours after the last dose of prasugrel.
Ticagrelor to prasugrel
A 60 mg loading dose of prasugrel should be administered 24 hours after the last dose of ticagrelor.
Prasugrel to ticagrelor
A 90 mg maintenance dose of ticagrelor should be administered twice daily 24 hours after the last prasugrel dose. If it has been fewer than 30 days since the patient’s PCI, a loading dose of 180 mg ticagrelor should be considered.
Guided de-escalation therapy by either platelet function testing or CYP2C19-directed genotyping may also be considered in select patients with acute coronary syndromes.58
Conclusion
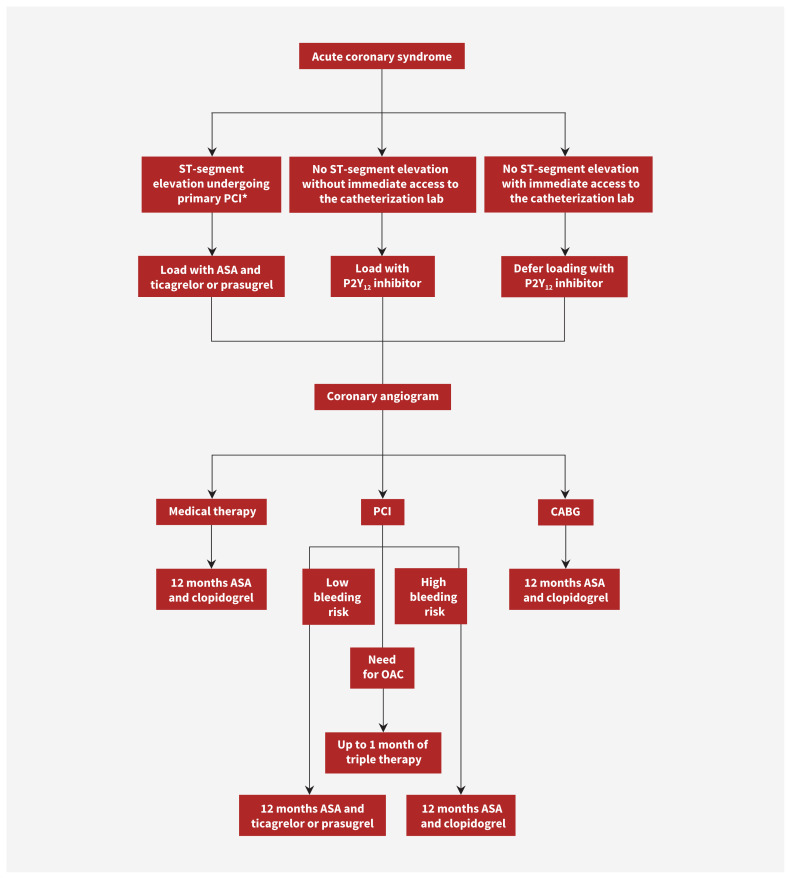
Prasugrel and ticagrelor are antiplatelet agents that are more effective than clopidogrel at decreasing the future risk of ischemic events in patients with acute coronary syndromes, but are more likely to cause bleeding. The choice of antiplatelet regimen is influenced by the ischemic and bleeding risk of each patient (Figure 3). Up to a month of triple therapy with ASA, clopidogrel and an oral anticoagulant should be considered in patients with acute coronary syndromes who also have atrial fibrillation.
Figure 3:
Flowchart for antiplatelet management in patients with acute coronary syndrome. Note: ASA = acetylsalicylic acid, CABG = coronary artery bypass grafting, OAC = oral anticoagulant, PCI = percutaneous coronary intervention. *Patients receiving fibrinolytic therapy should be loaded with ASA and clopidogrel. Switching to ticagrelor within 24 hours should be considered.
Footnotes
Competing interests: Alan Bell reports consulting fees from AstraZeneca, Bayer and Sanofi; speaker fees from AstraZeneca; and board membership with Thrombosis Canada, Hypertension Canada and the Canadian Cardiovascular Society. Sol Stern reports honoraria from Bayer, Pfizer, Bristol Myers Squibb and Sea Courses Inc. Shaun Goodman reports research grant support from Amgen, Anthos Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL Behring, Eli Lilly, Esperion, Ferring Pharmaceuticals, Merck, Novartis, Pfizer, Regeneron, Sanofi, Heart and Stroke Foundation of Ontario, Canadian Heart Research Centre and MD Primer, Canadian VIGOUR Centre, Cleveland Clinic Coordinating Center for Clinical Research, Duke Clinical Research Institute, New York University Clinical Coordinating Center, PERFUSE Research Institute and TIMI Study Group (Brigham Health). He also reports consulting honoraria from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL Behring, Eli Lilly, Ferring Pharmaceuticals, HLS Therapeutics, JAMP Pharma, Merck, Novartis, PendoPharm of Pharmascience, Pfizer, Regeneron, Sanofi, Servier, Valeo Pharma, Canadian Heart Research Centre and MD Primer, and speaking fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Ferring Pharmaceuticals, HLS Therapeutics, JAMP Pharma, Novartis, PendoPharm of Pharmascience, Pfizer, Regeneron, Sanofi, Servier, Valeo Pharm, Canadian Heart Research Centre and MD Primer. He sits on boards with American Regent of Daiichi-Sankyo and Novo Nordisk and is co-director of the Canadian VIGOUR Centre. All competing interests are outside the submitted work. No other competing interests were declared.
This article has been peer reviewed.
Contributors: All of the authors contributed to the conception and design of the work, drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
References
- 1.Alkhalil M. Mechanistic insights to target atherosclerosis residual risk. Curr Probl Cardiol 2021;46:100432. [DOI] [PubMed] [Google Scholar]
- 2.Ibanez B, James S, Agewall S, et al. ESC Scientific Document Group. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–77. [DOI] [PubMed] [Google Scholar]
- 3.Collet J-P, Thiele H, Barbato E, et al. ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42: 1289–367. [DOI] [PubMed] [Google Scholar]
- 4.Lewis HD, Jr, Davis JW, Archibald DG, et al. Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a Veterans Administration Cooperative Study. N Engl J Med 1983;309:396–403. [DOI] [PubMed] [Google Scholar]
- 5.Kuliczkowski W, Witkowski A, Polonski L, et al. Interindividual variability in the response to oral antiplatelet drugs: a position paper of the Working Group on antiplatelet drugs resistance appointed by the Section of Cardiovascular Interventions of the Polish Cardiac Society, endorsed by the Working Group on Thrombosis of the European Society of Cardiology. Eur Heart J 2009;30:426–35. [DOI] [PubMed] [Google Scholar]
- 6.Randomised trial of intravenous streptokinase, oral aspirin, both or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet 1988;2:349–60. [PubMed] [Google Scholar]
- 7.Yusuf S, Zhao F, Mehta SR, et al. Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494–502. [DOI] [PubMed] [Google Scholar]
- 8.Marquis-Gravel G, Mehta SR, Valgimigli M, et al. A critical comparison of Canadian and international guidelines recommendations for antiplatelet therapy in coronary artery disease. Can J Cardiol 2020;36:1298–307. [DOI] [PubMed] [Google Scholar]
- 9.Bertrand ME, Rupprecht HJ, Urban P, et al. CLASSIC Investigators. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: the clopidogrel aspirin stent international cooperative study (CLASSICS). Circulation 2000;102:624–9. [DOI] [PubMed] [Google Scholar]
- 10.Chen ZM, Jiang LX, Chen YP, et al. COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005;366:1607–21. [DOI] [PubMed] [Google Scholar]
- 11.Sabatine MS, Cannon CP, Gibson CM, et al. CLARITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med 2005;352:1179–89. [DOI] [PubMed] [Google Scholar]
- 12.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–57. [DOI] [PubMed] [Google Scholar]
- 13.Wiviott SD, Braunwald E, McCabe CH, et al. TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–15. [DOI] [PubMed] [Google Scholar]
- 14.Schüpke S, Neumann F-J, Menichelli M, et al. ISAR-REACT 5 Trial Investigators. Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med 2019;381:1524–34. [DOI] [PubMed] [Google Scholar]
- 15.Crea F, Thiele H, Sibbing D, et al. Debate: prasugrel rather than ticagrelor is the preferred treatment for NSTE-ACS patients who proceed to PCI and pretreatment should not be performed in patients planned for an early invasive strategy. Eur Heart J 2021;42:2973–85. [DOI] [PubMed] [Google Scholar]
- 16.Navarese EP, Khan SU, Kolodziejczak M, et al. Comparative efficacy and safety of oral P2Y12 inhibitors in acute coronary syndrome: network meta-analysis of 52 816 patients from 12 randomized trials. Circulation 2020;142:150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berwanger O, Lopes RD, Moia DDF, et al. Ticagrelor versus clopidogrel in patients with STEMI treated with fibrinolysis: TREAT trial. J Am Coll Cardiol 2019;73:2819–28. [DOI] [PubMed] [Google Scholar]
- 18.Valgimigli M, Bueno H, Byrne RA, et al. ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:213–60. [DOI] [PubMed] [Google Scholar]
- 19.Lindholm D, Varenhorst C, Cannon CP, et al. Ticagrelor vs. clopidogrel in patients with non-ST-elevation acute coronary syndrome with or without revascularization: results from the PLATO trial. Eur Heart J 2014;35:2083–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montalescot G, Bolognese L, Dudek D, et al. ACCOAST Investigators. Pretreatment with prasugrel in non-ST-segment elevation acute coronary syndromes. N Engl J Med 2013;369:999–1010. [DOI] [PubMed] [Google Scholar]
- 21.Tarantini G, Mojoli M, Varbella F, et al. DUBIUS Investigators; Italian Society of Interventional Cardiology. Timing of oral P2Y12 inhibitor administration in non-ST elevation acute coronary syndrome. J Am Coll Cardiol 2020;76:2450–9. [DOI] [PubMed] [Google Scholar]
- 22.Bonaca MP, Bhatt DL, Coeh M, et al. PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015;372:1791–800. [DOI] [PubMed] [Google Scholar]
- 23.Mauri L, Kereiakes DJ, Yeh RW, et al. DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014; 371:2155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan SU, Singh M, Valavoor S, et al. Dual antiplatelet therapy after percutaneous coronary intervention and drug-eluting stents: a systematic review and network meta-analysis. Circulation 2020;142:1425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hahn J-Y, Song YB, Oh J-H, et al. SMART-DATE investigators. 6-month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): a randomised, open-label, non-inferiority trial. Lancet 2018;391:1274–84. [DOI] [PubMed] [Google Scholar]
- 26.Valgimigli M, Frigoli E, Heg D, et al. MASTER DAPT Investigators. Dual antiplatelet therapy after PCI in patients at high bleeding risk. N Engl J Med 2021;385:1643–55. [DOI] [PubMed] [Google Scholar]
- 27.Koo B-K, Kang J, Park KW, et al. HOST-EXAM investigators. Aspirin versus clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): an investigator-initiated, prospective, randomised, open-label, multicentre trial. Lancet 2021;397:2487–96. [DOI] [PubMed] [Google Scholar]
- 28.Eikelboom JW, Connolly SJ, Bosch J, et al. COMPASS Investigators. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319–30. [DOI] [PubMed] [Google Scholar]
- 29.Valgimigli M, Bueno H, Byrne RA, et al. ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:213–60. [DOI] [PubMed] [Google Scholar]
- 30.Subherwal S, Bach RG, Chen AY, et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines) bleeding score. Circulation 2009;119:1873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehran R, Pocock SJ, Nikolsky E, et al. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol 2010;55:2556–66. [DOI] [PubMed] [Google Scholar]
- 32.Ducrocq G, Wallace JS, Baron G, et al. REACH Investigators. Risk score to predict serious bleeding in stable outpatients with or at risk of atherothrombosis. Eur Heart J 2010;31:1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeh RW, Secemsky EA, Kereiakes DJ, et al. DAPT Study Investigators. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA 2016;315:1735–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baber U, Mehran R, Giustino G, et al. Coronary thrombosis and major bleeding after PCI with drug-eluting stents: risk scores from PARIS. J Am Coll Cardiol 2016;67:2224–34. [DOI] [PubMed] [Google Scholar]
- 35.Costa F, van Klaveren D, James S, et al. PRECISE-DAPT Study Investigators. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet 2017;389:1025–34. [DOI] [PubMed] [Google Scholar]
- 36.Urban P, Gregson J, Owen R, et al. Assessing the risks of bleeding vs thrombotic events in patients at high bleeding risk after coronary stent implantation: the ARC-high bleeding risk trade-off model. JAMA Cardiol 2021;6:410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urban P, Mehran R, Colleran R, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the Academic Research Consortium for High Bleeding Risk. Eur Heart J 2019;40:2632–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Ascenzo F, De Filippo O, Gallone G, et al. PRAISE study group. Machine learning-based prediction of adverse events following an acute coronary syndrome (PRAISE): a modelling study of pooled datasets. Lancet 2021;397:199–207. [DOI] [PubMed] [Google Scholar]
- 39.Mihatov N, Secemsky EA, Kereiakes DJ, et al. Utility of the dual antiplatelet therapy score to guide antiplatelet therapy: a systematic review and meta-analysis. Catheter Cardiovasc Interv 2021;97:569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi SY, Kim MH, Cho Y-R, et al. Performance of PRECISE-DAPT score for predicting bleeding complication during dual antiplatelet therapy. Circ Cardiovasc Interv 2018;11:e006837. [DOI] [PubMed] [Google Scholar]
- 41.Capodanno D, Bhatt DL, Gibson CM, et al. Bleeding avoidance strategies in percutaneous coronary intervention. Nat Rev Cardiol 2022;19:117–32. [DOI] [PubMed] [Google Scholar]
- 42.Windecker S, Latib A, Kedhi E, et al. ONYX ONE Investigators. Polymer-based or polymer-free stents in patients at high bleeding risk. N Engl J Med 2020; 382:1208–18. [DOI] [PubMed] [Google Scholar]
- 43.Wärme J, Sundqvist M, Mars K, et al. Helicobacter pylori screening in clinical routine during hospitalization for acute myocardial infarction. Am Heart J 2021;231:105–9. [DOI] [PubMed] [Google Scholar]
- 44.Gimbel ME, Minderhoud SCS, Ten Berg JM. A practical guide on how to handle patients with bleeding events while on oral antithrombotic treatment. Neth Heart J 2018;26:341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhatt DL, Pollack CV, Weitz JI, et al. Antibody-based ticagrelor reversal agent in healthy volunteers. N Engl J Med 2019;380:1825–33. [DOI] [PubMed] [Google Scholar]
- 46.Baber U, Zafar MU, Dangas G, et al. Ticagrelor with or without aspirin after PCI: the TWILIGHT platelet substudy. J Am Coll Cardiol 2020;75:578–86. [DOI] [PubMed] [Google Scholar]
- 47.O’Donoghue ML, Murphy SA, Sabatine MS. The safety and efficacy of aspirin discontinuation on a background of a P2Y12 inhibitor in patients after percutaneous coronary intervention: a systematic review and meta-analysis. Circulation 2020;142:538–45. [DOI] [PubMed] [Google Scholar]
- 48.Valgimigli M, Gragnano F, Branca M, et al. P2Y12 inhibitor monotherapy or dual antiplatelet therapy after coronary revascularisation: individual patient level meta-analysis of randomised controlled trials. BMJ 2021;373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lip GYH, Collet J-P, Haude M, et al. Management of antithrombotic therapy in AF patients presenting with ACS and/or undergoing PCI: a summary of the joint consensus document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). Eur Heart J 2018;39:2847–50. [DOI] [PubMed] [Google Scholar]
- 50.Lopes RD, Heizer G, Aronson R, et al. AUGUSTUS Investigators. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med 2019;380:1509–24. [DOI] [PubMed] [Google Scholar]
- 51.Lopes RD, Leonardi S, Wojdyla DM, et al. Stent thrombosis in patients with atrial fibrillation undergoing coronary stenting in the AUGUSTUS trial. Circulation 2020;141:781–3. [DOI] [PubMed] [Google Scholar]
- 52.Alexander JH, Wojdyla D, Vora AN, et al. Risk/benefit tradeoff of antithrombotic therapy in patients with atrial fibrillation early and late after an acute coronary syndrome or percutaneous coronary intervention: insights from AUGUSTUS. Circulation 2020;141:1618–27. [DOI] [PubMed] [Google Scholar]
- 53.Gargiulo G, Goette A, Tijssen J, et al. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J 2019;40:3757–67. [DOI] [PubMed] [Google Scholar]
- 54.Alkhalil M, Shahmohammadi M, Spence MS, et al. Aspirin discontinuation in patients requiring oral anticoagulation undergoing percutaneous coronary intervention, the role of procedural complexity. Cardiovasc Drugs Ther 2020; 34:659–62. [DOI] [PubMed] [Google Scholar]
- 55.Yasuda S, Kaikita K, Akao M, et al. AFIRE Investigators. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med 2019;381: 1103–13. [DOI] [PubMed] [Google Scholar]
- 56.Zettler ME, Peterson ED, McCoy LA, et al. TRANSLATE-ACS Investigators. Switching of adenosine diphosphate receptor inhibitor after hospital discharge among myocardial infarction patients: Insights from the Treatment with Adenosine Diphosphate Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE-ACS) observational study. Am Heart J 2017;183:62–8. [DOI] [PubMed] [Google Scholar]
- 57.Angiolillo DJ, Rollini F, Storey RF, et al. International expert consensus on switching platelet P2Y12 receptor-inhibiting therapies. Circulation 2017; 136:1955–75. [DOI] [PubMed] [Google Scholar]
- 58.Sibbing D, Aradi D, Alexopoulos D, et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv 2019;12:1521–37. [DOI] [PubMed] [Google Scholar]