Abstract
Purpose
Neovascular age-related macular degeneration (nAMD) and diabetic macular edema (DME) patients treated with intravitreally injected anti-vascular endothelial growth factor (anti-VEGF) monotherapies achieve lower vision improvements compared with patients in clinical trials. This qualitative research study aimed to better understand the real-world anti-VEGF treatment experience from nAMD and DME patients’, caregivers’, and retina specialists’ perspectives.
Methods
One-time, semi-structured, individual interviews were conducted with adult patients with nAMD or DME treated with anti-VEGF injections for ≥12 months, their caregivers, and experienced retina specialists. Interview transcripts were analyzed qualitatively using a thematic analysis approach.
Results
A total of 49 nAMD and 46 DME patients, 47 nAMD and 33 DME caregivers, and 62 retina specialists were interviewed in the USA, Canada, France, Germany, Italy and Spain. Most (79%) patients and caregivers reported disruptions to their routine on the day before, the day of, or the day after anti-VEGF injection. Seven nAMD patients (14%) and 14 DME patients (30%) reported having missed an injection visit. The most frequently reported driver for adherence for patients was the doctor–patient relationship (n=66, 70%), whereas for caregivers, it was the ease of booking an appointment (n=25, 32%). Retina specialists reported patient education on the treatment (n=28, 45%) as the most important driver. Treatment barriers could be grouped into four categories: tolerability, clinical factors, logistical parameters and human factors. The most frequently reported barrier to adherence for patients and caregivers was related to side effects (pain/discomfort/irritation: n=63, 67% of patients; n=52, 66% of caregivers), whereas for retina specialists it was logistical parameters (travel logistics: n=44, 71%).
Conclusion
This study highlights the importance of the doctor–patient relationship and patient education as key drivers, and treatment tolerability and logistics as key barriers to treatment adherence. Improved doctor–patient relationship/communication and patient education together with new therapies offering convenience, long-acting effectiveness, and better tolerability may improve treatment adherence.
Keywords: qualitative interviews, treatment experience, adherence, neovascular age-related macular degeneration, diabetic macular edema
Introduction
Age-related macular degeneration (AMD) is a neurodegenerative disease of the central retina that causes irreversible destruction of the macula, leading to loss of fine-detail central vision required for activities such as driving, reading, seeing in color and recognizing faces.1 Neovascular AMD (nAMD) accounts for most of the cases of AMD‐related severe vision loss,2 and owing to age being a risk factor for nAMD, the incidence of nAMD is projected to increase with the aging of the population.3,4 Diabetic macular edema (DME) is a microvascular complication of diabetes mellitus, which is characterized by damage to retinal blood vessels secondary to hyperglycemia.5 Given the increasing prevalence of type 2 diabetes, the incidence of DME is projected to rise accordingly.6
People diagnosed with either nAMD or DME may experience significant reduction in functional status and quality of life due to difficulties performing daily activities and the impact of their disease on their emotional well-being.7–9 Intravitreally-injected anti-vascular endothelial growth factor (anti-VEGF) monotherapies (eg, ranibizumab, aflibercept) have been successful in improving and preserving vision and quality of life for patients living with nAMD or DME.1,10 However, both nAMD and DME require ongoing treatment with regular monitoring visits and treatment to control disease activity, and evidence suggests that real-world outcomes for patients living with nAMD or DME lag behind those documented in clinical trials.1,10 The burden of treatment related to nAMD and DME on patients, caregivers, and the healthcare system, which includes a dynamic interplay of practical, medical, psychological, financial and organizational factors, has been reported to result in patients receiving fewer anti-VEGF intravitreal (IVT) injections and less frequent monitoring than those participating in clinical trials.1,10 Studies on treatment preferences suggest that patients living with nAMD prefer treatments that will prevent visual acuity deterioration while adding the least regimen complexity as possible to their lives.11–13
Current literature on reasons for (non)-adherence, treatment preferences and satisfaction provided limited detailed information on, amongst other topics, co-morbid illnesses or psychological factors/emotional states that are likely to affect adherence. Most studies employed retrospective analysis of electronic health medical records to examine non-adherence. Cross-sectional and prospective observational studies relied mainly on surveys.11,14–16 Only a few studies included interviews.13,17–19 Several studies have investigated the impact of DME or nAMD and its management through anti-VEGF IVT on patients’ quality of life, but most studies were conducted in single countries (USA, China, Australia, United Kingdom, Sweden) using small samples of 7 to 25 patients.20–25
Given the limited scope of previous studies and the differences in the reimbursement systems across countries, a multinational study may provide interesting insights to the current field. Moreover, considering the challenges that patients experience with anti-VEGF IVT injections, identifying and examining the drivers and/or barriers to following anti-VEGF treatment plans, as well as patients’ treatment preferences, are of particular interest to ensure that the new technologies are acceptable to patients and lead to better real-world outcomes.
This study aimed to better understand the context of current anti-VEGF IVT injection standard of care including treatment experiences, retina specialists’ definitions of non-adherence, drivers and barriers to adherence in nAMD and DME from patients’, caregivers’, and retina specialists’ perspectives in a multinational setting, and to suggest how any future therapies might improve the patient treatment experience.
Materials and Methods
Study Procedures and Population
This was a multinational, non-interventional, cross-sectional research study that included qualitative interviews with patients with nAMD or DME, their caregivers, and retina specialists treating the two conditions. Concept elicitation interviews were conducted with the aim to better understand the participants’ experience with nAMD or DME and their treatment, specifically related to treatment experience, adherence, and preference. Participants were recruited in the USA, Canada, France, Italy, Germany, and Spain to capture potential differences in the perspectives of participants living in countries with diverse health systems.
It was initially targeted to recruit 10 participants per condition for every country. This target was not met across all countries, however qualitative studies typically use purposive sampling, rather than probability sampling used in quantitative research, with a focus on the quality and richness of the qualitative data rather than the number of participants. Moreover, concept saturation, the point at which no new concept-relevant data is uncovered by subsequent qualitative interviews26,27 was assessed during the coding process to determine the need for additional participants. On review of the data, additional interviews were not determined to be required.
Patients and caregivers, preferably dyads, were recruited by a recruitment agency through the agency’s internal panels/databases, word of mouth, internet advertising, email blasts, social media, patient associations, and health care professionals. Retina specialists (ie, experienced ophthalmologists) were recruited through the recruitment agency’s internal panels/databases. As per local ethical requirements and Institutional Review Board-approved process, no written consent was required.
The recruitment agency explained the study, addressed any questions or concerns participants had and screened them to confirm eligibility using the study-specific screening form.
Patient and Caregiver Inclusion and Exclusion Criteria
Patient eligibility was determined by proof of diagnosis of nAMD or DME (eg, a letter or a prescription from their physicians). Eligible patients had to be aged 18 years or older, have initiated an anti-VEGF IVT injection treatment regimen at least 12 months before the interview, and have received an anti-VEGF IVT injection within 6 months of the interview. Eligible caregivers had to be aged 18 years or older and the primary caregiver of an adult patient with a physician-confirmed diagnosis of either DME or nAMD. Caregivers were defined as the main person responsible for the patient’s daily care, who had contact with the patient on most days, for most of the time that the patient was at home during the past 12 months. Protocol criteria specified that these were not to be paid professional caregivers, but rather close contacts of the patient such as a family member or friend.
Potential patient and caregiver participants were excluded from participation if they met any of the following criteria: 1) any serious psychiatric disorder or other condition (eg, Alzheimer’s disease, dementia, schizophrenia) that impairs memory or cognitive function, 2) current or history of substance abuse, or appears to be impaired/under the influence, that could interfere with the participant providing informed consent, completing questionnaires or participating in the interview, 3) current enrollment in any clinical trial, 4) and if patients were receiving or had received anti-VEGF therapy in any clinical trial for retinal diseases.
Retina Specialist Eligibility Criteria
Eligible retina specialists were required to have a minimum of 5 years of experience practicing ophthalmology and to have treated at least 30 nAMD or DME patients with anti-VEGF IVT therapy over the past 12 months.
Qualitative Interview Conduct
One-to-one telephone interviews with patients and caregivers were conducted in local languages, while retina specialists’ telephone interviews were performed in English or in local languages upon request. Interviews were conducted by experienced qualitative interviewers following semi-structured interview guides for each participant type (ie, patient, caregivers, and retina specialists) and audio-recorded. Prior to conducting any interviews, all interviewers were trained by the study lead (JL) on the study protocol to understand the disease and treatment context (including IVT therapy) and overall objectives as well as on the semi-structured interview guides to ensure their understanding and address any questions they may have had.
Interviews with caregivers and patients were conducted separately. The interview guides were developed following a brief targeted literature review that was conducted to help identify and understand the factors that could influence adherence to intravitreally-injected nAMD and DME treatments, and patients’ treatment preferences and satisfaction in these indications. The interview guides were finalized after consulting with patient advocacy group representatives from various countries including the ones in scope for this qualitative study. The interview guides included open-ended questions such as “Are there any features of your treatment that affect how you receive your treatment?”, “What do you think about your current treatment?”, “How often do you miss receiving your anti-VEGF injection?”, “What are the reasons you have missed receiving your anti-VEGF injection?” or “Separate from how well a treatment might work, what would an ideal treatment look like?”. Those questions aimed to obtain descriptive answers that discussed various topics, such as disease experience, past, current and ideal treatments and adherence. All participants were compensated for their participation in the interviews, which lasted for up to one hour, as appropriate according to local regulations.
Qualitative Analysis
All interviews were audio-recorded and transcribed verbatim. Non-English interviews were translated into English during the transcription process (ie, no transcripts in the source language were done). De-identified verbatim transcriptions of the interviews were analyzed using the qualitative analysis software MAXQDA (VERBI Software, Germany, 2020).28
A thematic analysis approach based on grounded theory was used to analyze data collected during interviews, according to the principles described by Joffe and Yardley.29 This involved applying defined codes to pieces of text in the interview transcripts in order to categorize emergent themes and concepts. Codes were applied to both manifest and latent content, ie, things directly referred to by the participants and things which are implied by what the participant said. In line with the grounded theory tradition,29 analysts added new emerging concepts to the codebook as the analysis progressed. Analysts remained focused on the study objectives, added and organized codes in line with the interview guide’s structure. At the end of the analysis, cleaning and quality checks of the extracted data were performed, including merging codes that were conceptually similar or too detailed and further specifying codes that were too generic.
All data were coded by experienced qualitative researchers trained on the analysis approach specifically developed for the study. A subset of coded transcripts from all analysts were reviewed by senior members of the research team (SL and CEK) prior to commencing analysis of the full dataset to ensure correct understanding and application of the analysis approach. Interview data were synthesized in the form of concept frequencies. Only concepts that met a 10% threshold of the patient and caregiver subsamples or by a minimum of 5 retina specialists are reported in the results in order to focus on the most frequently reported concepts.
Results
Description of the Participants
A total of 94 patients, 79 caregivers, and 62 retina specialists were interviewed between June and October 2020. The sociodemographic and clinical characteristics of patients and caregivers are summarized in Table 1.
Table 1.
Sociodemographic and Clinical Characteristics of Patients and Caregivers
| Characteristics | Patients (n=94)ᵃ | Caregivers (n=79)ᵃ | ||
|---|---|---|---|---|
| nAMD (n=49) | DME (n=46) | nAMD (n=47) | DME (n=33) | |
| Gender, n (%) | ||||
| Female | 37 (76%) | 18 (39%) | 32 (68%) | 21 (64%) |
| Age, n (%) | ||||
| <60 years | 5 (10%) | 22 (48%) | 18 (38%) | 15 (45%) |
| 60–69 years | 10 (20%) | 18 (39%) | 17 (36%) | 15 (45%) |
| 70–79 years | 24 (49%) | 5 (11%) | 8 (17%) | 3 (9%) |
| ≥80 years | 10 (20%) | 1 (2%) | 4 (9%) | 0 (0%) |
| Working status, n (%) | ||||
| Retired | 40 (82%) | 15 (33%) | 21 (45%) | 11 (33%) |
| Working full/part time or self-employed | 3 (6%) | 15 (33%) | 18 (38%) | 16 (49%) |
| Looking after home or family | 1 (2%) | 2 (4%) | 2 (4%) | 0 (0%) |
| Disabled | 1 (2%) | 8 (17%) | 0 (0%) | 0 (0%) |
| Unemployed/ seeking work | 1 (2%) | 3 (7%) | 2 (4%) | 4 (12%) |
| Other | 2 (4%) | 1 (2%) | 3 (6%) | 2 (6%) |
| Not captured | 1 (2%) | 2 (4%) | 1 (2%) | 0 (0%) |
| Countries, n (%) | ||||
| Canada | 10 (20%) | 7 (15%) | 7 (15%) | 5 (15%) |
| France | 10 (20%) | 10 (22%) | 7 (15%) | 3 (9%) |
| Germany | 10 (20%) | 10 (22%) | 10 (21%) | 10 (30%) |
| Italy | 3 (6%) | 2 (4%) | 10 (21%) | 0 (0%) |
| Spain | 7 (14%) | 8 (17%) | 4 (9%) | 5 (15%) |
| USA | 9 (18%) | 9 (20%) | 9 (19%) | 10 (30%) |
| Number of anti-VEGF injections per year, n (%) | ||||
| 1 to 4 | 5 (10%) | 8 (17%) | 11 (23%) | 3 (9%) |
| 5 to 9 | 13 (27%) | 10 (22%) | 10 (21%) | 8 (24%) |
| 10 to 14 | 12 (24%) | 11 (24%) | 10 (21%) | 7 (21%) |
| 15 to 19 | 0 (0%) | 0 (0%) | 2 (4%) | 3 (9%) |
| 20 or moreb | 2 (4%) | 4 (9%) | 0 (0%) | 4 (12%) |
| Missing | 17 (35%) | 13 (28%) | 14 (30%) | 8 (24%) |
| Education level, n (%) | ||||
| Less than/Some high school | 8 (16%) | 8 (17%) | – | – |
| Completed high school or GED | 10 (20%) | 6 (13%) | – | – |
| Some college | 4 (8%) | 7 (15%) | – | – |
| Associate’s/Bachelor’s degree | 9 (18%) | 10 (22%) | – | – |
| Post-graduate degree | 6 (12%) | 4 (9%) | – | – |
| Otherc | 11 (22%) | 10 (22%) | – | – |
| Not captured | 1 (2%) | 2 (4%) | – | – |
| Comorbidities, n (%) | ||||
| Cataracts | 17 (35%) | 13 (28%) | – | – |
| High blood pressure | 9 (18%) | 6 (13%) | – | – |
| Diabetes | 7 (14%) | Not applicabled | – | – |
| Thyroid issues | 5 (10%) | 1 (2%) | – | – |
| Relationship to patient, n (%) | ||||
| Partner/spouse | - | - | 18 (38%) | 15 (45%) |
| Child | - | - | 17 (36%) | 3 (9%) |
| Parent | - | - | 2 (4%) | 8 (24%) |
| Friend | - | - | 4 (9%) | 2 (6%) |
| Othere | - | - | 6 (13%) | 5 (15%) |
| Living status, n (%) | ||||
| Lives with patient | - | - | 24 (51%) | 21 (64%) |
| Does not live with patient | - | - | 23 (49%) | 9 (27%) |
| Not captured | - | - | 0 (0%) | 3 (9%) |
Notes: Data as collected during the interview. ᵃThe total number of patients (n=94) and caregivers (n=79) is lower than that of the patient and caregiver nAMD and DME samples combined because one patient had both nAMD and DME. bOf these six patients, four (66%) had bilateral disease, while, for the remaining two patients, there was no mention in the transcript of unilateral or bilateral disease. cDue to local specificities/wording, the analyst could not categorize the level of education for all participants. dNot applicable as, per definition, DME is a complication of diabetes, and diabetes is not considered a comorbidity for DME patients. eIncludes: sister, brother, mother-in-law, nephew, uncle, work colleague, grandmother, neighbor, ex-husband.
Of the 94 patients interviewed, 49 were diagnosed with nAMD and 46 with DME (one patient was diagnosed with both nAMD and DME, and thus included in both groups). As shown in Table 1, the majority of the interviewed nAMD patients were female (n=37, 76%), retired (n=40, 82%), and aged 70 years or older (n=34, 69%). In contrast, 39% (n=18) of the interviewed DME patients were female and approximately one-third were retired (n=15, 33%). DME patients were younger than nAMD patients, with most patients being younger than 60 years (n=22, 48%) or between 60 and 69 years (n=18, 39%) old. Patients who reported the frequency of their injections most often reported receiving 5 to 14 anti-VEGF injections a year. Some patients had bilateral disease and received injections in both eyes, with both the injections counted separately.
Among the 79 caregivers, 47 were nAMD caregivers and 33 were DME caregivers. One caregiver cared for a patient diagnosed with both nAMD and DME. The majority of caregivers were female (nAMD, n=32, 68%; DME; n=21, 64%). Over one-third of nAMD caregivers (n=18, 38%) and approximately half of DME caregivers (n=16, 49%) reported working full-time/part-time or being self-employed. Caregivers were mostly partners/spouses (nAMD, n=18, 38%; DME, n=15, 45%), a child of a nAMD patient (n=17, 36%), or a parent of a DME patient (n=8, 24%). One-half of nAMD caregivers (n=24, 51%) and two-thirds of DME caregivers (n=21, 64%) lived with the patient.
As shown in Table 2, an experienced sample of retina specialists was interviewed, with expertise spanning both indications of interest. Roughly one-third of interviewed retina specialists each reported practicing retinal ophthalmology for 10 years or less (n=21, 34%), 11 to 20 years (n=22, 36%), or 21 years or more (n=18, 29%). Most of the retina specialists worked in a public practice or a practice attached to a hospital (n=38; 61%), while the remaining retina specialists worked in a private practice (n=29; 47%). Fifty-five percent of retina specialists (n=34) were seeing more than 100 nAMD patients monthly and 34% (n=21) administered more than 200 injections a month for nAMD patients. About half of the retina specialists (n=30, 48%) were seeing 50 DME patients or fewer) and 35% (n=22) administered less than 50 injections a month for DME patients.
Table 2.
Characteristics of Retina Specialists
| Characteristics | Retina Specialists (n=62) | ||
|---|---|---|---|
| Years practicing specialty (retinal ophthalmology), n (%) | |||
| Less than 5 years | 9 (15%) | ||
| 5 to 10 years | 12 (19%) | ||
| 11 to 15 years | 15 (24%) | ||
| 16 to 20 years | 7 (11%) | ||
| 21 to 25 years | 7 (11%) | ||
| 26 to 30 years | 7 (11%) | ||
| More than 30 years | 4 (6%) | ||
| Missing | 1 (2%) | ||
| Type of practice, n (%)a | |||
| Attached to a hospital or working in a public practice | 38 (61%) | ||
| Private practice | 29 (47%) | ||
| Countries, n (%) | |||
| Canada | 10 (16%) | ||
| France | 10 (16%) | ||
| Germany | 10 (16%) | ||
| Italy | 10 (16%) | ||
| Spain | 10 (16%) | ||
| USA | 12 (19%) | ||
| Availability of electronic medical records, n (%) | |||
| Yes | 51 (82%) | ||
| No | 1 (2%) | ||
| Missing | 10 (16%) | ||
| Number of patients seen monthly, n (%) | nAMD | DME | |
| 50 or less | 14 (23%) | 30 (48%) | |
| 51–99 | 6 (10%) | 10 (16%) | |
| 100–199 | 16 (26%) | 6 (10%) | |
| 200 or more | 18 (29%) | 7 (11%) | |
| Anti-VEGF IVT injection per month, n (%)b | |||
| 50 or less | 13 (21%) | 22 (35%) | |
| 51 to 99 | 12 (19%) | 12 (19%) | |
| 100 to 199 | 16 (26%) | 10 (16%) | |
| 200 or more | 21 (34%) | 5 (8%) | |
Notes: aRetina specialists may have mixed practice. bNormalized per month frequency, assuming 1 month = 4 weeks = 20 days (weekends not included).
Disease Experience
Patients reported currently experiencing a range of vision symptoms due to their disease, in particular loss of vision/weaker vision (nAMD, n=30, 61%; DME, n=24, 52%), blurred vision (nAMD, n=17, 35%; DME, n=26, 57%), and distorted vision (nAMD, n=16, 33%; DME, n=11, 24%).
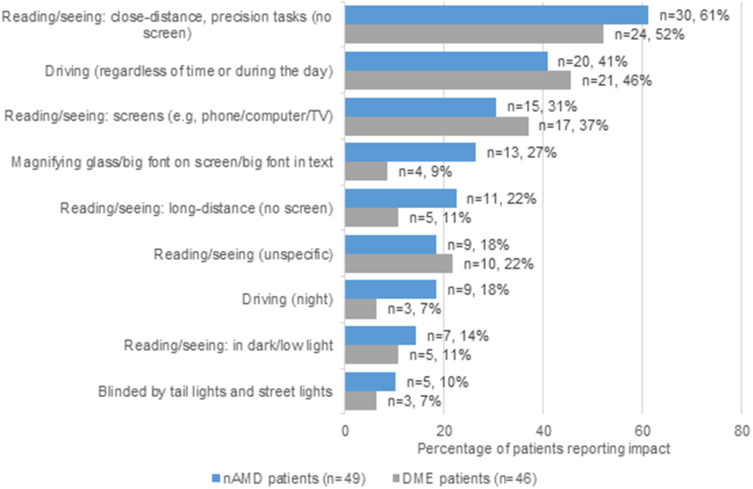
As shown in Figure 1, patients commonly reported having difficulties with various daily activities, such as close-distance reading/seeing (nAMD, n=30, 61%; DME, n=24, 52%) or driving at any time of the day (nAMD, n=20, 41%; DME, n=21, 46%).
Figure 1.
Impact of nAMD and DME on patients’ daily functioning (shown if at least 10% in one of the subsamples).
Caregivers reported a similar impact of the condition on patients’ quality of life and further added that patients needed help with errands/shopping (both nAMD and DME caregivers, n=20, 25%).
In terms of the impact of caregiving on their own wellbeing, about half of the caregivers (nAMD, n=26, 55%; DME, n=16, 49%) reported dealing with feelings of concern and worry for the patient. Some caregivers (nAMD, n=9, 19%; DME, n=4, 12%) discussed the emotional work of being a caregiver, managing their own feelings and, at the same time, helping alleviate patients’ anxiety or depression about their illness and treatment.
Illustrative quotes around disease experience are provided in Supplementary Table 1.
Anti-VEGF Intravitreal Injections Experience
Most nAMD/DME patients (n=76, 81%) and half of caregivers (n=35, 44%) reported that the anti-VEGF IVT injections negatively affected their daily routine on the day of treatment, the day before or the day after treatment. In some instances, patients described impacts on caregivers (and vice versa) during an interview but not necessarily impacts on themselves. When all reports are considered regardless of type of participant, the total number of patients/caregivers in all conditions who reported short-term impacts on either a patient’s or caregiver’s daily routine due to treatment was 137 (79%).
During the day of the anti-VEGF IVT injection, both patients and caregivers reported that they were unable to engage in caring activities (eg, caring for children, grandchildren, or pets) and had to adjust their day to make time for the appointment and ensure sufficient recovery time afterwards, including cancelling all social activities and avoiding going out to run errands. Patients explained that their daily routine was disrupted due to planning around travel to/from the appointment and the variable length of appointment duration (waiting time included). As shown in Table 1, three nAMD patients, 15 DME patients, 18 caregivers of nAMD patients and 16 caregivers of DME patients were still working. Overall, 26% (n=12) of nAMD caregivers and 39% (n=13) of DME caregivers reported a negative professional impact of taking time off work to attend treatment appointments, such as affecting productivity and career choices. Nine out of 46 DME patients (20%) and 3 out of 49 nAMD patients (6%) reported that their treatment had a negative impact on their work attendance. These small percentages are due to the low number of patients employed in a full-time or part-time job in our sample (33% of DME patients and 6% of nAMD, as indicated in Table 1).
Patients struggled in the days before the injection with anxiety about the procedure and in the one to two days after the injection with side effects. Patients and caregivers reported some form of pain/discomfort/irritation (n=77, 82% of patients; n=47, 59% of caregivers), followed by some accounts of bloodshot/red eye/bleeding (n=23, 24% of patients; n=17, 22% of caregivers) among patients due to anti-VEGF IVT injections. Patients described how after their injection they often felt fatigued or needed to rest, how they needed to be in a quiet and dark room to avoid exposure to sunlight, and how the injection made doing certain daily tasks difficult due to impaired vision (eg, cooking, driving, and reading).
Adherence to Anti-VEGF Intravitreal Injections
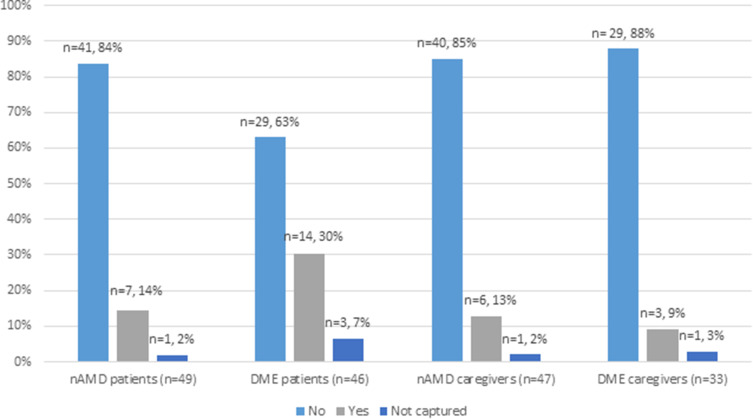
As shown in Figure 2, 14% (n=7) of nAMD patients and 30% (n=14) of DME patients reported having missed at least one injection in the past. Most participants mentioned they would never miss an injection. Where spontaneously reported, reasons for missing an appointment were diverse. Reasons included having scheduling conflicts, forgetting appointments, getting the time of the appointment wrong, having illness or bereavement in the family, or going on vacation.
Figure 2.
Patients and caregivers’ response to the question “Have you/the patient you care for ever missed an anti-VEGF injection?”.
Findings from the retina specialist interviews showed that there was no standard definition used to assess adherence in routine clinical practice. Retina specialists were more likely to use their intuition about a patient in order to determine adherence rather than use a precise definition.
Moreover, 42% (n=26) of retina specialists reported they did not categorize the adherence of patients at all. Retina specialists who did categorize the adherence of patients did so based on either the rate of missing injections/appointments (n=22, 36%) or other factors such as informal assessments or conversations with patients (n=9, 15%). Most of the retina specialists (n=46, 74%) estimated non-adherence rates to be between 0 and 20%.
Further illustrative quotes around adherence are provided in the Supplementary Table 1.
Drivers for Following Management Plan (Adherence)
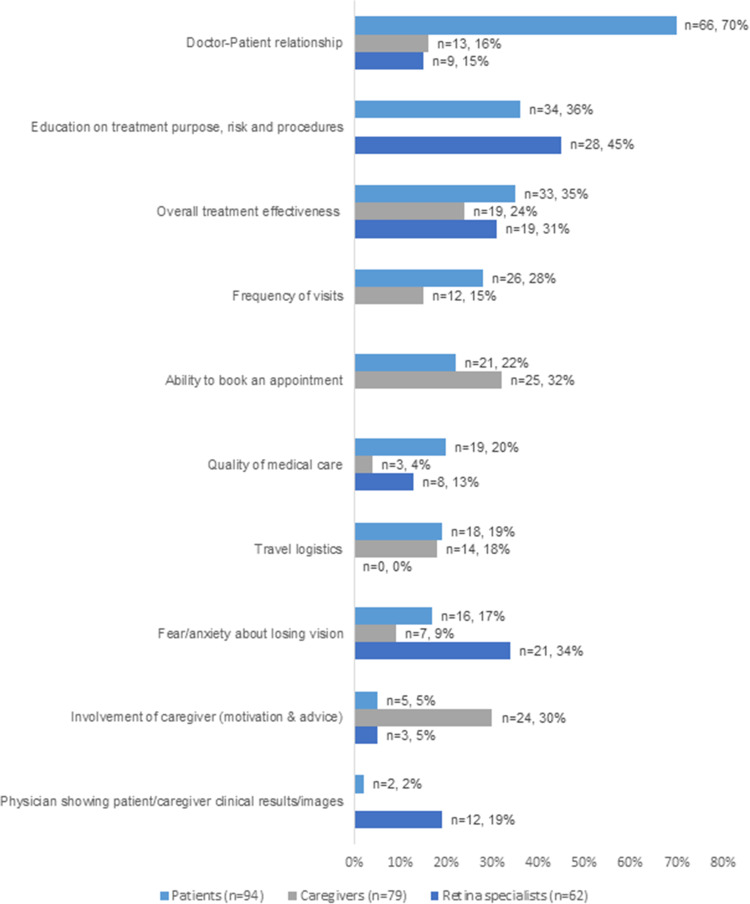
Figure 3 shows the frequency of reported drivers for adherence by participant type.
Figure 3.
Drivers to follow treatment management plan as reported by patients, caregivers, and retina specialists (shown if at least 10% in one of the subsamples).
The doctor-patient relationship was reported by 70% of patients (n=66) and was by far the most frequently patient-reported driver that would motivate them to follow the treatment management plan with anti-VEGF IVT injections. This domain included various aspects, such as getting along with the health care professionals, seeing the same health care professionals at each visit, being respectful to health care professional’s time and the health care professional providing personal and attentive care to the patient. In contrast, only 16% of caregivers (n=13) and 15% of retina specialists (n=9) reported the doctor-patient relationship as a driver for adherence.
Caregivers highlighted the ease of booking an appointment as the most important driver (n=25; 32%), followed closely by the caregiver’s support in helping the patient adhere to the treatment plan by motivating and advising the patient (n=24; 30%). However, retina specialists did not think the ease of booking an appointment (n=0; 0%) or the caregiver’s support to motivate and advise the patient (n=3; 5%) were the most important drivers to follow the treatment plan.
From the perspective of retina specialists, drivers of adherence were mostly related to patient education, in particular information provided on treatment purpose, risk and procedures (n=28; 45%). Additionally, retina specialists reported that a patients’ fear/anxiety about losing vision (n=21; 34%) and their perception of overall treatment effectiveness (n=19; 31%) could have a positive influence on adherence. The latter driver was corroborated by patients (n=33, 35%) and caregivers (n=19; 24%) as well.
The perspectives on drivers for adherence across conditions were similar as shown in Supplementary Table 2. Overall, doctor-patient relationships were reported as a driver by a majority of patients in both groups, followed by overall treatment effectiveness and frequency of visits. A higher percentage of nAMD patients reported logistics-related factors (ability to book appointment, travel logistics, frequency of visits, appointment frequency change) and feelings about injection as drivers. Conversely, education on treatment purpose, risk and procedures, quality of medical care, and feelings about losing vision were reported as drivers by a higher percentage of DME patients compared with nAMD patients. Drivers reported by both groups of caregivers were similar. Frequency of visits and feelings about losing vision were reported by more nAMD than DME caregivers and forgetting appointments/trouble remembering was reported by more DME than nAMD caregivers.
Illustrative quotes around drivers for following management plan are provided in the Supplementary Table 1.
Barriers to Following Management Plan (Adherence)
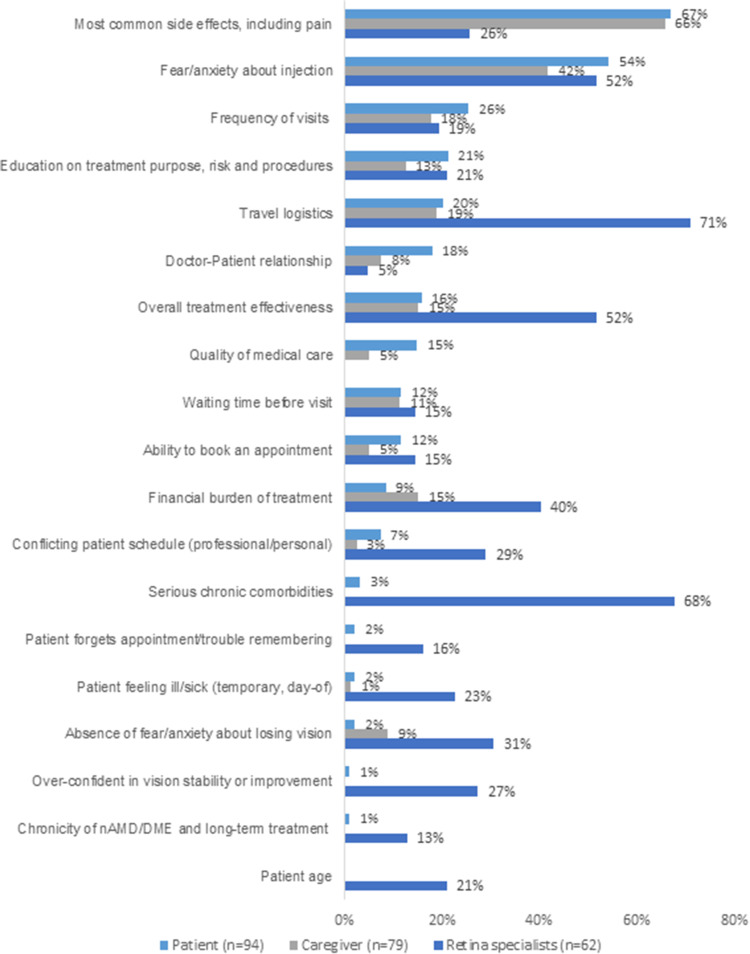
Barriers to adherence are shown in Figure 4 by participant type. They could be grouped into four main categories: 1) tolerability, 2) clinical factors, 3) logistical parameters, 4) and human factors.
Figure 4.
Treatment barriers with a potential negative impact on adherence to treatment management plan as reported by patients, caregivers, and retina specialists (shown if at least 10% in one of the subsamples).
According to patients and caregivers, the most impactful barriers were related to tolerability, in particular to treatment-related side effects, including pain/discomfort/irritation (67% of patients, n=63; and 66% of caregivers, n=52). Human factors were also considered a strong barrier for some patients and caregivers. These included fear/anxiety about injection (54% of patients, n=51; and 42% of caregivers, n=33), education on treatment (21% of patients, n=20; and 13% of caregivers, n=10), and doctor-patient relationships (18% of patients, n=17; and 8% of caregivers, n=6).
Other barriers were related to logistical parameters, such as the frequency of visits (26% of patients, n=24; and 18% of caregivers, n=14), travel logistics (20% of patients, n=19; and 19% of caregivers, n=15), waiting time before visit (12% of patients, n=11; and 11% of caregivers, n=9), ability to book an appointment (12% of patients, n=11; and 5% of caregivers, n=4), and financial burden (9% of patients, n=8; and 15% of caregivers, n=12), and clinical factors, such as patient perception of treatment effectiveness (16% of patients, n=15; and 15% of caregivers, n=12).
Similar barriers were identified during retina specialists’ interviews, although the reported aspects were given different weights. Most retina specialists reported barriers associated with logistical parameters, in particular travel logistics (n=44, 71%), financial burden (n=25, 40%) and frequency of visits (n=12, 19%). Most of them also highlighted the impact of clinical factors, such as serious chronic comorbidities (n=42, 68%), treatment effectiveness (n=32, 52%), patients feeling sick/ill temporarily on the day of the treatment (n=14, 23%), and patient age (n=13, 21%). Human factor-related barriers reported by patients and caregivers were also reported by retina specialists, including fear/anxiety related to injection (n=62, 52%), absence of fear about losing vision (n=19, 31%) and education on treatment (n=13, 21%).
The perspectives on barriers to adherence were found to be similar across conditions as shown in Supplementary Table 3. Overall, experiencing the most common side effects, including pain, and feeling fear/anxiety about injection were reported as barriers by the majority of participants in all respondent types. Financial burden of treatment and temporary decrease in visual acuity were reported as barriers by a higher percentage of DME caregivers compared with other groups of participants. The doctor-patient relationship was a more important barrier for nAMD patients compared with other groups.
When barriers to adherence were analyzed at the country level, patients’ reported treatment barriers were found to be generally consistent across countries, as shown in Supplementary Table 4. Treatment-related side effects, including pain (n=63, 67%), fear/anxiety about injection (n=51, 54%), and frequency of visits (n=24, 26%) were the most frequently identified barriers across countries. Some country differences were observed: the third most reported treatment barrier in Spain was cost associated with travelling to the appointment, including parking costs (n=6, 40%) whereas in Germany and Canada it was lack of patient education (n=6, 30%; and n=5, 31%, respectively).
Caregivers also similarly identified the same top two treatment barriers: treatment side effects (n=52, 66%) and fear/anxiety over injection (n=33, 42%), which were the top two treatment barriers observed across countries. A comparable proportion of caregivers found travel logistics (n=15, 19%) and frequency of visits (n=14, 18%) to be barriers to treatment. Canadian caregivers more often reported travel logistics (n=4, 36%) and frequency of visits as a barrier to treatment (n=4, 36%). Similarly, 44% of Spanish caregivers (n=4) also reported frequency of visits as a barrier. Moreover, in Germany overall treatment effectiveness was mentioned less often (n=1, 5%). In the USA, education and waiting time before visit were never reported by any caregivers. Supplementary Table 5 gives a detailed overview of the negative impacts described by caregivers across countries.
In terms of the country-related differences in the retina specialists’ data, similar treatment barriers were described overall, however some interesting differences could be noticed at a country level (shown in Supplementary Table 6). In particular, financial burden was considered an important barrier among US retina specialists (n=10, 83%), but less so in other countries (<40% among all other countries), and in contrast to patient and caregiver findings in the USA where <10% of patients and 21% of caregivers thought financial burden was an important barrier. Moreover, travel logistics barriers were found to be the most relevant overall barrier to treatment by retina specialists, especially according to those in Canada (n=7, 70%) and Spain (n=5, 50%). It should also be noted that, while both patients and caregivers strongly highlighted the importance of side effects, this was mentioned less often by retina specialists (n=7, 11%). Fear/anxiety about injection and overall effectiveness of treatment was not reported as barriers by any of the Canadian retina specialists.
Illustrative quotes around barriers to follow the management plan are provided in the Supplementary Table 1.
Ideal Treatment
Patients and caregivers reported similar characteristics as being desirable for an ideal treatment. They most frequently reported characteristics would include a self-administered oral treatment (46% of patients, n=43; and 62% of caregivers, n=49) and one that could be administered at home (46% of patients, n=43; and 61% of caregivers, n=48). Treatment effectiveness, especially the treatment response rate and its long-term effectiveness, were also identified as important characteristics for patients’ (n=34, 36%) and caregivers’ (n=33, 42%) ideal treatment.
The side effect profile of an ideal treatment was also a common consideration for patients (n=33, 35%) and caregivers (n=34, 43%), with participants stating that they would ideally like to not have to tolerate uncomfortable eye sensations and/or vision changes.
Discussion
This multinational qualitative study provides valuable insights on the treatment experience of patients with nAMD and DME and explored the impact of treatments on daily routine, quality of life and work attendance, current definitions and estimates of non-adherence, and drivers of and barriers to following treatment management plans. In-depth interviews were conducted with patients, caregivers, and retina specialists to gain a comprehensive understanding across relevant stakeholders in the patient’s treatment journey in the United States, Canada, France, Italy, Germany, and Spain.
Most patients reported that they understood the importance of their treatment for their condition. In the nAMD group, 7 (14%) patients reported missing an anti-VEGF injection compared to 14 (30%) patients in the DME group. Based on the current literature, conflicting results have been shown. One paper reported nAMD patients to be quite adherent to their treatment,14 although in another study focusing on pro re nata, the proportion of nAMD patients missing injection visits was quite high, with almost 40% of patients not able to strictly follow their treatment management plan.30 A previous study focusing on DME and nAMD also showed that more DME patients (46%) had at least one therapy break off (lateness >100 days) than nAMD patients (22%).19 Findings from interviews with retina specialists showed there is no standard definition of adherence, missed visits/injections are not always systematically tracked and even when they are, there is no agreed-upon threshold of missed visits/injections above which a patient is considered “non-adherent”. Still, retina specialists estimated 0 to 20% of their patients were not fully adherent to their treatment management plan. Note however, that in the absence of a clear definition for non-adherence, as reported by retina specialists when asked how they would categorize patients’ adherence in practice, it may be that missing one injection may not be considered by retina specialists as being non-adherent. Thus, the results seen in patients and caregivers’ interviews, where they were asked about missing an anti-VEGF injection as a measure of their adherence, may give an amplified non-adherence rate. Since there is an important difference in the ability to estimate adherence between real-world practice and clinical trials, where patients have defined visit schedules and delays or non-attendance are tracked, further work to accurately assess adherence could help to understand and address the reasons for non-adherence. For instance, in recent work, a definition of non-adherence and non-persistence was developed by a group of retinal experts, who classified reasons for treatment non-adherence/non-persistence in nAMD patients requiring IVT anti-VEGF therapy.31 This could provide a framework for future assessments in this group of patients.
The reported drivers for patients’ ability and willingness to follow their treatment management plan were associated with multiple aspects (education, treatment effectiveness, human factors) and were identified across patients, caregivers, and retina specialists. Commonly reported drivers of adherence for all participant groups included: overall effectiveness of current treatment, doctor–patient relationship and education (education was not mentioned by any caregivers). It is important to note that each population gave different weights to the various reported aspects depending on their personal perspective, therefore, compartmentalized approaches to developing treatment management strategies may not be effective. For example, from the point of view of patients, the doctor–patient relationship was the most frequently (70%) reported driver, but only 15% of retina specialists considered this relevant, so there is a clear mismatch regarding what is important. It may therefore be beneficial for retina specialists to improve the doctor–patient relationship, particularly with patients who are non-adherent. Moreover, caregivers most frequently (32%) mentioned the ability to book an appointment, which was also mentioned by patients (22%), but was not considered important by any retina specialist, so this is another area that retina specialists could consider improving. Caregivers also frequently (30%) mentioned their involvement with the patient’s treatment (providing motivation and advice), but this was mentioned less frequently (5%) by patients, which could reflect that not every patient needs a caregiver. Retina specialists thought that patient education and information provided by the doctor were the most important drivers for patients to follow their treatment management plan. Interestingly, although patients agreed that education on treatment purpose, risk, and procedures are drivers for adherence, only 2 (2%) patients mentioned that seeing clinical results/images are helpful compared with 12 (19%) clinicians. Clinical results/images may therefore need to be tied back to education around treatment purpose to further reinforce and motivate patients to be adherent. The importance of education corresponds with research from several studies that identified patient education as a central item in patient management, and acknowledged that the lack of education on the disease and its treatment could be a barrier for optimal patient care.22,23,25 Additionally, the Diabetic Retinopathy Barometer Study recognized fostering multidisciplinary communication between the primary care providers, ophthalmologists, and patients as an important need for individuals with diabetes undergoing eye treatment.32 Additional drivers mentioned by retina specialists were in line with more practical aspects that were specifically mentioned by patients and caregivers, such as the frequency of visits and ability to book an appointment.
Diverse barriers to adherence were identified, which could be grouped into four main categories: (1) tolerability (pain/discomfort/irritation linked to the injection, bloodshot/red eye/bleeding, floaters/black spots, sensitivity to light), (2) clinical factors (perception of treatment effectiveness, chronic comorbidities, patients feeling sick/ill temporarily on the day of the treatment, and patient age), (3) logistical parameters (financial burden, ability to book appointment, travel logistics, frequency of visits), and (4) human factors (relationship with physician, patient education, fear/anxiety of injection). All participants reported that fear/anxiety about injections and frequency of visits as barriers. Patients and caregivers more frequently identified side effects (including pain) as the main barrier to following treatment as planned, whereas retina specialists reported other barriers (including travel logistics, comorbidities, illness, overall treatment effectiveness, age, and financial burden) as most influential. These results on barriers to adherence support previously published interview-based qualitative and patient-reported outcomes measures-based quantitative findings that identified logistical constraints, increased financial costs and a decrease in psychosocial well-being as the main treatment-related burdens for nAMD patients and their caregivers.33–36 As every injection could result in an episode of fear/anxiety or pain/discomfort, reducing the number of injections needed to maintain vision could reduce the negative impact of these barriers. Moreover, reducing the number of visits could reduce the barriers for travel logistics as well. The impact of these barriers supports the value of more durable treatments, which could reduce the frequency of visits and discomfort to the patient. It is important to note that only a few patients reported that other comorbidities negatively impacted their adherence, which strongly contrasted with the view that was held by the majority of retina specialists who considered comorbidities to negatively impact adherence (as also reported in previous literature).19 This also applies to other specific drivers and barriers, which were given different weights across participants of this study, and could be due to each group of participants having a specific focus. For instance, doctors typically have a more clinical perspective, while patients may prioritize other aspects of the treatment experience that are different from those of caregivers. It could also be due to a sampling bias, whereby patients and caregivers that choose to take part in the study were feeling well enough to participate and by default did not experience comorbidities that had a significant detrimental impact on their health. In contrast, retina specialists would see a wider spectrum of patients including those with severe comorbidities.
Overall, nAMD and DME patients reported that their symptoms, in particular loss of vision/weaker vision, blurred and/or distorted vision, negatively affected their physical functioning. Indeed, both near and distant vision were impacted, as well as day and night vision. These led to impacts on patients’ quality of life, including difficulties with reading or seeing at a close-distance or driving at any time of the day. Similarly, when discussing their anti-VEGF IVT injection experience, most patients and caregivers also reported negative impacts to their daily routine, both due to treatment side effects and adjustments to, and difficulties with, daily or planned activities.
Our results are in line with results from other studies that investigated the impact of DME or nAMD and its management through anti-VEGF IVT injections on patients’ quality of life. For instance, a mixed-methods systematic review published in 2021 identified risk factors for non-adherence to treatment or monitoring appointments in nAMD.37 Overall, the six studies reviewed identified the following factors as having significant negative impacts on adherence: patient illness, fear of discomfort after injections, patients forgetting their appointment, and appointment frequency and inconvenience. The factors identified in the review matched our findings in nAMD and corroborated our grouping of barriers within the four aforementioned categories: tolerability, clinical factors, logistical parameters, and human factors. Moreover, a qualitative study by McCloud and Lake,24 conducted with 13 female and 12 male nAMD patients aged between 67 and 90 years of age, found that most participants associated regular anti-VEGF injections with fear, including apprehension at the thought of having a “needle” inserted into their eye. Several studies of patients with nAMD and DME have also recognized patient education as a central component of patient management, and acknowledged that the lack of education on the disease and its treatment could be a barrier to optimal patient care.22,23,25
Patients and caregivers had similar ideas of what constitutes an ideal treatment, with participants most frequently reporting that their ideal treatment would include a self-administered oral treatment that could be given at home and requires less frequent appointments. These characteristics tie in with our findings that show that majority of patients and caregivers reported disruption in their schedule on the days around treatment and an impact on caregivers’ attendance at work. Moreover, treatment effectiveness, especially the treatment response rate and its long-term effectiveness, were identified as particularly important characteristics of an ideal treatment from the viewpoint of patients and caregivers. Additionally, the side-effect profile of an ideal treatment was also a common consideration for patients and caregivers. These ideal treatment characteristics suggest that patients and caregivers would like more convenient and less frequent treatments, further supporting the value of durable products. Moreover, the desire for an oral treatment is consistent with our findings that side effects of injections and anxiety about injection are major barriers. While there may not be an oral medication currently available, reducing treatment frequency could reduce the burden and barriers related to fear/anxiety and side effects.
The current research benefited from a large, diverse sample of patients, caregivers and retina specialists from 6 countries, which, when compared to previous smaller scale studies in single countries (USA, China, Australia, United Kingdom, Sweden),20–25,37,38 supports the generalizability of the findings. Furthermore, this research was unique in that it captured the perspectives across major stakeholders involved in the patient’s treatment journey, including the patient, caregiver, and retina specialist. Health-related quality-of-life effects for caregivers and family members of ill patients (ie, “spill-over effects”), is an emerging topic of research that has not been deeply investigated and could help reveal a valuable, alternate perspective on the patient’s experience. To our knowledge, there are few qualitative studies that have investigated the experience of caregivers in nAMD/DME, highlighting how caregiver tasks, such as accompanying patients to the appointments for eye treatment, and the patient’s visual acuity in the better eye (a measure of the impact of the disease on the patient), were the biggest contributors to caregiver burden.15,34 Another study recruited caregivers of patients with wet AMD and measured their perceived burden, with 73% of them reporting little to no burden; however the authors did not further investigate the causes of the burden in the remaining caregivers.39
Nonetheless, this qualitative study has some limitations. First, the patient sample may underrepresent non-adherent patients who may have been less likely to volunteer to participate in the study. To minimize this selection bias, the recruitment of participants did not solely rely on physician referrals but used other approaches including word of mouth, internet advertising, email blasts, social media, and patient associations. Second, although interviews were conducted during the COVID-19 pandemic, the impact of the pandemic on treatment adherence was not captured in either the interviews or the analysis, as the focus of the study was on real-world adherence challenges, instead of adherence in the context of this exceptional event. Finally, despite the large number of participants recruited overall for a qualitative study, it is important to note that sample size in each individual country was smaller compared to those of previous studies.
Conclusions
This multinational qualitative study provides valuable insights into how to improve the nAMD and DME treatment experience for both patients and caregivers as well as how to improve patient adherence to treatment. This study elucidated which drivers and barriers to following treatment management plans are important for nAMD or DME patients, caregivers, and retina specialists. Major barriers for patients and caregivers were related to side effects, fear/anxiety about injection, and logistics. Furthermore, the doctor–patient relationship and education were the most important drivers of adherence reported by patients and retina specialists, respectively. Additionally, treatment logistics was identified as barrier to adherence by all groups, and convenience of treatment was desired in an ideal treatment. New solutions offering longer-acting treatments with fewer side effects and less frequent visits together with enhanced patient education could provide tangible improvements in the quality of life of nAMD and DME patients and their caregivers. These improvements could facilitate the ability and willingness of patients to follow their treatment management plan, leading to better vision outcomes, which may translate to greater independence and improved overall well-being.
Acknowledgments
We thank the patients, caregivers, and retina specialists, who participated in this study, as well as interviewers in all countries.
Funding Statement
F. Hoffmann-La Roche Ltd., Basel, Switzerland, provided financial support for the study, which was conducted by ICON plc, and participated in the review and approval of the manuscript. Funding was provided by F. Hoffmann-La Roche Ltd. for third-party writing assistance, which was provided by Andrea de Palma BSc, MSc, of ICON plc, and Sandra Boswell PhD, and Anne Nunn, PhD, CMPP, of Envision Pharma Group.
Abbreviations
AMD, age-related macular degeneration; nAMD, neovascular AMD; anti-VEGF, anti-vascular endothelial growth factor; DME, diabetic macular edema; IVT, intravitreal.
Data Sharing Statement
The data sets obtained and/or analysed during this study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
Salus IRB (Austin, TX) reviewed and approved the research for its conduct in the USA and Canada. Consultations with relevant European ethics committees confirmed the qualitative research did not meet the requirements for formal submission and approval in France, Germany, Italy, and Spain. Verbal consent was obtained from participants prior to their interview. All participants were informed about the purpose of the study, in accordance with the Declaration of Helsinki. Participants were informed that in any written reports or publications, they would not be identified or identifiable and only de-identified data would be presented.
Author Contributions
GCC, MM, BG, HL and JL contributed to the study design; CEK, JL and SL contributed to the qualitative analysis; All authors contributed to data interpretation, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
SL, CEK, JL, and HBL, were full time employees of ICON plc at the time this work was conducted. AGA is consultant for and reports personal fees from AbbVie, Alcon, Allergan, Bayer, Horus, Novartis, and Roche. AGL is consultant for and report personal fees from Allergan, Bayer, Novartis, Roche, and Thea. TP reports consultant fees from Roche/Genentech, Inc., Bayer, Novartis, Alimera, Heidelberg, Oxurion, Apellis. BG and GCC are employees of Genentech, Inc. MM is an employee of F. Hoffmann-La Roche Ltd. NMH reports consulting fees from Adverum, Allergan, Annexon, Apellis, Bayer, Lineage Cell Therapeutics, Clearside Biosciences, Gemini, Genentech, Gyroscope, Katalyst Surgical, Nacuity, Notal Vision, Novartis, Polyactiva, Regeneron, Stealth Biosciences; has been a member of Speakers Bureau for Allergan, Genentech, Novartis, Regeneron, and Spark; conducted contracted research for Genentech, Gemini, Gyroscope, Notal Vision; and holds intellectual property/patents: Katalyst Surgical. The authors report no other conflicts of interest in this work.
References
- 1.Holekamp NM. Review of neovascular age-related macular degeneration treatment options. Am J Manag Care. 2019;25(10 Suppl):S172–s181. [PubMed] [Google Scholar]
- 2.Wolf A, Langmann T. Anti-VEGF-A/ANG2 combotherapy limits pathological angiogenesis in the eye: a replication study. EMBO Mol Med. 2019;11(5). doi: 10.15252/emmm.201910362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gale RP, Mahmood S, Devonport H, et al. Action on neovascular age-related macular degeneration (nAMD): recommendations for management and service provision in the UK hospital eye service. Eye. 2019;33(Suppl 1):1–21. doi: 10.1038/s41433-018-0300-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Heal. 2014;2(2):e106–116. doi: 10.1016/S2214-109X(13)70145-1 [DOI] [PubMed] [Google Scholar]
- 5.Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care. 2003;26(9):2653–2664. doi: 10.2337/diacare.26.9.2653 [DOI] [PubMed] [Google Scholar]
- 6.MacKinnon JR, Forrester JV. Diabetic retinopathy. In: Was JAH, Shalet SM, editors. Oxford Textbook of Endocrinology and Diabetes. Oxford, U.K.: Oxford University Press; 2002:1764–1778. [Google Scholar]
- 7.Subhi Y, Sørensen TL. Neovascular age-related macular degeneration in the very old (≥90 years): epidemiology, adherence to treatment, and comparison of efficacy. J Ophthalmol. 2017;2017:7194927. doi: 10.1155/2017/7194927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miskala PH, Bass EB, Bressler NM, et al. Surgery for subfoveal choroidal neovascularization in age-related macular degeneration: quality-of-life findings: SST report no. 12. Ophthalmology. 2004;111(11):1981–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown MM, Brown GC, Sharma S, Shah G. Utility values and diabetic retinopathy. Am J Ophthalmol. 1999;128(3):324–330. doi: 10.1016/S0002-9394(99)00146-4 [DOI] [PubMed] [Google Scholar]
- 10.Dervenis N, Mikropoulou AM, Tranos P, Dervenis P. Ranibizumab in the treatment of diabetic macular edema: a review of the current status, unmet needs, and emerging challenges. Adv Ther. 2017;34(6):1270–1282. doi: 10.1007/s12325-017-0548-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Droege KM, Caramoy A, Kersten A, et al. Patient preference of ranibizumab treatment regimen for neovascular age-related macular degeneration - monthly injections versus pro re nata. Graefes Arch Clin Exp Ophthalmol. 2014;252(1):31–34. doi: 10.1007/s00417-013-2412-6 [DOI] [PubMed] [Google Scholar]
- 12.Jelin E, Wisløff T, Moe MC, Heiberg T. Development and testing of a patient-derived questionnaire for treatment of neovascular age-related macular degeneration: dimensions of importance in treatment of neovascular age-related macular degeneration. Acta Ophthalmol. 2018;96(8):804–811. doi: 10.1111/aos.13847 [DOI] [PubMed] [Google Scholar]
- 13.Mueller S, Agostini H, Ehlken C, Bauer-Steinhusen U, Hasanbasic Z, Wilke T. Patient preferences in the treatment of neovascular age-related macular degeneration: a discrete choice experiment. Ophthalmology. 2016;123(4):876–883. doi: 10.1016/j.ophtha.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 14.Droege KM, Muether PS, Hermann MM, et al. Adherence to ranibizumab treatment for neovascular age-related macular degeneration in real life. Graefes Arch Clin Exp Ophthalmol. 2013;251(5):1281–1284. doi: 10.1007/s00417-012-2177-3 [DOI] [PubMed] [Google Scholar]
- 15.Gohil R, Crosby-Nwaobi R, Forbes A, Burton B, Hykin P, Sivaprasad S. Caregiver burden in patients receiving ranibizumab therapy for neovascular age related macular degeneration. PLoS One. 2015;10(6):e0129361. doi: 10.1371/journal.pone.0129361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gohil R, Crosby-Nwaobi R, Forbes A, Burton BJ, Hykin P, Sivaprasad S. Treatment satisfaction of patients undergoing ranibizumab therapy for neovascular age-related macular degeneration in a real-life setting. Patient Prefer Adherence. 2016;10:949–955. doi: 10.2147/PPA.S105536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller S, Ehlken C, Bauer-Steinhusen U, et al. Treatment of age-related neovascular macular degeneration: the patient’s perspective. Graefes Arch Clin Exp Ophthalmol. 2017;255(11):2237–2246. doi: 10.1007/s00417-017-3739-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prenner JL, Halperin LS, Rycroft C, Hogue S, Williams Liu Z, Seibert R. Disease burden in the treatment of age-related macular degeneration: findings from a time-and-motion study. Am J Ophthalmol. 2015;160(4):725–731.e721. doi: 10.1016/j.ajo.2015.06.023 [DOI] [PubMed] [Google Scholar]
- 19.Weiss M, Sim DA, Herold T, et al. Compliance and adherence of patients with diabetic macular edema to intravitreal anti-vascular endothelial growth factor therapy in daily practice. Retina. 2018;38(12):2293–2300. doi: 10.1097/IAE.0000000000001892 [DOI] [PubMed] [Google Scholar]
- 20.Bian W, Wan J, Smith G, Li S, Tan M, Zhou F. Domains of health-related quality of life in age-related macular degeneration: a qualitative study in the Chinese cultural context. BMJ Open. 2018;8(4):e018756. doi: 10.1136/bmjopen-2017-018756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters CM, James AI, Tran I, et al. The impact of diabetic macular edema on the daily lives of diabetic adults—a qualitative study. Invest Ophthalmol Vis Sci. 2012;53(14):5449. [Google Scholar]
- 22.Burton AE, Shaw R, Gibson J. Experiences of patients with age-related macular degeneration receiving anti-vascular endothelial growth factor therapy: a qualitative study. Br J Vis Impair. 2013;31(3):178–188. doi: 10.1177/0264619613490517 [DOI] [Google Scholar]
- 23.Hartnett ME, Key IJ, Loyacano NM, Horswell RL, DeSalvo KB. Perceived barriers to diabetic eye care: qualitative study of patients and physicians. Arch Ophthalmol. 2005;123(3):387–391. doi: 10.1001/archopht.123.3.387 [DOI] [PubMed] [Google Scholar]
- 24.McCloud C, Lake S. Understanding the patient’s lived experience of neovascular age-related macular degeneration: a qualitative study. Eye. 2015;29(12):1561–1569. doi: 10.1038/eye.2015.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Granström T, Forsman H, Brorsson A-L, Granstam E, Leksell J. Patients’ experiences before starting anti-VEGF treatment for sight-threatening diabetic macular oedema: a qualitative interview study. Nord J Nurs Res. 2018;38(1):11–17. doi: 10.1177/2057158517709409 [DOI] [Google Scholar]
- 26.Hennink MM, Kaiser BN, Marconi VC. Code saturation versus meaning saturation: how many interviews are enough? Qual Health Res. 2017;27(4):591–608. doi: 10.1177/1049732316665344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerr C, Nixon A, Wild D. Assessing and demonstrating data saturation in qualitative inquiry supporting patient-reported outcomes research. Expert Rev Pharmacoecon Outcomes Res. 2010;10(3):269–281. doi: 10.1586/erp.10.30 [DOI] [PubMed] [Google Scholar]
- 28.VERBI Software. MAXQDA 2020 [computer software]. Berlin, Germany: VERBI Software; 2019. Available from: maxqda.com. Accessed February 25, 2022 [Google Scholar]
- 29.Joffe H, Yardley L. Content and thematic analysis. In: Research Methods for Clinical and Health Psychology. London: SAGE Publications, Ltd; 2004:56–68. [Google Scholar]
- 30.Polat O, Inan S, Özcan S, et al. Factors affecting compliance to intravitreal anti-vascular endothelial growth factor therapy in patients with age-related macular degeneration. Turk J Ophthalmol. 2017;47(4):205–210. doi: 10.4274/tjo.28003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okada M, Wong TY, Mitchell P, et al. Defining nonadherence and nonpersistence to anti-vascular endothelial growth factor therapies in neovascular age-related macular degeneration. JAMA Ophthalmol. 2021;139(7):769–776. doi: 10.1001/jamaophthalmol.2021.1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavan D, Makaroff L, da Rocha Fernandes J, et al. The diabetic retinopathy barometer study: global perspectives on access to and experiences of diabetic retinopathy screening and treatment. Diabetes Res Clin Pract. 2017;129:16–24. doi: 10.1016/j.diabres.2017.03.023 [DOI] [PubMed] [Google Scholar]
- 33.Soubrane G, Cruess A, Lotery A, et al. Burden and health care resource utilization in neovascular age-related macular degeneration: findings of a multicountry study. Arch Ophthalmol. 2007;125(9):1249–1254. doi: 10.1001/archopht.125.9.1249 [DOI] [PubMed] [Google Scholar]
- 34.Boyle J, Vukicevic M, Koklanis K, Itsiopoulos C, Rees G. Experiences of patients undergoing repeated intravitreal anti-vascular endothelial growth factor injections for neovascular age-related macular degeneration. Psychol Health Med. 2018;23(2):127–140. doi: 10.1080/13548506.2016.1274040 [DOI] [PubMed] [Google Scholar]
- 35.Gomi F, Toyoda R, Yoon AH, Imai K. Factors of anti-vascular endothelial growth factor therapy withdrawal in patients with neovascular age-related macular degeneration: implications for improving patient adherence. J Clin Med. 2021;10(14):3106. doi: 10.3390/jcm10143106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sobolewska B, Sabsabi M, Ziemssen F. Importance of treatment duration: unmasking barriers and discovering the reasons for undertreatment of anti-VEGF agents in neovascular age-related macular degeneration. Clin Ophthalmol. 2021;15:4317–4326. doi: 10.2147/OPTH.S325763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okada M, Mitchell P, Finger RP, et al. Nonadherence or nonpersistence to intravitreal injection therapy for neovascular age-related macular degeneration: a mixed-methods systematic review. Ophthalmology. 2021;128(2):234–247. doi: 10.1016/j.ophtha.2020.07.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krüger Falk M, Kemp H, Sørensen TL. Four-year treatment results of neovascular age-related macular degeneration with ranibizumab and causes for discontinuation of treatment. Am J Ophthalmol. 2013;155(1):89–95.e83. doi: 10.1016/j.ajo.2012.06.031 [DOI] [PubMed] [Google Scholar]
- 39.Senra H, Balaskas K, Mahmoodi N, Aslam T. Experience of anti-VEGF treatment and clinical levels of depression and anxiety in patients with wet age-related macular degeneration. Am J Ophthalmol. 2017;177:213–224. doi: 10.1016/j.ajo.2017.03.005 [DOI] [PubMed] [Google Scholar]