BACKGROUND:
We have sought to develop methodology for deriving optimal bispectral index (BIS) values (BISopt) for patients with moderate/severe traumatic brain injury, using continuous monitoring of cerebrovascular reactivity and bispectral electroencephalography.
METHODS:
Arterial blood pressure, intracranial pressure, and BIS (a bilateral measure that is associated with sedation state) were continuously recorded. The pressure reactivity index, optimal cerebral perfusion pressure (CPPopt), and BISopt were calculated. Using BIS values and the pressure reactivity index, a curve fitting method was applied to determine the minimum value for the pressure reactivity index thus giving the BISopt.
RESULTS AND CONCLUSIONS:
Identification of BISopt was possible in all of the patients, with both visual inspection of data and using our method of BISopt determination, demonstrating a similarity of median values of 44.62 (35.03–59.98) versus 48 (39.75–57.50) (p = 0.1949). Furthermore, our method outperformed common CPPopt curve fitting methods applied to BISopt with improved percent (%) yields on both the left side 52.1% (36.3–72.4%) versus 31.2% (23.0–48.9%) (p < 0.0001) and the right side 54.1% (35.95–75.9%) versus 33.5% (12.5–47.9%) (p < 0.0001). The BIS values and BISopt were compared with cerebral perfusion pressure, mean arterial pressure, and CPPopt. The results indicated that BISopt’s impact on pressure reactivity was distinct from CPPopt, cerebral perfusion pressure, or mean arterial pressure. Real-time BISopt can be derived from continuous physiologic monitoring of patients with moderate/severe traumatic brain injury. This BISopt value appears to be unassociated with arterial blood pressure or CPPopt, supporting its role as a novel physiologic metric for evaluating cerebral autoregulation. BISopt management to optimize cerebrovascular pressure reactivity should be the subject of future studies in moderate/severe traumatic brain injury.
Keywords: bispectral index, cerebrovascular reactivity, hemodynamic monitoring, neurocritical care, sedation, traumatic brain injury
Although current guideline-based treatments in moderate/severe traumatic brain injury (TBI) have improved outcomes over the past 25 years, mortality and morbidity remains high (1). Impaired cerebrovascular reactivity, a surrogate of cerebral autoregulation, has an emerging association with long-term outcomes in moderate/severe TBI, with recent work highlighting its independent link with 6-month outcomes (2–5). Evaluation of multimodal cerebral physiologic monitoring in TBI cohorts has demonstrated that a large portion of time spent in the ICU with physiologic dysfunction is dominated by impaired cerebrovascular reactivity (2, 3, 6–8). Despite improvements in the ability to reach intracranial pressure (ICP) and cerebral perfusion pressure (CPP) targets (8–10), current ICU-based therapeutic interventions in TBI have demonstrated little impact on continuously assessed cerebrovascular reactivity (1).
IV sedation is used as part of the standard treatment for moderate/severe TBI, to aid with mechanical ventilation, reduce cerebral metabolic demand, and attenuate ongoing secondary injury pathways (11, 12). However, evidence suggests that exposure to excessive sedation is linked with poor cognitive long-term outcomes in both TBI and general ICU populations (13–15), although the mechanisms for these associations is unclear. Recent work has suggested that titration of IV sedative agents has a negligible impact on cerebrovascular reactivity, both at the macrovascular and at the microvascular level (16–18). Yet, these studies have only compared dose-titration data directly to physiologic monitoring without accounting for patient-specific pharmacodynamic profiles that lead to variability in drug response (16–18). Thus, without objective quantifications of depth of sedation, the true impact of sedation on cerebrovascular reactivity is uncertain.
Finally, individualized optimal CPP (CPPopt) uses pressure reactivity index (PRx; correlation between ICP and mean arterial pressure [MAP]) (19) and CPP to demonstrate a parabolic relationship between CPP and PRx (20–22). Leveraging similar techniques, we have recently demonstrated that there is an association between individualized depth of sedation and cerebrovascular reactivity (23). Using continuous electroencephalogram-based entropy index (bispectral index [BIS]; BIS a potential route for depth of sedation monitoring) and PRx (19), we have shown that a parabolic relationship exists between BIS and PRx. The minimum of this parabolic curve represents the BIS value at which PRx was the most negative (i.e., cerebrovascular reactivity was most intact) and thus a potential route for individualized depth of sedation. However, it was unclear from this preliminary analysis whether this sedation effect on cerebrovascular reactivity was merely occurring through changes in MAP or acting as an independent physiologic metric.
We hypothesize that depth of sedation plays an important role in modulating cerebrovascular reactivity in TBI, independent from changes to CPP. The goal of this study is to explore the concept of optimal BIS (BISopt; a potential route for optimal sedation depth) based on BIS and PRx monitoring in a prospective cohort of moderate/severe TBI patients, highlighting: A) the presence of this novel target, B) its relationship with MAP and CPPopt, and C) preliminary attempts at continuous derivation.
MATERIALS AND METHODS
Ethics
Data were collected following full approval by the University of Manitoba Health Research Ethics Board (H2017:181, H2017:188, and B2019:065) and the Health Sciences Centre Research Impact Committee (R2019:072).
Patient Population and Data Collection
This was a retrospective observational study, inclusion criteria for the study were adult patients (> 16 yr old) with moderate/severe TBI (Glasgow Coma Scale 12 or less), requiring invasive ICP monitoring as determined by the Brain Trauma Foundation guidelines (11).
Admission demographic information were extracted following the existing prognostic models in moderate and severe TBI (24).
High-frequency arterial blood pressure, ICP, and BIS data were collected, see Supplementary File A (http://links.lww.com/CCX/A943) for more details. Note, BIS and BISopt values were obtained from the hemisphere that had no frontal lobe contusion, overlying hematoma, or subgaleal/scalp hematoma, with visual inspection of the electromyography signal of the frontalis indicating no large firing potentials, ensuring no muscle artifacts were present. Finally, no patients had neuromuscular blockade agents administered during the periods of recorded physiology and paroxysmal sympathetic hyperactivity was not actively monitored for or clinically detected in this cohort (as such active treatment was not administer), both may impact BIS values.
Signal Processing
Signal processing was done after the data was recorded in the ICU, with all signal artifacts removed using manual methods. All signal analysis work was conducted using Intensive Care Monitoring (ICM+) software (Cambridge Enterprise Ltd, Cambridge, UK, http://icmplus.neurosurg.cam.ac.uk) and R statistical computing. (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/) MAP and ICP were decimated over a 10-second nonoverlapping moving average filter. CPP was derived as MAP–ICP. PRx was derived using the standard Pearson correlation between 30 consecutive 10-second windows of ICP and MAP, updated every minute (1, 3, 25–27). CPPopt was determined in individual patients through the use of the published optimal Flex methodology, for more details, see Supplementary File B (http://links.lww.com/CCX/A943) (3, 28–30). All final data was output in a minute-by-minute updated frequency.
Optimal Depth of Sedation Determination (BISopt)
Similar to past work by Aries et al (20) in CPPopt determination, a custom-created automatic quadratic curve fitting method was applied to the binned 60-second BIS data and PRx to determine the BIS value with the lowest associated PRx values (for details, see Supplementary File C, http://links.lww.com/CCX/A943).
BISopt was first calculated over the entire recording period, for each patient, similar to the original CPPopt work by Steiner et al (21) (for results see Supplementary Files D and E, http://links.lww.com/CCX/A943).
Next, a continuous time trend of BISopt was calculated using the above-mentioned curve fitting methods, generated from a moving 4-hour time window updated every minute. The BISopt curve could be generated when at least 50% of the required data points of PRx were available, that is, after a minimum of 2 hours of monitoring, keeping with CPPopt (20–22, 29, 30).
Finally, to evaluate existing CPPopt algorithms, we used the multiwindow weighted optimal Flex methodology within ICM+ to determine a BISopt value. BIS values ranged from 20 to 80 arbitrary units (au) with a maximum window size of 20-hour window, where the BISopt was calculated. This was conducted to evaluate the performance of existing CPPopt methods when directly applied to our custom-created BISopt calculations.
Statistical Analysis
Statistical analysis was conducted using R statistical computing software (R Foundation for Statistical Computing). Descriptive analyses were of BISopt/BIS and its associations with other physiologic parameters of interest in TBI care. All physiologic variables were found to be non-parametric in nature via Shapiro-Wilk testing. Alpha was set at 0.05 with no correction for multiple comparisons given the exploratory nature of this study.
Initially, patient characteristics and BISopt values were summarized using descriptive techniques. Visually obtained BISopt values from the entire recording period, for each patient, were compared with those derived from our quadratic curve fitting algorithm using Wilcoxon signed-ranked test.
Next, we found the percentage of time that BISopt could be determined over each patient using a sliding 4-hour window that produced an updating value every minute. This included only time where BIS was sufficiently recorded to achieve at least 2-hour of interference-free data (20). Thus, we calculated the % yield for our algorithmic method and optimal Flex. Compared % yield of BISopt calculation between the two methods using Wilcoxon signed-ranked test, to determine which had a more optimal % yield.
Using our method of determining BISopt, we also performed a subgroup analysis by finding the % yield of BISopt and its association with all recorded sedative agents/dose and vasopressor agents used in this cohort. The data were separated for times which BISopt could be found (i.e., both BIS and PRx was available), and the sedative agents/dose over this time were added from bedside nursing charts. Due to the variability in dose and its weak association with sedation depth, three categories of sedation dose were chosen for each agent (high, moderate, and low) (31, 32). See Supplementary File G (http://links.lww.com/CCX/A943) for more information.
The association between BISopt and both MAP and CPPopt was determined between the minute-by-minute data for the entire recording period. First, using the entire recording period BISopt, CPPopt, and mean MAP were determined for each patient. Scatterplots were derived for BISopt versus MAP and BISopt versus CPPopt, for the entire population. Linear regression analysis was subsequently performed.
Next, using continuously derived PRx, we determined when the patient had intact (PRx < 0.2) versus impaired (PRx > 0.2) cerebrovascular reactivity (2, 26, 33). The data were then dichotomized with each state of cerebral reactivity, the BISopt (based on our method) was then compared with the minute-by-minute derived MAP and CPPopt over impaired and intact cerebrovascular reactivity using Kendall’s tau correlation (note the data dichotomization was only done comparing BIS relations to systemic pressure changes as the autoregulatory states may influence vascular response). Thus, we compared the minute-by-minute values of BIS to MAP using a Kendall’s tau correlation methodology over impaired and intact cerebrovascular reactivity, to confirm that the BIS values were sufficiently isolated from MAP phenomena.
Finally, BIS/BISopt were compared with ICP using the previously described methods for MAP/CPPopt, see Supplementary Files J and K (http://links.lww.com/CCX/A943) for details.
RESULTS
Demographics and Grand Averages of Monitored Modalities
Thirty-two patients were recruited with characteristics summarized in Table 1. The sedative regimens used were fentanyl and/or propofol to achieve a baseline sedation level with the addition of ketamine in three patients. The Richmond Agitation-Sedation Scale (RASS) was extracted from bedside charts and indicated to be –4 for nearly all time recorded, although this value was recorded often with over 4 hours of time between events and thus has a limited overall interpretation (34). The exact sedation regimen to reach the RASS goal of –4 was determined by the treating intensivist, without a defined algorithm present within our ICU.
TABLE 1.
Thirty-Two Patient Demographics
| Demographics | Median (IQR) or No. of Patients |
|---|---|
| Age | 43 (23–55) |
| Sex (% male) | 87.5 |
| Best admission GCS—total | 6.5 (4–10) |
| Best admission GCS—motor | 4 (2–5) |
| Number with hypoxia episode | 10 |
| Number with hypotension episode | 6 |
| Number with traumatic subarachnoid hemorrhage | 30 |
| Number with epidural hematoma | 3 |
| Pupils | |
| Bilateral unreactive | 3 |
| Unilateral unreactive | 6 |
| Bilateral reactive | 23 |
| Admission Marshall CT | |
| V | 17 |
| IV | 3 |
| III | 9 |
| II | 3 |
GCS = Glasgow Coma Score, IQR = interquartile range.
BISopt for Entire Recording
The mean recording time per patient after the removal of artifacts and empty BIS bilateral data was 0.80 days (0.0–5.15 d), with all patients at least having one hemisphere of usable data. Overall, visualization determination of an individual BISopt over the entire recording period was possible in all patients using visual inspection, 84.4% on the left side and 71.9% on the right side, see additional information in Supplementary File E (http://links.lww.com/CCX/A943).
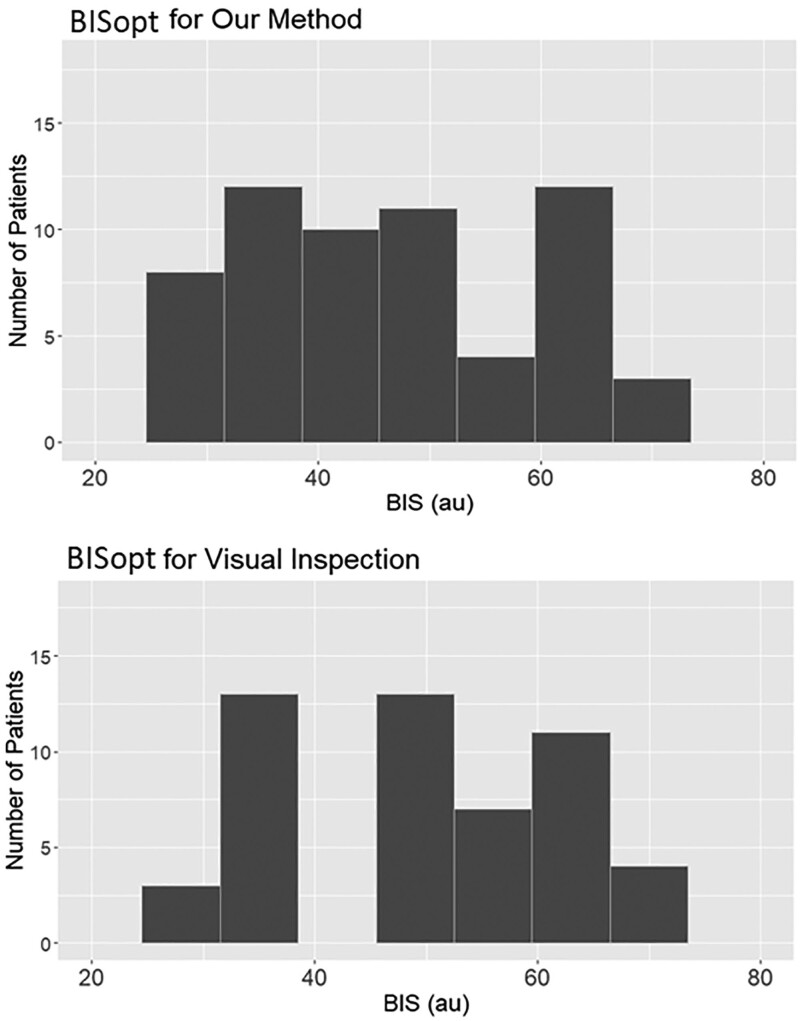
Using our custom algorithmic method, we could find a BISopt in all of the complete recordings (chosen as the most optimal bilateral BIS values) with Figure 1 demonstrating histograms of our algorithmic method BISopt values versus direct visualization BISopt values from error bar plots. Often the BISopt values were within 5 au from one another, with our algorithmic method versus direct visualization, still consistently close as to fail the Wilcoxon test 44.62 (35.03–59.98) versus 48 (39.75–57.50) (p = 0.1949).
Figure 1.
Optimal bispectral index (BISopt) through visual inspection and our method. The different bispectral index (BIS) values associated with each BISopt method. The Wilcoxon signed-ranked test of 0.1949 is the p value between these two histograms, demonstrating that the median values are similar. au = arbitrary units.
Continuous Derivation of BISopt—Comparison of Different Methods
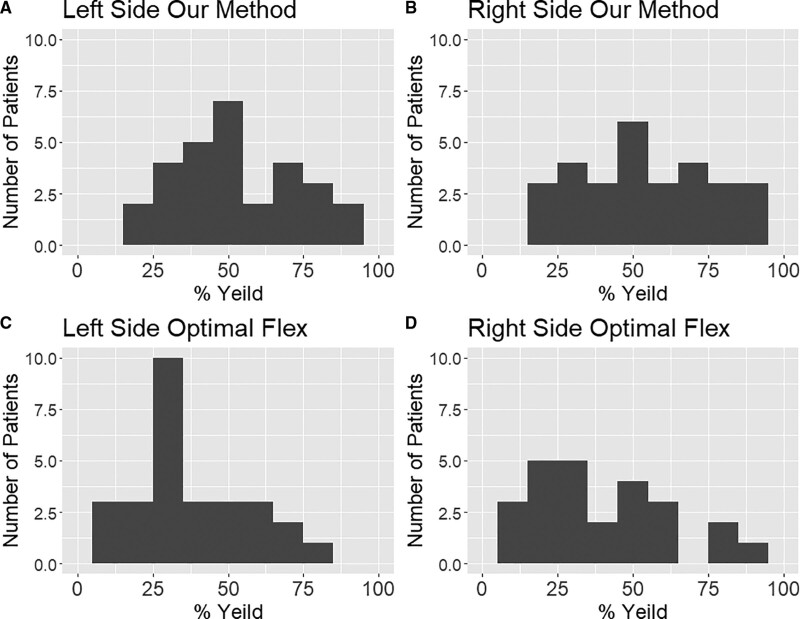
The optimal Flex method produced slightly inaccurate results (see Fig. 2 and Supplementary File F, http://links.lww.com/CCX/A943). When comparing the percent yield for BISopt over each patient, our method outperformed optimal Flex method as determined by % yield and a Wilcoxon test. The % yield for our method versus optimal flex on the left side was 52.1% (36.3–72.4%) versus 31.2% (23.0–48.9%) (p < 0.0001) and the right side 54.1% (35.95–75.9%) versus 33.5% (12.5–47.9%) (p < 0.0001).
Figure 2.
Histogram plots for % yield of different methods. A and B, The left and right side percent yield using our method for each patient. C and D, The left and right side of the percent yield of optimal Flex method for each patient.
Continuous Derivation of BISopt—Comparison of Different Agents
Supplementary File G (http://links.lww.com/CCX/A943) displays all the relationships between sedation dose/agents and vasopressor agents with our method of determining BISopt % yield. This analysis demonstrated that fentanyl and propofol whether together or separate attained roughly the same % yield in BISopt, likewise no matter the combination of vasopressor agents BISopt had a similar % yield. High levels of sedation demonstrated a slight increase in % yield of BISopt calculation, with low BIS values demonstrating an increase in PRx.
BISopt Association With ICP, MAP, and CPPopt—Entire Recording Period
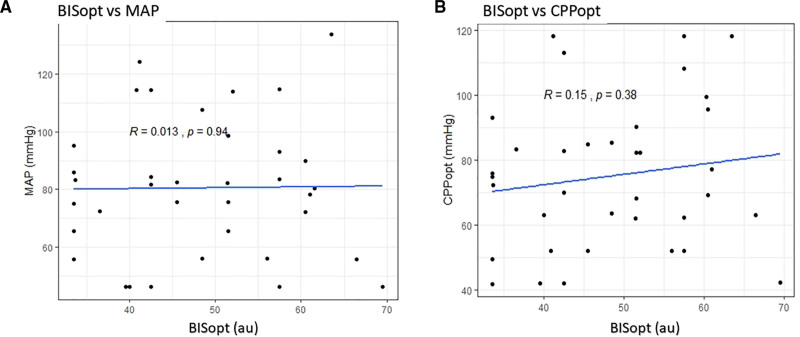
Figure 3 and Supplemen-tary Files I and J (http://links.lww.com/CCX/A943), displays the BISopt versus CPPopt/MAP/ICP relationships, derived over the entire recording period. Linear regression analysis failed to demonstrate any significant relationships between BISopt and either CPPopt/MAP/ICP.
Figure 3.
Scatter plot of optimal bispectral index (BISopt) and mean arterial pressure (MAP)/optimal cerebral perfusion pressure (CPPopt). A, BISopt versus mean MAP over the entire recording period for each patient with a Pearson correlation between the values, demonstrating no correlation between the values. B, BISopt versus CPPopt over the entire recording period for each patient with a Pearson correlation between the values, demonstrating no correlation between the values. au = arbitrary units, mm Hg = millimeter of mercury.
BISopt/BIS Association With ICP, MAP, and CPPopt—Minute-by-Minute Data
Through the comparisons of BISopt values to ICP, MAP, and CPPopt and comparisons of BIS values to ICP and MAP, using minute-by-minute data, there were no grossly significant relationships found through Kendall correlation analysis. Nearly, all correlation coefficients calculated were below 0.5, with no significant difference within the results. Analysis outputs can be found in Supplementary Files H and K (http://links.lww.com/CCX/A943).
DISCUSSION
This study assessed and confirmed the presence of a BISopt value in individual patients suffering moderate/severe TBI, expanding on our prior work (23). Further, we assessed the relationship between BISopt and other important physiologic aspects in TBI and critical care, namely MAP and CPPopt. By adapting previously described CPPopt curve fitting methods, we determined an BISopt value (a potential surrogate measure for depth of sedation) as attained by the lowest PRx value. This BISopt value was compared with ICP, MAP, and CPPopt in the entire cohort, showing no correlation. Individual BIS values were also compared with MAP and CPPopt using a Kendall’s tau test over the entire recording period for each patient and dichotomized for intact and impaired autoregulation. Thus, we were able to highlight that BISopt was sufficiently uncoupled from CPPopt, suggesting BISopt as a potential distinct metric for personalized physiologic targeting in critical care. Finally, we outlined a method that can be used to derive a continuously updating BISopt value from patient data. Although the results here remain preliminary, some important aspects deserve highlighting.
First, nearly all of the patients within our cohort displayed a unique BISopt value that confirms our previous findings (23) and highlights the novel relationship between BIS values and cerebrovascular reactivity. This is despite past studies that have assessed sedation and cerebral autoregulation, where results were indeterminate (16, 18, 19, 35, 36). Within these prior studies, sedation state was determined through nursing assessed depth of sedation scores, which have limited reliability when assessing patients who are heavily sedated, with extended epochs between each sedation score collected. Thus, the prior attempts at evaluating the association between sedation depth and cerebral autoregulation have been limited, warranting further investigation using methods described in our study.
Our work suggests that a parabolic relationship may exist between BIS and PRx, facilitating the derivation of a personalized BISopt value over time, which we propose is an BISopt level (which may represent an optimal depth of sedation) and could potentially balance the metabolic needs of cerebral vessels associated with an optimal PRx. It has been documented that the cerebrovascular response and blood flow are influenced by neurologic activity and metabolic demand, both of which are significantly linked to the sedation states of a patient (34, 36–40). Although the link between depth of sedation and cerebrovascular reactivity is still unclear, our work suggests the potential presence of an BISopt value where autoregulatory capacity remains the most intact. These findings may suggest that at very high levels of sedation (metabolic suppression levels) cerebral vessels may lose their innate ability to mediate vascular control and thus would demonstrated impaired PRx (39). In corollary, with less sedation in the post-TBI state, there may be an increase in metabolic demand, leading to the buildup of metabolic byproducts of anaerobic metabolism, which could cause vasodilation (39); alternatively, reduced sedation may increase sympathetic tone and vasoconstriction, both of these could have detrimental effects on cerebrovascular reactivity though this requires more study (16, 35, 41). BISopt may therefore offer an individualized threshold for sedation treatment and could lead to more effective management for critical care patients requiring sedation infusions as part of their ICU care (see the following for terminology Supplementary File L, http://links.lww.com/CCX/A943). This is supported by the fact that improved PRx values (PRx < 0.2) have been demonstrated to show improved outcomes in a large number of studies, both in TBI and non-TBI illness (1, 9, 21, 26, 28, 42–49). Further, it is highlighted through recent developments in personalized CPP derivation in TBI care (20–22, 28, 50).
Second, in the determination of BISopt, it should be noted that like CPPopt, the BISopt value index may fluctuate over the time of care. Therefore, we have endeavored to build a continuously updating method to determine BISopt throughout the entire patient recording period. Based on the similarity between our algorithmic method (Supplementary File C, http://links.lww.com/CCX/A943) and direct visual inspection, a simple quadratic function appears to be sufficient for most scenarios. Furthermore, the improved percent yield of BISopt using our algorithmic method versus optimal Flex indicates that when endeavoring to identify an BISopt value, not all methods currently applied to CPPopt derivation necessarily apply to BISopt derivation. Although we adapted previously documented CPPopt methods for the continuous determination of BISopt, future work is required to achieve the most BISopt value.
Finally, there was a disassociation between any BIS values/BISopt and ICP, MAP, or CPPopt value. This helps confirm that the BISopt values appear to be independent from other currently explored aspects of personalized care in the ICU, namely MAP or CPPopt directed targets. As such, the preliminary findings here potentially indicate that individual sedation depth and systemic blood pressure-based measures are sufficiently independent and highlights the idea that BISopt may in fact have a separate and entirely unique relationship to PRx, which is distinct from CPPopt.
First limitation is the small, heterogeneous cohort available in this study, which warrants larger datasets with collaborations like Collaborative European NeuroTrauma Effectiveness Research in TBI (13), as well as investigation of non-TBI populations (14, 51, 52). Second, the nature of the BIS values and its relationship to sedation type, sedation depth, systemic blood pressure, and other confounding factors was only preliminarily commented. Although BISopt from different sedative agents appeared to be similar, this requires further exploration. Also, all patients suffered from trauma-induced alterations in level of consciousness, which may impact BIS; thus, non-TBI populations are required to verify our findings. Third, we could not comment on the association of BISopt with outcomes. Analysis of the relationship between BISopt and long-term outcome requires more than the typical course outcome metric seen in TBI studies, often consisting of point measures of the Glasgow Outcome Score. Studies have shown that sedative dose exposure and time in ICU care are associated with worse long-term cognitive function (15–17), any comparison of BISopt with outcome would necessitate comprehensive neurocognitive assessments. Fourth, although we have shown that the BISopt, MAP, and CPPopt appear to function independently, titrated levels of sedation often have secondary impacts on systemic hemodynamics; similarly, it is unclear how the manipulation of MAP with vasoactive agents affects BISopt. Future interventional studies are warranted to understand how these metrics of cerebral autoregulation interact with each other in real-time. Finally, the capturing of BIS values can be problematic within the ICU over long periods of time. A consistent issue we encountered were artifacts or loss of signals due in part to the fact that the pads used to capture the electroencephalogram signals often lose sufficient scalp contact to capture signals. Such events require constant bedside attention to the electrodes and frequent changing of the disposable pads. If future large-scale adoption of BIS monitoring in the ICU is to occur, there will have to be improvements in disposable electrode design to facilitate longer artifact-free recording.
The method of CPPopt has completed its feasibly study, demonstrating that there were no significant differences between groups for therapy intensity level or for other safety endpoints, thus an individual and dynamic cerebral autoregulation-guided CPP is feasible and safe in TBI ICP patients (19). Based on these results, our similar method of BISopt may be equally feasible and safe.
Finally, future assessment in the association of BIS and other methods to sedation depth should be tested including other forms of the entropy electroencephalogram sedation measures, higher resolution full array electroencephalogram and entropy derivation at multiple channels. This should include the analysis of/ links to sedation, the impact of various cofactors as well as discrepancies within the methods. Such analysis will again require the evaluation of groups outside TBI populations and requires continuous physiologic and treatment information. Thus, future exploration into continuous data groups is advised.
CONCLUSIONS
Real-time BISopt can be identified during the continuous physiologic monitoring of patients with moderate/severe TBI. Real-time derivation of BISopt is possible, although future considerations on specific algorithmic methods for its calculation are required. BISopt appears to be unassociated with MAP or CPPopt, supporting it as a potentially unique individualized physiologic target in ICU care. BISopt management to optimize cerebrovascular pressure reactivity should be the subject of future studies in moderate/severe traumatic head-injury patients.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, through the Manitoba Public Insurance Neuroscience/Traumatic Brain Injury Research Endowment, the Health Sciences Centre Foundation Winnipeg, the University of Manitoba Research Grant Program, the University of Manitoba Thorlakson Chair in Surgical Research, a University of Manitoba Department of Surgery Geographical Full Time Grant, and the U.S. National Institutes of Health through the National Institute of Neurological Disorders and Stroke.
Mr. Froese is supported through the University of Manitoba (UofM)—Department of Surgery Geographical Full Time (GFT) Research Grant and the UofM Office of Research Services—University Research Grant Program. Mr. Stein is supported through the Manitoba Medical Services Foundation (MMSF)—Richard Hoeschen Memorial Award. Dr. Batson is supported through the Centre on Aging at the UofM. Dr. Mendelson is supported by the UofM Department of Internal Medicine New Investigator Grant. Dr. Zeiler receives research support from the Manitoba Public Insurance Neuroscience/Traumatic Brain Injury Research Endowment, the Health Sciences Centre Foundation Winnipeg, the U.S. National Institutes of Health through the National Institute of Neurologic Disorders and Stroke (Grant Number: R03NS114335-01), the Canada Foundation for Innovation (Project Number: 38583), Research Manitoba (Grant Number: 3906), the UofM Vice President Research and International Research Investment Fund, the UofM Centre on Aging, and the UofM Rudy Falk Clinician-Scientist Professorship. Dr. Gomez is supported through the UofM Clinician Investigator Program, the R. Samuel McLaughlin Research Fellowship, the MMSF Research and Education Fellowship Award, and the UofM Dean’s Fellowship Fund Award. Mr. Sainbhi is supported through the UofM—Department of Surgery GFT Research Grant and the UofM Graduate Student Association Award. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Donnelly J, Czosnyka M, Adams H, et al. : Twenty-five years of intracranial pressure monitoring after severe traumatic brain injury: A retrospective, single-center analysis. Neurosurgery 2019; 85:E75–E82 [DOI] [PubMed] [Google Scholar]
- 2.Zeiler FA, Ercole A, Beqiri E, et al. ; CENTER-TBI High Resolution ICU (HR ICU) Sub-Study Participants and Investigators: Association between cerebrovascular reactivity monitoring and mortality is preserved when adjusting for baseline admission characteristics in adult traumatic brain injury: A CENTER-TBI study. J Neurotrauma 2020; 37:1233–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorrentino E, Diedler J, Kasprowicz M, et al. : Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit Care 2012; 16:258–266 [DOI] [PubMed] [Google Scholar]
- 4.Zeiler FA, Aries M, Cabeleira M, et al. ; CENTER-TBI High Resolution ICU (HR ICU) Sub-Study Participants and Investigators: Statistical cerebrovascular reactivity signal properties after secondary decompressive craniectomy in traumatic brain injury: A CENTER-TBI pilot analysis. J Neurotrauma 2020; 37:1306–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathieu F, Zeiler FA, Whitehouse DP, et al. : Relationship between measures of cerebrovascular reactivity and intracranial lesion progression in acute TBI patients: An exploratory analysis. Neurocrit Care 2020; 32:373–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeiler FA, Ercole A, Cabeleira M, et al. : Univariate comparison of performance of different cerebrovascular reactivity indices for outcome association in adult TBI: A CENTER-TBI study. Acta Neurochir 2019; 161:1217–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavinio A, Ene-Iordache B, Nodari I, et al. : Cerebrovascular reactivity and autonomic drive following traumatic brain injury. Acta Neurochir Suppl 2008; 102:3–7 [DOI] [PubMed] [Google Scholar]
- 8.Budohoski KP, Czosnyka M, de Riva N, et al. : The relationship between cerebral blood flow autoregulation and cerebrovascular pressure reactivity after traumatic brain injury. Neurosurgery 2012; 71:652–660; discussion 660–661 [DOI] [PubMed] [Google Scholar]
- 9.Balestreri M, Czosnyka M, Steiner LA, et al. : Association between outcome, cerebral pressure reactivity and slow ICP waves following head injury. Acta Neurochir Suppl 2005; 95:25–28 [DOI] [PubMed] [Google Scholar]
- 10.Cabella B, Donnelly J, Cardim D, et al. : An association between ICP-derived data and outcome in TBI patients: The role of sample size. Neurocrit Care 2017; 27:103–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carney N, Totten AM, O’Reilly C, et al. : Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery 2017; 80:6–15 [DOI] [PubMed] [Google Scholar]
- 12.Kochanek PM, Tasker RC, Bell MJ, et al. : Management of pediatric severe traumatic brain injury: 2019 consensus and guidelines-based algorithm for first and second tier therapies. Pediatr Crit Care Med 2019; 20:269–279 [DOI] [PubMed] [Google Scholar]
- 13.Girard TD: Sedation, delirium, and cognitive function after critical illness. Crit Care Clin 2018; 34:585–598 [DOI] [PubMed] [Google Scholar]
- 14.Porhomayon J, El-Solh AA, Adlparvar G, et al. : Impact of sedation on cognitive function in mechanically ventilated patients. Lung 2016; 194:43–52 [DOI] [PubMed] [Google Scholar]
- 15.Stephens RJ, Dettmer MR, Roberts BW, et al. : Practice patterns and outcomes associated with early sedation depth in mechanically ventilated patients: A systematic review and meta-analysis. Crit Care Med 2018; 46:471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Froese L, Dian J, Batson C, et al. : Cerebrovascular response to propofol, fentanyl, and midazolam in moderate/severe traumatic brain injury: A scoping systematic review of the human and animal literature. Neurotrauma Rep 2020; 1:100–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeiler FA, Sader N, Gillman LM, et al. : The cerebrovascular response to ketamine: A systematic review of the animal and human literature. J Neurosurg Anesthesiol 2016; 28:123–140 [DOI] [PubMed] [Google Scholar]
- 18.Flower O, Hellings S: Sedation in traumatic brain injury. Emerg Med Int 2012; 2012:637171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Froese L, Dian J, Gomez A, et al. : Association between processed electroencephalogram-based objectively measured depth of sedation and cerebrovascular response: A systematic scoping overview of the human and animal literature. Front Neurol 2021; 12:692207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aries MJ, Czosnyka M, Budohoski KP, et al. : Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med 2012; 40:2456–2463 [DOI] [PubMed] [Google Scholar]
- 21.Steiner LA, Czosnyka M, Piechnik SK, et al. : Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med 2002; 30:733–738 [DOI] [PubMed] [Google Scholar]
- 22.Kramer AH, Couillard PL, Zygun DA, et al. : Continuous assessment of “optimal” cerebral perfusion pressure in traumatic brain injury: A cohort study of feasibility, reliability, and relation to outcome. Neurocrit Care 2019; 30:51–61 [DOI] [PubMed] [Google Scholar]
- 23.Froese L, Dian J, Gomez A, et al. : Sedation and cerebrovascular reactivity in traumatic brain injury: Another potential for personalized approaches in neurocritical care? Acta Neurochir 2021; 163:1383–1389 [DOI] [PubMed] [Google Scholar]
- 24.Dijkland SA, Helmrich IRAR, Nieboer D, et al. ; CENTER-TBI Participants and Investigators: Outcome prediction after moderate and severe traumatic brain injury: External validation of two established prognostic models in 1742 European patients. J Neurotrauma 2021; 38:1377–1388 [DOI] [PubMed] [Google Scholar]
- 25.Czosnyka M, Smielewski P, Kirkpatrick P, et al. : Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery 1997; 41:11–17; discussion 17–19 [DOI] [PubMed] [Google Scholar]
- 26.Zeiler FA, Lee JK, Smielewski P, et al. : Validation of intracranial pressure-derived cerebrovascular reactivity indices against the lower limit of autoregulation, part II: Experimental model of arterial hypotension. J Neurotrauma 2018; 35:2812–2819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Depreitere B, Citerio G, Smith M, et al. : Cerebrovascular autoregulation monitoring in the management of adult severe traumatic brain injury: A Delphi consensus of clinicians. Neurocrit Care 2021; 34:731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Maurits NM, Aries MJH, et al. : Monitoring of optimal cerebral perfusion pressure in traumatic brain injured patients using a multi-window weighting algorithm. J Neurotrauma 2017; 34:3081–3088 [DOI] [PubMed] [Google Scholar]
- 29.Depreitere B, Güiza F, Van den Berghe G, et al. : Pressure autoregulation monitoring and cerebral perfusion pressure target recommendation in patients with severe traumatic brain injury based on minute-by-minute monitoring data. J Neurosurg 2014; 120:1451–1457 [DOI] [PubMed] [Google Scholar]
- 30.Donnelly J, Czosnyka M, Adams H, et al. : Individualizing thresholds of cerebral perfusion pressure using estimated limits of autoregulation. Crit Care Med 2017; 45:1464–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haberland CM, Baker S, Liu H: Bispectral index monitoring of sedation depth in pediatric dental patients. Anesth Prog 2011; 58:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duchateau FX, Saunier M, Larroque B, et al. : Use of bispectral index to monitor the depth of sedation in mechanically ventilated patients in the prehospital setting. Emerg Med J 2014; 31:669–672 [DOI] [PubMed] [Google Scholar]
- 33.Zeiler FA, Donnelly J, Smielewski P, et al. : Critical thresholds of intracranial pressure-derived continuous cerebrovascular reactivity indices for outcome prediction in noncraniectomized patients with traumatic brain injury. J Neurotrauma 2018; 35:1107–1115 [DOI] [PubMed] [Google Scholar]
- 34.Ely EW, Truman B, Shintani A, et al. : Monitoring sedation status over time in ICU patients: Reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA 2003; 289:2983–2991 [DOI] [PubMed] [Google Scholar]
- 35.Froese L, Dian J, Batson C, et al. : The impact of vasopressor and sedative agents on cerebrovascular reactivity and compensatory reserve in traumatic brain injury: An exploratory analysis. Neurotrauma Rep 2020; 1:157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oddo M, Crippa IA, Mehta S, et al. : Optimizing sedation in patients with acute brain injury. Crit Care 2016; 20:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daffertshofer M, Hennerici M: Cerebrovascular regulation and vasoneuronal coupling. J Clin Ultrasound 1995; 23:125–138 [DOI] [PubMed] [Google Scholar]
- 38.Rossini PM, Altamura C, Ferretti A, et al. : Does cerebrovascular disease affect the coupling between neuronal activity and local haemodynamics? Brain 2004; 127:99–110 [DOI] [PubMed] [Google Scholar]
- 39.Venkat P, Chopp M, Chen J: New insights into coupling and uncoupling of cerebral blood flow and metabolism in the brain. Croat Med J 2016; 57:223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips AA, Chan HHN, Zheng MMZ, et al. : Neurovascular coupling in humans: Physiology, methodological advances and clinical implications. J Cereb Blood Flow Metab 2016; 36:647–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ter Laan M, van Dijk JM, Elting JW, et al. : Sympathetic regulation of cerebral blood flow in humans: A review. Br J Anaesth 2013; 111:361–367 [DOI] [PubMed] [Google Scholar]
- 42.Adams H, Donnelly J, Czosnyka M, et al. : Temporal profile of intracranial pressure and cerebrovascular reactivity in severe traumatic brain injury and association with fatal outcome: An observational study. PLoS Med 2017; 14:e1002353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caldas JR, Haunton VJ, Panerai RB, et al. : Cerebral autoregulation in cardiopulmonary bypass surgery: A systematic review. Interact Cardiovasc Thorac Surg 2018; 26:494–503 [DOI] [PubMed] [Google Scholar]
- 44.Hori D, Hogue CW, Jr, Shah A, et al. : Cerebral autoregulation monitoring with ultrasound-tagged near-infrared spectroscopy in cardiac surgery patients. Anesth Analg 2015; 121:1187–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zweifel C, Castellani G, Czosnyka M, et al. : Continuous assessment of cerebral autoregulation with near-infrared spectroscopy in adults after subarachnoid hemorrhage. Stroke 2010; 41:1963–1968 [DOI] [PubMed] [Google Scholar]
- 46.Jarus-Dziedzic K, Głowacki M, Warzecha A, et al. : Cerebrovascular reactivity evaluated by transcranial doppler sonography in patients after aneurysmal subarachnoid haemorrhage treated with microsurgical clipping or endovascular coiling technique. Neurol Res 2011; 33:18–23 [DOI] [PubMed] [Google Scholar]
- 47.Carrera E, Kurtz P, Badjatia N, et al. : Cerebrovascular carbon dioxide reactivity and delayed cerebral ischemia after subarachnoid hemorrhage. Arch Neurol 2010; 67:434–439 [DOI] [PubMed] [Google Scholar]
- 48.Hori D, Nomura Y, Ono M, et al. : Optimal blood pressure during cardiopulmonary bypass defined by cerebral autoregulation monitoring. J Thorac Cardiovasc Surg 2017; 154:1590–1598.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hogue CW, Brown CH, IV, Hori D, et al. ; Cerebral Autoregulation Study Group: Personalized blood pressure management during cardiac surgery with cerebral autoregulation monitoring: A randomized trial. Semin Thorac Cardiovasc Surg 2021; 33:429–438 [DOI] [PubMed] [Google Scholar]
- 50.Zeiler FA, Ercole A, Cabeleira M, et al. ; CENTER-TBI High Resolution (HR ICU) Sub-Study Participants and Investigators: Comparison of performance of different optimal cerebral perfusion pressure parameters for outcome prediction in adult traumatic brain injury: A Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study. J Neurotrauma 2019; 36:1505–1517 [DOI] [PubMed] [Google Scholar]
- 51.Maas AI, Menon DK, Steyerberg EW, et al. ; CENTER-TBI Participants and Investigators: Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): A prospective longitudinal observational study. Neurosurgery 2015; 76:67–80 [DOI] [PubMed] [Google Scholar]
- 52.Bernard F, Gallagher C, Griesdale D, et al. : The CAnadian High-Resolution Traumatic Brain Injury (CAHR-TBI) research collaborative. Can J Neurol Sci 2020; 47:551–556 [DOI] [PubMed] [Google Scholar]