Abstract
Immune hyperactivation has been linked to various vaccines. We present a potential association of new-onset systemic lupus erythematosus (SLE) post-COVID-19 immunization. The patient is a 54-year-old male admitted for evaluation of flu-like symptoms two weeks after receiving the second dose of the COVID-19 vaccine. Physical examination revealed high-grade fever, diffuse bilateral non-tender cervical lymphadenopathy, and erythematous maculopapular palpable purpuric lesions on bilateral feet. Laboratory evaluation showed a significant hypocomplementemia (C3 < 11 mg/dL, C4 < 3 mg/dL, and CH50 < 10 U/mL), high titer antinuclear antibody, anti-dsDNA antibodies, anti-Sjogren’s syndrome-related antigen A antibodies, anti-Sjogren’s syndrome-related antigen B antibodies, anti-Smith antibodies, anti-ribonucleoprotein antibodies, anti-histone antibodies with a negative malignancy, and infection workup. The patient was treated with a high dose of steroids with a positive response. This case highlights the possibility of SLE, a rare adverse event following COVID-19 vaccination.
Keywords: systemic lupus erythematosus, immunization, autoimmunity, covid-19 vaccination, covid-19
Introduction
Vaccinations are considered a safe and effective way to prevent severe viral illnesses and reduce the spread of infection in patients with autoimmune diseases. Regarding COVID-19, three vaccines are authorized for emergency use in the United States: mRNA vaccines (Pfizer and Moderna) and viral vector vaccines (Johnson & Johnson (J&J)). On August 23, 2021, the US Food and Drug Administration (FDA) approved the first COVID-19 vaccine (Pfizer-BioNTech) for individuals ≥ 16 years of age. The first vaccination round was administered in December 2020. As of December 22, 2021, a total of 62% of the population has been fully vaccinated [1]. The results from safety surveillance performed by the CDC are reassuring. First-month vaccine monitoring has revealed that 90.9% of people had nonserious adverse events including local or systemic reactions. Until January 2022, the reported serious adverse effects include anaphylaxis (approximately five cases per million doses administered), myocarditis, pericarditis, thrombosis with thrombocytopenia syndrome (TTS), Guillain-Barré syndrome (GBS), and rarely death. TTS was confirmed in 57 cases following Johnson & Johnson (J&J) and three cases following the Moderna vaccine; myocarditis was confirmed in 1,233 cases, mostly following mRNA vaccines. There are 302 preliminary reports of GBS following the J&J vaccine after more than 18 million vaccine doses were administered [2,3]. There is limited data on the emergence of systemic lupus erythematosus (SLE) with the COVID-19 vaccine [4]. In this case report, we have presented a patient with a history of well-controlled Sjogren’s syndrome diagnosed with systemic lupus erythematosus after receiving the second dose of the COVID-19 vaccine.
Case presentation
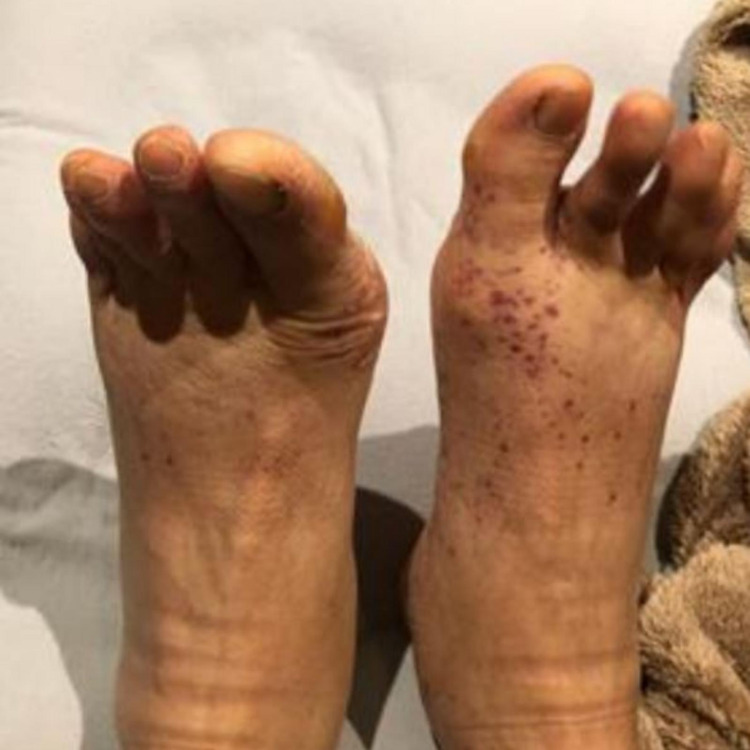
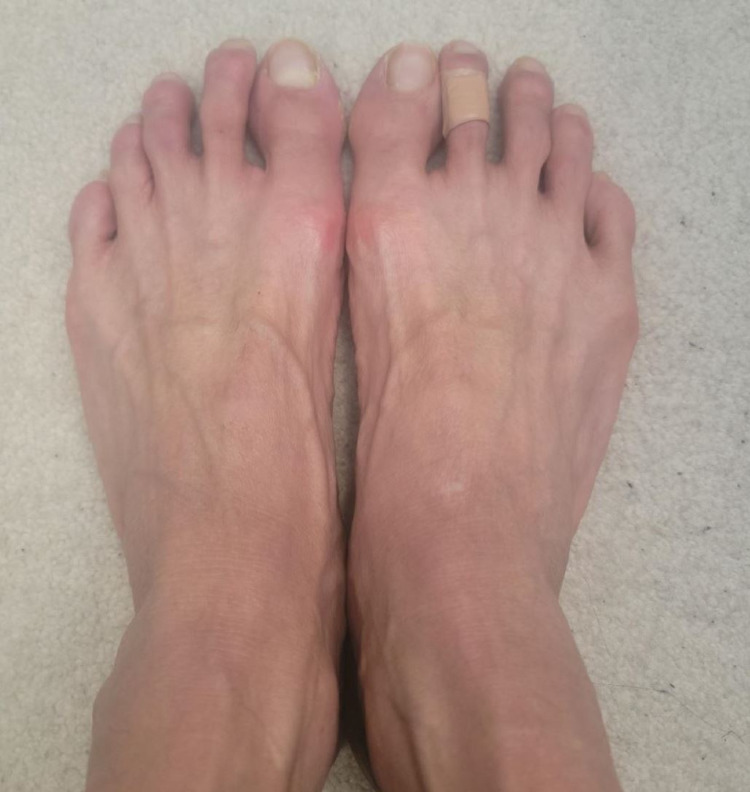
A 54-year-old Asian male was admitted for evaluation of flu-like symptoms two weeks after receiving the second dose of the Pfizer COVID-19 vaccine. The patient complained of fever, fatigue, generalized malaise, loss of appetite, unintentional weight loss, chest heaviness, shortness of breath, worsening of dry mouth and eyes, and burning and pain on bilateral feet. He denied joint pain, swelling or redness, focal weakness, or sensory loss. His only past medical history was stable Sjogren’s syndrome for around 30 years, manifested mainly by keratoconjunctivitis sicca. He was otherwise healthy. The patient denied a history of allergies. His medication list included artificial tears. He was a nonsmoker and occasional alcoholic. His maximum temperature during hospitalization was 101.3°F, and other vital signs remained stable. Physical examination revealed dry mouth, diffuse bilateral non-tender cervical lymphadenopathy, and tachycardia. Skin examination revealed non-pruritic erythematous maculopapular palpable purpuric lesions on the dorsal and plantar surface of the bilateral feet (Figure 1). Other examinations were unremarkable. On laboratory evaluation, the patient was found to have pancytopenia, hyponatremia, hypochloremia, elevated liver function tests, and significant hypocomplementemia (C3 < 11 mg/dL, C4 < 3 mg/dL, and CH50 < 10 U/mL) (Table 1). Malignancy workup including bone marrow biopsy and infection workup including COVID-19 RT-PCR was negative (Table 1). The urinalysis was positive for proteinuria (1.8 g/24 hours), hematuria, and pyuria. CT scan of the chest and abdomen was remarkable for diffuse lymphadenopathy. Chest X-ray and EKG were unremarkable. Additional serologies were positive for high titer antinuclear antibody (1:1,280), anti-dsDNA antibodies (>300 IU/mL), anti-Sjogren’s syndrome-related antigen A antibodies (>8 U/mL), anti-Sjogren’s syndrome-related antigen B antibodies (>8 U/mL), anti-Smith antibodies (>8.0 U/mL), anti-ribonucleoprotein antibodies (7.8 U), anti-histone antibodies (5.2 U), and anti-chromatin antibodies (>8 U/mL). Perinuclear and cytoplasmic antineutrophil cytoplasmic antibodies (P-ANCA and C-ANCA) were negative. According to the American College of Rheumatology (ACR) and Systemic Lupus International Collaborating Clinics (SLICC) criteria, the patient was classified as having SLE. Considering an evolution to SLE, the patient was started on prednisone 60 mg daily and mycophenolate mofetil 1,000 mg daily. He refused a renal biopsy. He improved significantly with the resolution of fever, fatigue, malaise, skin rash (Figure 2), pancytopenia, and transaminitis after initiation of treatment. Proteinuria went down to 500 mg/24 hours. After around a week, he developed confusion and recent memory amnesia. The neurology examination was non-focal. Lumbar puncture to rule out CNS infection, head CT, brain MRI, and EEG were unremarkable. He was started on Solu-Medrol 1,000 mg daily for three days, and mycophenolate mofetil was optimized to 3 g daily for the treatment of possible neuropsychiatric lupus. After two weeks of treatment, the patient stated a significant improvement of neuropsychiatric manifestations.
Table 1. Laboratory results.
L: low value; H: high value; WBC: white blood count; aPTT: activated partial thromboplastin clotting time; INR: international normalized ratio; AST: aspartate aminotransferase; ALT: alanine aminotransferase; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; ANA: antinuclear antibody; SSA: Sjogren’s syndrome-related antigen A; SSB: Sjogren’s syndrome-related antigen B; CCP: cyclic citrullinated peptides; TB: tuberculosis; HIV: human immunodeficiency virus; HTLV: human T-lymphotropic virus; EBV: Epstein–Barr virus
| Laboratory test | Value | Normal values |
| Complete blood count | ||
| WBC count | 1,700/mm3 (L) | 4,500–11,000/mm3 |
| Hemoglobin | 9.2 g/dL (L) | 13.5–17.5 g/dL |
| Platelet count | 77,000/μL (L) | 135,000– 317,000/μL |
| Absolute neutrophil count | 1,400/μL (L) | >1,500/μL |
| Absolute monocyte count | 100/μL (L) | 200–800/μL |
| Absolute lymphocyte count | 200/μL (L) | 1,000–4,800/μL |
| Absolute eosinophil and basophil count | 0 | <500/μL |
| Peripheral smear | There is pancytopenia. Red blood cells do not show any significant morphological abnormality. Platelet count is reduced in number. White blood cells do not show any blasts. Granulocytes show some blasts with the left shift. | - |
| CD4/CD8 ratio | 0.9 | >1 |
| Coagulation profile | ||
| aPTT | 44 seconds (H) | 25–35 seconds |
| INR | 1.06 | <1.1 |
| Prothrombin time | 13.5 seconds | 11–13.5 seconds |
| Comprehensive metabolic panel | ||
| Sodium | 122 mmol/L ( L) | 136–145 mEq/L |
| Potassium | 4.1 mmol/L | 3.5–5.0 mEq/L |
| Chloride | 94 mmol/L (L) | 95–105 mEq/L |
| Fasting glucose | 89 mg/dL | 70–110 mg/dL |
| Albumin | 2.3 g/dL ( L) | 3.5–5.5 g/dL |
| Bilirubin, total | 0.3 mg/dL | 0.1–1.0 mg/dL |
| Calcium | 7.1 mg/dL ( L) | 8.4–10.2 mg/dL |
| Creatinine | 0.8 mg/dL | 0.6–1.2 mg/dL |
| AST | 59 U/L (H) | 8–40 U/L |
| ALT | 28 U/L | 8–40 U/L |
| Other laboratory values | ||
| Ferritin | 3,223 μg/L (H) | 15–200 ng/mL |
| Triglyceride | 152 mg/dL (H) | 35–160 mg/dL |
| 24-hour urine protein | 1,830 mg (H) | <150 mg |
| Ceruloplasmin | 20 mg/dL | 14–40 mg/dL |
| Cortisol, baseline | 14.4 μg/dL | 5–23 μg/dL |
| TSH | 1.5 μU/mL | 0.5–5 μU/mL |
| Rheumatologic workup | ||
| CRP | 0.8 mg/dL | 0.0–0.8 mg/dL |
| ESR | 59 mmHg (H) | 0–15 mm/hour |
| ANA screen | Positive | Negative |
| ANA titer | 1:1,280 (H) | <1:160 |
| ANA pattern | Nuclear, speckled | - |
| C4 complement level | <3 mg/dL ( L) | 10–40 mg/dL |
| C3 complement level | <11 mg/dL (L) | 55–120 mg/dL |
| Total complement (CH50) | <10 U/mL ( L) | 37–55 U/mL |
| Anticardiolipin antibody | Negative | Negative |
| Antinuclear ribonucleoprotein antibodies | 7.8 U (positive) | Negative |
| Anti-Smith antibodies | >8.0 U/mL (positive) | Negative |
| SSA | >8 U/mL (positive) | Negative |
| SSB | >8 U/mL (positive) | Negative |
| Anti-DNA antibodies | >300 IU/mL (positive) | Negative |
| Anti-chromatin antibodies | >8 U/mL (positive) | Negative |
| Anti-histone antibodies | 5.2 U (positive) | Negative |
| Beta-2-glycoprotein 1 | Negative | Negative |
| Lupus anticoagulant | Negative | Negative |
| Anti-centromere antibodies | Negative | Negative |
| Anti-Jo-1 antibodies | Negative | Negative |
| Rheumatoid factor | Negative | <40 U/mL |
| Anti-CCP | Low titer | Negative |
| Cryoglobulins | Negative | Negative |
| Malignancy workup | ||
| Occult blood stool | Negative | Negative |
| Urine protein electrophoresis | No monoclonal protein identified | - |
| Bone marrow biopsy | Peripheral cytopenia of undetermined significance. Bone marrow showing maturing trilineage hematopoiesis with normal cellularity for age. No acute leukemia, lymphoma, or high-grade clonal stem cell disorder was identified. | - |
| Flow cytometry results | Viability is 86%. Lymphocytes comprise 21% of the sample, granulocytes 59%, and monocytes 1%. No increased blasts are identified. No monoclonal B lymphoid population is identified. No dropped pan T-cell antigens are identified. No significantly abnormal myeloid antigen expression is identified. | - |
| Final diagnosis | Peripheral blood smear showing pancytopenia. Flow cytometry does not identify any leukemia, lymphoma, or high-grade clonal stem cell disorder. | - |
| Infection workup | ||
| SARS-CoV-2 antibody total | Positive | Negative |
| Respiratory pathogen panel by RT-PCR with COVID-19 (COVID-19, influenza, parainfluenza, RSV, Bordetella pertussis, Bordetella parapertussis, Chlamydia pneumoniae, Mycoplasma pneumoniae, adenovirus, metapneumovirus, rhinovirus, enterovirus) | Not detected | Negative |
| Legionella antigen, urine | Negative | Negative |
| Ehrlichia antibody | Not detected | Negative |
| Lyme antibody screen | Negative | Negative |
| Chlamydia antibody panel | Not detected | Negative |
| Hepatitis acute panel | Not detected | Negative |
| QuantiFERON-TB Gold | Indeterminate | Negative |
| Rapid HIV | Non- reactive | Negative |
| HTLV antibody screen | Not detected | Negative |
| Cytomegalovirus antibody ( IgM) | Not detected | Negative |
| EBV antibody titers | Suggestive of a past EBV infection | Negative |
| Parvovirus B19 antibody titer | Negative | Negative |
| Malaria/Babesia smear | Negative | Negative |
| Blood culture | Negative | Negative |
Figure 1. Erythematous maculopapular palpable purpuric lesions on bilateral feet.
Figure 2. Rash resolution posttreatment.
Discussion
COVID-19 infection is associated with a flare-up of preexisting autoimmune rheumatic diseases or new-onset autoimmunity [5]. However, data regarding immune hyperactivation causing a disease flare or new onset of an autoimmune disorder post-COVID-19 vaccination is limited. Patients with autoimmune disorders were excluded from the vaccine trials, and guidelines regarding COVID-19 vaccination are based on expert opinion. There is a scarcity of long-term safety data in this patient population. A recent study in patients with SLE has assessed a mild to moderate disease flare in 11.4% of patients and severe flare in 1.3% of patients post-COVID-19 vaccination [6]. Hepatitis B, HPV, and influenza vaccines have increased the risk of autoimmunity as mentioned in a meta-analysis performed by Wang et al. [7]. The etiology is unclear; the proposed mechanism can be that inactive viral component or attenuated microorganism induces molecular mimicry or bystander activation in genetically predisposed individuals. Also, adjuvants added to enhance immunity in various vaccines can result in autoimmune events. Given the negative infectious and malignancy workup, a temporal relationship with COVID-19 vaccination, the biological plausibility of the vaccines causing autoimmunity, and clinical characteristics, the COVID-19 vaccine was considered as a possible trigger for the evolution of SLE in our patient. Skin lesions were thought to be secondary to possible lupus vasculitis. The cutaneous biopsy was deferred because of self-resolution with the treatment of SLE. This report illustrates that although the COVID-19 vaccine is considered safe to prevent the spread of disease among patients with preexisting autoimmune diseases, rare disease flares or evolution to a new autoimmune disease can be triggered. Nationwide studies demonstrating long-term COVID-19 vaccine safety in patients with preexisting well-controlled autoimmune diseases are needed to support the finding.
Conclusions
This case highlights the possibility of systemic lupus erythematosus, a rare adverse event following COVID-19 vaccination. Early recognition and treatment can prevent potentially serious complications related to SLE post-vaccination. Although the COVID-19 vaccine is highly recommended by experts in patients with autoimmune diseases, results from high-quality studies are warranted to demonstrate long-term safety in this patient population.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Centers for Disease Control and Prevention: COVID data tracker. [ Mar; 2020 ];CDC. (2020, March 28. https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total 2020
- 2.Centers for Disease Control and Prevention: First month of COVID-19 vaccine safety monitoring — United States, December 14, 2020-January 13, 2021. [ Feb; 2021 ];https://www.cdc.gov/mmwr/volumes/70/wr/mm7008e3.htm. 2021 doi: 10.15585/mmwr.mm7008e3. [DOI] [PMC free article] [PubMed]
- 3.Centers for Disease Control and Prevention: Selected adverse events reported after COVID-19 vaccination. [ Feb; 2021 ];https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html 2022
- 4.The emergence of new-onset SLE following SARS-CoV-2 vaccination. Nune A, Iyengar KP, Ish P, Varupula B, Musat CA, Sapkota HR. QJM. 2021;114:739–740. doi: 10.1093/qjmed/hcab229. [DOI] [PubMed] [Google Scholar]
- 5.Systemic lupus erythematosus manifestation following COVID-19: a case report. Zamani B, Moeini Taba SM, Shayestehpour M. J Med Case Rep. 2021;15:29. doi: 10.1186/s13256-020-02582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evaluation of immune response and disease status in systemic lupus erythematosus patients following SARS-CoV-2 vaccination. Izmirly PM, Kim MY, Samanovic M, et al. Arthritis Rheumatol. 2022;74:284–294. doi: 10.1002/art.41937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaccinations and risk of systemic lupus erythematosus and rheumatoid arthritis: a systematic review and meta-analysis. Wang B, Shao X, Wang D, Xu D, Zhang JA. Autoimmun Rev. 2017;16:756–765. doi: 10.1016/j.autrev.2017.05.012. [DOI] [PubMed] [Google Scholar]