Background:
Palmar hyperhidrosis (PH), a condition characterized by excess sweating of the palms, is a common concern that presents to the plastic surgeon, which can have major impacts on patient confidence and quality of life. While several studies summarize treatment options for hyperhidrosis in general, few outline the therapeutic options available specifically for PH.
Method:
The authors reviewed the current literature specific to the diagnostic workup and treatment of PH.
Results:
In this article, we show a practical approach to managing patients presenting with PH, summarize its main nonsurgical and surgical treatment options, provide a suggested treatment ladder, and outline emerging therapeutic approaches. We suggest that, after diagnosing PH and classifying its severity, nonsurgical treatments (ie, topical antiperspirants, iontophoresis, botulinum toxin A injection, and topical/oral anticholinergics) should be utilized in a stepwise manner. In patients with severe palmar hyperhidrosis who do not respond to nonsurgical treatments, surgical intervention may be warranted, generally in the form of sympathetic denervation.
Conclusion:
This article provides a clear overview of PH treatment options, stepwise guidelines for physicians, and educational video resources demonstrating botulinum toxin A injections with cryotherapy and nerve blocks.
Takeaways
Question: What are the most effective mechanisms to manage a new diagnosis of palmar hyperhidrosis (PH)?
Findings: PH should be classified as either mild, moderate, or severe. Treatment should be approached in a stepwise manner, beginning with nonsurgical treatments (ie, topical antiperspirants, iontophoresis, botulinum toxin A injection, and topical/oral anticholinergics). For severe PH that does not respond to nonsurgical treatments, surgical intervention may be warranted, generally in the form of sympathetic denervation.
Meaning: PH is a common condition that impacts patient quality of life. However, effective nonsurgical and surgical treatment options exist and may be used to reduce or eliminate its symptoms.
INTRODUCTION AND EPIDEMIOLOGY
Hyperhidrosis (HH) is a condition that is characterized by excess sweating, which occurs independent of the body’s need to initiate heat loss.1,2 In the United States, HH affects up to 4.8% of the population3 and is equally present in both men and women.4 However, given that only 51% of patients have discussed their symptoms with a healthcare professional,3 this prevalence is likely underestimated. Although HH can affect many parts of the body, up to 40% of hyperhidrotic patients indicate involvement of the palms, denoted specifically as palmar hyperhidrosis (PH), which highlights a major source of patient burden.3 Although research into nonsurgical and surgical management options for the condition have steadily increased over the past few decades, few articles outline the process for patient stratification and stepwise treatment specific to PH.
PH, especially in moderate to severe cases, has the potential to significantly impact patient quality of life.2 Increased palmar sweat can have both social and functional implications including social embarrassment and discomfort in school or the workplace.2 These symptoms can be exacerbated by increased stress, nervousness, fear, and anxiety, which further increase sweat production.2 Given the existence of effective nonsurgical and surgical management options,2,5 the provision of, or referral for, treatments is essential for improved patient well-being. As a result, an updated summary of current and emerging treatments is needed to provide physicians with evidence-based mechanisms to treat patients with PH.
While many reviews discuss the general condition of HH and summarize its most common subtypes,6–9 few offer a comprehensive, PH-specific overview, which includes practical approaches to treating patients presenting with the condition. To address this gap, this article describes the pathophysiology, classification, and diagnosis of PH, summarizes current nonsurgical and surgical treatment options, and recommends an approach to treating patients presenting with differing severities of the condition. Additionally, we provide supplemental video instruction for the injection of botulinum toxin type A (BoNTA) into the palms, with both cryoanesthesia and blocks of the median and ulnar nerve.
CLASSIFICATION AND PATHOPHYSIOLOGY OF HYPERHIDROSIS
HH is classified as being either primary or secondary, and generalized or focal.7,8 Primary HH is characterized by increased sweating that is independent of an underlying medical condition, and is often idiopathic in nature.2,7 Secondary HH occurs in response to an underlying pathological condition, which may be infectious, medication-related, neoplastic, neurologic, intoxication-related, alcohol-withdrawal-related, or endocrine in nature.9 Generalized HH typically affects the whole body, whereas focal HH affects only a limited portion of the body (e.g. PH or axillary HH).8 In those with HH, comorbidities described in the literature include increased rates of social anxiety disorder10 and depression,11 as well as athlete’s foot and an increased risk of skin infections, psoriasis, warts, and dermatophytes.12
HH generally occurs in areas of high eccrine gland density, which include the palms, as well as the axillae, soles of the feet, and craniofacial area.13 As eccrine glands are the sole sweat glands located in the palm,14 they are primarily responsible for the sweat released in PH.2,15 The pathophysiology of primary HH is related to the overactivity of the underlying neural network modulating the release of sweat, rather than an issue with the density or proportions of eccrine glands.16 In fact, a study investigating the ultrastructure of eccrine sweat glands in HH patients found a lack of any structural defects, supporting a picture more consistent with hyperactivity within the postganglionic sympathetic cholinergic fibers they are innervated by.17
Studies have shown that approximately two-thirds of patients report a family history of HH.18 Genetic testing has been conducted to try to determine the specific gene or genes associated with HH, and alterations of the BCHE gene have been identified in some small samples.19
DIAGNOSIS OF PH
A diagnosis of PH is performed through a thorough physical examination, patient history, and a definitive diagnostic questionnaire.2,8,20 As opposed to the other areas of HH, patients with PH typically first experience symptoms in childhood, which then worsen during puberty.2
For a formal diagnosis of PH to be made, patients must be experiencing visible and excess sweating on the palms, with at least two of the following2,8,20:
Sweating that is bilateral and relatively symmetric
Sweating that impairs the patient’s daily activities
Sweating frequency of at least one occurrence per week
Age of onset below 25 years
Positive family history
Lack of focal sweating during sleep
Additional support for the diagnosis of PH and further classification of potential treatments can be made through a quality of life measurement in relation to patients’ excess sweating.2 The Hyperhidrosis Disease Severity Scale (HDSS) is commonly used to do so (Table 1).2,21 Patients are asked to rate the severity of their HH, and are given a score from 1 to 4, with a score of 1 or 2 signifying mild or moderate primary HH, and scores of 3 or 4 signifying severe disease (Table 1).21,22 When re-scoring patients after treatment, success is denoted by a score decrease to 2 or 1, if they initially presented with severe HH, or from 2 to 1, if they initially presented with mild to moderate HH.21 Other validated surveys that may be used include the Dermatology Life Quality Index and the Keller questionnaire.2
Table 1.
HDSS for PH
| Severity | Score |
|---|---|
| My (palmar) sweating is never noticeable and never interferes with my daily activities | 1 |
| My (palmar) sweating is tolerable but sometimes interferes with my daily activities | 2 |
| My (palmar) sweating is barely tolerable and frequently interferes with my daily activities | 3 |
| My (palmar) sweating is intolerable and always interferes with my daily activities | 4 |
The HDSS is used to deduce the severity of PH in patients. Adapted from the recommendations of the Canadian Hyperhidrosis Advisory Committee.17
The Minor test, in which an iodine-containing solution, followed by starch, is applied to the hand, can also be used to visually assess affected areas.2,8 In the presence of sweat, a dark blue-purple color will appear, mapping areas of high perspiration.2,8 This technique is most often used in evaluating perspiration before and after treatment.
NONSURGICAL TREATMENTS FOR PH
A variety of nonsurgical treatment options exist for PH, including topical antiperspirants, BoNTA, and topical and systemic anticholinergics.
Topical Aluminum Salts
Topical antiperspirants may be used as a first step in the treatment of PH (Fig. 1). Aluminum chloride is a topical antiperspirant that functions to prevent the release of sweat.2,23 Aluminum ions precipitate with mucopolysaccharides, leading to damage within the sweat duct and the formation of a subsequent plug, preventing the excretion of sweat.23 Varying strengths of topical aluminum-chloride-based antiperspirants exist, ranging from 1% to up to 35% in concentration5,8; however, concentrations around 20% are the most widely used.25 Aluminum chloride hexahydrate can be prepared in salicylic gel, water, or absolute ethanol.5,22 Of these options, the salicylic acid gel formulation is generally more efficacious and is also associated with reduced irritation.24
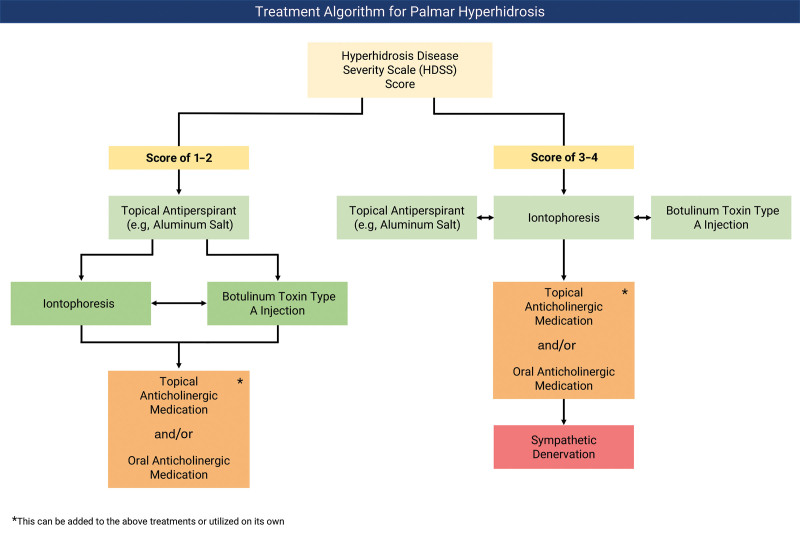
Fig. 1.
Treatment algorithm for PH. After scoring patients with PH through the HDSS, two treatment algorithms may be followed.5,17,22,23,24 Those with an HDSS score of 1 or 2 should start with a topical antiperspirant. If this fails, either iontophoresis or botulinum toxin A can be attempted. If either of these leads to unsatisfactory results, the other should be considered. If both iontophoresis and botulinum toxin A lead to unsatisfactory results, then topical or oral anticholinergic treatments, either alone or in combination with prior treatments, are recommended. In those with an HDSS score of 3 or 4, one may start with either a topical antiperspirant, iontophoresis, botulinum toxin type A, or a combination of any of these. For refractory cases, topical and/or oral anticholinergic medications may be utilized, either alone or in combination with prior treatments. If all of these fail, sympathetic denervation can be considered as a last resort.
In treating mild cases of PH, over-the-counter formulations, which contain aluminum zirconium trichlorohydrate, may help reduce or eliminate symptoms.5 In cases of moderate or severe PH, higher percentages of prescribed aluminum chloride hexahydrate may be needed.5 Aluminum chloride should be applied each night to the palms, ideally during periods free of sweat, for 6–8 hours at a time.5 Once the patient’s sweat goals have been achieved, the application interval can then be decreased.25,26 An important note is that the effects of aluminum chloride hexahydrate are not long-lasting, as its efficacy was seen to disappear in study participants after 48 hours of discontinued use.26 Additionally, patients often experience localized sensations of irritation and burning, a side effect that may be partially mitigated through topical hydrocortisone cream.8,20,25 If topical aluminum salts do not provide the patient with relief, treatment may be escalated to other nonsurgical options.
Iontophoresis
For PH that is resistant to aluminum chloride or other topical solutions, iontophoresis may represent the next step in the treatment ladder (Fig. 1).27 Iontophoresis is a technology that uses electrical current to pass ionized liquid into the skin.28 A fluid, often tap water, is placed in two shallow trays—one for each palm.29–31 A current is then applied, and patients place their hands in the trays.29–31 The current is then increased until discomfort is sensed, and then reduced slightly and maintained for approximately 10–30 minutes each day.31 The polarity should be reversed halfway through the treatment time to ensure both hands are affected equally by the device.30 Although not completely understood, the current literature suggests that iontophoresis may reduce sweating through mechanisms such as alterations in sodium reabsorption32 and increased acidity within the sweat gland, leading to its obstruction and dysfunction.33
Iontophoresis is an effective treatment option, reducing PH symptoms in most patients.29 A recent randomized controlled trial identified that patients treated with iontophoresis experienced a 78.6% improvement in quality of life and 91.8% reduction in the sweat secretion rate.29 Mild side effects include skin irritation, erythema, and/or pain.30,34,35 Iontophoresis can also be performed at home, with an approved device. Currently, the U.S. Food and Drug Administration (FDA) has registered several iontophoresis devices, such as the RA Fischer MD-1a and MD-2, and the Hidrex USA the Drionic.5,28 Although effective, iontophoresis is not a permanent solution, and maintenance therapy should occur at an interval dependent on the patient’s symptoms, often once to twice per week.31
Botulinum Toxin
As a local palmar injection, BoNTA is a potent second-line therapy for PH (Fig. 1).36 As a toxin produced by the bacterium Clostridium botulinum, BoNTA functions to inhibit the release of neurotransmitters (ie, acetylcholine) through a protease-mediated interaction with the presynaptic neuron.37,38 Numerous clinical studies, including single- and double-blind, placebo-controlled trials, have been performed using BoNTA, with efficacies ranging between 80% and 90%.14 BoNTA generally functions for 6 months before wearing off, but its effects can range from 4 to 12 months.14 Side effects of BoNTA are well-documented and include pain and swelling for 1–2 days postinjection, as well as transient numbness and mild hand weakness in some cases.39,40 In a study conducted by Kouris et al,41 local pain was reported in 58% of patients, and muscle weakness was seen in 21.5% of patients.
Currently, there is one botulinum toxin type B and four BoNTA formulations that are approved by the FDA,36,38,40 which include:
OnabotulinumtoxinA (BOTOX; Allergan Inc., Irvine, Calif.)36,38,40
AbobotulinumtoxinA (Dysport; Ipsen Biopharm LTD.; Wrexham, UK)36,38,40
IncobotulinumtoxinA (Xeomin; Merz Pharmaceuticals, Frankfurt/Main, Germany)36,38,40
PrabotulinumtoxinA (Jeuveau; Evolus Inc., Santa Barbara, Calif.)36,40
BoNTA is not currently approved for PH, and therefore is considered to be off-label, although it is still widely utilized and supported by positive outcomes.40 In preparing the patient for palmar BoNTA injection, the palm and fingers are marked in a grid-like pattern using a surgical marker, with spacing of 1–2 cm between each grid point.14,39,40 BoNTA is commonly diluted in 2–5 cm3 of preserved saline when used for PH and is then injected intradermally with a small gauge needle,14,39,40,42 adjacent to each grid point to prevent any risk of accidental tattooing (See Video 1 [online], which describes the technique for palmar BoNTA injection).40 While five types of BoNTA are approved by the FDA, their units are not interchangeable; therefore, specific conversion factors are needed.43 There is no current consensus on an optimal dilution of BoNTA, but for onabotulinumtoxinA, 1–2 units per 0.1 mL of BoNTA are generally used, with 0.05–0.2 mL delivered to each grid mark.5,39,40 Injections should be dermal or immediately subdermal to avoid inadvertent injection into the muscles, which can result in temporary weakness of the hand.5,40
{L-End}
Video 1. Palmar BoNTA injection. Video 1 from “A Practical Approach to the Diagnosis and Treatment of Palmar Hyperhidrosis”.
To minimize injection discomfort, ice can be applied to each site for approximately 10 seconds before injection. Alternatively, a median and ulnar nerve block may also be performed (See Video 2 [online], which describes the technique for median and ulnar nerve blocks for palmar BoNTA injection).39,40 In performing a median nerve block, locate the tendons of flexor carpi radialis and palmaris longus.44 For the block, approximately 2 mL of 1% plain lidocaine should be used, injecting approximately 0.3 mL above the fascia and 1.7 mL below the fascia, to target the palmar cutaneous nerve and main aspect median nerve, respectively.44 When performing an ulnar nerve block, first identify the tendon of flexor carpi ulnaris, which may be accomplished by asking the patient to ulnarly deviate their wrist in a fist and resist physician-applied extension.44 As with the median nerve block, approximately 2 mL of 1% lidocaine is injected, with approximately 0.3 mL above the fascia and 1.7 mL below the fascia, to target the dorsal sensory branch of the ulnar nerve and the main aspect of the ulnar nerve, respectively.44 Postinjection nonsteroidal antiinflammatory drugs or acetaminophen can be provided for pain relief.
{L-End}
Video 2. Median and ulnar nerve blocks for palmar BoNTA injection. Video 2 from “A Practical Approach to the Diagnosis and Treatment of Palmar Hyperhidrosis”.
Topical and Oral Anticholinergics
Topical and/or oral anticholinergics may be used in refractory cases of PH, when patients are unsatisfied after treatment attempts with topical antiperspirants, iontophoresis, and/or BoNTA injections (Fig. 1).40 Anticholinergic medications, existing in both topical and oral formulations for PH, act to antagonize muscarinic receptors within the sweat glands, reducing the amount of acetylcholine that can bind and cause downstream sweating.2 Glycopyrrolate, oxybutynin, and methantheline bromide are common examples of oral anticholinergic options that exist for PH.5,45 However, while several studies have looked at oral anticholinergics for primary HH, few have considered their PH-specific implications.5,38,45 As a result, most oral anticholinergics are currently considered to be off-label for PH.5 Nevertheless, evidence for their use is promising; a randomized controlled trial by Wolosker et al found that oxybutynin reduced PH symptoms in over 70% of patients and was associated with improved patient quality of life.46
Since oral anticholinergics are taken by mouth, side effects are more systemic in nature. The most common side effect is dry mouth, which often leads patients to discontinue treatment, and others include constipation, tachycardia, drowsiness, blurred vision, reduced whole-body sweating, and urinary retention.5,28,45 Up to one-third of patients taking oral anticholinergics will ultimately discontinue treatment.5 Further care must be taken when prescribing oral anticholinergic medications to patients who are elderly (drug–drug interactions) or pregnant (due to little existing evidence on anticholinergic safety during pregnancy).38
Topical anticholinergic medications remain understudied but exist as possible options for treating PH.5 Glycopyrronium tosylate was approved by the FDA for axillary HH in 2018, and was shown to be effective through patient-reported outcomes of sweating in phase 3 clinical trials.30,47 However, no published evidence currently exists for its efficacy specific to PH. Topical oxybutynin has been studied more widely for primary focal HH, and a recently published randomized double-blind placebo-controlled study tested its use for patients with axillary, palmar, and/or plantar HH, finding significant improvements in HDSS and Dermatology Life Quality Index scores.48 Due to overall limited evidence, however, side effects are not well characterized for PH.
SURGICAL TREATMENT FOR PH
Endoscopic Thoracic Sympathectomy
For patients with severe PH who do not respond to topical antiperspirants, iontophoresis, BoNTA, or topical or oral anticholinergics, surgical intervention may be indicated as a treatment option (Fig. 1).5,30 Surgical intervention for PH most commonly exists in the form of endoscopic thoracic sympathectomy (ETS).49 Ideal candidates are those with early age onset of HH who are generally healthy, do not sweat at night, have a body mass index below 28, and do not have bradycardia.50 ETS is an outpatient procedure commonly performed under general anesthetic, and involves physical interruption of the sympathetic chain (clamping, excision, ablation, or transection), disrupting the nerve signals that ultimately target eccrine sweat glands.5,8,51 Although the thoracic spinal nerve level is often used as a landmark in the literature, rib numbers were seen to be more accurate reference points due to patient mediastinal fat variations.50 For patients suffering solely from PH, the best outcomes are generally seen when it is performed superior to rib 3 or 4.50
In a 2018 study identifying the long-term impacts of ETS for PH, the procedure was shown to increase quality of life in 84% of patients, and leading to satisfaction in 97% of the respondents.49 It is important to note that there are many potential side effects of ETS such as pneumothorax, bradycardia, gustatory sweating, Horner’s syndrome, rhinitis, and compensatory HH, which affects up to 92% of patients.22,49,50 The specific side effects may be dependent on the rib level at which the procedure is performed, with procedures performed more superiorly generally causing more downstream effects.50 Compensatory HH is the most common postoperative complication, leading to increased sweating in other parts of the body to compensate for the lost capacity in the palms.49 While in many cases this compensatory HH is preferred to the original PH symptoms, if it is severe enough, it can also pose a major source of discomfort and dissatisfaction in patients.52
EMERGING TREATMENTS FOR PH
Given that the treatments for PH are still emerging, numerous experimental and novel treatment options exist, with promising preliminary results. These include:
Radiofrequency stimulation: A recent study performed by Lin et al in 2017 used pulsed radiofrequency stimulation at the T2 sympathetic trunk in rats.53 A significant reduction in palm humidity of the treated side was observed in rats who underwent treatment.53 As a result, radiofrequency stimulation represents a potential reversible treatment option for PH, which may reduce the potential for permanent compensatory sweating.53 Further research, including clinical trials, is necessary to elucidate the efficacy of this treatment option in humans.
Ultrasound stellate ganglion block: A case report published in 2018 investigated a noninvasive ultrasound stellate ganglion block as another potential treatment for PH.54 This technique uses ultrasound to block the stellate ganglion, a sympathetic nerve ganglion located caudal to the sternocleidomastoid muscle, under local anesthetic.54 The procedure, which was performed for one minute per day on alternating sides, led to effective results as confirmed by a Minor’s starch test, without the noted side effects seen in similar surgical procedures.54
BoNTA-coated microneedles: The use of microneedles coated with BoNTA is a novel and exciting procedure that may lead to positive results in treating PH.55 A study published by Shim et al in 2019 outlined a design for BoNTA-coated microneedles, and tested its use in the foot pad of mice in vivo, showing a significant reduction in the sweating response.55 While this method is a promising, and potentially less painful approach to treating PH, additional research is necessary to confirm its efficacy in humans.
Fractional CO2 laser-assisted BoNTA: Fractional CO2 laser-assisted BoNTA delivery is another newly emerging procedure in the treatment of PH.56,57 This procedure involves using an ablative fractional CO2 laser on the palm, then applying a topical BoNTA solution directly after, enabling transdermal BoNTA absorption.56 A study published in 2021, which performed fractional CO2 with 30 primary PH patients, showed clinical equivalency between this procedure, utilizing 75 units topically of BoNTA, and 50 units of injected BoNTA for the treatment of PH, with similar lengths of effectiveness but a reduction in pain intensity.56 Despite its recent emergence, this technique has already demonstrated promising results.
CONCLUSIONS
PH is a condition that can severely impact the day-to-day life of those it affects. As a common complaint received by plastic surgeons, the treatment of PH should be taken seriously, and with a stepwise approach. First, patients should be formally diagnosed, then scored for PH severity (Table 1). Based on this score, the PH treatment ladder should then be followed, working together with the patient to address their concerns to ultimately improve or eliminate their symptoms (Fig. 1).
Footnotes
Published online 7 March 2022.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Leung AK, Chan PY, Choi MC. Hyperhidrosis. Int J Dermatol. 1999;38:561–567. [DOI] [PubMed] [Google Scholar]
- 2.Romero FR, Haddad GR, Miot HA, et al. Palmar hyperhidrosis: clinical, pathophysiological, diagnostic and therapeutic aspects. An Bras Dermatol. 2016;91:716–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doolittle J, Walker P, Mills T, et al. Hyperhidrosis: an update on prevalence and severity in the United States. Arch Dermatol Res. 2016;308:743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strutton DR, Kowalski JW, Glaser DA, et al. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey. J Am Acad Dermatol. 2004;51:241–248. [DOI] [PubMed] [Google Scholar]
- 5.Gregoriou S, Sidiropoulou P, Kontochristopoulos G, et al. Management strategies of palmar hyperhidrosis: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walling HW, Swick BL. Treatment options for hyperhidrosis. Am J Clin Dermatol. 2011;12:285–295. [DOI] [PubMed] [Google Scholar]
- 7.Stashak AB, Brewer JD. Management of hyperhidrosis. Clin Cosmet Investig Dermatol. 2014;7:285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haider A, Solish N. Focal hyperhidrosis: diagnosis and management. CMAJ. 2005;172:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlereth T, Dieterich M, Birklein F. Hyperhidrosis—causes and treatment of enhanced sweating. Dtsch Arztebl Int. 2009;106:32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lessa Lda R, Luz FB, De Rezende RM, et al. The psychiatric facet of hyperhidrosis: demographics, disability, quality of life, and associated psychopathology. J Psychiatr Pract. 2014;20:316–323. [DOI] [PubMed] [Google Scholar]
- 11.Bahar R, Zhou P, Liu Y, et al. The prevalence of anxiety and depression in patients with or without hyperhidrosis (HH). J Am Acad Dermatol. 2016;75:1126–1133. [DOI] [PubMed] [Google Scholar]
- 12.Lenefsky M, Rice ZP. Hyperhidrosis and its impact on those living with it. Am J Manag Care. 2018;24(23 Suppl):S491–S495. [PubMed] [Google Scholar]
- 13.Lear W, Kessler E, Solish N, et al. An epidemiological study of hyperhidrosis. Dermatol Surg. 2007;33(1 Spec No.):S69–S75. [DOI] [PubMed] [Google Scholar]
- 14.Grunfeld A, Murray CA, Solish N. Botulinum toxin for hyperhidrosis: a review. Am J Clin Dermatol. 2009;10:87–102. [DOI] [PubMed] [Google Scholar]
- 15.Asahina M, Poudel A, Hirano S. Sweating on the palm and sole: physiological and clinical relevance. Clin Auton Res. 2015;25:153–159. [DOI] [PubMed] [Google Scholar]
- 16.Sato K, Kang WH, Saga K, et al. Biology of sweat glands and their disorders. II. Disorders of sweat gland function. J Am Acad Dermatol. 1989;20(5 Pt 1):713–726. [DOI] [PubMed] [Google Scholar]
- 17.Bovell DL, Clunes MT, Elder HY, et al. Ultrastructure of the hyperhidrotic eccrine sweat gland. Br J Dermatol. 2001;145:298–301. [DOI] [PubMed] [Google Scholar]
- 18.Ro KM, Cantor RM, Lange KL, et al. Palmar hyperhidrosis: evidence of genetic transmission. J Vasc Surg. 2002;35:382–386. [DOI] [PubMed] [Google Scholar]
- 19.Simes BC, Moore JP, Brown TC, et al. Genetic polymorphism analysis of patients with primary hyperhidrosis. Clin Cosmet Investig Dermatol. 2018;11:477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornberger J, Grimes K, Naumann M, et al.; Multi-Specialty Working Group on the Recognition, Diagnosis, and Treatment of Primary Focal Hyperhidrosis. Recognition, diagnosis, and treatment of primary focal hyperhidrosis. J Am Acad Dermatol. 2004;51:274–286. [DOI] [PubMed] [Google Scholar]
- 21.Solish N, Benohanian A, Kowalski JW; Canadian Dermatology Study Group on Health-Related Quality of Life in Primary Axillary Hyperhidrosis. Prospective open-label study of botulinum toxin type A in patients with axillary hyperhidrosis: effects on functional impairment and quality of life. Dermatol Surg. 2005;31:405–413. [PubMed] [Google Scholar]
- 22.Solish N, Bertucci V, Dansereau A, et al.; Canadian Hyperhidrosis Advisory Committee. A comprehensive approach to the recognition, diagnosis, and severity-based treatment of focal hyperhidrosis: recommendations of the Canadian Hyperhidrosis Advisory Committee. Dermatol Surg. 2007;33:908–923. [DOI] [PubMed] [Google Scholar]
- 23.Khademi Kalantari K, Zeinalzade A, Kobarfard F, et al. The effect and persistency of 1% aluminum chloride hexahydrate iontophoresis in the treatment of primary palmar hyperhidrosis. Iran J Pharm Res. 2011;10:641–645. [PMC free article] [PubMed] [Google Scholar]
- 24.Woolery-Lloyd H, Valins W. Aluminum chloride hexahydrate in a salicylic acid gel: a novel topical agent for hyperhidrosis with decreased irritation. J Clin Aesthet Dermatol. 2009;2:28–31. [PMC free article] [PubMed] [Google Scholar]
- 25.Jacob CI. Examining hyperhidrosis: an update on new treatments. Am J Manag Care. 2018;24(23 Suppl):S496–S501. [PubMed] [Google Scholar]
- 26.Goh CL. Aluminum chloride hexahydrate versus palmar hyperhidrosis. Evaporimeter assessment. Int J Dermatol. 1990;29:368–370. [DOI] [PubMed] [Google Scholar]
- 27.Hoorens I, Ongenae K. Primary focal hyperhidrosis: current treatment options and a step-by-step approach. J Eur Acad Dermatol Venereol. 2012;26:1–8. [DOI] [PubMed] [Google Scholar]
- 28.McConaghy JR, Fosselman D. Hyperhidrosis: management options. Am Fam Physician. 2018;97:729–734. [PubMed] [Google Scholar]
- 29.Kim DH, Kim TH, Lee SH, et al. Treatment of palmar hyperhidrosis with tap water iontophoresis: a randomized, sham-controlled, single-blind, and parallel-designed clinical trial. Ann Dermatol. 2017;29:728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wechter T, Feldman SR, Taylor SL. The treatment of primary focal hyperhidrosis. Skin Therapy Lett. 2019;24:1–7. [PubMed] [Google Scholar]
- 31.Kreyden OP. Iontophoresis for palmoplantar hyperhidrosis. J Cosmet Dermatol. 2004;3:211–214. [DOI] [PubMed] [Google Scholar]
- 32.Ohshima Y, Shimizu H, Yanagishita T, et al. Changes in Na+, K+ concentrations in perspiration and perspiration volume with alternating current iontophoresis in palmoplantar hyperhidrosis patients. Arch Dermatol Res. 2008;300:595–600. [DOI] [PubMed] [Google Scholar]
- 33.Sato K, Timm DE, Sato F, et al. Generation and transit pathway of H+ is critical for inhibition of palmar sweating by iontophoresis in water. J Appl Physiol (1985). 1993;75:2258–2264. [DOI] [PubMed] [Google Scholar]
- 34.Siah TW, Hampton PJ. The effectiveness of tap water iontophoresis for palmoplantar hyperhidrosis using a Monday, Wednesday, and Friday treatment regime. Dermatol Online J. 2013;19:14. [PubMed] [Google Scholar]
- 35.Reinauer S, Neusser A, Schauf G, et al. Iontophoresis with alternating current and direct current offset (AC/DC iontophoresis): a new approach for the treatment of hyperhidrosis. Br J Dermatol. 1993;129:166–169. [DOI] [PubMed] [Google Scholar]
- 36.Nawrocki S, Cha J. Botulinum toxin: Pharmacology and injectable administration for the treatment of primary hyperhidrosis. J Am Acad Dermatol. 2020;82:969–979. [DOI] [PubMed] [Google Scholar]
- 37.Breidenbach MA, Brunger AT. New insights into clostridial neurotoxin-SNARE interactions. Trends Mol Med. 2005;11:377–381. [DOI] [PubMed] [Google Scholar]
- 38.Hosp C, Hamm H. Safety of available and emerging drug therapies for hyperhidrosis. Expert Opin Drug Saf. 2017;16:1039–1049. [DOI] [PubMed] [Google Scholar]
- 39.Lakraj AA, Moghimi N, Jabbari B. Hyperhidrosis: anatomy, pathophysiology and treatment with emphasis on the role of botulinum toxins. Toxins (Basel). 2013;5:821–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu M, Burgess C, Solish N, et al. Botulinum toxin for the treatment of palmoplantar hyperhidrosis. In: Soft Tissue Augmentation. Fifth ed. (Ahead of Print). [Google Scholar]
- 41.Kouris A, Vavouli C, Markantoni V, et al. Muscle weakness in treatment of palmar hyperhidrosis with botulinum toxin type a: can it be prevented? J Drugs Dermatol. 2014;13:1315–1316. [PubMed] [Google Scholar]
- 42.Glogau RG. Hyperhidrosis and botulinum toxin A: patient selection and techniques. Clin Dermatol. 2004;22:45–52. [DOI] [PubMed] [Google Scholar]
- 43.Mannava S, Mannava KA, Nazir OF, et al. Treatment of palmar hyperhidrosis with botulinum neurotoxin a. J Hand Surg Am. 2013;38:398–400. [DOI] [PubMed] [Google Scholar]
- 44.Weinberg T, Solish N, Murray C. Botulinum neurotoxin treatment of palmar and plantar hyperhidrosis. Dermatol Clin. 2014;32:505–515. [DOI] [PubMed] [Google Scholar]
- 45.Cruddas L, Baker DM. Treatment of primary hyperhidrosis with oral anticholinergic medications: a systematic review. J Eur Acad Dermatol Venereol. 2017;31:952–963. [DOI] [PubMed] [Google Scholar]
- 46.Wolosker N, de Campos JR, Kauffman P, et al. A randomized placebo-controlled trial of oxybutynin for the initial treatment of palmar and axillary hyperhidrosis. J Vasc Surg. 2012;55:1696–1700. [DOI] [PubMed] [Google Scholar]
- 47.Nwannunu CE, Limmer AL, Coleman K, et al. Glycopyrronium Tosylate (Qbrexza) for Hyperhidrosis. Skin Therapy Lett. 2019;24:1–3. [PubMed] [Google Scholar]
- 48.Artzi O, Loizides C, Zur E, et al. Topical oxybutynin 10% gel for the treatment of primary focal hyperhidrosis: a randomized double-blind placebo-controlled split area study. Acta Derm Venereol. 2017;97:1120–1124. [DOI] [PubMed] [Google Scholar]
- 49.Horslen LC, Wilshire CL, Louie BE, et al. Long-term impact of endoscopic thoracic sympathectomy for primary palmar hyperhidrosis. Ann Thorac Surg. 2018;106:1008–1012. [DOI] [PubMed] [Google Scholar]
- 50.Cerfolio RJ, De Campos JR, Bryant AS, et al. The Society of Thoracic Surgeons expert consensus for the surgical treatment of hyperhidrosis. Ann Thorac Surg. 2011;91:1642–1648. [DOI] [PubMed] [Google Scholar]
- 51.Cinà CS, Cinà MM, Clase CM. Endoscopic thoracic sympathectomy for hyperhidrosis: technique and results. J Minim Access Surg. 2007;3:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chwajol M, Barrenechea IJ, Chakraborty S, et al. Impact of compensatory hyperhidrosis on patient satisfaction after endoscopic thoracic sympathectomy. Neurosurgery. 2009;64:511–8; discussion 518. [DOI] [PubMed] [Google Scholar]
- 53.Lin ML, Huang TR, Kao MC, et al. Pulsed radiofrequency stimulation suppresses palmar hyperhidrosis in an animal study. Brain Behav. 2017;7:e00833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heinig B, Koch A, Wollina U. Palmar hyperhidrosis treated by noninvasive ultrasound stellate ganglion block. Wien Med Wochenschr. 2018;168:250–253. [DOI] [PubMed] [Google Scholar]
- 55.Shim DH, Nguyen TT, Park PG, et al. Development of botulinum toxin A-coated microneedles for treating palmar hyperhidrosis. Mol Pharm. 2019;16:4913–4919. [DOI] [PubMed] [Google Scholar]
- 56.Agamia NF, Sobhy N, Abd-Elraouf A, et al. Fractional CO2 laser for transcutaneous drug delivery of onabotulinum toxin in palmar hyperhidrosis. Dermatol Surg. 2021;47:678–683. [DOI] [PubMed] [Google Scholar]
- 57.Junsuwan N, Manuskiatti W, Phothong W, et al. Fractional CO2 laser-assisted botulinum toxin type A delivery for the treatment of primary palmar hyperhidrosis. Lasers Med Sci. 2021;36:233–236. [DOI] [PubMed] [Google Scholar]