Abstract
Coronavirus disease 2019 (COVID-19) is an acute respiratory infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and it has become a public health concern worldwide. In addition to respiratory symptoms, some COVID‑19 patients also show various gastrointestinal symptoms and even consider gastrointestinal symptoms to be the first manifestation. A large amount of evidence has shown that SARS-CoV-2 infection could disrupt the gut microbiota balance, and disorders of the gut microbiota could aggravate the condition of COVID-19 patients. Therefore, maintaining the gut microbiota balance is expected to become a potential new therapeutic target for treating COVID-19. Traditional Chinese medicine (TCM) has significant effects in all stages of the prevention and treatment of COVID-19. It can adjust the gut microbiota and is an ideal intestinal microecological regulator. This review summarizes the advantages and clinical efficacy of TCM in the treatment of COVID-19 and expounds on the relationship between TCM and the gut microbiota, the relationship between COVID-19 and the gut microbiota, the mechanism of gut microbiota disorders induced by SARS-CoV-2, the relationship between cytokine storms and the gut microbiota, and the role and mechanism of TCM in preventing and treating COVID-19 by regulating the gut microbiota to provide new research ideas for TCM in the prevention and treatment of COVID-19.
Keywords: COVID-19, Traditional Chinese medicine, Gut microbiota, SARS-CoV-2, ACE2, Cytokine storm
Graphical Abstract

1. Introduction
In late December 2019, several health institutions reported patients with unexplained pneumonia related to epidemiology in Wuhan, Hubei Province, China. On January 7, 2020, Chinese scientists isolated the pathogenic coronavirus for the first time from respiratory tract samples of patients in Wuhan and sequenced the full length of the genome [1]. On February 11, the International Committee on Taxonomy of Viruses (ICTV) and the World Health Organization (WHO) named the virus and the disease that it caused severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease 2019 (COVID-19), respectively [2], [3]. COVID-19 is extremely harmful, with the characteristics of rapid transmission, strong pathogenicity, and poor prognosis. On March 12, the WHO declared that the outbreak of COVID-19 constituted a global "pandemic" [4]. Because of the lack of drugs targeting SARS-CoV-2 specifically, as of February 26, 2022, the cumulative number of COVID-19 cases globally has surpassed 433 million in 216 countries, areas, and territories and more than 5.96 million confirmed deaths [5]. Although significant progress has been made in clinical and vaccine research, many countries are still experiencing or have experienced a second or third wave of the COVID-19 pandemic, mainly due to mutations of SARS-CoV-2 [6], [7].
The initial symptoms of COVID-19 patients are mainly characterized by fever, fatigue, and a dry cough. Clinical studies have found that SARS-CoV-2 can also cause digestive symptoms, such as diarrhoea, abdominal pain, nausea, and vomiting [8], [9], [10], [11]. Clinical data have shown that a considerable proportion of COVID-19 patients have symptoms related to intestinal flora disturbance, indicating that the SARS-CoV-2 virus could cause the problem of intestinal microecological imbalance, especially in critically ill patients with poor prognoses [11], [12]. Compared with non-COVID-19 patients, the composition of the gut microbiota of COVID-19 patients has changed significantly, regardless of whether the patients have received medication [12], [13]. Therefore, there could be a close relationship between the intestinal flora and SARS-CoV-2 infection [14]. The diagnosis and treatment of COVID-19 (8th trial edition) [15] clearly mentions that "intestinal microecological regulators could be used to maintain intestinal microecological balance and prevent secondary infection". Studies have shown that traditional Chinese medicine (TCM) is an effective microecological regulator that has the functions of adjusting imbalances in the intestinal flora, promoting the growth of beneficial bacteria, and inhibiting the excessive reproduction of harmful bacteria. In the prevention and control of this epidemic, TCM has been deeply involved in the whole process of prevention, treatment, and rehabilitation, complementing and cooperating with Western medicine, making important contributions and playing a unique role in the comprehensive control of the COVID-19 epidemic [16]. We performed the comprehensive retrieval of electronic databases in both Chinese and English (the China National Knowledge Infrastructure Database(CNKI), VIP Chinese Science and Technology Journal Database(VIP), WangFang Database, Chinese BioMedical Literature Database (CBM), PubMed and Embase) for collecting the published research from inception to December 10, 2021. Eventually, we summarized 69 representative clinical trials, including 19 randomized, controlled trials (RCTs), 24 retrospective cohort studies (RCSs), 10 case-control studies (CCSs), and 16 others. This review mainly summarizes the clinical efficacy of TCM in the prevention and treatment of COVID-19; the relationship between the gut microbiota and TCM; COVID-19, SARS-CoV-2, and inflammatory storms; and how TCM regulates the gut microbiota to prevent and treat COVID-19 to provide new strategies and new targets for the prevention and treatment of COVID-19.
2. The advantages of TCM for COVID-19
COVID-19 has the characteristics of rapid onset, strong infectivity, and easy spread. It belongs to the category of “dampness toxin epidemics” in TCM, and the main nature of the disease is as a dampness toxin. According to the theory of TCM, the disease is mainly located in the lungs and spleen. The basic pathogenesis is “dampness, toxin, heat, phlegm, blood stasis and deficiency” [17], [18], [19], [20]. Early clinical manifestations of the disease can include fever or no fever, and fever is often accompanied by multiple symptoms of hiding fever, dry coughing, fatigue, vomiting, loose stools, diarrhoea, and other digestive symptoms. The tongue coating is generally greasy and has obvious dampness toxicity from the disease [21]. In the whole clinical process, attention must be paid to eliminating dampness, resolving turbidity, eliminating filth and removing toxicity, as well as the effects of blood stasis [19]. TCM has been used to treat epidemic diseases since ancient times and has accumulated abundant successful experience in the prevention and treatment of epidemic diseases for thousands of years [22], [23], [24]. The advantage of TCM is that, even if the cause of the disease is unknown, a set of corresponding prescriptions can be proposed based on the clinical symptoms under the guidance of the theory of syndrome differentiation and treatment, which can relieve clinical symptoms, shorten the course of the disease, and prevent deterioration due to the disease [25], [26].
3. Clinical efficacy of TCM in the prevention and treatment of COVID-19
In the prevention and treatment of COVID-19, TCM is an irreplaceable main force [27]. Zhang et al. [28] reported that early treatment with TCM could reduce the inflammatory response, regulate immune function, promote the absorption of pulmonary inflammation, and reduce mortality in patients with severe COVID-19. The integration of traditional Chinese and Western medicine had a good effect on COVID-19, and it could improve the rate of alleviation of fever, coughing, expectoration, fatigue, chest tightness, and anorexia; shorten the duration of fever and coughing; shorten the hospital stay; reduce the severity of illness; promote the absorption of pulmonary inflammation and nucleic acid negative conversion; and improve the overall response rate and cure rate [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43].
We independently searched the clinical trials of TCM treating COVID-19 in the relevant databases, including the CNKI, VIP, Wanfang Database, CMB, PubMed, and Embase. The search time was from inception to December 10, 2021. On the one hand, the following terms for COVID-19 were adopted: “novel coronavirus pneumonia”, “new coronary pneumonia”, “NCP”, “New Coronavirus”, “2019 novel coronavirus”, “COVID-19”, “2019-nCoV”, “coronavirus disease 2019”, “coronavirus disease-19”, “severe acute respiratory syndrome coronavirus 2”, and “SARS-CoV-2”. On the other hand, the search terms for TCM mainly included “traditional Chinese medicine”, “Chinese medicine”, “Chinese and Western medicine”, and “prescription”. A total of 69 representative clinical trials, including 19 randomized, controlled trials (RCTs), 24 retrospective cohort studies (RCSs), 10 case-control studies (CCSs), and 16 others, were completed and summarized [38], [44], [45], [46], [47], [48], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [95], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [114], [115], [116]. According to the available clinical data, there have been many clinical trials on the clinical advantages of TCM in the prevention and treatment of COVID-19, including suspected stages, mild stages, moderate stages, severe stages, critical stages, and convalescent stages, as shown in Fig. 1. The clinical evidence for TCM for COVID-19 is summarized in Table 1. In addition, the relationship of TCM with its constituents is depicted in Fig. 2.
Fig. 1.
Clinical advantages of TCM in the prevention and treatment of the COVID-19.
Table 1.
The clinical evidence of TCM for COVID-19.
| NO | Name of traditional Chinese medicine | Compositions | Type of study | Location of study | Intervention | Control | Severity classification | Treatment duration | Main findings | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | BuZhong Yiqi decoction (BZYQ) | Huangqi (Astragali Radix), Renshen (Ginseng Radix Et Rhizoma), Gancao (Glycyrrhizae Radix Et Rhizoma), Baizhu (Atractylodis Macrocephalae Rhizoma), Chenpi (Citri Reticulatae Pericarpium), Danggui (Angelicae Sinensis Radix), Shengma (Cimicifugae Rhizoma), Chaihu (Bupleuri Radix) | Case-control studies(CCS) | Hubei 672 Orthopedic Hospital of Integrated Traditional Chinese and Western Medicine | BZYQ+C(n=36) | WMST (n=35) | Mild cases | 10d |
|
[75] |
| 2 | Chaihu Ganlu decoction (CHGL) | Chaihu (Bupleuri Radix), Huangqin (Scutellariae Radix), Shichangpu (Acori Tatarinowii Rhizoma), Guang Huoxiang (Pogostemonis Herba), Doukou (Amomi Fructus Rotundus), Lianqiao (Forsythiae Fructus), Nanshashen (Adenophorae Radix), Yiyiren (Coicis Semen), Houpo (Magnoliae Officinalis Cortex), Fuling (Poria), Banxia (Pinelliae Rhizoma), Chenpi (Citri Reticulatae Pericarpium), Chantui (Cicadae Periostracum), Jiangcan (Bombyx Batryticatus), Kuxingren (Armeniacae Seman Amarum), Jiegeng (Platycodonis Radix), Maiya (Hordei Fructus Germinatus) | Before and after comparison | People’s Hospital of Wanzhou District of Chongqing | CHGL+WMST(n=46) | Asymptomatic cases | 5-22d |
|
[47] | |
| 3 | Danggui Shaoyao power (DGSY) | Danggui (Angelicae Sinensis Radix), Baishao (Paeoniae Radix Alba), Chuanxiong (Chuanxiong Rhizoma), Baizhu (Atractylodis Macrocephalae Rhizoma), Zexie (Alismatis Rhizoma), Fuling (Poria) | Before and after comparison | Wuhan Union Hospital | DGSY+WMST(n=100) | Severe cases | 10d |
|
[92] | |
| 4 | Ganlu Xiaodu decoction (GLXD) | Huashi (Talcum), Yinchen (Artemisiae Scopariae Herba), Huangqin (Scutellariae Radix), Shichangpu (Acori Tatarinowii Rhizoma), Mutong (Akebiae Caulis), Chuanbeimu (Fritillariae Cirrhosae Bulbus), Guanghuoxiang (Pogostemonis Herba), Doukou (Amomi Fructus Rotundus), Lianqiao (Forsythiae Fructus), Bohe (Menthae aplocalycis Herba), Shegan (Belamcandae Rhizoma), Shanyao (Dioscoreae Rhizoma), Gancao (Glycyrrhizae Radix Et Rhizoma) | Retrospective cohort studies(RCS) | Wuhan Third Hospital | GLXD+C(n=115) | WMST (n=115) | Moderate cases | 7-14d |
|
[63] |
| 5 | Gegen Qinlian pill (GGQL) | Huangqin (Scutellariae Radix), Huanglian (Coptidis Rhizoma), Gegen (Puerariae Lobatae Radix), Gancao (Glycyrrhizae Radix Et Rhizoma) | CCS | Hubei Provincial Hospital of traditional Chinese medicine | GGQL+C(n=58) | WMST(n=60) | All cases | 7-14d |
|
[106] |
| 6 | Huashi Baidu granule (HSBD) | Mahuang (Ehedraep Herba), Kuxingren (Armeniacae Seman Amarum), Shigao (Gypsum Fibrosum), Gancao (Glycyrrhizae Radix Et Rhizoma), Guanghuoxiang (Pogostemonis Herba), Houpo (Magnoliae Officinalis Cortex), Cangzhu (Atractylodis Rhizoma), Caoguo (Tsaoko Fructus), Banxia (Pinelliae Rhizoma), Fuling (Poria), Dahuang (Rhei Radix Et Rhizoma), Huangqi (Astragali Radix), Tinglizi (Descurainiae Semen Lepidii Semen), Chishao (Paeoniae Radix Rubra) | RCS | Wuhan Jinyintan Hospital | HSBD+C(n=23) | WMST(n=32) | Severe cases | 16d |
|
[90] |
| Randomized controlled trials(RCT) | Wuhan Jinyintan Hospital | HSBD+C(n=99) | WMST(n=96) | All cases | 14d |
|
[103] | |||
| CCS | TheThird People's Hospital of Yichanng | HSBD+C(n=25) | WMST(n=25) | Severe cases | 15d |
|
[88] | |||
| 7 | HuoXiang Zhengqi power (HXZQ) | Guanghuoxiang (Pogostemonis Herba), Cangzhu (Atractylodis Rhizoma), Chenpi (Citri Reticulatae Pericarpium), Fabanxia (Pinelliae Rhizoma Praeparatum), Fuling (Poria), Houpo (Magnoliae Officinalis Cortex), Jiegeng (Platycodonis Radix), Gancao (Glycyrrrhizae Radix Et Rhizoma) | Before and after comparison | The Red Cross Hospital of Yulin | HXZQ+WMST(n=11) | Moderate cases | 9-33d |
|
[60] | |
| 8 | Jiawei Yupingfeng powder (YPF) | Huangqi (Astragali Radix), Baizhu (Atractylodis acrocephalae Rhizoma), Fangfeng (Saposhnikoviae Radix), Fuling (Poria), Cangzhu (Atractylodis Rhizoma), Guanghuoxiang (Pogostemonis Herba), Zisugeng (Perillae Caulis), Banxia (Pinelliae Rhizoma), Sharen (Amomi Fructus), Shengjiang (Zingiberis Rhizoma Recens) | RCS | TheThird People's Hospital of Jiujiang | YPF+C(n=30) | WMST(n=24) | Mild or Moderate cases | 14d |
|
[73] |
| 9 | Jinbei oral liquid (JBOL) | Huangqi (Astragali Radix), Dangshen (Codonopsis Radix), Beishashen (Glehniae Radix), Danshen (Salviae Miltiorrhizae Radix Et Rhizoma), Danggui (Angelicae Sinensis Radix), Chuanxiong (Chuanxiong Rhizoma), Jinyinhua (Lonicerae Japonicae Flos), Lianqiao (Forsythiae Fructus), Huangqin (Scutellariae Radix), Chuanbeimu (Fritillariae Cirrhosae Bulbus), Banxia (Pinelliae Rhizoma), Gancao (Glycyrrhizae Radix Et Rhizoma) | RCS | The Third Hospital of Wuhan | JBOL+C (n=50) | WMST(n=46) | Mild or Moderate cases | 15 d |
|
[59] |
| 10 | Jinhua Qinggan granules (JHQG) | Jinyinhua (Lonicerae Japonicae Flos), Shigao (Gypsum Fibrosum), Niubangzi (Arctii Fructus), Zhebeimu (Fritillariae Thunbergii Bulbus), Kuxingren (Armeniacae Seman Amarum), Mahuang (Ehedraep Herba), Huangqin (Scutellariae Radix), Lianqiao (Forsythiae Fructus), Qinghao (Artemisiae Annuae Herba), Zhimu (Anemarrhenae Rhizoma), Bohe (Menthae Haplocalycis Herba), Gancao (Glycyrrrhizae Radix Et Rhizoma) | CCS | Hubei Provincial Hospital of Integrated Traditional Chinese and Western Medicine | JHQG+C(n=82) | WMST(n=41) | Mild cases | 5d |
|
[54] |
| RCS | Beijing YouAn Hospital Affiliated to Capital Medical University | JHQG+C(n=44) | WMST(n=36) | Moderate or Severe cases | 7d |
|
[110] | |||
| 11 | Jinqiang Xuanfei Jiedu mixture (JQXF) | Xuanshen (Scrophulariae Radix), Jinyinhua (Lonicerae Japonicae Flos), Lianqiao (Forsythiae Fructus), Kuxingren (Armeniacae Seman Amarum), Chenpi (Citri Reticulatae Pericarpium), Doukou (Amomi Fructus Rotundus), Huangqin (Scutellariae Radix), Shegan (Belamcandae Rhizoma), Bohe (Menthae aplocalycis Herba), Fangfeng (Saposhnikoviae Radix), Chaihu (Bupleuri Radix), Banlangen (Isatidis Radix), Gancao (Glycyrrhizae Radix Et Rhizoma), Banxia (Pinelliae Rhizoma), Jiegeng (Platycodonis Radix), Cangzhu (Atractylodis Rhizoma), Qianghuo (Notopterygii Rhizoma Et Radix), Sangbaipi (Mori Cortex), Baibu (Stemonae Radix) | RCS | Wuhan Union Hospital | JQXF+C(n=99) | WMST(n=60) | Mild or Moderate cases | 7d |
|
[62] |
| 12 | Jinyinhua oral liquids (JYH) | Jinyinhua (Lonicerae Japonicae Flos) | CCS | The First People's Hospital of Jiangxia District | JYH+C(n=100) | WMST(n=100) | Mild or Moderate cases | 10d |
|
[67] |
| CCS | The First People's Hospital of Jiangxia District | JYH+C(n=80) | WMST(n=40) | Moderate cases | 10d |
|
[65] | |||
| 13 | Lanxiang Jiedu oral liquid (LXJD) | Guanghuoxiang (Pogostemonis Herba), Peilan (Eupatorii Herba), Cangzhu (Atractylodis Rhizoma), Houpo (Magnoliae Officinalis Cortex), Chenpi (Citri Reticulatae Pericarpium), Shengjiang (Zingiberis Rhizoma Recens), Fuling (Poria), Banxia (Pinelliae Rhizoma), Jinyinhua (Lonicerae Japonicae Flos), Lianqiao (Forsythiae Fructus), Mianmaguanzhong (Dryopteridis Crassirhizomatis Rhizoma), Taoren (Persicae Semen), Jiegeng (Platycodonis Radix), Gancao (Glycyrrhizae Radix Et Rhizoma) | CCS | The Fifth Hospital of Shijiazhuang | LXJD+C(n=8) | WMST(n=8) | Asymptomatic cases | 7d |
|
[48] |
| 14 | Lianhua Qingke granule (LHQK) | Mahuang (Ehedraep Herba), Shigao (Gypsum Fibrosum), Lianqiao (Forsythiae Fructus), Huangqin (Scutellariae Radix), Sangbaipi (Mori Cortex), Kuxingren (Armeniacae Seman Amarum), Shanyinhua (Lonicerae Flos), Dahuang (Rhei Radix Et Rhizoma), Qianhu (Peucedani Radix), Banxia (Pinelliae Rhizoma), Chenpi (Citri Reticulatae Pericarpium), Zhebeimu (Fritillariae Thunbergii Bulbus), Niubangzi (Arctii Fructus), Jiegeng (Platycodonis Radix), Gancao (Glycyrrhizae Radix Et Rhizoma) | RCT | Tangshan Infectious Diseases Hospital; The Third People's Hospital of Hengshui City; Cangzhou People's Hospital | LHQK+C(n=32) | WMST(n=25) | Mild or Moderate cases | 14d |
|
[64] |
| 15 | Lianhua Qingwen granules (LHQW) | Jinyinhua (Lonicerae Japonicae Flos), Dahuang (Rhei Radix Et Rhizoma), Mahuang (Ehedraep Herba), Chenpi (Citri Reticulatae Pericarpium), Sangbaipi (Mori Cortex), Banxia (Pinelliae Rhizoma), Kuxingren (Armeniacae Seman Amarum), Shigao (Gypsum Fibrosum), Zhebeimu (Fritillariae Thunbergii Bulbus), Gancao (Glycyrrrhizae Radix Et Rhizoma), Qianhu (Peucedani Radix), Huangqin (Scutellariae Radix), Niubangzi (Arctii Fructus), Jiegeng (Platycodonis Radix) | RCT | Puren Hospital Affiliated to Wuhan University of Science and Technology; The Ninth Hospital of Wuhan; CR&WISCO General Hospital, Wuhan University of Science and Technology | LHQW+C(n=51) | WMST(n=51) | Moderate cases | 7d |
|
[52] |
| CCS | CR&WISCO General Hospital Affiliated to Wuhan University of Science and Technology | LHQW+C(n=63) | WMST(n=38) | Pediatric cases | 10d |
|
[44] | |||
| RCS | Xiangyang No.1 People's Hospital | LHQW+C(n=42) | WMST (n=41) | Pediatric cases | 5d |
|
[45] | |||
| RCS | The Ninth Hospital of Wuhan; CR&WISCO General Hospital, Wuhan University of Science and Technology | LHQW+C(n=21) | WMST (n=21) | Moderate cases | Unreported |
|
[53] | |||
| Before and after comparison | The Puren affiliated Hospital of Wuhan University of Science and Technology | LHQW+WMCT(n=54) | Moderate cases | 7d |
|
[51] | ||||
| RCS | Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology | LHQW+C(n=90) | WMST(n=158) | Moderate cases | 5-7d |
|
[79] | |||
| RCS | Ruijin Hospital and Tongji Hospital | LHQW+C(n=113) | WMST(n=49) | Moderate or Severe cases | Unreported |
|
[69] | |||
| 16 | Lung-toxin dispelling formula No.1 (LDF1) | Renshen (Ginseng Radix Et Rhizoma), Jingjie (Schizonepetae Herba), Fengfang (Vespae Nidus), Jinyinhua (Lonicerae Japonicae Flos), Lianqiao (Forsythiae Fructus), Zaojiaoci (Gleditsiae Spina), Xuanshen (Scrophulariae Radix), Kuxingren (Armeniacae Seman Amarum), Gancao (Glycyrrhizae Radix Et Rhizoma) | Before and after comparison | Zhumadian City of Chinese medicine Hospital and The First Affiliated Hospital of Henan University of Chinese Medicine | LDF1+WMST(n=6) | Severe or Critical cases |
|
[87] | ||
| 17 | Maxing Shigan decoction (MXS) | Mahuang (Ehedraep Herba), Kuxingren (Armeniacae Seman Amarum), Shigao (Gypsum Fibrosum), Gancao (Glycyrrhizae Radix Et Rhizoma) | Before and after comparison | Chongqing Three Gorges Central Hospital | MXS+WMST(n=40) | Moderate cases | 3d |
|
[57] | |
| 18 | Pneumonia No.1 (PN1) | Huangqin (Scutellariae Radix), Binglang (Arecae Semen), Zhimu (Anemarrhenae Rhizoma), Banxia (Pinelliae Rhizoma), Dangshen (Codonopsis Radix), Baishao (Paeoniae Radix Alba), Gancao (Glycyrrhizae Radix Et Rhizoma), Chaihu (Bupleuri Radix), Chenpi (Citri Reticulatae Pericarpium), Houpo (Magnoliae Officinalis Cortex), Gualou (Trichosanthis Fructus), Caoguo (Tsaoko Fructus), Huzhang (Polygoni Cuspidati Rhizoma Et Radix) | RCS | Hubei Provincial Hospital of Traditional Chinese medicine and Han-chuan People′s Hospital | PN1+C(n=47) | WMST(n=40) | Moderate or Severe cases | Unreported |
|
[91] |
| 19 | Qingfei decoction (QFD) | Gualou (Trichosanthis Fructus), Banxia (Pinelliae Rhizoma), Huangqin (Scutellariae Radix), Baibu (Stemonae Radix), Qianhu (Peucedani Radix), Baiqian (Cynanchi Stauntonii Rhizoma Et Radix), Gancao (Glycyrrhizae Radix Et Rhizoma) | Before and after comparison | Wuhan Jiangxia Fangcang Shelter Hospital | QFD+WMST(n=152) | Mild or Moderate cases | 14d |
|
[74] | |
| 20 | Qingfei Paidu decoction (QFPD) | Gancao (Glycyrrhizae Radix Et Rhizoma), Kuxingren (Armeniacae Seman Amarum), Shigao (Gypsum Fibrosum), Guizhi (Cinnamomi Ramulus), Zexie (Alismatis Rhizoma), Zhuling (Polyporus), Baizhu (Atractylodis Macrocephalae Rhizoma), Fuling (Poria), Shanyao (Dioscoreae Rhizoma), Chaihu (Bupleuri Radix), Huangqin (Scutellariae Radix), Banxia (Pinelliae Rhizoma), Ganjiang (Zingiberis Rhizoma), Guanghuoxiang (Pogostemonis Herba), Ziwan (Asteris Radix Et Rhizoma), Kuandonghua (Farfarae Flos), Shegan (Belamcandae Rhizoma), Xixin (Asari Radix Et Rhizoma), Zhishi (Aurantii Fructus Immaturus), Chenpi (Citri Reticulatae Pericarpium) | RCS | Department of Traditional Chinese Medicine,Union Hospital,Tongji Medical College,Huazhong University of Science and Technology | QFPD+C(n=12) | WMST(n=12) | Severe or Critical cases | 7d |
|
[83] |
| RCS | Xiangyang No. 1 People’s Hospital, affiliated hospital of Hubei University of Medicine | QFPD+C(n=37) | WMST(n=26) | Mild or Moderate cases | 6d |
|
[81] | |||
| RCS | The First Affiliated Hospital of Anhui University of Traditional Chinese Medicine | QFPD+WMST(n=782) | All cases | Unreported |
|
[108] | ||||
| CCS | Wuchang Makeshift Hospital; Jingkai Makeshift Hospital; JiangHan Makeshift Hospital; Xinzhou Makeshift Hospital; Qiankou Makeshift Hospital | QFPD+WMST(n=96) | QFPD(n=96) | Mild or Moderate cases | Unreported |
|
[50] | |||
| RCS | East Hospital of Renmin Hospital of Wuhan University | QFPD+C(n=43) | WMST(n=46) | All cases | 15d |
|
[107] | |||
| Before and after comparison | Infectious Disease Hospital of Heilongjiang Province; The First Hospital of Suihua City; Harbin Infectious Hospital; Jincheng People's Hospital; Mudanjiang Kangan Hospital; Suining Central Hospital; The Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University; Xingtai Hospital of Traditional Chinese Medicine; Chengde Hospital of Traditional Chinese Medicine; Yongwu Hospital, People's Hospital of Guangxi Zhuang Autonomous Region | QFPD+WMST(n=50) | Severe cases | 6d |
|
[38] | ||||
| Before and after comparison | 27 hospitals in 8 provinces(cities) (The fifth hospital of Shijiazhuang; Xingtai Hospital of Traditional Chinese Medicine; Chengde Hospital of Traditional Chinese Medicine;The First Hospital of Suihua City, ect.) | QFPD+WMST(n=119) | All cases | 6d |
|
[98] | ||||
| Before and after comparison | 36 hospitals in 9 provinces (municipalities) (The Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University; Harbin Infectious Hospital; Chongqing Hospital of Traditional Chinese Medicine, ect.) | QFPD+WMST(n=157) | All cases | 6d |
|
[97] | ||||
| Before and after comparison | 17 hospitals in eight provinces/autonomous regions/municipalities.Qiqihar Hospital of Infectious Diseases; The Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University; Mianyang Hospital of Traditional Chinese Medicine, ect) | QFPD+WMST(n=49) | All cases | 6d |
|
[101] | ||||
| Before and after comparison | 28 hospitals in eight provinces/autonomous regions/municipalities.(Qiqihar Hospital of Infectious Diseases; The Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University; Harbin Infectious Hospital, ect) | QFPD+WMST(n=124) | All cases | 6d |
|
[100] | ||||
| RCS | 14 hospitals (The Public Health Clinical Center of Chengdu, Sichuan Province; The First Hospital of Suihua City, Heilongjiang Province; ect) | QFPD+C(n=23) | WMST(n=22) | All cases | 6d |
|
[99] | |||
| 21 | Qingfei Touxie Fuzheng reccipe (QFTX) | Mahuang (Ehedraep Herba), Shigao (Gypsum Fibrosum), Kuxingren (Armeniacae Seman Amarum), Jinyinhua (Lonicerae Japonicae Flos), Lianqiao (Forsythiae Fructus), Lugen (Phragmitis Rhizoma), Yiyiren (Coicis Semen), Jiangcan (Bombyx Batryticatus), Chantui (Cicadae Periostracum), Huzhang (Polygoni Cuspidati Rhizoma Et Radix), Jianghuang (Curcumae Longae Rhizoma), Baishao (Paeoniae Radix Alba), Taizishen (Pseudostellariae Radix), Gancao (Glycyrrhizae Radix Et Rhizoma) | RCT | General Hospital of Central Theater Command of Chinese People’s Liberation Army | QFTX+C(n=51) | WMST(n=49) | All cases | 10d |
|
[112] |
| 22 | Qingre KangDu oral liquid (QRKD) | Shigao (Gypsum Fibrosum), Banlangen (Isatidis Radix), Shengma (Cimicifugae Rhizoma), Guanghuoxiang (Pogostemonis Herba), Pugongying (Taraxaci Herba), Huanglian (Coptidis Rhizoma), Mudanpi (Moutan Cortex),Baimaogen (Imperatae Rhizoma) | CCS | The Fifth Hospital of Shijiazhuang | QRKD+C(n=11) | WMST(n=11) | Moderate cases | 10d |
|
[48] |
| 23 | Reduning injection (RDN) | Qinghao (Artemisiae Annuae Herba), Jinyinhua (Lonicerae Japonicae Flos), Zhizi (Gardeniae Fructus) | RCT | Lianyungang Fourth People’s Hospital affiliated to Kangda College, Nanjing Medical University and The Third People’s Hospital of Yichang | RDN+C(n=27) | WMST(n=23) | Mild cases | 14d |
|
[70] |
| 24 | RCS | The second Affiliated Hospital of Chongqing Medical University | RDN+C(n=32) | WMST (n=26) | Severe cases | 5-7d |
|
[89] | ||
| 25 | Sanxiaoyin modified and subtracted prescription (SXY) | Caoguo (Tsaoko Fructus), Houpo (Magnoliae Officinalis Cortex), Huangqin (Scutellariae Radix), Baishao (Paeoniae Radix Alba), Zhimu (Anemarrhenae Rhizoma), Gancao (Glycyrrhizae Radix Et Rhizoma), Dahuang (Rhei Radix Et Rhizoma), Jiangcan (Bombyx Batryticatus), Chantui (Cicadae Periostracum), Mumianhua (Gossampini Flos), Niuxi (Achyranthis Bidentatae Radix) | RCS | The Second Hospital of Jingzhou | SXY+C(n=26) | WMST(n=26) | Mild or Moderate cases | 7d |
|
[66] |
| 26 | Shengmai injection (SM) | Hongshen (Ginseng Radix Et Rhizoma Rubra), Maidong (Ophiopogonis Radix), Wuweizi (Schisandrea Chinensis Fructus) | RCT | Hubei 672 Orthopedic Hospital of Integrated Traditional Chinese and Western Medicine | SM+C(n=34) | WMST(n=30) | Moderate or Severe cases | 7d |
|
[93] |
| 27 | Shenhuang granule (SHG) | Renshen (Ginseng Radix Et Rhizoma), Dahuang (Rhei Radix Et Rhizoma), Pugongying (Taraxaci Herba), Fuzi (Aconiti Lateralis Radix Praeparata), Shuizhi (Hirudo), Daxueteng (Sargentodoxae Caulis) | RCT | Leishenshan Hospital of Wuhan, Tongji Hospital, Wuhan Mental Health Center, and Huangshi Hospital of TCM. | SHG+C(n=57) | WMST(n=54) | Severe or Critical cases | 14d |
|
[86] |
| 28 | Shuanghuanglian oral liquid (SHL) | Jinyinhua (Lonicerae Japonicae Flos), Huangqin (Scutellariae Radix), Lianqiao (Forsythiae Fructus) | RCT | Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology ect. | SHL+C(n=176) | WMST(n=59) | All cases | 14d |
|
[109] |
| 29 | Shufeng Jiedu capsule (SFJD) | Huzhang (Polygoni Cuspidati Rhizoma Et Radix), Lugen (Phragmitis Rhizoma), Chaihu (Bupleuri Radix), Lianqiao (Forsythiae Fructus), Baijiangcao (Patriniae Herba), Mabiancao (Verbenae Herba), Banlangen (Isatidis Radix), Gancao (Glycyrrrhizae Radix Et Rhizoma) | RCS | Bozhou People’s Hospital | SFJD+C(n=40) | WMST(n=30) | Mild or Moderate cases | 10d |
|
[55] |
| Randomized controlled trials | Bozhou People’s Hospital | SFJD+C(n=40) | WMST(n=40) | Mild or Moderate cases | 10d |
|
[56] | |||
| RCT | The Central Hospital of Wuhan | SFJD+C(n=100) | WMST(n=100) | Mild cases | 14d |
|
[77] | |||
| 30 | Sodium Chloride Injection (SCI) | Before and after comparison | Jingzhou Hospital of Infectious Disease (Chest Hospital) of Hubei Province | SCI+WMST(n=40) | All cases | Unreported |
|
[105] | ||
| 31 | Tanreqing Capsule (TRQ) | Huangqin (Scutellariae Radix), Xiongdanfen (Bear BilePowder), Shanyangjiao (Cornu Caprae Hircus), Jinyinhua(Lonicerae Japonicae Flos), Lianqiao (Forsythiae Fructus) | RCS | Shanghai Public Health Clinical Center | TRQ+C(n=25) | WMST(n=57) | Mild or Moderate cases | Unreported |
|
[71] |
| 32 | Toujie Quwen granules (TJQW) | Lianqiao (Forsythiae Fructus), Shancigu (Cremastrae Pseudobulbus Pleiones Pseudobulbus), Jinyinhua (Lonicerae Japonicae Flos), Huangqin (Scutellariae Radix), Daqingye (Isatidis Folium), Chaihu (Bupleuri Radix), Qinghao (Artemisiae Annuae Herba), Chantui (Cicadae Periostracum), Qianhu (Peucedani Radix), Chuanbeimu (Fritillariae Cirrhosae Bulbus), Zhebeimu (Fritillariae Thunbergii Bulbus), Fuling (Poria), Wumei (Mume Fructus), Xuanshen (Scrophulariae Radix), Huangqi (Astragali Radix), Taizishen (Pseudostellariae Radix) | RCT | Guangzhou Eighth People's Hospital | TJQW+C(n=37) | WMST(n=36) | Mild or Moderate cases | 10d |
|
[61] |
| RCT | Guangzhou Eighth People's Hospital | TJQW+C(n=32) | WMST(n=33) | Moderate cases | 15d |
|
[80] | |||
| 33 | Wuye Lugen decoction (WYLG) | Bohe (Menthae aplocalycis Herba), Guanghuoxiang (Pogostemonis Herba), Heye (Nelumbinis Folium), Peilan (Eupatorii Herba), Pipaye (Eriobotryae Folium), Lugen (Phragmitis Rhizoma), Dongguazi (Benincasae Semen) | Before and after comparison | Jiangmen Wuyi Hospital of Traditional Chinese Medicine Affiliated to Ji'nan University | WYLG+CWMT(n=18) | Convalescent cases | moderate cases |
|
[116] | |
| 34 | Xiang Huo Spray (XHS) | Guanghuoxiang (Pogostemonis Herba), Xiangru (Moslae Herba), Aiye (Artemisiae Argyi Folium), Dingxiang (Caryophylli Flos), Bohe (Menthae aplocalycis Herba), Bingpian (Borneolum) | RCT | Hubei Provincial Hospital of Traditional Chinese medicine | XHS+C(n=60) | WMST(n=60) | Convalescent cases | 7d |
|
[114] |
| 35 | Xiaochaihu decoction combined with Yupingfeng powder (XCY) | Chaihu (Bupleuri Radix), Huangqin (Scutellariae Radix), Banxia (Pinelliae Rhizoma), Mianmaguanzhong (Dryopteridis Crassirhizomatis Rhizoma), Jinyinhua (Lonicerae Japonicae Flos), Lianqiao (Forsythiae Fructus), Taizishen (Pseudostellariae Radix), Huangqi (Astragali Radix), Fangfeng (Saposhnikoviae Radix), Baizhu (Atractylodis Macrocephalae Rhizoma), Gancao (Glycyrrhizae Radix Et Rhizoma) | RCS | Affiliated Hospital of Liaoning University of Traditional Chinese Medicine | XCY+C(n=18) | WMST(n=14) | Pediatric cases | 5d |
|
[46] |
| 36 | Xiyanping injection (XYP) | Andrographolide sulfonates | RCT | the Second Affiliated Hospital of Nanchang University ect. | XYP+C(n=65) | WMST(n=65) | Mild or Moderate cases | 28d |
|
[58] |
| 37 | Xuanfei Baidu decoction (XFBD) | Mahuang (Ehedraep Herba), Kuxingren (Armeniacae Seman Amarum), Shigao (Gypsum Fibrosum), Yiyiren (Coicis Semen), Cangzhu (Atractylodis Rhizoma), Guanghuoxiang (Pogostemonis Herba), Qinghao (Artemisiae Annuae Herba), Huzhang (Polygoni Cuspidati Rhizoma Et Radix), Mabiancao (Verbenae Herba), Lugen (Phragmitis Rhizoma), Tinglizi (Descurainiae Semen Lepidii Semen), Huajuhong (Citri Grandis Exocarpium), Gancao (Glycyrrhizae Radix Et Rhizoma) | RCT | Wuhan Pulmonary Hospital | XFBD+C(n=22) | WMST(n=22) | All cases | 7d |
|
[102] |
| 38 | Xuanfei Huazhuo prescription (XFHZ) | Mahuang (Ehedraep Herba), Lianqiao (Forsythiae Fructus), Qianhu (Peucedani Radix), Banxia (Pinelliae Rhizoma), Cangzhu (Atractylodis Rhizoma), Guanghuoxiang (Pogostemonis Herba), Qianghuo (Notopterygii Rhizoma Et Radix), Dahuang (Rhei Radix Et Rhizoma), Chenpi (Citri Reticulatae Pericarpium), Huangqin (Scutellariae Radix) | Before and after comparison | Affiliated Hospital o f Gansu University of Chinese Medicine | XFHZ+WMST(n=40) | All cases | 8-23d |
|
[104] | |
| 39 | Xue’s Fu Yang Zhu Shi decoction cutting (XFYZS) | Dangshen (Codonopsis Radix), Baizhu (Atractylodis Macrocephalae Rhizoma), Fuzi (Aconiti Lateralis Radix Praeparata), Fuling (Poria), Houpo (Magnoliae Officinalis Cortex), Banxia (Pinelliae Rhizoma), Ganjiang (Zingiberis Rhizoma), Chaihu (Bupleuri Radix), Shengma (Cimicifugae Rhizoma), Huangqi (Astragali Radix), Huangqin (Scutellariae Radix), Lugen (Phragmitis Rhizoma) | Before and after comparison | Chongqing public health medical treatment center | XFYZS+WMST(n=36) | Unreported | 14d |
|
[111] | |
| 40 | Xuebijing injection (XBJ) | Chishao (Paeoniae Radix Rubra), Honghua (Carthami Flos), Danggui (Angelicae Sinensis Radix), Chuanxiong (Chuanxiong Rhizoma), Danshen (Salviae Miltiorrhizae Radix Et Rhizoma) | RCT | The First People’s Hospital of Jingzhou | XBJ+C(n=30) | WMST(n=30) | Severe cases | 14d |
|
[84] |
| CCS | North Hospital of the First Hospital of Changsha City | XBJ+C(n=20) | WMST(n=20) | Severe cases | 7d |
|
[85] | |||
| RCT | Affiliated Dongfend Hospital, Hubei University of Medicine | XBJ+C(n=22) | WMST(n=22) | Moderate cases | 7d |
|
78] | |||
| 41 | Yidu-toxicity blocking lung decoction (YTBL) | Kuxingren (Armeniacae Seman Amarum), Shigao (Gypsum Fibrosum), Gualou (Trichosanthis Fructus), Dahuang (Rhei Radix Et Rhizoma), Mahuang (Ehedraep Herba), Tinglizi (Descurainiae Semen Lepidii Semen), Taoren (Persicae Semen), Caoguo (Tsaoko Fructus), Binglang (Arecae Semen), Cangzhu (Atractylodis Rhizoma) | RCT | The First Affiliated Hospital of Anhui Medical University | YTBL+C (n=15) | WMST (n=24) | Severe cases | 29d |
|
[95] |
| 42 | Yinqiao powder combined with modified Sanren decoction (YCS) | Pugongying (Taraxaci Herba), Lugen (Phragmitis Rhizoma), Yiyiren (Coicis Semen), Jinyinhua (Lonicerae Japonicae Flos), Lianqiao (Forsythiae Fructus), Chaihu (Bupleuri Radix), Huangqin (Scutellariae Radix), Kuxingren (Armeniacae Seman Amarum), Doukou (Amomi Fructus Rotundus), Jiegeng (Platycodonis Radix), Guanghuoxiang (Pogostemonis Herba) | RCS | the Second Hospital of Nanjing | YCS+C(n=20) | WMST(n=16) | Moderate cases | 7d |
|
[76] |
| 43 | Yiqi Yangyin granules (YQYY) | Dangshen (Codonopsis Radix), Maidong (Ophiopogonis Radix), Baihe (Lilii Bulbus), Fuling (Poria), Baizhu (Atractylodis Macrocephalae Rhizoma), Chenpi (Citri Reticulatae Pericarpium), Maiya (Hordei Fructus Germinatus), Hehuanpi (Albiziae Cortex), Digupi (Lycii Cortex), Gancao (Glycyrrhizae Radix Et Rhizoma) | RCT | Jiangxia Wuhan District Hospital of Traditional Chinese Medicine; The First Hospital of Wuhan; Huai’an Hospital of Traditional Chinese Medicine; Yangzhou Hospital of Traditional Chinese Medicine; The Third People's Hospital of Zhenjiang | YQYY+C(n=30) | WMST(n=30) | Convalescent cases | 14d |
|
[115] |
WMST: Western medicine routine symptomatic treatment.
Fig. 2.
The relationship of TCM with its constituents. Note: The green square nodes represent TCM. The red, yellow, and light red circular nodes represent the constituents of TCM. The size and darkness of the nodes represent the frequency of appearance of each constituent. The size from small to large and the darkness of the nodes from dark to light are illustrated in ascending order of frequency of appearance. The connecting lines indicate that each node is related. The width of the connecting lines was based on the frequency of appearance of constituents, and the colour and rule were the same as the circular nodes. JQXF: Jinqiang Xuanfei Jiedu mixture; LHQW: Lianhua Qingwen capsule; PN1: Pneumonia No.1; XBJ: Xuebijing injection; TJQW: Toujie Quwen granules; YPF: Jiawei Yupingfeng powder; GLXD: Ganlu Xiaodu decoction; XYP: Xiyanping injection; XCY: Xiaochaihu decoction combined with Yupingfeng powder; TRQ: Tanreqing injection; SHG: Shenhuang granule; YCS: Yinqiao powder combined with modified Sanren decoction; JHQG: Jinhua Qinggan granules; CHGL: Chaihu Ganlu decoction; QTF: Qingfei Touxie Fuzheng recipe; QFPDD: Qingfei Paidu decoction; SFJD: Shufeng Jiedu capsule; DGSY: Danggui Shaoyao power; XFBD: Xuanfei Baidu decoction; GGQL: Gegen Qinlian pill; JBOL: Jinbei oral liquid; SHL: Shuanghuanglian oral liquid; XFHZ: Xuanfei Huazhuo prescription; HSBD: Huashi Baudu decoction; QRKD: Qingre KangDu oral liquid; QFD: Qingfei decoction; LHQK: Lianhua Qingke granules; RDN: Reduning injection; LDF1: Lung-toxin dispelling formula No.1; BZYQ: BuZhong Yiqi decoction; HXZQ: HuoXiang Zhengqi power; JYH: Jinyinhua oral liquid; LXJD: Lanxiang Jiedu oral liquid; SM: Shengmai power; XFYZS: Xue’s Fu Yang Zhu Shi decoction cutting.
For suspected COVID-19 patients: 1) Lianhua Qingwen granules [44], [45] and Xiaochaihu decoction combined with Yupingfeng powder [46] significantly improved the clinical symptoms of fever, cough, phlegm, fatigue, and shortness of breath; Lianhua Qingwen granules [45] relieved gastrointestinal symptoms; and 2) Xiaochaihu decoction combined with Yupingfeng powder [46] reduced the level of C-reactive protein (CRP) and improved lung CT imaging. For asymptomatic COVID-19 patients, Chaihu Ganlu decoction [47] and Lanxiang Jiedu oral liquid [48] could shorten the nucleic acid negative conversion time and hospital stay and prevent deterioration due to the disease.
COVID-19 patients have the most typical clinical symptoms of fever, coughing, and fatigue. Therefore, alleviating these clinical symptoms is crucial for the prevention and treatment of COVID-19 [49]. For mild or moderate COVID-19 patients: 1) Qingfei Paidu decoction [50], Lianhua Qingwen granules [51], [52], [53], Jinhua Qinggan granules [54], Shufeng Jiedu capsules [55], [56], Maxing Shigan decoction [57], Xiyanping injection [58], Jinbei oral liquid [59], Huoxiang Zhengqi [60], Toujie Quwen granules [61], Jinqiang Xuanfei Jiedu mixture [62], Ganlu Xiaodu decoction [63], Lianhua Qingke granules [64], Jinyinhua oral liquid [65], and Sanxiaoyin modified and subtracted prescription [66] alleviated the main clinical symptoms of fever, coughing, and fatigue; Lianhua Qingwen granules [52], Ganlu Xiaodu decoction [63], Lianhua Qingke granules [64], Jinyinhua oral liquid [65], [67], and Jinye Baidu granules [68] reduced the rate of conversion from mild or moderate cases to severe or critical cases; 2) Lianhua Qingwen granules [69],Qingfei Paidu decoction [50], Shufeng Jiedu capsules [55], Maxing Shigan decoction [57], Reduning injection [70], Xiyanping injection [58], Tanreqing capsules [71], Reyanning mixture [72], Jinbei oral liquid [59], Jiawei Yupingfeng powder [73], Qingfei decoction[74], Ganlu Xiaodu decoction [63], Buzhong Yiqi decoction [75], Jinyinhua liquid [67], Yinqiao powder combined with modified Sanren decoction [76], and Sanxiaoyin modified and subtracted prescription [66]shortened nucleic acid negative time and the average hospitalization; Lianhua Qingwen granules[69], Qingfei Paidu decoction [50], Shufeng Jiedu capsules [56], [77], Reduning injection [70], Qingfei decoction [74], Ganlu Xiaodu decoction [63], Lianhua Qingke granules [64], Jinyinhua liquid [67], Xuebijing injection [78], Reyanning mixture [72], and Sanxiaoyin modified and subtracted prescription [66] promoted the absorption of pulmonary inflammation or improved chest CT imaging; and 3) Sanxiaoyin modified and subtracted prescription [66], Lianhua Qingwen granules [79], Jiawei Yupingfeng powder [73], Toujie Quwen granules [61], [80], and Buzhong Yiqi decoction [75] decreased the levels of erythrocyte sedimentation rate (ESR) or CRP, and increased lymphocyte count; Lianhua Qingwen granules [79] reduced the level of D-dimer and increased the levels of albumin and haemoglobin; Qingfei Paidu decoction [81] reduced the levels of CRP, creatine kinase (CK), creatine kinase-myocardial band (CK-MB), lactate dehydrogenase (LDH), and blood urea nitrogen (BUN); Maxing Shigan decoction [57] decreased the levels of CRP, interleukin-6 (IL-6), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and serum creatinine (Scr), and increased CD4 + T and CD8 + T cell counts; and Tanreqing capsule [71] increased CD3 + T cell counts.
Severe or critical COVID-19 patients have the main factors directly affecting mortality. Treating severe cases is crucial since they can easily develop into critical cases, or the patients can even eventually die without timely and effective treatment [26], [82]. The Diagnosis and Treatment Protocol for COVID-19 (trial version 8 revision) [15], formulated by the National Health Commission of the People’s Republic of China, pointed out that, in severe cases, dyspnoea and/or hypoxemia usually occur one week after disease onset, and some severe patients have rapid progression to acute respiratory distress syndrome (ARDS), septic shock, refractory metabolic acidosis, coagulation disorders, and multiple organ failure. Therefore, to reduce the mortality rate of COVID-19, it is necessary to apply more active and effective treatments to prevent the development of COVID-19. For severe or critical COVID-19 patients: 1) Qingfei Paidu decoction [38], [83], Xuebijing injection [84], [85], Shenhuang granules [86], Lung-toxin Dispelling Formula No. 1 [87], and Huashi Baidu decoction[88] improved the survival rate and prevented deterioration due to the disease; Xuebijing injection [84] and Reduning injection [89] shortened the length of intensive care unit (ICU) hospitalization; 2) Qingfei Paidu decoction [38], Huashi Baidu granules [90] Reduning injection [89], Xuebijing injection [84], [85], "Fei Yan No. 1" [91], and Danggui Shaoyao powder [92] shortened the time of nucleic acid negative conversion or improved the ratio of nucleic acid negative conversion; Qingfei Paidu decoction [38], [83], Huashi Baidu granules [90], Shengmai powder [93], Shenhuang granule [86], "Fei Yan No. 1"[91], and Danggui Shaoyao powder [92] promoted the absorption of pulmonary inflammation and improved chest CT imaging; and 3) Qingfei Paidu decoction [38], [83], Reduning injection [89], Xuebijing injection [84], and Huashi Baidu decoction [88] reduced the levels of IL-6, tumour necrosis factor-α (TNF-α), and CRP and increased the white blood cell (WBC) count; Qingfei Paidu decoction [38] decreased the levels of ALT, AST, LDH, ESR, and procalcitonin (PCT) and improved lymphocyte counts; "Fei Yan No. 1" [91] and Danggui Shaoyao powder [92] decreased the level of CRP and leukocyte counts; Shengmai powder [93], Xuebijing injection [85], and Huashi Baidu decoction [88] reduced the level of ESR; Xuebijing injection [94] improved the oxygenation index, PaCO2, and lymphocyte counts; and Yidu-toxicity blocking lung decoction [95] ameliorated inflammation by decreasing the levels of IL-6 and TNF-α.
For the clinical treatment period (mild, moderate, severe, and critical COVID-19 patients): 1) Lianhua Qingwen granules [96], Qingfei Paidu decoction [97], [98], [99], [100], [101], Xuanfei Baidu decoction [102], Huashi Baidu granules [103], Xuanfei Huazhuo prescription [104], and sodium chloride injection [105] relieved the clinical symptoms of fever, coughing, fatigue, chest tightness and loss of appetite; Gegen Qinlian pills [106] improved the clinical symptoms of coughing, chest tightness, vomiting and loose stools, and nausea; 2) Lianhua Qingwen granules [96], Qingfei Paidu decoction [97], [98], [99], [101], [107], [108], Gegen Qinlian pills [106], Shuanghuanglian oral liquid [109],Jinhua Qinggan granules [110],Xue's Fuyang Zhushi decoction [111], and sodium chloride injection [105] shortened the length of hospitalization or nucleic acid negative time; Lianhua Qingwen granules [96], Qingfei Paidu decoction [97], [98], [100], [101], [107], Shuanghuanglian oral liquid [109], Huashi Baidu granules [103], Xuanfei Huazhuo prescription [104], Jinhua Qinggan granules [110], Xue's Fuyang Zhushi decoction [111], and sodium chloride injection [105] promoted the absorption of pulmonary inflammation or improved lung CT imaging; and 3) Qingfei Paidu decoction [97], [99], [100], [101], [107] reduced the levels of IL-6, CRP, ALT, AST, CK, CK-MB, and LDH and increased lymphocytes, WBC, CD3 + T cell counts; and Qingfei Touxie Fuzheng recipe [112] decreased the levels of ESR, CRP, and IL-6; in addition, sodium chloride injection [105] and Gegen Qinlian pills [106] decreased the levels of high-sensitivity C-reactive protein (hs-CRP) and PCT and increased lymphocyte counts; Xuanfei Baidu decoction [102] increased lymphocyte and WBC counts and reduced the levels of CRP and ESR; and Shuanghuanglian oral liquid reduced the levels of N-terminal brain natriuretic peptide (NT-proBNP).
For convalescent COVID-19 patients, TCM can remove residual evil, help vital Qi, improve symptoms, promote the repair of injured organs and tissues, and expedite the process of rehabilitation [113]. Xiang Huo spray [114] improved the total clinical efficiency and alleviated the clinical symptoms of coughing, chest tightness, fatigue, sweating, muscle soreness, stuffy nose, and sore throat; Yiqi Yangyin granules [115] alleviated the clinical symptoms of fatigue, shortness of breath, dry mouth, vexing heat in the chest, palms, and soles, and dry pharynx; improved the expiratory peak flow rate value; and increased CD3 + T cell counts. Wuye Lugen decoction [116] could improve the clinical symptoms of fatigue, dry mouth, insomnia, and anxiety and increase lymphocyte and WBC counts and the levels of haemoglobin (HGL), CRP, and IgG.
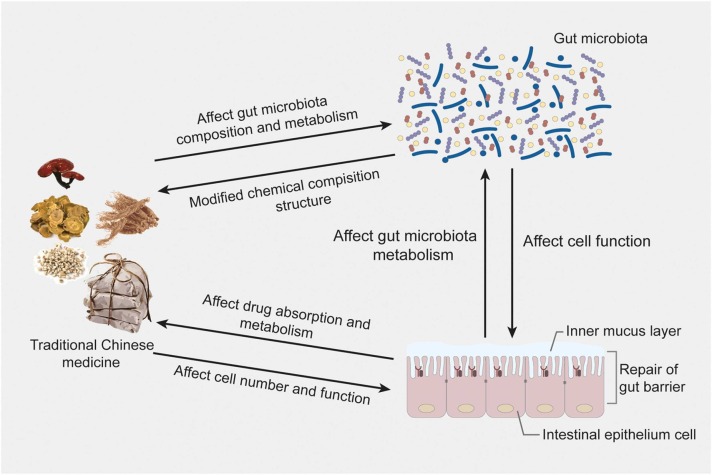
4. The relationship between the gut microbiota and TCM
The normal gut microbiota consists of bacteria, viruses, fungi, and archaea in numbers up to 1013-1014. On the one hand, under normal physiological conditions, the gut microbiota can interact with the host through complex dynamic network regulatory mechanisms, participate in regulating the host's material metabolism and immune defence, and protect the integrity of the intestinal mucosal barrier structure [117], [118], [119]. On the other hand, beneficial bacteria can produce antibacterial substances that are more beneficial to the body and resist the invasion of pathogens by competing for nutrients and occupying favourable sites [120]. When the homeostasis of the intestinal microecology is disrupted, beneficial bacteria decline, harmful bacteria increase, the intestinal barrier is damaged, and the function of the immune system is disrupted, thus triggering a variety of diseases and threatening human health. It has been reported that the gut microbiota is closely related to the inflammatory response induced by novel coronaviruses and could be highly correlated with individual susceptibility to COVID-19 [121]. Numerous studies have shown that respiratory virus infection of the host causes alteration of the gut microbiota, increasing the colonization of pathogenic bacteria and decreasing the number of probiotics. In turn, alteration of the gut microbiota can reduce the level of the host's antiviral immune response and aggravate the lung injury caused by viral infection. In addition, the gut microbiota can act directly on viruses through its structural components or metabolites or indirectly influence the body's immune response, ultimately affecting the outcome of viral infection [122]. Probiotics can enhance the antiviral ability of the body by regulating innate immunity, maintaining the integrity of the intestinal wall, and activating adaptive immune responses [123].
There is a close relationship between TCM and gut microbiota, as shown in Fig. 3. On the one hand, TCM can influence the composition and metabolism of the gut microbiota; on the other hand, the gut microbiota can metabolize Chinese herbal medicinal components [124]. A large number of studies have shown that TCM can significantly adjust the alteration of the gut microbiota, promote the growth of beneficial bacteria, inhibit the overproliferation of harmful bacteria, and balance the number of these probiotic and pathogenic bacteria, thus maintaining a healthy intestinal environment [125], [126]. Most TCM is administered orally and is selectively metabolized in the intestine by intestinal secretory enzymes into active or absorbable components; TCM thus exerts a therapeutic effect. In addition, the gut microbiota will be affected by TCM while transforming it. The composition of the gut microbiota will be changed under the effects of TCM. On the one hand, TCM can alleviate disease-induced gut microbiota disorders by regulating the composition of the gut microbiota and then treating the disease; on the other hand, changes in the composition of the gut microbiota can also lead to changes in its metabolic ability, thus triggering gut microbiota-mediated interactions of Chinese herbal medicinal components. The gut microbiota in the body plays an important role in the pharmacological effects of oral TCM, and TCM helps to maintain the balance of the gut microbiota. The interaction between them is of great significance in preventing and treating diseases and promoting health [127].
Fig. 3.
The interaction between TCM and gut microbiota.
5. The relationship between COVID-19 and gut microbiota
The initial symptoms of COVID-19 patients are mainly fever, coughing, and fatigue. With in-depth research on the disease, it has also not been uncommon to find gastrointestinal symptoms caused by SARS-CoV-2 infection in clinical practice [128], especially in critically ill patients with poor prognoses [9], [10], [11]. Available evidence suggests that SARS-CoV-2 can directly damage the digestive system, disrupt the intestinal microecological balance, and cause associated clinical manifestations [11], [129], [130]. Gastrointestinal symptoms are present in approximately 2–79.1% of patients [9], [49], [131], [132], [133], and these symptoms can appear early in the onset of the disease, even earlier than fever and respiratory symptoms [134], [135], [136]. By studying 1099 patients with COVID-19, Nanshan Zhong's team found that the clinical manifestations of gastrointestinal symptoms, such as nausea, vomiting, and diarrhoea, could be 8.7%, and gastrointestinal symptoms were more common in severe patients, accounting for 12.7% [49]. Another study [9] found that 11.4% of COVID-19-infected people presented with gastrointestinal symptoms, such as abdominal pain, diarrhoea, nausea, and vomiting. Moreover, a study [137] confirmed that some COVID-19 patients had their first symptoms in the digestive tract, suggesting that gastrointestinal symptoms are another major manifestation of COVID-19 patients, in addition to respiratory symptoms. The Renmin Hospital of Wuhan University reported "atypical" cases in which patients with COVID-19 presented with gastrointestinal symptoms only, without fever or respiratory symptoms [138]. Wang et al. [132] found that the proportion of COVID-19 patients with gastrointestinal symptoms as initial symptoms accounted for approximately 10.12%, and the patients only had diarrhoea symptoms at the onset of the disease, followed by fever, dyspnoea, and other symptoms. Currently, SARS-CoV-2 has been identified and isolated from the stool specimens of COVID-19 patients [139], [140], and disorders of the intestinal microecology have been detected in COVID-19 patients with gastrointestinal symptoms [130]. These findings suggest that SARS-CoV-2 might cause alterations in the gut microbiota along with pulmonary infection, which could be the main reason for the nausea, vomiting, diarrhoea, and other gastrointestinal symptoms in patients.
Alteration of the gut microbiota is closely related to the severity of COVID-19 patients [11], [141]. A study found that COVID-19 patients with diarrhoea were in more serious conditions and had higher rates of receiving noninvasive ventilation and transferring to the intensive care unit and a higher case fatality rate than those without diarrhoea [11], [142]. Another study that included 74 COVID-19 patients with gastrointestinal symptoms and 577 COVID-19 patients without gastrointestinal symptoms found that the proportion of COVID-19 patients with gastrointestinal symptoms who were in severe or critical condition was as high as 22.97% -- higher than the 8.14% of patients without gastrointestinal symptoms [9]. In 138 COVID-19 study subjects, the incidence of gastrointestinal symptoms was higher in severely ill patients than in ordinary patients, and severely ill patients presented with abdominal pain, whereas mildly ill patients did not [143]. Numerous retrospective studies have indicated that more than half of COVID-19 patients are complicated with underlying diseases, such as diabetes mellitus, hypertension, and hyperlipidaemia, with poor tolerance and rapid disease progression, and they are prone to becoming severe and critical high-risk patients [49], [131], [132]. In an interview with a CCTV reporter, the academician Li Lanjuan mentioned that severe and critical COVID-19 patients mostly have gut microbiota disorders. Severe and critical COVID-19 patients are predominantly elderly patients complicated with underlying diseases [144]. The intestinal microecological stability and diversity of elderly individuals decrease, and infection with SARS-CoV-2 causes gut microbiota disorders, which tend to affect the absorption of nutrients and reduce mucosal barrier immunity; the risk of nosocomial infections is thus increased [145]. In summary, there are many links between the gut microbiota and COVID-19. On the one hand, COVID-19 patients have gut microbiota disorders and impaired barrier function; could have gastrointestinal symptoms, such as nausea, vomiting, and diarrhoea; and could also have gastrointestinal symptoms as the first manifestation. On the other hand, gut microbiota disorders can aggravate the condition of COVID-19 patients.
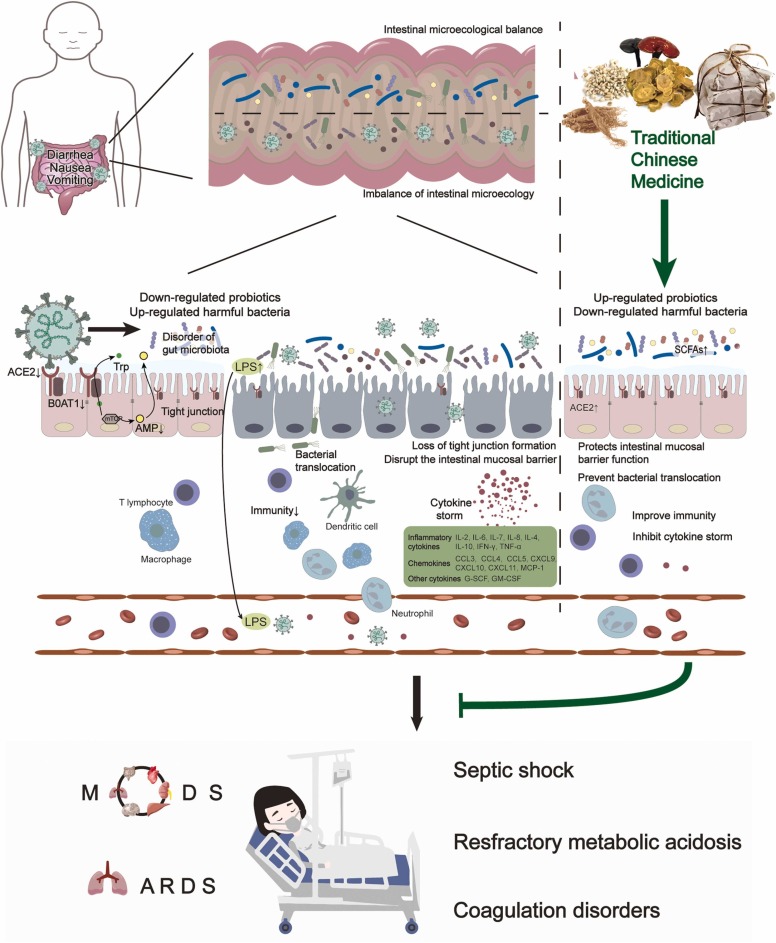
6. Mechanism of gut microbiota disorder induced by SARS-CoV-2
SARS-CoV-2 is the third coronavirus to emerge in humans over the past 20 years after severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) [146], [147]. Studies have shown that the pathogenic process of SARS-CoV-2 infection is associated with the binding of angiotensin-converting enzyme 2 (ACE2) [129]. Current studies have shown that, similar to SARS-CoV, SARS-CoV-2 uses ACE2 with the help of transmembrane protease serine 2 (TMPRSS2) as a cellular receptor in the host cell [148], [149], [150]. There are many ACE2 proteins in alveolar epithelial cells. After SARS-CoV-2 enters alveolar epithelial cells, virus replication causes an immune response in the body, and excessive cytokine production eventually forms a cytokine storm, in turn triggering inflammation, which is an important cause of lung injury, acute respiratory distress syndrome (ARDS), and multiple organ failure or even death [25], [151]. Notably, ACE2 is highly expressed not only in alveolar cells but also in oesophageal epithelial cells, gastrointestinal epithelial cells, and hepatobiliary duct cells [152], [153], [154]. The above evidence suggests that SARS-CoV-2 might not only infect the respiratory system but also directly infect the digestive system and cause related clinical manifestations [155]. The high expression of ACE2 in the intestine can regulate the composition of the gut microbiota [156]. ACE2 is necessary for the expression of the neutral amino acid transporter B0AT1 protein in intestinal epithelial cells, and it participates in the absorption of tryptophan [157]. When the level of ACE2 expression in the digestive tract is reduced, tryptophan is not efficiently absorbed, small intestinal mTOR pathway activity is reduced, and small intestinal antimicrobial peptide expression is inhibited, ultimately leading to alteration of the gut microbiota and an increase in endotoxin. These effects in turn trigger endotoxaemia and cytokine storms, leading to tissue damage and even ARDS and MODS [157], [158], [159]. Thus, the dysregulation of gut microbiota in COVID-19 might be related to decreased intestinal ACE2 expression caused by the binding of SARS-CoV-2 to the ACE2 receptor on the surface of intestinal epithelial cells, as shown in Fig. 4.
Fig. 4.
Mechanism of gut microbiota disorders induced by SARS-CoV-2 and the mechanism by which TCM regulates the gut microbiota to prevent and treat COVID-19. The binding of SARS-CoV-2 to the ACE2 receptor could reduce the expression of ACE2 and affect the absorption of tryptophan by small intestinal epithelial cells. It could also reduce the activity of the mTOR pathway in the small intestine and inhibit the expression of small intestinal antimicrobial peptides, leading to intestinal flora imbalance (a decrease in the number of beneficial bacteria and an increase in the number of harmful bacteria) and displacement, the destruction of the intestinal mucosal barrier, and an increase in intestinal endotoxins. The above process causes the excessive release of inflammatory mediators, the occurrence of endotoxaemia and cytokine storms, and tissue damage. In severe cases, ARDS and MODS can occur. However, TCM can help to suppress inflammatory storms and maintain the immune barrier function of the intestinal mucosa, thereby improving the body’s immunity and preventing the deterioration of the condition because of TCM’s ability to adjust the intestinal flora imbalance, promote the growth of beneficial bacteria, inhibit the excessive reproduction of harmful bacteria, and promote the production of SCFAs.
7. The relationship between cytokine storms and the gut microbiota
A cytokine storm, also known as a cytokine cascade or hypercytokinaemia, is essentially a more intense inflammatory response and an excessive immune response of the body to pathogenic microorganisms (such as bacteria and viruses) [160]. When pathogens invade the organism, a moderate immune response is activated, which can hinder viral replication and rapidly clear the pathogens. However, an excessive immune response leads to an imbalance in the immune regulatory network, and an excessive inflammatory response occurs in the body, prompting a large release of multiple inflammatory factors and cytokines into the body fluids in a short period and producing systemic inflammatory damage, resulting in lung injury, ARDS and multiple organ dysfunction syndromes (MODS) and even death [161]. Test reports of COVID-19 patients have shown that the absolute value of lymphocytes was reduced in most patients, suggesting that SARS-CoV-2 could mainly act on lymphocytes, especially T lymphocytes. The viral particles spread through the respiratory mucosa, infect other cells, induce a cytokine storm in vivo, produce a series of immune responses, and cause peripheral blood changes in immune cells, such as leukocytes and lymphocytes [131]. Clinical studies have shown that plasma levels of IL-2, IL-7, IL-10, granulocyte colony-stimulating factor (G-SCF), interferon-γ-inducible protein-10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), and TNF-α in COVID-19 patients are elevated [131], [151], and critically ill patients usually exhibit reduced peripheral blood T lymphocytes [132]. Abundant evidence has suggested that cytokine storms play an important role in the progression of COVID-19, especially in critically ill patients [11], [162], [163]. A recent COVID-19 clinical study [151] showed that a high level of inflammatory cytokine expression, the "cytokine storm", was found in critically ill patients infected with SARS-CoV-2, suggesting a possible correlation between cytokine storms and the severity of patients' infections. Cytokine storms are now considered a turning point in the progression of COVID-19 patients to severe and critical illnesses. Some patients with COVID-19 had mild disease in the early stages, with sudden aggravation in the later stages and eventual death from MODS, with exacerbation mainly due to a cytokine storm [164]. Reports have indicated that the cytokine storm in severe COVID-19 patients is closely associated with severe lung pathological injury and the occurrence and development of ARDS, and it is one of the main causes of death in patients [131], [151], [165].
Numerous studies in recent years have shown that intestinal microorganisms play important roles in regulating the immunity of the body and that changes in the gut microbiota are closely associated with local and systemic inflammation [166]. The microecological imbalance of the gut microbiota leads to the release of inflammatory mediators caused by intestinal endotoxin and gut microbiota displacement, directly or indirectly promoting the occurrence and development of the COVID-19 inflammatory storm. After SARS-CoV-2 infection, especially in elderly patients or those with underlying diseases, alteration of the gut microbiota increases the production of various toxins, including endotoxins, and impairs intestinal barrier function, causing a large number of toxins to enter the bloodstream. These toxins can directly damage lung structure and function, or they can aggravate the systemic and pulmonary inflammatory response by inducing inflammation and creating inflammatory factor storms, leading to severe disease. It was found that, among COVID-19 patients, the elderly and those with underlying diseases, such as diabetes, hypertensive disease, coronary heart disease, and extreme obesity, are susceptible to SARS-CoV-2, are prone to developing acute and critical illness, and have a higher risk of death [132], [167], [168], [169]. Previous studies [170], [171] have shown that the richness, evenness, and diversity of the gut microbiota in this population were significantly reduced, and the dominant flora was significantly decreased, with an imbalance in intestinal microecology. Infection with SARS-CoV-2 further aggravates gut microbiota disorders, leading to disruption of the intestinal mucosal barrier, the release of inflammatory mediators caused by intestinal endotoxin and gut microbiota displacement, and the formation of an inflammatory factor storm to exacerbate systemic and pulmonary inflammatory responses. It is now believed that the cause of severe pneumonia is not the virus itself but the excessive immune response induced by infection. The unbalanced inflammatory factor system in the body is an important cause of pneumonia and ALI [172], [173]. The above studies suggest that the gut microbiota is closely associated with the inflammatory storm in COVID-19 patients; that is, imbalance of the gut microbiota after SARS-CoV-2 infection, disruption of the intestinal mucosal barrier, and release of inflammatory mediators caused by intestinal endotoxin and gut microbiota displacement can directly or indirectly promote the occurrence and development of the COVID-19 "inflammatory storm".
8. TCM regulation of the gut microbiota to prevent and treat COVID-19
8.1. Regulation of gut microbiota structure
Many studies have been reported on the direct regulation of the gut microbiota composition by TCM, including the regulation of the gut microbiota by a single TCM or a single extracted component, and on the forms of Chinese herbal medicinal ingredients that act. Guo et al. [174] found that extracts of Ginseng radix et rhizome rubra and Coicis semen could promote the growth of probiotics such as Lactobacillus and Bifidobacterium and inhibit the growth of pathogenic bacteria such as Escherichia, Staphylococcus, and Salmonella in vitro. Chang et al. [175] reported the effect of adding Ganoderma extract to the food of mice fed a high-fat diet and found that Ganoderma extract not only reduced the elevated ratio of the Firmicutes/Bacteroides phylum and the level of endotoxin-producing Proteobacteria induced by a high-fat diet but also maintained the integrity of the intestinal mucosal barrier and reduced the occurrence of endotoxaemia. The compatibility of essential oil from Atractylodis macrocephalae rhizoma and total ginsenosides reduced the relative abundance of opportunistic pathogens, such as Bacteroides, Anaerotruncus, and Desulfovibrio [176]. Dong et al. [177] found that Shenling Baizhu powder could effectively regulate antibiotic-induced gut microbiota imbalances and reduce endotoxin production by promoting the proliferation of probiotics, such as Lactobacillus and Bifidobacterium, in the intestinal tract. The single Chinese medicines Ginseng radix et rhizoma, berberine, Lonicerae japonicae flos, Rehmanniae radix praeparata, Artemisiae argyi folium, and emodin had proliferative effects on intestinal probiotics such as Lactobacillus in the gut microorganisms of different disease models [127]. HuoXiang Zhengqi [178] could support the growth of normal intestinal flora and promote the proliferation of beneficial bacteria (Bifidobacterium and Lactobacillus). Gegen Qinlian decoction could reverse the reduction in the richness of the gut microbiota, change its structure, and significantly increase the relative abundances of short-chain fatty acid (SCFA)-producing bacteria, including Akkermansia, Bacteroides, Clostridium, Ruminococcus, and Phascolarctobacterium [179]. Coptidis rhizoma extracts could improve intestinal microecology. Pathogens such as Enterobacter and Enterococcus were inhibited, and probiotics such as Lactobacilli and Bifidobacteria were notably promoted by Coptidis rhizoma extracts [180]. Fermented Yupingfeng polysaccharides exhibited greater beneficial effects in improving the gut microbiota, including augmenting flora diversity and the abundance of cellulolytic bacteria and reducing the abundance of Enterococcus spp and Streptococcus spp [181]. Based on 16S rDNA sequencing, Wu et al. [182] found that Qingfei Paidu decoction significantly modulated the composition of rat gut microbiota, upregulating the relative abundance of Romboutsia, Turicibacter, and Clostridium_sensu_stricto_1 and downregulating the relative abundance of norank_f_Lachnospiraceae, suggesting that the clinical efficacy of Qingfei Paidu decoction might be related to the regulation of gut microbiota composition.
8.2. Regulation of the intestinal mucosal barrier to prevent bacterial translocation
In addition to influencing the composition and number of gut microbiota constituents, TCM can indirectly affect the positioning of the flora by protecting the intestinal mucosal barrier. The normal intestinal mucosal barrier effectively prevents intestinal bacteria and endotoxin from crossing the intestinal mucosa, avoiding gut-derived infection. A variety of single TCMs, Chinese herbal medicinal ingredients, and TCM preparations have been found to have significant protective effects on the intestinal mucosal barrier. A modern pharmacological study showed that Rhei radix et rhizoma can effectively block the cascade response of inflammatory mediators, reduce the number of intestinal Escherichia coli bacteria in severely infected rats, inhibit the expression of Toll-like receptor 2 (TLR2) and TLR4 mRNA, block the intestinal inflammatory response, maintain the function of the intestinal mucosal immune barrier, and inhibit the translocation of bacteria in the early stage of septicaemia, with important therapeutic effects on acute lung injury [183]. Chang et al. [175] found that Ganoderma lucidum was able to protect the barrier function of the intestinal mucosa in mice, thus resisting the invasion of pathogenic microorganisms and preventing bacterial translocation. Fermented Yupingfeng polysaccharides (FYP) maintain intestinal barrier integrity and functionality by upregulating the mRNA levels of ZO-1, claudin, polyimmunoglobulin receptor, trefoil factor, and epidermal growth factor in the jejunum and ileum, achieving a protective effect on the mechanical and immune barriers of the intestinal mucosa [184]. Rhei radix et rhizoma can maintain intestinal mucosal immune barrier function by regulating the gut microbiota and suppressing intestinal inflammatory responses [183].
8.3. Regulating immunity
Recent studies have suggested that the causes of exacerbation and even death in viral infectious diseases such as COVID-19 are not only related to pulmonary viral infection but also closely related to immune dysfunction of the body [185], [186]. Therefore, in the process of preventing and treating COVID-19, it is very important to improve the body's own immune ability and regulate the immune balance. In addition to the direct or indirect antiviral effects of TCM in the prevention and treatment of COVID-19, the greatest advantage of TCM lies in its excellent immunomodulatory effects and reduced toxicity and side effects [187]. TCM has played an important role in the prevention and treatment of COVID-19. Among its effects, the immunomodulatory effect of TCM cannot be ignored. There have been many reports on the direct regulation of immunity by TCM, not only in the form of a single extracted ingredient or single Chinese medicine but also in the form of a compound prescription of TCM.
8.3.1. Single extracted ingredients of Chinese medicine regulate immunity
Poria Cocos polysaccharides [188] could improve immunity by reducing the content of IL-10 and regulating the secretion of Th1/Th2. Radix glycyrrhizae polysaccharides [189] could regulate cellular immunity disorders in tumour-bearing mice by decreasing the proportion of Treg cells and increasing the spleen lymphocyte transformation ratio. Bordbar et al. [190] found that glycyrrhizin could enhance the expression of the surface differentiation antigens CD80 and CD86 and the major histocompatibility complex Ⅱ (MHC Ⅱ) of dendritic cells (DCs), improve the production of IL-12, enhance the proliferation of allogeneic T cells, increase the expression of IFN-γ and IL-10, and regulate the Th1-type immune response. Forsythia leaf polysaccharides [191] could significantly increase the thymus index, spleen index, macrophage phagocytosis, spleen lymphocyte proliferation, serum IL-2 and IL-4 levels, haemolysin content, and number of haemolytic plaques in immunosuppressed mice induced by cyclophosphamide. Baicalein [192] could play an immunomodulatory role by balancing CD4+/CD8+ and regulating the balance of T lymphocytes and their Th1/Th2 and Th17/Treg subsets. Chrysophanol [193] had an immunomodulatory effect in immunocompromised mice, which could enhance the proliferation of T and B lymphocytes, increase the spleen index and thymus index, upregulate the levels of serum IL-2 and IL-4, and promote an increase in haemolysin levels and the production of antibody cells. Flos Lonicerae polysaccharides [194] could regulate the immune function of immunosuppressed mice induced by cyclophosphamide, effectively increasing the organ index of the mice, enhancing the activity of NK cells and the phagocytosis of macrophages, promoting the proliferation of spleen lymphocytes, and enhancing the immunomodulatory activity of the immunosuppressed mice. In addition, Jia et al. [195] found that Flos Lonicerae polysaccharides could promote IFN-γ secretion by regulating immune function, stimulating an increase in the immune organ index and enhancing immune function. Liu et al. [196] found that Astragalus polysaccharides could improve the phagocytosis rate of macrophages and the spleen and thymus indices of mice.
8.3.2. Single Chinese medicines regulate immunity
Astragali radix, Scutellariae radix, Isatidis radix, Pulsatillae radix, Polygoni cuspidati rhizoma et radix, Herba hedyotidis, and Andrographis herba could regulate the balance of anti-inflammatory cytokines and proinflammatory cytokines in the lungs of mice infected with H1N1 influenza virus and then improve immune dysfunction, truncate the storm of cytokines, reduce the inflammatory injury of lung tissue, and promote the repair of inflammatory lesions of lung tissue [197], [198], [199], [200]. Astragali radix [197] reduced the mRNA expression of the proinflammatory cytokines TNF-α, IL-1, and IL-6, significantly increased the mRNA expression of the anti-inflammatory cytokines IL-10 and IFN-γ in the lungs of mice, and significantly inhibited and repaired immune-inflammatory injury in lung tissue in mice. Scutellariae radix [198] inhibited the protein and gene expression of TNF-α, IL-1 and IL-6 in the lungs, promoted the protein and gene expression of IL-10 and IFN-γ, reduced the immune-inflammatory injury of lung tissue, and promoted the repair of inflammatory lesions in lung tissue. Isatidis radix, Pulsatillae radix, Polygoni cuspidati rhizoma et tadix, and Herba hedyotidis can inhibit the expression of TNF-α, IL-1 and IL-6 and promote the expression of IL-10 and IFN-γ in the lung [199]. Andrographis herba [200] effectively regulated the concentrations of TNF-α, INF-γ and IL-10 in the peripheral blood of mice, increased the percentage of CD3+ T lymphocytes, regulated the CD4+/CD8+ ratio, improved the cellular immune function of mice, and regulated the balance of proinflammatory and anti-inflammatory factors.
8.3.3. Chinese medicine compound prescriptions regulate immunity
Clinical studies have shown that Qingfei Paidu decoction can significantly improve the CD3 + and CD4 + cell counts of COVID-19 patients and enhance the body's immune function [107], [201]. Toujie Quwen granules [80] could significantly increase the CD4 + count and the ratio of CD4 + /CD8 + in patients with COVID-19, promote the recovery of immune function and reduce the injury of cellular immunity. Lianhua Qingwen capsules [202] significantly inhibited the phosphorylation of p65 protein in the NF-κB pathway and significantly reduced the expression levels of TNF-α, IL-6, KC, MCP-1, IL-1β, and IP-10 in the lung tissue of H1N1-infected mice, suggesting that its immunomodulatory effect might be related to its inhibition of the NF-κB pathway. Jinhua Qinggan granules reduced the levels of serum CRP and IFN-γ in patients with influenza and improved immune function [203], [204]. Xuebijing injection [205] reduced the levels of TNF-α and IL-6 in the lungs of mice infected with the influenza virus. In addition, clinical studies showed that, compared with human immunoglobulin alone, Xuebijing injection combined with human immunoglobulin could significantly shorten the antipyretic time, coughing, and asthma regression time and significantly reduce the levels of hs-CRP, IL-6, and TNF-α in patients with severe viral pneumonia [206]. Reduning injection [207], [208] increased the level of IFN-γ in the lung tissue of virus-infected mice, decreased the levels of IL6 and TNF-α, decreased the levels of phosphorylated nuclear factor kappa B inhibitor protein (p-IκB) and NF-κB protein, decreased the expression of IL-1β mRNA and increased the gene expression of interferon-induced transmembrane protein 3 (IFITM3) and mitochondrial antiviral signal protein (MAVS). Tanreqing injection [209], [210], [211] could significantly improve the pathological injury of lung tissue in virus-infected mice, enhance the function of T and B lymphocytes, increase the content of IL-4 and IFN-γ in the lungs, downregulate the transcriptional activity of the transcription factor NF-κB, inhibit the content of TNF-α in the lungs, regulate the balance of Th1 and Th2 cells, reduce lung injury and corresponding inflammatory reactions, and enhance antiviral immune function. Buzhong Yiqi decoction [212] could significantly increase the serum CD3+ and CD4+ cell counts and the ratio of CD4+/CD8+ in patients with recurrent respiratory tract infections and improve the immune function of patients. Maxing Shigan decoction [213] upregulated the body mass, spleen index, and thymus index of mice infected with influenza virus, reduced the levels of TNF-α, IL-1β, and IL-6 in the lungs, downregulated the expression of MyD88 and tumour necrosis factor receptor-associated protein 6 (TRAF6) mRNA and protein in lung tissue, increased the level of IL-2 in lung tissue and decreased the levels of IL-4 and TNF-α. Wang et al. [214] found that Sijunzi decoction could increase the levels of acetic acid, propionic acid, and butyric acid in the intestine; reduce the levels of the splenic index and serum inflammatory factors IL-2 and IFN-γ; and increase the ratio of CD+ 4/CD+ 8 in spleen-deficient mice, suggesting that Sijunzi decoction could increase the major SCFAs in the intestine and improve immunity in spleen-deficient rats.
8.4. Regulation of gut microbiota and its metabolites
The gut microbiota and their metabolites can affect the local or systemic immune function of the host. SCFAs are important products of dietary fibre fermentation by beneficial intestinal bacteria. The most common SCFAs are propionic acid, acetic acid, and butyric acid, with Bacteroidetes producing mainly propionic acid and acetate and Firmicutes producing mainly butyrate [215]. SCFAs not only have an important function in the metabolism of the body but also play a role in regulating inflammation and immune homeostasis [216]. Immunomodulatory effects associated with SCFAs include enhancing the barrier function of intestinal epithelial cells, reducing the induction of proinflammatory cytokines, stimulating the production of regulatory T cells, promoting intestinal mucosal homeostasis, preventing colonic inflammation, and enhancing the proinflammatory state of intestinal epithelial cells and leukocytes [217], [218]. SCFAs can induce the production and differentiation of regulatory T cells, thus promoting the production of the anti-inflammatory cytokine IL-10, and they can strongly reduce the release of the proinflammatory chemokines CCL3, CCL4, CCL5, CXCL9, CXCL10, and CXCL11, thereby inhibiting leukocyte migration with a strong anti-inflammatory effect [219]. Studies have found that Firmicutes is an important source of butyrate, which is a major energy substrate for cells. The decrease in the ratio of Firmicutes to Bacteroidetes could directly affect the metabolism of dietary fibre by the gut microbiota, resulting in lower concentrations of SCFAs, and the decrease in Firmicutes could induce or exacerbate local inflammatory responses [220]. Prevotella, Ruminococcus, Coprococcus, Clostridium butyricum, and Lactobacillus all increase the content of SCFAs in the intestine, induce the production and differentiation of regulatory T cells in the intestine of mice, promote IL-10 production, and exert inflammatory inhibitory effects [221], [222], [223]. In contrast, overgrown Escherichia coli or Klebsiella pneumonia could promote the expression of chemokines such as CCL5 and CXCL10, thereby aggravating the degree of inflammatory injury [224]. A previous study showed that influenza A virus infection could cause structural disorders of the gut microbiota and immune dysfunction in mice, and Maxingshigan decoction had a protective effect against influenza virus-induced intestinal immune damage by upregulating the relative abundance of Firmicutes, Lactobacillus, and Coprococcus and downregulating the relative abundance of Proteobacteria and Escherichia, regulating the structure of the gut microbiota and affecting the production of chemokines [225]. Other studies have indicated that Chinese medicine could increase the production of SCFAs by modulating the gut microbiota to improve the barrier function of the intestine and inhibit the inflammatory response of the intestinal mucosa [179], [226]. Zhang et al. [227] reported that Shenling Baizhu powder could increase the relative abundance of SCFA-producing bacteria (including Bifidobacterium and anaerobic bacteria) in the intestine of obese rats, reduce the levels of serum endotoxin, TNF-α, and IL-1β, and reduce systemic inflammation. TCM regulates the gut microbiota to prevent and treat COVID-19, as shown in Fig. 4.
9. Conclusions and prospects
In the prevention and control of this epidemic, early intervention, full participation, and classified treatment of TCM have made important and lasting contributions to the comprehensive control of the COVID-19 epidemic. Clinical studies have confirmed that TCM has certain advantages in improving the clinical symptoms of COVID-19 patients, inhibiting the storm of inflammatory factors, promoting the absorption of pulmonary inflammation, shortening the course of treatment, and promoting recovery. Currently, the focus and difficulties in the treatment of COVID-19 concern mainly the more severe and critical cases, which are also the main factors directly affecting mortality [27]. There is no definite and effective treatment for the more severe and critical COVID-19 patients in modern medicine, but the intervention of TCM or integrated traditional Chinese and Western medicine according to the clinical symptoms of the more severe and critical COVID-19 patients can help to reduce mortality and improve prognosis [22], [25], [27], [228]. The gut microbiota in severe and critical COVID-19 patients is disordered, rendering it prone to bacterial infection and further aggravating the disease. Therefore, it is essential to maintain the balance of the gut microbiota.
There are many relationships between the gut microbiota and COVID-19. On the one hand, COVID-19 patients have gut microbiota disorders and intestinal barrier function damage; on the other hand, the gut microbiota can regulate the local and systemic immune system, becoming the turning point of the prognosis of the disease. Therefore, restoring and regulating the balance of gut microbiota are helpful for the rehabilitation of COVID-19 patients. In the Guideline on Diagnosis and Treatment of COVID-19 (trial 8th edition) [15], formulated by the National Health Commission of the People’s Republic of China, it is mentioned that, for more severe and critical COVID-19 patients, microecological regulators should be selected in a timely and reasonable manner to restore and maintain intestinal homeostasis and prevent bacterial infection. In addition, the "four antibodies and two balances" suggested by the academician Lanjuan Li, that is, anti-virus, anti-shock, anti-hypoxaemia, anti-secondary infection, maintaining water-electrolyte acid-base balance and microecological balance, also clearly indicate the importance of using microecological agents to maintain the balance of intestinal bacteria in improving patients' immunity and preventing secondary infection. TCM plays a positive role in promoting the prognoses of patients with coronavirus infection or COVID-19 by maintaining the dynamic balance between the type and number of the gut microbiota and improving body immunity, but its mechanism remains to be further elucidated. From the perspective of TCM, research on maintaining the gut microbiota balance and correcting gut microbiota imbalances and finding new targets and new ideas for the treatment of COVID-19, whether for the treatment of the sick or the health care of the undiseased, are of great significance.
CRediT authorship contribution statement
Shuping Wang was responsible for the entire manuscript; Zhihua Yang, Yangxi Liu, Lin Wang and Shanshan Lin conceived, designed, wrote and revised the manuscript; Zhihua Yang and Yangxi Liu have contributed equally to this work and shared first authorship; Xiangdong Dai, Haifeng Yan contributed to draw diagram; Zhao Ge, Qiuan Ren, Hui Wang, Feng Zhu revised the manuscript and discussed interpretation. All authors have read and approved the final submission..
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgments
The study was supported by the Tianjin Research Innovation Project for Postgraduate Students (No. 2021YJSB294) and Graduate Research innovation project of Tianjin University of Traditional Chinese Medicine (No. YJSKC-20211012).
References
- 1.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D., Sidorov I.A., Sola I., Ziebuhr J. Severe acute respiratory syndrome-related coronavirus: the species and its viruses – a statement of the coronavirus study group. bioRxiv. 2020 〈https://www.biorxiv.org/content/10.1101/2020.02.07.937862v1〉 [Google Scholar]
- 3.WHO, Novel Coronavirus (2019-nCoV) Situation Report-22 [EB/OL], 2020. 〈https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/〉.
- 4.WHO, Statement on the Second Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV), 2020. 〈https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov)〉. (Accessed 28 April 2020).
- 5.World Health Organization, WHO [EB/OL]. 〈https://www.who.int/emergencies/diseases/novel-coronavirus-2019〉.
- 6.Li M., Lou F., Fan H. SARS-CoV-2 variants of concern delta: a great challenge to prevention and control of COVID-19. Signal Transduct. Target. Ther. 2021;6(1):349. doi: 10.1038/s41392-021-00767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hakim A., Hasan M.M., Hasan M., Lokman S.M., Azim K.F., Raihan T., Chowdhury P.A., Azad A.K. Major insights in dynamics of host response to SARS-CoV-2: impacts and challenges. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.637554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren Z., Wang H., Cui G., Lu H., Wang L., Luo H., Chen X., Ren H., Sun R., Liu W., Liu X., Liu C., Li A., Wang X., Rao B., Yuan C., Zhang H., Sun J., Chen X., Li B., Hu C., Wu Z., Yu Z., Kan Q., Li L. Alterations in the human oral and gut microbiomes and lipidomics in COVID-19. Gut. 2021;70(7):1253–1265. doi: 10.1136/gutjnl-2020-323826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin X., Lian J.S., Hu J.H., Gao J., Zheng L., Zhang Y.M., Hao S.R., Jia H.Y., Cai H., Zhang X.L., Yu G.D., Xu K.J., Wang X.Y., Gu J.Q., Zhang S.Y., Ye C.Y., Jin C.L., Lu Y.F., Yu X., Yu X.P., Huang J.R., Xu K.L., Ni Q., Yu C.B., Zhu B., Li Y.T., Liu J., Zhao H., Zhang X., Yu L., Guo Y.Z., Su J.W., Tao J.J., Lang G.J., Wu X.X., Wu W.R., Qv T.T., Xiang D.R., Yi P., Shi D., Chen Y., Ren Y., Qiu Y.Q., Li L.J., Sheng J., Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du M., Cai G., Chen F., Christiani D.C., Zhang Z., Wang M. Multiomics evaluation of gastrointestinal and other clinical characteristics of COVID-19. Gastroenterology. 2020;158(8):2298–2301. doi: 10.1053/j.gastro.2020.03.045. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang L., Gu S., Gong Y., Li B., Lu H., Li Q., Zhang R., Gao X., Wu Z., Zhang J., Zhang Y., Li L. Clinical significance of the correlation between changes in the major intestinal bacteria species and COVID-19 severity. Engineering. 2020;6(10):1178–1184. doi: 10.1016/j.eng.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeoh Y.K., Zuo T., Lui G.C., Zhang F., Liu Q., Li A.Y., Chung A.C., Cheung C.P., Tso E.Y., Fung K.S., Chan V., Ling L., Joynt G., Hui D.S., Chow K.M., Ng S.S.S., Li T.C., Ng R.W., Yip T.C., Wong G.L., Chan F.K., Wong C.K., Chan P.K., Ng S.C. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70(4):698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaibani P., D’Amico F., Bartoletti M., Lombardo D., Rampelli S., Fornaro G., Coladonato S., Siniscalchi A., Re M.C., Viale P., Brigidi P., Turroni S., Giannella M. The gut microbiota of critically Ill patients with COVID-19. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.670424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao J., Wang C., Zhang Y., Lei G., Xu K., Zhao N., Lu J., Meng F., Yu L., Yan J., Bai C., Zhang S., Zhang N., Gong Y., Bi Y., Shi Y., Chen Z., Dai L., Wang J., Yang P. Integrated gut virome and bacteriome dynamics in COVID-19 patients. Gut Microbes. 2021;13(1):1–21. doi: 10.1080/19490976.2021.1887722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Health Commission of the People’s Republic of China, Diagnosis and treatment plan for COVID-19 (trial 8th edition), Chin. J. Clin. Infect. Dis., 14(02), 2021, pp. 81–88.
- 16.Huang Y.F., Bai C., He F., Xie Y., Zhou H. Review on the potential action mechanisms of Chinese medicines in treating Coronavirus Disease 2019 (COVID-19) Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y.R., Wang Y.G., Jiao Y.Q., Chen M., Cheng T.F., Liu X.T., Pan F., Liu Q.Q. Research progress of traditional Chinese medicine syndrome differentiation and treatment of coronavirus disease 2019. J. Tradit. Chin. Med. 2020;61(21):1846–1851. [Google Scholar]
- 18.Wang Y.G., Qi W.S., Ma J.J., Ruan L.G., Lu Y.R., Li X.C., Zhao X., Zhang Z.D., Liu Q.Q. Clinical features and syndrome differentiation of novel coronavirus pneumonia in traditional Chinese medicine. J. Tradit. Chin. Med. 2020;61(21):1846–1851. [Google Scholar]
- 19.Huang M., Yang F.W., Zhang L., Zheng W.K., Zhang J.H. Experiences and strategies of traditional Chinese medicine in the treatment of coronavirus disease 2019: academician ZHANG Boli’s view on anti-epidemic in Wuhan. J. Tradit. Chin. Med. 2020;16(24):2117–2120. [Google Scholar]
- 20.Lyu M., Fan G., Xiao G., Wang T., Xu D., Gao J., Ge S., Li Q., Ma Y., Zhang H., Wang J., Cui Y., Zhang J., Zhu Y., Zhang B. Traditional Chinese medicine in COVID-19. Acta Pharm. Sin. B. 2021;11(11):3337–3363. doi: 10.1016/j.apsb.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng W.K., Zhang J.H., Yang F.W., Huang M., Miao Q., Qi W.S., Wang Y.G., Liu Q.Q., Zhang B.L. Treatment of coronavirus disease 2019 ( COVID-19) from perspective of dampness-toxicity plagues. J. Tradit. Chin. Med. 2020;61(12):1024–1028. [Google Scholar]
- 22.Ren J.L., Zhang A.H., Wang X.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol. Res. 2020;155 doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang B.J., Zhang W., Zhou S., Peng W., Piao J.H., He J.Z., Liang T.X. Prevention and treatment of COVID-19 based on the theory of “acute deficiency syndrome”. J. Tradit. Chin. Med. 2021;62(09):826–828. [Google Scholar]
- 24.Li L., Wu Y., Wang J., Yan H., Lu J., Wan Y., Zhang B., Zhang J., Yang J., Wang X., Zhang M., Li Y., Miao L., Zhang H. Potential treatment of COVID-19 with traditional chinese medicine: what herbs can help win the battle with SARS-CoV-2? Engineering. 2021 doi: 10.1016/j.eng.2021.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Z.H., Wang B., Ma Q., Wang L., Lin Y.X., Yan H.F., Fan Z.X., Chen H.J., Ge Z., Zhu F., Wang H.J., Zhang B.N., Sun H.D., Feng L.M. Potential mechanisms of action of Chinese patent medicines for COVID-19: a review. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.668407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J. Pondering over COVID-19 prevented and treated by traditional Chinese medicine. J. Nanjing Univ. Tradit. Chin. Med. 2020;36(02):149–151. [Google Scholar]
- 27.Li Z.Y., Xie Z.J., Li H.C., Wang J.J., Wen X.H., Wu S.Y., Chen J., Zhang J.J., Li L., Guo Q.Q., Liu Q.P., Lan H., Jiang Y.P., Li D.M., Xu X.F., Song S.Y., Zhang M., Fang S., Lai W.D., Gao Y.N., Zhang F.Q., Luo W.Q., Lou Y., Chen W., Zhang X.F., Wang K.E., Zhou M.Q., He Y.F., Xi A.R., Gao Y., Zhang Y., Chen Y.L., Wen C.P. Guidelines on the treatment with integrated traditional Chinese medicine and western medicine for severe coronavirus disease 2019. Pharmacol. Res. 2021;174 doi: 10.1016/j.phrs.2021.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J., Shu T.T., Luo W.D., Huang M., Tang Y., Rao M.Y., Li H.Y., Wu D., Yang F.W., Liu J., Fan Y.B., Li X.C., Cheng C.X., Liu Q.Q., Zhang B.L. Association between early treatment with Chinese herbal medicine and clinical outcomes in patients with severe coronavirus disease 2019: a retrospective study. J. Tradit. Chin. Med. 2021;62(12) [Google Scholar]
- 29.Liu M., Gao Y., Yuan Y., Yang K., Shi S., Zhang J., Tian J. Efficacy and safety of integrated traditional Chinese and western medicine for corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S.Y., Xie Y., Wang J.J., Zhang X.J., Fu L.L., Sun J., Zhao J.L., Zhang H.R., Jia K., Zhao Q.X., Li B., Li J.S. Single arm clinical study on 46 severe or critical cases of COVID-19 treated by integrated traditional Chinese and western medicine. J. Tradit. Chin. Med. 2021;62(01):44–48. [Google Scholar]
- 31.Lian J., Zhang S.J., Li G.L., Shang D., Wang Q.Y., Xu L.S., Shi Y., Liu X.X. Retrospective analysis of 38 cases with coronavirus disease 2019 treated by integrated traditional Chinese and western medicine. J. Tradit. Chin. Med. 2020;61(24):2126–2130. [Google Scholar]
- 32.Xia W.G., An C.Q., Zheng C.J., Zhang J.X., Huang M., Wang Y., Yang F.W., Duan C., LI Z.L., Liu Q.Q., Zhang B.L. Clinical observation on 34 patients with novel coronavirus pneumonia ( COVID - 19) treated with intergrated traditional Chinese and western medicine. J. Tradit. Chin. Med. 2020;61(05):375–382. [Google Scholar]
- 33.Pan G.T., Du T., Liu Y.H., Liu Y., Cheng Y.F., Han F., Chen Z.Y., He Z.Q., Xia Z.F., Yang S.L. Integrated traditional Chinese and western medicine in the treatment of 40 cases of critical patients with COVID-19. Acta Med. Univ. Sci. Technol. Huazhong. 2020;49(02):202–207. [Google Scholar]
- 34.Yang Q., Sun Q.G., Jiang B., Xu H.J., Luo M., Xie P., Huang W., Cong Z.W. Retrospective clinical study on treatment of COVID-19 patients with integrated traditional Chinese and western medicine. Chin. Tradit. Herb. Drugs. 2020;51(08):2050–2054. [Google Scholar]
- 35.Wen G.Y., Lu S., Yang X.H., Han Q., Cheng M.Y. Systematic review of integrated traditional Chinese and western medicine in treatment of COVID-19. Chin. Tradit. Herb. Drugs. 2021;52(22):6953–6961. [Google Scholar]
- 36.Qin L.X., Lu W.L., Yang M., Xiao Y., Li H., Sun Q.G., Wei L., He D.C., Li X.G., Zeng Y., Fei X.Y., Huang C.Q., Zeng J.Q., Xiang Y., Xu X.H., Zhang S.Y., Wang R.L. Clinical characteristics, drug treatments and prognoses in 605 patients with severe and critical coronavirus disease 2019 in Hubei,China: a multi-center, retrospective, cohort study. Chin. Arch. Tradit. Chin. Med. 2021;39(03):89–95. [Google Scholar]
- 37.Jin W., Lu W., Zhao W., Tang S.Y., Sang X.Y., Zhang L.S., Li L.Y., Liu D.F., Gu Y.L., Cheng K.M., Tang J.Y., Xie C.G. The efficacy of ecommended treatments with integrated chinese and western medicine on coronavirus disease 2019 ( COVID-19) in Sichuan: a clinical trial observation. Pharmacol. Clin. Chin. Mater. Med. 2020;36(06):6–10. [Google Scholar]
- 38.Wang F., Guo Y., Jiao L.W., Zhang X.Y., Liang N., Guan L., Zong X.Y., Luan X.J., Zhang L., Wang J.Y., Chen R.B., Li H.Z., Fan Y.P., Zhao C., Xiao F.R., Huang J., Xiao Y.H., Wang Y.Z., Zhang L.S., Hu Y.H., Sun S., Wang M.X., Bai W.G., Ma Y., Shi N.N., Ge Y.W., Zhang H.M., Lin G., Wang Y.P., Wang Y.Y. Qingfei Paidu Decoction combined with conventional therapy for 50 severe cases of coronavirus disease 2019 (COVID-19): a retrospective study. J. Tradit. Chin. Med. 2021;62(20):1801–1805. [Google Scholar]
- 39.Han Y.Y., Zhao M.R., Shi Y., Song Z.H., Zhou S.P., He Y. Application of integrative medicine protocols in treatment of coronavirus disease 2019. Chin. Tradit. Herb. Drugs. 2020;51(04):878–882. [Google Scholar]
- 40.Li W.N., Ba Y.M., Wang L.Q., Tao R., Zuo X.H., Shi Q., Wang X.Q., Wu X., Lu D.B., Zhang X.R., Feng Y., Hu G.M., Jiang N., Yu B.B., Zhu X.Y., Xie L.H. The role of syndrome differentiation in traditional Chinese-western medicine combined diagnosis and treatment of 105 cases of COVID-19. J. Jinan Univ. Nat. Sci. Med. Ed. 2020;41(05):383–390. [Google Scholar]
- 41.Zhang A., Li Y.P., Qiu M., liu B.H., Chen Z.P., Wan P., Tao Y., Wang H., Wei D.R., Li Q.T., Qin Y.L. Impact of the timing of traditional Chinese medicine therapy on the therapeutic effect and prognosis of severe coronavirus disease 2019. Acta Acad. Med. Sin. 2020;42(04):521–530. doi: 10.3881/j.issn.1000-503X.12915. [DOI] [PubMed] [Google Scholar]
- 42.Su Q.W., Qiu M.Y., Tao C.H., Zhou T.T., Zhang S.C., Hu J. Clinical analysis of 75 cases of COVID-19 treated by integrated traditional Chinese and western medicine. Lishizhen Med. Mater. Med. Res. 2020;31(06):1450–1453. [Google Scholar]
- 43.Xiao M., Tian J., Zhou Y., Xu X., Min X., Lv Y., Peng M., Zhang Y., Yan D., Lang S., Zhang Q., Fan A., Ke J., Li X., Liu B., Jiang M., Liu Q., Zhu J., Yang L., Zhu Z., Zeng K., Li C., Zheng Y., Wu H., Lin J., Lian F., Li X., Tong X. Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: a randomized controlled trial. Pharmacol. Res. 2020;161 doi: 10.1016/j.phrs.2020.105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lyu R.B., Wang W.J., Li X. Clinical observation on Lianhua Qingwen granules combined with western medicine conventional therapy in the treatment of 63 suspected cases of coronavirus disease 2019. J. Tradit. Chin. Med. 2020;61(08):655–659. [Google Scholar]
- 45.Fang F., Yang L., Qin S.C., Jiao R. Clinical efficacy of traditional Chinese medicine Lianhua Qingwen granules in 42 suspected cases of children with Corona Virus Disease 2019. Chin. J. New Drugs. 2020;29(24) [Google Scholar]
- 46.Zheng J., L, Liu B.J. Clinical observation of Xiaochaihu Decoction combined with Yupingfeng powder in the treatment of suspected cases of COVID-19. J. Liaoning Univ. Chin. Med. 2021;23(02):134–137. [Google Scholar]
- 47.Xie X., Song Y., Ran C.S., Chen S.Y., Wei D.R., Wang C.H., Zhou Z.W., Ran J., Xiang Y., Yu Z.C., Zhao Y.H., Luo X.Q., Tao W.Q., Li Y.P., Li Y.P., Tang J., Liu H.B. Clinical research of Chaihu Ganlu Decoction on COVID-19 asymptomatic infected patients. J. Emerg. Tradit. Chin. Med. 2020;29(11):1888–1890. [Google Scholar]
- 48.Wang Y.L., Xue J., Dai E.H., Xu Z.G., Feng C.X., Liu H.D., Gao H.X., Zhao L. Clinical study on the treatment of patients with novel coronavirus pneumonia and asymptomatic infection with integrated traditional Chinese and western medicine. Hebei J. Tradit. Chin. Med. 2020;42(05):645–649. [Google Scholar]
- 49.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Zhong N.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18) doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun Y.N., Lu W.L., Li H., Xiao Y., Yang M., Yang H.J., Gao Q.H., Yang Z.Q., Shou Z.X., Hu J.C., Ma Y.G., Luo Z.W., Cheng B.J., Liu L., Sheng F., Zhang S.Y., Liu Z.Y., Xu X.H., Zhao Z., Zhang H.L., Long Y., Mei Q.Q., Shi R.W., Liu H.G. Qingfei Paidu Decoction for treatment of mild / moderate COVID-19 in 295 cases: a multi-centered. J. Tradit. Chin. Med. 2021;62(07):599–603. [Google Scholar]
- 51.Cheng D.Z., Li Y. Clinical effectiveness and case analysis in 54 NCP patients treated with Lanhuaqingwen granules. World Chin. Med. 2020;15(02):150–154. [Google Scholar]
- 52.Cheng D.Z., Wang W.J., Li Y., Wu X.D., Zhou B., Song Q.Y. Analysis of curative effect of 51 patients with novel coronavirus pneumonia treated with Chinese medicine Lianhua Qingwen: a multicentre retrospective study. Tianjin J. Tradit. Chin. Med. 2020;37(5):509–516. [Google Scholar]
- 53.Yao K.T., Liu M.Y., Li X., Huang J.H., Cai H.B. Retrospective clinical analysis on treatment of coronavirus disease 2019 with traditional chinese medicine Lianhua Qingwen. Chin. J. Exp. Tradit. Med. Formula. 2020;26(11):8–12. [Google Scholar]
- 54.Duan C., Xia W.G., Zheng C.J., Sun G.B., LI Z.L., Li Q.L., Li P., Zhang H.L., Yang W.G., Zhang B.L., Liu Q.Q. Clinical observation on Jinhua Qinggan granule combined with conventional western medicine therapy in treating mild cases of coronavirus disease 2019. J. Tradit. Chin. Med. 2020;61(17):1473–1477. [Google Scholar]
- 55.Qu X.K., Hao S.L., Ma J.H., Wei G.Y., Song K.Y., Tang C., Gao Y.F., Liang S.Q., Du W.J. Observation on clinical effect of Shufeng Jiedu capsule combined with arbidol hydrochloride capsule in treatment of COVID-19. Chin. Tradit. Herb. Drugs. 2020;51(05):1167–1170. [Google Scholar]
- 56.Qu X.K., Tang C., Hao S.L., Ma J.H., Wei G.Y., Song K.Y., Gao Y.F., Liang S.Q., Du W.J. Observation on the clinical effect of Shufeng Jiedu capsule combined with Arbidol in the treatment of COVID-19. J. China Prescr. Drug. 2021;19(03):6–8. [Google Scholar]
- 57.Qu Y.F., Fang W., Jin Y.Z., Qin C., Niu X.C., Zhang N., Zhang J.R. Forty cases of common COVID-19 treated with modified ephedra and apricot kernel and gypsum and licorice decoction combined with Western medicine routine treatment. Henan Tradit. Chin. Med. 2020;40(05):666–669. [Google Scholar]
- 58.Zhang X.Y., Lv L., Zhou Y.L., Xie L.D., Xu Q., Zou X.F., Ding Y., Tian J., Fan J.L., Fan H.W., Yang Y.X., Ye X.Q. Efficacy and safety of Xiyanping injection in the treatment of COVID-19: a multicenter, prospective, open-label and randomized controlled trial. Phytother. Res. 2021;35(8):4401–4410. doi: 10.1002/ptr.7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y.H., Zhang A.J., Wang J.K., Zhang Y.G., Suo X.Y., Wang J.Q., Zhang J.Z., Meng Z.Q. Clinical efficacy of Jinbei oral liquid in the treatment of COVID-19 by retrospective analysis. Pharmacol. Clin. Chin. Mater. Med. 2021;37(05):7–10. [Google Scholar]
- 60.Yang H.B., Li Y.X., Cao L.M., Feng X.P., Zhang J.J., Jiang S.Y., Su Z.J., Lai C.S., Zhou D.L. 11 Cases of COVID-19 treated by modified Huoxiang Zhengqi combined with Western Medicine, Guangxi. J. Tradit. Chin. Med. 2020;43(03):1–4. [Google Scholar]
- 61.Fu X.X., Lin L.P., Tan X.H. Clinical observation on effect of Toujie Quwen granules in treatment of COVID-19. Chin. J. Exp. Tradit. Med. Formula. 2020;26(12):44–48. [Google Scholar]
- 62.He H.X., Zhu X.W., Fan H. Clinical observation of Jinqiang Xuanfei Jiedu mixture in the treatment of COVID-19. Res. J. Integr. Tradit. Chin. West Med. 2021;13(03):178–180. [Google Scholar]
- 63.Chen L., Cheng Y.G., Cheng Z.Q., Liu F., Wu J.H., Xia Y., Sheng B. Retrospective analysis on clinical efficacy of Ganlu Xiaodu Decoction combined with western medicine in treatment of common COVID-19 patients. Chin. J. Exp. Tradit. Med. Formula. 2020;26(19):60–67. [Google Scholar]
- 64.Sun H.M., Xu F., Zhang L., Wei C., Chen J.Y., Wang Q.X., Jia Z.H. Study on clinical efficacy of Lianhua Qingke granule in treatment of mild and ordinary COVID-19. Chin. J. Exp. Tradit. Med. Formula. 2020;26(14):29–34. [Google Scholar]
- 65.Zhang Y.L., Lei L., Xu Y., Wei D.R., Hu F. Clinical efficacy of Jinyinhua oral liquid in the treatment of 80 patients with coronavirus disease 2019. China Pharm. 2020;29(09):23–26. [Google Scholar]
- 66.Luo Z.H., Wang K.X., Zhang Y.L., Chen Z.Q., Chen B., Chen J., Zhou T., Gu X.L., Li C.L., Yan P., Tian L.L., Xu C.C., Chen B.L., Chen S., Qi Q. Clinical efficacy of sanxiaoyin modified and subtracted prescription in treatment of mild or moderate COVID-19 patients: based on retrospective analysis. Chin. J. Exp. Tradit. Med. Formula. 2021 [Google Scholar]
- 67.Hu F., Guo A.H., Huang L., Yu W.X., Liu G.F., Gao X.S., Yin J., Liu T.M., Yang Y., Yang Y. Jinyinhua liquid at different dosage combined with western medicine conventional treatment for common type COVID-19: a multicenter trial of 187 cases. J. Tradit. Chin. Med. 2021;62(06):510–515. [Google Scholar]
- 68.Yu H.Y., Ren X.H., Qi X.X., Zuo Q., Liu D. Efficacy study of arbidol,Qingfei Paidu Decoction,Lianhua Qingwen Capsule,and Jinye Baidu granules in the treatment of mild /moderate COVID-19 in a Fangcang shelter hospital. Pharmacol. Clin. Chin. Mater. Med. 2020;210(06):2–6. [Google Scholar]
- 69.Fang J., Li H., Du W., Yu P., Guan Y.Y., Ma S.Y., Liu D., Chen W., Shi G.C., Bian X.L. Efficacy of early combination therapy with lianhuaqingwen and arbidol in moderate and severe COVID-19 patients: a retrospective cohort study. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.560209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma Q., Xie Y., Wang Z., Lei B., Chen R., Liu B., Jiang H., Wang Y., Liu Q., Yang Z. Efficacy and safety of ReDuNing injection as a treatment for COVID-19 and its inhibitory effect against SARS-CoV-2. J. Ethnopharmacol. 2021;279 doi: 10.1016/j.jep.2021.114367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang X., Xue Y., Chen X., Wu J.M., Su Z.J., Sun M., Liu L.J., Zhang Y.B., Zhang Y.L., Xu G.H., Shi M.Y., Song X.M., Lu Y.F., Chen X.R., Zhang W., Chen Q. Effects of tanreqing capsule on the negative conversion time of nucleic acid in patients with COVID-19: a retrospective cohort study. J. Integr. Med. 2021;19(1):36–41. doi: 10.1016/j.joim.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang M.B., Dang S.S., Huang S., Li Y.J., Guo Y.L. Multi-center clinical observation of reyanning mixture in treatment of COVID-19. Chin. J. Exp. Tradit. Med. Formula. 2020;26(14):7–12. [Google Scholar]
- 73.Ping X.H., Xu H.L., Fu D.F., Zhou Y.F., Liu L., Xu H.X. Clinical observation of Jiawei Yupingfeng powder combined with western medicine in the treatment of COVID-19. Med. Forum. 2021;25(02):149–151. [Google Scholar]
- 74.Li Y.Z., Yao Y.J., Ji N., Chang Y.Y., Chang C., Li J. The clinical effect of Qingfei Decoction in treating 152 patients with COVID-19 combined with hypertension. J. Zhejiang Univ. Chin. Med. 2020;44(08):729–733. [Google Scholar]
- 75.He Q., Zhang Q.J., Gan X.W., Li X.G. Efficacy analysis of Buzhong Yiqi Decoction in the treatment of mild COVID-19. J. Emerg. Tradit. Chin. Med. 2021;30(03):385–387. [Google Scholar]
- 76.Dai G.C., Zhang X.R., Gao W.W., Hu W.Y., Ge T.Q., Zhang X., Zeng Y. Clinical effect of Yinqiao powder combined with modified Sanren decoctionin treatment of common-type COVID-19 with dampness-toxicity stagnationin lung:an analysis of 20 cases. J. Anhui Univ. Chin. Med. 2020;39(05):1–4. [Google Scholar]
- 77.Xiao Q., Jiang Y.J., Wu S.S., wang Y., An J., Xu W.P., Wu J.J. Value analysis of Shufeng Jiedu capsule combined with Abidol in the treatment of mild COVID-19. J. Emerg. Tradit. Chin. Med. 2020;29(5):756–758. [Google Scholar]
- 78.Zhang C.Y., Li Z.H., Zhang S., Wang W., Jiang X.Q. Clinical observation of Xuebijing injection in the treatment of COVID-19. Chin. J. Hosp. Pharm. 2020;40(09):964–967. [Google Scholar]
- 79.Shen P., Li J., Tu S., Wu Y., Peng Y., Chen G., Chen C. Positive effects of Lianhuaqingwen granules in COVID-19 patients: a retrospective study of 248 cases. J. Ethnopharmacol. 2021;278 doi: 10.1016/j.jep.2021.114220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fu X.X., Lin L.P., Tan X.H. Clinical study on 37 case of COVID- 19 treated with integrated traditional Chinese and western medicine. Tradit. Chin. Drug Res. Clin. Pharmacol. 2020;31(05):600–604. [Google Scholar]
- 81.Xin S., Cheng X., Zhu B., Liao X., Yang F., Song L., Shi Y., Guan X., Su R., Wang J., Xing L., Xu X., Jin L., Liu Y., Zhou W., Zhang D., Liang L., Yu Y., Yu R. Clinical retrospective study on the efficacy of Qingfei Paidu decoction combined with Western medicine for COVID-19 treatment. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zou B.L., Li M., Fan T.B., Wang Y.Y., Bian Y.J., Chen S.P., Chen Y., Chen Y.Y., Cong X.D., Dong G.J., Guo J., Hu L.J., Huang L.Q., Jiang J.X., Leng L.X., Li B., Li D.X., Li H., Li J., Lu C., Lu W.L., Qi W.S., Miao Q., Shi J.H., Shi H.X., Wang B., Wang G., Wang J., Wang W., Xie X.L., Ying R.Y., Xu C.Y., Xu M., Yan B., Yang J.L., Yang Z.X., Zhang L., Zhou Z.Q., Zhou Z.Q., Zhu H.N. Experience summary and suggestion on diagnosis and treatment program of traditional Chinese medicine treating severe type coronavirus disease 2019. J. Tradit. Chin. Med. 2020;61(15):1289–1293. [Google Scholar]
- 83.Zhang P., Fan G.T. Clinical study on the improvement of inflammatory cytokines in critically Ill patients with COVID-19 treated by Qingfei Paidu Decoction. World Sci. Tech-Mod. Tradit. Chin. Med. 2021;23(02):391–395. [Google Scholar]
- 84.Luo Z., Chen W., Xiang M., Wang H., Xiao W., Xu C., Li Y., Min J., Tu Q. The preventive effect of Xuebijing injection against cytokine storm for severe patients with COVID-19: a prospective randomized controlled trial. Eur. J. Integr. Med. 2021;42 doi: 10.1016/j.eujim.2021.101305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wen L., Zhou Z., G, Jiang D.X., Huang K. Effect of Xuebijing injection on inflammatory markers and disease outcome of coronavirus disease 2019. Chin. Crit. Care Med. 2020;32(04):426–429. doi: 10.3760/cma.j.cn121430-20200406-00386. [DOI] [PubMed] [Google Scholar]
- 86.Zhou S., Feng J., Xie Q., Huang T., Xu X., Zhou D., Zhang W., Sun S., Liu X., Wu X., Che J., Fan T., Zou D., Wang J., Zhan D., Peng D., Feng Y., Yu G., Yuan Z., Fang B. Traditional Chinese medicine shenhuang granule in patients with severe/critical COVID-19: a randomized controlled multicenter trial. Phytomedicine. 2021;89 doi: 10.1016/j.phymed.2021.153612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li S.Y., Li G.Y., Zhang H.R., Li B., Ci Z.H. Clinical efficacy and experiences of lung-toxin dispelling formula No.1 treating patients of corona virus disease 2019 type severe/type extremely severe. Chin. J. Exp. Tradit. Med. Formula. 2020;26(11):13–20. [Google Scholar]
- 88.Liu Y.J. Analysis of clinical efficacy of combination of western medicine with huashibaidu decoction in the treatment of severe COVID-19. Lab. Med. Clin. 2021;18(08):1152–1153. [Google Scholar]
- 89.Qin Y.L., Song Y.Y., Zhou L., Cui Y., Zhang A. Clinical efficacy of reduning injection combined with methylprednisolone in the treatment of severe coronavirus disease 2019. China Pharm. 2020;29(09):19–22. [Google Scholar]
- 90.Wang Y., Lu C., Li H., Qi W., Ruan L., Bian Y., Shi H., Song H., Tu S., Zhang Y., Bai T., Cao R., Hong K., Li H., Liu L., Lu S., Rong N., Liu Y., Fang J., Shi J., Yang W., Zhao B., Yang Y., Zhao Y., Li S., Fan T., Rong P., Huang L. Efficacy and safety assessment of severe COVID-19 patients with Chinese medicine: a retrospective case series study at early stage of the COVID-19 epidemic in Wuhan, China. J. Ethnopharmacol. 2021;277 doi: 10.1016/j.jep.2021.113888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ai Z., Zhou S., Li W., Wang M., Wang L., Hu G., Tao R., Wang X., Shen Y., Xie L., Ba Y., Wu H., Yang Y. “Fei Yan No. 1" as a combined treatment for COVID-19: an efficacy and potential mechanistic study. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.581277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu Y.H., Pan G.T., Shang L.R., Zhou F.Y., Yang S.L. Clinical efficacy of Danggui Shaoyao power in COVID-19 and network pharmacology study of its mechanism. Nat. Prod. Res. Dev. 2021;33(03):490–499. [Google Scholar]
- 93.He Q., Zhang Q.J. Analysis of clinical efficacy of shengmai powder in the treatment of COVID-19 with Qi and Yin deficiency syndrome in the recovery period. Acta Chin. Med. Pharmacol. 2021;49(03):84–86. [Google Scholar]
- 94.Ma Q., Qiu M., Zhou H., Chen J., Yang X., Deng Z., Chen L., Zhou J., Liao Y., Chen Q., Zheng Q., Cai L., Shen L., Yang Z. The study on the treatment of Xuebijing injection (XBJ) in adults with severe or critical Corona Virus Disease 2019 and the inhibitory effect of XBJ against SARS-CoV-2. Pharmacol. Res. 2020;160 doi: 10.1016/j.phrs.2020.105073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao J., Yang X., Wang C., Song S., Cao K., Wei T., Ji Q., Zheng W., Li J., Zhou X., Liu J. Yidu-toxicity blocking lung decoction ameliorates inflammation in severe pneumonia of SARS-COV-2 patients with Yidu-toxicity blocking lung syndrome by eliminating IL-6 and TNF-a. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tian Y., Ma Z., R, Bi Y.F., Sun H.Y., Zhu Z.G., Su L.S., Zhang Q.X. Clinical study on the treatment of early COVID-19 based on Lianhua Qingwen capsule with integrated Chinese and Western medicine. Tianjin J. Tradit. Chin. Med. 2021;38(10):1236–1240. [Google Scholar]
- 97.Wang J.Y., LI H.Z., Guo Y.N., Jiao L.W., Zong X.Y., Liang N., Zhang H.L., Chen Y.X., Li X.L., Liu G.X., Ma Y., Shi N.N., Wang Y.P., Ge Y.W. Qingfei Paidu Decoction combined with western routine treatment for elderly patients with coronavirus disease 2019: a retrospective study. J. Tradit. Chin. Med. 2021;62(20):1788–1794. [Google Scholar]
- 98.Li H.Z., Wang J.Y., Guo Y.N., Jiao L.W., Zong X.Y., Liang N., Zhang H.L., Chen Y.X., Li X.L., Liu G.X., Ma Y., Shi N.N., Wang Y.P., Ge Y.W. Qingfei Paidu Decoction combined with western medicine routine treatment for coronavirus disease 2019 with hypertension: a retrospective study of 119 cases. J. Tradit. Chin. Med. 2021;62(20) [Google Scholar]
- 99.Chen R.B., Shi N.N., Li H.Z., Jiao L.W., Ma Y., Liu B., Xiong Y.B., Luan X.J., Wu G.H., Li J.K., Zhang X.Y., Zhu J.T., Xiao F.R., Wang J.Y., Li Q.T., Lei X., Huang J., Huang Q.H., Wang Y.D., Wang X.Y., Xiao Y.H., Liu H.D., Liu J.P., Hou Y.L., Zhang L.S., Xie H.J., Bai W.G., Zhang H.M., Wang Y.P., Wang Y.Y. Qingfei Paidu Decoction combined with western medicine routine treatment for coronavirus disease 2019 (COVID-19) concomitant with chronic hepatitis B: a multi-center retrospective study. J. Tradit. Chin. Med. 2021;62(19):1694–1701. [Google Scholar]
- 100.Zhang L., Wang F., Ma Y., Dong L., Liu S.H., Chen G.K., Gao H.J., Ge Y.W., Wang Y.P., Zhang H.M. A retrospective study on Qingfei Paidu Decoction combined with routine treatment for coronavirus disease 2019 (COVID-19) with cardiovascular and cerebrovascular diseases in 124 cases. J. Tradit. Chin. Med. 2021;62(19):1702–1708. [Google Scholar]
- 101.Jiao L.W., Guo Y.N., Zong X.Y., Gong Z.Y., Wang J.Y., Li H.Z., Liang N., Zhang H.L., Chen Y.X., Li X.L., Liu G.X., Ma Y., Shi N.N., Wang Y.P., Ge Y.W. Patients with coronavirus disease 2019(COVID-19)and type 2 diabetes treated by Qingfei Paidu Decoction in combination with western medicine routine method: a retrospective study of 49 cases. J. Tradit. Chin. Med. 2021;62(19):1709–1714. [Google Scholar]
- 102.Xiong W.Z., Wang G., Du J., Ai W. Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19:A pilot randomized clinical trial. Integr. Med. Res. 2020;9(3) doi: 10.1016/j.imr.2020.100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu J., Yang W., Liu Y., Lu C., Ruan L., Zhao C., Huo R., Shen X., Miao Q., Lv W., Li H., Shi H., Hu L., Yang Z., Zhang L., Wang B., Dong G., Xian Y., Li B., Zhou Z., Xu C., Chen Y., Bian Y., Guo J., Yang J., Wang J., Qi W., Chen S., Chen Y., Yan B., Wang W., Li J., Xie X., Xu M., Jiang J., Wang G., Cong X., Zhu H., Shi J., Leng L., Li D., Guo L., Huang L. Combination of Hua Shi Bai Du granule (Q-14) and standard care in the treatment of patients with coronavirus disease 2019 (COVID-19): A single-center, open-label, randomized controlled trial. Phytomedicine. 2021;91 doi: 10.1016/j.phymed.2021.153671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shi T.F., Zhou G.C., Zhang L.Y., Niu F., Ke Y.C., Zhou T., Wang Q.S., Jin X.J., Liu D.L., Wei B.J., Zhang W.Q., Zhang Z.M., Liu Y.Q. Clinical efficacy of Xuanfei Huazhuo prescription on 40 cases of COVID-19. Chin. J. Exp. Tradit. Med. Formula. 2020;26(16):26–31. [Google Scholar]
- 105.Yang M.W., Chen F., Zhu D.J., Li J.Z., Zhu J.L., Zeng W., Qu S.L., Zhang Y. Clinical efficacy of matrine and sodium chloride injection in treatment of 40 cases of COVID-19. China J. Chin. Mater. Med. 2020;45(10) doi: 10.19540/j.cnki.cjcmm.20200323.501. [DOI] [PubMed] [Google Scholar]
- 106.Wang L.Q., Li W.N., Huang W., Zhou Z.M., Deng Y.L., Hu Y.L., Tao R., Zuo X.H., Feng Y., Lu D.B., Yu B.B. Clinical study of Gegen Qinlian pill in treating COVID-19. World Sci Tech-Mod. Tradit. Chin. Med. 2020;22(10):3509–3514. [Google Scholar]
- 107.Yu X.Y., Zhang S., Yan F.F., Su D.Z. Comparison of clinical efficacy of Qingfei Paidu decoction combined with western medicine in 43 cases and single western medicine in 46 cases in the treatment of COVID-19. J. Shandong Univ. Health Sci. 2020;58(12):47–53. [Google Scholar]
- 108.Shi N., Liu B., Liang N., Ma Y., Ge Y., Yi H., Wo H., Gu H., Kuang Y., Tang S., Zhao Y., Tong L., Liu S., Zhao C., Chen R., Bai W., Fan Y., Shi Z., Li L., Liu J., Gu H., Zhi Y., Wang Z., Li Y., Li H., Wang J., Jiao L., Tian Y., Xiong Y., Huo R., Zhang X., Bai J., Chen H., Chen L., Feng Q., Guo T., Hou Y., Hu G., Hu X., Hu Y., Huang J., Huang Q., Huang S., Ji L., Jin H., Lei X., Li C., Wu G., Li J., Li M., Li Q., Li X., Liu H., Liu J., Liu Z., Ma Y., Mao Y., Mo L., Na H., Wang J., Song F., Sun S., Wang D., Wang M., Wang X., Wang Y., Wang Y., Wu W., Wu L., Xiao Y., Xie H., Xu H., Xu S., Xue R., Yang C., Yang K., Yang P., Yuan S., Zhang G., Zhang J., Zhang L., Zhao S., Zhao W., Zheng K., Zhou Y., Zhu J., Zhu T., Li G., Wang W., Zhang H., Wang Y., Wang Y. Association between early treatment with Qingfei Paidu decoction and favorable clinical outcomes in patients with COVID-19: a retrospective multicenter cohort study. Pharmacol. Res. 2020;161 doi: 10.1016/j.phrs.2020.105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ni L., Wen Z., Hu X., Tang W., Wang H., Zhou L., Wu L., Wang H., Xu C., Xu X., Xiao Z., Li Z., Li C., Liu Y., Duan J., Chen C., Li D., Zhang R., Li J., Yi Y., Huang W., Chen Y., Zhao J., Zuo J., Weng J., Jiang H., Wang D.W. Effects of Shuanghuanglian oral liquids on patients with COVID-19: a randomized, open-label, parallel-controlled, multicenter clinical trial. Front. Med. 2021;15(5):704–717. doi: 10.1007/s11684-021-0853-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu Z., Li X., Gou C., Li L., Luo X., Zhang C., Zhang Y., Zhang J., Jin A., Li H., Zeng Y., Li T., Wang X. Effect of Jinhua Qinggan granules on novel coronavirus pneumonia in patients. J. Tradit. Chin. Med. 2020;40(3):467–472. doi: 10.19852/j.cnki.jtcm.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 111.Liu M.J., Tao Y., Wan P., Luo A. Clinical observation of 36 COVID-19 patients whose nucleic acid testing was turned negative after treatment with “Xue’s Fu Yang Zhu Shi Decoction cutting. J. Southwest Univ. Nat. Sci. Ed. 2020:31–35. [Google Scholar]
- 112.Ding X.J., Zhang Y., He D.C., Zhang M.Y., Tan Y.J., Yu A.R., Yu Q., Wu W., Yang W.C., Huang H.S., Liu L. Clinical effect and mechanism of Qingfei Touxie Fuzheng ecipe in the treatment of COVID-19. Herb. Med. 2020;39(05):640–644. [Google Scholar]
- 113.Liu Q.Q., XIA W.G., An C.Q., Liu X.C., Wang Y.G., Miao Q., Yang F.W., Zhang B.L. Refection on effects of integrated traditional chinese and western medicine on coronavirus disease 2019. J. Tradit. Chin. Med. 2020;61(06):463–464. [Google Scholar]
- 114.Yang H.Z., Lin R.C., Dong X., Guo G.H., Zhang B.E., Li R., Tian F.N., Li X.D. Xiang Huo spray combined with basic rehabilitation therapy in treating 60 cases of COVID-19 with syndrome of residual toxin during convalescence period. J. Tradit. Chin. Med. 2021;62(17):1509–1513. [Google Scholar]
- 115.Shi S.F., Fang Z.Y., Xiong K., Ye D.L., Wang W.M., Wu H., Chen Y.C., Wu D., Li Y.Z., Zhang Y.Q., Cui L., Chen M.Q., Wang B.H. Clinical study on comprehensive therapy of traditional Chinese Medicine in the treatment of 30 cases of deficiency of both Qi and Yin in COVID-19 ’s convalescent period. Jiangsu J. Tradit. Chin. Med. 2021;53(01):25–28. [Google Scholar]
- 116.Ma C.C., Li Y.M., Wu J.H. Observation of clinical culative effect before and after application of modified Wuye Lugen Decoction combined with moxibustionin patients with COVID-19 in recovery period. Asia-Pac. Tradit. Med. 2021;17(21):113–117. [Google Scholar]
- 117.Maynard C.L., Elson C.O., Hatton R.D., Weaver C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489(7415):231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hooper L.V., Dan R.L., Macpherson A.J. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Heiss C.N., Olofsson L.E. Gut microbiota-dependent modulation of energy metabolism. J. Innate Immun. 2018;10(3):163–171. doi: 10.1159/000481519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Donaldson G.P., Lee S.M., Mazmanian S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016;14(1):20–32. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Iddir M., Brito A., Dingeo G., Fernandez Del Campo S.S., Samouda H., La Frano M.R., Bohn T. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. 2020;12(6) doi: 10.3390/nu12061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Groves H.T., Cuthbertson L., James P., Moffatt M.F., Cox M.J., Tregoning J.S. Respiratory disease following viral lung infection alters the murine gut microbiota. Front. Immunol. 2018;9:182. doi: 10.3389/fimmu.2018.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hao Q., Dong B.R., Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2015;2:Cd006895. doi: 10.1002/14651858.CD006895.pub3. [DOI] [PubMed] [Google Scholar]
- 124.Feng W., Ao H., Peng C., Yan D. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol. Res. 2019;142:176–191. doi: 10.1016/j.phrs.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 125.Jia Q., Wang L., Zhang X., Ding Y., Li H., Yang Y., Zhang A., Li Y., Lv S., Zhang J. Prevention and treatment of chronic heart failure through traditional Chinese medicine: role of the gut microbiota. Pharmacol. Res. 2020;151 doi: 10.1016/j.phrs.2019.104552. [DOI] [PubMed] [Google Scholar]
- 126.Jin D.X., He J.F., Zhang K.Q., Luo X.G., Zhang T.C. EtOAc extract of H. attenuatum Choisy inhibits inflammation by suppressing the NF-κB and MAPK pathways and modulating the gut microbiota. Phytomedicine. 2019;57:292–304. doi: 10.1016/j.phymed.2018.12.037. [DOI] [PubMed] [Google Scholar]
- 127.Xu J., Chen H.B., Li S.L. Understanding the molecular mechanisms of the interplay between herbal medicines and gut microbiota. Med. Res. Rev. 2017;37(5):1140–1185. doi: 10.1002/med.21431. [DOI] [PubMed] [Google Scholar]
- 128.Cipriano M., Ruberti E., Giacalone A. Gastrointestinal infection could be new focus for coronavirus diagnosis. Cureus. 2020;12(3) doi: 10.7759/cureus.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rosa C.P., Pereira J.A., Cristina de Melo Santos N., Brancaglion G.A., Silva E.N., Tagliati C.A., Novaes R.D., Corsetti P.P., de Almeida L.A. Vancomycin-induced gut dysbiosis during Pseudomonas aeruginosa pulmonary infection in a mice model. J. Leukoc. Biol. 2020;107(1):95–104. doi: 10.1002/JLB.4AB0919-432R. [DOI] [PubMed] [Google Scholar]
- 131.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lin L., Jiang X., Zhang Z., Huang S., Zhang Z., Fang Z., Gu Z., Gao L., Shi H., Mai L., Liu Y., Lin X., Lai R., Yan Z., Li X., Shan H. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69(6):997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 134.Antonelli M., Martin-Loeches I., Dimopoulos G., Gasbarrini A., Vallecoccia M.S. Clostridioides difficile (formerly Clostridium difficile) infection in the critically ill: an expert statement. Intensive Care Med. 2020;46(2):215–224. doi: 10.1007/s00134-019-05873-x. [DOI] [PubMed] [Google Scholar]
- 135.Pan X., Zhou J., Chen Y., Xie X., Rao C., Liang J., Zhang Y., Peng C. Classification, hepatotoxic mechanisms, and targets of the risk ingredients in traditional Chinese medicine-induced liver injury. Toxicol. Lett. 2020;323:48–56. doi: 10.1016/j.toxlet.2020.01.026. [DOI] [PubMed] [Google Scholar]
- 136.Redd W.D., Zhou J.C., Hathorn K.E., McCarty T.R., Bazarbashi A.N., Thompson C.C., Shen L., Chan W.W. Prevalence and characteristics of gastrointestinal symptoms in patients with severe acute respiratory syndrome coronavirus 2 infection in the United States: a multicenter cohort study. Gastroenterology. 2020;159(2):765–767. doi: 10.1053/j.gastro.2020.04.045. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schmulson M., Dávalos M.F., Berumen J. Beware: gastrointestinal symptoms can be a manifestation of COVID-19. Rev. Gastroenterol. Mex. 2020;85(3):282–287. doi: 10.1016/j.rgmx.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.P. An, H. Chen, X. Jiang, J. Su, Y. Xiao, Y. Ding, H. Ren, M. Ji, Y. Chen, W. Chen, Clinical Features of 2019 Novel Coronavirus Pneumonia Presented Gastrointestinal Symptoms But without Fever Onset, Social Science Electronic Publishing, 2020. 〈https://ssrn.com/abstract=3532530〉.
- 139.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K., Washington State-nCo V.C.I.T. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gu J., Han B., Wang J. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020;158(6):1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vodnar D.C., Mitrea L., Teleky B.E., Szabo K., Calinoiu L.F., Nemes S.A., Martau G.A. Coronavirus disease (COVID-19) caused by (SARS-CoV-2) infections: a real challenge for human gut microbiota. Front. Cell. Infect. Microbiol. 2020;10 doi: 10.3389/fcimb.2020.575559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wan Y., Li J., Shen L., Zou Y., Hou L., Zhu L., Faden H.S., Tang Z., Shi M., Jiao N., Li Y., Cheng S., Huang Y., Wu D., Xu Z., Pan L., Zhu J., Yan G., Zhu R., Lan P. Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. Lancet Gastroenterol. Hepatol. 2020;5(6):534–535. doi: 10.1016/S2468-1253(20)30118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zuo T., Zhang F., Lui G.C.Y., Yeoh Y.K., Li A.Y.L., Zhan H., Wan Y., Chung A.C.K., Cheung C.P., Chen N., Lai C.K.C., Chen Z., Tso E.Y.K., Fung K.S.C., Chan V., Ling L., Joynt G., Hui D.S.C., Chan F.K.L., Chan P.K.S., Ng S.C. Alterations in gut microbiota of patients With COVID-19 during time of hospitalization. Gastroenterology. 2020;159(3):944–955. doi: 10.1053/j.gastro.2020.05.048. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hu K., Lin L., Liang Y., Shao X., Hu Z., Luo H., Lei M. COVID-19: risk factors for severe cases of the delta variant. Aging. 2021;13(20):23459–23470. doi: 10.18632/aging.203655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Perlman S. Another decade, another coronavirus. N. Engl. J. Med. 2020;382(8):760–762. doi: 10.1056/NEJMe2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Munster V.J., Koopmans M., van Doremalen N., van Riel D., de Wit E., Novel A. Coronavirus emerging in China - key questions for impact assessment. N. Engl. J. Med. 2020;382(8):692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 148.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ge X.Y., Li J.L., Yang X.L., Chmura A.A., Zhu G., Epstein J.H., Mazet J.K., Hu B., Zhang W., Peng C., Zhang Y.J., Luo C.M., Tan B., Wang N., Zhu Y., Crameri G., Zhang S.Y., Wang L.F., Daszak P., Shi Z.L. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503(7477):535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang M., Hu Z. Bats as animal reservoirs for the SARS coronavirus: hypothesis proved after 10 years of virus hunting. Virol. Sin. 2013;28(6):315–317. doi: 10.1007/s12250-013-3402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W., China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang H., Kang Z., Gong H., Xu D., Xu H. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. 2020 gutjnl-2020-320953. [Google Scholar]
- 155.Liang W., Feng Z., Rao S., Xiao C., Xue X., Lin Z., Zhang Q., Qi W. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69(6):1141–1143. doi: 10.1136/gutjnl-2020-320832. [DOI] [PubMed] [Google Scholar]
- 156.Hashimoto T., Perlot T., Rehman A., Trichereau J., Ishiguro H., Paolino M., Sigl V., Hanada T., Hanada R., Lipinski S., Wild B., Camargo S.M., Singer D., Richter A., Kuba K., Fukamizu A., Schreiber S., Clevers H., Verrey F., Rosenstiel P., Penninger J.M. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487(7408):477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vuille-dit-Bille R.N., Camargo S.M., Emmenegger L., Sasse T., Kummer E., Jando J., Hamie Q.M., Meier C.F., Hunziker S., Forras-Kaufmann Z., Kuyumcu S., Fox M., Schwizer W., Fried M., Lindenmeyer M., Gotze O., Verrey F. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids. 2015;47(4):693–705. doi: 10.1007/s00726-014-1889-6. [DOI] [PubMed] [Google Scholar]
- 158.Singer D., Camargo S.M., Ramadan T., Schafer M., Mariotta L., Herzog B., Huggel K., Wolfer D., Werner S., Penninger J.M., Verrey F. Defective intestinal amino acid absorption in Ace2 null mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;303(6):G686–G695. doi: 10.1152/ajpgi.00140.2012. [DOI] [PubMed] [Google Scholar]
- 159.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833. doi: 10.1053/j.gastro.2020.02.055. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu Q., Zhou Y.H., Yang Z.Q. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol. Immunol. 2016;13(1):3–10. doi: 10.1038/cmi.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. MMBR. 2012;76(1):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Konig M.F., Powell M.A., Staedtke V., Bai R.Y., Bettegowda C. Preventing cytokine storm syndrome in COVID-19 using α-1 adrenergic receptor antagonists. J. Clin. Investig. 2020;130(7) doi: 10.1172/JCI139642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir. Med. 2020;8(6):e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang F.S., Zhang C. What to do next to control the 2019-nCoV epidemic? Lancet. 2020;395(10222):391–393. doi: 10.1016/S0140-6736(20)30300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., Akdis C.A., Gao Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 166.Belkaid Y., Hand T.W. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhou F. Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China, Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi, 41(2), 2020, pp. 145–151. [DOI] [PubMed]
- 170.Xu R., Tan C., Zhu J., Zeng X., Gao X., Wu Q., Chen Q., Wang H., Zhou H., He Y., Pan S., Yin J. Dysbiosis of the intestinal microbiota in neurocritically ill patients and the risk for death. Crit. Care. 2019;23(1):195. doi: 10.1186/s13054-019-2488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dickson R.P., Singer B.H., Newstead M.W., Falkowski N.R., Erb-Downward J.R., Standiford T.J., Huffnagle G.B. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat. Microbiol. 2016;1(10):16113. doi: 10.1038/nmicrobiol.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hawlisch H., Belkaid Y., Baelder R., Hildeman D., Gerard C., Kohl J. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity. 2005;22(4):415–426. doi: 10.1016/j.immuni.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 173.Kohl J. Anaphylatoxins and infectious and non-infectious inflammatory diseases. Mol. Immunol. 2001;38(2–3):175–187. doi: 10.1016/s0161-5890(01)00041-4. [DOI] [PubMed] [Google Scholar]
- 174.Guo M., Ding S., Zhao C., Gu X., He X., Huang K., Luo Y., Liang Z., Tian H., Xu W. Red Ginseng and Semen Coicis can improve the structure of gut microbiota and relieve the symptoms of ulcerative colitis. J. Ethnopharmacol. 2015;162:7–13. doi: 10.1016/j.jep.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 175.Chang C.J., Lin C.S., Lu C.C., Martel J., Ko Y.F., Ojcius D.M., Tseng S.F., Wu T.R., Chen Y.Y., Young J.D., Lai H.C. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015;6:7489. doi: 10.1038/ncomms8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang J., Feng W., Zhang S., Chen L., Sheng Y., Tang F., He J., Xu X., Ao H., Peng C.. Ameliorative effect of Atractylodes macrocephala essential oil combined with Panax ginseng total saponins on 5-fluorouracil induced diarrhea is associated with gut microbial modulation. J. Ethnopharmacol. 2019 doi: 10.1016/j.jep.2019.111887. [DOI] [PubMed] [Google Scholar]
- 177.Dong K.Z., Gao Y.S., Qin N.E.J., Su L. Effects of Senlin Baishu San on the dysbacteriosis induced by antibiotics in mices. Chin. J. Exp. Tradit. Med. Formula. 2015;21(01):154–157. [Google Scholar]
- 178.Yang R., Ye Z.L., Chen C.L. Effect of Huoxiang ZhengQi on modulation of intestinal flora in mice. Chin. J. Ethnomed. Ethnopharm. 2019;28(24):34–36. [Google Scholar]
- 179.Liu C.S., Liang X., Wei X.H., Jin Z., Chen F.L., Tang Q.F., Tan X.M. Gegen Qinlian decoction treats diarrhea in piglets by modulating gut microbiota and short-chain fatty acids. Front. Microbiol. 2019;10:825. doi: 10.3389/fmicb.2019.00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cui X., Tao J.H., Jiang S., Wei X.Y., Xu J., Qian D.W., Duan J.A. Research on interaction between coptidis rhizoma extracts and intestinal bacteria. Chin. Tradit. Herb. Drugs. 2018;49(09):2103–2107. [Google Scholar]
- 181.Su C., Fan D., Pan L., Lu Y., Wang Y., Zhang M. Effects of Yu-Ping-Feng polysaccharides (YPS) on the immune response, intestinal microbiota, disease resistance and growth performance of Litopenaeus vannamei. Fish Shellfish Immunol. 2020;105:104–116. doi: 10.1016/j.fsi.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wu G.S., Zhong J., Zheng N.N., Wang C.R., Jin H.L., Ge G.B., Han J.Y., Gao Y., Sheng L.L., Zhang W.D., Li H.K. Investigation of modulating effect of Qingfei Paidu Decoction on host metabolism and gut microbiome in rats. China J. Chin. Mater. Med. 2020;45(15):3726–3739. doi: 10.19540/j.cnki.cjcmm.20200609.201. [DOI] [PubMed] [Google Scholar]
- 183.Yao P., Cui M., Li Y., Deng Y., Wu H. Effects of rhubarb on intestinal flora and toll-like receptors of intestinal mucosa in rats with severe acute pancreatitis. Pancreas. 2015;44(5):799–804. doi: 10.1097/MPA.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 184.Sun H., Ni X., Song X., Wen B., Zhou Y., Zou F., Yang M., Peng Z., Zhu H., Zeng Y., Wang H., Fu X., Shi Y., Yin Z., Pan K., Jing B., Zeng D., Wang P. Fermented Yupingfeng polysaccharides enhance immunity by improving the foregut microflora and intestinal barrier in weaning rex rabbits. Appl. Microbiol. Biotechnol. 2016;100(18):8105–8120. doi: 10.1007/s00253-016-7619-0. [DOI] [PubMed] [Google Scholar]
- 185.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang B., Zhou X., Qiu Y., Song Y., Feng F., Feng J., Song Q., Jia Q., Wang J. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15(7) doi: 10.1371/journal.pone.0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ma H.D., Deng Y.R., Tian Z., Lian Z.X. Traditional Chinese medicine and immune regulation. Clin. Rev. Allergy Immunol. 2013;44(3):229–241. doi: 10.1007/s12016-012-8332-0. [DOI] [PubMed] [Google Scholar]
- 188.Liu Y.Y., Chen Y.X., Hou A.J. Effect of carboxycyl pachymaran cytokines derived from T cells in mice. Pharmacol. Clin. Chin. Mater. Med. 2006;Z1:71–73. [Google Scholar]
- 189.Li X.B., He X.J., Liu B., Xu L., Ju D.H., Jiang M., Lv A.P. Immunoregulatory function of radix glycyrrhizae polsaccharide in tumor-bearing mice. J. Chin. Integr. Med. 2010;8(04):363–367. doi: 10.3736/jcim20100411. [DOI] [PubMed] [Google Scholar]
- 190.Bordbar N., Karimi M.H., Amirghofran Z. The effect of glycyrrhizin on maturation and T cell stimulating activity of dendritic cells. Cell Immunol. 2012;280(1):44–49. doi: 10.1016/j.cellimm.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 191.Zhang X.X., Cai Y., Wu Z.M., Gao Y. Investigation on the influence of polysaccharide from Forsythia suspensa leaves on immunological function of mice. Food Res. Dev. 2015;36(23):25–28. [Google Scholar]
- 192.Wu Y., Jin Y.Z., Wu J., Yu X.F., Hao Y. Antiviral effects of major constituents of Radix Scutellariae on influenza A virus (FM1) in vitro. J. Beijing Univ. Chin. Med. 2010;33(08):541–545. [Google Scholar]
- 193.Song B.C., Jiang B., J.M. M, Han Y., Ma L., Chen Z.B. Immunoprotection effect of chrysophanol on cyclophosphamide induced immunosuppression in mice. J. Heilongjiang Bayi Agric. Univ. 2019;31(06):66–71. [Google Scholar]
- 194.Zhou X., Dong Q., Kan X., Peng L., Xu X., Fang Y., Yang J. Immunomodulatory activity of a novel polysaccharide from Lonicera japonica in immunosuppressed mice induced by cyclophosphamide. PLoS One. 2018;13(10) doi: 10.1371/journal.pone.0204152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jia W., Mao S.M., Zhang P.P., Yan G.L., Jin J., Liu Y.H. Study on antiviral effect of Lonicera japonica thumb polysaccharide in vivo. J. Liaoning Univ. Chin. Med. 2018;20(06):25–27. [Google Scholar]
- 196.Liu Y.H., Zhao Z.Q., Liu S.Y., Zhang Q.B. Effects of astragalin polysaccharides on immune function in vivo. Hebei Med. J. 2015;37(04):485–487. [Google Scholar]
- 197.Xu H.R., Wang C.X., Wang L., Zhou P.A., Yin R.Y., Jiang L.D., Wang H.F. Mechanism of tonifying Qi traditional Chinese medicines contained in Yiqi Qingwen Jiedu mixture against influenza immune inflammatory injury. China J. Chin. Mater. Med. 2014;39(20):4020–4026. [PubMed] [Google Scholar]
- 198.Xu R.H., Li Y.L., Wang C.X., Liu G.X., Liu C., Zhang L.L., Li Q.M., Li M., Cao H.Y., Cheng M., Wang H.P. Effect of Scutellariae Radix on expression of inflammatory cytokine protein and gene in lung of mice with viral pneumonia caused by influenza virus FM1 infection. China J. Chin. Mater. Med. 2019;44(23):5166–5173. doi: 10.19540/j.cnki.cjcmm.201910.09.401. [DOI] [PubMed] [Google Scholar]
- 199.Wang C.X., Cao H.Y., Cheng M., Xu H.R., Wang M.Z., Xu L.L., Yu H.Y., Li Q. The influence of representative herbs of clearing and detoxifying drugs effect on inflammatory cytokines expression of mice lung homogenate infected by influenza virus FM1. Chin. J. Exp. Clin. Virol. 2012;26(06):415–418. [PubMed] [Google Scholar]
- 200.Fan Q.M., Pan X., He Y.T., Zhu Z.F., Xiao M.F., Zhou J., Zhou Y.Q., Deng K.X., Meng L., He F.Y. Research progress on immunomodulatory effect of Chinese materia medica and prescriptions on viral pneumonia. Chin. Tradit. Herb. Drugs. 2020;51(08):2065–2074. [Google Scholar]
- 201.Wang Q.L., Sun L.F., Zhao M.F., Li Y.Y., Zhu Y.L. The application effect of Qingfei Paidu decoction in the treatment of patients with COVID-19 with the syndrome of deficiency of both Qi and Yin. J. Tradit. Chin. Med. 2021;36(05):910–914. [Google Scholar]
- 202.Mo H.Y., Yang Z.F., Zheng J., P, Mo Z.Y., Li Y.M., Xiao Z.L. Experimental study of Lianhua Qingwen capsule on prevention and treatment of Influenza virus FM1 infection in mice. Chin. Tradit. Pat. Med. 2008;31(08):1230–1233. [Google Scholar]
- 203.Li G.Q., Zhao J., Tu Z.T., Li J.B., LIU Q.Q., Shi L.Q., Miao Q., Yuan H.Q., Liu X.Q., Long Y.Y., Liu Z.G., Zhao T., Li L., Tang Q.H., He Y.C., Bian Y.J., Hu J.Q. Treating influenza patients of wind-heat affecting Fei syndrome by Jinhua Qinggan granule: a double-blinded randomized control trial. Chin. J. Integr. Tradit. West Med. 2013;33(12):1631–1635. [PubMed] [Google Scholar]
- 204.Qi J.P., Qi X.Y., Wang X.J. Clinical efficacy of different doses of Jinhuaqinggan granule on influenza and serum levels of cytokines. Mod. Med. J. 2016;44(12):1664–1669. [Google Scholar]
- 205.Ma Y.X., Guo Y.L., Liu J., Wang Y.G. Study on treatment of influenza A H1N1 induced severe pneumonia by Xuebijing injection. World Chin. Med. 2015;10(02):243–246. [Google Scholar]
- 206.Wu H.L., Song X., Shen Y.Y. Clinical study on Xuebijing Injection combined with human immunoglobulin in treatment of severe viral pneumonia. Drugs Clin. 2016;31(11):1725–1728. [Google Scholar]
- 207.Wang Z.Z., Bao L.L., Sun L., BI Y.A., Zhou J., Xiao W. Mechanism of reduning injection against influenza A/H1N1 virus. Chin. Tradit. Herb. Drugs. 2014;45(01):90–93. [Google Scholar]
- 208.Tang L.P., Mao Z.F., Li X.X., Chen M., Li S.B., Tsoi B., Cao L.F., Li L., Zeng J.M., Wang Z.W., Yu G.Y., Xiao W., Li Y.F., Kurihara H., He R.R. ReDuNing, a patented Chinese medicine, reduces the susceptibility to H1N1 influenza of mice loaded with restraint stress. Eur. J. Integr. Med. 2014;6(6):637–645. [Google Scholar]
- 209.Zheng L.S., Gu L.G. Study on anti-virus effect of Tanreqing injection on the mice with influenza virus FM1 infected. China J. Tradit. Chin. Med. 2009;24(07):851–854. [Google Scholar]
- 210.Zheng J.L., Gu L.G., Li P., Zhao H. Effect of Tanreqing injection on immune function of influenza virus FM1 infected mice. J. Beijing Univ. Chin. Med. 2006;29(11):756–759. [Google Scholar]
- 211.Meng M., Chen D.Z., Wu B.Y., Xu W.J., Wu S.H., Liu C.Y. Study of the effect of tanreqing injection on immune function of the mice infected with influenza virus FM1. J. Hebei Univ. Nat. Sci. Ed. 2006;26(05):529–535. [Google Scholar]
- 212.Chen Y., Zhou J. Effect of Buzhong Yiqi Decoction on immune function in children with recurrent respiratory tract infection. Hainan Med. J. 2020;31(03):354–356. [Google Scholar]
- 213.Li L., WEI K., Lu F.G., Cai L., Zhang B., Zhang S.Y., Gao Q., Dai B. Effect of Maxing Shigan Decoction against type A influenza virus infection in mice induced by viral lung injury based on TLR4-MyD88-TRAF6 signal pathways. Chin. Tradit. Herb. Drugs. 2017;48(08):1591–1596. [Google Scholar]
- 214.Wang R.J., Peng Y., Meng H., Li X.B. Protective effect of polysaccharides fractions from Sijunzi decoction in reserpine-induced spleen deficiency rats. RSC Adv. 2016;6(65):60657–60665. [Google Scholar]
- 215.den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54(9):2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Dang A.T., Marsland B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019;12(4):843–850. doi: 10.1038/s41385-019-0160-6. [DOI] [PubMed] [Google Scholar]
- 217.Park J., Kim M., Kang S.G., Jannasch A.H., Cooper B., Patterson J., Kim C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8(1):80–93. doi: 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rooks M.G., Garrett W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016;16(6):341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Nastasi C., Candela M., Bonefeld C.M., Geisler C., Hansen M., Krejsgaard T., Biagi E., Andersen M.H., Brigidi P., Odum N., Litman T., Woetmann A. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci. Rep. 2015;5:16148. doi: 10.1038/srep16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kho Z.Y., Lal S.K. The human gut microbiome – a potential controller of wellness and disease. Front. Microbiol. 2018;9:1835. doi: 10.3389/fmicb.2018.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Koh A., De Vadder F., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 222.Louis P., Hold G.L., Flint H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014;12(10):661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 223.Yu H.N., Liu Z.H. Recent progress in intestinal microbiota and mucosal immunity. Chin. J. Immunol. 2019;35(16):1921–1930. [Google Scholar]
- 224.Karlsson I., Hagman R., Guo Y., Humblot P., Wang L., Wernersson S. Pathogenic Escherichia coli and lipopolysaccharide enhance the expression of IL-8, CXCL5, and CXCL10 in canine endometrial stromal cells. Theriogenology. 2015;84(1):34–42. doi: 10.1016/j.theriogenology.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 225.Wang P., Zhao C., Lu F.G., Wu T., Zhang X.G., Chen C.J., Xiao R., Ning Y., Wei K., Li L., Cai K., Tian W.Y. Effects of Maxing Shigan Decoction on intestinal flora and chemokines CCL5 and CXCL10 in mice infected with influenza virus. Chin. Tradit. Herb. Drugs. 2021;52(01):160–175. [Google Scholar]
- 226.Shao S., Wang D., Zheng W., Li X., Zhang H., Zhao D., Wang M. A unique polysaccharide from Hericium erinaceus mycelium ameliorates acetic acid-induced ulcerative colitis rats by modulating the composition of the gut microbiota, short chain fatty acids levels and GPR41/43 respectors. Int. Immunopharmacol. 2019;71:411–422. doi: 10.1016/j.intimp.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 227.Zhang Y., Tang K., Deng Y., Chen R., Liang S., Xie H., He Y., Chen Y., Yang Q. Effects of shenling baizhu powder herbal formula on intestinal microbiota in high-fat diet-induced NAFLD rats. Biomed. Pharmacother. 2018;102:1025–1036. doi: 10.1016/j.biopha.2018.03.158. [DOI] [PubMed] [Google Scholar]
- 228.Sun Q.G., An X.D., Xie P., Jiang B., Tian J.X., Yang Q., Li X.Y., Luo M., Liu P., Zhao S.H., Duan L.Y., Lang S.P., Fan A., Luo P.C., Lian F.M., Huang X.D., Tong X.L. Traditional Chinese medicine decoctions significantly reduce the mortality in severe and critically Ill patients with COVID-19: a retrospective cohort study. Am. J. Chin. Med. 2021;49(5):1063–1092. doi: 10.1142/S0192415X21500518. [DOI] [PubMed] [Google Scholar]