Abstract
Background:
Typical thermoregulatory responses to elevated temperatures among healthy individuals include reduced blood pressure and perspiration. Individuals with end-stage kidney disease (ESKD) are susceptible to systemic fluctuations caused by ambient temperature changes that may increase morbidity and mortality. We investigated whether pre-dialysis systolic blood pressure (preSBP) and interdialytic weight gain (IDWG) can independently mediate the association between ambient temperature, all-cause hospital admissions (ACHA), and all-cause mortality (ACM).
Methods:
The study population consisted of ESKD patients receiving hemodialysis treatments at Fresenius Medical Care facilities in Philadelphia County, PA, from 2011 to 2019 (n = 1981). Within a time-to-event framework, we estimated the association between daily maximum dry-bulb temperature (TMAX) and, as separate models, ACHA and ACM during warmer calendar months. Clinically measured preSBP and IDWG responses to temperature increases were estimated using linear mixed effect models. We employed the difference (c-c’) method to decompose total effect models for ACHA and ACM using preSBP and IDWG as time-dependent mediators. Covariate adjustments for exposure-mediator and total and direct effect models include age, race, ethnicity, blood pressure medication use, treatment location, preSBP, and IDWG. We considered lags up to two days for exposure and 1-day lag for mediator variables (Lag 2-Lag 1) to assure temporality between exposure-outcome models. Sensitivity analyses for 2-day (Lag 2-only) and 1-day (Lag 1-only) lag structures were also conducted.
Results:
Based on Lag 2- Lag 1 temporal ordering, 1 °C increase in daily TMAX was associated with increased hazard of ACHA by 1.4% (adjusted hazard ratio (HR), 1.014; 95% confidence interval, 1.007–1.021) and ACM 7.5% (adjusted HR, 1.075, 1.050–1.100). Short-term lag exposures to 1 °C increase in temperature predicted mean reductions in IDWG and preSBP by 0.013–0.015% and 0.168–0.229 mmHg, respectively. Mediation analysis for ACHA identified significant indirect effects for all three studied pathways (preSBP, IDWG, and preSBP + IDWG) and significant indirect effects for IDWG and conjoined preSBP + IDWG pathways for ACM. Of note, only 1.03% of the association between temperature and ACM was mediated through preSBP. The mechanistic path for IDWG, independent of preSBP, demonstrated inconsistent mediation and, consequently, potential suppression effects in ACHA (15.5%) and ACM (6.3%) based on combined pathway models. Proportion mediated estimates from preSBP + IDWG pathways achieved 2.2% and 0.3% in combined pathway analysis for ACHA and ACM outcomes, respectively. Lag 2 discrete-time ACM mediation models exhibited consistent mediation for all three pathways suggesting that 2-day lag in IDWG and preSBP responses can explain 2.11% and 4.41% of total effect association between temperature and mortality, respectively.
Conclusion:
We corroborated the previously reported association between ambient temperature, ACHA and ACM. Our results foster the understanding of potential physiological linkages that may explain or suppress temperature-driven hospital admissions and mortality risks. Of note, concomitant changes in preSBP and IDWG may have little intermediary effect when analyzed in combined pathway models. These findings advance our assessment of candidate interventions to reduce the impact of outdoor temperature change on ESKD patients.
Keywords: End-stage kidney disease, Dry-bulb temperature, Mediation analysis, Blood pressure, Inter-dialytic weight gain, Suppression effects
1. Introduction
Warming ambient temperatures can influence physiological changes within the general population. More specifically, renal-related health effects, such as acute kidney failure, heatstroke, as well as fluid and electrolyte imbalances, are typical causes of hospital admissions and mortality among older adults exposed to heatwaves (Bobb et al., 2014; Borg et al., 2017; Hansen et al., 2008). Such symptoms are frequent sequelae of heat stress and dehydration during periods of extreme heat (Borg et al., 2017). At the proximal level, typical thermoregulatory responses to elevated ambient temperature among healthy individuals can include reduced blood pressure and loss of extracellular fluid through perspiration and dehydration (Wang et al., 2017). As such, projected elevated temperature and increased frequency of extreme heat related to climate change can pose a substantive public health threat to individuals with chronic renal dysfunction such as end-stage kidney disease (ESKD).
ESKD management includes frequent dialysis treatments to prevent the accumulation of harmful levels of electrolytes, wastes, and fluids (National Institute of Diabetes and Digestive and Kidney Diseases, 2018). As an added health care safety concern, ESKD patients are susceptible to life-threatening complications resulting from fluctuations in weight and blood pressure (Iseki et al., 1997; Klassen et al., 2002; Port et al., 1999). Blood pressure stability is critical to improving survival, irrespective of initial blood pressure levels (Raimann et al., 2012). Increased fluid-based weight gains between hemodialysis (HD) treatments, measured by inter-dialytic weight gain (IDWG), can increase fluid overload complications resulting in hospitalization or death (Wong et al., 2017). The accumulation of fluids between treatments is known to cause increased blood pressure. As such, dialysis patients can develop hypertension and other cardiovascular-related complications. Clinical studies have shown that increased IDWG changes is associated with higher pre-dialysis blood pressure (Inrig et al., 2007). Also, sudden changes in blood pressure can compromise physiological stability among ESKD patients (Lertdumrongluk et al., 2015; Raimann et al., 2012).
Previously, we observed increased risks of all-cause hospitalization and mortality during extreme heat events for ESKD patients residing in selected northeastern American cities (Remigio et al., 2019). From unpublished work, we found that extreme heat events can, on average, reduce systolic blood pressure (1.6 mmHg; 95% CI: 1.8, 1.4) and IDWG percentage decline (0.14%; 95% CI: 0.21%, 0.18%) when compared to non-extreme heat events (Remigio et al., 2018). IDWG is the percentage of weight gain, usually fluid accumulation, between two consecutive HD treatments. These observations present an interesting paradox for ESKD patients where both reduced blood pressure and weight changes can co-occur during extreme heat days (Kalantar-Zadeh et al., 2009a; Kalantar-Zadeh et al., 2009b; Wong et al., 2017). Concomitant changes in IDWG and preSBP might have opposing effects in heat-related adverse risks in mortality and hospitalization. Changes in IDWG due to temperature changes could increase blood pressure variability among ESKD patients and subsequently enhance complications and result in hospitalization and premature death (Iseki et al., 1997; Klassen et al., 2002; Port et al., 1999; Wong et al., 2017). Between decreasing IDWG and reduced blood pressure from heat exposure, the primary uncertainty is determining whether concomitant changes in blood pressure and IDWG may have a reduced or enhanced influence on the total effect. Underlying pathways for temperature-related effects is relatively unclear for the ESKD population. Investigating the role of blood pressure and interdialytic weight gain as mediators between warmer temperature exposures and mortality and hospitalization endpoints could potentially identify plausible interventions to protect ESKD patients from heat exposures.
This study aims to understand the role of intermediate effects due to ambient temperature exposures during warmer calendar months between May to September (MJJAS) within Philadelphia county-a mostly continuously urbanized area-with a high chronic renal disease prevalence (Boyle et al., 2020). By leveraging individual-level repeated clinical measurements, preSBP (mmHg), and IDWG change (%), we can begin to describe mechanistic linkages to heat-related hospital and mortality events among ESKD patients. This work also linked discrete-time temperature exposures to individual-level time-varying intermediate responses using repeated clinical measurements from HD patients. To attain this objective, we tested the working hypothesis that preSBP and IDWG change, when modeled together as mediators, can each independently mediate the association between ambient temperature exposure and hospitalization and mortality outcomes (Bellavia and Valeri, 2018). Through mediation analysis, we can better understand the extent of physiological mediators on temperature exposure-response pathways. Operating through these mediators, we also estimated its independent and conjoined effects on ESKD patients. The findings from this research could inform potential interventions to minimize the impact of extreme heat among ESKD patients.
2. Methods
2.1. Study area and participants
We conducted a retrospective cohort study that included ESKD patients undergoing hemodialysis treatment at Fresenius Medical Care-North American (FMC-NA) clinics in Philadelphia County, PA, USA, from 2011 to 2019 (n = 1981). Philadelphia, located in southeastern Pennsylvania, has a subtropical climate that consists of wet and hot summers and mild winters (NOAA National Centers for Environmental Information, 2020). During MJJAS months from 2000 to 2021, Philadelphia can achieve up to 30 C averaged monthly maximum temperature (Supplemental Fig. 1). Average monthly relative humidity ranges from 64.9% to 69.2%, and averaged total monthly rainfall is most pronounced between June and August. Monthly summaries for maximum daily dry-bulb temperature, averaged daily relative humidity, and total precipitation are shown in supplemental materials.
Philadelphia includes one of the largest county-wide ESKD populations within the northeastern United States serviced by FMC. Patient information on socio-demographic and medical information pertaining to sex, race, ethnicity, age, clinic site, and history of prescribed cardioprotective drugs, along with routine clinical anthropometric clinical treatment measurements were included. The study used deidentified information and was determined exempt by the University of Maryland Institutional Review Board (1337900-2).
2.2. Meteorological data
Dry-bulb temperature is typically known as air temperature and is not influenced by moisture. Often, temperature-related epidemiological studies use dry-bulb temperature for shared exposure observations for a given population (NOAA National Centers for Environmental Information, 2020). For this work, archived dry-bulb temperature data was extracted using the R interface package rnooa through the National Climatic Data Center at the National Oceanic and Atmospheric Agency (NOAA) (Chamberlain, 2020). We identified two meteorological stations at Philadelphia International Airport (39.87327°, 75.22678°) and Northeast Philadelphia Airport (40.07892°, 75.01341°) as local data sources. Observations were aggregated at the county-level and expressed as daily maximum air temperatures (TMAX) during warmer calendar months, May to September (MJJAS), from 2011 to 2019. We elected to use maximum daily dry bulb temperature to capture the strongest temperature-mortality and temperature-hospital admissions signals specific for Philadelphia (Davis et al., 2016). Also, we initially adjusted for air moisture measures like relative humidity but found that its inclusion did not result in model improvement and did not provide any additional information that might explain exposure-outcome and exposure-mediator relationships. Consequently, related variables were not included in our analysis.
2.3. Outcome measures
We obtained all-cause hospital admission (ACHA) and all-cause mortality (ACM) medical records among HD patients from the Renal Research Institute (RRI). Patient medical records for ACHA and ACM events are considered highly accurate since records were tracked through FMC-NA billing and payment requirements and subject to Centers for Medicare & Medicaid (CMS) reporting. ACHA includes first and recurrent visits for each patient from the study population.
2.4. Blood pressure, inter-dialytic weight gain mediators
Blood pressure (BP) and fluid balance measures are routine diagnostics for determining clinical decisions for fluid volume removal during a dialysis session. We used preSBP as a proxy for blood pressure. Measurements were made using an oscillometric method when a patient is in a sitting position after 5 min (Raimann et al., 2012). To avoid concerns with collinearity, we did not include diastolic blood pressure in the analysis (Mange, 2004). The IDWG change is the difference between current pre-dialysis weight and previous post-dialysis weight divided by the patient’s dry weight expressed as a percentage. Clinical studies have shown that higher IDWG change is associated with higher pre-dialysis blood pressure (Hägg-Holmberg et al., 2019; Mange, 2004). More often, dialysis patients can develop hypertension and other cardiovascular-related complications due to IDWG-related fluid overloading (Kalantar-Zadeh, Regidor, Kovesdy, Van Wyck, Bunnapradist, Horwich and Fonarow, 2009b). We also adjusted for prescribed anti-hypertensive medication (Yes/No) as a confounder, given the expected higher prevalence of cardiovascular-related co-morbidities among patients (Gronlund et al., 2018).
We specified potential confounders as race and ethnicity (Asian, Hispanic, non-Hispanic Black, non-Hispanic White, Other/Did Not Specify), sex (Male or Female), treatment facility site (27 identified HD treatment locations within Philadelphia), and age (continuous) into models. The selection of covariates was made a priori based on research and clinical findings that suggested potential confounding between exposure and outcome, and mediator and outcome pathways.
2.5. Analytical approach
Given the lack of reported temperature effects among ESKD patients for blood pressure and IDWG, we estimated temperature-responses using the sample population. Random-intercept linear mixed-effects models were used to calculate mean patient responses for preSBP and IDWG change measures. Crude and adjusted models for lag structure specifications were explored.
We assessed whether TMAX could acutely affect ACM and ACHA outcomes by using time-to-event methods. Discrete-time Cox proportional hazards regression models with time-dependent covariates were used to estimate temperature effects by generating hazard ratios (HR) based on specified lag periods for exposure and mediators. Despite the historically favored case-crossover design for characterizing short-term effects, a cohort design approach allows for studying subject-level effects such as physiological responses to temperature using subject-specific characteristics associated with hospitalization and mortality. This approach also increases our statistical power by including right-censored patients that may generally not be included in case-crossover studies (Peters et al., 2006).
Cox models were generated for ACHA and ACM outcomes. Since hospital admissions are recurrent events, generalized Cox models that account for within-person correlation and differently timed event occurrences are required (Amorim and Cai, 2015). In this work, we specified Prentice, Williams, and Peterson modeling with a gap time (PWP-GT) measuring scaled times between sequential recurrent events. PWP-GT assumes that within-subject recurrent events are related and that baseline hazards vary from event to event (Amorim and Cai, 2015; Mills, 2010). Studies related to ESKD and HD research typically often apply Andersen-Gill models when using ACHA outcomes (Chan et al., 2009; Go et al., 2004; Harel et al., 2015). However, using stratified PWP-GT is preferable since the effect of time-varying covariates can result in different risk intervals in subsequent events for each participant. For ACM, we selected unshared frailty modeling to account for unmeasured heterogeneity not explained by selected covariates (Mills, 2010; Ratcliffe et al., 2004). This approach accounts for individuals with higher failure predispositions by including random effects to account for their individual-specific vulnerabilities. We specified a Gaussian distribution for the frailty and used a restricted maximum likelihood (REML) approximation (Balan and Putter, 2020).
Through sequential model building, we estimated TMAX effects (crude total effect), followed by the inclusion of baseline covariates (adjusted total effect), and then finally time-varying mediators (adjusted direct effect) for each outcome. Robust standard errors for all model specifications were calculated. All statistical tests were two-tailed and based on an alpha of 0.05. All analyses were conducted using R statistical software version 3.6.1 (R Core Team, 2019). Statistical software for Cox modeling and extensions are available from the survival R package through CRAN (Therneau, 2020). Lastly, we assessed proportional hazards assumption through Schoenfeld hypothesis testing and through plotting scaled Schoenfeld residuals for ACM models. We note that holding the assumption for recurrent events generally may not hold for traditional PH Cox models given the extended period for time at risk (Twisk, 2005). However, the specification of PWP-GT stratified models does address the violation of a common baseline hazard for all events by allowing different baselines for each event by stratifying by event and defining event-specific baselines (Kelly and Lim, 2000).
2.6. Mediation analysis
To quantify the extent of the total effect of TMAX exposure on ACHA or ACM operating through preSBP and IDWG, we tested preSBP and IDWG (separate and combined) as selected mediators using the difference method (c-c’) to estimate indirect effects (IE) (Burgos Ochoa et al., 2020; VanderWeele, 2016). Indirect effects for these analyses represent the change in daily maximum temperature β coefficients between adjusted total effect (c, no mediator) and adjusted direct effect (c’, with mediator(s)) regression models. We calculated IEs for preSBP, IDWG, and both preSBP and IDWG as concomitant mediators.
We used IEs to characterize proportions of the total effect contributed by preSBP, IDWG, and preSBP and IDWG combined, as shown in Fig. 1 (Burgos Ochoa et al., 2020; VanderWeele, 2016). The proportion mediated statistic gives insight on the relative effect of exposure on the outcome that works through a mediator or mediators (MacKinnon et al., 2000). Pathway proportions were assessed through preSBP, IDWG, and preSBP and IDWG as individually separate mediated pathways and as combined pathways. Bootstrapping at 1000 repetitions was used to generate 95% confidence intervals for indirect associations. Regression analysis quantifying mediator-outcome associations were also conducted.
Fig. 1.
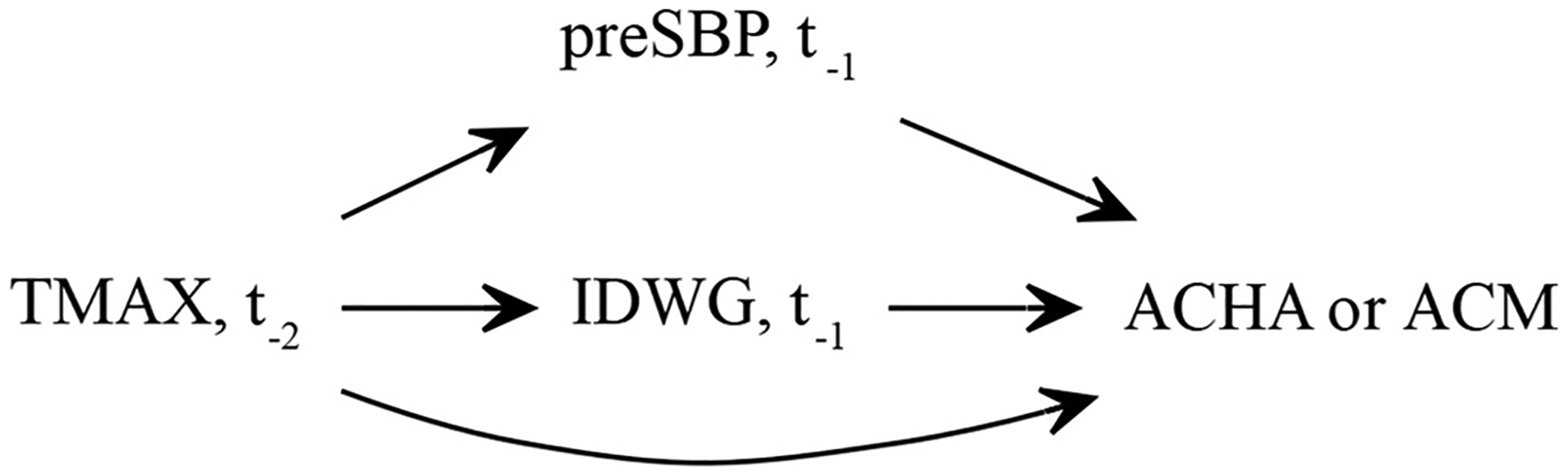
Directed acyclic graph (DAG) depicting mechanistic pathways between time-dependent daily maximum temperature exposures (TMAX), IDWG, preSBP, and all-cause mortality (ACM) and all-cause hospital admission (ACHA) outcomes. The DAG represents Lag 2-Lag1 specific pathways using two-lagged exposures (t−2) and one-day lagged mediators (t−1) that correspond to outcome events.
2.7. Lag structures
The study considered lag structures for exposures and mediator variables for up to two days (two-day lag [Lag 2]) before ACHA or ACM events. This parameterization aided in characterizing potential delayed effects associated with temperature. To assure temporal precedence, we focused on two-day lagged TMAX exposure and one-day lagged mediator (Lag 2-Lag 1) models to explore direct and indirect effects between our exposure, mediators, and outcomes (Fig. 1). Additional discrete-time lagged models (Lag 2-only and Lag 1-only, Fig. S4) are explored as associational models for sensitivity analysis.
2.8. Missing data
While there were no missing data for baseline measurements, we noted up to 30% missingness for preSBP and IDWG change values for subsequent visits. Through aggregated mean comparisons between missing and not missing data, we determined that physiological data were missing at random (Supplemental Table 1). Missing covariate data were imputed by using multivariate imputation by chain equations (MICE) as recommended (Carroll et al., 2020; Marshall et al., 2010; Murad et al., 2020; Tan et al., 2018). We generated 5 imputed data sets using 5 iterations for each imputed data set and used predictive mean matching. All model coefficients and standard errors were pooled from imputed data sets by using the mice package in R (Van Buuren and Groothuis-Oudshoorn, 2011).
3. Results
Study participants (n = 1981) contributed approximately a total of 4764 person-years. We estimated a median value of 518 days for person-time contributions with an interquartile range (IQR) of 1095 days by each participant. During the study period, the overall median daily maximum air temperature was 28.3 °C (IQR = 6.1 °C) with a range of 11.7 °C–38.9 °C. Among the 1981 HD patients treated within Philadelphia (Table 1), we studied 934 patients with recurrent hospital admissions (1+ hospital admissions) followed by 439 patients who reported one hospital admission visit. The remainder reported no hospital admissions during MJJAS months. Among cases with recurrent ACHAs, non-Hispanic (NH) Black (59%) and male (55%) patients made up most of the ACHA-based cohort. Mean preSBP (148 mm Hg, SD = 25 mm Hg) and IDWG percentage (3.05%, SD = 1.89%) measures also exhibit non-significant elevated mean levels for patients with recurrent hospital admissions compared to patients with no reported hospital admission visits. There were 276 cases of death (13.9%) within the sample population. Similar to the ACHA cohort, NH-Black (59%) and male (57%) patients were over-represented. The mean levels of blood pressure (146 mm Hg, SD = 27.1) and IDWG (3.11%, SD = 1.93) were slightly different among ACM cases when compared to censored patients (preSBP = 147.0 mm Hg, SD = 23.9; IDWG = 2.85%, SD = 1.81).
Table 1.
Clinical and demographic characteristics of ESKD Philadelphia cohort stratified by hospital admissions and mortality status.
| Characteristics | Total | Hospital Admissions | Mortality | |||
|---|---|---|---|---|---|---|
| None | 1-only | 1+ | No | Yes | ||
| Patients, n (%) | 1981 | 608 (31) | 439 (22) | 934 (47) | 1705 (86) | 276 (14) |
| Age, mean (SD) | 60.1 (14.1) | 60.2 (14.5) | 60.5 (14.3) | 59.9 (13.8) | 59.5 (14.3) | 64.1 (12.5) |
| Race/ethnicity, n (%) | ||||||
| Asian | 30 (2) | 9 (1) | 8 (2) | 13 (1) | 26 (2) | 4 (1) |
| Hispanic | 142 (7) | 33 (5) | 39 (9) | 70 (7) | 118 (7) | 24 (9) |
| NH-Black | 1079 (54) | 307 (50) | 221 (50) | 551 (59) | 916 (54) | 163 (59) |
| NH-White | 390 (2) | 119 (20) | 87 (20) | 184 (20) | 322 (19) | 68 (25) |
| Other, Not-reported | 340 (17) | 140 (23) | 84 (19) | 116 (12) | 323 (19) | 17 (6) |
| Blood Pressure Medication | ||||||
| Yes | 739 (37%) | 264 (43) | 278 (63) | 314 (34) | 663 (39) | 76 (28) |
| No | 1242 (63%) | 344 (57) | 161 (37) | 620 (66) | 1042 (61) | 200 (72) |
| Sex, n (%) | ||||||
| Female | 828 (42%) | 243 (40) | 169 (38) | 416 (45) | 708 (42) | 120 (43) |
| Male | 1153 (58%) | 365 (60) | 270 (62) | 518 (55) | 997 (58) | 156 (57) |
| Treatment clinics, n | 27 | 24 | 22 | 25 | 27 | 19 |
| Daily Maximum Temperature, median (IQR) | 28.3 (6.1) | 28.3 (6.1) | 28.3 (6.1) | 28.3 (6.1) | 28.3 (6.1) | 28.3 (6.7) |
| preSBP, mean (SD) | 147.0 (24.3) | 145 (23.7) | 147.0 (23.7) | 148.0 (25.0) | 147.0 (23.9) | 146 (27.1) |
| IDWG %, mean (SD) | 2.89 (1.83) | 2.68 (1.73) | 2.84 (1.81) | 3.05 (1.89) | 2.85 (1.81) | 3.11 (1.93) |
As depicted in Table 2, individual-level clinical measures (preSBP, IDWG) were negatively associated with increasing TMAX. Decreases in mean pre-SBP responses ranged between 2.33 mmHg and 1.68 mmHg across lag models in fully adjusted models. Likewise, the mean reduction in IDWG ranged between 0.19% and 0.13% for each 10 °C increase in dry-bulb temperature. Mediator-outcome analyses are provided in supplemental tables (Tables S10–S13).
Table 2.
Crude and adjusted IDWG and preSBP mean changes to 10 °C increase in maximum daily temperature increase based on linear mixed effects regression. Crude models contain daily maximum temperature. Adjusted models contain daily maximum temperature, age, race/ethnicity, treatment clinic, sex, and blood pressure medication use covariates.
| Crude | Adjusted | ||||
|---|---|---|---|---|---|
| Clinic measure | Lag | mean effect | Std Error | mean effect | Std Error |
| IDWG | Lag 2 | −0.145 | 0.003 | −0.149 | 0.003 |
| Lag 1 | −0.188 | 0.003 | −0.189 | 0.003 | |
| Lag 0 | −0.138 | 0.003 | −0.13 | 0.003 | |
| preSBP | Lag 2 | −1.693 | 0.038 | −1.68 | 0.038 |
| Lag 1 | −2.35 | 0.038 | −2.337 | 0.038 | |
| Lag 0 | −2.381 | 0.039 | −2.287 | 0.038 | |
Based on unadjusted total temperature association models (Model 1), 1 °C increase in daily maximum temperature was associated with 7.5% greater ACHA risk (Table 3) and 6.9% greater ACM risk (Table 4). In this work, risk is defined as hazards. Slight incremental changes in coefficient estimates occurred across partially adjusted (confounders-only, Model 2; confounders and one mediator, Models 3 &4) and fully adjusted (confounders and mediators, Model 5) models. One percent increase in IDWG change resulted in an 8% increase in the risk of hospital admission (HR, 1.082, 95%CI: 1.050–1.115) and 19% increase in the risk of mortality (HR, 1.193, 95%CI: 1.106–1.287) in fully adjusted direct effect models (Model 5). A one-unit increase in preSBP resulted in small but significant protection against hospital admissions risk (HR, 0.998, 95%CI: 0.997–0.999) shown in Model 3. Compared to Non-Hispanic Black and female patients, Hispanic (HR, 1.371, 95%CI: 1.090–1.724) and male (HR, 0.866, 95%CI: 0.773–0.970) patients appear to have a higher risk of hospital admissions. Whereas non-Hispanic White (HR, 1.515, 95%CI: 1.137–2.018) patients and age (HR, 1.034, 95%CI: 1.025–1.044) are positively associated with increased mortality risk. On average, patients prescribed blood pressure medication exhibit approximately 49% protective effects from death (HR, 0.514, 95%CI: 0.396–0.667) as shown in Model 5 (Table 4). Associational estimates for Lag 2 and Lag 1 lag model structures are consistent with the main results (Supplemental Tables 2–5). Also, we note all ACM models did not violate assumptions for proportional hazards based on global tests. And, as expected, all ACHA models suggested non-proportionality for hazards corresponding to recurrent outcomes.
Table 3.
Hazard ratio (HR) and 95% confidence intervals for the association of daily maximum daily temperature and all-cause hospital admissions (ACHA) based on Lag 2- Lag 1 discrete-time model structure. Due to the large number of categorical factors, treatment clinics associations were not reported. Bold indicates statistically significant hazard ratio at p < 0.05.
| Model 1: unadjusted total temperature association | Model 2: adjusted total temperature association | Model 3: adjusted direct temperature association (preSBP) | Model 4: adjusted direct temperature association (IDWG %) | Model 5: adjusted direct temperature association (preSBP + IDWG%) | |
|---|---|---|---|---|---|
| Daily Max Temp (Lag2) | 1.013 (1.007–1.020) | 1.012 (1.005–1.019) | 1.012 (1.006–1.019) | 1.014 (1.007–1.021) | 1.014 (1.007–1.021) |
| preSBP (Lag1) | 1.001 (0.999–1.002) | 1.000 (0.999–1.002) | |||
| IDWG change (Lag1) | 1.081 (1.058–1.105) | 1.081 (1.058–1.104) | |||
| Age (yr) | 0.994 (0.992–0.997) | 0.995 (0.992–0.997) | 0.996 (0.993–0.998) | 0.996 (0.993–0.998) | |
| Race/Ethnicity | |||||
| Non-Hispanic Black | 1.000 | 1.000 | 1.000 | 1.000 | |
| Asian | 0.963 (0.750–1.238) | 0.958 (0.745–1.231) | 0.903 (0.702–1.162) | 0.903 (0.702–1.162) | |
| Hispanic | 1.104 (0.993–1.228) | 1.105 (0.994–1.229) | 1.090 (0.980–1.211) | 1.090 (0.980–1.212) | |
| Non-Hispanic White | 1.141 (1.050–1.240) | 1.143 (1.052–1.242) | 1.134 (1.235–1.520) | 1.133 (1.042–1.232) | |
| Other/Not Reported | 1.351 (1.218–1.498) | 1.353 (1.220–1.501) | 1.370 (1.235–1.520–1.135) | 1.371 (1.236–1.521) | |
| Blood Pressure Medication | |||||
| No | 1.000 | 1.000 | 1.000 | 1.000 | |
| Yes | 0.625 (0.586–0.666) | 0.621 (0.582–0.662) | 0.627 (0.589–0.669) | 0.627 (0.587–0.669) | |
| Sex | |||||
| Female | 1.000 | 1.000 | 1.000 | 1.000 | |
| Male | 0.959 (0.904–1.018) | 0.961 (0.906–1.019) | 0.945 (0.891–1.003) | 0.945 (0.891–1.003) |
Table 4.
Hazard ratio (HR) and 95% confidence intervals for the association of daily maximum daily temperature and all-cause mortality (ACM) based on Lag 2- Lag 1 discrete-time model structure. Due to a large number of categorical factors, treatment clinics associations were not reported. Bold indicates a statistically significant hazard ratio at p < 0.05.
| Model 1: unadjusted total temperature association | Model 2: adjusted total temperature association | Model 3: adjusted direct temperature association (preSBP) | Model 4: adjusted direct temperature association (IDWG %) | Model 5: adjusted direct temperature association (preSBP + IDWG%) | |
|---|---|---|---|---|---|
| Daily Max Temp (Lag2) | 1.069 (1.045–1.094) | 1.071 (1.047–1.096) | 1.071 (1.046–1.096) | 1.076 (1.051–1.101) | 1.075 (1.050–1.100) |
| preSBP (Lag1) | 0.997 (0.992–1.003) | 0.996 (0.991–1.002) | |||
| IDWG change (Lag1) | 1.186 (1.100–1.280) | 1.193 (1.106–1.287) | |||
| Age (yr) | 1.0334 (1.024–1.043) | 1.033 (1.023–1.043) | 1.035 (1.025–1.044) | 1.034 (1.025–1.044) | |
| Race/Ethnicity | |||||
| Non-Hispanic | 1.000 | 1.000 | 1.000 | 1.000 | |
| Black | |||||
| Asian | 1.128 (0.418–3.048) | 1.140 (0.411–3.165) | 0.977 (0.359–2.654) | 0.987 (0.365–2.674) | |
| Hispanic | 1.516 (0.985–2.334) | 1.533 (0.980–2.396) | 1.412 (0.916–2.177) | 1.436 (0.932–2.212) | |
| Non-Hispanic White | 1.575 (1.182–2.099) | 1.567 (1.163–2.110) | 1.527 (1.144–2.036) | 1.515 (1.137–2.018) | |
| Other/Not Reported | 0.769 (0.459–1.289) | 0.769 (0.456–1.297) | 0.796 (0.475–1.336) | 0.798 (0.476–1.337) | |
| Blood Pressure Medication | |||||
| No | 1.000 | 1.000 | 1.000 | 1.000 | |
| Yes | 0.493 (0.381–0.638) | 0.499 (0.385–0.646) | 0.504 (0.388–0.654) | 0.514 (0.396–0.667) | |
| Sex | |||||
| Female | 1.000 | 1.000 | 1.000 | 1.000 | |
| Male | 1.027 (0.807–1.307) | 1.017 (0.799–1.294) | 1.010 (0.792–1.288) | 0.994 (0.781–1.267) |
In Lag 2-Lag1 single mediator models, pathways through IDWG-only and preSBP + IDWG mediators for hospital admission and mortality were statistically significant as observed by respective estimated indirect effects (Tables 5 and 6). We found that 15.46% of the association between TMAX and ACHA path was mediated through preSBP and approximately 6.26% between TMAX and mortality. Negative indirect effect estimates suggest inconsistent mediation. As a result, negative proportions mediated between total and direct effect models imply potential suppression effects for temperature and outcome variables. We note that the IDWG and preSBP + IDWG pathways in Lag 2-only (Table S6) and Lag 1-only (Table S8) ACHA models also demonstrated negative proportion mediated estimates.
Table 5.
Effect size estimates and bootstrap-generated two-sided 95% confidence intervals for mediation effects of the association between daily maximum temperature and all-cause hospital admissions (ACHA) based on Lag 2- Lag 1 discrete-time model structure. Bold indicates statistically significant estimate at p < 0.05.
| Estimate | Model 1: Temperature and confounders only | Model 2: Temperature, confounders, and SBP | Model 3: Temperature, confounders, and IDWG | Model 4: Temperature, confounders, IDWG, and SBP |
|---|---|---|---|---|
| Temperature β | 0.0120 | 0.0123 | 0.0138 | 0.0139 |
| Indirect Effect | – | −0.0003 (−0.0009, 0.0002) | −0.0019 (−0.0029, −0.0009) | −0.0019 (−0.0031, −0.0009) |
| Percent Mediated | – | −2.42% | −15.46% | −15.71% |
Table 6.
Effect size estimates and bootstrap-generated two-sided 95% confidence intervals for mediation effects of the association between daily maximum temperature and all-cause mortality (ACM) based on Lag 2- Lag 1 discrete-time model structure. Bold indicates statistically significant estimate at p < 0.05.
| Estimate | Model 1: Temperature and confounders only | Model 2: Temperature, confounders, and SBP | Model 3: Temperature, confounders, and IDWG | Model 4: Temperature, confounders, IDWG, and SBP |
|---|---|---|---|---|
| Temperature β | 0.0689 | 0.06817 | 0.0732 | 0.07228 |
| Indirect Effect | – | 0.0007 (−0.0012, 0.00111) | −0.0043 (−0.0052, −0.0015) | −0.0034 (−0.0061, −0.0016) |
| Percent Mediated | – | 1.03% | −6.26% | −4.94% |
In combined pathway analysis, the total association between ambient temperature with ACHA (Fig. 2) and ACM (Fig. 3) outcomes is explained by the preSBP + IDWG pathway (independent of preSBP and IDWG pathways) at 15.71% and 0.30%, respectively. There is a notable change when comparing proportion mediated for preSBP + IDWG as a single pathway (Table 5, Model 4)to a combined (Fig. 2) pathway. Tables S5–S8 provide mediation results for Lag 2 and Lag 1 discrete time-dependent models. Inconsistent mediation was observed in ACHA models. In comparison, Lag 2-ACM models demonstrated consistent mediation for all single pathways. In combined pathway models (Figs. S5–S8), we noted that the preSBP, independent of IDWG, pathway in Lag 2 explained about 4.41% of the total effect between temperature and mortality (Fig. S6). Whereas Lag 1 models for IDWG, independent of preSBP, pathway yielded 5.54% and 4.51% for ACHA (Fig. S7) and ACM (Fig. S8) outcomes, respectively.
Fig. 2.
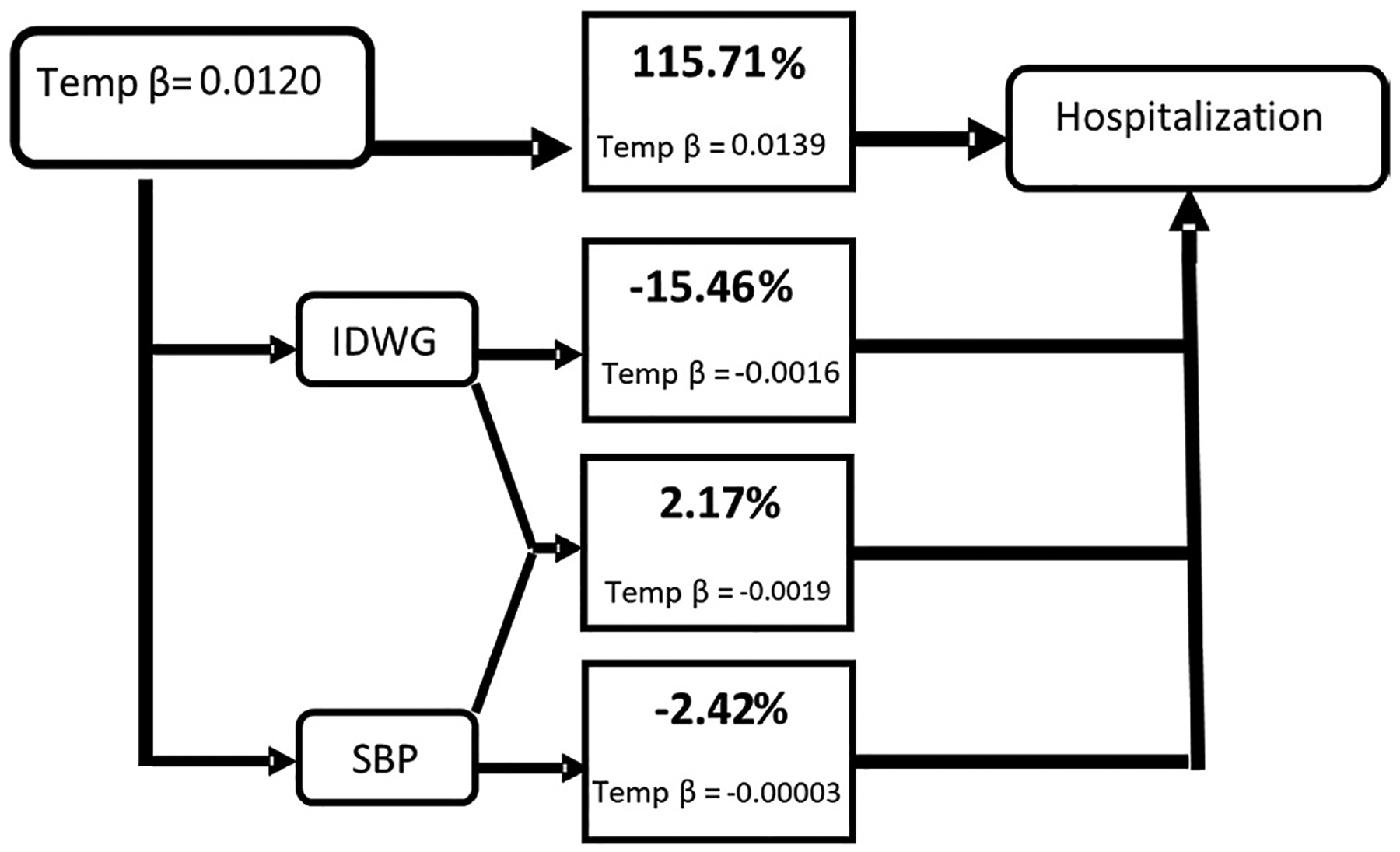
Combined pathway analysis of the association between daily maximum temperature and all-cause hospital admissions (ACHA) and mediating paths for combined mediators based on Lag 2- Lag 1 discrete-time model structure. For indirect paths: IDWG path is considered independent of preSBP, and preSBP indirect effect is independent of IDWG, and IDWG + preSBP path is independent of IDWG and preSBP indirect effects. Direct effects greater than 100% can occur when inconsistent mediation is present in one or more of the paths.
Fig. 3.
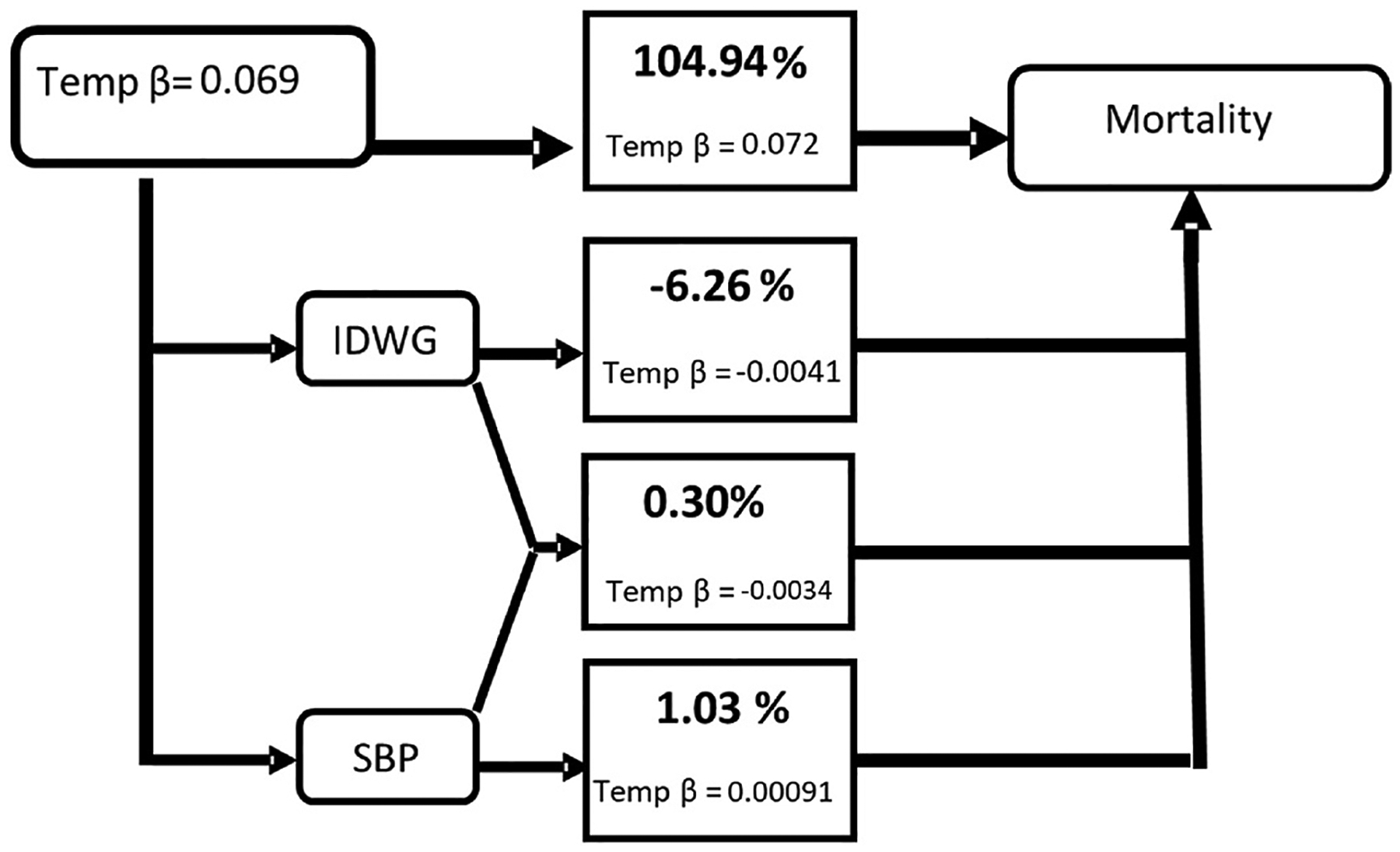
Combined pathway analysis of the association between daily maximum temperature and all-cause mortality (ACM) and mediating paths for combined mediators based on Lag 2- Lag 1 discrete-time model structure For indirect paths: IDWG path is considered independent of preSBP, and preSBP indirect effect is independent of IDWG, and IDWG + preSBP path is independent of IDWG and preSBP indirect effects. Direct effects greater than 100% can occur when inconsistent mediation is present in one or more of the paths.
4. Discussion
Among ESKD patients (n = 1981) treated in Philadelphia County, PA from 2011 to 2019, our study confirmed that increased ambient temperature exposure is associated with an increased risk of mortality and hospital admission. Our study also found significant mean changes in blood pressure and IDWG responses inversely related to temperature, suggesting plausible proximate physiological responses. We found evidence of significant pathways for all three (preSBP, IDWG, and preSBP + IDWG) and two of the three (IDWG and preSBP + IDWG) studied pathways for ACM and ACHA event outcomes, respectively. However, indirect effect estimates demonstrated inconsistent mediation and suggested suppression effects for certain mechanistic pathways.
The association between TMAX and increased risk of ACHA and ACM rates are consistent with other work related to ambient temperature exposure (Borg et al., 2017; Lavados et al., 2018; Tasian et al., 2014; Weinberger et al., 2019; Zhang et al., 2014). One study devoted to ESKD population found that seasonal changes can influence physiological responses and mortality, particularly in more temperate regions where climatic fluctuations are most pronounced (Usvyat et al., 2012). A recent case-crossover study found consistent and significant increased mortality and hospital admissions rates associated with two-day cumulative exposure of extreme heat events in Boston and New York, and non-significant positive associations in Philadelphia (Remigio et al., 2019). The results observed in this study relates to the use of a climate-based dichotomous exposure metric for an upper percentile threshold a continuous meteorological measure.
In this work, we measured the extent of thermoregulatory responses (IDWG, preSBP) as mediators for hospital admissions and death events and explored the interesting paradox of both reduced blood pressure and IDWG changes co-occurring in ESKD patients. Our findings offer insight into the total effect decomposition between exposure-outcome relationships by estimating indirect effects through intermediated physiological responses. Our results found significant indirect effects for most IDWG (independent of preSBP) and IDWG + preSBP pathways. Proportion mediated estimates for individual mediators ranged from 15.71% to 0.86% and 6.26%–5.97% for ACHA and ACM outcomes. We found evidence of indirect mediation resulting from negative indirect effects between total effects and direct effects models. For many direct effect models, mediators yielded increased temperature β-coefficient magnitudes (Tables 4–5 & S2–S5) and confirmed a third variable effect known as suppression (MacKinnon et al., 2000; Shrout and Bolger, 2002). As such, pathways between exposure and outcome variables displayed a combination of consistent and inconsistent mediation effects.
Despite the relatively lower estimates of a proportion mediated, several significant pathways exhibited potential suppression effects. In the case of the IDWG pathway, independent of preSBP, we note that indirect effects operating through IDWG had an opposite effect with respect to total effect (Figs. 2 and 3). This Lag 2- Lag 1 pathway also yielded the most dominant indirect effect among studied lag structures. For example, exposure to increased TMAX one-day before an ACM event may have had a slightly protective effect. This inverse relationship was confirmed in the exposure-mediator analysis, where one-day lag exposure to increased TMAX can reduce IDWG. Deductively, if increased IDWG can result in ESKD complications, we could infer that IDWG could act as a modest suppressor from temperature-related health risks. Though, a serial mediation pathway between IDWG and blood pressure needs to be included. And more broadly, for Lag2-Lag 1 ACM mediation studied pathways (Fig. 2), there was approximately 5% suppression of the overall effect between temperature and mortality when considering all three pathways. It appears that in this causal structure, using IDWG, preSBP, and combined IDWG + preSBP pathways may not adequately explain the total effect of temperature on mortality. This is similar for hospital admissions, where the three combined pathways demonstrate approximately 16% suppression.
As shown previously, mean preSBP and IDWG percent changes inversely respond to increased ambient temperatures. This an expected observation as typical thermoregulatory responses to elevated temperature among healthy individuals include reduced blood pressure (Wang et al., 2017). Physiologically, fluid loss due to increased perspiration and restricted fluid replacement is plausibly attributed to reduced IDWG percent changes among the ESKD population. Though, such changes from increased temperatures do pose unintended health concerns. The literature on ESKD patient health found that blood pressure changes can enhance complications and result in hospitalization and premature death (Iseki et al., 1997; Klassen et al., 2002; Port et al., 1999; Wong et al., 2017). Also, increased fluid-based weight gains between HD treatments can increase fluid overflow complications resulting in hospitalization or death (Wong et al., 2017). In sequence, increased changes to IDWG can raise blood pressure, and any sudden changes in blood pressure can compromise physiological stability (Raimann et al., 2012). The minor reduction in IDWG needs to be explored further as a potential independent protective pathway and as an antecedent mediator before blood pressure.
Concerning clinical implications, we found that blood pressure and interdialytic weight gain, while mechanistically significant, may not have substantive mediation effects. Instead, proportion mediated estimates were found to be minor, and pathways mostly demonstrated suppression effects. The goal of determining an explanation of the observed relationship between TMAX and mortality/hospital admissions for potential clinical interventions was not necessarily achieved. However, considering additional passive heat responses related to electrolyte disorders is worth pursuing. Also, since patients using hypertension medication had nearly 50% reduction in all-cause mortality and all-cause hospital admission events in TMAX-adjusted models (Tables 2, S2, S4), a stratified analysis might provide enhanced statistical insight on the role of blood pressure medication in reducing mortality risk.
A major strength of this study includes statistical robustness from using a longitudinal study design. As a study novelty, we inferred physiological responses using high-density patient-level health records specific to ESKD patients. Using repeated measurements of individual-level time-varying covariates, we established temporal precedence with mediator and outcome responses by considering two-day lag TMAX as the exposure and one-day lag preSBP and IDWG as proximal mediators. This time-varying approach assured a one-day separation between each time-dependent variable. We adjusted for multiple explanatory factors that are traditionally associated with increased risks of mortality and hospital admissions. Also, we considered seasonality by restricting our analyses to warmer calendar months (May through September, MJJAS) as part of our exposure assessment. This approach effectively captured heat-related exposures, and by extension, potential temperature increases that are warmer season-appropriate. Long-term trends to account for climate, population, health care practices changes were not considered.
More notably, we incorporated hypertension prescription use to account for patients with high blood pressure. Lastly, we applied a practical mediation analysis approach as an exploratory method to understand the role of environmental exposures such as temperature.
There are some limitations to this work. First, this study was based on a simplified causal path model that included only two measured mediators. Testing additional physiological responses as candidate mediators is needed to understand temperature-related causes of mortality and morbidity. Though, for now, it was a crucial step to establish and confirm potential pathways driven by regularly recorded clinical measures before dialysis treatments. We did not consider a U-shaped relationship between blood pressure and mortality among HD patients (Zager et al., 1998) since traditional mediation methods assume linearity. In a re-analysis, dichotomizing clinical mediators using clinically meaningful or individual-based thresholds or trends could be explored (Raimann et al., 2012) and may inform candidate mechanistic responses (e.g., suppression). In the current analysis, we assumed independent, parallel pathways with preSBP and IDWG mediators. IDWG might be considered as both an intermediate confounder that precedes blood pressure and a mediator since changes in IDWG could also influence blood pressure. Also, since we restricted exposure and outcome events to warmer months, we did not capture cold-temperature-related effects. Similarly, In this work, we acknowledge that built environment features within Philadelphia can influence the urban heat island effect (UHIE) while increasing temperature variability due to climate change can also compound neighborhood-level heat intensity and duration. But due to data privacy concerns, our study could not apply more localized shared exposure estimates for patients. Additionally, we not did consider clinical measures on dialysis vintage (length of time on dialysis), estimated glomerular filtration rate (eGFR), and albumin to creatinine ratio (ACR) in this work. Lastly, more advanced causal mediation approaches are needed to capture more complex pathways among multiple mediators and intermediate confounders while upholding rigorous assumptions to infer causal relationships (Pazzagli et al., 2018; Tchetgen Tchetgen Eric, 2011).
5. Conclusions
This study found that increased ambient temperature is associated with reduced blood pressure and IDWG and increased mortality and hospital admissions risks. When combined in mediation analysis, we observed modest suppression effects operating through most proximal pathways in defined lag model structures. Our findings suggest that, statistically, blood pressure and IDWG may not fully explain the relationship between TMAX and mortality/hospital admissions; instead, depending on selected lags, those responses could exhibit protective effects. This suggests that causes of mortality and morbidity effects may involve other time-varying and time-invariant risk factors, and more complex mechanistic pathways to describe temperature effects specific to the ESKD population.
Supplementary Material
Acknowledgements
We thank Rachel Lasky and Sheetal Chaudhuri for performing data extractions from Fresenius Kidney Care medical records database.
Funding support
This work was supported by the Agency for Healthcare Research and Quality (grant number R36HS027716). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The work was also supported by NRT-INFEWS: UMD Global STEWARDS (STEM Training at the Nexus of Energy, WAter Reuse and FooD Systems) that was awarded to the University of Maryland School of Public Health by the National Science Foundation National Research Traineeship Program (award number 1828910). Rodman Turpin was supported by the University of Maryland Prevention Research Center cooperative agreement from the Centers for Disease Control and Prevention (grant number U48DP006382)
Footnotes
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Drs. Raimann and Kotanko reported being an employees of the Renal Research Institute (a wholly owned subsidiary of Fresenius Medical Care [FMC]). Dr. Kotanko reported receiving honoraria from UpToDate and owning stock in FMC. Dr. Maddux reported owning stock in and being employed by FMC. No other disclosures were reported.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2021.112127.
References
- Amorim LD, Cai J, 2015. Modelling recurrent events: a tutorial for analysis in epidemiology. Int. J. Epidemiol 44, 324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan TA, Putter H, 2020. A tutorial on frailty models. Stat. Methods Med. Res 29, 3424–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavia A, Valeri L, 2018. Decomposition of the total effect in the presence of multiple mediators and interactions. Am. J. Epidemiol 187, 1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Obermeyer Z, Wang Y, Dominici F, 2014. Cause-specific risk of hospital admission related to extreme heat in older adults. J. Am. Med. Assoc 312, 2659–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg M, Bi P, Nitschke M, Williams S, McDonald S, 2017. The impact of daily temperature on renal disease incidence: an ecological study. Environ. Health 16, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle SM, Zhao Y, Chou E, Moore K, Harhay MN, 2020. Neighborhood context and kidney disease in Philadelphia. SSM-population health 12, 100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos Ochoa L, Rijnhart JJ, Penninx BW, Wardenaar KJ, Twisk JW, Heymans MW, 2020. Performance of methods to conduct mediation analysis with time-to-event outcomes. Stat. Neerl 74, 72–91. [Google Scholar]
- Carroll OU, Morris TP, Keogh RH, 2020. How are missing data in covariates handled in observational time-to-event studies in oncology? A systematic review. BMC Med. Res. Methodol 20, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain S, 2020. rnoaa:’NOAA’Weather Data from R. R Package Version 1.1. 0 https://cran.r-project.org/web/packages/rnoaa/.
- Chan KE, Lazarus JM, Wingard RL, Hakim RM, 2009. Association between repeat hospitalization and early intervention in dialysis patients following hospital discharge. Kidney Int 76, 331–341. [DOI] [PubMed] [Google Scholar]
- Davis RE, Hondula DM, Patel AP, 2016. Temperature observation time and type influence estimates of heat-related mortality in seven US cities. Environ. Health Perspect 124, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-Y, 2004. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med 351, 1296–1305. [DOI] [PubMed] [Google Scholar]
- Gronlund CJ, Sheppard L, Adar SD, O’Neill MS, Auchincloss A, Madrigano J, Kaufman J, Diez Roux AV, 2018. Vulnerability to the cardiovascular effects of ambient heat in six US cities: results from the multi-ethnic study of atherosclerosis (MESA). Epidemiology 29, 756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägg-Holmberg S, Dahlstrom EH, Forsblom CM, Harjutsalo V, Liebkind R, Putaala J, Tatlisumak T, Groop P-H, Thorn LM, 2019. The role of blood pressure in risk of ischemic and hemorrhagic stroke in type 1 diabetes. Cardiovasc. Diabetol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AL, Bi P, Ryan P, Nitschke M, Pisaniello D, Tucker G, 2008. The effect of heat waves on hospital admissions for renal disease in a temperate city of Australia. Int. J. Epidemiol 37, 1359–1365. [DOI] [PubMed] [Google Scholar]
- Harel Z, Wald R, McArthur E, Chertow GM, Harel S, Gruneir A, Fischer HD, Garg AX, Perl J, Nash DM, Silver S, Bell CM, 2015. Rehospitalizations and emergency department visits after hospital discharge in patients receiving maintenance hemodialysis. J. Am. Soc. Nephrol 26, 3141–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inrig JK, Patel UD, Gillespie BS, Hasselblad V, Himmelfarb J, Reddan D, Lindsay RM, Winchester JF, Stivelman J, Toto R, Szczech LA, 2007. Relationship between interdialytic weight gain and blood pressure among prevalent hemodialysis patients. Am. J. Kidney Dis 50, 108–118 e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseki K, Miyasato F, Tokuyama K, Nishime K, Uehara H, Shiohira Y, Sunagawa H, Yoshihara K, Yoshi S, Toma S, Kowatari T, Wake T, Oura T, Fukiyama K, 1997. Low diastolic blood pressure, hypoalbuminemia, and risk of death in a cohort of chronic hemodialysis patients. Kidney Int 51, 1212–1217. [DOI] [PubMed] [Google Scholar]
- Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, Fonarow GC, 2009a. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation 119, 671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, Fonarow GC, 2009b. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation 119, 671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PJ, Lim LLY, 2000. Survival analysis for recurrent event data: an application to childhood infectious diseases. Stat. Med 19, 13–33. [DOI] [PubMed] [Google Scholar]
- Klassen PS, Lowrie EG, Reddan DN, DeLong ER, Coladonato JA, Szczech LA, Lazarus JM, Owen WF Jr., 2002. Association between pulse pressure and mortality in patients undergoing maintenance hemodialysis. Jama 287, 1548–1555. [DOI] [PubMed] [Google Scholar]
- Lavados PM, Olavarría VV, Hoffmeister L, 2018. Ambient temperature and stroke risk. Stroke 49, 255–261. [DOI] [PubMed] [Google Scholar]
- Lertdumrongluk P, Streja E, Rhee CM, Sim JJ, Gillen D, Kovesdy CP, Kalantar-Zadeh K, 2015. Changes in pulse pressure during hemodialysis treatment and survival in maintenance dialysis patients. Clin. J. Am. Soc. Nephrol 10, 1179–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Krull JL, Lockwood CM, 2000. Equivalence of the mediation, confounding and suppression effect. Prev. Sci 1, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mange KC, 2004. Blood pressure and the survival of renal allografts from living donors. J. Am. Soc. Nephrol 15, 187–193. [DOI] [PubMed] [Google Scholar]
- Marshall A, Altman DG, Holder RL, 2010. Comparison of imputation methods for handling missing covariate data when fitting a Cox proportional hazards model: a resampling study. BMC Med. Res. Methodol 10, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills M, 2010. Introducing Survival and Event History Analysis Sage. [Google Scholar]
- Murad H, Dankner R, Berlin A, Olmer L, Freedman LS, 2020. Imputing missing time-dependent covariate values for the discrete time Cox model. Stat. Methods Med. Res 29, 2074–2086. [DOI] [PubMed] [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases, 2018. Hemodialysis 2018.
- NOAA National Centers for Environmental Information, 2020. Climate Data Online Data Tools https://www.ncdc.noaa.gov/cdo-web/datatools.
- Pazzagli L, Linder M, Zhang M, Vago E, Stang P, Myers D, Andersen M, Bahmanyar S, 2018. Methods for time-varying exposure related problems in pharmacoepidemiology: an overview. Pharmacoepidemiol. Drug Saf 27, 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Von Klot S, Berglind N, Hörmann A, Löwel H, Nyberg F, Pekkanen J, Perucci CA, Stafoggia M, Sunyer J, Tiittanen P, Forastiere F, 2006. Epidemiol. Perspect. Innovat 3, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port FK, Hulbert-Shearon TE, Wolfe RA, Bloembergen WE, Golper TA, Agodoa LY, Young EW, 1999. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am. J. Kidney Dis 33, 507–517. [DOI] [PubMed] [Google Scholar]
- R Core Team, R., 2019. A Language and Environment for Statistical Computing https://www.R-project.org/.
- Raimann JG, Usvyat LA, Thijssen S, Kotanko P, Rogus J, Lacson E Jr., Levin NW, 2012. Blood pressure stability in hemodialysis patients confers a survival advantage: results from a large retrospective cohort study. Kidney Int 81, 548–558. [DOI] [PubMed] [Google Scholar]
- Ratcliffe SJ, Guo W, Ten Have TR, 2004. Joint modeling of longitudinal and survival data via a common frailty. Biometrics 60, 892–899. [DOI] [PubMed] [Google Scholar]
- Remigio RV, Jiang C, Raimann J, Kotanko P, Usvyat L, Maddux FW, Kinney P, Sapkota A, 2019. Association of extreme heat events with hospital admission or mortality among patients with end-stage renal disease. JAMA Network Open 2, e198904–e198904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remigio RV, Raimann JG, Kotanko P, Maddux F, Usvyat L, Sapkota A, Kinney P, 2018. Impact of extreme heat on end-stage renal disease patients in the northeast US using selected clinical outcomes. ISEE Conf. Abstract 2018, 2018. [Google Scholar]
- Shrout PE, Bolger N, 2002. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol. Methods 7, 422. [PubMed] [Google Scholar]
- Tan FE, Jolani S, Verbeek H, 2018. Guidelines for multiple imputations in repeated measurements with time-dependent covariates: a case study. J. Clin. Epidemiol 102, 107–114. [DOI] [PubMed] [Google Scholar]
- Tasian GE, Pulido JE, Gasparrini A, Saigal CS, Horton BP, Landis JR, Madison R, Keren R, Project U.D.i.A., 2014. Daily mean temperature and clinical kidney stone presentation in five US metropolitan areas: a time-series analysis. Environ. Health Perspect 122, 1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchetgen Tchetgen Eric J, 2011. On causal mediation analysis with a survival outcome. Int. J. Biostat 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therneau T, 2020. A Package for Survival Analysis in R Vol. R Package Version 3,.2–7. [Google Scholar]
- Twisk JWR, 2005. Applied analysis of recurrent events: a practical overview. J. Epidemiol. Community Health 59, 706–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usvyat LA, Carter M, Thijssen S, Kooman JP, van der Sande FM, Zabetakis P, Balter P, Levin NW, Kotanko P, 2012. Seasonal variations in mortality, clinical, and laboratory parameters in hemodialysis patients: a 5-year cohort study. Clin. J. Am. Soc. Nephrol 7, 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buuren S, Groothuis-Oudshoorn K, 2011. Multivariate imputation by chained equations. J. Stat. Software 45, 1–67. [Google Scholar]
- VanderWeele TJ, 2016. Mediation analysis: a practitioner’s guide. Annu. Rev. Publ. Health 37, 17–32. [DOI] [PubMed] [Google Scholar]
- Wang Q, Li C, Guo Y, Barnett AG, Tong S, Phung D, Chu C, Dear K, Wang X, Huang C, 2017. Environmental ambient temperature and blood pressure in adults: a systematic review and meta-analysis. Sci. Total Environ 575, 276–286. [DOI] [PubMed] [Google Scholar]
- Weinberger KR, Spangler KR, Zanobetti A, Schwartz JD, Wellenius GA, 2019. Comparison of temperature-mortality associations estimated with different exposure metrics. Environ. Epidemiol 3, e072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MM, McCullough KP, Bieber BA, Bommer J, Hecking M, Levin NW, McClellan WM, Pisoni RL, Saran R, Tentori F, Tomo T, Port FK, Robinson BM, 2017. Interdialytic weight gain: trends, predictors, and associated outcomes in the international dialysis outcomes and practice patterns study (DOPPS). Am. J. Kidney Dis 69, 367–379. [DOI] [PubMed] [Google Scholar]
- Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, Van Stone J, Levey A, Meyer KB, Klag MJ, Johnson HK, Clark E, Sadler JH, Teredesai P, for the Medical Directors of Dialysis Clinic, I., 1998. “U” curve association of blood pressure and mortality in hemodialysis patients. Kidney Int 54, 561–569. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li S, Pan X, Tong S, Jaakkola JJ, Gasparrini A, Guo Y, Wang S, 2014. The effects of ambient temperature on cerebrovascular mortality: an epidemiologic study in four climatic zones in China. Environ. Health 13, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


