Abstract
BACKGROUND:
Bortezomib-based triplet regimens, specifically bortezomib, lenalidomide and dexamethasone (VRD) and bortezomib, cyclophosphamide and dexamethasone (VCD) are the two most common induction regimens used in transplant-eligible patients with NDMM, with conflicting data on comparative efficacy and outcomes in this population.
OBJECTIVES:
We compared long-term outcomes of multiple myeloma (MM) patients receiving VRD vs. VCD induction prior to autologous stem cell transplant (ASCT).
STUDY DESIGN:
Patients registered with Center for International Blood and Marrow Transplant Registry were included if they underwent ASCT for MM from 01/2013 to 12/2018 within 6 months of diagnosis, received VRD or VCD induction and achieved pre-transplant ≥ partial response. Of 1,135 patients, 914 received VRD and 221 received VCD.
RESULTS:
Patients receiving VCD were more likely to have renal impairment and ISS stage III disease and less likely to receive full dose melphalan (200 mg/m2) conditioning (69% vs 80%, p<0.001). Very good partial response rates pre-transplant, post-transplant and at best response in VRD vs. VCD were not significantly different. Maintenance use was more common after VRD (88% vs. 76%, p<0.001) with lenalidomide being the most common agent (80% vs 63%). Patients in the VRD group had higher rates of renal recovery, 74% vs. 43% p<0.001, which may be due to rapid reduction of light chains in the VRD group or improvement in renal function with VCD, which allowed switch over to VRD as patients who switched were classified in the VRD group. Patients receiving VRD had better survival on univariate analysis, with median progression-free survival (PFS) from transplant of 44.6 vs 34.1 months, p=0.004 and 5-year overall survival (OS) of 79% and 60%, p<0.001, respectively. On multivariate analysis there was no significant survival difference, with hazard ratio (VCD vs. VRD induction) for PFS being 1.22 (95% CI: 0.96–1.55, p=0.10) and OS being 1.33 (95% CI: 0.93–1.92, p=0.12). Maintenance use was independently associated with superior PFS and OS, along with ISS stage, cytogenetics and pre-transplant response (PFS only).
CONCLUSIONS:
In patients with MM undergoing upfront transplant after VRD or VCD induction, no independent survival difference was seen based on the induction therapy received after adjusting for other prognostic factors. The use of maintenance treatment was uniformly associated with superior outcomes.
INTRODUCTION:
Novel agent triplet regimens have been associated with improved progression free survival (PFS) and overall survival (OS) in patients with newly diagnosed multiple myeloma (NDMM)(1, 2) and have become standard of care treatment. Amongst the triplet regimens, bortezomib-based triplet regimens, specifically bortezomib, lenalidomide and dexamethasone (VRD) and bortezomib, cyclophosphamide and dexamethasone (VCD) are the two most common induction regimens used in transplant-eligible patients with NDMM. (1, 3–6) VCD is often used in patients with renal failure, given the challenges with use of lenalidomide in patients with fluctuating renal function.(7) Published data comparing outcomes with VCD and VRD induction in phase II trials (8, 9) and retrospective studies (6, 10–12) have shown variable results. It is unclear whether VRD induction has any significant advantage over VCD induction in patients receiving upfront ASCT.
A phase III randomized clinical trial comparing these two regimens is unlikely to be planned and no definitive conclusion can be made regarding the comparative efficacy of VCD vs VRD from current data. Therefore, a larger study is needed to compare these two regimens. The objective of this study was to compare outcomes of patients receiving VRD vs. VCD induction prior to transplant using the CIBMTR (Center for International Blood and Marrow Transplant Research) database. We also aimed to account for key confounding variables that may impact choice of induction regimen or impact survival outcomes, such as renal failure and post-transplant maintenance. (13–15).
METHODS:
The Center for International Blood and Marrow Transplant Research® (CIBMTR®) registry was used to identify patients and collect data. The CIBMTR registry is a prospectively maintained registry of patients undergoing autologous and allogeneic stem cell transplant.(16) For a sub-group of patients obtained using a weighted randomization algorithm,(17) detailed case report forms are reported to CIBMTR, which capture demographics, co-morbidities, laboratory data, disease characteristics, treatment details (including induction and maintenance therapy) and outcomes. Studies using CIBMTR registry data are conducted under approved by the Institutional Review Boards of the Medical College of Wisconsin, Milwaukee and National Marrow Donor Program, Minneapolis.
Patients were included in this study if they underwent upfront ASCT for MM from January 2013 to December 2018 and within six months of myeloma diagnosis, received VRD or VCD induction therapy and achieved at least a partial response prior to transplant. Induction therapy was coded in a hierarchical manner, and any patients who started on VD or VCD and switched to VRD induction before transplant were included in the VRD group. Additional inclusion criteria included use of melphalan only conditioning for ASCT and at least 3 months of follow-up for alive patients. Overall, 1,135 patients met inclusion criteria (Supplementary data: consort flow diagram), of which 914 patients received VRD induction and 221 patients received VCD induction. Two-year follow-up data were available in over 90% of patients.
High-risk cytogenetics were defined as presence of at least one of the following abnormalities: t(4;14), t(14;16), t(14;20), deletion 17p, gain or amplification of 1q (18) on FISH and/or conventional cytogenetics. Patients were categorized by International Staging System (ISS) as previously described.(19) Hematopoietic Cell Transplant Comorbidity Index (HCT-CI) scores were evaluated.(20) Renal-adjusted HCT-CI scores were used as described before,(7) which excluded renal co-morbidity as renal insufficiency was studied separately as a covariate. Renal insufficiency was defined based on estimated glomerular filtration rate (eGFR). Patients were categorized as having moderate to severe renal impairment if eGFR was < 60 ml/min/1.73 m2 or normal renal function/mild impairment if eGFR was ≥ 60 ml/min/1.73 m2. Response and disease progression were defined per International Myeloma Working Group criteria. (21)
Univariable analysis for categorical variables were carried out using chi-square tests and for continuous variables using Wilcoxon rank-sum test/Kruskal Wallis test. Survival analysis was done using Kaplan Meier method, and log-rank test was used to compare survival curves. The cumulative incidence of relapse/progression was estimated using the cumulative incidence function and tested using Gray’s test, accounting for death without preceding relapse/progression as competing risk. Estimates of outcomes were reported with 95% confidence interval (CI). PFS was defined as time from ASCT to progression or death and OS was defined as time from ASCT to death or last follow-up. Cox proportional hazards models were created for multivariable survival analysis. Hazard ratios (HR) with 95% confidence intervals (CI) are reported. The following covariates were considered in the multivariable models: induction regimen (main effect), age, sex, race, performance score, renally adjusted HCT-CI score, eGFR at diagnosis, immunoglobulin subtype, cytogenetics, ISS stage, response status at transplant, conditioning melphalan dose, and maintenance therapy. A step-wise approach was used to identify the variables to be included in the final model. All p-values were 2-sided and the difference between two variables was consider significant if p<0.05.
RESULTS:
Amongst patients who met the inclusion criteria, 914 patients received VRD induction and 221 patients received VCD induction prior to undergoing upfront ASCT. As shown in Table 1, patients in the VRD and VCD cohorts had similar age (median age: 60.3 and 61.4 years) and sex distribution (males: 55% and 54%). Patients in the VRD group were more likely to be African American (30% vs 19%, p=0.004). Patients in both groups had a similar performance status at diagnosis. Karnofsky performance status ≥ 90 was seen in 51% and 59% of patients in the VRD and VCD cohorts, p=0.09. There was no difference in renal-adjusted HCT-CI scores (score of ≥ 2: 55% and 49%, p=0.74), and distribution of high-risk cytogenetics (37% and 35%, p=0.89) amongst patients in VRD and VCD groups, respectively. Patients in the VCD group were more likely to have renal impairment, ISS stage III disease and light chain myeloma. Moderate to severe renal insufficiency at diagnosis with an eGFR < 60 mL/min/1.73m2 was seen in 26% of patients receiving VRD vs. 48% of patients receiving VCD induction, p=<0.001. Similarly, ISS stage III was seen in 17% vs. 34% (p=<0.001) of patients, respectively. Light chain myeloma was seen in 19% of patients in the VRD group and 27% patients in the VCD group, p=0.07. We also observed a change over time, with use of VRD therapy becoming more frequent from 2013 to 2018 compared to VCD therapy. (Supplementary Table 1) Black or African American patients were more likely to receive VRD therapy, likely related to the fact that Black/African American patients in our cohort were younger and less likely to have renal impairment. (Supplementary Table 2)
Table 1.
Patient and disease related characteristics in patients receiving VRD and VCD induction therapy before autologous transplant for newly diagnosed multiple myeloma
| Variable | VRD(N=914; 80.5%) Median (range) or N (%) |
VCD (N=221;19.5%) Median (range) or N (%) |
P-value |
|---|---|---|---|
|
| |||
| Age, years | 60 (30 – 82) | 61 (32 – 79) | 0.06 |
|
| |||
| Sex, Male | 503 (55) | 119 (54) | 0.75 |
|
| |||
| Self-reported race | 0.004 | ||
| Caucasian | 576 (63) | 162 (73) | |
| African-American | 278 (30) | 41 (19) | |
| Other/Missing | 60 (6) | 18 (8) | |
|
| |||
| Karnofsky performance score, ≥90% | 470 (51) | 131 (59) | 0.09 |
|
| |||
| Renal adjusted HCT-CI Score # | 0.74 | ||
| 0 | 279 (31) | 77 (35) | |
| 1 | 136 (15) | 35 (16) | |
| 2+ | 498 (55) | 109 (49) | |
|
| |||
| International Staging System (ISS) | <0.001 | ||
| I | 296 (32) | 49 (22) | |
| II | 309 (34) | 52 (24) | |
| III | 158 (17) | 76 (34) | |
| Missing | 151 (17) | 44(20) | |
|
| |||
| Cytogenetics | 337 (37) | 78 (35) | 0.89 |
| High-risk [t(4;14), t(14;16), t(14;20), del17p, +1q, HR2] | |||
| Standard risk, including normal | 544 (60) | 134 (61) | |
| Missing/not done | 33 (4) | 9 (4) | |
|
| |||
| Myeloma subtype | 0.07 | ||
| IgG | 515 (56) | 106 (48) | |
| IgA | 210 (23) | 52 (24) | |
| Light chain myeloma | 172 (19) | 60 (27) | |
| Non-secretory/other | 17 (2) | 3 (1) | |
|
| |||
| eGFR at diagnosis, <60 mL/min/1.73m2: ## | 241 (26) | 105 (48) | <0.001 |
|
| |||
| Serum creatinine at diagnosis, ≥ 2 mg/dl | 74 (8) | 64 (29) | <0.001 |
|
| |||
| eGFR prior to HCT, < 60 mL/min/1.73m2 ## | 78 (9) | 67 (30) | <0.001 |
|
| |||
| Serum creatinine prior to transplant, ≥ 2 mg/dl | 14 (2) | 26 (12) | <0.001 |
|
| |||
| Pre-transplant response | 0.21 | ||
| CR | 152 (17) | 38 (17) | |
| VGPR | 438 (48) | 92 (42) | |
| PR | 324 (35) | 91 (41) | |
|
| |||
| Pre-transplant response, ≥ VGPR | 590 (65) | 130 (59) | 0.11 |
Table Abbreviations: CR: complete response, eGFR: estimated glomerular filtration rate, PR: partial response, VCD: bortezomib, cyclophosphamide and dexamethasone, VGPR: very good partial response, VRD: bortezomib, lenalidomide and dexamethasone.
Renal HCT-CI score missing in one patient in the VRD group.
eGFR at diagnosis missing in 59 patients (6%) in VRD group and 18 (8%) in VCD group. eGFR prior to HCT missing in 1 patient in VRD group (0%) and 3 patients in VCD group. Therefore numbers do not add to 100%.
Treatment and Response:
Median cycles of induction therapy was similar in both groups at 4, p=0.30. (missing in n=138). As shown in Table 1, pre-transplant response in the VRD cohort was: complete response (CR)- 17%, very good partial response (VGPR)- 48% and partial response (PR)- 35%. Response in the VCD cohort was: CR- 17%, VGPR- 42% and PR- 41%. Rates of VGPR or better response pre-transplant in the VRD vs VCD group were 65% vs. 59%, p=0.11. Table 2 describes transplant and post-transplant differences between the two groups. A higher proportion of patients in the VRD group received full dose melphalan conditioning (melphalan 200 mg/m2) compared to the VCD group, 80% vs. 69%, p=<0.001. Rates of VGPR or better response post-transplant in the VRD and VCD group at day 100 and best response were similar at 74% vs. 75%, p=0.47 and 85% vs. 89%, p=0.17, respectively. Post-transplant CR rates in the VRD and VCD groups were 63% vs. 57%, p=0.07, respectively.
Table 2:
Transplant and maintenance in patients receiving VRD and VCD induction therapy before autologous transplant for newly diagnosed multiple myeloma
| Variable | VRD(N=914, 80.5%) Median (range) or N (%) |
VCD (N=221,19.5%) Median (range) or N (%) |
P-value |
|---|---|---|---|
|
| |||
| Conditioning melphalan dose, 200 mg/m2 | 732 (80) | 152 (69) | <0.001 |
|
| |||
| Post-transplant response (day 100)## | 0.07 | ||
| CR | 404 (45) | 81 (38) | |
| VGPR | 277 (31) | 85 (39) | |
| PR | 181 (20) | 38 (18) | |
| SD or worse | 40 (5) | 12 (6) | |
|
| |||
| Post-transplant response, ≥ VGPR | 681(76) | 166 (77) | 0.68 |
|
| |||
| Best response to transplant## | 0.07 | ||
| CR | 571 (63) | 125 (57) | |
| VGPR | 213 (23) | 70 (32) | |
| PR | 101 (11) | 21 (10) | |
| SD or worse | 26 (3) | 4 (2) | |
|
| |||
| Best response, ≥ VGPR | 784 (86) | 195 (89) | 0.31 |
|
| |||
| Maintenance Therapy, Yes | 806 (88) | 169 (76) | <0.001 |
|
| |||
| Maintenance Regimen# | <0.001 | ||
| Lenalidomide based (+/−bortezomib) | 732 (80) | 139 (63) | |
| Bortezomib (+/− other) | 50 (5) | 25 (11) | |
| Other (including carfilzomib) | 24 (3) | 4 (1) | |
| None | 108 (12) | 52 (24) | |
|
| |||
| Response to Maintenance### | 0.46 | ||
| CR | 455 (60) | 84 (54) | |
| VGPR | 166 (22) | 40 (25) | |
| PR | 73 (10) | 15 (10) | |
| SD or worse | 59 (8) | 16 (11) | |
Table Abbreviations: CR: complete response, PR: partial response, SD: stable disease, VCD: bortezomib, cyclophosphamide and dexamethasone, VGPR: very good partial response, VRD: bortezomib, lenalidomide and dexamethasone.
Data missing for one patient in the VCD group
Missing response (best response: 4 patients, day 100 response: 17patients, maintenance response: 67 patients)
Not applicable (no maintenance): 160 patients
Maintenance Therapy:
The majority of patients received maintenance therapy, though maintenance was more common in patients receiving VRD induction (VRD: 88%, VCD: 76%, p <0.001), with lenalidomide based maintenance being most frequently used regimen. The median time from transplant to start of maintenance therapy was 4 months (inter-quartile range 2.99–4.34 months) in both groups. Maintenance in the VRD group was as follows- lenalidomide based (+/− bortezomib): 80% (n=732), bortezomib based: 5% (n=50), other: 3% (n=24) and no maintenance in 12% of patients. In the VCD group, maintenance was as follows- lenalidomide based: 63% (n=139), bortezomib based: 11% (n=25), other- 1% (n=4) and no maintenance: 24% (n=52). Response to maintenance therapy in the VRD vs. VCD group was VGPR or better in 68% vs. 56% of patients, p 0.66, respectively. In the no maintenance group, 18 patients reported progression before the first 4 months after transplant.
Renal function recovery kinetics:
We evaluated recovery of renal function from diagnosis to ASCT as shown in Table 3. This was defined as improvement of renal function at diagnosis from moderate/severe renal dysfunction (eGFR < 60 ml/min/1.73m2) to mild impairment/normal renal function (eGFR ≥ 60 ml/min/1.73m2) before transplant. Paired data on renal function was available in 1,040 patients, of whom 344 patients had at least moderate renal impairment (eGFR<60) at baseline. Median (range) eGFR at diagnosis in the VRD and VCD groups was 75.7 (2.2–56.5) ml/min and 56.5 (2.3–167.3) ml/min (p<0.001), while eGFR prior to transplant in the two cohorts was 95.6 (7.8–276.7) ml/min and 82.0 (5.2–191.7) ml/min, respectively (p<0.001). Amongst patients with renal dysfunction (eGFR < 60) at diagnosis, median eGFR in the VRD vs. VCD group was 45 ml/min and 22 ml/min, respectively, p <0.001. Overall, 64% (222/344) of patients experienced improvement in renal function, including 74% in the VRD group and 43% in the VCD group, p=<0.001. There was no difference in receipt of maintenance therapy based on renal function recovery. Maintenance therapy was given in 86% (191/222) of patients with renal function improvement (eGFR increased from < 60 to ≥ 60 ml/min), which was similar to rates of maintenance in patients who had eGFR ≥ 60 at both time points (86%, 602/696), p=0.86. Amongst patients who did not recover renal function, maintenance was given in 81% (99/122) of patients.
Table 3:
Renal Function Recovery Kinetics (Renal recovery is defined as improvement in eGFR at diagnosis from < 60 ml/min to > = 60 ml/min at pre-transplant)
| VRD N=845 N (%) | VCD N=195 N (%) | Overall N=1040 N (%) | |
|---|---|---|---|
| Diagnosis eGFR ≥ 60 ml/min and pre-transplant eGFR ≥ 60 ml/min (Normal Renal Function) | 604 (71.5%) | 92 (47%) | 696 (67%) |
| Diagnosis eGFR < 60 ml/min and pre-transplant eGFR ≥ 60 ml/min (Recovered Renal Function) | 178 (21%) | 44 (23%) | 222 (21%) |
| Diagnosis eGFR < 60 ml/min and pre-transplant eGFR < 60 ml/min (Non-recovered Renal Function) | 63 (7.5%) | 59 (30%) | 122 (12%) |
Table Abbreviations: eGFR: Estimated glomerular filtration rate
Survival:
Median follow-up of survivors in the VRD group was 25 months (range: 3–82) and that in the VCD group was 38 months (range: 3–77). On unadjusted analysis, patients receiving VRD induction had superior outcomes compared to VCD induction, with median PFS from transplant of 44.6 (95% CI: 38.0–55.8) months vs 34.1 ( 95% CI: 25.8–44.7) months, p=0.004 respectively. (Figure 1) Median OS was not reached in either group, with 5-year OS being 79% in the VRD cohort and 60% in the VCD cohort, p <0.001. Univariable PFS and OS outcomes at 2 and 5 years by induction regimen are shown in Table 4. We further evaluated survival outcomes after excluding 52 patients who were initially started on VD/VCD and switched to VRD. Outcomes after excluding these patients were similar to that observed in the entire group, with better PFS and OS observed in the VRD group on unadjusted analysis, as shown in Supplementary Table 3. We also analyzed survival outcomes based on renal function at diagnosis, as shown in Table 5 and Figure 2. In patients with eGFR ≥ 60, patients in the VRD group had a longer PFS than those receiving VCD. Median PFS from transplant in the VRD vs. VCD group for patients with eGFR ≥ 60 was: 42.1 vs 31.6 months, p=0.003. Median OS was not reached in either group, p=0.075. At 5 years, OS in the VRD vs VCD patients with eGFR > 60 was 78% vs 61%, p=0.017. In patients with eGFR < 60, there was no difference in the median PFS in the VRD vs VCD group, : 48.7 vs 38.5 months, p=0.555. OS was superior in patients with eGFR < 60 who received VRD (p=0.042). 5-year OS was 84% vs 60%, p=0.008.
Figure 1:
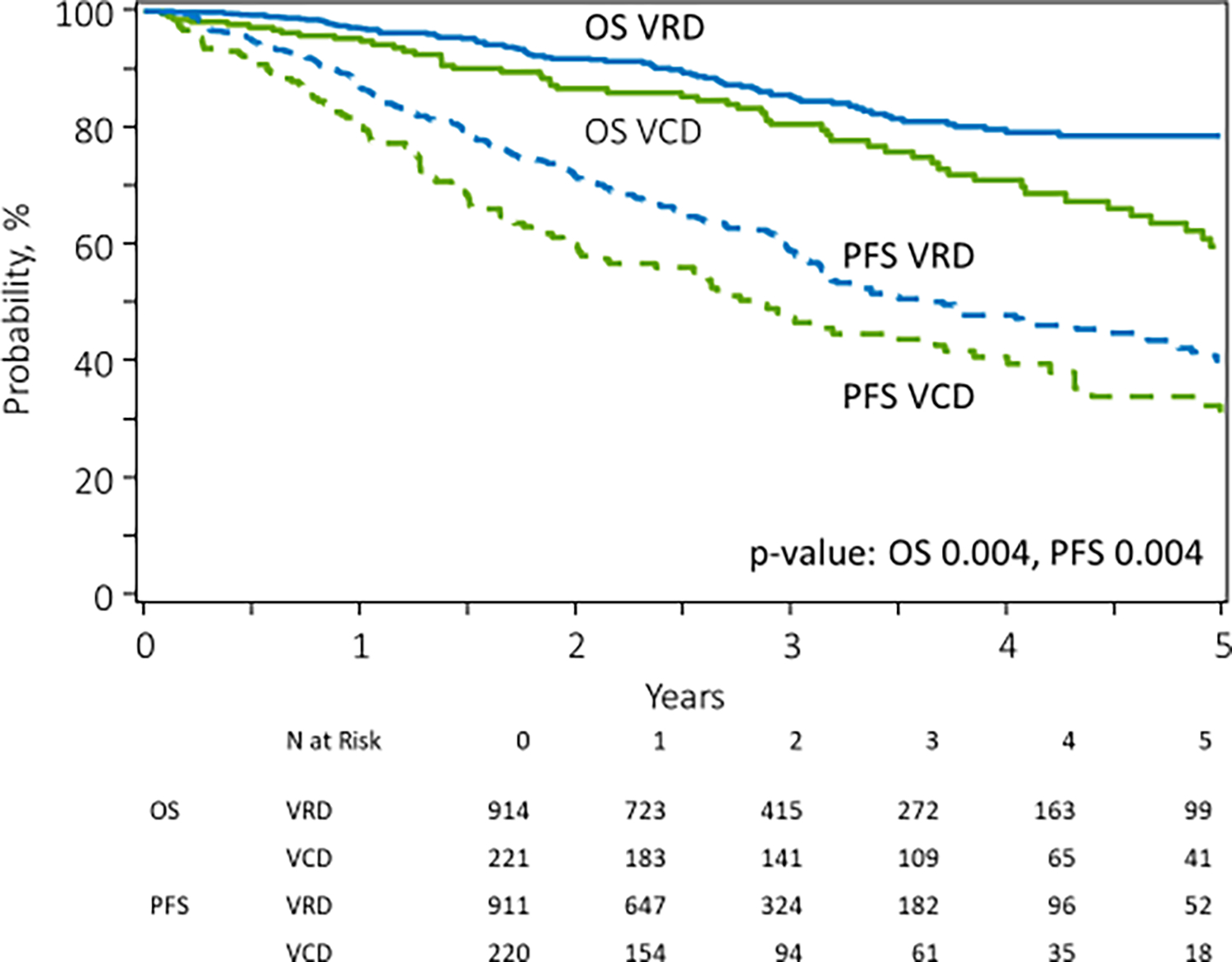
Unadjusted progression free survival and overall survival in patients receiving VRD (N=914, 80.5%) vs VCD (N=221, 19.5%) induction therapy for newly diagnosed multiple myeloma.
Table 4.
Univariate survival outcomes in patients receiving VCD and VRD induction. Probabilities with 95% confidence intervals are shown
| VRD, (n=914;80.5%) | VCD, (n=221; 19.5%) | p-value | |
|---|---|---|---|
|
| |||
| PFS (%) | 0.004 | ||
| 2-year | 72 (68–75) | 60 (53–67) | 0.004 |
| 5-year | 40 (34–46) | 32 (24–41) | 0.152 |
|
| |||
| OS (%) | 0.004 | ||
| 2-year | 92 (90–94) | 87 (81–91) | 0.056 |
| 5-year | 79 (74–83) | 60 (50–69) | <0.001 |
Table Abbreviations: OS: overall survival, PFS: progression free survival, VCD: bortezomib, cyclophosphamide and dexamethasone VRD: bortezomib, lenalidomide and dexamethasone.
Multi-variate Z test based on the pointwise estimates and standard errors was used to calculate P-value at each timepoint. Overall P-value for PFS and OS calculated using Log Rank Test
Table 5.
Univariate survival outcomes in patients receiving VCD and VRD induction, stratified by eGFR. Probabilities with 95% confidence intervals are shown
| VRD, eGFR≥60 (n=614) | VRD, eGFR<60 (n=241) | VCD, eGFR≥60 (n=98) | VCD, eGFR<60 (n=105) | p-value | |
|---|---|---|---|---|---|
|
| |||||
| PFS (%) | 0.021 | ||||
| 2-year | 73 (68–77) | 71 (64–78) | 57 (46–67) | 64 (53–74) | 0.033 |
| 5-year | 41 (33–48) | 39 (27–51) | 28 (17–40) | 39 (25–53) | 0.342 |
|
| |||||
| OS (%) | 0.053 | ||||
| 2-year | 92 (89–95) | 93 (89–96) | 90 (83–95) | 85 (76–92) | 0.250 |
| 5-year | 78 (72–84) | 84 (76–90) | 61 (48–73) | 60 (44–75) | 0.003 |
Table Abbreviations: OS: overall survival, PFS: progression free survival, VCD: bortezomib, cyclophosphamide and dexamethasone VRD: bortezomib, lenalidomide and dexamethasone.
Figure 2:
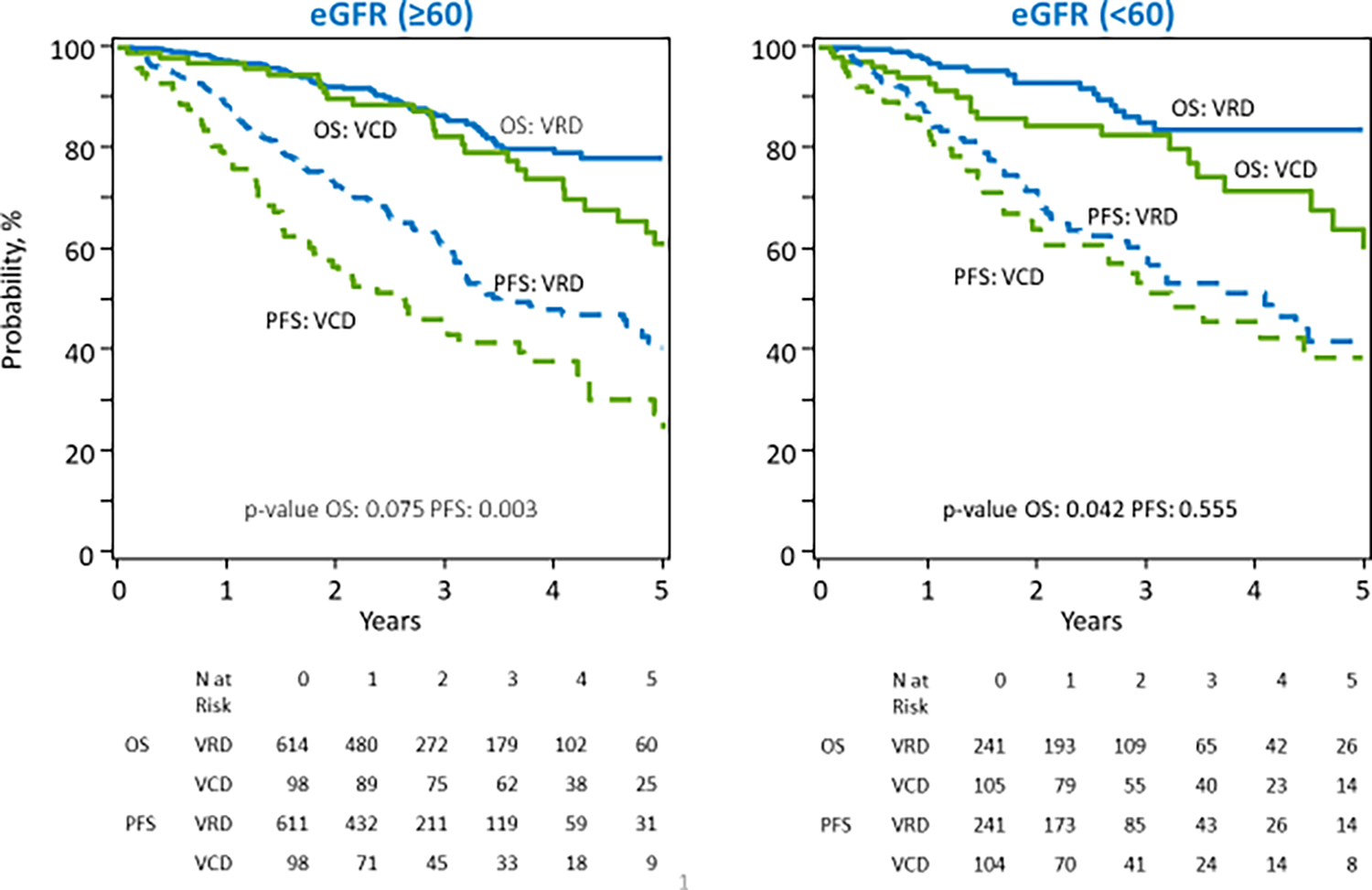
Unadjusted progression free survival and overall survival in patients receiving VRD vs VCD induction therapy by eGFR at diagnosis for newly diagnosed multiple myeloma.
Multivariable survival analysis is shown in Table 6. Variables considered in the multivariable analysis are described under the methods section. Using a step-wise approach, the following variables were included: induction regimen, ISS stage, cytogenetics, maintenance therapy and pre-transplant response. After adjusting for these variables in this model, there was no statistically significant difference between the two induction regimens, with hazard ratio (HR) for PFS in the VCD vs VRD group being 1.20 (95% CI: 0.95–1.53, p=0.13). ISS stage, cytogenetics and maintenance were independent prognostic factors for PFS. Similarly, on multivariable analysis for OS, there was no statistically significant difference in the two groups, with HR for OS in the VCD vs. VRD group being 1.33 (95% CI: 0.92–1.9, p=0.13). ISS stage, cytogenetics and maintenance were independent prognostic factors for OS. Specifically, hazard ratio for maintenance no vs. yes was as follows, PFS: 1.74, 95% CI: 1.33–2.28, p<0.001 and OS: 2.28, 95% CI: 1.53–3.38, p <0.0001. We also conducted a sub-group analysis in patients receiving full dose melphalan 200 mg/m2 conditioning (N=821). (Table 7) Results were similar, and there was no statistically significant difference between the two induction regimens with HR for PFS in the VCD vs VRD group being 1.20 (0.91–1.57), p=0.19 and HR for OS in the VCD vs VRD group being 1.24 (0.81–1.88), p=0.32.
Table 6.
Multivariate analysis of survival outcomes in patients receiving VRD and VCD induction therapy. Hazard ratios with 95% confidence intervals are shown
| Outcomes | N | HR (95% CI) | P-value |
|---|---|---|---|
| Progression free survival | |||
| Induction Therapy | 0.13 | ||
| VRD | 852 | Reference | |
| VCD | 202 | 1.20 (0.95–1.53) | 0.13 |
| ISS Stage at Diagnosis | 0.001 | ||
| Stage I | 330 | Reference | |
| Stage II | 346 | 1.20 (0.90–1.59) | 0.22 |
| Stage III | 220 | 1.81 (1.34–2.46) | <0.001 |
| Missing | 158 | 1.41 (1.00–1.97) | 0.047 |
| Cytogenetics | <0.001 | ||
| No Abnormality | 180 | Reference | |
| High Risk | 393 | 1.45 (1.07–1.99) | 0.02 |
| Standard Risk | 447 | 0.87 (0.64–1.18) | 0.36 |
| Missing | 34 | 1.44 (0.86–2.42) | 0.17 |
| Disease Status Prior to Transplant | <0.001 | ||
| sCR/CR | 175 | Reference | |
| VGPR | 499 | 1.23 (0.88–1.73) | 0.23 |
| PR | 380 | 1.79 (1.28–2.51) | 0.001 |
| Maintenance Therapy | <0.001 | ||
| Yes | 904 | Reference | |
| No | 150 | 1.74 (1.33–2.28) | <0.001 |
| Overall survival | |||
| Induction Therapy | 0.13 | ||
| VRD | 852 | Reference | |
| VCD | 202 | 1.33 (0.92–1.9) | 0.13 |
| ISS Stage at Diagnosis | 0.002 | ||
| Stage I | 330 | Reference | |
| Stage II | 346 | 1.31 (0.80–2.14) | 0.29 |
| Stage III | 220 | 2.25 (1.36–3.72) | 0.002 |
| Missing | 158 | 2.26 (1.31–3.89) | 0.003 |
| Cytogenetics | <0.001 | ||
| No Abnormality | 180 | Reference | |
| High Risk | 393 | 2.21 (1.33–3.66) | 0.002 |
| Standard Risk | 447 | 0.80 (0.46–1.40) | 0.44 |
| Missing | 34 | 2.01 (0.94–4.27) | 0.07 |
| Disease Status Prior to Transplant | 0.053 | ||
| sCR/CR | 175 | Reference | |
| VGPR | 499 | 1.12 (0.65–1.93) | 0.68 |
| PR | 380 | 1.66 (0.97–2.84) | 0.06 |
| Maintenance Therapy | <0.001 | ||
| Yes | 904 | Reference | |
| No | 150 | 2.28 (1.53–3.38) | <0.001 |
The following covariates were considered in the multivariate models: induction regimen (main effect), age, sex, race, performance score, hematopoietic cell transplant-comorbidity index (HCT-CI), estimated glomerular filtration rate (eGFR) at diagnosis, immunoglobulin subtype, cytogenetics, ISS stage, response status at transplant, melphalan dose, and maintenance therapy. A step wise approach was used to narrow down the variables in the model
Table Abbreviations: CI: confidence interval, CR: complete response, HR: hazard ratio, PR: partial response, sCR: stringent complete response, VCD: bortezomib, cyclophosphamide and dexamethasone, VGPR: very good partial response, VRD: bortezomib, lenalidomide and dexamethasone.
Table 7:
Multivariate analysis in subgroup of patients receiving full dose melphalan conditioning, 200 mg/m2 (N=821)
| Outcomes | N | HR (95% CI) | p-value |
|---|---|---|---|
| Progression Free Survival | |||
| Induction therapy | 0.19 | ||
| VRD | 682 | Reference | |
| VCD | 139 | 1.20 (0.91–1.57) | 0.19 |
| ISS stage at diagnosis | 0.001 | ||
| Stage I | 268 | Reference | |
| Stage II | 278 | 1.20 (0.88–1.64) | 0.26 |
| Stage III | 152 | 1.93 (1.38–2.70) | <0.001 |
| Missing | 123 | 1.40 (0.97–2.03) | 0.08 |
| Cytogenetics | 0.0541 | ||
| No Abnormality | 147 | Reference | |
| High risk | 313 | 1.31 (0.93–1.85) | 0.12 |
| Standard risk | 334 | 0.92 (0.66–1.30) | 0.65 |
| Test not done/ Unknown | 27 | 1.37 (0.78–2.39) | 0.27 |
| Disease status prior to transplant | 0.001 | ||
| sCR/CR | 140 | Reference | |
| VGPR | 384 | 1.43 (0.97–2.09) | 0.07 |
| PR | 297 | 1.99 (1.36–2.92) | <0.001 |
| Maintenance therapy | 0.001 | ||
| Yes | 702 | Reference | |
| No | 119 | 1.66 (1.23–2.22) | 0.001 |
| Overall Survival | |||
| Induction therapy | 0.32 | ||
| VRD | 682 | Reference | |
| VCD | 139 | 1.24 (0.81–1.88) | 0.32 |
| ISS Stage at diagnosis | 0.006 | ||
| Stage I | 268 | Reference | |
| Stage II | 278 | 1.20 (0.69–2.09) | 0.53 |
| Stage III | 152 | 2.34 (1.33–4.10) | 0.003 |
| Missing | 123 | 2.09 (1.12–3.90) | 0.02 |
| Cytogenetics | 0.001 | ||
| No Abnormality | 147 | Reference | |
| High risk | 313 | 2.45 (1.34–4.48) | 0.004 |
| Standard risk | 334 | 1.05 (0.55–1.99) | 0.89 |
| Test not done/ Unknown | 27 | 1.96 (0.82–4.65) | 0.13 |
| Disease status prior to transplant | 0.009 | ||
| sCR/CR | 140 | Reference | |
| VGPR | 384 | 1.54 (0.78–3.01) | 0.21 |
| PR | 297 | 2.46 (1.27–4.78) | 0.008 |
| Maintenance therapy | <0.001 | ||
| Yes | 702 | Reference | |
| No | 119 | 2.44 (1.58–3.77) | <0.001 |
The following covariates were considered in the multivariate models: induction regimen (main effect), age, sex, race, performance score, hematopoietic cell transplant-comorbidity index (HCT-CI), estimated glomerular filtration rate (eGFR) at diagnosis, immunoglobulin subtype, cytogenetics, ISS stage, response status at transplant and maintenance therapy. A step wise approach was used to narrow down the variables in the model
Table Abbreviations: CI: confidence interval, CR: complete response, HR: hazard ratio, PR: partial response, sCR: stringent complete response, VCD: bortezomib, cyclophosphamide and dexamethasone, VGPR: very good partial response, VRD: bortezomib, lenalidomide and dexamethasone.
DISCUSSION
In patients with MM undergoing upfront transplant, VRD induction was associated with longer PFS and OS on univariable analysis. Importantly, however, there was no difference in VGPR or better response rates in both groups pre- or post-transplant and survival outcomes were similar after adjusting for key prognostic factors. The use of maintenance treatment was uniformly associated with superior outcomes.
Severe or moderate renal impairment at diagnosis was more common in the VCD group compared to patients receiving VRD induction, which aligns with a higher proportion of patients with ISS stage III in this group due to decreased renal clearance of beta-2-microglobulin and a higher proportion of patients with light chain myeloma, which increases the likelihood of cast nephropathy.(22, 23) This is similar to findings seen in a comparativeness effective study of VRD vs. VCD induction in NDMM patients, where patients in the VCD group were more likely to have renal dysfunction.(24) While dose adjusted lenalidomide can be given with renal dysfunction,(25, 26) it can be challenging to administer lenalidomide with fluctuating renal function and therefore physicians may elect to start VCD in such patients. It has been previously shown that PFS and OS outcomes in patients with moderate to severe renal dysfunction undergoing ASCT are similar to that observed in patients with normal renal function, and other factors such as maintenance can independently impact survival outcomes in this population.(7) We noted higher renal improvement rates in the VRD group compared to the VCD group. This could be due to two reasons. The first being that patients who show improvement on VCD are often transitioned to VRD therapy, and such patients were categorized in the VRD group in our study. Second, it is possible that achieving a rapid response with VRD therapy can result in higher likelihood of renal recovery. Significant out of pocket costs for lenalidomide for some patients can also play a role in treatment selection and that may have contributed to choice of triplet induction in some patients.
Previous studies comparing VRD and VCD induction or similar regimens have found differing results. In the IFM 2014–04 trial comparing four cycles of VTD (bortezomib, thalidomide and dexamethasone) induction to VCD induction, rates of VGPR or better response were higher in the VTD arm, 66% vs 56%, p=0.05.(8) Post-transplant response or survival data from this trial are not yet available. The EVOLUTION trial was a randomized phase II trial of VCD, VRD and VCRD quadruplet therapy in both transplant-eligible and ineligible patients.(9) In contrast to findings from the IFM 2013–04 trial, there was no difference in response rates or one year PFS in the VRD or VCD groups.(9) Some institutional retrospective studies have demonstrated similar response rates and survival outcomes with VCD vs. VRD induction, while others have demonstrated that VRD induction is associated with deeper response and survival.(6, 10–12, 24) It is important to note that maintenance use was infrequent in studies reporting superior outcomes with VRD over VCD induction therapy. Uttervall et al. reported superior outcomes with VRD therapy, however, only 8% patients received maintenance in their cohort.(12) Chakraborty et al. observed that VRD induction may be associated with superior OS after controlling for baseline prognostic factors. However, only 20% of patients received maintenance therapy in that study(10), likely because the role of maintenance was not established in the era during which most patients were treated. Kumar et al. reported data from a randomized trial of 125 patients receiving VRD or VCD induction conducted in India.(27) The primary endpoint was VGPR rate after 4 cycles of treatment and there was a trend towards superiority in the VRD arm (61.5 vs 48.3%, p=0.09). CR rates were also higher in the VRD arm (35.4 vs 18.3, p<0.02). Survival data was not reported.
In this current study, VGPR or better response rates after induction were similar in the VRD and VCD cohorts. (65% vs 59%, p=0.11). Response rates in our study are comparable to prior reports where VRD induction has been associated with VGPR rates of 45% to 70%.(3, 4, 10, 28, 29) Similarly, VCD induction has been associated with VGPR rates of 40% to 61%.(5, 8–10, 24, 30) Patients with MM spend the longest therapeutic period in the maintenance phase. Therefore, it is not surprising to see that maintenance was more prognostic than choice of induction therapy. Our findings of greater impact of maintenance therapy over induction therapy are in line with previous reports. In a CIBMTR study evaluating impact of doublet or triplet novel induction regimens in patients undergoing transplant from 2008 to 2013, post-transplant maintenance was noted to have a greater impact on disease outcomes than doublet vs. triplet induction therapy.(31) Paquin et al. also reported that choice of induction therapy did not impact OS in patients with NDMM.(6) Prospective trials have demonstrated that maintenance therapy with lenalidomide is associated with improved PFS and OS in MM.(15) A recent long-term follow-up update of the STAMINA trial, a BMT CTN multicenter phase 3 trial, demonstrated inferior PFS in patients who discontinued lenalidomide maintenance at 38 months post-transplant, underlining the role of long-term continuous maintenance.(32) It is reassuring to see that VCD induction was not an independent predictor of inferior PFS in our study, likely due to the eventual transition to maintenance therapy in the majority of patients. Renal function improved in two-thirds of patients following induction therapy and lenalidomide-based therapy was most common maintenance therapy. We did not observe a difference in rates of maintenance based on recovery of renal function. Our findings are different from that seen in the FORTE trial, where the cohort of patients receiving KRD + transplant had superior outcomes compared to the group receiving KCD + transplant, including deeper response rates and better PFS.(33) There could be several reasons why our results differ from the FORTE trial, including (i) exclusion of patients in our study who had primary refractory disease i.e those who did not achieve at least a partial response and (ii) inclusion of only fit, younger patients with normal renal function in the FORTE trial, whereas our ‘real-world’ study population included all patients, including those with impaired renal function.
At present, VRD induction remains the standard of care induction choice in patients with NDMM, and its role was further cemented with the findings from the ENDURANCE randomized clinical trial,(34) which demonstrated similar PFS in patients receiving VRD vs. KRD (carfilzomib, lenalidomide and dexamethasone) induction therapy, despite deeper responses in the KRD group. Daratumumab-based quadruplets are being investigated in clinical trials.(8, 35) It remains to be seen whether daratumumab-VRD will become a standard of care over VRD in the future based on long term follow-up data. Taken together with available evidence, our findings suggest that lenalidomide exposure is important in optimizing survival outcomes in NDMM. If patients are started on VCD therapy, maintenance therapy, typically lenalidomide based maintenance should be considered following transplant.
Our study has the advantage of comparing outcomes with VRD and VCD induction in large cohort of patients undergoing transplant in the current era. However, given the retrospective design, there are inherent limitations. We observed clear confounding differences between the two groups, with impaired renal function and lower rates of maintenance therapy being the most obvious differences. We attempted to account for these through multivariable analysis incorporating known prognostic variables. Our analysis was not intention to treat and patients who switched from initial VCD during inpatient hospitalization or initial cycle to VRD were analyzed in the VRD group. We excluded patients not achieving at least a partial response before transplant {4% of patients meeting other eligibility criteria (Supplementary data, consort flow diagram)}. However, it is impossible to know whether sub-optimal response in a particular treatment group excluded patients from proceeding to transplant in a timely manner since our data are limited to transplanted patients only and results of our study should be interpreted in that context.
In conclusion, we did not observe any difference in VGPR or better response rates with VRD or VCD induction therapy amongst patients who achieved at least a partial response and proceeded to stem cell transplant. In patients who proceed to stem cell transplant, the two regimens were found to have associated with comparable survival outcomes after adjusting for maintenance therapy and other known prognostic variables. Similar findings were observed in the subset of patients receiving full dose melphalan conditioning. Maintenance use was more important than the choice of bortezomib-based triplet induction in patients with MM undergoing upfront transplant. As the CIBMTR data does not capture patients who do not proceed to transplant, these results do not indicate the superiority or equivalence of one induction regimen over another for all newly diagnosed patients with MM.
Supplementary Material
ACKNOWLEDGMENTS:
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); U24HL138660 from NHLBI and NCI; OT3HL147741, and U01HL128568 from the NHLBI; HHSH250201700006C, and HHSH250201700007C from the Health Resources and Services Administration (HRSA); and N00014-20-1-2705 and N00014-20-1-2832 from the Office of Naval Research; Additional federal support is provided by P01CA111412, R01CA152108, R01CA215134, R01CA218285, R01CA231141, R01AI128775, R01HL126589, R01HL129472, R01HL130388, R01HL131731, U01AI069197, U01AI126612, UG1HL06924, and BARDA. Support is also provided by Be the Match Foundation, Boston Children’s Hospital, Dana Farber, St. Baldrick’s Foundation, Stanford University, the Medical College of Wisconsin the National Marrow Donor Program, and from the following commercial entities: Actinium Pharmaceuticals, Inc.; Adienne SA; Allovir, Inc.; Amgen, Inc.; Angiocrine Bioscience; Astellas Pharma US; bluebird bio, Inc.; Bristol Myers Squibb Co.; Celgene Corp.; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; ExcellThera; Fate Therapeutics; Gamida-Cell, Ltd.; Genentech Inc; Incyte Corporation; Janssen/Johnson & Johnson; Jazz Pharmaceuticals, Inc.; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Merck Sharp & Dohme Corp.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; Novartis Pharmaceuticals Corporation; Omeros Corporation; Oncoimmune, Inc.; Orca Biosystems, Inc.; Pfizer, Inc.; Pharmacyclics, LLC; Sanofi Genzyme; Stemcyte; Takeda Pharma; Vor Biopharma; Xenikos BV. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
CONFLICT OF INTEREST DISCLOSURE:
Surbhi Sidana: Consultancy: Janssen, Research Funding: Janssen, Magenta Therapeutics and Allogene.
Anita D’Souza: Research funding: Takeda, Sanofi, TeneoBio, Ad Board: Imbrium, Pfizer, Akcea, Consulting: Janssen
Dr. Kumar reports grants and other from BMS/Celgene, grants and other from Takeda, grants and other from Abbvie, grants and other from Roche, grants from Medimmune, grants from Tenebio, grants from Carsgen, personal fees from Oncopeptides, grants and other from Janssen, outside the submitted work.
Dr. Usmani reports grants and personal fees from Amgen, personal fees from Abbvie, grants from BMS, grants and personal fees from Celgene, personal fees from MundiPharma, grants from Pharmacyclics, grants and personal fees from Sanofi, grants and personal fees from Seattle Genetics, grants and personal fees from Janssen, grants and personal fees from Takeda, grants and personal fees from SkylineDX, grants and personal fees from Merck, outside the submitted work.
Dr. Shah reports grants from Celgene/BMS, Janssen, Bluebird Bio, Sutro Biopharma, Teneobio, Poseida, Nektar, personal fees from GSK, Amgen, Indapta Therapeutics, Sanofi, BMS, CareDx, Kite, Karyopharm, outside the submitted work.
Dr. Dhakal reports personal fees from Celgene/BMS, personal fees from Janssen, personal fees from Amgen, personal fees from Takeda, personal fees from GSK, personal fees from Sanofi, outside the submitted work.
Dr. Dholaria reports and Institutional research support from Takeda, Janssen, Angiocrine, Pfizer, Celgene.
Dr. Sidana reports grants from Magenta Therapeutics, grants from Allogene, grants and consultancy fees from Janssen, outside the submitted work.
Dr. Anderson reports personal fees from Celgene/BMS, personal fees from Amgen, personal fees from GSK, personal fees from Oncopeptides, personal fees from Karyopharm, personal fees from Janssen, outside the submitted work.
Dr. Giralt reports personal fees and other from Celgene, personal fees and other from Janssen, personal fees and other from BMS, personal fees and other from Sanofi, personal fees and other from Actinium, personal fees and other from Amgen, personal fees and other from Pfizer, personal fees and other from GSK, personal fees and other from Jazz, personal fees and other from OMEROS, outside the submitted work.
Dr. Hashmi reports other from Pfizer, other from Novartis, other from Therakos, other from Janessen, other from MSD, outside the submitted work.
DATA SHARING:
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
FUNDING:
This study was funded through the CIBMTR. Surbhi Sidana was supported by: KL2TR003143, KL2 Mentored Career Development Program, Stanford Clinical Translational Science Award Program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Durie BG, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet (London, England). 2017;389(10068):519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durie BGM, Hoering A, Sexton R, Abidi MH, Epstein J, Rajkumar SV, et al. Longer term follow-up of the randomized phase III trial SWOG S0777: bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT). Blood Cancer J. 2020;10(5):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N Engl J Med. 2017;376(14):1311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosiñol L, Oriol A, Rios R, Sureda A, Blanchard MJ, Hernández MT, et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood. 2019;134(16):1337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Einsele H, Engelhardt M, Tapprich C, Müller J, Liebisch P, Langer C, et al. Phase II study of bortezomib, cyclophosphamide and dexamethasone as induction therapy in multiple myeloma: DSMM XI trial. British journal of haematology. 2017;179(4):586–97. [DOI] [PubMed] [Google Scholar]
- 6.Paquin AR, Kumar SK, Buadi FK, Gertz MA, Lacy MQ, Dispenzieri A, et al. Overall survival of transplant eligible patients with newly diagnosed multiple myeloma: comparative effectiveness analysis of modern induction regimens on outcome. Blood Cancer J. 2018;8(12):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahindra A, Hari P, Fraser R, Fei M, Huang J, Berdeja J, et al. Autologous hematopoietic cell transplantation for multiple myeloma patients with renal insufficiency: a center for international blood and marrow transplant research analysis. Bone marrow transplantation. 2017;52(12):1616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreau P, Hulin C, Macro M, Caillot D, Chaleteix C, Roussel M, et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013–04 trial. Blood. 2016;127(21):2569–74. [DOI] [PubMed] [Google Scholar]
- 9.Kumar S, Flinn I, Richardson PG, Hari P, Callander N, Noga SJ, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119(19):4375–82. [DOI] [PubMed] [Google Scholar]
- 10.Chakraborty R, Muchtar E, Kumar S, Buadi FK, Dingli D, Dispenzieri A, et al. The impact of induction regimen on transplant outcome in newly diagnosed multiple myeloma in the era of novel agents. Bone marrow transplantation. 2017;52(1):34–40. [DOI] [PubMed] [Google Scholar]
- 11.Kumar SK, Engebretson AE, Buadi FK, Lacy MQ, Dispenzieri A, Duh MS, et al. Comparable Outcomes With Bortezomib-Cyclophosphamide-Dexamethasone (VCD) and Bortezomib-Lenalidomide-Dexamethasone (VRD) For Initial Treatment Of Newly Diagnosed Multiple Myeloma (MM). Blood. 2013;122(21):3178-. [Google Scholar]
- 12.Uttervall K, Borg Bruchfeld J, Gran C, Wålinder G, Månsson R, Lund J, et al. Upfront bortezomib, lenalidomide, and dexamethasone compared to bortezomib, cyclophosphamide, and dexamethasone in multiple myeloma. Eur J Haematol. 2019;103(3):247–54. [DOI] [PubMed] [Google Scholar]
- 13.Tsakiris DJ, Stel VS, Finne P, Fraser E, Heaf J, de Meester J, et al. Incidence and outcome of patients starting renal replacement therapy for end-stage renal disease due to multiple myeloma or light-chain deposit disease: an ERA-EDTA Registry study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2010;25(4):1200–6. [DOI] [PubMed] [Google Scholar]
- 14.Augustson BM, Begum G, Dunn JA, Barth NJ, Davies F, Morgan G, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United kingdom Medical Research Council trials between 1980 and 2002--Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005;23(36):9219–26. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy PL, Holstein SA, Petrucci MT, Richardson PG, Hulin C, Tosi P, et al. Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. J Clin Oncol. 2017;35(29):3279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horowitz M The role of registries in facilitating clinical research in BMT: examples from the Center for International Blood and Marrow Transplant Research. Bone marrow transplantation. 2008;42 Suppl 1:S1–s2. [DOI] [PubMed] [Google Scholar]
- 17.D’Souza A, Fretham C, Lee SJ, Arora M, Brunner J, Chhabra S, et al. Current Use of and Trends in Hematopoietic Cell Transplantation in the United States. Biology of Blood and Marrow Transplantation. 2020;26(8):e177–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127(24):2955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–20. [DOI] [PubMed] [Google Scholar]
- 20.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e46. [DOI] [PubMed] [Google Scholar]
- 22.Bridoux F, Arnulf B, Karlin L, Blin N, Rabot N, Macro M, et al. Randomized Trial Comparing Double Versus Triple Bortezomib-Based Regimen in Patients With Multiple Myeloma and Acute Kidney Injury Due to Cast Nephropathy. J Clin Oncol. 2020;38(23):2647–57. [DOI] [PubMed] [Google Scholar]
- 23.Royal V, Leung N, Troyanov S, Nasr SH, Écotière L, LeBlanc R, et al. Clinicopathologic predictors of renal outcomes in light chain cast nephropathy: a multicenter retrospective study. Blood. 2020;135(21):1833–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar SK, Ma E, Engebretson AE, Buadi FK, Lacy MQ, Dispenzieri A, et al. Treatment outcomes, health-care resource utilization and costs of bortezomib and dexamethasone, with cyclophosphamide or lenalidomide, in newly diagnosed multiple myeloma. Leukemia. 2016;30(4):995–8. [DOI] [PubMed] [Google Scholar]
- 25.Chen N, Lau H, Kong L, Kumar G, Zeldis JB, Knight R, et al. Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. Journal of clinical pharmacology. 2007;47(12):1466–75. [DOI] [PubMed] [Google Scholar]
- 26.Mikhael J, Manola J, Dueck AC, Hayman S, Oettel K, Kanate AS, et al. Lenalidomide and dexamethasone in patients with relapsed multiple myeloma and impaired renal function: PrE1003, a PrECOG study. Blood Cancer J. 2018;8(9):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar L, Chellapuram Sk, Sahoo R, Gupta R VRd versus VCd as induction therapy for newly diagnosed multiple myeloma: A Phase III, randomized study. Clinical Lymphoma, Myeloma and Leukemia. 2019;19(10):e361. [Google Scholar]
- 28.Joseph NS, Kaufman JL, Dhodapkar MV, Hofmeister CC, Almaula DK, Heffner LT, et al. Long-Term Follow-Up Results of Lenalidomide, Bortezomib, and Dexamethasone Induction Therapy and Risk-Adapted Maintenance Approach in Newly Diagnosed Multiple Myeloma. J Clin Oncol. 2020;38(17):1928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sidiqi MH, Aljama MA, Bin Riaz I, Dispenzieri A, Muchtar E, Buadi FK, et al. Bortezomib, lenalidomide, and dexamethasone (VRd) followed by autologous stem cell transplant for multiple myeloma. Blood Cancer J. 2018;8(11):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reeder CB, Reece DE, Kukreti V, Chen C, Trudel S, Hentz J, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia. 2009;23(7):1337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cornell RF, D’Souza A, Kassim AA, Costa LJ, Innis-Shelton RD, Zhang MJ, et al. Maintenance versus Induction Therapy Choice on Outcomes after Autologous Transplantation for Multiple Myeloma. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2017;23(2):269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hari P, Pasquini MC, Stadtmauer EA, Fraser R, Fei M, Devine SM, et al. Long-term follow-up of BMT CTN 0702 (STaMINA) of postautologous hematopoietic cell transplantation (autoHCT) strategies in the upfront treatment of multiple myeloma (MM). Journal of Clinical Oncology. 2020;38(15_suppl):8506-. [Google Scholar]
- 33.Gay F, Musto P, Rota Scalabrini D, Galli M, Belotti A, Zamagni E, et al. Survival Analysis of Newly Diagnosed Transplant-Eligible Multiple Myeloma Patients in the Randomized Forte Trial. Blood. 2020;136(Supplement 1):35–7. [Google Scholar]
- 34.Kumar SK, Jacobus SJ, Cohen AD, Weiss M, Callander N, Singh AK, et al. Carfilzomib or bortezomib in combination with lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma without intention for immediate autologous stem-cell transplantation (ENDURANCE): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voorhees PM, Kaufman JL, Laubach J, Sborov DW, Reeves B, Rodriguez C, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136(8):936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


