Abstract
Autism spectrum disorder is a construct used to describe individuals with a specific combination of impairments in social communication and repetitive behaviours, highly restricted interests and/or sensory behaviours beginning early in life. The worldwide prevalence of autism is just under 1%, but estimates are higher in high-income countries. Although gross brain pathology is not characteristic of autism, subtle anatomical and functional differences have been observed in post-mortem, neuroimaging and electrophysiological studies. Initially, it was hoped that accurate measurement of behavioural phenotypes would lead to specific genetic subtypes, but genetic findings have mainly applied to heterogeneous groups that are not specific to autism. Psychosocial interventions in children can improve specific behaviours, such as joint attention, language and social engagement, that may affect further development and could reduce symptom severity. However, further research is necessary to identify the long-term needs of people with autism, and treatments and the mechanisms behind them that could result in improved independence and quality of life over time. Families are often the major source of support for people with autism throughout much of life and need to be considered, along with the perspectives of autistic individuals, in both research and practice.
Autism spectrum disorder (ASD), also known as autism, is a common, highly heritable and heterogeneous neurodevelopmental disorder that has underlying cognitive features and commonly co-occurs with other conditions. The behaviours, strengths and challenges of people with autism have attracted the attention of scientists and clinicians for at least 500 years (FIG. 1). Autism is a heterogeneous disorder and, reflecting this heterogeneity, the term autism has been used in various ways to describe both a broader presentation as well as a specific diagnosis following its consideration as a subgroup within the general diagnostic category of ‘pervasive developmental disorders’ (PDDs). PDDs are a group of disorders introduced in the Diagnostic and Statistical Manual of Mental Disorders, Third Edition (DSM-III) in 1980 to convey the idea of a broader spectrum of social communication deficits. Owing to a lack of clear borders between the PDDs and difficulties in reliably distinguishing them, the most recent diagnostic systems, the International Classification of Diseases 11th Revision (ICD-11) and DSM-5 use the umbrella term ‘ASD’, and differentiate individuals using additional clinical specifiers and modifiers. In this Primer, we use the term ‘autism’ to refer to ASD in general, both for brevity and out of respect for the preferences of self-advocates.
Fig. 1 |. Theories and findings regarding autism mechanisms, outcomes and heterogeneity.
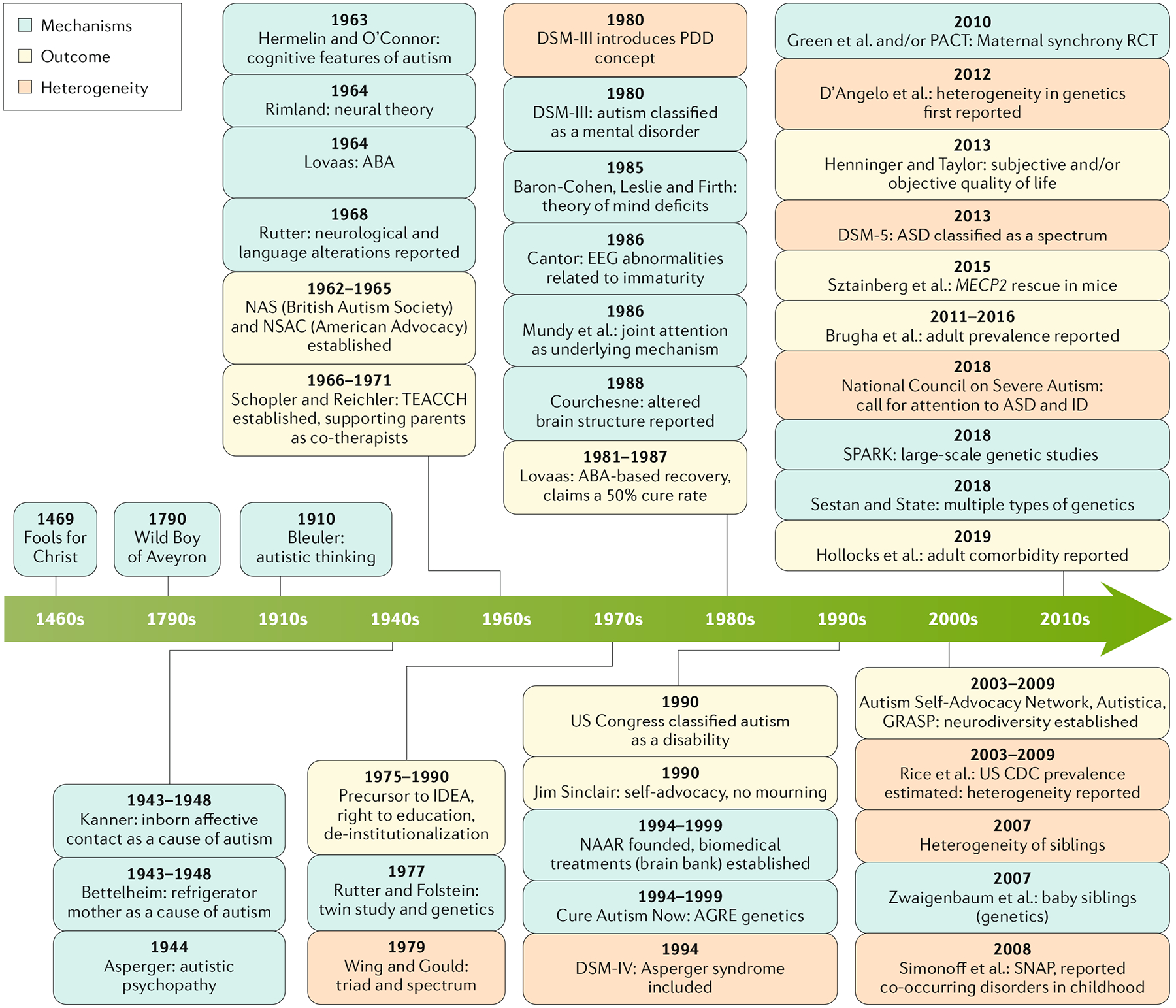
Original descriptions of the cardinal features of autism were attributed to a range of causes, including being raised by wolves (the Wild Boy of Aveyron), inborn limitations in affective contact and unfeeling parenting (such as ‘refrigerator mothers’), and holy people (such as Fools for Christ)257. Conceptualizations of autism as a common highly heritable neurodevelopmental disorder with underlying cognitive features began with the recognition of differences in brain function and cognition in the 1960s258–261 and the first twin study in the 1970s262. Other proposed mechanisms include maturational lags in neurophysiology94 and cognitive mechanisms such as joint engagement176,263. With the search for pathways to and sometimes out of autism on many levels, conceptualization of positive outcomes has been more recent but has also varied markedly. In the 1970s, autism societies and collaborative clinical programmes focused on community integration and de-institutionalization (such as National Autistic Society (NAS) and National Society for Autistic Children (NSAC))264. Priorities shifted in the 1980s and 1990s, with still unreplicated claims of ‘recovery’ in children who participated in intensive behavioural interventions179, new advocacy groups focusing on biomedical discoveries to yield potential biological treatments and even ‘cures’ (such as National Alliance for Autism Research (NAAR) and Cure Autism Now257), and the neurodiversity movement265, which rejected ‘cures’ and called for adaptation of environments to support autistic people, using terminology preferred by self-advocates and community participation. Recognition of the marked heterogeneity within autism began in the 1970s, with the triad of impairments in language, play and social interaction characterizing many children with intellectual disabilities (ID) or those with classical autism266. The first twin study demonstrated that monozygotic twin pairs, although concordant for difficulties associated with autism, differed in specific characteristics and co-occurring conditions, including ID262. More recently, phenotypic heterogeneity has been the rule in most, although not all, gene-first phenotypic studies267. Thus, developmental aspects of differences in strengths, difficulties and trajectories as well as biological factors require highly personalized conceptualizations of the needs of autistic individuals and their families. ABA, applied behaviour analysis; AGRE, Autism Genetic Resource Exchange; ASD, autism spectrum disorder; DSM, Diagnostic and Statistical Manual of Mental Disorders; EEG, electroencephalography; GRASP, Global and Regional Asperger Syndrome Partnership; IDEA, Individuals with Disabilities Education Act; MECP2, the gene associated with Rett syndrome; PACT, Preschool Autism Communication Trial; PDD, pervasive developmental disorder; RCT, randomized controlled trial; SNAP, Special Needs and Autism Project; SPARK, Simons Foundation Powering Autism Research for Knowledge; TEACCH, Treatment and Education of Autistic and Related Communication-handicapped Children.
Manifestations of autism include impairments in social communication and interaction, sensory anomalies, repetitive behaviours and varying levels of intellectual disability (BOX 1). Together with these core symptoms, co-occurring psychiatric or neurological disorders are common in people with autism, of which hyperactivity and attention disorders (such as attention-deficit/hyperactivity disorder (ADHD)), anxiety, depression and epilepsy are fairly prevalent. A diagnosis of autism is reached after obtaining a detailed developmental history, often from the parents, and observation of the individual interacting with parents or other individuals1,2. Early intervention for children with autism is key owing to common difficulties in communication. The types of interventions used change throughout life and include parent-mediated interventions and/or therapist-delivered interventions in childhood, and school-based strategies and techniques to promote independence in adulthood. Pharmacological therapies can be used to treat some of the associated symptoms of autism, such as irritability, and comorbidities, such as anxiety.
Box 1 |. ASD as defined in DSM-5a.
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria for autism spectrum disorder (ASD) comprise five symptom clusters (A–E).
- Social communication and social interaction
- Must have evidence across multiple contexts of all of the following three subdomains currently or by history:
- Social reciprocity
- Non-verbal communication
- Developing, maintaining and understanding relationships
- Restricted, repetitive behaviours and interests
- Must have evidence of two of four of the following subdomains currently or by history:
- Stereotyped, repetitive behaviours
- Insistence on sameness
- Highly restricted, fixed interests
- Hypersensitivity or hyposensitivity or interest in sensory inputs
Symptoms must be present in early development but may not fully manifest until later or may be masked later in life by learned strategies
Symptoms must cause clinically significant impairment in current functioning
Not better explained by intellectual disability or global developmental delay
Note: previously established DSM-IV diagnoses of any pervasive developmental disorder, including Asperger’s disorder, should be assumed to be equivalent to DSM-5 ASD. ASD may co-occur with many other disorders, including attention-deficit/hyperactivity disorder, intellectual disability, language delay and genetic syndromes.
a Adapted from REF.125.
This Primer discusses the epidemiology and mechanisms of autism, together with the diagnosis and treatment of people with this condition. Three themes are addressed: mechanisms of causality and change over time, heterogeneity within and between individuals with autism, and outcomes across the lifespan.
Epidemiology
Prevalence
Epidemiological administrative and community-based studies have suggested that autism is more common in males than in females, with reported ratios ranging from 2:1 to 5:1, with an estimate of 4:1 in the 2010 Global Burden of Disease study3,4. The sex ratio is slightly lower in studies that use population-wide testing to find community cases within a population than in the more common passive case-finding studies that review administrative data (for example, medical or special educational records), which may result in less plausible associations and may, therefore, artificially increase prevalence estimates5. Active case-finding that does not rely on administrative records has demonstrated an equivalent community rate of autism in men and women with moderate-to-profound intellectual disability4. Thus, even the most widely accepted tenet of our understanding of factors associated with autism is far from straightforward.
Estimates of the prevalence of autism in various populations and settings differ according to the method of ascertainment used in the study, including definition, sampling and the extent of independent population case assessment in contrast to administratively based sources. Of note, the Global Burden of Disease study uses all known data from administrative and community survey sources on a disease or disorder to model associations (particularly with time) to examine trends. In the 2010 Global Burden of Disease study, an estimated 52 million people had autism globally, equating to a prevalence of 1 in 132 individuals6. Worldwide, little interpretable variation in the prevalence of autism between regions, ethnicities or services and resource provision has been reported. Indeed, one systematic review did not find a strong effect of ethnic, cultural or socioeconomic factors on the prevalence of autism7. However, statistical power to detect any effects was limited in the available data sets, particularly in low-income countries. An increased prevalence of autism has been reported in migrant groups in some studies8, with few clear factors that might contribute to a greater prevalence in an Afro-Caribbean population in higher-income countries9–11 in the absence of any evidence of geographical variation7. However, a survey of adults in the general population has shown that rates of autism in black and minority ethnic groups may be lower than in the rest of the population12; data from indigenous and Aboriginal cultures are very limited.
Many individuals and groups presume that rates of autism are increasing over time, but this supposition is based on data from administrative records rather than community-based studies. Indeed, after accounting for methodological variations between studies, there was no clear evidence of a change in the prevalence of autism in the community between 1990 and 2010 (REF.13). In addition, general population and systematic case-finding community-based surveys (including testing of representative populations) have also confirmed the lack of significant change in prevalence rates in childhood14 and adulthood15 over time. No significant evidence is available supporting that autism is rarer in older people, which provides further evidence against the suggestion that autism is increasing in prevalence over time4. Even in high-income countries with strong autism public health policies, there is evidence that autism in adults goes largely unrecognized, whereas administratively recorded diagnoses in children increase year by year16. This finding highlights the importance of obtaining information on autism rates in settings where professionals may be able to improve its recognition. The prevalence of autism in mental health inpatient settings is estimated to be far higher than in the general population, ranging from 4% to 9.9%17.
Environmental factors
One review of systematic reviews and meta-analyses of environmental risk factors for autism included a comprehensive coverage of the literature, a discussion of the limitations of research and the need for long-term prospective cohort-based studies to begin to address these limitations18 (FIG. 2). This review and other studies identified environmental risk factors for autism as advanced parental age19 and birth trauma, particularly if due to proxies of hypoxia18. Moreover, maternal obesity, a short interval between pregnancies, gestational diabetes mellitus and valproate use during pregnancy have all been associated with increased risk of autism (FIG. 2). However, it should be noted that these factors cannot be considered causal, but could be reactive, independent or contributory for autism. Studies evaluating risk factors for autism that have reported an absence of association are equally, if not more, important to note, including clear evidence that autism is not associated with vaccination20. Other negative associations include prolonged labour, delivery by caesarean section or assisted vaginal delivery, premature rupture of membranes and the use of assisted reproductive technologies, among other factors (FIG. 2). Environmental risk factors could trigger the risk of autism through several complex underlying mechanisms such as genetic and epigenetic effects (see Mechanisms/pathophysiology, below), inflammation and oxidative stress, or hypoxic and ischaemic damage18.
Fig. 2 |. Environmental risk factors for autism.
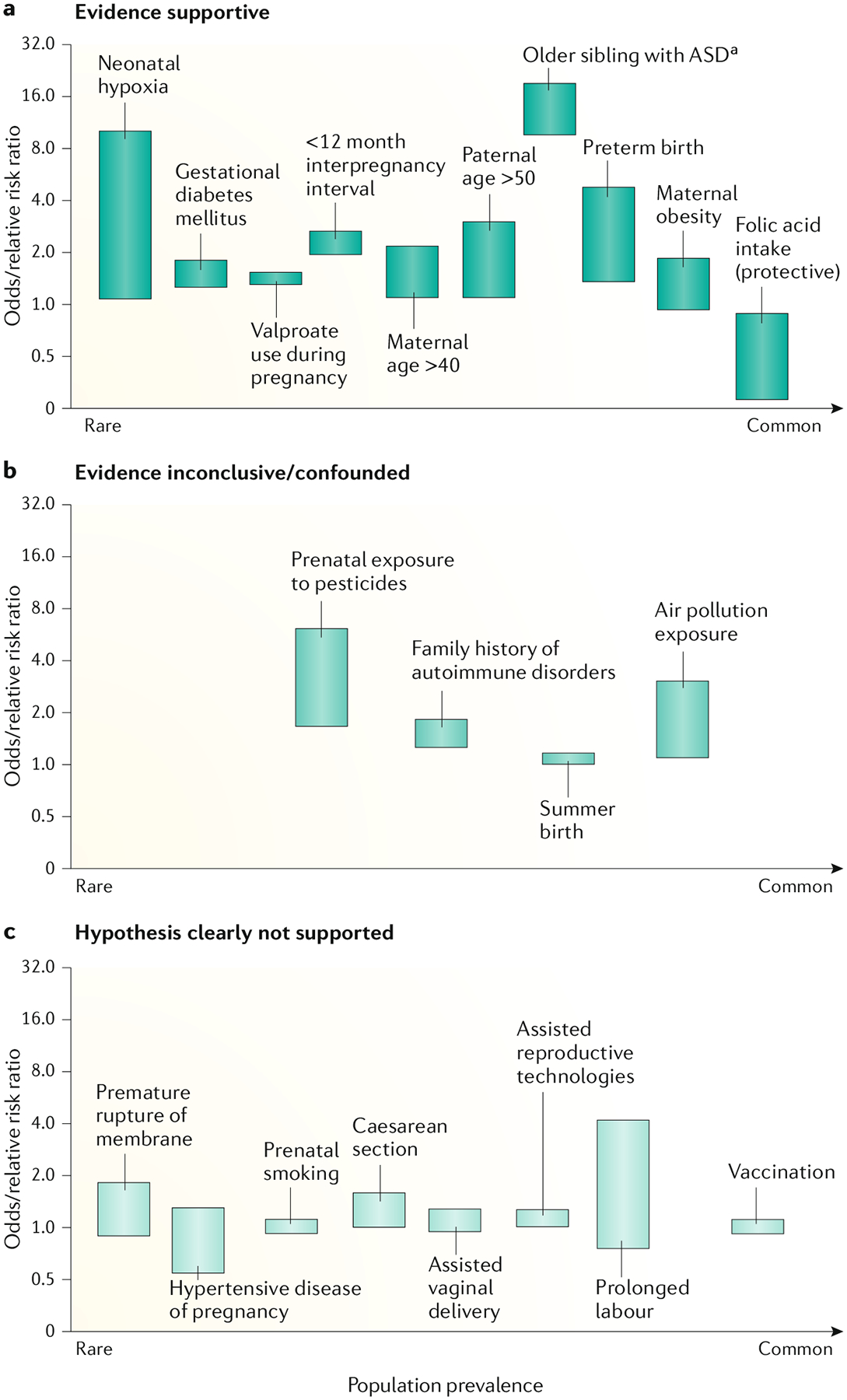
Data from studies aiming to identify risk factors for autism can be broadly split into three categories: those with evidence supporting an association (a), those with inconclusive evidence (b) and, importantly, those with no supporting evidence (c). Bars represent ranges. aRepresents recurrence risk. Figure adapted from REF.18 with added findings from select reviews and empirical papers: neonatal hypoxia estimate268, childhood vaccines20, valproate use during pregnancy269, parent age estimates270, preterm birth estimate271,272, maternal obesity estimate273, folic acid intake estimate274, siblings estimate275,276, interpregnancy interval estimate277, assisted reproductive technologies estimate278,279, pesticide and air pollution estimate280, and caesarean section estimate281. Adapted from REF.18, CC-BY-4.0 (https://creativecommons.org/licenses/by/4.0/).
Mechanisms/pathophysiology
Many cognitive theories have been suggested to underlie the behavioural and developmental manifestations of autism, although the prominence and the consensus on the potential explanatory value of these theories have declined in the past decade. These theories range from ‘social first’ theories, such as the theory of mind (or mentalizing) and social motivational deficit theories, to global processing deficit theories, including attentional control, executive dysfunction and weak central coherence or enhanced perceptual processing theories21,22. Although many of these theories had a useful descriptive role and provide potential insights into differences in how autistic individuals might process and experience the world around them, the theories pertain to neurodevelopmental disorders in general and lack specificity for autism, they are largely non-developmental, applying only to a single point in time, and lack evidence as explanatory models. Nevertheless, they have been useful in clinical practice and underlie some recently proposed interventions, such as cognitive behavioural therapy (CBT)-oriented treatments for anxiety23.
Following cohorts of infants from gestation or birth to 2 or 3 years of age (that is, to an age when a diagnosis of autism can be established) enables the study of the brain and behavioural manifestations of autism as they emerge24. Indeed, prospective studies of infants with a relative with autism have yielded several insights into the mechanisms of this disorder. For example, infants who develop autism later in childhood have substantially typical profiles of interest in faces25 and eyes26 at 6 months of age, which have cast doubt on social orienting theories in which autism originates from a primary deficit in innate patterns of subcortically mediated social orienting27. In addition, subtle but diffuse differences in electroencephalography (EEG) and other measures of brain function have been demonstrated in autistic people (see Findings from electrophysiological studies, below), which could represent alternative pathways to a common end-state phenotype or to whole-brain alterations in synaptic signalling pathways that have effects on development28. Such considerations highlight the limitations of deterministic models of autism, in which a genetic change leads to a synaptic change that relates to a canonical symptom29. Rather, there is probably a complex set of developmental interactions in which the child’s emerging brain activity and behaviour have bidirectional relationships to synaptic signalling and gene expression30.
Genetics
Twin and family studies consistently demonstrate that autism has a particularly large genetic contribution, with estimated heritability ranging from ~40% to 90%31,32. In addition, one analysis demonstrated that autism is among the most heritable common medical conditions33. More than 100 genes and genomic regions have now been confidently associated with autism34,35, mostly based on the study of heterozygous, germline, de novo mutations. These genetic changes range in size from a single base (or nucleotide)36–38 to submicroscopic segments of DNA of thousands to millions of bases (also known as copy number variations (CNVs))39,40. Whether these genetic changes lead to alterations in the sequence of DNA or in the structure of the chromosome, changes that have a functional effect on protein-coding regions of the genome have the strongest and most reliable association with autism risk. Collectively, these de novo heterozygous mutations are rare and confer relatively large risks of autism41. With genetic studies now including cohorts of up to tens of thousands of individuals and the associated increase in statistical power, common, transmitted alleles of modest effect size, mostly corresponding to the non-coding regions of the genome, have begun to be identified42.
Studies of the genetics of autism contrast broadly with those of adult-onset psychiatric disorders in which most successful gene discovery has emerged from genome-wide association studies assessing common alleles of small effect size. Indeed, the earliest successes in autism presaged a more general finding that the contribution of rare, de novo mutations in coding regions of the genome is relatively greater among a range of early-onset disorders43–45 than for typically later-onset common conditions such as schizophrenia and bipolar disorder, although there is also a surprising degree of overlap in genetic risk for overtly disparate neuropsychiatric phenotypes that remains to be further elucidated31.
The extent to which rare, high effect size mutations account for autism risk raises some important definitional issues. Considering the overall population, the contribution of de novo mutations to autism risk is quite small (~3%)32. Indeed, most individuals who harbour a genetic risk for a common condition, particularly those with variants of small effect size, will never develop symptoms or need clinical attention. By contrast, there is a marked enrichment of individuals with rare and de novo mutations in the clinical autism population. Conservative estimates are that 10–20% of people with autism harbour a de novo rare point mutation or CNV contributing to their presentation34,46,47. If the clinical population is constrained to those with autism who have a combination of factors, including being female, having intellectual disability, multiple unaffected siblings, or seizures, ~20–30% have a rare de novo mutation; if an individual has several of these risk factors, the yield of de novo sequence and structural mutations would be expected to be even higher34.
However, irrespective of the precise proportion of risk conveyed by these mutations, their most substantial contribution to the understanding of autism is likely to be in elaborating the mechanisms of this disorder48,49. In autism, a single de novo germline heterozygous loss-of-function point mutation can convey more risk than the cumulative effect of the top decile of polygenic risk for schizophrenia47,50. Unfortunately, although manifestly more tractable than modelling hundreds of alleles simultaneously, addressing a single autism mutation at a time is not synonymous with an easy avenue to clinical care of most people with autism.
Molecular pathophysiology.
Over the past decade, there have been many studies using model systems to recapitulate so-called single gene (or monogenic) versions of autism, such as fragile X syndrome and tuberous sclerosis complex, which are cumulatively estimated to account for <10% of clinical cases of autism51. In addition, more recent studies have modelled the effects of rare and de novo mutations identified in idiopathic autism. This literature is too vast to review comprehensively here52,53. Although the study of autism risk genes in model systems has revealed a great deal about general biology, how these findings relate to the pathophysiology of autism is less clear48,49. In general, autism risk genes tend to have a role in multiple functions in many brain regions that unfold in a spatiotemporally defined manner across development. Consequently, although manipulation of a single risk gene in a model system may lead to interesting phenotypes, including social-behavioural phenotypes in evolutionarily distant organisms, it does not necessarily illuminate its contribution to human social disability. Moreover, although a single mutation can confer a several-fold increase in the risk of autism, these variants do not demonstrate the type of causal clarity that is associated with classic monogenic neurodevelopmental disorders such as fragile X syndrome, Angelman syndrome, Rett syndrome or tuberous sclerosis complex. In addition, the well-established sexual dimorphism of social disability adds yet another dimension to the expansive search space that exists between risk genes and human behaviour48. The challenges of disentangling the spatiotemporal dynamics of risk gene expression and protein function are made even more difficult by the reality that these may play out differently in males and females.
Owing to these challenges, multiple approaches have emerged focusing on convergence38,40,54–57, that is, searching for points of commonality across different autism risk genes, with the reasoning that this approach could identify shared pathological mechanisms. In fact, the earliest successes in gene discovery quickly revealed important general properties that have held up well over time, including that, prima facie, most proteins encoded by autism risk genes are involved in either synaptic structure and function or chromatin modification and regulation of gene expression38,46,47,58 (FIG. 3). More recently, there has been an additional focus on spatiotemporal convergence and several studies have supported a nexus in mid-fetal, glutamatergic neurons during cortical development, with modestly divergent findings regarding deep56 versus superficial54 cortical layers. With improvements in technology, additional regions, including the striatum, have also begun to emerge as points of potential risk convergence for autism59.
Fig. 3 |. Encoded proteins associated with autism risk.
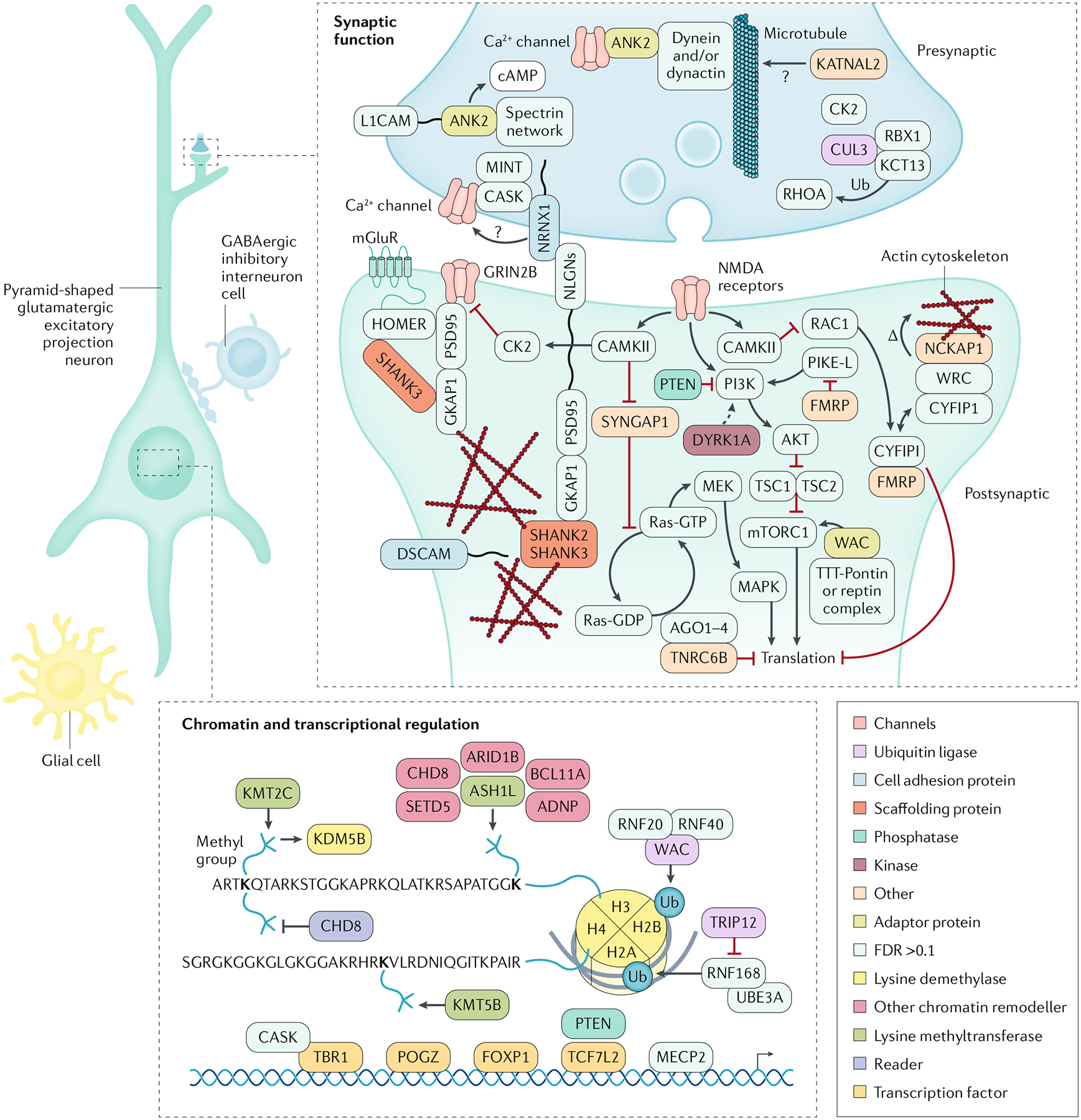
Simplified schematic of the major cellular components of a neural circuit in the cerebral cortex, with a focus on pyramid-shaped glutamatergic excitatory projection neurons. Proteins encoded by selected high-confidence (false discovery rate < 0.1) autism risk genes34 and proteins encoded by selected syndromic autism genes have a role in these neurons during development. These proteins have a diverse intracellular distribution: those at the synapse have roles in cell adhesion, scaffolding and signalling. In addition, some of these proteins are localized to the nucleus and have been shown, broadly, to mediate chromatin modification and transcriptional control. Syndromic autism genes include FMR1 (encoding fragile X mental retardation protein; fragile X syndrome), UBE3A (encoding ubiquitin-protein ligase E3A; Angelman syndrome), TSC1 and TSC2 (encoding hamartin and tuberin; tuberous sclerosis complex), PTEN (encoding phosphatase and tensin homologue) and MECP2 (encoding methyl-CpG-binding protein 2; Rett syndrome). GABA, γ-aminobutyric acid; Ub; ubiquitin. Adapted with permission from REF.48, Elsevier.
The ability to constrain future experiments to examine mutations in specific risk-associated regional, cellular and developmental contexts should allow narrowing in on relevant mechanisms. Of note, one study used single-cell technologies to examine specific cell types and developmental stages using brain tissue from people with autism60, and demonstrated changes in transcription in multiple cell types, including upper-layer cortical neurons. These types of post-mortem studies ask important but somewhat broader questions from the approaches described above regarding the underlying pathology and how the brain changes and responds to pathology over time. In these studies, similar to any cross-sectional study, it can be challenging to differentiate cause from effect. Consequently, the pursuit and intersection of studies that seek to define convergence early in development and those that examine subsequent molecular, cellular and circuit level changes will be critical to illuminating pathological mechanisms.
Indeed, given the success and US FDA approval of gene therapy for early-onset neurological disorders, particularly spinal muscular atrophy type 1 (REFS61,62), targeting single genes of large effect in both idiopathic and monogenic autism is being viewed as increasingly plausible. As rare syndromes such as fragile X syndrome, Angelman syndrome and Rett syndrome have offered some of the earliest insights into autism biology, these disorders are also likely to lead the way in illuminating the practical and important ethical challenges that will attend such efforts for idiopathic autism. Efforts aimed at the highest confidence risk genes identified in idiopathic autism, such as SCN2A and CHD8 (REFS34,35), are almost certain to soon follow on attempts at gene therapy for monogenic neurodevelopmental disorders in light of the growing list of well-defined large-effect targets, the increasing options for addressing haploinsufficiency63, the ability to manipulate gene products without leaving a DNA ‘scar’ (REFS63,64) and the increasing ability to readily detect mutations — and intervene — in utero and very early in postnatal development.
Neurobiology
Findings from MRI studies.
MRI can facilitate understanding of how the brain structurally and functionally develops differently in people with autism, although, to date, MRI results in autism are not definitive. Although neuroimaging is typically more expensive than EEG and studies are limited by issues of replication (sometimes this is related to head motion occurring during the scan, which can erode signal65), structural studies including those using diffusion tensor imaging66 and functional MRI (fMRI)67 have accelerated our understanding of how altered neural circuits relate to clinical symptoms of autism68,69. Studying circuitry in childhood that is specifically associated with the social brain (a network of brain areas involved with processing social information), including visual areas, areas of the prefrontal cortex, sub-cortex and areas integrating information (such as temporal parietal function and superior temporal sulcus), could also offer insight into the neural mechanisms of autism70. In addition, MRI may facilitate understanding of the heterogeneity of autism, demonstrating subgroups of individuals with specific neurobiological alterations that could account for their symptomology. The summary of MRI studies in this section focuses on 0–2 years of age and addresses biomarkers for autism. Prospective study designs are largely covered as they represent a substantial portion of MRI research in autism.
The first MRI studies of autism focused on cerebral and cerebellar grey matter and white matter volumes in young children71,72, although these studies were limited by studying toddlers and children aged ≥18 months, missing the opportunity to detect biomarkers of autism in the first year of life. More recently, longitudinal studies have obtained multiple brain MRIs of infants at high risk of developing autism (that is, those with a sibling with autism; known as baby sibling studies) during their first 2 years of life and assessed these children for autism at this age. In these studies, detectable differences in brain structure were observed at 6 months of age in the fractional anisotropy trajectories for 12 of 15 neural fibre tracts in the brain in children diagnosed with autism at 2 years of age compared with children not diagnosed73. Furthermore, abnormal growth in the cortical surface between 6 and 12 months of age and greater brain volume between 12 and 24 months of age were seen in children who were later diagnosed with autism, compared with those not diagnosed with autism74 (FIG. 4). In addition, white matter integrity in the genu pathway at 6 months of age predicted the presence of restricted and repetitive behaviours at 2 years of age75 and computational work demonstrated that whole-brain functional connectivity at 6 months of age predicted a diagnosis of autism at 2 years of age76. Collectively, these studies suggest the presence of disrupted neural pathways before the emergence of behavioural symptoms in children with autism and might provide clues about the underlying neural mechanisms of autism. Although data from MRI studies have revealed differences in neurobiology between young children diagnosed with autism and those without autism77, given that replication has been particularly difficult in these studies, more work is required before MRI can be used as a reliable biomarker of autism78.
Fig. 4 |. Longitudinal trajectories of total brain volume, surface area and cortical thickness in autism.
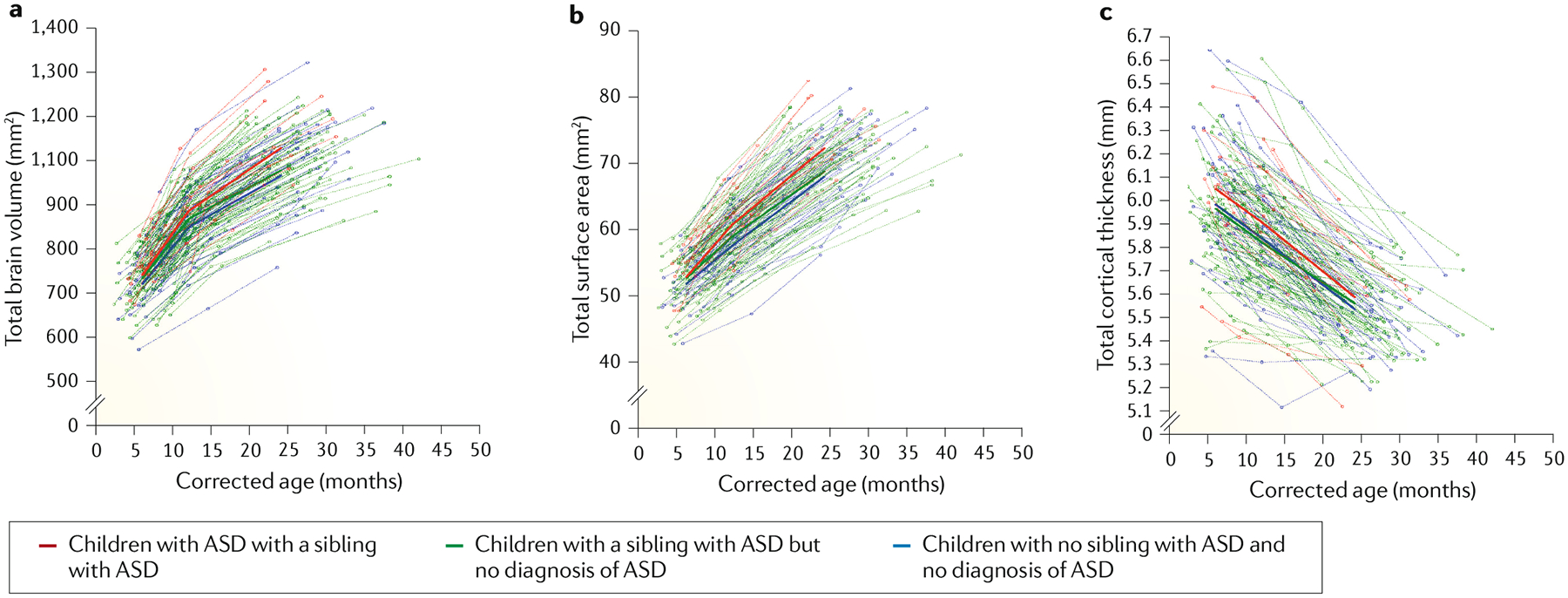
Brain trajectories from 6 to 24 months of age for total brain volume (a), total surface area (b) and total cortical thickness (c). Toddlers diagnosed with autism spectrum disorder (ASD) had significantly greater surface area growth from 6 to 12 months than infants who were high risk for ASD but did not receive a diagnosis as well as than typically developing infants. Differences in surface area growth became more pronounced from 12 to 24 months of age for toddlers who received an ASD diagnosis. Corrected age refers to the age corrected by length (body size). Adapted from REF.74, Springer Nature Limited.
Task-based fMRI studies investigate circuits that are responsible for core challenges in autism, such as language production and comprehension79, and have demonstrated hyper-activation of the superior temporal gyrus and inferior frontal gyrus as well as hypoactivation of the bilateral middle temporal gyrus76. In addition, these studies have demonstrated challenges in processing emotions in faces and the ‘social brain’ (REF.78) and deficits in attention79. Studies have also demonstrated greater sensitivity to sensory information. Increased connectivity between the anterior insula and sensorimotor areas, and the anterior insula and amygdala, together was associated with greater sensitivity to slightly aversive sounds and tactile information80. Although this area of research has revealed similarities or differences in people with autism in relation to comparison groups, it has been limited by averaging data across many individuals, which can mask heterogeneity and differences across age groups. In addition, the work has been limited by small sample sizes and problems with replication probably caused by the many challenges with MRI data collection in people with autism, such as differences in data processing, inter-subject variability and data quality80. Longitudinal imaging81 as well as associating neuroimaging data with longitudinal behavioural outcomes82 can address some of these limitations characterizing differences within participants.
Resting state functional connectivity MRI studies that require participants to look at a blank screen with no task demands have been used to study intrinsic connections in the human brain. Large data sets, such as the Autism Brain Imaging Date Exchange (ABIDE)83, have enabled researchers to pool data to allow more highly powered studies to address known limitations of small sample sizes, and many data sets have relied on resting state studies to study neural connectivity in autism. In these studies, evidence has emerged of both hyper-connectivity and hypo-connectivity in short-range and long-range connections throughout the brain84,85. Differences in results between studies could be due to the age of the participants86, sex differences, heterogeneity, methodological concerns87 or that both connectivity states exist in autism.
In future, MRI could be well suited to categorize subgroups of autism88 as well as parsing out commonalities and distinctions among other developmental disorders89. Using MRI to better understand the differences between boys and girls on the spectrum90, such as differences in whole-brain connectivity90 or the social brain91 — a field in its infancy — or as a marker of biological change due to treatment, has growing interest92.
Findings from electrophysiological studies.
EEG has historically been used for the diagnosis of comorbid epilepsy in people with autism93, although it can also be used to study the mechanisms of autism. Compared with MRI, EEG is more economical, easier to use and less invasive, which is particularly important for paediatric populations, while granting access to brain dynamics at millisecond timescales. Magnetoencephalography (MEG), although more expensive, provides higher spatial resolution than EEG.
Since the early recordings, the first focus of quantitative EEG was to study people with autism in task-free conditions. Pioneering studies have revealed alterations in oscillatory activity during resting state in people with autism, with more slow waves and less alpha waves as well as less intra-hemispheric and inter-hemispheric asymmetry than in people without autism94. More recent work has demonstrated the presence of developmental trajectories as revealed through increasingly sophisticated spatiospectral analyses, and has shown how differences in the trajectories of EEG power in high-risk infants may represent endophenotypes of autism95,96.
In terms of mechanisms, other studies have started to focus on the task-based modulation of cognitive function such as low-level perceptual anomalies and action observation that relate to the autism phenotype. One theory proposing a specific failure in autism of the ability of the brain to ‘mirror’ observed actions of another person (thereby named the ‘broken mirror’ theory) was based on altered mu wave suppression in autism97 but was later questioned both theoretically98,99 and empirically100,101, pointing towards a more complex picture of dysfunctional executive functions and visual attention102. Other studies, particularly those assessing event-related potentials, have demonstrated the modulation of sensory processing in people with autism, with observed changes in sensitivities and latency103. Differences in auditory and visual processing could have a role in the development of core features of autism, such as language delay and difficulty in emotion recognition, although this hypothesis requires further study. Although perceptual processes appear different in people with autism, the electrophysiological underpinning is still far from clear regarding the main event-related potentials such as mismatch negativity104 or the N170 (REF.105). Although data from meta-analyses have suggested smaller mismatch negativity amplitudes and delayed N170 latencies, on average, in people with autism compared with typically developing controls, additional studies are required that account for the large heterogeneity of this disorder by moving away from averaging the data to focus either on specific subgroups106 or refined modelling strategies that can capture individual differences in developmental trajectories95. Although this avenue of research has not yet been fully explored, interactive tasks that encompass real-time social interaction could allow the study of brain activity in experimental contexts that are more relevant for core autism symptoms, rather than the more passive tasks that are used in most functional imaging studies107. Experiments focusing on human–human interaction108 and human–machine interaction109 have been under-taken but, so far, no study has ever made explicit use of such methods to study the electrophysiology of autism.
In a further search for mechanisms of autism, prospective baby sibling studies have suggested that the gradual emergence of behavioural symptoms of autism is preceded by earlier subtle alterations in the activity of regions and networks of the social brain24. For example, early work on a small group of 5–6-month-old infants who later developed autism observed faster but less prolonged neural activation and delayed sensitization responses to faces compared with infants who did not develop autism110, and one report demonstrated that newborns with an increased familial likelihood of autism showed higher signal homogeneity within core social brain networks (the right fusiform and left parietal cortex111). By comparison, reduced frontal power, particularly in the high-alpha band, during quiet play at 3 months of age112 and cortical hyperexcitability in the right tempo-parietal region during auditory repetition of pure tones at 9–10 months of age have been found in babies at familial risk for autism113, suggesting that atypical patterns occur in brain regions other than those involved in social processing. Such alterations could have a cascading effect on social learning and contribute to the later emergence of behavioural symptoms of autism, although a causal link remains to be demonstrated. Replications across different research centres are needed because many of these studies had small sample sizes, different definitions of groups, and varied measures and time points.
Interestingly, results from MEG and EEG studies jointly point towards two physiological mechanisms of autism: excitation/inhibition imbalance and alteration of large-scale functional interactions of brain systems as quantified through connectivity analysis114. An excitation/inhibition imbalance is supported by results from computational modelling of how reductions in the amount of inhibition can account for the previously observed perceptual consequences of autism115 and by transcranial magnetic stimulation studies demonstrating a neurophysiological deficit in γ-aminobutyric acid (GABA) receptor-mediated function in people with autism116. In parallel, decreased long-range functional connectivity has also crystalized as a consistent mechanism117. MEG studies have suggested a complex functional connectivity pattern in the somatosensory cortex with reductions in the feedback (top-down) direction but increased in the feedforward (bottom-up) direction118. Clarifying the extent to which this pattern is a methodological artefact that could result from the predominant average-brain approach, as suggested by fMRI studies, is critical119.
Beyond use to understand the pathophysiology of autism, the scalability and accessibility of EEG suggest that this technique could be an ideal candidate for use as a brain-based biomarker. Measures from information theory have already provided promising case–control classification120, but developing generalizable biomarkers may require a combination of multiple EEG measures supported by robust machine learning methods121. Against the background of the current reproducibility crisis that characterizes many studies122 as well as the defining heterogeneity of autism, the next breakthrough will certainly demand large-scale collaboration between researchers and clinicians.
Diagnosis, screening and prevention
Diagnosis of autism is made on the basis of behavioural presentation. Although substantial heterogeneity exists between and within individuals across development, a set of core diagnostic features of autism (covering social interaction, communication, and restricted, repetitive or sensory behaviours) can be reliably identified by trained clinicians123,124.
Diagnostic criteria
The reformulation of the diagnostic criteria for ASD in DSM-5 (REF.125) (BOX 1), which is similar to the criteria in ICD-11 (REF.126), contains several changes from previous editions that were based on good empirical and clinical evidence127. First, the subclassification of ‘Asperger’s disorder’ was subsumed under the unitary term ASD as the diagnosis was inconsistently applied even by expert groups128. This change is controversial, yet the evidence supporting the inclusion of Asperger’s disorder as a separate condition is very weak129. The important issues are how to better consider the factors that characterize differences among autistic individuals and ensuring that these differences are measured and addressed using neurobiological and clinical research, rather than contained within very poorly defined categories of Asperger’s and PDD not otherwise specified (NOS), as defined in DSM-IV130. In addition, some individuals with social communication problems but not restricted and repetitive behaviours who would previously have fallen into the now-removed subcategory of PDD-NOS now receive a different diagnosis of social communication disorder, which is not yet well validated. Although these changes have led to concerns that the DSM-5 ASD criteria are more restrictive than those in DSM-IV, many clinicians feel that the changes better reflect clinical consensus and practice. Second, the social and communication domains of the diagnostic criteria were unified to reflect the factor structure of symptomatology. Third, sensory anomalies (hypersensory and hyposensory responsiveness and sensation-seeking) in DSM-5 were included under the ‘restricted, repetitive behaviours and interests’ domain to reflect their pervasiveness131. Fourth, the DSM-IV criteria required symptoms to be present in the first 3 years of life, but criteria in DSM-5 recognize symptom onset during the early developmental period with the caveat that symptoms might not fully manifest until social demands exceed limited capacities. This change recognizes the developmental nature of autism, wherein for some individuals, clear manifestation of autism might not be apparent until mid-childhood, adolescence or even adulthood. In addition, late diagnosis (that is, diagnosis beyond early childhood) can occur even in those who received intensive early monitoring132. The DSM-5 criteria support the use of specifiers that can denote those with dual diagnoses such as individuals with ASD and ADHD or other psychiatric disorders as well as with genetic conditions such as fragile X syndrome or Down syndrome. Beyond the clinic, these changes have implications for large-scale data pooling efforts, in considering domains of behaviour to be modelled, and in identifying shared and distinct developmental pathways to conditions such as autism and ADHD.
Diagnosis and screening in children
The two core elements of the diagnostic process of autism in children are a detailed developmental history that is usually obtained from parents, covering first concerns and early history to the present day, and an observation of the child’s interactions with their parents and with unfamiliar adults during a combination of structured and unstructured assessments. Ideally, observations of the young person in peer-group settings such as school or nursery would also form part of the diagnostic process. Of note, in one population-based study in the UK, girls with similar levels of symptom expression to boys were less likely to receive a diagnosis of autism from clinical services133. This finding might reflect sociocultural factors in the application of the diagnostic criteria, greater resilience or protective factors in girls that reduce the need for clinical services at a given symptom level, or the need for the revision of instruments used to identify symptoms to more fully cover female autistic traits127.
A number of structured diagnostic interviews and observational assessments for autism exist, but only a limited number have been rigorously tested for diagnostic accuracy relative to the gold standard of expert clinician judgement. Although these interviews and assessments have reasonably robust sensitivity, specificity and reliability (see134 for a review) and are widely used in some services in communities135, there are also challenges to the widespread adoption of the best validated instruments: the Autism Diagnostic Interview-Revised (ADI-R)136 and the Autism Diagnostic Observation Schedule-2nd Edition (ADOS-2)123. These challenges include the cost of the instruments and training, the time required to complete them and the need for substantial training to use them reliably137. Although expert clinical judgement was previously believed to be more reliable than reliance on instrument scores alone for the diagnosis of autism138, more recent evidence suggests that this may not be true at least in toddlers and preschool children139. The need to take a global perspective on autism is driving attempts to develop more scalable tools but this work is currently in its infancy140 (BOX 2).
Box 2 |. Global challenges in autism research.
Recently, there have been calls for more attention to global issues in autism research251 (Global Research on Developmental Disabilities Collaboration – Lancet Global Health, 2016), including a number of related issues with somewhat different potential solutions. For example, broader populations should be included in autism research, including individuals from low-income and middle-income countries, but also inclusive representation of the ethnic, linguistic and socioeconomic diversity of many high-income countries and people whose autism is unrecognized. Moreover, there should be the creation of opportunities to carry out research in low-income and middle-income countries253. Open source and shared databanks, including autism-specific resources such as the Simons Simplex Collection and Autism Brain Imaging Data Exchange (ABIDE), as well as broader collaborations, such as PsychENCODE, could assist in promoting international research. In addition, the science of autism should be disseminated in ways that are useful for practice in all countries but with particular attention to the needs of communities and families with fewer resources254,255. More immediately, searches for scalable methods of identification, and perhaps intervention, with children and adults with autism140,256 have begun. However, the need to develop scalable global practices highlights how little is known about when we need population-wide testing for autism versus broader neurodevelopmental disorders, the minimal intensity and duration needed for effective interventions, behavioural mechanisms behind changes in behaviour, and which treatments work with which children, adults and families, all of which have a bearing on interventions locally and globally. In addition, global issues of stigma, governance and paucity of resources also have to be considered253.
The stability of a diagnosis of autism from the preschool years to mid-childhood is relatively high1. However, although diagnostic systems currently pre-suppose that autism is a lifelong condition, there is a growing recognition that autism has a heterogeneous developmental time course141. Indeed, subgroups of individuals with autism and improving or worsening symptoms over time can be identified142,143. Such developmental trajectories might be a more meaningful phenotype on which to map aetiological mechanisms than a static case–control dichotomy74,144,145. Some individuals diagnosed as children have no clinically meaningful (or even detectable) impairment later in life (so-called optimal outcome146,147); one critical question in identifying mechanisms is whether this profile is associated with the successful effects of early intervention or is an aetiologically distinct subtype of autism.
Screening and early identification.
The potential for early testing to prospectively identify children with autism at a young age has considerable interest and several studies have evaluated the performance of parent-report instruments between 14 and 24 months of age such as the Modified Checklist for Autism in Toddlers (M-CHAT) and the Early Screening of Autistic Traits (ESAT)134,148,149. However, there are contrasting views on the strength of the evidence for universal population-wide testing, also known colloquially as screening150,151. Of note, research is lacking on the effectiveness of therapeutic interventions in those identified with autism through universal screening. In addition, although it is possible to identify some children with autism before parents or professionals have identified concerns, diagnosis is missed in many children152, and most tested cohorts have not been systematically followed up to identify later-onset autism in children who initially tested negatively153. Screening also often identifies children with broader developmental difficulties as well as those with autism154. In general, such instruments could be more useful for identifying possible signs and symptoms of autism in high-risk populations, for example, in young children with older siblings with autism155 or in those referred for speech or other developmental concerns to community paediatric services156. In addition, population-wide testing may also have a role in improving awareness and recognition of the early signs and symptoms of autism of both professionals and the general public, which alongside ongoing developmental surveillance pathways in community services, could help to bring down the age of recognition and diagnosis. These principles also apply in low-income and middle-income countries in which testing for autism and other neurodevelopmental disabilities has only just begun to be implemented154. Very little research has been devoted to cultural and ethnic differences in either child early presentation and parents’ understanding or the experience of autism, which may affect how screening instruments work and, therefore, affect parents and families as much as autistic individuals.
Early developmental profiles.
Understanding of onset patterns of autism has dramatically expanded over the past 10 years through work on infants with a first-degree relative with autism who, due to the high heritability of the condition, have a 20% chance of developing autism themselves25. Symptoms of autism have a gradual developmental onset. Indeed, although the average age of autism diagnosis remains at ~4–5 years of age157, parents typically report first concerns to health professionals at ~2 years of age158. In many individuals, symptoms emerge during the second and third year of life (although, as per the DSM-5 onset criteria above, in others, onset might not be noticed until the child reaches school-age or later), whereas in others, symptoms become apparent after a seeming period of typical development, including a period of regression or stasis. To this end, conceptualization of what has been called ‘regression’ prior to 2 years of age has been reconsidered159,160. Over the first 2 years of life, a substantial proportion of infants who later receive autism diagnoses show gradually accumulating delays across social, communication and language domains, suggesting that ‘regression’ represents a spectrum ranging from frank loss of acquired skills, to a gradual erosion (or ‘plateauing’) of developmental potential to individuals in whom these skills never emerge161.
Diagnosis and screening in adults
Information on diagnostic methods to identify autism in adulthood is in its infancy, with little methodologically acceptable evaluation of interview methods or screening questionnaires (including self-completion questionnaires). Clinical approaches rely heavily on extending methods developed for use in childhood to adulthood. These methods tend to rely on childhood developmental data, although validation research from adult general population-wide testing suggests good specificity and sensitivity for the observationally based ADOS Module 4 (REF.162). However, typically, much research has depended on the judgement of expert clinicians and on standardized data collection of early child development that is unlikely to be obtainable for many older adults. Given that (undiagnosed) autistic adults presenting for an autism assessment are also more likely to have co-occurring adult mental health disorders, any method of assessment must be capable of differentiating such abnormalities in symptoms and behaviour from abnormalities due to autism. This point has led to the suggestion that clinical examination methods to identify adult psychopathology could be extended to include autism in addition to depression, anxiety and psychosis, among other disorders163. Semi-structured adult psychopathology interviewing has been fruitful in the assessment of closely related neurodevelopmental disorders in adults, most notably ADHD164. Given that most people in the world who are autistic are adults, and as many of these individuals have not received a diagnosis of autism4,15, the development and evaluation of such adult assessment approaches is an urgent research priority.
Co-occurring disorders
In addition to the core features of autism, co-occurring difficulties or disorders (FIG. 5) are much more widely recognized in research165,166, although they are not necessarily adequately addressed in clinical practice167. For preschool children with autism, language delays, motor problems, epilepsy, difficulties with sleep and eating, and high levels of activity are most commonly observed168,169. By comparison, ADHD, anxiety, obsessive–compulsive disorder (OCD), intellectual disability, academic challenges, irritability and disruptive behaviours become more apparent in school-aged children170. The proportion of individuals with depressive symptoms becomes higher in adolescents and adults171, whereas other issues often remain. Moreover, growing evidence (although it is reliant on administrative case-finding data) suggests that people with autism have premature mortality172,173 and increased risk of self-harm and possibly suicide, although the mechanisms involved have yet to be elucidated. Studies using electronic health records have demonstrated that adults with autism are more likely to be diagnosed with many physical health conditions, such as immune conditions, sleep disorders and obesity, than adults in the general population167.
Fig. 5 |. Co-occurring disorders.
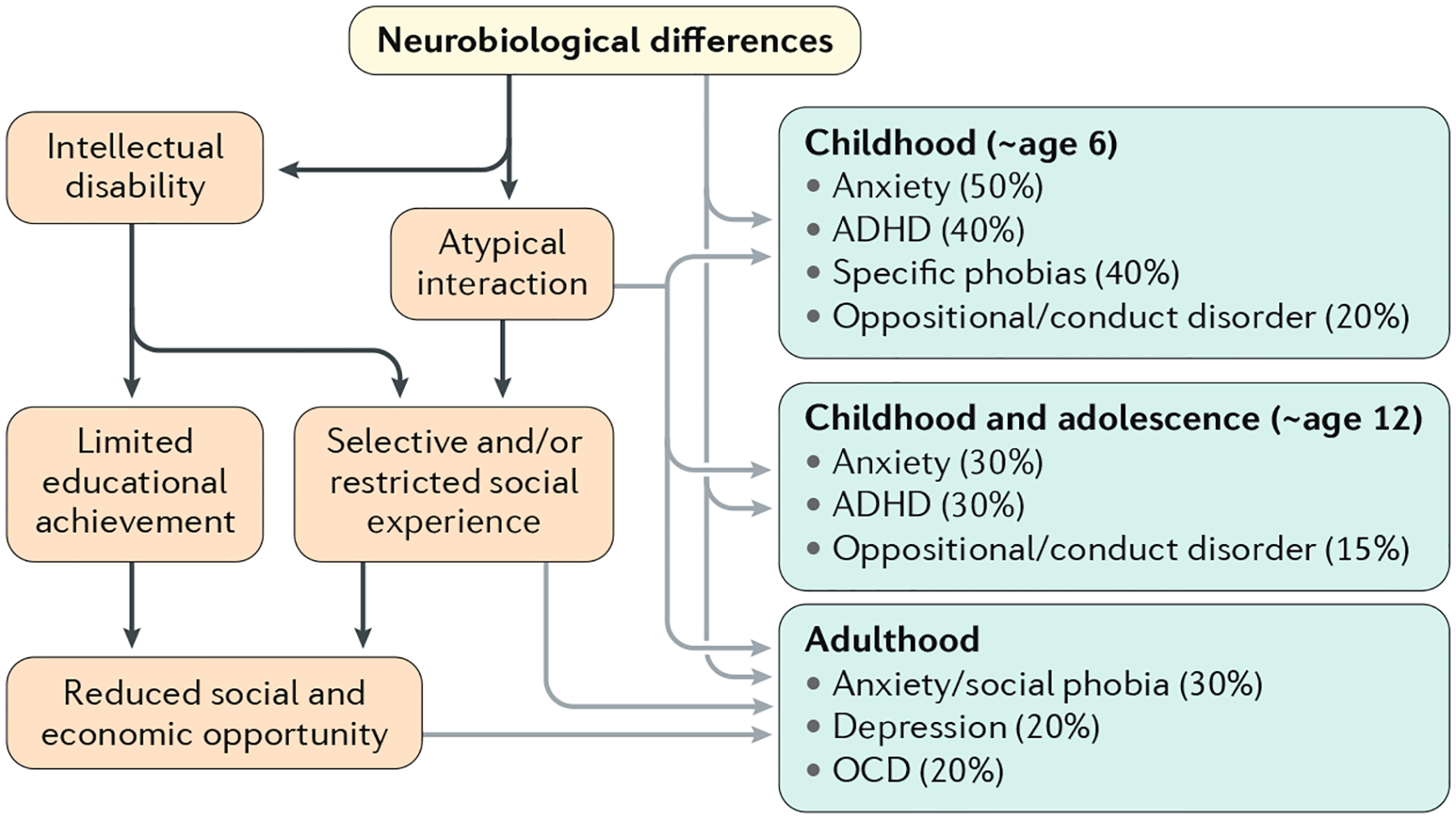
Primary and secondary disorders and disadvantage can accumulate through development in people with autism. These disorders can form additional targets for treatment and policy. Prevalence estimates are preliminary, derived from QUEST282, SNAP138,283 and EDX147 cohorts, but are limited by the fact that many are based on clinical populations or data that are inherently biased (such as US Medicaid data, in which billing for treatment is contingent on having a non-autism diagnosis) and few well-designed population studies exist. ADHD, attention-deficit/hyperactivity disorder; OCD, obsessive–compulsive disorder.
Collectively, these difficulties and disorders contribute to autism severity174 as well as independence and well-being at each age175. However, it is important to note, in the context of heterogeneity, that the prevalence of each of these co-occurring conditions varies considerably with the context of the sample (such as from psychiatry referrals, neurological referrals or schools) and the methodology used (administrative, self-report or assessed) as well as with age, level of cognitive function and perhaps region166. As many of these conditions are treatable, they are very important as clinical considerations but are also more complex than sometimes conveyed.
Management
Early intervention
Early intervention is seen as a priority because many young children with autism struggle to communicate and interact with others, restricting their opportunities to learn and affecting their parents, who can find their child’s behaviour perplexing and challenging to manage. Thus, outcomes of such interventions include changes in the individual’s availability for learning and increased parent understanding. Intervention delivered in the preschool years at an age when there is increased brain plasticity might lead to additional benefit, although this theory has not yet been empirically supported.
The primary models of psychological intervention for preschool children with autism are developmental and behavioural. Although some consensus has been reached on the interventions that have more supporting evidence (termed naturalistic developmental behavioural interventions176), there is some uncertainty and disagreement about the strength of evidence for different approaches, with almost no direct comparisons of treatments or studies to assess which child should receive what treatment or the treatment intensity. Indeed, clinical trials in autism are limited by cost, time, placebo effects and limited outcome measures, and are far behind much other research. This gap leaves parents and practitioners with no options other than what is available and sometimes marketed in their region. Indeed, access to early intervention services is variable in most communities, including in high-income countries, and is mostly carried out by non-specialists supervised by specially trained professionals. In low-income and middle-income countries, most children and young people with autism — similar to those with intellectual and developmental disabilities — will not receive specialized services177, although a number of groups have begun to test community delivery of early intervention in such settings178.
Many current interventions build on the original applied behavioural analysis (ABA)179 and have shifted to more natural, child-initiated developmentally appropriate strategies and tasks instead of dependence on repeated ‘discrete trials’ (known as discrete trial training (DTT)). In addition, considerable variation exists between different intervention models in terms of mode of delivery (for example, parent mediated versus therapist implemented), length (12-week versus 2-year programmes), intensity (from a few hours a week to ~15 hours per week) and the balance between the developmental or dyadic versus behavioural components.
Lower-intensity approaches include parent-mediated interventions whereby parents are coached to become more attuned to their child’s communication signals and style (which are considered an intermediate child outcome) and to facilitate more joint engagement in play and everyday activities designed to increase social and communication skills in the child180. Some studies, such as the 12-week Joint Attention Symbolic Play Engagement and Regulation (JASPER) programme, both when delivered by parents in the home181 and by teaching assistants in school182, have demonstrated enhanced joint engagement and joint attention (which are considered important intermediate child outcomes) with these lower-intensity approaches in preschool children compared with a control group. However, other lower-intensity, time-limited parent-mediated interventions, such as focus playtime intervention (FPI)183, have not improved child outcomes (such as social orienting and joint attention), although some interventions have increased parental responsiveness184. A longer programme (Preschool Autism Communication Trial (PACT)), which consists of fortnightly parent–therapist sessions for 6 months, then monthly sessions for another 6 months, demonstrated improvements in parent and child dyadic behaviours such as parental synchrony and child initiations when interacting with each other (those close to the intervention target) but not symptom reduction at immediate follow-up185. A subsequent 6-year follow-up to mid-childhood at age 7–11 years identified modest reductions in overall autism symptoms using the ADOS over the whole course of the study; these reductions were not detectable at the immediate end point, suggesting that a longer-term perspective is critical in considering outcomes186.
A higher intensity, more comprehensive approach is the Early Start Denver Model (ESDM), which combines behavioural and developmental or dyadic approaches. The ESDM is delivered by therapists for ~15 hours per week, and as part of this programme, parents are trained to improve social communication and interaction with their child. A small-scale trial demonstrated improvements in child developmental and adaptive outcomes, primarily in the language and communication domains, following 2 years of ESDM compared with treatment as usual187. One larger multi-site trial found attenuated benefits with improvement in language outcomes at two of the three trial sites, but no differences between the treatment as usual and ESDM groups in overall developmental ability, adaptive behaviour or autism severity188,189.
Many of these early intervention approaches are based on models of typical development. Increasingly, studies are using a combination of methods to define treatment outcomes and to better understand the mechanisms and models of change of interventions. These methods include analysis of the degree to which changes in the direct target of the intervention (for example, parent behaviour) mediate later changes in child behaviour186 and the use of experimental methods such as EEG to examine whether there are accompanying changes in relevant brain networks190. Many parents seek complementary medical approaches, which, to date, have not been supported and are sometimes dangerous191. A note of general caution is that, even in the context of significant treatment differences between groups, individual outcomes are very variable, and some children do not improve, although reliable predictors of response to treatment have not been demonstrated in rigorous, randomized controlled trials. As autism is a heterogeneous developmental condition, different interventions may be required at diverse stages throughout life and different individuals might benefit from different interventions. One area that many consider to hold much promise — neurobiologically or biomarker ‘informed’ psychological intervention — is on the horizon but such targeted therapies have not yet been developed.
School-age children and adolescents
Many children and young people with autism can also benefit from interventions at later ages. A number of programmes and approaches are available that focus on the core social communication difficulties of autism, for example, social skills training programmes, for which moderate evidence of benefit exists192,193. In addition, non-verbal young people with autism can benefit from the use of augmentative communication systems such as the Picture Exchange Communication System (PECS) and more-sophisticated speech generating devices that use picture symbols and behavioural training methods to allow children to request and make choices194 or of other technology-based augmentative communication systems. Increasingly, more generic interventions that target co-occurring emotional and behavioural problems are being adapted for youths with autism, with initial studies suggesting moderate benefits195. These interventions include modified CBT for anxiety (modified, for example, to include parents, increase the duration of sessions, use more visual materials and specific work on understanding one’s own emotional states)196 and parent-mediated interventions for disruptive behaviour and ADHD197. More recently, there have been efforts to develop and test interventions that target aspects of parental wellbeing, such as parental stress and self-efficacy198. Increasingly, interventions for school-age children and young people with autism are being delivered within the school environment, rather than the clinic, which has natural advantages for programmes that consist of groups or peer-to-peer interactions with an emphasis on social skills. Indeed, it is hoped that this approach may facilitate generalization of the skills learned199,200.
Adult services
As individuals with autism progress into and through adulthood, the focus of management shifts from treating the core symptoms of autism to addressing associated symptoms or behaviours and promoting independence. However, there are few intervention studies to guide treatment options in adulthood. Indeed, a 2012 systematic review identified only 32 studies published between 1980 and 2010 that evaluated treatment studies for adolescents and young adults with autism201. A more recent review identified 41 studies of interventions targeting social functioning in adults over a 37-year period202.
Despite the low number of treatment studies, there is some evidence supporting treatment efficacy for a limited number of symptoms, behaviours and functional outcomes such as employment, social skills and anxiety; however, in general, the evidence base is weak201,202. For example, only three randomized controlled trials (all of which included small cohort sizes) that tested job interviewing skills curricula have been published. Social skills interventions have a somewhat more robust literature base (see202 for review), but most of these studies had very small sample sizes and were not well controlled. In addition, it is unclear whether social skills interventions can be generalized to other social settings and situations, that is, whether skills learned in the treatment context are used by the participants in other settings such as with peers or at work. There is some evidence for the use of CBT for effectively treating anxiety in people with autism who have sufficient cognitive and language skills to participate in current programmes203. However, nearly all of the existing research has been conducted with children and adolescents rather than with adults202, and individuals with substantial communication challenges are excluded from CBT studies. Furthermore, in contrast to the general population, CBT has not yet been shown to be effective for the treatment of depression in individuals with autism. Given this weak evidence base, it may be fruitful to explore therapies and treatments tested in other groups that may benefit those with autism.
Formal service systems and social care can help fill in the treatment gaps. Indeed, although many adults with autism do not receive adequate services and support204, their receipt can improve outcomes across a number of domains163. For example, transportation services can allow adults with autism to engage in employment and access therapies and programmes in the community. In addition, comprehensive job support services can promote finding and maintaining employment, particularly for adults with more severe impairments205. Public health insurance can increase access to psychiatric care for those with co-occurring mental health problems and income support can reduce dependence on families.
Medications
All medications that have evidence of benefit for autism treat the associated symptoms or co-occurring diagnoses, rather than the symptoms of autism directly (including social communication or repetitive behaviours). As mentioned earlier, autism is an extremely heterogeneous disorder, and individuals with autism can have a number of common co-occurring disorders that can also vary in severity.
Risperidone and aripiprazole (both of which are often termed ‘atypical antipsychotics’) are approved in the USA to treat irritability and agitation, including aggression, self-injury and tantrums, in children and adolescents with autism206–208. However, both treatments are associated with adverse events, including sedation, risk of movement disorders and weight gain, which limit their use to people with severe irritability with agitation208. The anti-diabetic drug metformin has been shown to limit weight gain from these medications, possibly broadening their safe use209.
As mentioned previously (see Co-occurring disorders, above), co-occurring mental health conditions are common in people with autism. Methylphenidate, atomoxetine and guanfacine are beneficial for ADHD symptoms in people with autism210–212 (TABLE 1). Although serotonin reuptake inhibitors, such as fluoxetine and citalopram, are used for the treatment of depression, anxiety and OCD in the general population, their efficacy in people with autism is less well established. Indeed, although fluoxetine improves symptoms of OCD in adults with autism213, citalopram has demonstrated poor tolerability and no benefit for repetitive behaviour in children with autism214. Medications for depression or anxiety have not been tested in people with autism.
Table 1 |.
Evidence-based medication in autism
| Medication | FDA or EMA, indication and age | Effect size (d)286 | Common adverse effects |
|---|---|---|---|
| Typically used for ADHD symptoms | |||
| Methylphenidate | FDA and EMA approval for ADHD (not specific for autism) in those ≥6 years of age | d = 0.78 (teacher rated) | Sleep disruption and decreased appetite |
| Atomoxetine | FDA and individual country approval for ADHD (not specific for autism) in those ≥6 years of age | d = −0.68 to 0.084 | Decreased appetite, nausea and irritability |
| Guanfacine | FDA and EMA approval for ADHD (not specific for autism) in those 6–17 years of age | d = 1.67 | Fatigue, sedation, and decreased pulse and blood pressure |
| Typically used to treat agitation and irritability | |||
| Risperidone | FDA approval for irritability associated with autism and EMA approval only for other indications in those 5–17 years of age | d = 0.94 | Increased appetite, sedation and weight gain |
| Aripiprazole | FDA approval for irritability associated with autism and EMA approval only for other indications in those 6–17 years of age | d = 0.87 | Nausea and weight gain |
ADHD, attention-deficit/hyperactivity disorder; EMA, European Medicines Agency.
Some excitement has accompanied the recent studies of medications targeting the neurohormonal oxytocin or vasopressin systems, both of which modulate social behaviour across species. Underpowered studies of intranasal oxytocin have demonstrated mixed results that are overall not supportive of a large effect size215,216, with results from adequately powered studies (NCT01944046) still pending. In addition, a pilot study of intranasal vasopressin suggested possible benefit in people with autism, although this study was underpowered217. A large trial of balovaptan, a vasopressin AVPR1A antagonist, in adults with autism showed negative results on its primary outcome (a general rating of autism symptoms), with suggestive results on a key secondary parent report measure of adaptive behaviour, including social and communication behaviour218. A few studies have also focused on the hypothesis that, at the level of neural circuits, autism may result from excessive excitation or insufficient inhibition219, with some promising but inconclusive results for medicines that target the GABAergic system220. Medications targeting genetic syndromes that can cause autism have not yet yielded consistent improvement221,222, but there is much hope for a precision medicine approach that links genetic subgroups with neurobiology-based treatments.
Quality of life
Objective and subjective measures
Several aspects of intervention research speak straight to the heart of current debates within the clinical field and broader autism community, including how a good outcome is defined for an individual with autism as well as who should decide what outcomes are used in intervention studies223. This point is aligned both with the debates about medical versus social models of disability but also with a more general shift in medicine away from focusing on symptom reduction to improving the wellbeing and quality of life (QOL) of patients. QOL research in adults with autism has focused on two aspects: objective and subjective QOL. Objective QOL encompasses social achievements such as employment, adequate living conditions, supportive relationships, and good physical and mental health224, whereas subjective QOL focuses on individuals’ perceptions and subjective assessments of their own lives225. Both subjective and objective QOL are often related but not synonymous, and both are important to take into account when considering outcomes for individuals with autism (TABLE 2).
Table 2 |.
Factors that affect QOL
| Type of QOL | Factor | Description |
|---|---|---|
| Objective QOL | Early language | Follow-up studies of adults with autism who were diagnosed as children have examined the amount of spoken language during early childhood. Individuals with autism who had fluent speech are more likely to have higher levels of objective QOL in adulthood than those with phrase speech or those with no speech or who spoke in single words |
| Indicators of intelligence | Studies examining IQ scores using standardized IQ tests administered in both early childhood and adulthood find that individuals with autism and higher IQ scores have higher levels of objective QOL than those with lower IQ scores. Other, less-standardized measures of intelligence (such as those used in large cohort studies) have similar findings | |
| Adaptive behaviour | Higher levels of adaptive behaviour — and particularly more activities of daily living — are associated with better objective QOL in people with autism. Adaptive behaviour is a challenge for many individuals with autism, who have scores below what would be expected based on IQ287. Adaptive behaviour is changeable, making it a promising avenue for interventions to improve objective QOL | |
| Autism symptom severity | Individuals with more severe autism symptoms tend to have lower objective QOL in adulthood | |
| Challenging behaviours | Higher levels of challenging behaviours in people with autism, which can include both internalizing problems and externalizing problems, are related to lower objective QOL | |
| Sex or gender | Sex or gender associations with objective QOL have been demonstrated in terms of employment or post-secondary education; indeed, women with autism obtain employment and post-secondary educational positions at the same rate as men with autism but have a more difficult time maintaining those positions over time | |
| Subjective QOL | Perceived stress | Many adults with autism perceive high levels of stress in their own lives; these perceptions are related to lower subjective QOL |
| Supports | Several different types of supports have been related to subjective QOL, including formal services, support from family members (most often parents) and more general social support from others |
QOL, quality of life.
Objective QOL.
Adults with autism tend to have poor objective QOL. Unemployment is high in this population, and even among those employed, individuals are often working below their skills and abilities226,227. Moreover, independent living can be a challenge, and adults often lack meaningful relationships with peers228. When aggregating across these domains of life, many adults with autism have ‘poor’ or ‘very poor’ outcomes229,230.
Autism is a highly heterogeneous condition and several factors have been associated with higher versus lower objective QOL. Most of the studied factors associated with higher objective QOL have been characteristics of the individuals (versus families, service system or communities), and consistent predictors of higher objective QOL include better early language development, higher IQ and adaptive behaviour scores, less severe autism symptoms and fewer challenging behaviours231. In addition, more recent research suggests that women with autism may have a more difficult time maintaining employment positions232, and are more likely to ‘camouflage’ their autism symptoms than men, which can lead to mental health challenges233.
Subjective QOL.
Meta-analyses have suggested that, across the lifespan, subjective QOL tends to be lower among individuals with autism than among typically developing peers234 but is often more positive than indicators of objective QOL229,235. Predictors of subjective QOL tend to be inconsistent across studies, except for perceived stress and support, the latter of which encompasses services, family and social support236–238.
Self-advocate perspective
It is clear that autism has heterogeneous outcomes and biological underpinnings; what is less clearcut are the differing and nuanced views of autistic people regarding how autism should be approached and researched (BOX 3, Autistica239, see also Ontario Brain Institute240). Indeed, some people with a diagnosis see autism as being a fundamental part of their identity, whereas other people do not. In addition, many people feel that social change is required241, whereas others want therapies to meet a range of their needs242. The key is respect for a variety of views and ultimately respect for autistic people. Researchers can demonstrate respect by considering how autism as a topic is distinct from, for example, cancer. To this end, terms such as ‘disease’ are inappropriate and are scientifically inaccurate when referring to autism. Ultimately, active participation in the design, implementation and interpretation of research studies, clear consideration of research ethics, and the consequences of research involvement and broad consultation of autistic people in research is key to authentically addressing the substantial inequalities that autistic people face as a group and ensuring that they live long, healthy and happy lives.
Box 3 |. Top questions for autism research from autistic people, families and professionalsa.
Which interventions improve mental health or reduce mental health problems in people with autism? How should mental health interventions be adapted for the needs of people with autism?
Which interventions are effective in the development of communication and language skills in autism?
What are the most effective ways to support or provide social care for autistic adults?
Which interventions reduce anxiety in autistic people?
Which environment supports are most appropriate in terms of achieving the best education, life and social skills outcomes in autistic people?
How can parents and family members be supported and/or educated to care for and better understand an autistic relative?
How can autism diagnostic criteria be made more relevant for the adult population? And how do we ensure that autistic adults are appropriately assessed and diagnosed?
How can we encourage employers to apply person-centred interventions and support to help autistic people maximize their potential and performance in the workplace?
How can sensory processing in autism be better understood?
How should service delivery for autistic people be improved and adapted in order to meet their needs?
aBased on REF.239.
Family perspectives
Families of people with autism are also heterogeneous, yet, as a group, they experience lower QOL than families with a member with other neurodevelopmental conditions, even before receiving the formal diagnosis243. Thus, it is essential that parents, other family members, clinicians, educators and the entire external support system coalesce around common goals for outcomes while accessing and maximizing resources for the betterment of the child and family. Parents are typically at the centre of this support network and carry much of the responsibility of direct care, coordination and advocacy, over and above typical parental responsibilities244,245. The exact parental roles are dependent on the child’s strengths and challenges, and frequently shift over time (FIG. 6). During this process, it is important that parents maintain motivation by setting realistic goals and tracking progress to experience the many achievements that their loved one with autism can attain.
Fig. 6 |. Major parental milestones in advocating and supporting their child with autism.
Families of children and adults with autism have many decisions and expectations across the lifespan of their children, from seeking initial diagnostic evaluation and intervention to preparing for ageing-related services. These decisions vary across different cultures, regions and countries, and depend on many factors, including the resources and services available. However, several decisions are common across all regions, including low-income and middle-income countries, such as choices about who will care for their child if the parents are temporarily unable, the amount of time parents and other family members can spend with the child with autism versus meeting other needs, ways to modify their home environment to ensure the safety and independence of the individual with autism and the kinds of behavioural expectations that are most helpful for their child or adult. Of note, for many families, these choices and responsibilities are lifelong and are relevant for children, adolescents, adults and elders with autism.
Effective parents often work closely with experienced providers who can track the development of the child with autism and can provide guidance on next actions246. Early in childhood, this role includes identifying and engaging with early and school-based interventions. It is never too early for parents to begin planning for the adult transition process, including (dependent on the capacity of the person with autism) promoting self-advocacy, preparation for life after secondary education, vocational training and employment support, living needs, community participation and long-term financial considerations. During adulthood, for cognitively able adults, parental roles might shift to more traditional relationships247, whereas for those with cognitive disability, parental caregiving often continues and culminates in planning for late life needs248. Although the journey can be challenging, for many parents, it can be incredibly rewarding and a source of life meaning.
Many parents recognize the need to give back to the community through research. Accordingly, it is crucial that researchers foster this desire carefully, communicating with parents to ensure that any potential immediate or future risks or benefits are clear. Even if the study period is brief, in many cases, the goal should be to develop a positive longer-term relationship, as this can lead to parents and people with autism continually re-engaging in and developing positive feelings about the research process.
Outlook
Autism research has substantially expanded in the past 50 years, and particularly in the past 20 years, as reflected in the websites listed in BOX 4. Although it seems unlikely that the incidence of autism is truly rising at the rate suggested in administrative prevalence studies, these data have increased the awareness and the numbers of diagnosed children in schools and clinics, although adult services and recognition run far behind. The lives of people with autism diagnoses have improved at least in some high-income countries, with a greater proportion of children using some language249, more adults with educational qualifications and less institutionalization249, although the changing nature of diagnoses has to be considered when interpreting historical trends. Some risk factors for autism have been identified, which has implications at least for more careful follow-up. In addition, the genetics of autism has yielded surprising discoveries, with substantial implications for heritable neurodevelopmental disorders such as ADHD, language delay and named syndromes associated with profound intellectual disability. The perceived value of routine genetic screening for autism diagnosis is disputed, with American medical academies strongly in favour, whereas those in other countries are much more selective. Studies of brain structure and function have added similarly intriguing findings that are just beginning to be integrated into both developmental and more mechanistic models of behaviour with possible targets or markers for change. Despite the intellectual contribution of these studies to research, at this point, neither EEG nor imaging are recommended as part of standard practice for the diagnosis of autism but can be used for other neurological indicators (such as if there are concerns beyond autism symptoms that merit an EEG or imaging). In this field, replication of findings across sites and even within individuals as well as larger samples through collaboration are the promise of the future.
Box 4 |. Examples of autism websites.
Sites for health-care professionals or research scientists
American Academy of Paediatrics: https://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/Pages/autism-initiatives.aspx
International Society for Autism Research: https://www.autism-insar.org
National Autistic Society: https://www.autism.org.uk
Royal College of General Practitioners: https://www.rcgp.org.uk/clinical-and-research/resources/toolkits/asd-toolkit.aspx
Information about treatment, research and advocacy for people with autism and their families
Autism Canada: https://autismcanada.org
Research Autism: http://www.researchautism.net/
Autism Europe: https://www.autismeurope.org/
Autism India: http://www.autism-india.org
WHO: https://www.who.int/news-room/fact-sheets/detail/autism-spectrum-disorders
Autism Spain: http://www.autismo.org.es
Autism Speaks: www.autismspeaks.org
Autismus Deutschland: https://www.autismus.de
Autistica: https://www.autistica.org.uk
Information about research funding and up-to-date information for people with autism and families
Simons Foundation: https://www.sfari.org
US NIH: https://www.nimh.nih.gov/health/topics/autism-spectrum-disorders-asd/index.shtml
Autism Science Foundation: https://autismsciencefoundation.org
Epidemiological and surveillance information
Adult Population Autism Surveys, England: https://digital.nhs.uk/data-and-information/publications/statistical/adult-psychiatric-morbidity-survey/adult-psychiatric-morbidity-survey-survey-of-mental-health-and-wellbeing-england-2014
Mental Health of Children and Young People (including autism) in England, 2017: https://digital.nhs.uk/data-and-information/publications/statistical/mental-health-of-children-and-young-people-in-england/2017/2017
The University of Queensland, Queensland Centre for Mental Health Research: Global Burden of Disease Programme (autism): https://www.uq.edu.au/news/article/2014/08/autism-rates-steady-two-decades
One way of bringing the three themes of mechanisms, heterogeneity and outcomes of autism together is to consider the trajectories of this disorder over time (FIG. 7), how knowledge of these trajectories can contribute to investigations of the biological and cognitive underpinnings of autism, and how treatments and supports could make the lives of children and adults with autism more positive.
Fig. 7 |. Changes in daily living scores as predicted by IQ scores and autistic symptoms.
Changes in independent daily living skills can be observed in people with autism over time. This sample consists of ~100 young adults with autism with a mean age of 26 years, who were evaluated at 2, 3 and 9 years of age and followed up to 26 years of age. Daily living scores are very diverse, ranging from age-appropriate levels of independence at adulthood (represented by a daily living score of 100, assessed using Vineland II284) to very limited skills (represented by a score of <30). Increasing divergence shows where measurement after 2 years of age is additionally predictive, with the line thickness indicating the proportion of early referred children that followed each trajectory. Heterogeneity in intellectual functioning and severity of autistic symptoms (social communication and restricted, repetitive, sensory behaviours) can be observed. In addition, improvements and worsening of autistic symptoms and intellectual functioning can occur over time. a,b | Referred children had verbal IQs predominantly <50 (over 3 standard deviations below average) but could show improvement in daily living standard scores from 2 to 3 years of age that were indicative of eventual greater independence in adulthood. Relatively less early change in non-verbal IQ is seen but, like verbal IQ, by adulthood the association with eventual adult daily living skills is strong. c,d | Variation in autism symptom severity in social communication (CSS refers to the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) comparison scores) showed a stronger association with adult independence than restricted, repetitive behaviours and continued to change over the lifespan following more divergent pathways than intellectual functioning. Data from the EDX cohort compiled from REFS1,147,285.
In terms of mechanisms, despite earlier hopes for simple genetic explanations of autism, we have instead identified many single-gene, germline loss-of-function point mutations yielding some initial models of disruption in very basic molecular patterns as well as common genes with small effects that are just beginning to emerge29. Attempts to study genes first have shown heterogeneity even within highly specific CNVs, with a few exceptions. In addition, hope exists that genetically based interventions for autism may be possible, although this will probably involve much further research. Data from genetic approaches that might yield targeted genetic interventions may be most relevant to rare and severe neurodevelopmental difficulties in general rather than autism as a specific entity. With more information about the differing developmental trajectories of autism, more continuous measures of symptoms and measures of language and intellectual function, behavioural phenotypes and changes over time can be quantified across different neurobiologically defined subgroups. This approach could potentially identify different ‘routes’ to different outcomes, whether autism or not, and could have a practical benefit in terms of selecting and monitoring appropriate treatments. In addition, with the heterogeneity of autism, our growing understanding of mechanisms, be they causal or mechanisms for change, needs to be linked to trajectories in development and not considered as static250. Researchers modelling autism in other species might find the incorporation of early developmental manifestations, such as regressions or motor delays, more tractable than the current focus on autism-related social communication symptoms seen in humans. With collaborations and studies of sufficient sample sizes, investigators have begun to focus on findings within different developmental periods that could provide insight into trajectories and targets for intervention. Thus, more study of the development of autism, both in studies of human behaviours and in animal models, might influence the identification and treatment of autism as a neurodevelopmental disorder. Prospective studies, including epidemiological and direct behavioural work across developmental periods, moving beyond very young children to later childhood, adolescence and adulthood are needed.
Similarly, limited findings about adult development and patterns that lead into autism (FIGS 5,7) call for measurement of different outcomes that respect individual differences in autistic people and in families (BOX 3). By young adulthood, available supports for places to live, employment and mental health services are needed for individuals who have a range of skill levels, with supports not always well matched to the needs of individuals; however, comparisons of treatments or treatment intensities have not historically been made, even though they are continually called for. The types and specific goals of treatments differ greatly for autistic people who are verbally fluent versus those who have difficulty speaking for themselves, such that alternative systems need to be in place that consider co-occurring conditions, strengths, preferences and challenges. More studies of well-defined, more homogeneous subgroups of autistic children and adults over time would provide different and more useful information about real-life issues (TABLE 2) than large-scale surveys of very heterogeneous samples251.
Progress in the biology of more generally defined neurodevelopmental disorders may have the greatest yield for children with autism in their early years. Clinical trials that compare known treatments (both psychosocial and biological) with new ones and treatment-as-usual would allow us to build on previous findings in a more meaningful way and begin to address the priorities listed in BOX 3, which, strikingly, are seldom priorities in autism research. To move from science to practice, including evaluation and treatment, autism researchers need to find a way to select and fund studies of more mundane, but critical evidence gaps in understanding heterogeneity, mechanisms of change and outcomes that affect practice in any circumstance, not just internationally, within academic systems that reward creativity and novelty. Unique methodologies, including the baby sibling studies, accumulation of large data sets (such as ABIDE and the Simons Simplex Collection (SSC)), prospective epidemiological studies and mechanistic studies of intermediate biomarkers may begin to bring together information from molecular to pathophysiological to cognitive and behavioural levels. However, for now, as for other neurodevelopmental and psychiatric disorders including schizophrenia252, the distance between science and practice remains great, and the amount of research that attempts to address solvable problems for autistic people alive today and their families remains modest.
Acknowledgements
The authors thank J. McCauley, S. Gaspar, K. Byrne and A. Holbrook from UCLA for help with manuscript preparation. S. Tromans is thanked for his updated review of the epidemiology literature. We recognize the many investigators who contributed research that we cannot cite due to space limitations. C.L. is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHHD; R01 HD081199), the National Institute of Mental Health (NIMH; R01MH081873-01A1) and the Simons Foundation. T.S.B. is supported by grants from the Health and Social Care Information Centre, Leeds, and the National Institute for Health Research (NIHR HTA; grant ref. NIHR127337). T.C. is supported by grants from Innovative Medicines Initiative 2 (no. 777394), the Medical Research Council (MRC; grants MR/K021389/1) and the NIHR (grant 13/119/18). J.C. is funded by Autistica. G.D. is supported by the Institut Pasteur. T.F. is supported by the Autism Speaks Foundation. E.J.H.J. is supported by grants from the Economic and Social Research Council (ESRC; ES/R009368/1), the Innovative Medicines Initiative 2 (no. 777394), the MRC (MR/K021389/1) and the Simons Foundation (609081). R.M.J. acknowledges the Mortimer D. Sackler Family and the NIMH (R01MH114999). J.L.T. is supported by grants from the FAR fund and the NIMH (R34 MH104428, R03 MH 112783 and R01 MH116058). A.P. is partially supported by the Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London and the NIHR (NF-SI-0617-10120). M.W.S. is supported by the National Institutes of Health (NIH; MH106934, MH109901, MH110928, MH116487 MH102342, MH111662, MH105575 and MH115747), the Overlook International Foundation and the Simons Foundation. J.V.-V. is supported by the NIH (MH016434 and MH094604), the Simons Foundation and the New York State Psychiatric Institute. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Competing Interests
C.L. acknowledges the receipt of royalties from Western Psychological Services for the sale of the Autism Diagnostic Interview-Revised (ADIR), the Autism Diagnostic Observation Schedule (ADOS) and the Social Communication Questionnaire (SCQ). T.S.B. has received royalties from Cambridge University Press and Oxford University Press. T.C. has served as a consultant to F. Hoffmann-La Roche. and has received royalties from Guilford Publications and Sage Publications. T.F. has received federal funding research support from, acted as a consultant to, received travel support from, and/or received a speaker’s honorarium from the Brain and Behaviour Research Foundation, Bristol-Myers Squibb, the Cole Family Research Fund, EcoEos, Forest Laboratories, Ingalls Foundation, IntegraGen, Kugona LLC, the National Institutes of Health, Roche Pharma, Shire Development and the Simons Foundation. J.L.T. receives compensation from Sage Publishers for editorial work. A.P. receives royalties from Imperial College Press, Oxford University Press and Western Psychological Services. M.W.S. serves on the scientific advisory boards and has stock or stock options for Arett Pharmaceuticals and BlackThorn Therapeutics. J.V.-V. has consulted or served on an advisory board for Novartis, Roche Pharmaceuticals and SynapDx, has received research funding from Forest, Novartis, Roche Pharmaceuticals, Seaside Therapeutics, SynapDx, and has received an editorial stipend from Springer and Wiley. All other authors declare no competing interests.
References
- 1.Lord C et al. Autism from 2 to 9 years of age. Arch. Gen. Psychiatry 63, 694–701 (2006). [DOI] [PubMed] [Google Scholar]; This paper establishes that autism is a stable diagnosis (as a spectrum) beginning at least by 2 years of age. The paper also establishes parent interview and clinician observation as predictive of autism at 9 years of age. Finally, it is the first paper that shows that the specific DSM-IV-TR diagnoses is unstable across childhood but that the instability is almost all shifting across categories not outside the spectrum.
- 2.Risi S et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. J. Am. Acad. Child Adolesc. Psychiatry 45, 1094–1103 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Loomes R, Hull L & Mandy WPL What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry 56, 466–474 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Brugha TS et al. Epidemiology of autism in adults across age groups and ability levels. Br. J. Psychiatry 209, 498–503 (2016). [DOI] [PubMed] [Google Scholar]; This paper uses active case-finding to provide representative estimates of the prevalence of autism and demonstrated that rates of autism in men and women are equivalent in adults with moderate-to-profound intellectual disability.
- 5.Brugha T, Bankart J, McManus S & Gullon-Scott F CDC autism rate: misplaced reliance on passive sampling? Lancet 392, 732–733 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Baxter AJ et al. The epidemiology and global burden of autism spectrum disorders. Psychol. Med 45, 601–613 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Elsabbagh M et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res 5, 160–179 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magnusson C et al. Migration and autism spectrum disorder: population-based study. Br. J. Psychiatry 201, 109–115 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Goodman R & Richards H Child and adolescent psychiatric presentations of second-generation Afro-Caribbeans in Britain. Br. J. Psychiatry 167, 362–369 (1995). [DOI] [PubMed] [Google Scholar]
- 10.Dyches TT, Wilder LK, Sudweeks RR, Obiakor FE & Algozzine B Multicultural issues in autism. J. Autism Dev. Disord 34, 211–222 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Keen DV, Reid FD & Arnone D Autism, ethnicity and maternal immigration. Br. J. Psychiatry 196, 274–281 (2010). [DOI] [PubMed] [Google Scholar]
- 12.McManus S, Bebbington P, Jenkins R & Brugha T Adult Psychiatric Morbidity Survey: mental health and wellbeing in England, 2014. NHS https://digital.nhs.uk/data-and-information/publications/statistical/adult-psychiatric-morbidity-survey/adult-psychiatric-morbidity-survey-survey-of-mental-health-and-wellbeing-england-2014 (2016). [Google Scholar]
- 13.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789–1858 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcheselli F et al. Mental health of children and young people in England, 2017. NHS https://digital.nhs.uk/data-and-information/publications/statistical/mental-health-of-children-and-young-people-in-england/2017/2017 (2018). [Google Scholar]
- 15.Brugha TC et al. Autism Spectrum Disorder, Adult Psychiatric Morbidity Survey 2014. (2014).
- 16.Lundstrom S, Reichenberg A, Anckarsater H, Lichtenstein P & Gillberg C Autism phenotype versus registered diagnosis in Swedish children: prevalence trends over 10 years in general population samples. BMJ 350, h1961 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tromans S, Chester V, Kiani R, Alexander R & Brugha T The prevalence of autism spectrum disorders in adult psychiatric inpatients: a systematic review. Clin. Pract. Epidemiol. Ment. Health 14, 177–187 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Modabbernia A, Velthorst E & Reichenberg A Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol. Autism 8, 13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu S et al. Advanced parental age and autism risk in children: a systematic review and meta-analysis. Acta Psychiatr. Scand 135, 29–41 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Taylor LE, Swerdfeger AL & Eslick GD Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine 32, 3623–3629 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Lai M-C, Lombardo MV & Baron-Cohen S Autism. Lancet 383, 896–910 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Velikonja T, Fett A-K & Velthorst E Patterns of nonsocial and social cognitive functioning in adults with autism spectrum disorder: a systematic review and meta-analysis. JAMA Psychiatry 76, 135–151 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNally Keehn RH, Lincoln AJ, Brown MZ & Chavira DA The coping cat program for children with anxiety and autism spectrum disorder: a pilot randomized controlled trial. J. Autism Dev. Disord 43, 57–67 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones EJH, Gliga T, Bedford R, Charman T & Johnson MH Developmental pathways to autism: a review of prospective studies of infants at risk. Neurosci. Biobehav. Rev 39, 1–33 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozonoff S et al. Recurrence risk for autism spectrum disorders: a baby siblings research consortium study. Pediatrics 128, e488–e495 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones RM & Lord C Diagnosing autism in neurobiological research studies. Behav. Brain Res 251, 113–124 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson MH Autism: demise of the innate social orienting hypothesis. Curr. Biol 24, R30–R31 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Johnson MH, Jones EJH & Gliga T Brain adaptation and alternative developmental trajectories. Dev. Psychopathol 27, 425–442 (2015). [DOI] [PubMed] [Google Scholar]
- 29.The Lancet Psychiatry. Of mice and mental health. Lancet Psychiatry 6, 877 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Nelson CA et al. An integrative, multidisciplinary approach to the study of brain-behavior relations in the context of typical and atypical development. Dev. Psychopathol 14, 499–520 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Cross-Disorder Group of the Psychiatric Genomics Consortium et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat. Genet 45, 984–994 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaugler T et al. Most genetic risk for autism resides with common variation. Nat. Genet 46, 881–885 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang K, Gaitsch H, Poon H, Cox NJ & Rzhetsky A Classification of common human diseases derived from shared genetic and environmental determinants. Nat. Genet 49, 1319–1325 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanders SJ et al. Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron 87, 1215–1233 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satterstrom FK et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Preprint at 10.1101/484113(2019). [DOI] [PMC free article] [PubMed]
- 36.Sanders SJ et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485, 237–241 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neale BM et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485, 242–245 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Roak BJ et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485, 246–250 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanders SJ et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 70, 863–885 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levy D et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron 70, 886–897 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Sebat J et al. Strong association of de novo copy number mutations with autism. Science 316, 445–449 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper is the first to focus explicitly on simplex autism and show the importance of de novo CNVs in simplex cases, versus familial cases, versus controls.
- 42.Grove J et al. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet 51, 431–444 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willsey J et al. De novo coding variants are strongly associated with Tourette syndrome. Eur. Neuropsychopharmacol 29, S737 (2019). [Google Scholar]
- 44.Epi4K Consortium. Epi4K: gene discovery in 4,000 genomes. Epilepsia 53, 1457–1467 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jamain S et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet 34, 27–29 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first paper to show a de novo loss-of-function mutation in a synaptic gene associated with non-syndromic autism and was a harbinger for many of the findings that came after.
- 46.Iossifov I et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 515, 216–221 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Rubeis S et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515, 209–215 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sestan N & State MW Lost in translation: traversing the complex path from genomics to therapeutics in autism spectrum disorder. Neuron 100, 406–423 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.State MW & Sestan N The emerging biology of autism spectrum disorders. Science 337, 1301–1303 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Devlin B & Scherer SW Genetic architecture in autism spectrum disorder. Curr. Opin. Genet. Dev 22, 229–237 (2012). [DOI] [PubMed] [Google Scholar]
- 52.de la Torre-Ubieta L, Won H, Stein JL & Geschwind DH Advancing the understanding of autism disease mechanisms through genetics. Nat. Med 22, 345–361 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.SFARI Gene Website. https://gene.sfari.org/ (2019).
- 54.Parikshak NN et al. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell 155, 1008–1021 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ben-David E & Shifman S Combined analysis of exome sequencing points toward a major role for transcription regulation during brain development in autism. Mol. Psychiatry 18, 1054–1056 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Willsey AJ et al. Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell 155, 997–1007 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pinto D et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature 466, 368–372 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gilman SR et al. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron 70, 898–907 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fuccillo MV Striatal circuits as a common node for autism pathophysiology. Front. Neurosci 10, 27 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Velmeshev D et al. Single-cell genomics identifies cell type-specific molecular changes in autism. Science 364, 685–689 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mendell JR et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med 377, 1713–1722 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Mercuri E et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N. Engl. J. Med 378, 625–635 (2018). [DOI] [PubMed] [Google Scholar]
- 63.Matharu N et al. CRISPR-mediated activation of a promoter or enhancer rescues obesity caused by haploinsufficiency. Science 363, eaau0629 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abudayyeh OO et al. RNA targeting with CRISPR–cas13. Nature 550, 280–284 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Power JD et al. Customized head molds reduce motion during resting state fMRI scans. NeuroImage 189, 141–149 (2019). [DOI] [PubMed] [Google Scholar]
- 66.Solso S et al. Diffusion tensor imaging provides evidence of possible axonal overconnectivity in frontal lobes in autism spectrum disorder toddlers. Biol. Psychiatry 79, 676–684 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clements CC et al. Evaluation of the social motivation hypothesis of autism: a systematic review and meta-analysis. JAMA Psychiatry 75, 797–808 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ecker C Brain anatomy and its relationship to behavior in adults with autism spectrum disorder: a multicenter magnetic resonance imaging study. Arch. Gen. Psychiatry 69, 195–209 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Langen M et al. Changes in the development of striatum are involved in repetitive behavior in autism. Biol. Psychiatry 76, 405–411 (2014). [DOI] [PubMed] [Google Scholar]
- 70.Elsabbagh M & Johnson MH Autism and the social brain: the first-year puzzle. Biol. Psychiatry 80, 94–99 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Courchesne E et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology 57, 245–254 (2001). [DOI] [PubMed] [Google Scholar]
- 72.Hazlett HC et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch. Gen. Psychiatry 62, 1366–1376 (2005). [DOI] [PubMed] [Google Scholar]
- 73.Wolff JJ et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am. J. Psychiatry 169, 589–600 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hazlett HC et al. Early brain development in infants at high risk for autism spectrum disorder. Nature 542, 348–351 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This seminal paper, through careful recruitment and methodology, was the first to show significant early differences that may contribute to our understanding of developmental features in neural structure and circuits.
- 75.Wolff JJ et al. Neural circuitry at age 6 months associated with later repetitive behavior and sensory responsiveness in autism. Mol. Autism 8, 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Emerson RW et al. Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age. Sci. Transl. Med 9, eaag2882 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith E et al. Cortical thickness change in autism during early childhood: CT in early childhood ASD. Hum. Brain Mapp 37, 2616–2629 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uddin LQ, Dajani DR, Voorhies W, Bednarz H & Kana RK Progress and roadblocks in the search for brain-based biomarkers of autism and attention-deficit/hyperactivity disorder. Transl. Psychiatry 7, e1218 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herringshaw AJ, Ammons CJ, DeRamus TP & Kana RK Hemispheric differences in language processing in autism spectrum disorders: a meta-analysis of neuroimaging studies. Autism Res 9, 1046–1057 (2016). [DOI] [PubMed] [Google Scholar]
- 80.He Y, Byrge L & Kennedy DP Non-replication of functional connectivity differences in autism spectrum disorder across multiple sites and denoising strategies. Preprint at 10.1101/640797 (2019). [DOI] [PMC free article] [PubMed]
- 81.Lawrence KE, Hernandez LM, Bookheimer SY & Dapretto M Atypical longitudinal development of functional connectivity in adolescents with autism spectrum disorder. Autism Res 12, 53–65 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Plitt M, Barnes KA, Wallace GL, Kenworthy L & Martin A Resting-state functional connectivity predicts longitudinal change in autistic traits and adaptive functioning in autism. Proc. Natl Acad. Sci. USA 112, E6699–E6706 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Di Martino A et al. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol. Psychiatry 19, 659–667 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doyle-Thomas KAR et al. Atypical functional brain connectivity during rest in autism spectrum disorders. Ann. Neurol 77, 866–876 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Supekar K et al. Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep 5, 738–747 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dajani DR & Uddin LQ Local brain connectivity across development in autism spectrum disorder: a cross-sectional investigation. Autism Res 9, 43–54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hull JV et al. Resting-state functional connectivity in autism spectrum disorders: a review. Front. Psychiatry 7, 205 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lombardo MV et al. Different functional neural substrates for good and poor language outcome in autism. Neuron 86, 567–577 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carlisi CO et al. Disorder-specific and shared brain abnormalities during vigilance in autism and obsessive-compulsive disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 644–654 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alaerts K, Swinnen SP & Wenderoth N Sex differences in autism: a resting-state fMRI investigation of functional brain connectivity in males and females. Soc. Cogn. Affect. Neurosci 11, 1002–1016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kirkovski M, Enticott PG, Hughes ME, Rossell SL & Fitzgerald PB Atypical neural activity in males but not females with autism spectrum disorder. J. Autism Dev. Disord 46, 954–963 (2016). [DOI] [PubMed] [Google Scholar]
- 92.Venkataraman A et al. Pivotal response treatment prompts a functional rewiring of the brain among individuals with autism spectrum disorder. NeuroReport 27, 1081–1085 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Levisohn PM The autism-epilepsy connection. Epilepsia 48, 33–35 (2007). [DOI] [PubMed] [Google Scholar]
- 94.Cantor DS, Thatcher RW, Hrybyk M & Kaye H Computerized EEG analyses of autistic children. J. Autism Dev. Disord 16, 169–187 (1986). [DOI] [PubMed] [Google Scholar]
- 95.Lefebvre A et al. Alpha waves as a neuromarker of autism spectrum disorder: the challenge of reproducibility and heterogeneity. Front. Neurosci 12, 662 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tierney AL, Gabard-Durnam L, Vogel-Farley V, Tager-Flusberg H & Nelson CA Developmental trajectories of resting EEG power: an endophenotype of autism spectrum disorder. PLOS ONE 7, e39127 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oberman LM et al. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cogn. Brain Res 24, 190–198 (2005). [DOI] [PubMed] [Google Scholar]
- 98.Fan Y-T, Decety J, Yang C-Y, Liu J-L & Cheng Y Unbroken mirror neurons in autism spectrum disorders. J. Child Psychol. Psychiatry 51, 981–988 (2010). [DOI] [PubMed] [Google Scholar]
- 99.Southgate V & Hamilton AF Unbroken mirrors: challenging a theory of autism. Trends Cogn. Sci 12, 225–229 (2008). [DOI] [PubMed] [Google Scholar]
- 100.Bernier R, Aaronson B & McPartland J The role of imitation in the observed heterogeneity in EEG mu rhythm in autism and typical development. Brain Cogn 82, 69–75 (2013). [DOI] [PubMed] [Google Scholar]
- 101.Raymaekers R, Wiersema JR & Roeyers H EEG study of the mirror neuron system in children with high functioning autism. Brain Res 1304, 113–121 (2009). [DOI] [PubMed] [Google Scholar]
- 102.Dumas G, Soussignan R, Hugueville L, Martinerie J & Nadel J Revisiting mu suppression in autism spectrum disorder. Brain Res 1585, 108–119 (2014). [DOI] [PubMed] [Google Scholar]; This paper replicates the mu suppression deficits in autism during action observation but questions, through high-density spectral analyses and source reconstruction, its previously drawn relation to the mirror neuron system.
- 103.Marco EJ, Hinkley LBN, Hill SS & Nagarajan SS Sensory processing in autism: a review of neurophysiologic findings. Pediatr. Res 69, 48R–54R (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schwartz S, Shinn-Cunningham B & Tager-Flusberg H Meta-analysis and systematic review of the literature characterizing auditory mismatch negativity in individuals with autism. Neurosci. Biobehav. Rev 87, 106–117 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kang E et al. Atypicality of the N170 event-related potential in autism spectrum disorder: a meta-analysis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 657–666 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bonnet-Brilhault F et al. GABA/glutamate synaptic pathways targeted by integrative genomic and electrophysiological explorations distinguish autism from intellectual disability. Mol. Psychiatry 21, 411–418 (2016). [DOI] [PubMed] [Google Scholar]
- 107.Schilbach L Towards a second-person neuropsychiatry. Phil. Trans. R. Soc. B 371, 20150081 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This review supports that psychiatric disorders are more commonly characterized by impairments of social interaction rather than social observation, and advocates for an interactive turn in neuropsychiatry.
- 108.Barraza P et al. Implementing EEG hyperscanning setups. MethodsX 6, 428–436 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dumas G, de Guzman GC, Tognoli E & Kelso JA The human dynamic clamp as a paradigm for social interaction. Proc. Natl Acad. Sci. USA 111, E3726–E3734 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jones EJH et al. Reduced engagement with social stimuli in 6-month-old infants with later autism spectrum disorder: a longitudinal prospective study of infants at high familial risk. J. Neurodev. Disord 8, 7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ciarrusta J et al. Social brain functional maturation in newborn infants with and without a family history of autism spectrum disorder. JAMA Netw. Open 2, e191868 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Levin AR, Varcin KJ, O’Leary HM, Tager-Flusberg H & Nelson CA EEG power at 3 months in infants at high familial risk for autism. J. Neurodev. Disord 9, 34 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kolesnik A et al. Increased cortical reactivity to repeated tones at 8 months in infants with later ASD. Transl. Psychiatry 9, 46 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rippon G, Brock J, Brown C & Boucher J Disordered connectivity in the autistic brain: challenges for the ‘new psychophysiology’. Int. J. Psychophysiol 63, 164–172 (2007). [DOI] [PubMed] [Google Scholar]
- 115.Rosenberg A, Patterson JS & Angelaki DE A computational perspective on autism. Proc. Natl Acad. Sci. USA 112, 9158–9165 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Masuda F et al. Motor cortex excitability and inhibitory imbalance in autism spectrum disorder assessed with transcranial magnetic stimulation: a systematic review. Transl. Psychiatry 9, 110 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.O’Reilly C, Lewis JD & Elsabbagh M Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies. PLOS ONE 12, e0175870 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Khan S et al. Somatosensory cortex functional connectivity abnormalities in autism show opposite trends, depending on direction and spatial scale. Brain 138, 1394–1409 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen H, Nomi JS, Uddin LQ, Duan X & Chen H Intrinsic functional connectivity variance and state-specific under-connectivity in autism. Hum. Brain Mapp 38, 5740–5755 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Catarino A, Churches O, Baron-Cohen S, Andrade A & Ring H Atypical EEG complexity in autism spectrum conditions: a multiscale entropy analysis. Clin. Neurophysiol 122, 2375–2383 (2011). [DOI] [PubMed] [Google Scholar]
- 121.Engemann DA et al. Robust EEG-based cross-site and cross-protocol classification of states of consciousness. Brain 141, 3179–3192 (2018). [DOI] [PubMed] [Google Scholar]
- 122.Open Science Collaboration. Psychology. Estimating the reproducibility of psychological science. Science 349, aac4716 (2015). [DOI] [PubMed] [Google Scholar]
- 123.Lord C et al. Autism diagnostic observation schedule: ADOS-2 (Western Psychological Services, 2012). [Google Scholar]
- 124.Regier DA et al. DSM-5 field trials in the United States and Canada, part II: test-retest reliability of selected categorical diagnoses. Am. J. Psychiatry 170, 59–70 (2013). [DOI] [PubMed] [Google Scholar]
- 125.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edn (American Psychiatric Association, 2013). [Google Scholar]
- 126.World Health Organization. International classification of diseases for mortality and morbidity statistics (11th Revision). https://icd.who.int/browse11/l-m/en (WHO, 2018). [Google Scholar]
- 127.Constantino JN & Charman T Diagnosis of autism spectrum disorder: reconciling the syndrome, its diverse origins, and variation in expression. Lancet Neurol 15, 279–291 (2016). [DOI] [PubMed] [Google Scholar]
- 128.Lord C A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch. Gen. Psychiatry 69, 306–313 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Miller JN & Ozonoff S The external validity of Asperger disorder: lack of evidence from the domain of neuropsychology. J. Abnorm. Psychol 109, 227–238 (2000). [PubMed] [Google Scholar]
- 130.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edn (American Psychiatric Association, 1994). [Google Scholar]
- 131.Green D, Chandler S, Charman T, Simonoff E & Baird G Brief report: DSM-5 sensory behaviours in children with and without an autism spectrum disorder. J. Autism Dev. Disord 46, 3597–3606 (2016). [DOI] [PubMed] [Google Scholar]
- 132.Ozonoff S et al. Diagnosis of autism spectrum disorder after age 5 in children evaluated longitudinally since infancy. J. Am. Acad. Child Adolesc. Psychiatry 57, 849–857.e2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Russell G, Steer C & Golding J Social and demographic factors that influence the diagnosis of autistic spectrum disorders. Soc. Psychiatry Psychiatr. Epidemiol 46, 1283–1293 (2011). [DOI] [PubMed] [Google Scholar]
- 134.Charman T & Gotham K Measurement issues: screening and diagnostic instruments for autism spectrum disorders—lessons from research and practice. Child Adolesc. Ment. Health 18, 52–63 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ashwood KL, Buitelaar J, Murphy D, Spooren W & Charman T European clinical network: autism spectrum disorder assessments and patient characterisation. Eur. Child Adolesc. Psychiatry 24, 985–995 (2015). [DOI] [PubMed] [Google Scholar]
- 136.Rutter M, LeCouteur A & Lord C Autism Diagnostic Interview-Revised (ADI-R). (Western Psychological Services, 2003). [Google Scholar]
- 137.Durkin MS et al. Autism screening and diagnosis in low resource settings: challenges and opportunities to enhance research and services worldwide. Autism Res 8, 473–476 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This position paper highlights the challenges to translating knowledge on better awareness, understanding, identification and diagnosis (and then treatments) from the past two decades of clinical research in high-income countries into low-income and middle-income countries.
- 138.Baird G et al. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP). Lancet 368, 210–215 (2006). [DOI] [PubMed] [Google Scholar]
- 139.Luyster R et al. The autism diagnostic observation schedule — toddler module: a new module of a standardized diagnostic measure for autism spectrum disorders. J. Autism Dev. Disord 39, 1305–1320 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.de Vries PJ Thinking globally to meet local needs: autism spectrum disorders in Africa and other low-resource environments. Curr. Opin. Neurol 29, 130–136 (2016). [DOI] [PubMed] [Google Scholar]
- 141.Georgiades S, Bishop SL & Frazier T Editorial perspective: longitudinal research in autism—introducing the concept of ‘chronogeneity’. J. Child Psychol. Psychiatry 58, 634–636 (2017). [DOI] [PubMed] [Google Scholar]
- 142.Fountain C, Winter AS & Bearman PS Six developmental trajectories characterize children with autism. Pediatrics 129, e1112–e1120 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim SH et al. Variability in autism symptom trajectories using repeated observations from 14 to 36 months of age. J. Am. Acad. Child Adolesc. Psychiatry 57, 837–848.e2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bussu G et al. Latent trajectories of adaptive behaviour in infants at high and low familial risk for autism spectrum disorder. Mol. Autism 10, 13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zerbi V et al. Dysfunctional autism risk genes cause circuit-specific connectivity deficits with distinct developmental trajectories. Cereb. Cortex 28, 2495–2506 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fein D et al. Optimal outcome in individuals with a history of autism. J. Child Psychol. Psychiatry 54, 195–205 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Anderson DK, Liang JW & Lord C Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J. Child Psychol. Psychiatry 55, 485–494 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chlebowski C, Robins DL, Barton ML & Fein D Large-scale use of the modified checklist for autism in low-risk toddlers. Pediatrics 131, e1121–e1127 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stenberg N et al. Identifying children with autism spectrum disorder at 18 months in a general population sample. Paediatr. Perinat. Epidemiol 28, 255–262 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pierce K, Courchesne E & Bacon E To screen or not to screen universally for autism is not the question: why the task force got it wrong. J. Pediatr 176, 182–194 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Siu AL et al. Screening for autism spectrum disorder in young children: US Preventive Services Task Force recommendation statement. JAMA 315, 691–696 (2016). [DOI] [PubMed] [Google Scholar]
- 152.Øien RA et al. Clinical features of children with autism who passed 18-month screening. Pediatrics 141, e20173596 (2018). [DOI] [PubMed] [Google Scholar]
- 153.Sánchez-García AB, Galindo-Villardón P, Nieto-Librero AB, Martín-Rodero H & Robins DL Toddler screening for autism spectrum disorder: a meta-analysis of diagnostic accuracy. J. Autism Dev. Disord 49, 1837–1852 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Marlow M, Servili C & Tomlinson M A review of screening tools for the identification of autism spectrum disorders and developmental delay in infants and young children: recommendations for use in low- and middle-income countries. Autism Res 12, 176–199 (2019). [DOI] [PubMed] [Google Scholar]
- 155.Raza S et al. Brief report: evaluation of the short quantitative checklist for autism in toddlers (Q-CHAT-10) as a brief screen for autism spectrum disorder in a high-risk sibling cohort. J. Autism Dev. Disord 49, 2210–2218 (2019). [DOI] [PubMed] [Google Scholar]
- 156.Charman T et al. Testing two screening instruments for autism spectrum disorder in UK community child health services. Dev. Med. Child Neurol 58, 369–375 (2016). [DOI] [PubMed] [Google Scholar]
- 157.Brett D, Warnell F, McConachie H & Parr JR Factors affecting age at ASD diagnosis in UK: no evidence that diagnosis age has decreased between 2004 and 2014. J. Autism Dev. Disord 46, 1974–1984 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zuckerman KE, Lindly OJ & Sinche BK Parental concerns, provider response, and timeliness of autism spectrum disorder diagnosis. J. Pediatr 166, 1431–1439.e1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Boterberg S, Charman T, Marschik PB, Bölte S & Roeyers H Regression in autism spectrum disorder: a critical overview of retrospective findings and recommendations for future research. Neurosci. Biobehav. Rev 102, 24–55 (2019). [DOI] [PubMed] [Google Scholar]
- 160.Pearson N, Charman T, Happé F, Bolton PF & McEwen FS Regression in autism spectrum disorder: reconciling findings from retrospective and prospective research. Autism Res 11, 1602–1620 (2018). [DOI] [PubMed] [Google Scholar]
- 161.Ozonoff S & Iosif A-M Changing conceptualizations of regression: what prospective studies reveal about the onset of autism spectrum disorder. Neurosci. Biobehav. Rev 100, 296–304 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; Despite its potential importance as a biological marker and/or subgroup of ASD, developmental regression has remained very poorly understood. This paper outlines recent data and reconceptualization about patterns of onset (and loss) that chime with a more contemporaneous understanding of ASD as a heterogeneous condition in terms of its manifestation both within and across individuals.
- 162.Brugha TS et al. Validating two survey methods for identifying cases of autism spectrum disorder among adults in the community. Psychol. Med 42, 647–656 (2012). [DOI] [PubMed] [Google Scholar]
- 163.Brugha TS The Psychiatry of Adult Autism and Asperger Syndrome: a Practical Guide (Oxford Univ. Press, 2018). [Google Scholar]
- 164.Epstein J, Johnson DE & Conners CK Conners Adult ADHD Diagnostic Interview for DSM-IV (CAADID) (MHS, 2001). [Google Scholar]
- 165.Lai M-C et al. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatry 6, 819–829 (2019). [DOI] [PubMed] [Google Scholar]
- 166.Havdahl A & Bishop S Heterogeneity in prevalence of co-occurring psychiatric conditions in autism. Lancet Psychiatry 6, 794–795 (2019). [DOI] [PubMed] [Google Scholar]
- 167.Croen LA et al. The health status of adults on the autism spectrum. Autism 19, 814–823 (2015). [DOI] [PubMed] [Google Scholar]
- 168.Mannion A, Leader G & Healy O An investigation of comorbid psychological disorders, sleep problems, gastrointestinal symptoms and epilepsy in children and adolescents with autism spectrum disorder. Res. Autism Spectr. Disord 7, 35–42 (2013). [Google Scholar]
- 169.Soke GN, Maenner MJ, Christensen D, Kurzius-Spencer M & Schieve LA Prevalence of co-occurring medical and behavioral conditions/symptoms among 4- and 8-year-old children with autism spectrum disorder in selected areas of the United States in 2010. J. Autism Dev. Disord 48, 2663–2676 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chandler S et al. Emotional and behavioural problems in young children with autism spectrum disorder. Dev. Med. Child Neurol 58, 202–208 (2016). [DOI] [PubMed] [Google Scholar]
- 171.Pezzimenti F, Han GT, Vasa RA & Gotham K Depression in youth with autism spectrum disorder. Child Adolesc. Psychiatr. Clin. N. Am 28, 397–409 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hwang YIJ, Srasuebkul P, Foley KR, Arnold S & Trollor JN Mortality and cause of death of Australians on the autism spectrum. Autism Res 12, 806–815 (2019). [DOI] [PubMed] [Google Scholar]
- 173.Hirvikoski T et al. Premature mortality in autism spectrum disorder. Br. J. Psychiatry 208, 232–238 (2016). [DOI] [PubMed] [Google Scholar]
- 174.Havdahl KA et al. Multidimensional influences on autism symptom measures: implications for use in etiological research. J. Am. Acad. Child Adolesc. Psychiatry 55, 1054–1063.e3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nicolaidis C et al. Comparison of healthcare experiences in autistic and non-autistic adults: a cross-sectional online survey facilitated by an academic-community partnership. J. Gen. Intern. Med 28, 761–769 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schreibman L et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J. Autism Dev. Disord 45, 2411–2428 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tomlinson M et al. Setting global research priorities for developmental disabilities, including intellectual disabilities and autism: setting research priorities for developmental disabilities. J. Intellect. Disabil. Res 58, 1121–1130 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rahman A et al. Effectiveness of the parent-mediated intervention for children with autism spectrum disorder in South Asia in India and Pakistan (PASS): a randomised controlled trial. Lancet Psychiatry 3, 128–136 (2016). [DOI] [PubMed] [Google Scholar]
- 179.Lovaas OI Behavioral treatment and normal educational and intellectual functioning in young autistic children. J. Consult. Clin. Psychol 55, 3–9 (1987). [DOI] [PubMed] [Google Scholar]
- 180.Nevill RE, Lecavalier L & Stratis EA Meta-analysis of parent-mediated interventions for young children with autism spectrum disorder. Autism 22, 84–98 (2018). [DOI] [PubMed] [Google Scholar]
- 181.Kasari C et al. Randomized controlled trial of parental responsiveness intervention for toddlers at high risk for autism. Infant Behav. Dev 37, 711–721 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shire SY et al. Hybrid implementation model of community-partnered early intervention for toddlers with autism: a randomized trial. J. Child Psychol. Psychiatry 58, 612–622 (2017). [DOI] [PubMed] [Google Scholar]
- 183.Siller M, Hutman T & Sigman M A parent-mediated intervention to increase responsive parental behaviors and child communication in children with ASD: a randomized clinical trial. J. Autism Dev. Disord 43, 540–555 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rogers SJ et al. Effects of a brief early start denver model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J. Am. Acad. Child Adolesc. Psychiatry 51, 1052–1065 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Green J et al. Parent-mediated communication-focused treatment in children with autism (PACT): a randomised controlled trial. Lancet 375, 2152–2160 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pickles A et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet 388, 2501–2509 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dawson G et al. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics 125, e17–e23 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Charman T Editorial: trials and tribulations in early autism intervention research. J. Am. Acad. Child Adolesc. Psychiatry 58, 846–848 (2019). [DOI] [PubMed] [Google Scholar]
- 189.Rogers SJ et al. A multisite randomized controlled two-phase trial of the early start denver model compared to treatment as usual. J. Am. Acad. Child Adolesc. Psychiatry 58, 853–865 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dawson G et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. J. Am. Acad. Child Adolesc. Psychiatry 51, 1150–1159 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Myers SM, Johnson CP & The Council on Children With Disabilities. Management of children with autism spectrum disorders. Pediatrics 120, 1162–1182 (2007). [DOI] [PubMed] [Google Scholar]
- 192.Laugeson EA, Frankel F, Gantman A, Dillon AR & Mogil C Evidence-based social skills training for adolescents with autism spectrum disorders: the UCLA PEERS program. J. Autism Dev. Disord 42, 1025–1036 (2012). [DOI] [PubMed] [Google Scholar]
- 193.Reichow B, Servili C, Yasamy MT, Barbui C & Saxena S Non-specialist psychosocial interventions for children and adolescents with intellectual disability or lower-functioning autism spectrum disorders: a systematic review. PLOS Med 10, e1001572 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Brignell A et al. Communication interventions for autism spectrum disorder in minimally verbal children. Cochrane Database Syst. Rev 11, CD012324 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tarver J et al. Child and parent outcomes following parent interventions for child emotional and behavioral problems in autism spectrum disorders: a systematic review and meta-analysis. Autism 23, 1630–1644 (2019). [DOI] [PubMed] [Google Scholar]
- 196.Keefer A et al. Exploring relationships between negative cognitions and anxiety symptoms in youth with autism spectrum disorder. Behav. Ther 49, 730–740 (2018). [DOI] [PubMed] [Google Scholar]
- 197.Bearss K et al. Effect of parent training vs parent education on behavioral problems in children with autism spectrum disorder: a randomized clinical trial. JAMA 313, 1524–1533 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Da Paz NS & Wallander JL Interventions that target improvements in mental health for parents of children with autism spectrum disorders: a narrative review. Clin. Psychol. Rev 51, 1–14 (2017). [DOI] [PubMed] [Google Scholar]
- 199.Kasari C et al. Children with autism spectrum disorder and social skills groups at school: a randomized trial comparing intervention approach and peer composition. J. Child Psychol. Psychiatry 57, 171–179 (2016). [DOI] [PubMed] [Google Scholar]
- 200.Marshall D et al. Social stories in mainstream schools for children with autism spectrum disorder: a feasibility randomised controlled trial. BMJ Open 6, e011748 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Taylor JL et al. A systematic review of vocational interventions for young adults with autism spectrum disorders. Pediatrics 130, 531–538 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pallathra AA, Cordero L, Wong K & Brodkin ES Psychosocial interventions targeting social functioning in adults on the autism spectrum: a literature review. Curr. Psychiatry Rep 21, 5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.White SW et al. Psychosocial treatments targeting anxiety and depression in adolescents and adults on the autism spectrum: review of the latest research and recommended future directions. Curr. Psychiatry Rep 20, 82 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Shattuck PT, Wagner M, Narendorf S, Sterzing P & Hensley M Post-high school service use among young adults with an autism spectrum disorder. Arch. Pediatr. Adolesc. Med 165, 141–146 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wehman P et al. Effects of an employer-based intervention on employment outcomes for youth with significant support needs due to autism. Autism 21, 276–290 (2017). [DOI] [PubMed] [Google Scholar]
- 206.McCracken JT et al. Risperidone in children with autism and serious behavioral problems. N. Engl. J. Med 347, 314–321 (2002). [DOI] [PubMed] [Google Scholar]
- 207.Owen R et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics 124, 1533–1540 (2009). [DOI] [PubMed] [Google Scholar]
- 208.McPheeters ML et al. A systematic review of medical treatments for children with autism spectrum disorders. Pediatrics 127, e1312–e1321 (2011). [DOI] [PubMed] [Google Scholar]
- 209.Anagnostou E et al. Metformin for treatment of overweight induced by atypical antipsychotic medication in young people with autism spectrum disorder: a randomized clinical trial. JAMA Psychiatry 73, 928–937 (2016). [DOI] [PubMed] [Google Scholar]
- 210.Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch. Gen. Psychiatry 62, 1266–1274 (2005). [DOI] [PubMed] [Google Scholar]
- 211.Handen BL et al. Atomoxetine, parent training, and their combination in children with autism spectrum disorder and attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 54, 905–915 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Scahill L et al. Extended-release guanfacine for hyperactivity in children with autism spectrum disorder. Am. J. Psychiatry 172, 1197–1206 (2015). [DOI] [PubMed] [Google Scholar]
- 213.Hollander E et al. A double-blind placebo-controlled trial of fluoxetine for repetitive behaviors and global severity in adult autism spectrum disorders. Am. J. Psychiatry 169, 292–299 (2012). [DOI] [PubMed] [Google Scholar]
- 214.King BH et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch. Gen. Psychiatry 66, 583–590 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Anagnostou E et al. Intranasal oxytocin in the treatment of autism spectrum disorders: a review of literature and early safety and efficacy data in youth. Brain Res 1580, 188–198 (2014). [DOI] [PubMed] [Google Scholar]
- 216.Guastella AJ et al. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: a randomized controlled trial. J. Child Psychol. Psychiatry 56, 444–452 (2015). [DOI] [PubMed] [Google Scholar]
- 217.Parker KJ et al. A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Sci. Transl. Med 11, eaau7356 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bolognani F et al. A phase 2 clinical trial of a vasopressin V1a receptor antagonist shows improved adaptive behaviors in men with autism spectrum disorder. Sci. Transl. Med 11, eaat7838 (2019). [DOI] [PubMed] [Google Scholar]
- 219.Rubenstein JLR & Merzenich MM Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav 2, 255–267 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Veenstra-VanderWeele J et al. Arbaclofen in children and adolescents with autism spectrum disorder: a randomized, controlled, phase 2 trial. Neuropsychopharmacology 42, 1390–1398 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Berry-Kravis E et al. Mavoglurant in fragile X syndrome: results of two randomized, double-blind, placebo-controlled trials. Sci. Transl. Med 8, 321ra5 (2016). [DOI] [PubMed] [Google Scholar]
- 222.Krueger DA et al. Everolimus for treatment of tuberous sclerosis complex-associated neuropsychiatric disorders. Ann. Clin. Transl. Neurol 4, 877–887 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Georgiades S & Kasari C Reframing optimal outcomes in autism. JAMA Pediatr 172, 716–717 (2018). [DOI] [PubMed] [Google Scholar]
- 224.Bishop-Fitzpatrick L et al. Characterizing objective quality of life and normative outcomes in adults with autism spectrum disorder: an exploratory latent class analysis. J. Autism Dev. Disord 46, 2707–2719 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.The WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol. Med 28, 551–558 (1998). [DOI] [PubMed] [Google Scholar]
- 226.Gotham K et al. Characterizing the daily life, needs, and priorities of adults with autism spectrum disorder from interactive autism network data. Autism 19, 794–804 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Taylor JL & Seltzer MM Employment and post-secondary educational activities for young adults with autism spectrum disorders during the transition to adulthood. J. Autism Dev. Disord 41, 566–574 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Orsmond GI, Shattuck PT, Cooper BP, Sterzing PR & Anderson KA Social participation among young adults with an autism spectrum disorder. J. Autism Dev. Disord 43, 2710–2719 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Henninger NA & Taylor JL Outcomes in adults with autism spectrum disorders: a historical perspective. Autism 17, 103–116 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Howlin P & Moss P Adults with autism spectrum disorders. Can. J. Psychiatry 57, 275–283 (2012). [DOI] [PubMed] [Google Scholar]
- 231.Farley MA et al. Twenty-year outcome for individuals with autism and average or near-average cognitive abilities. Autism Res 2, 109–118 (2009). [DOI] [PubMed] [Google Scholar]
- 232.Taylor JL, Henninger NA & Mailick MR Longitudinal patterns of employment and postsecondary education for adults with autism and average-range IQ. Autism 19, 785–793 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lai M-C et al. Quantifying and exploring camouflaging in men and women with autism. Autism 21, 690–702 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.van Heijst BF & Geurts HM Quality of life in autism across the lifespan: a meta-analysis. Autism 19, 158–167 (2015). [DOI] [PubMed] [Google Scholar]
- 235.Moss P, Mandy W & Howlin P Child and adult factors related to quality of life in adults with autism. J. Autism Dev. Disord 47, 1830–1837 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bishop-Fitzpatrick L, Mazefsky CA & Eack SM The combined impact of social support and perceived stress on quality of life in adults with autism spectrum disorder and without intellectual disability. Autism 22, 703–711 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kamio Y, Inada N & Koyama T A nationwide survey on quality of life and associated factors of adults with high-functioning autism spectrum disorders. Autism 17, 15–26 (2013). [DOI] [PubMed] [Google Scholar]
- 238.Mason D et al. Predictors of quality of life for autistic adults. Autism Res 11, 1138–1147 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Autistica. Your questions shaping future autism research. https://www.autistica.org.uk/downloads/files/Autism-Top-10-Your-Priorities-for-Autism-Research.pdf (2016).
- 240.Ontario Brain Institute. Community priorities for research on neurodevelopmental disorders. http://braininstitute.ca/img/JLA-NDD-Final-Report.pdf (2018).
- 241.den Houting J Neurodiversity: an insider’s perspective. Autism 23, 271–273 (2018). [DOI] [PubMed] [Google Scholar]
- 242.Szatmari P Risk and resilience in autism spectrum disorder: a missed translational opportunity? Dev. Med. Child Neurol 60, 225–229 (2018). [DOI] [PubMed] [Google Scholar]
- 243.Markowitz LA et al. Development and psychometric evaluation of a psychosocial quality-of-life questionnaire for individuals with autism and related developmental disorders. Autism 20, 832–844 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ryan S & Cole KR From advocate to activist? Mapping the experiences of mothers of children on the autism spectrum. J. Appl. Res. Intellect. Disabil 22, 43–53 (2009). [Google Scholar]
- 245.McCann D, Bull R & Winzenberg T The daily patterns of time use for parents of children with complex needs: a systematic review. J. Child Health Care 16, 26–52 (2012). [DOI] [PubMed] [Google Scholar]
- 246.Karst JS & Van Hecke AV Parent and family impact of autism spectrum disorders: a review and proposed model for intervention evaluation. Clin. Child Fam. Psychol. Rev 15, 247–277 (2012). [DOI] [PubMed] [Google Scholar]
- 247.Lounds J, Seltzer MM, Greenberg JS & Shattuck PT Transition and change in adolescents and young adults with autism: longitudinal effects on maternal well-being. Am. J. Ment. Retard 112, 401–417 (2007). [DOI] [PubMed] [Google Scholar]
- 248.Burke M & Heller T Individual, parent and social-environmental correlates of caregiving experiences among parents of adults with autism spectrum disorder. J. Intellect. Disabil. Res 60, 401–411 (2016). [DOI] [PubMed] [Google Scholar]
- 249.Kim SH, Bal VH & Lord C Longitudinal follow-up of academic achievement in children with autism from age 2 to 18. J. Child Psychol. Psychiatry 59, 258–267 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lord C, Bishop S & Anderson D Developmental trajectories as autism phenotypes. Am. J. Med. Genet. C Semin. Med. Genet 169, 198–208 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Global Research on Developmental Disabilities Collaborators. Developmental disabilities among children younger than 5 years in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancel Glob. Health 6, e1100–e1121 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kahn RS et al. Schizophrenia. Nat. Rev. Dis. Primers 1, 15067 (2015). [DOI] [PubMed] [Google Scholar]
- 253.Patel V et al. Addressing the burden of mental, neurological, and substance use disorders: key messages from Disease Control Priorities, 3rd edition. Lancet 387, 1672–1685 (2016). [DOI] [PubMed] [Google Scholar]
- 254.Franz L, Chambers N, von Isenburg M & de Vries PJ Autism spectrum disorder in sub-Saharan Africa: a comprehensive scoping review. Autism Res 10, 723–749 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.World Health Organization. Training parents to transform children’s lives. https://www.who.int/mental_health/maternal-child/PST/en/ (WHO, 2019). [Google Scholar]
- 256.Naslund JA et al. Digital innovations for global mental health: opportunities for data science, task sharing, and early intervention. Curr. Treat. Options Psychiatry 10.1007/s40501-019-00186-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sadowsky J, Donvan J & Zucker C In a different key: the story of autism. J. Hist. Behav. Sci 54, 66–67 (2018). [Google Scholar]; This paper presents a different, broad overview of the changes in perspective about autism and ASD over the years.
- 258.Rutter M, Greenfeld D & Lockyer L A five to fifteen year follow-up study of infantile psychosis. II. Social and behavioural outcome. Br. J. Psychiatry 113, 1183–1199 (1967). [DOI] [PubMed] [Google Scholar]
- 259.Hermelin B & O’Connor N Psychological Experiments with Autistic Children (Pergamon Press, 1970). [Google Scholar]
- 260.Rimland B Infantile Autism: the Syndrome and its Implications for a Neural Theory of Behaviour (Meredith Publishing Company, 1964). [Google Scholar]
- 261.Frith U Studies in pattern detection in normal and autistic children: I. Immediate recall of auditory sequences. J. Abnorm. Psychol 76, 413–420 (1970). [DOI] [PubMed] [Google Scholar]
- 262.Folstein S & Rutter M in Autism (eds. Rutter M & Schopler E) 219–241 (Springer, 1978). [Google Scholar]
- 263.Mundy P, Sigman M & Kasari C A longitudinal study of joint attention and language development in autistic children. J. Autism Dev. Disord 20, 115–128 (1990). [DOI] [PubMed] [Google Scholar]
- 264.Schopler E & Reichler RJ Parents as cotherapists in the treatment of psychotic children. J. Autism Child. Schizophr 1, 87–102 (1971). [DOI] [PubMed] [Google Scholar]
- 265.Sinclair J Don’t mourn for us. Autism Network International http://www.autreat.com/dont_mourn.html (1993). [Google Scholar]
- 266.Wing L & Gould J Severe impairments of social interaction and associated abnormalities in children: epidemiology and classification. J. Autism Dev. Disord 9, 11–29 (1979). [DOI] [PubMed] [Google Scholar]
- 267.Chawner S et al. A genetic first approach to dissecting the heterogeneity of autism: phenotypic comparison of autism risk copy number variants. Eur. Neuropsychopharmacol 29 (Suppl. 3), S783–S784 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Modabbernia A, Mollon J, Boffetta P & Reichenberg A Impaired gas exchange at birth and risk of intellectual disability and autism: a meta-analysis. J. Autism Dev. Disord 46, 1847–1859 (2016). [DOI] [PubMed] [Google Scholar]
- 269.Christensen J et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 309, 1696–1703 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Xie F, Peltier M & Getahun D Is the risk of autism in younger siblings of affected children moderated by sex, race/ethnicity, or gestational age? J. Dev. Behav. Pediatr 37, 603–609 (2016). [DOI] [PubMed] [Google Scholar]
- 271.Guy A et al. Infants born late/moderately preterm are at increased risk for a positive autism screen at 2 years of age. J. Pediatr 166, 269–275.e3 (2015). [DOI] [PubMed] [Google Scholar]
- 272.Schendel D & Bhasin TK Birth weight and gestational age characteristics of children with autism, including a comparison with other developmental disabilities. Pediatrics 121, 1155–1164 (2008). [DOI] [PubMed] [Google Scholar]
- 273.Windham GC et al. Maternal pre-pregnancy body mass index and gestational weight gain in relation to autism spectrum disorder and other developmental disorders in offspring. Autism Res 12, 316–327 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Schmidt RJ et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am. J. Clin. Nutr 96, 80–89 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Conde-Agudelo A, Rosas-Bermudez A & Norton MH Birth spacing and risk of autism and other neurodevelopmental disabilities: a systematic review. Pediatrics 137, e20153482 (2016). [DOI] [PubMed] [Google Scholar]
- 276.Lyall K et al. The changing epidemiology of autism spectrum disorders. Annu. Rev. Public Health 38, 81–102 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Cheslack-Postava K, Liu K & Bearman PS Closely spaced pregnancies are associated with increased odds of autism in California sibling births. Pediatrics 127, 246–253 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Conti E, Mazzotti S, Calderoni S, Saviozzi I & Guzzetta A Are children born after assisted reproductive technology at increased risk of autism spectrum disorders? A systematic review. Hum. Reprod 28, 3316–3327 (2013). [DOI] [PubMed] [Google Scholar]
- 279.Lehti V et al. Autism spectrum disorders in IVF children: a national case-control study in Finland. Hum. Reprod 28, 812–818 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Rossignol DA, Genuis SJ & Frye RE Environmental toxicants and autism spectrum disorders: a systematic review. Transl. Psychiatry 4, e360 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Curran EA et al. Research review: birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J. Child Psychol. Psychiatry 56, 500–508 (2015). [DOI] [PubMed] [Google Scholar]
- 282.Chandler S, Howlin P, Simonoff E, Kennedy J & Baird G Comparison of parental estimate of developmental age with measured IQ in children with neurodevelopmental disorders. Child Care Health Dev 42, 486–493 (2016). [DOI] [PubMed] [Google Scholar]
- 283.Charman T et al. IQ in children with autism spectrum disorders: data from the Special Needs and Autism Project (SNAP). Psychol. Med 41, 619–627 (2011). [DOI] [PubMed] [Google Scholar]
- 284.Sparrow SS, Cicchetti D & Balla DA Vineland Adaptive Behavior Scales, 2nd Edn. 10.1037/t15164-000 (AGS, 2005). [DOI] [Google Scholar]
- 285.Jones RM, Pickles A & Lord C Evaluating the quality of peer interactions in children and adolescents with autism with the Penn Interactive Peer Play Scale (PIPPS). Mol. Autism 8, 28 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Lord C, Elsabbagh M, Baird G & Veenstra-Vanderweele J Autism spectrum disorder. Lancet 392, 508–520 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Duncan AW & Bishop SL Understanding the gap between cognitive abilities and daily living skills in adolescents with autism spectrum disorders with average intelligence. Autism 19, 64–72 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]


