Purpose of review
The ‘gut-liver axis’ is thought to play an important role in pathogenesis of sepsis. Despite a wealth of experimental data to support the concept of reciprocal crosstalk between gut and liver through bacterial translocation and shaping of the microbiome by liver-derived molecules, for example bile acids, clinical data, and in particular diagnostic and therapeutic options, are limited.
Recent findings
Assessment of organ failure in the current definition of sepsis is operationalized by means of the Sequential Organ Failure Assessment (SOFA) score, including exclusively bilirubin to reflect the complex functions of the liver but ignoring the gut. However, our understanding of the intestinal microbiome and how it is affected by critical illness has clearly improved. Microbiota maintain gut-barrier function and modulate the innate and adaptive immune system. The best-defined intervention affecting the gut microbiome, that is selective decontamination of the digestive tract (SDD) is clinically studied regarding prevention of nosocomial lung infection and antibiotic resistance patterns, although its impact on liver function has not been systematically evaluated in critical illness.
Summary
Characterization of liver function beyond bilirubin and the microbiome can be achieved with contemporary sequencing and metabolomic techniques. Such studies are essential to understand how gut-liver crosstalk and ‘dysbiosis’ affect susceptibility to and outcome of sepsis.
Keywords: gut-liver axis, holobiont, microbiome, sepsis
INTRODUCTION
Microorganisms can be considered as the evolutionary origin of all higher organisms and with more than 1030 microbial cells by far outnumber all other organisms on earth [1]. Moreover, over the last decades, a dramatic shift in the understanding of a healthy human being gradually evolved in theoretical biology. The concept of the ‘holobiont’ defines most living beings, including humans, as ‘entities composed of a host and all of its symbiotic microbes’ [2], that is constituting an ‘ecosystem’ of man and associated microorganisms. An immense quantity of high-ranked articles has identified a role for the microbiome, primarily the microbiome of the gut, in an ever-increasing spectrum of diseases, in which the key role of the microbiome came as a surprise, including cancer [3], arteriosclerosis [4], metabolic syndrome [5], neurodegenerative diseases [6] and autism [7], to name but a few. These diseases are often associated with microbiome perturbations, that is dysbiosis.
Box 1.
no caption available
THE MICROBIOME AND PATHOGENESIS OF SEPSIS
The effects of ‘critical illness’ on human microbial habitats have been well documented. Predominant changes include a marked decrease in microbial diversity, which has been attributed to, for example, the use of broad-spectrum antibiotics [8]. However, even in the absence of antibiotics, physiological changes, such as fasting and stress, as well as therapeutic interventions, such as proton-pump inhibitors and the associated change in pH, strongly influence the human microbiome in patients admitted to the ICU. Potential further factors that alter microbiome composition are decreased immigration of food-associated bacteria and decreased nutritional supply for commensals and slowing of the transit through the gastrointestinal tract by endogenous as well as therapeutic factors [9]. The use of broad-spectrum antibiotics is, however, associated with a shift towards fungal species, collectively referred to as the ‘mycobiome’ and a selection of several bacterial species that have a deleterious effect on vitality at least in a model of gut colonization in Caenorhabditis elegans as a simple model organism [10]. It has long been acknowledged that in many cases, species identified as presumably causal pathogens in the septic host are derived from the host microbiome, as prototypically known for methicillin-resistant Staphylococcus aureus (MRSA) [11]. Vice versa, the frequent clinically ‘septic’ appearance of the critically ill patient with a primary sterile systemic inflammation, for example in the case of trauma or acute necrotizing pancreatitis, has led to the heavily debated concept of gut and liver as ‘source and motor’ of multiple organ failure [12], a concept that can now be rigorously tested by ‘next generation sequencing’ [13].
CHANGING THE PARADIGM – NOVEL CONCEPTS FOR THE ‘GUT-LIVER-AXIS’ IN HEALTH AND DISEASE
Crosstalk between gut and liver is essential for digestion, metabolism of nutrients and clearance of, for example, bacterial products. It thus does not come as surprise that diseases of the liver are often associated with dysbiosis, a term summarizing a disruption of homeostasis of microbiota due to altered functional composition and metabolic activities [14]. A reciprocal interaction of changes in the gut microbiome and function of parenchymal and nonparenchymal liver cells has also been demonstrated in a variety of liver diseases. For instance, Ma et al.[3] demonstrated recently that commensal Clostridium species prevent an effective immune response to primary liver tumours and liver metastases not by their own metabolites, but by microbial modification of host-produced bile acids.
Similarly, a key role for bile acids as regulators of the host-pathogen interaction has been demonstrated in patients with chronic liver disease by Leonhardt et al.[15▪▪]. The authors demonstrated that binding of bile acids to TGR-5, a G-protein coupled receptor on monocytes, can profoundly alter critical immune functions that protect the host, leading to prognosis-limiting deterioration of hepatic and extrahepatic organ function. A multitude of gut microbiome effects on metabolic liver functions have been described [16]. For example, high-fat diet can result in dysbiosis and intestinal bacterial overgrowth. The resulting altered microbial metabolism leads to choline deficiency, which contributes to the development of non-alcoholic fatty liver disease (NAFLD) and hepatic steatosis. Also, excessive alcohol intake is associated with intestinal dysbiosis, characterized by decreased abundance of Lactobacilli and increased microbial production of ethanol and acetaldehyde. These metabolites can lead to increased intestinal permeability and, together with transport of microbial products to the liver that induce an inflammatory response, can cause alcoholic liver disease.
Whereas so far focus has been mainly placed on gut barrier integrity as regulator of gut-liver interactions, recent evidence suggests that the interface between microbiome and liver is not exclusively depending on the lining of the gastrointestinal wall, but also consists of a ‘downstream’ interface that depends on the endothelial lining of the liver sinusoids [17▪▪,18▪▪].
As outlined above, there is evidence to support a role for shifts in the gut microbiome to trigger acute-on-chronic liver failure (ACLF) and admission to the ICU of patients with chronic liver disease. Recent reports indicate that such alterations translate into biomarkers that are associated with ACLF and death in hospitalized patients [19▪]. Moreover, the ample evidence to support a modulatory role of (non)absorbable antibiotics, probiotics, prebiotics and synbiotics on progression of chronic liver disease, most notably cirrhosis [20–22] allow to speculate that similar mechanisms might apply in the acute setting of patients without chronic liver disease as well.
LESSONS LEARNED FROM CHRONIC LIVER DISEASE FOR THE ICU
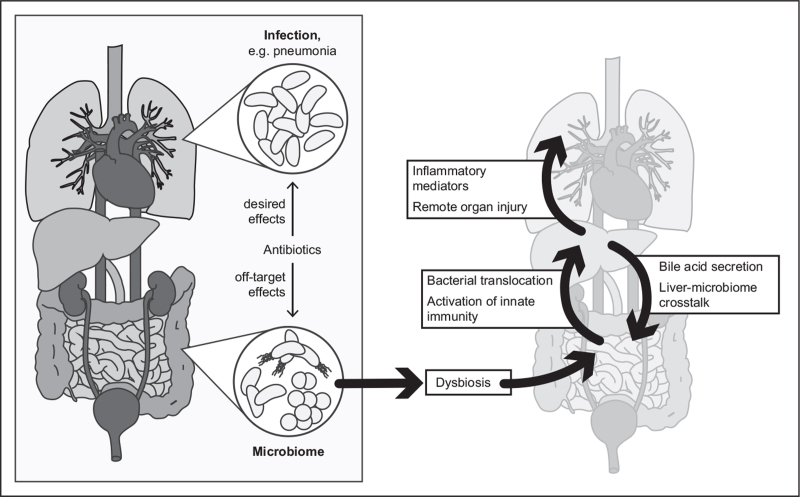
How do these concepts translate into the concept of care for the patient without preexisting liver disease? Metabolic adaptation is a central determinant for survival in the ICU. Metabolomic analysis in septic and nonseptic critically ill patients showed that deviations, independent of direction, in plasma levels of lipid metabolites were associated with sepsis mortality suggesting a ‘corridor of safety’ for hepatic metabolism in critical illness [23]. The crosstalk through gut-derived hormones and neuronal signals as well as the gradient of nutrients and substrates to the liver affects the ‘zonation’ of metabolic functions including bile production [24], making the gut-liver-axis a vulnerable target due to reciprocal regulation of metabolism and microbiome. Thus, off-target effects of antibiotics (and that of other less obvious modulators discussed above) on the microbiome should be an obvious concern in caring for the septic patient on the ICU. These interventions profoundly affect the host's ‘second genome’, that is a diverse set of genomes carried by the microbiota and will have concomitant substantial effects on the metabolism of the host. Thus, net effects on fitness of the host that result from desired effects at sites of infection and untoward side effects on the microbiome are difficult to predict (Fig. 1).
FIGURE 1.
The gut-liver axis in sepsis – an interface of the human host and its microbiome. The understanding of the human being as a ‘holobiont’ requires reappraisal of antiinfective therapy regarding net effects on the host.
Nonetheless, approaches to alter the microbiome are of high interest for the development of treatment approaches (see, e.g. [25] for an extensive review). The best-studied example for broad intervention that targets the microbiome is selective decontamination of the digestive tract (SDD). SDD consists in topical or parenteral administration of antibiotics and can be considered standard of care on ICUs in the Netherlands [26]. This approach to remove potentially pathogenic microbes results in different outcomes, depending amongst others on the prevalence of antimicrobial resistance [26], and still produces controversial discussions and data [26–28]. Importantly, potential side effects on the beneficial microbiome or the liver are largely ignored. The plain opposite of this approach, the administration of beneficial microbes as probiotics or via faecal microbiome transfer (FMT) has been scrutinized as well, with equally ambivalent results [29,30]. However, regarding the gut-liver axis, experimental studies that demonstrated beneficial effects of FMT on sepsis-mediated liver inflammation and injury are of particular interest [31▪▪,32▪▪]. The observed discrepancies can be interpreted as a result of the still poorly understood complexity of microbiome-host interactions and underline that ‘one size fits all’ treatment approaches are likely to fail.
Interestingly, antibiotics often fail in clinical practice to resolve organ failure despite evidence of infection and, even more concerning, are frequently administered not to miss an infection in patients with organ dysfunction, that is suspected sepsis. For instance, the EPIC-III investigators reported that while only 54% of ICU patients had suspected or proven infection, as many as 70% received at least one antibiotic [33].
Although the resulting increase of multiresistant bacteria or occurrence of Clostridium difficile is perceived as a problem, the negative impact of antibiotics on the ‘holobiont’ is nowadays probably not receiving the deserved attention by the medical community including ICU practitioners. More to the point, considerations in intensive care are still more reflecting the concepts of the early days of introduction of antimicrobial therapy when Paul Ehrlich propagated the concept of ‘therapia sterilisans magna’ wherein only ‘parasitotropic’ effects in the absence of ‘organotropic’ effects of drugs were envisioned and a healthy host was considered to be essentially devoid of microbes [34]. With the general acceptance of commensal microbes, the concept of a tight intestinal barrier that limits the systemic dissemination of microbes and their toxins and failure becomes apparent as ‘bacterial translocation’ was coming into focus [35]. The concept of the human being as a ‘holobiont’ will even further move our understanding towards a presence of microbes in body compartments currently thought to be sterile and will further shape our understanding of sepsis as a failed host-microbial interaction.
IN A NUTSHELL
Sepsis and multiorgan failure represent common conditions of critically ill patients in ICUs, responsible for immense global mortality and economic burden [36]. Most ICU patients receive antibiotics, causing depletion of commensal gut bacteria [37], enrichment of opportunistic pathogens [8] and disturbance of immune response and physiological activity, which in turn influence organ functions such as bile production in the liver [38,39]. Perturbation of the gut microbiome even with a short antibiotic administration can persist for months [40], whereas broad-spectrum antibiotic usage can cause extreme and long-lasting disbalance with an unknown impact on the recovery of critically ill patients [29]. In fact, a prolonged stay in the ICU likely causes the emergence of ultra-low-diversity communities in many patients, including pathogenic bacteria and fungi [8]. Therapeutic manipulation of the gut microbiome such as faecal transplantation and administration of probiotics have shown promising results in preventing and treating bacterial infections and organ dysfunctions as well as reducing the length of ICU stays [41], at least in some of the studies. However, we understand little about how microbiota confer resistance to organ dysfunction, in particular liver damage, and how they improve longevity. Furthermore, these microbiome-based interventions are currently a ‘one drug for all’ approach, while clearly personalized therapy and precision medicine is required for critically ill patients.
CONCLUSION
In connection to sepsis, several comparatively recent findings challenge the long-standing paradigm ‘Hit hard and hit early’ and call for a different perspective:
-
(1)
Antibiotic resistance is on the rise due to its excessive use.
-
(2)
The rapidly increasing field of microbiome research allows a more differentiated approach to benefits and threats of antibiotics, including disturbance of the microbiome.
-
(3)
The human body is increasingly perceived as a ‘holobiont’ – a notion that is additionally fuelled by the finding that long-thought sterile tissues harbour an extensive microbiome as well (e.g. [42,43]) and the gut-liver axis reflects a most significant interface that determines prognosis in the critically ill.
Acknowledgements
We thank Margit Leitner for technical help with figure design.
Financial support and sponsorship
M.B. is supported by the Deutsche Forschungsgemeinschaft (German Research Foundation) under Germany's Excellence Strategy (EXC 2051).
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Lennon JT, Locey KJ. The underestimation of global microbial diversity. mBio 2016; 7:e01298–e1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Theis KR, Dheilly NM, Klassen JL, et al. Getting the hologenome concept right: an eco-evolutionary framework for hosts and their microbiomes. mSystems 2016; 1:e00028-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma C, Han M, Heinrich B, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018; 360:eaan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun 2017; 8:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pascale A, Marchesi N, Marelli C, et al. Microbiota and metabolic diseases. Endocrine 2018; 61:357–371. [DOI] [PubMed] [Google Scholar]
- 6.Dinan TG, Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol 2017; 595:489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012; 13:701–712. [DOI] [PubMed] [Google Scholar]
- 8.Zaborin A, Smith D, Garfield K, et al. Membership and behavior of ultra-low-diversity pathogen communities present in the gut of humans during prolonged critical illness. MBio 2014; 5:e01361-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickson RP. The microbiome and critical illness. Lancet Respir Med 2016; 4:59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marfil-Sánchez A, Zhang L, Alonso-Pernas P, et al. An integrative understanding of the large metabolic shifts induced by antibiotics in critical illness. Gut Microbes 2021; 13:1993598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner NA, Sharma-Kuinkel BK, Maskarinec SA, et al. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol 2019; 17:203–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrico CJ, Meakins JL, Marshall JC, et al. Multiple-organ-failure syndrome. Arch Surg 1986; 121:196–208. [DOI] [PubMed] [Google Scholar]
- 13.Wang C, Li Q, Ren J. Microbiota-immune interaction in the pathogenesis of gut-derived infection. Front Immunol 2019; 10:1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lobo LA, Benjamim CF, Oliveira AC. The interplay between microbiota and inflammation: lessons from peritonitis and sepsis. Clin Transl Immunol 2016; 5:e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪▪.Leonhardt J, Haider RS, Sponholz C, et al. Circulating bile acids in liver failure activate TGR5 and induce monocyte dysfunction. Cell Mol Gastroenterol Hepatol 2021; 12:25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]; Specific bile acids have marked capacity to activate TGR5; This activity compromises monocyte function, leading to reduced release of pro-inflammatory cytokines in response to bacterial challenge. The study reveals certain bile acid profiles as risk factors for fatal outcome of liver disease and provide insight into gut-liver crosstalk that modulates immune dysfunction in liver disease.
- 16.Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014; 146:1513–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17▪▪.Wang Y, Zhang Y, Liu Y, et al. Gut-liver axis: liver sinusoidal endothelial cells function as the hepatic barrier in colitis-induced liver injury. Front Cell Dev Biol 2021; 9:702890. [DOI] [PMC free article] [PubMed] [Google Scholar]; Whereas the literature provides extensive data on the importance of the ‘leaky gut’ in the gut-liver-axis, this study demonstrated that an additional barrier is in place in the liver, in which LSECs play a central role.
- 18▪▪.Gola A, Dorrington MG, Speranza E, et al. Commensal-driven immune zonation of the liver promotes host defence. Nature 2021; 589:131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study describes immune zonation of the liver and reveals underlying mechanisms as well as functional importance.
- 19▪.Bajaj JS, Reddy KR, O’Leary JG, et al. Serum levels of metabolites produced by intestinal microbes and lipid moieties independently associated with acute-on-chronic liver failure and death in patients with cirrhosis. Gastroenterology 2020; 159:1715–1730. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors performed large-scale metabolomics and stool microbiome analyses in hospitalized cirrhosis patients. Using the measured parameters, the authors developed predictive models for progression to ACLF and death. Important predictors included metabolites of microbial origin and lipid moieties. In addition, an association with the composition of faecal microbiomes could be demonstrated.
- 20.Lee NY, Yoon SJ, Han DH, et al. Lactobacillus and Pediococcus ameliorate progression of nonalcoholic fatty liver disease through modulation of the gut microbiome. Gut Microbes 2020; 11:882–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duseja A, Acharya SK, Mehta M, et al. High potency multistrain probiotic improves liver histology in nonalcoholic fatty liver disease (NAFLD): a randomised, double-blind, proof of concept study. BMJ Open Gastroenterol 2019; 6:e000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajaj JS, Heuman DM, Hylemon PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol 2014; 60:940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khaliq W, Großmann P, Neugebauer S, et al. Lipid metabolic signatures deviate in sepsis survivors compared to nonsurvivors. Comput Struct Biotechnol J 2020; 18:3678–3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunningham RP, Porat-Shliom N. Liver zonation: revisiting old questions with new technologies. Front Physiol 2021; 12:732929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adelman MW, Woodworth MH, Langelier C, et al. The gut microbiome's role in the development, maintenance, and outcomes of sepsis. Crit Care 2020; 24:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wittekamp BHJ, Oostdijk EAN, Cuthbertson BH, et al. Selective decontamination of the digestive tract (SDD) in critically ill patients: a narrative review. Intensive Care Med 2020; 46:343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonten MJ. Selective decontamination is safe and efficacious for critically ill patients. Crit Care Med 2020; 48:736–738. [DOI] [PubMed] [Google Scholar]
- 28.Hurley JC. Selective digestive decontamination is neither safe nor efficacious for critically ill patients. Crit Care Med 2020; 48:732–735. [DOI] [PubMed] [Google Scholar]
- 29.Wischmeyer PE, McDonald D, Knight R. Role of the microbiome, probiotics, and ’dysbiosis therapy’ in critical illness. Curr Opin Crit Care 2016; 22:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yelin I, Flett KB, Merakou C, et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat Med 2019; 25:1728–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31▪▪.Liu Z, Li N, Fang H, et al. Enteric dysbiosis is associated with sepsis in patients. FASEB J 2019; 33:12299–12310. [DOI] [PMC free article] [PubMed] [Google Scholar]; Apart from confirming that sepsis patients show severe disruption of the gut microbiome, Liu et al.[31▪▪] also demonstrated that faecal transplantation of microbiota from septic patients to mice led to more severe hepatic inflammation and injury from subsequent sepsis (CLP) when compared to mice receiving FMT from healthy controls prior to sepsis induction.
- 32▪▪.Gong S, Yan Z, Liu Z, et al. Intestinal microbiota mediates the susceptibility to polymicrobial sepsis-induced liver injury by Granisetron generation in mice. Hepatology 2019; 69:1751–1767. [DOI] [PubMed] [Google Scholar]; The study explicitly addressed the role of the gut microbiota in sepsis-induced liver injury. Using a sepsis mouse model, sensitive or resistant phenotypes were distinguished. Faecal microbiota transplantation (FMT) resulted in markedly more severe liver damage in animals that received FMT from sensitive donors. Metabolomics analyses of the different phenotypes revealed differences in granisetron levels. This compound not only protected CLP-mice from death and liver injury, but also was negatively correlated with ALT/AST levels in septic patients.
- 33.Vincent JL, Sakr Y, Singer M, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA 2020; 323:1478–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehrlich P. Chemotherapeutics: scientific principles, methods, and results. Lancet 1913; 182:445–451. [Google Scholar]
- 35.Marshall JC, Christou NV, Meakins JL. The gastrointestinal tract. The ‘undrained abscess’ of multiple organ failure. Ann Surg 1993; 218:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet 2010; 376:1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blaser M. Antibiotic overuse: stop the killing of beneficial bacteria. Nature 2011; 476:393–394. [DOI] [PubMed] [Google Scholar]
- 38.Weis S, Carlos AR, Moita MR, et al. Metabolic adaptation establishes disease tolerance to sepsis. Cell 2017; 169:1263–1275. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Press AT, Babic P, Hoffmann B, et al. Targeted delivery of a phosphoinositide 3-kinase γ inhibitor to restore organ function in sepsis. EMBO Mol Med 2021; 13:e14436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A 2011; 108: (Suppl 1): 4554–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siempos II, Ntaidou TK, Falagas ME. Impact of the administration of probiotics on the incidence of ventilator-associated pneumonia: a meta-analysis of randomized controlled trials. Crit Care Med 2010; 38:954–962. [DOI] [PubMed] [Google Scholar]
- 42.Charlson ES, Bittinger K, Haas AR, et al. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med 2011; 184:957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roquilly A, Torres A, Villadangos JA, et al. Pathophysiological role of respiratory dysbiosis in hospital-acquired pneumonia. Lancet Respir Med 2019; 7:710–720. [DOI] [PubMed] [Google Scholar]