Abstract
Introduction
Velsecorat (AZD7594) is a non-steroidal, selective, glucocorticoid receptor modulator (SGRM), being developed for the treatment of asthma. This article reports the initial, first-in-human, single and repeat dose-escalating study in healthy male volunteers.
Methods
The study comprised two parts, a single ascending dose part (n=47) and a multiple ascending dose part (n=26). Inhaled velsecorat was administered by nebulization as one single dose in the first part of the study and as a single dose with subsequent multiple daily doses (day 5–16) for 12 days once daily in the second part of the study. At each dose level, participants were randomized to velsecorat (n=6) or placebo (n=2/3). The safety, pharmacokinetics (PK) and pharmacodynamics (PD) of velsecorat were evaluated.
Results
Inhaled velsecorat was safe and well tolerated up to and including the highest dose tested (1872 µg). Plasma exposure suggested dose proportional PK. The terminal half-life following repeated dosing was 25–31 hours and steady state conditions for velsecorat in plasma were generally reached within 4 doses. The accumulation ratio was low (≤2), and data did not indicate any time-dependent PK. There were dose-related effects on 24-hour plasma cortisol, plasma cortisol after ACTH stimulation and osteocalcin, systemic PD markers of glucocorticoid activity. There were no effects on other biomarkers tested (DHEA-S and 4βOH-cholesterol).
Conclusion
The early clinical evaluation of inhaled velsecorat suggests that this novel SGRM is well tolerated in the dose range investigated. It shows dose proportional plasma exposure, low accumulation, and has dose-dependent effects on markers of glucocorticoid activity.
Keywords: AZD7594, velsecorat, pharmacokinetics, non-steroidal glucocorticoid receptor modulator, PK
Introduction
Inhaled glucocorticosteroids alone or in combination with inhaled long-acting β2-agonists are currently approved for the treatment of respiratory diseases such as asthma and chronic obstructive pulmonary disease.1 Velsecorat (AZD7594) (3-(5-((1R,2S)-2-(2,2-Difluoropropanamido)-1-(2,3-dihydrobenzo-[b][1,4]dioxin-6-yl)propoxy)-1H-indazol-1-yl)-N-((R)-tetrahydrofuran-3-yl)benzamide) (Supplementary Figure 1) is a novel inhaled, potent, non-steroidal selective glucocorticoid receptor modulator (SGRM) being developed as a maintenance anti-inflammatory treatment for asthma,2 and has recently completed Phase 2b development.3
Velsecorat modulates the glucocorticoid receptor (GR) in a novel way compared with reference glucocorticosteroids. Velsecorat binds potently to the human GR, and demonstrates selectivity over androgen, mineralocorticoid, progesterone and the oestrogen α and β-receptors (ERα and β) by >10,000-fold.2 The compound was designed to have prolonged exposure in the lung, based on slow dissolution due to low solubility, allowing for once daily dosing.2 Velsecorat was also designed to have rapid clearance from the systemic circulation and low oral bioavailability, to give opportunity for a favorable concentration gradient between the lung and systemic circulation.2 It is predominantly metabolized by CYP3A4 with some contribution of CYP2C9.4
Toxicokinetic evaluation of velsecorat after inhalation in rat and dog showed that velsecorat was absorbed into systemic circulation within 30 minutes after dose and displayed multiphasic plasma decay. Repeat dose studies showed a dose proportional increase in exposure. In rat, a high plasma clearance and low oral bioavailability was concluded (data on file). In a pre-clinical rat model, measuring inhibition of Sephadex-induced lung edema, velsecorat demonstrated dose dependent anti-inflammatory effects in the airways.2
Taken together, these properties suggest that velsecorat has a potential for an improved therapeutic ratio compared to current standard of care inhaled corticosteroids.2 Subsequent clinical studies have shown that it is well tolerated in healthy volunteers of Japanese descent4 and adolescents with asthma.5 In phase 2 studies in adults with asthma, velsecorat delivered via dry powder inhaler has also shown efficacy on lung function, asthma control, use of rescue medication, fractional exhaled nitric oxide (FeNO), and CompEx, a novel composite endpoint that accurately mirrors the response to treatment seen with severe exacerbations.3,6,7
Here, we describe the first-in-human clinical study of velsecorat, evaluating single and multiple ascending doses in healthy volunteers, prior further studies in patients with asthma. The primary objective of the study was to investigate the safety and tolerability of inhaled velsecorat. Exposure limits were predefined by the maximum exposure obtained in the preclinical toxicological studies, as was a maximum tolerated lung deposited dose. Secondary objectives included a characterisation of the pharmacokinetics (PK), to assess dose proportionality, the time required to reach steady state and the degree of accumulation, and to thoroughly investigate the pharmacodynamics (PD) by assessing effects on the HPA axis (measured as 24 hour cortisol concentration profiles and ACTH/Synacthen test) and other relevant biomarkers of systemic activity.
Methods
Study Design and Participants
This was a randomized first-in-human, double-blind, placebo-controlled, single-center study in healthy male volunteers (NCT01636024, ClinicalTrials.gov). The trial was conducted in compliance with Good Clinical Practice and the Declaration of Helsinki and all participants gave their written informed consent before participating in the trial. The investigators obtained institutional review board approvals on 19 September 2012 at NRES Committee, Surrey Borders, London, UK for the study protocol, REC Reference: 12/LO/1170, IRAS Project ID: 111,060. The study was conducted at Quintiles Drug Research Unit at Guy’s Hospital, London, UK, from 25 September 2012 to 7 June 2013. Participants were included that met the following criteria: aged between 18 and 45 years with veins suitable for cannulation or repeated venepuncture; a body mass index between 18 and 30 kg/m2 weighing at least 50 kg and no more than 100 kg. Participants were excluded if they had a history of any clinically significant disease or disorder, had used systemic glucocorticosteroids within 6 weeks of enrolment.
Randomization and Interventions
The study comprised two parts; the first investigating single ascending doses of inhaled velsecorat/placebo and the second investigating repeated once-daily dosing of velsecorat/placebo; all administered via the Spira Electra nebulizer. A randomization scheme was generated using validated internal software, allocating the participants to velsecorat or placebo. Safety findings, as well as PK and cortisol data were evaluated by a Safety Review committee prior to any dose escalations.
Single Ascending Dose Study
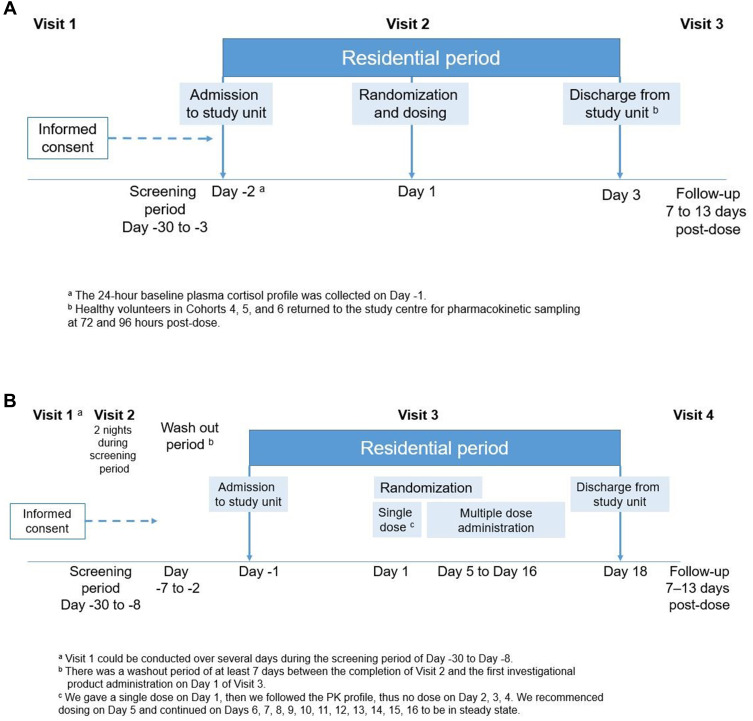
In each cohort, two participants were dosed on the first day (one received velsecorat and one received placebo). Safety and tolerability data were reviewed by the investigator before the remainder of the cohort were sequentially dosed (velsecorat n=6 and placebo n=2 per dose level). The starting dose was set based on the toxicological screening program no observable adverse effect level (NOAEL) using the FDA guidance, 2005 and set 10-fold lower than the estimated predicted therapeutic lung deposited dose. The dose range, set at a delivered dose of 7.4 μg with up to 9 planned dose levels not to exceed a delivered dose of 1872 μg (1200 μg lung deposited dose), equal to the maximum allowed lung deposited dose as attained in the toxicological screening program. The study comprised 3 visits: enrolment (within 30 days of dosing), a residential study period with dosing on Day 1 and discharge on Day 3 and a follow-up visit 7–13 days post-dose (Figure 1A).
Figure 1.
Study design: (A) single ascending dose; (B) multiple ascending dose.
Multiple Ascending Dose Part
Three cohorts were planned with one optional additional cohort. Three cohorts were dosed (velsecorat n=6; placebo n=3 each cohort). A single inhaled dose of velsecorat/placebo was administered on Day 1, followed by once-daily dosing on Days 5 through 16 (for 12 days). The starting dose was a delivered dose of 312 μg. The daily delivered dose for Cohort 2 was 1248 μg. Cohort 3 received a daily delivered dose of 1872 μg equal to the maximum allowed lung deposited dose as attained in the toxicological screening program (Figure 1B). The study consisted of 3 visits: screening (within 30 days of dosing), a residential study period with dosing on Day 1 and discharge on Day 18 and a follow-up visit 7–13 days following the last dose.
Assessments
In both study parts, safety and tolerability were assessed during the study as adverse events (AEs), vital signs, ECG, physical examination, laboratory assessments and lung function test (spirometry). Venous blood samples for analysis of plasma velsecorat concentrations were taken on Day 1 (in both study parts) and Day 16 at pre-dose, 5, 15, 30 minutes, and 1, 2, 4, 6, 9, 12, 18, 36, 48 and 96 hours post-dose and on Days 6 through 15 at pre-dose in the multiple dose part.
To assess the effects of velsecorat on the HPA axis, venous blood samples for analysis of plasma cortisol concentrations were collected on Day −1, Day 1 (in both study parts) and Day 16 at pre-dose (0), 2, 4, 6, 8, 10, 12, 16, 20, 22 and 24 h post dose. All sampling commenced at approximately 8 in the morning.
For the ACTH Stimulation test, venous blood samples for determination of plasma cortisol were taken immediately before (baseline), and after 30±1 minutes and 60±1 minutes following Synacthen injection at screening and on Day 17 of the residential period. Synacthen (0.25 mg) was injected at approximately 8 am. A plasma cortisol level of >150 nmol/L at baseline, and a maximal value above 400 nmol/L post-Synacthen or an increase by Synacthen of >200 nmol/L were regarded as normal.
In the multiple dose part, venous blood samples for analysis of plasma DHEA-S and osteocalcin were taken on Day 1 at pre-dose and Day 16 at 24 h after dose and venous blood samples for β hydroxycholesterol were taken pre-dose and on Day 16.
Investigational Products and Administration
Velsecorat and placebo were characterized with respect to aerodynamic particle size distribution and delivered dose. The aerodynamic droplet size distribution was measured using a chilled new-generation multi-stage impactor operated at 15 L/min.8 Untransformed impactor data were fitted to a bimodal distribution using Thiel’s method of nonlinear regression.9 The lower mode (smaller particles) was assumed to be log-normally distributed and characterized by mass median aerodynamic diameter (MMAD) and a geometric standard deviation (GSD). The MMAD was determined to be 4.4 [2.2] – 6.0 [2.1] µm (range, n = 10, [GSD]) for the investigated suspensions. Optimal size for lung deposition of inhaled medications is in the range 1–5 µm.10,11
Suspension for inhalation ranging from 0.14 to 4.0 mg/g micronized velsecorat or placebo was administered via a dosimetric jet nebulizer (Spira Electra 2™, Respiratory Care Center, Hameenlinna, Finland). The volunteers were instructed to inhale a pre-determined number of breaths (ranging between 4 to 36 breaths) by slow tidal breathing (inhaled flow rate about 18L/min to a total volume of >1 L and free exhalation). Training in the inhalation technique was given during screening and on admission to the study. Delivered dose, defined as the nominal dose delivered from the mouthpiece of the nebulizer, ranged from 7 µg to 1872 µg in the single ascending dose study and 312 µg to 1872 µg in the multiple ascending dose study. Following inhalation of velsecorat/placebo, in order to minimize systemic absorption, the volunteers were instructed to rinse their mouth three times and to spit out the water, prior to first PK blood draw.
Bioanalytical Methods
Velsecorat was quantitated in human plasma samples using a validated bioanalytical method at Covance Laboratories Limited, Harrogate, UK. The plasma method employs protein precipitation and liquid chromatography with tandem mass spectrometric (LC-MS/MS) detection in the negative ion mode. The method was validated in the range 10.0 to 10,000 pmol/L and the lower limit of quantification (LLOQ) set at 10.0 pmol/L using 200 µL plasma. The bioanalytical method for velsecorat is described in detail in a separate publication.4
Plasma cortisol concentrations were determined by a validated bioanalytical method at York Bioanalytical Solutions, North Yorkshire, UK. The plasma cortisol assay involved liquid extraction followed by LC-MS/MS detection, validated in the range 5 to 1000 ng/mL and with a LLOQ set at 5 ng/mL using 100 µL plasma.
Dehydroepiandrosterone-sulfate (DHEA-S) and osteocalcin concentrations in plasma were analysed by validated bioanalytical methods.
Statistical Analysis
PK evaluation was performed using noncompartmental analysis (Phoenix WinNonlin Version 6.4). AUC was calculated as AUC0–last+Clast/λz in which Clast is the last observed quantifiable concentration. λz is the rate constant estimated from individual linear regression of the terminal part of the log concentration versus time curve. The t½ was calculated by 1n(2)/λz. If t½ was greater than half of the total sampling interval (48 hrs) or the percent extrapolated AUC was >30%, the terminal elimination phase dependent parameters (t½ and AUC) were excluded from descriptive statistics. Observations below LLOQ were set to missing and thus ignored in the analysis.
Due to the exploratory nature of the study, the sample sizes were not based on formal statistical considerations, but rather on experience from previous similar Phase I studies with other compounds. Descriptive statistics are presented throughout. Dose proportionality was analyzed based on a graphical analysis of dose-adjusted AUC and Cmax and by using the power model approach. The intercept α and the slope β (in [AUC or Cmax]=α*doseβ) together with associated 90% confidence intervals (CI) were estimated and presented for AUC and Cmax, and dose proportionality was concluded if the CI of the slope included one and had a reasonable range (Hummel et al Pharmaceutical Stat 2009). The power model parameters were estimated using least squares regression. For plasma cortisol, AUC0–24 was calculated, and the AUC ratios of treatment over baseline were compared between treatments using a multiplicative analysis of covariance (ANCOVA). The ratio was also log transformed prior to analysis, with treatment included as the fixed factor and the baseline included as a covariate. Data from healthy volunteers who received placebo were pooled across cohorts in all analyses.
Results
In the single dose part, 47 healthy male volunteers were randomized into six cohorts and the following delivered doses were administered as a single dose: 7 μg (Cohort 1); 38 μg (Cohort 2); 187 μg (Cohort 3); 624 μg (Cohort 4); 1248 μg (Cohort 5); and 1872 μg (Cohort 6). All healthy volunteers randomized to the single dose part completed the study. In the multiple dose part, 26 participants were randomized into 3 cohorts and the following delivered doses were administered as a single dose on Day 1 and multiple doses from Day 5 to Day 16 (1 dose per day): 312 μg (Cohort 1); 1248 μg (Cohort 2);and 1872 μg (Cohort 3). Twenty-five out of the 26 healthy volunteers randomized completed the multiple dose part. The one withdrawal, which was in Cohort 1, was due to personal reasons. The demographic and key baseline characteristics are summarized in Table 1.
Table 1.
The Demographic and Key Baseline Characteristics of the Healthy Volunteers
| Single Dose Part | Multiple Dose Part | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pooled Placebo N=12 | 7 μg N=6 | 38 μg N=6 | 187 μg N=6 | 624 μg N=5 | 1248 μg N=6 | 1872μg N=6 | Pooled Placebo N=9 | 312 μg N=6 | 1248 μg N=5 | 1872 μg N=6 | |
| Age in years, Mean (range) | 28 (20–37) | 30 (21–43) | 32 (22–41) | 31 (21–42) | 35 (27–45) | 30 (20–40) | 30 (20–44) | 27 (20–33) | 31 (23–42) | 36 (31–44) | 34 (27–40) |
| Height in cm Mean (SD) | 178.1 (5.6) | 177.0 (5.5) | 175.8 (8.9) | 173.3 (7.4) | 177.6 (3.6) | 175.3 (5.2) | 176.8 (8.3) | 179.4 (6.6) | 175.4 (6.8) | 171.2 (9.5) | 176.2 (4.4) |
| Weight in kg Mean (SD) | 82.1 (11.0) | 77.8 (8.7) | 73.7 (12.4) | 74.2 (7.5) | 80.9 (6.7) | 73.1 (7.0) | 74.5 (4.5) | 77.6 (13.1) | 80.2 (5.7) | 72.5 (13.4) | 80.1 (10.6) |
| BMI in kg/m2 Mean (SD) | 25.83 (2.81) | 24.94 (3.49) | 23.67 (2.32) | 24.73 (2.48) | 25.60 (1.49) | 23.82 (2.62) | 23.90 (1.43) | 23.96 (2.59) | 26.05 (1.25) | 24.63 (3.35) | 25.74 (2.75) |
| Race (n [%]) | |||||||||||
| Asian | 1 (8.3) | 0 (0.0) | 1 (16.7) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (22.2) | 1 (16.7) | 0 (0.0) | 0 (0.0) |
| Black or African American | 1 (8.3) | 3 (50.0) | 1 (16.7) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 3 (33.3) | 1 (16.7) | 0 (0.0) | 2 (33.3) |
| White | 10 (83.3) | 3 (50.0) | 4 (66.7) | 3 (50.0) | 5 (100.0) | 6 (100.0) | 5 (83.3) | 4 (44.4) | 4 (66.7) | 5 (100.0) | 4 (66.7) |
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Safety and Tolerability
During the study, there were no deaths reported, nor any AEs that led to discontinuation. There were only a few AEs, reported and the majority were of mild intensity. There was no particular pattern of AEs evident and no increase in the incidence of AEs with increasing doses of velsecorat. All AEs resolved during the study.
Single Ascending Dose Part
There were no serious AEs reported. The most frequently reported AE was cough, reported for three volunteers, all on velsecorat (624 μg, 1248 μg, and 1872 μg). One of the cough events reported in the 1248 μg cohort was considered moderate. This event was reported more than one day after the velsecorat administration and the other two events were reported within one hour of the velsecorat administration. Oropharyngeal pain was reported for two volunteers overall (one on placebo and one in the 1248 µg cohort). All other AEs were each only reported for one volunteer overall, and a list of AEs is available in Table 2A.
Table 2.
Number of Individuals Who Reported at Least One AE During (A) the Single Ascending Dose Part and (B) the Multiple Ascending Dose Part of the Study
| A: | ||||||||||||
| Summary of number (%) of participants who had at least 1 adverse event in any category | ||||||||||||
| Velsecorat | ||||||||||||
| Adverse event category |
Pooled placebo N=12 |
7 µg N=6 |
38 µg N=6 |
187 µg N=6 |
624 µg N=5 |
1248 µg N=6 |
1872 µg N=6 |
All velsecorat N=35 |
||||
| Any AE | 3 (25.0) | 1 (16.7) | 1 (16.7) | 1 (16.7) | 1 (20.0) | 2 (33.3) | 1 (16.7) | 7 (20.0) | ||||
| Any serious AE (including events with outcome=death) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||
| Summary of number (%) of participants who had at least 1 adverse event, by Preferred Term, arranged by System Organ Class | ||||||||||||
| System Organ Class Preferred Term |
Pooled placebo N=12 |
7 µg N=6 |
38 µg N=6 |
187 µg N=6 |
624 µg N=5 |
1248 µg N=6 |
1872 µg N=6 |
All velsecorat N=35 |
||||
| Ear and labyrinth disorders | 1 (8.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||
| Ear pain | 1 (8.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||
| Gastrointestinal disorders | 1 (8.3) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.9) | ||||
| Abdominal discomfort | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||
| Salivary hypersecretion | 1 (8.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||
| Infections and infestations | 1 (8.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.9) | ||||
| Nasopharyngitis | 1 (8.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||
| Rhinitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | ||||
| Musculoskeletal and connective tissue disorders | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.9) | ||||
| Musculoskeletal chest pain | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.9) | ||||
| B: | ||||||||||||
| Summary of number (%) of participants who had at least 1 adverse event in any category | ||||||||||||
| Velsecorat | ||||||||||||
| Adverse event category | Pooled placebo N=9 |
312 µg N=6 |
1248 µg N=5 |
1872 µg N=6 |
All AZD7594 N=17 |
|||||||
| Any AE | 6 (66.7) | 3 (50.0) | 1 (20.0) | 3 (50.0) | 7 (41.2) | |||||||
| Any serious AE (including events with outcome=death) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (5.9) | |||||||
| Summary of number (%) of subjects who had at least 1 adverse event, by Preferred Term, arranged by System Organ Class | ||||||||||||
| System Organ Class Preferred Term | Pooled placebo N=9 |
312 µg N=6 |
1248 µg N=5 |
1872 µg N=6 |
All AZD7594 N=17 |
|||||||
| Gastrointestinal disorders | 3 (33.3) | 3 (50.0) | 0 (0.0) | 1 (16.7) | 4 (23.5) | |||||||
| Abdominal pain | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | |||||||
| Diarrhea | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 1 (5.9) | |||||||
| Dyspepsia | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||
| Gingival bleeding | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 1 (5.9) | |||||||
| Mouth ulceration | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 1 (5.9) | |||||||
| Nausea | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 1 (5.9) | |||||||
| Oral pain | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||
| Toothache | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||
| General disorders and administration site conditions | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||
| Local swelling | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||
| Infections and infestations | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||
| Nasopharyngitis | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||
| Musculoskeletal and connective tissue disorders | 2 (22.2) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (5.9) | |||||||
| Muscle twitching | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||
| Myalgia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (5.9) | |||||||
| Pain in extremity | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||
| Nervous system disorders | 4 (44.4 | ) 0 (0.0) | 1 (20.0) | 1 (16.7) | 2 (11.8) | |||||||
| Headache | 4 (44.4) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (5.9) | |||||||
| Dizziness | 0 (0.0) | 0 (0.0) | 1 (20.0) | 0 (0.0) | 1 (5.9) | |||||||
| Dysgeusia | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||
| Psychiatric disorders | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||
| Restlessness | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||
| Respiratory, thoracic, and mediastinal disorders | 2 (22.2) | 2 (33.3) | 0 (0.0) | 0 (0.0) | 2 (11.8) | |||||||
| Dyspnea | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||
| Hiccups | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 1 (5.9) | |||||||
| Oropharyngeal pain | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 1 (5.9) | |||||||
| Rhinorrhea | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||
| Sneezing | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||
| Vascular disorders | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (5.9) | |||||||
| Thrombosisa | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (5.9) | |||||||
Multiple Ascending Dose Part
There was one serious AE reported: severe abdominal pain, reported in the 1872 μg cohort during the follow-up period at 2.5 days after the last dose of velsecorat. The event was not considered to be related to the study drug. All other AEs were mild in severity. The most frequently reported AE was headache, reported for five volunteers: four on placebo and one in the 1872 μg cohort. All other AEs were each only reported for one volunteer overall, and a full list of AEs is available in Table 2B.
There were no clinically relevant treatment-related changes or trends in any individual or mean vital signs (supine pulse rate and blood pressure), 12-lead ECG findings (heart rate, QRS, QTcF, or PR intervals). For QTcF, no healthy volunteer showed an increase from baseline of >30 msec or QTcF values >450 msec.
In the single dose part, no clinically important changes in haematology, biochemistry, or urinalysis parameters were reported for any participant.
In the multiple dose part, no clinically important changes in haematology or urinalysis parameters were reported for any participant. One participant who received velsecorat at the 799 μg dose level, showed an increase in alanine aminotransferase (ALT) and to a lesser extent aspartate aminotransferase (AST) during the study (maximum ALT value 109 IU on Day 14) which resolved after the end of dosing. Although a drug effect could not be excluded, other study-related factors such as dietary change might have been the cause.
No abnormal laboratory values were reported as an AE. Variation, but no relevant trends, was observed over time and between treatments in mean and median laboratory values.
Pharmacokinetics
Single Dose Plasma Exposure Data
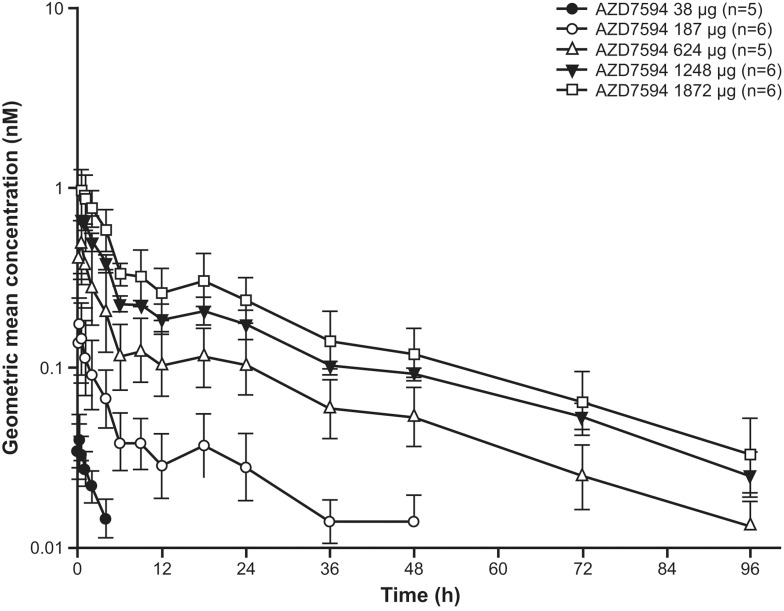
Plasma concentration time data after a single dose of velsecorat are given in Figures 2 and 3, Day 1. The PK profile of inhaled velsecorat was characterised by an initial rapid absorption from the lung, a relatively fast initial concentration decline and a slow terminal elimination from plasma. The data indicates dose proportional plasma exposure: In a power model, dose adjusted analysis of the data from a single dose, including the first dose in the multiple dose study, with doses 38–1872 µg, the 95% CI of the slope included 1 both for AUC (0.88 to 1.05) and Cmax (0.85 to 1.01). Hence, within this dose range, data supports dose proportional plasma exposure of velsecorat.
Figure 2.
Geometric mean (+SD geometric mean) plasma concentrations of velsecorat (AZD7594) following a single dose (Day 1). Figures illustrate semi-logarithmic data, and doses are depicted as µg delivered dose.
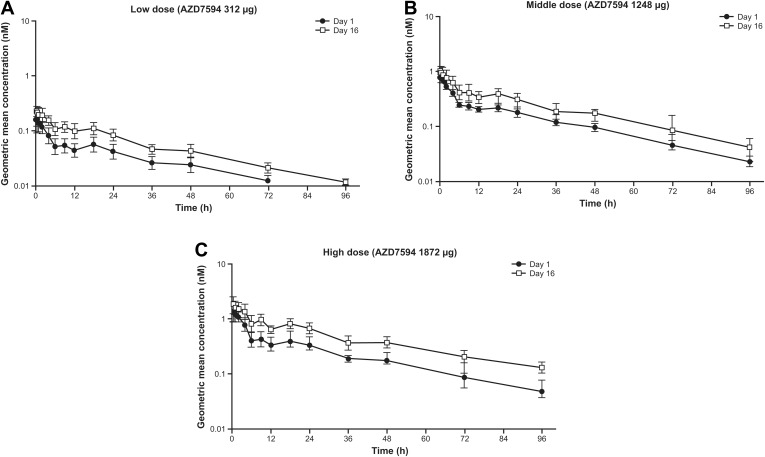
Figure 3.
Geometric mean (+SD) plasma concentrations of velsecorat (AZD7594) on Day 1 and Day 16 during once-daily inhalation. Figures illustrate semi-logarithmic data, and doses are depicted as µg delivered dose. (A) Low dose (velsecorat 312 µg), (B) middle dose (velsecorat 1248 µg), (C) high dose (velsecorat 1872 µg).
Multiple Dose Plasma Exposure Data and Pharmacokinetics
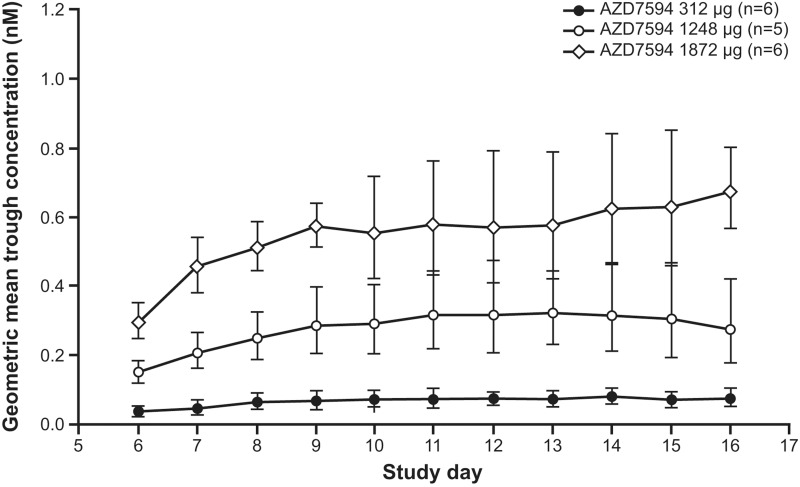
Plasma exposure increased with dose following multiple dosing (Figure 3, Day 16). PK parameters are provided in Table 3. Overall, after 12 days of once daily treatment with velsecorat, there was no relevant deviation from dose proportional PK . In a power model analysis of doses 312–1872 µg, the 95% CI of the slope included 1 for AUC (0.95 to 1.27) and nearly for Cmax (1.02 to 1.33). The terminal half-life following repeated dosing was 25–31 hours (Figure 3) and steady state conditions for velsecorat in plasma were generally reached within 4 doses (Figure 4). The accumulation ratio was low (≤2), and data did not indicate any time-dependent PK.
Table 3.
Delivered Doses and PK Parameters of Velsecorat After a Single Inhaled Dose from Part A (A) and After Inhaled Dosing for 12 Days in Part B (B) Geometric Mean (CV%)
| A: | ||||||||
|---|---|---|---|---|---|---|---|---|
| Delivered Dose (µg) | n | tmax (Hours)e | Cmax (nmol/L) | AUC (nmol·h/L) | t½λz (Hours)f | |||
| 38 | 6 | 0.27 (0.27–0.55)b | 0.039 (33.8)b | NDc | 3.24 (0.681)d | |||
| 187 | 6 | 0.28 (0.25–0.50) | 0.176 (59.3) | 2.36 (26.6)a | 21.9 (3.33)a | |||
| 624 | 6 | 0.25 (0.08–0.52)b | 0.528 (45.4)b | 6.84 (40.9)b | 24.3 (2.60)b | |||
| 1248 | 6 | 0.27 (0.10–0.35) | 0.853 (9.60) | 12.9 (6.80) | 28.1 (8.20) | |||
| 1872 | 6 | 0.42 (0.10–1.02) | 0.992 (30.1) | 17.3 (32.8) | 26.3 (4.95) | |||
| B: | ||||||||
| Delivered Dose (µg) | n | tmax (Hours)e | Cmax (nmol/L) | AUC0–24, ss (nmol·h/L) | t½λz (Hours)f | Racg | ||
| 312 | 6 | 0.08 (0.08–1.02)b | 0.251 (17.8)b | 2.88 (25.3)b | 24.6 (3.56)b | 1.98 (1.64–2.40)b | ||
| 1248 | 6 | 0.25 (0.25–0.25)b | 1.08 (35)b | 10.9 (34.6)b | 25.1 (5.17)b | 1.59 (1.32–1.93)b | ||
| 1872 | 6 | 0.25 (0.25–0.25) | 2.20 (24.2) | 22.4 (23.4) | 31.4 (2.85) | 1.79 (1.50–2.13) | ||
Notes: an=4. bn=5. cn=0. dn=3. eMedian and range. fArithmetic mean (sd). g90% CI, definition AUC0–24, ss/AUC0–24 day 1.
Abbreviation: ND, not determined.
Figure 4.
Geometric mean (±SD) trough plasma concentration of velsecorat (AZD7594) versus study days during once-daily dosing for 12 days. Figure illustrates semi-logarithmic data presented by delivered dose.
Pharmacodynamics
Effects on the HPA Axis
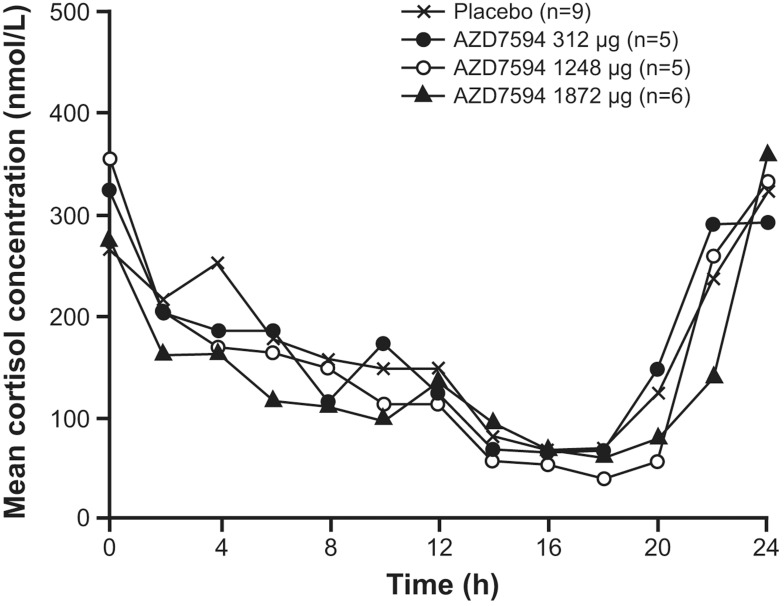
Following single and multiple dose administration of velsecorat, the active/placebo ratio of baseline-adjusted AUEC(0–24) of plasma cortisol decreased with increasing dose (Table 4). Suppression of the HPA-axis based on plasma cortisol AUEC(0–24) was observed following a single dose of 1248 μg and single and multiple dose administration of velsecorat at the highest dose, 1872 μg. The degree of cortisol suppression of the 1872 µg dose, compared to placebo, was 14% after a single dose and 23% after 12 days of once daily dosing in the multiple dose study (Table 4, Figure 5). At the lower dose levels, the plasma cortisol suppression was comparable with placebo.
Table 4.
Geometric Mean and 95% CI of Velsecorat versus Placebo 0- to 24-hr Plasma Cortisol Ratios After Single (Day 1) and Repeated, Once-Daily Dosing for 12 Days (Day 16)
| Study/ Day | Parameter | Treatment | n | Geometric LS Mean | Pairwise Comparison to Placebo | |
|---|---|---|---|---|---|---|
| Ratio (%) | 95% CI | |||||
| Single dose part | ||||||
| Day 1 | Post dose/baseline ratio for AUEC(0–24) | Placebo | 12 | 1.071 | ||
| 7 ug | 6 | 1.041 | 97.18 | (88.41, 106.82) | ||
| 38 µg | 6 | 1.074 | 100.27 | (91.03, 110.45) | ||
| 187 µg | 6 | 0.9745 | 91.01 | (82.54, 100.36) | ||
| 624 µg | 5 | 1.035 | 96.66 | (87.52, 106.74) | ||
| 1248 µg | 6 | 0.9721 | 90.79 | (82.50, 99.90) | ||
| 1872 µg | 6 | 0.9238 | 86.28 | (78.23, 95.15) | ||
| Multiple dose part | ||||||
| Day 16 | Postdose/baseline ratio for AUEC(0–24) | Placebo | 8 | 0.9011 | ||
| 312 ug | 5 | 0.8562 | 95.01 | (77.93, 115.84) | ||
| 1248 ug | 5 | 0.7830 | 86.89 | (71.45, 105.67) | ||
| 1872 ug | 5 | 0.6976 | 77.42 | (63.66, 94.15) | ||
Figure 5.
Mean plasma cortisol concentration versus time by treatment following 12 days of once daily morning (8 am) dosing of velsecorat (AZD7594).
Participants underwent an Synacthen test at baseline and after 12 days of treatment with velsecorat to test adrenal functional capacity. The maximum response following Synacthen was suppressed by ~28% in the high dose (1872 µg) cohort, with a pairwise comparison to placebo ratio of 71.9% (95% CI 53.2, 97.0) (Supplementary Table 1). At the lower doses the effect of ACTH stimulation was comparable with placebo, with the ratio 116.8 (95% CI 87.3, 156.4) for the 312 µg cohort and 102.7 (95% CI 76.1, 138.7) for the 1248 µg cohort.
Other Biomarkers
A dose dependent decrease in osteocalcin was observed following 12 days dosing of velsecorat. Post-dose versus baseline ratios were 76.9% (95% CI of 65.6–90.2) and 53.1% (95% CI of 46.3–60.9) for the two highest doses respectively (Supplementary Table 2). At the lower dose level, plasma osteocalcin levels were comparable with placebo.
There was no effect on DHEA-S nor β-hydroxycholesterol following 12 days of treatment with inhaled velsecorat (Supplementary Table 2).
Discussion
This study showed that inhaled velsecorat, delivered by nebulization was well tolerated in healthy male volunteers and raised no safety concerns throughout the investigated dose range – up to 1872 µg given as single dose and once daily for 12 days. The single and multiple ascending dose parts of the study both indicate dose proportional plasma exposure of velsecorat. Steady state kinetics were reached in 4 days with a low (≤2) accumulation ratio.
Further dose escalation was not possible in this ascending dose study as the highest dose defined in the earlier toxicological program was reached by the 1872 μg delivered dose. Hence, a maximum tolerated dose could not be defined.
PD analyses showed that only at the highest doses of inhaled velsecorat, 1248 μg and 1872 μg, were there pharmacological effects on the HPA axis (0–24 hour cortisol data), a the highest dose only, an effect on adrenal capacity and function (Synacthen challenge data). An effect on osteocalcin was observed at the two highest doses following repeated dosing, which indicates a systemic PD effect of velsecorat. No effect was seen on DHEA-S, a steroidal adrenal hormone affected by exogenous corticosteroid exposure but with the advantage for a biomarker of lacking diurnal variation.12 In addition, in the tested dose range, inhaled velsecorat did not appear to have any potential for drug-drug interactions through CYP3A4 induction, as 4β-hydroxycholesterol – an endogenous marker of CYP3A4 induction – was unaffected by treatment. The in-vitro investigation of velsecorat metabolism indicate that the compound is mainly metabolised by CYP3A4 with some contribution of CYP2C9. Although in-vitro studies with velsecorat showed inhibition of CYP isoforms (with lowest IC50 for 2C9) and transporters P-gp, BCRP and OATP1B1, this was observed at concentrations considerably higher than clinically relevant. The potential for velsecorat to induce CYP isoenzymes was low for the same reason. Velsecorat’s DDI liability when co-administered with CYP3A4 inhibitors warrants further investigation in the clinic.
It should be borne in mind that the data sets reported herein are small, and that effects may have been distinguished in other and/or larger cohorts of participants.
The observed effects on markers of adrenal capacity and HPA axis function appear to occur at doses higher than those used in the subsequent Phase 2a6 and Phase 2b3 studies of velsecorat, which administered 800 µg or less. In both phase 2 studies evaluating velsecorat in adults with asthma, efficacy was seen on key measures such as lung function, FeNO, and CompEx yet no statistically significant differences in plasma cortisol level was observed between velsecorat and placebo.3,6
In conclusion, this first clinical evaluation of nebulized velsecorat suggests that this novel SGRM is well tolerated in healthy male volunteers when given as single doses 7–1872 µg and multiple (12 days once-daily) doses 312–1872 µg. Velsecorat shows dose proportional plasma exposure, low accumulation, and has a dose dependent effect on systemic markers of glucocorticoid activity. As velsecorat continues to show promising outcomes in future studies in patients, it may comprise a new option in the available range of inhaled anti-inflammatory agents for the treatment of respiratory diseases such as asthma. Though the data in this report are based on a small number of healthy volunteers, and larger, confirmatory clinical trials are warranted to fully characterize the efficacy, safety and therapeutic benefit of this novel treatment regimen.
Acknowledgments
We thank the volunteers who participated in this study as well as the investigators and site staff at the Quintiles Drug Research Unit at Guy’s Hospital (London, UK). We thank Ulrika Wählby Hamrén (AstraZeneca, Gothenburg, Sweden) for her contribution to data interpretation. Editorial assistance was provided by Lee Wulund of AstraZeneca, as well as David Candlish and Sophieanne Wastling of inScience Communications, Springer Healthcare Ltd, UK, which was funded by AstraZeneca in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Funding Statement
This study was funded by AstraZeneca. Quintiles received funding from AstraZeneca for the conduct of the study.
Data Sharing Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy, described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
S. Prothon, M.Aurivillius, U.Tehler, U. G. Eriksson: Employee of AstraZeneca and holds shares in AstraZeneca. A. Aggarwal: was an employee of AstraZeneca at the time of study conduct and is now a current employee of CereXis Inc. Y. Chen: was an employee of AstraZeneca at the time of study conduct is now a current employee of Epizyme. The authors report no other conflicts of interest in this work.
References
- 1.Global Initiative for Asthma. Global strategy for asthma management and prevention. GINA; 2019. Available from: http://wwwginasthmaorg/. Accessed January 7, 2022.
- 2.Hemmerling M, Nilsson S, Edman K, et al. Selective nonsteroidal glucocorticoid receptor modulators for the inhaled treatment of pulmonary diseases. J Med Chem. 2017;60(20):8591–8605. doi: 10.1021/acs.jmedchem.7b01215 [DOI] [PubMed] [Google Scholar]
- 3.Chupp G, Beeh KM, Jauhiainen A, et al. Results of a phase 2b dose finding study of velsecorat, an inhaled non-steroidal, selective Glucocorticoid Receptor Modulator in Asthma (GRANIT). Am Thorac Soc Int Conf. 2021;203(A1202 [abstract]):A1202. [Google Scholar]
- 4.Prothon S, Wählby Hamrén U, Tehler U, et al. Safety, pharmacokinetics and pharmacodynamics of the selective glucocorticoid receptor modulator AZD7594, following inhalation in healthy Japanese volunteers. Drug Des Devel Ther. 2019;13:3845–3853. doi: 10.2147/DDDT.S215170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Psallidas I, Necander S, Thurman L, et al. Results and learnings from AMBER, a decentralized Phase 1 study assessing the pharmacokinetic profile of inhaled velsecorat, a non-steroidal, selective glucocorticoid receptor modulator, in adolescents with asthma. Am Thorac Soc Int Conf. 2021;203(A3239 [abstract]):A3239. [Google Scholar]
- 6.Brown MN, Fuhr R, Beier J, et al. Efficacy and safety of AZD7594, an inhaled non-steroidal selective glucocorticoid receptor modulator, in patients with asthma: a phase 2a randomized, double blind, placebo-controlled crossover trial. Respir Res. 2019;20(1):37. doi: 10.1186/s12931-019-1000-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuhlbrigge AL, Bengtsson T, Peterson S, et al. A novel endpoint for exacerbations in asthma to accelerate clinical development: a post-hoc analysis of randomised controlled trials. Lancet Respir Med. 2017;5(7):577–590. doi: 10.1016/S2213-2600(17)30218-7 [DOI] [PubMed] [Google Scholar]
- 8.Berg E, Svensson JO. Establishing standardised methods for comparing aqueous droplet inhalers. Pharmeur Sci Notes. 2006;2006(2):9–15. [PubMed] [Google Scholar]
- 9.Thiel CG. Cascade impactor data and the lognormal distribution: nonlinear regression for a better fit. J Aerosol Med. 2002;15(4):369–378. doi: 10.1089/08942680260473443 [DOI] [PubMed] [Google Scholar]
- 10.de Boer AH, Gjaltema D, Hagedoorn P, Frijlink HW. Can ‘extrafine’ dry powder aerosols improve lung deposition? Eur J Pharm Biopharm. 2015;96:143–151. doi: 10.1016/j.ejpb.2015.07.016 [DOI] [PubMed] [Google Scholar]
- 11.Demoly P, Hagedoorn P, de Boer AH, Frijlink HW. The clinical relevance of dry powder inhaler performance for drug delivery. Respir Med. 2014;108(8):1195–1203. doi: 10.1016/j.rmed.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 12.Sahiner UM, Cetinkaya S, Ozmen S, Arslan Z. Evaluation of adrenocortical function in 3–7 aged asthmatic children treated with moderate doses of fluticasone propionate: reliability of dehydroepiandrosterone sulphate (dhea-s) as a screening test. Allergol Immunopathol. 2011;39(3):154–158. doi: 10.1016/j.aller.2010.06.005 [DOI] [PubMed] [Google Scholar]