Abstract
Recombinant human granulocyte colony-stimulating factor (rhG-CSF) is used to counter chemotherapy-induced neutropenia. Our previous study showed an inverse correlation between serum rhG-CSF levels and the number of circulating neutrophils in cancer patients (H. Takatani, H. Soda, M. Fukuda, M. Watanabe, A. Kinoshita, T. Nakamura, and M. Oka, Antimicrob. Agents Chemother. 40:988–991, 1996). The aim of this study was to clarify the relationship between rhG-CSF clearance and G-CSF receptors on circulating neutrophils. In five cancer patients receiving chemotherapy, a bolus dose of rhG-CSF (5 μg/kg) was injected intravenously during defined phases of posttreatment neutropenia and neutrophilia. Serum rhG-CSF levels were measured by a chemiluminescence enzyme immunoassay and analyzed by moment analysis. G-CSF receptors on neutrophils were detected by flow cytometry with biotinylated rhG-CSF. rhG-CSF clearance was significantly higher at neutrophilia than at neutropenia (1,497 ± 132 versus 995 ± 266 ml/h; P < 0.01). The percentage of G-CSF receptor-positive neutrophils, reflecting the number of G-CSF receptors per cell, was low at neutropenia without rhG-CSF therapy (44.5% ± 22.1%) and high at neutrophilia with rhG-CSF therapy (73.0% ± 11.4%; P < 0.01). rhG-CSF clearance closely correlated with the percentage of G-CSF receptor-positive neutrophils (r2 = 0.91; P < 0.0001) and neutrophil count (r2 = 0.72; P < 0.005). Our results indicate that, in cancer patients receiving chemotherapy, rhG-CSF increases the number of G-CSF receptors per cell as well as circulating neutrophil counts, resulting in modulation of its own clearance.
Recombinant human granulocyte colony-stimulating factor (rhG-CSF) is used to increase the number of neutrophils during intensive chemotherapy. Results of recent studies, however, have not always demonstrated a clear clinical benefit from rhG-CSF therapy in cancer patients. Therefore, it is necessary to identify those patients who will clearly benefit from rhG-CSF therapy (3). The levels of rhG-CSF achieved in serum do not always reflect the effects on neutrophils (13, 14). Nevertheless, the pharmacokinetics and pharmacodynamics of rhG-CSF in cancer patients are not fully understood. Serum rhG-CSF levels may vary due to several factors such as the dose or route of rhG-CSF administration (6, 13), renal function (1), number of circulating neutrophils (13, 14), cellularity of myeloid cells in the bone marrow (18), circulating proteases, anti-G-CSF antibodies, soluble G-CSF receptor, and G-CSF receptor antagonists (9).
Previous studies, including those from our laboratories, have demonstrated that serum rhG-CSF levels after subcutaneous administration in cancer patients receiving chemotherapy were inversely correlated with the number of circulating neutrophils (13, 14). Furthermore, Nicola et al. (8) suggested that murine G-CSF was processed intracellularly through G-CSF receptors on neutrophils in vitro. Thus, the G-CSF receptors on neutrophils may modulate the clearance of rhG-CSF. However, previous studies had two limitations: the influence of release of rhG-CSF from the subcutaneous tissue and no evaluation of G-CSF receptors on circulating neutrophils. In the present study, to exclude the kinetics in the subcutaneous tissue, rhG-CSF was administered intravenously in cancer patients. We then measured the number of G-CSF receptors on neutrophils and investigated the relationship between these receptors and rhG-CSF clearance.
MATERIALS AND METHODS
Patient selection.
Our study was conducted according to the ethical standards of Nagasaki University. Patients were consecutively selected if they fulfilled the following criteria: (i) presence of histologically or cytologically confirmed malignancy; (ii) no prior chemotherapy or radiotherapy; (iii) Eastern Cooperative Oncology Group performance status of 2 or better; (iv) eligibility for chemotherapy; (v) absence of metastases in bones and pleural and ascitic fluid; (vi) adequate bone marrow function with neutrophil counts of >2,000/μl, platelet counts of >100,000/μl, and hemoglobin level of >10 g/dl; (vii) normal hepatic and renal function; and (viii) informed consent of the patient to this study. All tests and analytical procedures were performed during the first course of chemotherapy.
rhG-CSF administration.
Following chemotherapy, a bolus dose of 5 μg of rhG-CSF (lenograstim; Chugai Pharmaceutical Co., Tokyo, Japan) per kg of body weight was injected intravenously at 9 a.m. from the first day of neutrophil count of <1,000/μl (defined as neutropenia) to the first day when neutrophil count was >5,000/μl (defined as neutrophilia). The dose of rhG-CSF was selected based on results of a dose determination study in Japan. The number of neutrophils was determined three times weekly by the method reported previously (14).
Serum rhG-CSF levels.
Blood samples were obtained just before and 0.5, 1, 2, 4, 6, and 8 h after rhG-CSF administration on the first day (neutropenia) and last day (neutrophilia) of rhG-CSF therapy. The samples were immediately centrifuged, and sera were stored at −20°C until assayed. Serum G-CSF levels were measured by a chemiluminescence enzyme immunoassay which has higher sensitivity than the standard enzyme immunoassay (4).
Serum G-CSF levels were analyzed by moment analysis (automated pharmacokinetic analysis system; Nankodo Co., Tokyo, Japan) (20). The parameters calculated were area under the concentration-time curve (AUC0–∞), elimination half-life (t1/2), systemic clearance (CL), and volume of distribution (Vd).
G-CSF receptors on neutrophils.
The density of G-CSF receptors on neutrophils was estimated by flow cytometric analysis with biotinylated rhG-CSF, as previously described (10). For biotin labeling of rhG-CSF, 50 μg of rhG-CSF was diluted in 2 ml of labeling buffer (0.01 M phosphate, 0.15 M NaCl, pH 7.45). Biotinylation was carried out by the addition of 2 μl of biotinyl N-hydroxysuccinimide ester (EOY Laboratories, San Mateo, Calif.) in dimethyl formamide to yield a final concentration of 50 μg/ml. The reaction lasted for 3 h with continuous shaking at room temperature. The unbound reagent was then removed with a PD10 column equilibrated with 25 ml of labeling buffer supplemented with 0.1% bovine serum albumin. Sodium azide was added to the recovered sample at a final concentration of 0.1%.
For preparation of leukocytes, blood samples were obtained from patients on the days of neutropenia and neutrophilia before rhG-CSF administration and also from three healthy individuals as a control. Accordingly, the samples from patients contained rhG-CSF at neutrophilia but not at neutropenia. Leukocytes were separated from each 8-ml sample by density gradient centrifugation with Ficoll-Hypaque (Dainippon Pharmaceutical. Co., Tokyo, Japan). Erythrocytes were removed by using a hemolysis buffer containing 155 mM ammonium chloride, 10 mM potassium hydrogen carbonate, and 0.1 mM EDTA-2Na.
To prepare cells for flow cytometric analysis, 5 × 105 cells in a 50-μl volume were added to microtubes containing 25 ng of biotinylated rhG-CSF in a total volume of 100 μl. In a competition binding assay, both biotinylated rhG-CSF and a 40-fold excess of unlabeled rhG-CSF (1 μg) were added to the above cell suspension. After a 30-min incubation at 4°C, cells were washed three times with ice-cold binding buffer and incubated with 10 ng of streptavidin-phycoerythrin conjugate (Becton Dickinson, Mountain View, Calif.) for 30 min at 4°C. Then, the cells were washed and resuspended in 500 μl of binding buffer. Detection of G-CSF receptors was performed with an EPICS Elite flow cytometer (Coulter Corp., Hialeah, Fla.).
Flow cytometric analysis.
Flow cytometric data are usually reduced to percent positive cells, which is a count-independent measure of the number of fluorescent cells. The number of G-CSF receptor-positive neutrophils was obtained by comparing the histogram in the absence of unlabeled G-CSF (total binding) with that in the presence of excess unlabeled G-CSF (nonspecific binding). The fraction of cells that was shifted to a greater fluorescence intensity after specific ligand binding represented the percentage of G-CSF receptor-positive neutrophils: (number of G-CSF receptor-positive neutrophils)/(10,000 neutrophils analyzed) × 100 (%). The fluorescence level that delineated fluorescent cells from nonfluorescent cells was selected, and then the percentage of cells with an equivalent or higher fluorescence level was calculated. The flow cytometric analysis of each sample was performed in triplicate. The coefficient of variation for assay error was less than 5%.
The percentage of cells positive for G-CSF receptors is thought to reflect the number of G-CSF receptors per cell, because a good positive correlation is observed between the number of G-CSF receptors per cell in the radioisotopic binding assay and that in flow cytometric analysis (11, 16).
Statistical analysis.
Serum G-CSF concentrations and the percentage of G-CSF receptor-positive neutrophils on the days of neutropenia and neutrophilia were compared by the paired t test. The number of circulating neutrophils was log transformed for normalization. Correlations between the rhG-CSF clearance and the above values were analyzed by linear regression. The coefficient of determination (r2) was used to assess variability in rhG-CSF clearance. A two-tailed test value of P < 0.05 was considered significant.
RESULTS
Patient characteristics.
Five patients comprising four with lung cancer and one with ovarian cancer met our inclusion criteria (Table 1). The median duration of chemotherapy-induced neutropenia (<1,000/μl), neutrophil count at nadir, and duration of rhG-CSF therapy were 3 days, 552/μl, and 5 days, respectively. The numbers of erythrocytes, platelets, monocytes, and lymphocytes and creatinine clearance did not change significantly during rhG-CSF therapy (data not shown).
TABLE 1.
Patient characteristicsa
| No. | Sex/age (yr) | Site of cancer | Chemotherapy | Degree of neutropenia
|
Duration of rhG-CSF therapy (days) | |
|---|---|---|---|---|---|---|
| Duration of <1,000/μl (days) | Nadir (per μl) | |||||
| 1 | M/62 | Lung | CBDCA–CPT-11 | 3 | 884 | 7 |
| 2 | M/81 | Lung | CBDCA–VP-16 | 3 | 253 | 5 |
| 3 | M/68 | Lung | MVP | 3 | 552 | 4 |
| 4 | M/59 | Lung | MVP | 3 | 930 | 4 |
| 5 | F/63 | Ovary | CDDP | 7 | 252 | 13 |
M, male; F, female; CBDCA, carboplatin; CPT-11, irinotecan; VP-16, etoposide; MVP, mitomycin-vindesine-cisplatin; CDDP, cisplatin.
Pharmacokinetics of rhG-CSF.
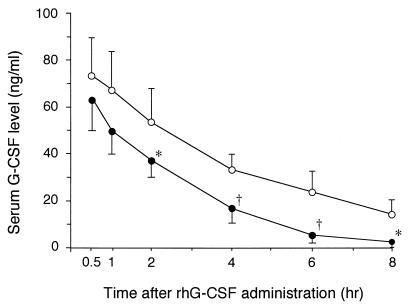
Two hours after administration of rhG-CSF, serum rhG-CSF levels were significantly lower at neutrophilia than at neutropenia (Fig. 1). Serum G-CSF concentrations before rhG-CSF injection were 0.084 ± 0.059 (mean ± standard deviation [SD]) ng/ml at neutropenia and 0.517 ± 0.248 ng/ml at neutrophilia. G-CSF levels before rhG-CSF injection were too small to influence the difference in serum G-CSF levels after administration. The individual pharmacokinetic parameters of rhG-CSF are summarized in Table 2. AUC0–∞ and t1/2 were significantly smaller (P < 0.01) and shorter (P < 0.05), respectively, at neutrophilia than at neutropenia. In contrast, the CL was significantly higher at neutrophilia than at neutropenia (P < 0.01). There was no significant difference in Vd between the period of neutropenia and that of neutrophilia.
FIG. 1.
Serum G-CSF levels on the days of neutropenia and neutrophilia after intravenous bolus injection of 5 μg of rhG-CSF per kg. Open circles, neutropenia; closed circles, neutrophilia. Data are expressed as means ± SDs (n = 5). ∗, P < 0.05, and †, P < 0.01, versus neutropenia value.
TABLE 2.
Pharmacokinetic parameters of rhG-CSF in cancer patients at neutropenia and neutrophilia
| Patient no. | Neutropenia
|
Neutrophilia
|
||||||
|---|---|---|---|---|---|---|---|---|
| AUC0–∞ (μg · h/ml) | t1/2 (h) | CL (ml/h) | Vd (ml) | AUC0–∞ (μg · h/ml) | t1/2 (h) | CL (ml/h) | Vd (ml) | |
| 1 | 0.37 | 2.61 | 1,080 | 3,771 | 0.23 | 1.89 | 1,696 | 4,774 |
| 2 | 0.37 | 4.79 | 836 | 5,174 | 0.18 | 1.17 | 1,383 | 3,273 |
| 3 | 0.49 | 2.68 | 700 | 2,627 | 0.19 | 1.94 | 1,504 | 4,449 |
| 4 | 0.25 | 2.38 | 1,398 | 3,189 | 0.13 | 0.73 | 1,582 | 2,466 |
| 5 | 0.35 | 4.09 | 961 | 4,619 | 0.19 | 1.46 | 1,321 | 3,072 |
| Mean ± SD | 0.37 ± 0.09 | 3.31 ± 1.07 | 995 ± 266 | 3,876 ± 1,034 | 0.18 ± 0.04a | 1.44 ± 0.51b | 1,497 ± 132a | 3,607 ± 971 |
P < 0.01 versus neutropenia value.
P < 0.05 versus neutropenia value.
rhG-CSF clearance and G-CSF receptors on neutrophils.
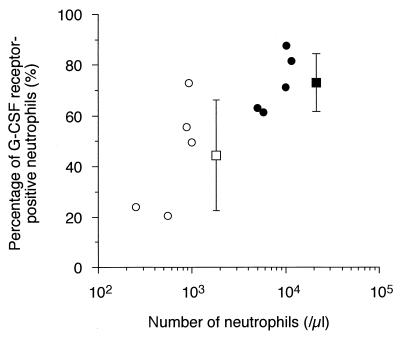
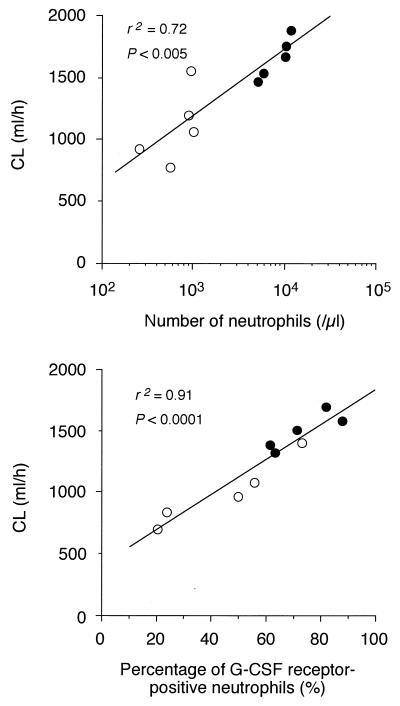
In three healthy individuals, the percentage of G-CSF receptor-positive neutrophils was 84.7% ± 0.02% (mean ± SD). The percentage at the time of chemotherapy-induced neutropenia without rhG-CSF therapy was 44.5% ± 22.1%, which was significantly lower than that in healthy individuals (P < 0.05). Furthermore, the percentage at neutrophilia following rhG-CSF therapy (73.0% ± 11.4%) was significantly higher than that at neutropenia (44.5% ± 22.1%; P < 0.01) (Fig. 2). As shown in Fig. 3, there was a positive correlation among CL of rhG-CSF and the number of circulating neutrophils (r2 = 0.72; P < 0.005) and the percentage of G-CSF receptor-positive neutrophils (r2 = 0.91; P < 0.0001).
FIG. 2.
Relationship between number of circulating neutrophils and percentage of G-CSF receptor-positive neutrophils. P is < 0.01 between the percentages at neutropenia (open circles) and neutrophilia (closed circles).
FIG. 3.
Correlation between rhG-CSF clearance and number of circulating neutrophils (upper panel) and percentage of G-CSF receptor-positive neutrophils (lower panel). Open circles, neutropenia; closed circles, neutrophilia.
DISCUSSION
The present study demonstrated that treatment with rhG-CSF following chemotherapy increased the number of G-CSF receptors per cell as well as the number of neutrophils in cancer patients. The rhG-CSF clearance closely correlated with both the number of G-CSF receptors per cell and that of circulating neutrophils. Results of previous studies indicated that hematopoietic cytokines exhibit common regulatory mechanisms, and a similar relationship between cytokine clearance and the number of target cells has been described elsewhere for rhG-CSF, recombinant human macrophage CSF, recombinant human granulocyte-macrophage CSF, and rhEPO (9). In addition, upregulation of receptor mRNA by the ligand has also been reported for G-CSF and interleukin 2 (12, 17, 19). However, to our knowledge, no in vivo study has previously reported the relationship between G-CSF receptor and neutrophils or rhG-CSF clearance.
rhG-CSF is eliminated through saturable and unsaturable mechanisms. In a rat model, a high dose of rhG-CSF decreases clearance to a plateau level, and clearance of a high dose of rhG-CSF is abrogated by nephrectomy (15). In humans, a high intravenous dose of rhG-CSF (≥10 μg/kg) also decreases clearance to a plateau level (6). Clearance of rhG-CSF administered subcutaneously is inversely correlated with circulating neutrophil counts (13, 14), and clearance of rhG-CSF administered intravenously decreases in patients with renal failure (1). These findings indicate that saturable and unsaturable clearance mechanisms of rhG-CSF mainly involve the neutrophils and kidneys, respectively. In this study, since creatinine clearance did not change during rhG-CSF therapy, the kidney was probably not involved in the observed change in rhG-CSF clearance.
At the time of chemotherapy-induced neutropenia, the number of G-CSF receptors per cell decreased to almost half the level found in healthy individuals. G-CSF receptors are normally present on myeloid progenitor cells to peripheral neutrophils (2). The number of receptors per cell increases with differentiation, and neutrophils in bone marrow have fewer receptors than do peripheral neutrophils (1). On the other hand, chemotherapy has been shown to inhibit the functions of peripheral neutrophils, but to our knowledge, no study of G-CSF receptors has been reported. There are two possible explanations for the reduced number of G-CSF receptors per cell observed in the present study: (i) increased release of neutrophils from bone marrow and (ii) direct inhibition by chemotherapy.
Treatment with rhG-CSF increased the percentage of G-CSF receptor-positive neutrophils to 73.0%. In a series of preliminary studies, when the neutrophil count spontaneously returned to the prechemotherapy level without rhG-CSF therapy, the percentage of G-CSF receptor-positive neutrophils remained low (51.5% ± 11.8%; n = 4). Other investigators have shown that rhG-CSF enhances the expression of G-CSF receptor mRNA in human neutrophils in vitro (17), supporting our results in vivo. Steinman and Tweardy (12) reported that the upregulation of murine G-CSF receptor mRNA by rhG-CSF is rapid and due to transcriptional activation without the synthesis of new protein. rhG-CSF increased not only the number of circulating neutrophils but also the density of neutrophil G-CSF receptors, which accelerated the increase in the total number of G-CSF receptors. We also showed that rhG-CSF clearance closely correlated with the number of G-CSF receptors per neutrophil. Accordingly, rhG-CSF is thought to be eliminated through G-CSF receptors on neutrophils rather than by nonspecific endocytosis by neutrophils.
G-CSF receptors are also expressed on platelets, monocytes, endothelial cells, and certain cancer cell lines (2). Soluble G-CSF receptors, anti-G-CSF antibodies, proteases, and G-CSF receptor antagonists such as complement component C5a are present in peripheral blood (2, 9). Accordingly, these receptors and substances may modify G-CSF clearance. However, platelet and monocyte counts did not change significantly in this study, and the density of G-CSF receptors on neutrophils accounted for as much as 91% of rhG-CSF clearance. In rats treated with cyclophosphamide, rhG-CSF enhanced the expression of G-CSF receptor mRNA on bone marrow cells but not that of soluble G-CSF receptors when serum rhG-CSF levels dropped (7). Although rhG-CSF therapy induces circulating anti-G-CSF antibodies, the antibodies do not inhibit cytokine function (5). To our knowledge, no study has previously reported the in vivo role of circulating proteases, receptor antagonists, or G-CSF receptors in cancer cell lines. These findings suggest that G-CSF receptors on cells other than neutrophils and circulating G-CSF-related substances are unlikely to contribute to changes in rhG-CSF clearance following chemotherapy.
In conclusion, rhG-CSF increased the density of G-CSF receptors on neutrophils, which closely correlated with rhG-CSF clearance. The pharmacokinetics of rhG-CSF are probably modulated in a complex fashion, and further studies are necessary to determine the optimal usage of rhG-CSF.
REFERENCES
- 1.Akizawa T, Shishido K, Koshikawa S. The effects and pharmacokinetics of rhG-CSF in patients with chronic renal failure. Artif Organs. 1995;19:1251–1257. doi: 10.1111/j.1525-1594.1995.tb02295.x. [DOI] [PubMed] [Google Scholar]
- 2.Avalos B R. Molecular analysis of the granulocyte colony-stimulating factor receptor. Blood. 1996;88:761–777. [PubMed] [Google Scholar]
- 3.Hoelzer D. Hematopoietic growth factors—not whether, but when and where. N Engl J Med. 1997;336:1822–1824. doi: 10.1056/NEJM199706193362510. [DOI] [PubMed] [Google Scholar]
- 4.Kiriyama R, Chichibu K, Matsuno T, Ohsawa N. Sensitive chemiluminescent immunoassay for human granulocyte colony-stimulating factor (G-CSF) in clinical applications. Clin Chim Acta. 1993;220:201–209. doi: 10.1016/0009-8981(93)90048-9. [DOI] [PubMed] [Google Scholar]
- 5.Laricchia-Robbio L, Moscato S, Genua A, Liberati A M, Revoltella R P. Naturally occurring and therapy-induced antibodies to human granulocyte colony-stimulating factor (G-CSF) in human serum. J Cell Physiol. 1997;173:219–226. doi: 10.1002/(SICI)1097-4652(199711)173:2<219::AID-JCP25>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Layton J E, Hockman H, Sheridan W P, Morstyn G. Evidence for a novel in vivo control mechanism of granulopoiesis: mature cell-related control of a regulatory growth factor. Blood. 1989;74:1303–1307. [PubMed] [Google Scholar]
- 7.Matsuzaki G, Li X-Y, Ohyama Y, Nomoto K. Kinetics of serum granulocyte-colony stimulating factor (G-CSF) concentration and G-CSF receptor expression during G-CSF treatment of cyclophosphamide-treated mice. Int J Immunopharmacol. 1996;18:363–369. doi: 10.1016/s0192-0561(96)00039-2. [DOI] [PubMed] [Google Scholar]
- 8.Nicola N A, Peterson L, Hilton D J, Metcalf D. Cellular processing of murine colony-stimulating factor (multi-CSF, GM-CSF, G-CSF) receptors by normal hemopoietic cells and cell lines. Growth Factor. 1988;1:41–49. doi: 10.3109/08977198809000245. [DOI] [PubMed] [Google Scholar]
- 9.Piscitelli S C, Reiss W G, Figg W D, Petros W. Pharmacokinetics studies with recombinant cytokines: scientific issues and practical considerations. Clin Pharmacokinet. 1997;32:368–381. doi: 10.2165/00003088-199732050-00003. [DOI] [PubMed] [Google Scholar]
- 10.Shimoda K, Okamura S, Harada N, Niho Y. Detection of the granulocyte colony-stimulating factor receptor using biotinylated granulocyte colony-stimulating factor: presence of granulocyte colony-stimulating factor receptor on CD34-positive hematopoietic progenitor cells. Res Exp Med. 1992;192:245–255. doi: 10.1007/BF02576281. [DOI] [PubMed] [Google Scholar]
- 11.Shinjo K, Takeshita A, Ohnishi K, Ohno R. Expression of granulocyte colony-stimulating factor receptor increases with differentiation in myeloid cells by a newly-devised quantitative flow-cytometric assay. Br J Haematol. 1995;91:783–794. doi: 10.1111/j.1365-2141.1995.tb05390.x. [DOI] [PubMed] [Google Scholar]
- 12.Steinman R A, Tweardy D J. Granulocyte colony-stimulating factor receptor mRNA upregulation is an immediate early marker of myeloid differentiation and exhibits dysfunctional regulation in leukemic cells. Blood. 1994;83:119–127. [PubMed] [Google Scholar]
- 13.Stute N, Santana V M, Rodman J H, Schell M J, Ihle J N, Evans W E. Pharmacokinetics of subcutaneous recombinant human granulocyte colony-stimulating factor in children. Blood. 1992;79:2849–2854. [PubMed] [Google Scholar]
- 14.Takatani H, Soda H, Fukuda M, Watanabe M, Kinoshita A, Nakamura T, Oka M. Levels of recombinant human granulocyte colony-stimulating factor in serum are inversely correlated with circulating neutrophil counts. Antimicrob Agents Chemother. 1996;40:988–991. doi: 10.1128/aac.40.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka H, Tokiwa T. Influence of renal and hepatic failure on the pharmacokinetics of recombinant human granulocyte colony-stimulating factor (KRN8601) in the rat. Cancer Res. 1990;50:6615–6619. [PubMed] [Google Scholar]
- 16.Tatsumi N, Yamane T, Tsuda I, Okuda K, Chaisiripoomkere W. Flow cytometric detection of recombinant human granulocyte-colony stimulating factor binding to leukemic cells. J Clin Lab Anal. 1993;7:86–90. doi: 10.1002/jcla.1860070203. [DOI] [PubMed] [Google Scholar]
- 17.Tsuruta T, Tani K, Shimane M, Ozawa K, Takahashi S, Tsuchimoto D, Takahashi K, Nagata S, Sato N, Asano S. Effects of myeloid cell growth factors on alkaline phosphatase, myeloperoxidase, defensin and granulocyte colony-stimulating factor receptor mRNA expression in haemopoietic cells of normal individuals and myeloid disorders. Br J Haematol. 1996;92:9–22. doi: 10.1046/j.1365-2141.1996.299833.x. [DOI] [PubMed] [Google Scholar]
- 18.Watari K, Ozawa K, Takahashi S, Tojo A, Tani K, Kamachi S, Asano S. Pharmacokinetic studies of intravenous glycosylated recombinant human granulocyte colony-stimulating factor in various hematological disorders: inverse correlation between the half-life and bone marrow myeloid cell pool. Int J Hematol. 1997;66:57–67. doi: 10.1016/s0925-5710(97)00576-8. [DOI] [PubMed] [Google Scholar]
- 19.Wilson A, Corthesy P, Reichenbach P, MacDonald H R, Nabholz M. Interleukin (IL)-1 and IL-2 receptor alpha and beta expression in immature thymocytes. Eur J Immunol. 1994;24:1729–1735. doi: 10.1002/eji.1830240802. [DOI] [PubMed] [Google Scholar]
- 20.Yamaoka K, Nakagawa T, Uno T. Statistical moments in pharmacokinetics. J Pharmacokinet Biopharm. 1978;6:547–558. doi: 10.1007/BF01062109. [DOI] [PubMed] [Google Scholar]