Abstract
Introduction
Many countries are administering a third dose of COVID-19 vaccines, but the evaluation of vaccine-induced immunity is insufficient. In addition, there are few reports of long-term observation of anti-spike IgG antibody titers after the vaccination in the Japanese population. This study aimed to evaluate anti-spike IgG levels in the Japanese health care workers six months after the BNT162b2 vaccination.
Methods
Dynamics of anti-spike IgG levels were assessed over a six-month period following the second vaccination in 49 participants (Analysis-1). A cross-sectional assessment of anti-spike IgG levels six months after the second vaccination was performed in 373 participants (Analysis-2).
Results
In Analysis-1, the geometric mean titer of anti-spike IgG was lower in the older age group and decreased consistently after the second vaccination regardless of age. In Analysis-2, the anti-spike IgG level was significantly negatively associated with age (r = −0.35, p < 0.01). This correlation remained statistically significant (r = −0.28, p < 0.01) after adjustment for sex, BMI, smoking habits, alcohol drinking habits, allergies, and fever or other adverse reactions at the time of vaccination. Additionally, participants who drank alcohol daily had significantly lower anti-spike IgG levels than participants who had never drunk alcohol. Sex, smoking habits, allergy, and fever and other side effects after vaccination did not show a significant association with anti-spike IgG levels.
Conclusions
Six months post-vaccination, the anti-spike IgG level was substantially lower in older persons and daily alcohol drinkers. This may be an indication for an additional vaccine dose for these at-risk categories.
Keywords: COVID-19, BNT162b2, Anti-spike IgG antibody
Abbreviations
- COVID-19
coronavirus disease 2019
- IgG
immunoglobulin G
- AU/ml
arbitrary units per milliliter
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- γ-GTP
γ-glutamyl transpeptidase
The mRNA coronavirus disease 2019 (COVID-19) vaccines, BNT162b2 (Pfizer/BioNTech) and mRNA-1273 (Moderna), have been highly effective in preventing COVID-19 in real-world practice [1,2]. However, many countries are currently experiencing a resurgence of COVID-19, dominated first by the Delta (B.1.617.2) variant and then by Omicron (B.1.1.529) variant of SARS-CoV-2 [3,4]. The increase in infections and hospitalizations of vaccinated individuals potentially stems from a combination of waning vaccine immunity over time and possible reduced vaccine effectiveness against the delta and omicron variants [[5], [6], [7], [8]]. In response to the resurgence of COVID-19, many countries are commencing administration of a third vaccination dose, and Israel was the first country to implement this in July 2021 [9,10]. The decrease in immunoglobulin G (IgG) levels to the spike protein six months after the second vaccination has been cited as a rationale for the third vaccination. However, the IgG level needed to prevent COVID-19 infection remains unclear. In addition, although the vaccine's effectiveness in preventing symptomatic infection in the early stages after the third vaccination has been reported, it is also unclear how long the effect will last [9,10]. There are some reports that the effect of preventing severe disease is maintained even six months after the second vaccination [6,11]. However, it is also unclear what IgG level is enough to avert severe COVID-19 disease. In addition, there are few reports describing the long-term dynamics of IgG levels in the Japanese population. This study aimed to investigate the dynamics of the anti-spike IgG levels in the Japanese health care workers over a six-month period after the second vaccination and identify indicators to determine which groups of people should be prioritized for an additional vaccination dose.
Study participants were recruited from health care workers in Haradoi Hospital, a care-mix hospital in Fukuoka. Of the 485 health care workers in this hospital, 368 (75.9%) participated in this study. All participants were free of any immunosuppressants and had not been affected by COVID-19 during the study period. Most of the study participants were nurses, and approximately 80% were women. All participants provided written informed consent prior to enrollment. All studies were carried out in accordance with the principles of the Declaration of Helsinki, as revised in 2008, and approved by the Haradoi hospital institutional ethics review committee prior to data collection (Approval No. 2020–08).
This study consisted of two sub-studies. The first study evaluated the dynamics of anti-spike IgG levels before the first vaccination, three weeks after the first vaccination (just before the second vaccination), and one, two, four, and six months after the second vaccination; 49 participants were included (Analysis-1). The second study evaluated IgG antibody levels six months after the second vaccination and 368 participants were included (Analysis-2). Because the exposure of interest was age, we categorized participants into age groups. In Analysis-1, 49 participants were categorized into three groups: those in their 20s and 30s (n = 22), those in their 40s and 50s (n = 23), and those who were 60 and over (n = 4). The categories were derived from the median age of this population, which was 40 years, but those over 60 years were analyzed as a separate group because of their significantly lower IgG antibody levels. In Analysis-2, 368 participants were categorized into groups of 10 years each. All participants were offered first and second doses of the vaccine between March and April 2021. Participants also provided information on their height, weight, smoking habits (current, past, or never), drinking habits (daily, often, or never), allergies, medical history, medications, whether they had had adverse reactions to vaccination (such as fever), and whether they had needed antipyretics.
Levels of anti-spike IgG were quantified using the SARS-CoV-2 IgG II Quant assay (Abbott Diagnostics, Chicago, IL, USA). Participants in Analysis-1 underwent blood testing to quantitatively assess anti-spike IgG six times. All participants in Analysis-2 underwent blood testing to quantitatively assess anti-spike IgG in October 2021, six months after the second vaccination. The results of anti-spike IgG are expressed as arbitrary units per milliliter (AU/ml) (positive threshold: 50 AU/ml; upper limit: 40,000 AU/ml). Participants in Analysis-1 also had blood tests for total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (γ-GTP), and serum creatinine, using standard enzymatic methods.
All analyses were performed using SAS version 9.4 (SAS Institute Inc). The Mann–Whitney U test was used to compare two groups, and the Kruskal–Wallis test was used to compare three or more groups. The Tukey–Kramer method was used for each two-group comparison among three or more groups. Anti-spike IgG levels, with adjustment factors, were determined by the least means square method. Univariate analysis for categorical variables was carried out using the Chi-square or Fisher's exact test. Odds ratios for each factor for anti-spike IgG levels of ≥2150 AU/ml at six months were determined by univariate and multivariate logistic analyses. Based on the correlation with the plaque reduction neutralization test, the anti-spike IgG level of 2150 AU/ml is considered to be approximately 80% effective in preventing infection [12]. In the multivariate logistic regression analysis, the adjustment factors were sex, age, smoking habits, alcohol drinking habits, allergies, and the interval days between the second vaccination and anti-spike IgG measurement. The statistical significance threshold was set at 5%.
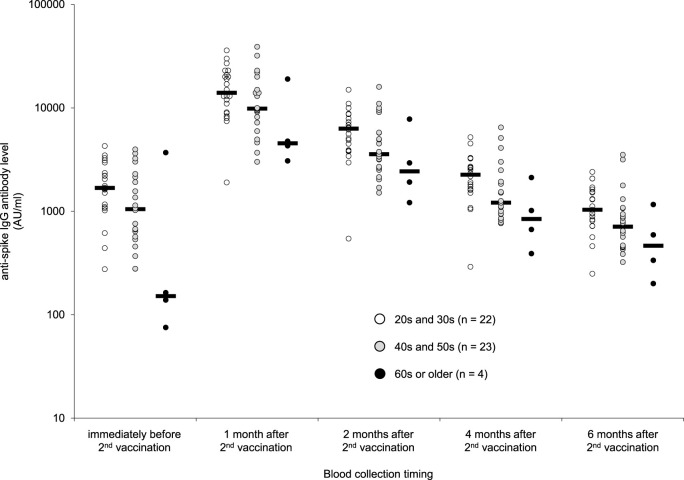
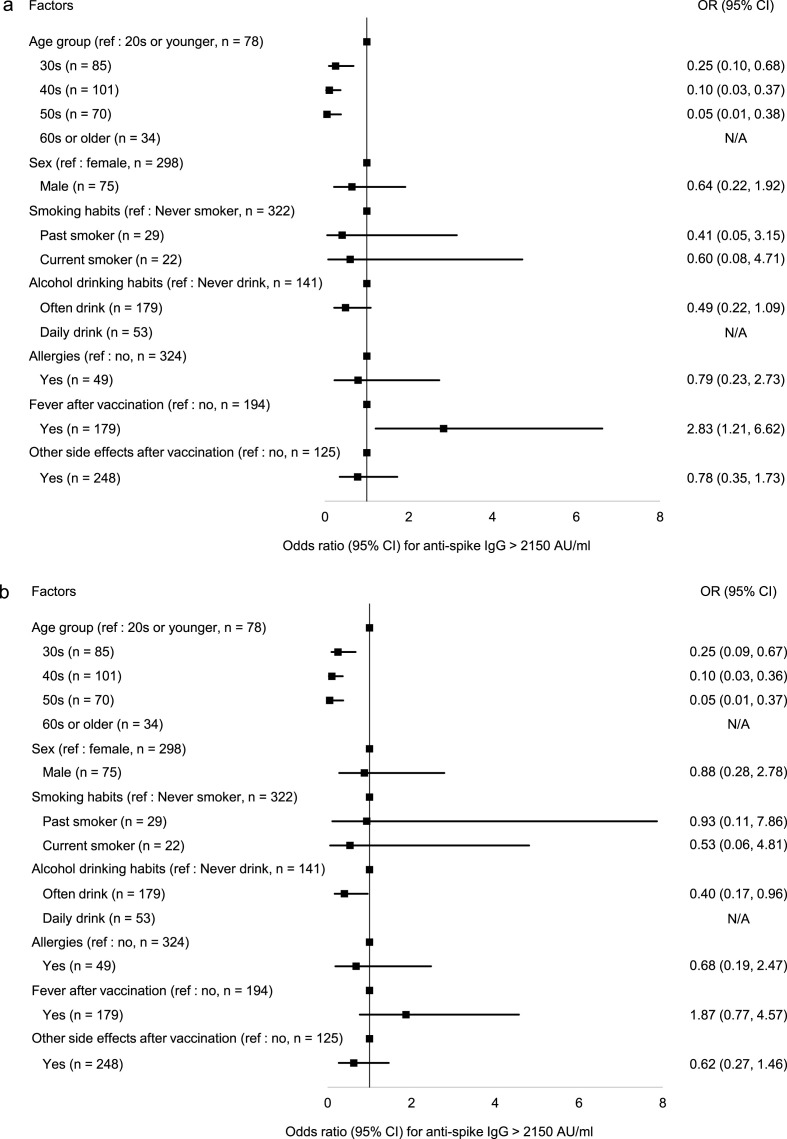
Baseline characteristics of the 49 participants of Analysis-1 and the 368 participants of Analysis-2 were shown in Table 1 . The median age was approximately 40 years and 80% of the participants were women in both analyses. The median interval between the date of the second vaccination and the date of blood collection in Analysis-2 was 185 days. The dynamics in anti-spike IgG level in Analysis-1 by age group are shown in Fig. 1 . The geometric mean anti-spike IgG levels after the first vaccination were higher in the younger age groups than the older age groups and this trend continued through 6 months after the second vaccination. In this analysis, the anti-spike IgG level peaked one month after the second vaccination: the geometric mean IgG levels were 13883 AU/ml in the 20s and 30s group, 10736 AU/ml in the 40s and 50s group, and 5887 AU/ml in the group over 60 years. The anti-spike IgG decline rate from one month to six months after the second vaccination was approximately 90% and there was no difference among age groups. The geometric mean anti-spike IgG levels six months after the second vaccination were 1044 AU/ml in the 20s and 30s group, 770 AU/ml in the 40s and 50s group, and 464 AU/ml in the group over 60 years. The anti-spike IgG level of the Analysis-2 participants showed a statistically significant negative correlation with age (r = −0.35, p < 0.01). This correlation remained statistically significant (r = −0.28, p < 0.01) even after adjusting for sex, BMI, smoking habits, alcohol drinking habits, allergies, and the presence of fever or other adverse reactions at the time of vaccination. Logistic regression analyses were performed to determine the factors that led to anti-spike IgG levels above 2150 AU/ml six months after vaccination (Fig. 2 ). In univariate analyses, the factors that significantly influenced anti-spike IgG levels above 2150 AU/ml were age and fever after vaccination (Fig. 2a). In multivariate analyses, age and alcohol drinking habits affected anti-spike IgG levels above 2150 AU/ml (Fig. 2b). None of the 53 participants with daily alcohol drinking habits had anti-spike IgG levels of 2150 AU/ml or higher.
Table 1.
Baseline characteristics of participants in Analysis-1 and Analysis-2a.
| Analysis-1 (n = 49) | Analysis-2 (n = 368) | |
|---|---|---|
| Demographics | ||
| Age – years | 41 [31, 47] | 42 [32, 51] |
| Sex | ||
| Female – no. (%) | 42 (85.7) | 293 (79.6) |
| Male – no. (%) | 7 (14.3) | 75 (20.4) |
| Body mass indexb– kg/m2 | 21.2 [19.2, 23.4] | 21.2 [19.6, 23.5] |
| Smoking habit – no. (current/past/never) | 0/6/43 | 22/29/317 |
| Alcohol drinking habit – no. (daily/often/never) | 5/29/15 | 53/178/137 |
| Allergies – no. (%) | 9 (18.4) | 48 (13.1) |
| Comorbidities | ||
| Number of comorbidities – no. | 0 [0, 1] | 0 [0, 1] |
| Hypertension – no. (%) | 6 (12.2) | 41 (11.1) |
| Diabetes – no. (%) | 4 (8.2) | 10 (2.7) |
| Dyslipidemia – no. (%) | 6 (12.2) | 34 (9.2) |
| Hyperuricemia – no. (%) | 2 (4.1) | 10 (2.7) |
| Coronary heart disease – no. (%) | 0 (0.0) | 1 (0.3) |
| Arrhythmia – no. (%) | 3 (6.1) | 4 (1.0) |
| Stroke – no. (%) | 0 (0.0) | 0 (0.0) |
| Lung disease – no. (%) | 2 (4.1) | 24 (6.5) |
| Thyroid disease – no. (%) | 1 (2.0) | 13 (3.5) |
| Atopic dermatitis – no. (%) | 3 (6.1) | 37 (10.1) |
| Autoimmune disease – no. (%) | 0 (0.0) | 2 (0.1) |
| Cancer – no. (%) | 0 (0.0) | 13 (3.5) |
| Laboratory results | ||
| Total bilirubin – mg/dl | 0.60 [0.50, 0.85] | N/A |
| Aspartate aminotransferase – IU/l | 20.0 [15.5, 23.0] | N/A |
| Alanine aminotransferase – IU/l | 14.5 [11.5, 21.0] | N/A |
| γ-glutamyl transpeptidase – IU/l | 16.0 [14.0, 24.5] | N/A |
| Serum creatinine – mg/dl | 0.63 [0.57, 0.71] | N/A |
| Side effect of vaccination | ||
| Fever – no. (%) | 27 (55.1) | 178 (48.4) |
| Other side effects – no. (%) | 40 (81.6) | 243 (66.0) |
| Antipyretics – no. (%) | 35 (71.4) | 203 (55.2) |
N/A, not assessed.
Continuous variables are presented as median [1st quartile, 3rd quartile], and categorical variables are presented as number (%).
Body mass index was calculated using the following equation: body weight (kg)/height (m)/height (m).
Fig. 1.
Distribution of anti-spike IgG levels after the 2nd vaccination.
Distribution of anti-spike IgG levels by age group is shown. The anti-spike IgG levels were measured three weeks after the first vaccination (just before the second vaccination) and one, two, four, and six months after the second vaccination. Dots represent individual serum samples. Black bars indicate geometric mean anti-spike IgG levels.
Fig. 2.
Contributed factor for anti-spike IgG level ≥ 2150 AU/ml.
Odds ratios for anti-spike IgG level ≥ 2150 AU/ml (2a for univariate and 2b for multivariate) are shown. Adjusted factors in the multivariate logistic model were sex, generation, smoking habit, drinking habit, allergy status, and the interval days between the second vaccination and anti-spike IgG measurement. In the multivariate analyses, age and alcohol drinking habits significantly affected the anti-spike IgG level ≥ 2150 AU/ml.
Vaccination against SARS-CoV-2 has been considered to be a highly effective strategy for reducing COVID-19 infection, however, it is still unclear how long the protective effect remains post-vaccination. In addition, there are few reports regarding long-term observation of IgG antibody titers after the vaccination against SARS-CoV-2 in the Japanese population. Therefore, we conducted one prospective study (Analysis-1) and one cross-sectional study (Analysis-2) on the Japanese population. In the prospective study, we found significant waning of anti-spike IgG levels in the six months after receiving the second dose of the BNT162b2 vaccine. The anti-spike IgG levels six months after the second vaccination were less than 10% of those one month after the second vaccination. Our results are consistent with previous studies with European or Japanese, which have also reported that the anti-spike IgG level drops to approximately 10% within six months of vaccination [13,14]. The cross-sectional study found that anti-spike IgG levels were associated with age and alcohol drinking habits. Compared to the group that was 20 years or younger, the odds ratio of an anti-spike IgG level over 2150 six months after the second vaccination were less than 0.3 in other age groups. Furthermore, none of those aged 60 years or older had anti-spike IgG levels of 2150 or higher. This trend did not change even after adjustment for sex, smoking habits, alcohol drinking habits, allergies, and days since vaccination. Participants with daily and occasional alcohol drinking habits had a significantly lower odds ratio of anti-spike IgG levels of 2150 or higher after six months compared to those without alcohol drinking habits. This was particularly true for those who drank alcohol daily, none of whom had anti-spike IgG levels of 2150 or higher. These results are also consistent with previous studies with the Japanese population [14]. In addition, we have previously reported that the Japanese elderly aged 60 years or older had a lower anti-spike IgG level after vaccination than younger Japanese people. We measured anti-spike IgG levels before first and second vaccinations, and one month after the second dose of BNT162b2 in 185 participants including outpatients, nursing home residents, and inpatients of long-term care units older than 60 years. The median IgG levels one month after the second vaccination in nursing home residents or inpatients were only 16% and levels in outpatients were only 38% of that of the medical staff who were younger than 60 years (2085.9 AU/mL, 4891.2 AU/mL, and 13000 AU/mL, respectively) [15]. Based on these results, the effectiveness of vaccination in the Japanese population may be lower in those aged 60 years or older than in younger people.
Some limitations of this study should be noted. We only assessed anti-spike IgG levels and not neutralizing antibodies and cellular immunity, which prevent severe disease. The sample size of the cohort was small and only 10% of the participants were over 60 years old. In addition, most of the participants did not have any comorbidities. Further studies, including participants with various backgrounds, and assessing neutralizing antibodies and cellular immunity, are necessary.
In conclusions, the anti-spike IgG level was substantially lower six months after vaccination, particularly among persons 60 years of age or older and among persons with alcohol drinking habits. A third dose of mRNA vaccine would be recommended especially for those persons.
Authorship
All authors meet the ICMJE authorship criteria as below. The conception and design of the study was carried out by HI, HN, and NS. Data acquisition was done by all the authors. Measurement was done by HI. The data were analyzed by HI and interpreted by all the authors. All authors contributed to the drafting of the paper and its revision and are responsible for the intellectual content and the final approval of the version to be published. HI is the guarantor of this study.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
Acknowledgments
The authors thank Ms. Ryoko Nakashima for managing the dataset and Drs. Kahori Miyoshi, Yuichi Hara, Jun Hayashi, and Hiroshi Hara for scientific advice.
References
- 1.Pritchard E., Matthews P.C., Stoesser N., Eyre D.W., Gethings O., Vihta K.D., et al. Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom. Nat Med. 2021;27:1370–1378. doi: 10.1038/s41591-021-01410-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paris C., Perrin S., Hamonic S., Bourget B., Roué C., Brassard O., et al. Effectiveness of mRNA-BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19 vaccines against COVID-19 in healthcare workers: an observational study using surveillance data. Clin Microbiol Infect. 2021;27:1699. doi: 10.1016/j.cmi.2021.06.043. e5–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pouwels K.B., Pritchard E., Matthews P.C., Stoesser N., Eyre D.W., Vihta K.D., et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021;27:2127–2135. doi: 10.1038/s41591-021-01548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karim S.S.A., Karim Q.A. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398:2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Planas D., Veyer D., Baidaliuk A., Staropoli I., Guivel-Benhassine F., Rajah M.M., et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 8.Kato H., Miyakawa K., Ohtake N., Go H., Yamaoka Y., Yajima S., et al. Antibody titers against the Alpha, Beta, Gamma, and Delta variants of SARS-CoV-2 induced by BNT162b2 vaccination measured using automated chemiluminescent enzyme immunoassay. J Infect Chemother. 2022;28(2):273–278. doi: 10.1016/j.jiac.2021.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barda N., Dagan N., Cohen C., Hernán M.A., Lipsitch M., Kohane I.S., et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398:2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbott Diagnostics. Architect SARS-COV-2 IgG II quant instructions for use, H18566R01. Abbott Diagnostics, IL, USA.
- 13.Naaber P., Tserel L., Kangro K., Sepp E., Jürjenson V., Adamson A., et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10:100208. doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato H., Miyakawa K., Ohtake N., Yamaoka Y., Yajima S., Yamazaki E., et al. medRxiv; 2021 Oct 30. Vaccine-induced humoral and cellular immunity against SARS-CoV-2 at 6 months post BNT162b2 vaccination. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikezaki H., Hara Y., Hayashi J., Hara H., Nomura H., Shimono N. Low IgG antibody production in the elderly Japanese population after fully BNT162b2 vaccination. J Hosp Gen Med. 2022;4(1):25–28. [Google Scholar]