Abstract
Aim
Inconsistent investigations of the risk factors for all-cause mortality in patients undergoing peritoneal dialysis (PD) were reported. The present meta-analysis aimed to assess the impact of some clinical characteristics on the risk of mortality in PD patients.
Methods
PubMed and Embase were systematically searched for studies evaluating the risk factors for all-cause mortality in PD patients. Hazard ratio (HR) and 95% confidence interval (CI) were derived using a random-effect or fixed-effect model considering the heterogeneity across studies.
Result
A total of 26 studies were included in this meta-analysis in accordance with the inclusion and exclusion criteria. Age, primary cardiovascular diseases, diabetes mellitus, and high level of alkaline phosphatase showed significant positive associations with elevated risk of all-cause and cardiovascular mortality in PD patients, while hemoglobin acted as a benefit factor. Furthermore, early onset of peritonitis, high peritoneal transport status, elevated body mass index and high-sensitivity C-reactive protein could also considerably increase the risk of all-cause mortality. The absolute serum level of magnesium, potassium, and uric acid required to improve survival in PD patients should be verified further.
Conclusions
Multiple factors could affect the risk of mortality in PD patients.
Keywords: Cardiovascular mortality, meta-analysis, mortality, peritoneal dialysis, risk factor
Introduction
Peritoneal dialysis (PD) is one of the major renal replacement therapies for patients with end-stage kidney disease (ESKD) [1]. The number of PD patients has been increasing in numerous developing countries. However, the long-term survival rate of PD patients remains low [2]. Additionally, cardiovascular disease (CVD) and death are highly prevalent in patients with ESKD [3,4]. These findings may be attributed to chronic inflammation, disturbed mineral metabolism, primary CVD, and other physical conditions. For instance, commonly accepted nutritional markers, such as serum albumin (ALB) level, serum creatinine (Cr) level, hemoglobin (Hb) level, and body mass index (BMI), might be used to assess prognosis of patients with chronic kidney disease (CKD) [5,6]. However, other studies reported a poor predictive value of serum ALB, serum Cr, and other characteristics of PD patients for all-cause mortality or cardiovascular outcomes [7,8]. Furthermore, the prognostic values of various physiological ions have not been well determined [9–12]. This meta-analysis aimed to identify the risk factors for mortality in PD patients to improve prognosis.
Materials and methods
Search strategy
This meta-analysis was conducted following the meta-analysis of observational studies in epidemiology (MOOSE) guidelines [13]. PubMed and Embase were searched for studies conducted from January 2000 to December 2020. Studies evaluating the risk factors for all-cause mortality or cardiovascular mortality in patients undergoing PD satisfied the inclusion criteria in the present meta-analysis. We used ((((‘peritoneal dialysis’ OR ‘renal dialysis’ OR ‘renal replacement therapy’ OR ‘chronic kidney disease’ OR ‘end-stage kidney disease’ OR ‘ESKD’) AND (‘mortality’ OR ‘death’ OR ‘survival’) AND (‘potassium’ OR ‘magnesium’ OR ‘peritonitis’ OR ‘body mass index’ OR ‘albumin’ OR ‘hemoglobin’) AND Clinical Trial[ptyp]) as the search terms. Moreover, the references of all the relevant original articles were manually reviewed to identify additional eligible studies.
Selection criteria
Respective study was assessed by two independent reviewers, and any disagreements between the two reviewers were resolved by another independent reviewer. The inclusion criteria were as follows: (1) studies including patients undergoing PD; (2) studies mentioning at least one of the risk factors, namely, age, Hb level, serum ALB level, BMI, diabetes mellitus (DM), serum potassium level, serum magnesium level, peritonitis, peritoneal transport characteristics, alkaline phosphatase (ALP) level, high-sensitivity C-reactive protein (hs-CRP) level, and uric acid (UA) level; (3) studies evaluating all-cause mortality; (4) studies reporting statistical data including hazard ratio (HR) and 95% confidence interval (CI). Studies without adjustment for specific potential confounders and non-English studies were excluded. Finally, a total of 26 studies were included.
Statistical analysis
All statistical analyses were conducted in Review Manager 5.3 software. HR adjusted for confounding variables and 95% CI were extracted from included studies. Each HR was transformed into a log HR, and the standard error was calculated based on the corresponding 95% CI. Statistical heterogeneity among studies was evaluated using the I2 index [14]. A random-effect model was adopted if the I2 index was >50%, demonstrating substantial heterogeneity; otherwise, a fixed-effect model was employed. A p value <0.05 was considered statistically significant. Furthermore, Egger’s tests were performed to assess potential publication bias, and a sensitivity analysis was conducted to determine the robustness of the conclusion by excluding each article from the meta-analysis.
Results
Literature search
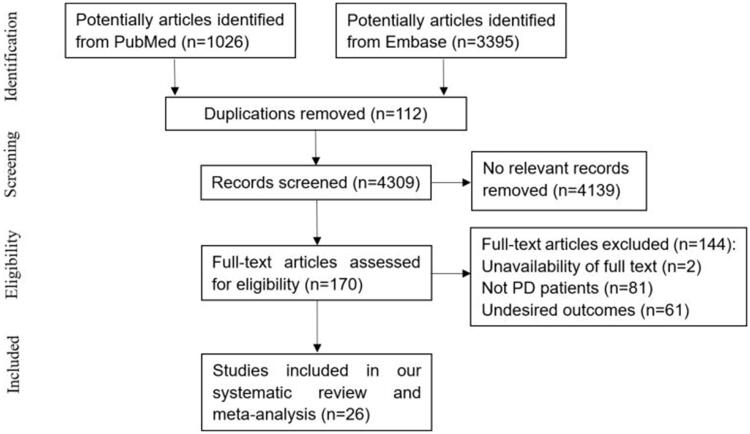
A total of 4421 potentially relevant articles were retrieved from PubMed and Embase. After screening titles and abstracts, 112 duplicate studies and 4139 non-relevant studies were excluded. The remaining 170 studies were subjected to full-text assessment, and 144 trials were further removed based on the following criteria: (i) unavailable full-text, (ii) non-inclusion of PD patients, or (iii) unavailable desired outcomes. Finally, 26 papers were included in our systematic review [9–12,15–36]. This meta-analysis had a good inter-reviewer agreement (κ = 0.851). A flowchart summarizing the selection process is presented in Figure 1.
Figure 1.
Flow diagram of the trail selection process.
Study characteristics
The baseline characteristics of included studies are shown in Table 1. Each study included 102-10,692 patients, and median follow-up duration ranged from 13.0 months to 52.8 months. Among these studies, 26 trials ascertained all-cause mortality, and 15 studies enrolled the outcomes of cardiovascular mortality. All studies reported HR adjusted for possible confounders, such as age, sex, BMI, DM, and laboratory indices.
Table 1.
Baseline of characteristics of studies of systematic review and meta-analysis.
| Study | Country | Type | Sample size | Mean age (year) |
Percentage Male (%) |
Percentage DM (%) |
Follow-up duration (month) | Reported outcome | Adjusted factors |
|---|---|---|---|---|---|---|---|---|---|
| Ye H 2017 [15] | China | Retrospec-tive | 1321 | 48.1 ± 15.3 | 58.7 | 23.5 | 34 (21–48) | All-cause and cardiovas-cular mortality | age, sex, DM, CVD, ALB, 24 h urine output, Hb, P |
| Xiang S 2019 [16] |
China | Retrospec-tive | 9405 | 52.5 ± 14.6 | 54.9 | 12.2 | 34.5 ± 23.2 | All-cause and cardiovas-cular mortality |
DM, CVD, Ca, RRF, Hb, Cr, ALB, PTH, potassium, natrium, P, fasting plasma glucose |
| Xue Y 2019 [17] | China | Retrospec-tive | 748 | 50.1 | 6.23 (2.423– 11.652) |
All-cause and cardiovas-cular mortality |
age, Cr, urea, ALB, Hb, Ca, DM, P, DBP, CHD, eGFR | ||
| Hwang SD 2019 [18] | Korea | Retrospec-tive | 6071 | 65.8 ± 13.7 | 57.5 | 36.1 | 90.96 ± 54.34 | All-cause mortality | age, sex, DM, ALB, Hb, SBP, DBP, PNA, cholesterol, total body water, urine duration, Kt/Vurea week total, Cr clearance weekly |
| Lai KJ 2018 [19] | China | Retrospec-tive | 492 | 53.5 ± 15.3 | 48 | 34.6 | 36.4 | All-cause and cardiovas-cular mortality |
age, sex, BMI, smoking, medication, comorbidi-ties, PD related parameters, laboratory data |
| Jung HY 2018 [20] | Korea | Prospective | 953 | 57.2 ± 12.8 | 58.6 | 45 | 36.0 ± 16.2 | All-cause and cardiovas-cular mortality | age, sex, BMI, SBP, DBP, DM, smoking, myocardial infarction, LDL, HDL, total cholesterol, dialysis vintage |
| Liu Y 2017 [21] | China | Retrospec-tive | 667 | 42.9 | 24.7 | 32.64 | All-cause and cardiovas-cular mortality |
age, sex, 24 h urinary output, Hb, ALB, Ca, AST, P, ALT, PTH | |
| Rhee CM 2014 [22] | USA | Retrospec-tive | 9244 | 54 ± 15 | 52 | 49 | 32.4 (15.6–51.6) | All-cause mortality | age, sex, race, DM, BMI, CVD, smoking, ferritin, dialysis vintage, primary insurance, marital status |
| Liu X 2014 [23] | China | Retrospec-tive | 1021 | 47.5 ± 15.5 | 59.1 | 22.8 | 31 (19–45) | All-cause and cardiovas-cular mortality |
age, sex, 24 h urinary output, BP, ALB, Hb, comorbidity score, ALT, AST, P, Ca, iPTH, phosphate binders use |
| Li W 2017 [24] | China | Retrospec-tive | 1228 | 46.96 ± 14.9 | 61.2 | 25.5 | 35 (18.7–52.3) | All-cause and cardiovas-cular mortality |
age, sex, BMI, DM, CVD, Hb, ALB, TG, uric acid, MAP, rGFR |
| Lee S 2017 [25] | Korea | Prospective | 1152 | 54 (45–64) | 56.5 | 34.8 | 52.8 ± 20.4 | All-cause mortality | age, sex, MCCI, BMI, ALB, ALP, Ca, P, SGA, uric acid |
| Kim YK 2014 [26] | Korea | Prospective | 900 | 56 ± 12 | 57 | 32 | 24 (14 − 27) | All-cause mortality | age, sex, DM, Davies comorbidity score |
| Prasad N 2014 [27] | India | Prospective | 328 | 52.6 ± 12.6 | 73.8 | 53.7 | 20.0 ± 14.3 | All-cause mortality | age, SGA, DM, ALB, comorbidities, rGFR |
| Tian Y 2016 [28] | China | Retrospec-tive | 294 | 50.8 ± 14.0 | 62.2 | 14.3 | 33.3 (17.3–52.8) | All-cause mortality | age, time to first peritonitis, cholesterol, DM, eGFR, ALB, CCI score |
| Feng S 2016 [29] | China | Retrospec-tive | 189 | 57.5 ± 15.9 | 56.1 | 51.3 | All-cause mortality | age, DM, ALB, CRP, RRF | |
| Liu X 2016 [30] | China | Retrospec-tive | 1778 | 47.4 ± 15.6 | 59.5 | 25.3 | 35 (17–56) | All-cause and cardiovas-cular mortality |
age, sex, PD inception |
| Yang X 2016 [9] | China | Retrospec-tive | 10692 | 56 ± 16 | 55 | 40 | 13 (7–23) | All-cause mortality | age, sex, DM, race, primary insurance, geographic location, ESRD reason, prior transplant, comorbidities, laboratory |
| Cai K 2016 [10] | China | Retrospec-tive | 253 | 58 ± 16 | 55.3 | 22.9 | 29 (4–120) | All-cause and cardiovas-cular mortality |
age, sex, DM, rGFR, MAP, urinary output, sodium, Cr clearance, malnutrition, bone disorder-related factors |
| Wu X 2016 [31] | China | Retrospec-tive | 1068 | 48.0 ± 15.4 | 58.8 | 23.1 | 21.7 ± 14.1 | All-cause mortality | age, heart disease, DM, stroke, MAP, Hb, ALB, hs-CRP, time to first peritonitis |
| Xiong L 2015 [32] | China | Retrospec-tive | 1263 | 47.8 ± 15.0 | 58.6 | 24.1 | 25.3 (3.03– 82.07) |
All-cause and cardiovas-cular mortality |
age, sex, DM, CVD, MAP, Hb, ALB, hs-CRP, TG, rGFR, Kt/Vurea, total cholesterol |
| Xu Q 2014 [12] | China | Retrospec-tive | 886 | 48.5 ± 15.4 | 57.1 | 23.9 | 31 (0.5–81.0) | All-cause and cardiovas-cular mortality | age, sex, BMI, DM, Hb, ALB, CCI, hs-CRP |
| Dong J 2014 [33] | China | Retrospec-tive | 2264 | 58.1 ± 15.5 | 49.1 | 37.7 | 26.5 (13.6–43.6) | All-cause and cardiovas-cular mortality |
age, sex, RRF, ALB, Hb, P, CRP, CVD, DM, MAP, BMI, LDL |
| Torlen K 2012 [34] | Sweden | Retrospec-tive | 10468 | 56 ± 16 | 53 | 49 | 27 | All-cause and cardiovas-cular mortality |
age, sex, DM, race, dialysis vintage, Hb, primary insurance, marital status, BMI, smoking, CVD, cancer, ferritin, ALP, WBC, ALB, Cr, Ca, P, PTH, total iron binding capacity |
| Angela 2003 [35] |
China | Prospective | 246 | 55 ± 12 | 52 | 31 | 24 (2–34) | All-cause and cardiovas-cular mortality |
age, weight, height, BMI, CVD, DBP, LV mass index, LV end-diastolic diameter, LV ejection fraction, LV fractional shortening |
| Guan JC 2015 [36] |
China | Retrospec-tive | 102 | 57.8 | 22.6 | All-cause mortality | age, ALB, Hb, glycated Hb A1c, clearance of Cr, RRF, PNA | ||
| Rumpsfe-ld M 2006 [37] | Australia | Retrospec-tive | 3702 | 59.4 ± 14.8 | 53.9 | 38.1 | All-cause mortality | age, sex, race, smoking, BMI, weekly Kt/V, RRF, vintage year, PD modality, hyperten-sion,chronic lung disease, DM, coronary artery disease, peripheral vascular disease, cerebrovas-cular disease |
Hb: hemoglobulin; PTH: parathyroid hormone; DM: diabetes mellitus; RRF: residual renal function; ALB: albumin; hs-CRP: hypersensitive C-reactive protein; CVD: cardiovascular disease; TG: total triglyceride; WBC: white blood cell; LDL: low density lipoprotein; HDL: high density lipoprotein; BMI: body mass index; BP: blood pressure; SBP: systolic blood pressure; DBP: diastolic blood pressure; Ca: calcium; Cr: creatinine; eGFR: estimated glomerular filtration rate; rGFR: residual glomerular filtration rate; MAP: mean arterial pressure; SGA: subjective global assessment; PNA: protein equivalent of total nitrogen appearance; MCCI, modified Charlson comorbidity index.
Risk factors for all-cause mortality in patients undergoing PD
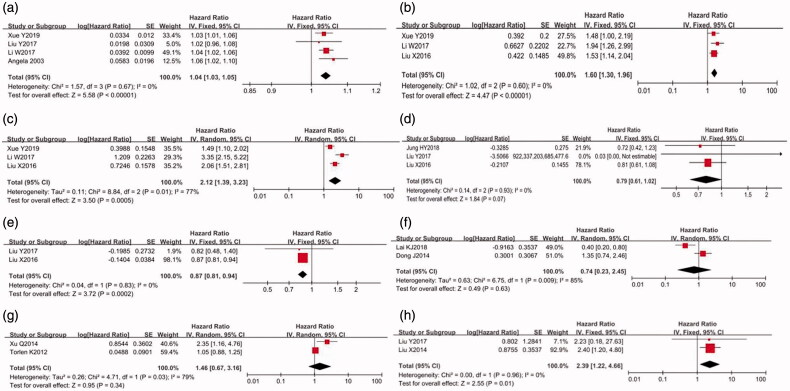
As shown in Table 2 and Figure 2, age (HR: 1.04, 95% CI: 1.04–1.05, p < 0.00001), DM (HR: 1.58, 95% CI: 1.41–1.78, p < 0.00001), primary CVD (HR: 1.72, 95% CI: 1.17–2.52, p = 0.006), high BMI (HR: 1.15, 95% CI: 1.04–1.28, p = 0.005), ALP level (HR: 2.11, 95% CI: 1.86–2.39, p < 0.00001), early onset of peritonitis (HR: 1.83, 95% CI: 1.26–2.64, p = 0.001), high hs-CRP level (HR: 1.37, 95% CI: 1.04–1.82, p = 0.03), and high peritoneal transport status (HR: 1.39, 95% CI: 1.10–1.76, p = 0.006) showed significant positive associations with all-cause mortality in PD patients. A fixed-effect model was applied to analyze variables because the I2 index was <50%. Sensitivity analysis was conducted in CVD and hs-CRP, given the substantial heterogeneity, while the conclusion was not affected. Egger’s test revealed no significant publication bias for any abovementioned risk factors (p for age = 0.712, p for DM = 0.458, p for CVD = 0.801, p for high BMI = 0.738, p for hs-CRP = 0.113, and p for ALP = 0.221).
Table 2.
Factors influencing the risk of all-cause mortality in PD patients.
| Risk factors | HR (95% CI) |
p Value |
p Value for heterogeneity (p, I2) |
|---|---|---|---|
| Age (per 1 year increase) |
1.04 (1.04–1.05) | p < 0.00001*** |
p = 0.30 I2=17% |
| CVD | 1.72 (1.17–2.52) |
p = 0.006** |
p < 0.0001 I2=87% |
| DM | 1.58 (1.41–1.78) |
p < 0.00001*** |
p = 0.09 I2=46% |
| Hs-CRP (mg/l) | 1.37 (1.04–1.82) |
p = 0.03 |
p < 0.00001 I2=94% |
| Hb (g/dl) | 0.87 (0.83–0.90) |
p < 0.00001*** |
p = 0.41 I2=0% |
| ALB (mg/dl) | 0.71 (0.42–1.21) |
p = 0.21 |
p < 0.00001 I2=93% |
| High BMI (kg/m2) | 1.17 (1.04–1.31) |
p = 0.007 |
p = 0.40 I2=0% |
| Low BMI (kg/m2) | 1.20 (0.83–1.73) |
p = 0.34 |
p = 0.009 I2=71% |
| ALP (U/l) | 2.11 (1.86–2.39) |
p < 0.00001*** |
p = 0.95 I2=0% |
| Magnesium (high vs low) | 0.19 (0.02–2.22) |
p = 0.18 |
p = 0.03 I2=73% |
| Uric acid (high vs low) | 0.93 (0.48–1.79) |
p = 0.83 |
p < 0.0001 I2=91% |
| Potassium (low vs high) | 1.25 (0.97–1.60) |
p = 0.08 |
p = 0.10 I2=56% |
| Early on-set of peritonitis |
1.83 (1.26–2.64) |
p = 0.001*** |
p = 0.41 I2=0% |
| Peritoneal transport status (high vs low) | 1.39 (1.10–1.76) |
p = 0.006 |
p = 0.21 I2=36% |
HR: hazard ratio; CI: confidence interval; ALB: albumin; DM: diabetes mellitus; CVD: cardiovascular diseases; hs-CRP: hypersensitive C-reactive protein; Hb: hemoglobulin; ALP: alkaline phosphatases; GFR: glomerular filtration rate; BMI: body mass index.
**p < 0.01.
***p < 0.001.
Figure 2.
Forest plots for the hazard risk (HR) between risk factors and all-cause mortality in PD patients (a) age (per 1 year increase); (b) cardiovascular disease; (c) diabetes mellitus; (d) hypersensitive-C reaction protein (mg/l); (e) hemoglobin (g/dl); (f) albumin (mg/dl); (g) high body mass index; (h) low body mass index; (i) alkaline phosphatases (U/l); (j) magnesium (high vs low); (k) uric acid (high vs low); (l) potassium (low vs high); (m) early on-set of peritonitis; (n) peritoneal transport status (high vs low)).
Furthermore, the pooled HR suggested a significant association between Hb level (HR: 0.87, 95% CI: 0.83-0.90, p < 0.00001) and a low risk of all-cause mortality in PD patients. Egger’s test revealed no significant publication bias for Hb (p = 0.876).
Meanwhile, this study identified no associations between serum ALB, low BMI, high UA level, high magnesium level, low potassium level, and the risk of all-cause mortality in PD patients.
Risk factors for cardiovascular mortality in patients undergoing PD
As shown in Figure 3, the pooled HR indicated that age (HR: 1.04, 95% CI: 1.03–1.050, p < 0.00001), primary CVD (HR: 2.12, 95% CI: 1.39–3.23, p = 0.0005), DM (HR: 1.60, 95% CI: 1.30–1.96, p < 0.00001), and high ALP (HR: 2.39, 95% CI: 1.22–4.66, p = 0.01) might elevate the risk of cardiovascular mortality in PD patients. Moreover, Hb level (HR: 0.87, 95% CI: 0.81–0.94, p = 0.0002) acted as a protective factor for cardiovascular mortality. Egger’s test revealed no significant publication bias (p for age = 0.95, p for DM = 0.574, and P for CVD = 0.292). Serum ALB level, high UA level, and low potassium level did not significantly affect the risk of cardiovascular mortality (Table 3).
Figure 3.
Forest plots for the hazard risk (HR) between risk factors and cardiovascular mortality in PD patients (a) age (per 1 year increase); (b) diabetes mellitus; (c) cardiovascular disease; (d) albumin (mg/dl); (e) hemoglobin (g/dl); (f) uric acid (high vs low); (g) potassium (low vs high); (h) alkaline phosphatases (U/l)).
Table 3.
Factors influencing the risk of cardiovascular mortality in PD patients.
| Risk factors | HR (95% CI) |
p Value | p Value for heterogeneity (p, I2) |
|---|---|---|---|
| Age (per 1 year increase) |
1.04 (1.03-1.05) |
p < 0.00001*** |
p = 0.67 I2=0% |
| DM | 1.60 (1.30-1.96) |
p < 0.00001*** |
p = 0. 60 I2=0% |
| CVD | 2.12 (1.39-3.23) |
p = 0.0005*** |
p = 0. 01 I2=77% |
| ALB (g/dl) | 0.79 (0.61-1.02) |
p = 0.07 |
p = 0.93 I2=0% |
| Hb (g/dl) | 0.87 (0.81-0.94) |
p = 0.0002*** |
p = 0.83 I2=0% |
| Uric acid (high vs low) | 0.74 (0.23-2.45) |
p = 0.63 |
p = 0.009 I2=85% |
| Potassium (low vs high) | 1.46 (0.67-3.16) |
p = 0.34 |
p = 0.03 I2=79% |
| ALP (U/l) | 2.39 (1.22-4.66) |
p = 0.01** |
p = 0.96 I2=0% |
HR: hazard ratio; CI: confidence interval; ALB: albumin; Hb: hemoglobulin; DM: diabetes mellitus; CVD: cardiovascular diseases; ALP: alkaline phosphatases.
**p < 0.01.
***p < 0.001.
Discussion
The present meta-analysis of 26 studies, including a total of 66,735 patients, was considered as the first meta-analysis exploring the risk factors for all-cause and cardiovascular mortality in patients undergoing PD. It covered five prospective studies. Our results indicated that age, primary CVD, DM, and ALP level negatively affected the risk of all-cause and cardiovascular mortality. Furthermore, early onset of peritonitis, obesity, high hs-CRP level, and membrane transport status might elevate the risk of all-cause mortality. Hb level was found to have a beneficial impact on the risk of all-cause mortality, while serum ALB level had a slight beneficial impact.
In this meta-analysis, only articles with sufficient data to calculate HR and adjusted ones were included. In the existing study, functional status of patients undergoing PD might predict the risk of mortality, such as employment status and family education [37,38]. The use of medicine, such as angiotensin receptor blockers and oral active vitamin D, could also reduce the risk of major cardiovascular events and total mortality [39]. Notably, the associations between several clinical and laboratory characteristics of PD patients and all-cause mortality were still under debate.
In the present meta-analysis, age, primary CVD, DM, and high level of ALP were found to be the risk factors for both all-cause and cardiovascular mortality in patients undergoing PD. Additionally, patients with high BMI, high peritoneal transport status, high level of hs-CRP, and early onset of peritonitis also had an elevated risk of all-cause mortality. Primary DM and CVD might impair vascular endothelial cells and cause chronic inflammation, primarily leading to arterial stif-fness; it might predict the risk of fatal cardiovascular events and all-cause mortality. Furthermore, poor glycemic control was found to be an independent risk factor for mortality in PD patients [40,41]. A correlation between an increased BMI and improved outcomes in hemodialysis patients has been reported, although such a relationship is so far unknown in PD patients [42]. In this meta-analysis, we found that patients in the highest BMI group experienced poorer survival outcomes compared to those in the normal BMI group. Although a U-shaped relationship between underweight and mortality was observed previously [43], the association was not significant in our analysis. Obesity was shown to be related to a decline of residual renal function (RRF), adversely affecting survival [44]. However, the prognostic value of BMI might depend on race, total cholesterol level, inflammation status, and comorbidities. Mineral metabolism disorders are highly prevalence among patients with CKD, such as abnormal serum calcium, phosphorus, and parathyroid hormone concentrations. Hyperphosphatemia was shown to be correlated with elevated apoptosis and poor immune response, which might increase risk for mortality [45]. Serum ALP level is generally considered to indicate renal bone disease in patients with CKD. Additionally, high level of ALP might be associated with vascular calcification and osteomalacia. In this meta-analysis, high peritoneal membrane transport, assessed by the peritoneal equilibration test, was also found to be a risk factor for mortality. An increased rate of peritoneal membrane solute transport might enhance protein losses through peritoneal membrane leading to a malnutrition status. Guan et al. [35] reported that higher peritoneal transport status was not an independent predictor after adjusting for ALB level, Hb level, and RRF. Large-scale randomized clinical trials are required to verify this relationship. In addition, we showed that early onset of peritonitis and elevated hs-CRP level increased the risk of all-cause mortality significantly although there was significant heterogeneity among studies in terms of hs-CRP level. Two prospective and one retrospective study were included for hs-CRP as a continuous variable, with different sample sizes, country, and primary status of patients, which might account for the heterogeneous. Meanwhile, elevated Hb level was found to be a protective factor for all-cause mortality. Lower Hb level was commonly regarded as malnutrition, demonstrating the absence of intravenous iron or erythropoiesis-stimulating agent therapy.
Hypokalemia is common in PD patients, primarily affecting the cardiovascular system. Furthermore, patients with hypokalemia may have more comorbidities. We included three studies exploring the relationship between serum potassium level and mortality, which was presented as a categorical variable. Xu et al. [12] and Lee et al. [25] reported a serum potassium level of <3 mEq/L or <4.5 mmol/L to be an independent risk factor for all-cause mortality in PD patients. However, the baseline values in another study did not show this relationship [11]. The change or fluctuation in serum potassium level during PD might be more reliable to predict death risk in PD patients and should be investigated further. Magnesium, the fourth most abundant cation in the body, plays a key role in various biological processes. Tubular injuries might cause renal magnesium wasting. Serum magnesium level of <1.8 mg/dL was reported to be a risk factor after adjusting for baseline demographic characteristics and comorbidities, but the risk was attenuated after further adjustment for laboratory indices [9]. Furthermore, the beneficial effect of a serum magnesium level of >0.7 mmol/L might be different between male and female individuals [10]. Considering that lower serum magnesium is associated with a poor nutrition status and increased inflammation, the independent relationship between serum magnesium and mortality in PD patients should be evaluated,and an appropriate treatment to maintain the right serum magnesium level needs to be determined. UA is the final product of nucleotide metabolism, and it is primarily excreted from the kidney by glomerular filtration. High level of serum UA could be an endothelial toxin and aggravated endothelial function by activating the inflammatory pathway [46]; it is also associated with RRF loss [47]. However, we did not observe any significant association between hyperuricemia and mortality. Xiang et al. [16] studied the impact of high serum UA level on mortality in PD patients by divided them into five groups according to their serum UA level. Patients with UA > 7.28 mg/dL had a higher all-cause mortality compared to those in the middle group. Furthermore, UA < 6.06 mg/dL was found to be a risk factor only in an unadjusted model. The other two studies divided patients into three groups according to their UA level. However, Lai et al. [19] reported high serum UA level to be a protective factor for mortality. Serum UA level reflects patients’ nutrition condition, and a low serum UA level might lead to inflammation. Considering a potential U-shaped association between UA and mortality, a proper range of serum UA and treatments to lower serum UA level should be determined to improve survival. However, these outcomes might not be feasible because there are only few studies reporting such indices. To confirm this association, more studies were required.
Importantly, the present meta-analysis is the first one to explore the risk factors for all-cause mortality in PD patients. This study had a large enough sample size and HR adjusted for possible confounders. There was a debate about different risk factors in previous studies, and this meta-analysis might be more reliable to predict all-cause and cardiovascular mortality in PD patients.
This study has several limitations. First, individual studies adjusted for the different potential confounders, which might result in a high heterogeneity. Second, the present meta-analysis might have missed some unpublished articles without conference abstracts in line with the selection criteria. Third, although we observed a significant heterogeneity in the impact of CVD on mortality across studies, we did not perform a subgroup analysis according to race. Moreover, we applied a random-effect model in this meta-analysis. Fourth, this meta-analysis was not registered in PROSPERO, which might lead to a bias. However, importantly, this meta-analysis was conducted in accordance with the standards of systematic review. Furthermore, more data regarding the respective risk factors are recommended.
In conclusion, the present meta-analysis revealed that a considerable number of risk factors displayed significant associations with an elevated risk of all-cause and cardiovascular mortality in PD patients. A proper threshold for serum magnesium, potassium, and UA should be determined to improve the survival in PD patients. Since data on several indices were limited, more studies are required to confirm the findings of this meta-analysis.
Supplementary Material
Funding Statement
This work was supported by National Natural Science Foundation of China [81670673] and Beijing Natural Science Foundation [7182060].
Author contributions
Han Li and Shixiang Wang conceived and designed the experiment; Jialing Zhang searched the literature and acquired the data primarily, while Xiangxue Lu secondarily; Han Li settled any inconsistencies between these two authors; Han Li and Jialing Zhang analyzed and interpreted the data; Han Li, Jialing Zhang and Xiangxue Lu wrote the paper; Han Li obtained the funding. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data of this study are available from the corresponding author.
References
- 1.Tian Y, Xie X, Xiang S, et al. . Risk factors and outcomes of early-onset peritonitis in Chinese peritoneal dialysis patients. Kidney Blood Press Res. 2017;42(6):1266–1276. [DOI] [PubMed] [Google Scholar]
- 2.Collins AJ, Foley RN, Chavers B, et al. . United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2012;59(1 Suppl 1):A7, e1–420. [DOI] [PubMed] [Google Scholar]
- 3.Foley RN, Murray AM, Li S, et al. . Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. JASN. 2005;16(2):489–495. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Lopez E, Carrero JJ, Suliman ME, et al. . Risk factors for cardiovascular disease in patients undergoing peritoneal dialysis. Perit Dial Int. 2007;27(2_suppl):205–209. [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Kopple JD.. Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. Am J Kidney Dis. 2001;38(6):1343–1350. [DOI] [PubMed] [Google Scholar]
- 6.Pifer TB, McCullough KP, Port FK, et al. . Mortality risk in hemodialysis patients and changes in nutritional indicators. DOPPS. Kidney Int. 2002;62(6):2238–2245. [DOI] [PubMed] [Google Scholar]
- 7.Lee MJ, Shin DH, Kim SJ, et al. . Progression of aortic arch calcification over 1 year is an independent predictor of mortality in incident peritoneal dialysis patients. PloS One. 2012;7(11):e48793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai L, Yu J, Yu J, et al. . Prognostic value of inflammation-based prognostic scores on outcome in patients undergoing continuous ambulatory peritoneal dialysis. BMC Nephrol. 2018;19(1):297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Soohoo M, Streja E, et al. . Serum magnesium levels and hospitalization and mortality in incident peritoneal dialysis patients: a cohort study. Am J Kidney Dis. 2016;68(4):619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai K, Luo Q, Dai Z, et al. . Hypomagnesemia is associated with increased mortality among peritoneal dialysis patients. PloS One. 2016;11(3):e0152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torlén K, Kalantar-Zadeh K, Molnar MZ, et al. . Serum potassium and cause-specific mortality in a large peritoneal dialysis cohort. CJASN. 2012;7(8):1272–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Q, Xu F, Fan L, et al. . Serum potassium levels and its variability in incident peritoneal dialysis patients: associations with mortality. PloS One. 2014;9(1):e86750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, et al. . Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, et al. . Measuring inconsistency in meta-analyses. BMJ (Clin Res ed). 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye H, Zhou Q, Fan L, et al. . The impact of peritoneal dialysis-related peritonitis on mortality in peritoneal dialysis patients. BMC Nephrol. 2017;18(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang S, Zhang X, Xie X, et al. . High serum uric acid level is a mortality risk factor in peritoneal dialysis patients: a retrospective cohort study. Nutr Metabol. 2019;16:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue Y, Xu B, Su C, et al. . Cardiorenal syndrome in incident peritoneal dialysis patients: what is its effect on patients’ outcomes? PLoS One. 2019;14(6):e0218082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang SD, Lee JH, Jhee JH, et al. . Impact of body mass index on survival in patients undergoing peritoneal dialysis: Analysis of data from the Insan Memorial End-Stage Renal Disease Registry of Korea (1985–2014). Kidney Res Clin Pract. 2019;38(2):239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai KJ, Kor CT, Hsieh YP.. An inverse relationship between hyperuricemia and mortality in patients undergoing continuous ambulatory peritoneal dialysis. JCM. 2018;7(11):416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung HY, Kim SH, Jang HM, et al. . Individualized prediction of mortality using multiple inflammatory markers in patients on dialysis. PloS One. 2018;13(3):e0193511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Zhu JG, Cheng BC, et al. . An association between time-varying serum alkaline phosphatase concentrations and mortality rate in patients undergoing peritoneal dialysis: a five-year cohort study. Sci Rep. 2017;7(1):43314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhee CM, Molnar MZ, Lau WL, et al. . Comparative mortality-predictability using alkaline phosphatase and parathyroid hormone in patients on peritoneal dialysis and hemodialysis. Perit Dial Int. 2014;34(7):732–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Guo Q, Feng X, et al. . Alkaline phosphatase and mortality in patients on peritoneal dialysis. CJASN. 2014;9(4):771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li W, Xiong L, Fan L, et al. . Association of baseline, longitudinal serum high-sensitive C-reactive protein and its change with mortality in peritoneal dialysis patients. BMC Nephrol. 2017;18(1):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S, Kang E, Yoo KD, et al. . Lower serum potassium associated with increased mortality in dialysis patients: a nationwide prospective observational cohort study in Korea. PloS One. 2017;12(3):e0171842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim YK, Kim SH, Kim HW, et al. . The association between body mass index and mortality on peritoneal dialysis: a prospective cohort study. Perit Dial Int. 2014;34(4):383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prasad N, Sinha A, Gupta A, et al. . Effect of body mass index on outcomes of peritoneal dialysis patients in India. Perit Dial Int. 2014;34(4):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian Y, Xie X, Xiang S, et al. . Risk factors and outcomes of high peritonitis rate in continuous ambulatory peritoneal dialysis patients: a retrospective study. Medicine. 2016;95(49):e5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng S, Wang Y, Qiu B, et al. . Impact of early-onset peritonitis on mortality and technique survival in peritoneal dialysis patients. SpringerPlus. 2016;5(1):1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Huang R, Wu H, et al. . Patient characteristics and risk factors of early and late death in incident peritoneal dialysis patients. Sci Rep. 2016;6(1):32359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu X, Yang X, Liu X, et al. . Patient survival and technique failure in continuous ambulatory peritoneal dialysis patients with prior stroke. Perit Dial Int. 2016;36(3):308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiong L, Cao S, Xu F, et al. . Association of body mass index and body mass index change with mortality in incident peritoneal dialysis patients. Nutrients. 2015;7(10):8444–8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong J, Han QF, Zhu TY, Ren YP, et al. . The associations of uric acid, cardiovascular and all-cause mortality in peritoneal dialysis patients. PloS One. 2014;9(1):e82342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang AY, Woo J, Lam CW, et al. . Is a single time point C-reactive protein predictive of outcome in peritoneal dialysis patients? Journal of the American Society of Nephrology: JASN. 2003;14(7):1871–1879. [DOI] [PubMed] [Google Scholar]
- 35.Guan JC, Bian W, Zhang XH, et al. . Influence of peritoneal transport characteristics on nutritional status and clinical outcome in Chinese diabetic nephropathy patients on peritoneal dialysis. Chinese Medical Journal. 2015;128(7):859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rumpsfeld M, McDonald SP, Johnson DW.. Higher peritoneal transport status is associated with higher mortality and technique failure in the Australian and New Zealand peritoneal dialysis patient populations. JASN. 2006;17(1):271–278. [DOI] [PubMed] [Google Scholar]
- 37.Shah S, Leonard AC, Thakar CV.. Functional status, pre-dialysis health and clinical outcomes among elderly dialysis patients. BMC Nephrol. 2018;19(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang ZK, Han QF, Zhu TY, et al. . The associations between the family education and mortality of patients on peritoneal dialysis. PloS One. 2014;9(5):e95894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang CW, Tzeng NS, Yin YJ, et al. . Angiotensin receptor blockers decrease the risk of major adverse cardiovascular events in patients with end-stage renal disease on maintenance dialysis: a nationwide matched-cohort study. PloS One. 2015;10(10):e0140633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duong U, Mehrotra R, Molnar MZ, et al. . Glycemic control and survival in peritoneal dialysis patients with diabetes mellitus. CJASN. 2011;6(5):1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abe M, Hamano T, Hoshino J.. Glycemic control and survival in peritoneal dialysis patients with diabetes: a 2-year nationwide cohort study. Sci Rep. 2019;9(1):3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, et al. . Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81(3):543–554. [DOI] [PubMed] [Google Scholar]
- 43.Kiran VR, Zhu TY, Yip T, et al. . Body mass index and mortality risk in Asian peritoneal dialysis patients in Hong Kong-impact of diabetes and cardiovascular disease status. Perit Dial Int. 2014;34(4):390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson DW, Mudge DW, Sturtevant JM, et al. . Predictors of decline of residual renal function in new peritoneal dialysis patients. Perit Dial Int. 2003;23(3):276–283. [PubMed] [Google Scholar]
- 45.Yoon JW, Gollapudi S, Pahl MV, et al. . Naive and central memory T-cell lymphopenia in end-stage renal disease. Kidney Int. 2006;70(2):371–376. [DOI] [PubMed] [Google Scholar]
- 46.Karbowska A, Boratynska M, Kusztal M, et al. . Hyperuricemia is a mediator of endothelial dysfunction and inflammation in renal allograft recipients. Transpl Proc. 2009;41(8):3052–3055. [DOI] [PubMed] [Google Scholar]
- 47.Park JT, Kim DK, Chang TI, et al. . Uric acid is associated with the rate of residual renal function decline in peritoneal dialysis patients. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association. Eur Renal Assoc. 2009;24(11):3520–3525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of this study are available from the corresponding author.