Purpose of review
Invasive pulmonary aspergillosis (IPA) can affect patients with severe coronavirus disease 2019 (COVID-19), but many questions remain open about its very variable incidence across the world, the actual link between the viral infection and the fungal superinfection, the significance of Aspergillus recovery in a respiratory sample, and the management of such cases. This review addresses these questions and aims at providing some clues for the practical diagnostic and therapeutic approaches of COVID-19-associated pulmonary aspergillosis (CAPA) in a clinical perspective.
Recent findings
Definitions have been proposed for possible/probable/proven CAPA, but distinction between colonization and invasive fungal infection is difficult and not possible in most cases in the absence of histopathological proof or positive galactomannan in serum. Most importantly, the recovery of an Aspergillus by a direct (culture, PCR) or indirect (galactomannan) test in a respiratory sample is an indicator of worse outcome, which justifies a screening for early detection and initiation of preemptive antifungal therapy in such cases.
Summary
The COVID-19 pandemic has increased our awareness of IPA among ICU patients. Although current recommendations are mainly based on experts’ opinions, prospective studies are needed to get more evidence-based support for the diagnostic approach and management of CAPA.
Keywords: Aspergillus fumigatus, bronchoalveolar lavage fluid, bronchoscopy, intensive care, severe acute respiratory syndrome - coronavirus - 2
INTRODUCTION
Invasive mold infections, such as invasive pulmonary aspergillosis (IPA), mainly affect patients with severe immune defects, such as those with hematologic cancer and chemotherapy-induced neutropenia or transplant recipients. However, the clinical spectrum of IPA has expanded over the two last decades with the emergence of new categories of patients ‘at risk’. Notably, IPA has been recognized as a life-threatening complication among ICU patients without preexisting immunosuppressive conditions [1,2]. The influenza H1N1 pandemic of 2009–2010 has highlighted the high risk of IPA among patients with severe influenza requiring ICU admission and mechanical ventilation [3,4]. More recently, the devastating pandemic of the novel coronavirus disease 2019 (COVID-19) led to a similar concern about IPA complicating the course of patients with the severe respiratory form of the disease [5,6]. Diagnosis of IPA in the ICU is challenging, as the current definitions of the European Organization for Research and Treatment of Cancer and Mycoses Study Group (EORTC-MSG) are not appropriate in this setting [7]. Several consensus definitions have been proposed for IPA in the ICU, including specific definitions for influenza-associated pulmonary aspergillosis (IAPA) and COVID-19-associated pulmonary aspergillosis (CAPA) [4,8,9▪,10]. This approach implicitly recognizes that IPA in the ICU is a complex topic with possibly distinct entities.
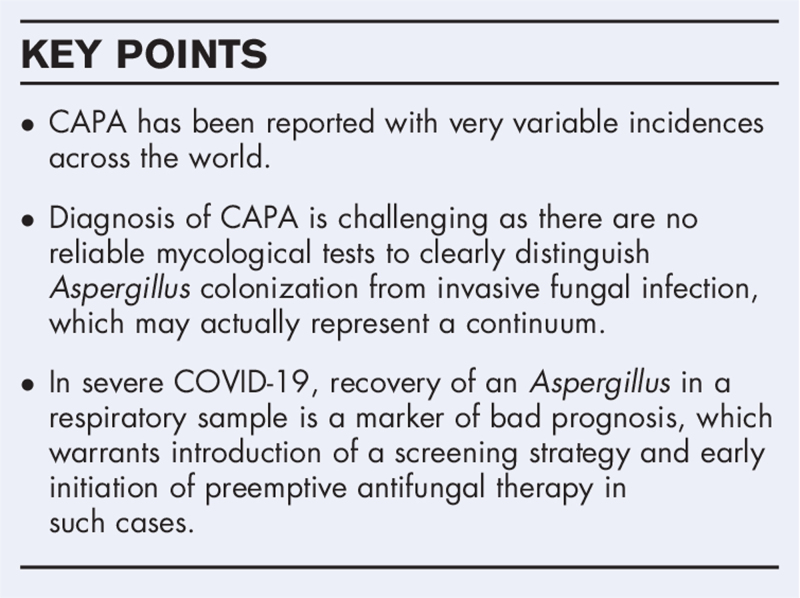
Box 1.
no caption available
The aim of this review is to discuss the important questions that remain open regarding CAPA and to provide some clues for its diagnostic approach and management.
What is the actual prevalence of coronavirus disease 2019-associated pulmonary aspergillosis?
Although the eventuality of IPA complicating COVID-19 has been recognized early during the pandemic, a very variable prevalence of CAPA (ranging from 2 to 30%) has been reported since that time [11,12▪▪]. This large heterogeneity could be attributed to differences in the local epidemiological context, definitions of IPA (i.e. distinction between ‘true’ IPA and lung colonization) or strategies of screening. However, these factors are probably not sufficient to explain such variability. The impact of the pandemic on healthcare systems may have a predominant role in these variations. For instance, the opening of new dedicated COVID-19 units in overwhelming situations, as well as the implementation of negative air room pressure to protect the medical staff, can result in higher risk of air contamination with molds [13]. This could explain the high incidence of CAPA in geographical regions (e.g. Northern Italy) that were strongly affected by the first COVID-19 wave [14▪▪]. Finally, it should be reminded that such geographical or seasonal variations of incidence has also been reported for IAPA, irrespective of the influenza type or any other identified parameter [15].
Is there a specific association between coronavirus disease 2019 and invasive pulmonary aspergillosis?
There are a lot of data showing that IPA can complicate the course of severe COVID-19. However, it is not clear whether the severe acute respiratory syndrome - coronavirus - 2 infection per se is a distinct predisposing factor to IPA. Indeed, prolonged ICU stay and mechanical ventilation may constitute the major predisposing underlying condition. The hypothesis of an association between COVID-19 and IPA originates from previous observations demonstrating a direct causal link between severe influenza and IPA [4]. Such comparative study is currently lacking for CAPA. Moreover, an important proportion of CAPA cases are classified as putative/possible rather than proven/probable IPA [11]. Indeed, Kula et al.[16▪▪] found proven CAPA in only 2% of COVID-19 autopsies, while such histopathological proof is much more frequent in IAPA [17]. Similarly, the rate of positive serum galactomannan, a marker of angio-invasion, is low (<15%) in COVID-19 compared with influenza (about 60%) [5].
Although there are similarities between the physiopathology of Influenza and COVID-19, some notable differences should be outlined [5]. Influenza pneumonia is characterized by important destruction of the respiratory epithelium, which favors the development of Aspergillus tracheobronchitis and subsequent lung invasion [18]. Although epithelial cell damage is also observed in COVID-19, acute respiratory distress syndrome (ARDS) seems mainly related to endothelial cell damage leading to increase permeability and edema [19]. Indeed, Aspergillus tracheobronchitis is less commonly observed in COVID-19 compared with influenza (10–20% versus 50–60%, respectively), although it could be underreported [20]. Most importantly, the inflammatory responses of Influenza and COVID-19 are relatively distinct [5]. Influenza is associated with important immune paralysis and increased levels of IL-10, which is supposed to favor IPA, while the cytokine profile of COVID-19 is not the same [21,22].
Which are the risk factors for coronavirus disease 2019-associated pulmonary aspergillosis?
CAPA almost exclusively happens among ICU patients under mechanical ventilation [12▪▪,23▪].
Patients with CAPA tend to be older [12▪▪,24▪] and more likely to have underlying pulmonary diseases [24▪,25▪]. Some immunosuppressive or debilitating conditions, such as solid organ transplantation, multiple myeloma, solid tumors or liver disease were identified as a risk factor of CAPA in some studies [25▪,26]. However, most patients with a diagnosis of presumed CAPA have no underlying immunosuppressive conditions according to the EORTC-MSG criteria [5,7,14▪▪,23▪]. Previous exposure to corticosteroids (>3 weeks), even at lower doses than those defined by the EORTC-MSG criteria [7], appears to be a significant predisposing condition [14▪▪,24▪,26]. However, there is currently no evidence of an increased risk of CAPA among patients treated with short-course (10 days) corticosteroids as recommended for the management of severe COVID-19 [14▪▪,24▪,27]. Regarding the impact of immunodulatory therapies of COVID-19, a significant association between the use of tocilizumab and CAPA has been reported in some studies [12▪▪,27], but not all [14▪▪,24▪]. Surprisingly, one study suggested an association with azithromycin [28], but this treatment is no longer recommended for the treatment of COVID-19.
Are there specific clinical patterns of coronavirus disease 2019-associated pulmonary aspergillosis?
IPA in ICU is characterized by the nonspecificity of clinical and radiological signs. The imaging of IPA in nonneutropenic patients includes a wide spectrum of radiological patterns that can mimic bacterial superinfection (e.g. lobar or segmental consolidation) or the viral infection (e.g. ground-glass opacities, tree-in-bud pattern). Patients with severe COVID-19 typically exhibit patchy areas of ground-glass opacities, which can also be observed in IPA. The presence of a cavitation should increase the suspicion of IPA [10], but is observed at later stages. Aspergillus tracheobronchitis is characterized by the presence of plaques and pseudomembranes, but is not a common manifestation of CAPA [20]. As a consequence, the eventuality of CAPA should be considered in any intubated COVID-19 patient with worsening respiratory conditions or lack of improvement despite antibacterial therapy.
How to distinguish coronavirus disease 2019-associated pulmonary aspergillosis from Aspergillus airway colonization?
The diagnosis of IPA is usually graded on a scale of probability (i.e. possible, probable, proven) according to the EORTC-MSG criteria [7]. These definitions rely on the presence of host factors as the entry criterion and therefore do not apply for ICU patients [8]. In this later setting, the direct or indirect microbiological documentation of an Aspergillus represents the unique criterion of IPA in the absence of any reliable clinical or radiological hint. Because these patients are less immunocompromised compared with neutropenic or transplant patients, the interpretation of a positive microbiological test for Aspergillus is more difficult, as it may reflect either colonization or true infection. Several definitions have been proposed for the distinction between colonization and putative/possible or probable IPA in the ICU, including adapted definitions for IAPA and CAPA [4,8,9▪,10]. Some important points should be outlined about this approach. First, the pathophysiology of IPA in the ICU (including IAPA and CAPA) should be regarded as a continuum and a dynamic situation, which can evolve gradually and more or less rapidly from colonization toward infection. Second, with the exception of serum galactomannan, there is no reliable microbiological marker for the distinction between colonization and IPA. In CAPA, a positive serum galactomannan is present in a minority of cases [5,23▪] and the diagnosis only relies on the interpretation of mycological tests from respiratory samples [9▪]. Albeit not discriminative per se, deep samples obtained by bronchoscopy [e.g. bronchoalveolar lavage (BAL) fluid] represent the cornerstones of IPA diagnosis in ICU [4,8,10]. During the COVID-19 pandemic, a more restrictive approach was applied regarding the use of bronchoscopy because of the concern about contagiousness from aerosolization [29]. As a consequence, the CAPA definitions have been adapted to consider results of nonbronchoscopic samples (e.g. nondirected BAL or tracheal aspirates) for the diagnosis of possible IPA [9▪,10]. However, there is little experience to support the reliability of this approach [30,31]. In particular, the cut-offs for galactomannan in non-BAL samples are debated [9▪]. Finally, it should be emphasized that there is a near complete lack of data about the performance (i.e. sensitivity and specificity) of these mycological tests (culture, PCR, galactomannan) in both bronchoscopic or nonbronchoscopic samples for nonimmunocompromised patients in the ICU. In the absence of a reliable gold standard of CAPA, these criteria based on experts’ recommendations remain speculative and have the major interest to propose a standardized approach for defining CAPA in clinical trials and epidemiological surveys [9▪].
What is the impact of Aspergillus recovery in a respiratory sample of a coronavirus disease 2019 patient?
Although there is virtually no reliable marker for the distinction between CAPA and colonization, we can question the actual impact of the recovery of an Aspergillus spp. (by culture, PCR or galactomannan) in a respiratory sample on patients’ outcome. The largest studies or meta-analysis reaching sufficient statistical power to address this question found an association between presumed CAPA and mortality, which remained significant after adjustment for other predictors of mortality [12▪▪,14▪▪,24▪]. An impact of CAPA on the overall morbidity and the course of COVID-19 (i.e. higher severity scores, longer time to improvement, duration of mechanical ventilation and hospital stay) was also demonstrated in some studies [12▪▪,25▪]. It should be emphasized that a meta-analysis showed that patients who subsequently developed CAPA had higher sequential organ failure assessment score at ICU admission [24▪]. Therefore, it is unclear whether this worse outcome in presumed CAPA is a direct consequence of true IPA or whether Aspergillus colonization may actually represent an index of disease severity occurring among the most critically ill patients. It might be that both cause-effect hypotheses play a role in a vicious circle with the most severe patients being predisposed to Aspergillus colonization and further evolution toward CAPA and death.
Which should be the diagnostic approach of coronavirus disease 2019-associated pulmonary aspergillosis?
In the absence of suggestive clinical or radiological signs, CAPA should be suspected in any COVID-19 patient under mechanical ventilation whose conditions are not improving or deteriorating. Therefore, there is a rationale to consider a systematic screening approach for such patients, as proposed in Table 1. Monitoring of fungal biomarkers (galactomannan or beta-glucan) in serum (e.g. twice weekly) has been implemented in some centers [25▪,32]. However, because of the low rate of positive serum galactomannan (<15%) in CAPA and the lack of specificity of beta-glucan positivity in ICU, this strategy cannot be firmly recommended except for patients with EORTC-MSG host criteria [7] or in centers/regions with high CAPA incidence. Importantly, respiratory samples that are regularly collected via the orotracheal tube for the detection of ventilator-associated pneumonia should be processed for specific fungal cultures (and Aspergillus PCR if available) in addition to standard bacterial cultures. Although the diagnostic value of fungal cultures or PCR in nonbronchoscopic samples is questionable, these samples are easy to collect for a screening and a positive result may trigger further diagnostic work-up by bronchoscopy with BAL. Although the use of bronchoscopy has been restricted during the early COVID-19 pandemic, physicians should not refrain from performing this procedure when clinically indicated, as preventive measures have been standardized and improved over time. Indeed, experts currently recommend that management of ARDS should be the same among ICU patients, regardless of a COVID-19 diagnosis [33].
Table 1.
Proposed diagnostic approach for COVID-19-associated pulmonary aspergillosis (CAPA) in ICU mechanically-ventilated patients
| Respiratory samples | ||
| Serum samples | Non-bronchoscopica | Bronchoscopic (BAL) |
| GM screening (e.g. 2/week) if: high local CAPA incidence (>10%) OR EORTC-MSG host criteria present Punctual GM testing if: clinical suspicion or positive fungal marker in respiratory sample | Fungal culture and/or Af PCR screening (e.g. 2/week) (GM possible in non-directed BAL samples, but lack of reliable interpretive criteria) | Bronchoscopy with fungal culture, Af PCR and GM in BAL if: Deteriorating respiratory conditions OR In case of positive fungal marker in a non-bronchoscopic sample or serum |
Tracheal aspirates or non-directed BAL samples.
Af PCR: Aspergillus fumigatus specific polymerase chain reaction, BAL, bronchoalveolar lavage fluid; CAPA, coronavirus disease 2019-associated pulmonary aspergillosis; EORTC-MSG, European Organization for Research and Treatment of Cancer and Mycoses Study Group; GM, galactomannan.
Which patients should receive antifungal therapy?
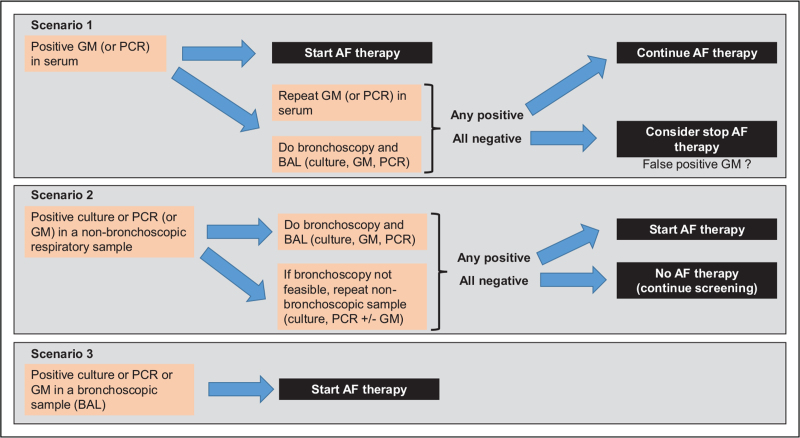
Although the diagnosis of putative/possible or even probable CAPA remains uncertain, the threshold to start antifungal therapy in these very frail patients should be low. Indeed, antifungal therapy should be considered not only as a targeted therapy (e.g. proven or probable IPA), but also as a preemptive approach in patients with possible CAPA or even colonization, since we admit that these patterns can evolve toward true CAPA and/or represent per se a factor of overall bad prognosis. Figure 1 shows a proposed algorithm for the management of CAPA, where we can distinguish three clinical scenarios, according to the first positive microbiological index of IPA (i.e. galactomannan/PCR in serum, culture/PCR/galactomannan in nonbronchoscopic or bronchoscopic respiratory samples).
FIGURE 1.
Proposed therapeutic approach for coronavirus disease 2019-associated pulmonary aspergillosis. AF, antifungal; BAL, bronchoalveolar lavage fluid; GM, galactomannan.
A positive galactomannan in serum (scenario 1) is an index of angio-invasive disease and should prompt immediate initiation of antifungal therapy. However, such result should be confirmed by a second serum sample (collected just before the start of antifungal therapy) and trigger further investigations by bronchoscopy. Indeed, considering the low rate of positive serum galactomannan in CAPA and the limited specificity of the test, especially in the ICU, the probability of a false positive result on a single test is relatively high. In case of a negative second serum galactomannan test and negative fungal markers in BAL, discontinuation of antifungal therapy can be considered.
A positive fungal marker (culture, PCR or possibly galactomannan) in a nonbronchoscopic sample (scenario 2) should prompt further investigations by bronchoscopy. These nonbronchoscopic samples are more prone to reflect colonization or contamination compared with bronchoscopic samples, but they can be used for screening because of their noninvasive procedure. In situations where bronchoscopy is not feasible, repeated isolation of Aspergillus by culture or PCR (especially when the quantitative burden is high) in nonbronchoscopic samples should trigger initiation of antifungal therapy.
Finally, a positive fungal marker (culture, PCR or galactomannan) in a BAL obtained by bronchoscopy is representative of the presence of the pathogen in the distal airways with a high probability of infection. Such finding should trigger initiation of antifungal therapy in all cases since several studies have demonstrated that it was an indicator of worse prognosis [14▪▪,24▪,25▪,27,28].
A role for antifungal prophylaxis in severe COVID-19 is also under consideration. This approach has been recently assessed for patients with severe influenza in a randomized open-label trial, which failed to demonstrate a significant benefit of posaconazole prophylaxis in preventing IPA in this setting [34]. These nonconclusive results can be explained by the low number of inclusions and the high incidence of early IPA (i.e. occurring during the first 48 h from ICU admission) in this study. In COVID-19, the time-window of IPA diagnosis is more variable with a substantial proportion of late cases (i.e. beyond 7 days) [5], which may be a rationale for a role of antifungal prophylaxis. Two single-center observational studies reported a significantly lower CAPA incidence among ICU COVID-19 patients having received prophylaxis with intravenous posaconazole or inhaled amphotericin B versus those without prophylaxis [35,36]. These results should be interpreted cautiously as they were biased by the nonrandomized assignment of patients and/or comparison between different periods. Nonetheless, and not surprisingly, antifungal prophylaxis seems to result in a decrease of CAPA incidence and/or Aspergillus lung colonization, although its impact on mortality is not demonstrated. As for other populations at risk of IPA, the choice between antifungal prophylaxis versus a preemptive strategy should be assessed on the basis of the local epidemiology [37], which is extremely variable for CAPA. A more than 10% CAPA incidence may be considered as the threshold, at which this measure may result in some significant benefit and cost-effectiveness.
How and how long should we treat coronavirus disease 2019-associated pulmonary aspergillosis?
The choice of antifungal therapy of CAPA should not basically differ from that of IPA in other setting, but can be influenced by specific characteristics of the ICU population, including pharmacokinetic alterations (e.g. volume of distribution, clearance), drug-drug interactions, and mainly toxicity issues related to the high occurrence of liver or renal dysfunction among severe COVID-19 patients [38]. The local prevalence of azole resistance among Aspergillus fumigatus should also be taken into account. Although there is no comparative study about efficacy, both triazoles (voriconazole, isavuconazole or posaconazole) and liposomal amphotericin B seem to be valid options and therapeutic choices should be assessed individually.
Despite abundant literature on the topic, the question of the duration of antifungal therapy has been rarely addressed for CAPA and IPA in ICU in general. As above mentioned, IPA in ICU represents a distinct pathophysiological and clinical entity compared with IPA in classical immunocompromised populations. Moreover, the dysregulated immune response (e.g. ‘cytokine storm’) in CAPA occurs within a short time-window compared with the chronic stages of immunosuppression following anticancer chemotherapy or transplantation. Although there is no evidence-based recommendations, the following pragmatic approach could be proposed. In patients with Aspergillus recovered in respiratory samples who do not have clear evidence of CAPA (i.e. possible or putative IPA), antifungal therapy may be considered as mostly preemptive and could be interrupted following extubation and ICU discharge, provided that a control chest CT-scan does not show any specific abnormality (e.g. cavitation or well circumscribed mass). For other patients with higher suspicion of CAPA (i.e. probable or proven CAPA), antifungal therapy should be continued for at least 4–6 weeks (or even longer in case of cavitation), according to evolution in radiological follow-up.
CONCLUSION
CAPA has emerged as a novel form of IPA, for which several questions remain open regarding the actual association with the viral disease, the unexplained variations of incidence, the pathophysiology, the diagnosis and management. Although there is some controversy about this entity, notably about the case definitions, there is sufficient evidence to support the fact that Aspergillus recovery in a respiratory sample of a severe COVID-19 patient may affect its prognosis and should be considered seriously. The aim of this article was to provide some practical hints for the diagnostic approach and management of such cases, which should be adapted for each individual center on the basis of their local epidemiology. Prospective studies focusing on the above discussed questions should be undertaken to provide higher level of evidence-based support to the current diagnostic and therapeutic recommendations of CAPA.
Acknowledgements
None.
Financial support and sponsorship
None to declare in relationship with the present article.
Conflicts of interest
F.L. declares research funding from the Swiss National Science Foundation, The Santos-Suarez Foundation, Novartis, MSD and Pfizer, outside of the submitted work, and honoraria for advisory boards from Gilead.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Garbino J, Fluckiger U, Elzi L, et al. Survey of aspergillosis in nonneutropenic patients in Swiss teaching hospitals. Clin Microbiol Infect 2011; 17:1366–1371. [DOI] [PubMed] [Google Scholar]
- 2.Meersseman W, Vandecasteele SJ, Wilmer A, et al. Invasive aspergillosis in critically ill patients without malignancy. Am J Respir Crit Care Med 2004; 170:621–625. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Vidal C, Barba P, Arnan M, et al. Invasive aspergillosis complicating pandemic influenza A (H1N1) infection in severely immunocompromised patients. Clin Infect Dis 2011; 53:e16–e19. [DOI] [PubMed] [Google Scholar]
- 4.Schauwvlieghe A, Rijnders BJA, Philips N, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med 2018; 6:782–792. [DOI] [PubMed] [Google Scholar]
- 5.Lamoth F, Lewis RE, Walsh TJ, Kontoyiannis DP. Navigating the uncertainties of COVID-19 associated aspergillosis (CAPA): a comparison with influenza associated aspergillosis (IAPA). J Infect Dis 2021; 224:1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marr KA, Platt A, Tornheim JA, et al. Aspergillosis complicating severe coronavirus disease. Emerg Infect Dis 2021; 27:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 2020; 71:1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blot SI, Taccone FS, Van den Abeele AM, et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med 2012; 186:56–64. [DOI] [PubMed] [Google Scholar]
- 9▪.Koehler P, Bassetti M, Chakrabarti A, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis 2021; 21:e149–e162. [DOI] [PMC free article] [PubMed] [Google Scholar]; Proposition of standardized diagnostic definitions and classification of coronavirus disease 2019 (COVID-19) associated pulmonary aspergillosis (CAPA).
- 10.Verweij PE, Rijnders BJA, Bruggemann RJM, et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med 2020; 46:1524–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fekkar A, Neofytos D, Nguyen MH, et al. COVID-19-associated pulmonary aspergillosis (CAPA): how big a problem is it? Clin Microbiol Infect 2021; 27:1376–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪▪.Prattes J, Wauters J, Giacobbe DR, et al. Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients-a multinational observational study by the European Confederation of Medical Mycology. Clin Microbiol Infect 2021; [In press, online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; Large multicenter study highlighting some important baseline characteristics and risk factors of CAPA and the impact of CAPA on outcome.
- 13.Ichai P, Saliba F, Baune P, et al. Impact of negative air pressure in ICU rooms on the risk of pulmonary aspergillosis in COVID-19 patients. Crit Care 2020; 24:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14▪▪.Bartoletti M, Pascale R, Cricca M, et al. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study. Clin Infect Dis 2021; 73:e3606–e3614. [DOI] [PMC free article] [PubMed] [Google Scholar]; Large series of probable or putative cases of CAPA, which shows an association of CAPA with mortality in a logistic regression model after adjustment for confounding factors.
- 15.Schwartz IS, Friedman DZP, Zapernick L, et al. High rates of influenza-associated invasive pulmonary aspergillosis may not be universal: a retrospective cohort study from Alberta, Canada. Clin Infect Dis 2020; 71:1760–1763. [DOI] [PubMed] [Google Scholar]
- 16▪▪.Kula BE, Clancy CJ, Hong Nguyen M, Schwartz IS. Invasive mould disease in fatal COVID-19: a systematic review of autopsies. Lancet Microbe 2021; 2:e405–e414. [DOI] [PMC free article] [PubMed] [Google Scholar]; Large autopsy series showing an actual low incidence of proven invasive aspergillosis in deceased COVID-19 patients.
- 17.Vanderbeke L, Spriet I, Breynaert C, et al. Invasive pulmonary aspergillosis complicating severe influenza: epidemiology, diagnosis and treatment. Curr Opin Infect Dis 2018; 31:471–480. [DOI] [PubMed] [Google Scholar]
- 18.Nyga R, Maizel J, Nseir S, et al. Invasive tracheobronchial aspergillosis in critically ill patients with severe influenza. A clinical trial. Am J Respir Crit Care Med 2020; 202:708–716. [DOI] [PubMed] [Google Scholar]
- 19.Pfortmueller CA, Spinetti T, Urman RD, et al. COVID-19-associated acute respiratory distress syndrome (CARDS): current knowledge on pathophysiology and ICU treatment – a narrative review. Best Pract Res Clin Anaesthesiol 2021; 35:351–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Veerdonk FL, Bruggemann RJM, Vos S, et al. COVID-19-associated Aspergillus tracheobronchitis: the interplay between viral tropism, host defence, and fungal invasion. Lancet Respir Med 2021; 9:795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roilides E, Sein T, Roden M, et al. Elevated serum concentrations of interleukin-10 in nonneutropenic patients with invasive aspergillosis. J Infect Dis 2001; 183:518–520. [DOI] [PubMed] [Google Scholar]
- 22.Yu X, Zhang X, Zhao B, et al. Intensive cytokine induction in pandemic H1N1 influenza virus infection accompanied by robust production of IL-10 and IL-6. PLoS One 2011; 6:e28680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23▪.Salmanton-Garcia J, Sprute R, Stemler J, et al. COVID-19-associated pulmonary aspergillosis. Emerg Infect Dis 2021; 27:1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]; Multicenter collection of CAPA cases, which is representative of the variable incidence of CAPA and its related epidemiological features.
- 24▪.Chong WH, Saha BK, Neu KP. Comparing the clinical characteristics and outcomes of COVID-19-associate pulmonary aspergillosis (CAPA): a systematic review and meta-analysis. Infection 2021; [In press, online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; This meta-analysis of 8 cohort studies, including 729 critically ill COVID-19 patients, of which 109 were diagnosed with CAPA, provides important information about the clinical characteristics and outcomes associated with CAPA.
- 25▪.Permpalung N, Chiang TP, Massie AB, et al. COVID-19 associated pulmonary aspergillosis in mechanically ventilated patients. Clin Infect Dis 2021; [In press, online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; Large cohort investigating the clinical characteristics and outcomes associated with CAPA.
- 26.Fekkar A, Lampros A, Mayaux J, et al. Occurrence of invasive pulmonary fungal infections in patients with severe COVID-19 admitted to the ICU. Am J Respir Crit Care Med 2021; 203:307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segrelles-Calvo G, de SAGR, Llopis-Pastor E, et al. Prevalence of opportunistic invasive aspergillosis in COVID-19 patients with severe pneumonia. Mycoses 2020; 64:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delliere S, Dudoignon E, Fodil S, et al. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort. Clin Microbiol Infect 2020; 27:790.e1–790.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wahidi MM, Lamb C, Murgu S, et al. American Association for Bronchology and Interventional Pulmonology (AABIP) statement on the use of bronchoscopy and respiratory specimen collection in patients with suspected or confirmed COVID-19 infection. J Bronchology Interv Pulmonol 2020; 27:e52–e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Biesen S, Kwa D, Bosman RJ, Juffermans NP. Detection of invasive pulmonary aspergillosis in COVID-19 with nondirected bronchoalveolar lavage. Am J Respir Crit Care Med 2020; 202:1171–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White PL, Dhillon R, Cordey A, et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin Infect Dis 2020; 73:e1634–e1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamoth F, Glampedakis E, Boillat-Blanco N, et al. Incidence of invasive pulmonary aspergillosis among critically ill COVID-19 patients. Clin Microbiol Infect 2020; 26:1706–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffiths M, Meade S, Summers C, et al. RAND appropriateness panel to determine the applicability of UK guidelines on the management of acute respiratory distress syndrome (ARDS) and other strategies in the context of the COVID-19 pandemic. Thorax 2021; [In press, online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 34.Vanderbeke L, Janssen NAF, Bergmans D, et al. Posaconazole for prevention of invasive pulmonary aspergillosis in critically ill influenza patients (POSA-FLU): a randomised, open-label, proof-of-concept trial. Intensive Care Med 2021; 47:674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatzl S, Reisinger AC, Posch F, et al. Antifungal prophylaxis for prevention of COVID-19-associated pulmonary aspergillosis in critically ill patients: an observational study. Crit Care 2021; 25:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Ackerbroeck S, Rutsaert L, Roelant E, et al. Inhaled liposomal amphotericin-B as a prophylactic treatment for COVID-19-associated pulmonary aspergillosis/Aspergillus tracheobronchitis. Crit Care 2021; 25:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect 2018; 24 Suppl 1:e1–e38. [DOI] [PubMed] [Google Scholar]
- 38.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]