Abstract
Purpose
To investigate the effects of ultrasound-guided thoracic paravertebral block combined with general anaesthesia or general anaesthesia alone for thoracoscopic lung adenocarcinoma surgery, and to provide new thoughts for improving the clinical outcomes.
Methods
This was a retrospective study. Data were retrieved for 195 patients with lung adenocarcinoma undergoing elective radical lobectomy via video-assisted thoracoscopy between January 2018 and August 2019 in The Second Hospital of Shandong University, including 86 patients who received thoracic paravertebral block (TPVB) combined with general anaesthesia (group TG), and 109 patients who received general anaesthesia alone (group GA). All patients were given self-controlled intravenous analgesia pump for 48 h after surgery. The primary outcome was the recurrence-free survival 2 years postoperatively (the time between surgery and the earliest date of recurrence, metastasis or lung cancer-cause death). The secondary outcomes included the average numeric rating scale (NRS) scores within 48 h postoperatively, the first time of postoperative ambulation, duration of chest tube drainage, length of postoperative hospitalization, perioperative opioid consumption and the postoperative side effects.
Results
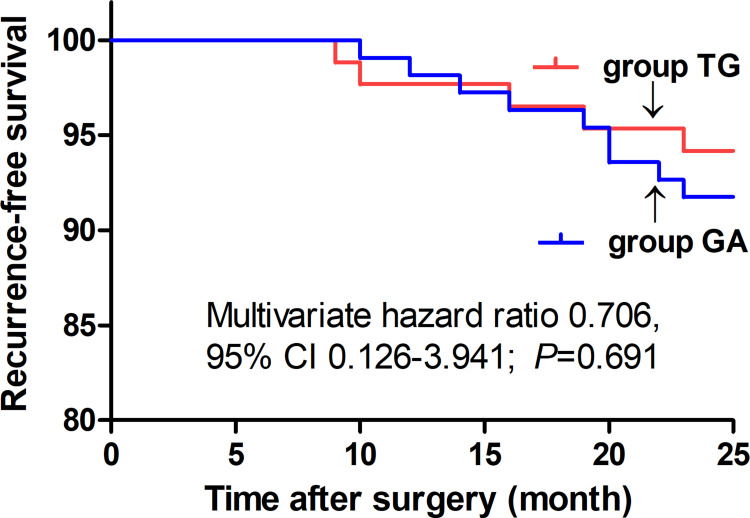
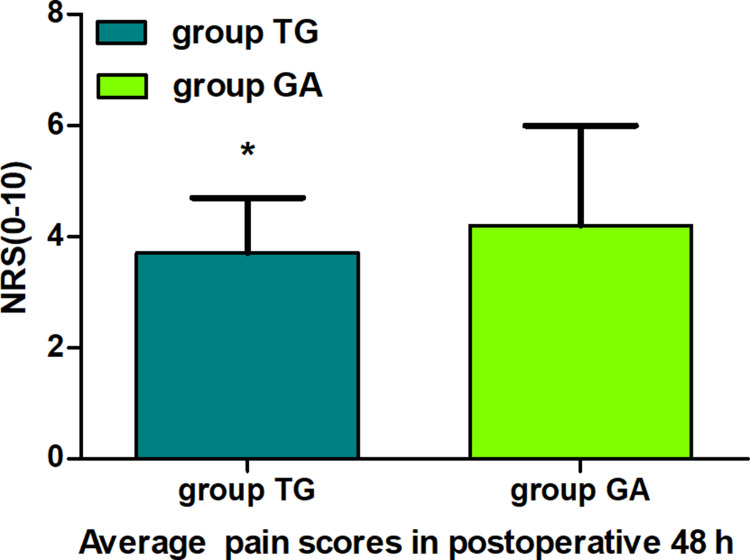
There were no statistical differences in the recurrence-free survival 2 years postoperatively between groups (Multivariate hazard ratio 0.706, 95% CI 0.126–3.941, P=0.691). The average NRS scores within 48 h postoperatively were significantly lower in group TG (P<0.05). The first time of postoperative ambulation, duration of chest tube drainage, and length of postoperative hospitalization in group TG were significantly reduced (P<0.05). Opioid consumption was significantly decreased in group TG (P<0.01). Finally, the incidence of postoperative nausea and vomiting (PONV) was significantly lower in group TG (P<0.05).
Conclusion
TPVB for thoracoscopic lung adenocarcinoma surgery did not improve the recurrence-free survival 2 years postoperatively compared with general anaesthesia alone, but it significantly enhanced the postoperative analgesia effect, reduced opioid consumption as well as side effects, and accelerated postoperative early recovery.
Clinical Trial Registration Number
The Chinese Clinical Trial Registry (ChiCTR-2100050454).
Keywords: lung adenocarcinoma, prognosis, video-assisted thoracoscopic surgery, thoracic paravertebral block, ultrasound guidance
Introduction
Lung cancer is a malignant tumour with the highest mortality among all cancers worldwide, and adenocarcinoma has been the most common histological subtype in the past few decades. At present, video-assisted thoracoscopic surgery (VATS) remains the best option for cure and favourable prognosis in the early stages, especially in Asian non-smoking related lung cancer screening program.1–5 Even if the neoplasm is completely resected, involving systemic lymph node dissection, the possibility of tumour recurrence or metastasis remains high because of undetected micro-metastasis.6 Development of cancer recurrences depends heavily on the balance between the anti-tumour immune activity of the body and the metastatic potential of cancer cells. Nevertheless, both surgery and anaesthesia can impair the host defense against malignant tumours.7 Surgical procedures release cancerous cells into the circulation, and surgery-related stress responses damage immunity, which all promote the growth of the neoplasm.8,9
For a long time, opioids have played an important role in perioperative analgesia. However, several studies have reported that they might cause deterioration of cell-mediated immunity and promote the development of tumours.10–12 Previous studies have shown that the appropriate choice of anaesthesia methods could reduce the stress response and protect the immune system, which would facilitate recovery from surgery.13,14 Although general anaesthesia is the most frequently approach to the surgery, it causes higher levels of inflammation and stronger immunosuppressive effects than regional anaesthesia.15 Studies have demonstrated that regional block analgesia technology, as an important method of multimodal analgesia, could generate efficient analgesic properties, lower the response to surgical stress, and also reduce immunosuppression through afferent nerve blockade. It might also potentially delay postoperative tumour recurrence.16–19
Along with the progress of ultrasound technology, thoracic paravertebral block (TPVB) is increasingly being used for analgesia in thoracic surgery. Moreover, it has many advantages such as providing effective analgesia, alleviating the inflammatory response as well as immunosuppression, stabilizing haemodynamics, reducing side effects, and accelerating postoperative rehabilitation.20–24 Two recent randomized trials investigated the effect of TPVB in breast cancer patients with cancer recurrence as the primary outcome, which shown that neither identified a recurrence benefit from TPVB.25,26 To a certain degree that regional analgesia might preserve host defense against cancer, the benefit is most likely in patients having operations that cause considerable tissue injury and are painful. Lung cancer surgery is far more invasive than breast surgery and thus triggers a far greater stress response, and more opioids are generally needed. In a retrospective cohort study, Lee et al27 study demonstrated that TPVB was not related to lung cancer recurrence, but it had a beneficial effect on overall survival of the patients. However, to our knowledge, no studies have compared the surgical outcomes between ultrasound-guided TPVB combined with general anaesthesia and general anaesthesia alone in VATS lung adenocarcinoma surgery. Here, in this retrospective study, we therefore tested the primary hypothesis that ultrasound-guided TPVB combined with general anaesthesia could improve recurrence-free survival compared with general anesthesia alone with intravenous analgesia in patients having potentially curative lung adenocarcinoma surgery. Secondarily, we tested the hypotheses that ultrasound-guided TPVB combined with general anaesthesia might enhance the postoperative early recovery.
Materials and Methods
Ethical Statement for Collecting Clinical Information
This is a retrospective study, which was approved by the Research Ethics Committee of the Second Hospital of Shandong University (No: KYLL-2021LW-065), registered in the Chinese Clinical Trial Registry (ChiCTR2100050454), and carried out in accordance with the Declaration of Helsinki. The written informed consent was waived because of no damage to patients or conflicts of interest. The confidentiality of patient data was guaranteed according to the requirements of the Ethics Committee.
Study Design and Patient Selection
A total of 195 lung adenocarcinoma patients undergoing elective radical lobectomy via VATS in the second hospital of Shandong University between January 2018 and August 2019 were included in this study. The data were extracted from the clinical electronic information system, patients and/or their family members who were familiar with the patients’ information were contacted by phone, and then the patients were divided into two groups according to the anaesthesia type: TPVB combined with general anaesthesia group (group TG, n=86) and general anaesthesia alone group (group GA, n=109). Inclusion criteria were: (1) ages 18 to 80 years; American Society of Anesthesiologists (ASA) grade I~III; (2) the diagnosis of lung adenocarcinoma (I~III A) by postoperative pathology; (3) the same surgical technique applied to all patients (all patients had undergone lobectomy and/or lymph node dissection by 2-VATS, which consisted of both surgical incision and observation incision. A 3~4 cm incision was made along the anterior axillary line in the 4th intercostal space to create the utility port, and another 1 cm incision was made along the midaxillary line in the 7th intercostal interspace for the observation port; (4) general anaesthesia alone or combined with TPVB was applied in all operations; (5) all anaesthesia and surgical management were carried out by the same team; (6) patients signed the informed consent for 48-h patient-controlled intravenous analgesia (PCIA), and sufentanil was used for emergency analgesia if PCIA could not be achieved satisfactorily. Exclusion criteria were: (1) severe neurological conditions (eg preoperative cognitive dysfunction), severe cardiopulmonary dysfunction, hepatic renal disease (Child-Pugh grade C and serum creatinine greater than 442 mol/L), autoimmune diseases, organ replacement therapy, haematological system disease, preoperative infectious diseases; (2) distant metastasis, other malignant tumours; (3) history of previous lung surgery, preoperative chemoradiotherapy or immune therapy, use of glucocorticoid or opioid analgesics within 6 months; (4) history of surgery/anaesthesia within 6 months; (5) contraindications or complications in response to PVB in group TG, including coagulation dysfunction, local infection, and local anaesthetic toxicity; (6) blood transfusion therapy or any allergic reaction during hospitalization; (7) conversion to thoracotomy; (8) failure to complete data collection and follow-up; (9) other serious accidents and complications during hospitalization (eg cardiac failure, respiratory failure, reoperation for postoperative bleeding, pulmonary embolism.).
Anaesthesia Methods
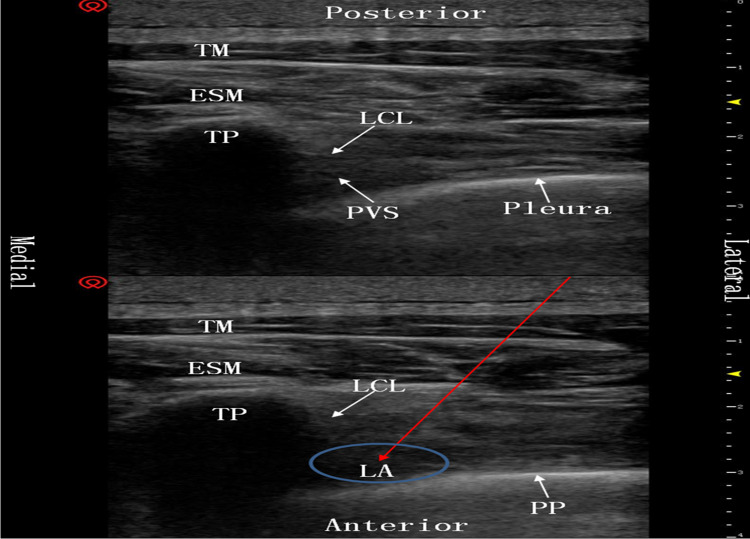
All patients were regularly monitored by electrocardiography (ECG), pulse oxygen saturation (SPO2), invasive blood pressure (IBP), end-tidal carbon dioxide (ETCO2) concentration, body temperature and bispectral index (BIS) values. Before induction of general anaesthesia, the unilateral TPVB20 was performed under ultrasonic guidance in group TG. Patients were placed in the lateral position, and a high-frequency linear array probe (11MHz, Vivid S70N, GE Healthcare) was applied in a parasagittal orientation to distinguish the intended transverse processes (T4 and T7). After identifying the pleura and superior costotransverse ligament, a 21 G, 100 mm needle (Facet NanoLine) was advanced slowly with an in-plane technique from a lateral to medial direction, until the tip punctured the superior costotransverse ligament and entered the paravertebral space. Then, the position of the needle tip was confirmed by hydrodissection. Following confirmation, 10 mL of 0.375% ropivacaine solution was injected, and depression of the pleura was clearly seen on the ultrasound image (Figure 1). After 10–30 min of injection, if the local blocks were satisfactory, general anaesthesia was performed in group TG. Both patient groups were given double-lumen endotracheal intubation anaesthesia (one-lung ventilation was implemented, as needed) with propofol, sevoflurane, sufentanil, and rocuronium bromide, and 48-h PCIA was performed immediately after surgery. The formula was: sufentanil 2 μg/kg and tropisetron 5 mg diluted to 100 mL with normal saline, the background dose was 2 mL/h, initial load was 5 mL, locking time was 15 min, and additional amount was 0.5 mL.
Figure 1.
Ultrasound image of the thoracic paravertebral block. The red arrow denotes the puncture path of the needle. Compared with the picture above, the pleura in the picture below is being pressed down by the local anaesthetics.
Abbreviations: TM, trapezius muscle; ESM, erector spinae muscle; LCL, lateral costotransverse ligamentum; TP, transverse process; PVS, paravertebral space; LA, local anaesthetics; PP, pleura is pressed down by local anaesthetics.
Outcomes
The primary outcome was the recurrence-free survival at postoperative year 2, which was defined as the time between surgery and the earliest date of recurrence, metastasis or lung cancer-cause death, whichever came first.
(2) The secondary outcomes were as follows: I. The average numerical rating scale (NRS) scores (from 0 to 10: 0 as painless, 10 as extremely painful) within 48 h after surgery. II. The recovery of the patients, including the first time of postoperative ambulation, duration of chest tube drainage, and length of postoperative hospitalization. III. Opioid analgesics used during intraoperative and postoperative 48-h sufentanil dosage. IV. The side effects within 48 h after surgery, including lethargy, postoperative nausea and vomiting (PONV), itching, urinary retention, hypotension and bradycardia.
Statistical Analysis
All statistical analyses were performed using SPSS 23.0 software (IBM Corp, Armonk, NY, USA). Quantitative variables were presented as mean ± standard deviation (SD), and qualitative variables were presented as numbers (proportion). The independent-sample t-test, Mann–Whitney U-test, chi-square (χ2) test and Fisher’s exact test were applied to analyse the differences between groups according to the type of data. Recurrence-free survival at postoperative year 2 was analysed using a Kaplan–Meier estimator with differences between groups assessed by Log rank test. A Cox proportional hazard model was used to analyse the factors predetermined according to clinical importance and included sex, age, smoking status, ASA, intraoperative and postoperative sufentanil dosage, tumour stage, treatment regime. Effect size was expressed as hazard ratio and 95% CI. The interactions between treatment effect and predefined factors as above were assessed separately with Cox proportional hazard models. All comparisons were two-sided and differences were regarded as statistically significant (P<0.05).
Results
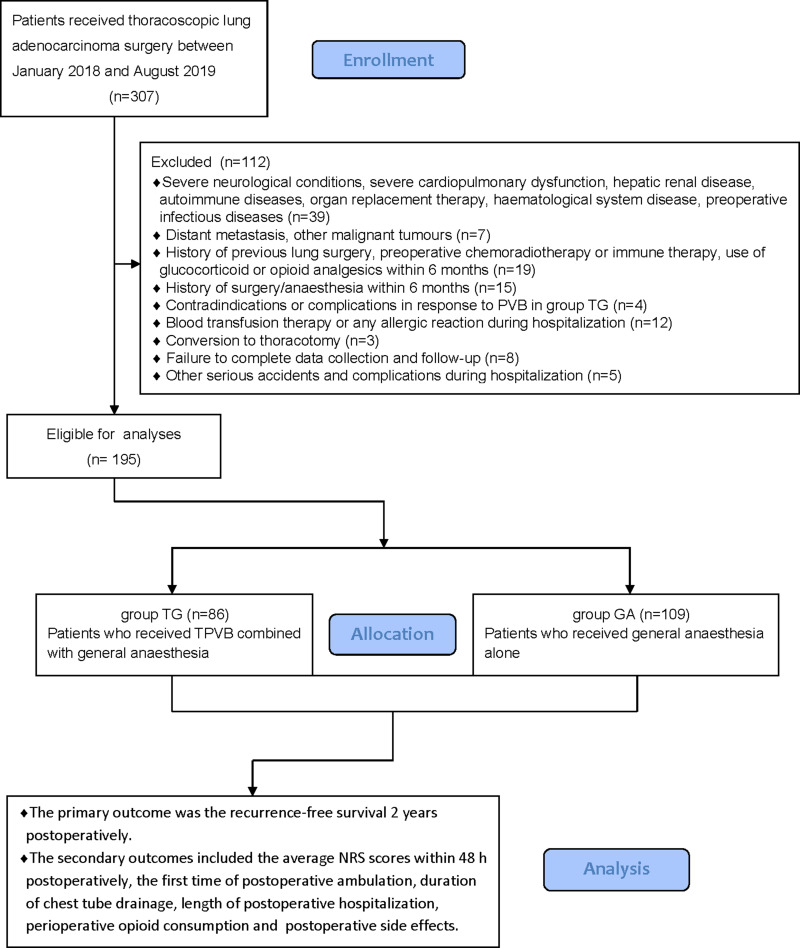
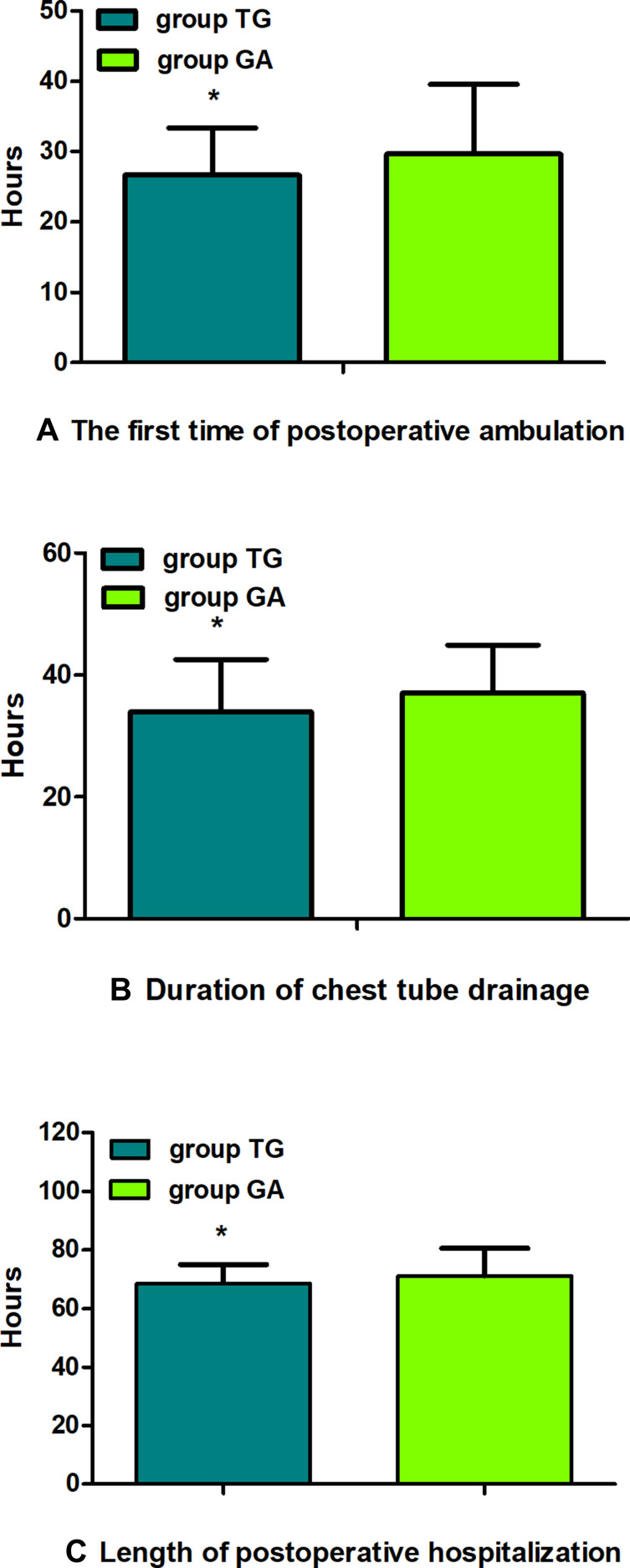
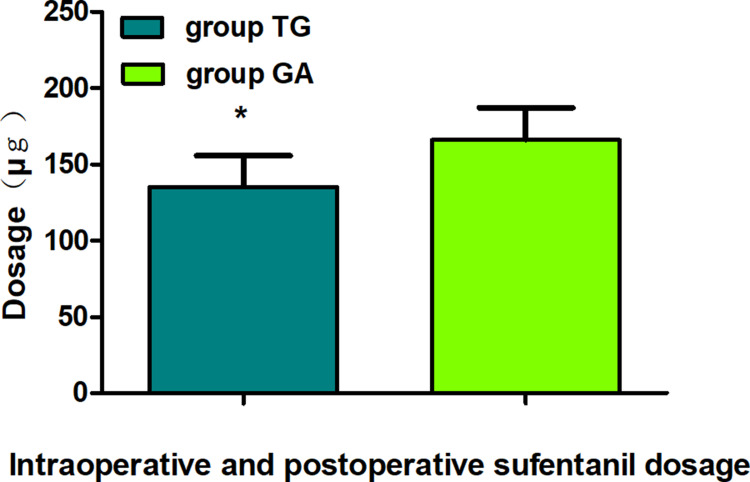
Eventually, between January 02, 2018, and August 30, 2019, a total of 195 patients with thoracoscopic lung adenocarcinoma surgery were enrolled, including 86 patients in group TG and 109 patients in group GA who met the criteria for this study (Figure 2). Baseline characteristics were generally comparable in the two groups. The patients in both the groups did not differ significantly in terms of sex, age, weight, body mass index (BMI), smoking status, ASA, PaO2 (partial pressure of arterial oxygen), PaCO2 (partial pressure of arterial carbon dioxide), preoperative haemoglobin level, sevoflurane, propofol, duration of anaesthesia, duration of surgery, intraoperative blood loss, urine volume, type of lobectomy, tumour stage, or treatment regime (P>0.05, Table 1). There were no statistically significant or clinically meaningful differences in recurrence-free survival at postoperative year 2 between groups (Multivariate hazard ratio 0.706, 95% CI 0.126–3.941, P=0.691, Figure 3). We also conducted the Cox regression analyses for the total study to determine clinically significant risk factors for lung adenocarcinoma recurrence. As a result, the tumour stage (III) and treatment regime (other treatment) were found to be associated with higher risks of the cancer recurrence (Table 2).The average NRS score within 48 h postoperatively in group TG was significantly lower compared with that of group GA (P<0.05, Figure 4). Regarding postoperative early recovery, the first time of postoperative ambulation, duration of chest tube drainage, and length of postoperative hospitalization in group TG were significantly reduced compared with those in group GA (P<0.05, Figure 5). The dosage of intraoperative and postoperative sufentanil in group TG was significantly lower than in group GA (P<0.01, Figure 6). The group TG had a significantly lower incidence of PONV (P<0.05, Table 3). In addition, no significant differences were noted in other side effects between the two groups (P>0.05, Table 3).
Figure 2.
Flow diagram of the study.
Table 1.
Comparison of Demographic Data and Characteristics in the Two Groups
| Characteristics | Group TP (n=86) | Group GA (n=109) | P value |
|---|---|---|---|
| Age, (year) | 57.6±8.7 | 60.1±9.8 | 0.06# |
| Sex, n (%) | |||
| Male | 51 (59.3) | 57 (52.3) | 0.33£ |
| Female | 35 (40.7) | 52 (47.7) | |
| Weight (kg) | 65.2±11.2 | 63.0±10.6 | 0.16# |
| BMI (kg/m2) | 23.1±1.4 | 22.8±1.1 | 0.07# |
| Smoking status, n (%) | 26(30.2) | 37(33.9) | 0.65£ |
| ASA grade, n (%) | |||
| I | 12 (14.0) | 11 (10.1) | 0.67£ |
| II | 66 (76.7) | 89 (81.6) | |
| III | 8 (9.3) | 9 (8.3) | |
| PaO2 (mmHg) | 86.6±7.9 | 84.6±8.4 | 0.10# |
| PaCO2 (mmHg) | 41.3±5.6 | 42.5±4.9 | 0.12# |
| Haemoglobin (g/L) | 134.8±18.3 | 133.1±18.6 | 0.52# |
| Sevoflurane (mL) | 42.1±6.5 | 43.3±5.9 | 0.18# |
| Propofol (mg) | 906.7±213.9 | 901.1±198.0 | 0.85# |
| Duration of anaesthesia (min) | 166.1 ± 22.3 | 171.3 ± 23.1 | 0.12# |
| Duration of surgery (min) | 134.9 ± 22.2 | 138.7 ± 19.1 | 0.19# |
| Intraoperative blood loss (mL) | 69.7±23.1 | 73.6±27.6 | 0.29# |
| Urine volume (mL) | 123.6±26.8 | 118.7±24.2 | 0.19# |
| Type of lobectomy, n (%) | |||
| Left upper | 15 (17.4) | 21 (19.3) | 0.51£ |
| Left lower | 12 (14.0) | 17 (15.6) | |
| Right upper | 20 (23.3) | 29 (26.6) | |
| Right middle | 14 (16.3) | 22 (20.2) | |
| Right lower | 25 (29.0) | 20 (18.3) | |
| Tumour stage, n (%) | |||
| I | 61 (70.9) | 70 (64.2) | 0.36£ |
| II | 15 (17.5) | 18 (16.5) | |
| III A | 10 (11.6) | 21 (19.3) | |
| Treatment regime, n (%) | |||
| Surgery only | 57 (66.3) | 75 (68.8) | 0.58Δ |
| Surgery &chemotherapy | 16(18.6) | 13 (11.9) | |
| Surgery &radiation | 1 (1.2) | 2 (1.8) | |
| Surgery&chemotherapy and radiation | 0 | 0 | |
| Other treatment | 12 (14.0) | 19 (17.4) |
Notes: The data are given as mean ± SD or n (%). Quantitative variables were compared by #independent-sample t; Qualitative variables were compared by £Chi-square (χ2) or ΔFisher’s exact test.
Abbreviations: TG, thoracic paravertebral block combined with general anaesthesia alone; GA, general anaesthesia alone; BMI, body mass index; ASA, American Society of Anesthesiologists; PaO2, partial pressure of arterial oxygen; PaCO2, partial pressure of arterial carbon dioxide; SD, standard deviation.
Figure 3.
Comparison of Kaplan–Meier survival curves for the 2-year postoperative recurrence-free survival between groups. There were no statistical differences in the 2-year postoperative recurrence-free survival between groups (multivariate hazard ratio 0.706, 95% CI 0.126–3.941, P=0.691).
Abbreviations: group TG, thoracic paravertebral block combined with general anaesthesia; group GA, general anaesthesia alone.
Table 2.
Cox Regression Analysis for Recurrence-Free Survival at Postoperative Year 2
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P value | HR | 95% CI | P value |
| Type of anaesthesia | ||||||
| GA | Reference | Reference | ||||
| TG | 0.702 | 0.235–2.096 | 0.527 | 0.706 | 0.126–3.941 | 0.691 |
| Tumour stage | ||||||
| I | Reference | Reference | ||||
| II | 0.851 | 0.184–3.940 | 0.837 | 0.136 | 0.015–1.743 | 0.077 |
| III | 1.467 | 0.397–5.419 | 0.566 | 0.022 | 0.001–0.734 | 0.033 |
| Treatment regime | ||||||
| Surgery only | Reference | Reference | ||||
| Surgery & chemotherapy | 0.979 | 0.212–4.532 | 0.978 | 5.372 | 0.438–65.935 | 0.189 |
| Surgery & radiation | 5.394 | 0.682–42.657 | 0.110 | 17.585 | 0.641–6482.186 | 0.080 |
| Other treatment | 0.960 | 0.207–4.443 | 0.958 | 159.206 | 1.953–12,377.670 | 0.024 |
| ASA status | ||||||
| I | Reference | Reference | ||||
| II | 0.856 | 0.288–2.548 | 0.780 | 1.207 | 0.119–12.236 | 0.874 |
| III | 0.722 | 0.089–5.866 | 0.760 | 1.170 | 0.119–11.507 | 0.893 |
| Sex | ||||||
| Male | Reference | Reference | ||||
| Female | 0.576 | 0.200–1.660 | 0.307 | 0.682 | 0.183–2.545 | 0.569 |
| Smoking status | ||||||
| Yes | Reference | Reference | ||||
| No | 0.838 | 0.263–2.670 | 0.764 | 0.682 | 0.183–2.545 | 0.569 |
| Age, yr | ||||||
| Age <60 | Reference | Reference | ||||
| Age ≥60 | 1.154 | 0.400–3.326 | 0.791 | 1.243 | 0.374–4.131 | 0.722 |
| IPSD | 1.016 | 0.996–1.036 | 0.122 | 1.018 | 0.990–1.047 | 0.192 |
Notes: Only the clinically meaningful clinicopathologic and perioperative variables were included in the univariate and multivariate ananlysis, which were analysed by Cox proportional hazards regression. Associations with P < 0.05 were considered statistically significant.
Abbreviations: TG, thoracic paravertebral block combined with general anaesthesia alone; GA, general anaesthesia alone; ASA, American Society of Anesthesiologists; HR, hazard ratio; CI, confidence interval; IPSD, intraoperative and postoperative sufentanil dosage.
Figure 4.
Comparison of the average pain scores 48 h postoperatively between groups. The data are given as mean ± SD, compared with group GA, *P<0.05. Data were compared by independent-sample t-test.
Abbreviations: group TG, thoracic paravertebral block combined with general anaesthesia; group GA, general anaesthesia alone; NRS, numeric rating scale.
Figure 5.
Comparison of postoperative early recovery between groups. The first time of postoperative ambulation (A), the duration of chest tube drainage (B), the length of postoperative hospitalization (C). The data are given as mean ± SD, compared with group GA, *P<0.05. Data were compared by independent-sample t-test.
Abbreviations: group TG, thoracic paravertebral block combined with general anaesthesia; group GA, general anaesthesia alone.
Figure 6.
Comparison of intraoperative and postoperative sufentanil dosage between groups. The data are given as mean ± SD, compared with group GA, *P<0.01. Data were compared by independent-sample t-test.
Abbreviations: group TG, thoracic paravertebral block combined with general anaesthesia; group GA, general anaesthesia alone.
Table 3.
Comparison of Side Effects After Operation (n (%))
| Indicators | Group TG (n=86) | Group GA (n=109) | P value |
|---|---|---|---|
| Drowsiness | 2 (2.3%) | 5 (4.6%) | 0.47 |
| PONV | 3 (3.5%)* | 13 (11.9%) | 0.03 |
| Itching | 1 (1.1%) | 7 (6.9%) | 0.08 |
| Respiratory depression | 1 (1.1%) | 4 (3.4%) | 0.39 |
| Urinary retention | 0 | 5 (10.3%) | 0.07 |
| Hypotension | 0 | 2 (3.4%) | 0.50 |
| Bradycardia | 0 | 1 (%) | 1.00 |
Notes: The data are given as n (%). Qualitative variables were compared by Fisher’s exact test. Compared with group GA, *P<0.05.
Abbreviations: TG, thoracic paravertebral block combined with general anaesthesia alone; GA, general anaesthesia alone; PONV, postoperative nausea and vomiting.
Discussion
The most important findings of this study were that the ultrasound-guided TPVB in thoracoscopic lung adenocarcinoma surgery did not improve the recurrence-free survival at postoperative year 2, but it significantly enhanced the postoperative analgesia effect, reduced opioid consumption as well as side effects, and accelerated postoperative early recovery.
Surgical resection is the preferred technique for lung adenocarcinoma treatment, yet surgical trauma can lead to a range of systemic metabolic, inflammatory, and neuroendocrine responses. As a result, the immune surveillance function of patients is suppressed, which may promote cancer progression after surgery.28 It was recently reported that besides surgical trauma, various perioperative factors can influence the anti-tumour immune response and are linked with cancer recurrence. Among these factors, anaesthesia methods are receiving increasing attention.29 According to the current relevant literature, regional anaesthesia influences cancer recurrence, metastasis and survival, generally through three main mechanisms: abrogation of the adrenergic-inflammatory response to surgical insult and systemic effects of local anaesthetic agents and/or opioid-sparing analgesia. However these proposals remain controversial.6,10,30 Although some observational studies in cancer patients revealed beneficial effects of regional anaesthesia, most others did not.31,32 In a retrospective 12-year follow-up after breast cancer surgery, Kairaluoma et al33 demonstrated that TPVB did not improve the prognosis of patients with breast cancer. A 5-year follow-up of a randomized controlled trial by Karmakar et al26 suggested that TPVB had little to no appreciable effect on local recurrence, metastasis or mortality after breast cancer surgery. Furthermore, in a recent randomized trial, Xu et al7 reported that the overall survival was comparable in 400 lung patients undergoing pulmonectomy with combined epidural–general anaesthesia or general anaesthesia alone. Sessler et al34 showed the regional anaesthesia-analgesia (paravertebral block) did not reduce breast cancer recurrence after potentially curative surgery compared with volatile anaesthesia (sevoflurane) and opioids in a randomized controlled trial. However, a retrospective analysis by Exadaktylos et al35 suggested that TPVB and postoperative analgesia can reduce the risk of recurrence and metastasis in breast cancer patients during the initial years of follow-up after mammectomy. In a randomized, controlled clinical trial, Chu et al6 demonstrated that TPVB attenuated matrix metalloproteinase-9 (MMP-9) response to VATS lobectomy, which may be beneficial for patients by reducing postoperative tumour recurrence. To date, there are few studies on the effect of TPVB on lung adenocarcinoma, nevertheless our outcome firstly showed that TPVB had no effect on the recurrence-free survival at postoperative year 2 in patients with lung adenocarcinoma. The most likely factors are as follows: shorter surgery time, lower stress response, fewer opioids, relatively stable perioperative immune function, earlier pathological stage and shorter follow-up time.
VATS is a commonly performed minimally invasive procedure that has helped to reduce levels of pain, but pain remains an issue associated with VATS, especially for the first 3 days after surgery, and it requires analgesia that blocks both visceral and somatic nerve fibres for more effective pain control so as to improve postoperative outcome.36–38 It has been reported that regional techniques combined with general anaesthesia provide better perioperative analgesia by completing the somatosensorial and sympathetic block and have an opioid analgesic sparing effect.39 The results of our study suggest that the average NRS score within 48 h postoperatively in group TG was significantly lower than that in group GA (P<0.05), which is consistent with the results of Qiu et al40 who found that TPVB provided analgesia that was superior to general anaesthesia alone during the initial 2 h after VATS in a noninferiority randomized trial. Our results are also similar to those from a study by Chu et al6 who demonstrated that TPVB could provide statistically better pain relief in VATS lobectomy in a randomized clinical trial. What is more, Turhanet al.37 reported that TPVB appeared to be the preferable method with more successful analgesia of the erector spinae plane block (ESPB) and intercostal nerve block (ICNB) in thoracoscopic surgery.
There are a variety of factors affecting the speed of early postoperative recovery, including the extent of surgical trauma, postoperative analgesia, early postoperative activities, duration of chest tube drainage, and length of postoperative hospitalization.41 Our findings showed that these parameters (the first time of postoperative ambulation, duration of chest tube drainage, and length of postoperative hospitalization) were significantly reduced in group TG in comparison with those in group GA (P<0.05). The results are similar to those of Ma et al42 who demonstrated shorter drainage duration and reduced postoperative hospital stay in the TPVB group versus the control group after VATS. A randomized, double-blind study by Kang et al22 suggested that TPVB could significantly promote postoperative early rehabilitation in patients who received thoracoscopic radical lung cancer surgery compared with GA. In a randomized trial, Zhang et al43 study revealed that TPVB could enhance recovery, protect against independent lung injury and cellular immunity. In other words, this is in line with the concept of enhanced recovery after surgery (ERAS) to restore preoperative organ function, reduce perioperative stress response, and as a consequence, accelerate recovery.
The dosage of intraoperative and postoperative sufentanil in group TG was significantly lower than in group GA (P<0.01). This indicated that TPVB could significantly reduce perioperative pain, which was consistent with the results of Qiu et al40 who found that PTVB reduced the use of sufentanil during surgery by about 30%, and that patients required smaller amounts of rescue drugs within 24 h after operation. Zhang et al43 demonstrated that the reduced consumption of remifentanil occurred in response to TPVB in patients undergoing pulmonary surgery in a randomized trial. In addition, Turhanet al.37 reported that TPVB appeared to lower the morphine requirement in contrast to ICNB or ESPB in thoracoscopic surgery.
Surgical factors, residual anaesthetics and postoperative analgesics may cause adverse reactions, such as drowsiness, PONV, itching, urinary retention, bradycardia and hypotension. The results showed that the incidence of adverse reactions (PONV) after surgery in group TG was significantly lower than those in group GA (P<0.05), which was consistent with previous studies.40,44,45 All these results implied that the TPVB and the opioid were secure.
There were several limitations in this study. Firstly, this study was retrospective and from a single centre; incomplete documentation and recall bias regarding history added to the complexity of the study, and a multicentre randomized prospective trial should be conducted for further validation of these results. Secondly, the follow-up period of postoperative analgesic efficacy was only 48h, and a longer follow-up period is needed to validate the findings. Thirdly, very few patients might undergo lobectomy and segmental (wedge) resection simultaneously, which probably affects the accuracy of the outcomes. Finally, since our conclusion was based on a single centre study, a multi-centre study with more cases might further confirm the validity of our results.
Conclusions
This retrospective study demonstrated that TPVB for thoracoscopic lung adenocarcinoma surgery did not improve the recurrence-free survival at postoperative year 2 compared with general anaesthesia alone, but it significantly enhanced the postoperative analgesia effect, reduced opioid consumption as well as side effects, and accelerated postoperative early recovery.
Acknowledgments
The authors thank all the research assistants and patients for their time and efforts in this retrospective study. Additionally, we thank Professor Liyuan Liu for her help in statistics.
Funding Statement
This work was supported by the scientific research fund of Shandong Medical Association under Grant No. YXH2020ZX021. This study was also supported by clinical medicine science and technology innovation plan of Jinan Science and Technology Bureau, Shandong Province, China (No. 202019127). Additional, This research was supported by the cultivation fund of the second hospital of Shandong University, China (No. 2022YP08).
Data Sharing Statement
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declared no conflicts of interest for this work and no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Tang D, Luo H, Xie A, et al. Silencing LMNB1 contributes to the suppression of lung adenocarcinoma development. Cancer Manag Res. 2021;13:2633–2642. doi: 10.2147/CMAR.S275874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang ML, How CH, Hung MH, et al. Long-term outcomes after nonintubated versus intubated thoracoscopic lobectomy for clinical stage I non-small cell lung cancer: a propensity-matched analysis. J Formos Med Assoc. 2021;120(11):1949–1956. doi: 10.1016/j.jfma.2021.04.018 [DOI] [PubMed] [Google Scholar]
- 4.Wu FZ, Kuo PL, Huang YL, et al. Differences in lung cancer characteristics and mortality rate between screened and non-screened cohorts. Sci Rep. 2019;9(1):19386. doi: 10.1038/s41598-019-56025-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu FZ, Huang YL, Wu CC, et al. Assessment of selection criteria for low-dose lung screening CT among asian ethnic groups in Taiwan: from mass screening to specific risk-based screening for non-smoker lung cancer. Clin Lung Cancer. 2016;17(5):e45–e56. doi: 10.1016/j.cllc.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 6.Chu H, Dong H, Wang Y, et al. Effects of ultrasound-guided paravertebral block on MMP-9 and postoperative pain in patients undergoing VATS lobectomy: a randomized, controlled clinical trial. BMC Anesthesiol. 2020;20(1):59. doi: 10.1186/s12871-020-00976-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu ZZ, Li HJ, Li MH, et al. Epidural anesthesia-analgesia and recurrence-free survival after lung cancer surgery: a randomized trial. Anesthesiology. 2021;135(3):419–432. doi: 10.1097/ALN.0000000000003873 [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi K, Takagi Y, Aoki S, et al. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann Surg. 2000;232(1):58–65. doi: 10.1097/00000658-200007000-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melamed R, Rosenne E, Shakhar K, et al. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a β-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. 2005;19(2):114–126. doi: 10.1016/j.bbi.2004.07.004 [DOI] [PubMed] [Google Scholar]
- 10.Cata JP, Gottumukkala V, Thakar D, et al. Effects of postoperative epidural analgesia on recurrence-free and overall survival in patients with nonsmall cell lung cancer. J ClinAnesth. 2014;26(1):3–17. doi: 10.1016/j.jclinane.2013.06.007 [DOI] [PubMed] [Google Scholar]
- 11.Feng M, Feng Q, Chen Y, et al. Effect of dezocine on the ratio of Th1/Th2 cytokines in patients receiving postoperative analgesia following laparoscopic radical gastrectomy: a prospective randomised study. Drug Des Devel Ther. 2021;15:2289–2297. doi: 10.2147/DDDT.S306120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tedore T. Regional anaesthesia and analgesia: relationship to cancer recurrence and survival. Br J Anaesth. 2015;115Suppl 2:ii34–45. doi: 10.1093/bja/aev375 [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Sun Y, Chen H, et al. Effects of two different anesthetic methods on cellular immunity of patients after liver cancer resection. J Biol Regul Homeost Agents. 2016;30(4):1099–1106. [PubMed] [Google Scholar]
- 14.Plücker J, Wirsik NM, Ritter AS, et al. Anaesthesia as an influence in tumour progression. Langenbecks Arch Surg. 2021;406(5):1283–1294. doi: 10.1007/s00423-021-02078-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez-Gonzalez O, Cuellar-Guzman LF, Soliz J, et al. Impact of regional anesthesia on recurrence, metastasis, and immune response in breast cancer surgery: a systematic review of the literature. Reg Anesth Pain Med. 2017;42(6):751–756. doi: 10.1097/AAP.0000000000000662 [DOI] [PubMed] [Google Scholar]
- 16.Wahal C, Kumar A, Pyati S. Advances in regional anaesthesia: a review of current practice, newer techniques and outcomes. Indian J Anaesth. 2018;62(2):94–102. doi: 10.4103/ija.IJA_433_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novak-Jankovič V, Markovič-Božič J. Regional anaesthesia in thoracic and abdominal surgery. ActaClin Croat. 2019;58(Suppl 1):96–100. doi: 10.20471/acc.2019.58.s1.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Chang C-L, Lu C-Y, et al. Paravertebral block in regional anesthesia with propofol sedation reduces locoregional recurrence in patients with breast cancer receiving breast conservative surgery compared with volatile inhalational without propofol in general anesthesia. Biomed Pharmacother. 2021;142:111991. doi: 10.1016/j.biopha.2021.111991 [DOI] [PubMed] [Google Scholar]
- 19.Cui X, Zhu C, Chen P, et al. Effect of pectoral nerve block type II under general anesthesia on the immune function of patients with breast cancer. Am J Surg. 2020;220(4):938–944. doi: 10.1016/j.amjsurg.2020.03.008 [DOI] [PubMed] [Google Scholar]
- 20.Chu H, Dong H, Wang Y, et al. Effects of ultrasound-guided paravertebral block on MMP-9 and postoperative pain in patients undergoing VATS lobectomy: a randomized, controlled clinical trial. BMC Anesthesiol. 2020;20(1):59. doi: 10.1186/s12871-020-00976-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang XL, An R, Chen Q, et al. The analgesic effects of thoracic paravertebral block versus thoracic epidural anesthesia after thoracoscopic surgery: a meta-analysis. J Pain Res. 2021;14:815–825. doi: 10.2147/JPR.S299595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang K, Meng X, Li B, et al. Effect of thoracic paravertebral nerve block on the early postoperative rehabilitation in patients undergoing thoracoscopic radical lung cancer surgery. World J SurgOncol. 2020;18(1):298. doi: 10.1186/s12957-020-02071-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niesen AD, Jacob AK, Law LA, et al. Complication rate of ultrasound-guided paravertebral block for breast surgery. Reg Anesth Pain Med. 2020;45(10):813–817. doi: 10.1136/rapm-2020-101402 [DOI] [PubMed] [Google Scholar]
- 24.Wei W, Zheng X, Gu Y, et al. Effect of general anesthesia with thoracic paravertebral block on postoperative delirium in elderly patients undergoing thoracoscopic lobectomy: a randomized-controlled trial. BMC Anesthesiol. 2022;22(1):1. doi: 10.1186/s12871-021-01532-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cata JP, Forget P. Paravertebral block with propofol anaesthesia does not improve survival compared with sevoflurane anaesthesia for breast cancer surgery: independent discussion of a randomised controlled trial. Br J Anaesth. 2020;124(1):19–24. doi: 10.1016/j.bja.2019.09.039 [DOI] [PubMed] [Google Scholar]
- 26.Karmakar MK, Samy W, Lee A, et al. Survival analysis of patients with breast cancer undergoing a modified radical mastectomy with or without a thoracic paravertebral block: a 5-year follow-up of a randomized controlled trial. Anticancer Res. 2017;37(10):5813–5820. doi: 10.21873/anticanres.12024 [DOI] [PubMed] [Google Scholar]
- 27.Lee EK, Ahn HJ, Zo JI, et al. Paravertebral block does not reduce cancer recurrence, but is related to higher overall survival in lung cancer surgery: a retrospective cohort study. Anesth Analg. 2017;125(4):1322–1328. doi: 10.1213/ANE.0000000000002342 [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, Guo K, Sun X, et al. Impact of anesthesia methods on perioperative systemic inflammation and long-term outcomes in patients undergoing surgery for hepatocellular carcinoma: a propensity score-matched analysis. Ann Transl Med. 2021;9(1):49. doi: 10.21037/atm-20-3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiller JG, Perry NJ, Poulogiannis G, et al. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol. 2018;15(4):205–218. doi: 10.1038/nrclinonc.2017.194 [DOI] [PubMed] [Google Scholar]
- 30.Dubowitz J, Hiller J, Riedel B. Anesthetic technique and cancer surgery outcomes. Curr Opin Anaesthesiol. 2021;34(3):317–325. doi: 10.1097/ACO.0000000000001002 [DOI] [PubMed] [Google Scholar]
- 31.Lusty AJ, Hosier GW, Koti M, et al. Anesthetic technique and oncological outcomes in urology: a clinical practice review. Urol Oncol. 2019;37(12):845–852. doi: 10.1016/j.urolonc.2019.08.004 [DOI] [PubMed] [Google Scholar]
- 32.Cata JP. Outcomes of regional anesthesia in cancer patients. Curr Opin Anaesthesiol. 2018;31(5):593–600. doi: 10.1097/ACO.0000000000000636 [DOI] [PubMed] [Google Scholar]
- 33.Kairaluoma P, Mattson J, Heikkilä P, et al. Perioperative Paravertebral Regional Anaesthesia and Breast Cancer Recurrence. Anticancer Res. 2016;36(1):415–418. [PubMed] [Google Scholar]
- 34.Sessler DI, Pei L, Huang Y, et al. Recurrence of breast cancer after regional or general anaesthesia: a randomised controlled trial. The Lancet. 2019;394(10211):1807–1815. doi: 10.1016/S0140-6736(19)32313-X [DOI] [PubMed] [Google Scholar]
- 35.Exadaktylos AK, Buggy DJ, Moriarty DC, et al. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105(4):660–664. doi: 10.1097/00000542-200610000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shim JG, Ryu KH, Kim PO, et al. Evaluation of ultrasound-guided erector spinae plane block for postoperative management of video-assisted thoracoscopic surgery: a prospective, randomized, controlled clinical trial. J Thorac Dis. 2020;12(8):4174–4182. doi: 10.21037/jtd-20-689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turhan Ö, Sivrikoz N, Sungur Z, et al. Thoracic paravertebral block achieves better pain control than erector spinae plane block and intercostal nerve block in thoracoscopic surgery: a randomized study. J Cardiothorac Vasc Anesth. 2021;35(10):2920–2927. doi: 10.1053/j.jvca.2020.11.034 [DOI] [PubMed] [Google Scholar]
- 38.Wu Z, Fang S, Wang Q, et al. Patient-controlled paravertebral block for video-assisted thoracic surgery: a randomized trial. Ann Thorac Surg. 2018;106(3):888–894. doi: 10.1016/j.athoracsur.2018.04.036 [DOI] [PubMed] [Google Scholar]
- 39.Mogahed MM, Elkahwagy MS. Paravertebral block versus intercostal nerve block in non-intubated uniportalvideo-assisted thoracoscopic surgery: a randomised controlled trial. Heart Lung Circ. 2020;29(5):800–807. doi: 10.1016/j.hlc.2019.04.013 [DOI] [PubMed] [Google Scholar]
- 40.Qiu Y, Wu J, Huang Q, et al. Acute pain after serratus anterior plane or thoracic paravertebral blocks for video-assisted thoracoscopic surgery: a noninferiority randomised trial. Eur J Anaesthesiol. 2021;38(Suppl 2):S97–S105. doi: 10.1097/EJA.0000000000001450 [DOI] [PubMed] [Google Scholar]
- 41.Cavallaro P, Bordeianou L. Implementation of an ERAS pathway in colorectal surgery. Clin Colon Rectal Surg. 2019;32(2):102–108. doi: 10.1055/s-0038-1676474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma H, Song X, Li J, et al. Postoperative pain control with continuous paravertebral nerve block and intercostal nerve block after two-port video-assisted thoracic surgery. Wideochir Inne Tech Maloinwazyjne. 2021;16(1):273–281. doi: 10.5114/wiitm.2020.99349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang W, Cong X, Zhang L, et al. Effects of thoracic nerve block on perioperative lung injury, immune function, and recovery after thoracic surgery. Clin Transl Med. 2020;10(3):e38. doi: 10.1002/ctm2.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu L, Xu X, Tian H, et al. Effect of single-injection thoracic paravertebral block via the intrathoracic approach for analgesia after single-port video-assisted thoracoscopic lung wedge resection: a randomized controlled trial. Pain Ther. 2021;10(1):433–442. doi: 10.1007/s40122-020-00231-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Z, Fang S, Wang Q, et al. Patient-controlled paravertebral block for video-assisted thoracic surgery: a randomized trial. Ann Thorac Surg. 2018;106(3):888–894. doi: 10.1016/j.athoracsur.2018.04.036 [DOI] [PubMed] [Google Scholar]