Abstract
Functional proteins are the most versatile macromolecules. They can be obtained by extraction from natural sources or by genetic engineering technologies. The outstanding selectivity, specificity, binding activity, and biocompatibility endow engineered proteins with outstanding performance for disease therapy. Nevertheless, their stability is dramatically impaired in blood circulation, hindering clinical translations. Thus, many strategies have been developed to improve the stability, efficacy, bioavailability, and productivity of therapeutic proteins for clinical applications. In this review, we summarize the recent progress in the fabrication and application of therapeutic proteins. We first introduce various strategies for improving therapeutic efficacy via bioengineering and nanoassembly. Furthermore, we highlight their diverse applications as growth factors, nanovaccines, antibody-based drugs, bioimaging molecules, and cytokine receptor antagonists. Finally, a summary and perspective for the future development of therapeutic proteins are presented.
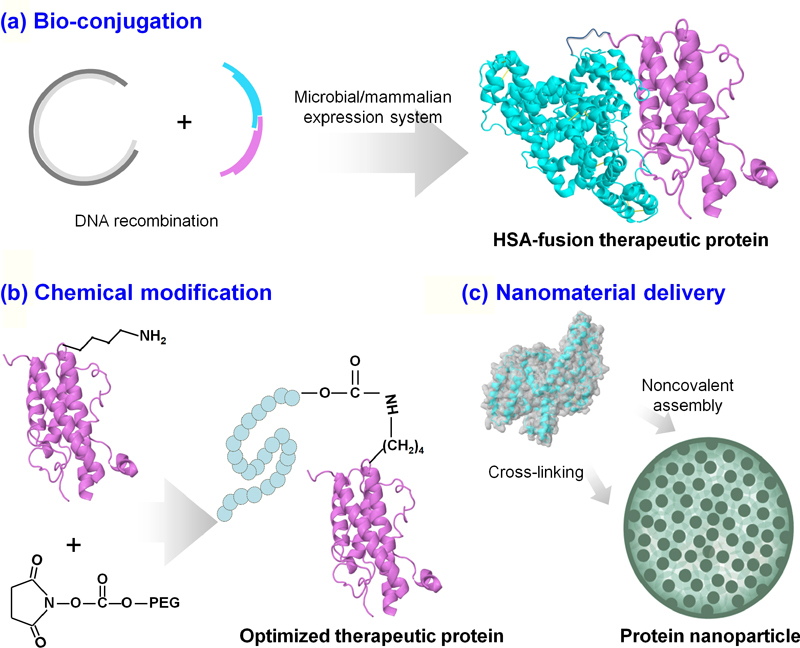
Keywords: therapeutic proteins, nanodrug, genetic engineering, structural modification, therapeutic efficacy
Acknowledgements
This research was supported by the National Key Research and Development Program of China (Nos. 2020YFA0908900, 2018YFA0902600, and 2020YFA0712102), the National Natural Science Foundation of China (Nos. 21877104, 21834007, 22107097, 21878258, 22020102003, and 22125701), K. C. Wong Education Foundation (No. GJTD-2018-09), and the Youth Innovation Promotion Association of the Chinese Academy (CAS, No. 2021226).
Contributor Information
Jingjing Li, Email: jjingli@ciac.ac.cn.
Kai Liu, Email: kai.liu@tsinghua.edu.cn.
References
- [1].Johnson-Léger C, Power C A, Shomade G, Shaw J P, Proudfoot A E. Protein therapeutics-lessons learned and a view of the future. Expert Opin. Biol. Ther. 2006;6:1–7. doi: 10.1517/14712598.6.1.1. [DOI] [PubMed] [Google Scholar]
- [2].Wen F, Rubin-Pitel S B, Zhao H. Engineering of therapeutic proteins. In: Park S J, Cochran J R, editors. Protein Engineering and Design. Boca Raton: CRC Press; 2009. pp. 153–177. [Google Scholar]
- [3].Cheng L, Yang L, Meng F H, Zhong Z Y. Protein nanotherapeutics as an emerging modality for cancer therapy. Adv. Healthc. Mater. 2018;7:1800685. doi: 10.1002/adhm.201800685. [DOI] [PubMed] [Google Scholar]
- [4].Usmani S S, Bedi G, Samuel J S, Singh S, Kalra S, Kumar P, Ahuja A A, Sharma M, Gautam A, Raghava G P S. THPdb: Database of FDA-approved peptide and protein therapeutics. PLoS One. 2017;12:e0181748. doi: 10.1371/journal.pone.0181748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Werle M, Bernkop-Schnürch A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids. 2006;30:351–367. doi: 10.1007/s00726-005-0289-3. [DOI] [PubMed] [Google Scholar]
- [6].Zaman R, Islam R A, Ibnat N, Othman I, Zaini A, Lee C Y, Chowdhury E H. Current strategies in extending half-lives of therapeutic proteins. J. Control. Release. 2019;301:176–189. doi: 10.1016/j.jconrel.2019.02.016. [DOI] [PubMed] [Google Scholar]
- [7].Bajracharya R, Song J G, Back S Y, Han H K. Recent advancements in non-invasive formulations for protein drug delivery. Comput. Struct. Biotechnol. J. 2019;17:1290–1308. doi: 10.1016/j.csbj.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang S Y, Duan Y O, Zhang Q Z, Komarla A, Gong H, Gao W W, Zhang L F. Drug targeting via platelet membrane-coated nanoparticles. Small Struct. 2020;1:2000018. doi: 10.1002/sstr.202000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kozlowski H N, Mohamed M A A, Kim J, Bell N G, Zagorovsky K, Mubareka S, Chan W C W. A Colorimetric test to differentiate patients infected with influenza from COVID-19. Small Struct. 2021;2:2100034. doi: 10.1002/sstr.202100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nguyen V H, Lee B J. Protein corona: A new approach for nanomedicine design. Int. J. Nanomed. 2017;12:3137–3151. doi: 10.2147/IJN.S129300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hong S Y N, Choi D W, Kim H N, Park C G, Lee W, Park H H. Protein-based nanoparticles as drug delivery systems. Pharmaceutics. 2020;12:604. doi: 10.3390/pharmaceutics12070604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang N, Mei K, Guan P, Hu X, Zhao Y L. Protein-based artificial nanosystems in cancer therapy. Small. 2020;16:1907256. doi: 10.1002/smll.201907256. [DOI] [PubMed] [Google Scholar]
- [13].Corchero J L, Vázquez E, García-Fruitós E, Ferrer-Miralles N, Villaverde A. Recombinant protein materials for bioengineering and nanomedicine. Nanomedicine (Lond) 2014;9:2817–2828. doi: 10.2217/nnm.14.153. [DOI] [PubMed] [Google Scholar]
- [14].Uzunalli G, Guler M O. Peptide gels for controlled release of proteins. Ther. Deliv. 2020;11:193–211. doi: 10.4155/tde-2020-0011. [DOI] [PubMed] [Google Scholar]
- [15].Sandra F, Khaliq N U, Sunna A, Care A. Developing protein-based nanoparticles as versatile delivery systems for cancer therapy and imaging. Nanomaterials (Basel) 2019;9:1329. doi: 10.3390/nano9091329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ferrer-Miralles N, Rodríguez-Carmona E, Corchero J L, García-Fruitós E, Vázquez E, Villaverde A. Engineering protein self-assembling in protein-based nanomedicines for drug delivery and gene therapy. Crit. Rev. Biotechnol. 2015;35:209–221. doi: 10.3109/07388551.2013.833163. [DOI] [PubMed] [Google Scholar]
- [17].Hassanin I A, Elzoghby A O. Self-assembled non-covalent protein-drug nanoparticles: An emerging delivery platform for anti-cancer drugs. Expert Opin. Drug Deliv. 2020;17:1437–1458. doi: 10.1080/17425247.2020.1813713. [DOI] [PubMed] [Google Scholar]
- [18].Yang Y Y, Chen Q L, Lin J Y, Cai Z, Liao G C, Wang K, Bai L, Zhao P, Yu Z Q. Recent advance in polymer based microspheric systems for controlled protein and peptide delivery. Curr. Med. Chem. 2019;26:2285–2296. doi: 10.2174/0929867326666190409130207. [DOI] [PubMed] [Google Scholar]
- [19].Patra J K, Das G, Fraceto L F, Campos E V R, Rodriguez-Torres M D P, Acosta-Torres L S, Diaz-Torres L A, Grillo R, Swamy M K, Sharma S, et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018;16:71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Flintegaard T V, Thygesen P, Rahbek-Nielsen H, Levery S B, Kristensen C, Clausen H, Bolt G. N-glycosylation increases the circulatory half-life of human growth hormone. Endocrinology. 2010;151:5326–5336. doi: 10.1210/en.2010-0574. [DOI] [PubMed] [Google Scholar]
- [21].Strohl W R. Fusion proteins for half-life extension of biologics as a strategy to make biobetters. BioDrugs. 2015;29:215–239. doi: 10.1007/s40259-015-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Beals J M, Shanafelt A B. Enhancing exposure of protein therapeutics. Drug Discov. Today Technol. 2006;3:87–94. doi: 10.1016/j.ddtec.2006.03.001. [DOI] [PubMed] [Google Scholar]
- [23].Ekladious I, Colson Y L, Grinstaff M W. Polymer-drug conjugate therapeutics: Advances, insights and prospects. Nat. Rev. Drug Discov. 2019;18:273–294. doi: 10.1038/s41573-018-0005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Feng L D, Yang L, Li L J, Xiao J Y, Bie N N, Xu C, Zhou J, Liu H M, Gan L, Wu Y Z. Programmed albumin nanoparticles regulate immunosuppressive pivot to potentiate checkpoint blockade cancer immunotherapy. Nano Res. 2022;15:593–602. doi: 10.1007/s12274-021-3525-6. [DOI] [Google Scholar]
- [25].Kontermann R E. Half-life extended biotherapeutics. Expert Opin. Biol. Ther. 2016;16:903–915. doi: 10.1517/14712598.2016.1165661. [DOI] [PubMed] [Google Scholar]
- [26].Sleep D. Albumin and its application in drug delivery. Expert Opin. Drug Deliv. 2015;12:793. doi: 10.1517/17425247.2015.993313. [DOI] [PubMed] [Google Scholar]
- [27].Cohen-Barak O, Sakov A, Rasamoelisolo M, Bassan M, Brown K, Mendzelevski B, Spiegelstein O. Safety, pharmacokinetic and pharmacodynamic properties of TV-1106, a long-acting GH treatment for GH deficiency. Eur. J. Endocrinol. 2015;173:541–551. doi: 10.1530/EJE-15-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Santagostino E, Martinowitz U, Lissitchkov T, Pan-Petesch B, Hanabusa H, Oldenburg J, Negrier C, Pabinger I, Prondzinski M V D, Altisent C, et al. Long-acting recombinant coagulation factor IX albumin fusion protein (rIX-FP) in hemophilia B: Results of a phase 3 trial. Blood. 2016;127:1761–1769. doi: 10.1182/blood-2015-09-669234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shi Y N, Sun X F, Zhang L P, Sun K X, Li K K, Li Y X, Zhang Q. Fc-modified exenatide-loaded nanoparticles for oral delivery to improve hypoglycemic effects in mice. Sci. Rep. 2018;8:726. doi: 10.1038/s41598-018-19170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jazayeri J A, Carroll G J. Fc-based cytokines: Prospects for engineering superior therapeutics. BioDrugs. 2008;22:11. doi: 10.2165/00063030-200822010-00002. [DOI] [PubMed] [Google Scholar]
- [31].Pridgen E M, Alexis F, Kuo T T, Levy-Nissenbaum E, Karnik R, Blumberg R S, Langer R, Farokhzad O C. Transepithelial transport of Fc-targeted nanoparticles by the neonatal fc receptor for oral delivery. Sci. Transl. Med. 2013;5:213ra167. doi: 10.1126/scitranslmed.3007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liu L M. Pharmacokinetics of monoclonal antibodies and Fc-fusion proteins. Prot. Cell. 2018;9:15–32. doi: 10.1007/s13238-017-0408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nestorov I, Zitnik R, DeVries T, Nakanishi A M, Wang A, Banfield C. Pharmacokinetics of subcutaneously administered etanercept in subjects with psoriasis. Br. J. Clin. Pharmacol. 2006;62:435–445. doi: 10.1111/j.1365-2125.2006.02581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wells J A, Glassman A R, Ayala A R, Jampol L M, Bressler N M, Bressler S B, Brucker A J, Ferris F L, Hampton G R, Jhaveri C, et al. Aflibercept, Bevacizumab, or Ranibizumab for diabetic macular edema: Two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123:1351–1359. doi: 10.1016/j.ophtha.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rath T, Baker K, Dumont J A, Peters R T, Jiang H Y, Qiao S W, Lencer W I, Pierce G F, Blumberg R S. Fc-fusion proteins and FcRn: Structural insights for longer-lasting and more effective therapeutics. Crit. Rev. Biotechnol. 2015;35:235–254. doi: 10.3109/07388551.2013.834293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Do B H, Kang H J, Song J A, Nguyen M T, Park S, Yoo J, Nguyen A N, Kwon G G, Jang J, Jang M, et al. Granulocyte colony-stimulating factor (GCSF) fused with Fc domain produced from E. coli is less effective than Polyethylene Glycol-conjugated GCSF. Sci. Rep. 2017;7:6480. doi: 10.1038/s41598-017-06726-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Han J A, Kang Y J, Shin C, Ra J S, Shin H H, Hong S Y, Do Y, Kang S. Ferritin protein cage nanoparticles as versatile antigen delivery nanoplatforms for dendritic cell (DC)-based vaccine development. Nanomed. Nanotechnol. Biol. Med. 2014;10:561–569. doi: 10.1016/j.nano.2013.11.003. [DOI] [PubMed] [Google Scholar]
- [38].Deshpande S, Masurkar N D, Girish V M, Desai M, Chakraborty G, Chan J M, Drum C L. Thermostable exoshells fold and stabilize recombinant proteins. Nat. Commun. 2017;8:1442. doi: 10.1038/s41467-017-01585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jeon J O, Kim S, Choi E, Shin K, Cha K, So I, Kim S I, Jun E, Kim D J, Ahn H J, et al. Designed nanocage displaying ligand-specific peptide bunches for high affinity and biological activity. ACS Nano. 2013;7:7462–7471. doi: 10.1021/nn403184u. [DOI] [PubMed] [Google Scholar]
- [40].Georgiev I S, Joyce M G, Chen R E, Leung K, McKee K, Druz A, Van Galen J G, Kanekiyo M, Tsybovsky Y, Yang E S, et al. Two-component ferritin nanoparticles for multimerization of diverse trimeric antigens. ACS Infect. Dis. 2018;4:788–796. doi: 10.1021/acsinfecdis.7b00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Almine J F, Bax D V, Mithieux S M, Nivison-Smith L, Rnjak J, Waterhouse A, Wise S G, Weiss A S. Elastin-based materials. Chem. Soc. Rev. 2010;39:3371–3379. doi: 10.1039/b919452p. [DOI] [PubMed] [Google Scholar]
- [42].MacEwan S R, Chilkoti A. Elastin-like polypeptides: Biomedical applications of tunable biopolymers. Biopolymers. 2010;94:60–77. doi: 10.1002/bip.21327. [DOI] [PubMed] [Google Scholar]
- [43].Koria P, Yagi H, Kitagawa Y, Megeed Z, Nahmias Y, Sheridan R, Yarmush M L. Self-assembling elastin-like peptides growth factor chimeric nanoparticles for the treatment of chronic wounds. Proc. Natl. Acad. Sci. USA. 2011;108:1034–1039. doi: 10.1073/pnas.1009881108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang W, Despanie J, Shi P, Edman M C, Lin Y A, Cui H G, Heur J M, Fini M E, Hamm-Alvarez S F, MacKay J A. Lacritin-mediated regeneration of the corneal epithelia by protein polymer nanoparticles. J. Mater. Chem. B. 2014;2:8131–8141. doi: 10.1039/C4TB00979G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Caliceti P, Veronese F M. Pharmacokinetic and biodistribution properties of poly(ethylene glycol)-protein conjugates. Adv. Drug Deliv. Rev. 2003;55:1261–1277. doi: 10.1016/S0169-409X(03)00108-X. [DOI] [PubMed] [Google Scholar]
- [46].Qi Y Z, Chilkoti A. Protein-polymer conjugation-moving beyond PEGylation. Curr. Opin. Chem. Biol. 2015;28:181–193. doi: 10.1016/j.cbpa.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Steiert E, Radi L, Fach M, Wich P R. Protein-based nanoparticles for the delivery of enzymes with antibacterial activity. Macromol. Rapid Commun. 2018;39:1800186. doi: 10.1002/marc.201800186. [DOI] [PubMed] [Google Scholar]
- [48].Grozdanovic M, Laffey K G, Abdelkarim H, Hitchinson B, Harijith A, Moon H G, Park G Y, Rousslang L K, Masterson J C, Furuta G T, et al. Novel peptide nanoparticle-biased antagonist of CCR3 blocks eosinophil recruitment and airway hyperresponsiveness. J. Allergy Clin. Immunol. 2019;143:669–680.e12. doi: 10.1016/j.jaci.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li J J, Li B, Sun J, Ma C, Wan S K, Li Y X, Göstl R, Herrmann A, Liu K, Zhang H J. Engineered near-infrared fluorescent protein assemblies for robust bioimaging and therapeutic applications. Adv. Mater. 2020;32:2000964. doi: 10.1002/adma.202000964. [DOI] [PubMed] [Google Scholar]
- [50].Ma C, Li B, Zhang J R, Sun Y, Li J J, Zhou H C, Shen J L, Gu R, Qian J, Fan C H, et al. Significantly improving the bioefficacy for rheumatoid arthritis with supramolecular nanoformulations. Adv. Mater. 2021;33:2100098. doi: 10.1002/adma.202100098. [DOI] [PubMed] [Google Scholar]
- [51].Song Q X, Song H H, Xu J R, Huang J L, Hu M, Gu X, Chen J, Zheng G, Chen H Z, Gao X L. Biomimetic ApoE-reconstituted high density lipoprotein nanocarrier for blood-brain barrier penetration and amyloid beta-targeting drug delivery. Mol. Pharm. 2016;13:3976–3987. doi: 10.1021/acs.molpharmaceut.6b00781. [DOI] [PubMed] [Google Scholar]
- [52].Song Q X, Huang M, Yao L, Wang X L, Gu X, Chen J, Chen J, Huang J L, Hu Q Y, Kang T, et al. Lipoprotein-based nanoparticles rescue the memory loss of mice with Alzheimer’s disease by accelerating the clearance of amyloid-beta. ACS Nano. 2014;8:2345. doi: 10.1021/nn4058215. [DOI] [PubMed] [Google Scholar]
- [53].Kim S K, Foote M B, Huang L. The targeted intracellular delivery of cytochrome C protein to tumors using lipid-apolipoprotein nanoparticles. Biomaterials. 2012;33:3959–3966. doi: 10.1016/j.biomaterials.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lee H J, Park H H, Kim J A, Park J H, Ryu J, Choi J, Lee J, Rhee W J, Park T H. Enzyme delivery using the 30Kc19 protein and human serum albumin nanoparticles. Biomaterials. 2014;35:1696–1704. doi: 10.1016/j.biomaterials.2013.11.001. [DOI] [PubMed] [Google Scholar]
- [55].Jiang Y Y, Lu H X, Chen F, Callari M, Pourgholami M, Morris D L, Stenzel M H. PEGylated albumin-based polyion complex micelles for protein delivery. Biomacromolecules. 2016;17:808–817. doi: 10.1021/acs.biomac.5b01537. [DOI] [PubMed] [Google Scholar]
- [56].Pham D T, Saelim N, Tiyaboonchai W. Alpha mangostin loaded crosslinked silk fibroin-based nanoparticles for cancer chemotherapy. Colloids Surf. B Biointerf. 2019;181:705–713. doi: 10.1016/j.colsurfb.2019.06.011. [DOI] [PubMed] [Google Scholar]
- [57].Pham D T, Saelim N, Cornu R, Béduneau A, Tiyaboonchai W. Crosslinked fibroin nanoparticles: Investigations on biostability, cytotoxicity, and cellular internalization. Pharmaceuticals (Basel) 2020;13:86. doi: 10.3390/ph13050086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wang S H, Xu T, Yang Y H, Shao Z Z. Colloidal stability of silk fibroin nanoparticles coated with cationic polymer for effective drug delivery. ACS Appl. Mater. Interfaces. 2015;7:21254–21262. doi: 10.1021/acsami.5b05335. [DOI] [PubMed] [Google Scholar]
- [59].Kundu J, Chung Y I, Kim Y H, Tae G, Kundu S C. Silk fibroin nanoparticles for cellular uptake and control release. Int. J. Pharm. 2010;388:242–250. doi: 10.1016/j.ijpharm.2009.12.052. [DOI] [PubMed] [Google Scholar]
- [60].Bessa P C, Balmayor E R, Hartinger J, Zanoni G, Dopler D, Meinl A, Banerjee A, Casal M, Redl H, Reis R L, et al. Silk fibroin microparticles as carriers for delivery of human recombinant bone morphogenetic protein-2: In vitro and in vivo bioactivity. Tissue Eng. Part C Methods. 2010;16:937–945. doi: 10.1089/ten.tec.2009.0486. [DOI] [PubMed] [Google Scholar]
- [61].Wang F, Zhang Y Q. Bioconjugation of silk fibroin nanoparticles with enzyme and peptide and their characterization. Adv. Protein Chem. Struct. Biol. 2015;98:263–291. doi: 10.1016/bs.apcsb.2014.11.005. [DOI] [PubMed] [Google Scholar]
- [62].Kim W J, Islam R, Kim B S, Cho Y D, Yoon W J, Baek J H, Woo K M, Ryoo H M. Direct delivery of recombinant Pin1 protein rescued osteoblast differentiation of Pin1-deficient cells. J. Cell. Physiol. 2017;232:2798–2805. doi: 10.1002/jcp.25673. [DOI] [PubMed] [Google Scholar]
- [63].Huang M, Hu M, Song Q X, Song H H, Huang J L, Gu X, Wang X L, Chen J, Kang T, Feng X Y, et al. GM1-modified lipoprotein-like nanoparticle: Multifunctional nanoplatform for the combination therapy of Alzheimer’s disease. ACS Nano. 2015;9:10801. doi: 10.1021/acsnano.5b03124. [DOI] [PubMed] [Google Scholar]
- [64].Zhang Q, Song Q X, Gu X, Zheng M N, Wang A T, Jiang G, Huang M, Chen H, Qiu Y, Bo B, et al. Multifunctional nanostructure RAP-RL rescues Alzheimer’s cognitive deficits through remodeling the neurovascular unit. Adv. Sci. 2021;8:2001918. doi: 10.1002/advs.202001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wang H N, Zou Q, Boerman O C, Nijhuis A W G, Jansen J A, Li Y B, Leeuwenburgh S C G. Combined delivery of BMP-2 and bFGF from nanostructured colloidal gelatin gels and its effect on bone regeneration in vivo. J. Control. Release. 2013;166:172–181. doi: 10.1016/j.jconrel.2012.12.015. [DOI] [PubMed] [Google Scholar]
- [66].Ortiz-Guerrero J M, Polanco M C, Murillo F J, Padmanabhan S, Elías-Arnanz M. Light-dependent gene regulation by a coenzyme B12-based photoreceptor. Proc. Natl. Acad. Sci. USA. 2011;108:7565–7570. doi: 10.1073/pnas.1018972108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wang R, Yang Z G, Luo J R, Hsing I M, Sun F. B12-dependent photoresponsive protein hydrogels for controlled stem cell/protein release. Proc. Natl. Acad. Sci. USA. 2017;114:5912–5917. doi: 10.1073/pnas.1621350114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bessa P C, Machado R, Nürnberger S, Dopler D, Banerjee A, Cunha A M, Rodríguez-Cabello J C, Redl H, Van Griensven M, Reis R L, et al. Thermoresponsive self-assembled elastin-based nanoparticles for delivery of BMPs. J. Control. Release. 2010;142:312–318. doi: 10.1016/j.jconrel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- [69].Pokorski J K, Hovlid M L, Finn M G. Cell targeting with hybrid Qβ virus-like particles displaying epidermal growth factor. ChemBioChem. 2011;12:2441–2447. doi: 10.1002/cbic.201100469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Matsumoto R, Hara R, Andou T, Mie M S Y S, Kobatake E. Targeting of EGF-displayed protein nanoparticles with anticancer drugs. J. Biomed. Mater. Res. B. 2014;102:1792–1798. doi: 10.1002/jbm.b.33162. [DOI] [PubMed] [Google Scholar]
- [71].Li X, Pan C, Sun P, Peng Z H, Feng E L, Wu J, Wang H L, Zhu L. Orthogonal modular biosynthesis of nanoscale conjugate vaccines for vaccination against infection. Nano Res. 2022;15:1645–1653. doi: 10.1007/s12274-021-3713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Raman S, Machaidze G, Lustig A, Aebi U, Burkhard P. Structure-based design of peptides that self-assemble into regular polyhedral nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2006;2:95–102. doi: 10.1016/j.nano.2006.04.007. [DOI] [PubMed] [Google Scholar]
- [73].Pimentel T A P F, Yan Z, Jeffers S A, Holmes K V, Hodges R S, Burkhard P. Peptide nanoparticles as novel immunogens: Design and analysis of a prototypic severe acute respiratory syndrome vaccine. Chem. Biol. Drug Des. 2009;73:53–61. doi: 10.1111/j.1747-0285.2008.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kanekiyo M, Wei C J, Yassine H M, McTamney P M, Boyington J C, Whittle J R R, Rao S S, Kong W P, Wang L S, Nabel G J. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature. 2013;499:102–106. doi: 10.1038/nature12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Marcandalli J, Fiala B, Ols S, Perotti M, De Van Der Schueren W, Snijder J, Hodge E, Benhaim M, Ravichandran R, Carter L, et al. Induction of potent neutralizing antibody responses by a designed protein nanoparticle vaccine for respiratory syncytial virus. Cell. 2019;176:1420–1431.e17. doi: 10.1016/j.cell.2019.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Boyoglu-Barnum S, Ellis D, Gillespie R A, Hutchinson G B, Park Y J, Moin S M, Acton O J, Ravichandran R, Murphy M, Pettie D, et al. Quadrivalent influenza nanoparticle vaccines induce broad protection. Nature. 2021;592:623–628. doi: 10.1038/s41586-021-03365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Deng L, Mohan T, Chang T Z, Gonzalez G X, Wang Y, Kwon Y M, Kang S M, Compans R W, Champion J A, Wang B Z. Double-layered protein nanoparticles induce broad protection against divergent influenza A viruses. Nat. Commun. 2018;9:359. doi: 10.1038/s41467-017-02725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Lizotte P H, Wen A M, Sheen M R, Fields J, Rojanasopondist P, Steinmetz N F, Fiering S. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol. 2016;11:295–303. doi: 10.1038/nnano.2015.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lebel M È, Daudelin J F, Chartrand K, Tarrab E, Kalinke U, Savard P, Labrecque N, Leclerc D, Lamarre A. Nanoparticle adjuvant sensing by TLR7 enhances CD8+ T cellmediated protection from Listeria monocytogenes infection. J. Immunol. 2014;192:1071–1078. doi: 10.4049/jimmunol.1302030. [DOI] [PubMed] [Google Scholar]
- [80].Jovčevska I, Muyldermans S. The therapeutic potential of nanobodies. BioDrugs. 2020;34:11–26. doi: 10.1007/s40259-019-00392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Jiang S B, Hillyer C, Du L Y. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41:355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Xiang Y F, Nambulli S, Xiao Z Y, Liu H, Sang Z, Duprex W P, Schneidman-Duhovny D, Zhang C, Shi Y. Versatile and multivalent nanobodies efficiently neutralize SARS-CoV-2. Science. 2020;370:1479–1484. doi: 10.1126/science.abe4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lee C, Choi M, MacKay J A. Live long and active: Polypeptide-mediated assembly of antibody variable fragments. Adv. Drug Deliv. Rev. 2020;167:1–18. doi: 10.1016/j.addr.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Alam M K, Brabant M, Viswas R S, Barreto K, Fonge H, Ronald Geyer C. A novel synthetic trivalent single chain variable fragment (tri-scFv) construction platform based on the SpyTag/SpyCatcher protein ligase system. BMC Biotechnol. 2018;18:55. doi: 10.1186/s12896-018-0466-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Beck A, Goetsch L, Dumontet C, Corvaïa N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug. Discov. 2017;16:315–337. doi: 10.1038/nrd.2016.268. [DOI] [PubMed] [Google Scholar]
- [86].Govindan S V, Cardillo T M, Sharkey R M, Tat F, Gold D V, Goldenberg D M. Milatuzumab-SN-38 conjugates for the treatment of CD74+ cancers. Mol. Cancer Ther. 2013;12:968–978. doi: 10.1158/1535-7163.MCT-12-1170. [DOI] [PubMed] [Google Scholar]
- [87].Sandland J, Boyle R W. Photosensitizer antibody-drug conjugates: Past, present, and future. Bioconjugate Chem. 2019;30:975–993. doi: 10.1021/acs.bioconjchem.9b00055. [DOI] [PubMed] [Google Scholar]
- [88].Hoffman R M. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat. Rev. Cancer. 2005;5:796–806. doi: 10.1038/nrc1717. [DOI] [PubMed] [Google Scholar]
- [89].Guan X G, Li C, Wang D, Sun W Q, Gai X D. A tumortargeting protein nanoparticle based on Tat peptide and enhanced green fluorescent protein. RSC Adv. 2016;6:9461–9464. doi: 10.1039/C5RA27411G. [DOI] [Google Scholar]
- [90].Matlashov M E, Shcherbakova D M, Alvelid J, Baloban M, Pennacchietti F, Shemetov A A, Testa I, Verkhusha V V. A set of monomeric near-infrared fluorescent proteins for multicolor imaging across scales. Nat. Commun. 2020;11:239. doi: 10.1038/s41467-019-13897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Yu D, Baird M A, Allen J R, Howe E S, Klassen M P, Reade A, Makhijani K, Song Y Q, Liu S M, Murthy Z, et al. A naturally-monomeric infrared fluorescent protein for protein labeling in vivo. Nat. Methods. 2015;12:763–765. doi: 10.1038/nmeth.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Zhang J R, Li B, Zuo J L, Gu R, Liu B, Ma C, Li J J, Liu K. An engineered protein adhesive with properties of tissue integration and controlled release for efficient cartilage repair. Adv. Healthc. Mater. 2021;10:2100109. doi: 10.1002/adhm.202100109. [DOI] [PubMed] [Google Scholar]
- [93].Wang S D, Li B, Zhang H L, Chen J Y, Sun X, Xu J, Ren T, Zhang Y Y, Ma C, Guo W, et al. Improving bioavailability of hydrophobic prodrugs through supramolecular nanocarriers based on recombinant proteins for osteosarcoma treatment. Angew. Chem., Int. Ed. 2021;60:11252. doi: 10.1002/anie.202101938. [DOI] [PubMed] [Google Scholar]
- [94].Su J J, Lu S, Jiang S J, Li B, Liu B, Sun Q N, Li J J, Wang F, Wei Y. Engineered protein photo-thermal hydrogels for outstanding in situ tongue cancer therapy. Adv. Mater. 2021;33:2100619. doi: 10.1002/adma.202100619. [DOI] [PubMed] [Google Scholar]
- [95].Xiao L L, Wang Z L, Sun Y, Li B, Wu B H, Ma C, Petrovskii V S, Gu X Q, Chen D, Potemkin I I, et al. An artificial phase-transitional underwater bioglue with robust and switchable adhesion performance. Angew. Chem., Int. Ed. 2021;60:12082–12089. doi: 10.1002/anie.202102158. [DOI] [PubMed] [Google Scholar]
- [96].Kobayashi M, Squires G R, Mousa A, Tanzer M, Zukor D J, Antoniou J, Feige U, Poole A R. Role of interleukin-1 and tumor necrosis factor α in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52:128–135. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- [97].Nuki G, Bresnihan B, Bear M B, McCabe D. Long-term safety and maintenance of clinical improvement following treatment with anakinra (recombinant human interleukin-1 receptor antagonist) in patients with rheumatoid arthritis: Extension phase of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:2838–2846. doi: 10.1002/art.10578. [DOI] [PubMed] [Google Scholar]
- [98].Shamji M F, Betre H, Kraus V B, Chen J, Chilkoti A, Pichika R, Masuda K, Setton L A. Development and characterization of a fusion protein between thermally responsive elastin-like polypeptide and interleukin-1 receptor antagonist: Sustained release of a local antiinflammatory therapeutic. Arthritis Rheum. 2007;56:3650–3661. doi: 10.1002/art.22952. [DOI] [PubMed] [Google Scholar]
- [99].Cai R, Chen C Y. The Crown and the Scepter: Roles of the protein corona in nanomedicine. Adv. Mater. 2019;31:1805740. doi: 10.1002/adma.201805740. [DOI] [PubMed] [Google Scholar]
- [100].Azizi M, Ghourchian H, Yazdian F, Bagherifam S, Bekhradnia S, Nyström B. Anti-cancerous effect of albumin coated silver nanoparticles on MDA-MB 231 human breast cancer cell line. Sci. Rep. 2017;7:5178. doi: 10.1038/s41598-017-05461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Yousefpour P, Ahn L, Tewksbury J, Saha S, Costa S A, Bellucci J J, Li X H, Chilkoti A. Conjugate of doxorubicin to albumin-binding peptide outperforms aldoxorubicin. Small. 2019;15:1804452. doi: 10.1002/smll.201804452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Schöttler S, Becker G, Winzen S, Steinbach T, Mohr K, Landfester K, Mailänder V, Wurm F R. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nanotechnol. 2016;11:372–377. doi: 10.1038/nnano.2015.330. [DOI] [PubMed] [Google Scholar]
- [103].Zhang Z, Guan J, Jiang Z X, Yang Y, Liu J C, Hua W, Mao Y, Li C, Lu W Y, Qian J, et al. Brain-targeted drug delivery by manipulating protein corona functions. Nat. Commun. 2019;10:3561. doi: 10.1038/s41467-019-11593-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Chen J Q, Qi J, Chen C, Chen J H, Liu L J, Gao R K, Zhang T T, Song L, Ding D, Zhang P, et al. Tocilizumab-conjugated polymer nanoparticles for NIR-II photoacoustic-imaging-guided therapy of rheumatoid arthritis. Adv. Mater. 2020;32:2003399. doi: 10.1002/adma.202003399. [DOI] [PubMed] [Google Scholar]


