Abstract
Appreciating that varied stimuli belong to different categories requires that attention be differentially allocated to relevant and irrelevant features of those stimuli. Such selective attention ought to be definable and measurable in both humans and nonhuman animals. We first discuss the definition and methods of assessing attention in animals. We then introduce new experimental and computational tools for assessing attention in pigeons both during and after category learning. Deploying these tools, we have found that, like humans, pigeons attend more to relevant than to irrelevant stimulus features during category learning. Nonetheless, unlike humans, post-acquisition assessment reveals that pigeons less selectively attend to deterministic than to probabilistic features of category members, indicating that pigeons’ attention is more distributed. Fresh opportunities now exist for more effectively understanding the evolution and mechanisms of categorical cognition.
Keywords: category learning, attention, attention assessment, animal cognition, pigeons
Pay attention! This exhortation is often given if an individual’s upcoming task is likely to be challenging or if their behavior suggests insufficient involvement in an ongoing task. However, for researchers studying the role of attention in animal cognition, such exhortations are largely beside the point. When animals are properly motivated, we presume they will fully engage in the given task.
Of course, the complexities of exploring attention in animals transcend their proper motivation. First, we must define attention behaviorally—introspective reports are off the table. Second, we must devise suitable paradigms to assess attention; until recently, most of the methods used in human research have been of limited help.
Bushnell (1998) made these points, observing that, “relatively little effort has been directed toward meaningful comparisons of attention across species, in part because of inconsistent operational definitions of the processes in any species and a lack of process-specific assessment tools for humans upon which animal models may be based (p. 232).” We will first consider these issues before discussing some recent advances in the area.
Defining attention
William James (1890/1950) famously stressed the phenomenology of selectively attending to some stimuli while simultaneously ignoring others. Attention, he said, “is the taking possession by the mind, in clear and vivid form, of one out of what seem several simultaneously possible objects or trains of thought (pp. 403-404).” James’ definition was later behaviorally grounded by Herbert Spencer Jennings (1906/1976), for whom attention was not a conscious mental state; rather, “at the basis of attention lies objectively the phenomenon that the organism may react to only one stimulus even though other stimuli are present which would, if acting alone, likewise produce a response (p. 330).”
Although many operational definitions of attention have since been offered (Sutherland & Mackintosh, 1971), the most general and established may be that of Reynolds (1961). He proposed that, “an organism attends to an aspect of the environment if independent variation or independent elimination of that aspect brings about variation in the organism’s behavior (p. 203).” Reynolds closely adhered to B. F. Skinner’s (1953) proposition that attention represents a controlling relation between a discriminative stimulus and a response.
Assessing attention
Reynolds empirically assessed attention in his well-known demonstration of attention in the pigeon. He first trained two pigeons on a go/no go discrimination task to peck a key when it displayed a white triangle on a red background (the positive discriminative stimulus, S+), but not to peck the key when it displayed a white circle on a green background (the negative discriminative stimulus, S−). Reynolds later gave the pigeons post-acquisition tests with the triangle, circle, red background, and green background separately and randomly displayed. One pigeon responded only to the triangle, whereas the second pigeon responded only to the red background (cf. Wilkie & Masson, 1976). Reynolds’ pioneering project thus showed “that a pigeon may attend to only one of several aspects of a discriminative stimulus. Every part of the environment that is present when a reinforced response occurs may not subsequently be an occasion for the emission of that response (p. 208).”
Post-acquisition tests like Reynolds’ have remained the predominant means of assessing attention in animals. Indeed, as we later document, when enhanced with contemporary computational modeling tools, such tests can still yield interesting and important results. Yet, post-acquisition tests have a notable limitation: they measure the consequence, but not the process of deploying attention.
One way to assess attention during rather than after learning is to train with a Multiple Necessary Cues (MNC) task (Soto & Wasserman, 2010). Here, a set of several compound stimuli is created from cues lying along two or more dimensions. In the simplest case of two dimensions (A and B), two values along each dimension (A1 and A2; B1 and B2) are factorially combined, with only one of the four resulting compound stimuli paired with reinforcement: A1B1+, A1B2−, A2B1−, A2B2−. Control by dimensions A and B can be continuously monitored by separately calculating two discrimination ratios:
| (1) |
| (2) |
Ratio 1 assesses control by dimension A and Ratio 2 assesses control by dimension B. If dimension A were eventually to exert sole control over responding, then Ratio 1 (contrasting compounds containing A1 vs. A2) would rise from .50 to 1.00, whereas Ratio 2 (contrasting compounds containing B1 vs. B2) would remain near .50.
All of our research (using touchscreen technology; Gibson, Wasserman, Frei, & Miller, 2004) has considerably expanded the MNC task. For example, Vyazovska, Teng, and Wasserman (2014) gave pigeons a go/no go discrimination task reinforcing their pecks to only one of sixteen different compound stimuli created from all possible combinations of two values along four visual dimensions: shape (circle/square), size (large/small), line orientation (horizontal/vertical), and brightness (dark/light). Pigeons readily mastered the task, with their rates of responding to each of the fifteen S-s eventually falling to less than 15% of their rate of responding to the sole S+. Converting these sixteen rates to four discrimination ratios, as described earlier, indicated that pigeons’ responding was controlled by all four stimulus dimensions. Across all pigeons, learning rates were similar for each dimension, although individual birds exhibited slightly different speeds of learning the different dimensions. Also, learning was faster the more dimensions along which the S-s differed from the S+, documenting the added benefit arising from increasing perceptual disparities between the S-s and the S+.
Most interestingly—and in agreement with the familiar notions of selective attention and limited capacity (Kruschke & Johansen, 1999; Pashler, 1998)—clear attentional tradeoffs among the four dimensions also occurred during learning. These tradeoffs were typically drops in discrimination accuracy to previously-learned dimensions when later-learned dimensions were being acquired (Teng, Vyazovska, & Wasserman, 2015).
It is important to appreciate that, as was true in Reynolds’ work, the compound visual stimuli we used do involve interpretive intricacies: most notably, that such integral stimuli permit both elemental and configural cues to control behavior and attention (Soto & Wasserman, 2010). Computer modeling has revealed that, in the case of the MNC task, many theoretical accounts must hypothesize unique configural cues associated with each compound stimulus in order to explain the full details of performance (Vyazovska et al., 2014). Other tasks involving separable stimulus elements are less subject to this intricacy.
Attention to the separable elements of compound visual stimuli is often monitored in humans by gaze direction, although looking at a discrete stimulus may not be sufficient for behavioral control by the stimulus. That said, within the area of attention and categorization, compelling evidence attests to the intimate interrelation between gaze direction and attention to relevant visual features in humans (Blair et al., 2009; Rehder & Hoffman, 2005a, 2005b).
As to Bushnell’s (1998) hope of developing an effective process-specific assessment tool on which animal models of attention might be based, such monitoring of visual gaze appears to be particularly promising. Empirically, Rehder and Hoffman (2005a) reported that human research participants: (a) learned to optimally allocate their attention to stimulus dimensions in the process of discriminating categories, (b) initially fixated on all of the dimensions, and (c) selectively directed their eye fixations toward the relevant dimensions after they had greatly reduced their categorization errors. Encouraged by these results, we began a series of investigations exploring peck tracking and visual category learning in pigeons also using tasks involving separable stimulus elements.
Peck tracking and visual category learning
Concern with attentional precursors to successful discrimination behavior is not new (Dinsmoor, 1985). Spence (1940) deemed it vital that, if an animal is to learn a visual discrimination, then it must also engage in suitable receptor-orienting acts: “[The] animal must learn to orient and fixate its head and eyes so as to receive the … relevant stimulus aspects (p. 277).” To Spence (1950): “Such learning is itself an active trial-and-error process with those adjustments being learned that lead to reception of stimulus-cues (p. 169).”
In our research, we both temporally and spatially separated the animal’s stimulus-reception response from its final categorization response; doing so allowed us to isolate two important aspects of performance that would otherwise be conflated if the animal were to respond directly to the stimulus to be categorized—the customary behavioral measure. We called this task and monitoring system peck tracking.
In Castro and Wasserman (2014), our initial peck tracking project, we trained pigeons to classify stimuli from two different artificial visual categories (Figure 1, left). Each category exemplar contained two relevant features (perfect predictors of category membership) and two irrelevant features. There were two relevant features for Category A and two different relevant features for Category B. The irrelevant features were common to both Categories A and B, preventing them from predicting category membership. Each of the relevant and irrelevant features appeared equally often in each of the four corner locations: top-left, top-right, bottom-left, or bottom-right. So, spatial location could not signal where the relevant features would be presented.
Figure 1.
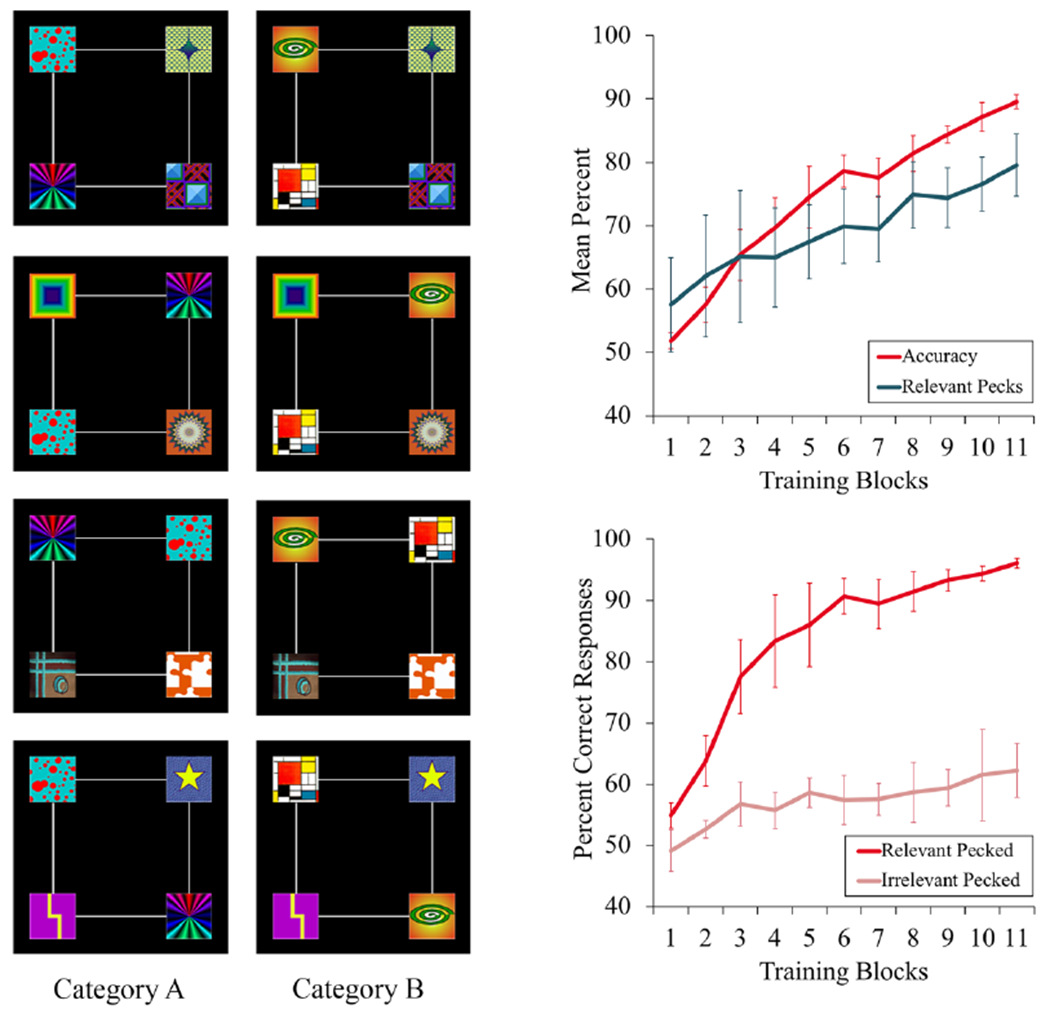
On the left, examples of Category A and Category B training exemplars in Castro and Wasserman (2014). There were two relevant features for Category A (the rainbow and the red bubbles) and two different relevant features for Category B (the green spiral and the Mondrian-colored squares). The other stimuli were irrelevant features, common to Categories A and B. On the right, top, mean percent accuracy and relevant pecks across training in Experiment 1 of Castro and Wasserman. On the right, bottom, accuracy scores across training depending on whether a relevant or an irrelevant feature had been pecked just before making the choice response. Error bars indicate the standard error of the means.
When each exemplar was presented on the computer screen, pigeons had to peck it multiple times. Critically, only the areas occupied by the relevant and irrelevant features were ‘active’ for monitoring pecks; pecks at the black background did not count. But pigeons were free to peck any of the features, relevant or irrelevant. We recorded the location of pigeons’ pecks, in order to determine whether or not they selectively directed their pecks to the relevant features of the category exemplars.
After pigeons completed the observing response requirement, two report buttons appeared: one to the left and one to the right of the category exemplar. Birds had to peck one of the report buttons in order to classify the exemplar as belonging to Category A or Category B. Correct choices were followed by food reinforcement; incorrect choices were not followed by food, and correction trials continued until pigeons made the correct response.
As training proceeded, categorization accuracy increased, as expected. Importantly, pigeons also increasingly pecked the relevant stimulus features (Figure 1, top right), suggesting that they were tracking the relevant information for solving the task.
This performance parallel prompted another question: Did attention to the relevant features rise first and promote category learning or did categorization accuracy rise before pigeons selectively attended to the relevant features? Rehder and Hoffman (2005a) found that people’s eye movements toward the relevant stimulus elements followed rather than preceded improvements in accuracy; correct responses were already high before people fully deployed attention to the relevant category features. In our case, two findings similarly suggested that pigeons’ tracking the relevant stimuli followed increases in categorization accuracy.
First, pigeons’ categorization accuracy was considerably higher after they pecked the relevant category features than after they pecked the irrelevant features (Figure 1, bottom right). Second, using a state-space model (Smith et al., 2004), Castro and Wasserman (2016) mathematically confirmed that increases in peck tracking significantly followed increases in categorization accuracy. So, if pigeon make a correct choice response and consequently receive reinforcement, then they will pay increased attention to the chosen feature on future trials; attention correspondingly shifts to those features that prove to be more reliable predictors of reinforcement, in accord with several prominent attentional learning models (e.g., George & Pearce, 2012; Kruschke, 2001; Mackintosh, 1975).
Pigeons’ attention to relevant and irrelevant attributes of training exemplars can therefore be effectively monitored during category learning, indicating that peck tracking can serve as a useful measure of attention in pigeons, much as eye tracking is a useful measure of attention in humans (Castro & Wasserman, 2017; Sheridan et al., 2019).
Computational modeling of post-acquisition performance
“Attention is more than looking at something …. An organism is attending to a detail of a stimulus whether or not its receptors are oriented to produce the most clear-cut reception, if its behavior is predominantly under control of that detail (Skinner, 1953, p. 124).” Although we have found that peck tracking can serve as a useful proxy for the online assessment of visual attention, we are cognizant that establishing the controlling relation between the details of a stimulus and the organism’s response always remains primary.
In this connection, we have recently seen that fresh inroads into assessing that controlling relation can be made with the aid of computational modeling. This approach is also amenable to online assessment, although we have thus far limited our work to post-acquisition assessment in order to validate its analytical utility.
In our peck tracking experiments, one or more visual features were always perfect predictors of category membership. Under these circumstances, pigeons discovered the perfectly predictive features and their attention was directed to them (Lea et al., 2009; Wills et al., 2009). But it is important to appreciate that a category discrimination can also be accomplished by perceiving the overall similarity or family resemblance of the exemplars in each category; in this case, attention may be more widely distributed among multiple features.
For example, Deng and Sloutsky (2016; Smith & Kemler, 1977) reported that infants learn the statistical co-occurrence of several features within exemplars of different categories, suggesting that their attention is distributed rather than focused on specific diagnostic features. What about pigeons’ attentional selectivity? Would they predominantly attend to a perfect predictor, as prior studies suggest? Or, if other features were also to have some but lesser predictive value, would pigeons attend more diffusely, similar to the behavior of young children?
To find out, Castro et al. (2020) gave human adults and pigeons a categorization task in which there was a single rule-like deterministic feature that perfectly predicted category membership accompanied by six other features that only probabilistically predicted category membership. So, the task could be learned on the basis of either one deterministic feature (encouraging selective, focused attention) or multiple probabilistic features (encouraging distributed attention).
Overall accuracy scores suggested that both humans and pigeons relied on the deterministic feature to categorize the stimuli, although humans may have done so to a greater degree. To gain a clearer understanding of humans’ and pigeons’ attention and categorization behavior, we tested them with new exemplars in which the deterministic feature was absent but the probabilistic features were present, as well as with new exemplars in which the deterministic feature was present but one or more of the probabilistic features were absent. Then, we used a modeling approach to determine their attentional profiles during testing.
We used Nosofsky’s (1986) Generalized Context Model (GCM), a computational model which assumes that organisms represent categories by storing individual training exemplars in memory. Later classification of novel testing exemplars is based on similarity comparisons between the novel exemplars and the stored exemplars. This estimation of similarity is context-dependent and influenced by the features to which the organism attends. Attention allocated to particular features—attentional weights—changes during training. Thus, we used GCM to estimate the attentional weights that best accounted for the responses of humans and pigeons on categorization testing trials.
We computed each subject’s attentional weight to each feature, and then summarized each subject’s attentional profile by calculating the entropy of their attentional weights. Because we were interested in determining whether our subjects focused on one feature or distributed their attention to some or all of them, we deemed that entropy, a measure of variety or diversity provided by information theory (Shannon & Weaver, 1949), was a good candidate for this purpose. Entropy measures the amount of informational diversity by computing a weighted average of the amount of information that, in our case, each of the features in an exemplar provides. When only one feature carries all of the information (in our case, w = {1, 0, 0, 0, 0, 0, 0}), there is no informational diversity, so entropy is 0. When some or all of the features carry some amount of information, entropy is larger. Entropy is maximal when each of the features carry equal amounts of information (in our case, w = {.14, .14, .14, .14, .14, .14, .14}).
Once the entropy score for each subject was obtained, it was normalized based on the maximum possible entropy given the number of features. Thus, normalized entropy was bound between 0.00 and 1.00, with 0.00 representing maximal selectivity (when the attentional weight for one of the features equals 1.00, whereas the remaining weights for all of the other features equals 0.00), and with 1.00 representing minimal selectivity or maximal distribution of attention (when all of the attentional weights are equal).
Most of the adults exhibited maximal selectivity—fully focusing on the deterministic feature—whereas pigeons distributed their attention among several features (Figure 2). Therefore, under these training conditions, pigeons do not appear to strongly focus attention on deterministic information and filter less predictive information, as do human adults. Pigeons, much like young children (Smith & Kemler, 1977; Deng & Sloutsky, 2016), tend to distribute their attention among various elements of a stimulus.
Figure 2.
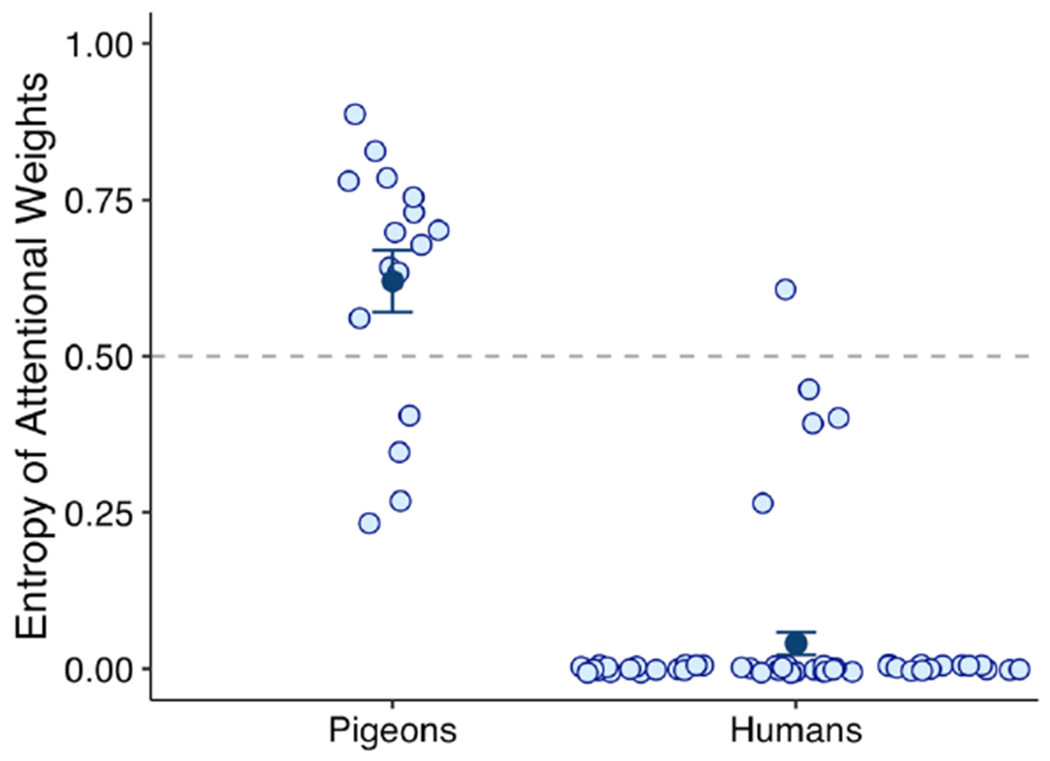
Normalized entropy of GCM’s best-fitting attentional weights for each individual subject in Castro et al. (2020). A value of 0 represents maximal selectivity (when the attentional weight for one of the features equals 1 and the remaining weights for all other features equals 0), whereas a value of 1 represents minimal selectivity or maximal distribution of attention (when all weights are equal). The dark blue points indicate the mean of each of the distributions. The dashed line, at 0.50, represents the middle value and is simply included as a reference.
Our peck tracking experiments (Castro & Wasserman, 2014, 2016, 2017; Sheridan et al., 2019) thus indicated that pigeons selectively attend to the relevant features of category exemplars. In apparent contrast, the study by Castro et al. (2020) indicated that pigeons distribute their attention among multiple stimulus features. Reconciling these apparently contradictory conclusions requires examining the predictive values of the stimulus features in the different studies. In the peck tracking experiments, the relevant features had perfect predictive value, whereas the irrelevant features had no predictive value. In Castro et al., the deterministic feature was a perfect category predictor, but each of the probabilistic features also predicted category membership, albeit imperfectly (each was associated on 66% of the trials with the correct category response). It is thus possible that pigeons can learn to ignore entirely irrelevant features, but have more difficulty disengaging from features that have some, but lesser predictive value than other perfectly predictive features.
Final remarks
Pigeons’ pecking behavior can importantly inform us about the process and consequence of visual attention. Touchscreen technology has allowed us to see that, during learning, pigeons track those features that allow them to successfully solve a challenging categorization task. In this way, we can measure the ongoing process of attention in visual category learning. Critically, strong empirical parallels between pigeons and humans reveal that peck tracking can be a worthwhile measure of pigeons’ visual attention, much as eyetracking is considered a worthwhile measure of visual attention in human category learning (Rehder & Hoffman, 2005b). In addition, computational modeling techniques allow us to gain a better understanding of the consequences of visual attention even after learning has taken place.
These experimental strategies and computational methods facilitate and encourage a truly comparative study of the interplay between attention and categorization in humans and animals. Comparing diverse species should offer substantial insights into the evolutionary roots of categorization, specifically, and the nature of cognition, generally.
Acknowledgments
This research was supported by National Institutes of Health Grant P01HD080679 to EAW.
References
- Blair M, Watson MR, Walshe RC, & Maj F (2009). Extremely selective attention: Eyetracking studies of the dynamic allocation of attention to stimulus features in categorization. Journal of Experimental Psychology: Learning, Memory, and Cognition, 35, 1196–1206. doi: 10.1037/a0016272 [DOI] [PubMed] [Google Scholar]
- Bushnell PJ (1998). Behavioral approaches to the assessment of attention in animals. Psychopharmacology, 138, 231–259. doi: 10.1007/s002130050668 [DOI] [PubMed] [Google Scholar]
- Castro L, & Wasserman EA (2014). Pigeons’ tracking of relevant attributes in categorization learning. Journal of Experimental Psychology: Animal Learning and Cognition, 40, 195–211. doi: 10.1037/xan0000022 [DOI] [PubMed] [Google Scholar]
- Castro L, & Wasserman EA (2016). Attentional shifts in categorization learning: Perseveration but not learned irrelevance. Behavioural Processes, 123, 63–73. doi: 10.1016/j.beproc.2015.11.001 [DOI] [PubMed] [Google Scholar]
- Castro L, & Wasserman EA (2017). Feature predictiveness and selective attention in pigeons’ categorization learning. Journal of Experimental Psychology: Animal Learning and Cognition, 43, 231–242. doi: 10.1037/xan0000146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro L, Savic O, Navarro V, Wasserman EA, & Sloutsky VM (2020). Selective and distributed attention in human and pigeon category learning. Cognition, 204. doi: 10.1016/j.cognition.2020.104350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, & Sloutsky VM (2016). Selective attention, diffused attention, and the development of categorization. Cognitive Psychology, 91, 24–62. doi: 10.1016/j.cogpsych.2016.09.00 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinsmoor JA (1985). The role of observing and attention in establishing stimulus control. Journal of the Experimental Analysis of Behavior, 43, 365–381. doi: 10.1901/jeab.1985.43-365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George DN, & Kruschke JK (2012). Contextual modulation of attention in human category learning. Learning & Behavior, 40, 530–541. doi: 10.3758/s13420-012-0072-8 [DOI] [PubMed] [Google Scholar]
- George DN, & Pearce JM (2012). A configural theory of attention and associative learning. Learning & Behavior, 40, 241–254. doi: 10.3758/s13420-012-0078-2 [DOI] [PubMed] [Google Scholar]
- Gibson BM, Wasserman EA, Frei L, & Miller K (2004). Recent advances in operant conditioning technology: A versatile and affordable computerized touch screen system. Behavior Research Methods, Instruments and Computers, 36, 355–362. doi: 10.3758/BF03195582 [DOI] [PubMed] [Google Scholar]
- James W (1890/1955). Principles of psychology, vol. 1. Dover, New York. [Google Scholar]
- Jennings HS (1906/1976). Behavior of the lower organisms. Indiana University Press, Bloomington. [Google Scholar]
- Kruschke JK (2001). Towards a unified model of attention in associative learning. Journal of Mathematical Psychology, 45, 812–863. doi: 10.1006/jmps.2000.1354 [DOI] [Google Scholar]
- Kruschke JK, & Johansen MK (1999). A model of probabilistic category learning. Journal of Experimental Psychology: Learning, Memory, and Cognition, 25, 1083–1119. doi: 10.1037/0278-7393.25.5.1083 [DOI] [PubMed] [Google Scholar]
- Lea SEG, Wills AJ, Leaver LA, Ryan CME. Bryant CML, & Millar L (2009). A comparative analysis of the categorization of multidimensional stimuli: II. Strategic information search in humans (Homo sapiens) but not in pigeons (Columba livia). Journal of Comparative Psychology, 123, 406–420. doi: 10.1037/a0016851 [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ (1975). A theory of attention: Variations in the associability of stimuli with reinforcement. Psychological Review, 82, 276–298. doi: 10.1037/h0076778 [DOI] [Google Scholar]
- Nosofsky RM (1986). Attention, similarity, and the identification–categorization relationship. Journal of Experimental Psychology: General, 115, 39–57. doi: 10.1037/0096-3445.115.1.39 [DOI] [PubMed] [Google Scholar]
- Pashler HE (1998). The psychology of attention. Cambridge, MA: MIT Press. [Google Scholar]
- Rehder B, & Hoffman AB (2005a). Eyetracking and selective attention in category learning. Cognitive Psychology, 51, 1–41. doi: 10.1016/j.cogpsych.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Rehder B, & Hoffman AB (2005b). Thirty-something categorization results explained: Selective attention, eyetracking, and models of category learning. Journal of Experimental Psychology: Learning, Memory, and Cognition, 31, 811–829. doi: 10.1037/0278-7393.31.5.811 [DOI] [PubMed] [Google Scholar]
- Reynolds GS (1961). Attention in the pigeon. Journal of the Experimental Analysis of Behavior, 4, 203–208. doi: 10.1901/jeab.1961.4-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley DA, & Leith CR (1976). Multidimensional psychophysics and selective attention in animals. Psychological Bulletin, 83, 138–160. doi: 10.1037/0033-2909.83.1.138 [DOI] [Google Scholar]
- Shannon CE, & Weaver W (1949). The mathematical theory of communication. Urbana: University of Illinois Press. [Google Scholar]
- Sheridan CL, Castro L, Fonseca S, & Wasserman EA (2019). The role of category density in pigeons’ tracking of relevant information. Learning and Behavior, 47, 234–244. doi: 10.3758/s13420-019-00372-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner FB (1953). Science and human behavior. Macmillan, New York. [Google Scholar]
- Smith AC, Frank LM, Wirth S, Yanike M, Hu D, Kubota Y, Graybiel AM, Suzuki WA, & Brown EN (2004). Dynamic analysis of learning in behavioral experiments. Journal of Neuroscience, 24, 447–461. doi: 10.1523/jneurosci.2908-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LB, & Kemler DG (1977). Developmental trends in free classification: Evidence for a new conceptualization of perceptual development. Journal of Experimental Child Psychology, 24, 279–298. doi: 10.1016/0022-0965(77)90007-8 [DOI] [Google Scholar]
- Soto FA, & Wasserman EA (2010). Integrality/separability of stimulus dimensions and multidimensional generalization in pigeons. Journal of Experimental Psychology: Animal Behavior Processes, 36, 194–205. doi: 10.1037/a0016560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence KW (1940). Continuous versus non-continuous interpretations of discrimination learning. Psychological Review, 47, 271–288. doi: 10.1037/h0054336 [DOI] [Google Scholar]
- Spence KW (1950). Cognitive versus stimulus-response theories of learning. Psychological Review, 57, 159–172. doi: 10.1037/h0058250 [DOI] [PubMed] [Google Scholar]
- Sutherland NS, & Mackintosh NJ (1971). Mechanisms of animal discrimination learning. Academic Press, New York. [Google Scholar]
- Teng Y, Vyazovska OV, & Wasserman EA (2015). Selective attention and pigeons’ multiple necessary cues discrimination learning. Behavioural Processes, 112, 61–71. doi: 10.1016/j.beproc.2014.08.004 [DOI] [PubMed] [Google Scholar]
- Vyazovska OV, Teng Y, & Wasserman EA (2014). Attentional tradeoffs in the pigeon. Journal of the Experimental Analysis of Behavior, 101, 337–354. doi: 10.1002/jeab.82 [DOI] [PubMed] [Google Scholar]
- Wilkie DM, & Masson ME (1976). Attention in the pigeon: A reevaluation. Journal of the Experimental Analysis of Behavior, 26, 207–212. doi: 10.1901/jeab.1976.26-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills AJ, Lea SEG, Leaver LA, Osthaus B, Ryan CME, Suret MB, Bryant CML, Chapman SJA, & Millar L (2009). A comparative analysis of the categorization of multidimensional stimuli: I. Unidimensional classification does not necessarily imply analytic processing; evidence from pigeons (Columba livia), squirrels (Sciurus carolinensis), and humans (Homo sapiens). Journal of Comparative Psychology, 123, 391–405. doi: 10.1037/a0016216 [DOI] [PubMed] [Google Scholar]
Recommended Readings
- Güntürkün O, Koenen C, Iovine F, Garland A, & Pusch R (2018). The neuroscience of perceptual categorization in pigeons: A mechanistic hypothesis. Learning & Behavior, 46, 229–241. doi: 10.3758/s13420-018-0321-6. [DOI] [PubMed] [Google Scholar]; Recent review focused on the neurobiological foundations of visual categorization learning in pigeons and other avian species.
- Rehder B, & Hoffman AB (2005). Thirty-something categorization results explained: Selective attention, eyetracking, and models of category learning. Journal of Experimental Psychology: Learning, Memory, and Cognition, 31, 811–829. doi: 10.1037/0278-7393.31.5.811. [DOI] [PubMed] [Google Scholar]; Comprehensive evaluation of different results in attention and category learning in humans, using eyetracking and computational modeling techniques.
- Washburn DA, & Taglialatela LA (2012). The competition for attention in humans and other animals. In Zentall TR & Wasserman EA (Eds.), Oxford handbook of comparative cognition (pp. 100–116). Oxford University Press. doi: 10.1093/oxfordhb/9780195392661.013.0007. [DOI] [Google Scholar]; A clear and wide-ranging review of different aspects of attention in human and nonhuman animals.


