Abstract
The penetration by moxifloxacin of human neutrophils (polymorphonuclear leukocytes [PMN]) and tissue-cultured epithelial cells (McCoy cells) was evaluated by a fluorometric assay. At extracellular concentrations of 5 mg/liter, the cellular-to-extracellular concentration ratios (C/E) of moxifloxacin in PMN and McCoy cells were 10.9 ± 1.0 and 8.7 ± 1.0, respectively (20 min; 37°C). The uptake of moxifloxacin by PMN was rapid, reversible, nonsaturable (at extracellular concentrations ranging from 1 to 50 μg/ml), and not affected by cell viability. The uptake of moxifloxacin was affected by external pH and the environmental temperature. The incubation of PMN in the presence of sodium fluoride, sodium cyanide, and carbonyl cyanide m-chlorophenylhydrazone significantly decreased the C/E of this agent. Neither PMN stimulation nor phagocytosis of opsonized Staphylococcus aureus significantly affected the uptake of moxifloxacin by human PMN. This agent, at concentrations of 0.5, 1, and 5 mg/liter, induced a significant reduction in the survival of intracellular S. aureus in human PMN. In summary, moxifloxacin reaches much higher intracellular concentrations within phagocytic and nonphagocytic cells than extracellular ones, remaining active inside the neutrophils.
Fluoroquinolones are able to concentrate intracellularly in human phagocytic cells, fibroblasts, and epithelial and endothelial cells (10). Moreover, these agents are not affected by the intracellular environment and remain active against different facultative and obligate intracellular pathogens, such as Staphylococcus aureus (1, 12), Legionella spp. (17), Mycobacterium spp. (9), and Chlamydia spp. (7).
Moxifloxacin (BAY 12-8039) is a new 8-methoxyquinolone with a bicyclic amine substituent at the 7 position and with a broad spectrum of activity against both gram-negative and gram-positive bacteria (2). Moxifloxacin has been found to be 2 to 16 times more active than ciprofloxacin and ofloxacin against staphylococci, streptococci, enterococci, anaerobes, and Chlamydia spp. (3, 6, 18).
The purpose of this study was to evaluate the uptake of moxifloxacin by human polymorphonuclear leukocytes (PMN) and tissue-cultured epithelial cells. The mechanism involved in the penetration by this agent of human PMN and its intracellular activity compared with those of ofloxacin and ciprofloxacin were also evaluated.
MATERIALS AND METHODS
Isolation of PMN.
PMN were recovered from heparinized venous blood of healthy donors by using dextran sedimentation and a Ficoll-Hypaque gradient and purified by previously described methods (16). PMN preparations were 97% pure. Final cell suspensions were adjusted to 5 × 106 PMN per ml in Hanks balanced salt solution (HBSS) containing 1% gelatin. PMN were 95% viable as determined by trypan blue exclusion.
Tissue culture cells.
McCoy cells (Flow Laboratories, Irvine, United Kingdom) were grown in minimal essential medium (Flow) supplemented with 1 mM HEPES (Flow) and containing 10% fetal calf serum (Flow) without antibiotics. For each experiment, the cells were detached from tissue culture bottles with trypsin-EDTA (Flow), washed once with minimal essential medium containing fetal calf serum (10%), and suspended in HBSS at a concentration of 5 × 106 per ml.
Moxifloxacin uptake by cells.
A previously described fluorometric assay was used to measure quinolone uptake by human PMN and epithelial cells (11). Moxifloxacin was kindly supplied by Bayer AG, Leverkusen, Germany. In these experiments, PMN or tissue cells were incubated in HBSS containing different concentrations of antimicrobial agent (1 to 50 mg/liter). After different incubation times at 37°C, cells were separated from the extracellular solution by centrifugation through a water-impermeable silicone-oil barrier (density, 1,029 g/cm3) in a microcentrifuge tube. The entire cell pellet, obtained by cutting off the portion of the microcentrifuge tube containing the pellet, was placed in 2 ml of 0.1 M glycine-HCl buffer (pH 3.0) and agitated vigorously in a vortex shaker. Incubation for 2 h at room temperature was sufficient to release the intracellular antimicrobial agent fully (11). Samples were centrifuged for 5 min at 5,600 × g, and the amount of antimicrobial agent was determined by fluorescence emission of supernatants with an F 2000 fluorescence spectrophotometer (Hitachi, Tokyo, Japan). The fluorescence excitation and emission maxima in 0.1 M glycine-HCl (pH 3.0) were 295 and 498 nm, respectively. Controls without antimicrobial agents were always used to determine the background fluorescence.
Intracellular water space was measured by using tritiated water and the extracellular marker [14C]polyethylene glycol (1.4 mCi/g; New England Nuclear Corp., Boston, Mass.). Cells were incubated with these radiolabeled compounds for 2 min at 37°C, separated from extracellular fluid by velocity gradient centrifugation as described above, and counted in a liquid scintillation counter. Total water content of the cell pellet was corrected for trapped extracellular water, i.e., polyethylene glycol space, to obtain the intracellular water space. From the values obtained by this procedure, cell-associated antimicrobial agent concentrations were calculated and expressed as ratios of the cellular concentration to extracellular concentration (C/E ratios) (8).
The efflux of reversibility of the binding of PMN or tissue cell-associated moxifloxacin was also studied. Cells were incubated for 20 min at 37°C with moxifloxacin (extracellular concentration, 5 mg/liter), collected by centrifugation, and rapidly suspended in quinolone-free medium. Cell-associated moxifloxacin was quantitated at various intervals (1, 5, 10, 20, and 30 min) after removal of the extracellular antimicrobial agent. All assays were performed in duplicate with PMN from five donors.
Characterization of moxifloxacin uptake.
Further studies to elucidate the mechanism of moxifloxacin uptake by PMN were performed as described previously (11). The importance of cell viability was studied by using PMN killed by exposure to 10% formalin for 30 min. These cells were washed and then suspended in fresh medium. Moreover, the influences of environmental temperature, pH, and metabolic inhibitors were evaluated. The influence of temperature was examined by comparing antimicrobial uptake at 4 and 37°C. The pH profiles of moxifloxacin uptake in media preadjusted to different external pHs (pH 5, 6, 7, and 8) by the addition of 10 N HCl or 10 N NaOH were measured. An inhibitor of glycolysis (sodium fluoride, 1.5 × 10−3 M) (Sigma Chemical Co., St. Louis, Mo.), an inhibitor of mitochondrial oxidative metabolism (sodium cyanide, 1.5 × 10−3 M) (Sigma), a blocker of the proton gradient (carbonyl cyanide m-chlorophenylhydrazone [CCCP]; 1.5 × 10−5 M) (Sigma), and an uncoupler of oxidative phosphorylation (2,4-dinitrophenol; 1 × 10−4 M) (Sigma) were used as metabolic inhibitors. PMN in HBSS with and without metabolic inhibitors were incubated for 30 min at 37°C. Moxifloxacin (final concentration, 5 mg/liter) was then added, and the uptake was measured as described above.
In a series of experiments, moxifloxacin (extracellular concentration, 5 mg/liter) uptake by human PMN was measured after the stimulation of cells with 200 nM phorbol myristate acetate (PMA; Sigma) and after the phagocytosis of S. aureus ATCC 25923 opsonized in 5% pooled human serum (15 min, 37°C) at a 10/1 ratio of bacteria to PMN. PMA or opsonized bacteria were added to PMN suspensions at the same time as the antimicrobial agent, and the uptake was measured as described above. Controls were always used to evaluate the effects of inhibitors and substrates on the fluorescence of moxifloxacin in cell-free systems. All assays were performed in duplicate with PMN from five different donors.
Organisms and susceptibility testing.
S. aureus ATCC 25923 was used for the killing assays. Susceptibility studies were determined by dilution assay. The MICs and minimum bactericidal concentrations of ciprofloxacin (Bayer AG), ofloxacin (Hoechst AG), and moxifloxacin for this strain were 0.25, 0.25, and 0.06 mg/liter, respectively.
Intracellular activity of antimicrobial agents.
To evaluate the intracellular activity of antimicrobial agents, a previously described method was used (15). Briefly, 0.1 ml of bacterial suspension preopsonized in 5% pooled human serum (5 × 107 CFU/ml) and 0.1 ml of PMN (5 × 106 per ml) were combined in a series of polypropylene biovials (Beckman) and the vials were incubated in a shaker (50 rpm) for 60 min at 37°C. After incubation, the mixtures were washed three times with 2.5 ml of ice-cold phosphate-buffered saline (pH 7.2) by using differential centrifugation (160 × g; 5 min at 4°C) to remove the extracellular bacteria. The cells were then suspended in 0.2 ml of RPMI medium (Sigma). At this time (designated time zero) different concentrations of the different fluoroquinolones (0.125 to 5 mg/liter) were added, and the vials were reincubated in a shaker (50 rpm) at 37°C. The vials were removed at time zero (control without antimicrobial agents) and after 3 h of incubation (control without antimicrobial agents and samples with antimicrobial agents). Cells were lysed in distilled water, and samples were diluted and pour plated in agar. Colonies were counted after 24 h of incubation at 37°C. The data were expressed as percentages of staphylococci surviving, compared with the levels in the controls (without antimicrobial agents) at 3 h. In addition to determining bacterial survival, morphologic studies were also routinely performed at time zero and after 3 h of incubation to evaluate the disposition of bacteria (cell associated or extracellular). Samples (50 μl) were removed from biovials and were deposited on glass slides. After being stained with Wright stain, the samples were examined by light microscopy. All assays were performed in duplicate with PMN from five different donors.
Statistical analysis of data.
Data were expressed as means ± standard deviations. Differences among groups were compared by analysis of variance, used to assess statistical significance at a P value of ≤0.05.
RESULTS
Uptake of moxifloxacin by PMN and tissue-cultured epithelial cells.
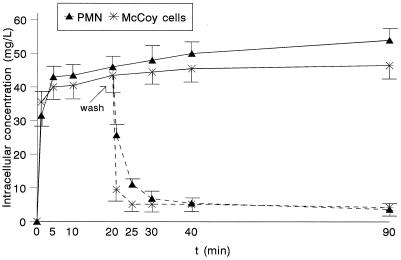
Figure 1 shows the kinetics of uptake of moxifloxacin by both human PMN and tissue-cultured epithelial cells. Moxifloxacin uptake by these cells was rapid and high. With extracellular concentrations of 5 mg/liter, the C/E ratios were higher than 6 after 1 min of incubation. The kinetics of efflux of moxifloxacin in both types of cells are also shown in Fig. 1. The reversibility of binding of moxifloxacin was rapid, with 75 and 88% of the cell-associated drug being lost by 5 min in PMN and McCoy cells, respectively.
FIG. 1.
Moxifloxacin uptake by both human PMN and McCoy cells and efflux of cell-associated moxifloxacin after the removal of the extracellular drug (n = 5). The extracellular concentration was 5 mg/liter.
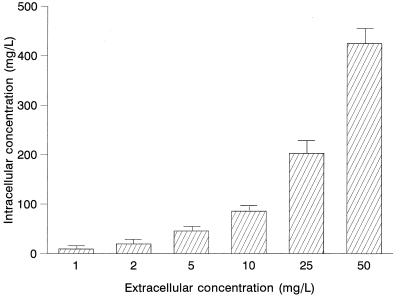
The effect of extracellular concentrations of moxifloxacin on uptake by PMN is presented in Fig. 2. Cell-associated moxifloxacin was not saturable at concentrations ranging from 1 to 50 mg/liter. Similar results were observed when McCoy cells were used.
FIG. 2.
Effect of extracellular concentrations on the intracellular penetration of moxifloxacin in human PMN (n = 5).
The effects of environmental temperature, cell viability, and pH on the uptake of moxifloxacin by human PMN are shown in Table 1. The intracellular penetration of moxifloxacin was significantly impaired at 4°C but was not affected by cell viability. The C/E values also decreased at pH 5, 6, and 8.
TABLE 1.
Effect of environmental temperature, cell viability, and external pH on the uptake of moxifloxacin by human PMN
| Conditionsa | C/E ratio |
|---|---|
| Viable cells at 37°C (control) | 10.9 ± 1.0 |
| Viable cells at 4°C | 2.3 ± 0.9b |
| Formalin-killed cells | 11.1 ± 1.4 |
| pH 5 | 7.2 ± 0.2b |
| pH 6 | 9.1 ± 0.3 |
| pH 7 | 9.4 ± 0.2 |
| pH 8 | 8.4 ± 0.2b |
Experiments were carried out for 20 min at an extracellular concentration of 5 mg/liter with PMN from five subjects.
P ≤ 0.05 compared to viable cells at 37°C.
The effects of different metabolic inhibitors on moxifloxacin uptake by human PMN are shown in Table 2. Among the inhibitors evaluated, sodium fluoride, sodium cyanide, and CCCP significantly impaired the intracellular penetration of this quinolone. The stimulation of PMN by a membrane activator (PMA) and the phagocytosis of opsonized S. aureus did not affect the intracellular penetration by moxifloxacin of PMN (Table 2).
TABLE 2.
Effect of metabolic inhibitors and membrane stimuli on the uptake of moxifloxacin by human PMN
| Conditiona | C/E ratio |
|---|---|
| Viable cells at 37°C (control) | 10.9 ± 1.0 |
| Metabolic inhibitor | |
| Sodium fluoride | 6.8 ± 1.3b |
| Sodium cyanide | 5.8 ± 0.7b |
| CCCP | 5.7 ± 1.3b |
| 2,4-Dinitrophenol | 9.9 ± 1.0 |
| Stimulus | |
| PMA | 11.0 ± 0.9 |
| S. aureus | 9.1 ± 0.3 |
Experiments were carried out for 20 min at an extracellular concentration of 5 mg/liter with PMN from five subjects.
P ≤ 0.05 compared to viable cells at 37°C.
Intracellular activity of moxifloxacin against S. aureus.
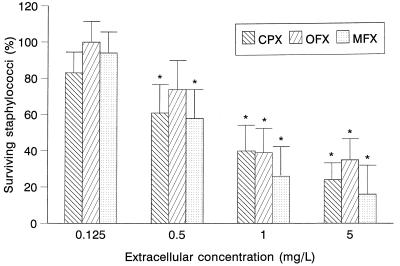
The intracellular activity of moxifloxacin against S. aureus ATCC 25923 compared with those of ciprofloxacin and ofloxacin was evaluated by a 3-h assay (Fig. 3). At extracellular concentrations of 0.5, 1, and 5 mg/liter, moxifloxacin showed significant intracellular activity against that microorganism compared to a control without antimicrobial agents. This activity was similar to that observed for ciprofloxacin and ofloxacin.
FIG. 3.
Intracellular activity of moxifloxacin against S. aureus in human PMN in a 3-h assay (n = 5). Data are expressed as percentages of intracellular surviving staphylococci compared to controls without antimicrobial agents (100%). ∗, P ≤ 0.05 compared with control without antimicrobial agent. CPX, ciprofloxacin; OFX, ofloxacin; MFX, moxifloxacin.
DISCUSSION
The intracellular penetration by moxifloxacin of human PMN and tissue-cultured epithelial cells was evaluated. At therapeutic extracellular concentrations, moxifloxacin reached intracellular concentrations in PMN 9 or more times higher than extracellular ones. The C/E ratio of this quinolone was slightly higher than those observed previously for ciprofloxacin, ofloxacin, sparfloxacin, and other quinolones (4, 5, 12, 13).
Moxifloxacin also reached high intracellular concentrations in tissue-cultured epithelial cells. These values are higher than those observed for ofloxacin, levofloxacin, and lomefloxacin and similar to those observed for trovafloxacin and sparfloxacin (4, 12–14). The greater uptake of trovafloxacin and sparfloxacin by these cells may be related to the higher hydrophobicity of these molecules, which allows them to cross the cytoplasmic membranes more easily. This phenomenon, however, would not explain the high accumulation of moxifloxacin, whose hydrophobicity is similar to that ofloxacin. Since moxifloxacin showed high intrinsic activity against Chlamydia spp. and since these microorganisms can multiply within epithelial cells, these results could reinforce the potential use of this agent against chlamydial infections (18).
The penetration by moxifloxacin of human PMN was rapid, nonsaturable, and reversible. In contrast to the case with most quinolones, the uptake of moxifloxacin by PMN was significantly decreased at acidic pHs (3, 4, 10, 13). This may be related to the fact that this quinolone displays a bicyclic amine and a metoxy substituent at the 7 and 8 positions, respectively. The uptake of moxifloxacin was affected by environmental temperature and some metabolic inhibitors, as has been described for ofloxacin and BAY Y 3118 (5, 11). The uptake of other quinolones, such as sparfloxacin and trovafloxacin, was not affected by any of these parameters (4, 14). The mechanisms whereby quinolones accumulate in cells are not yet known, and no simple model can be presented in view of the findings presented above. Although most data point towards a passive mechanism, some are typical of an active one. Sparfloxacin and trovafloxacin seem to use a passive mechanism, probably related to their high hydrophobicities, which allow easier penetration of cell membranes. Other less hydrophobic quinolones, such as ofloxacin, BAY Y 3118, and moxifloxacin, seem partially to require an active process. As has been described previously for other quinolones (4, 14), neither the phagocytosis of S. aureus nor the stimulation of the cell membrane affected the intracellular accumulation of moxifloxacin in PMN.
Moxifloxacin showed high intracellular activity against S. aureus, similar to that of ciprofloxacin and ofloxacin. This activity was dose dependent and probably related to intrinsic activity against the strain used, its ability to concentrate within phagocytes, and the fact that it is not affected by the intracellular environment. Although the intrinsic activity of moxifloxacin was superior to that of ciprofloxacin and ofloxacin, its intracellular activity was only slightly higher than theirs. This discrepancy could be related to different degrees of quinolone activity in the intracellular compartment or to limitations in the method of detecting differences of activity in compounds offering very high intracellular activity.
In summary, moxifloxacin penetrates phagocytic and nonphagocytic cells, reaching intracellular concentrations several times higher than extracellular ones, while it remains active intracellularly in human PMN. The high intracellular activity of this agent and its broad spectrum of activity, in addition to the properties observed in this study, enhance the potential uses of moxifloxacin.
ACKNOWLEDGMENTS
We thank Patricia Hidalgo and Janet Dawson for preparation of the manuscript.
This study was partially supported by Bayer AG.
REFERENCES
- 1.Buggy B P, Schaberg D R, Swartz R D. Intraleukocytic sequestration as a cause of persistent Staphylococcus aureus peritonitis in continuous ambulatory peritoneal dialysis. Am J Med. 1984;76:1035–1040. doi: 10.1016/0002-9343(84)90854-4. [DOI] [PubMed] [Google Scholar]
- 2.Dalhoff A, Petersen U, Endermann R. In vitro activity of BAY 12-8039, a new 8-methoxyquinolone. Chemotherapy (Basel) 1996;42:410–425. doi: 10.1159/000239474. [DOI] [PubMed] [Google Scholar]
- 3.Fass R J. In vitro activity of BAY 12-8039, a new 8-methoxyquinolone. Antimicrob Agents Chemother. 1997;41:1818–1824. doi: 10.1128/aac.41.8.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.García I, Pascual A, Guzmán M C, Perea E J. Uptake and intracellular activity of sparfloxacin in human polymorphonuclear leukocytes and tissue culture cells. Antimicrob Agents Chemother. 1992;36:1053–1056. doi: 10.1128/aac.36.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García I, Pascual A, Perea E J. Intracellular penetration and activity of BAY Y 3118 in human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1994;38:2426–2499. doi: 10.1128/aac.38.10.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein E, Citron D M, Hudspeth M, Gerardo S H, Merriam C V. In vitro activity of BAY 12-8039, a new 8-methoxyquinolone, compared to activities of 11 other oral antimicrobial agents against 390 aerobic and anaerobic bacteria isolated from human and animal bite wound skin and soft tissue infection in humans. Antimicrob Agents Chemother. 1997;41:1552–1557. doi: 10.1128/aac.41.7.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones, R. B., B. Van der Pol, and R. B. Johnson. 1997. Susceptibility of Chlamydia trachomatis to trovafloxacin. J. Antimicrob. Chemother. 39(Suppl. B):63–65. [DOI] [PubMed]
- 8.Klempner M S, Styrt B. Clindamycin uptake by human neutrophils. J Infect Dis. 1989;144:472–475. doi: 10.1093/infdis/144.5.472. [DOI] [PubMed] [Google Scholar]
- 9.Mor N, Vanderkoek J, Heifeits L. Inhibitory and bactericidal activities of levofloxacin against Mycobacterium tuberculosis in vitro and in human macrophages. Antimicrob Agents Chemother. 1994;38:1161–1164. doi: 10.1128/aac.38.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pascual A. Uptake and intracellular activity of antimicrobial agents in phagocytic cells. Rev Med Microbiol. 1995;6:228–235. [Google Scholar]
- 11.Pascual A, García I, Perea E J. Fluorometric measurement of ofloxacin uptake by human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1989;33:653–656. doi: 10.1128/aac.33.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pascual A, García I, Perea E J. Uptake and intracellular activity of an optically active ofloxacin isomer in human neutrophils and tissue culture cells. Antimicrob Agents Chemother. 1990;34:277–280. doi: 10.1128/aac.34.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pascual A, García I, Perea E J. Effect of lomefloxacin and temafloxacin into human neutrophils, peritoneal macrophages and tissue culture cells. Diagn Microbiol Infect Dis. 1992;15:393–398. doi: 10.1016/0732-8893(92)90079-9. [DOI] [PubMed] [Google Scholar]
- 14.Pascual A, García I, Ballesta S, Perea E J. Uptake and intracellular activity of trovafloxacin in human phagocytes and tissue-cultured epithelial cells. Antimicrob Agents Chemother. 1997;41:274–277. doi: 10.1128/aac.41.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pascual A, Tsakayama D, Kovarik J, Gekker G, Peterson P K. Uptake and activity of rifapentine in human peritoneal macrophages and polymorphonuclear leukocytes. Eur J Clin Microbiol. 1987;6:152–157. doi: 10.1007/BF02018197. [DOI] [PubMed] [Google Scholar]
- 16.Peterson P K, Verhoef J, Schmeling D, Quie P G. Kinetics of phagocytosis and bacterial killing by human polymorphonuclear leukocytes and monocytes. J Infect Dis. 1977;136:502–509. doi: 10.1093/infdis/136.4.502. [DOI] [PubMed] [Google Scholar]
- 17.Walz A, Nichterlein T, Hof H. Excellent activity of newer quinolones on Legionella pneumophila in J774 macrophages. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1997;285:431–439. doi: 10.1016/s0934-8840(97)80009-6. [DOI] [PubMed] [Google Scholar]
- 18.Woodcock J M, Andrews J M, Boswell F J, Brenwald N P, Wise R. In vitro activity of BAY 12-8039, a new fluoroquinolone. Antimicrob Agents Chemother. 1997;41:101–106. doi: 10.1128/aac.41.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]