Abstract
Background and Objectives
Neurofilament light chain (NfL) in blood is a sensitive but nonspecific marker of brain injury. This study sought to evaluate associations between NfL concentration and MRI findings of vascular brain injury in older adults.
Methods
A longitudinal cohort study included 2 cranial MRI scans performed about 5 years apart and assessed for white matter hyperintensities (WMH) and infarcts. About 1 year before their second MRI, 1,362 participants (median age 77 years, 61.4% women) without a history of TIA or stroke had measurement of 4 biomarkers: NfL, total tau, glial fibrillary acidic protein (GFAP), and ubiquitin carboxyl-terminal hydrolase L1. Most (n = 1,279) also had the first MRI scan, and some (n = 633) had quantitative measurements of hippocampal and WMH. In primary analyses, we assessed associations of NfL with a 10-point white matter grade (WMG) and prevalent infarcts on second MRI and with worsening WMG and incident infarct comparing the 2 scans. A p value <0.0125 (0.05/4) was considered significant for these analyses. We also assessed associations with hippocampal and WMH volume.
Results
In fully adjusted models, log2(NfL) concentration was associated with WMG (β = 0.27; p = 2.3 × 10−4) and worsening WMG (relative risk [RR] 1.24; p = 0.0022), but less strongly with prevalent brain infarcts (RR 1.18; p = 0.013) and not with incident brain infarcts (RR 1.18; p = 0.18). Associations were also present with WMH volume (β = 2,242.9, p = 0.0036). For the other 3 biomarkers, the associations for log2 (GFAP) concentration with WMG and worsening WMG were significant.
Discussion
Among older adults without a history of stroke, higher serum NfL concentration was associated with covert MRI findings of vascular brain injury, especially the burden of WMH and its worsening. Whether these results offer opportunities for the use of NfL as a noninvasive biomarker of WMH or to control vascular risk factors remains to be determined.
Neurofilament light chain (NfL), detectable in the CSF and more recently in blood, is a neuron-specific structural protein and a sensitive but nonspecific marker of neuroaxonal damage regardless of whether the mechanism of brain injury is inflammatory, degenerative, traumatic, or vascular.1 Relatively few investigations of NfL have been performed with vascular brain injury (VBI), and most have considered overt, clinically defined events, such as acute stroke. Even less is known about its association with subclinical or covert imaging-defined evidence of VBI, specifically white matter hyperintensities (WMH) and infarcts on cranial MRI scans in people without a history of stroke. Covert MRI-defined VBI has been associated with clinical manifestations such as incoordination, depressed mood, and cognitive impairment and with the risk of outcomes such as stroke, dementia, and death.2-4 We measured blood levels of NfL along with 3 other emerging biomarkers of brain pathology: total tau, a microtubule associated protein found predominantly in cortical nonmyelinated axons, mostly studied in degenerative brain injury; glial fibrillary acid protein (GFAP), an intermediate filament protein expressed in astrocytic glial cells and a nonspecific biomarker of brain injury; and ubiquitin carboxy-terminal hydrolase L1 (UCH-L1), an enzyme expressed in the cytoplasm of neurons and also proposed as a biomarker for brain injury.5 The focus of this work is the association of blood NfL in this multiplexed assay with the covert MRI findings of WMH and infarcts in older adults. The Cardiovascular Health Study (CHS), a community-based longitudinal cohort study of older participants with an initial and follow-up cranial MRI scan, offers a unique opportunity to address questions about the association of serum NfL concentration and MRI findings of VBI. The goal of this work is to help clarify whether NfL could serve as a noninvasive biomarker for identifying and monitoring people with active or ongoing VBI—the ones most likely to benefit from aggressive conventional6,7 or investigational interventions.8
Methods
Cohort
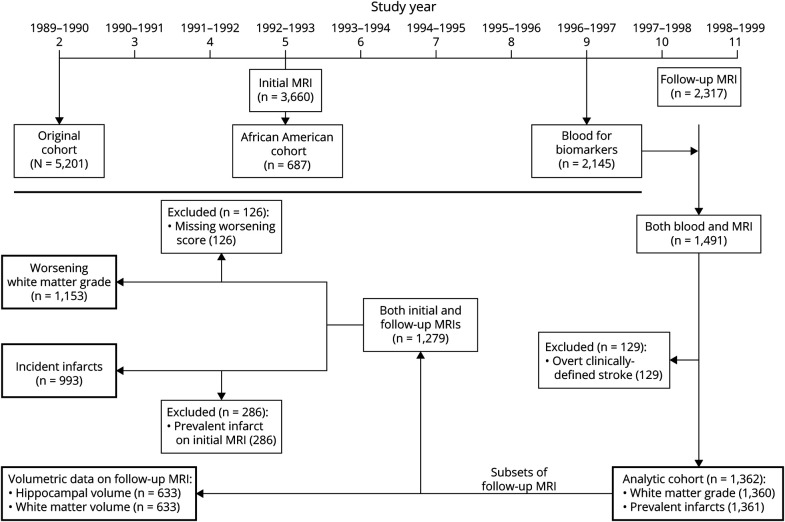
The CHS is a population-based, longitudinal cohort of 5,888 men and women aged 65 years or older at enrollment.9 In year 2 of the study (1989–1990), 5,201 participants were recruited from 4 communities: Forsyth County, North Carolina; Washington County, Maryland; Sacramento County, California; and Pittsburgh, Pennsylvania. In year 5, an additional cohort of 687 predominantly African American participants was added (Figure). Examinations were performed at baseline and repeated annually until year 11 and included measures of subclinical disease and risk factors for cardiovascular outcomes.
Figure. Timeline and Flowchart for Participants From the Cardiovascular Health Study Included in These Analyses.
The boxes with the heavier borders include the particular outcome for each analysis and the number of participants in each analysis.
Brain Imaging
Two cranial MRI scans were performed in CHS, as described previously.10-12 The initial scan was performed in 3,660 participants in years 4–6, and the follow-up scan was performed in years 10–11 in 2,317 participants (Figure). Both scans were performed in 2,116 participants with a median and mean of 5 years between the initial and follow-up scans. The scanning protocols for the initial and follow-up MRI scans included sagittal T1-weighted localizer sequences, and axial T1, spin-density, and T2-weighted images.11,13 Radiologic images were sent to a single reading center, where neuroradiologists with training in the CHS protocol evaluated the images using a standardized protocol. Neuroradiologists were blinded to the participants' age, sex, race, ethnicity, and clinical information.11,13 To determine WMG, the burden of periventricular and subcortical white matter signal abnormality on either axial T2-weighted or spin-density images was compared to a series of 8 images that had successively increased white matter changes from barely detectable to extensive.13 WMH was graded on a 10-point semiquantitative scale with a white matter grade (WMG) of 0 being the least severe and 9 being the most severe. WMG had an interreader intraclass correlation coefficient of 0.76 and an intrareader coefficient of 0.89.13 For determining change in WMG, neuroradiologists reread all scans side by side while blinded to previous grade determination and order of scans.11 These reads had an intrareader reliability kappa of 0.59, and interreader reliability kappa of 0.36, so certain scans were reviewed and adjudicated, as detailed previously.11 In addition, neuroradiologists identified brain infarcts 3 mm or greater. Incident infarct was defined as at least one infarct on the follow-up scan in a participant whose initial scan was free of any infarcts.10
Some of the follow-up MRI scans, which were all performed on 1.5T scanners, also included 3D T1-weighted spoiled gradient-recalled sequences, as detailed previously.14 Volumetric assessments were performed in these higher-resolution images of the follow-up scans using FreeSurfer in 633 participants, as described elsewhere.15 Briefly, the FreeSurfer software reformatted T1-weighted images to 1 × 1 × 1 mm3 voxels, and generated a surface-based reconstruction of the brain, which included the hippocampus and WMH on T1-weighted sequences. The latter has shown strong correlation with WMH on fluid-attenuated inversion recovery sequences.16
Biomarker Measurement
The current analyses included participants who had serum biomarkers measured in year 9 and a follow-up cranial MRI scan performed in years 10–11 (Figure). Frozen serum was used from participants who attended the year 9 examination and who completed an oral glucose tolerance test as part of a study identifying prevalent diabetes; those on medication for diabetes were excluded because their diabetes status was known. Of the 2,297 participants who had an in-person year 9 clinic visit and a follow-up MRI, 310 were excluded from participating in oral glucose testing due to diabetes medication or not fasting and another 118 declined to participate. Of the 1,668 participants who completed the oral glucose test, biomarkers were measured in participants with fresh fasting serum available for analysis. The assay used was the single-molecule array (SIMOA) Human Neurology 4-Plex A assay (Quanterix), which includes 4 biomarkers of brain injury: NfL, total tau, GFAP, and UCH-L1. The SIMOA 4-plex has high sensitivity and reproducibility; of these biomarkers, UCH-L1 has the poorest sensitivity. The interassay coefficients of variation were NfL, 9.3%; tau, 10.1%; GFAP, 8.2%; and UCH-L1, 21.6%. Serum biomarkers were evaluated independently from brain images and all other clinical information.
Covariates
Serum used for the NfL assays and all covariates for analysis were assessed during the year 9 examination when the biomarkers were measured. Race, ethnicity, and sex were self-reported. Due to low numbers of participants reporting Asian/Pacific Islander, American Indian/Alaska Native, and other race/ethnicities (n = 7), analyses are conducted comparing Black to not Black participants. Diabetes mellitus was defined as fasting glucose of 126 mg/dL or higher or nonfasting glucose 200 mg/dL or higher. Those on a glucose-lowering medication were excluded. Smoking was assessed as current, former, or never. Atrial fibrillation was defined based on ECG, or presence of a single hospital discharge diagnosis from CHS hospitalization or Medicare data, or inpatient, outpatient, or physician claim from Medicare data. The eGFR was calculated using cystatin C. Hypertension was defined as systolic blood pressure of 140 mm Hg or more, diastolic blood pressure of 90 mm Hg or more, or history of hypertension and on antihypertensive medications. Participants with an adjudicated TIA or stroke prior to the follow-up MRI scan were excluded from analysis.
Statistical Methods
We assessed the association of NfL with 4 primary outcomes in participants with a follow-up MRI scan (Figure): (1) WMG in the follow-up scan; (2) prevalent brain infarct in the follow-up scan; (3) worsening WMG in the follow-up compared with initial scan; and (4) incident brain infarct in the follow-up scan in participants without any on the initial scan. We determined the association of serum log2 transformed NfL concentration with the 10-point WMG using linear regression with robust standard errors and with the 3 binary outcomes using relative risk (RR) regression with robust standard errors. These analyses were adjusted for time between when blood was collected and the follow-up scan was performed, age, sex, race, study site, BMI, systolic blood pressure, diastolic blood pressure, eGFR, medication use for hypertension, diabetes mellitus, smoking status, and presence of prior myocardial infarction, congestive heart failure, and atrial fibrillation. For analyses including the initial and follow-up scans, we also adjusted for time between the brain scans. For these 4 primary outcomes, we accounted for multiple comparisons using Bonferroni correction. Thus, a p value less than 0.0125 (0.05/4) was considered significant for these analyses.
In secondary analyses, we examined the association between log2 transformed NfL concentration and measures in the follow-up scan of hippocampal volume and WMH volume using linear regression with robust standard errors. Besides the covariates already mentioned, we also adjusted these volumetric analyses for total intracranial volume.
In sensitivity analyses, we explored interactions by age, sex, and hypertension for the associations of log2 transformed NfL concentration with the WMG and prevalent infarcts on the follow-up scan. Age was divided at the median, and we compared participants under 77 years to those older. In addition, prior CHS articles have suggested that the combination of both high WMH burden and infarcts are more strongly related to manifestations and outcomes than either alone.17,18 Thus, we performed relative risk regression with robust standard errors of the association with log2 transformed NfL concentration. The outcome variable was a profile of VBI and was divided into 4 groups based upon findings in the follow-up MRI scan: (1) low WMG 0–2 and absent infarcts; (2) low WMG 0–2 and present infarcts; (3) high WMG 3–9 and absent infarcts; and (4) high WMG 3–9 and present infarcts. Each of the groups was compared to the low WMG 0–2 and absent infarcts as the reference group.
Finally, although NfL was the focus of this work, we also assessed the other biomarkers of brain injury in the Human Neurology 4-Plex A assay: total tau, GFAP, and UCH-L1. Thus, we repeated the analyses of the 4 primary outcomes for the 3 other biomarkers. We did not account for multiple comparisons in any secondary analyses due to their exploratory nature.
We conducted all analyses in RStudio (R version 3.6.3), using packages that included “lmtest” and “sandwich.”19,20
Standard Protocol Approvals, Registrations, and Patient Consents
Institutional review boards at the University of Washington and at each study site approved the study. All CHS participants provided written informed consent.
Data Availability
The data that support the findings of this study are available upon reasonable request through the CHS Coordinating Center (CHS-NHLBI.org).
Results
The CHS cohort included 1,491 participants with both measurements of NfL and follow-up MRI scans. Participants with TIA or stroke prior to the follow-up MRI scan were excluded (n = 129), so all of the findings on the MRI scans were covert. The remaining 1,362 participants formed the analytic cohort (Figure) and their year 9 characteristics are described in Table 1, along with results for the biomarker assays and the MRI findings. Of the 1,153 participants with WMG measured on both scans, 315 (27.3%) had WMG worsening in the follow-up scan. Of the 993 participants with both scans and no infarct identified on the initial scan, 163 (16.4%) had incident brain infarct identified in the follow-up scan. Pairwise associations of each covariate and log2 transformed NfL concentration are presented in eTable 1, links.lww.com/WNL/B722.
Table 1.
Characteristics of 1,362 Participants in the Cardiovascular Health Study With Serum Neurofilament Light Chain Measured in Study Year 9, With Follow-up MRI in Study Year 10–11, and Without Stroke Prior to the MRI
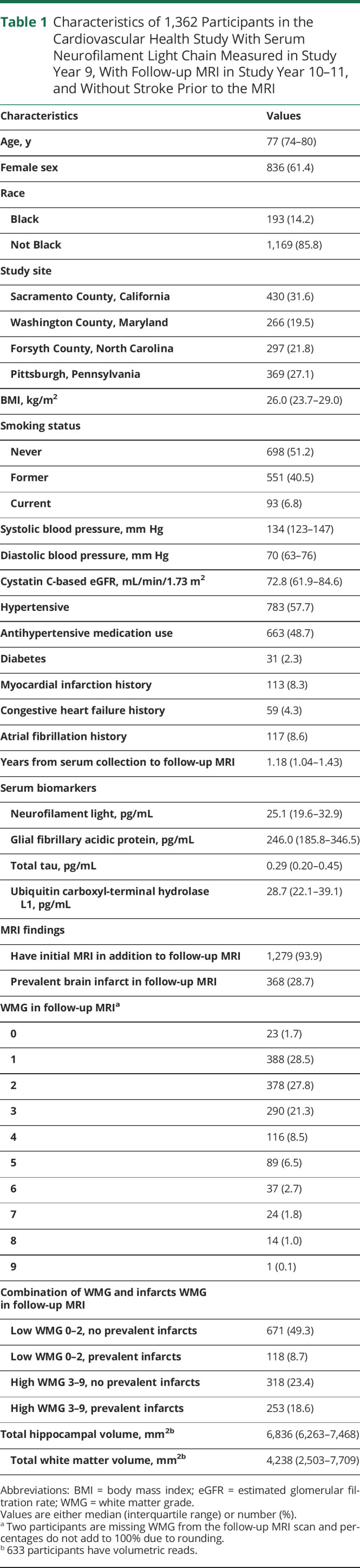
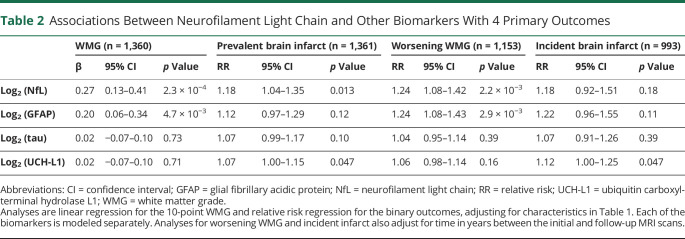
In fully adjusted models of findings on the follow-up scan (Table 2), log2 transformed NfL concentration was significantly associated with a higher or worse WMG in the follow-up scan (β = 0.27, p = 2.3 × 10−4) but not with prevalent brain infarct (RR 1.18, p = 0.013) after accounting for multiple comparisons. Thus, every doubling of NfL serum concentration was associated with a 0.27-point higher or worse score in the 10-point WMG. Results for all covariates in the models are presented in eTable 2, links.lww.com/WNL/B722. We did not identify interactions by sex, age, or hypertension for the associations between NfL concentration and the primary outcomes of WMG and prevalent infarct in the follow-up MRI (eTable 3). Limiting the infarcts to small, subcortical infarcts did not change associations between serum NfL and either prevalent or incident infarcts (data not shown).
Table 2.
Associations Between Neurofilament Light Chain and Other Biomarkers With 4 Primary Outcomes
We determined the RR in 3 fully adjusted models using as the reference the group with low WMG 0–2 and absent infarcts (eTable 4, links.lww.com/WNL/B722). The strongest association with log2 transformed NfL concentration was comparing groups with high WMG 3–9 and present infarcts vs low WMG 0–2 and absent infarct (RR 1.32, 95% CI 1.15–1.52, p = 1.1 × 10−4).
In fully adjusted models of findings in participants with both scans (also Table 2), log2 transformed NfL concentration was significantly associated with worsening WMG (RR = 1.24, p = 0.0022) but not with incident brain infarct (RR 1.18, p = 0.18). Thus, every doubling of NfL concentration was associated with a 1.24-fold higher risk of WMG worsening between the initial and follow-up scan. Results for all covariates in the models are presented in eTable 5, links.lww.com/WNL/B722.
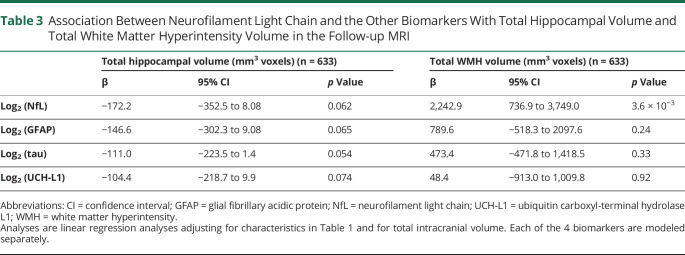
In fully adjusted models of volume measures on the follow-up scan (Table 3), higher log2 transformed NfL concentration was not associated with smaller total hippocampal volume (β = −172.2 mm3, p = 0.06) but was associated with larger total WMH volume (β = 2,242.9 mm3, p = 0.004).
Table 3.
Association Between Neurofilament Light Chain and the Other Biomarkers With Total Hippocampal Volume and Total White Matter Hyperintensity Volume in the Follow-up MRI
As shown in Table 2, concentrations of log2 transformed total tau were not associated with any of the 4 primary outcomes in fully adjusted models. Higher concentration of log2 transformed GFAP was associated with WMG in the follow-up scan and with worsening WMG between scans but not with prevalent or incident infarcts. Higher concentration of log2 transformed UCH-L1 was marginally associated with prevalent and incident brain infarcts. As shown in Table 3, none of these 3 biomarkers was significantly associated with total hippocampal or total WMH volumes.
Because log (GFAP) was significantly associated with WMG and worsening WMG, we included it in a multivariate model of the association between log (NfL) and these outcomes. The magnitude and strength of the association between log (NfL) and WMG and worsening WMG did not change substantially when including log (GFAP) as a covariate, though the association with worsening WMG was weakened; every doubling of log (NfL) was associated with a 0.26 unit increase in WMG (95% CI 0.11–0.42; p = 9.6 × 10−4) and a RR of 1.15 (95% CI 0.99–1.34; p = 0.069) for risk of worsening WMG in the follow-up MRI.
Discussion
This study leveraged a large, well-characterized, population-based, longitudinal cohort of older adults to evaluate the association of serum NfL concentration with findings on a cranial MRI performed on average 1.2 years after the assay and with changes from a prior MRI scan performed on average 5 years earlier. In this population of older adults without a history of stroke, the major findings based on the primary analysis are that NfL concentration was associated with WMG on the follow-up scan and with risk of worsening WMG comparing the initial and follow-up scans. Serum NfL concentration was only marginally associated with prevalent brain infarcts. For every doubling of serum NfL concentration, WMG was 0.27 points higher on the 10-point scale, and the relative risk of the WMG worsening between the initial and follow-up scan was 24% higher. The clinical significance of these findings is difficult to judge. Secondary analyses suggested that the strongest association with NfL concentration was in those with both a high WMH burden and infarcts compared with those with neither. In a subset of participants with quantitative measures on the follow-up MRI, higher NfL concentration was associated with higher total WMH volume. The results with total WMH volume are not surprising given its strong correlation with WMG.21 These results show that in this population higher NfL concentration is more strongly associated with WMG than brain infarcts and is more strongly associated with WMH volume, which reflects covert VBI, than with hippocampal volume, which reflects degenerative brain injury, although difference in measurement precision and analyses might also explain the difference in these associations.
Of the 3 other biomarkers of brain injury, only UCH-L1 concentration was marginally associated with prevalent or incident brain infarcts, and none of the biomarkers was significantly associated with total hippocampal or WMH volume. Only GFAP concentration was associated with WMG on the follow-up scan and with risk of worsening WMG comparing the initial and follow-up scans. The finding that associations were stronger with NfL than with the other biomarkers supports the notion that NfL concentration may be related more to VBI than degenerative brain injury.22
An association between NfL concentration and the burden of WMH on MRI was first described in 2001 based on an assay of CSF.23 Since the development of a reliable assay of NfL concentration in blood, testing in patients with VBI has become more common, often in patients with overt clinically defined stroke, where concentration has been shown to be elevated following acute ischemic stroke.1 Several such studies of patients with acute ischemic stroke have shown associations of blood NfL concentration with the burden of WMH24-26 and integrity of white matter tracts,27 and have suggested that blood NfL concentration may be a marker for progressive small vessel disease.24,27,28 The current study suggests the same may be true for older adults who have not had an overt clinically defined stroke.
Some studies have addressed these issues in select groups of participants. Data from the Alzheimer's Disease Neuroimaging Initiative in select older adults without dementia have also shown associations of plasma NfL concentration with WMH and their progression,29,30 as well as microbleeds and lacunar infarcts.30 In the Swiss-Atrial Fibrillation Study, a longitudinal study of 1,379 patients with atrial fibrillation and a mean age of 72 years, serum NfL concentration was significantly associated with WMH volume after controlling for age and cardiovascular risk factors, including when participants with a history of stroke were excluded.31 Interestingly, serum NfL concentration was also associated with large noncortical or cortical infarcts but not small noncortical infarcts.
Few longitudinal cohort studies have examined the association between blood NfL concentration and MRI findings. Among 4,444 participants without dementia with a mean age of 72 years, investigators from the Rotterdam Study found that higher plasma levels of NfL and lower plasma levels of β-amyloid 42 identified participants without dementia who were at increased risk to develop all-cause dementia and Alzheimer disease dementia during follow-up.32 MRI findings of vascular brain imaging were not addressed in that article. Among 335 participants without dementia from the Austrian Stroke Prevention Family Study with a mean age of 65 years, serum NfL was correlated with MRI findings of normalized brain volume but not with WMH volume after adjusting for age, perhaps because patients with a history of stroke or an infarct on cranial MRI were excluded.33 Among 268 participants from the Memory and Morbidity in Augsburg Elderly study, with a mean age of 72, serum NfL was associated in adjusted models with MRI findings of WMH and any lacunar infarcts.34
The stronger association in the current study of serum NfL concentration with the burden of WMH than with brain infarcts may simply reflect the amount of tissue injured with each finding, the exclusion of patients with treated diabetes, and the exclusion of participants with a history of clinically defined stroke prior to their follow-up MRI scan. Also, analyses of incident brain infarcts on the follow-up scan were disadvantaged by excluding participants whose initial MRI scan showed a prevalent infarct. This conservative approach was necessary because in CHS specific infarcts were not tracked between the initial and follow-up scans.10
The CHS has many strengths, including its longitudinal design, its banked blood samples allowing biomarker studies, its 2 cranial MRIs separated by about 5 years, and its well-characterized participants. It also has factors that limited the generalizability of these findings and other weaknesses. We have probably underestimated associations because participants getting MRI scans were healthier than those who did not, especially those getting both the initial and follow-up MRI scans.11 Also, the selection of participants for the serum assay at study year 9 was such that those on medications for diabetes were excluded. Thus, we cannot generalize our results to include those with diabetes, which is related to the development of small vessel disease. Furthermore, we have not accounted for all possible injuries to the nervous system that could increase serum NfL. The NfL was not measured at the time of the follow-up MRI but rather about a year before and was not available at the time of the initial MRI. Thus, we cannot evaluate how changes in NfL level correlate with changes on the brain images or if changes in NfL levels precede or result from changes in brain structure. The MRIs performed in CHS were from the 1990s and lacked other findings of VBI such as microbleeds, enlarged perivascular spaces, diffusion tensor imaging, peak width of skeletonized mean diffusivity, and others—some of which have been shown to be related to blood NfL concentration in other studies and will surely be the focus of future investigations.27,35,36 Although the scanners used to collect the brain images may have differed across the sites and between time points, all images were evaluated at a central reading center with standardized protocols. When measures of change between the initial and follow up MRIs were evaluated, both scans were read together side by side. The current study does not address the etiology of WMH, which are thought to have a vascular basis. Other etiologies have been proposed, including that an early vascular injury sparks an immune reaction, perhaps against NfL, that contributes to the progressive disease.37 Finally, the current study examined associations between serum NfL concentration and MRI findings of VBI, which are presumed to mediate the outcomes of interest, including stroke, dementia, and death.3 Evaluating the associations of serum NfL concentration with these outcomes is needed.
Elevated blood concentration of NfL in older adults may serve as a noninvasive biomarker to identify those with ongoing brain injury, while a cranial MRI may suggest the mechanism of that brain injury. The MRI findings of WMH and infarcts would suggest a covert vascular mechanism, which we have shown can be associated with elevated blood NfL concentration, and would prompt aggressive conventional or investigational interventions.8 Continued monitoring of blood NfL concentrations could be used to judge the success of those interventions. A degenerative process could also be present but would be especially likely if the MRI lacked findings of VBI in the setting of an elevated blood NfL concentration or if blood β-amyloid 42 was also found to be depressed in the blood.32 Future work will need to examine whether blood NfL concentrations are associated with manifestations of covert VBI, such as incoordination, depressed mood, and cognitive impairment, and are predictive of subsequent outcomes, such as overt clinically defined stroke, dementia, and death. The MRI findings of WMH and infarcts, as well as other findings reflecting covert VBI, may well be found to mediate such associations of blood NfL concentrations with manifestations and outcomes. Much work remains to be done to determine the utility, if any, of measuring blood NfL concentrations in routine care of older adults.
Acknowledgment
A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Glossary
- CHS
Cardiovascular Health Study
- GFAP
glial fibrillary acidic protein
- NfL
neurofilament light chain
- RR
relative risk
- UCH-L1
ubiquitin carboxyl-terminal hydrolase L1
- VBI
vascular brain injury
- WMG
white matter grade
- WMH
white matter hyperintensities
Appendix. Authors
Study Funding
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, N01HC15103, and 75N92021D00006, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurologic Disorders and Stroke. Additional support was provided by R01AG20098, R01AG023629, and R01AG053325 from the National Institute on Aging. K.J.M. was supported by K24AG065525 from the NIA.
Disclosure
B.M. Psaty serves on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson. All other authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Barro C, Chitnis T, Weiner HL. Blood neurofilament light: a critical review of its application to neurologic disease. Ann Clin Transl Neurol. 2020;7(12):2508-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Agtmaal MJM, Houben A, Pouwer F, Stehouwer CDA, Schram MT. Association of microvascular dysfunction with late-life depression: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(7):729-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Debette S, Schilling S, Duperron MG, Larsson SC, Markus HS. Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta-analysis. JAMA Neurol. 2019;76(1):81-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meinel TR, Kaesmacher J, Roten L, Fischer U. Covert brain infarction: towards precision medicine in research, diagnosis, and therapy for a silent pandemic. Stroke. 2020;51(8):2597-2606. [DOI] [PubMed] [Google Scholar]
- 5.Korley FK, Yue JK, Wilson DH, et al. Performance evaluation of a multiplex assay for simultaneous detection of four clinically relevant traumatic brain injury biomarkers. J Neurotrauma. 2018;36:182-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katsanos AH, Lioutas VA, Charidimou A, et al. Statin treatment and accrual of covert cerebral ischaemia on neuroimaging: a systematic review and meta-analysis of randomized trials. Eur J Neurol. 2020;27(6):1023-1027. [DOI] [PubMed] [Google Scholar]
- 7.Nasrallah IM, Pajewski NM, Auchus AP, et al. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA. 2019;322(6):524-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith EE, Markus HS. New treatment approaches to modify the course of cerebral small vessel diseases. Stroke. 2020;51(1):38-46. [DOI] [PubMed] [Google Scholar]
- 9.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263-276. [DOI] [PubMed] [Google Scholar]
- 10.Longstreth WT Jr, Dulberg C, Manolio TA, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2002;33(10):2376-2382. [DOI] [PubMed] [Google Scholar]
- 11.Longstreth WT Jr, Arnold AM, Beauchamp NJ Jr, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36(1):56-61. [DOI] [PubMed] [Google Scholar]
- 12.Longstreth WT Jr, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol. 1998;55(9):1217-1225. [DOI] [PubMed] [Google Scholar]
- 13.Longstreth WT Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: the Cardiovascular Health Study. Stroke. 1996;27(8):1274-1282. [DOI] [PubMed] [Google Scholar]
- 14.Raji CA, Lopez OL, Kuller LH, et al. White matter lesions and brain gray matter volume in cognitively normal elders. Neurobiol Aging. 2012;33(4):834e7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei K, Tran T, Chu K, et al. White matter hypointensities and hyperintensities have equivalent correlations with age and CSF β-amyloid in the nondemented elderly. Brain Behav. 2019;9(12):e01457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longstreth WT Jr., Diehr P, Manolio TA, et al. Cluster analysis and patterns of findings on cranial magnetic resonance imaging of the elderly: the Cardiovascular Health Study. Arch Neurol. 2001;58(4):635-640. [DOI] [PubMed] [Google Scholar]
- 18.Longstreth WT Jr, Diehr P, Beauchamp NJ, Manolio TA. Patterns on cranial magnetic resonance imaging in elderly people and vascular disease outcomes. Arch Neurol. 2001;58(12):2074. [DOI] [PubMed] [Google Scholar]
- 19.Zeileis A, Hothorn T. Diagnostic checking in regression relationships. R News. 2002;2(3):7-10. [Google Scholar]
- 20.Zeileis A. Econometric computing with HC and HAC covariance matrix estimators. J Stat Softw. 2004;11:1-17. [Google Scholar]
- 21.Fornage M, Debette S, Bis JC, et al. Genome-wide association studies of cerebral white matter lesion burden: the CHARGE consortium. Ann Neurol. 2011;69(6):928-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajan KB, Aggarwal NT, McAninch EA, et al. Remote blood biomarkers of longitudinal cognitive outcomes in a population study. Ann Neurol. 2020;88(6):1065-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sjogren M, Blomberg M, Jonsson M, et al. Neurofilament protein in cerebrospinal fluid: a marker of white matter changes. J Neurosci Res. 2001;66(3):510-516. [DOI] [PubMed] [Google Scholar]
- 24.Gattringer T, Pinter D, Enzinger C, et al. Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology. 2017;89(20):2108-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinter D, Gattringer T, Enzinger C, et al. Longitudinal MRI dynamics of recent small subcortical infarcts and possible predictors. J Cereb Blood Flow Metab. 2019;39(9):1669-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uphaus T, Bittner S, Groschel S, et al. NfL (neurofilament light chain) levels as a predictive marker for long-term outcome after ischemic stroke. Stroke. 2019;50(11):3077-3084. [DOI] [PubMed] [Google Scholar]
- 27.Tiedt S, Duering M, Barro C, et al. Serum neurofilament light: a biomarker of neuroaxonal injury after ischemic stroke. Neurology. 2018;91(14):e1338-e47. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen A, Stanne TM, Nilsson S, et al. Circulating neurofilament light in ischemic stroke: temporal profile and outcome prediction. J Neurol. 2019;266(11):2796-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Y, Tan L, Xu W, et al. Plasma neurofilament light and longitudinal progression of white matter hyperintensity in elderly persons without dementia. J Alzheimers Dis. 2020;75(3):729-737. [DOI] [PubMed] [Google Scholar]
- 30.Qu Y, Tan CC, Shen XN, et al. Association of plasma neurofilament light with small vessel disease burden in nondemented elderly: a longitudinal study. Stroke. 2021;52:896-904. [DOI] [PubMed] [Google Scholar]
- 31.Polymeris AA, Coslovksy M, Aeschbacher S, et al. Serum neurofilament light in atrial fibrillation: clinical, neuroimaging and cognitive correlates. Brain Commun 2020;2(2):fcaa166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Wolf F, Ghanbari M, Licher S, et al. Plasma tau, neurofilament light chain and amyloid-beta levels and risk of dementia; a population-based cohort study. Brain. 2020;143(4):1220-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khalil M, Pirpamer L, Hofer E, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun. 2020;11(1):812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rübsamen N, Maceski A, Leppert D, et al. Serum neurofilament light and tau as prognostic markers for all-cause mortality in the elderly general population: an analysis from the MEMO study. BMC Med. 2021;19(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duering M, Konieczny MJ, Tiedt S, et al. Serum neurofilament light chain levels are related to small vessel disease burden. J Stroke. 2018;20(2):228-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mielke MM, Syrjanen JA, Blennow K, et al. Plasma and CSF neurofilament light: relation to longitudinal neuroimaging and cognitive measures. Neurology. 2019;93(3):e252-e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zmira O, Halpern AI, Drori T. Anti-neurofilament antibodies and neurodegeneration: markers and generators. J Neuroimmunol. 2020;344:577248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request through the CHS Coordinating Center (CHS-NHLBI.org).