Abstract
The application of antiviral coatings to masks and respirators is a potential mitigating step toward reducing viral transmission during the SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) pandemic. The use of appropriate masks, social distancing, and vaccines is the immediate solution for limiting the viral spread and protecting people from this virus. N95 respirator masks are effective in filtering the virus particles, but they cannot kill or deactivate the virus. We report a possible approach to deactivating SARS-CoV-2 by applying an antimicrobial coating (Goldshield 75) to masks and respirators, rendering them suitable for repeated use. Masks coated with Goldshield 75 demonstrated continuous inactivation of the Alpha and Beta variants of the SARS-CoV-2 over a 3-day period and no loss of inactivation when stored at temperatures at 50 °C.
Keywords: Antiviral coatings, SARS-CoV-2 virus deactivation, Alpha and beta variants, N95 respirator masks, Toxicity
Graphical abstract
1. Introduction
The SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) pandemic has stimulated the need for personal protective equipment, specifically filtering face masks such as N95 masks, to be worn as part of a strategy that includes social distancing and vaccinations. Typically, N95 masks or respirators contain electrostatically charged melt-blown polypropylene fabrics embedded between top and bottom layers of spun-bond polypropylene (SP) materials. The charged fabrics filter virus effectively, and the large porosity in the fabrics enable easy breathing [1]. To ensure suitable performance of N95 respirator/mask which filters at least 95% of non-oil-containing ∼0.3 μm size particles as defined in the Code of Federal Regulations (CFR Title 42 Chapter I Subchapter G part 84 subpart K) [2] over their shelf life commercial N95 masks are overdesigned and initially filter ∼ 99.97% particles such that after any decay (up to 70 °C temperature, 85% humidity and 24 h of exposure time), the filters still meet or exceed the required 95% performance criteria [2,3]. Several types of masks, including N95 masks and cloth masks, have been demonstrated to limit the inhalation of the virus that causes the novel coronavirus disease (COVID-19); however, they cannot deactivate the virus and when contaminated presents as a potential fomite and source of infection [4,5]. The role that fomites play in the spread of the virus has been demonstrated in Syrian hamsters [6,7], and although the likelihood is thought to be low, fomites cannot be ruled out as a potential source of infection. Hence, there is a critical need to develop an antiviral coating that can be either sprayed on or embedded in masks for deactivating the virus.
Given the current SARS-CoV-2 pandemic and the use of face masks as a community and professional countermeasure against spreading the virus, there has been a growing interest in antimicrobial coatings for face masks [[10], [8], [9]]. Several approaches to applying antiviral coatings to face coverings have been demonstrated in the literature, including the use of metal ions such as Ag and Cu, along with other coatings, such as benzalkonium chloride, polymers, metal oxides, and functional nanomaterials [[11], [12], [13], [14], [15]]. The main issue with some of these coatings is that they can create serious consequences for the user and the surroundings [[16], [17], [18]]. It would be advantageous to develop an antiviral coating that is user-friendly as well as effective in deactivating the virus. The main goal of this research is to study GS75 (∼0.75% active ingredient) as a unique, nonleaching water-soluble antiviral spray that imparts residual antiviral activity to N95 masks, cotton cloth masks, N95 polypropylene fabrics/filters, and nonwoven SP fabric surfaces. Here, we have successfully demonstrated the use of an antiviral coating (Goldshield 75 [GS75]), on masks and mask materials that is effective for deactivating both Alpha and Beta variants of the SARS-CoV-2.
2. Methods
GS75 is based on the formulation of an organosilane water-stabilized quaternary ammonium chloride formulation in long alkyl chains consisting of a nonionic surfactant, a siloxane molecule that forms a nonpolar covalent bond between the surfaces of masks and filters and that will cross-link to the inert materials. GS75 contains a positively charged nitrogen atom, in a quaternary ammonium group that attracts microbes to the surfaces of masks or filters to which it has bonded and a long alkyl chain that dissolves in and disrupts the lipid layers that are typically on the surfaces of microorganisms and allows the cellular contents to leak out of the microbes, leading to their demise (Fig. 1 ). Thus, the antiviral functionality is an electrochemical action that is expected to provide durable, residual protection against SARS-CoV-2 and subsequent variants that arise.
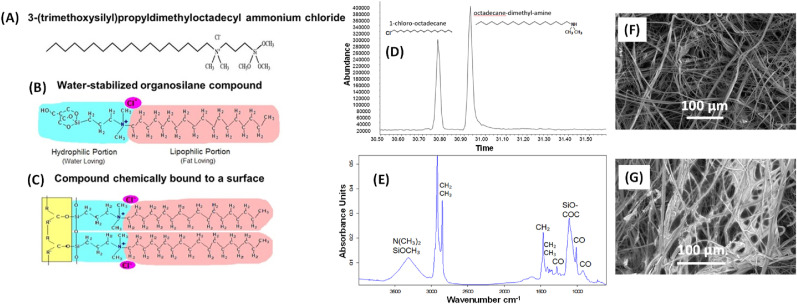
Fig. 1.
(A) Structure of (3-trihydroxysilyl)propyldimethyl octadecyl ammonium chloride, the active ingredient in Goldshield GS75, which was coated onto the N95 filters and masks to determine whether it would inactivate Alpha and Beta variants of the SARS-CoV-2. (B) Structure of water-stabilized organosilane compound. (C) Structure of the compound chemically bound to a surface. (D) The Gas Chromatography Mass Spectrometry (GCMS) profile of the GS75 solution confirming the presence of two fragmented peaks. (E) The Fourier Transform Infrared spectroscopy (FTIR) spectrum is consistent with a fatty quaternary ammonium structure, confirming the composition of GS75 [[1], [2], [3], [4], [5]]. The micrographs are SEM images of (F) uncoated and (G) GS-75-coated N95 polypropylene fabrics. See also Figs. S6 and S7. [1] G. Socrates, Infrared and Raman Characteristic Group Frequencies – Tables and Charts, 3rd Edition 2005. [2] Know-It-All Spectral Analysis Software, John C. Wiley and Sons, 2020. [3] Know-It-All Library Search, John C. Wiley and Sons, 2020. [4] C.J. Brinker and G.W Scherer, Sol-Gel Science, Academic Press, 1990 pp582-583. [5] R.K. Iler, The Chemistry of Silica, John C. Wiley and Sons, 1979, p639.
The antiviral water-stabilization technology was originally developed by Liebeskind and Allred at Emory University (Atlanta, Georgia) and was initially licensed to AP Goldshield LLC [[19], [20], [21]]. The scientists at AP Goldshield, Higgins and Shlisky, further advanced the chemistry by developing nonionic compounds that break down the interfacial tension on substrates, permitting the antimicrobial substance to disperse over the substrates more evenly, providing enhanced anti-viral activity [22]. The technology is currently registered with the US Environmental Protection Agency (85556–1 and 85556–2). Previous work has demonstrated that Goldshield products have bactericidal properties [[23], [24], [25]]. For example, Goldshield 5 used as a coating on masks had been shown to be an effective antibacterial agent against both gram-positive and gram-negative bacteria [26].
For use in this study, GS75 was used without any dilution. We obtained several types of test materials and tested their abilities to inactivate SARS-CoV-2 when coated with GS75. The coating was done by spraying the GS75 solution onto various masks and fabrics by a simple hand held plastic spray bottle with a nozzle on both sides. The coatings left the mask surfaces visibly damp and they were left to air dry. The spray coating followed by air drying was repeated twice for preparing double coated masks. Electrostatic and other types of spraying pumping methods will be developed for scale up. Materials include NIOSH-approved N95 masks, 100% cotton cloth masks, melt-blown filter media produced at the Carbon Fiber Technology Facility (CFTF) at Oak Ridge National Laboratory (ORNL) from Exxon polypropylene (Ex-FM), and SP materials obtained from an industry partner (Cummins). Filtration efficiency of N95 fabrics with double coated (99.6%) and uncoated (99.7%) were measured using a TSI automated filter tester 8130A standard testing media by penetration of the filter media by a sodium chloride aerosol particle generator. The typical airflow rate used during the test was 50 L per minute. It was determined that the coating does not affect the filtration efficiency of the N95 fabrics.
Test materials coated with GS75 were screened for the ability to inactivate SARS-CoV-2 in cell-based assays. After the virus was exposed to these materials for various lengths of time, the solution containing the virus was removed and introduced to Vero E6 cells. Viral activity was measured through the release of the cytosolic enzyme lactate dehydrogenase (LDH) into the culture media (CyQUANTTM LDH Cytotoxicity, ThermoFisher), which is an indicator of viral cytopathic effects (CPEs); high LDH levels would indicate little to no viral inactivation, and low LDH levels comparable to uninfected control would indicate viral inactivation. Where possible, uncoated test materials were used as controls. Presence of the virus and CPE was observed on a laser scanning confocal microscope following immunofluorescent staining of Vero E6 cells exposed to the virus. SARS-CoV-2 was visualized using an anti-SARS spike protein antibody; the Vero E6 cells were stained with the nuclear stain DAPI, and actin was stained with phalloidin. Two SARS-CoV-2 variants were used: (1) the USA-WA1/2020 (lineage A), which was isolated from a patient in Washington, USA, who had traveled to China, and (2) the hCoV-19/South African/KRISP-EC-K005321/2020 (lineage B.1.351), which was isolated from a patient in South Africa.
2.1. Viral stocks and cultures
SARS-CoV-2 was obtained through BEI Resources, NIAID, NIH: SARS-Related Coronavirus 2 lineage A, Isolate USA-WA1/2020, NR-52281 and SARS-Related Coronavirus 2 lineage B.1.351, Isolate hCoV-19/South Africa/KRISP-EC-K005321/2020, NR-54008, contributed by Alex Sigal and Tulio de Oliveira. Virus was propagated in confluent monolayers of Vero E6 cells (ATCC CRL-1586) cultured in Dulbecco's Modified Eagle Medium (DMEM) containing 5% FBS at 37 °C under 5% CO2. Following passage and viral amplification in Vero E6 cells aliquots were stored at −80 °C.
2.2. Anti-viral testing
Vero E6 cells were plated out in 96 well tissue culture plates at a cell density of 2.0 x 104 cells per well in a 100 μl volume the day prior to infection with SARS-CoV-2. Nine mm punches of the test fabrics were cut from sheets or masks and placed into the wells of a separate 96 or 48 well tissue culture plates; test fabrics included those coated with Goldshield and those not coated with Goldshield. Additionally, separate wells were coated with 10 μl of the Goldshield solutions and left to dry. Viral stocks of the USA-WA1/2020 variant were diluted in PBS to 1.0 x 106 PFU/ml and 100 μl of these viral solutions were pipetted onto the test materials in the 96 well plates or in the case of 48 well plates 250 μl of a 4.0 x 105 PFU/ml viral solution in PBS was used. For the experiments with the B.1.351 variant in 48 well tissue culture plates, 250 μl of a 2.0 x 104 PFU/ml solution in PBS was used. Less virions of the B.1.351 variant were used because of limited viral stocks available. Forty-eight well plates were used in later experiments to accommodate the thicker more rigid mask materials used. The virus solutions were left on the fabrics for various lengths of time out to 24 h in a 37 °C CO2 incubator. After interacting with the test materials, 10 μl of the USA-WA1/2020 virus solutions or 20 μl of the South African virus solutions were pipetted from the test material wells onto the Vero E6 cells in 96 well tissue culture plates; prior to adding the virus solutions to the Vero cells the old cell culture media was removed and replaced with 200 μl of fresh cell culture media. The entire portion of the virus inoculum was not carried over to the Vero cells from the test materials because of absorption of these solutions by the test materials. Controls included uninfected cells and mock infected cells with 10,000–4000 PFUs SARS-CoV-2 USA-WA1/2020 variant or in the case of the B.1.351 variant 400 PFUs; 10,000 PFUs was used during the earlier materials testing done in 96 well plates and 4000 PFUs with the later materials testing in 48 well plates with the USA-WA1/2020 virus. These PFUs represent the maximum PFUs that could be transferred over to the Vero cells if no inactivation of virus had occurred during the materials testing phase and would ensure prolific development of CPE based on previous work were as little as 50 PFUs resulted in complete CPE of Vero cell monolayers over a 3-day period (data not shown). Two hours after applying the virus, the media with virus was removed from the Vero E6 cells and the cells were washed once with PBS and then 200 μl of DMEM with 10% FBS and 100 units/ml penicillin and streptomycin was added. The Vero E6 cells were maintained at 37 °C and 5% CO2 for 3–4 days for viral cytopathic effects (CPE) to develop. When CPE was evident, viral cytotoxicity was measured through an LDH cytotoxicity assay following the manufacturer's recommended protocol (CyQUANTTM LDH Cytotoxicity Assay, Invitrogen). Viral activity was calculated using the formula: % viral cytotoxicity = [(test material LDH activity – uninfected control LDH activity)/(mock infected control LDH activity – uninfected control LDH activity)]∗100. Representative images were taken with a bright-field microscope.
Additional testing including high temperature storage mimicking, where the test materials were held at 50 °C for 48 h prior to exposing these materials to virus. With the remaining testing proceeded as described above. To test whether these materials could inactivate repeated applications of virus, test materials were exposed to virus for 24 h, and then this virus was removed and tested for inactivation. Additional virus was then placed onto these materials for another 24 h and tested for inactivation. This cycle of virus application and testing occurred three times. Testing of inactivation followed the above protocol using CyQUANTTM LDH Cytotoxicity Assay.
2.3. Microscopy
Vero E6 cells were plated at 100,000 cells per well in a 24 well plate containing coverslips and infected with virus incubated with each test material, as described for the anti-viral testing. After incubation, they were fixed in 4% PFA. Once fixed, they were washed twice with PBS and incubated in a blocking buffer of 10 μg/ml donkey gammaglobulin for an hour at room temperature. The target of the antibody used was a mouse monoclonal against the spike glycoprotein of the Urbani strain of SARS-CoV. Primary antibody staining was performed by diluting anti-SARs-CoV-2 (BEI) 1:200 in a buffer of BSA and saponin for an hour at room temperature and then washed three times in PBS. Secondary antibodies directed against primary host species (Jackson ImmunoResearch) were incubated with coverslips for an additional hour incubation at a 1:200 dilution in the BSA/saponin buffer. Polymerized actin was detected by staining with Alexa 660 fluorophore conjugated phalloidin (Invitrogen) at a 1/100 dilution. Nuclei were stained using 5 μg/ml DAPI. Secondary antibody and additional stains were then removed via washing with PBS three times, after which the coverslips were mounted to a slide using a mountant. Coverslips were imaged using inverted confocal Olympus Fluoview 2000 with 60× apochromatic objective with final image preparation with ImageJ (NIH Image).
2.4. Mammalian cell cytotoxicity
A549 cells were plated out in 96 well tissue culture plates at a cell density of 2.0 x 104 cells per well in a 100 μl volume the day prior to exposure to media incubated with the test materials. Nine mm punches of the test fabrics were cut from sheets or masks and placed into the wells of a separate 48 well tissue culture plates; test fabrics included those coated with Goldshield and those not coated with Goldshield. Additionally, separate wells were coated with 10 μl of the Goldshield solutions and left to dry. 500 μL of 2% FBS DMEM was added to each well with a test material and incubated overnight at 37 °C. The next day, the media on the cells was removed and replaced with media incubated with the test materials and incubated for 24 h at 37 °C. Cellular cytotoxicity was measured through an LDH cytotoxicity assay following the manufacturer's recommended protocol (CyQUANTTM LDH Cytotoxicity Assay, Invitrogen). Percent cytotoxicity was calculated by dividing the sample readout by the average of the readings for the maximum LDH output, and multiplying by 100.
2.5. Gas Chromatography – mass spectrometry (GCMS)
GCMS analysis was performed using an Agilent 6890 Gas Chromatograph equipped with 7673 Mass selective detector. The Goldshield sample was dissolved in methanol diluted 100 mg to 5 mL. The column was an Rxi-5MS from Restek. The temperature profile was 50 °C for 10 min followed by 10 °C per min ramp to the final 320 °C temperature and kept at that temperature for 30 min. The inlet temperature was 340 °C, the MS transfer line was 320 °C. The flow was held at 1 ml per min with a 10/1 split ratio. The injection volume was 1 μl. The GCMS data for the Goldshield solution is reported in Fig. 1d. Since the Goldshield is a salt and not volatile, it will not chromatograph as a single peak. Only couple of fragment peaks are visible. The Fig. 1d chromatographic profile shows two fragmented peaks that are consistent with the known structure (Fig. 1).
2.6. Fourier Transform Infrared (FTIR) spectroscopy
FTIR was collected over the range 4000 to 600 cm−1 to confirm the composition of the Goldshield solution. The measurements were conducted on a Bruker Tensor 27 instrument equipped with a Hyperion 1000 microscope. The sample was smeared onto a low E (emissivity) microscope slide and allowed to evaporate to dryness. A thin film was leveled out until a good signal, generally 0 to 1 absorbance unit could be collected. A reflectance spectrum in absorbance was obtained on the instrument. The FTIR spectra for the Goldshield solution is shown in Fig. 1e.
2.7. Inductively coupled plasma optical emission spectroscopy (ICP-OES)
ICP-OES analysis was performed on the Goldshield solution and it confirmed the presence of 1 mol of silicon (Si) present per mole of Goldshield solution.
2.8. Scanning Electron Microscopy (SEM)
SEM was used to determine the microstructure of both uncoated and GS75 coated N-95 polypropylene fabrics and the images were obtained in a Carl Zeiss Merlin instrument operating at 1 kV. Energy Dispersive X-Ray Spectroscopy (EDS) elemental mapping images have been acquired for both samples with a system from Bruker Nano Gmbh using a XFlash detector 5030.
3. Results and discussion
Initial testing of the SP (Cummins) material that makes up the outer material of N95 masks with the USA-WA1/2020 virus showed that the material on its own in the absence of GS75 resulted in prolific Vero E6 cell CPEs, indicating that material has little impact on inactivating this virus (Fig. S1). In the case of SP material coated with the GS75 product, it was observed that a double coating resulted in better virus inactivation. With a double GS75 coating, a complete lack of Vero E6 cell CPEs was observed after a 6 h exposure of the virus to the material, indicating complete inactivation of the applied virus. In contrast, the virus needed a 12 h exposure to the single-coated material for complete inactivation and resultant absence of Vero E6 cell CPEs.
The GS75 product in the absence of material coating as a control step indicated viral inactivation with 6 h of exposure to the virus. A black cotton cloth mask with a single GS75 coating resulted in incomplete viral inactivation in 2 h of exposure and complete viral inactivation in 6 h (An uncoated mask was not tested in conjunction with these tests.)
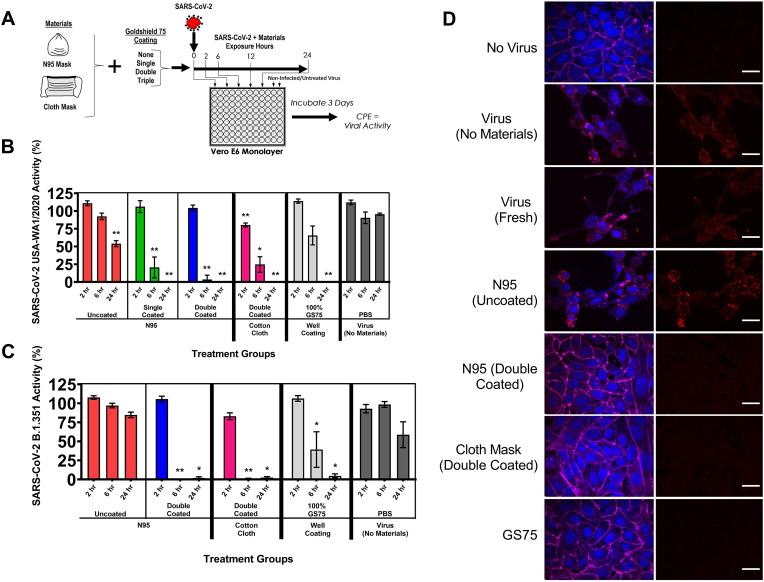
Further testing with the USA-WA1/2020 virus, this time using N95 masks coated with the GS75 product, indicated viral inactivation (Fig. 2 B). An uncoated N95 mask showed a significant decrease in viral CPEs following 24 h of exposure to the virus; the coated N95 masks (single- and double-coated) resulted in complete inhibition of viral CPEs following 24 h of exposure to the virus. Both masks also showed significant reductions in viral CPEs following 6 h of exposure to the virus. When applied to a double-coated black cloth mask, GS75 was observed to completely inactivate the virus with 24 h of exposure to the virus, and lesser degrees of viral inactivation with 2 and 6 h of exposure to the virus. Immunofluorescent-stained cover slips with Vero E6 cells having been exposed to the virus showed the absence of the virus when GS75-coated materials after exposure times of 6 and 24 h; virus was observed in all treatments with a 2 h exposure (Fig. S2). Ample virus was visualized when the materials used lacked GS75 coating. We observed similar results when this testing technique was applied to the B.1.351 lineage virus (Fig. 2C). The uncoated N95 mask resulted in a viral CPE consistent with the virus control in Vero E6 cells. Complete inactivation of the virus was observed with 6 and 12 h of exposure to the double-coated N95 mask. We observed similar results with a double-coated cloth mask. Immunofluorescent staining showed an absence of virus within the Vero E6 cells when GS75-coated materials were used to inactivate the virus with a 24 h exposure time (Fig. 2D).
Fig. 2.
Inactivation of SARS-CoV-2 by GS75 coated materials. (A) Schematic of experimental plan for combinations of materials tested, in which the materials are exposed to the virus and subsequently given an antiviral assessment using Vero E6 tissue culture cells. Masks and materials, both uncoated and coated with Goldshield GS75, were exposed to the virus in phosphate buffered saline (PBS) for various durations and were tested for inactivation by infecting Vero E6 cells and measuring viral cytopathic effects via LDH release into the cell culture media. Viral activity was calculated using the formula % viral cytotoxicity = [(test material LDH activity – uninfected control LDH activity)/(mock infected control LDH activity – uninfected control LDH activity)]∗100. (B) Inactivation of SARS-CoV-2 USA/WA1 strain by various materials and GS75 coating combinations. (C) Inactivation of SARS-CoV-2 (South African/KRISP-EC-K005321/2020) by various materials and GS75 coating combinations. Values represent the means ± the standard error of mean. One-way analysis of variance with Dunnett's comparison of means between test materials and virus (no materials). Statistical significance from untreated viral samples is indicated. (∗P < 0.01, ∗∗P < 0.001). (D) Fluorescence confocal laser scanning microscopy images of Vero E6 cells exposed to SARS-CoV-2 (South African/KRISP-EC-K005321/2020) following a 24 h inactivation period by materials coated with GS75. Blue: DAPI-tained nuclei. Magenta: Phalloidin-stained actin. Red: anti-SARS-CoV-2 antibody. Scale represents 20 μm.
The Exxon material used as the internal filter of N95 masks showed antiviral activity when coated with GS75 (Fig. S3). Uncoated Exxon material, like the uncoated N95 material, resulted in substantial viral CPEs in Vero E6 cells regardless of exposure time. We observed a significant decrease in Vero E6 cell CPEs with 6 h of viral exposure and an absence of CPEs after 24 h of exposure to Exxon material that was triple-coated with the GS75 product. A 2-h exposure of the USA-WA1/2020 virus to double coated Exxon material resulted in a 2.74 TCID50 log reduction, which corresponds to a 99.8% inactivation of this virus; however, no further reduction in viral inactivation was noted with a 6-h exposure to the treated materials (S4).
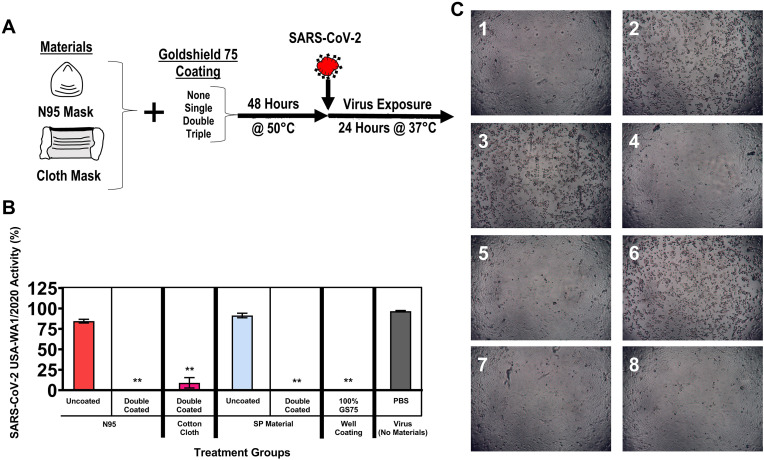
The same methods were used to test the ability of the GS75 coating to retain its antiviral activity following high-temperature storage (which could occur during shipping). The materials were held at 50 °C for 48 h and then tested for the ability to inactivate SARS-CoV-2 (USA-WA1/2020) (Fig. 3 ). The double-coated N95 mask, cloth mask, and SP material were all observed to retain antiviral activity following the high-temperature storage. LDH cytotoxicity results showed levels comparable to the uninfected control for the double-coated N95 mask, the double-coated cloth mask, the double-coated SP material, and GS75 well coating (Fig. 3B). Microscopic observation showed CPEs only in the materials lacking GS75 coating (Fig. 3C).
Fig. 3.
Pretreatment of coated materials at elevated temperature. (A) Experimental design schematic for evaluating stability of materials held at 50 °C for 48 h prior to use. Materials were cooled and exposed to SARS-CoV-2 for 24 h at 37 °C. (B) Viral inactivation was quantified by infecting Vero E6 cells and measuring viral cytopathic effects via LDH release into the cell culture media. Viral activity was calculated using the formula % viral cytotoxicity = [(test material LDH activity – uninfected control LDH activity)/(mock infected control LDH activity – uninfected control LDH activity)]∗100. Values represent the means ± the SEM. One-way analysis of variance with Dunnett's comparison of means between test materials and virus (no materials), ∗∗p < 0.001. (C) Representative brightfield microscopy images show the presence or absence of viral CPEs. 1: No virus. 2: Virus (maintained in PBS for 24 h). 3: N95 (uncoated). 4: N95 (double-coated with GS75). 5: Cloth mask (double-coated with GS75). 6: SP material (uncoated). 7: SP material (double-coated with GS75). 8: GS75 coated well (10 μl).
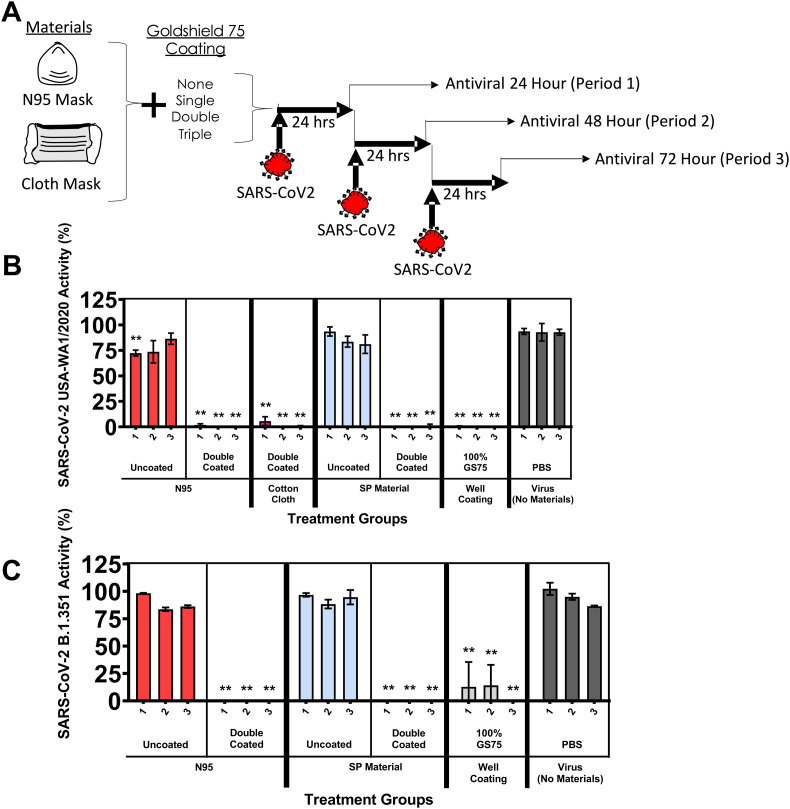
Having a material that can inactivate SARS-CoV-2 continuously would be highly desirable. To test these materials under repeated conditions, fresh virus (USA-WA1/2020) was added to the materials every 24 h, and Vero E6 cells and the CyQUANT LDH assay were used to test for inactivation (Fig. 4 A). As expected, the uncoated N95 mask and SP material had a minimal impact of viral activity when tested on the Vero E6 cells. An N95 mask, a cotton cloth mask, and SP material were double-coated with GS75 and exposed to the USA-WA1/2020 virus were observed to inactivate all virus cycled over 3 days. We observed similar results when we repeated the experiment with the B.1.351 lineage virus (Fig. 4B). The double-coated N95 mask and double-coated SP material were observed to inactivate the virus over three consecutive applications.
Fig. 4.
(A) Schematic of repeated exposure of coated masks and materials to fresh virus and subsequent antiviral testing. Repeated use of materials against continued exposure to fresh virus were evaluated for CPE induction with Vero E6 cells and measuring viral cytopathic effects via LDH release into the cell culture media. Material was exposed to fresh virus in 24 h cycles. Viral activity was calculated using the formula % viral cytotoxicity = [(test material LDH activity – uninfected control LDH activity)/(mock infected control LDH activity – uninfected control LDH activity)]∗100. (B) SARS-CoV-2 (USA-WA1/2020). (C) SARS-CoV-2 (hCoV-19/South African/KRISP-EC-K005321/2020). Values represent the means ± the SEM. One-way analysis of variance with Dunnett's comparison of means between test materials and virus (no materials), ∗∗P ≤ 0.001.
Safety is always a concern when a chemical may come in contact with skin. To test the safety of the GS75 product, test materials were incubated in cell culture media for 24 h, and then the media were applied to A549 cells, and cytotoxicity was measured with the CyQUANT LDH cytotoxicity assay (Fig. S5). SEM images of both uncoated and double coated N95 polypropylene fabrics with EDS elemental mapping data is presented in Figs. S6 and S7. Materials tested for cytotoxicity in mammalian cell lines were the uncoated and double-coated N95 masks, a double-coated cotton cloth mask, uncoated and double-coated SP material, and the GS75 solution. The only one found to elicit cytotoxicity was the double-coated cloth mask. Unfortunately, an uncoated cloth mask was not tested in parallel, but it would seem from the results that it would be a material or chemical unique to the cloth mask that is causing this cytotoxicity and not the GS75 coating because none of the other materials coated with GS75 elicited a cytotoxic response.
4. Conclusion
In this study, we have demonstrated that GS75 coating on masks made from a variety of materials can safely inactivate SARS-CoV-2. The GS75 coating maintains its antiviral properties when stored at 50 °C, and the coated masks will continuously inactivate virus over a 3-day period. These results show that using GS75 to coat masks, as well as potentially other fabrics/materials, adds to our arsenal in combating the SARS-CoV-2 pandemic.
Notice
This manuscript has been authored in part by UT-Battelle, LLC, under contract DE-AC05-00OR22725 with the US Department of Energy (DOE). The US government retains and the publisher, by accepting the article for publication, acknowledges that the US government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for US government purposes. DOE will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
Data availability
The datasets generated during and/or analyzed during this study are available from the corresponding author on request.
Credit author statement
M.P.P, M.T., A.M.L., R.J.L., and B.H.B. designed the study. N.P.B., K.R.L., and K.S.P. performed the experiments. T.S., D.K.H., and A.L.M. performed the analysis. M.P.P., N.P.B., M.T., A.M.L., R.J.L., and B.H.B. analyzed the data. M.P.P., N.P.B., and B.H.B. wrote the initial draft, and all authors edited the final version.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by the DOE Office of Science through the National Virtual Biotechnology Laboratory (NVBL), a consortium of DOE national laboratories focused on response to COVID-19, with funding provided by the Coronavirus CARES Act and Office of Energy Efficiency and Renewable Energy, Advanced Manufacturing Office. Scanning Electron Microscopy (SEM) microstructural characterizations and Fourier Transform Infrared spectroscopy ( FTIR) measurements were conducted at the Center for Nanophase Materials Sciences, which is a US Department of Energy Office of Science User Facility. Part of the coating research (MPP) was supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, Materials Sciences and Engineering Division. We thank Mr. Thomas Higgins, CEO at Goldshield Technologies for providing Goldshield GS75 solution. We thank Cummins for providing materials and help with testing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mtadv.2022.100228.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hossain E., et al. Recharging and rejuvenation of decontaminated N95 masks. Phys. Fluids. 2020;32 doi: 10.1063/5.0023940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larsen G.S., et al. Polymer, additives, and processing effects on N95 filter performance. ACS Appl. Polym. Mater. 2021;3:1022–1031. doi: 10.1021/acsapm.0c01294. [DOI] [PubMed] [Google Scholar]
- 3.Wang P.L., et al. Recent developments in filtration media and respirator technology in response to COVID-19. MRS Bull. 2021;1–10 doi: 10.1557/s43577-021-00173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueki H., et al. Effectiveness of face masks in preventing airborne transmission of SARS-CoV-2. mSphere. 2020;5 doi: 10.1128/mSphere.00637-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasloff S.B., Leung A., Strong J.E., Funk D., Cutts T. Stability of SARS-CoV-2 on critical personal protective equipment. Sci. Rep. 2021;11:984. doi: 10.1038/s41598-020-80098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Port J.R., et al. SARS-CoV-2 disease severity and transmission efficiency is increased for airborne compared to fomite exposure in Syrian hamsters. Nat. Commun. 2021;12:4985. doi: 10.1038/s41467-021-25156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onakpoya I.J., et al. SARS-CoV-2 and the role of fomite transmission: a systematic review. F1000Res. 2021;10:233. doi: 10.12688/f1000research.51590.1. 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stokes K., et al. Enhanced medical and community face masks with antimicrobial properties: a systematic review. J. Clin. Med. 2021;10 doi: 10.3390/jcm10184066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pullangott G., Kannan U., Kiran G.S.D.V., Maliyekkal S.M. A comprehensive review on antimicrobial face masks: an emerging weapon in fighting pandemics. RSC Adv. 2021;11:6544–6576. doi: 10.1039/d0ra10009a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zorko D.J., et al. Decontamination interventions for the reuse of surgical mask personal protective equipment: a systematic review. J. Hosp. Infect. 2020;106:283–294. doi: 10.1016/j.jhin.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung S., et al. Copper-coated polypropylene filter face mask with SARS-CoV-2 antiviral ability. Polymers. 2021;13 doi: 10.3390/polym13091367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martí M., et al. Protective face mask filter capable of inactivating SARS-CoV-2, and methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis. Polymers. 2021;13 doi: 10.3390/polym13020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balagna C., Perero S., Percivalle E., Nepita E.V., Ferraris M. Virucidal effect against coronavirus SARS-CoV-2 of a silver nanocluster/silica composite sputtered coating. Open Ceramics. 2020;1 [Google Scholar]
- 14.Zhou J., Hu Z., Zabihi F., Chen Z., Zhu M. Progress and perspective of antiviral protective material. Adv. Fiber Mater. 2020;2:123–139. doi: 10.1007/s42765-020-00047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pemmada R., et al. Science-based strategies of antiviral coatings with viricidal properties for the COVID-19 like pandemics. Materials. 2020;13:4041. doi: 10.3390/ma13184041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bamal D., et al. Silver nanoparticles biosynthesis, characterization, antimicrobial activities, applications, cytotoxicity and safety issues: an updated review. Nanomaterials. 2021;11 doi: 10.3390/nano11082086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ameh T., Sayes C.M. The potential exposure and hazards of copper nanoparticles: a review. Environ. Toxicol. Pharmacol. 2019;71:103220. doi: 10.1016/j.etap.2019.103220. [DOI] [PubMed] [Google Scholar]
- 18.Lai X., et al. Intranasal delivery of copper oxide nanoparticles induces pulmonary toxicity and fibrosis in C57bl/6 mice. Sci. Rep. 2018;8:4499. doi: 10.1038/s41598-018-22556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liebeskind L.S., Allred G.D. U.S. Patent and Trade Office; 2003. Methods for Using Water-Stabalized Organosilanes. (U.S. Patent No. 6,632,805) [Google Scholar]
- 20.Liebeskind L.S., Allred G.D. U.S. Patent and Trademark Office.; 2001. Water-stabilized Organosilane Compounds and Methods for Using the Same. (U.S. Patent No. 6,221,944) [Google Scholar]
- 21.Liebeskind L.S., Allred G.D. U.S. Patent and Trademark Office; 1999. Water-stabilized Organosilane Compounds and Methods for Using the Same. (U.S. Patent No. 5,959,014) [Google Scholar]
- 22.Higgins T.L., Shlisky T. U.S. Patent and Trade Office.; 2015. Organosilane-nonionic Water Stable Quaternary Ammonium Compositions and Methods. (U.S. Patent No. 9,089,138) [Google Scholar]
- 23.Baxa D., et al. In vitro evaluation of a novel process for reducing bacterial contamination of environmental surfaces. Am. J. Infect. Control. 2011;39:483–487. doi: 10.1016/j.ajic.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Perez V., Mena K.D., Watson H.N., Prater R.B., McIntyre J.L. Evaluation and quantitative microbial risk assessment of a unique antimicrobial agent for hospital surface treatment. Am. J. Infect. Control. 2015;43:1201–1207. doi: 10.1016/j.ajic.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Murray J., et al. Evaluation of bactericidal and anti-biofilm properties of a novel surface-active organosilane biocide against healthcare associated pathogens and Pseudomonas aeruginosa biolfilm. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tseng C.-C., Pan Z.-M., Chang C.-H. Application of a quaternary ammonium agent on surgical face masks before use for pre-decontamination of nosocomial infection-related bioaerosols. Aerosol. Sci. Technol. 2016;50:199–210. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during this study are available from the corresponding author on request.