Abstract
Inhaled antibiotics are a common and valuable therapy for patients suffering from chronic lung infection, with this particularly well demonstrated for patients with cystic fibrosis. However, in vitro tests to predict patient response to inhaled antibiotic therapy are currently lacking. There are indications that antimicrobial susceptibility testing (AST) may have a role in guidance of therapy, but which tests would correlate best still needs to be researched in clinical studies or animal models. Applying the principles of European Committee on Antimicrobial Susceptibility Testing methodology, the analysis of relevant and reliable data correlating different AST tests to patients’ outcomes may yield clinical breakpoints for susceptibility, but these data are currently unavailable. At present, we believe that it is unlikely that standard determination of minimum inhibitory concentration will prove the best predictor.
Keywords: antimicrobial resistance, bronchiectasis, chronic pulmonary infection, cystic fibrosis, inhaled antibiotics, susceptibility breakpoints
Inhaled antimicrobial therapy is a recommended option in the treatment of chronic lung infection in a number of diseases, the advantages of such topical therapy compared to systemic therapy being 3-fold [1, 2]: (1) Higher concentrations are achieved at the site of infection, which may overcome decreased antibiotic susceptibility of the infecting bacteria; (2) by specifically targeting the lungs of the patient, a lower systemic exposure and toxicity can be achieved; and (3) for antibiotics with low oral bioavailability, inhalation therapy may replace intravenous administration [3, 4].
Antimicrobial susceptibility testing (AST) is predictive for the outcome of antibiotic treatment in many bacterial infections [5, 6], and susceptibility testing is the basis for guidance of most antibiotic therapies, both in establishing the optimal therapy at the patient level and in guiding the preferred empirical regimens at the population level. AST determines the minimum inhibitory concentration (MIC) of an antibiotic for a bacterial isolate, that is, the minimum concentration to inhibit visible bacterial growth under standardized circumstances. To interpret these laboratory results, breakpoints are set. From a clinical perspective, (clinical) breakpoints refer to those MICs which separate strains into those where there is a high likelihood of treatment success versus those where treatment is more likely to fail. Additionally, microbiologists define epidemiological cutoffs, which separate the wild-type populations of bacteria from bacteria with acquired resistance mechanisms [6].
AST is widely used to guide systemic antibiotic therapy. However, evidence on the correlation between results of current AST methods and response to inhaled antibiotics is scarce and the role of AST is widely questioned. Patients infected with “resistant” bacteria often still benefit from treatment with inhaled therapy [7]. In practice, activity will be established by the assessment of the clinical response to a chosen regimen. Presumed higher antibiotic concentrations reached at the site of infection have been proposed as explanation for the discrepancy between AST and response to treatment [8]. It has been suggested that inhaled antibiotic therapy may require separate (higher) susceptibility breakpoints or different AST methods [9]. Currently, standardized testing methods and clinical breakpoints are largely unavailable for topical antibiotic therapy, and none exist for inhaled therapy.
This manuscript outlines a route to establish AST clinical breakpoints, describes the issues likely to be encountered defining these breakpoints for inhaled therapy, and signposts the studies needed to develop standardized susceptibility tests and breakpoints for inhaled antibiotic therapy.
INHALED ANTIBIOTICS: FORMULATIONS, CHARACTERISTICS, AND DRUG CONCENTRATIONS
Antibiotics may be inhaled as nebulized solutions or as dry powders [4]. Nebulizers disperse antibiotics in solution as a mist of aerosolized droplets, which the patient breathes in. The technique is time-consuming, and the dispersal devices require regular cleaning to avoid contamination [10]. With dry powder inhalation (DPI), the patient actively inspires the antibiotic. DPI requires a minimum inspiratory effort; it is therefore less suitable for patients with a limited residual lung function, during acute exacerbations or for patients on mechanical ventilation. DPI is more rapid, and the dispersal devices do not require a power source or extensive cleaning.
Inhalation therapy is aimed at to deliver antibiotics to the small airways of the lungs, which are considered the main site of infection [10]. However, only a fraction of the applied dose will reach them: a part is retained in the inhalation device and another part is deposited in the upper airways (mostly ending up in the gastrointestinal tract). Under ideal circumstances, in healthy volunteers, up to a third of the dose may be effectively delivered to the lungs [11, 12], but this fraction has also been reported as low as 5% [12]. Data on antibiotic concentrations in the small airways after inhalation are limited. Methods to determine these concentrations have included direct measurements in induced sputum, bronchoalveolar lavage (BAL) fluid, epithelial lining fluid (ELF), and resected pulmonary tissue, as well as measurements by gamma scintigraphy [13].
The concentrations measured in sputum are typically higher than those in BAL. For instance, the maximum concentration (Cmax) of tobramycin after inhalation has been reported to be between 3.6 and 5.5 mg/L in lung and ELF, and as high as 1048 mg/L in sputum. Colistin concentrations have been reported to reach 6.7 mg/L in ELF and 40 mg/L in sputum [14, 15]. Large differences may be found between different inhalation techniques, and between different formulations, on top of the variability in patients and test subjects [13].
INHALED ANTIBIOTICS IN CYSTIC FIBROSIS AND BRONCHIECTASIS
The use of inhaled antibiotics to suppress or eradicate respiratory pathogens in cystic fibrosis (CF) has been shown to reduce infectious exacerbations, slow the deterioration in lung function, and increase quality of life; it has therefore become one of the mainstays of therapy in the treatment of CF [2]. Inhaled antibiotics are also widely used for bronchiectasis (BE) due to other diseases, but the evidence in these patient groups is more limited.
The main pathogen associated with acute infective exacerbations and deterioration of lung function in CF and BE is Pseudomonas aeruginosa [16], and the antibiotics used in inhalation therapy therefore all possess antipseudomonal activity. A decrease in P aeruginosa density in airway secretions has, to some extent, been correlated with clinical response in patients using inhaled antibiotic therapy [17]. However, a clear correlation between susceptibility of the cultured microorganisms and the response to inhaled or intravenous antibiotic therapy has not been demonstrated in CF chronic lung infection [18, 19]. The lack of understanding of the contribution of separate pathogens to infections in CF and BE is a major issue encountered when attempting to use conventional AST to predict response to therapy, and hence to apply clinical breakpoints.
The microenvironment in the CF lung adds to the complexity; for example, the anaerobic conditions in sputum may diminish or increase the effect of the antibiotics, as may components of the mucus layer itself [20]. Furthermore, the bacteria in this microenvironment grow in biofilms, while reference standard AST assesses the effect of antibiotics on planktonic bacterial growth.
INHALED ANTIBIOTICS IN ACUTE PULMONARY INFECTION
Treatment of acute pulmonary infections with inhaled antibiotics has been attempted, in particular with patients on mechanical ventilation in the intensive care unit (ICU) [21]. ICU patients are at a higher risk of infection with resistant microorganisms and are more likely to suffer from organ failure or toxicity from their medication; treatment of an infected lung with high localized antibiotic concentrations but with low systemic exposure may therefore offer significant advantages. Furthermore, mechanical ventilation enables administration of nebulized antibiotics. Clinical studies have failed to demonstrate efficacy of inhaled antibiotics for acute pulmonary infections [21]. One possible explanation may be found in the airway obstruction occurring in diseased areas of the lungs, limiting penetration of inhaled drugs into those areas [3].
The same may apply to acute exacerbations of chronic lung infections in CF. Typically, these do not respond to inhalation antibiotic therapy and are treated with systemic therapy [22]. It is unclear whether exacerbations originate from spontaneous disturbances in the lung microbiome, from obstructions, or from external triggers such as viral infections. Exacerbations therefore do not necessarily reflect nonsusceptibility of the suppressed bacteria in the lung.
BREAKPOINTS AND BREAKPOINT DEVELOPMENT FOR AST
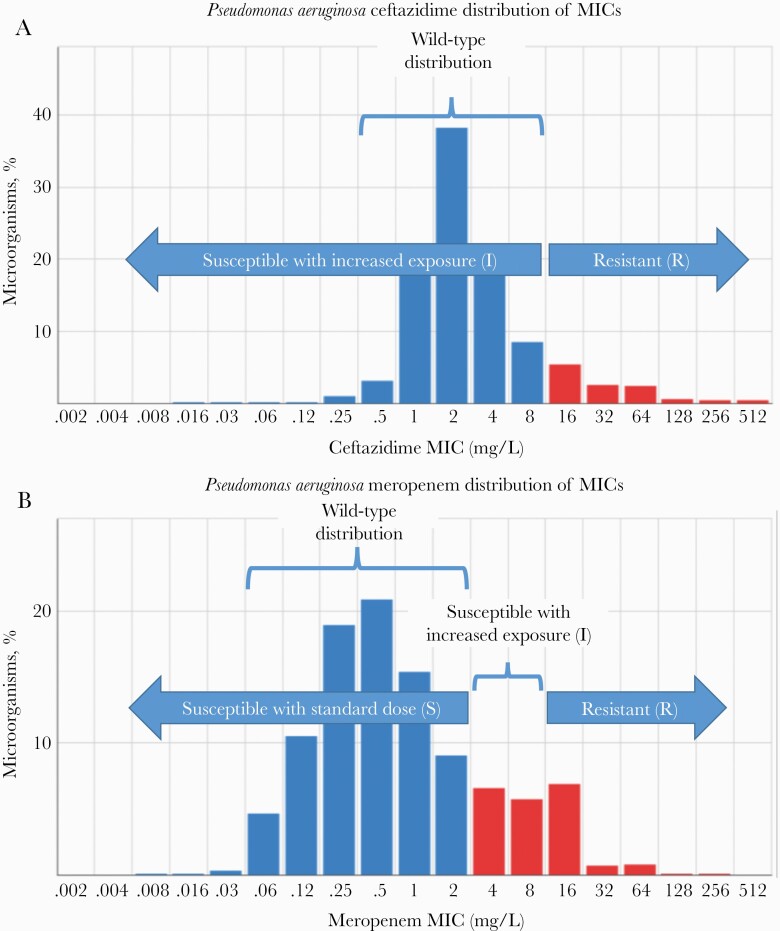
Breakpoint development for AST is a systematic process that follows a predefined set of steps [6, 23]. First, the wild-type distribution of the MICs of an antibiotic against a bacterial species is determined, that is, the MIC distribution of strains without acquired resistance mechanisms. The MIC is the result of a standardized test that determines the antibiotic concentration required to inhibit growth of planktonic bacteria in exponential growth phase. As a general rule, the wild-type MIC distribution will be bell-shaped and will span 3–5 two-fold dilutions. Beyond this bell-shaped distribution, strains with mechanisms causing decreased susceptibility are found (Figure 1). The highest MIC of the wild-type distribution is designated the epidemiological cutoff (ECOFF) [24].
Figure 1.
A, Wild-type and I (susceptible with increased exposure) category coincide; no category for S (susceptible at standard dose) is defined. Strains with minimum inhibitory concentrations (MICs) above the wild-type range are defined as R (resistant). Based on aggregated data from 32 276 observations in the European Committee on Antimicrobial Susceptibility Testing (EUCAST) database (https://mic.eucast.org/search/). B, Activity of meropenem beyond the wild-type distribution is likely with higher exposure. The wild-type category coincides with S (susceptible with standard dose). A non-wild-type I category (susceptible with increased exposure) is defined. Based on aggregated data from 57 615 observations in the EUCAST database (https://mic.eucast.org/search/).
As a second step, the pharmacokinetic/pharmacodynamic (PK/PD) target of the antibiotic against infections with the microorganism is determined. Preferably, this target is established based on outcome data from clinical studies; as an alternative, it can be based on data acquired from animal models or from in vitro models. One of 2 main PK/PD targets may be defined: either a percentage of time above MIC (%T > MIC) for time-dependent antibiotics, or an area under the curve for the unbound antibiotic divided by the MIC (fAUC0–24/MIC) for exposure-dependent antibiotics. Of note: the PK/PD relation for efficacy is usually based on the (free) plasma concentration of antibiotics, not on the concentration in the infected tissue, where the antimicrobial activity actually takes place.
Finally, using computer simulations for a diverse population, the dosage that is required to achieve the PK/PD target for the highest MIC value of the wild-type population in 95%–99% of the patients is calculated—that is, to achieve a probability of target attainment (PTA) of 95%–99% at the ECOFF. If this required dosage can be safely administered to patients, the ECOFF is often set as the susceptibility breakpoint, although sometimes a concentration 1 or 2 dilutions higher may be chosen. If such as dosage is not deemed achievable, it is considered that the antibiotic is not reliably effective and no susceptibility breakpoint is defined [25].
For some drug/microorganism combinations, different clinical breakpoints may be defined depending on the site of infection, the dosage, or the route of drug administration. Some of these situations have been addressed by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) with the “I” category, which is defined as “susceptible with increased exposure” next to the “S” category, which is defined as “susceptible with standard dose” [26]. Similarly, to some extent, the Clinical and Laboratory Standards Institute (CLSI) uses the category “susceptible dose-dependent” when alternative dosing regimens may overcome higher MICs in the pathogens, and the intermediate (I) category when successful treatment of uncomplicated urinary tract infections is still likely with antibiotics that accumulate in urine [27].
The EUCAST I category, with its corresponding dosage (or corresponding infection site), may apply to all susceptible isolates of a drug/species combination. In this case, an increased exposure (also referred to as “high dose”) is required to achieve a 95%–99% PTA in the wild-type population, and only I and R categories exist; this is, for instance, the case with the combination of ceftazidime and P aeruginosa (Figure 1A). In other cases, a 95%–99% PTA is achieved with the “standard dose” in the wild-type, but a part of the population that falls just above the wild-type may be properly treated with an increased dose. These drug/microorganism combinations have S, I, and R categories defined, as is the case with the combination of meropenem and P aeruginosa (Figure 1B).
A number of bottlenecks in breakpoint development for inhaled therapy are evident when applying the previously described process:
When there is not enough clear correlation of MIC values with clinical outcomes, breakpoints are often set at the ECOFF, to distinguish the wild-type population from bacteria with acquired resistance mechanisms, while the intention of defining separate clinical breakpoints for inhaled therapy is to identify those strains with MICs above the breakpoint for systemic therapy (ie, the ECOFF) that may still be effectively treated.
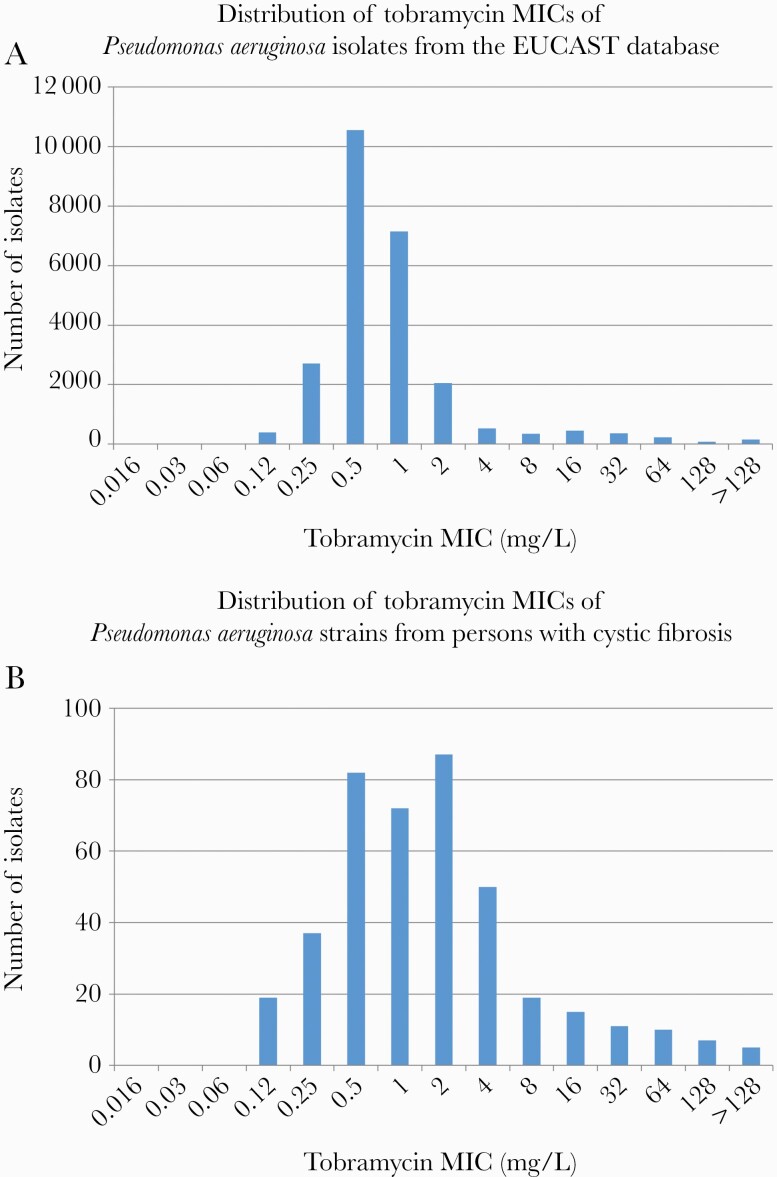
In fact, since CF P aeruginosa strains are regularly exposed to antibiotics, the shape of their MIC distribution usually does not allow a wild-type population to be defined (Figure 2).
Data on antibiotic concentrations reached by inhalation at the infection site are still scarce and the validity of some measurements is at times questionable. Serum concentrations are not suitable as proxy indicators (as they are in systemic therapy).
Although data are limited, it is clear that with inhalation therapy, large variations in concentrations attained occur between different subjects, disease states, and technologies used. Simulations to ensure effective concentrations for pathogens at the highest “susceptible” MIC in 95%–99% of patients will lead to either excessively high dosages or low MIC cutoffs.
The standardized circumstances under which AST is performed, with planktonic bacteria in log-phase, are likely to be more predictive of behavior of bacteria in acute infections than in chronic biofilm infections.
Figure 2.
A, Data were obtained from the European Committee on Antimicrobial Susceptibility Testing (EUCAST) database (https://mic.eucast.org/search/, accessed 2 December 2020). B, Data were adapted from Ekkelenkamp et al [28]. Epidemiological cutoff (ECOFF), as determined in non–cystic fibrosis (CF) isolates = 2 mg/L; Susceptible with standard dose (S) ≤2 mg/L; Resistant (R) >2 mg/L. Comparison of A and B shows how the distribution of the minimum inhibitory concentrations (MICs) has been skewed toward higher MICs for the Pseudomonas aeruginosa strains of persons with CF, likely due to adaptation of these strains to antibiotic exposure; in B, it is therefore not possible to establish a clear ECOFF.
Additionally:
6. A mixed population of different bacteria is present in the CF and BE lung. Conventional culture results may not accurately reflect the microorganisms that need to be targeted. In particular, P aeruginosa may not be the (only) microorganism that requires treatment [29]. Furthermore, the (in vitro) antimicrobial susceptibility of a clonal bacterial population can change when grown in the presence of other bacterial species.
7. The endpoints used to determine clinical breakpoints for AST are generally bimodal and short-term, whereas those to assess effectivity of inhalation therapy are continuous and long-term.
EUCAST REPORT ON TOPICAL THERAPY
In a report in November 2019 [30], EUCAST noted the lack of supporting data to set breakpoints for topical therapy, in particular the lack of pharmacokinetic data. The committee suggested a set of screening breakpoints for phenotypic resistance, based on the ECOFFs, but only for the treatment of superficial skin, eye, and ear infections. Most of these breakpoints are identical to those defined for systemic therapy with some differing by a single 2-fold dilution. This EUCAST document explicitly excluded inhaled antibiotics. CLSI has defined breakpoints only for inhaled amikacin in the treatment of Mycobacterium avium complex (MAC) infections [31]. A CLSI working group established to define breakpoints for aerosolized antimicrobials was disbanded due to the lack of data to accurately determine these breakpoints.
DATA FROM CLINICAL STUDIES/CORRELATION OF MICs WITH CLINICAL OUTCOMES OF INHALED ANTIBIOTIC THERAPY
Assessing the relation between in vitro susceptibility and clinical outcome is complex for inhaled antibiotic therapy. The study endpoints usually evaluated to determine AST breakpoints are generally clear cut, short-term, and binomial: survival, clinical cure, or microbiological cure. On the contrary, study endpoints used to assess antibiotic inhalation therapy are continuous and long-term: the time to first exacerbation or hospitalization, the frequency of exacerbations or hospitalizations, and the change in FEV1 (forced expiratory volume in 1 second) or FVC (forced vital capacity). Additionally, during such an extended treatment period, the susceptibility of the pathogens may change. Understandably, correlating AST results to outcome is complex under these circumstances. However, there are some indications that in vitro tests may predict clinical outcome.
A recent meta-analysis concluded that long-term inhaled suppressive antipseudomonal antibiotic therapy reduced hospitalizations and improved lung function in CF [32]. Of the 11 randomized placebo-controlled trials conducted in this field, 10 had P aeruginosa colonization as an inclusion criterion, and a relation between P aeruginosa MIC and response to therapy was found in a limited set of analyses [17]. An additional argument for a relationship between MIC and outcome is that the use of inhaled antibiotics led to significant increase in resistance in these studies; this was also found in observational data from the CF registry [33]. Evidence for a relationship between susceptibility and efficacy of treatment also comes from longitudinal observational studies that correlate P aeruginosa resistance to lower pulmonary function at baseline and more rapid decline in FEV1 [34, 35]. Finally, in 2 phase 3 trials in patients with BE, inhaled ciprofloxacin resulted in a significant reduction in frequency of exacerbations only in the subgroup of patients who were free from ciprofloxacin-resistant microorganisms at baseline [36, 37].
A quite different example where in vitro testing correlates with clinical outcomes of inhaled therapy concerns the use of the inhaled aminoglycoside amikacin for the treatment of MAC. Amikacin is active against a variety of nontuberculous mycobacteria, and the inhaled form of the drug is used in combination regimens against infections by diverse species, in particular Mycobacterium abscessus and MAC. EUCAST has not yet defined AST methods for mycobacteria, and has only established breakpoints for new antimycobacterial drugs in the treatment of Mycobacterium tuberculosis [38]. The CLSI breakpoints for amikacin and M abscessus are ≤16 mg/L for susceptibility and >32 mg/L for resistance, identical to those for P aeruginosa and Enterobacterales. However, separate CLSI breakpoints have been established for inhaled therapy against MAC: ≤64 mg/L for susceptibility and >64 mg/L for resistance [31]. The breakpoint of >64 mg/L for resistance correlates best with the presence or development of resistance mutations, and with efficacy of both inhaled and systemic administration [39]; this cutoff would also be the most congruent with the MIC90 of amikacin against MAC, which was 32 mg/L in unexposed strains [40]. In summary, the activity of inhaled amikacin may be predicted by AST, but with breakpoints identical to those that predict the effect of systemic therapy.
DATA FROM BIOFILM MODELS
Current methods for AST use planktonic bacteria in pure culture and in exponential growth phase. This simplified model may be less predictive for chronic infections, in particular for those chronic infections where bacteria persist in biofilms [41]. It has been suggested that susceptibility testing in biofilm models could correlate more closely to efficacy of inhaled antibiotics, as they would constitute a closer approximation of the in vivo situation in the lung.
The biofilm model for AST most commonly used is the Calgary biofilm model. The Calgary biofilm model allows the determination of 2 measures of biofilm antibiotic susceptibility: the minimum biofilm inhibitory concentration (MBIC) and the minimum biofilm eradication concentration (MBEC). To determine the MBIC and MBEC, plastic pegs coated with hydroxyapatite are incubated in bacterial suspensions to allow biofilms to form on the pegs. Subsequently, the pegs are suspended in microtiter plate wells containing growth medium and 2-fold dilutions of antibiotics. The MBIC is the minimum concentration required to inhibit the growth of the bacteria on the biofilm formed on the peg; in technical terms this is defined as the lowest concentration of an antibiotic that results in an optical density at 650 nm difference at or below 10% of the positive control (a 1-log10 difference in growth) after 6 hours of incubation. The MBEC is the lowest concentration of an antibiotic that prevents visible growth in the recovery medium used to collect biofilm cells [42].
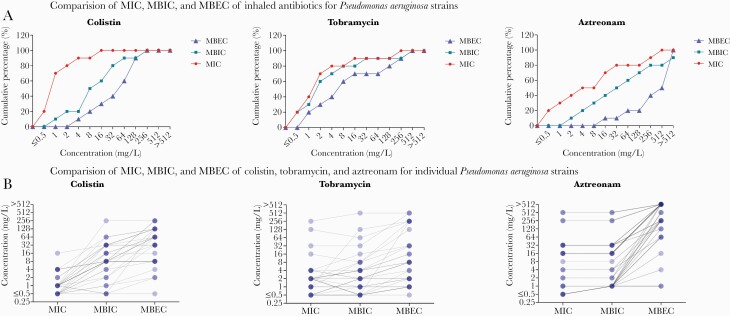
Figure 3 shows the relationship between MICs, MBICs, and MBECs for 32 P aeruginosa strains recovered from persons with CF [42]. As is apparent from the figure, the MBICs are higher than the MICs, and the MBECs are even higher: Antibiotic concentrations needed to inhibit bacteria are higher when these are growing in a biofilm, and higher even if the bacteria in the biofilm are to be eradicated. In Figure 3B, it is also apparent that this is not a fixed shift of the inhibitory concentration, but that the relationship between the 3 parameters differs from strain to strain. Furthermore, this relationship differs between antibiotics: Tobramycin MBICs are on average <2-fold higher than the MICs, whereas colistin MBICs are generally 8-fold higher or more. Assuming that the MBIC and MBEC values are true representations of the activity of antimicrobials within the biofilm, ECOFFs could be determined for these values, which would define the wild-type biofilm bacterial population and the population with mechanisms that contribute to antimicrobial tolerance in biofilm. A subsequent step would be to validate whether these ECOFFs are predictive for therapeutic response in inhalation therapy and whether they are useful in guiding inhaled antibiotic therapy in chronic lung infections [44].
Figure 3.
Comparison of the minimum inhibitory concentration (MIC), minimum biofilm inhibitory concentration (MBIC), and minimum biofilm eradication concentration (MBEC) for 53 Pseudomonas aeruginosa strains isolated from persons with cystic fibrosis. A, The relation between MIC, MBIC, and MBEC varies between the different inhaled antibiotics; for tobramycin, the relative difference between these values is smaller than for colistin and aztreonam. B, Superimposed strains are represented by darker shades or lines. This figure shows that the differences between antimicrobial susceptibility testing parameters are isolate-specific; 1 isolate may have both a higher MIC and a lower MBEC than another isolate. Both figures were originally published by Díez-Aguilar et al [43].
DATA FROM ANIMAL MODELS
In preclinical antibiotic drug development, dose-fractionation studies with animal infection models have been used to determine the PK/PD index and the PK/PD target for various infections [45], and to establish dosing regimens for clinical trials. The most commonly applied model for PK/PD antimicrobial efficacy studies is the thigh infection model with neutropenic mice. Other animal models, usually involving rodents or rabbits—including those for acute bacterial pneumonia, skin and soft tissue infection, sepsis, meningitis, urinary tract infection, infectious endocarditis, and peritonitis—all assess acute infection. Chronic lung infection, such as in CF and BE, does not occur naturally in rodents; lung infections are either rapidly fatal or are cleared by the animals.
To establish chronic lung infection in rodents, the lungs and airways of the animals need to be artificially damaged or obstructed to avoid clearance of the bacteria; this has commonly been accomplished by installing colonized agarose or alginate beads in the lungs. Animal CFTR-knockout models have been developed with mice, rats, rabbits, ferrets, and pigs [46]. CFTR-knockout ferrets and pigs have been found to spontaneously develop chronic lung infection; these animals rapidly develop bacterial colonization of the lungs, mostly with enteric microorganisms, and subsequent lung disease [47, 48]. In CFTR-knockout ferrets, systemic antibiotic therapy has been shown to slow disease progression and expand the life expectancy 10-fold [48]. Another model of the CF lung currently in development is that of β-ENaC mice. β-ENaC mice have been genetically modified to overexpress the epithelial Na+ channel specifically in the lungs, where overabsorption of Na+ occurs, mimicking the pathophysiology of the CF lung [49].
Thus far, animal models of chronic CF-like lung infections have been used primarily to study disease pathogenesis. However, as development and understanding progress, they may become available to study pathogen–antibiotic relations and aid in determining PK/PD parameters and targets. However, P aeruginosa has not been found to form part of the microbiome of CFTR-knockout pigs and ferrets [47, 50], and such differences with the human CF/BE lung will need to be addressed or compensated.
INHALED ANTIBIOTICS TARGETING ONLY P AERUGINOSA
Antibiotics currently used for inhalation therapy do not only target P aeruginosa, but have activity against most aerobic gram-negative bacteria, and often also against some gram-positive bacteria. Uncertainty about which microorganisms are targeted interferes in establishing a PK/PD relation between the drug and the pathogens. Currently, murepavadin, a peptidomimetic antibiotic that targets only P aeruginosa, is in clinical development for inhalation therapy in CF and BE. If the development of this drug proceeds to phase 3, it would offer the possibility to explore the role of P aeruginosa in chronic infections and to investigate whether treatment of this pathogen alone suffices. Furthermore, establishing PK/PD relations and breakpoints with a single pathogen–antibiotic combination is far more straightforward than in the context of a collection of potential pathogens.
FUTURE DIRECTIONS AND SCIENCE AGENDA
To provide supportive clinical data for breakpoint development, appropriately designed new studies are essential. The main characteristics of such studies would be (1) the proper drawing of samples and culture methods prior to initiation of therapy, (2) reliable estimates of the local pharmacokinetics of the drugs in the patients—if possible based on measurements, and (3) the inclusion of novel AST methods, as a minimum to determine MBIC and MBEC.
Furthermore, (4) valid endpoints to define treatment success will be required, preferably bimodal. These may be either microbiological (eg, reduction in bacterial counts, bacterial eradication) or clinical (eg, short-term improvement of FEV1). Data may be acquired in both randomized and nonrandomized studies. Preferably, various inhalation regimens and dosages would be used within a single study, to establish exposition–response curves for different PK/PD relations.
With these data, the main pharmacokinetic and pharmacodynamic parameters can be combined to examine which, if any, correlates best with the defined outcome, and what the corresponding PK/PD target values would be. These could be fAUC/MIC, fAUC/MBIC, fAUC/MBEC, T > MIC, T > MBIC, or T > MBEC, or perhaps a novel parameter yet to be explored. The ratio between AUC and mutant prevention concentration has, for instance, been proposed to establish PK/PD targets [51]. Still, it must be noted that a major hurdle in this process will be to identify the contribution of the different bacteria within the lung microbiome. It needs to be determined to what extend such analysis may be limited to the treatment of P aeruginosa.
In summary: At present, standardized AST methods are very limited in their prediction of therapeutic success of inhalation therapy. To establish the optimal laboratory methods, different AST modalities will need to be validated with pathogens obtained from well-documented infections. Furthermore, robust criteria for treatment failure and success will need to be defined. Though certainly feasible, this will require a major effort from the respiratory, microbiological, and pharmacological scientific community.
Notes
Financial support. This work has received support from the Innovative Medicines Initiative Joint Undertaking (grant agreement number 115721-2), resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and European Federation of Pharmaceutical Industries and Associations companies’ in-kind contributions.
Potential conflicts of interest. M. M. T. has been involved in grants from Antabio (payment to institution) and has received consulting fees from Shionogi. J. S. E. has received grants from Vertex for educational activities and honoraria from Vertex and Gilead for talks. R. C. is clinical data coordinator of the European Committee on Antimicrobial Susceptibility Testing. He has been involved in grants from MSD (payment to institution) and has received honoraria for participation in educational programs organized by MSD, Pfizer, and Shionogi. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50:1700629. [DOI] [PubMed] [Google Scholar]
- 2. Castellani C, Duff AJA, Bell SC, et al. ECFS best practice guidelines: the 2018 revision. J Cyst Fibros 2018; 17:153–78. [DOI] [PubMed] [Google Scholar]
- 3. Karampitsakos T, Papaioannou O, Kaponi M, et al. Low penetrance of antibiotics in the epithelial lining fluid. The role of inhaled antibiotics in patients with bronchiectasis. Pulm Pharmacol Ther 2020; 60:101885. [DOI] [PubMed] [Google Scholar]
- 4. Wenzler E, Fraidenburg DR, Scardina T, Danziger LH.. Inhaled antibiotics for gram-negative respiratory infections. Clin Microbiol Rev 2016; 29:581–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Henderson A, Bursle E, Stewart A, et al. A systematic review of antimicrobial susceptibility testing as a tool in clinical trials assessing antimicrobials against infections due to gram negative pathogens. Clin Microbiol Infect 2021; 27:1746–53. [DOI] [PubMed] [Google Scholar]
- 6. Turnidge J, Paterson DL.. Setting and revising antibacterial susceptibility breakpoints. Clin Microbiol Rev 2007; 20:391–408, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. LiPuma JJ. Microbiological and immunologic considerations with aerosolized drug delivery. Chest 2001; 120(3 Suppl):118S–23S. [DOI] [PubMed] [Google Scholar]
- 8. Waters VJ, Kidd TJ, Canton R, et al. Antimicrobial resistance international working group in cystic fibrosis. Reconciling antimicrobial susceptibility testing and clinical response in antimicrobial treatment of chronic cystic fibrosis lung infections. Clin Infect Dis 2019; 69:1812–6. [DOI] [PubMed] [Google Scholar]
- 9. Morosini MI, García-Castillo M, Loza E, Pérez-Vázquez M, Baquero F, Cantón R.. Breakpoints for predicting Pseudomonas aeruginosa susceptibility to inhaled tobramycin in cystic fibrosis patients: use of high-range Etest strips. J Clin Microbiol 2005; 43:4480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tiddens HA, Bos AC, Mouton JW, Devadason S, Janssens HM.. Inhaled antibiotics: dry or wet? Eur Respir J 2014; 44:1308–18. [DOI] [PubMed] [Google Scholar]
- 11. Weers J, Metzheiser B, Taylor G, Warren S, Meers P, Perkins WR.. A gamma scintigraphy study to investigate lung deposition and clearance of inhaled amikacin-loaded liposomes in healthy male volunteers. J Aerosol Med Pulm Drug Deliv 2009; 22:131–8. [DOI] [PubMed] [Google Scholar]
- 12. Newhouse MT, Hirst PH, Duddu SP, et al. Inhalation of a dry powder tobramycin PulmoSphere formulation in healthy volunteers. Chest 2003; 124:360–6. [DOI] [PubMed] [Google Scholar]
- 13. Dalhoff A. Pharmacokinetics and pharmacodynamics of aerosolized antibacterial agents in chronically infected cystic fibrosis patients. Clin Microbiol Rev 2014; 27:753–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Athanassa ZE, Markantonis SL, Fousteri MZ, et al. Pharmacokinetics of inhaled colistimethate sodium (CMS) in mechanically ventilated critically ill patients. Intensive Care Med 2012; 38:1779–86. [DOI] [PubMed] [Google Scholar]
- 15. Ratjen F, Rietschel E, Kasel D, et al. Pharmacokinetics of inhaled colistin in patients with cystic fibrosis. J Antimicrob Chemother 2006; 57:306–11. [DOI] [PubMed] [Google Scholar]
- 16. Garcia-Clemente M, de la Rosa D, Máiz L, et al. Impact of Pseudomonas aeruginosa infection on patients with chronic inflammatory airway diseases. J Clin Med 2020; 9:3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moss RB. Long-term benefits of inhaled tobramycin in adolescent patients with cystic fibrosis. Chest 2002; 121:55–63. [DOI] [PubMed] [Google Scholar]
- 18. Somayaji R, Parkins MD, Shah A, et al. ; Antimicrobial Resistance in Cystic Fibrosis International Working Group. Antimicrobial susceptibility testing (AST) and associated clinical outcomes in individuals with cystic fibrosis: a systematic review. J Cyst Fibros 2019; 18:236–43. [DOI] [PubMed] [Google Scholar]
- 19. Burns JL, Van Dalfsen JM, Shawar RM, et al. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. J Infect Dis 1999; 179:1190–6. [DOI] [PubMed] [Google Scholar]
- 20. Bos AC, Passé KM, Mouton JW, Janssens HM, Tiddens HA.. The fate of inhaled antibiotics after deposition in cystic fibrosis: how to get drug to the bug? J Cyst Fibros 2017; 16:13–23. [DOI] [PubMed] [Google Scholar]
- 21. Schreiber MP, Shorr AF.. Inhaled antibiotics for the treatment of pneumonia. Curr Opin Pulm Med 2019; 25:289–93. [DOI] [PubMed] [Google Scholar]
- 22. Elborn JS. Cystic fibrosis. Lancet 2016; 388:2519–31. [DOI] [PubMed] [Google Scholar]
- 23. Mouton JW, Brown DF, Apfalter P, et al. The role of pharmacokinetics/pharmacodynamics in setting clinical MIC breakpoints: the EUCAST approach. Clin Microbiol Infect 2012; 18:E37–45. [DOI] [PubMed] [Google Scholar]
- 24. Kahlmeter G. The 2014 Garrod Lecture: EUCAST—are we heading towards international agreement? J Antimicrob Chemother 2015; 70:2427–39. [DOI] [PubMed] [Google Scholar]
- 25. European Committee on Antimicrobial Susceptibility Testing. SOP 1.3: setting breakpoints for new antimicrobial agents. 2019. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/EUCAST_SOPs/EUCAST_SOP_1.3_Setting_breakpoints_new_agents_20191023.pdf. Accessed 1 December 2021.
- 26. Giske CG, Turnidge J, Cantón R, Kahlmeter G.. Update from the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [manuscript published online ahead of print 4 August 2021]. J Clin Microbiol 2021. doi: 10.1128/JCM.00276-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. M100. 30th ed. Wayne, PA: CLSI; 2020. [Google Scholar]
- 28. Ekkelenkamp MB, Cantón R, Díez-Aguilar M, et al. Susceptibility of Pseudomonas aeruginosa recovered from cystic fibrosis patients to murepavadin and 13 comparator antibiotics. Antimicrob Agents Chemother 2020; 64:e01541–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chmiel JF, Aksamit TR, Chotirmall SH, et al. Antibiotic management of lung infections in cystic fibrosis. I. The microbiome, methicillin-resistant Staphylococcus aureus, gram-negative bacteria, and multiple infections. Ann Am Thorac Soc 2014; 11:1120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. European Committee on Antimicrobial Susceptibility Testing. Antimicrobial susceptibility tests on groups of organisms or agents for which there are no EUCAST breakpoints. 2016. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Organisms_and_agents_without_breakpoints_20160626.pdf. Accessed 1 December 2021.
- 31. Clinical and Laboratory Standards Institute. Performance Standards for Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes. CLSI supplement M62. 1st ed. Wayne, PA: CLSI; 2018. [PubMed] [Google Scholar]
- 32. Smith S, Rowbotham NJ, Charbek E.. Inhaled antibiotics for pulmonary exacerbations in cystic fibrosis. Cochrane Database Syst Rev 2018; 10:CD008319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Merlo CA, Boyle MP, Diener-West M, Marshall BC, Goss CH, Lechtzin N.. Incidence and risk factors for multiple antibiotic-resistant Pseudomonas aeruginosa in cystic fibrosis. Chest 2007; 132:562–8. [DOI] [PubMed] [Google Scholar]
- 34. Lechtzin N, John M, Irizarry R, Merlo C, Diette GB, Boyle MP.. Outcomes of adults with cystic fibrosis infected with antibiotic-resistant Pseudomonas aeruginosa. Respiration 2006; 73:27–33. [DOI] [PubMed] [Google Scholar]
- 35. Konstan MW, Wagener JS, Vandevanter DR, et al. Risk factors for rate of decline in FEV1 in adults with cystic fibrosis. J Cyst Fibros 2012; 11:405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aksamit T, De Soyza A, Bandel TJ, et al. RESPIRE 2: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J 2018; 51:1702053. [DOI] [PubMed] [Google Scholar]
- 37. De Soyza A, Aksamit T, Bandel TJ, et al. RESPIRE 1: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J 2018; 51:1702052. [DOI] [PubMed] [Google Scholar]
- 38. European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 11.0.2021. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf. Accessed 1 December 2021.
- 39. Brown-Elliott BA, Woods GL.. Antimycobacterial susceptibility testing of nontuberculous mycobacteria. J Clin Microbiol 2019; 57:e00834–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brown-Elliott BA, Iakhiaeva E, Griffith DE, et al. In vitro activity of amikacin against isolates of Mycobacterium avium complex with proposed MIC breakpoints and finding of a 16S rRNA gene mutation in treated isolates. J Clin Microbiol 2013; 51:3389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Coenye T, Goeres D, Van Bambeke F, Bjarnsholt T.. Should standardized susceptibility testing for microbial biofilms be introduced in clinical practice? Clin Microbiol Infect 2018; 24:570–2. [DOI] [PubMed] [Google Scholar]
- 42. Macià MD, Rojo-Molinero E, Oliver A.. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin Microbiol Infect 2014; 20:981–90. [DOI] [PubMed] [Google Scholar]
- 43. Díez-Aguilar M, Ekkelenkamp M, Morosini MI, et al. Anti-biofilm activity of murepavadin against cystic fibrosis Pseudomonas aeruginosa isolates. J Antimicrob Chemother 2021; 76:2578–85. [DOI] [PubMed] [Google Scholar]
- 44. Moskowitz SM, Emerson JC, McNamara S, et al. Randomized trial of biofilm testing to select antibiotics for cystic fibrosis airway infection. Pediatr Pulmonol 2011; 46:184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhao M, Lepak AJ, Andes DR.. Animal models in the pharmacokinetic/pharmacodynamic evaluation of antimicrobial agents. Bioorg Med Chem 2016; 24:6390–400. [DOI] [PubMed] [Google Scholar]
- 46. Rosen BH, Chanson M, Gawenis LR, Liu J, Sofoluwe A, Zoso A, Engelhardt JF.. Animal and model systems for studying cystic fibrosis. J Cyst Fibros 2018; 17:S28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stoltz DA, Meyerholz DK, Pezzulo AA, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med 2010; 2:29ra31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rosen BH, Evans TIA, Moll SR, et al. Infection is not required for mucoinflammatory lung disease in CFTR-knockout ferrets. Am J Respir Crit Care Med 2018; 197:1308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou Z, Duerr J, Johannesson B, et al. The ENaC-overexpressing mouse as a model of cystic fibrosis lung disease. J Cyst Fibros 2011; 10(Suppl 2):S172–82. [DOI] [PubMed] [Google Scholar]
- 50. Sun X, Olivier AK, Liang B, et al. Lung phenotype of juvenile and adult cystic fibrosis transmembrane conductance regulator-knockout ferrets. Am J Respir Cell Mol Biol 2014; 50:502–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hesje CK, Tillotson GS, Blondeau JM.. MICs, MPCs and PK/PDs: a match (sometimes) made in hosts. Expert Rev Respir Med 2007; 1:7–16. [DOI] [PubMed] [Google Scholar]