Abstract
Background:
The SARS-CoV-2 (COVID-19) pandemic has impacted many facets of critical care delivery.
Methods:
An electronic survey was distributed to explore the pandemic’s perceived impact on neurocritical care delivery between June 2020 and March 2021. Variables were stratified by World Bank country income level, presence of a dedicated neurocritical care unit (NCCU) and experiencing a COVID-19 patient surge.
Results:
Respondents from 253 hospitals (78.3% response rate) from 47 countries (45.5% low/middle income countries; 54.5% with a dedicated NCCU; 78.6% experienced a first surge) participated in the study. Independent of country income level, NCCU and surge status, participants reported reductions in NCCU admissions (67%), critical care drug shortages (69%), reduction in ancillary services (43%) and routine diagnostic testing (61%), and temporary cancellation of didactic teaching (44%) and clinical/basic science research (70%). Respondents from low/middle income countries were more likely to report lack of surge preparedness (odds ratio [OR], 3.2; 95% confidence interval [CI], 1.8-5.8) and struggling to return to prepandemic standards of care (OR, 12.2; 95% CI, 4.4-34) compared with respondents from high-income countries. Respondents experiencing a surge were more likely to report conversion of NCCUs and general-mixed intensive care units (ICUs) to a COVID-ICU (OR 3.7; 95% CI, 1.9-7.3), conversion of non-ICU beds to ICU beds (OR, 3.4; 95% CI, 1.8-6.5), and deviations in critical care and pharmaceutical practices (OR, 4.2; 95% CI 2.1-8.2). Respondents from hospitals with a dedicated NCCU were less likely to report conversion to a COVID-ICU (OR, 0.5; 95% CI, 0.3-0.9) or conversion of non-ICU to ICU beds (OR, 0.5; 95% CI, 0.3-0.9).
Conclusion:
This study reports the perceived impact of the COVID-19 pandemic on global neurocritical care delivery, and highlights shortcomings of health care infrastructures and the importance of pandemic preparedness.
Key Words: COVID-19, care delivery, neurocritical care, pandemic, resources, SARS-Cov-2
As of August 10, 2021, the SARS-CoV-2 (COVID-19) pandemic had affected >200 million individuals across 188 countries; 3.9 million deaths had been reported worldwide since the start of the pandemic.1 With up to 38% of COVID-19 patients requiring admission to an intensive care unit (ICU), and 75% to 88% of critically ill COVID-19 patients requiring mechanical ventilation,2–7 the pandemic has necessitated changes to the structure and function of critical care services in hospitals and health care systems worldwide. The delivery of care to patients requiring neurological/neurosurgical critical care (hereby referred to as NCC patients) may be disrupted as scarce neurocritical care resources were reallocated due to shifting priorities8,9 in preparation for managing projected surges of patients with COVID-19 disease.10
NCC patients may be admitted to a dedicated neurocritical care unit (NCCU) or a mixed-general intensive care unit (gen-ICU).11 Although the Neurocritical Care Society9 and the American Academy of Neurology12 provided urgent guidelines for maintaining care to critically ill neurological and neurosurgical patients during a pandemic, and despite initiatives to preserve care for NCC patients,13–21 the global impact of the pandemic on the delivery of neurocritical care is unknown.12,22 We conducted an international survey-based study to examine perceptions of neurocritical care providers regarding the impact of the pandemic on neurocritical care delivery. We inquired about the following metrics: changes to preexisting NCCU/gen-ICU location, ICU staffing, ICU admissions, critical care medication shortages, diagnostic testing, ancillary services, and research and academic activity. We specifically explored the global impact of the pandemic based on World Bank country income level divided into low/middle income countries (LMICs) and high-income countries (HICs), presence of a dedicated NCCU, and surge status. The primary goal of the study was to determine the perceived impact of the COVID-19 pandemic on neurocritical care delivery worldwide, and to provide information to optimize care delivery planning for future pandemic scenarios.
METHODS
This study received approval from the institutional review board of the University of Washington (STUDY00010502). Response to the survey implied consent to participate in the study.
Study Design, Participants, and Sample Size
A 176-question survey available in English, Spanish, and Brazilian Portuguese was developed using the Institute of Translational Health Sciences’ REDCap database management system. Survey items covered the Neurocritical Care Society’s recommendations on neurocritical care resource utilization in a pandemic.9 The full questionnaire is available in the supplementary material (Supplemental Digital Content [SDC] 1: Survey questionnaire, http://links.lww.com/JNA/A461). The survey was pilot-tested amongst members of the NCC-COVID study project committee. Our target population was healthcare providers that routinely deliver critical care to neurological and neurosurgical patients,11,23 with responses limited to one provider per hospital. The survey was disseminated through large established networks including neurocritical care fellowship directors,24 the Latin-American Brain Injury Consortium (LABIC),25 and Sociedad Argentina De Terapia Intensiva (SATI, Argentina),26 in wide geographical locations (North America, Latin America, Europe, Asia, Africa, Middle East, and Oceania).
Representatives from 323 hospitals received 5 emails between July 10, 2020 and September 30, 2020, inviting participation. The email invitation described the purpose of the study, emphasized the importance of obtaining a global perspective, and highlighted that participation was voluntary and without any financial incentive. The survey participants were made aware that their responses would be included in this study and that their response implied consent to participate. A follow-up semistructured interview was conducted between September 2020 and March 2021 (SDC 2: Semi-structured interview questionnaire, http://links.lww.com/JNA/A462). The response rate to the initial survey was defined as the return of completed surveys from recipients of the email invitations that did not bounce back because of incorrect e-mail addresses.
Statistical Analysis
Descriptive statistics (count, percentages) were used to report categorical variables. Continuous data are presented as mean (SD) or median (interquartile range [IQR]). Differences in observations between LMICs and HICs,27 between NCCU and gen-ICU,11 and self-reported first COVID-19 surge status (surge vs. no surge) were examined using the Fisher exact test and reported as relative risk (RR) with 95% confidence intervals (CI). A multivariate nominal logistic fit model included World Bank country income level, NCCU, and first surge status to generate odds ratios (ORs). We used the Dedoose software application28 to codify free-text responses into six principal and 25 minor codes for the follow-up semi-structured interview. Stata version 1529 was used for statistical analysis. A P-value <0.05 indicated statistical significance.
RESULTS
Respondents from 253 hospitals (78.3% response rate) located in 47 countries (Fig. 1) completed the initial survey questionnaire; 115 respondents (45.4%) were from LMICs, 138 respondents (54.5%) represented institutions with a dedicated NCCU, and 199 (78.6%) had experienced a first surge of COVID-19 patients by the time of their response. The respondents represented hospitals in North America (n=83, 32.8%), Latin America (n=59, 23.3%), Europe (n=44, 17.4%), Asia (n=41, 16.2%), Africa (n=12, 4.7%), and Oceania (n=8, 3.2%). The majority of respondents represented academic medical centers (n=219, 78%) with >600 hospital beds (n= 139, 55%) and 1 to 100 ICU beds (n=186, 74%). The survey was most often completed by physicians (n=245, 96.9%), and a small proportion by pharmacists (n=7, 2.8%) and nurses (n=1, 0.4%), all representing their institution’s general or neurocritical care service. Institutional and critical-care team characteristics of the participating hospitals are presented in Table 1. Of the 70 nonrespondents, 23 (32.8%) were located in LMICs.
FIGURE 1.
World map showing the location of the 253 hospitals in 47 countries participating in the neurocritical care-COVID study.
TABLE 1.
Institutional and Critical Care Team Characteristics of the 253 Hospitals Participating in the Study by Country Income Level, Dedicated Neurocritical Care Unit Status, and by Surge of COVID-19 Patients
| Institutional Characteristics | Overall | Low/Middle-income Countries | High-income Countries | Dedicated Neurocritical Care Unit (NCCU) | General-mixed ICU (Gen-ICU) | Surge of COVID-19 Patients | No-surge of COVID-19 Patients |
|---|---|---|---|---|---|---|---|
| Total, n (%) | n=253 | 115 (45.5) | 138 (54.5) | 138 (54.5) | 115 (45.5) | 199 (78.6) | 54 (21.3) |
| Hospital beds | |||||||
| 1-200 | 31 (12.3) | 29 (25.2) | 2 (1.5) | 8 (5.8) | 23 (20) | 19 (9.6) | 12 (22.2) |
| 201-400 | 34 (13.4) | 24 (20.9) | 10 (7.3) | 10 (7.3) | 24 (20.9) | 24 (12.1) | 10 (18.5) |
| 401-600 | 49 (19.4) | 18 (15.7) | 31 (22.5) | 29 (21) | 20 (17.4) | 40 (20.1) | 9 (16.7) |
| >600 | 139 (54.9) | 44 (38.3) | 95 (68.8) | 91 (65.9) | 48 (41.7) | 116 (58.3) | 23 (42.6) |
| ICU beds | |||||||
| 1-50 | 108 (42.9) | 76 (66.1) | 32 (23.4) | 27 (19.7) | 81 (70.4) | 78 (39.2) | 30 (56.6) |
| 51-100 | 78 (30.9) | 23 (20) | 55 (40.2) | 53 (38.7) | 25 (21.7) | 67 (33.7) | 11 (20.8) |
| 101-150 | 34 (13.5) | 6 (5.2) | 28 (20.4) | 30 (21.9) | 4 (3.5) | 27 (13.6) | 7 (13.2) |
| >150 | 32 (12.7) | 10 (8.7) | 22 (16.1) | 27 (19.7) | 5 (4.4) | 27 (13.6) | 5 (9.4) |
| Academic Medical Center | 219 (86.6%) | 89 (77.4%) | 130 (94.2) | 93 (80.9) | 126 (91.3) | 172 (86.4) | 47 (87) |
| Level I NCCU* | 198 (77.5) | 72 (62.6) | 124 (89.9) | 120 (87) | 76 (66.1) | 156 (78.4) | 40 (74.1) |
| Comprehensive Stroke Center | 123 (48.6) | 34 (29.6) | 89 (64.5) | 77 (55.8) | 46 (40) | 103 (51.8) | 20 (37) |
| Level I Trauma Center | 119 (47) | 32 (27.8) | 87 (63) | 76 (55.1) | 43 (37.4) | 96 (48.2) | 23 (42.6) |
| NCCU beds (11-20 beds) | 53 (38.4) | 9 (20.4) | 44 (46.8) | 53 (38.7) | NA | 41 (37.3) | 12 (42.9) |
| Gen-ICU beds (11-20 beds) | 36 (31.3) | 29 (40.3) | 7 (16.3) | NA | 35 (31) | 25 (27.8) | 11 (44) |
| Neurocritical Care Fellowship | 97 (38.3) | 24 (20.9) | 73 (52.9) | 80 (58) | 17 (14.8) | 80 (40) | 17 (31.5) |
| Attending intensivists in NCCU | |||||||
| Neurology | 118 (46.6) | 24 (20.9) | 94 (68.1) | 91 (65.9) | 27 (23.5) | 96 (48.2) | 22 (40.7) |
| Anesthesiology | 92 (36.3) | 27 (23.5) | 65 (47.1) | 70 (50.7) | 22 (19.1) | 74 (37.2) | 18 (33.3) |
| Pulmonary | 66 (26.1) | 36 (31.3) | 30 (21.7) | 31 (22.5) | 35 (30.4) | 53 (26.6) | 13 (24.1) |
| General Surgery | 45 (17.8) | 23 (20) | 22 (15.9) | 28 (20.3) | 17 (14.8) | 40 (20) | 5 (9.3) |
| Emergency Medicine | 35 (13.8) | 9 (7.8) | 26 (18.8) | 25 (18.1) | 10 (8.7) | 30 (15.1) | 5 (9.3) |
| Pediatrics | 5 (2%) | 5 (4.4) | 0 | 1 (0.7) | 4 (3.5) | 4 (2) | 1 (1.9) |
| Attending intensivist in Gen-ICU | |||||||
| Pulmonary | 93 (36.8) | 52 (45.2) | 41 (29.7) | 26 (18.8) | 67 (58.3) | 73 (36.7) | 20 (37) |
| Anesthesiology | 75 (29.6) | 34 (29.6) | 41 (29.7) | 26 (18.8) | 49 (42.6) | 58 (29.2) | 17 (31.5) |
| Emergency Medicine | 45 (17.8) | 30 (26.1) | 15 (10.9) | 12 (8.7) | 33 (28.7) | 31 (15.6) | 14 (26) |
| General Surgery | 37 (14.6) | 16 (13.9) | 21 (15.2) | 10 (7.3) | 27 (23.5) | 31 (15.6) | 6 (11.1) |
| Other NCCU/gen-ICU staff | |||||||
| Advanced practice providers | 170 (67.5) | 74 (64.9) | 96 (69.6) | 101 (73.7) | 69 (60) | 136 (68.7) | 34 (63) |
| Critical care pharmacist | 114 (46.7) | 20 (18.7) | 94 (68.6) | 81 (61.8) | 33 (29.2) | 94 (48.7) | 20 (39.2) |
| Neurocritical care fellows | 97 (38.3) | 24 (20.9) | 73 (52.9) | 80 (58) | 17 (14.8) | 80 (40.2) | 17 (31.5) |
| Nonneurocritical critical care fellows | 149 (58.9) | 52 (45.2) | 97 (70.3%) | 91 (65.9) | 58 (50.4) | 121 (60.8) | 28 (51.9) |
| Rotating residents | 231 (91.3) | 96 (83.5) | 135 (97.8) | 133 (96.4) | 98 (85.2) | 182 (91.5) | 49 (90.7) |
| Rotating fellows | 191 (75.5%) | 73 (63.5) | 118 (86.1) | 120 (87.6) | 71 (61.7) | 155 (78.3) | 36 (66.7) |
Level 1 NCCUs offer a full complement of advanced monitoring, surgical and medical treatments, and have the capability to provide physician fellowship and advanced practice professional training as defined by the Neurocritical Care Society.
gen-ICU indicates general/mixed critical care unit; ICU, intensive care unit; NCCU, dedicated neurocritical care unit.
Epidemiology of the COVID-19 Patient Surge
At the time of the survey, most respondents (199, 79%) were in the midst of surge of COVID-19 patients or had already experienced a first surge. The average duration of the first surge was longer in HICs compared with LMICs (4.8, IQR 2.4 vs. 2.6, IQR 3.2 wk, respectively; P<0.001). During the follow-up semi-structured interview survey, 75 (52%) respondents reported having experienced 2 or more surges at their hospitals. These self-reported surge data aligned with publicly available data from the Institute for Health Metrics and Evaluation30 and World Health Organization31 on temporal trends in COVID-19 deaths by country (SDC 3: Surges in SARS-COV-2 deaths between 2020 and 2021 by country income level, http://links.lww.com/JNA/A463).
Changes to Neurocritical Care Delivery
Changes to neurocritical care delivery by World Bank country income level, NCCU and COVID-19 surge status are shown in Figure 2. Independent of country income level, NCCU and surge status, hospitals reported reductions in NCCU admissions (67%) and critical care drug shortages (69%), including shortages of analgesics and sedatives (n=149, 59%), neuromuscular blocking agents (n=105, 42%), bronchodilators (n=50, 20%), anti-epileptic drugs (n=50, 20%), and anti-hypertensive agents (n=14, 6%). In univariate analysis, hospitals in LMICs were more likely to report a shortage of anti-epileptic drugs (RR, 4.3; 95% CI, 2.9-7.9), respondents who had experienced the first surge were more likely to report a shortage of bronchodilators (RR, 2.6; 95% CI, 1.5-4.8), and hospitals with NCCUs were more likely to report a shortage of neuromuscular blocking agents (RR, 2.1; 95% CI, 1.3-3.5) (SDC 4: Impact of the SARS-CoV-2 pandemic on drug shortages by country income level, dedicated NCCU and surge of COVID-19 patients, http://links.lww.com/JNA/A464).
FIGURE 2.
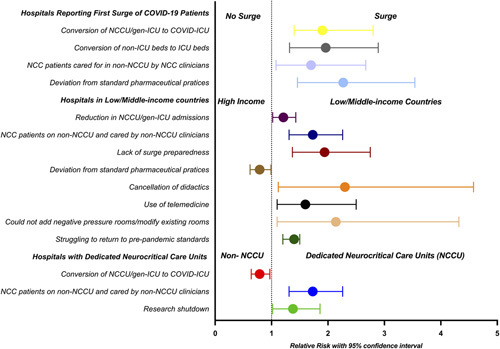
Univariate analysis highlighting changes to neurocritical care delivery by country income level, dedicated neurocritical care unit and surge of COVID-19 patient status. gen-ICU indicates general/mixed critical care unit; ICU, intensive care unit; NCCU, dedicated neurocritical care unit.
Respondents commonly reported deviations from standard pharmaceutical practices (n=144, 56%); these included use of bolus/infusion of benzodiazepines (n =90, 34%), enteral opioids for sedation (n=45, 18%), ketamine for analgo-sedation (n=50, 20%), fentanyl infusion (n=45, 18%), remifentanil infusion (n=20, 8%), inhalational anesthetics for sedation (n=3, 3.2%), switching from propofol-based to dexmedetomidine-based sedation (n=50, 20%), and use of nonformulary drugs (n=14, 6%). Other major adjustments included reduction in ancillary services (n=95, 43%), reduction in diagnostic testing (n=154, 61%; SDC 5: Diagnostic testing during the COVID-19 pandemic by country income level, dedicated NCCU and surge status, http://links.lww.com/JNA/A465), temporary cancellation of didactic teaching and other educational opportunities (n=141, 56%), temporary suspension of clinical and basic science research (n=177, 70%), and increased use of telemedicine (n=85, 34%).
Hospitals from LMICs were more likely to report a lack of adequate surge preparedness (OR, 3.2; 95% CI, 1.8-5.8), less likely to report conversion of non-ICU beds to ICU beds (OR, 0.5; 95% CI, 0.3-0.9), and more likely to report struggling to return to prepandemic standards of care (OR, 12.2; 95% CI, 4.4-34). Hospitals that had experienced the first surge of COVID-19 patients were more likely to report conversion of NCCU/gen-ICU beds to COVID-ICU beds (OR, 3.7; 95% CI, 1.9-7.3), conversion of non-ICU beds to ICU beds (OR, 3.4; 95% CI, 1.8-6.5), and deviations from standard critical care pharmaceutical practices (OR, 4.2; 95% CI, 2.1-8.2). Hospitals with a dedicated NCCU were less likely to report conversion to a COVID-ICU (OR, 0.5; 95% CI, 0.3-0.9) and less likely to report conversion of non-ICU to ICU beds (OR, 0.5; 95% CI, 0.3-0.9) (Fig. 2).
Semi-structured Interview
Of the 253 respondents to the initial survey, 148 (58%) participated in the follow-up semi-structured interview; 57 (39%) were from LMICs, 93 (63%) represented hospitals with a dedicated NCCU, 107 (73%) had experienced a first surge, 52 (36%) had experienced only one surge, and 75 (52%) had experienced 2 or more surges.
Respondents from 142 hospitals (96% of the participants in the semistructured interview) reported that the pandemic had substantially impacted neurocritical care delivery in their institution. The major themes that emerged from the semistructed interview were: diversion of NCC patients, admission of NCC patients to a non-NCCU or to an “isolation ICU,” a sentiment/perception that NCC patients might not have received the standard of care traditionally provided during the golden hour of a neurological emergency, burnout/low morale amongst health care providers, changes in nursing care (loss of supplemental nurse staffing pool/junior nurses not familiar with NCC patients), delay in presentation to hospital or for diagnostic testing, limited family visitation/mistrust of providers, and lack of exposure of residents/students to neurological cases and neurological examinations.
The majority of respondents (n=103, 70%) reported impact on research activities; 79 (55%) reported a temporary suspension of most research activities during the initial surge, 91 (63%) reported prioritization of COVID-19 research, and 86 (46%) reported reduced screening and enrollment in clinical trials. By the time of the interview, 85 (56%) respondents reported that research had not returned to prepandemic levels in their institution. Eighty-six (60%) respondents reported that the pandemic had affected the teaching of medical students and residents, and 81 (56%) that academic productivity of the critical care faculty had suffered because of the pandemic.
Only 37 (26%) respondents reported that they were better prepared to handle surges and only 26 (18%) that there was improved cohorting of COVID-19 patients in their hospitals. However, respondents also reported that the pandemic had changed some of their systems for the better; “virtual meetings were a possibility” (n=93, 65%), there was “improved critical care crosstalk between various ICUs and teams” (n=22, 15%), and “neurocritical care practitioners were more comfortable in managing severe injury and able to use new drugs for sedation” (n=13, 9%). One hundred twenty-four respondents (86%) across country income level, NCCU and surge status reported that they “struggled with lack of a new provider pool and were not able to add new equipment/space” (Fig. 2). Other areas highlighted from the semi-structured interviews are summarized in Figure 3; specific quotations related to the major themes (divided into opportunities and challenges) are included in the supplementary material (SDC 6: Quotations related to the impact of the SARS-CoV-2 pandemic on neurocritical care delivery, http://links.lww.com/JNA/A466).
FIGURE 3.
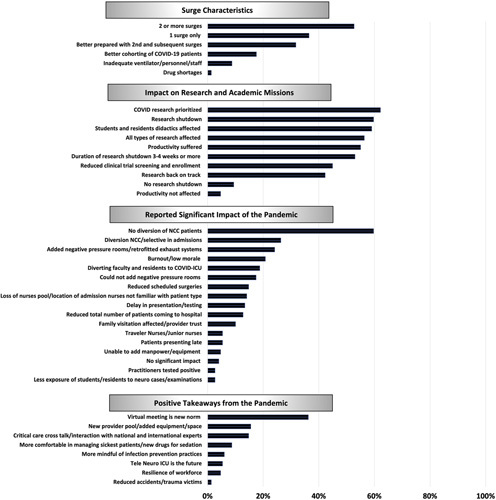
Themes regarding the impact of the COVID-19 pandemic on neurocritical care delivery. ICU indicates intensive care unit; NCC, neurocritical care.
DISCUSSION
This global survey explored the impact of COVID-19 pandemic on delivery of care to patients requiring neurological/neurosurgical critical care, stratified by World Bank country income level, presence of dedicated NCCU and COVID-19 surge status. The main findings are: (1) independent of country income level, dedicated NCCU and surge status, hospitals across the world reported fundamental changes to neurocritical care delivery, including reductions in critical resources, diagnostic testing, education and academic activities, during the pandemic; (2) hospitals in LMICs were more likely to be insufficiently prepared to deal with COVID-19 surges and struggled to return to prepandemic standards than those in HICs; (3) dedicated NCCUs were less likely to be converted partially or completely to a COVID-19 ICU; (4) hospitals experiencing surge(s) were more likely to accommodate patients with COVID-19 by conversion of NCCUs and gen-ICUs to COVID-ICUs and by increasing the number of non-ICU beds, and were more likely to report drug shortages and deviation from standard critical care pharmaceutical practices; (5) the pandemic continues to challenge the healthcare workforce by introducing conflicts in care paradigms while at the same time presenting opportunities to improve future care delivery, and; (6) the pandemic affected healthcare provider morale with many survey respondents self-reporting concerns about burnout. This is the first description of the global impact of the COVID-19 pandemic on neurocritical care delivery.
Impact of the COVID-19 Pandemic on Neurocritical Care Delivery by Country Income Level
Respondents from LMICs were more likely to report inadequate surge preparedness and a struggle to return to pre-pandemic standards of care than those from HICs. The pandemic may affect hospitals from LMICs differently, partly because of baseline differences in availability of health care resources and neurocritical care organizations compared with HICs.11 Study participants raised concerns regarding disruption of access to healthcare in already challenged health systems in LMICs.32 Physicians may face the arduous task of triaging resources based on a patient’s age, premorbid status and disease severity. Moreover, once a NCC patient is admitted to an ICU in a LMIC, they may not be routinely cared for by a neurocritical care specialist because dedicated NCCUs are less prevalent in LMICs.11 While the impact of the COVID-19 pandemic on NCC patient outcomes based on country income level status is not known, non-neurological patients in LMICs have been reported to have worse outcomes than those in HICs during the pandemic.33 Moving forwards, the information gathered in this study complements other reports, and supports calls for policymakers and governments to advocate for investment in critical care health service delivery.
Impact of the COVID-19 Pandemic on Neurocritical Care Delivery by Dedicated NCCU Status
Our study found that dedicated NCCUs (predominantly present in HICs) were less likely than gen-ICUs to be partially or completely converted to a COVID-ICU. However, many respondents perceived that NCC patients might not have received the usual standards of care traditionally provided during the golden hour of a neurological emergency compared with prepandemic standards of care. This study reignites the discussion of whether outcomes are improved when NCC patients are admitted to a dedicated NCCU13,34,35 or managed by a dedicated neurocritical care service.36 The association between admissions during the COVID-19 surge and NCC patient outcomes deserves further investigation, especially as this study suggests that the presence of a dedicated NCCU may help preserve care for patients requiring neurological and neurosurgical critical care and prevent marginalization.
Impact of the COVID-19 Pandemic on Neurocritical Care Delivery by Surge Status
Our study findings are in agreement with other reports on COVID-19 surges.37 When facing the anticipated first surge, many ICUs shifted priorities to accommodate COVID-19 patients,38 balancing the care of critically ill COVID-19 and non-COVID-19 patients.39 There are conflicting data on disease severity and mortality in the pandemic period compared with prepandemic trends.40–43 Early in the pandemic, critical care capacity for NCC patients was preserved by a temporary suspension of elective non-neurological and neurological surgeries, including elective endovascular, cranioplasty, and open cerebrovascular procedures.44,45 Strategies to generate additional staffing for ICU teams included: (a) reallocation or redeployment of existing ICU clinicians by specialty and subspecialty training; (b) utilization of non-ICU based clinicians, such as registered certified nurse anesthetists, to participate in routine critical care delivery; (c) return to work of retired physicians and nurses,9 and; (d) recruitment of traveler nurses or per diem personnel. Medication shortages were reported by more than two-thirds of respondents’ that had experienced a first surge of COVID-19 patients; the reported pharmaceutical practices confirmed shortages and strategies to mitigate shortages as previously published.46–51 In light of the increasing necessity to navigate medication shortages in the ICU, including alternative medication administration strategies, formulary management, and medication procurement from nontraditional manufacturers or wholesalers, the pandemic has confirmed the importance of the role of critical care pharmacists.48 Further analysis of the impact of the significant changes in patient volume and hospital services on morbidity and mortality is warranted.
According to our study participants, the impact of the pandemic was felt long after the first surge when many hospitals struggled to return to prepandemic care standards. We hypothesize that in addition to the location of a hospital by country income level status, the peak (number of deaths) and the tail (how long) of the surge may have played an important role in how quickly hospitals could recover from the effects of a surge in COVID-19 patients.
Preserving Neurocritical Care Delivery Amidst Global Health Crises
The results of our study validate pandemic preparedness recommendations.9 While the pandemic tested resolve and resilience, it also provided opportunities for improvements to prepandemic standards of critical care workflow. There was a wide range of emotions expressed related to the pandemic’s impact and, as much as there are opportunities, there remain conflicts. The major opportunities include implementation of tele-medicine into neurocritical care services for patients admitted to hospitals with limited or no infrastructure to handle neurocritical care emergencies. Widespread use and familiarity with virtual conferencing have provided opportunities to build bridges with local, regional, national and international experts. Hospitals may consider investments in creating healthcare resource pools allowing subspeciality clinicians to focus on neurocritical care while maintaining adequate resources for COVID-19 patients. There are significant challenges to preserving care standards in resource-limited countries, and this is something to which the world needs to pay close attention.
Strengths and Limitations
The main strengths of this study are the gathering of information from all 7 geographical regions of the world, the high response rate and clustering of results by the institution. The limitations include those of a survey-based study, specifically that our results represent perceptions regarding critical care delivery which may be biased. Respondents from different regions may over/underestimate both positive and negative findings based on cultural biases and differences in perceptions, and have a tendency to over or under-report shortages and concerns. Also, though we made every effort to obtain information over a long period to account for the timing of the various COVID-19 surges that occurred worldwide, differences in timing between the survey and individual institutional surges may have affected the results due to recall bias. The self-reported data related to the surges are corroborated by data received from the Institute for Health Metrics and the World Health Organization regarding the worldwide distribution of deaths associated with COVID-19. Most respondents reported working primarily at large academic medical centers, so our results may not be generalizable to all health care institutions. Further quantitative studies from individual institutions as well as at a regional and national levels are needed. The survey’s primary language was English. Although we presented translated versions to respondents from non–English-speaking countries, this may have affected the overall response rate and the responses to survey questions from countries where English is not the first language. Since most of the participants were physicians, the perspectives of nurses, pharmacists and other critical care clinicians and hospital administration leadership are unknown and deserve further examination. Given the length of the survey, questions were asked only once and in a specific order, with limited internal consistency testing. The nature of the response options may lend itself to acquiescence bias. The more qualitative questions (eg, whether or not adequate time was available to plan) may be viewed as highly subjective, and responses to them may vary individually and culturally even within a single country.
CONCLUSIONS
This study reports the perceived impact of the COVID-19 pandemic on global neurocritical care delivery; it highlights shortcomings in health care infrastructure, the importance of pandemic preparedness, and the key concerns of neurocritical care providers. Our findings can serve as a guideline to prepare for future surges, especially given the likelihood of emergence of more transmissible variants of SARS-CoV-2.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jnsa.com.
ACKNOWLEDGMENTS
The authors would like to acknowledge the following prelicensure nursing students from the Rush University College of Nursing who assisted with data collection: Brielle Cobb, Troy Meikle, Andrei Collins, Courtney Adler, Laurencia Ouedraogo, Nhung Vo, Andrew McFee, Kellie Garvey, Jennifer Goodfriend, Maria Menjivar & Sabrina Jose, and the following for contributing data: Fabricio J. Vera, Ecuador, Alexandra M. Saraguro Orozco, Ecuador, Luis F. Mamani Cruz, Paraguay, María A. Cruz, Brazil, Sotomayor Ramírez Francisco J., Argentina, Nadia S. Garófalo Sarro, Argentina, Joel IS Villalba, Argentina.
APPENDIX
NCC-COVID Study Collaborators: North America: H.E. Hinson, Oregon Health Sciences University, Portland, OR. Casey M. Olm-Shipman, University of North Carolina, Chapel Hill, NC. Ivan Da Silva, Rush University Medical Center, Chicago, IL. Anna M. Cervantes-Arslanian, Boston University School of Medicine, Boston, MA. Andrew P. Carlson, The University of New Mexico, Albuquerque, NM. Sanjeev Sivakumar, The University of South Carolina School of Medicine, Greenville, SC. Vishank A. Shah, The University of Arkansas for Medical Sciences, Little Rock, AR. Jordan B. Bonomo, University of Cincinnati, Cincinnati, OH. Kevin W. Hatton, University of Kentucky. Lexington, KY. Gregory Kapinos, NYC Kings County and SUNY Downstate College of Medicine, Brooklyn, NY. Christopher G. Hughes, Vanderbilt University Medical Center, Nashville, TN. Gloria M Rodríguez-Vega, HIMA San Pablo Caguas, San Juan, Puerto Rico. Shraddha Mainali, Ohio State University, Columbus, OH. Cherylee W.J. Chang, The Queen’s Medical Center, Honolulu, HI. Jonathan Dissin, Einstein Medical Center Philadelphia, PA. Jing Wang, Inova Fairfax Hospital, Chantilly, VA. Patrick T Mailloux, Maine Medical Center, ME. Rajat Dhar, Washington University in St. Louis, St. Louis, MO. Bhiken I. Naik, University of Virginia, Charlottesville, VA. Aarti Sarwal, Wake Forest Baptist Health, Winston-Salem, NC. Susanne Muehlschlegel, University of Massachusetts Medical School, Boston, MA. Christa O’Hana S. Nobleza, University of Mississippi Medical Center, Jackson, MS. Angela Hays Shapshak, University of Alabama, Birmingham, AL. David A. Wyler, Thomas Jefferson University Hospital and the Jefferson Hospital for Neuroscience, Philadelphia, PA. Julius Gene S Latorre, SUNY Upstate Medical University, Syracuse, NY. Panayiotis N Varelas, Albany Medical College, Albany NY. Safdar A Ansari, University of Utah, Salt Lake City, UT. Vijay Krishnamoorthy, Duke University, Durham, NC. Shyam S. Rao, Alpert Medical School of Brown University, Providence, RI. Demetrios J Kutsogiannis The University of Alberta, Edmonton, Canada. Ivan Da Silva, Rush University Medical Center, Chicago, IL. Yama Akbari, University of California, Irvine, CA, USA. Kathryn Rosenblatt, Johns Hopkins University School of Medicine, Baltimore, MD. Debra E Roberts, University of Rochester Medical Center, Rochester, NY. Jennifer A. Kim, Yale University, New Haven, CT. Ayush Batra, Feinberg School of Medicine, Northwestern University, Chicago, IL. Vasisht Srinivasan, University of Cincinnati, Cincinnati. OH. Craig A. Williamson, University of Michigan, Ann Arbor, MI. Xuemei Cai, Tufts Medical Center, Boston, MA. Pravin George, Cleveland Clinic Cerebrovascular Center, Cleveland, OH. Michael A. Pizzi, University of Florida, Gainesville, FL. K H Kevin Luk, Mayo Clinic, Arizona, USA. Karen Berger, New York-Presbyterian Hospital/Weill Cornell Medical Center, New York City, NY. Marc-Alain Babi, University of Florida, Gainesville, FL. Karen G. Hirsch, Stanford University, Stanford, CA. Cappi C. Lay, Mount Sinai School of Medicine, New York City, NY. Gabriel V. Fontaine, Intermountain Healthcare, Intermountain Medical Center, Ariane Lewis, NYU Langone Medical Center, New York City, NY. Amanda B Lamer-Rosen, Cedars-Sinai Medical Center, Los Angeles, CA. Atul Kalanuria, University of Pennsylvania, Philadelphia, PA. Ayaz M. Khawaja, Wayne State University, Detroit, MI. Alejandro A. Rabinstein, Mayo Clinic, Rochester, MN. Charles M. Andrews, Medical University of South Carolina, SC. Neeraj Badjatia, University of Maryland School of Medicine, Baltimore, MD. David L. McDonagh, UT Southwestern, TX. Venkatakrishna Rajajee, University of Michigan, Ann Arbor, MI. Keith E. Dombrowski, USF/TGH Tampa General Hospital, Tampa, FL. Justin D. Daniels, University of Kansas Medical Center, Kansas City, KS. Kristine H O’Phelan, University of Miami, Miller School of Medicine, Miami, FL. Kara L. Birrer, Orlando Regional Medical Center/Orlando Health, Orlando, FL. Nicole C Davis, The Mount Sinai Hospital, NY. Kaylee K. Marino, Brigham and Women’s Hospital, Boston, MA. Fanny Li, University of California San Francisco, CA. Archit Sharma University of Iowa Hospitals and Clinics, Iowa City, IA. Eljim P. Tesoro, University of Illinois Health, Chicago, IL. Ofer Sadan, University School of Medicine, Atlanta GA. Yatin B. Mehta, Geisinger Medical Center, PA. Myles Dustin Boone, Dartmouth Hitchcock Medical Center, NH. Colleen Barthol, University Health System, San Antonio, TX. Hubiel J. López Delgado, CEDIMAT, Dominican Republic. García Arellano Maricela, Instituto Mexicano del Seguro Social Centenario Hospital Miguel Hidalgo, Mexico. Julio C. Mijangos-Mendez, Hospital Civil de Guadalajara “Fray Antonio Alcalde”, Guadalajara, Jalisco Mexico. Jose A. Lopez-Pulgarin, Hospital Civil de Guadalajara, Guadalajara, México. Luke A. Terrett, Saskatchewan Health Authority, University of Saskatchewan, Saskatchewan, Canada. Andrea Rigamonti, St. Michael’s Hospital, Unity Health Toronto, University of Toronto, Canada. Philippe Couillard, University of Calgary, Calgary, Canada. Michaël Chassé, University of Montreal Health Center (CHUM), Montreal, Canada. Hosam M Al-Jehani, Imam Abdulrahman bin Faisal University, and McGill University, Canada. Latin America: Eleonora R. Cunto, Médica, Jefa de Terapia Intensiva del Hospital de Infecciosas F J Muñiz, Buenos Aires, Argentina. Luis M. Villalobos, Medicina Vascular Sanatorio Mariano Pelliza, Buenos Aires, Argentina. Nicolás S. Rocchetti, Hospital Eva Peron, Granadero Baigorria, Santa Fe, Argentina. Gabriela Aparicio, Hospital de Niños Sor María Ludovica, La Plata, Argentina. Gustavo G. Domeniconi, Sanatorio de la Trinidad San Isidro, Argentina. Nicolas A. Gemelli, Terapia Intensiva del Hospital Italiano de Buenos Aires, Argentina. Mariana F. Badano, Hospital Italiano de La Plata, La Plata, Argentina. Cesar M. Costilla, Sanatorio Güemes, Buenos Aires, Argentina. Paula Caporal, Hospital del Niños, La Plata, Argentina. Sebastián Camerlingo, Universidade de Buenos Aires, Argentina. Carina Balasini, Hospital Pirovano, CABA, Argentina. Rossana G. López, ICU Clínica Pueyrredón, Mar del Plata, Buenos Aires, Argentina. Mauri Mario, Hospital Nacional Prof. A. Posadas, El palomar, Argentina. Santiago A. Ilutovich, Sanatorio de la Trinidad Mitre, Buenos Aires, Argentina. Gabriela V. Torresan, Hospital Nodal de Venado Tuerto, Argentina. Ana M Mazzola, Hospital San Felipe, San Nicolas, Argentina. Daniela E. Olmos K., Príncipe de Asturias, Córdoba, Argentina. Roberto Mérida Maldonado, Hospital San Juan de Dios, Tarija, Bolivia. Gustavo La Fuente Zerain. Universitario Japones, Bolivia. Wellingson Silva Paiva, University of Sao Paulo Medical School, São Paulo, Brazil. Antônio Eiras Falcão, UNICAMP -University of Campinas, São Paulo, Brazil. Salomón Rojas, Beneficência Portuguesa Hospital, São Paulo, Brazil. Gilberto Paulo Pereira Franco, Hospital Universitario Júlio Muller, Brazil. Renata A. Azevedo, Real Hospital Portugues de Beneficência, Pernambuco, Brazil. Pedro Kurtz, Instituto Estadual do Cérebro Paulo Niemeyer, Rio de Janeiro, Brazil. Flor G. Balbo, Hospital Regional de Antofagasta, Chile. Jose N. Carreno, Fundacion Santa Fe de Bogotá, Colombia. Andres M. Rubiano, El Bosque University, Bogota, Colombia. Juan Diego Ciro, Clínica de Las Americas, Medellín, Colombia. Zulma Urbina C, Hospital Meoz, Cúcuta, Colombia. Diego Barahona Pinto, Hospital de los Valles, Quito, Ecuador. Pedro César Gutiérrez Gómez, Llano A. Miguel Hospital de las FF. AA., Quito, Ecuador. Castillo L, Catholic University, ICU Hospital Barra Luco Santiago de Chile, Chile. Jorge Luis Ranero, Instituto Guatemalteco de Seguridad Social, Guatemala. Julio C. Apodaca, Hospital de Trauma Manuel Giagni, Asunción, Paraguay. Natalia E. Gómez Arriola, Hospital de Emergências Médicas, Paraguay. Rocío Nájar Reátegui, Médico Intensivista, Lima, Peru. Maria M Chumbe, Instituto Nacional de Ciências Neurológicas, Lima, Perú. dra Xandra Yanina Rodriguez Tucto, Hospital Edgardo Rebagliati, Lima, Perú. Rafael E Davila Flores, Jacobo E, Mora, Hospital Universitario Luís Razetti, Barcelona, Venezuela. Middle East: Faisal Abdulrahman Al-Suwaidan, King Fahad Medical City—Central Second Health Cluster, Riyadh, Saudi Arabia. Yasser B. Abulhasan, Kuwait University, Kuwait. Africa: Hanna Demissie Belay, College of Health Science, Addis Ababa University, Tikur Anbesa Specialized Hospital, Ethiopia. Dawit K. Kebede, College of Health Sciences, Addis Ababa University, Tikur Anbessa Specialized Hospital, Ethiopia. Mulugeta Biyadgie Ewunetu Tebebe Ghion specialized hospital, Ethiopia. Sisay Molla, Hiwot Fana Specialized Teaching Hospital, Ethiopia. Fitsum Alemu Tulu, Yekatit 12 hospital medical college, Addis Ababa, Ethiopia. Senay A. Gebremariam, Mekelle University, College of Health Sciences, Ethiopia. Houyam Tibar, Regional hospital of Guelmim. Guelmim, Morocco. Fasika Tesfaneh Yimer, University of Namibia, Namibia. Temitope Hannah Farombi, University of Ibadan, University College Hospital, Ibadan, Nigeria. Nshimiyimana Francios Xavier, University of Rwanda (UR), University Teaching Hospital of Kigali (CHUK), Rwanda. Jama Osman, Hargeisa Group hospital, Somaliland. Llewellyn C. Padayachy, University of Pretoria, Steve Biko Academic Hospital, South Africa. Europe: Margot J. Vander Laenen, Ziekenhuis Oost Limburg, Genk, Belgium. Tomislav Breitenfeld, Sestre milosrdnice University Hospital Center, Zagreb, Croatia. Riikka Takala, Turku University Hospital, Turku, Finland. Sigismond Lasocki, Département Anesthésie Réanimation, CHU Angers, Francs. Patrick Czorlich, University Medical Center Hamburg-Eppendorf, Department of Neurosurgery, Hamburg, Germany. Sven Poli, Hertie Institute for Clinical Brain Research, University Hospital Tübingen, Tübingen, Germany. Bernhard Neumann University of Regensburg, Bezirksklinikum, Regensburg, Germany. Piergiorgio Lochner, Saarland University Medical Center Homburg, Germany. Sanjay Menon, Klinikum Aschaffenburg-Alzenau, Germany. Katja E. Wartenberg, University of Leipzig, Leipzig, Germany. Stefan Wolf, Charite University Medicine, Berlin, Germany. Nima Etminan, University Hospital Mannheim, University of Heidelberg, Germany. Juergen Konczalla, Goethe-University Hospital Frankfurt, Germany. Gerrit A. Schubert, RWTH Aachen University, Aachen, Germany. Matthias Wittstock, University Medicine Rostock, Rostock, Germany. Julian Bösel, Klinikum Kassel, Kassel, Germany. Chiara Robba, Policlinico San Marrtino, IRCCS for Oncology and Neuroscience, Genova Italy. Alessandro De Cassai, University Hospital of Padova, Padova, Italy. Daniela Alampi, Sapienza University of Rome, Italy. Nicola Zugni, University of Brescia at Spedali Civili Hospital, Brescia, Italy. Ennio Fuselli, San Camillo-Forlanini, Rome, Italy. Federico Bilotta, Rome University “Sapienza”, Rome, Italy. Eleonora Stival, MD Fondazione Policlinico Agostino Gemelli Roma, Italy. Carlo Alberto Castioni. Ospedale San Giovanni Bosco, Torino, Italy. Eleonora Tringali, Dirigente Medico Anestesia Catania, Sicountry income levely, Italy. Domenico Gelormini, A.O.U.I. Verona Università Cattolica del Sacro Cuore, Italy. Celeste Dias, Centro Hospitalar São João, Porto, Portugal. Rafael Badenes. Hospital Clínic Universitari de Valencia. Spain. Luis A. Ramos-Gómez. Hospital General de La Palma. Canary Islands, Spain. Juan A. Llompart-Pou. Hospital Universitari Son Espases, Palma, Spain. Susana Altaba Tena, Hospital General Universitario De Castellan, Spain. Paolo Merlani, Ente Ospedaliero Cantonale, Ticino, Switzerland. Walter M. van den Bergh, University Medical Center Groningen, University of Groningen, The Netherlands. Cornelia W. Hoedemaekers, Institute: Radboudumc, Nijmegen, The Netherlands. Wilson F. Abdo, Institute: Radboudumc, Nijmegen, The Netherlands. Mathieu van der Jagt, University Medical Center Rotterdam, The Netherlands. Sergii Gorbachov, Zaporozhye State Medical University, Ukraine. J E. Dinsmore, St Georges Healthcare NHS Trust, London, England, UK. Ugan Reddy, National Hospital for Neurology and Neurosurgery, London, UK. L Tattum, London, United Kingdom. Anders Aneman, Liverpool Hospital, Liverpool, UK. Jonathan K.J. Rhodes, University of Edinburgh, and NHS Lothian, UK. Asia: Pak Sopheak, Khmer-Soviet Friendship Hospital, Phnom Penh, Cambodia. Song Jian The General Hospital of Chinese People’s Liberation Army Central Theater Command, Wuhan, China. Matthew TV Chan, The Chinese University of Hong Kong, Hong Kong, China. Masao Nagayama, International University of Health and Welfare Graduate School of Medicine, Narita City, Japan. Hidenori Suzuki, Mie University Hospital, Tsu, Japan. Ankur Luthra, PGIMER, Chandigarh, India. Kapil G. Zirpe, Ruby Hall Hospitals, Pune, India. Pratheema R. Apollo Specialty hospitals, Chennai, India. Manikandan Sethuraman, SCTIMST, Trivandrum, India. Swagata Tripathy, AIIMS Bhubaneswar, India. Charu Mahajan, AIIMS, New Delhi, India. Kallol Deb, Institute of Neurosciences Kolkata, India. Devendra Gupta, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India. Nidhi Gupta, Indraprastha Apollo Hospitals, New Delhi, India. Indu Kapoor, AIIMS, New Delhi, India. Monica S. Tandon, G.B. Pant Institute of Postgraduate Medical Education and Research, New Delhi, India. Vasudha Singhal, Medanta- the Medicity, Gurugram, India. Anil Parakh, Global Hospital, Mumbai, India. Srilata Moningi, Nizam’s institute of medical sciences, Hyderabad, India. Mudit Garg, Max Multi-Specialty Hospital in Dehradun, India. Kavita Sandhu, Max Superspeciality Hospital, Saket, New Delhi, India. Zulfiqar Ali, Sher-i-Kashmir Institute of Medical Sciences, Soura, Srinagar, Jammu and Kashmir, India. Dr (Col) Vivek Bharti Sharma, Army Hospital R & R, New Delhi, India. Subodh Kumar, GMCH, Chandigarh, India. Prashant Kumar, Pt. BDS PGIMS at University of Health Sciences, Rohtak. Haryana. India. Deepesh G. Aggarwal, Saifee Hospital, Mumbai, India. Urvi B Shukla, Symbiosis University Hospital and Research Centre and Symbiosis Medical College for Women, India. Subhal Dixit, Sanjeevan Hospital, Pune, India. Shahriar Nafissi, Hariati Hospital, Tehran University of Medical Sciences, Tehran, Iran. Majid Mokhtari, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Gentle S. Shrestha, Tribhuvan University Teaching Hospital, Maharaj Gunj, Kathmandu, Nepal. Shanmugam Puvanendiran, General Teaching Hospital, Sri Lanka. Sarunkorn Sakchinabut, Phahonphonphayuhasena Hospital, Kanchanaburi, Thailand. Jeerawat Kaewwinud, Surin Hospital, Surin, Thailand. Porntip Thirapattaraphan, Sorayouth Chumnanvej, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand. Suttasinee Petsakul, Prince of Songkla University, Songkla, Thailand. Pruchwilai Nuchpramool, Vajira Hospital, Thailand. Phongsak Nitikaroon, Prapokklao Hospital, Chanthaburi, Thailand. Niyutta Thaksin, Uttaradit Hospital, Uttaradit, Thailand. Jirapong Vongsfak, Chiang Mai University, Chiang Mai, Thailand. Gemmalynn B. Sarapuddin, The Medical City and Quirino Memorial Medical Center, Quezon City, Philippines. Tuan Van Bui, MD, Cho Ray Hospital, Vietnam. Oceania: Ian M. Seppelt, The University of Sydney, Sydney, Australia. Deepak Bhonagiri, Western Sydney University, Macquarie University, Sydney, Australia. James R. Winearls, Gold Coast University Hospital, Brisbane, Australia. Oliver J. Flower, Royal North Shore Hospital, Sydney, Australia. Torgeir A. Westerlund, John Hunter Hospital and University of Newcastle, Australia. Wout Van Oosterwyck, Royal Adelaide Hospital, Adelaide, Australia.
Footnotes
A.V.L.: study design, data collection, data analysis, interpretation of results, manuscript draft, review, and submission, and approval of the submitted manuscript. S.W., B.A., M.A.G., S.H.-Y.C., G.C., S.E., C.D.-N., D.J.G., M.K.-T., N.K., M.A.K., M.L., S.L.L., J.M.-M., K.M., H.M., L.T., C.P.V.R., A.A.U., W.V., and A.M.M.: study design, interpretation of results, manuscript draft, review, and approval of the submitted manuscript.
The NCC-COVID Study Collaborators are listed in the Appendix.
A.V.L. reports receiving research support from Aqueduct Critical Care and salary support from LifeCenter Northwest. D.J.G. receives research support from a Centers for Biomedical Research Excellence grant from the National Institute of General Medical Sciences (1P20GM139745-01). A.A.U. has received in-kind clinical trial support (study consumables) from Integra Lifesciences. S.H.-Y.C. reports research funding from the National Center for Advancing Translational Sciences (NCATS) UL1 TR001857, the National Institutes of Neurological Disorders and Stroke (NINDS) R21NS113037, and the University of Pittsburgh. S.L.L. reports receiving consulting fees from Lombardi-Hill/Stroke Challenges LLC. The remaining authors have no conflicts of interest to declare.
Contributor Information
Abhijit V. Lele, Email: abhijit2@uw.edu.
Sarah Wahlster, Email: wahlster@uw.edu.
Bhunyawee Alunpipachathai, Email: bhunyav@hotmail.com.
Meron Awraris Gebrewold, Email: merianair@yahoo.com.
Sherry H.-Y. Chou, Email: sherry.chou@northwestern.edu.
Gretchen Crabtree, Email: gretchen.crabtree@gmail.com.
Shane English, Email: senglish@toh.ca.
Caroline Der-Nigoghossian, Email: cad9105@nyp.org.
David J. Gagnon, Email: DGagnon@mmc.org.
May Kim-Tenser, Email: May.Kim@med.usc.edu.
Navaz Karanjia, Email: nkaranjia@health.ucsd.edu.
Matthew A. Kirkman, Email: matthew.kirkman@gmail.com.
Massimo Lamperti, Email: docmassimomd@gmail.com.
Sarah L. Livesay, Email: sarahlynnlivesay@yahoo.com.
Jorge Mejia-Mantilla, Email: jorge.mejia.m@me.com.
Kara Melmed, Email: kara.melmed@gmail.com.
Hemanshu Prabhakar, Email: prabhakaraiims@yahoo.co.in.
Leandro Tumino, Email: leandrotumino@gmail.com.
Chethan P. Venkatasubba Rao, Email: cprao@bcm.edu.
Andrew A. Udy, Email: andrew@udy.com.
Walter Videtta, Email: wvidetta@gmail.com.
Asma M. Moheet, Email: asmamoheet@gmail.com.
REFERENCES
- 1.World Health Organization. Coronavirus disease (COVID-19), Situation Report-135. 2021. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed August 14, 2021.
- 2.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji Y, Ma Z, Peppelenbosch MP, et al. Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob Health. 2020;8:e480. doi: 10.1016/S2214-109X(20)30068-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moheet AM, Shapshak AH, Brissie MA, et al. Neurocritical care resource utilization in pandemics: a statement by the Neurocritical Care Society. Neurocrit Care. 2020;33:13–19. doi: 10.1007/s12028-020-01001-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moghadas SM, Shoukat A, Fitzpatrick MC, et al. Projecting hospital utilization during the COVID-19 outbreaks in the United States. Proc Natl Acad Sci U S A. 2020;117:9122–9126. doi: 10.1073/pnas.2004064117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suarez JI, Martin RH, Bauza C, et al. Worldwide organization of neurocritical care: results from the PRINCE study part 1. Neurocrit Care. 2019;32:172–179. doi: 10.1007/s12028-019-00750-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leira EC, Russman AN, Biller J, et al. Preserving stroke care during the COVID-19 pandemic: potential issues and solutions. Neurology. 2020;95:124–133. doi: 10.1212/WNL.0000000000009713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bershad EM, Feen ES, Hernandez OH, et al. Impact of a specialized neurointensive care team on outcomes of critically ill acute ischemic stroke patients. Neurocrit Care. 2008;9:287–292. doi: 10.1007/s12028-008-9051-5 [DOI] [PubMed] [Google Scholar]
- 14.Fuentes B, Alonso de Lecinana M, Calleja-Castano P, et al. Impact of the COVID-19 pandemic on the organisation of stroke care. Madrid Stroke Care Plan. Neurologia. 2020;35:363–371. doi: 10.1016/j.nrl.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghoreishi A, Arsang-Jang S, Sabaa-Ayoun Z, et al. Stroke care trends during COVID-19 pandemic in Zanjan Province, Iran. From the CASCADE Initiative: Statistical Analysis Plan and Preliminary Results. J Stroke Cerebrovasc Dis. 2020;29:105321. doi: 10.1016/j.jstrokecerebrovasdis.2020.105321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markus HS, Brainin M. COVID-19 and stroke-A global World Stroke Organization perspective. Int J Stroke. 2020;15:361–364. doi: 10.1177/1747493020923472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer D, Meyer BC, Rapp KS, et al. A stroke care model at an academic, comprehensive stroke center during the 2020 COVID-19 pandemic. J Stroke Cerebrovasc Dis. 2020;29:104927. doi: 10.1016/j.jstrokecerebrovasdis.2020.104927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montaner J, Barragan-Prieto A, Perez-Sanchez S, et al. Break in the stroke chain of survival due to COVID-19. Stroke. 2020;51:2307–2314. doi: 10.1161/STROKEAHA.120.030106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sylaja PN, Srivastava MVP, Shah S, et al. The SARS-CoV-2/COVID-19 pandemic and challenges in stroke care in India. Ann N Y Acad Sci. 2020;1473:3–10. doi: 10.1111/nyas.14379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Chen Y, Li Z, et al. Providing uninterrupted care during COVID-19 pandemic: experience from Beijing Tiantan Hospital. Stroke Vasc Neurol. 2020;5:180–184. doi: 10.1136/svn-2020-000400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao J, Li H, Kung D, et al. Impact of the COVID-19 epidemic on stroke care and potential solutions. Stroke. 2020;51:1996–2001. doi: 10.1161/STROKEAHA.120.030225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao J, Rudd A, Liu R. Challenges and potential solutions of stroke care during the coronavirus disease 2019 (COVID-19) outbreak. Stroke. 2020;51:1356–1357. doi: 10.1161/STROKEAHA.120.029701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wahlster S, Wijdicks EFM, Patel PV, et al. Brain death declaration: practices and perceptions worldwide. Neurology. 2015;84:1870–1879. doi: 10.1212/WNL.0000000000001540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.United Councountry income level of Neurologic S. UCNS Fellowship Directory (Neurocritical Care). 2020. Available at: https://www.ucns.org/Online/Fellowship_Directory/Online/Fellowship_Directory.aspx?hkey=ca0abd99-aad7-4e31-b735-6baddea75ca2. Accessed May 15, 2020.
- 25.Latinamerican Brain Injury Consortium. 2020. Available at: http://labic.org/. Accessed June 1, 2020.
- 26.SATI—Sociedad Argentina De Terapia Intensiva. 2020. Available at: https://www.sati.org.ar/ Published 2020. Accessed June 1, 2020.
- 27.The World Bank. World Bank Country and Lending Groups. The World Bank Group. 2021. Available at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed May 15, 2020.
- 28.Dedoose. A web application for managing, analyzing, and presenting qualitative and mixed method research data [computer program]. Version 8.0 35. Los Angeles, CA: SocioCultural Research Consultants, LLC 2018.
- 29.Stata Statistical Software, Release 15 [computer program]. College Station, TX, 2017.
- 30.Institute for Health Metrics and Evaluation (IHME). COVID-19 projections. Population Health Building/Hans Rosling Center. 2021. Available at: https://covid19.healthdata.org/global?view=total-deaths&tab=trend. Accessed March 15, 2021.
- 31.World Health Organization. COVID-19 explorer. 2021. Available at: https://worldhealthorg.shinyapps.io/covid/. Accessed August 14, 2021.
- 32.Okereke M, Ukor NA, Adebisi YA, et al. Impact of COVID-19 on access to healthcare in low- and middle-income countries: Current evidence and future recommendations. Int J Health Plann Manage. 2021;36:13–17. doi: 10.1002/hpm.3067 [DOI] [PubMed] [Google Scholar]
- 33.Chew NW, Ow ZGW, Teo VXY, et al. The global impact of the COVID-19 pandemic on STEMI care: a systematic review and meta-analysis. Can J Cardiol. 2021. doi: 10.1016/j.cjca.2021.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diringer MN, Edwards DF. Admission to a neurologic/neurosurgical intensive care unit is associated with reduced mortality rate after intracerebral hemorrhage. Crit Care Med. 2001;29:635–640. doi: 10.1097/00003246-200103000-00031 [DOI] [PubMed] [Google Scholar]
- 35.Sarpong Y, Nattanmai P, Schelp G, et al. Improvement in quality metrics outcomes and patient and family satisfaction in a neurosciences intensive care unit after creation of a dedicated neurocritical care team. Crit Care Res Pract. 2017;2017:6394105. doi: 10.1155/2017/6394105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burns JD, Green DM, Lau H, et al. The effect of a neurocritical care service without a dedicated neuro-ICU on quality of care in intracerebral hemorrhage. Neurocrit Care. 2013;18:305–312. doi: 10.1007/s12028-013-9818-1 [DOI] [PubMed] [Google Scholar]
- 37.Kadri SS, Sun J, Lawandi A, et al. Association between caseload surge and COVID-19 survival in 558 US hospitals, March to August 2020. Ann Intern Med. 2021;174:1240–1251. doi: 10.7326/M21-1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathews KS, Seitz KP, Vranas KC, et al. Variation in initial US hospital responses to the coronavirus disease 2019 pandemic. Crit Care Med. 2021;49:1038–1048. doi: 10.1097/CCM.0000000000005013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bravata DM, Perkins AJ, Myers LJ, et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs Hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4:e2034266. doi: 10.1001/jamanetworkopen.2020.34266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lund S, MacArthur T, Fischmann MM, et al. Impact of COVID-19 governmental restrictions on emergency general surgery operative volume and severity. Am Surg. 2021. 31348211011113. doi: 10.1177/00031348211011113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Algattas HN, McCarthy D, Kujawski B, et al. Impact of coronavirus disease 2019 shutdown on neurotrauma volume in Pennsylvania. World Neurosurg. 2021. doi: 10.1016/j.wneu.2021.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maman Y, Lee Goldstein A, Neeman U, et al. The impact of the COVID-19 pandemic on an Israeli Acute Care Surgery Unit: fewer patients, more disease. Am Surg. 2021. 31348211011132. doi: 10.1177/00031348211011132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cano-Valderrama O, Morales X, Ferrigni CJ, et al. Reduction in emergency surgery activity during COVID-19 pandemic in three Spanish hospitals. Br J Surg. 2020;107:e239. doi: 10.1002/bjs.11667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel PD, Kelly KA, Reynolds RA, et al. Tracking the volume of neurosurgical care during the coronavirus disease 2019 pandemic. World Neurosurg. 2020;142:e183–e194. doi: 10.1016/j.wneu.2020.06.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castelnuovo P, Turri-Zanoni M, Karligkiotis A, et al. Skull-base surgery during the COVID-19 pandemic: the Italian Skull Base Society recommendations. Int Forum Allergy Rhinol. 2020;10:963–967. doi: 10.1002/alr.22596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shuman AG, Fox E, Unguru Y. Preparing for COVID-19-related drug shortages. Ann Am Thorac Soc. 2020;17:928–931. doi: 10.1513/AnnalsATS.202004-362VP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pulk RA, Leber M, Tran L, et al. Dynamic pharmacy leadership during the COVID-19 crisis: optimizing patient care through formulary and drug shortage management. Am J Health Syst Pharm. 2020;77:1874–1884. doi: 10.1093/ajhp/zxaa219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanji S, Burry L, Williamson D, et al. Therapeutic alternatives and strategies for drug conservation in the intensive care unit during times of drug shortage: a report of the Ontario COVID-19 ICU Drug Task Force. Can J Anaesth. 2020;67:1405–1416. doi: 10.1007/s12630-020-01713-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elbeddini A, Hooda N, Yang L. Role of Canadian pharmacists in managing drug shortage concerns amid the COVID-19 pandemic. Can Pharm J (Ott). 2020;153:198–203. doi: 10.1177/1715163520929387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Badreldin HA, Atallah B. Global drug shortages due to COVID-19: impact on patient care and mitigation strategies. Res Social Adm Pharm. 2021;17:1946–1949. doi: 10.1016/j.sapharm.2020.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ammar MA, Sacha GL, Welch SC, et al. Sedation, analgesia, and paralysis in COVID-19 patients in the setting of drug shortages. J Intensive Care Med. 2021;36:157–174. doi: 10.1177/0885066620951426 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jnsa.com.


